Abstract
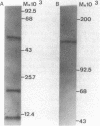
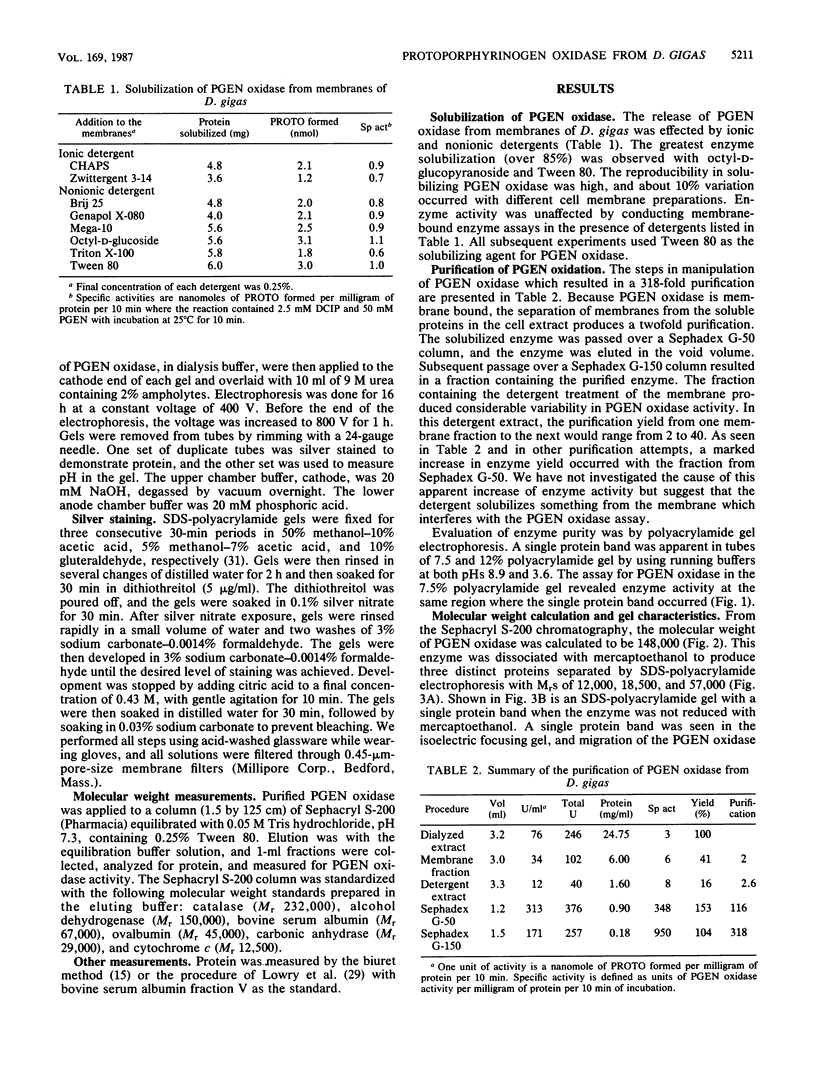
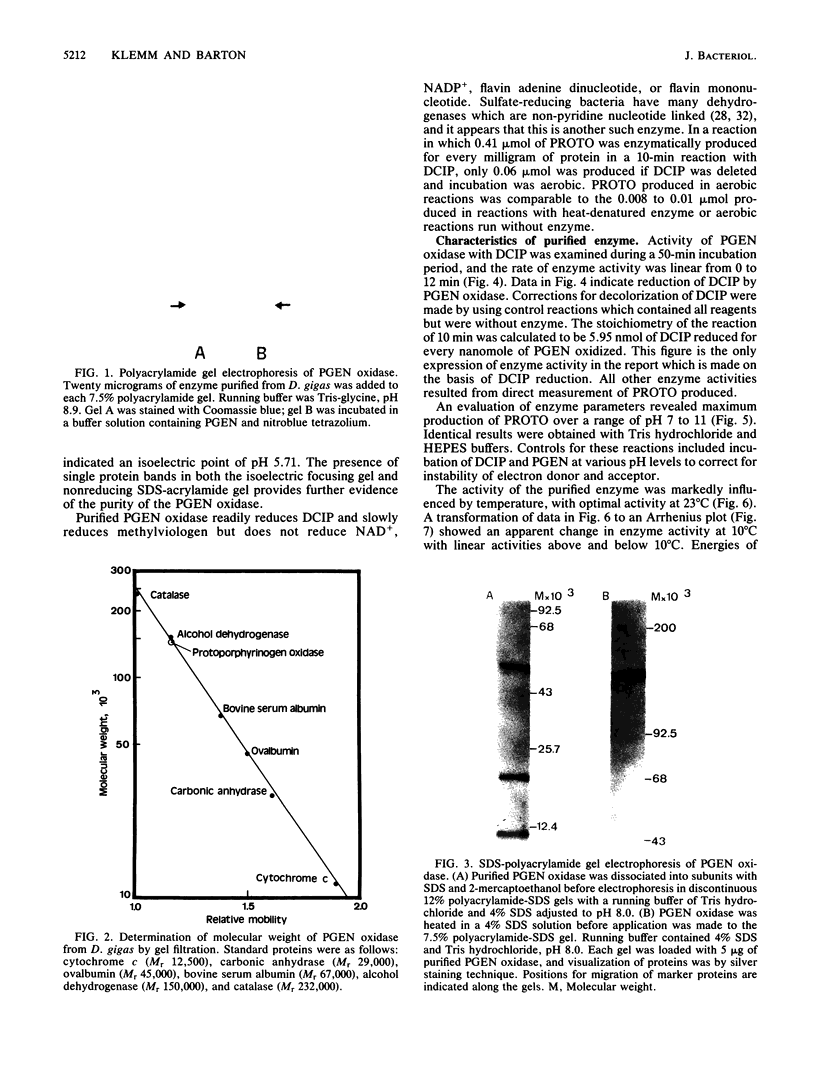
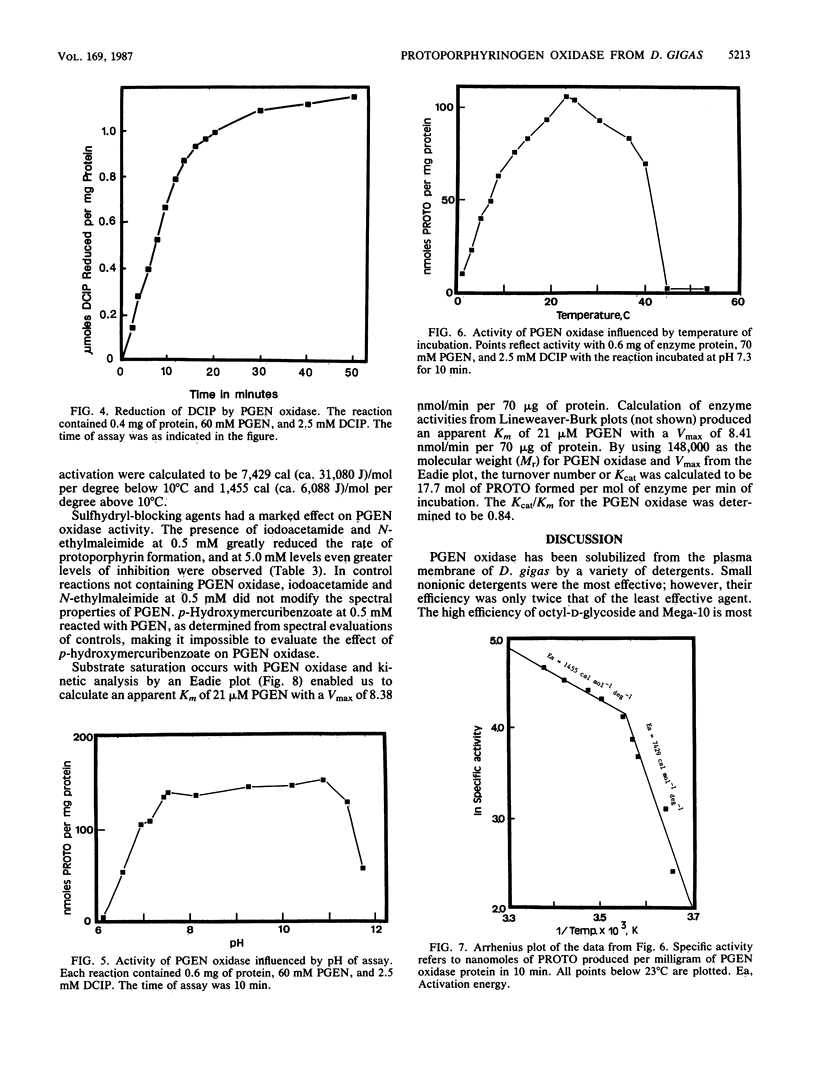
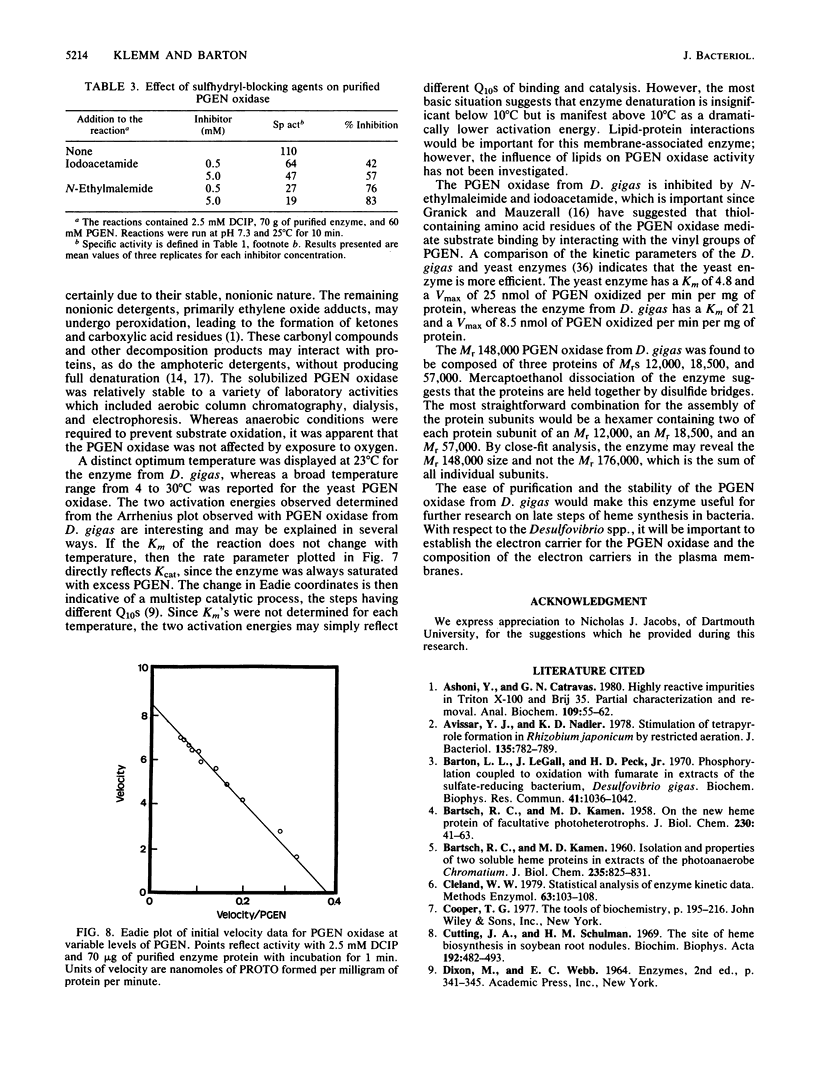
Protoporphyrinogen oxidase has been solubilized from plasma membranes of Desulfovibrio gigas. The enzyme was purified to apparent homogeneity with single silver-stained protein bands on isoelectric focusing and sodium dodecyl sulfate-polyacrylamide gels. This protoporphyrinogen oxidase has a molecular weight (Mr) of 148,000 and is composed of three dissimilar subunits of Mrs 12,000, 18,500, and 57,000, which are held together by sulfhydryl bonds. Unlike other protoporphyrinogen oxidases, which use molecular oxygen as an electron acceptor, this enzyme does not couple to oxygen. The protoporphyrinogen oxidase donates electrons to 2,6-dichlorophenol-indophenol but not to NAD+, NADP+, flavin adenine dinucleotide, or flavin mononucleotide. The natural physiological electron acceptor of the protoporphyrinogen oxidase from D. gigas is unknown. By using 2,6-dichlorophenol-indophenol as the electron acceptor, the Km and Vmax values for oxidation of protoporphyrinogen were determined to be 21 microM and 8.38 nmol/min per 70 micrograms of protein, respectively. The catalytic rate constant, Kcat, was calculated to be 17.7 mol of protoporphyrin formed per mole of enzyme per min of incubation, and the Kcat/Km was 0.84. Energies of activation were calculated from Arrhenius plots with 7,429 cal (ca. 31,080 J)/mol per degree below 10 degrees C and 1,455 cal (ca. 6,088, J)/mol per degree above 10 degrees C. Optimum enzyme activity was at 23 degrees C, and inhibition was observed with both N-ethylmaleimide and iodoacetamide.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashani Y., Catravas G. N. Highly reactive impurities in Triton X-100 and Brij 35: partial characterization and removal. Anal Biochem. 1980 Nov 15;109(1):55–62. doi: 10.1016/0003-2697(80)90009-3. [DOI] [PubMed] [Google Scholar]
- Avissar Y. J., Nadler K. D. Stimulation of tetrapyrrole formation in Rhizobium japonicum by restricted aeration. J Bacteriol. 1978 Sep;135(3):782–789. doi: 10.1128/jb.135.3.782-789.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTSCH R. G., KAMEN M. D. Isolation and properties of two soluble heme proteins in extracts of the photoanaerobe Chromatium. J Biol Chem. 1960 Mar;235:825–831. [PubMed] [Google Scholar]
- BARTSCH R. G., KAMEN M. D. On the new heme protein of facultative photoheterotrophs. J Biol Chem. 1958 Jan;230(1):41–63. [PubMed] [Google Scholar]
- Barton L. L., Le Gall J., Peck H. D., Jr Phosphorylation coupled to oxidation of hydrogen with fumarate in extracts of the sulfate reducing bacterium, Desulfovibrio gigas. Biochem Biophys Res Commun. 1970 Nov 25;41(4):1036–1042. doi: 10.1016/0006-291x(70)90189-0. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. Statistical analysis of enzyme kinetic data. Methods Enzymol. 1979;63:103–138. doi: 10.1016/0076-6879(79)63008-2. [DOI] [PubMed] [Google Scholar]
- Cutting J. A., Schulman H. M. The site of heme synthesis in soybean root nodules. Biochim Biophys Acta. 1969 Dec 30;192(3):486–493. doi: 10.1016/0304-4165(69)90398-5. [DOI] [PubMed] [Google Scholar]
- Fuller M. D., Caldwell D. R. Tetrapyrrole utilization of Bacteroides fragilis. Can J Microbiol. 1982 Dec;28(12):1304–1310. doi: 10.1139/m82-195. [DOI] [PubMed] [Google Scholar]
- GIBSON J. Cytochrome pigments from the green photosynthetic bacterium Chlorobium thiosulphatophilum. Biochem J. 1961 Apr;79:151–158. doi: 10.1042/bj0790151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANICK S., MAUZERALL D. Pbrphyrin biosynthesis in erythrocytes. II. Enzymes converting gamma-aminolevulinic acid to coproporphyrinogen. J Biol Chem. 1958 Jun;232(2):1119–1140. [PubMed] [Google Scholar]
- Gonenne A., Ernst R. Solubilization of membrane proteins by sulfobetaines, novel zwitterionic surfactants. Anal Biochem. 1978 Jun 15;87(1):28–38. doi: 10.1016/0003-2697(78)90565-1. [DOI] [PubMed] [Google Scholar]
- HORIO T., KAMEN M. D. Preparation and properties of three pure crystalline bacterial haem proteins. Biochim Biophys Acta. 1961 Apr 1;48:266–286. doi: 10.1016/0006-3002(61)90476-0. [DOI] [PubMed] [Google Scholar]
- Hjelmeland L. M. A nondenaturing zwitterionic detergent for membrane biochemistry: design and synthesis. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6368–6370. doi: 10.1073/pnas.77.11.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBS N. J., WOLIN M. J. Electron-transport system of Vibrio succinogenes. I. Enzymes and cytochromes of electron-transport system. Biochim Biophys Acta. 1963 Jan 1;69:18–28. doi: 10.1016/0006-3002(63)91221-6. [DOI] [PubMed] [Google Scholar]
- Jacobs N. J., Jacobs J. M. Assay for enzymatic protoporphyrinogen oxidation, a late step in heme synthesis. Enzyme. 1982;28(2-3):206–219. doi: 10.1159/000459103. [DOI] [PubMed] [Google Scholar]
- Jacobs N. J., Jacobs J. M., Brent P. Characterization of the late steps of microbial heme synthesis: conversion of coproporphyrinogen to protoporphyrin. J Bacteriol. 1971 Jul;107(1):203–209. doi: 10.1128/jb.107.1.203-209.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs N. J., Jacobs J. M., Brent P. Formation of protoporphyrin from coproporphyrinogen in extracts of various bacteria. J Bacteriol. 1970 May;102(2):398–403. doi: 10.1128/jb.102.2.398-403.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs N. J., Jacobs J. M. Fumarate as alternate electron acceptor for the late steps of anaerobic heme synthesis in Escherichia coli. Biochem Biophys Res Commun. 1975 Jul 8;65(1):435–441. doi: 10.1016/s0006-291x(75)80112-4. [DOI] [PubMed] [Google Scholar]
- Jacobs N. J., Jacobs J. M. Microbial oxidation of protoporhydrinogen, an intermediate in heme and chlorphyll biosynthesis. Arch Biochem Biophys. 1979 Oct 15;197(2):396–403. doi: 10.1016/0003-9861(79)90261-3. [DOI] [PubMed] [Google Scholar]
- Jacobs N. J., Jacobs J. M. Nitrate, fumarate, and oxygen as electron acceptors for a late step in microbial heme synthesis. Biochim Biophys Acta. 1976 Oct 13;449(1):1–9. doi: 10.1016/0005-2728(76)90002-5. [DOI] [PubMed] [Google Scholar]
- Klemm D. J., Barton L. L. Oxidation of protoporphyrinogen in the obligate anaerobe Desulfovibrio gigas. J Bacteriol. 1985 Oct;164(1):316–320. doi: 10.1128/jb.164.1.316-320.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McCall D. R., Caldwell D. R. Tetrapyrrole utilization by Bacteroids ruminocola. J Bacteriol. 1977 Sep;131(3):809–814. doi: 10.1128/jb.131.3.809-814.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- ORLANDO J. A. Rhodopseudomonas spheroides-cytochrome-553. Biochim Biophys Acta. 1962 Feb 26;57:373–375. doi: 10.1016/0006-3002(62)91133-2. [DOI] [PubMed] [Google Scholar]
- Odom J. M., Peck H. D., Jr Localization of dehydrogenases, reductases, and electron transfer components in the sulfate-reducing bacterium Desulfovibrio gigas. J Bacteriol. 1981 Jul;147(1):161–169. doi: 10.1128/jb.147.1.161-169.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulson R., Polglase W. J. The enzymic conversion of protoporphyrinogen IX to protoporphyrin IX. Protoporphyrinogen oxidase activity in mitochondrial extracts of Saccharomyces cerevisiae. J Biol Chem. 1975 Feb 25;250(4):1269–1274. [PubMed] [Google Scholar]
- Poulson R. The enzymic conversion of protoporphyrinogen IX to protoporphyrin IX in mammalian mitochondria. J Biol Chem. 1976 Jun 25;251(12):3730–3733. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]