Abstract
Two mechanisms of H+ ion secretion in the proximal tubule that mediate bicarbonate reabsorption have been identified: the brush border Na/H exchanger and electrogenic H+ ion secretion. Angiotensin II (AII) has been shown to be a regulator of the luminal Na+/H+ exchanger and the basolateral Na+/HCO3− cotransporter. In the present study, we examined the effects of AII on H+-ATPase activity in isolated proximal tubule fragments. H+-ATPase activity was assessed by monitoring intracellular pH after Na+ removal from the bath. In addition, we investigated the effects on pH recovery of the proton pump inhibitor bafilomycin A1, removal of Cl−, and of colchicine. pH was continuously measured with the pH-sensitive fluorescent dye 2′, 7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein (BCECF). Recovery of cell pH was observed in the absence of external Na+ and was significantly accelerated by AII. The AII-stimulated pH recovery was completely abolished by bafilomycin A1, by removal of Cl−, by NPPB [5-nitro-2-(3-phenylpropylamino)-benzoate; a potent Cl− channel blocker], and by colchicine. We conclude from these studies that AII stimulates proton extrusion via H+-ATPase by a Cl−-dependent process involving brush border insertion of vesicles. This process may contribute to up-regulation of HCO3− reabsorption along the proximal tubule when tubules are exposed to AII.
Keywords: pH/vesicular trafficking/proximal tubule/hormones/acid-base
The proximal tubule is the main site of reabsorption of filtered bicarbonate (1), a process that has been shown to depend on the secretion of H+ ions across the brush border membrane. This secretion of H+ involves two major mechanisms: Na+-H+ exchange and H+ secretion by H+-ATPase (2–4). Both of these mechanisms have been shown to be involved in adaptive processes of HCO3− reabsorption (1, 4–6) after acid-base disturbances. Angiotensin II (AII) has been found to stimulate HCO3− reabsorption by up-regulation of both apical Na+-H+ exchange and basolateral Na/HCO3− cotransport (7).
The goal of the present study was to determine whether AII also might modify Na+-independent H+ ion secretion by H+-ATPase. We demonstrate that AII in physiological concentrations significantly stimulates H+ secretion by inducing insertion of H+-ATPase-containing vesicles into the brush border membrane.
MATERIALS AND METHODS
Proximal tubules from Sprague–Dawley rats (200 g) were isolated by the method of Schafer et al. (8). Rats were killed by pentobarbital injection, and the kidneys were removed and decapsulated and slices 1–2 mm in thickness prepared. After removal of the pelvis and the inner part of the medulla, slices were incubated in a digestion solution (0.8 mg of collagenase/24 mg of trypsin inhibitor/5 mM glycine/4 ml MEM medium; GIBCO) for 40 min at 37°C. Digestion was stopped by adding ice-cold 1% BSA Ringer solution (Sigma). Tubules were kept on ice in Ringer solution. For experiments, tubules were transferred to a perfusion chamber that contained coverslips prepared with the biological adhesive Cell-Tak (Becton Dickinson). The temperature of the chamber was maintained at 37 ± 0.5°C by an electronic feedback circuit. The control bath solution was initially a Ringer solution, flowing continuously at ≈5 ml/min. The chamber volume was ≈180 μl. Single fragments of proximal tubules were loaded for 15 min with the pH-sensitive dye 2′,7′-bis(2-carboxylethyl)-5(6)-carboxyfluorescein (BCECF) as described (7). pHi was measured microfluorometrically by alternately exciting the dye with a 10-μm diameter spot of light at 420 and 495 nm while monitoring the emission at 532 nm (7). The resulting fluorescence excitation ratios were converted to pHi values as described (9), using the high-[K+]/nigericin calibration technique (10). The Ringer solution consisted of 125 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1.8 mM MgSO4, 2 mM NaH2PO4, 32.2 mM Hepes, and 5 mM glucose. Na+ was replaced mole-for-mole with N-methyl-d-glucammonium (Sigma); when chloride was removed, it was replaced with gluconate. All solutions were titrated to pH 7.4 by using either HCl or Tris and the osmolality of the solution adjusted to 300 mOsm. AII (Asn1, Val5), bafilomycin A1, and colchicine were obtained from Sigma. NPPB was a kind gift from R. Greger, Freiburg, Germany. pH-sensitive dye 2′,7′-bis(2-carboxylethyl)-5(6)-carboxyfluorescein (BCECF) was purchased from Molecular Probes. Data are presented as mean ± SEM. We tested significance by using the unpaired t test. In averaging ratios, we assumed a log-normal distribution.
RESULTS
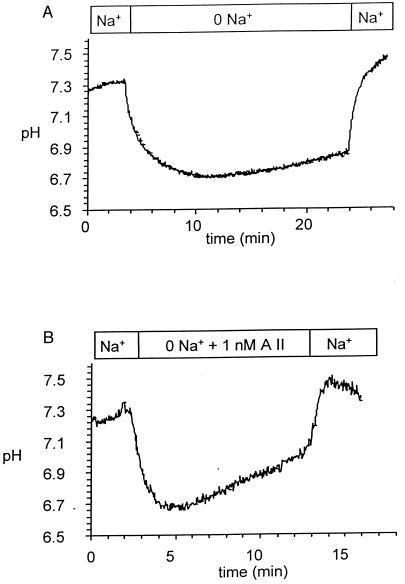
Proximal tubule cells superfused with Ringer solution had a mean initial pHi of 7.24 (see Table 1). As demonstrated in Fig. 1A, removal of Na+ from the bath caused a decrease of intracellular pH to 6.72 ± 0.04 pH × 10−4 sec−1. pHi slowly recovered in sodium-free conditions at a rate of 1.41 pH × 10−4 sec−1. pHi returned to resting pHi values despite the continued absence of Na+ in ≈10–15 min (data of full recovery not shown). Readdition of Na+ led to a rapid rise in pH and a slight alkalinization beyond the resting pH value (7.32, P < 0.001). The rate of Na+-dependent pHi recovery was 24.3 ± 1.6 pH × 10−4 sec−1 units pH/min.
Table 1.
AII-dependent pHi recovery rates
| Condition | 0 Na+
|
Na+ readdition
|
||
|---|---|---|---|---|
| Initial pH | ΔpH × 10−4/sec | ΔpH × 10−4/sec | Number | |
| Control | 7.24 ± 0.02 | 1.41 ± 0.3 | 24.3 ± 1.6 | 19 |
| 1 nM AII | 7.22 ± 0.01 | 4.65 ± 0.8 | 79.3 ± 13.3 | 26 |
| Bafilomycin | 7.19 ± 0.12 | 0.51 ± 0.1 | 24.3 ± 1.7 | 20 |
| Bafilomycin + AII | 7.24 ± 0.003 | 0.02 ± 0.2 | 67.1 ± 13.3 | 10 |
| 0 Cl− + AII | 7.30 ± 0.04 | 0.51 ± 0.3 | 67.3 ± 20.0 | 4 |
| NPPB + AII | 7.27 ± 0.02 | 0.68 ± 0.3 | 54.0 ± 11.6 | 8 |
| Colchicine + AII | 7.28 ± 0.03 | 1.56 ± 0.3 | 52.3 ± 6.7 | 5 |
For calculating the rate of the Na+-dependent pHi recovery rate, we only used the initial steeper slope because the function of the Na+/H+ exchanger is pH dependent. The range for calculating the rates of recovery for all dpH/dT values was from 6.7 to 6.9.
Figure 1.
Effect of 1 nM AII on the rate of recovery of pHi after removal of Na+ from the bath. (A) Recovery in sodium-free conditions in the absence of AII. (B) Effects of 1 nM AII. Note acceleration of both Na+-independent and Na+-dependent pHi recovery.
As demonstrated in Fig. 1B, addition of 1 nM AII in the absence of Na+ significantly accelerated the rate of pH recovery. In this series of experiments, initial pH was 7.22 and Na+ deletion lowered this value to 6.74. Addition of AII to the sodium-free solution significantly accelerated the rate of pHi 3.4 fold (see Table 1). Moreover, the Na+-dependent pHi recovery also was increased, from a value of 24.3–79.3 pH × 10−4 sec−1. The observation that AII-stimulated Na+-dependent pHI recovery was similar to that previously described (7).
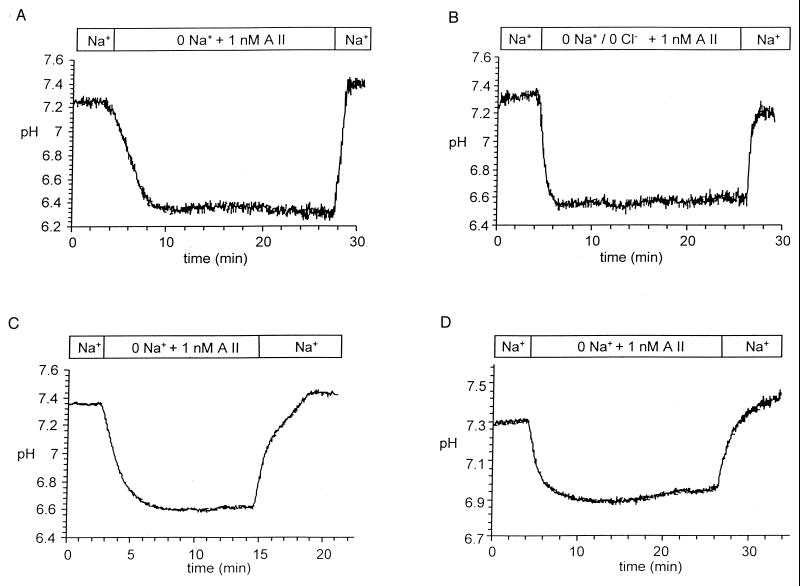
To test whether the enhanced rate of pHi recovery in the absence of sodium was mediated by H+-ATPase activity, tubules were incubated for 45 min before experiments with the specific H+-ATPase inhibitor bafilomycin A1 (10 μM) (11). As seen in Fig. 2A and in Table 1, this agent significantly reduced the rate of pHi recovery in Na+-free conditions and completely abolished the effect of AII on pH recovery (see Table 1). As shown in Table 1, the rate of Na+-dependent pHi recovery was not affected by bafilomycin A1.
Figure 2.
Inhibition of the effects of AII on H+-ATPase-mediated pH recovery. (A) Effects of H+-ATPase inhibitor bafilomycin A1. (B) Removal of Cl− in addition to Na+. (C) Preincubation with the Cl−-channel blocker NPPB. (D) Effects of preincubation with colchicine.
In view of evidence that the activity of H+-ATPase is affected by Cl− (2, 11, 12), experiments were carried out in which either Cl− was removed from the perfusion fluid (Fig. 2B) or AII was added in the presence of the potent anion channel blocker NPPB (see Fig. 2C). As can be seen from inspection of Table 1, the initial mean pH of cells in a Cl−-free medium was 7.30 and fell to a value of 6.78 after the simultaneous removal of Na+ and Cl−, values not significantly different from those cells in which Cl− was present. However, the rate of sodium-independent pHi recovery in the absence of Cl− after AII addition was significantly reduced to a value of 0.51 pH × 10−4 sec−1 (P < 0.001). It can be seen in Fig. 2C and Table 1 that NPPB similarly led to almost complete abolishment of recovery in Na+-free conditions. In contrast, the rate of Na+-dependent pHi recovery was not affected by removal of Cl− or by addition of NPPB.
In a final set of experiments the effects of colchicine, an inhibitor of microtubule- dependent vesicular trafficking, were investigated. Proximal tubules were preincubated with 10 μM colchicine for 2 h before the experiments. As shown in Fig. 2D and Table 1, this treatment prevented the AII-induced up-regulation of the pHi recovery. Exposure to colchicine for shorter periods did not completely inhibit Na+-dependent pH recovery (data not shown). As in the previous experiments, the Na+-dependent pH recovery was not affected. In two experimental conditions, NPPB and colchicine treatment, the rate of pH recovery in the Na+-containing solutions declined at higher pH levels (Fig. 2 C and D). This result was not observed in the presence of bafilomycin A1 or the absence of Cl−. The reason for the different behavior of pH recovery is not known but may be related to specific effects of these two agents on the pH sensitivity of the Na+-H+ exchanger.
DISCUSSION
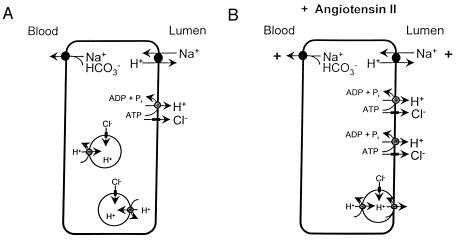
It is well established that bicarbonate reabsorption in the proximal tubule depends on two proton translocating processes; Na+-H+ exchange and ATP-dependent electrogenic H+ ion transport (1, 2, 4, 13–15). Both operations are subject to regulation, and AII has been shown to enhance Na+-H+ exchange and bicarbonate reabsorption (7, 13, 16). In the present study, we demonstrate that AII also stimulates sodium-independent H+ ion secretion in rat proximal tubule. Fig. 3 shows a cell model of a proximal tubule cell including the proposed mechanism of AII-induced stimulation of H+-ion transport.
Figure 3.
Cell Model of a proximal tubule cell in the absence (A) and in the presence of AII (B). Note the insertion of vesicles containing both H+-ATPase and Cl− channels into the apical membrane.
Several lines of evidence have demonstrated the presence of an H+-ATPase in the apical membrane of proximal tubules. First, microperfusion of proximal tubules in the rat in vivo indicates that a significant fraction of bicarbonate transport continues in the absence of Na+ in the lumen (17). Second, brush border vesicle studies show the presence of proton-pumping ATPases and further demonstrate significant up-regulation of their activity in membranes from rats with chronic metabolic acidosis (18, 19). Increased H+-ATPase activity also was observed in brush border vesicles harvested from rats in which proximal reabsorption of bicarbonate was enhanced by raising the tubule load of bicarbonate (5). Third, characterization of H+-ion secretion in proximal tubule apical membranes indicates sensitivity to a variety of specific inhibitors of vacuolar H+-ATPase (16, 20–23) and to cytosolic ATP (24). Finally, morphological studies have shown a pool of cytosolic and brush border H+-ATPase. H+-ATPase has been localized in intracellular vesicles and apical membranes, and redistribution from subapical cytosol to brush border membranes has been observed after acute and chronic acid loading (3–5, 25).
We demonstrate that Na+-independent H+ ion secretion is stimulated by physiological concentrations of AII as shown by the acceleration of cell pH recovery after deletion of Na+ from the extracellular fluid. The stimulation of pH recovery by AII was inhibited by: (i) specific inhibitors of H+-ATPase pump, (ii) deletion of Cl−, and (iii) agents known to disrupt microtubules. We conclude that AII activates proton extrusion by H+-ATPase, a process exclusively localized to the brush border membrane (16, 20–28).
In the present study, we also found that Cl− ions play an important role in proximal H+ ion secretion and its stimulation by AII. Cl− sensitivity of electrogenic H+ ion transport resembles H+ transport in distal nephron segments such as the cortical collecting duct (23, 29) and cultured renal tubule cell preparations (4, 14, 21). Two mechanisms have been suggested to account for the Cl− dependence of H+-ATPase activity. First, Cl− ions are necessary as cotransported anions to maintain electric neutrality during H+ ion transport (12, 20, 22–24). The observation that the deletion of Cl− and exposure to Cl− channel blockers abolishes AII-stimulated pH recovery after deletion of Na+ supports this view. A second mechanism involves the reported direct stimulation by Cl− of proton ATPases of the renal medulla (23, 29). Because the present experiments were done in the absence of bicarbonate, it is unlikely that lowering Cl− accelerated basolateral Cl− exit by involving carrier-mediated anion exchange, but it is most reasonable to assume that cell Cl− concentration declined during exposure of tubules to a chloride-free superfusion medium.
Stimulation of proton transport by AII in the absence of Na+ also has been shown to be sensitive to inhibition by colchicine. This agent disrupts microtubular structures and has been implicated in vesicular trafficking of H+-ATPase along the nephron, especially in the translocation of intracellular vesicles to the apical membranes (21, 22, 29). Relevant examples include the marked redistribution of H+-ATPase containing vesicles from a subapical pool to the apical membrane, a process shown to be effectively blocked by colchicine. The fact that AII-induced pH recovery was abolished by colchicine in the present experiments strongly suggests that vesicular insertion of H+-ATPase-containing vesicles was involved in the stimulation of Na+-independent pH recovery.
Acknowledgments
This work was supported by National Institutes of Health Grants DK-17433, DK-14669, and DK-50230 to J. P.G., DK-17433 to G.G., and by a Deutsches Forschung Gemeinschaft grant to F.L. C.A.W. had a scholarship of the Studienstiftung des Deutschen Volkes and was supported by a grant from the Freunde der Universität Tübingen.
ABBREVIATIONS
- AII
Angiotensin II
- NPPB
5-nitro-2-(3-phenylpropylamino)-benzoate
References
- 1.Hamm L J, Alpern R J. In: The Kidney. Seldin D, Giebisch G, editors. New York: Raven; 1992. pp. 2581–2626. [Google Scholar]
- 2.Chen L K, Boron W F. Kidney Int. 1991;40:S11–S17. [PubMed] [Google Scholar]
- 3.Zimolo Z, Montrose M H, Murer H. J Membr Biol. 1992;126:19–26. doi: 10.1007/BF00233457. [DOI] [PubMed] [Google Scholar]
- 4.Gluck S L, Underhill D M, Iyori M, Holliday L S, Kostrominova T Y, Lee B S. Annu Rev Physiol. 1996;58:427–445. doi: 10.1146/annurev.ph.58.030196.002235. [DOI] [PubMed] [Google Scholar]
- 5.Maddox D A, Barnes W D, Gennari F J. Kidney Int. 1997;52:446–453. doi: 10.1038/ki.1997.351. [DOI] [PubMed] [Google Scholar]
- 6.Liu F Y, Cogan M G. J Clin Invest. 1987;80:272–275. doi: 10.1172/JCI113059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geibel J, Giebisch G, Boron W F. Proc Natl Acad Sci USA. 1990;87:7917–7920. doi: 10.1073/pnas.87.20.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schafer J A, Watkins M L, Li L, Herter P, Haxelmans S, Schlatter E. Am J Physiol. 1997;273:F650–F657. doi: 10.1152/ajprenal.1997.273.4.F650. [DOI] [PubMed] [Google Scholar]
- 9.Boyarsky G, Ganz G B, Sterzel B, Boron W F. Am J Physiol. 1988;255:C844–C856. doi: 10.1152/ajpcell.1988.255.6.C844. [DOI] [PubMed] [Google Scholar]
- 10.Thomas J A, Buchsbaum R N, Zimniak A, Racker E. Biochemistry. 1979;81:2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- 11.Bowman E J, Siebers A, Altendorf K. Proc Natl Acad Sci USA. 1988;85:7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hilden S A, Johns C A, Madias N E. Am J Physiol. 1988;255:F885–F897. doi: 10.1152/ajprenal.1988.255.5.F885. [DOI] [PubMed] [Google Scholar]
- 13.Harris P J. Clin Exp Pharmacol Physiol. 1992;19:213–222. doi: 10.1111/j.1440-1681.1992.tb00441.x. [DOI] [PubMed] [Google Scholar]
- 14.Brown D, Hirsch S, Gluck S. J Clin Invest. 1988;82:2114–2126. doi: 10.1172/JCI113833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geibel J. Cell Physiol Biochem. 1993;3:34–41. [Google Scholar]
- 16.Baum M, Quigley R, Quan A. Am J Physiol. 1997;273:F595–F600. doi: 10.1152/ajprenal.1997.273.4.F595. [DOI] [PubMed] [Google Scholar]
- 17.Chan Y L, Giebisch G. Am J Physiol. 1981;240:F222–F230. doi: 10.1152/ajprenal.1981.240.3.F222. [DOI] [PubMed] [Google Scholar]
- 18.Chambrey R, Paillard M, Podevin R-A. J Biol Chem. 1994;269:3243–3250. [PubMed] [Google Scholar]
- 19.Bastani B, Purcell H, Hemken P, Trigg D, Gluck S. J Clin Invest. 1991;88:126–136. doi: 10.1172/JCI115268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jehmlich K, Sablotini, Simon B J, Burckhardt G. Kidney Int. 1991;40:S64–S70. [PubMed] [Google Scholar]
- 21.Brown D, Sabolic I, Gluck S. Kidney Int. 1991;40:S79–S83. [PubMed] [Google Scholar]
- 22.Khadouri C, Cheval L, Marsy S, Barlet-Bas C, Doucet A. Kidney Int. 1991;40:S71–S78. [PubMed] [Google Scholar]
- 23.Stone D K, Crider B, Xie X-S. Kidney Int. 1990;39:649–653. doi: 10.1038/ki.1990.255. [DOI] [PubMed] [Google Scholar]
- 24.Sabolic I, Haase W, Burckhardt G. Am J Physiol. 1985;248:F835–F844. doi: 10.1152/ajprenal.1985.248.6.F835. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz G, Al-Awqati Q. J Clin Invest. 1985;75:1638–1644. doi: 10.1172/JCI111871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown D, Gluck S, Hartwig J. J Cell Biol. 1987;105:1637–1648. doi: 10.1083/jcb.105.4.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madsen K M, Verlander J W, Kim J, Tisher C. Kidney Int. 1991;40:S57–S63. [PubMed] [Google Scholar]
- 28.Tojo A, Tisher C C, Madsen K M. Am J Physiol. 1994;267:F1045–F1051. doi: 10.1152/ajprenal.1994.267.6.F1045. [DOI] [PubMed] [Google Scholar]
- 29.Stone D K, Xie X-S. Kidney Int. 1988;33:767–774. doi: 10.1038/ki.1988.65. [DOI] [PubMed] [Google Scholar]