Abstract
Families bearing mutations in the presenilin 1 (PS1) gene develop Alzheimer’s disease. Previous studies have shown that the Alzheimer-associated mutations in PS1 increase production of amyloid β protein (Aβ1–42). We now show that PS1 also regulates phosphorylation of the microtubule-associated protein tau. PS1 directly binds tau and a tau kinase, glycogen synthase kinase 3β (GSK-3β). Deletion studies show that both tau and GSK-3β bind to the same region of PS1, residues 250–298, whereas the binding domain on tau is the microtubule-binding repeat region. The ability of PS1 to bring tau and GSK-3β into close proximity suggests that PS1 may regulate the interaction of tau with GSK-3β. Mutations in PS1 that cause Alzheimer’s disease increase the ability of PS1 to bind GSK-3β and, correspondingly, increase its tau-directed kinase activity. We propose that the increased association of GSK-3β with mutant PS1 leads to increased phosphorylation of tau.
The neuropathological diagnosis of Alzheimer’s disease (AD) requires the presence of both senile plaques and neurofibrillary tangles (NFT) (1). Senile plaques are largely composed of amyloid β protein (Aβ), whereas NFT are composed of hyperphosphorylated tau organized into filamentous structures termed paired helical filaments (2–4). Mutations on the presenilin 1 (PS1) gene cause an early onset form of AD with an autosomal dominant inheritance pattern (5–7). The role of PS1 in AD is particularly interesting because it has a strong causal relationship to the disease; genetic studies show that mutations for PS1 exhibit 100% penetrance in causing AD (8). Although, the mechanism through which PS1 causes AD is unclear. Mutations in presenilins affect Aβ processing. Recent studies indicate that cell lines, transgenic mice, or patients expressing mutant forms of PS1 show a selective increase in production of Aβ1–42 (9–12). Mutations in the presenilins also activate apoptotic pathways and render neurons more vulnerable to stressors, such as Aβ neurotoxicity (13–16). The ability of PS1 to potentiate Aβ toxicity raises the possibility that PS1 interacts with glycogen synthase kinase 3β (GSK-3β), which we previously have shown to be involved in Aβ-mediated cell death (17–20). The enzyme GSK-3β also has been implicated in AD because this kinase is one of a group of proline-directed kinases that can phosphorylate the microtubule-associated protein tau, to generate a precursor to NFTs, termed paired helical filaments-tau (21, 22). PS1 (23–26) and GSK-3β (27, 28) can be found in association with NFTs in the Alzheimer brain, which further suggests that there may be a physiological connection between PS1, GSK-3β, and tau. To pursue these intriguing connections, we investigated whether PS1 might directly associate with GSK-3β and tau.
MATERIALS AND METHODS
Preparation of Brain Samples.
Human brain cortex was obtained at autopsy from patients ranging in age from 44 to 88 years old. Twenty-one samples, from donors age 44–79, showed no evidence of neurological disorders, whereas two samples, from donors ages 81 and 88, showed neuropathological and clinical evidence of AD. The brain samples were homogenized in Tris-buffered saline (TBS) with a teflon glass dounce. TBS consists of 50 mM Tris⋅HCl, pH 7.4, 150 mM NaCl, plus protease and phosphatase inhibitors (1 mM phenylmethylsulfonyl fluoride/1 μg/ml each of leupeptin, pepstatin, and aprotinin/5 μM okadaic acid/0.1 mM sodium orthovanadate). The homogenates were centrifuged at 12,000 × g; the pellets were extracted with 1% Triton X-100 in TBS and recentrifuged at 12,000 × g, and the supernatants were collected. The resulting proteins were separated by 13% SDS/PAGE and transferred to nitrocellulose membranes. Samples were not heated before loading. The membranes were blocked with 10% milk, PBS, and 0.1% Tween 20 and incubated overnight at 4°C in primary antibody diluted in 5% milk and PBS and developed by using peroxidase conjugated secondary antibodies (Zymed) with chemiluminescent detection (ECL, Amersham).
cDNA Constructs.
Mutations were produced by PCR-based site-directed mutagenesis using wild-type PS1 lacking the VRSQ motif as a template. The human 4 repeat tau cDNA (htau40; 4R) was a gift from M. Goedert, MRC Laboratory of Molecular Biology, Cambridge, U.K. Three repeats (3R), no repeats (ΔR), and NΔR were produced by PCR-based site-directed mutagenesis by using the 4R tau as a template. After subcloning the products into the pCIneo vector (Promega), the sequences were confirmed by sequencing the entire region.
Transfection.
COS-7 cells were cultured in DMEM and 10% fetal bovine serum. For transient expression, 5 × 105 cells were plated on 10-cm2 dishes and grown overnight. Each cDNA (2 μg/ml) was transfected into COS-7 cells by using LipofectAMINE (4 μl/ml; GIBCO/BRL), and the cells were harvested after 48 hr in TBS and 1% Triton X-100. The lysates were then centrifuged at 15,000 rpm at 4°C, the supernatant was collected and used for experiments.
Immunoprecipitation.
For immunoprecipitation, the cell extracts from each transfection were preincubated with protein G Sepharose (Pharmacia) and then incubated with the indicated antibodies at 4°C overnight. The immunocomplexes were precipitated by incubation with protein G Sepharose at room temperature for 2 hr, washed five times in TBS and 1% Triton X-100, and eluted with 0.1 M glycine (pH 2.5). For detection of tau and GSK-3β, the immunoprecipitates were eluted by incubation with Laemli sample buffer at 95°C for 3 min.
Immuno-Affinity Column.
The mAb MKAD3.4 was purified from conditioned medium on protein G (Pharmacia) coupled to CNBr-activated Sepharose 4B (Pharmacia). Triton X-100-extracted brain samples were prepared as stated above and loaded overnight at 4°C. After washing with 10-column volumes TBS and 1% Triton X-100, the proteins were eluted in 0.1 M glycine, pH 2.7 (500 μl/fraction), and the samples were neutralized immediately with 1 M Tris, pH 9.0.
Treatment of Alkaline Phosphatase.
Eluent (47 μg protein/ml) of MKAD3.4 immuno-affinity column was incubated with buffer (10 mM Tris[chem]HCl, pH 8.9/500 μM MgCl) containing alkaline phosphatase (130 units/ml; Takara) at 37°C for 1 hr. The control sample was treated in the same manner while omitting the phosphatase.
RESULTS
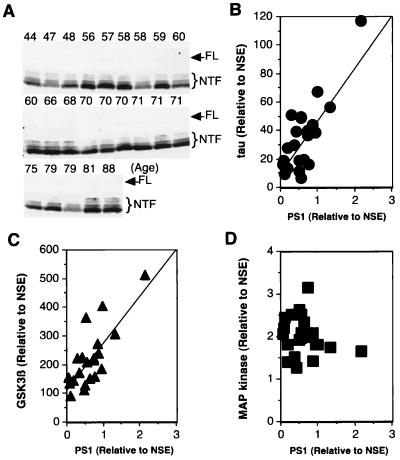
Quantitation of the levels of PS1, tau, and the tau kinase GSK-3β in 23 human brains of varying ages (44- to 88-years-old) suggests that the levels of these three proteins are linked (Fig. 1). The levels of PS1 were quantified by using anti-PS1 antibody, MKAD3.4, which was raised against the N-terminal hydrophilic region of PS1 (29–31). In human brain extracts, this antibody recognizes a faint 48-kDa band, which corresponds to full length PS1, and a strong 28–35 kDa series of bands, which correspond to the N-terminal fragments of PS1 (Fig. 1A). The level of PS1 was determined including full and N-terminal fragments, which normalizing with neuron-specific enorase (NSE) tended to increase with age. We also determined the amount of tau in these same lysates by using a rabbit polyclonal antibody (JM) raised against recombinant human tau, which recognizes both phosphorylated and nonphosphorylated tau. Like PS1, the levels of tau normalizing with NSE also increased with age. Next, we quantified the levels of PS1 and tau, seen in the immunoblots, normalized the values to the levels of NSE in each sample, and then compared the normalized levels of tau and PS1. Compared with these fragments as a positive control, their levels showed a good correlation (correlation coefficiency was >0.9). Plots of tau and PS1 levels also showed good correlation with a coefficiency of 0.8 (Fig. 1B). A similar analysis of GSK-3β, a putative tau kinase (32, 33), also showed good correlation with PS1 levels (Fig. 1C, correlation coefficiency = 0.78). On the other hand, comparison of PS1 levels with those of another kinase that can phosphorylate tau, mitogen-activated protein kinase (MAPK) (34), failed to show any significant correlation (Fig. 1D). The correlations of levels of PS1, tau, and GSK-3β suggest to us a possible physiological link between the proteins.
Figure 1.
Correlation between levels of PS1, tau, GSK-3β, and MAPK in human brains of various ages. The Triton X-100 soluble fraction of brain homogenate (60 μg total protein/lane) was examined by Western analysis using anti-PS1 antibody (MKAD3.4) (1:5), anti-tau antibody (JM) (1:5,000), anti-GSK-3β (Transduction Laboratories, Lexington, KY) (1:1,000), anti-MAPK (Santa Cruz) (1:2,000), and anti-NSE (Dako) (1:500). (A) Western blot using the mAb MKAD3.4. Top of each lane shows age of each person. MKAD3.4 reacts with a faint 47 kDa full-length PS1 (FL) and 28–35 kDa N-terminal fragments (NTF). The reactive band was quantified with a Densitograph Lumino-CCD (ATTO Corporation, Tokyo, Japan). The levels of PS1, tau, GSK-3β, and MAPK were normalized to the level of NSE in each sample, and the levels of tau (B), GSK-3β (C), and MAPK(D) were plotted against the corresponding PS1 levels. There were significant correlations between both PS1 and tau (n = 23, slope of regression line = 41.8, R2 = 0.65, P < 0.0001), as well as GSK-3β (n = 23, slope of regression line = 110.9, R2 = 0.61, P < 0.0001). In contrast, a linear regression showed no significant correlation between PS1 and MAPK (n = 23, slope of regression line = −0.2, R2 = 0.04, P > 0.1).
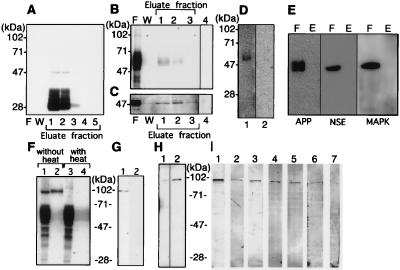
To investigate the interaction of PS1 with tau and GSK-3β in vivo, we investigated whether these proteins could be found in the association with each other during immunoprecipitations. PS1 was immunoprecipitated from Triton X-100 extracts of human brain by using a MKAD3.4 affinity column. PS1 was eluted from the column in fractions 1 and 2, using a buffer containing 0.1 M glycine and HCl (pH 2.5) (Fig. 2A); no PS1 was observed in the flow through or wash (F or W, Fig. 2A). The same fractions were then examined by using the anti-tau antibody, JM (Fig. 2B), or the anti-GSK-3β antibody (Fig. 2C). Although most of the tau and GSK-3β reactivity was in the flow-through, a small amount of reactivity for both proteins coeluted with PS1 (Figs. 2 B and C). The JM reactive bands that coeluted with PS1 showed a set of bands in the 50–70 kDa characteristic of tau (Fig. 2B). The anti-GSK-3β reactivity that coeluted with PS1 showed a 47-kDa band characteristic of GSK-3β (Fig. 2C). Omission of primary antibody from the immunoblots eliminated the bands (Fig. 2 B and C, lane 4). Pre-absorption of the JM antibody with recombinant human tau eliminated all reactivity (Fig. 2D, lane 2). Probing the eluates with an unrelated antibodies, anti-NSE, anti-amyloid protein precursor (APP), and anti-MAPK (Fig. 2E), produced no reaction in eluate fraction, indicating that the tau and GSK-3β reactivity that was present represented genuine associations with PS1. Although Xia W. et al. (35) reported that APP directly bonded to PS1, our experiment didn’t show any immunoreactivity for APP (Fig. 2E). Possibly this result is because the affinity of PS1 with tau, and GSK-3β are higher than that of PS1 with APP. Interestingly, when the eluates were not heated before immunoblotting, the tau reactivity migrated as a 95-kDa band (Fig. 2F), which was absorbed by preincubation of JM with human recombinant tau (Fig. 2G). Omission of the heating results in less stringent denaturing conditions, which might allow tau dimers to remain associated. To confirm that the 95-kDa band was indeed tau protein, we probed unheated eluate from the PS1 column with six different anti-phospho-tau antibodies, PS199, PS202, PS262, PS396, PS404, and PS413 (33, 36). As shown in Fig. 2I, all of these antibodies reacted with the 95-kDa band, and reactivity was eliminated by preabsorption with corresponding antigen peptide (Fig. 2I, lane 7). Although probing the same fractions with a tau antibody, Tau-1, that recognizes only nonphosphorylated tau, yielded only a weak band at 95 kDa (Fig. 2H, lane 1), although pretreatment of these fractions with alkaline phosphatase enhanced the reactivity of this band (Fig. 2H, lane 2). These data indicate that the 95-kDa band represents dimerized tau that is phosphorylated, a transition characteristic of paired helical filaments-tau.
Figure 2.
Analysis of PS1-associated tau and GSK-3β in human brain. Triton X-100 soluble fractions (10 ml) of clinically normal human brain (2 g) was loaded onto a MKAD3.4 immuno-affinity column to purify PS1 and associated proteins. (A) Purification profiles show the flow-through fraction (20 μl) (F), the fraction of last wash (20 μl/total 5 ml) by TBS containing 1% Triton X-100 (W), and purified fraction (20 μl per lane was loaded from total 500 μl eluate) (Eluate). The immuno-detection of each fraction by using MKAD3.4 (1:5) did not show any reactivity in the flow-through or the wash. Eluate fractions 1 and 2 revealed abundant reactivity corresponding to the 47-kDa full length and 28-kDa N-terminal fragment of PS1. JM (1:3,000) was used for immuno-detection of tau (B), and anti-GSK-3β (1:1,000) was used to detect GSK-3β (Transduction Laboratories) (C). Most of the tau and GSK-3β was recovered in the flow through fractions (20 μl). The JM antibody reacted with bands at 50–70 kDa, whereas anti-GSK-3β reacted with a band at 47 kDa. Reactivity for both antibodies was recovered in fractions 1 and 2 (20 μl). Only secondary antibody incubation, as negative control, did not show any visible bands in the eluate fraction 1 (20 μl) (lane 4). (D) The JM reactive band in eluate fraction 1 (20 μl) (lane 1) was eliminated by preabsorption with human recombinant tau (lane 2). No reactivity is seen in lane 2. (E) Eluate fraction 1 (lane E) and flow-through fraction (lane F) were incubated with anti-NSE, anti-APP, and anti-MAPK. No reactivities are seen in eluate fraction. (F) The 95-kDa band in the eluate from fraction 1 (20 μl) could be eliminated by heating before loading on the gel (lane 1, lysate without heating; lane 2, eluate without heating; lane 3, lysate with heating; lane 4, lysate without heating). The heating process appeared to convert the 95-kDa JM-reactive band into a set of JM-reactive bands in 50- to 70-kDa region, which is characteristic of tau. (G) The 95-kDa band (lane 1) in eluate fraction 1 was absorbed away by preabsorption of JM with human recombinant tau (lane 2). (H) The 95-kDa band was weakly reactive with a nonphosphorylation-dependent anti-tau antibody (tau-1; 1:5,000), (20 μl of a 5-fold concentrated eluate was used) (lane 1). Alkaline phosphatase (130 units/ml, Takara Shuzo, Kyoto) treatment of the same sample for 1 hr at 4°C enhanced the immunoreactivity of tau-1 (lane 2). (I) The phosphorylation sites present in the 95-kDa band was determined by using well characterized phosphorylation-dependent anti-tau antibodies (33, 35). The phosphorylation sites on tau (in the numbering of htau40) identified by each antibody correspond to: lane 1, PS199 (1:200), Ser-199; lane 2, PS202 (1:200), Ser-202; lane 3, PS262 (1:200), Ser-262; lane 4, PS396 (1:200), Ser-396; lane 5, PS404 (1:200), Ser-404; and lane 6, PS413 (1:30), Ser-413. Use of PS396 (lane 7) that had been preabsorbed with its corresponding antigen peptide eliminated all reactivity.
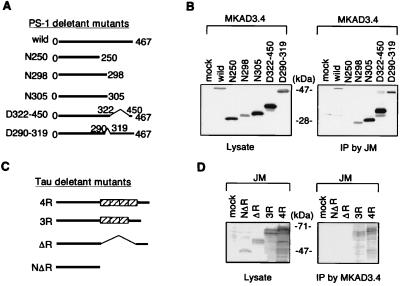
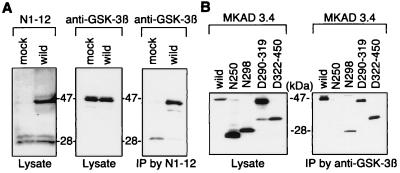
To elucidate the nature of the interaction among PS1, tau, and GSK-3β, we generated a number of PS1 and tau cDNA constructs containing varying deletions (Figs. 3A and C). The N305, N298, and N250 are PS1 constructs that have the C terminus deleted (Fig. 3A). The D290–319 construct contains a deletion corresponding to exon 9 of the PS1 gene and is therefore not cleaved (7, 37), and the D322–450 construct contains a deletion after the PS1 cleavage site (Fig. 3A). COS-7 cells were transfected with each PS1 construct and a construct coding for full length tau (ht40). Tau was then immunoprecipitated by using the anti-human tau antibody JM, and the subsequent immunoblots were probed by using the anti-PS1 antibody, MKAD3.4. All of the PS1 constructs coimmunoprecipitated with tau with the exception of N250 (Fig. 3B). This result indicates that PS1 directly binds tau protein, and the site of the interaction is between residues 250 and 298 of PS1. Next, we analyzed the ability of full length PS1 to immunoprecipitate tau deletion constructs. We generated four different forms of human tau containing 3R, 4R, ΔR, or NΔR (Fig. 3C). Each of these tau constructs were cotransfected with full length PS1 into COS-7 cells; PS1 was immunoprecipitated from the cell lysates with MKAD3.4, and the subsequent immunoblots were probed with the anti-tau antibody JM. The tau proteins containing the repeat domains, 3R and 4R, coprecipitated with PS1, whereas the ΔR and NΔR proteins did not coprecipitate with PS1 (Fig. 3D). These results indicate that the microtubule-binding repeat region in tau protein is necessary for binding to PS1. Finally, we also examined whether PS1 directly binds GSK-3β. Wild type of PS1 constructs was transfected into COS-7 cells, when PS1 was immunoprecipitated from the lysates with the anti-PS1 polyclonal antibody N1–12, and the immunoblots were probed with a mAb directed against GSK-3β. Overexpression of wild-type PS1 did not affect the level of GSK-3β (Fig. 4A). However, GSK-3β did immunoprecipitate with PS1 (Fig. 4A). Full length PS1 as well as N298, D290–319, and D322–450 were all able to bind GSK-3β, but N250 did not bind GSK-3β (Fig. 4B). This result indicates that residues 250–298 are necessary for the association of PS1 with GSK-3β. Thus, tau and GSK-3β both bind to the same region of PS1, residues 250–298. Moreover, the ability of PS1 to bring tau and GSK-3β into close physical proximity suggests that PS1 could play an important role in regulating the phosphorylation of tau by GSK-3β.
Figure 3.
Direct binding of PS1 with tau. (A) Schematic drawing of PS1 deletant mutants. Wild-type PS1 consists of 467 aa (wild). The N250, N298, and N305 deletion constructs have the C terminus of PS1 deleted beyond residue 250, 298, and 305, respectively. The D322–450 and D290–319 deletion constructs contain deletions between residues 322–450 and 290–319, respectively. (B) These wild or mutant PS1 constructs and a full length human tau construct (ht40) were transiently cotransfected into COS-7 cells. The expression of each PS1 was analyzed by using 1/10 volume of total cell lysate (Left). The rest of each cell lysate was used for immunoprecipitation by the anti-tau antibody (JM) and analyzed for formation of immunocomplexes of tau with PS1 deletant mutants (Right). N298, N305, D322–450, and D290–319 coprecipitated with tau, whereas N250 but did not. (C) Schematic drawing of tau deletion mutants, containing ΔR, 3R, or 4R or NΔR. (D) These different forms of tau and human wild-type PS1 were cotransfected in COS-7 cells. The expression of each tau was analyzed by using 1/10 volume of total cell lysate (Left). The rest of each cell lysate was used for immunoprecipitation by the anti-PS1 antibody (MKAD3.4) and analyzed for the formation of immunocomplexes of PS1 with tau deletant mutants (Right). 3R and 4R tau were recovered in the immunocomplexes with PS1, whereas NΔR and ΔR were not recovered.
Figure 4.
Direct binding of PS1 with GSK-3β. (A) Wild-type PS1 was transiently transfected into COS-7. The expression of PS1 and endogenous GSK-3β in cell lysates (1/10 volume of total cell lysate) was confirmed by using rabbit polyclonal anti-PS1 antibody (N1–12) (Left) and mouse monoclonal anti-GSK-3β antibody (1:1,000) (Center). PS1 in mock transfected cells showed a faint 47-kDa full length and 28-kDa N-terminal fragment. PS1 transfection showed the increases the level of full-length PS1, but did not affect the level of GSK-3β. Immunoprecipitation with N1–12 from the cell lysates revealed a faint GSK-3β in mock-transfected cells and the increased levels of GSK-3β in PS1 transfected cells. (B) To determine the binding site of PS1, we used PS1 deletion mutants as shown in Fig. 3A. The mutant PS1 constructs were transiently transfected into COS-7 cells. The expression of PS1 was analyzed by using 1/10 volume of total cell lysate (Left). The remainder of each cell lysate was used for immunoprecipitation with the rabbit polyclonal anti-GSK-3β antibody (C1) and analyzed for the formation of immunocomplexes of GSK-3β with the PS1 deletion constructs (Right). N298, N305, D322–450, and D290–319 were recovered in the immunocomplex of GSK-3β, whereas N250 was not recovered.
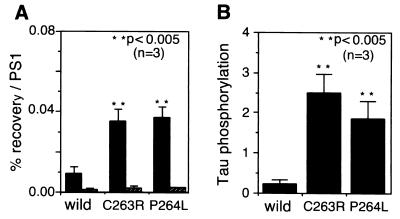
Because the region of 250–298 on PS1 is a “hot spot” for Alzheimer mutations (38), we sought to determine whether the Alzheimer-associated mutations in this region of PS1 might affect the interaction of PS1 with GSK-3β or tau. Each of the PS1 constructs containing point mutations in the 250–298 region, C263R and P264L (8, 38, 39) were cotransfected into COS-7 cells with the full length tau construct. PS1 was immunoprecipitated from the lysates with MKAD3.4 and the immunoblots were probed with either the anti-GSK-3β antibody or the anti-tau antibody JM (Fig. 5A). Interestingly, each of the PS1 mutations increased the PS1/GSK-3β association by more than three times that of the wild-type PS1 association (Fig. 5A). The mutations did not significantly increase the binding to tau, though (Fig. 5A). Next, we sought to determine whether the increased GSK-3β-binding activity of the PS1 mutants might affect the tau-directed kinase activity of GSK-3β. Lysates from the PS1/tau cotransfections were probed with the phospho-tau-specific antibody PS199. Tau lacking interaction with PS1 (ΔR) did not show immunoreactivity for PS199 (data not shown). Interestingly, tau (4R) phosphorylation was greatly increased in the mutant of the C263R and P264L PS1 transfections (Fig. 5B). The results suggest that PS1 mutations may enhance the ability of GSK-3β to phosphorylate tau.
Figure 5.
Effect of PS1 mutants on tau phosphorylation. (A) Constructs coding for FAD-associated mutant forms of PS1 was transfected into COS-7 cells. Ten percent of each lysate was used for immunoblotting with MKAD3.4, and the remainder of each lysate was used for analyzing the coimmunoprecipitation of GSK-3β with PS1, using MKAD3.4. Constructs coding for four repeat tau and FAD-associated mutant forms of PS1 were cotransfected into COS-7 cells. Each lysate was then immunoprecipitated by using MKAD3.4 and immunoblotted by using anti-tau antibody (JM). Recovery of GSK-3β (closed bar) and tau (striped bar) is presented as the percentage (%) of recovery normalized to the corresponding expression levels of PS1 (mean ± SD; n = 3). The lower percentage recovery/PS1 on tau is because of underestimation of the cotransfection results. ∗∗, P < 0.005 (two-tailed t test). (B) Constructs coding for four repeat tau and FAD-associated mutant forms of PS1 were cotransfected into COS-7 cells. Each lysate (30 μg) was then immunoblotted by using the phosphorylation-dependent antibody (PS199). The immunoreactive bands were quantified with a Lumino-CCD Densitograph (Atto). The level of tau phosphorylation is presented as mean ± SD (n = 3), ∗∗, P < 0.005 (two-tailed t test).
DISCUSSION
The functional consequences of presenilin mutations on the biology of the cell are only beginning to be understood. Expression of mutant presenilins increases production of Aβ1–42, which is presumed highly neurotoxic because of its tendency to aggregate (9–12). In this study, we have shown that PS1 binds GSK-3β and its substrate, tau. Small amounts of tau and GSK-3β (<1% of flow through fraction) were coimmunoprecipitated with PS1 in human brain samples, although transfected cells showed ≈10% of GSK-3β and tau bound to PS1. Actually, >10% of tau may bind to PS1 in transfected cells because the amount of tau bound to PS1 (10% of total tau) was estimated from results obtained from cotransfection of tau and PS1. A possible reason of the difference between human brain and transfected cells is that we used postmortem samples. The dissociation if it does occur may be caused by the postmortem condition. Alzheimer-associated mutations in PS1 located in the tau-binding region, residues 250–298, increase the binding to GSK-3β and its tau-directed kinase activity. This increase suggests that PS1 may be acting to control the association of GSK-3β with its substrates, and, by increasing binding of GSK-3β, mutations in PS1 may enhance the ability of GSK-3β to phosphorylate its targets. Irving and Miller (40) have reported that FAD PS1 constructs do not increase tau phosphorylation in COS cells. A discrepancy may be caused by each mutation of PS1 used for transfection. The extent of tau phosphorylation by PS1 mutant may vary according to each mutation.
Recently, it has been reported (35, 41) that PS1 binds to delta-catenin and APP. Both proteins also are substrate for GSK-3β (42, 43). Thus, PS1 may act as a molecular tether, connecting GSK-3β with important substrates, much like the kinase-binding proteins AKAP and RACK1 modulate activity by controlling the association of protein kinases A and C with their substrates (44, 45).
Increasing GSK-3β activity could have important consequences for neurons. One clear consequence is to increase tau phosphorylation and enhance NFT formation. However, GSK-3β also plays an important role in the apoptotic cascades. It is one of the principle targets of the kinase Akt, which mediates the actions of PI3 kinase. Inhibition of PI3 kinase or Akt activity leads to activation of GSK-3β and apoptosis; this mechanism appears to be particularly important in neurons (46, 47). In regulating GSK-3β activity, PS1 could play a critical role in regulating the Akt/GSK-3β apoptotic cascade. Increases in activation of GSK-3β associated with PS1 mutations might render the cell more vulnerable to stressful, pro-apoptotic stimuli, such as neurotoxicity due to the Aβ peptide, which we have already shown to operate as a killer via a mechanism involving PI3 kinase and GSK-3β (48). Interestingly, recent work indicates that PS2 acts as an inhibitor on the apoptotic cascade mediated by c-Jun Kinase and NF-κB (B. Wolozin, personal communication). The Alzheimer-associated mutations reduce PS2 function, leading to enhanced c-Jun kinase responsiveness, which could also render neurons more vulnerable to stressors. Our findings in this study provide an intriguing parallel to the work on PS2 and support the hypothesis that PS1 and PS2 play important roles in regulating cell death pathways. Abnormal regulation of these cascades, caused by mutations in the presenilins, could lead to AD.
Acknowledgments
We are grateful to Dr. M. Ito for his support and to Dr. K. Ishiguro and Ms. C. Park for providing us with the phosphorylation dependent anti-tau antibodies and anti-GSK-3β antibody (C1).
ABBREVIATIONS
- PS1
presenilin 1
- AD
Alzheimer’s disease
- Aβ
amyloid β protein
- GSK-3β
glycogen synthase kinase-3β
- NSE
neuron-specific enorase
- NFT
neurofibrillary tangles
- APP
amyloid protein precursor
- MAPK
mitogen-activated protein kinase
- 3R
three repeats tau
- 4R
four repeats tau
- ΔR
no repeats
- NΔR
C terminus deleted through the repeat region
- TBS
Tris-buffered saline
References
- 1.Selkoe D J. Annu Rev Neurosci. 1994;17:489–517. doi: 10.1146/annurev.ne.17.030194.002421. [DOI] [PubMed] [Google Scholar]
- 2.Ihara Y, Nukina N, Miura R, Ogawara M. J Biochem. 1986;99:1807–1810. doi: 10.1093/oxfordjournals.jbchem.a135662. [DOI] [PubMed] [Google Scholar]
- 3.Grundke-Iqbal I, Iqbal K, Quinlan M, Tung Y C, Zaidi M S, Wisniewski H M. J Biol Chem. 1986;261:6084–6089. [PubMed] [Google Scholar]
- 4.Grundke-Iqbal I, Iqbal K, Tung Y C, Quinlan M, Wisniewski H M, Binder L I. Proc Natl Acad Sci USA. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sherrington R, Rogaev E I, Liang Y, Rogaev E A, Levesque G, Ikeda M, Chi H, Lin C, Li G, Holman K, et al. Nature (London) 1995;375:754–760. doi: 10.1038/376775a0. [DOI] [PubMed] [Google Scholar]
- 6.Levy-Lehad E, Wasco W, Poorkaj P, Romano D M, Oshima J, Pettingell W H, Yu C, Jondro P D, Schmidt S D, Wang K, et al. Science. 1995;269:973–977. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- 7.Alzheimer’s Disease Collaborative Group. Nat Genet. 1995;11:219–222. doi: 10.1038/ng1095-219. [DOI] [PubMed] [Google Scholar]
- 8.Hardy J. Trends Neurosci. 1997;20:154–159. doi: 10.1016/s0166-2236(96)01030-2. [DOI] [PubMed] [Google Scholar]
- 9.Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird T D, Hardy J, Hutton M, Kukull W, et al. Nat Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- 10.Duff K, Eckman C, Zehr C, Yu X, Prada C-M, Perez-tur J, Hutton M, Buee L, Harigaya Y, Yager D, et al. Nature (London) 1996;383:710–713. doi: 10.1038/383710a0. [DOI] [PubMed] [Google Scholar]
- 11.Borchelt D R, Thinakaran G, Eckman C B, Lee M K, Davenport F, Ratovitsky T, Prada C-M, Kim G, Seekins S, Yager D, et al. Neuron. 1996;17:1005–1013. doi: 10.1016/s0896-6273(00)80230-5. [DOI] [PubMed] [Google Scholar]
- 12.Lemere C A, Lopera F, Kosik K S, Lendon C L, Ossa J, Saido T C, Yamaguchi H, Ruiz A, Martinez A, Madrigal L, et al. Nat Med. 1996;2:1146–1150. doi: 10.1038/nm1096-1146. [DOI] [PubMed] [Google Scholar]
- 13.Wolozin B, Iwasaki K, Vito P, Ganjei J K, Lacana E, Sunderland T, Zhao B, Kusiak J W, Wasco W, D’Adamio L. Science. 1996;274:1710–1713. doi: 10.1126/science.274.5293.1710. [DOI] [PubMed] [Google Scholar]
- 14.Vito P, Wolozin B, Ganjei J K, Iwasaki K, Lacana E, D’Adamio L. J Biol Chem. 1996;271:31025–31028. doi: 10.1074/jbc.271.49.31025. [DOI] [PubMed] [Google Scholar]
- 15.Deng G, Pike C, Cotman C. FEBS Lett. 1996;397:50–54. doi: 10.1016/s0014-5793(96)01142-8. [DOI] [PubMed] [Google Scholar]
- 16.Guo Q, Furukawa K, Sopher B L, Pham D G, Xie J, Robinson N, Martin G M, Mattson M P. J Neurosci. 1997;17:4212–4222. doi: 10.1523/JNEUROSCI.17-11-04212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takashima A, Noguchi K, Sato K, Hoshino T, Imahori K. Proc Natl Acad Sci USA. 1993;90:7789–7793. doi: 10.1073/pnas.90.16.7789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takashima A, Yamaguchi H, Noguchi K, Michel G, Ishiguro K, Sato K, Hoshino T, Hoshi M, Imahori K. Neurosci Lett. 1995;198:83–86. doi: 10.1016/0304-3940(95)11964-x. [DOI] [PubMed] [Google Scholar]
- 19.Hoshi M, Takashima A, Noguchi K, Murayama M, Sato K, Kondo S, Saitoh Y, Ishiguro K, Hoshino T, Imahori K. Proc Natl Acad Sci USA. 1996;93:2719–2723. doi: 10.1073/pnas.93.7.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoshi M, Takashima A, Murayama M, Yasutake K, Yoshida N, Ishiguro K, Hoshino T, Imahori K. J Biol Chem. 1997;272:2038–2041. doi: 10.1074/jbc.272.4.2038. [DOI] [PubMed] [Google Scholar]
- 21.Lee V M-Y, Balin B J, Otvos L, Trojanowski J Q. Science. 1991;251:675–678. doi: 10.1126/science.1899488. [DOI] [PubMed] [Google Scholar]
- 22.Morishima-Kawashima M, Hasegawa M, Takio K, Suzuki M, Yoshida H, Titani K, Ihara Y. J Biol Chem. 1995;270:823–829. doi: 10.1074/jbc.270.2.823. [DOI] [PubMed] [Google Scholar]
- 23.Levey A I, Heilman C J, Lah J J, Nash N R, Rees H D, Wakai M, Mirra S S, Rye D B, Nochlin D, Bird T D, et al. Ann Neurol. 1997;41:742–753. doi: 10.1002/ana.410410610. [DOI] [PubMed] [Google Scholar]
- 24.Murphy G M, Jr, Forno L S, Ellis W G, Nochlin D, Levy-Lahad E, Poorkaj P, Bird T D, Jiang Z, Cordell B. Am J Pathol. 1996;149:1839–1846. [PMC free article] [PubMed] [Google Scholar]
- 25.Busciglio J, Hartmann H, Lorenzo A, Wong C, Baumann K, Sommer B, Staufenbiel M, Yankner B A. J Neurosci. 1997;17:5101–5107. doi: 10.1523/JNEUROSCI.17-13-05101.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamada T, Takashima A. Exp Neurol. 1997;148:10–12. doi: 10.1006/exnr.1997.6661. [DOI] [PubMed] [Google Scholar]
- 27.Yamaguchi Y, Ishiguro K, Uchida T, Takashima A, Lemere C A, Imahori K. Acta Neuropathol. 1996;92:232–241. doi: 10.1007/s004010050513. [DOI] [PubMed] [Google Scholar]
- 28.Shiurba R A, Ishiguro K, Takahashi M, Sato K, Spooner E T, Mercken M, Yoshida R, Wheelock T R, Yanagawa H, Imahori K, et al. Brain Res. 1996;737:119–132. doi: 10.1016/0006-8993(96)00717-2. [DOI] [PubMed] [Google Scholar]
- 29.Mercken M, Takahashi H, Honda T, Sato K, Murayama M, Nakazato Y, Noguchi K, Imahori K, Takashima A. FEBS Lett. 1996;389:297–303. doi: 10.1016/0014-5793(96)00608-4. [DOI] [PubMed] [Google Scholar]
- 30.Takashima A, Sato M, Mercken M, Tanaka S, Kondo S, Honda T, Sato K, Murayama M, Noguchi K, Nakazato Y, Tkahashi H. Biochem Biophys Res Comm. 1996;227:423–426. doi: 10.1006/bbrc.1996.1523. [DOI] [PubMed] [Google Scholar]
- 31.Murayama O, Honda T, Mercken M, Murayama M, Yasutake K, Nihonmatu N, Nakazato Y, Michel G, Shaochuen S, Sato K, et al. Neurosci Lett. 1997;229:61–64. doi: 10.1016/s0304-3940(97)00417-5. [DOI] [PubMed] [Google Scholar]
- 32.Ishiguro K, Omori A, Takamatsu M, Sato K, Arioka M, Uchida T, Imahori K. Neurosci Lett. 1992;148:202–206. doi: 10.1016/0304-3940(92)90839-y. [DOI] [PubMed] [Google Scholar]
- 33.Michel G, Mercken M, Murayama M, Yasutake K, Ishiguro K, Imahori K, Takashima A. Biochim Biophys Acta. 1998;1380:177–182. doi: 10.1016/s0304-4165(97)00139-6. [DOI] [PubMed] [Google Scholar]
- 34.Drewes G, Lichtenberg-Kraag B, Doring F, Mandelkow E-M, Biernat J, Goris J, Doree M, Mandelkow E. EMBO J. 1992;11:2131–2138. doi: 10.1002/j.1460-2075.1992.tb05272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xia W, Perez R, Koo E, Selkoe D. Proc Natl Acad Sci USA. 1997;94:8208–8213. doi: 10.1073/pnas.94.15.8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ishiguro K, Sato K, Takamatsu M, Park J, Uchida T, Imahori K. Neurosci Lett. 1995;202:81–84. doi: 10.1016/0304-3940(95)12206-0. [DOI] [PubMed] [Google Scholar]
- 37.Thinakaran G, Borchelt D R, Lee M K, Slunt H H, Spitzer L, Kim G, Ratovisky T, Davenport F, Norstedt C, Seeger M, et al. Neuron. 1996;17:181–190. doi: 10.1016/s0896-6273(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 38.Van Broeckhoven C. Nat Genet. 1995;11:230–232. doi: 10.1038/ng1195-230. [DOI] [PubMed] [Google Scholar]
- 39.Gomez-Isla T, Wasco W, Pettingell W P, Gurubhagavatula S, Schmidt S D, Jondro P D, McNamara M, Rodes L A, DiBlasi T, Growdon W, et al. Ann Neurol. 1997;41:809–813. doi: 10.1002/ana.410410618. [DOI] [PubMed] [Google Scholar]
- 40.Irving N G, Miller C C. Neurosci Lett. 1997;222:71–74. doi: 10.1016/s0304-3940(97)13343-2. [DOI] [PubMed] [Google Scholar]
- 41.Zhou J, Liyanage U, Medina M, Ho C, Simmons A D, Lovett M, Kosik K S. NeuroReport. 1997;8:1489–1494. doi: 10.1097/00001756-199704140-00033. [DOI] [PubMed] [Google Scholar]
- 42.Aplin A E, Gibb G M, Jacobsen J S, Gallo J M, Anderton B H. J Neurochem. 1996;67:699–707. doi: 10.1046/j.1471-4159.1996.67020699.x. [DOI] [PubMed] [Google Scholar]
- 43.Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitso S, Polakis P. Science. 1996;272:1023–1025. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- 44.Battaini F, Pascale A, Paoletti R, Govoni S. Trends Neurosci. 1997;20:410–415. doi: 10.1016/s0166-2236(97)01084-9. [DOI] [PubMed] [Google Scholar]
- 45.Coghlan V M, Perrino B A, Howard M, Langeberg L K, Hicks J B, Gallatin W M, Scott J D. Science. 1995;267:108–111. doi: 10.1126/science.7528941. [DOI] [PubMed] [Google Scholar]
- 46.Hemmings B A. Science. 1997;275:628–630. doi: 10.1126/science.275.5300.628. [DOI] [PubMed] [Google Scholar]
- 47.Dudek H, Datta S R, Franke T F, Birnbaum M J, Yao R, Cooper G M, Segal R A, Kaplan D R, Greenberg M E. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 48.Takashima A, Noguchi K, Michel G, Mercken M, Hoshi M, Ishiguro K, Imahori K. Neurosci Lett. 1996;203:33–36. doi: 10.1016/0304-3940(95)12257-5. [DOI] [PubMed] [Google Scholar]