Abstract
To investigate the role of aquaporin-1 (AQP1) water channels in proximal tubule function, in vitro proximal tubule microperfusion and in vivo micropuncture measurements were done on AQP1 knockout mice. The knockout mice were generated by targeted gene disruption and found previously to be unable to concentrate their urine in response to water deprivation. Unanesthetized knockout mice consumed 2.8-fold more fluid than wild-type mice and had lower urine osmolality (505 ± 40 vs. 1081 ± 68 milliosmolar). Transepithelial osmotic water permeability (Pf) in isolated microperfused S2 segments of proximal tubule from AQP1 knockout [−/−] mice was 0.033 ± 0.005 cm/s (SE, n = 6 mice, 37°C), much lower than that of 0.15 ± 0.03 cm/s (n = 8) in tubules from wild-type [+/+] mice (P < 0.01). In the presence of isosmolar luminal perfusate and bath solutions, spontaneous fluid absorption rates (nl/min/mm tubule length) were 0.31 ± 0.12 (−/−, n = 5) and 0.64 ± 0.15 (+/+, n = 8). As determined by free-flow micropuncture, the ratios of tubular fluid-to-plasma concentrations of an impermeant marker TF/P in end proximal tubule fluid were 1.36 ± 0.05 (−/−, n = 8 mice [53 tubules]) and 1.95 ± 0.09 (+/+, n = 7 mice [40 tubules]) (P < 0.001), corresponding to 26 ± 3% [−/−] and 48 ± 2% [+/+] absorption of the filtered fluid load. In collections of distal tubule fluid, TF/P were 2.8 ± 0.3 [−/−] and 4.4 ± 0.5 [+/+], corresponding to 62 ± 4% [−/−] and 76 ± 3% [+/+] absorption (P < 0.02). These data indicate that AQP1 deletion in mice results in decreased transepithelial proximal tubule water permeability and defective fluid absorption. Thus, the high water permeability in proximal tubule of wild-type mice is primarily transcellular, mediated by AQP1 water channels, and required for efficient near-isosmolar fluid absorption.
Keywords: water transport/AQP1/urinary concentrating mechanism/kidney/micropuncture
An important function of the kidney proximal tubule is the near-isosmolar reabsorption of a significant fraction of fluid that is filtered by the glomerulus. The proximal tubule also reabsorbs nearly all of the filtered glucose, amino acids, and bicarbonate. The apical and basolateral plasma membranes of proximal tubule cells contain water channel protein aquaporin-1 (AQP1), which is thought to provide an important water-selective pathway for transcellular fluid transport (1–3). However, there is conflicting evidence that significant paracellular water transport occurs (4), and it has been suggested that other water channels (AQP7, ref. 5) and transporters (glucose transporter GLUT1, refs. 6, 7; sodium-glucose cotransporter SGLT1, ref. 8) might contribute to transcellular water movement. It is generally believed, but without direct evidence, that high proximal tubule water permeability is important to permit the efficient coupling of solute and water transport to accomplish near-isosmolar fluid absorption.
The AQP1 water channel is a water-selective transporter (9, 10) that is found in membranes as tetramers (11) in which each functionally independent monomer (12) contains six transmembrane, tilted helical domains surrounding a putative aqueous pore (13–15). In kidney, AQP1 is strongly expressed in apical and basolateral plasma membranes of epithelial cells in proximal tubule and thin descending limb of Henle and in endothelial cells of descending vasa recta (1–3, 16, 17). Recently, a transgenic AQP1 knockout mouse was generated by targeted gene disruption and shown to manifest a severe defect in urinary concentrating ability (18). When given access to water, the mice appeared grossly normal except for mild growth retardation compared with wild-type mice. When deprived of water, the mice were unable to concentrate their urine and conserve fluid, resulting in marked dehydration and serum hyperosmolality in 1–2 days.
The purpose of this study was to define the role of AQP1 in proximal tubule water transport and fluid reabsorption. Isolated tubule microperfusion was used to measure transepithelial osmotic water permeability and fluid absorption under defined in vitro conditions. Free-flow micropuncture was used to determine the in vivo consequences of decreased proximal tubule water permeability. A remarkable decrease in proximal tubule water permeability and fluid reabsorption was found in the AQP1 knockout mice. The results have important implications regarding the mechanisms of proximal tubule fluid reabsorption.
METHODS
Transgenic Mice.
Transgenic knockout mice deficient in AQP1 protein were generated by targeted gene disruption as described (18). Measurements were done in litter-matched mice (6–10 weeks of age) produced by intercrossing of CD1 AQP1 heterozygotes. Genotype analysis of tail DNA was done by PCR at age 5 days. The investigators were blinded to genotype information for microperfusion and micropuncture measurements.
Isolated Tubule Microperfusion.
Proximal straight tubules (S2 segments, length 0.4–0.8 mm) were microdissected with fine forceps from medullary rays of freshly excised kidneys. Cortical tissue was taken from each mouse for AQP1 immunoblot analysis to confirm genotype. Individual tubule segments were mounted on glass pipettes and perfused in vitro at 37°C as first described by Burg (19). The tubule lumen was perfused with 295 milliosmolar (mosM) solution containing (in mM): 114 NaCl, 25 NaHCO3, 2 K2HPO2, 2CaCl2, 1.2 MgSO2, 5.5 glucose, 6 alanine, 4 sodium lactate, 1 sodium citrate, 2 sodium acetate, 4 glycine, and 1 heptanoic acid, pH 7.4. In the first experimental period in which spontaneous fluid absorption (Jv, nl/min/mm tubule length) was measured, the peritubular bath solution was identical to the perfusate except for addition of 6 g/dl BSA to the bath solution. In the second period, osmotic water permeability (Pf, cm/s) was measured from the transepithelial water flux in response to a 50 mosM bath-to-lumen osmotic gradient. The peritubular bath was the same as the perfusate except that 45 mM raffinose (345 mosM) and 6 g/dl BSA was added. The luminal solution also contained 0.5 mM fluorescein isothiocyanate-dextran (10,000 MW, Molecular Probes) as a volume marker. The fluorescence of the perfusate in the collected fluid was determined by a continuous flow ultramicrofluorometer (20). Pf was calculated from measured tubule length and diameter, initial lumen flow rate, and perfused and collected marker concentrations.
Micropuncture.
Measurements were made on 7 [+/+] (five male, two female) and eight AQP1 [−/−] (six male, two female) mice maintained on standard rodent chow and tap water. Mice were anesthetized with thiobutabarbital (inactin, 100 mg/kg i.p.) and ketamine (100 mg/kg, i.m.) (21). Body temperature was maintained at 38°C by using a heating plate. The trachea was cannulated and 100% oxygen was blown toward the tube throughout the experiment. The femoral artery was cannulated with polyethylene tubing (≈300 μm outer diameter) for blood pressure measurement and blood sample withdrawal. The femoral vein was cannulated for continuous maintenance infusion of 2.25 g/dl BSA in saline at a rate of 0.35 ml/hr (1.25–1.4 ml/hr per 100 g of body weight). Urine was collected by using a bladder catheter. The left kidney was approached from a flank incision, freed of adherent fat and connective tissue, placed in a lucite cup, and covered with mineral oil.
To determine nephron filtration and absorption rates, mice were infused with methoxy-[3H]inulin (New England Nuclear) at ≈60 μCi/hr (1 Ci = 37 GBq) (for 2 [+/+] and 2 [−/−] mice) or [125I]iothalamate (Glofil, Cypros Pharmaceutical, Carlsbad, CA) at ≈40 μCi/hr (for 5 [+/+] and 6 [−/−] mice). The first blood samples were obtained after 45-min equilibration. Free-flow micropuncture was performed according to techniques used previously in rats (22). Briefly, end-proximal and distal segments were identified by injecting a bolus of artificial tubular fluid stained with FD&C green from a 3–4-μm tip pipette connected to a pressure manometer. This pipette remained in place during the collections to permit control of intratubular pressure. All of the proximal collections were done in the last surface segment whereas distal collections were restricted to early sites defined as the first of two surface segments. Timed fluid collections in 2–9 proximal and 1–3 distal segments per experiment were made with oil-filled pipettes over 3.5–5 min. Fluid volume was determined from column length in a constant bore capillary. Blood samples were collected in heparinized 5-μl microcaps at the beginning, after 45–60 min, and after 110–120 min. Experiments did not extend beyond 2 hr. [3H]inulin or [125I]iothalamate radioactivities were measured in duplicate by using 0.5-μl samples of plasma and urine. Statistical significance was assessed by unpaired t test.
RESULTS
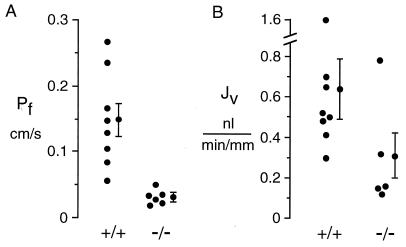
Fig. 1A summarizes osmotic water permeability coefficients (Pf) measured in isolated microperfused proximal tubules from wild-type [+/+] and AQP1 knockout [−/−] mice. Pf was determined from the volume transported out of the lumen in response to a 50-mM bath-to-lumen osmotic gradient. Averaged Pf in [+/+] mice was 0.15 ± 0.03 cm/s at 37°C, in agreement with previous published data (23, 24). Pf was decreased by 78% in [−/−] mice (0.033 ± 0.005 cm/s) (P < 0.01). It was noted that proximal tubule cells from several [+/+] mice showed intracellular vacuole formation during perfusion with the hyperosmolar bath. This phenomenon, which has been referred to as “proximal rot,” is thought to result from the nonphysiologically high transcellular volume flows required in tubule perfusion measurements.
Figure 1.
Pf and Jv in isolated microperfused proximal tubules. S2 segments of proximal tubule were dissected from wild-type [+/+] and AQP1 knockout [−/−] mice and microperfused in vitro at 37°C as described in Methods. (A) Osmotic water permeability. Tubules were perfused with 295 mosM buffer and bathed in a 345 mosM buffer to give a 50 mosM bath-to-lumen osmotic gradient. Pf was computed from the increase in concentration of a membrane-impermeant marker at the distal end of the tubule. (B) Near-isosmolar fluid reabsorption. Tubules were perfused and bathed in an isosmolar buffer. Jv was computed from the increase in luminal marker concentration. For A and B, each point is the averaged data from 1 or 2 tubules from one mouse. Averaged data with SEs (n = number of different mice) is shown at the right of each data set.
To determine whether AQP1 deletion affects spontaneous, actively driven fluid reabsorption from the lumen, proximal tubules were perfused and bathed with identical isosmolar solutions. Jv was measured from the increase in concentration of a luminal volume marker (Fig. 1B). Jv in proximal tubules from [−/−] mice (0.31 ± 0.12 nl/min/mm tubule length) was lower than that from [+/+] mice (0.64 ± 0.15 nl/min/mm), although there was considerable variability in the results from different tubules.
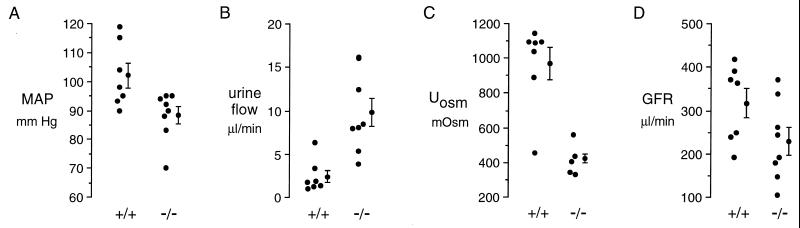
Free-flow micropuncture was done to determine whether the decrease in proximal tubule reabsorption suggested by the in vitro microperfusion measurements occurs in vivo. Fig. 2 and Table 1 summarize key parameters for the mice that underwent micropuncture. As reported previously (18), body and kidney weights in AQP1 [−/−] mice were slightly lower than in age-matched controls. Although kidney weight was proportionately decreased in [−/−] mice, wet-to-dry weight ratios did not differ. In the anesthetized mice prepared for micropuncture, mean arterial blood pressure (Fig. 2A) was significantly lower in [−/−] mice (88 ± 3 mm Hg vs. 102 ± 4 mm Hg, P = 0.019), which is probably related to extracellular fluid volume depletion. Urine flow (Fig. 2B) was significantly increased in [−/−] mice, and urine osmolality was significantly decreased (Fig. 2C). Glomerular filtration rate (GFR, Fig. 2D) for both kidneys averaged 317 ± 34 μl/min in [+/+] and 230 ± 33 μl/min in [−/−] mice (P = 0.08). For comparison, in eight age-matched, unanesthetized mice given free access to water, urine osmolalities were 1081 ± 68 mosM [+/+] and 505 ± 40 mosM [−/−] (P < 0.01). The unanesthetized knockout mice were polydipsic and polyuric, consuming 14.6 ± 2.0 ml fluid/day [−/−] vs. 5.2 ± 0.6 ml fluid/day [+/+] (P < 0.01).
Figure 2.
Blood pressure and urinary parameters in anesthetized mice prepared for micropuncture. (A) Mean arterial blood pressure (MAP). (B) Urine flow. (C) Urine osmolality. (D) Glomerular filtration rate. Each point represents averaged measurements from one mouse. Averaged data with SEs is shown at the right of each data set. Significance values are given in Table 1.
Table 1.
Summary of micropuncture data
| Units | +/+ | −/− | P value | |
|---|---|---|---|---|
| Physiological data | ||||
| Body weight | g | 29.0 ± 2.0 | 24.9 ± 1.1 | 0.078 |
| Kidney weight | mg | 207 ± 20 | 180 ± 10 | 0.23 |
| Kidney dry weight | % total weight | 22.9 ± 0.9 | 22.3 ± 0.8 | 0.66 |
| MAP | mm Hg | 102 ± 4.2 | 88.4 ± 3.0 | 0.019 |
| Uosm | mosM | 972 ± 92 | 423 ± 25 | <0.001 |
| Urine flow | μl/min | 2.4 ± 0.7 | 9.8 ± 1.6 | 0.0014 |
| U/P marker | 185 ± 32 | 32.3 ± 8.7 | 0.0003 | |
| GFR | μl/min | 317 ± 34 | 230 ± 33 | 0.08 |
| GFP/kidney weight | μl/min/g | 811 ± 118 | 629 ± 73 | 0.19 |
| Proximal puncture (end proximal tubule fluid collection) | ||||
| TF/P | 1.95 ± 0.09 | 1.36 ± 0.05 | <0.001 | |
| SNGFR | nl/min | 9.6 ± 1.5 | 8.1 ± 0.7 | 0.37 |
| Flow | nl/min | 4.9 ± 0.7 | 6.0 ± 0.6 | 0.2 |
| Absorption | nl/min | 4.7 ± 0.9 | 2.1 ± 0.3 | 0.011 |
| % absorption | 48.0 ± 2.5 | 25.6 ± 2.5 | <0.001 | |
| Distal puncture (distal tubule fluid collection) | ||||
| TF/P | 4.4 ± 0.5 | 2.8 ± 0.3 | 0.02 | |
| SNGFR | nl/min | 11.1 ± 1.6 | 5.1 ± 0.4 | 0.001 |
| Flow | nl/min | 2.63 ± 0.45 | 1.95 ± 0.29 | 0.14 |
| Absorption | nl/min | 8.5 ± 1.3 | 3.1 ± 0.3 | 0.0013 |
| % absorption | 76.2 ± 2.7 | 61.9 ± 3.9 | 0.018 | |
Data are mean ± SE for measurements as described in Methods. Physiological and proximal puncture measurements were done on seven [+/+] and 8 [−/−] mice; distal puncture was done on five [+/+] and 6 [−/−] mice. Abbreviations: MAP, mean arterial pressure; Uosm, urine osmolality; U/P marker, ratio of marker concentration in urine vs. plasma; TF/P, ratio of tubular fluid-to-plasma marker concentration.
Micropuncture data are summarized in Fig. 3 and Table 1. Data are given as the average of experimental means from separate mice, not as the average of tubules. The fractional absorption along the proximal tubule calculated from end-proximal fluid-to-plasma marker ratios averaged 48 ± 2.5% in [+/+] mice and 25.6 ± 2.5% in [−/−] mice (P < 0.001). Fractional fluid absorption before the early distal tubule averaged 76.2 ± 2.7% in [+/+] mice and 61.9 ± 3.9% in [−/−] mice (P = 0.018). The absolute rate of absorption along the proximal tubule was also lower in [−/−] than in [+/+] mice (2.1 ± 0.3 nl/min vs. 4.7 ± 0.9 nl/min, P = 0.01), and this difference persisted when the loop of Henle was included (3.1 ± 0.3 nl/min vs. 8.5 ± 1.3 nl/min, P = 0.001). Interestingly, flow rates in the distal nephron were not different between [+/+] and [−/−] mice (2.63 ± 0.45 nl/min vs. 1.95 ± 0.29 nl/min, P = 0.14). The normal flow rates in [−/−] mice despite greatly reduced absorption rates are related to a marked reduction in single nephron GFR (SNGFR) calculated from distal fluid collections (11.1 ± 1.6 nl/min [+/+] vs. 5.1 ± 0.4 nl/min [−/−], P = 0.001). As explained in the Discussion, the decreased SNGFR is probably related to tubuloglomerular feedback (TGF). In contrast, SNGFR based on proximal fluid collections was not significantly different (9.6 ± 1.5 nl/min [+/+] vs. 8.1 ± 0.7 nl/min [−/−], P = 0.37). There can be no TGF in proximal tubule collections because the macula densa is not perfused.
Figure 3.
Free-flow micropuncture data. Averaged data shown for each mouse that underwent micropuncture with end proximal tubule fluid collection (A) and distal tubule fluid collection (B). See Methods for details and Table 1 for significance values. TF/P, ratio of marker in collected tubule fluid vs. plasma; SNGFR, single nephron GFR; % abs, percentage fluid absorption.
DISCUSSION
This study used transgenic AQP1 knockout mice to determine the role of AQP1 in transepithelial osmotic water permeability and net fluid absorption in the proximal tubule. The mice were shown previously to lack detectable AQP1 protein in kidney and other tissues and to maintain a low urine osmolality even when deprived of water (18). It was proposed that the defect in urinary concentrating ability was caused by a combination of defective proximal tubule reabsorptive capacity, resulting in increased distal fluid delivery, and defective countercurrent exchange, resulting in low medullary interstitial osmolality. The data here indicate that AQP1 deletion produced a 78% decrease in osmotic water permeability across the proximal tubule epithelium whereas net fluid reabsorption both in vitro and in vivo was reduced by ≈50%.
The decreased water permeability in microperfused proximal tubules from the knockout mice provides strong evidence that the major pathway for osmotically driven water transport is transcellular and mediated by AQP1 water channels. It was reported previously that osmotic water permeability was decreased by 89% at 10°C in purified apical plasma membrane vesicles from proximal tubule in AQP1 knockout vs. wild-type mice and that the remaining low water permeability in vesicles from knockout mice was not inhibited by mercurials (18). To relate the transepithelial Pf of 0.033 cm/s in the knockout mice to an intrinsic membrane water permeability, the highly convoluted structure of the apical and basolateral plasma membranes in proximal tubule cells must be considered. By using a folding-factor of ≈10 (25) and assuming equal apical and basolateral membrane water permeabilities, the Pf of 0.033 cm/s suggests an intrinsic membrane Pf of ≈0.006 cm/s at 37°C. This low membrane Pf is consistent with water movement exclusively across the lipid portion of the membrane. It is concluded that other aquaporin-type water channels and nonaquaporin transporters make little or no contribution to proximal tubule water permeability. The results also indicate that < 20% of osmotically driven transepithelial water transport in proximal tubule is paracellular. This conclusion is in agreement with previous measurements showing strong inhibition of proximal tubule water permeability by mercurials (26) and with a theoretical analysis based on the apparent size of paracellular pores (23). Our conclusion does not agree with data suggesting similar transcellular and paracellular water permeabilities based on fast video measurements of apical and basolateral membrane Pf in perfused proximal tubules (4). It is possible that membrane Pf and thus the transcellular water permeability was underestimated in those studies or there may be species or other differences in the preparations.
Net fluid absorption of isolated tubules in the nominal absence of a transepithelial osmotic gradient was reduced by ≈50% in AQP1 knockout mice, and the same reduction in fluid absorption was observed by free-flow micropuncture in situ. It is noted that the 50% decrease in proximal tubule fluid reabsorption is less than the 78% decrease in proximal tubule water permeability. This observation suggests that luminal hypotonicity must be greater in the proximal tubules of the AQP1 knockout mice compared with wild-type mice. Further studies are needed to determine osmolalities, ion concentrations, and transepithelial membrane potential along the tubule axis to define the driving forces for NaCl and water absorption in AQP1 deficiency.
In view of the inhibition of fluid absorption along the proximal tubule and of the increased urine flow in AQP1 knockout mice, it was surprising that micropuncture evidence was not found for increased distal fluid delivery. The transit time of dye through the loop of Henle was not found to be accelerated, the lumen of distal tubules appeared appropriately narrow, and collections of fluid confirmed the apparent low flow state in distal tubules in AQP1 knockout mice. Thus, at least in superficial nephrons, inhibition of fluid absorption along proximal tubules and loops of Henle does not cause increased delivery of water and NaCl into the distal nephron. The distal micropuncture studies indicated that a decrease in superficial nephron GFR was responsible for normal distal flows despite decreased proximal absorption. The decrease in SNGFR is probably caused by activation of the TGF mechanism (27). SNGFR was similar in wild-type and knockout mice as determined from proximal fluid collections, whereas SNGFR determined from distal fluid collections, with macula densa segment perfusion intact, was reduced in the AQP1 knockout mice. The mechanism of TGF activation in the knockout mice is not clear. The NaCl delivery signal at the macula densa may be elevated because of decreased NaCl and water absorption in the proximal tubule, and/or AQP1 deficiency may be associated with resetting of the TGF response curve because of chronic extracellular volume depletion. Volume depletion and dehydration has been shown to increase TGF sensitivity, in part because of activation of the renin-angiotensin system (21). It is noted that the ≈50% reduction in superficial nephron GFR is greater than the ≈30% decrease in kidney GFR, suggesting that GFR of deeper nephrons may be less affected in the knockout mice. Nevertheless, decreased GFR in AQP1 knockout mice seems to be an appropriate compensatory response to the threat of NaCl depletion caused by defective proximal tubule reabsorption. Further studies are indicated to determine whether other compensatory responses occur, such as upregulation of tubule ion transporters or changes in interstitial barrier properties.
The increased urinary flow rates despite normal distal delivery suggests that the diuresis seen in AQP1 knockout mice results primarily from reduced fluid absorption in the collecting duct. Given the normally high expression of AQP1 in descending limb of Henle and descending vasa recta, it is likely that AQP1 deletion results in a major defect in the countercurrent mechanism that prevents the formation of a hyperosmolar medullary interstitium. This conclusion is supported by the finding that in water-deprived AQP1 knockout mice, DDAVP stimulation of collecting duct water permeability (that should nearly equalize urine and medullary interstitial osmolalities) did not increase urine osmolality (18). Direct measurements of interstitial osmolalities and mathematical modeling of the countercurrent mechanism are indicated for further analysis of the concentrating defect in AQP1 knockout mice. It is noted that unlike nephrogenic diabetes insipidus in which urine osmolality is generally quite low, the urine can be mildly concentrated in AQP1 deficiency because salt transporters are functional and the collecting duct can be water permeable. It may be for this reason that no overt abnormalities were found in AQP1-deficient humans not subject to a water deprivation stress (28).
Acknowledgments
We thank Drs. Maurice Burg and Kenneth Spring for critical reading of the manuscript. This work was supported by grants DK37448, DK40042, DK35124, HL42368, HL59198, and DK43840 from the National Institutes of Health, Gene Therapy Core Center Grant DK47766, RDP Grant R613 from the National Cystic Fibrosis Foundation, and intramural funds from the National Heart, Lung and Blood Institute.
ABBREVIATIONS
- AQP1
aquaporin-1
- mosM
milliosmolar
- GFR
glomerular filtration rate
- SNGFR
single nephron GFR
- TGF
tubuloglomerular feedback
- Pf
osmotic water permeability
- TF/P
ratio of tubular fluid-to-plasma concentrations of an impermeant marker
- Jv
volume reabsorption
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
A commentary on this article begins on page 9061.
References
- 1.Sabolic I, Valenti J, Verbavatz J M, Van Hoek A N, Verkman A S, Ausiello D A, Brown D. Am J Physiol. 1992;263:C1225–C1233. doi: 10.1152/ajpcell.1992.263.6.C1225. [DOI] [PubMed] [Google Scholar]
- 2.Nielsen S, Smith B L, Christensen E I, Knepper M A, Agre P. J Cell Biol. 1993;120:371–383. doi: 10.1083/jcb.120.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang R, Skach W, Hasegawa H, Van Hoek A N, Verkman A S. J Cell Biol. 1993;120:359–369. doi: 10.1083/jcb.120.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carpi-Medina P, Whittembury G. Pflügers Arch. 1988;412:66–74. doi: 10.1007/BF00583732. [DOI] [PubMed] [Google Scholar]
- 5.Ishibashi K, Kuwahara M, Gu Y, Kageyama Y, Tohsaka M, Marumo F, Sasaki S. J Biol Chem. 1997;272:20782–20786. doi: 10.1074/jbc.272.33.20782. [DOI] [PubMed] [Google Scholar]
- 6.Fischbarg J, Kunyan K, Vera J C, Arant S, Silverstein S, Loike J, Rosen O M. Proc Natl Acad Sci USA. 1990;87:3244–3247. doi: 10.1073/pnas.87.8.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang R, Alper S, Thorens B, Verkman A S. J Clin Invest. 1991;88:1553–1558. doi: 10.1172/JCI115466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loo D D F, Zeuthen T, Chandy G, Wright E M. Proc Natl Acad Sci USA. 1996;93:13367–13370. doi: 10.1073/pnas.93.23.13367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Hoek A N, Verkman A S. J Biol Chem. 1992;267:18267–18269. [PubMed] [Google Scholar]
- 10.Zeidel M L, Ambudkar S V, Smith B L, Agre P. Biochemistry. 1992;31:7436–7440. doi: 10.1021/bi00148a002. [DOI] [PubMed] [Google Scholar]
- 11.Verbavatz J M, Brown D, Sabolic I, Valenti G, Ausiello D A, Van Hoek A N, Ma T, Verkman A S. J Cell Biol. 1993;123:605–618. doi: 10.1083/jcb.123.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi L B, Skach W, Verkman A S. J Biol Chem. 1994;269:10417–10422. [PubMed] [Google Scholar]
- 13.Cheng A, Van Hoek A N, Yaeger M, Verkman A S, Mitra A K. Nature (London) 1997;387:627–630. doi: 10.1038/42517. [DOI] [PubMed] [Google Scholar]
- 14.Walz T, Hirai T, Murata K, Heymann J B, Mitsuoka K, Fujiyoshi Y, Smith B L, Agre P, Engel A. Nature (London) 1997;387:624–627. doi: 10.1038/42512. [DOI] [PubMed] [Google Scholar]
- 15.Li H L, Lee S, Jap B K. Nat Struct Biol. 1997;4:263–265. doi: 10.1038/nsb0497-263. [DOI] [PubMed] [Google Scholar]
- 16.Maeda Y, Smith B L, Agre P, Knepper M A. J Clin Invest. 1995;95:422–428. doi: 10.1172/JCI117672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pallone T L, Kishore B K, Nielsen S, Agre P, Knepper M A. Am J Physiol. 1997;41:F587–F596. doi: 10.1152/ajprenal.1997.272.5.F587. [DOI] [PubMed] [Google Scholar]
- 18.Ma T, Yang B, Gillespie A, Carlson E J, Epstein C J, Verkman A S. J Biol Chem. 1998;273:4296–4299. doi: 10.1074/jbc.273.8.4296. [DOI] [PubMed] [Google Scholar]
- 19.Burg M B. Yale J Biol Med. 1972;45:321–326. [PMC free article] [PubMed] [Google Scholar]
- 20.Wall S M, Han J S, Chou C L, Knepper M A. Am J Physiol. 1992;262:F980–F998. doi: 10.1152/ajprenal.1992.262.6.F989. [DOI] [PubMed] [Google Scholar]
- 21.Schnermann J, Traynor T, Yang T, Huang Y G, Oliverio M I, Coffman T, Briggs J P. Am J Physiol. 1997;41:F315–F320. doi: 10.1152/ajprenal.1997.273.2.F315. [DOI] [PubMed] [Google Scholar]
- 22.Briggs J P, Schubert G, Schnermann J. Am J Physiol. 1984;247:808–817. doi: 10.1152/ajprenal.1984.247.5.F808. [DOI] [PubMed] [Google Scholar]
- 23.Preisig P, Berry C A. Am J Physiol. 1985;249:F124–F141. doi: 10.1152/ajprenal.1985.249.1.F124. [DOI] [PubMed] [Google Scholar]
- 24.Green R, Giebisch G. Am J Physiol. 1989;257:F658–F668. doi: 10.1152/ajprenal.1989.257.4.F658. [DOI] [PubMed] [Google Scholar]
- 25.Welling L M, Welling D J. Kidney Int. 1975;8:343–348. doi: 10.1038/ki.1975.125. [DOI] [PubMed] [Google Scholar]
- 26.Berry C A, Verkman A S. J Membr Biol. 1988;105:33–43. doi: 10.1007/BF01871104. [DOI] [PubMed] [Google Scholar]
- 27.Schnermann J, Briggs J P. In: The Kidney: Physiology and Pathophysiology. 2nd Ed. Seldin D W, Giebisch G, editors. Vol. 1. New York: Raven; 1992. pp. 1249–1289. [Google Scholar]
- 28.Preston G M, Smith B L, Zeidel M L, Moulds J J, Agre P. Science. 1994;265:1585–1587. doi: 10.1126/science.7521540. [DOI] [PubMed] [Google Scholar]