Abstract
We report that the actin assembly inhibitor latrunculin-A (LAT-A) causes complete disruption of the yeast actin cytoskeleton within 2–5 min, suggesting that although yeast are nonmotile, their actin filaments undergo rapid cycles of assembly and disassembly in vivo. Differences in the LAT-A sensitivities of strains carrying mutations in components of the actin cytoskeleton suggest that tropomyosin, fimbrin, capping protein, Sla2p, and Srv2p act to increase actin cytoskeleton stability, while End3p and Sla1p act to decrease stability. Identification of three LAT-A resistant actin mutants demonstrated that in vivo effects of LAT-A are due specifically to impairment of actin function and implicated a region on the three-dimensional actin structure as the LAT-A binding site.
LAT-A was used to determine which of 19 different proteins implicated in cell polarity development require actin to achieve polarized localization. Results show that at least two molecular pathways, one actindependent and the other actin-independent, underlie polarity development. The actin-dependent pathway localizes secretory vesicles and a putative vesicle docking complex to sites of cell surface growth, providing an explanation for the dependence of polarized cell surface growth on actin function. Unexpectedly, several proteins that function with actin during cell polarity development, including an unconventional myosin (Myo2p), calmodulin, and an actin-interacting protein (Bud6/Aip3p), achieved polarized localization by an actin-independent pathway, revealing interdependence among cell polarity pathways. Finally, transient actin depolymerization caused many cells to abandon one bud site or mating projection and to initiate growth at a second site. Thus, actin filaments are also required for maintenance of an axis of cell polarity.
In the budding yeast, Saccharomyces cerevisiae, there are two types of actin filament-based structures, cytoplasmic cables, and cortical patches (Kilmartin and Adams, 1984). Throughout the yeast cell cycle, precisely choreographed changes in the organization of the actin cytoskeleton underlie spatial control of cell surface growth and thereby determine cell morphology (Kilmartin and Adams, 1984; Adams and Pringle, 1984; Novick and Botstein, 1995; Lew and Reed, 1993). Important questions concern the mechanism by which actin cytoskeleton organization is controlled and how actin-based structures facilitate polarized cell surface growth.
Recently, expression of green fluorescent protein–labeled actin cytoskeleton proteins in yeast has shown that actin cortical patches are in constant motion within the plane of the cell cortex (Waddle et al., 1996; Doyle and Botstein, 1996). This patch motility might facilitate programmed changes in actin organization. An alternative possibility is that disassembly of actin patches in one region of the cell cortex and assembly of new patches in different regions underlie changes in actin organization. Also, the mechanism by which actin cortical patches move has not been determined. Movement might be driven by myosin motors or by an actin assembly-coupled process akin to the movement of Listeria bacteria and cell surface comets (Tilney and Portnoy, 1989; Tilney et al., 1990; Theriot and Mitchison, 1992; Forscher et al., 1992). A possible problem with models proposing actin assembly dynamics in cortical patches is the suggestion that the pool of free actin monomers in yeast is too low to be compatible with dynamic actin assembly and disassembly (Karpova et al., 1995).
Nevertheless, there are indications that dynamic assembly and disassembly of actin filaments is a characteristic of actin in all eukaryotes. First, actin from all organisms has an intrinsic ATPase activity, indicating that all actins have the capacity to assemble and disassemble dynamically. Second, all eukaryotic cells, including yeast, are endowed with a full complement of proteins including cofilin (Moon et al., 1993), profilin (Haarer et al., 1990), and Arp2 (Moreau et al., 1996), which are implicated in the dynamic turnover of actin filaments. Third, the yeast cortical actin cytoskeleton appears to have the capacity to continuously nucleate actin filament assembly (Li et al., 1995). Presumably, this assembly would be balanced by continuous disassembly. Clearly, knowing whether actin filaments undergo rapid cycles of assembly and disassembly in yeast will greatly help to resolve the issues discussed here and will provide insights into regulation of actin-mediated morphogenetic processes in nonmotile cells.
Here, we characterize the effects on yeast of a drug, latrunculin-A (LAT-A)1, which had previously been shown to disrupt the actin cytoskeleton in vertebrate cells (Spector et al., 1989). Our results lead us to conclude that the yeast actin cytoskeleton undergoes rapid cycles of assembly and disassmbly in vivo and provide novel insights into the contributions of a variety of proteins to modulation of cytoskeleton integrity.
We also used LAT-A to investigate the role of actin in the establishment and maintenance of cell polarity. Based on a multitude of studies, it has been hypothesized that functional hierarchies govern the generation of cell polarity in eukaryotic cells as diverse as budding yeast and mammalian epithelia (reviewed by Drubin and Nelson, 1996). That is, certain proteins must function at the right place and time before other proteins involved in polarity establishment function properly. Numerous proteins have been identified in yeast which accumulate at a specific area of the cell cortex before bud emergence. This area has been termed the presumptive bud site. Several of the proteins localizing to this site have been shown to be important for the formation of the bud or for subsequent cytokinesis of the bud from the mother cell, while the specific roles for many other proteins located at the presumptive bud site are not known. However, the interdependencies between the many polarized proteins for localization and subsequent function have not been intensively investigated.
While actin is essential for polarized cell growth in yeast (Novick and Botstein, 1985), other proteins are postulated to act upstream of actin in the hierarchy of cell polarity establishment. Three polarity establishment proteins are Cdc24p, Cdc42p, and Bem1p. At the nonpermissive temperature, temperature-sensitive cdc24, cdc42, and bem1 mutants accumulate as large, round, unbudded cells (Sloat et al., 1981; Adams et al., 1990; Bender and Pringle, 1991; Chant et al., 1991). In cdc24 and cdc42 mutant cells, neither the neck filament–associated septin proteins nor proteins of the actin cytoskeleton are polarized (Adams and Pringle, 1984; Pringle et al., 1995), which is in contrast to the wild-type situation in which both of these cytoskeletal elements localize to the bud site before bud formation (Kilmartin and Adams, 1984; Ford and Pringle, 1991; Kim et al., 1991). These observations suggest that both septins and proteins associated with the actin cytoskeleton require Cdc24p and Cdc42p for localization at the bud site. Thus, the cytoskeletal proteins would appear to function downstream from the polarity establishment proteins. However, the ability of polarity establishment proteins such as Bem1p and Cdc42p to achieve their normal polarized organization, which is at the presumptive bud site (Ziman et al., 1993; Pringle et al., 1995), in the absence of the cytoskeletal structures, has not been tested. Therefore, localization of cytoskeleton proteins and Cdc42p and Bem1p may be interdependent processes. Furthermore, the proteins functioning downstream of actin in cell polarity development pathways remain to be defined. Our studies reveal that actin-dependent and actin-independent pathways function downstream of Cdc42p and Bem1p to facilitate polarity development, and that the actin-dependent pathway organizes elements of the secretory pathway to facilitate polarized cell surface growth.
Materials and Methods
Materials
Unless otherwise stated, chemicals used were obtained from Sigma Chem. Co. (St. Louis, MO) or Fisher Scientific, (Pittsburgh, PA). LAT-A was purified as described below. However, it is now also available from Molecular Probes (Eugene, OR).
Animal Material
The three sponges which yielded compounds used in this study were as follows: Jasplakinolide was isolated from Jaspis johnstoni (Collection No. 93100); Latrunculin-A was isolated from Cacospongia mycofijiensis (Collection No. 93102); Swinholide A was isolated from Stelleta clavosa (Collection No. 95004). All three sponges were collected by SCUBA at depths of 40–100 feet from vertical reef habitats in Milne Bay, Papua New Guinea.
Actin Inhibitors
For preparation of jasplakinolide (Inman and Crews, 1989) and swinholide-A (Kitagawa et al., 1990) sponges were preserved by being immersed in an EtOH-H20 (1:1) solution. After ∼24 h, this solution was decanted and discarded. The damp organisms were separately placed in Nalgene bottles and shipped back to the home laboratory at ambient temperature. Next, 100% MeOH was added and the organisms were soaked for at least 24 h after which time the extraction solution was decanted and evaporated at room temperature in vacuo. This procedure was repeated twice. The MeOH extract was then successively partitioned between equal volumes of aqueous MeOH (percent adjusted to produce a biphasic solution) and hexanes (FH fraction) followed by CH2Cl2 (FD fraction). This FD fraction afforded semi-pure oil which was then subjected to Sephadex LH20 chromatography using MeOH:CH2Cl2 (70:30) as an eluent to yield the pure compound. Comparison of the 1H and 13C NMR spectra of jasplakinolide (Inman and Crews, 1989) and swinholide-A (Kitagawa et al., 1990), respectively, matched those reported in the literature. For preparation of latrunculin-A (Quiñoà et al., 1988), the FD fraction was obtained from the sponge as described above. This was then fractionated using silica flash chromatography (35 mm × 50 cm) using 4.7% MeOH:CH2Cl2 as an eluent. The structure of the pure compound was confirmed by comparison of the NMR spectra to those reported in the literature (Quinoa et al., 1988). Latrunculin-A, swinholide-A, and jasplakinolide were made up as 10 mM or 20 mM stocks in DMSO.
Mycalolide-B was a gift from Helen Yin (Southwestern Medical School, Dallas, TX) and Hideaki Karaki (Department of Veterinary Pharmacology, University of Tokyo). It was made up as a 10 mM stock in 50% ethanol. Latrunculin-B was from LC Laboratories (Woburn, MA) and was made up as a 10-mM stock in DMSO.
Yeast Strains, Growth Conditions, Measuring Cell Growth, and Viability
Yeast strains used in this study are listed in Table I. Unless otherwise stated cells were grown with rotary shaking at 25°C in liquid YPD medium (1% yeast extract, 2% bacto peptone, 2% glucose) except for plasmid carrying strains which were grown on synthetic medium with appropriate selection as described by Kaiser et al. (1994). Cell growth was measured using a counter (Coulter Electronics Ltd., Luton, Bedfordshire, UK) and by measuring the turbidity at OD 600 nm of yeast in suspension. The viability of cells following the addition of LAT-A to a cell suspension was assessed by micromanipulating 120 cells for each condition onto a YPD-agar plate using a micromanipulation needle. Viability was scored as the number of cells that were able to form a colony.
Table I.
Yeast Strains Used in This Study
| Strain | Genotype | Source | ||
|---|---|---|---|---|
| DDY182 | MAT a ura3-52 | 1 | ||
| DDY1132 | MAT a/α ura3-52/ura3-52 | 1 | ||
| R757 | MAT α ura3-52, his4-15, lys9 | 2 | ||
| R2563 | MAT α ura3-52, his4-15, lys9, Δerg6 | 2 | ||
| DDY186 | MAT α ura3-52, leu2-3,112, | 1 | ||
| DDY321 | MAT a his3-200, leu2-3,112, ura3-52, Δabp1::LEU2 | 1 | ||
| DDY332 | MAT a, ura3-52, his3-Δ200, Δsla1-Δ1::URA3 | 1 | ||
| DDY544 | MAT α ura3-52, ade2, leu2-3,112, Δsla2-Δ1::URA3 | 1 | ||
| DDY771 | MAT α, his4 ura3-52, Δsac6::URA3 | 1 | ||
| RH144-3D | MAT a, ura3-52, leu2-3,112, his4, bar1-1 | 3 | ||
| RH266-1D | MAT a, ura3-52, leu2-3,112, his4, bar1-1, end3-1 | 3 | ||
| DDY 816 | MAT α, his3-200, leu2-3,112, ura3-52 | 1 | ||
| DDY 817 | MAT α, his3-200, leu2-3,112, ura3-52, Δsrv2/CAP::HIS3 | 1 | ||
| DDY199 | MAT a his3, leu2, ura3, ade2, ade3, can1, sap3 | 4 | ||
| DDY200 | MAT a his3, leu2, ura2, ade3, can1, sap3, Δtpm1::URA3 | 4 | ||
| YJC093 | MAT a rho +, his3-11, leu2-3,112, trp1-1, ura3-1 | 5 | ||
| YJC108 | MAT a rho +, his3-11, leu2-3,112, trp1-1, ura3-1, cap2-Δ1::HIS3 | 5 | ||
| DDY280 | MATα ura3-62, leu2-3,112, trp1-1, his4-619, bem1Δ::LEU2 | 6 | ||
| DDY1157 | MATa/α ura3-52/ura3-52, Δsec8::URA3/ Δsec8::URA3, leu2,3-112::LEU2-Sec8-myc/ leu2-3,112:LEU2-Sec8-myc | 7 | ||
| JZ414 | MAT a/α ura3-52/ura3-52, his3Δ200/ his3Δ200, leu2-Δ1/leu2-Δl lys2-801. trp1-Δ1/trp1-Δ1 + pBUD6, 2μ URA3 | 8 | ||
| M-442 | MATa/α ura3-52/ura3-52, his3Δ200/ his3Δ200, leu2-Δ1/leu2-Δ1 lys2-801/lys2-801, trp1-Δ1/trp1-Δ1, gin4Δ::HIS3/gin4Δ::HIS3, GIN4-GST::URA3/GIN4-GST::URA3 | 8 | ||
| *DDY336 | MAT a, act1-133::HIS3, can1-1, cry1 | 1 | ||
| *DDY337 | MAT a, act1-108::HIS3, can1-1, cry1 | 1 | ||
| *DDY338 | MAT a, act1-101::HIS3, can1-1, cry1 | 1 | ||
| *DDY339 | MAT a, act1-102::HIS3, ade2-101, can1-1 | 1 | ||
| *DDY340 | MAT α, act1-104::HIS3, can1-1 | 1 | ||
| *DDY341 | MAT a, act1-111::HIS3, ade2-101, cry1 | 1 | ||
| *DDY342 | MAT a, act1-113::HIS3, can1-1 | 1 | ||
| *DDY343 | MAT α, act1-115::HIS3, ade2-201, can1-1 | 1 | ||
| *DDY344 | MAT α, act1-116::HIS3, can1-1 | 1 | ||
| *DDY345 | MAT a, act1-117::HIS3, ade2-101, cry1 | 1 | ||
| *DDY346 | MAT a, act1-119::HIS3, ade2-101, cry1 | 1 | ||
| *DDY347 | MAT a, act1-120::HIS3, can1-1, cry1 | 1 | ||
| *DDY348 | MAT a, act1-123::HIS3, ade2-101, can1-1, cry1 | 1 | ||
| *DDY349 | MAT α, act1-124::HIS3 | 1 | ||
| *DDY350 | MAT α, act1-125::HIS3 | 1 | ||
| *DDY351 | MAT α, act1-129::HIS3, can1-1 | 1 | ||
| *DDY352 | MAT a, act1-132::HIS3, ade2-101, can1-1, cry1 | 1 | ||
| *DDY353 | MAT α, act1-135::HIS3 | 1 | ||
| *DDY354 | MAT a, ACT1::HIS3, cry1 | 1 | ||
| *DDY355 | MAT a, act1-112::HIS3, ade2-101, ade4, cry1 | 1 | ||
| *DDY356 | MAT α, act1-105::HIS3, can1-1 | 1 | ||
| *DDY357 | MAT a, act1-136::HIS3, | 1 | ||
| *DDY654 | MAT α, act1-121::HIS3, can1-1 | 1 | ||
| 972 | h- wild-type Schizosaccharomyces pombe | 9 |
These strains are a congenic collection of act1 alleles made as described by Wertman et al. (1992). Only alleles that vary between strains are listed above. All strains are his3-Δ200, ura3-52, leu2-3,112, and tub2-201. Sources of strains listed. (1). This lab; (2) R. Gaber (Northwestern University, Evanston, IL); (3) H. Riezman (Biozentrum, University of Basel, Switzerland); (4) A. Bretscher (Cornell University, Ithaca, NY); (5) J. Cooper (Washington University, St. Louis, MO); (6) I. Herskowitz (UCSF, San Francisco, CA); (7) Dan TerBush, Peter Novick (Yale University School of Medicine, New Haven, CT); (8) J. Pringle (University of North Carolina, Chapel Hill, NC); (9) Paul Nurse (ICRF, London, UK).
LAT-A Treatment of Cells in Culture
For study of the kinetics of F-actin disruption, cells were grown to log phase and LAT-A was added from a 10-mM DMSO stock to a final concentration of 200 μM. For those experiments in which recovery from LAT-A treatment was monitored, cells that were treated for a specified time with LAT-A were washed three times before release into fresh media.
For those experiments observing the effect of LAT-A on shmoo formation, an asynchronous culture of wild-type cells (DDY182) was taken and α-factor added to 2 μg/ml. When >80% cells were observed to have shmoos, LAT-A (or DMSO as a control) was added to 200 μM for 5 min. Cells were then washed and released back into fresh media containing α-factor.
Halo Assays and Relative Apparent Sensitivity
An overnight culture of yeast was grown in YPD medium. 10-μl cells were added to 2 ml of 2× YPD, and then 2 ml molten 1% agar (cooled to 55°C) was added and the cell suspension was poured onto the surface of a YPD plate. Dilutions of the drugs (or dilutions of solvent alone as a control) were made into dH2O and 10 μl of each dilution of the drug was pipetted onto the center of a sterile 6-mm filter disk (Fisher). The disk was then placed on the top agar. The plate was inverted and left at 25°C for 24–48 h before halos were clearly visible. The diameters of halos formed were measured for the various concentrations of drugs applied to the disks. Relative apparent sensitivities were calculated as described by Reneke et al. (1988). For mutant strains, comparison was always made to a congenic wild-type strain.
Yeast Actin Purification
Yeast actin was purified using a procedure modified from that described by Kron et al. (1992). Yeast (6 liters) was grown in YPD to stationary phase (OD 600 nm 6–8). Cells were pelleted and resuspended in a minimal volume of G buffer (10 mM Tris, pH 7.5, 0.2 mM CaCl2, 2 mM DTT, 0.5 mM ATP) and then frozen in liquid N2 by squirting through a syringe. 100-g cells were lysed with liquid N2 in a Waring blender (McCormack et al., 1996).The yeast lysate was then thawed and 100 ml G buffer + protease inhibitors (0.5 μg/ml each of antipain, leupeptin, pepstatin A, chymostatin, and aprotinin and 1 mM PMSF) was added. The lysate was spun in an SLA-1500 rotor (Sorvall/DuPont, Wilmington, DE) at 12,000 rpm (13,400 g), 4°C, 30 min. The supernatant was then spun in a 45Ti rotor (Beckman Instruments Inc, Fullerton, CA), 120 min, 4°C, 45,000 rpm. The supernatant was filtered through cheesecloth and then loaded onto a pre-equilibrated 20-ml DNase1 column at ∼1 ml/min. DNase 1 (Boehringer Mannheim, Indianapolis, IN) was coupled to Affigel-10 (BioRad, Richmond, CA). The column was washed with 25 ml G buffer + PIs + 10% formamide; with 25 ml G buffer + PIs + 10% formamide + 0.2M ammonium chloride; with 25 ml G buffer + PIs. The column was eluted with 25 ml G-buffer + PIs + 50% formamide at 0.5 ml/min. Ammonium sulfate was added to the eluate at 375 mg/ml; the mixture was gently inverted until the ammonium sulfate was dissolved. The ammonium sulfate mix was transferred to a 30-ml Corex tube and spun at 10,000 rpm (10,300 g) in a Sorvall SA-600 rotor, for 20 min at 4°C. The pellet was gently resuspended in 0.4 ml G buffer and 0.5 ml of this was desalted using a G-25 column (NAP-10 column, Pharmacia, Piscataway, NJ).
Nucleotide Exchange Assay
The fluorescent signal provided by etheno-ATP (ε-ATP; Molecular Probes, Eugene, OR) bound to actin was used to measure the rate of exchange of actin nucleotide (Waechter and Engel, 1975) as described by GoldschmidtClermont et al. (1991) with the modifications noted (Goldschmidt-Clermont et al., 1992). Excitation was at 360 nm and emission at 410 nm. Various concentrations of LAT-A were pre-incubated for 20 min with the reaction buffer (2 mM Tris-HCl, pH 7.5, 1 μM ATP, 200 μM ε-ATP, 0.5 μM CaCl2, 1 mM EGTA, 1 mM MgCl2, 50 mM KCl, and 2 mM DTT). Yeast actin was added at 3 μM to initiate the reaction.
Actin Polymerization
Actin (3 μM) was assembled by the addition of one tenth volume of 10× polymerization-inducing solution (20 mM MgCl2, 5 mM ATP, 1 M KCl, pH 7.0) in a final volume of 100 μl. LAT-A (3 μM), or an equal volume of solvent, was added to the actin before addition of polymerization salts. Polymerization was allowed to proceed for 2 h at room temperature. Filaments were then pelleted at 90 K rpm in a TLA table top centrifuge using a TLA100 rotor (Beckman Instruments Inc.). Supernatants and pellets were visualized on Coomassie-stained SDS-PAGE gels. Analysis of band intensity was performed using a IS-1000 digital imaging system (Alpha Innotech Corp., San Leandro, CA).
Growth of Cells from G0 in the Presence or Absence of LAT-A
200 μl of an overnight yeast culture was spread onto a YPD-agar plate and the cells were allowed to form a lawn (1–2 d at 30°C). Cells were scraped from the plate into YPD liquid medium, pelleted, and then resuspended in 20 ml 1 M Sorbitol, 50% YPD. Cells were spun for 1 min, at 500 g. This step preferentially pellets budded cells. The supernatant was retained. The percentage of unbudded cells was checked under the microscope. Cells were repeatedly centrifuged as above until there was an essentially uniform unbudded population of cells. These cells were then pelleted, resuspended in YPD at 0.25–0.5 × 107 cells/ml, and allowed to grow out of stationary phase (G0) at 25°C over the next few h in the presence or absence of 100 μM LAT-A (added to the media from a 20-mM stock in DMSO). An equal volume of DMSO alone was added to the control population of cells.
Purification of Bem1 Antibodies
Antisera raised against a Bem1-TrpE fusion protein and the Bem1-TrpE plasmid construct were generous gifts from Erfei Bi and John Pringle (University of North Carolina, Chapel Hill, NC). Antibodies against Bem1p were affinity purified using a micro-affinity purification technique as described by Pringle et al. (1991). The insoluble Bem1-TrpE fraction obtained in the induction of the fusion protein in E. coli was blotted onto nitrocellulose and used for the affinity purification. Overexpression of the Bem1-TrpE fusion was as described by Koerner et al. (1991). To further purify the antibodies following micro-affinity purification, the antibodies were pre-absorbed against bem1 null cells.
Fluorescence Procedures
Rhodamine-phalloidin (Rd-phalloidin) staining of actin in yeast was performed as described by Pringle et al. (1989). Rd-phalloidin staining of actin filaments in vitro was performed according to Drubin et al. (1993). For analyzing protein localization in cells emerging from stationary phase, 1-ml samples of yeast cells were removed at 1-h intervals following release from G0. Cells were processed for immunofluorescence essentially as described by Pringle et al. (1991) and Ayscough and Drubin (1997). The cold methanol/acetone step (Pringle et al., 1991) was required to observe actin, Aip3p/ Bud6p, Abp1p, Arp2p, Cdc10p, Cdc11p, cofilin, calmodulin, Gin4p, Myo2p, Sla2p, and Spa2. To observe Sla1p, cells were treated as in Pringle (1991) except that they were fixed in formaldehyde for 10 min rather than 60 min. To visualize Cdc42p, Bem1p, Sec4p, Sec8p, and Smy1p, we followed the protocol described by Ziman et al. (1993) in which the methanol/acetone step was replaced with an incubation of the cells in 0.5% SDS for 5 min. Table II lists the primary antibodies used in this study and the dilutions used. Secondary antibodies used were fluorescein-isothiocyanate (FITC) conjugated goat anti–mouse and FITC goat anti–rabbit (Cappell/ Organon Technika, Malvern, PA) at a dilution of 1:1,000 and CY3-conjugated sheep anti–rabbit (Sigma Chem. Co.) at a dilution of 1:200. Cells were viewed with a Zeiss Axioskop fluorescence microscope with a 100 W mercury lamp and a Zeiss 100X Plan-NeoFluar oil immersion objective. Images were recorded using T-MAX 400 film (Kodak). In addition, images were captured electronically using a 200-E CCD camera (Sony Electronics Inc. San José, CA) and displayed on a Micron 133 computer (Micron Electronics Inc., Nampa, ID) using Northern Exposure software (Phase 3 Imaging Systems, Milford, MA). All images show cells after 4 h release from the G0 arrest.
Table II.
Primary Antibodies Used in This Study
| Antigen | Raised in | Dilution used | Source | |||
|---|---|---|---|---|---|---|
| actin | Rabbit | 1/40 | 1 | |||
| Cdc42p | Rabbit | 1/300 | 2 | |||
| Bem1p | Rabbit | 1/10 | 3 | |||
| Cdc10p | Rabbit | 1/10 | 3 | |||
| Cdc11p | Rabbit | 1/10 | 3 | |||
| Abp1p | Rabbit | 1/200 | 1 | |||
| cofilin | Rabbit | 1/150 | 1 | |||
| Sla1p | Rabbit | 1/7.5 | 1 | |||
| Sla2p | Rabbit | 1/50 | 1 | |||
| Bud6p/Aip3p | Rabbit | 1/2 | 3 | |||
| Arp2p | Rabbit | 1/30 | 4 | |||
| Sec4p | Mouse | 1/10 | 5 | |||
| Myc 9E10 | Mouse | 1/50 | 6 | |||
| (for Sec8p) | ||||||
| Smy1p | Rabbit | 1/10 | 7 | |||
| Myo2p | Rabbit | 1/20 | 7 | |||
| calmodulin | Rabbit | 1/50 | 8 | |||
| Bni4p | Rabbit | 1/100 | 3 | |||
| GST (for Gin4p) | Rabbit | 1/20 | 1 | |||
| Spa2p | Rabbit | 1/400 | 9 |
Sources: (1) Drubin lab (Drubin et al., 1988; Moon et al., 1993; Ayscough, K., and S. Yang, unpublished observations); (2) D. Johnson (University of Vermont, Burlington, VT; Ziman et al., 1993); (3) J. Pringle (University of North Carolina, Chapel Hill, NC; Ford and Pringle, 1991); (4) B. Winsor (Strasbourg, France; Moreau et al., 1996); (5) P. Novick (Yale University School of Medicine, New Haven, CT; Novick and Brennwald, 1993); (6) Santa Cruz Biotechnology Inc., Santa Cruz, CA; (7) S. Brown (University of Michigan Medical School, Ann Arbor, MI; Lillie and Brown, 1994); (8) M. Stark (Biochemistry Department, University of Dundee, Scotland, UK); (9) M. Snyder (Department of Biology, Yale University, New Haven, CT; Snyder, 1989).
Counting Cells and Analyzing Polarized Cell Staining
The staining patterns for each antibody, in the presence or absence of LAT-A, were examined at each time point after release from G0. In each case, the time course was repeated three times and the number of cells counted was at least 200 for each time point. Polarized staining was indicated by an intense patch or ring at one end of the ellipsoidal diploid cell.
Results
The Action of Actin Disrupting Drugs in Yeast
We tested the effect of six actin disrupting drugs on the growth of wild-type S. cerevisiae cells by pipetting different concentrations of the drugs onto filter disks which were then placed onto nascent lawns of wild-type yeast cells. Cytochalasin B (see Cooper, 1987, and references therein), jasplakinolide (Bubb et al., 1994; Senderowicz et al., 1995), and swinholide-A (Bubb et al., 1995) did not cause zones of inhibited growth at any concentration tested (1 μM–1 mM; Table II). However, cells in which the ERG6 gene has been deleted (Δerg6) to increase cell permeability (Gaber et al., 1989), were sensitive to swinholide-A (Table III). In addition, when the Δerg6 cells were exposed to 500 μM swinholide-A in suspension, all filamentous-actin (F-actin) structures detectable by rhodamine-phalloidin staining were rapidly lost (data not shown).
Table III.
Testing Actin Inhibitors for Effects on Yeast Cells In Vivo
| Halo diameter | ||||||
|---|---|---|---|---|---|---|
| Drug | Wild-type (ERG6) | Δerg6 | S. pombe | |||
| mm | ||||||
| Latrunculin-A | 13 | 24 | 28 | |||
| Latrunculin-B | 10 | 22 | 23 | |||
| Mycalolide-B | 11 | 19 | 13 | |||
| Swinholide-A | − | 10 | − | |||
| Jasplakinolide | − | − | − | |||
| Cytochalasin-B | − | − | − | |||
Halo assays were used to determine whether the indicated drugs were able to inhibit growth in either wild-type S. cerevisiae cells or in S. cerevisiae cells in which the ERG6 gene was deleted to increase cell permeability. Results for wild-type S. pombe are also presented. The results presented here are the halo size (mm) obtained when 1 mM of each drug was tested.
LAT-A, LAT-B, and mycalolide-B all caused zones of inhibited growth in lawns of wild-type cells (Table III) and all disrupted the actin cytoskeleton (not shown). We used LAT-A for subsequent studies because its reported mechanism of actin assembly inhibition, via actin monomer binding and sequestration (Coué et al., 1987), best suited the objectives of our studies (see below), and because yeast were more sensitive to LAT-A than LAT-B. Diploid cells are slightly more sensitive (1.7 fold) than congenic haploid cells to LAT-A (data not shown), and both haploid and diploid cells show <15% difference in sensitivity to LAT-A between 14°C and 37°C. Finally, LAT-A, LAT-B, and mycalolide B all caused inhibition of growth of the fission yeast Schizosaccharomyces pombe (Table III).
The Effect of LAT-A on the Actin Cytoskeleton in S. cerevisiae
To determine the effect of LAT-A on actin itself rather than its effect on the growth of cells on plates, we added the drug to cells in suspension at the lowest concentration at which a halo was formed. Diploid cells were used for this characterization because they are larger, facilitating visualization of actin structures. After 15-min or 2-h incubations of log phase cells in 200 μM LAT-A (or an equivalent volume of DMSO, our solvent for LAT-A, as a control) at 25°C, samples were fixed for Rd-phalloidin staining of filamentous actin structures. No actin structures were seen in cells at either time point (see Fig. 1 for t = 2 h; t = 15 min, data not shown) indicating that LAT-A had caused a complete disruption of the actin cytoskeleton. We also stained these cells with antibodies to tubulin and observed normal mitotic spindles and cytoplasmic microtubules in LAT-A– treated cells indicating that the tubulin cytoskeleton was not grossly affected by the drug (data not shown).
Figure 1.
The effect of LAT-A on actin structures in yeast cells. Rd-phalloidin was used to visualize the effects of LAT-A addition to yeast cells. (a) Cells incubated in the absence of LAT-A. (b) Cells incubated for 2 h in the presence of 200 μM LAT-A. The kinetics of the effect of LAT-A on actin structures in yeast cells. (c) Cells were fixed at various time points after the addition of LAT-A. The cells were then processed for visualization of actin using Rd-phalloidin. (d) After 15 min of treatment with LAT-A, the cells were washed twice with fresh medium, resuspended in medium, and allowed to resume growth. Cells were fixed at various time points after washing and processed as above. (▴) actin cables, (○) depolarized cortical actin patches, and (•) polarized actin patches. In both c and d, in each of three repeated experiments, 500 cells were analyzed for their actin structures at each time point. Bar (b) 5 μm.
Kinetics of the LAT-A Effect Suggest a High Rate of Actin Filament Turnover
To determine the rate of the disruption of the actin cytoskeleton, LAT-A was added to cells and samples were fixed at time points from 1–15 min after addition. Cells were then stained using Rd-phalloidin to observe F-actin structures. The percentage of cells containing actin cables or cortical actin patches was determined (Fig. 1 c). Whether the cortical structures were polarized within the cell was also noted. The actin cables disappeared rapidly with only 5% of cells having any observable cables after 1 min. No cables were seen after 2 min. Cortical patches were observed in only 5% of cells after 5 min and in <1% of cells after 10 min. Interestingly, many of the patches appeared to become depolarized before they were fully disassembled. The effect of LAT-A was also shown to be fully reversible with cells requiring ∼60 min to regain their normal polarized actin cytoskeleton (Fig. 1 d).
The rapidity with which actin filaments disassembled in yeast treated with LAT-A suggests that actin filaments turnover very rapidly in living yeast cells. However, this conclusion is based on the assumption that LAT-A inhibits assembly by binding to actin monomers and not by actively causing disassembly through a mechanism such as filament severing. Two lines of evidence reported by Coué et al. (1987) suggested that LAT-A binds to actin monomers to form an assembly-incompetent complex. The first was observation of a kinetic lag in polymerization which increased with increasing LAT-A concentration. The second was that at steady state, levels of F-actin assembled in the presence of varying concentrations of LAT-A were consistent with formation of a 1:1 LAT-A:actin complex incapable of assembly.
We repeated the analysis of Coué et al. (1987) with yeast actin and obtained similar results (not shown). In addition, we incubated F-actin filaments below the critical concentration for polymerization in the presence of LAT-A (or DMSO) and monitored F-actin levels by light scattering. If LAT-A were capable of actively promoting disassembly via a mechanism such as severing, we would have expected the level of F-actin to fall dramatically when incubated with the drug under our experimental protocol (see Bubb et al., 1995), but we did not observe this. Rather, we detected a gradual decline in F-actin levels, at a rate only slightly greater than that of the control (data not shown). These data confirmed that LAT-A inhibits assembly by forming an assembly-incompetent complex with monomeric yeast actin, and that it does not actively promote disassembly.
Specificity of the LAT-A Effect
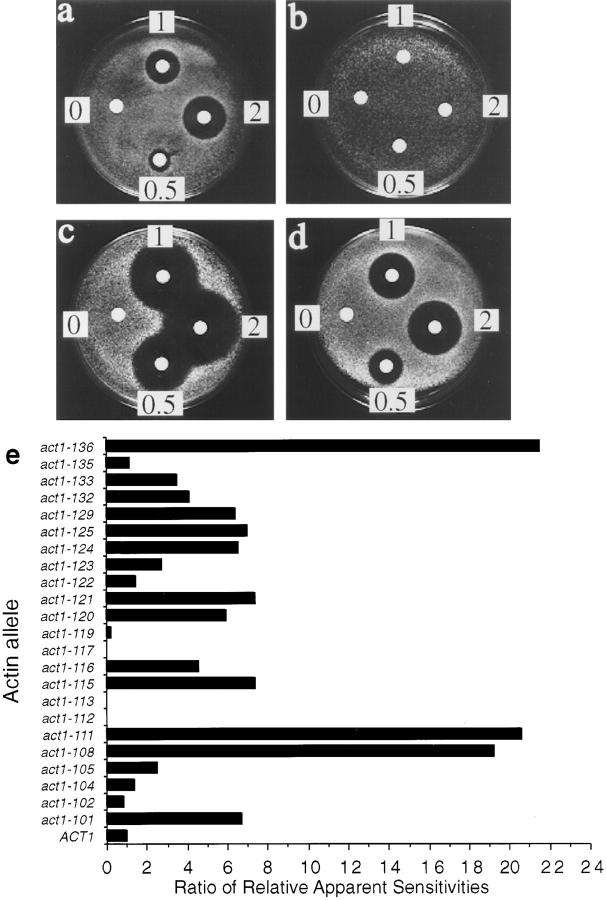
As LAT-A provided an opportunity to determine for the first time the consequences of a total lack of filamentous actin in yeast, it was necessary to first demonstrate the specificity of the drug in living yeast cells. To demonstrate LAT-A specificity, we took advantage of a congenic collection of actin mutants which was generated by a charged-to-alanine mutagenesis scan of ACT1, the single, yeast conventional actin gene (Wertman et al., 1992). Each allele in the collection expresses a mutant form of actin as the sole source of actin in the cell. The collection, comprising 23 viable strains, was previously used to map the binding sites on the actin surface for phalloidin (Drubin et al., 1993), for yeast fimbrin (Holtzman et al., 1994), and for several other actin-associated proteins (Amberg et al., 1995). The LAT-A sensitivities of all of the strains in the collection were compared using the halo assay. Fig. 2, a–d shows the halo assays of several strains in the collection to illustrate the variation in sensitivity among the alleles. In addition, the LAT-A sensitivity of each allele was compared to the sensitivity of cells containing wild-type actin. This comparison is shown graphically in Fig. 2 e.
Figure 2.
Sensitivity of a set of congenic actin mutants to LAT-A. Halo assays were used to assess the sensitivity of 23 actin mutants to LAT-A. (a–d) Representative examples of LAT-A halo assays. Concentrations measured 0.5 mM, 1 mM, and 2 mM. (a) ACT1 (wild-type actin), (b) act1-117, (c) act1-129, (d) act1-116. (e) Summary bar graph of the relative apparent sensitivities of all of the actin alleles tested compared to the wild-type.
The graph clearly shows that there is a large variation in the response of the different alleles to LAT-A. The first observation is that the LAT-A sensitivity does not correlate with salt or heat sensitivity (phenotypes described by Wertman et al., 1992). It is therefore not simply that the sickest strains are the most sensitive to LAT-A. Indeed, one of the sickest strains in the collection, act1-112, which can only grow between 20°C and 30°C, and has very restricted growth on salt-containing media, is actually resistant to LAT-A. In all, there were four strains in the collection of mutants which showed some resistance to LAT-A. act1-119 was partially resistant, while act1-112, act1-113, and act1-117 showed complete resistance at all levels of LAT-A tested. The three mutants that were completely resistant to LAT-A showed variation in their other gross phenotypic properties. As already mentioned, cells carrying the act1-112 mutation are very sick with a very restricted range of temperatures for growth. Another strain, expressing mutation act1-113, shows slight temperature sensitivity and also exhibits salt sensitivity at low temperatures. The third strain, expressing act1-117, has been called a pseudo-wild-type strain as it can grow under the same conditions of temperature and salt concentration as wildtype cells (Wertman et al., 1992).
The existence of actin alleles showing resistance to LAT-A in an otherwise congenic background was very strong evidence that the LAT-A was acting in a very specific manner on actin in yeast cells. However, a final caveat was that these three actin mutations might impair general uptake mechanisms for drugs such as LAT-A. Halo assays performed with two other drugs, mycalolide-B and swinholide-A, indicated that this is not likely to be the case. With mycalolide-B, act1-113 and act1-117 cells showed a similar sensitivity to wild-type cells, and act1-112 cells were in fact more sensitive (data not shown). Wild-type cells are resistant to swinholide-A at all levels tested, however, all three of the mutants resistant to LAT-A were sensitive to this drug (data not shown) demonstrating that their lack of sensitivity to LAT-A is unlikely to be due to impaired uptake mechanisms.
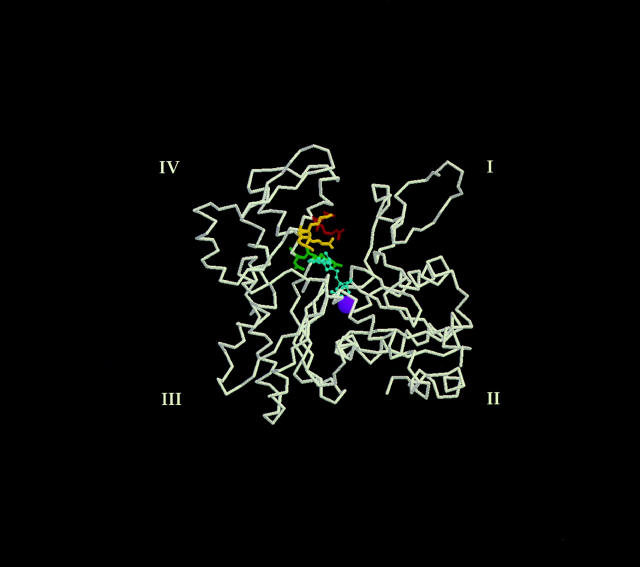
Thus, the halo assay results were highly indicative of a specific interaction of LAT-A with actin in yeast cells. To investigate the possibility that resistance was due to disruption of the normal LAT-A binding site on the actin molecule, we looked at the position of the three resistant alleles within the actin crystal structure. As shown in Fig. 3, the alleles define a small patch on actin adjacent to the nucleotide binding cleft. Residues which have been mutated in the three alleles (act1-112 = K213A, E214A, K215A; act1-113 = R210A, D211A; act1-117 = R183A, D184A) are thought to either directly contact through salt bridges or hydrogen bonds, the nucleotide itself, or, to form salt bridges to position other residues which themselves bind the nucleotide (Kabsch et al., 1990). We therefore determined the effect of LAT-A on nucleotide exchange.
Figure 3.
Mapping the three LAT-A resistant alleles on the actin molecular structure. Backbone of the actin monomer from the coordinates of rabbit muscle actin as determined by Kabsch et al. (1990). Subdomains are marked I to IV. The side chains of the residues mutated to alanine in the LAT-A resistant mutants are shown and are color coded by their allele designation (Wertman et al., 1992). act1112 (yellow), act1-113 (red), act1-117 (green). The adenine nucleotide (cyan) is shown in the prominent cleft as a ball and stick model, and the divalent cation (purple) as a Van der Waal's sphere.
We tested the nucleotide exchange properties of wildtype yeast actin in the presence of increasing concentrations of LAT-A by monitoring the increase in fluorescence observed when ε-ATP binds to actin. As shown in Fig. 4, the addition of LAT-A inhibits nucleotide exchange in actin in a dose-dependent manner.
Figure 4.
The effect of LAT-A on actin nucleotide exchange. Purified wild-type yeast actin was added to buffer containing a fluorescent nucleotide analogue (ε-ATP) and increasing concentrations of LAT-A. The kinetics of nucleotide exchange were monitored using ε-ATP fluorescence as described in the Materials and Methods section.
As a final test of LAT-A specificity, we purified actin from two of the LAT-A resistant strains, act1-113 and act1-117. We did not attempt to isolate actin from act1-112 cells because they are very sick and we therefore thought the likelihood that they would yield an actin sufficiently stable to allow in vitro analysis was low. Indeed, actin from an act1-113 mutant polymerized very poorly in vitro (<10% could be pelleted under conditions that nearly quantitatively pellet polymerized wild-type actin), even though act1-113 strains are considerably more healthy than act1112 strains. Since it would have been difficult to analyze data from assembly of the act1-113 actin, we proceeded with only act1-117 actin. G-actin from ACT1 or act1-117 strains was allowed to polymerize in the absence or presence of LAT-A. F-actin was pelleted, and the resulting supernatants and pellets were analyzed by SDS-PAGE (Fig. 5 a). The actin protein bands on this gel were analyzed by densitometry (Fig. 5 b). The results from these experiments show that incubation of LAT-A with an equimolar concentration of wild-type actin, but not actin from act1117 cells, resulted in inhibition of filament assembly. The act1-117 mutation itself appears to affect the ability of actin to form filaments. Only ∼40% of act1-117 actin was pelleted using the conditions which resulted in >90% pelleting for wild-type actin. However, the addition of LAT-A to this actin did not significantly affect its ability to polymerize. To verify that pelleting of the mutant actin was due to filament formation, rather than aggregation of actin monomers, we observed the mutant actin filaments, labeled with Rh-phalloidin, directly by fluorescence microscopy (Fig. 5 c) and found that both wild-type and act1-117 actin were able to form filaments, but only act1-117 actin was able to form filaments in the presence of LAT-A.
Figure 5.
The effect of LAT-A on actin polymerization. Purified actin from ACT1 or act1-117 cells was incubated with LAT-A before addition of polymerization salts. (a) After polymerization actin was spun to pellet filamentous actin. Supernatants and pellets were run on gels and actin was visualized using Coomassie staining. (b) Quantitation of the data in a using densitometry. (c) After polymerization, Rd-phalloidin–labeled actin filaments were visualized using fluorescence microscopy. Bar, 5 μM.
Effects of LAT-A on Growth, Viability, Morphogenesis and Endocytosis
Having determined that the effects of LAT-A on actin in yeast cells are rapid, reversible, and specific, we next determined how total disruption of actin filaments would affect cellular processes. The growth of cells in rich medium was monitored by cell counting following the addition of LAT-A (Fig. 6 a). After addition of the drug there was almost no increase in cell number. However, when cell growth was assessed by measuring turbidity (OD 600 nm), there was an increase after LAT-A addition to about 2.5 times the original OD reading (Fig. 6 b). This indicated a growth in cell volume that was not matched by increasing cell number. The mutant alleles act1-113 and act1-117 were also assessed for growth in the presence of LAT-A. Neither strain showed detectable alteration of its growth rate in the presence of the drug (Fig. 6, c and d). In addition, both act1-113 and act1-117 mutants were stained for F-actin in the presence of LAT-A. Cortical actin patches were clearly present in these cells throughout the 1-h time course (data not shown).
Figure 6.
The effect of LAT-A on growth and viability of cells. Growth of wild-type cells in the presence (•) or absence (○) of LAT-A assessed by cell counting (a) or by OD measurements (b). Growth of mutant cells in the presence (•) or absence (○) of LAT-A assessed by cell counting, (c) act1-117, and (d) act1-113. (e) Viability of wild-type cells following addition of 100 μM LAT-A to a log phase culture.
The viability of cells following treatment with 100 μM LAT-A was assessed by counting the number of treated cells that could form colonies following a timed incubation (Fig. 6 e). Cells were treated with LAT-A in suspension, struck onto a plate, and 120 cells were dissected out from this population using a micromanipulator. The ability of each manipulated cell to form a colony was assessed. The viability of cells following addition of LAT-A did not drop significantly over the 4-h time course, with 93% of cells treated for 4 h with LAT-A able to form apparently normal colonies when plated. In the control population of cells which were not treated with LAT-A, all of the colonies that formed were roughly equal in size. At each time point after LAT-A addition, 2–5% of colonies were much smaller than in the control case, being just visible by eye (data not shown). The reason for the small colonies is not clear. It is possible that they are petites with the temporary loss in F-actin somehow affecting mitochondrial genome inheritance.
Having established that cell viability remained high for extended periods in LAT-A, we could now use the drug to analyze effects on cell physiology. Thus, we first looked for effects on cell morphogenesis and observed that during incubation in LAT-A cells increase considerably in size but they did not arrest with uniform morphology. When assessed over a 4-h time course, the percentage of unbudded cells was observed to increase from 38% unbudded in the absence of LAT-A (39% small budded and 23% largebudded) to 77% unbudded in the presence of LAT-A (12% small budded, 11% large budded) demonstrating defects in bud formation. In addition, many arrested cells contained buds which were still attached to the mother cell. These buds could be separated from the mother cells by enzymatic cell wall digestion indicating that actin is not required for cytokinesis, but is necessary for the complete degradation of septal material between mother and daughter cells.
Endocytosis in yeast has been shown to be dependent on the presence of an intact actin cytoskeleton (Kübler and Riezman, 1993). We assessed endocytosis by monitoring the uptake of the fluid phase marker lucifer yellow (LY; Dulic et al., 1991). In the absence of LAT-A, there is specific uptake of LY into vacuoles. However, in the presence of LAT-A, while the vacuoles were still visible by Nomarski optics, there was no uptake of LY, indicating inhibition of endocytosis (data not shown).
The Sensitivity to LAT-A of Strains with a Disrupted Actin Cytoskeleton
To gain new insights into the role of actin cytoskeleton proteins in modulating cytoskeletal integrity and function, we investigated the effect on LAT-A sensitivity of mutations in genes encoding various proteins that are important for actin cytoskeleton function. We performed halo assays on each mutant strain, and also on the congenic parent strain. This control was necessary because we noticed some variation in LAT-A sensitivity between wild-type strains of different strain backgrounds. There was a range of sensitivities of the mutant cells to LAT-A when assessed by halo assays. Two mutants showed a slight resistance to LAT-A. These carried a deletion of the SLA1 gene, and a mutation in the END3 gene (end3-1), with ratios of apparent sensitivities compared to controls of 0.5 and 0.3, respectively (see Materials and Methods). An increased sensitivity to LAT-A was shown by several mutants including Δsrv2, Δcap2, Δtpm1, Δsac6p, and Δsla2 with ratios of apparent sensitivities of 2.9, 4.0, 2.8, 1.8, and 2.6, respectively. Strains carrying the Δabp1 mutation showed a barely detectable increase in resistance. These results highlight differences among cytoskeletal proteins in their contributions to cytoskeleton stability.
The Role of Actin in the Development of Polarity at the Presumptive Bud Site
We used sensitivity to LAT-A to determine which of 19 proteins that normally localize to the presumptive bud site depend on an intact actin cytoskeleton for localization. We used diploid cells because they are more sensitive than haploids to LAT-A, their larger size facilitates observations made by immunofluorescence microscopy and they have an ellipsoidal shape allowing the ends of a cell to be distinguished. Being able to identify the ends of a cell is important so one can determine whether a bud site has formed at the correct position (see Drubin and Nelson, 1996). Cells were released from G0, as described in Materials and Methods, in the presence or absence of LAT-A. At each time point, immunofluorescence was used to localize the proteins of interest.
Actin Is Required for Bud Formation in Cells Exiting G0
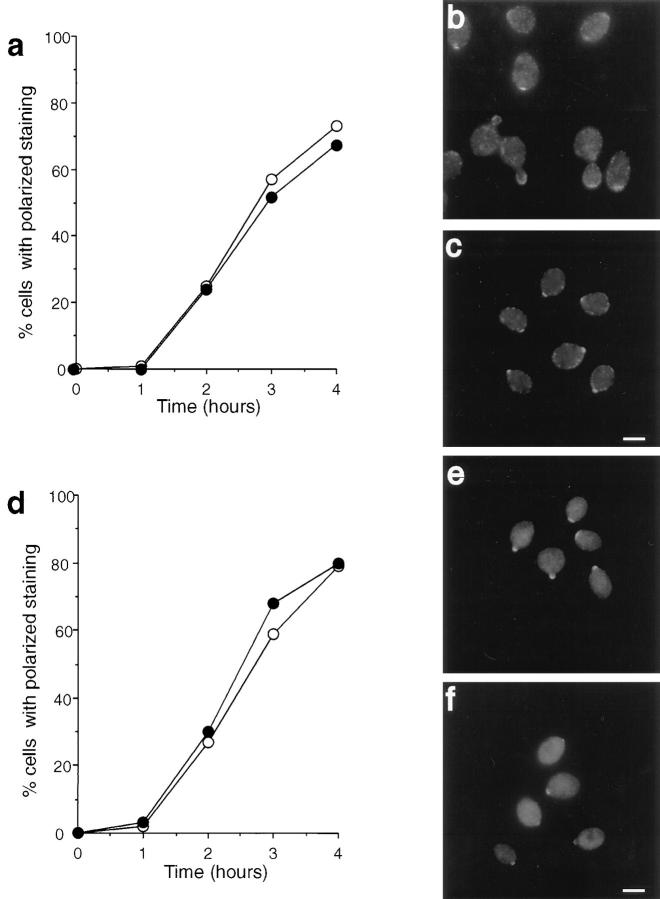
To establish a baseline for comparisons, we determined the rate at which polarization of actin was achieved in cells exiting G0. Hourly time points were taken from G0 cells released into fresh media in the presence or absence of LAT-A. Fig. 7 a shows the percentage of cells with polarized actin staining obtained by this procedure. Polarization of actin staining in the absence of LAT-A was clear after 2 h and increased to a peak of 80% cells with polarized staining after 4 h. In longer time courses, the number of cells with polarized staining did not get higher than 80%, presumably because the level of synchrony in the cells was not sufficiently high (i.e., the remaining cells are at early G1 or late G2/mitosis, stages in which the cortical actin cytoskeleton is depolarized). Fig. 7 c shows the anti-actin immunofluorescence staining pattern for cells after 4 h of growth following the G0 release in the absence of LAT-A. During this 4-h period, cell morphology was also monitored. Although growth in culture after release was not completely synchronous, there was a significant enrichment for smallbudded cells at 2–3 h after release, and for medium- to large-budded cells at 4 h after the release (Fig. 7 b).
Figure 7.
Immunolocalization of actin in cells exiting G0 at 25°C in the absence or presence of LAT-A. (a) Quantification of immunofluorescence to assess the percentage of cells incubated in the presence (•) or absence (○) of LAT-A that exhibited polarized actin staining. (b) Morphology of cells exiting G0 in the absence of LAT-A. Cells were classified as unbudded (○), small-budded (•), or medium- to large-budded (□). (c) Immunolocalization of actin in cells grown for 4 h in the absence of LAT-A. (d) Immunolocalization of actin in cells grown for 4 h in the presence of LAT-A. Note the presence in some cells of actin bars and actin in the nucleus (as confirmed by DAPI staining, not shown). Bar, 5 μm.
In the presence of LAT-A there was no observable polarization of the actin cytoskeleton at any time (Fig. 7, a and d). At early time points (0–1 h), no actin structures were seen. However, after this stage an increasing number of cells contained actin bars (Fig. 7 d). Similar bars have been reported previously in mutant strains that have actin defects (Johnston et al., 1991; Holtzman et al., 1993; Welch and Drubin, 1993). The actin bars do not stain with the F-actin staining dye rhodamine-phalloidin and so have been proposed to be aggregates of monomeric actin. At the later time points, smaller dots of actin staining can also be seen which appear to be similar in size to cortical patches. However, these aggregates do not label with Rd-phalloidin, nor, in most cases, do they appear to be at the cortex, indicating that these structures are most likely aggregates of monomeric actin. In addition, in a small percentage of cells (<5%), actin was seen to stain within the nucleus.
Because no filamentous actin could be detected in LATA–treated cells, any proteins that were able to achieve a polarized staining pattern in the presence of LAT-A could be concluded to be doing so in the absence of F-actin.
Polarity Establishment Proteins, Cdc42p and Bem1p
To determine whether the actin cytoskeleton has any role in the early stage of polarity establishment involving localization of Cdc42p and Bem1p at the cell cortex, we analyzed the localization of Cdc42p and Bem1p upon exit from G0 in the presence or absence of LAT-A. As depicted in Fig. 8, a and d, in the absence of LAT-A, Cdc42p and Bem1p became polarized with kinetics similar to actin. As reported in the literature (Ziman et al., 1993; Pringle et al., 1995), both proteins showed highly localized staining at the presumptive bud site and at the tip of small buds (Fig. 8, b and e). In cells with larger buds, the staining was more diffuse but still polarized. Staining was also seen at the mother-bud neck before cytokinesis.
Figure 8.
Immunolocalization of polarity establishment proteins in cells exiting stationary phase. Quantification of immunofluorescence to assess the percentage of cells incubated in the presence (•) or absence (○) of LAT-A that exhibited polarized Cdc42p staining (a) or Bem1p staining (d). Immunolocalization of Cdc42p (b) or Bem1p (e) in cells grown in the absence of LAT-A. Immunolocalization of Cdc42p (c) or Bem1p (f) in cells grown in the presence of LAT-A. Bar, 5 μm.
In the absence or presence of LAT-A, Cdc42p and Bem1p achieved polarized cellular localization and did so with similar kinetics (Fig. 8). In the LAT-A–treated cells, staining was in the form of a bright spot at one end of the ellipsoidal diploid cell (Fig. 8, c and f) which was the appropriate position for the formation of the bud site. In the case of Cdc42p localization in the presence of LAT-A, in addition to the bright spot of staining at the end of the cell, a low level of diffuse cortical staining could also be seen. To further address whether the polarized localization of Bem1p and Cdc42p reflected elements of a natural pathway for presumptive bud site assembly, we performed double labeling with antibodies to another presumptive bud-site protein, Cdc11p (see following section for description of Cdc11p localization in LAT-A). The patterns of staining of the proteins are different, a small patch of staining is observed for Cdc42p or Bem1p, while a ring is observed for Cdc11p staining, so the patterns of staining could be distinguished. In the presence of LAT-A, staining could be observed to be coincident (a Cdc11p ring encircled the Cdc42p or Bem1p patch) in 99% of the cells (400 cells counted) suggesting further that Cdc42p, Bem1p, and Cdc11p had localized to what was a natural bud site except for the absence of actin and associated proteins.
Actin-binding Proteins and Proteins Implicated Genetically in Actin Function
The polarized localization of the actin-binding proteins Abp1p and cofilin in cells released from G0 was found to depend on the presence of F-actin structures. The percentage of cells with polarized staining in the presence and absence of LAT-A was quantified for each protein and these data are illustrated graphically in Fig. 13. In contrast, both Sla1p and Sla2p which also colocalize with cortical actin structures (Holtzman et al., 1993; Ayscough, K., and S. Yang, unpublished observations), were able to localize to the cell cortex and were in patch-like structures in the absence of F-actin (data not shown). In a small population of cells, these proteins appeared to have a polarized distribution.
Figure 13.
Polarization of presumptive bud site proteins in the absence (▒⃞ ) or presence (▪) of LAT-A. A summary of data collected for all the proteins analyzed in this study. The cell counts shown here are those at the 4-h time point after release of cells from the stationary phase.
In the absence of LAT-A, Aip3p/Bud6p, a protein which interacts with yeast actin in a two-hybrid screen and which localizes to cortical sites (Amberg et al., 1996), achieved its normal polarized staining pattern with staining being observed at the presumptive bud site, at the bud tip, and, in large budded cells, at the mother-bud neck (see above; Fig. 13). In the presence of LAT-A, the number of cells showing localized staining was reduced. However, it was clear that in a significant number of cells, Aip3p/Bud6p was still able to localize to the cell cortex, and in many cases it showed a polarized localization. 22% of cells showed polarized staining at the ends of the cell (Fig. 13) whereas in other cases, the staining was confined to discrete patches which were not at the end of the ellipsoidal cells (3.5%), or there were two patches at the cortex with at least one patch not being at the end of the cell (9.5%).
Kelleher (1995) proposed that an Arp2/Arp3 heterodimer might serve as a nucleus for actin polymerization. It was therefore of interest to determine whether Arp2 in yeast could function independently of actin and achieve its normal polar localization in the absence of conventional actin. In the absence of LAT-A, Arp2p has a polarized cortical patch-like staining pattern (Moreau et al., 1996) with kinetics of localization similar to actin. However, in the presence of LAT-A, Arp2p did not polarize, though it did show a different distribution from actin (see Fig. 13 for summary of data). In the presence of LAT-A, actin, but not Arp2p, localizes to bar structures and, in some cases, to the nucleus. In contrast to actin, a significant amount of Arp2 staining appeared to be associated with the cell cortex where its staining was diffuse. This was particularly apparent at early time points (1–2 h) after release of cells from G0.
Proteins Involved in Secretory Processes
We localized several proteins (Sec4p, Sec8p, Myo2p, calmodulin, and Smy1p) implicated in secretion in the absence and presence of LAT-A. Sec4p is a member of the Rab-GTPase family of proteins and acts at a late stage of the secretory pathway between the Golgi apparatus and the plasma membrane (Salminen and Novick, 1987). Sec4p localizes to the presumptive bud site and the tip of smallbudded cells (Novick and Brennwald, 1993). Sec8p is a component of a protein complex that localizes to the presumptive bud site and the bud tip, and may be involved in the docking of vesicles at the plasma membrane (TerBush and Novick, 1995; TerBush, D., personal communication). Myo2p is an unconventional myosin with calmodulin as an associated subunit (Johnston et al., 1991; Brockerhoff et al., 1994). Mutations in Myo2p and calmodulin can cause vesicle accumulation, delocalized cell wall synthesis, chitin deposition and often an arrest as unbudded cells (Johnston et al., 1991; Brockerhoff and Davis, 1992; Brockerhoff et al., 1994; Lillie and Brown, 1994; Govindan, et al., 1995). Smy1p is a kinesin-like protein which was identified as a dosage suppressor of a mutation in MYO2. (Lillie and Brown, 1994).
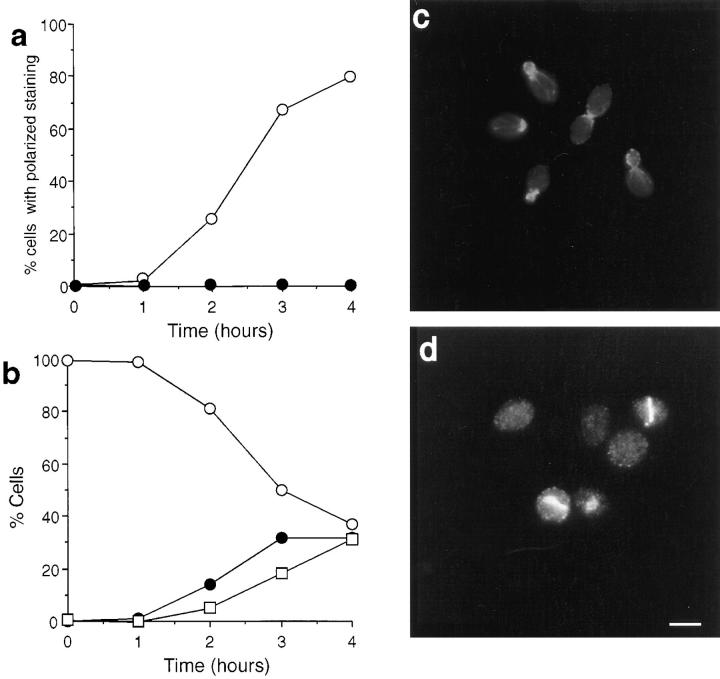
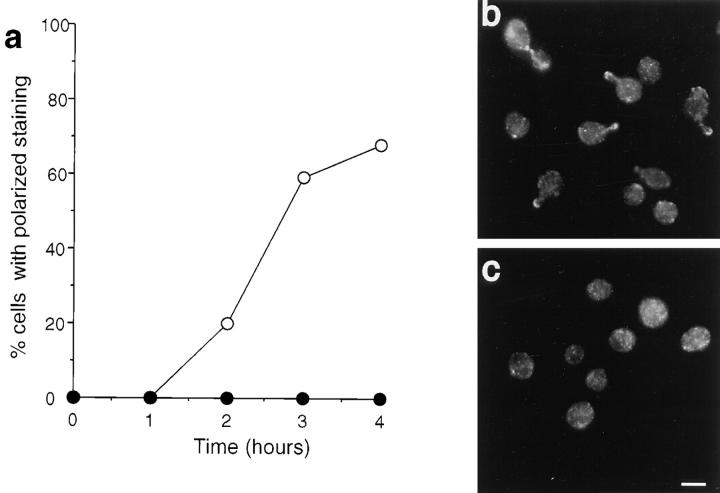
We found that actin is required for the localization of Sec4p, Sec8p, and Smy1p to the presumptive bud site. In contrast, both Myo2p and calmodulin were able to localize to this region in the absence of actin, albeit to a lesser extent than in control cells. Examples of kinetic and photographic data are shown for Sec4p and calmodulin (Figs. 9 and 10). In cells growing out from G0, Sec4p became highly polarized at the presumptive bud site, remaining at the bud tip in small-budded cells and then becoming slightly more diffuse as the bud grows. It was not clearly visible in most medium- to large-budded cells but was observed at the mother-bud neck before cytokinesis (Fig. 9, a and b). However, when LAT-A was added to the growth medium during the exit from stationary phase, Sec4p did not exhibit polarized localization (Fig. 9, a and c).
Figure 9.
Immunolocalization of Sec4p in cells exiting stationary phase in the absence or presence of LAT-A. (a) Quantification of immunofluorescence to assess the percentage of cells incubated in the presence (•) or absence (○) of LAT-A that exhibited polarized Sec4p staining. Immunolocalization of Sec4p in cells grown in the absence (b) or presence (c) of LAT-A. Bar, 5 μm.
Figure 10.
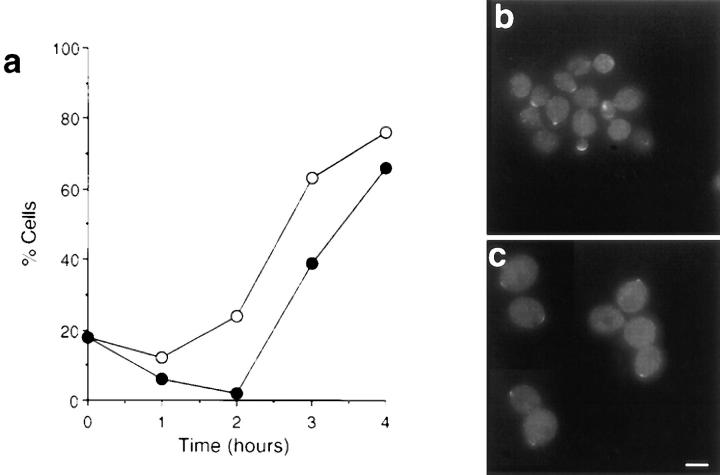
Immunolocalization of calmodulin in cells exiting stationary phase in the absence or presence of LAT-A. (a) Quantification of immunofluorescence to assess the percentage of cells that exhibited polarized calmodulin staining when incubated in the presence (•) or absence (○) of LAT-A. Immunolocalization of calmodulin in cells grown in the absence (b) or presence (c) of LAT-A. Bar, 5 μm.
In the absence of LAT-A, calmodulin and Myo2p localized to the presumptive bud site, the tip of small-budded cells and the septal region. Staining in medium- to large- budded cells was fainter (Fig. 10, a and b; only calmodulin data is shown since Myo2p staining was essentially identical). In the presence of LAT-A, there was a high level of cytoplasmic staining with most of the cells showing no polarization of staining. However, in ∼30% of cells, calmodulin appeared in a discrete patch located at the end of the ellipsoidal cell, a position that is appropriate for the bud site (Fig. 10 c). When cells were followed for longer than 4 h, the number of cells with this staining pattern increased slightly but did not rise above 35% of cells observed.
Proteins Required for Cytokinesis
Several proteins become localized to the presumptive bud site but are most important for processes that occur subsequent to bud formation. The yeast septins, required for cytokinesis and proposed to be subunits of the filaments observed at the mother-bud neck by electron microscopy, are such proteins (Hartwell, 1971; Haarer and Pringle, 1987; Ford and Pringle, 1991; Kim et al., 1991).
We examined the localization of two septin proteins, Cdc10p and Cdc11p. Because the results were indistinguishable for these two proteins, we have only presented the data for Cdc11p. In both the absence and presence of LAT-A, Cdc11p became polarized and the kinetics of localization were similar (Fig. 11). The staining pattern was a bright ring structure at the presumptive bud site. In the absence of LAT-A, cells with larger buds were seen to contain a double ring of staining at the bud neck (Fig. 11 b). In dividing cells, one ring appeared to remain with the mother and the other with the daughter cell. In the presence of LAT-A, only single rings were seen, indicating that ring duplication is dependent on bud formation (Fig. 11 c).
Figure 11.
Immunolocalization of Cdc11p in cells exiting stationary phase in the absence or presence of LAT-A. (c) Quantification of immunofluorescence to assess the percentage of cells that exhibited polarized Cdc11p staining when incubated in the presence (•) or absence (○) of LAT-A. Immunolocalization of Cdc11p in cells grown in the absence (b) or presence (c) of LAT-A. Note the lack of double ring structures in the cells incubated with LAT-A. Bar, 5 μm.
Other Proteins of the Pre-Bud Site
Several other proteins have been localized to the presumptive bud site, but their cellular activities are not currently understood well enough to permit them to be classified functionally.
Gin4p and Bni4p localize to a ring structure at the presumptive bud site which persists at the mother-bud neck throughout bud growth (Longtine, M., D. DeMarini, J. Pringle, personal communication). In the presence of LAT-A, both Gin4p and Bni4p achieved a polarized localization (Fig. 13).
Spa2p localizes to the presumptive bud site and to the tip of mating projections (Snyder, 1989; Snyder et al., 1991). Cells deleted for SPA2 exhibit no defects in the ability to bud but show a defect in bud site selection (Snyder, 1989). In the absence of LAT-A, Spa2p became polarized with similar kinetics to actin (Fig. 12, a and b). In the presence of LAT-A, Spa2p was still able to polarize. However, the kinetics of polarization were delayed (Fig. 12, a and c). In three separate experiments, polarized localization of Spa2p was always 1–2 h slower than in the absence of LAT-A.
Figure 12.
Immunolocalization of Spa2p in cells exiting stationary phase in the absence or presence of LAT-A. (a) Quantification of immunofluorescence to assess the percentage of cells that exhibited polarized Spa2p staining when incubated in the presence (•) or absence (○) of LAT-A. Immunolocalization of Spa2p in cells grown in the absence (b) or presence (c) of LAT-A. Bar, 5 μm.
A Role for Actin in the Maintenance of Cell Polarity
To determine whether actin plays a role in the maintenance of the axis of cell polarity, we released both haploid and diploid cells from stationary phase to a stage where more than 50% of cells had small buds. We then treated these cells with LAT-A for 5 min to completely disrupt the actin cytoskeleton, washed the cells, and allowed them to resume growth. After 2 h, we analyzed the morphology of the cells (Fig. 14). For both haploid and diploid cells, the disruption of the actin cytoskeleton caused a significant percentage of cells to become two-budded. Interestingly, in nearly all cases, this second bud was correctly placed with respect to the expected position of bud site selection. That is, haploid buds were adjacent to one another, and diploid buds were either at both poles or at the same pole of the cell (for a discussion of budding patterns, see Drubin and Nelson, 1996).
Figure 14.
The formation of two-budded cells after incubation of wild-type cells with LAT-A. Cells were released from stationary phase and allowed to grow until more than 50% of the population was small budded. Cells were then treated with LAT-A for 5 min (or with an equal volume of DMSO in the control case). They were then washed to remove LAT-A and allowed to resume growth. After 2 1/2 h the morphology of cells was assessed. Examples of two-budded haploid cells (a) and diploid cells (c) are shown. Cells were classified as unbudded, single-budded, or two-budded in the haploid (b) or diploid (d) populations. Control cells (▪); cells treated with LAT-A (▨ ).
In a similar set of experiments, haploid MATa cells were exposed to α-factor until more than 90% of the population had formed a mating projection. The population was then exposed to LAT-A for 5 min to completely disrupt the actin cytoskeleton, washed, and released into fresh media. α-Factor was present at all stages. In a control population to which DMSO was added at the intermediate stage, two shmoos were observed in 19% cells. By contrast, 53% of cells exposed to LAT-A contained two shmoos.
Discussion
LAT-A Action is Rapid and Reversible Indicating Actin Filaments Undergo Rapid Cycles of Assembly and Disassembly in S. cerevisiae
The rapidity of the disappearance of actin structures upon treatment of yeast with LAT-A suggests two mechanistic possibilities. LAT-A might (1) actively destabilize actin filaments, or, (2) it might bind to monomers, preventing the assembly step in a rapid cycle of assembly and disassembly. Previously published data are most consistent with the latter possibility, a sequestering mechanism via the formation of a 1:1 complex with actin monomer (Coué et al., 1987). We obtained similar data with yeast actin, verifying the conclusions of the earlier studies and demonstrating that they apply to yeast actin. We also observed that LAT-A did not induce rapid F-actin disassembly in vitro, arguing further against a filament-destabilizing or severing mode of action. Thus, the data we generated, and the previously reported data, indicate that the most likely mode of action for LAT-A is monomer sequestration. Such a sequestration mechanism for LAT-A action, in combination with the rapidity of its action in vivo, would indicate that the actin cytoskeleton in yeast is highly dynamic. The observations that an inherent capacity for dynamic turnover is a universal property of actin, and that a nonmotile organism has a dynamic actin cytoskeleton, lead us to suggest that rapid assembly and disassembly is a fundamental property of eukaryotic actin cytoskeletons.
The LAT-A Sensitivity of Actin-associated Protein Mutants
Several actin cytoskeleton-associated protein mutants showed significantly increased sensitivity to LAT-A compared with wild-type cells. Where previously derived information about the contributions of individual proteins to cytoskeletal integrity was available, LAT-A sensitivities confirmed previously reached conclusions. For example, mutations in capping protein (encoded by the CAP1 and CAP2 genes) and yeast fimbrin (Sac6p) lead to decreased F-actin and increased G-actin in cells (Karpova et al., 1995). With an already depleted polymer pool, it is expected that sensitivity to LAT-A would be heightened in these mutants. Indeed, these mutants showed elevated sensitivity to the drug. Tropomyosin is also postulated to stabilize actin cables in yeast (Liu and Bretscher, 1992; Drees et al., 1995). tpm1 mutants showed elevated sensitivity to LAT-A, providing further evidence that tropomyosin stabilizes actin structures in vivo. While the above examples validated this approach to analysis of cytoskeleton protein function, the opportunity to obtain novel insights into cytoskeleton protein function comes from the application of this approach to the remaining proteins.
Srv2p has an actin monomer binding activity (Freeman et al., 1995) so one might predict that deletion of SRV2 would lead to an increased resistance to LAT-A. However, the deletion of SRV2 causes increased LAT-A sensitivity. This result could be explained if Srv2p plays a role in facilitating filament assembly, perhaps by delivering actin monomers to the ends of actin filaments.
Two of the mutants assayed, Δsla1 and end3-1, had a slightly increased resistance to LAT-A compared with wildtype cells. These mutants have similar actin phenotypes to one another, with the cortical patches being larger, though fewer, than in the wild-type situation (Holtzman et al., 1993; Benedetti et al., 1994). In addition, actin cables appear to be similarly prominent or even more pronounced compared to wild-type. These actin phenotypes might suggest that the mutant cells have increased levels of F-actin. In light of the elevated resistance of the Δsla1 and end3-1 mutant strains to LAT-A, we propose that Sla1p and End3p might promote destabilization of the yeast actin cytoskeleton.
Actin Mutants Resistant to LAT-A Demonstrate Specificity and Suggest a Binding Site
The demonstration that mutation of actin was sufficient to make a strain resistant to LAT-A provided powerful evidence for a highly specific interaction between LAT-A and actin. Furthermore, the amino acid substitutions in the three resistant alleles mapped to a distinct region on the actin molecule, implicating this region as the binding site for LAT-A. This region was adjacent to the nucleotide binding cleft. In vitro assays tested the prediction that LAT-A might impair nucleotide exchange and demonstrated that this was the case. In addition, actin purified from a LAT-A resistant mutant was resistant to the action of LAT-A in vitro, adding further support to the proposal that the mutations identified the LAT-A binding site on actin.
A Functional Hierarchy of Proteins Is Involved in the Establishment of Cell Polarity
Fig. 15 shows a hierarchy of protein functions required for cell polarity development. Previous studies suggested that Cdc42p and Bem1p function early in the pathways leading to polarity establishment. Temperature-conditional cdc42 and bem1 mutations caused the actin cytoskeleton and septins to be delocalized at the nonpermissive temperature, indicating that cytoskeleton localization depends on the functions of the polarity establishment proteins (Adams et al., 1990; Bender and Pringle, 1991; Chant et al., 1991; Pringle et al., 1995). We showed here that neither Cdc42p nor Bem1p requires actin for localization, an observation consistent with the conclusion that Cdc42p becomes localized independent of actin and then organizes the actin cytoskeleton.
Figure 15.

Model depicting the development of polarity at the presumptive bud site. Cdc42p and Bem1p localize to the presumptive bud site in an actin-independent manner. After localization of these polarity establishment proteins, other proteins associated with the development of cell polarity are able to localize. Proteins associated with secretion require actin in order to achieve their polarized localization. Other proteins do not require actin for localization and therefore occupy a separate branch in polarity development. Note that in the case of Cdc10p, Cdc11p, and Spa2p, a pathway parallel to the actin/secretion pathway is indicated by results from previous studies (see text). Additional experiments are required to elucidate dependency relationships for localization of Cdc10p, Cdc11p, Spa2p, Bni4p, and Gin4p.
Proteins That Require Actin to Achieve Their Normal Polarized Localization
As may have been predicted, proteins that bind directly to actin, Abp1p and cofilin, require actin to achieve their normal localization at the presumptive bud site. However, Sla1p, Sla2p, and Arp2p, which in control cells colocalize with actin cortical patches, show a level of actin-independent association with the cell cortex, but not polarized localization. This indicates that these proteins localize to the cortex in an actin-independent manner but require actin to polarize to the presumptive bud site.
Actin was shown to be required for the localization of Sec4p, Sec8p, and Smy1p (discussed further below) to the presumptive bud site. In contrast, Myo2p and calmodulin were able to localize to this region in the absence of actin, albeit to a lesser extent than in control cells. This observation indicates that Myo2p/calmodulin complexes are able to interact with proteins other than actin at the presumptive bud site. Interestingly, previous studies on the localization of these proteins in actin mutants indicated that both Myo2p (Lillie and Brown, 1994) and calmodulin (Brockerhoff and Davis, 1992) showed actin-dependent localization. Both of these studies were performed in actin mutants in which asynchronous cultures of log phase cells were shifted to the nonpermissive temperature. Brockerhoff and Davis (1992) reported that, at the nonpermissive temperature, no budded cells show polarized calmodulin whereas in 5% of the unbudded cells, polarized staining is seen. In our studies we have assessed the ability of cells to polarize proteins during entry into the cell cycle and so, have focused on the unbudded population of cells. An explanation of the results which could encompass the previous findings (Brockerhoff and Davis, 1992; Lillie and Brown, 1994) would be that localization of calmodulin and Myo2p to the presumptive bud site can occur in the absence of actin. However, stabilization of this association throughout the cell cycle, and/or at elevated temperatures, requires the presence of an intact actin cytoskeleton.
A Role for Actin in Polarized Secretion
The formation of new buds involves localized secretion (Field and Schekman, 1980) and is an actin-dependent process (Novick and Botstein, 1985). Several proteins previously shown to be involved in vesicle trafficking and secretion were demonstrated here to require actin to become localized to the presumptive bud site. This observation strongly suggests that actin facilitates bud growth by orienting spatially elements of the secretory pathway. Our data establish that actin functions at at least two levels to mediate polarized exocytosis. We demonstrated for the first time that actin is necessary to localize secretory vesicles, marked by Sec4p, to the bud site, a role which had been postulated previously (Adams and Pringle, 1984; Kilmartin and Adams, 1984; Johnston et al., 1991). In addition, we have established that actin is involved in localization of the putative vesicle docking complex, marked by Sec8p, to the presumptive bud site.
Proteins That Do Not Require Actin for Localization to the Presumptive Bud Site
The septins are a group of related proteins that localize to the presumptive bud site (Haarer and Pringle, 1987; Ford and Pringle, 1991; Kim et al., 1991; Longtine et al., 1996). Septin mutants are able to polarize their actin cytoskeletons, indicating independence of actin and septin cytoskeletal components for localization (Adams and Pringle, 1984). Earlier studies by Ford and Pringle (1991) investigated localization of septins and actin at the presumptive bud site. Their results suggested that actin may arrive at this site slightly before Cdc11p. However, a few cells in the population were observed with only septins or only actin localized at the presumptive bud site, suggesting that localization of actin and septins at the site might be independent processes. Here, using LAT-A to disrupt F-actin structures, we demonstrated unequivocally that septin localization is independent of actin localization. Thus, while both actin and the septins are delocalized in the absence of Cdc42p, they are not dependent on one another for their own localization. This allows us to demonstrate a bifurcation point in the hierarchy of polarity establishment functions with actin-dependent, and actin-independent functions defining the division (Fig. 15).
The proteins Bni4p and Gin4p have immunofluorescence staining patterns at the presumptive bud site similar to those of the septins, and, like the septins, neither Bni4p nor Gin4p required the presence of an intact actin cytoskeleton for localization. Their actin-independent localization suggests that the primary role of these proteins is not in bud formation processes but is most likely in subsequent processes, possibly associated with cytokinesis.
Finally, we showed that localization of Spa2p at the presumptive bud site was not dependent on an intact actin cytoskeleton, but there was a kinetic delay in its polarized localization in cells incubated with LAT-A. It is possible that Spa2p, or a protein that binds Spa2p, normally uses actinbased structures to localize at the presumptive bud site. However, when at the bud site, Spa2p makes interactions with other proteins not associated with actin, and in LATA–treated cells these interactions are responsible for Spa2p localization. Alternatively, the kinetic delay might reflect perturbation by LAT-A of a mechanism to coordinate temporally actin and Spa2p localization.
A Role for Actin in Maintaining an Axis of Cell Polarity
We also investigated whether actin might have a role in the maintenance of cell polarity. When actin filaments were transiently disrupted in small-budded cells, a significant number of cells subsequently generated a second bud. Previous studies showed that disruption of a MAP kinase pathway, but not transient disorganization of the actin cytoskeleton, results in formation of second buds (Brewster and Gustin, 1994). The difference between results reported here and those of Brewster and Gustin (1994) indicates that analysis of cells with disorganized actin is not the same as analysis of cells lacking actin filaments, and may not reveal the full functional repertoire of actin. An axis of cell polarity is thought to be marked by the local activation of the small GTP-binding proteins Rsr1p and Cdc42p (reviewed by Drubin and Nelson, 1996). We speculate that induction of actin assembly in the proximity of Cdc42p-GTP (Li et al., 1995) might make permanent the mark on the cell surface so that hydrolysis of GTP by Cdc42p does not result in loss of the axis of cell polarity. In some cells, possibly at a particular phase of the cell cycle, disruption of actin might cause the cue marking the correct axis of polarity to be destabilized. Thus, following LAT-A treatment, the existing bud site or mating projection is no longer marked and the cells select a new axis of cell polarity.
Acknowledgments
We thank Helen Yin and Hideaki Karaki for the mycalolide-B, Morgan Conn, Pekka Lappaleinen, and Jamie Cope for assistance with the computer modeling for mapping the actin alleles on the molecular structure of actin, D. Johnson, J. Pringle, B. Winsor, P. Novick, D. TerBush, S. Brown, M. Stark, and M. Snyder for sending antibodies and reagents used in this work, Keith Kozminski, Lisa Belmont, Pekka Lappaleinen and Bruce Goode for helpful comments and for critical reading of the manuscript. Special thanks go to D. TerBush, D. Amberg, and J. Pringle for communicating unpublished results.
This work was supported by grants to David Drubin from the National Institute of General Medical Sciences (GM-42759) and the American Cancer Society (CB-106, FRA-442). Kathryn Ayscough is an International Prize Travelling Fellow of the Wellcome Trust (038110/Z/93/Z). Financial support at the University of California at Santa Cruz was from National Institutes of Health grant CA47135 and nuclear magnetic resonance equipment grants from National Science Foundation BIR-94-19409 and the Elsa U. Pardee Foundation.
Abbreviations used in this paper
- F-actin
filamentous actin
- LAT-A
latrunculin-A
- LAT-B
latrunculin-B
- LY
lucifer yellow
- Rd-phalloidin
rhodamine phalloidin
Footnotes
Received for publication 19 September 1996 and in revised form 7 February 1997.
Please address all correspondence to D.G. Drubin, Department of Molecular and Cell Biology, 401 H.A. Barker Hall, University of California, Berkeley, CA 94720-3202. Tel.: (510) 642-3692. Fax: (510) 642-6420. E-Mail: drubin@mendel.berkeley.edu
References
- Adams AEM, Pringle JR. Relationship to actin and tubulin distribution to bud growth in wild type and morphogenetic mutant Saccharomyces cerevisiae. . J Cell Biol. 1984;98:934–945. doi: 10.1083/jcb.98.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams AE, Johnson DI, Longnecker RM, Sloat BF, Pringle JR. CDC42 and CDC43 , two additional genes involved in budding and the establishment of cell polarity in the yeast Saccharomyces cerevisiae. . J Cell Biol. 1990;111:131–142. doi: 10.1083/jcb.111.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amberg DC, Basart E, Botstein D. Defining protein interactions with yeast actin in vivo. Nature Structural Biology. 1995;2:28–35. doi: 10.1038/nsb0195-28. [DOI] [PubMed] [Google Scholar]
- Amberg, D.C., J.E. Zahner, J.W. Mulholland, J.R. Pringle, and D. Botstein. 1997. Aip3p/Bud6p, a yeast actin-interacting protein that is involved in morphogenesis and the selection of bipolar budding sites. Mol. Biol. Cell. In press. [DOI] [PMC free article] [PubMed]
- Ayscough, K.R., and D.G. Drubin. 1997. Immunofluorescence microscopy of yeast cells. In Cell Biology: A Laboratory Handbook. 2nd Edition. J.E. Celis, Editor. Academic Press, San Diego, CA. In press.
- Bender AEM, Pringle JR. Use of a screen for synthetic lethal and multicopy suppressee mutants to identify two new genes involved in morphogenesis in Saccharomyces cerevisiae. . Mol Cell Biol. 1991;11:1295–1305. doi: 10.1128/mcb.11.3.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti H, Raths S, Crausaz F, Riezman H. The END3gene encodes a protein that is required for the internalization step of endocytosis and for actin cytoskeleton organization in yeast. Mol Biol Cell. 1994;5:1023–1037. doi: 10.1091/mbc.5.9.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botstein, D., A.E.M. Adams, D. Amberg, D.G. Drubin, T. Huffaker, J. Mulholland, and T. Stearns. 1997. The yeast cytoskeleton. In The Molecular Biology of the Yeast Saccharomyces. Cold Spring Harbor Press, Cold Spring Harbor, NY. In press.
- Brewster JL, Gustin MC. Positioning of cell growth and division after osmotic stress requires a MAP kinase pathway. Yeast. 1994;10:425–439. doi: 10.1002/yea.320100402. [DOI] [PubMed] [Google Scholar]
- Brockerhoff S, Davis TN. Calmodulin concentrates at regions of cell growth in Saccharomyces cerevisiae. . J Cell Biol. 1992;118:619–629. doi: 10.1083/jcb.118.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockerhoff S, Stevens R, Davis TN. The unconventional myosin, Myo2p, is a calmodulin target at sites of cell growth in Saccharomyces cerevisiae. . J Cell Biol. 1994;124:315–323. doi: 10.1083/jcb.124.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubb M, Senderowicz A, Sausville E, Duncan K, Korn E. Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F actin. J Cell Biol. 1994;269:14869–14871. [PubMed] [Google Scholar]
- Bubb M, Spector I, Bershadsky A, Korn E. Swinholide A is a microfilament disrupting marine toxin that stabilizes actin dimers and severs actin filaments. J Biol Chem. 1995;270:3463–3466. doi: 10.1074/jbc.270.8.3463. [DOI] [PubMed] [Google Scholar]
- Chant J, Corrado K, Pringle J, Herskowitz I. Yeast BUD5, encoding a putative GDP-GTP exchange factor, is necessary for bud site selection and interacts with bud formation gene BEM1. . Cell. 1991;65:1213–1224. doi: 10.1016/0092-8674(91)90016-r. [DOI] [PubMed] [Google Scholar]
- Chant J, Pringle JR. Patterns of bud site selection in the yeast Saccharomyces cerevisiae. . J Cell Biol. 1995;129:751–765. doi: 10.1083/jcb.129.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. Effects of cytochalasin and phalloidin on actin. J Cell Biol. 1987;105:1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coué M, Brenner S, Spector I, Korn E. Inhibition of actin polymerization by Latrunculin A. Febs Lett. 1987;213:316–318. doi: 10.1016/0014-5793(87)81513-2. [DOI] [PubMed] [Google Scholar]
- Doyle T, Botstein D. Movement of yeast cortical actin cytoskeleton visualized in vivo. . Proc Natl Acad Sci USA. 1996;93:3886–3891. doi: 10.1073/pnas.93.9.3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drees B, Brown C, Barrell B, Bretscher A. Tropomyosin is essential in yeast, yet the TPM1 and TPM2products perform distinct functions. J Cell Biol. 1995;128:383–392. doi: 10.1083/jcb.128.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drubin DG, Nelson WJ. Origins of cell polarity. Cell. 1996;84:335–344. doi: 10.1016/s0092-8674(00)81278-7. [DOI] [PubMed] [Google Scholar]
- Drubin DG, Miller KG, Botstein D. Yeast actin binding proteins: evidence for a role in morphogenesis. J Cell Biol. 1988;107:2551–2561. doi: 10.1083/jcb.107.6.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drubin D, Jones H, Wertman K. Actin structure and function: roles in mitochondrial organization and morphogenesis in budding yeast and identification of the phalloidin-binding site. Mol Biol Cell. 1993;4:1277–1294. doi: 10.1091/mbc.4.12.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulic V, Egerton M, Elgundi I, Raths S, Singer B, Riezman H. Yeast endocytosis assays. Methods Enzymol. 1991;194:697–710. doi: 10.1016/0076-6879(91)94051-d. [DOI] [PubMed] [Google Scholar]
- Field C, Schekman R. Localized secretion of acid phosphatase reflects the pattern of cell surface growth in Saccharomyces cerevisiae. . J Cell Biol. 1980;86:123–128. doi: 10.1083/jcb.86.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford S, Pringle JR. Cellular morphogenesis in the Saccharomyces cerevisiae cell cycle: localization of the CDC11gene product and the timing of events at the budding site. Dev Genet. 1991;12:281–292. doi: 10.1002/dvg.1020120405. [DOI] [PubMed] [Google Scholar]
- Forscher P, Lin C, Thompson C. Novel form of growth cone motility involving site directed actin filament assembly. Nature (Lond) 1992;357:515–518. doi: 10.1038/357515a0. [DOI] [PubMed] [Google Scholar]
- Freeman NL, Chen Z, Horenstein J, Weber A, Field J. An actin monomer binding activity localizes to the carboxyl half of the Saccharomyces cerevisiaecyclase associated protein. J Biol Chem. 1995;270:5680–5695. doi: 10.1074/jbc.270.10.5680. [DOI] [PubMed] [Google Scholar]
- Gaber RF, Copple DM, Kennedy BK, Vidal M, Bard M. The yeast gene ERG6 is required for normal membrane function but is not essential for the biosynthesis of the cell-cycle-sparking sterol. Mol Cell Biol. 1989;9:3447–3456. doi: 10.1128/mcb.9.8.3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt-Clermont P, Furman M, Wachsstock D, Safer D, Nachmias V, Pollard T. The control of actin nucleotide exchange by thymosin β4 and profilin. A potential regulatory mechanism for actin polymerization in cells. Mol Biol Cell. 1992;3:1015–1024. doi: 10.1091/mbc.3.9.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt-Clermont P, Machesky L, Doberstein S, Pollard T. Mechanism of the interaction of human platelet profilin with actin. J Cell Biol. 1991;113:1081–1089. doi: 10.1083/jcb.113.5.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindan B, Bowser R, Novick P. The role of Myo2, a yeast class V myosin, in vesicular transport. J Cell Biol. 1995;128:1055–1068. doi: 10.1083/jcb.128.6.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haarer B, Pringle JR. Immunofluorescence localization of the CDC12gene product to the vicinity of the 10 nm filaments in the mother bud neck. Mol Cell Biol. 1987;7:3678–3687. doi: 10.1128/mcb.7.10.3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haarer BK, Lillie SH, Adams AEM, Magdolen V, Bandlow W, Brown S. Purification of profilin from Saccharomyces cerevisiaeand analysis of profilin deficient cells. J Cell Biol. 1990;110:105–114. doi: 10.1083/jcb.110.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. Genetic control of the cell division cycle in yeast. Genes controlling bud emergence and cytokinesis. Exp Cell Res. 1971;69:265–276. doi: 10.1016/0014-4827(71)90223-0. [DOI] [PubMed] [Google Scholar]
- Holtzman D, Wertman K, Drubin D. Mapping actin surfaces required for functional interactions in vivo. J Cell Biol. 1994;126:423–432. doi: 10.1083/jcb.126.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman DA, Yang S, Drubin DG. Synthetic-lethal interactions identify two novel genes, SLA1 and SLA2, that control membrane cytoskeleton assembly in Saccharomyces cerevisiae. . J Cell Biol. 1993;122:635–644. doi: 10.1083/jcb.122.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman W, Crews P. Novel marine sponge derived amino acids 8. Conformational analysis of Jasplakinolide. J Amer Chem Soc. 1989;111:2822–2829. [Google Scholar]
- Johnston G, Prendergast J, Singer R. The Saccharomyces cerevisiae MYO2gene encodes an essential myosin for vectorial transport of vesicles. J Cell Biol. 1991;113:539–551. doi: 10.1083/jcb.113.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabsch W, Mannherz H, Suck D, Pai E, Holmes K. Atomic Structure of the actin:DNase1 complex. Nature (Lond) 1990;347:37–44. doi: 10.1038/347037a0. [DOI] [PubMed] [Google Scholar]
- Kaiser, C., S. Michaelis, and A. Mitchell. 1994. Methods in yeast genetics: a laboratory course manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Karpova T, Tatchell K, Cooper J. Actin filaments in yeast are unstable in the absence of capping protein. J Cell Biol. 1995;131:1483–1493. doi: 10.1083/jcb.131.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher J, Atkinson S, Pollard T. Sequences, structural models and cellular localization of the actin related proteins Arp2 and Arp3 from Acanthamoeba. . J Cell Biol. 1995;131:385–397. doi: 10.1083/jcb.131.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmartin JV, Adams AEM. Structural rearrangements of tubulin and actin during the cell cycle of the yeast, Saccharomyces. . J Cell Biol. 1984;98:922–939. doi: 10.1083/jcb.98.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Haarer B, Pringle JR. Cellular morphogenesis in the Saccharomyces cerevisiaecell cycle: localization of the CDC3 gene product and the timing of events at the budding site. J Cell Biol. 1991;112:535–544. doi: 10.1083/jcb.112.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa I, Kobayashi M, Katori T, Yamashita M, Tanaka J, Doi M, Ishida T. Absolute stereostructure of Swinholide A, a potent cytotoxic macrolide from the Okinawan marine sponge Theonella swinhoei. . J Amer Chem Soc. 1990;112:3710–3712. [Google Scholar]
- Koerner T, Hill J, Myers A, Tzagoloff A. High expression vectors with multiple cloning sites for construction of trpEfusion genes: pATH vectors. Methods Enzymol. 1991;194:477–490. doi: 10.1016/0076-6879(91)94036-c. [DOI] [PubMed] [Google Scholar]
- Kron SJ, Drubin DG, Botstein D, Spudich JA. Yeast actin filaments display ATP-dependent sliding movement over surfaces coated with rabbit muscle myosin. Proc Natl Acad Sci USA. 1992;89:4466–4470. doi: 10.1073/pnas.89.10.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kübler E, Riezman H. Actin and fimbrin are required for the internalization step of endocytosis in yeast. EMBO (Eur Mol Biol Organ) J. 1993;12:2855–2862. doi: 10.1002/j.1460-2075.1993.tb05947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew D, Reed S. Morphogenesis in the yeast cell cycle: regulation by Cdc28 and Cyclins. J Cell Biol. 1993;120:1305–1320. doi: 10.1083/jcb.120.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Zheng Y, Drubin DG. Regulation of cortical actin assembly during polarized cell growth in budding yeast. J Cell Biol. 1995;128:599–615. doi: 10.1083/jcb.128.4.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie S, Brown S. Immunofluorescence localization of the unconventional myosin, Myo2p, and the putative kinesin-related protein, Smy1p to the same regions of polarized growth in Saccharomyces cerevisiae. . J Cell Biol. 1994;125:825–842. doi: 10.1083/jcb.125.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HP, Bretscher A. Characterization of TPM1disrupted yeast cells indicates an involvement of tropomyosin in directed vesicular transport. J Cell Biol. 1992;118:285–299. doi: 10.1083/jcb.118.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine M, DeMarini D, Valencik M, Al-Awar O, Fares H, DeVirgilio C, Pringle JR. The Septins: roles in cytokinesis and other processes. Curr Opin Cell Biol. 1996;8:106–119. doi: 10.1016/s0955-0674(96)80054-8. [DOI] [PubMed] [Google Scholar]
- Matsui Y, Matsui R, Akada R, Toh A -e. Yeast src-homology region 3 domain-binding proteins involved in bud formation. J Cell Biol. 1996;133:865–878. doi: 10.1083/jcb.133.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack AL, Schieltz DM, Goode B, Yang S, Barnes G, Drubin D, Yates JR., III Direct analysis and identification of proteins in mixtures by LC/MS/MS and database searching at the low femtomole level. J Anal Chem. 1996;69:767–776. doi: 10.1021/ac960799q. [DOI] [PubMed] [Google Scholar]
- Moon AL, Janmey PA, Louie A, Drubin DG. Cofilin is an essential component of the yeast cortical actin cytoskeleton. J Cell Biol. 1993;120:421–435. doi: 10.1083/jcb.120.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau V, Madania A, Martin R, Winsor B. The Saccharomyces cerevisiaeactin related protein Arp2 is involved in the actin cytoskeleton. J Cell Biol. 1996;134:117–132. doi: 10.1083/jcb.134.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P, Botstein D. Phenotypic analysis of temperature sensitive yeast actin mutants. Cell. 1985;40:405–416. doi: 10.1016/0092-8674(85)90154-0. [DOI] [PubMed] [Google Scholar]
- Novick P, Brennwald P. Friends and family: the role of the Rab GTPases in vesicular traffic. Cell. 1993;75:597–601. doi: 10.1016/0092-8674(93)90478-9. [DOI] [PubMed] [Google Scholar]
- Preuss D, Mulholland J, Franzusoff A, Segev N, Botstein D. Characterization of the SaccharomycesGolgi complex through the cell cycle by immunoelectron microscopy. Mol Biol Cell. 1992;3:789–803. doi: 10.1091/mbc.3.7.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle JR, Preston RA, Adams AEM, Stearns T, Drubin DG, Haarer BK, Jones EW. Fluorescence microscopy methods for yeast. Methods Cell Biol. 1989;31:357–435. doi: 10.1016/s0091-679x(08)61620-9. [DOI] [PubMed] [Google Scholar]
- Pringle JR, Adams AEM, Drubin DG, Haarer B. Immunofluorescence methods for yeast. Methods Enzymol. 1991;194:565–602. doi: 10.1016/0076-6879(91)94043-c. [DOI] [PubMed] [Google Scholar]
- Pringle JR, Bi E, Harkins H, Zahner J, DeVirgilio C, Chant J, Corrado K, Fares H. Establishment of cell polarity in yeast. Cold Spring Harbor Symp Quant Biol. 1995;60:729–744. doi: 10.1101/sqb.1995.060.01.079. [DOI] [PubMed] [Google Scholar]
- Quiñoà E, Kakou Y, Crews P. Fijianolides, polyketide heterocycles from a marine sponge. J Org Chem. 1988;53:3642–3644. [Google Scholar]
- Reneke J, Blumer K, Courchesne W, Thorner J. The carboxy-terminal segment of the yeast α-factor receptor is a regulatory domain. Cell. 1988;55:221–234. doi: 10.1016/0092-8674(88)90045-1. [DOI] [PubMed] [Google Scholar]
- Salminen A, Novick P. A ras-like protein is required for a postGolgi event in yeast secretion. Cell. 1987;49:527–538. doi: 10.1016/0092-8674(87)90455-7. [DOI] [PubMed] [Google Scholar]
- Senderowicz A, Kaur G, Sainz E, Laing C, Inman WD, Rodriguez J, Crews P, Malspeis L, Grever M, Sausville E, Duncan K. Jasplakinolide's inhibition of the growth of prostate carcinoma cells in vitro with disruption of the actin cytoskeleton. J Natl Cancer Inst. 1995;87:46–51. doi: 10.1093/jnci/87.1.46. [DOI] [PubMed] [Google Scholar]
- Sloat B, Adams A, Pringle JR. Roles of the CDC24 gene product in cellular morphogenesis during the Saccharomyces cerevisiaecell cycle. J Cell Biol. 1981;89:395–405. doi: 10.1083/jcb.89.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M. The SPA2protein of yeast localizes to sites of cell growth. J Cell Biol. 1989;108:1419–1429. doi: 10.1083/jcb.108.4.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M, Gehrung S, Page B. Studies concerning the temporal and genetic control of cell polarity in Saccharomyces cerevisiae. . J Cell Biol. 1991;114:515–532. doi: 10.1083/jcb.114.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector I, Shochet N, Blasberger D, Kashman Y. Latrunculinsnovel marine macrolides that disrupt microfilament organization and affect cell growth: 1. Comparison with cytochalasin D. Cell Motil Cytoskeleton. 1989;13:127–144. doi: 10.1002/cm.970130302. [DOI] [PubMed] [Google Scholar]
- TerBush D, Novick P. Sec6, Sec8, and Sec15 are components of a multisubunit complex which localizes to small bud tips in Saccharomyces cerevisiae. . J Cell Biol. 1995;130:299–312. doi: 10.1083/jcb.130.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theriot J, Mitchison T. The rate of actin-based motility of intracellular Listeria monocytogenesequals the rate of actin polymerization. Nature (Lond) 1992;357:257–260. doi: 10.1038/357257a0. [DOI] [PubMed] [Google Scholar]
- Tilney L, Portnoy D. Actin filaments and the growth, movement and spread of the intracellular bacterial parasite, Listeria monocytogenes. . J Cell Biol. 1989;109:1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L, Connelly P, Portnoy D. Actin filament nucleation by the bacterial pathogen, Listeria monocytogenes. . J Cell Biol. 1990;11:2979–2988. doi: 10.1083/jcb.111.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waechter F, Engel J. The kinetics of the exchange of G-actin bound:N6-ethenoadenosine 5′-triphosphate with ATP as followed by fluorescence. Eur J Biochem. 1975;57:453–459. doi: 10.1111/j.1432-1033.1975.tb02320.x. [DOI] [PubMed] [Google Scholar]
- Waddle JA, Karpova TS, Waterson RH, Cooper JA. Movement of cortical actin patches in yeast. J Cell Biol. 1996;132:861–870. doi: 10.1083/jcb.132.5.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch M, Drubin D G. A nuclear protein with sequence similarity to proteins implicated in human acute leukaemias is important for cellular morphogenesis and actin cytoskeletal function in Saccharomyces Cerevisiae. . Mol Biol Cell. 1993;5:617–632. doi: 10.1091/mbc.5.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertman K F, Drubin D G, Botstein D. Systematic mutational analysis of the yeast ACT1gene. Genetics. 1992;132:337–350. doi: 10.1093/genetics/132.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziman M, Preuss D, Mulholand J, O'Brien J, Botstein D, Johnson D. Subcellular localization of Cdc42p, a Saccharomyces cerevisiaeGTPbinding protein involved in the control of cell polarity. Mol Biol Cell. 1993;4:1307–1316. doi: 10.1091/mbc.4.12.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]