Abstract
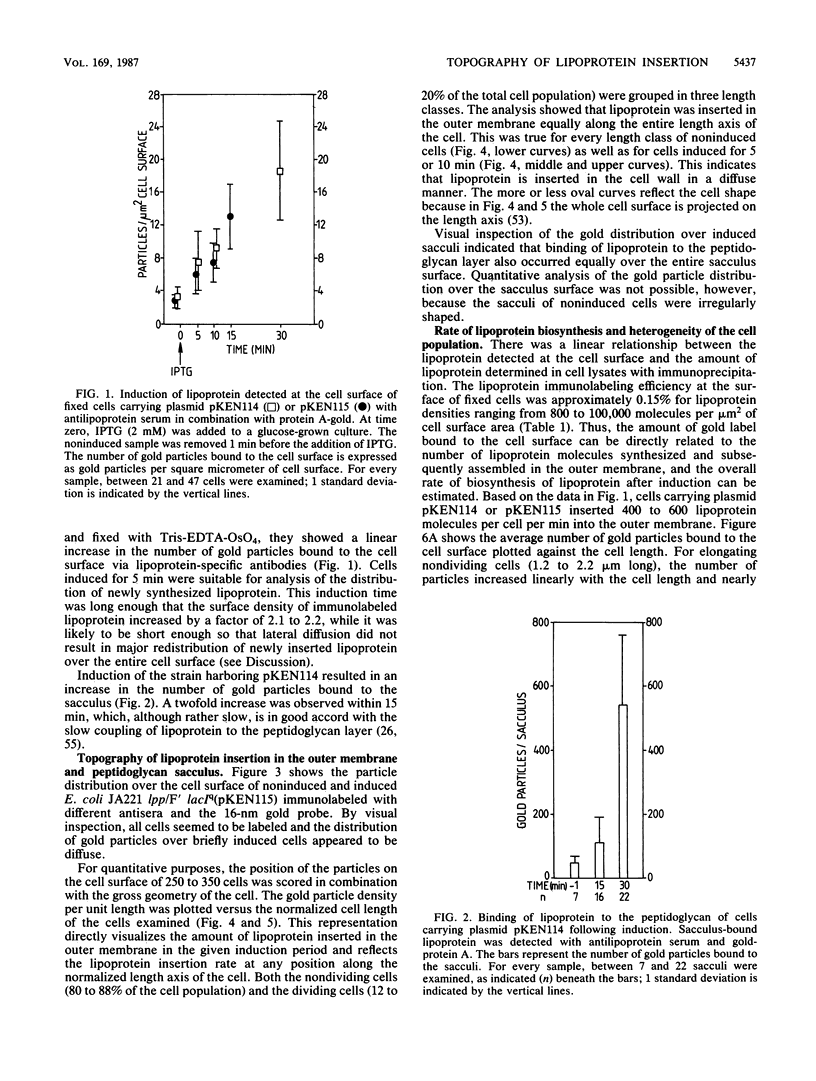
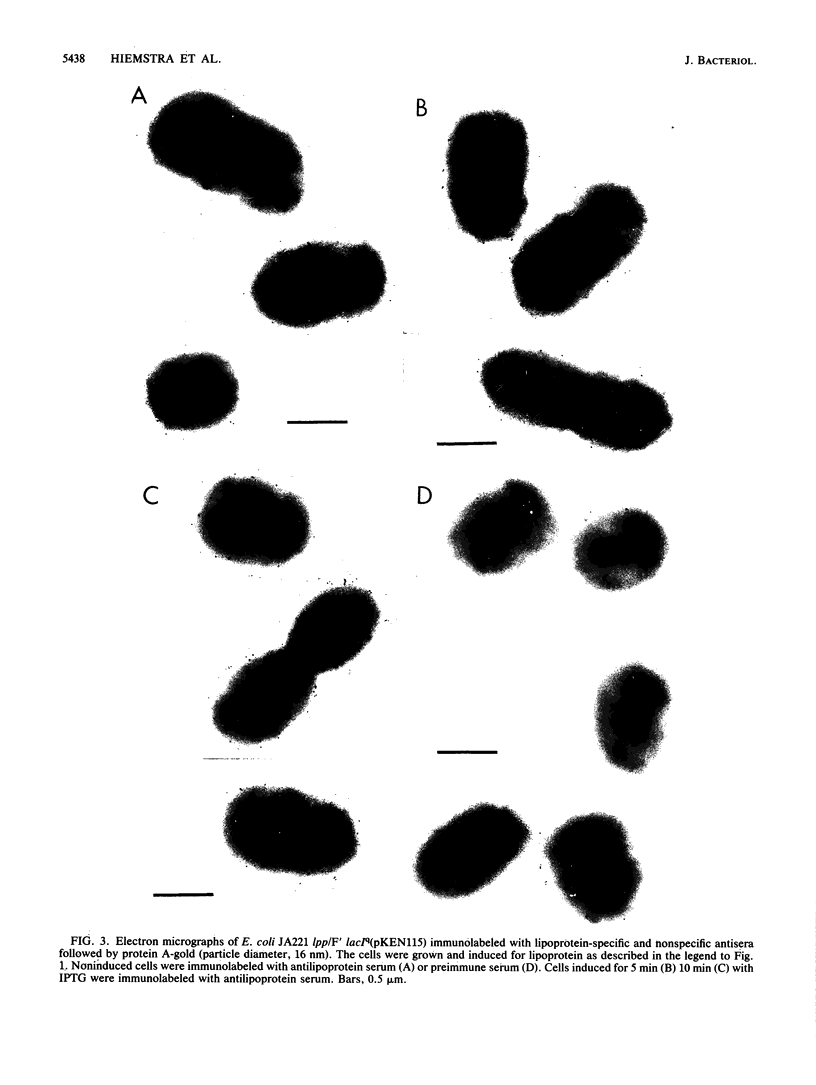
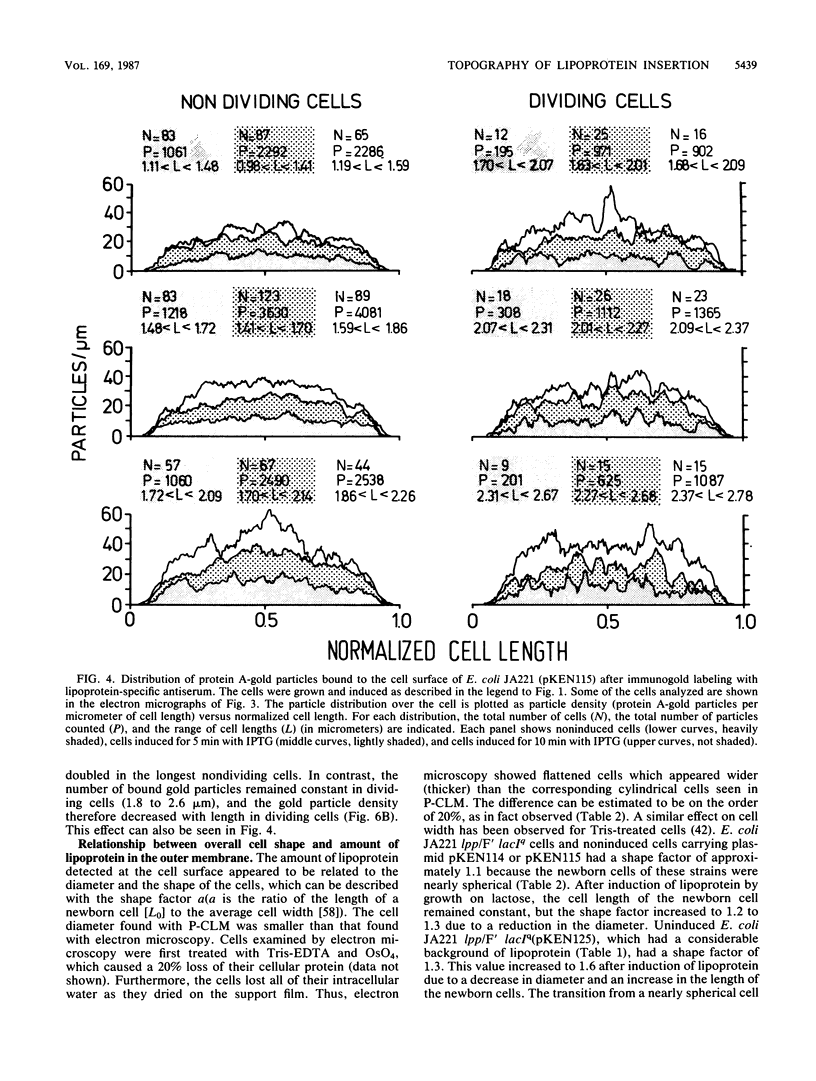
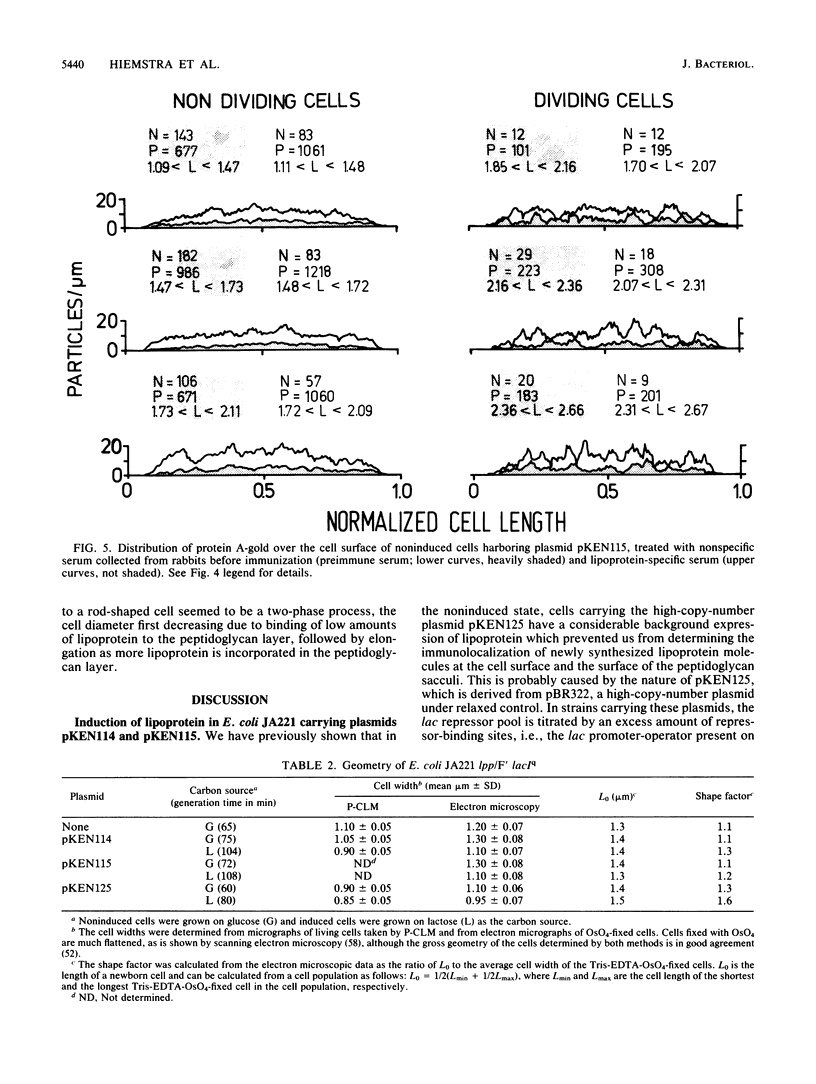
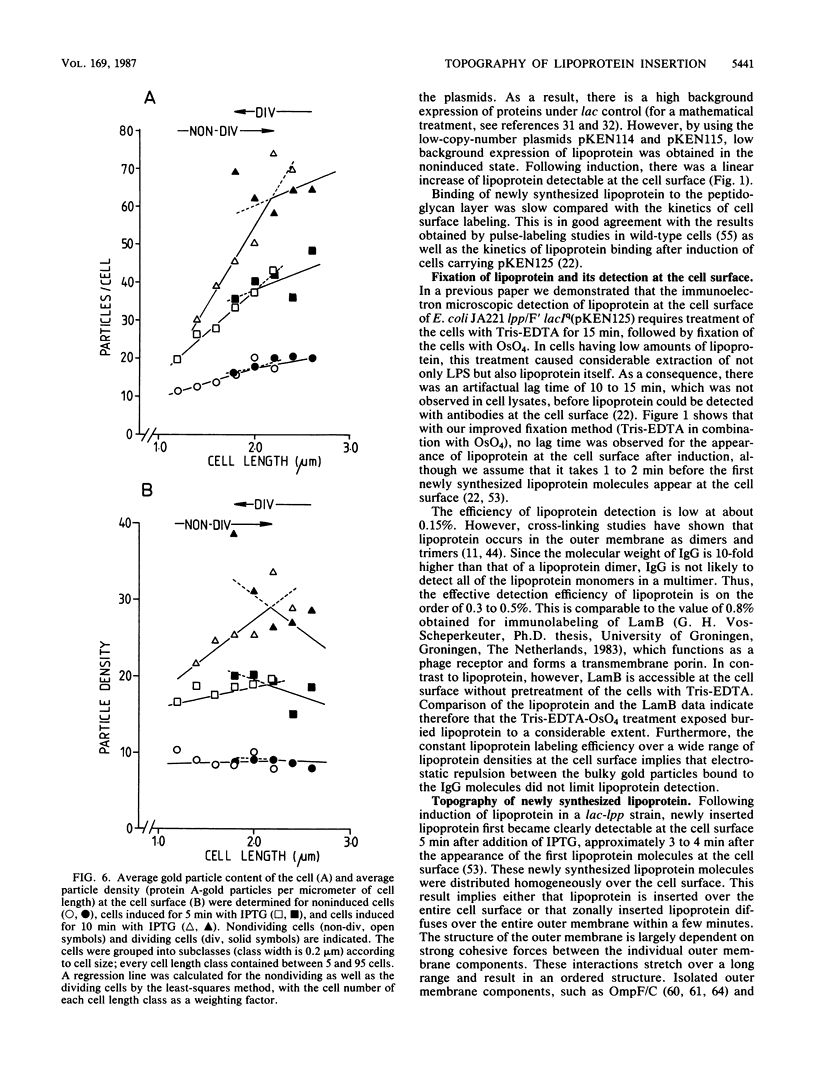
The insertion of newly synthesized lipoprotein molecules into the cell wall of Escherichia coli was studied topographically by immunoelectron microscopy. Lipoprotein was briefly induced with isopropyl-beta-D-thiogalactopyranoside in cells carrying lac-lpp on a low-copy-number plasmid in an E. coli lpp host. Specific antibodies bound to the newly inserted lipoprotein molecules, which were exposed at the cell surface after treatment of the cells with Tris-EDTA, were detected with a protein A-gold probe. The average distribution of the gold particles over the cell surface of noninduced cells was determined for cells induced for 5 and 10 min. Analysis of 250 to 350 cells showed that the distribution of newly synthesized lipoprotein over the cell surface was homogeneous in both cases. The binding of lipoprotein to the peptidoglycan layer was studied by the same technique, and visual inspection again revealed a homogeneous distribution of bound lipoprotein over the entire sacculus surface. It is therefore concluded that free lipoprotein is inserted equally over the entire cell wall of E. coli, while binding to peptidoglycan also occurs over the entire cell surface. The rate of lipoprotein synthesis increased with cell length in nondividing cells, whereas it was constant in cells which had initiated constriction. Analysis of cells having different amounts of lipoprotein in their cell wall revealed that the cell shape depended on the total lipoprotein content of the cell. Cells having no or only a small amount of lipoprotein grew as spheres, whereas cells with increasing numbers of lipoprotein molecules gradually changed their shape to short rods.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer M. E., Leive L. Effect of ethylenediaminetetraacetate upon the surface of Escherichia coli. J Bacteriol. 1977 Jun;130(3):1364–1381. doi: 10.1128/jb.130.3.1364-1381.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg K. J. Cell surface growth in Escherichia coli: distribution of matrix protein. J Bacteriol. 1978 Aug;135(2):307–310. doi: 10.1128/jb.135.2.307-310.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg K. J., Doanachie W. D. Growth of the Escherichia coli cell surface. J Bacteriol. 1977 Mar;129(3):1524–1536. doi: 10.1128/jb.129.3.1524-1536.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg K. J., Donachie W. D. Concentration of a major outer membrane protein at the cell poles in Escherichia coli. J Gen Microbiol. 1984 Sep;130(9):2339–2346. doi: 10.1099/00221287-130-9-2339. [DOI] [PubMed] [Google Scholar]
- Begg K. J., Donachie W. D. Topography of outer membrane growth in E. coli. Nat New Biol. 1973 Sep 12;245(141):38–39. doi: 10.1038/newbio245038a0. [DOI] [PubMed] [Google Scholar]
- Boyd A., Holland I. B. Regulation of the synthesis of surface protein in the cell cycle of E. coli B/r. Cell. 1979 Oct;18(2):287–296. doi: 10.1016/0092-8674(79)90048-5. [DOI] [PubMed] [Google Scholar]
- Brass J. M., Higgins C. F., Foley M., Rugman P. A., Birmingham J., Garland P. B. Lateral diffusion of proteins in the periplasm of Escherichia coli. J Bacteriol. 1986 Mar;165(3):787–795. doi: 10.1128/jb.165.3.787-795.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Bosch V., Klumpp E. R., Neff I., Mayer H., Schlecht S. Antigenic determinants of murein lipoprotein and its exposure at the surface of Enterobacteriaceae. Eur J Biochem. 1976 Mar 1;62(3):555–566. doi: 10.1111/j.1432-1033.1976.tb10190.x. [DOI] [PubMed] [Google Scholar]
- Braun V., Rehn K. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur J Biochem. 1969 Oct;10(3):426–438. doi: 10.1111/j.1432-1033.1969.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Calcott P. H., Calcott K. N. Involvement of outer membrane proteins in freeze--thaw resistance of Escherichia coli. Can J Microbiol. 1984 Mar;30(3):339–344. doi: 10.1139/m84-050. [DOI] [PubMed] [Google Scholar]
- Choi D. S., Yamada H., Mizuno T., Mizushima S. Trimeric structure and localization of the major lipoprotein in the cell surface of Escherichia coli. J Biol Chem. 1986 Jul 5;261(19):8953–8957. [PubMed] [Google Scholar]
- Davison M. T., Garland P. B. Immunochemical demonstration of zonal growth of the cell envelope of Escherichia coli. Eur J Biochem. 1983 Feb 15;130(3):589–597. doi: 10.1111/j.1432-1033.1983.tb07190.x. [DOI] [PubMed] [Google Scholar]
- DeMartini M., Inouye M. Interaction between two major outer membrane proteins of Escherichia coli: the matrix protein and the lipoprotein. J Bacteriol. 1978 Jan;133(1):329–335. doi: 10.1128/jb.133.1.329-335.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endermann R., Henning U. Nearest neighbors of major proteins in the outer membrane of Escherichia coli K12. FEBS Lett. 1979 Jan 15;97(2):339–342. doi: 10.1016/0014-5793(79)80117-9. [DOI] [PubMed] [Google Scholar]
- Fung J., MacAlister T. J., Rothfield L. I. Role of murein lipoprotein in morphogenesis of the bacterial division septum: phenotypic similarity of lkyD and lpo mutants. J Bacteriol. 1978 Mar;133(3):1467–1471. doi: 10.1128/jb.133.3.1467-1471.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson P., Wolf-Watz H., Lind L., Johansson K. E., Nordström K. Binding between the par region of plasmids R1 and pSC101 and the outer membrane fraction of the host bacteria. EMBO J. 1983;2(1):27–32. doi: 10.1002/j.1460-2075.1983.tb01375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasunuma K., Sekiguchi M. Replication of plasmid pSC101 in Escherichia coli K12: requirement for dnaA function. Mol Gen Genet. 1977 Sep 9;154(3):225–230. doi: 10.1007/BF00571277. [DOI] [PubMed] [Google Scholar]
- Henning U. L. Determination of cell shape in bacteria. Annu Rev Microbiol. 1975;29:45–60. doi: 10.1146/annurev.mi.29.100175.000401. [DOI] [PubMed] [Google Scholar]
- Hirashima A., Inouye M. Specific biosynthesis of an envelope protein of Escherichia coli. Nature. 1973 Apr 6;242(5397):405–407. doi: 10.1038/242405a0. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Suzuki H., Nishimura Y., Yasuda S. On the process of cellular division in Escherichia coli: a mutant of E. coli lacking a murein-lipoprotein. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1417–1420. doi: 10.1073/pnas.74.4.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobot J. A., Carlemalm E., Villiger W., Kellenberger E. Periplasmic gel: new concept resulting from the reinvestigation of bacterial cell envelope ultrastructure by new methods. J Bacteriol. 1984 Oct;160(1):143–152. doi: 10.1128/jb.160.1.143-152.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M., Hirashima A., Lee N. Discussion paper: biosynthesis and assembly of a structural lipoprotein in the envelope of Escherichia coli. Ann N Y Acad Sci. 1974 May 10;235(0):83–90. doi: 10.1111/j.1749-6632.1974.tb43258.x. [DOI] [PubMed] [Google Scholar]
- Irvin R. T., MacAlister T. J., Chan R., Costerton J. W. Citrate-tris(hydroxymethyl)aminomethane-mediated release of outer membrane sections from the cell envelope of a deep-rough (heptose-deficient lipopolysaccharide) strain of Escherichia coli O8. J Bacteriol. 1981 Mar;145(3):1386–1396. doi: 10.1128/jb.145.3.1386-1396.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James R., Gudas L. J. Cell cycle-specific incorporation of lipoprotein into the outer membrane of Escherichia coli. J Bacteriol. 1976 Jan;125(1):374–375. doi: 10.1128/jb.125.1.374-375.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppes L. J., Nanninga N. Positive correlation between size at initiation of chromosome replication in Escherichia coli and size at initiation of cell constriction. J Bacteriol. 1980 Jul;143(1):89–99. doi: 10.1128/jb.143.1.89-99.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N., Scandella C., Inouye M. Spin labeling of a cysteine residue of the Escherichia coli outer membrane lipoprotein in its membrane environment. Proc Natl Acad Sci U S A. 1978 Jan;75(1):127–130. doi: 10.1073/pnas.75.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leive L. Release of lipopolysaccharide by EDTA treatment of E. coli. Biochem Biophys Res Commun. 1965 Nov 22;21(4):290–296. doi: 10.1016/0006-291x(65)90191-9. [DOI] [PubMed] [Google Scholar]
- Lenard J., Singer S. J. Alteration of the conformation of proteins in red blood cell membranes and in solution by fixatives used in electron microscopy. J Cell Biol. 1968 Apr;37(1):117–121. doi: 10.1083/jcb.37.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luftig R. B., McMillan P. N. The importance of adequate fixation in preservation of membrane ultrastructure. Int Rev Cytol Suppl. 1981;12:309–325. doi: 10.1016/b978-0-12-364373-5.50017-2. [DOI] [PubMed] [Google Scholar]
- Maupin-Szamier P., Pollard T. D. Actin filament destruction by osmium tetroxide. J Cell Biol. 1978 Jun;77(3):837–852. doi: 10.1083/jcb.77.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movva N. R., Katz E., Asdourian P. L., Hirota Y., Inouye M. Gene dosage effects of the structural gene for a lipoprotein of the Escherichia coli outer membrane. J Bacteriol. 1978 Jan;133(1):81–84. doi: 10.1128/jb.133.1.81-84.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mug-Opstelten D., Witholt B. Preferential release of new outer membrane fragments by exponentially growing Escherichia coli. Biochim Biophys Acta. 1978 Apr 4;508(2):287–295. doi: 10.1016/0005-2736(78)90331-0. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Masui Y., Inouye M. Use of a lac promoter-operator fragment as a transcriptional control switch for expression of the constitutive lpp gene in Escherichia coli. J Mol Appl Genet. 1982;1(4):289–299. [PubMed] [Google Scholar]
- Nichol C. P., Davis J. H., Weeks G., Bloom M. Quantitative study of the fluidity of Escherichia coli membranes using deuterium magnetic resonance. Biochemistry. 1980 Feb 5;19(3):451–457. doi: 10.1021/bi00544a008. [DOI] [PubMed] [Google Scholar]
- Nogami T., Mizushima S. Outer membrane porins are important in maintenance of the surface structure of Escherichia coli cells. J Bacteriol. 1983 Oct;156(1):402–408. doi: 10.1128/jb.156.1.402-408.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordström K. Control of plasmid replication: a synthesis occasioned by the recent EMBO Workshop "Replication of Prokaryotic DNA," held at de Eemhof, The Netherlands, May 1982 (organizers: Veltkamp and Weisbeek). Plasmid. 1983 Jan;9(1):1–7. doi: 10.1016/0147-619x(83)90026-4. [DOI] [PubMed] [Google Scholar]
- Palva E. T. Protein interactions in the outer membrane of Escherichia coli. Eur J Biochem. 1979 Feb 1;93(3):495–503. doi: 10.1111/j.1432-1033.1979.tb12848.x. [DOI] [PubMed] [Google Scholar]
- Ryter A., Shuman H., Schwartz M. Intergration of the receptor for bacteriophage lambda in the outer membrane of Escherichia coli: coupling with cell division. J Bacteriol. 1975 Apr;122(1):295–301. doi: 10.1128/jb.122.1.295-301.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara Y., Yuasa S. Continuous synthesis of the dnaA gene product of Escherichia coli in the cell cycle. Mol Gen Genet. 1982;186(1):87–94. doi: 10.1007/BF00422917. [DOI] [PubMed] [Google Scholar]
- Schindler M., Osborn M. J., Koppel D. E. Lateral mobility in reconstituted membranes--comparisons with diffusion in polymers. Nature. 1980 Jan 24;283(5745):346–350. doi: 10.1038/283346a0. [DOI] [PubMed] [Google Scholar]
- Sonntag I., Schwarz H., Hirota Y., Henning U. Cell envelope and shape of Escherichia coli: multiple mutants missing the outer membrane lipoprotein and other major outer membrane proteins. J Bacteriol. 1978 Oct;136(1):280–285. doi: 10.1128/jb.136.1.280-285.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Nishimura Y., Yasuda S., Nishimura A., Yamada M., Hirota Y. Murein-lipoprotein of Escherichia coli: a protein involved in the stabilization of bacterial cell envelope. Mol Gen Genet. 1978 Nov 16;167(1):1–9. doi: 10.1007/BF00270315. [DOI] [PubMed] [Google Scholar]
- Trueba F. J., Woldringh C. L. Changes in cell diameter during the division cycle of Escherichia coli. J Bacteriol. 1980 Jun;142(3):869–878. doi: 10.1128/jb.142.3.869-878.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos-Scheperkeuter G. H., Pas E., Brakenhoff G. J., Nanninga N., Witholt B. Topography of the insertion of LamB protein into the outer membrane of Escherichia coli wild-type and lac-lamB cells. J Bacteriol. 1984 Aug;159(2):440–447. doi: 10.1128/jb.159.2.440-447.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensink J., Witholt B. Conversion of free lipoprotein to the murein-bound form. Eur J Biochem. 1981 Jun;117(1):207–212. doi: 10.1111/j.1432-1033.1981.tb06323.x. [DOI] [PubMed] [Google Scholar]
- Wensink J., Witholt B. Identification of different forms of the murein-bound lipoprotein found in isolated outer membranes of Escherichia coli. Eur J Biochem. 1981 Jan;113(2):349–357. doi: 10.1111/j.1432-1033.1981.tb05073.x. [DOI] [PubMed] [Google Scholar]
- Witholt B., Boekhout M., Brock M., Kingma J., Heerikhuizen H. V., Leij L. D. An efficient and reproducible procedure for the formation of spheroplasts from variously grown Escherichia coli. Anal Biochem. 1976 Jul;74(1):160–170. doi: 10.1016/0003-2697(76)90320-1. [DOI] [PubMed] [Google Scholar]
- Witholt B. Method for isolating mutants overproducing nicotinamide adenine dinucleotide and its precursors. J Bacteriol. 1972 Jan;109(1):350–364. doi: 10.1128/jb.109.1.350-364.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldringh C. L., de Jong M. A., van den Berg W., Koppes L. Morphological analysis of the division cycle of two Escherichia coli substrains during slow growth. J Bacteriol. 1977 Jul;131(1):270–279. doi: 10.1128/jb.131.1.270-279.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H., Mizushima S. Interaction between major outer membrane protein (O-8) and lipopolysaccharide in Escherichia coli K12. Eur J Biochem. 1980 Jan;103(1):209–218. doi: 10.1111/j.1432-1033.1980.tb04305.x. [DOI] [PubMed] [Google Scholar]
- Yamada H., Mizushima S. Reconstitution of an ordered structure from major outer membrane constituents and the lipoprotein-bearing peptidoglycan sacculus of Escherichia coli. J Bacteriol. 1978 Sep;135(3):1024–1031. doi: 10.1128/jb.135.3.1024-1031.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H., Nogami T., Mizushima S. Arrangement of bacteriophage lambda receptor protein (LamB) in the cell surface of Escherichia coli: a reconstitution study. J Bacteriol. 1981 Aug;147(2):660–669. doi: 10.1128/jb.147.2.660-669.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yem D. W., Wu H. C. Physiological characterization of an Escherichia coli mutant altered in the structure of murein lipoprotein. J Bacteriol. 1978 Mar;133(3):1419–1426. doi: 10.1128/jb.133.3.1419-1426.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F., Mizushima S. Roles of lipopolysaccharide and outer membrane protein OmpC of Escherichia coli K-12 in the receptor function for bacteriophage T4. J Bacteriol. 1982 Aug;151(2):718–722. doi: 10.1128/jb.151.2.718-722.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Smet M. J., Kingma J., Witholt B. The effect of toluene on the structure and permeability of the outer and cytoplasmic membranes of Escherichia coli. Biochim Biophys Acta. 1978 Jan 4;506(1):64–80. doi: 10.1016/0005-2736(78)90435-2. [DOI] [PubMed] [Google Scholar]