Abstract
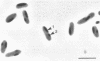
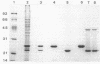
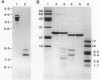
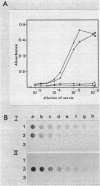
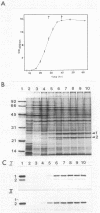
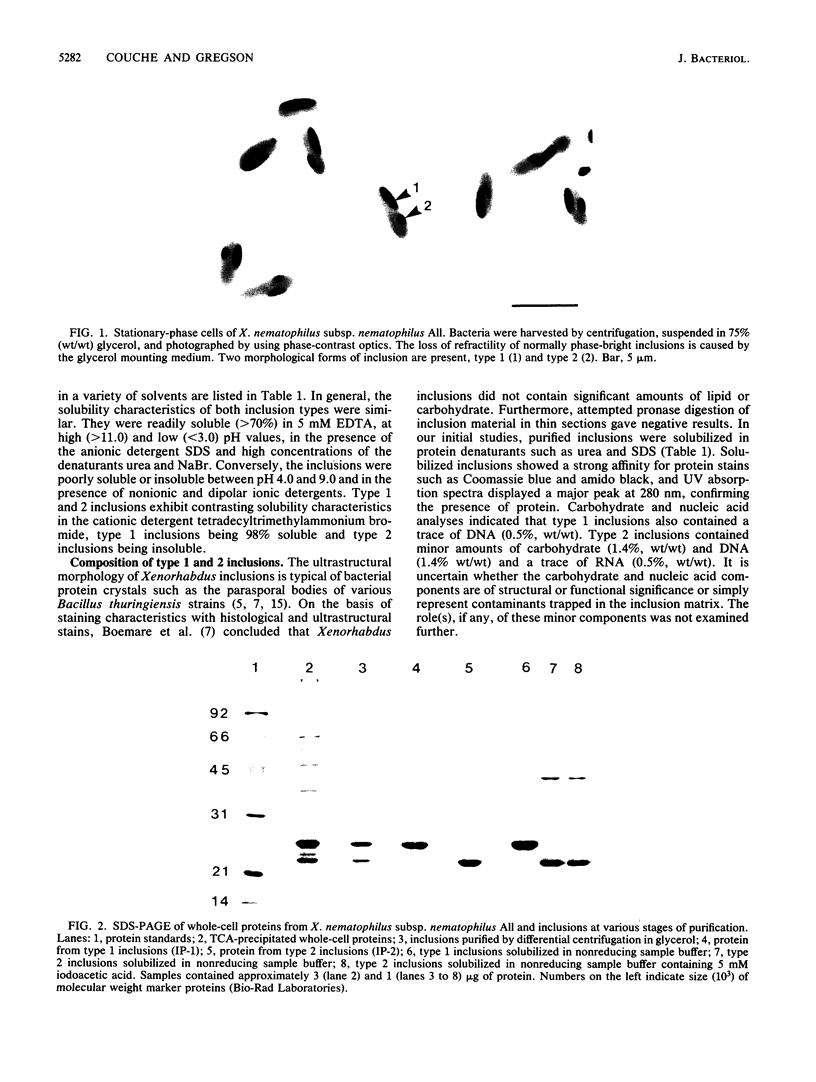
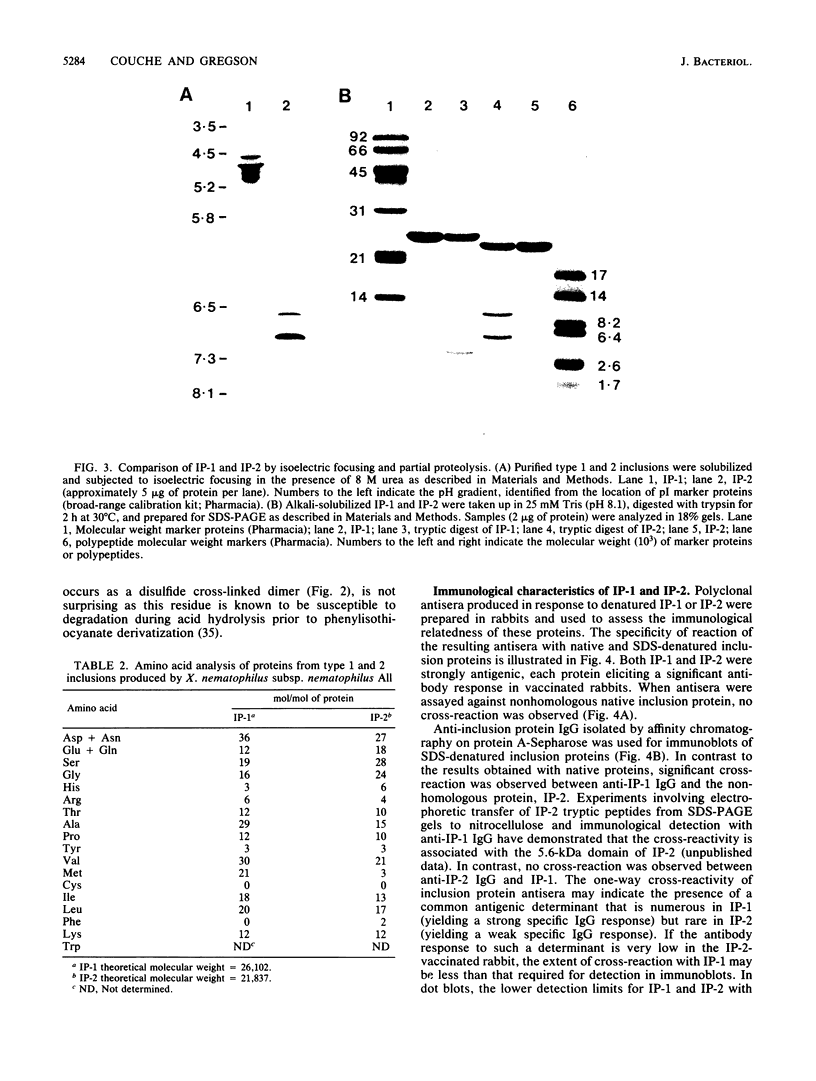
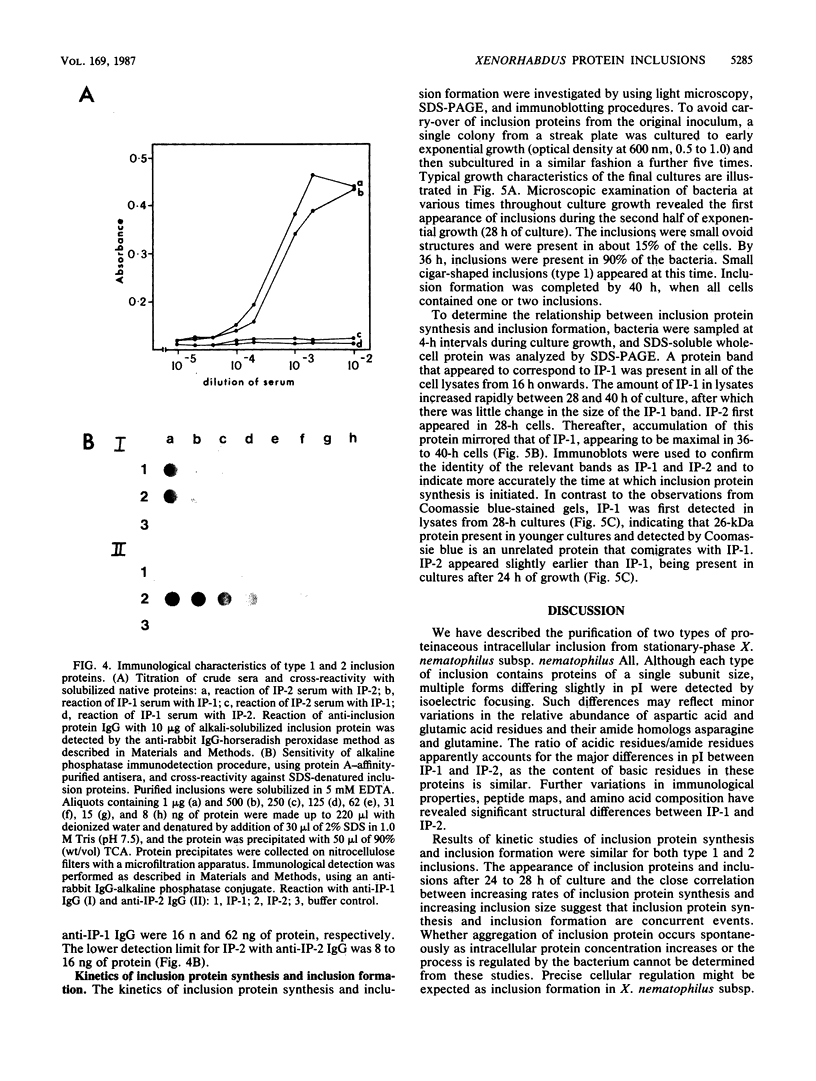
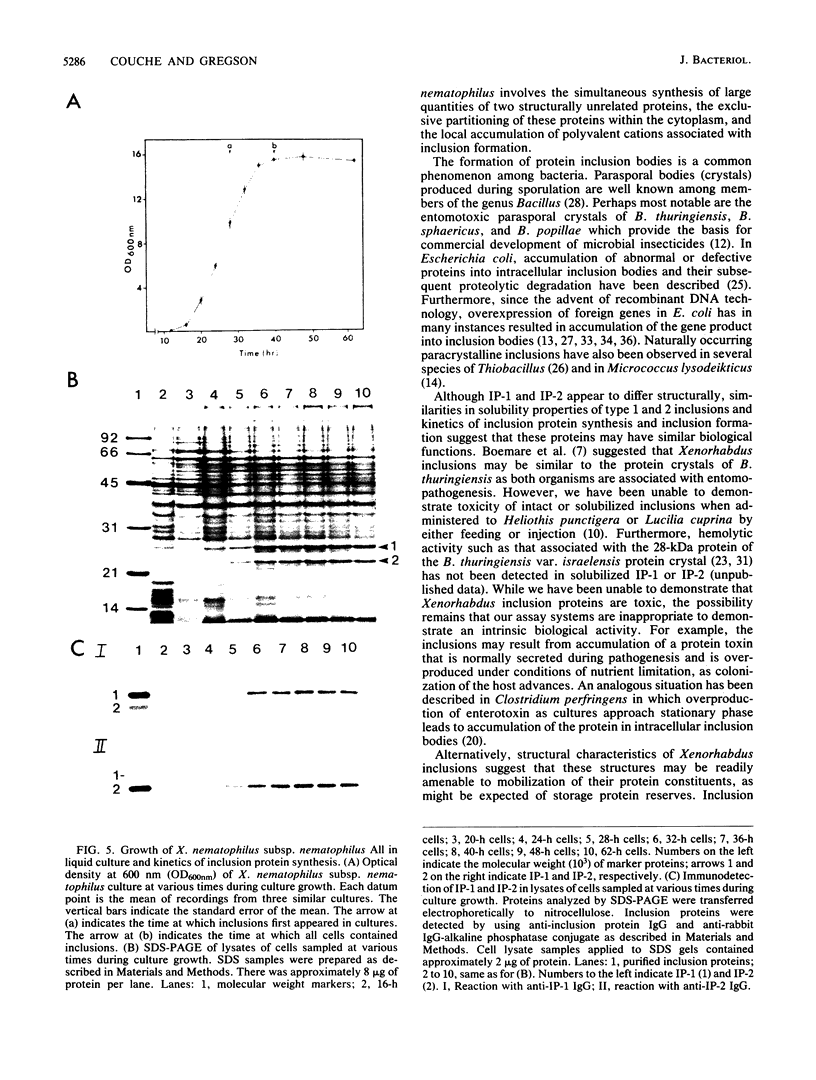
The entomopathogenic bacterium Xenorhabdus nematophilus subsp. nematophilus produces two types of intracellular inclusion bodies during in vitro culture. Large cigar-shaped inclusions (designated type 1) and smaller ovoid inclusions (designated type 2) were purified from cell lysates, using differential centrifugation in discontinuous glycerol gradients and isopycnic density gradient centrifugation in sodium diatrizoate. The inclusions, composed almost exclusively of protein, are readily soluble at high and low pH values and in the presence of cation chelators such as EDTA, anionic detergents (sodium dodecyl sulfate), or protein denaturants (urea, NaBr). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of purified inclusions revealed a single 26-kilodalton protein (IP-1) in type 1 inclusions and a 22-kilodalton protein (IP-2) in type 2 inclusions. Analysis of these proteins by isoelectric focusing in the presence of 8 M urea showed that IP-1 is acidic and IP-2 is neutral. Furthermore, each protein occurred in multiple forms differing slightly in isoelectric point. Other variations in peptides released by trypsin digestion, immunological properties, and amino acid composition revealed significant structural differences between IP-1 and IP-2. Kinetic studies using light microscopy, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and immunoblotting procedures showed that inclusion protein synthesis occurs only during the second half of exponential culture growth. Synthesis of inclusion proteins and their aggregation to form inclusions occurred concurrently. Possible functions for these abundant proteins are discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akhurst R. J. Antibiotic activity of Xenorhabdus spp., bacteria symbiotically associated with insect pathogenic nematodes of the families Heterorhabditidae and Steinernematidae. J Gen Microbiol. 1982 Dec;128(12):3061–3065. doi: 10.1099/00221287-128-12-3061. [DOI] [PubMed] [Google Scholar]
- Aronson A. I., Beckman W., Dunn P. Bacillus thuringiensis and related insect pathogens. Microbiol Rev. 1986 Mar;50(1):1–24. doi: 10.1128/mr.50.1.1-24.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cohen S. A., Bidlingmeyer B. A., Tarvin T. L. PITC derivatives in amino acid analysis. Nature. 1986 Apr 24;320(6064):769–770. doi: 10.1038/320769a0. [DOI] [PubMed] [Google Scholar]
- Dastidar P. G., Nickerson K. W. Interchain crosslinks in the entomocidal Bacillus thuringiensis protein crystal. FEBS Lett. 1979 Dec 15;108(2):411–414. doi: 10.1016/0014-5793(79)80575-x. [DOI] [PubMed] [Google Scholar]
- Friedberg I., Avigad G. Structures containing polyphosphate in Micrococcus lysodeikticus. J Bacteriol. 1968 Aug;96(2):544–553. doi: 10.1128/jb.96.2.544-553.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Löffler A., Labbé R. Characterization of a parasporal inclusion body from sporulating, enterotoxin-positive Clostridium perfringens type A. J Bacteriol. 1986 Feb;165(2):542–548. doi: 10.1128/jb.165.2.542-548.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poinar G. O., Jr, Thomas G. M. Significance of Achromobacter nematophilus Poinar and Thomas (Achromobacteraceae: Eubacteriales) in the development of the nematode, DD-136 (Neoaplectana sp. Steinernematidae). Parasitology. 1966 May;56(2):385–390. doi: 10.1017/s0031182000070980. [DOI] [PubMed] [Google Scholar]
- Prouty W. F., Goldberg A. L. Fate of abnormal proteins in E. coli accumulation in intracellular granules before catabolism. Nat New Biol. 1972 Nov 29;240(100):147–150. doi: 10.1038/newbio240147a0. [DOI] [PubMed] [Google Scholar]
- Shively J. M., Decker G. L., Greenawalt J. W. Comparative ultrastructure of the thiobacilli. J Bacteriol. 1970 Feb;101(2):618–627. doi: 10.1128/jb.101.2.618-627.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas W. E., Ellar D. J. Bacillus thuringiensis var israelensis crystal delta-endotoxin: effects on insect and mammalian cells in vitro and in vivo. J Cell Sci. 1983 Mar;60:181–197. doi: 10.1242/jcs.60.1.181. [DOI] [PubMed] [Google Scholar]
- Weis J. H., Enquist L. W., Salstrom J. S., Watson R. J. An immunologically active chimaeric protein containing herpes simplex virus type 1 glycoprotein D. Nature. 1983 Mar 3;302(5903):72–74. doi: 10.1038/302072a0. [DOI] [PubMed] [Google Scholar]
- Williams D. C., Van Frank R. M., Muth W. L., Burnett J. P. Cytoplasmic inclusion bodies in Escherichia coli producing biosynthetic human insulin proteins. Science. 1982 Feb 5;215(4533):687–689. doi: 10.1126/science.7036343. [DOI] [PubMed] [Google Scholar]