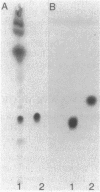
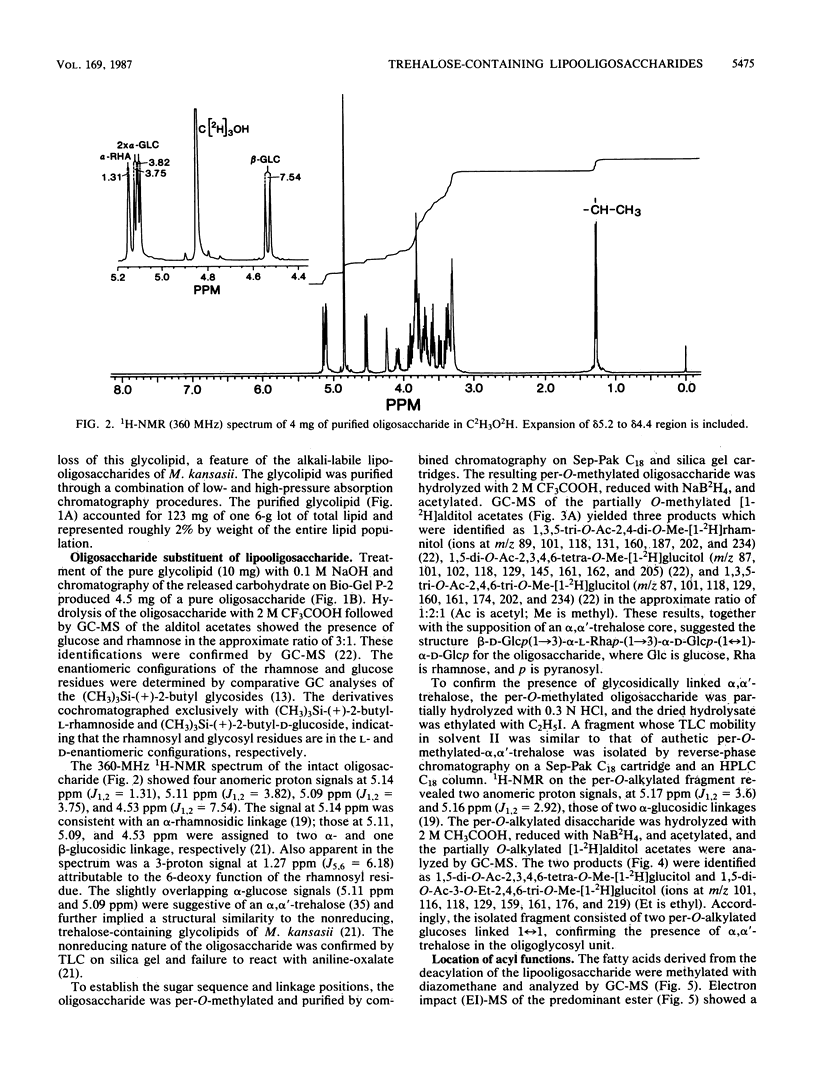
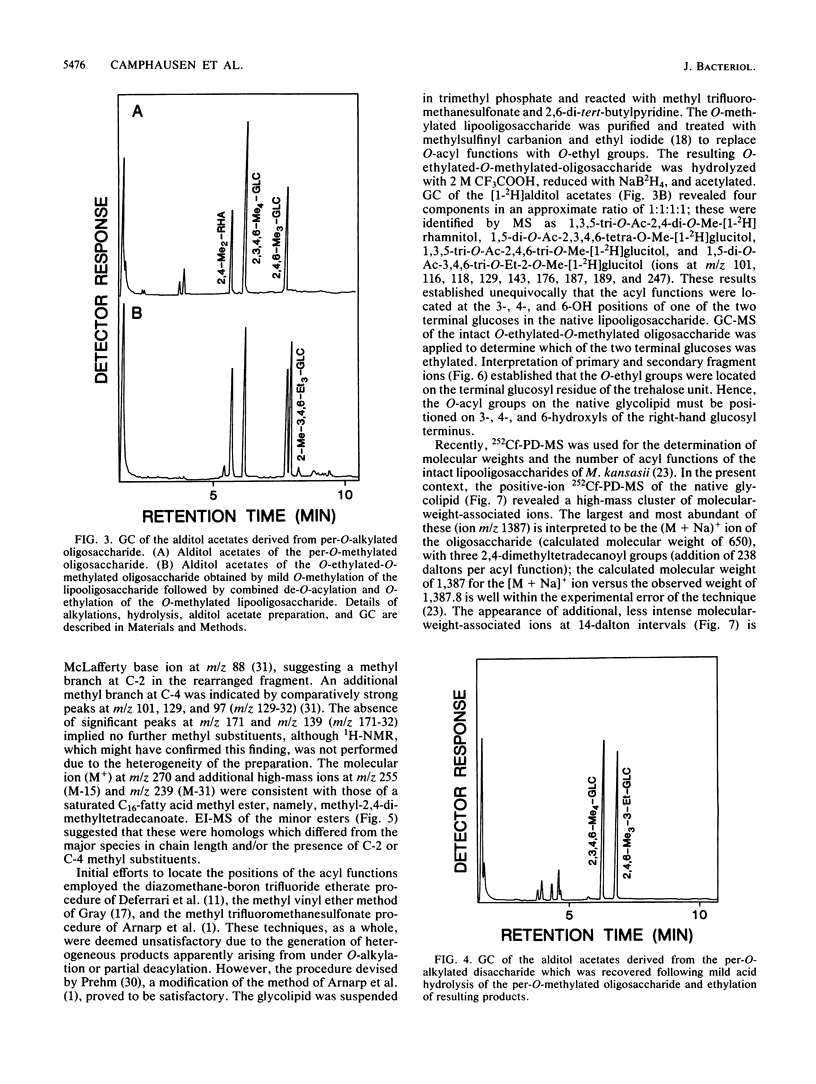
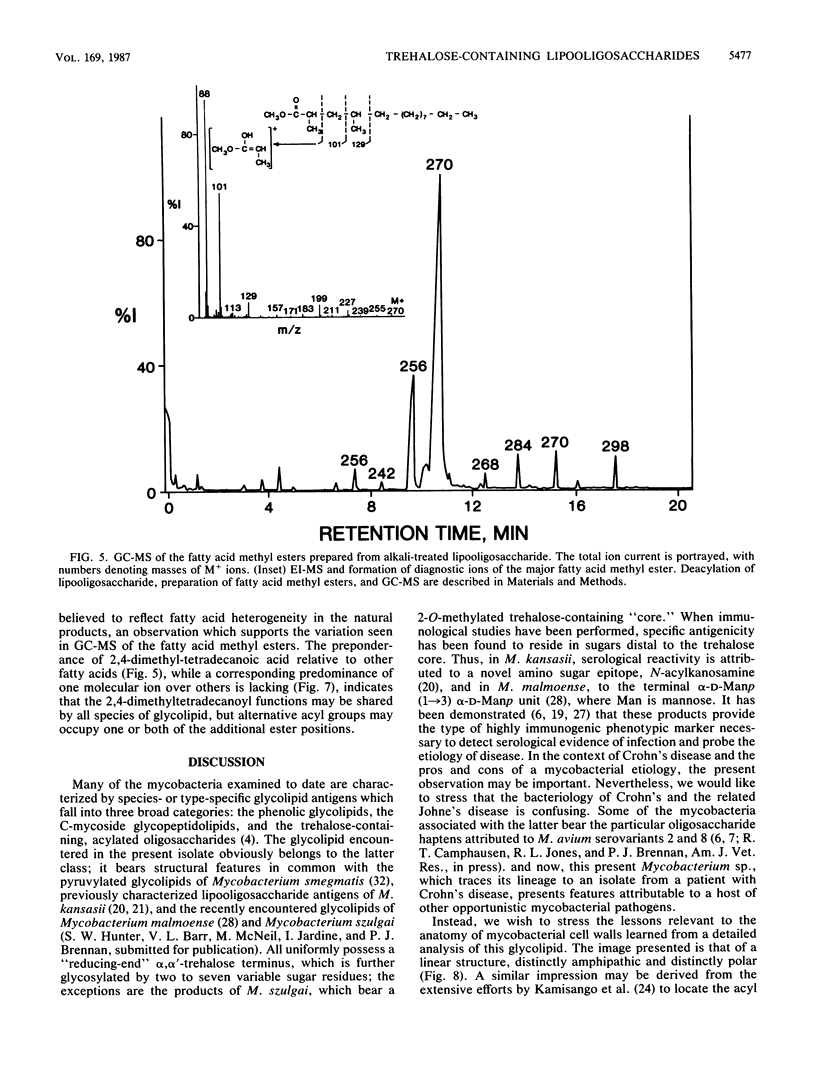
Abstract
A variant of a Mycobacterium sp. originating in a patient with Crohn's disease, but not necessarily implicated in the disease, provided a simple version of a newer class of species-specific surface glycolipids, the trehalose-containing lipooligosaccharides. A combination of high-resolution 1H nuclear magnetic resonance, methylation, ethylation, and absolute configurational analysis established the structure of the oligosaccharide unit as beta-D-Glcp(1----3)-alpha-L-Rhap(1----3)-alpha-D-Glcp(1----1)-alph a-D-Glcp (where Glc is glucose, Rha is rhamnose, and p is pyranosyl), and gas chromatography-electron impact mass spectrometry allowed identification of the fatty acyl esters as primarily 2,4-dimethyltetradecanoate. The relative simplicity of the glycolipid combined with the application of a mild methylation procedure and californium-252 plasma desorption mass spectrometry allowed recognition of three such acyl residues on the 3-, 4-, and 6-hydroxyl positions of the terminal glucosyl residue of the trehalose unit. Thus, the glycolipid is decidedly amphipathic yet is clearly not membranous. This observation leads to speculation about the role of these novel lipooligosaccharides in contributing to the outer segment of the hydrophobic barrier of the cell wall of certain mycobacteria.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auer I. O., Röder A., Wensinck F., van de Merwe J. P., Schmidt H. Selected bacterial antibodies in Crohn's disease and ulcerative colitis. Scand J Gastroenterol. 1983 Mar;18(2):217–223. doi: 10.3109/00365528309181586. [DOI] [PubMed] [Google Scholar]
- Blaser M. J., Miller R. A., Lacher J., Singleton J. W. Patients with active Crohn's disease have elevated serum antibodies to antigens of seven enteric bacterial pathogens. Gastroenterology. 1984 Oct;87(4):888–894. [PubMed] [Google Scholar]
- Brennan P. J., Goren M. B. Structural studies on the type-specific antigens and lipids of the mycobacterium avium. Mycobacterium intracellulare. Mycobacterium scrofulaceum serocomplex. Mycobacterium intracellulare serotype 9. J Biol Chem. 1979 May 25;254(10):4205–4211. [PubMed] [Google Scholar]
- Camphausen R. T., Jones R. L., Brennan P. J. A glycolipid antigen specific to Mycobacterium paratuberculosis: structure and antigenicity. Proc Natl Acad Sci U S A. 1985 May;82(10):3068–3072. doi: 10.1073/pnas.82.10.3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camphausen R. T., Jones R. L., Brennan P. J. Structure and relevance of the oligosaccharide hapten of Mycobacterium avium serotype 2. J Bacteriol. 1986 Nov;168(2):660–667. doi: 10.1128/jb.168.2.660-667.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodini R. J., Van Kruiningen H. J., Merkal R. S., Thayer W. R., Jr, Coutu J. A. Characteristics of an unclassified Mycobacterium species isolated from patients with Crohn's disease. J Clin Microbiol. 1984 Nov;20(5):966–971. doi: 10.1128/jcm.20.5.966-971.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodini R. J., Van Kruiningen H. J., Thayer W. R., Coutu J. A. Spheroplastic phase of mycobacteria isolated from patients with Crohn's disease. J Clin Microbiol. 1986 Sep;24(3):357–363. doi: 10.1128/jcm.24.3.357-363.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodini R. J., Van Kruiningen H. J., Thayer W. R., Merkal R. S., Coutu J. A. Possible role of mycobacteria in inflammatory bowel disease. I. An unclassified Mycobacterium species isolated from patients with Crohn's disease. Dig Dis Sci. 1984 Dec;29(12):1073–1079. doi: 10.1007/BF01317078. [DOI] [PubMed] [Google Scholar]
- Graham D. Y., Markesich D. C., Yoshimura H. H. Mycobacteria and inflammatory bowel disease. Results of culture. Gastroenterology. 1987 Feb;92(2):436–442. doi: 10.1016/0016-5085(87)90139-9. [DOI] [PubMed] [Google Scholar]
- HAKOMORI S. A RAPID PERMETHYLATION OF GLYCOLIPID, AND POLYSACCHARIDE CATALYZED BY METHYLSULFINYL CARBANION IN DIMETHYL SULFOXIDE. J Biochem. 1964 Feb;55:205–208. [PubMed] [Google Scholar]
- Hunter S. W., Fujiwara T., Brennan P. J. Structure and antigenicity of the major specific glycolipid antigen of Mycobacterium leprae. J Biol Chem. 1982 Dec 25;257(24):15072–15078. [PubMed] [Google Scholar]
- Hunter S. W., Jardine I., Yanagihara D. L., Brennan P. J. Trehalose-containing lipooligosaccharides from mycobacteria: structures of the oligosaccharide segments and recognition of a unique N-acylkanosamine-containing epitope. Biochemistry. 1985 May 21;24(11):2798–2805. doi: 10.1021/bi00332a030. [DOI] [PubMed] [Google Scholar]
- Hunter S. W., Murphy R. C., Clay K., Goren M. B., Brennan P. J. Trehalose-containing lipooligosaccharides. A new class of species-specific antigens from Mycobacterium. J Biol Chem. 1983 Sep 10;258(17):10481–10487. [PubMed] [Google Scholar]
- Jardine I., Hunter S. W., Brennan P. J., McNeal C. J., Macfarlane R. D. Heterogeneity of bacterial antigenic lipooligosaccharides determined by californium-252 plasma desorption mass spectrometry. Biomed Environ Mass Spectrom. 1986 Jun;13(6):273–276. doi: 10.1002/bms.1200130603. [DOI] [PubMed] [Google Scholar]
- Kamisango K., Saadat S., Dell A., Ballou C. E. Pyruvylated glycolipids from Mycobacterium smegmatis. Nature and location of the lipid components. J Biol Chem. 1985 Apr 10;260(7):4117–4121. [PubMed] [Google Scholar]
- Kim K. S., Salton M. R., Barksdale L. Ultrastructure of superficial mycosidic integuments of Mycobacterium sp. J Bacteriol. 1976 Feb;125(2):739–743. doi: 10.1128/jb.125.2.739-743.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil M., Tsang A. Y., Brennan P. J. Structure and antigenicity of the specific oligosaccharide hapten from the glycopeptidolipid antigen of Mycobacterium avium serotype 4, the dominant Mycobacterium isolated from patients with acquired immune deficiency syndrome. J Biol Chem. 1987 Feb 25;262(6):2630–2635. [PubMed] [Google Scholar]
- McNeil M., Tsang A. Y., McClatchy J. K., Stewart C., Jardine I., Brennan P. J. Definition of the surface antigens of Mycobacterium malmoense and use in studying the etiology of a form of mycobacteriosis. J Bacteriol. 1987 Jul;169(7):3312–3320. doi: 10.1128/jb.169.7.3312-3320.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadat S., Ballou C. E. Pyruvylated glycolipids from Mycobacterium smegmatis. Structures of two oligosaccharide components. J Biol Chem. 1983 Feb 10;258(3):1813–1818. [PubMed] [Google Scholar]
- Takayama K., Qureshi N., Mascagni P. Complete structure of lipid A obtained from the lipopolysaccharides of the heptoseless mutant of Salmonella typhimurium. J Biol Chem. 1983 Nov 10;258(21):12801–12803. [PubMed] [Google Scholar]
- Thayer W. R., Jr, Coutu J. A., Chiodini R. J., Van Kruiningen H. J., Merkal R. S. Possible role of mycobacteria in inflammatory bowel disease. II. Mycobacterial antibodies in Crohn's disease. Dig Dis Sci. 1984 Dec;29(12):1080–1085. doi: 10.1007/BF01317079. [DOI] [PubMed] [Google Scholar]
- Yoshimura H. H., Graham D. Y., Estes M. K., Merkal R. S. Investigation of association of mycobacteria with inflammatory bowel disease by nucleic acid hybridization. J Clin Microbiol. 1987 Jan;25(1):45–51. doi: 10.1128/jcm.25.1.45-51.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]