Abstract
A new class of 16-membered macrolides, the epothilones (Epos), has been synthesized and evaluated for antitumor potential in vitro and in vivo. Recent studies in these and other laboratories showed that epothilones and paclitaxel (paclitaxel) share similar mechanisms of action in stabilizing microtubule arrays as indicated by binding-displacement studies, substitution for paclitaxel in paclitaxel-dependent cell growth, and electron microscopic examinations. The present study examined cell growth-inhibitory effects in two rodent and three human tumor cell lines and their drug-resistant sublines. Although paclitaxel showed as much as 1,970-fold cross-resistance to the sublines resistant to paclitaxel, adriamycin, vinblastine, or actinomycin D, most epothilones exhibit little or no cross-resistance. In multidrug-resistant CCRF-CEM/VBL100 cells, IC50 values for EpoA (1), EpoB (2), desoxyEpoA (3) (dEpoA), desoxyEpoB (4) (dEpoB), and paclitaxel were 0.02, 0.002, 0.012, 0.017, and 4.14 μM, respectively. In vivo studies, using i.p. administration, indicated that the parent, EpoB, was highly toxic to mice and showed little therapeutic effect when compared with a lead compound, dEpoB. More significantly, dEpoB (25–40 mg/kg, Q2Dx5, i.p.) showed far superior therapeutic effects and lower toxicity than paclitaxel, doxorubicin, camptothecin, or vinblastine (at maximal tolerated doses) in parallel experiments. For mammary adenocarcinoma xenografts resistant to adriamycin, MCF-7/Adr, superior therapeutic effects were obtained with dEpoB compared with paclitaxel when i.p. regimens were used. For ovarian adenocarcinoma xenografts, SK-OV-3, dEpoB (i.p.), and paclitaxel (i.v.) gave similar therapeutic effects. In nude mice bearing a human mammary carcinoma xenograft (MX-1), marked tumor regression and cures were obtained with dEpoB.
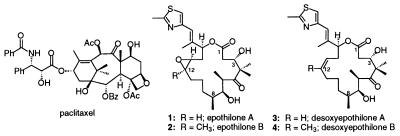
The isolation of the naturally occurring macrolides epothilone A and epothilone B (EpoA and EpoB) from the myxobacteria Sorangium cellulosum (1, 2) and the subsequent demonstration of their ability to stabilize microtubule arrays in vitro have elicited considerable interest in this class of compounds (3, 8–11). We and others (4–8) recently have conducted total syntheses of these natural products (Fig. 1). In our lab more than 45 related analogs (12–14) have been prepared to investigate their chemical structure–biological activity relationships (5). Our studies allowed us to dissect the epothilone structure into three zones. Thus, in the C-1∼8 acyl sector, structural changes are not tolerated in terms of in vitro cytotoxicity and microtubule stabilizing ability. This stands in contrast to the C-9∼15 O-alkyl sector and the C-15 pendant aryl sectors wherein considerable modification of structures is tolerated (5, 12). In the present study, we describe results of in vitro and in vivo experiments on the Z-12,13-desoxy version of EpoB (see dEpoB, 4).
Figure 1.
Chemical structures of paclitaxel, epothilones A and B, and desoxyepothilones A and B.
It has been shown that the natural epothilones 1 and 2 have a similar mechanism of action to paclitaxel (paclitaxel), although the agents differ a great deal in their structures (5, 9, 15, 16). Paclitaxel, isolated from the Pacific yew tree (Taxus brevifolia), has been widely used clinically to treat a variety of solid cancers including neoplasms of ovary, breast, colon, and lung (16–20). Epothilones A and B as well as paclitaxel stabilize microtubule assemblies as demonstrated by binding displacement, substitution for paclitaxel in paclitaxel-dependent cell growth, and electron microscopic examinations (3). The epothilones are more water soluble than paclitaxel, thereby offering potentially distinct advantages for formulation. Furthermore, the Epos are more potent than paclitaxel in inhibiting cell growth, especially against cells expressing P-glycoprotein (Pgp) that are multidrug-resistant (MDR), including cross-resistance to paclitaxel (3, 5).
MATERIALS AND METHODS
Chemicals.
All epothilones used in this study were obtained in our laboratory through total syntheses as described in previous publications (4, 5, 9, 12, 13). For in vitro studies, paclitaxel (paclitaxel), etoposide (VP-16), teniposide (VM), camptothecin (CPT), actinomycin D (AD), and vinblastine sulfate (VBL) were purchased from Sigma. All stock solutions of the above (except VBL in saline) were prepared by using dimethyl sulfoxide (DMSO) as a solvent and were further diluted to desired concentrations for experimental use. The final concentration of DMSO in tissue culture was 0.25% (vol/vol) or less to avoid solvent cytotoxicity. For in vivo studies, paclitaxel (paclitaxel) in Cremophor-EtOH was obtained from Bristol-Myers Squibb and further diluted with DMSO as needed. Vinblastine sulfate (Velban) (Eli Lilly) and doxorubicin (Adriamycin) HCl (DX or Adr) (Pharmacia) in saline were diluted with DMSO as needed. DMSO was used as a vehicle for epothilones. Each mouse received ≤40 μl DMSO in all experiments.
Cell Lines.
The CCRF-CEM human T cell acute lymphoblastic leukemia cell line and its vinblastine-resistant (CCRF-CEM/VBL100) and teniposide-resistant (CCRF-CEM/VM1) sublines (19, 20) were obtained from W. T. Beck, University of Illinois, Chicago. CCRF-CEM/paclitaxel was developed in this laboratory (T.-C.C.) after continuous exposure of CCRF-CEM cells with increasing concentrations of paclitaxel (at IC50–IC90) for 10 months. The fresh medium with paclitaxel was replenished every week. The CCRF-CEM/paclitaxel cell lines exhibited 57-fold resistance to paclitaxel (IC50 = 0.12 μM) when compared with original CCRF-CEM cells at the beginning of the experiment (IC50 = 0.0021 μM, see Table 1). The DC-3F hamster lung fibroblast cell line and its actinomycin D-selected resistant sublines (DC-3F/ADII and DC-3F/ADX) were obtained from J. L. Biedler of the Memorial Sloan–Kettering Cancer Center. The murine leukemic P388/0 and its doxorubicin-selected subline (P388/DX) as well as human neuroblastoma SK-N-As and its doxorubicin-selected subline (SK-N-FI/Adr) were obtained from F. A. Schmid of the Memorial Sloan–Kettering Cancer Center.
Table 1.
Susceptibility of CCRF-CEM and its drug-resistant sublines to epothilone derivatives
| Compound | (A) CCRFCEM | (B) CCRFCEM/VBL100 | (C) CCRF-CEM/paclitaxel | (D) CCRF-CEM/VM1 | (B)/(A) | (C)/(A) | (D)/(A) |
|---|---|---|---|---|---|---|---|
| IC50, μM* | |||||||
| 1 | 0.0027 | 0.020 | 0.0037 | 0.0061 | 7.4 | 1.4 | 2.3 |
| 2 | 0.00035 | 0.0021 | 0.0011 | 0.0013 | 6.1 | 3.1 | 3.6 |
| 3 | 0.0220 | 0.012 | 0.0150 | 0.013 | 0.55 | 0.7 | 0.59 |
| 4 | 0.0095 | 0.017 | 0.0162 | 0.014 | 1.8 | 1.7 | 1.5 |
| Paclitaxel | 0.0021 | 4.140 | 0.120 | 0.0066 | 1,971 | 57 | 3.1 |
| Vinblastine | 0.00063 | 0.332 | 0.0069 | 0.00041 | 527 | 10.9 | 0.7 |
| Etoposide | 0.290 | 10.30 | 1.32 | 34.4 | 35 | 4.5 | 117 |
| Adriamycin | 0.036 | 1.74 | 0.082 | 0.128 | 48 | 2.3 | 3.6 |
| Actinomycin D | 0.00035 | 0.038 | 0.0013 | 0.00027 | 109 | 3.7 | 0.8 |
The drug-resistant cell lines were cultured continuously in the presence of the selecting agent, AD, DX, VBL, or VM to maintain the drug-resistant phenotypes. Each sub-cell line was cultured for one to two passages in an appropriate concentration (e.g., IC50) of the drug, which was then removed from the media, and the cells were resuspended in fresh media for a minimum of 4 days before each assay. All cells were cultured in RPMI 1640 medium/10%FBS at 37°C, 5% CO2 (see below).
Cytotoxicity Assays.
The cells were cultured at an initial density of 5 × 104 cells/ml. They were maintained in a 5% CO2-humidified atmosphere at 37°C in RPMI 1640 medium (GIBCO/BRL) containing penicillin (100 units/ml), streptomycin (100 μg/ml) (GIBCO/BRL), and 10% heat-inactivated fetal bovine serum. Cytotoxicity studies for cells in suspension (such as for CCRF-CEM, P388, and sublines) were performed by the XTT-microculture tetrazonium method (21) in duplicate in 96-well microtiter plates. 2′,3′-bis(methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) was prepared at 1 mg/ml in prewarmed (37°C) medium without serum. Phenazine methosulfate (PMS) and fresh XTT were mixed together to obtain 0.025 mM PMS-XTT solution (25 μl of the stock 5 mM PMS was added per 5 ml of 1 mg/ml XTT). After a 72-h incubation, 50 μl of the assay aliquots was added to each well of the cell culture. After incubation at 37°C for 4 h, absorbance at 450 and 630 nm was measured with a microplate reader (EL340, Bio-Tek, Wincoski, VT).
The cytotoxicity of the drug toward the monolayer cell cultures (such as DC-3F, MCF-7, SK-N-As, and sublines) was determined in 96-well microtiter plates by the SRB method as described by Skehan and coworkers (22) for measuring the cellular protein content. Cultures were fixed with trichloroacetic acid and then stained for 30 min with 0.4% sulforhodamine B dissolved in 1% acetic acid. Unbound dye was removed by acetic acid washes, and the protein-bound dye was extracted with an unbuffered Tris base [tris(hydroxy-methyl)aminomethane] for determination of absorbance at 570 nm in a 96-well microtiter plate reader. The experiments were carried out in duplicate. Each run entailed six to seven concentrations of the tested drugs. Data were analyzed with the median-effect plot (23) by using a previously described computer program (24).
In Vivo Antitumor Effects.
Athymic nude mice (nu/nu) were used for MX-1, MCF-7/Adr, and SK-OV3 human mammary and ovarian carcinoma xenografts. Mice were obtained from Taconic Farms (outbred, Swiss background). Male mice, 6–8 weeks old, weighing 20–25 g, were used. All studies were conducted in accordance with the guidelines of the National Institutes of Health “Guide for the Care and Use of Animals,” and after protocol review by the Memorial Sloan–Kettering Cancer Center Institutional Care and Use Committee. For the humane treatment of tumor-bearing animals, mice were euthanized when tumors reached ≥10% of their total body weight.
RESULTS
In Vitro Comparisons of Structure–Activity Relationships.
To extend our recent study on structure–activity relationships of epothilones (5), we examined the susceptibility of CCRF-CEM leukemic cells and the respective drug-resistant sublines CCRF-CEM/VBL100 (Pgp-MDR cells) (19) and CCRF/CEM/VM1 (cells with a mutated topo II enzyme) (20) to epothilones 1 and 2 and desoxyepthilones (3, 4) (Table 1). Although/VBL100 is 527-fold resistant to VBL and 1,971-fold resistant to paclitaxel, the epothilones (1, 2) exhibited only 6.1- to ∼7.4-fold resistance, whereas desoxyepothilones (3, 4) evidenced only 0.6- to ∼1.8-fold resistance. Using paclitaxel as the selecting agent, a cell line was obtained (CCRF-CEM/paclitaxel) that was 57-fold resistant to paclitaxel and found to be 10.9-fold resistance to VBL. By contrast, DX, AD, and VP-16 showed only 2.3- to 4.5-fold resistance, and, interestingly, 1 and 2 showed very little resistance (i.e., 1.4- to ∼3.1-fold) and compounds 3 and 4 displayed almost no resistance (i.e., 0.7- to ∼1.7-fold) (Table 1). It is also of interest to note that CCRF-CEM/VM1 cells that were 117-fold resistant to etoposide were sensitive to all Epos or dEpos listed in Table 1 with only 0.6- to 3.6-fold resistance.
In Vitro Effects Against Various Tumor Sublines.
Further susceptibility evaluations were conducted for EpoA, EpoB, dEpoA, and dEpoB in four additional tumor cell lines and four of their drug-resistant sublines (Table 2). Hamster lung tumor cells, DC-3F/ADX, which were selected 13,000-fold resistant to AD, were found to be 337-fold resistant to paclitaxel and 124-fold resistant to DX when compared with the parent cell line (DC-3F). In contrast, compounds 1, 2, and 3 showed only 3.9- to ∼28-fold resistance, and compound 4 showed no cross-resistance (0.9-fold resistance).
Table 2.
Comparison of in vitro growth inhibition potency of epothilone derivatives against various parent and drug-resistant tumor cell lines
| Compound | DC-3F | DC-3F/ADX | P388/0 | P388/Adr | SK-N-As | SK-N-FI | MCF-7 | MCF-7/Adr |
|---|---|---|---|---|---|---|---|---|
| IC50, μM* | ||||||||
| 1 | 0.0037 | 0.053 | 0.0018 | 0.0010 | 0.012 | 0.023 | 0.0030 | 0.0094 |
| (14.5×) | (5.3×) | (1.9×) | (3.1×) | |||||
| 2 | 0.0006 | 0.017 | 0.00029 | 0.0016 | 0.004 | 0.010 | 0.0005 | 0.0027 |
| (28×) | (5.5×) | (25×) | (5.4×) | |||||
| 3 | 0.011 | 0.042 | 0.0213 | 0.0125 | 0.073 | 0.223 | 0.032 | 0.144 |
| (3.9×) | (0.59×) | (3.1×) | (4.5×) | |||||
| 4 | 0.00097 | 0.00091 | 0.0068 | 0.0042 | 0.021 | 0.046 | 0.0029 | 0.0071 |
| (0.9×) | (0.62×) | (2.2×) | (2.4×) | |||||
| Paclitaxel | 0.095 | 32.0 | 0.0029 | 0.326 | 0.0016 | 0.130 | 0.0033 | 0.150 |
| (338×) | (111×) | (80×) | (46×) | |||||
| Actinomycin D | 0.00044 | 0.572 | 0.00015 | 0.0012 | 0.00085 | 0.0119 | 0.00068 | 0.00167 |
| (13,000×) | (8×) | (14×) | (2.5×) | |||||
| Adriamycin | 0.018 | 2.236 | 0.0055 | 2.65 | 0.077 | 1.42 | 0.057 | 0.216 |
| (124×) | (482×) | (18.4×) | (3.8×) | |||||
Numbers in parentheses are folds of resistance based on the IC50 ratio when compared with the corresponding parent cell lines except for P388/0 and P388/Adr, and XTT assay (21) was used.
Murine leukemic P388/Adr cells that were 482-fold resistant to DX, were found to be 111-fold resistant to paclitaxel. However, compounds 1 and 2 showed less than 6-fold resistance, and for 3 and 4 there was no cross-resistance (0.6-fold resistance).
Human neuroblastoma cells, SK-N-F1, that were selected as 18-fold resistant to DX were found to be 80-fold resistant to paclitaxel. By contrast, EpoB (2) was 25-fold resistant, whereas the resistance of 1, 3, and 4 was only between 1.9 and 3.1.
Human mammary carcinoma cells, MCF-7/Adr, that were selected 3.8-fold resistant to DX were found to be 46-fold resistant to paclitaxel. In contrast, compounds 1, 2, and 3 were 3.1- to ∼5.4-fold resistant, and dEpoB (4) showed only 2.4-fold resistance.
Overall, dEpoB 4 was the least cross-resistant among Epos and dEpos in various drug-resistant tumor sublines. By contrast, paclitaxel suffers from marked cross-resistance in tumor cells that were selected to be resistant to VBL, DX, or AD. In three out of five cell lines studied, cross-resistance to paclitaxel was even greater than that of the selecting agents.
A Study of the Toxicity of dEpoB and EpoB.
The toxicity of EpoB and dEpoB was compared in normal athymic nude mice on the daily i.p. schedule (see Table 3). EpoB (2) at 0.6 mg/kg, QDX4, i.p. led to lethality in all eight mice. In contrast, in the group treated with dEpoB (4) 25 mg/kg, QDx5, i.p., none of six mice died. It was also observed that the vehicle-treated control group showed a steady increase in body weight and the dEpoB treated mice maintained approximately the same average body weight, whereas the EpoB treated group showed steady decreases in body weight until death. These results indicated a higher toxicity for EpoB given daily than in tumor-bearing nude mice when the treatment was given every other day, i.p. (see Table 4). In the preliminary studies, for the non-tumor-bearing nude mice receiving EpoB 0.6 mg/kg or dEpoB 25 mg/kg, QDx4, i.p., there were no apparent changes in hematological cell counts or blood chemistry parameters except for a 43% decrease in lymphocytes. Similar leukopenia was found with paclitaxel. Some obstructive fecal mass in the large intestine was noted after Epo treatments in the preliminary study. No gross pathological abnormalities were observed in other organs. Further studies are being organized and will be described in due course.
Table 3.
Toxicity of epothilone B and desoxyepothilone B in normal nude mice
| Group | Dose, schedule, and route of administration | Mice, n | Mice that died, n |
|---|---|---|---|
| Control | 4 | 0 | |
| 2 | 0.6 mg/kg, QD × 4, i.p. | 8 | 8* |
| 4 | 25 mg/kg, QD × 4, i.p. | 6 | 0 |
Mice died of toxicity on days 5, 6, 6, 7, 7, 7, 7, and 7.
Table 4.
Therapeutic effect of desoxyepothilone B, epothilone B, paclitaxel, vinblastine, and camptothecin in nude mice bearing human MX-1 xenograft
| Drug | Dose, mg/kg | Average body weight change, g
|
Average tumor size, T/C
|
Toxicity death | n | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 7 | 11 | 13 | 15 | 17 | Day 11 | 13 | 15 | 17 | ||||
| Control | 27.2 | +0.8 | +1.1 | +1.9 | +0.6 | 1.00 | 1.00 | 1.00 | 1.00 | 0/8 | 8 | |
| 4 | 15 | 27.1 | +0.8 | +1.1 | +1.6 | +1.5 | 0.65 | 0.46* | 0.49* | 0.41** | 0/6 | 6 |
| 25† | 27.0 | +0.4 | +0.7 | +1.0 | +0.7 | 0.38* | 0.11** | 0.05*** | 0.04**** | 0/6 | 6 | |
| 2 | 0.3 | 26.9 | +0.5 | +0.4 | −0.3 | −1.2 | 1.00 | 0.71 | 0.71 | 0.84 | 0/7 | 7 |
| 0.6‡ | 27.4 | −0.3 | −1.3 | −2.1 | −2.1 | 1.08 | 0.73 | 0.81 | 0.74 | 3/7‖ | 7 | |
| Paclitaxel | 5 | 26.9 | −0.1 | +0.4 | +1.1 | +1.2 | 0.54 | 0.46 | 0.40* | 0.45** | 0/7 | 7 |
| 10§ | 27.6 | −2.7 | −1.1 | −0.3 | +2.2 | 0.43 | 0.37 | 0.12 | 0.11 | 4/7‖ | 7 | |
| Vinblastine | 0.2 | 25.7 | +0.6 | +1.4 | +2.3 | +2.9 | 0.65 | 0.54 | 0.56 | 0.88 | 0/7 | 7 |
| 0.4¶ | 26.4 | +0.8 | +0.5 | +1.9 | +2.1 | 0.80 | 0.56 | 0.83 | 0.88 | 1/7‖ | 7 | |
| Campothecin | 1.5 | 27.4 | −0.9 | −0.7 | −0.4 | +1.0 | 0.61 | 0.45* | 0.32* | 0.36** | 0/7 | 7 |
MX-1 tissue, 50 μl/mouse, was implanted s.c. on day 0. Every other day in treatments were given on days 7, 9, 11, 13, and 15. The average tumor volumes of the control group on day 11, 13, 15, and 17 were 386 ± 120, 915 ± 245, 1,390 ± 324, and 1,903 ± 319 mm3 (mean ± SEM), respectively; *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001.
One of six mice with no detectable tumor on day 35.
Three mice died of drug toxicity on day 17.
Four mice died of drug toxicity on days 13, 13, 13, and 15.
One mouse died of drug toxicity on day 15.
P values were not shown because of toxic lethality.
Therapeutic Effects Against MX-1 Xenografts.
Our in vitro results suggested that the naturally occurring Epo B (2) was the most potent of the epothilone drugs. However, early in vivo probes (5) pointed to a worrisome toxicity that raised serious concerns as to the tolerability of this drug. Therefore, we wondered whether a less potent but still highly in vitro active congener such as 12,13-desoxyepo B (4) might provide a more useful therapeutic index. Accordingly, we launched a direct comparison of the in vivo performance of these two fully synthetic drugs. To evaluate these Epo agents in a broader context, we included paclitaxel in our comparisons as well as two mechanistically different chemotherapeutic agents, VBL and CPT.
Therapeutic effects of the various drugs were evaluated in athymic nude mice bearing human mammary adenocarcinoma MX-1 xenografts (Table 4). Compound 4 at a 15 mg/kg dose i.p. on days 7, 9, 11, 13, and 15 produced a 50–60% tumor volume reduction when compared with the control group. A higher dose of drug, 25 mg/kg, produced as much as 96% average tumor volume reduction measured 2 days after the last drug treatment (i.e., on day 17). These effects were achieved with no lethality nor significant body weight reduction. Furthermore, with a 25-mg/kg dose, one of six mice was tumor-free on day 35 after tumor implantation (i.e., on day 35). In contrast, after treatment with EpoB (0.3 mg/kg or 0.6 mg/kg, i.p., on days 7, 9, 11, 13, and 15), the average body weight decreased over 1 gm and 2 gm, respectively. In the case of 0.6 mg/kg treatment, three of seven mice died of toxicity. Despite the apparent toxicity at these doses, EpoB appeared to have only marginal therapeutic effect, as only 16–26% tumor volume reduction was observed (Table 4). The parallel experiments for paclitaxel in the i.p. setting led to a lower therapeutic effect. In animals treated with paclitaxel, 5 mg/kg i.p., there was 55% reduction in tumor volume and no decrease in average body weight. At a dose of 10 mg/kg i.p., paclitaxel showed a 89% tumor reduction; however, four of seven mice died of toxicity. In this survey, we also included doxorubicin (2–3 mg/kg) and camptothecin (1.5–3 mg/kg), i.e., near the maximal tolerated doses, and found that inferior results were obtained (see Table 4). Thus, even well below the maximal tolerated dose, dEpoB had the best therapeutic effect among the five compounds studied under these experimental conditions.
We note, however, that paclitaxel was not evaluated in the optimal context (i.e., i.v. in a Cremophor formulation). We carried out our study in the i.p. mode because Epos 2 and 4 had exhibited considerable toxicity in i.v. injection. Only in the i.p. regime do the epothilones manifest useful therapeutic effects. Accordingly, the comparison reported herein is not intended to address the ultimate promise of epothilones versus paclitaxel in their respective optimal clinical settings.
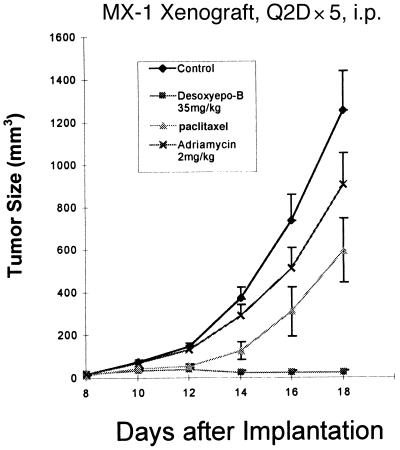
In a separate experiment, MX-1 xenograft-bearing mice were treated with dEpoB (4) 35 mg/kg, Q2Dx5, i.p. beginning on day 8 after tumor implantation (Fig. 2). On day 16, 2 of 10 mice had no detectable tumor. These 10 mice were further treated with compound 4, 40 mg/kg, Q2Dx5 beginning on day 18. At the end of treatment on day 26, 5 of 10 mice had no detectable tumor, and three remained tumor-free on day 60. There was modest body weight reduction during treatments, but no lethality occurred.
Figure 2.
Therapeutic effect of dEpoB, paclitaxel, and Adriamycin in nude mice bearing the human mammary carcinoma MX-1 xenograft. MX-1 tissue preparation, 100 μl per mouse, was implanted s.c. on day 0. Every other day i.p. treatments were given on days 8, 10, 12, 14, and 16 with 35 mg/kg dEpoB (■), 5 mg/kg paclitaxel (▴), 2 mg/kg Adriamycin (X), and vehicle (DMSO, 30 μl)-treated control (♦). For paclitaxel, 2 of 10 mice died of toxicity on day 18. For Adriamycin, 1 of 10 mice died of toxicity on day 22. For dEpoB, 10 of 10 mice survived and were subjected to the second cycle of treatment at 40 mg/kg on days 18, 20, 22, 24, and 26. This led to 3 of 10 mice tumor-free up to day 80, whereas 7 of 10 mice were with markedly suppressed tumors and were sacrificed on day 50.
In a parallel experiment, 10 mice were treated with paclitaxel 5 mg/kg, Q2Dx5, i.p. from day 8 to day 16, followed by a second cycle of treatment in the same manner from day 18 to day 26. The tumor sizes were reduced but continued to grow during treatment, and, by day 24, the average tumor size was 2,285 ± 597 mm3 (n = 10). In a further experiment, DX was given 2 mg/kg, Q2Dx5, i.p. (Fig. 2). The therapeutic effect was much weaker when compared with dEpoB or paclitaxel. It should be noted that in Fig. 2, no data after day 18 are shown because the tumor burden in the control group was excessive and the mice in this group were sacrificed.
Therapeutic Effects Against MCF-7/Adr Xenografts.
The therapeutic effects of dEpoB also were evaluated in nude mice bearing xenografts of human mammary adenocarcinoma resistant to DX (MCF-7/Adr) (Table 5). For reference purposes, paclitaxel, DX, and CPT also were included in this study. The background findings for this work were the in vitro data shown in Table 2. Thus, MCF-7/Adr cells selected to be 3.8-fold resistant to DX had been found to be 46-fold resistant to paclitaxel and only 2.4-fold resistant to dEpoB (4). In the in vivo studies, each drug was given Q2Dx5 i.p. beginning on day 8 after tumor implantation. Paclitaxel (12 mg/kg) and DX (3 mg/kg) were highly toxic to the nude mice with 3/7 and 3/6 lethality, respectively. CPT 3 mg/kg led to moderate toxicity without lethality. By contrast, 35 mg/kg dEpoB showed negligible toxicity as shown by minimal body weight changes (Table 5).
Table 5.
Therapeutic effects of desoxyepothilone B, paclitaxel, adriamycin, and camptothecin in nude mice bearing MDR human MCF-7/Adr tumor
| Drug | Dose, mg/kg | Average body weight change, g
|
Average tumor size, T/C
|
Toxicity death | n | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 8 | 11 | 13 | 15 | 17 | Day 11 | 13 | 15 | 17 | ||||
| Control | 0 | 25.0 | +2.0 | +2.6 | +3.1 | +3.7 | 1.00 | 1.00 | 1.00 | 1.00 | 0/8 | 8 |
| dEpoB | 35 | 25.0 | 0.3 | +0.7 | +0.6 | +0.8 | 0.31** | 0.27*** | 0.30*** | 0.34* | 0/8 | 8 |
| Paclitaxel | 6 | 25.3 | +1.7 | +1.8 | +0.8 | +0.9 | 0.57 | 0.66 | 0.85 | 0.90 | 0/7 | 7 |
| 12 | 24.5 | +0.7 | −1.3 | −2.4 | 0 | 0.50 | 0.51 | 0.32 | 0.40 | 3/7 | 7† | |
| Adriamycin | 2 | 25.6 | +0.2 | −0.4 | −0.6 | −0.4 | 0.70 | 0.68 | 0.84 | 0.78 | 0/8 | 8 |
| 3 | 24.6 | +0.5 | −1.3 | −3.2 | −1.6 | 0.66 | 0.83 | 0.57 | 0.53 | 3/6 | 6† | |
| Campothecin | 1.5 | 24.4 | +1.1 | +0.9 | +1.7 | +1.4 | 1.08 | 0.72 | 0.61 | 0.72 | 0/8 | 8 |
| 3 | 24.5 | −0.6 | −0.4 | −0.8 | −0.9 | 0.95 | 0.76 | 0.61 | 0.43* | 0/6 | 6 | |
MCF-7/Adr cell 3 × 106/mouse was implanted s.c. on day 0. Every other day i.p. treatments were given on days 8, 10, 12, 14, and 16. The average tumor size of control group on days 11, 13, 15, and 17 was 392 ± 84, 916 ± 210, 1,499 ± 346, and 2,373 ± 537 mm3, respectively (mean ± SEM).
*P < 0.05, **P < 0.01, ***P < 0.005.
P values were not shown because of toxic lethality.
In these studies it was found that 6 mg/kg paclitaxel and 2 mg/kg DX produced only slight growth suppression of this drug-resistant tumor, which was not significantly different from the control group (see Table 5). However, dEpoB at 35 mg/kg significantly suppressed tumor size by 66–73% when compared with the control group (P < 0.005–0.05), and CPT at 3 mg/kg reduced 57% of tumor size on day 17 (P < 0.05 when compared with control group). Thus, dEpoB (4) stands out as the superior drug prospect among the four agents tested against this drug-resistant tumor.
Therapeutic Effects Against SK-OV3 Ovarian Adenocarcinoma and a Comparison of i.p. and i.v. Administration.
Nude mice bearing human ovarian adenocarcinoma, SK-OV3, were treated with dEpoB, both i.p. (DMSO as solvent) and i.v. (Cremophor and EtOH, 1:1) injections. For comparison, paclitaxel, i.p. and i.v. (clinical samples in Cremophor and EtOH as specified by the manufacturer), and EpoB, i.v. (Cremophor and EtOH, 1:1), also were included in this experiment. As shown in Table 6, for Q2Dx5 schedule, dEpoB, i.p. (35 mg/kg), and paclitaxel, i.v. (15 mg/kg), both yield significant therapeutic effects against SK-OV3 with the tumor size on day 21, treated/control = 0.28 in both cases. By contrast, dEpoB, i.v. (15 mg/kg), paclitaxel, i.p. (5 mg/kg), and EpoB, i.v. (0.6 mg/kg), showed more toxicity and less therapeutic effect.
Table 6.
Therapeutic effects of desoxyepothilone B, Epo B, and paclitaxel in nude mice bearing SK-OV-3 tumors using different vehicles and different routes of administration
| Drug/route | Dose, mg/kg | Average body weight change, g
|
Average tumor size, T/C
|
Tumor disappearance | Toxicity death | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 11 | 15 | 17 | 19 | 21 | Day 15 | 17 | 19 | 21 | ||||
| Control | 0 | 26.4 | −0.2 | −0.4 | +0.2 | +0.8 | 1.00 | 1.00 | 1.00 | 1.00 | 0/6 | 0/6 |
| 4/i.p. | 35 | 27.8 | −1.7 | −2.1 | −2.1 | −2.4 | 0.57 | 0.33 | 0.35 | 0.28 | 0/6 | 0/6 |
| 4/i.v. | 15 | 27.0 | 0 | −0.6 | −1.1 | −2.6 | 0.86 | 0.56 | 0.50 | 0.44 | 0/6 | 4/6* |
| 2/i.v. | 0.6 | 27.0 | −0.9 | −0.5 | −3.3 | −3.4 | 0.75 | 0.69 | 0.88 | 0.77 | 0/6 | 0/6 |
| Paclitaxel/i.p. | 5 | 27.4 | −1.1 | −2.0 | −1.0 | −0.6 | 0.69 | 0.60 | 0.49 | 0.40 | 0/6 | 0/6 |
| Paclitaxel/i.v. | 15 | 27.2 | −0.6 | −0.8 | −0.8 | −0.9 | 0.97 | 0.67 | 0.42 | 0.28 | 0/6 | 0/6 |
Fifty-microgram tumor tissue was implanted s.c. on day 0. Every other day i.p. or i.v. treatments were given on days 11, 13, 15, 17, and 19. The average tumor size of control group (right side) on day 15, 17, 21, and 23 was 170, 392, 659, 1,003, and 1,280 mm3, respectively. i.p. route used DMSO, and i.v. route used Cremophor + EtOH (1:1) as vehicles.
Four of six mice died of drug toxicity on days 23, 23, 23, and 25.
Further studies of dEpoB, both i.p. and i.v., were conducted with the MX-1 adenocarcinoma (see Table 7). In the Q2Dx5 schedule, dEpoB, i.p. (35 mg/kg), and paclitaxel, i.v. (15 mg/kg), gave potent therapeutic effects, as shown earlier. However, dEpoB, i.v. (15 mg/kg), and paclitaxel, i.p. (5 mg/kg), again showed high toxicity and little therapeutic value against the MX-1 tumor. Thus, dEpoB showed the best results when given i.p. and paclitaxel gave the best results when given i.v. (Cremophor and EtOH, 1:1). Attempts will be made to explore optimal formulations of the epothilones so that the i.v. route can be used routinely.
Table 7.
Therapeutic effects of desoxyepothilone B, Epo B, and paclitaxel in nude mice bearing MX-1 tumors using different vehicles and different routes of administration
| Drug/route | Dose, mg/kg | Average body weight change, g
|
Average tumor size, T/C
|
Tumor disappearance | Toxicity death | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 9 | 13 | 15 | 17 | 19 | Day 13 | 15 | 17 | 19 | ||||
| Control | 0 | 26.4 | −0.2 | −0.4 | +0.2 | +0.8 | 1.00 | 1.00 | 1.00 | 1.00 | 0/6 | 0/6 |
| 4/i.p. | 35 | 27.8 | −1.7 | −2.1 | −2.1 | −2.4 | 0.35 | 0.14 | 0.04 | 0.02 | 3/6 | 0/6 |
| 4/i.v. | 15 | 27.0 | 0 | −0.6 | −1.1 | −2.6 | 0.47 | 0.30 | 0.10 | 0.04 | 0/6 | 4/6* |
| 2/i.v. | 0.6 | 27.0 | −0.9 | −0.5 | −3.3 | −3.4 | 0.67 | 0.63 | 0.61 | 0.51 | 0/6 | 0/6 |
| Paclitaxel/i.p. | 5 | 27.4 | −1.1 | −2.0 | −1.0 | −0.2 | 0.59 | 0.72 | 0.59 | 0.55 | 0/6 | 0/6 |
| Paclitaxel/i.v. | 15 | 27.2 | −0.6 | −0.8 | −0.08 | −0.9 | 0.36 | 0.13 | 0.04 | 0.01 | 3/6 | 0/6 |
Fifty-microgram tumor tissue was implanted s.c. on day 0. Every other day i.p. or i.v. treatments were given on days 9, 11, 13, 15, and 17. The average tumor size of control group (left side) on days 13, 15, 17, and 19 was 274, 378, 677, and 1,139 mm3, respectively. i.p. route used DMSO, and i.v. route used Cremophor + ETOH (1:1) as vehicles.
Four of six mice died of drug toxicity on days 23, 23, 23, and 25.
DISCUSSION
Two classes of naturally occurring compounds, Epos and paclitaxel, which apparently are structurally dissimilar, show similar mechanisms of action in stabilizing microtubule assemblies (3, 5, 9–14). These similarities include binding tubulin, substitution for paclitaxel in maintaining paclitaxel-dependent cell growth in a resistant cell line, and similar morphologic changes as determined by electron microscopic examination of the drug–microtubule complex. There are, however, differences between the two classes of compounds. These differences are most strikingly exhibited by the lack of cross-resistance in cytotoxicity between the Epos and paclitaxel even in CCRF-CEM/paclitaxel cells (Table 1). Furthermore, CCRF/CEM/VBL100, which are 527-fold resistant to vinblastine and 1,971-fold resistant to paclitaxel, were only 6.1-fold resistant to EpoB and 1.8-fold resistant to dEpoB (Table 1). In DC-3F/ADX cells, there was 13,000-fold resistance to actinomycin D and 338-fold resistance to paclitaxel. However, these cells were only 28-fold resistant to EpoB and had no resistance to dEpoB (i.e., 0.9-fold resistant or collateral sensitivity) (Table 2). It is of interest to note that paclitaxel showed a higher degree of cross-resistance in these cell lines than other MDR drugs such as doxorubicin, actinomycin D, vinblastine, or etoposide. In some cases, the degrees of resistance to paclitaxel were even greater than those of the resistance-selecting agent (e.g., CCRF-CEM/VBL100 in Table 1 and SK-N-FI and MCF/7-Adr in Table 2). In contrast, among all compounds tested, dEpoB showed the least cross-resistance in several drug-resistant cell lines (e.g., DC-3F/Adr).
It should be noted that in this study we performed parallel cancer chemotherapeutic studies for EpoB, dEpoB, paclitaxel, and other drugs under the same experimental conditions (i.e., treatment schedule, Q2D; solvent vehicle, DMSO; and route of administration, i.p.), except for the studies shown in Tables 6 and 7, where the i.p. and i.v. routes were compared directly. Further studies using different schedules, different vehicles, and different routes of administration with different tumors are being organized and will be described in due course.
In summary, our results already indicate that even though EpoB 2 is the most potent of the epothilones in vitro, it is by no means the optimal candidate for cancer therapy in terms of therapeutic index (i.e., the therapeutic efficacy at tolerable dosage, or the ratio of toxic dose vs. the therapeutic dose). Compound 4, lacking the epoxide functionality, exhibited far superior therapeutic results in vivo as compared with the more potent EpoB 2. Similarly, the present therapeutic results for dEpoB (4) in MX-1 xenografts were far better than those for EpoB (2), paclitaxel, doxorubicin, vinblastine, or camptothecin when these drugs were administered i.p. In addition, the effects of 4 on MCF-7/Adr xenografts were significantly better than those for paclitaxel, doxorubicin, and camptothecin. Desoxyepothilone B (4) also showed moderate therapeutic effect, similar to paclitaxel, against the human ovarian adenocarcinoma, SK-OV3.
Thus far, we regard dEpoB (4) as our lead compound for potential development. Further evaluations of other fully synthetic epothilone analogs are planned. Indeed, some modified epothilones available by total synthesis are evidencing rather promising early in vitro results. Through the experiments reported herein, it was found that i.p. administration of dEpoB (4) is far better tolerated than the i.v. method. We currently are surveying methods of improving i.v. administration of the epothilones. In view of the finding that Epos have little or no cross-resistance against MDR tumor cells in vitro, the special therapeutic advantage of such compounds might be beneficial against MDR tumors.
Acknowledgments
This research was supported by the National Institutes of Health [Grants CA-28824 (S.J.D.) and CA-GM 72231 (A.B.)]. D.M. gratefully acknowledges an Army Breast Cancer Predoctoral Fellowship (F33 US ARMY DAMD 17-97-1-7146).
ABBREVIATIONS
- Epo
epothilone
- dEpoB
desoxyepothilone B
- MDR
multidrug resistant
- DX
doxorubicin (Adriamycin)
- VBL
vinblastine
- VM
teniposide
- CPT
camptothecin
- AD
actinomycin D
References
- 1.Höfle G, Bedorf N, Steinmetz H, Schumburg D, Gerth K, Reichenbach H. Angew Chem Int Ed Engl. 1996;35:1567–1569. [Google Scholar]
- 2.Gerth K, Bedorf N, Höfle G, Irschik H, Reichenbach H. J Antibiot. 1996;49:560–563. doi: 10.7164/antibiotics.49.560. [DOI] [PubMed] [Google Scholar]
- 3.Bollag D M, McQuency P A, Zhu J, Hensens O, Koupal L, Liesch J, Goetz M, Lazarides E, Woods C M. Cancer Res. 1995;55:2325–2333. [PubMed] [Google Scholar]
- 4.Balog A, Meng D, Kamenecka T, Bertinato P, Su D-S, Sorensen E J, Danishefsky S J. Angew Chem Int Ed Engl. 1996;35:2801–2803. [Google Scholar]
- 5.Su D-S, Balog A, Meng D, Bertinato P, Danishefsky S J, Zheng Y-H, Chou T-C, He L, Horwitz S B. Angew Chem Int Ed Engl. 1997;36:2093–2096. [Google Scholar]
- 6.Nicolaou K C, Winssinger N, Pastor J A, Ninkovic S, Sarabia F, He Y, Vourloumis D, Yang Z, Li T, Giannakakou P, Hamel E. Nature (London) 1997;387:268–272. doi: 10.1038/387268a0. [DOI] [PubMed] [Google Scholar]
- 7.Ynag Z, He Y, Vourloumis D, Vallberg H, Nicolaou K C. Angew Chem Int Ed Engl. 1997;36:166–168. [Google Scholar]
- 8.Schinzer D, Limberg A, Bauer A, Böhm O M, Cordes M. Angew Chem Int Ed Engl. 1997;36:523–524. [Google Scholar]
- 9.Meng D, Su D-S, Balog A, Bertinato P, Sorensen E J, Danishefsky S J, Zheng Y-H, Chou T-C, He L, Horwitz S B. J Am Chem Soc. 1997;119:2733–2734. [Google Scholar]
- 10.Muhlradt P F, Sasse F. Cancer Res. 1997;57:3344–3346. [PubMed] [Google Scholar]
- 11.Service R E. Science. 1996;274:2009. doi: 10.1126/science.274.5295.2009. [DOI] [PubMed] [Google Scholar]
- 12.Meng D, Bertinato P, Balog A, Su D-S, Kamenecka T, Sorensen E J, Danishefsky S J. J Am Chem Soc. 1997;119:10073–10092. [Google Scholar]
- 13.Su D-S, Meng D, Bertinato P, Balog A, Sorensen E J, Danishefsky S J, Zheng Y-H, Chou T-C, He L, Horwitz S B. Angew Chem Int Ed Engl. 1997;36:757–759. [Google Scholar]
- 14.Chou T-C, Zhang X-G, Danishefsky S J. Proc Am Assoc Cancer Res. 1998;39:163–164. [Google Scholar]
- 15.Schiff P B, Fant J, Horwitz S B. Nature (London) 1979;277:665–667. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- 16.Landino L M, MacDonald T L. In: The Chemistry and Pharmacology of paclitaxel and Its Derivatives. Favin V, editor. New York: Elsevier; 1995. , Chapter 7, p. 301. [Google Scholar]
- 17.Rose W C. Anti-Cancer Drugs. 1992;3:311–321. [PubMed] [Google Scholar]
- 18.Rowinsky E K, Eisenhauer E A, Chaudhry V, Arbuck S G, Donehower R C. Seminars Oncol. 1993;20:1–15. [PubMed] [Google Scholar]
- 19.Cass C E, Janowska-Wieczorck A, Lynch M A, Sheinin H, Hindenburg A, Beck W T. Cancer Res. 1989;49:5798–5804. [PubMed] [Google Scholar]
- 20.Danks M K, Yalowich J C, Beck W T. Cancer Res. 1987;47:1297–1301. [PubMed] [Google Scholar]
- 21.Scudiero D A, Shoemaker R H, Paull K D, Monks A, Tierney S, Nofziger T H, Currens M J, Seniff D, Boyd M R. Cancer Res. 1988;48:4827–4833. [PubMed] [Google Scholar]
- 22.Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren J T, Bokesch H, Kenny S, Boyd M R. J Natl Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 23.Chou T-C, Talalay P T. Adv Enzyme Reg. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 24.Chou J, Chou T-C. Dose-Effect Analysis with Microcomputers: Quantitation of ED50, Synergism, Antagonism, Low-Dose Risk, Reception-Ligand Binding and Enzyme Kinetics, IBM-PC software and manual. Cambridge, U.K.: Biosoft; 1987. [Google Scholar]