Abstract
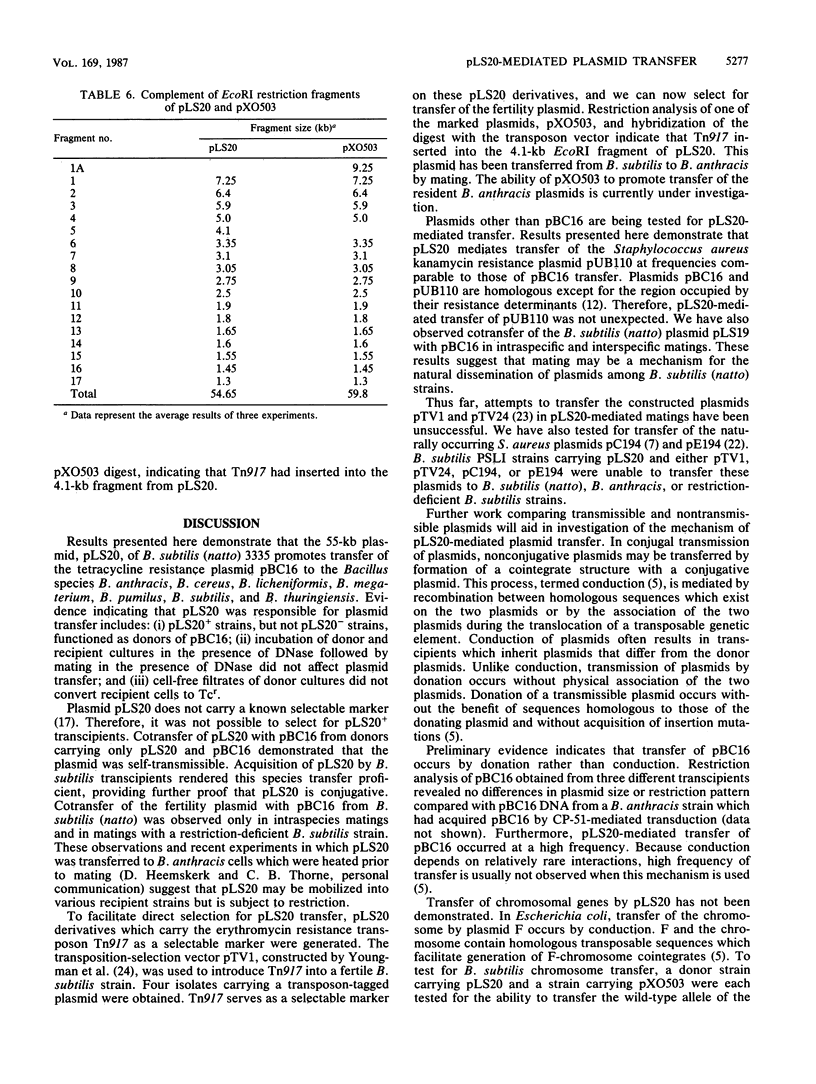
The 55-kilobase plasmid, pLS20, of Bacillus subtilis (natto) 3335 promotes transfer of the tetracycline resistance plasmid pBC16 from B. subtilis (natto) to the Bacillus species B. anthracis, B. cereus, B. licheniformis, B. megaterium, B. pumilus, B. subtilis, and B. thuringiensis. Frequency of pBC16 transfer ranged from 2.3 x 10(-6) to 2.8 x 10(-3). Evidence for a plasmid-encoded conjugationlike mechanism of genetic exchange includes (i) pLS20+ strains, but not pLS20- strains, functioned as donors of pBC16; (ii) plasmid transfer was insensitive to the presence of DNase; and (iii) cell-free filtrates of donor cultures did not convert recipient cells to Tcr. Cotransfer of pLS20 and pBC16 in intraspecies matings and in matings with a restriction-deficient B. subtilis strain indicated that pLS20 was self-transmissible. In addition to mobilizing pBC16, pLS20 mediated transfer of the B. subtilis (natto) plasmid pLS19 and the Staphylococcus aureus plasmid pUB110. The fertility plasmid did not carry a selectable marker. To facilitate direct selection for pLS20 transfer, plasmid derivatives which carried the erythromycin resistance transposon Tn917 were generated. Development of this method of genetic exchange will facilitate the introduction of plasmid DNA into nontransformable species by use of transformable fertile B. subtilis or B. subtilis (natto) strains as intermediates.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battisti L., Green B. D., Thorne C. B. Mating system for transfer of plasmids among Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis. J Bacteriol. 1985 May;162(2):543–550. doi: 10.1128/jb.162.2.543-550.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhard K., Schrempf H., Goebel W. Bacteriocin and antibiotic resistance plasmids in Bacillus cereus and Bacillus subtilis. J Bacteriol. 1978 Feb;133(2):897–903. doi: 10.1128/jb.133.2.897-903.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S., Cohen S. N. High frequency transformation of Bacillus subtilis protoplasts by plasmid DNA. Mol Gen Genet. 1979 Jan 5;168(1):111–115. doi: 10.1007/BF00267940. [DOI] [PubMed] [Google Scholar]
- Clark A. J., Warren G. J. Conjugal transmission of plasmids. Annu Rev Genet. 1979;13:99–125. doi: 10.1146/annurev.ge.13.120179.000531. [DOI] [PubMed] [Google Scholar]
- Green B. D., Battisti L., Koehler T. M., Thorne C. B., Ivins B. E. Demonstration of a capsule plasmid in Bacillus anthracis. Infect Immun. 1985 Aug;49(2):291–297. doi: 10.1128/iai.49.2.291-297.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryczan T. J., Contente S., Dubnau D. Characterization of Staphylococcus aureus plasmids introduced by transformation into Bacillus subtilis. J Bacteriol. 1978 Apr;134(1):318–329. doi: 10.1128/jb.134.1.318-329.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T., Aumayr A., Fujio Y., Ueda S. Elimination of plasmid-linked polyglutamate production by Bacillus subtilis (natto) with acridine orange. Appl Environ Microbiol. 1982 Dec;44(6):1456–1458. doi: 10.1128/aem.44.6.1456-1458.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak J., Novick R. P. Closely related plasmids from Staphylococcus aureus and soil bacilli. Plasmid. 1982 Mar;7(2):152–162. doi: 10.1016/0147-619x(82)90074-9. [DOI] [PubMed] [Google Scholar]
- Reddy A., Battisti L., Thorne C. B. Identification of self-transmissible plasmids in four Bacillus thuringiensis subspecies. J Bacteriol. 1987 Nov;169(11):5263–5270. doi: 10.1128/jb.169.11.5263-5270.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ruhfel R. E., Robillard N. J., Thorne C. B. Interspecies transduction of plasmids among Bacillus anthracis, B. cereus, and B. thuringiensis. J Bacteriol. 1984 Mar;157(3):708–711. doi: 10.1128/jb.157.3.708-711.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- THORNE C. B. Transduction in Bacillus subtilis. J Bacteriol. 1962 Jan;83:106–111. doi: 10.1128/jb.83.1.106-111.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Koshikawa T. Isolation and characterization of four types of plasmids from Bacillus subtilis (natto). J Bacteriol. 1977 Aug;131(2):699–701. doi: 10.1128/jb.131.2.699-701.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne C. B. Transducing bacteriophage for Bacillus cereus. J Virol. 1968 Jul;2(7):657–662. doi: 10.1128/jvi.2.7.657-662.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne C. B. Transduction in Bacillus thuringiensis. Appl Environ Microbiol. 1978 Jun;35(6):1109–1115. doi: 10.1128/aem.35.6.1109-1115.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisblum B., Graham M. Y., Gryczan T., Dubnau D. Plasmid copy number control: isolation and characterization of high-copy-number mutants of plasmid pE194. J Bacteriol. 1979 Jan;137(1):635–643. doi: 10.1128/jb.137.1.635-643.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngman P., Perkins J. B., Losick R. Construction of a cloning site near one end of Tn917 into which foreign DNA may be inserted without affecting transposition in Bacillus subtilis or expression of the transposon-borne erm gene. Plasmid. 1984 Jul;12(1):1–9. doi: 10.1016/0147-619x(84)90061-1. [DOI] [PubMed] [Google Scholar]