Abstract
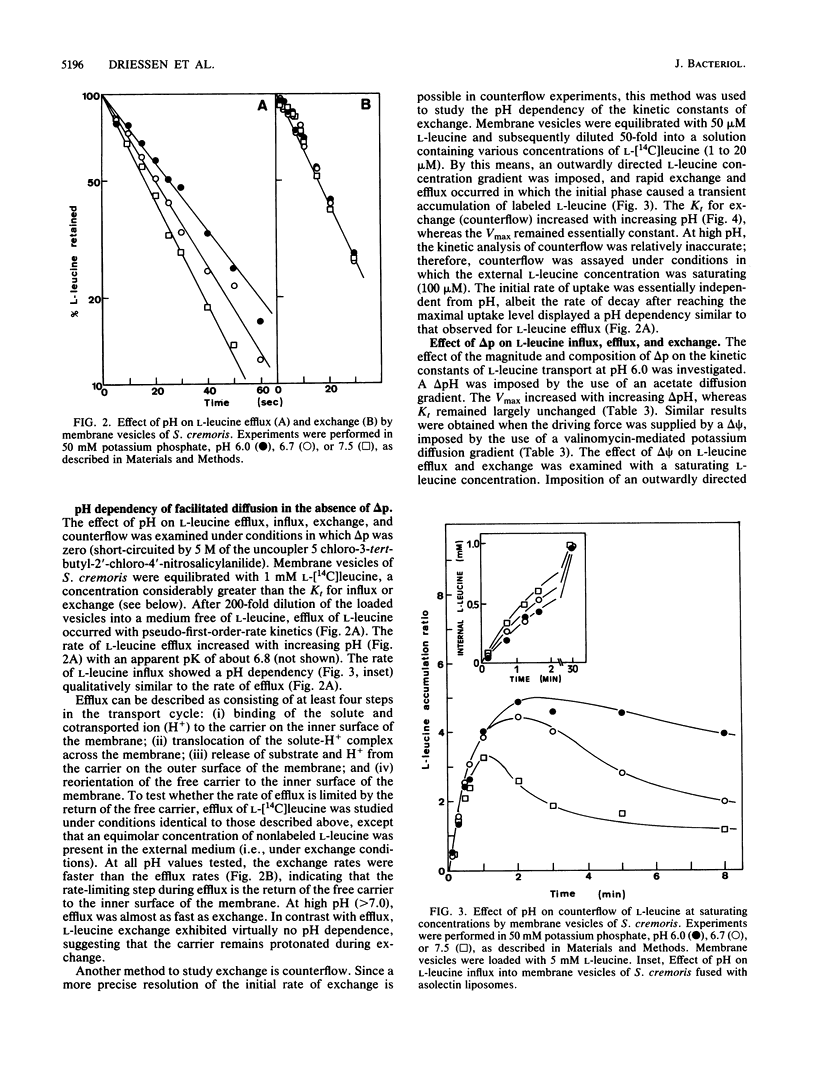
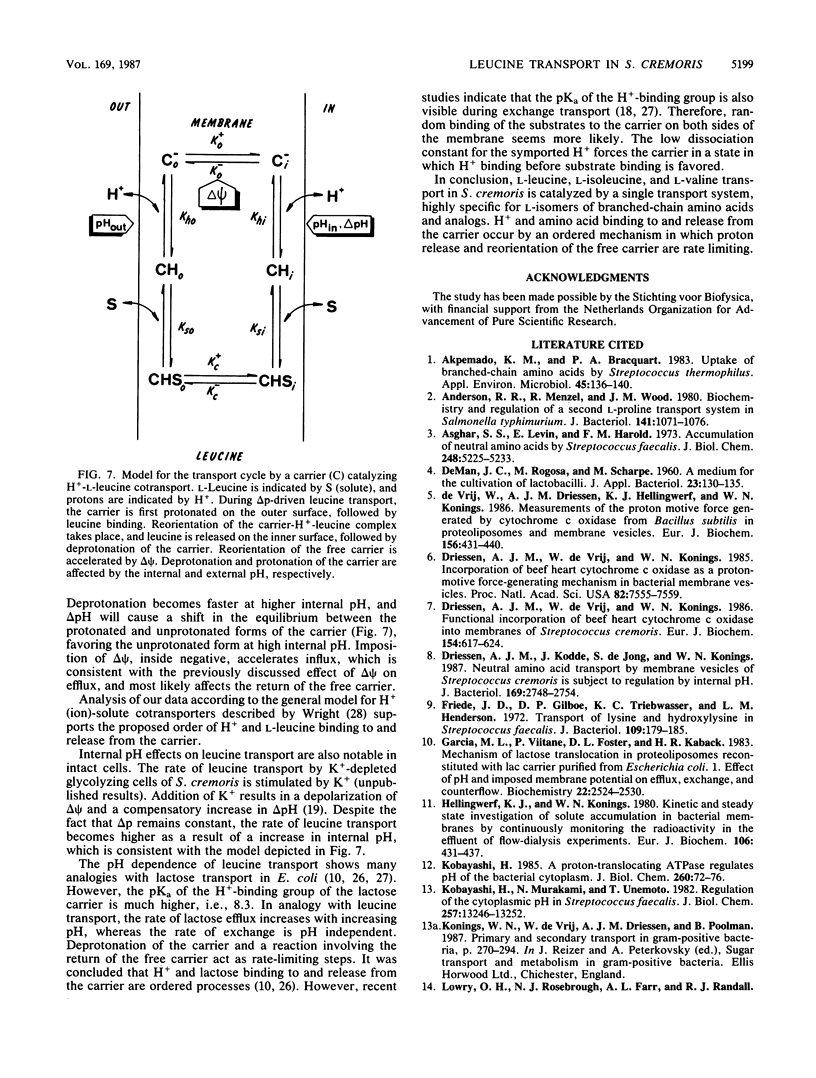
The kinetics, specificity, and mechanism of branched-chain amino acid transport in Streptococcus cremoris were studied in a membrane system of S. cremoris in which beef heart mitochondrial cytochrome c oxidase was incorporated as a proton motive force (delta p)-generating system. Influx of L-leucine, L-isoleucine, and L-valine can occur via a common transport system which is highly selective for the L-isomers of branched chain amino acids and analogs. The pH dependency of the kinetic constants of delta p-driven L-leucine transport and exchange (counterflow) was determined. The maximal rate of delta p-driven transport of L-leucine (Vmax) increased with increasing internal pH, whereas the affinity constant increased with increasing external pH. The affinity constant for exchange (counterflow) varied in a similar fashion with pH, whereas Vmax was pH independent. Further analysis of the pH dependency of various modes of facilitated diffusion, i.e., efflux, exchange, influx, and counterflow, suggests that H+ and L-leucine binding and release to and from the carrier proceed by an ordered mechanism. A kinetic scheme of the translocation cycle of H+-L-leucine cotransport is suggested.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akpemado K. M., Bracquart P. A. Uptake of Branched-Chain Amino Acids by Streptococcus thermophilus. Appl Environ Microbiol. 1983 Jan;45(1):136–140. doi: 10.1128/aem.45.1.136-140.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R. R., Menzel R., Wood J. M. Biochemistry and regulation of a second L-proline transport system in Salmonella typhimurium. J Bacteriol. 1980 Mar;141(3):1071–1076. doi: 10.1128/jb.141.3.1071-1076.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asghar S. S., Levin E., Harold F. M. Accumulation of neutral amino acids by Streptococcus faecalis. Energy coupling by a proton-motive force. J Biol Chem. 1973 Aug 10;248(15):5225–5233. [PubMed] [Google Scholar]
- Driessen A. J., Kodde J., de Jong S., Konings W. N. Neutral amino acid transport by membrane vesicles of Streptococcus cremoris is subject to regulation by internal pH. J Bacteriol. 1987 Jun;169(6):2748–2754. doi: 10.1128/jb.169.6.2748-2754.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen A. J., de Vrij W., Konings W. N. Functional incorporation of beef-heart cytochrome c oxidase into membranes of Streptococcus cremoris. Eur J Biochem. 1986 Feb 3;154(3):617–624. doi: 10.1111/j.1432-1033.1986.tb09443.x. [DOI] [PubMed] [Google Scholar]
- Driessen A. J., de Vrij W., Konings W. N. Incorporation of beef heart cytochrome c oxidase as a proton-motive force-generating mechanism in bacterial membrane vesicles. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7555–7559. doi: 10.1073/pnas.82.22.7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friede J. D., Gilboe D. P., Triebwasser K. C., Henderson L. M. Transport of lysine and hydroxylysine in Streptococcus faecalis. J Bacteriol. 1972 Jan;109(1):179–185. doi: 10.1128/jb.109.1.179-185.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M. L., Viitanen P., Foster D. L., Kaback H. R. Mechanism of lactose translocation in proteoliposomes reconstituted with lac carrier protein purified from Escherichia coli. 1. Effect of pH and imposed membrane potential on efflux, exchange, and counterflow. Biochemistry. 1983 May 10;22(10):2524–2531. doi: 10.1021/bi00279a033. [DOI] [PubMed] [Google Scholar]
- Hellingwerf K. J., Konings W. N. Kinetic and steady-state investigations of solute accumulation in bacterial membranes by continuously monitoring the radioactivity in the effluent of flow-dialysis experiments. Eur J Biochem. 1980 May;106(2):431–437. doi: 10.1111/j.1432-1033.1980.tb04589.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi H. A proton-translocating ATPase regulates pH of the bacterial cytoplasm. J Biol Chem. 1985 Jan 10;260(1):72–76. [PubMed] [Google Scholar]
- Kobayashi H., Murakami N., Unemoto T. Regulation of the cytoplasmic pH in Streptococcus faecalis. J Biol Chem. 1982 Nov 25;257(22):13246–13252. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Moran J. W. Branched-chain amino acid transport in Streptococcus agalactiae. Appl Environ Microbiol. 1980 Jul;40(1):25–31. doi: 10.1128/aem.40.1.25-31.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto R., Lageveen R. G., Veldkamp H., Konings W. N. Lactate efflux-induced electrical potential in membrane vesicles of Streptococcus cremoris. J Bacteriol. 1982 Feb;149(2):733–738. doi: 10.1128/jb.149.2.733-738.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto R., Sonnenberg A. S., Veldkamp H., Konings W. N. Generation of an electrochemical proton gradient in Streptococcus cremoris by lactate efflux. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5502–5506. doi: 10.1073/pnas.77.9.5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M. G. The role of protons in the mechanism of galactoside transport via the lactose permease of Escherichia coli. Biochim Biophys Acta. 1987 Feb 12;897(1):112–126. doi: 10.1016/0005-2736(87)90319-1. [DOI] [PubMed] [Google Scholar]
- Poolman B., Hellingwerf K. J., Konings W. N. Regulation of the glutamate-glutamine transport system by intracellular pH in Streptococcus lactis. J Bacteriol. 1987 May;169(5):2272–2276. doi: 10.1128/jb.169.5.2272-2276.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman B., Smid E. J., Konings W. N. Kinetic properties of a phosphate-bond-driven glutamate-glutamine transport system in Streptococcus lactis and Streptococcus cremoris. J Bacteriol. 1987 Jun;169(6):2755–2761. doi: 10.1128/jb.169.6.2755-2761.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston R. L., Schaeffer J. F., Curran P. F. Structure-affinity relationships of substrates for the neutral amino acid transport system in rabbit ileum. J Gen Physiol. 1974 Oct;64(4):443–467. doi: 10.1085/jgp.64.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Panos C. Transport of alpha-aminoisobutyric acid by Streptococcus pyogenes and its derived L-form. J Bacteriol. 1982 Jan;149(1):211–220. doi: 10.1128/jb.149.1.211-220.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. Characteristics and energy requirements of an alpha-aminoisobutyric acid transport system in Streptococcus lactis. J Bacteriol. 1976 Aug;127(2):719–730. doi: 10.1128/jb.127.2.719-730.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombe M. C., Lanéelle G., Sicard A. M. Characterization of a Streptococcus pneumoniae mutant with altered electric transmembrane potential. J Bacteriol. 1984 Jun;158(3):1109–1114. doi: 10.1128/jb.158.3.1109-1114.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viitanen P., Garcia M. L., Foster D. L., Kaczorowski G. J., Kaback H. R. Mechanism of lactose translocation in proteoliposomes reconstituted with lac carrier protein purified from Escherichia coli. 2. Deuterium solvent isotope effects. Biochemistry. 1983 May 10;22(10):2531–2536. doi: 10.1021/bi00279a034. [DOI] [PubMed] [Google Scholar]
- Wright J. K. Experimental analysis of ion/solute cotransport by substrate binding and facilitated diffusion. Biochim Biophys Acta. 1986 Jan 29;854(2):219–230. doi: 10.1016/0005-2736(86)90114-8. [DOI] [PubMed] [Google Scholar]
- Wright J. K., Riede I., Overath P. Lactose carrier protein of Escherichia coli: interaction with galactosides and protons. Biochemistry. 1981 Oct 27;20(22):6404–6415. doi: 10.1021/bi00525a019. [DOI] [PubMed] [Google Scholar]
- Wright J. K. The kinetic mechanism of galactoside/H+ cotransport in Escherichia coli. Biochim Biophys Acta. 1986 Mar 13;855(3):391–416. doi: 10.1016/0005-2736(86)90085-4. [DOI] [PubMed] [Google Scholar]
- Yu C., Yu L., King T. E. Studies on cytochrome oxidase. Interactions of the cytochrome oxidase protein with phospholipids and cytochrome c. J Biol Chem. 1975 Feb 25;250(4):1383–1392. [PubMed] [Google Scholar]
- de Vrij W., Driessen A. J., Hellingwerf K. J., Konings W. N. Measurements of the proton motive force generated by cytochrome c oxidase from Bacillus subtilis in proteoliposomes and membrane vesicles. Eur J Biochem. 1986 Apr 15;156(2):431–440. doi: 10.1111/j.1432-1033.1986.tb09600.x. [DOI] [PubMed] [Google Scholar]



