Abstract
Leukocyte adhesion to vascular endothelium under flow involves an adhesion cascade consisting of multiple receptor pairs that may function in an overlapping fashion. P-selectin glycoprotein ligand-1 (PSGL-1) and L-selectin have been implicated in neutrophil adhesion to P- and E-selectin under flow conditions. To study, in isolation, the interaction of PSGL-1 with P-and E-selectin under flow, we developed an in vitro model in which various recombinant regions of extracellular PSGL-1 were coupled to 10-μm-diameter microspheres. In a parallel plate chamber with well defined flow conditions, live time video microscopy analyses revealed that microspheres coated with PSGL-1 attached and rolled on 4-h tumor necrosis factor-α–activated endothelial cell monolayers, which express high levels of E-selectin, and CHO monolayers stably expressing E-or P-selectin. Further studies using CHO-E and -P monolayers demonstrate that the first 19 amino acids of PSGL-1 are sufficient for attachment and rolling on both E- and P-selectin and suggest that a sialyl Lewis x–containing glycan at Threonine-16 is critical for this sequence of amino acids to mediate attachment to E- and P-selectin. The data also demonstrate that a sulfated, anionic polypeptide segment within the amino terminus of PSGL-1 is necessary for PSGL-1–mediated attachment to P- but not to E-selectin. In addition, the results suggest that PSGL-1 has more than one binding site for E-selectin: one site located within the first 19 amino acids of PSGL-1 and one or more sites located between amino acids 19 through 148.
Acritical step in the recruitment of leukocytes to a site of inflammation is the adhesion of the leukocytes to the vascular endothelium in the fluid dynamic environment of the microcirculation. This adhesion process involves a cascade of events including initial attachment, rolling, spreading, and ultimately transendothelial migration (11, 20, 28, 50). In vivo and in vitro studies have shown that the inducible endothelial cell adhesion molecules P-selectin (CD62P) and E-selectin (CD62E) are involved in leukocyte initial attachment and rolling on activated vascular endothelium (1, 20–22, 29–31). The third known member of the selectin family, L-selectin (CD62L), is expressed only on leukocytes and is also involved in leukocyte recruitment (4, 16, 22–24, 46, 50). The expression of E-selectin requires de novo protein synthesis and can be elicited by a 4-h treatment of cultured endothelium with certain Gram-negative endotoxins and cytokines such as IL-1 and tumor necrosis factor-α (TNF-α)1 (7). P-selectin is stored in the Weibel-Palade bodies of endothelial cells and is rapidly mobilized to the cell surface by secretagogues such as thrombin and histamine (32). The cytokine activation pathway may also elicit limited P-selectin expression on cultured human umbilical vein endothelium (29, 30). A notable feature of the selectins is their NH2-terminal, lectin-like domain that binds carbohydrate moieties in a Ca2+-dependent manner (6). Thus, several carbohydrate ligands for P- and E-selectin have been proposed including the sialyl Lewis x (SLex) tetrasaccharide and related glycans (15, 41, 53).
Recent studies have focused on identifying the underlying leukocyte proteins which present carbohydrate ligands for binding to E- and P-selectin. Perhaps the best characterized of these is P-selectin glycoprotein ligand-1 (PSGL-1), which was first isolated from HL-60 cells (33) and subsequently cloned from an HL-60 cell cDNA library (43). PSGL-1, a homodimer of disulfide-linked subunits with an apparent molecular mass of 120 kD each (33), is present on a variety of leukocytes including neutrophils, monocytes, and lymphocytes (35). PSGL-1 is extensively glycosylated with N-linked glycans and closely spaced O-linked glycans, a portion of which are modified by SLex (34, 37, 54). L-selectin expressed on myeloid leukocytes also has been proposed as a ligand for E- and P-selectin since, similarly to PSGL-1, it displays SLex-type structures and is localized on the tips of microvilli (22, 35, 39).
In vitro flow adhesion assays have investigated PSGL-1– and L-selectin–mediated leukocyte adhesion to E- and P-selectin under fluid flow. Patel et al. (38) measured neutrophil accumulation on CHO cell monolayers stably expressing P-selectin (CHO-P) or E-selectin (CHO-E). Based on mAb blocking studies, the authors inferred that PSGL-1 is necessary for attachment of neutrophils to P-selectin and for optimal attachment to E-selectin. In contrast, they also reported that neutrophils pretreated (in the presence of cytochalasin D to prevent shape change) with fMLP, a chemoattractant known to induce endoproteolytic cleavage of L-selectin, did not accumulate on CHO-P or -E monolayers but did retain functional PSGL-1–dependent binding sites for P-selectin (38). A separate study (22) reported that the accumulation of neutrophils on soluble E-selectin under flow was inhibited (∼90%) by mAbs to L-selectin. The authors inferred that neutrophil attachment to E-selectin under flow requires L-selectin, implying that PSGL-1 is not sufficient to mediate attachment to E-selectin under flow. mAb approaches have suggested the involvement of PSGL-1 in neutrophil rolling, subsequent to attachment, on E- and P-selectin (35, 38). However, since attachment is a prerequisite for rolling, if the attachment step is blocked by an mAb to PSGL-1 (35, 38), it is difficult to determine if PSGL-1 can mediate adhesive events subsequent to attachment such as rolling. Therefore, while it appears that PSGL-1 is involved in neutrophil attachment to E- and P-selectin, it is unclear if PSGL-1 is sufficient to mediate attachment and rolling.
Previous studies have investigated PSGL-1 regions necessary for recognition of P- and E-selectin (42, 44). These studies have revealed that an anionic polypeptide segment containing three tyrosine residues, at least one of which is sulfated, is located within the amino terminus of mature PSGL-1 (42, 44). This region is necessary for fluid phase PSGL-1 recognition of cell surface–expressed P-selectin but is unnecessary for fluid phase PSGL-1 recognition of cell surface–expressed E-selectin (44). However, the structure–function relationship for PSGL-1 and E- and P-selectin has not been investigated in the more relevant (49) in vitro flow assay.
To examine isolated PSGL-1 attachment and rolling on E- and P-selectin, we have studied the adhesion of 10-μmdiam nondeformable microspheres, coated with various extracellular regions of PSGL-1, to TNF-α–activated cultured human endothelium and to CHO-E and -P monolayers under defined flow conditions. The results demonstrate that: (a) PSGL-1 is sufficient to mediate microsphere attachment and rolling on TNF-α–activated endothelial monolayers and on CHO-E and -P monolayers; (b) the first 19 amino acids of PSGL-1 are sufficient for attachment and rolling on both E- and P-selectin; (c) an SLex containing O-glycan at Threonine-16 is critical for the first 19 amino acids of PSGL-1 to mediate attachment to E- and P-selectin; (d) the anionic polypeptide segment within the amino terminus of PSGL-1 is necessary for PSGL-1–mediated attachment to P- but not E-selectin; and (e) PSGL-1 has more than one binding site for E-selectin: one site located within the first 19 amino acids of PSGL-1 and one or more sites located between amino acids 19 through 148.
Materials and Methods
Materials
RPMI-1640 containing 1 mM l-glutamine and 20 mM Hepes, Alpha media, DPBS with (DPBS+) or without Ca2+ and Mg2+, were obtained from Biowhittaker (Walkersville, MD). FBS and dialyzed FBS were obtained from Hyclone Labs (Urem, UT). O-sialoglycoprotein endopeptidase (OSGE) was obtained from Accurate Chemicals (Westbury, NY). Neuraminidase from Vibrio cholerae was obtained from Boehringer Mannheim Corp. (Indianapolis, IN). BSA was obtained from Sigma Chemical Co. (St. Louis, MO). All other chemicals were of the highest grade available from J.T. Baker, Inc. (Phillipsburg, NJ).
Antibodies
Function blocking murine mAb to E-selectin 7A9 (IgG1) was obtained from American Type Culture Collection (Rockville, MD) (clone HB 10135) and used as F(ab′)2 (10 μg/ml). Nonblocking murine mAb to E-selectin H4/18 (IgG1) was used as F(ab′)2 (10 μg/ml). Leukocyte function blocking murine anti–P-selectin mAb, HPDG2/3 (IgG1) (43), was used as F(ab′)2 (10 μg/ml), and nonblocking murine anti–P-selectin mAb, HPDG2/1 (IgG1) (43), was used as purified IgG1 (10 μg/ml). The following antibodies recognize human PSGL-1 and have been described previously: murine mAb PSL-275 (IgG1; purified IgG, 20 μg/ml) (43, 48) and polyclonal rabbit sera, Rb3026 (1:20 dilution) (43, 48). Normal rabbit sera (1:20 dilution) was used as a control for Rb3026. mAbs KPL1 and KPL2 (Snapp, K.R., F.W. Luscinskas, R. Warnke, and G.S. Kansas. 1997. J. Allergy Clin. Immunol. 99:s459) recognize PSGL-1 and were used as ascites (1:200 dilution for adhesion assays and 1:500 for flow cytometric analysis) and purified IgG. Murine antihuman IgG1 Fc specific (CALTAG Laboratories, San Francisco, CA) was used as a control for the purified KPL1. Murine anti– ICAM-1 mAb Hu5/3 (IgG1) was used as purified IgG (20 μg/ml). Murine antibody to human Class I, W6/32, (IgG2a) was used as F(ab′)2 (10 μg/ml). FITC-labeled goat F(ab′)2 secondary antibodies (each at 1:50 dilution) to rabbit IgG, mouse IgG, and human Fc were obtained from CALTAG Laboratories. FITC-labeled goat F(ab′)2 secondary antibody (1:50 dilution) to mouse Fc and unlabeled goat F(ab′)2 were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA).
Cell Culture
CHO cells stably expressing human E-selectin (CHO-E) or human P-selectin (CHO-P) were prepared and cultured in Alpha media containing 10% dialyzed FBS as previously detailed (43). Human umbilical vein endothelial cells (HUVEC) were isolated and cultured as described previously (28). For induction of adhesion molecule expression, HUVEC monolayers were activated by a 4-h treatment with 25 ng/ml TNF-α (29).
Soluble Forms of PSGL-1
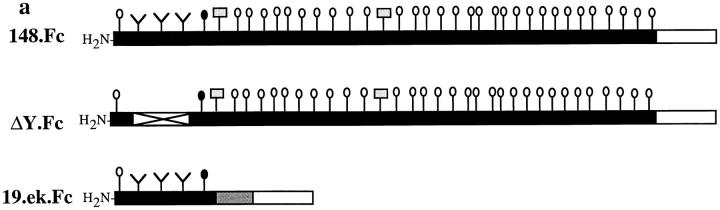
The PSGL-1 molecules used in these studies are chimeras consisting of various truncated extracellular regions of mature PSGL-1 fused to the heavy chain CH2-CH3 (Fc) region of human IgG1. Their construction, expression, and selectin-binding properties have been described previously (44). In the present studies, plasmids encoding two of these chimeras, 148.Fc and ΔY148.Fc (abbreviated to ΔY.Fc here), were transfected into COS cells as described except for the substitution of a plasmid encoding an α(1,3)fucosyltransferase-VII (Fuc-TVII) enzyme (36, 45) for the FucTIII plasmid used in earlier cotransfections (44). The resulting conditioned media were supplemented with 0.002% NaN3 and 10 μM PMSF and then clarified by centrifugation. Fc chimeras were purified by protein A–Sepharose (Pharmacia LKB Biotechnology, Inc., Piscataway, NJ) chromatography per the manufacturer's instructions and quantified by an anti-Fc enzyme-linked immunosorbent assay using human IgG1 (Sigma Chemical Co.) as a standard. The 19.Fc form of PSGL-1 described earlier (44) was mutated to include an enterokinase cleavage site (19) located between the PSGL-1 and IgG Fc regions and is termed 19.ek.Fc. A plasmid encoding this construct was stably transfected into CHO cells that were engineered to also express Fuc-TVII and β(1,6)-N-acetylglucosaminyltransferase (“core2” [9]) activities (17, 25). 19.ek.Fc was purified from conditioned media and quantified as above. Similar to COS produced 19.Fc, 19.ek.Fc is tyrosine sulfated and glycosylated with SLex-type containing O-glycans and, in E- and P-selectin binding experiments (44), is functionally indistinguishable (data not shown). The nomenclature for microspheres coated with various extracellular regions of PSGL-1 is as follows: the 148.Fc construct, 148.Fc microspheres; the 19.ek.Fc construct, 19.ek.Fc microspheres; the ΔY.Fc construct, ΔY.Fc microspheres (see Fig. 6 a).
Figure 6.
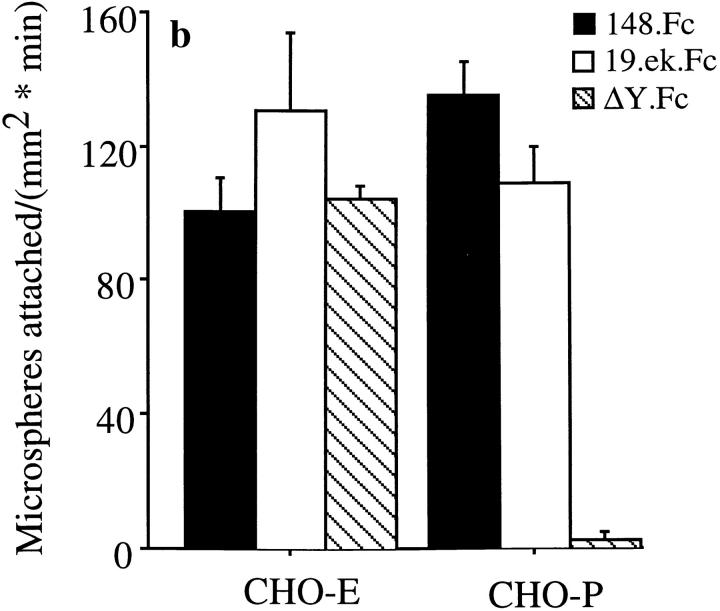
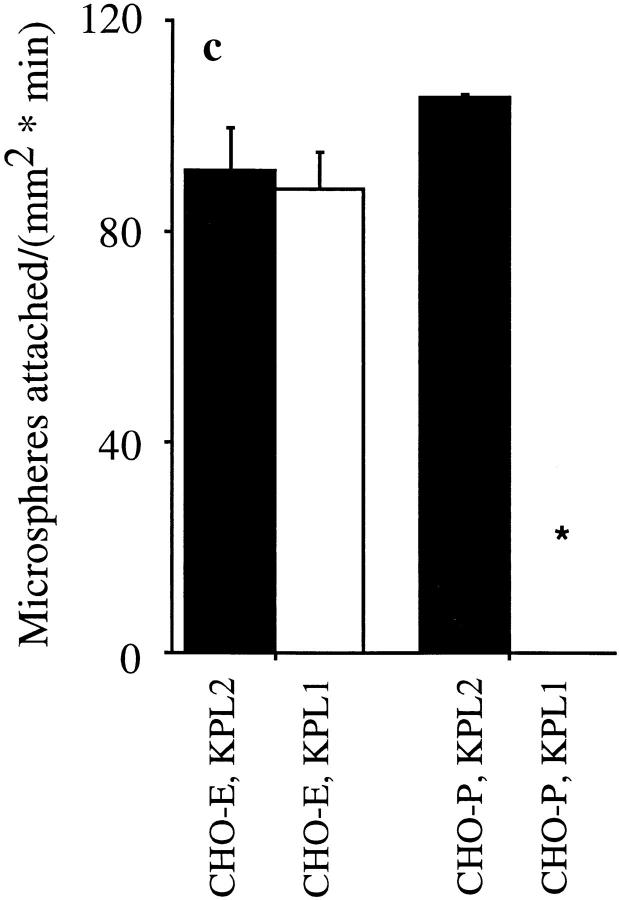
The first 19 amino acids of PSGL-1 are sufficient for attachment to CHO-E and -P monolayers, and the NH2-terminal anionic polypeptide segment of PSGL-1 is necessary for 148.Fc-mediated attachment to CHO-P, but not CHO-E monolayers. (a) Schematic representation of the PSGL-1.Fc constructs. Closed bars, PSGL-1 segments; open bars, human Fc segments; open bar with X, an internal deletion of amino acids 5–11 within the NH2-terminal anionic polypeptide region; shaded bar, the enterokinase cleavage site; Y, amino-terminal tyrosine; vertical lines with open circles, the approximate number and location of potential O-linked glycosylation sites; vertical lines with closed circles, O-linked site at amino terminal threonine 16; vertical lines with shaded rectangles, potential N-linked glycosylation sites. (Drawing not to scale.) (b) Microspheres coated with the 19.ek.Fc mutant (open bars), containing the first 19 amino acids of PSGL-1, attached to CHO-P and -E monolayers at a rate similar to 148.Fc microspheres (dark bars). Microspheres coated with the ΔY.Fc mutant, which consists of the first 148 amino acids of PSGL-1 with an internal deletion of the residues in positions 5–11, attached to CHO-E monolayers at a rate similar to 148.Fc microspheres but did not attach to CHO-P monolayers (crosshatched bars). (Shear stress = 2 dynes/cm2; n = 2). (c) An mAb to PSGL-1, KPL1, which requires amino acids 5–11 to recognize PSGL-1 (Table I), did not affect attachment of 148.Fc microspheres to CHO-E monolayers but eliminated attachment to CHO-P monolayers. 148.Fc microspheres treated with mAb KPL1 attached to CHO-E monolayers at a rate similar to that observed for 148.Fc microspheres treated with control mAb KPL2 but did not attach to CHO-P monolayers. (Shear stress = 2 dynes/cm2; *P < 0.05; n = 2). (d) mAb KPL1 eliminated attachment of 19.ek.Fc microspheres to both CHO-E and -P monolayers. (Shear stress = 2 dynes/cm2; *P < 0.05; n = 2).
Preparation, Flow Cytometric Analysis, and Enzymatic Treatment of PSGL-1 Microspheres
Preparation.
10-μm nondeformable polystyrene microspheres (Polysciences Inc., Warrington, PA) were washed twice in 0.1 M NaHCO3, pH 9.2, and incubated (2.5 × 107 microspheres/ml) overnight at room temperature (RT) with 300 μg/ml protein A (Zymed Labs, South San Francisco, CA) in 0.1 M NaHCO3, pH 9.2, with end-to-end rotation. Microspheres were washed twice with DPBS and blocked for 30 min at RT with DPBS containing 1% BSA. After the blocking step, the microspheres (2 × 108 microspheres/ml) were incubated with a PSGL-1.Fc extracellular construct or human IgG1 diluted in DPBS for 1 h at RT with agitation. The microspheres were washed with DPBS+, 1% BSA and blocked with DPBS+, 1% BSA containing 200 μg/ml human IgG1 kappa or lambda (Sigma Chemical Co.). Microspheres were held in this buffer at 1 × 108 microspheres/ml at RT for 1 h before use in the assays.
Indirect Immunofluorescence and Flow Cytometric Analysis of PSGL-1 Microspheres.
After the human IgG1 blocking step, replicate aliquots (∼5 × 105) of microspheres were washed in DPBS+ containing 1% BSA and incubated for 20 min at RT with 20 μg/ml primary mAb in DPBS+, 1% BSA. After incubation, microspheres were washed and blocked with unlabeled goat F(ab′)2 fragments (100 μg/ml), and the primary mAb was detected with FITC-labeled F(ab′)2 fragments of goat anti–mouse Fc-specific secondary antibody. The microspheres were incubated for 20 min at RT, washed twice in DPBS+, 1% BSA, once in DPBS+, and fixed with 2% formaldehyde in DPBS+. FITC fluorescence was determined on a FACScan® flow cytometer (Becton-Dickinson Immunocytometry Sys., Mountain View, CA) by counting the fluorescence of 5,000 or 10,000 microspheres. The data are presented as single parameter histograms on a four-decade scale.
OSGE Treatment of PSGL-1 Microspheres.
Microspheres were prepared as described above up to blocking with human IgG1. A suspension of PSGL-1–coated microspheres (1 × 108/ml) was incubated in DPBS+, 0.2% FBS, 0.05% NaN3, 25 mM Hepes, pH 7.4, with or without 160 μg/ml OSGE for 30 min at 37°C. The microspheres were then washed with DPBS+, 1% BSA and blocked with DPBS+, 1% BSA containing 200 μg/ml human IgG1 at 1 × 108 microspheres/ml. The microspheres used in the flow cytometric analysis to detect human Fc were incubated with 0.2% normal rabbit serum instead of human IgG1, blocked with 100 μg/ml unlabeled goat (Fab′)2 fragments, and then stained with goat FITC-labeled (Fab′)2 fragments of antibodies to either human Fc or mouse IgG.
Sialidase Treatment of PSGL-1 Microspheres.
Microspheres were prepared with the 19.ek.Fc construct as described above. 19.ek.Fc microspheres were then washed twice in RPMI-1640, resuspended to 1 × 108 in RPMI-1640 with or without 0.1 U/ml neuraminidase, and incubated for 30 min at 37°C. Microspheres were then washed twice in DPBS+, 1% BSA and blocked (1 × 108 microspheres/ml) with DPBS+, 1% BSA containing 200 μg/ml human IgG1.
Parallel Plate Flow Chamber Analysis
The parallel plate flow chamber apparatus used in these studies has been described in detail (28). Temperature was maintained at 37°C by a heating plate. The flow apparatus was mounted on an inverted microscope (model Diaphot; Nikon Inc., Melville, NY), and the entire perfusion period was recorded on videotape by a video camera and VCR. Microspheres were drawn through the chamber at 5 × 105 microspheres/ml in DPBS+, 0.5% BSA, and microsphere attachment rates were quantified by observing a 10× field of view for ∼1 min and 15 s. The number of microspheres that attached throughout this time period was determined and divided by the time of observation and the area of the field of view to yield the rate of attachment per unit area. For a given coverslip, an attachment rate was determined at two different fields of view and the two values were averaged to give an n = 1. Microsphere accumulation was determined by counting the number of microspheres interacting with the substrate in a particular field of view (10× magnification). This value was determined for three to five different fields of view between 3.5 to 4 min after initiation of microsphere perfusion. These numbers were averaged and normalized to the area of the field of view. Rolling velocities were determined manually by measuring the distance a microsphere traveled in a given amount of time.
Antibody Blocking Experiments
All control or blocking antibodies to molecules on the cell monolayers were diluted (10 μg/ml) in the appropriate media and added to the monolayers 20 min before the adhesion assays described above. For mAb blocking of the PSGL-1 microspheres, 10–15 μl of a 1 × 108 microsphere/ml suspension was mixed 1:1 with DPBS+, 1% BSA containing a 1:100 dilution of ascites mAb KPL1 or KPL2. This suspension was allowed to incubate for 15 min at RT, diluted to 5 × 105 microspheres/ml in DPBS+, 0.5% BSA, and used immediately in the flow chamber assay.
Statistics
All differences were evaluated by a two-tailed Student's t test. P values represent the results of these t tests and values ⩽0.05 were considered significant. All error bars represent standard deviations.
Results
A PSGL-1.Fc Chimera Can Be Coupled to Polystyrene Microspheres Precoated with Protein A
Previous investigators have developed methods to couple biotinylated SLex (10) or various mAbs (18, 40) to microspheres and used the resulting microspheres as model cells in adhesion assays. We adapted these techniques to study the interaction of PSGL-1, in isolation, with E- and P-selectin under fluid flow. Sako et al. (44) have generated and described recombinant PSGL-1.Fc constructs that consist of various portions of the extracellular region of PSGL-1 fused to the Fc region of human IgG1. The presence of the Fc region led to the idea of coupling the PSGL-1.Fc constructs to microspheres precoated with protein A. Such an approach allows for the “correct” orientation of PSGL-1.Fc chimera on the microsphere, i.e., the PSGL-1 portion of the PSGL-1.Fc chimera oriented away from the microsphere and available for binding to other molecules and the Fc portion of the chimera coupled to the protein A adsorbed to the surface of the microsphere. Although recombinant forms of PSGL-1 may differ slightly from native PSGL-1, other investigators have used recombinant forms of PSGL-1 to gain significant insights into the biochemistry of PSGL1–E- and –P-selectin interactions (25, 42, 44) . In addition, Sako et al. (44) have documented that recombinant and native PSGL-1 exhibit similar function in a variety of biochemical assays.
In initial studies, 148.Fc, a truncated PSGL-1 construct consisting of the first 148 amino acids of PSGL-1 linked to the Fc region of human IgG1 (44), was coupled to 10-μmdiam polystyrene microspheres precoated with protein A to generate 148.Fc microspheres. The 148.Fc version of PSGL-1 was used, as it was demonstrated previously to exhibit high affinity binding to both E- and P-selectin under static conditions when produced in the presence of an SLex-generating fucosyltransferase (44). We chose to use 10-μm-diam microspheres since such microspheres have a density (1.05 g/ml) and diameter similar to that of leukocytes. The presence of the 148.Fc chimera on the surface of the 148.Fc microsphere was detected with an mAb to PSGL-1, PSL-275 (Fig. 1, a–e). The amount of 148.Fc chimera bound to the microspheres was concentration dependent (Fig. 1, a–e). An isotype-matched control mAb, Hu5/3, did not recognize microspheres coated with 100 μg/ml 148.Fc, the highest coating concentration used (Fig. 1 f). From this data we conclude that the 148.Fc chimera was bound to the protein A–coated microspheres. Preliminary studies indicated that microspheres prepared with 50 μg/ml of the 148.Fc construct gave consistent results in the flow adhesion assays. Hence, a coating concentration of 50 μg/ml was used in the remainder of the studies unless otherwise noted.
Figure 1.
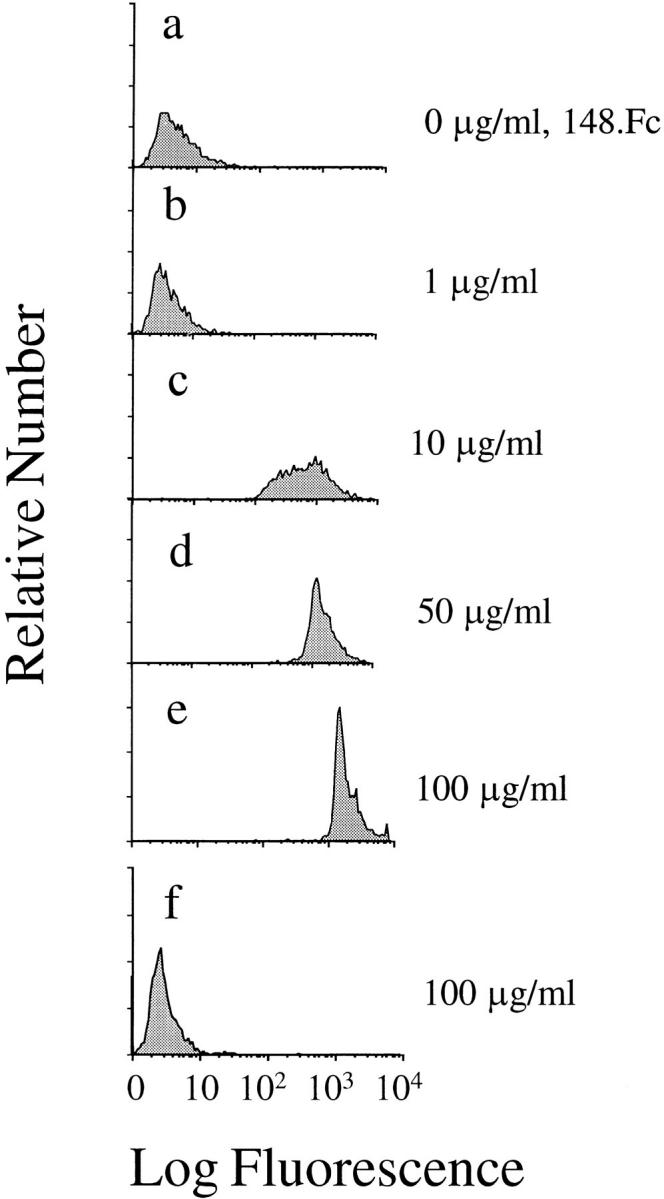
A PSGL-1.Fc chimera molecule can be coupled to protein A microspheres. 10-μm microspheres were precoated with protein A. These microspheres were then incubated with various concentrations of the 148.Fc chimera. The amount of 148.Fc chimera bound to the microspheres was detected with an mAb to PSGL-1 (a–e), PSL-275, and an appropriate FITC-labeled secondary antibody. Isotype-matched control mAb to ICAM-1, Hu5/3, did not recognize the 148.Fc chimera (f ).
148.Fc Microspheres Attach and Roll on TNF-α–stimulated HUVEC
Previous studies have revealed that 4-h TNF-α–stimulated cultured HUVEC express high levels of E-selectin (7) and that E-selectin is involved in neutrophil accumulation on cytokine-activated HUVEC under in vitro flow conditions (1). While PSGL-1 has been implicated in neutrophil accumulation on CHO-E monolayers (38), it is unclear if PSGL-1 is sufficient to mediate attachment and rolling on E-selectin (22, 38). In addition, there is very little data on PSGL-1 interactions with TNF-α–stimulated cultured endothelium. Hence, we sought to determine if PSGL-1 is sufficient to mediate attachment and rolling on TNF-α– stimulated HUVEC.
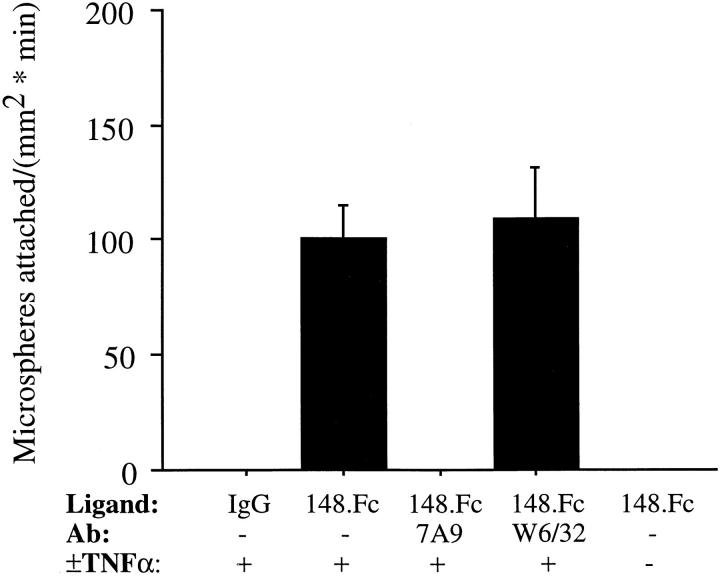
At 2 dynes/cm2, 148.Fc microspheres attached to 4-h TNF-α–activated HUVEC at a rate of 101 ± 14 microspheres/mm2/min (Fig. 2). The attachment events were inhibited by F(ab′)2 fragments of a blocking anti–E-selectin mAb, 7A9, but unaffected by F(ab′)2 fragments of an endothelial cell binding control mAb anti–Class I, W6/32, indicating that the attachment was mediated by E-selectin (Fig. 2). Unstimulated HUVEC, which do not express E-selectin (7), did not support attachment of 148.Fc microspheres (Fig. 2). Microspheres coated with human IgG1 did not attach to TNF-α–stimulated HUVEC (Fig. 2). Subsequent to attachment, >95% of the attached 148.Fc microspheres rolled in a manner similar to that described for leukocytes (Fig. 3), i.e., a low-velocity, high-variance translation (12). Thus, it appears that the first 148 amino acids of PSGL-1 are sufficient to support initial attachment and subsequent rolling on TNF-α–activated endothelium and that the attachment step is mediated by E-selectin.
Figure 2.
148.Fc microspheres attached and rolled on TNF-α–activated HUVEC monolayers under flow. 148.Fc or IgG microspheres were perfused across TNF-α– (4 h, 25 ng/ml) activated or –unactivated HUVEC monolayers. 148.Fc microspheres attached and rolled on TNF-α–activated HUVEC monolayers while IgG microspheres did not attach to the TNF-α–activated HUVEC monolayers. 148.Fc microsphere attachment was blocked by an mAb to E-selectin (7A9) but unaffected by a negative isotypematched control endothelial cell binding mAb to Class I (W6/32). 148.Fc microspheres did not attach to unactivated HUVEC monolayers. (Shear stress = 2 dynes/cm2; n = 3).
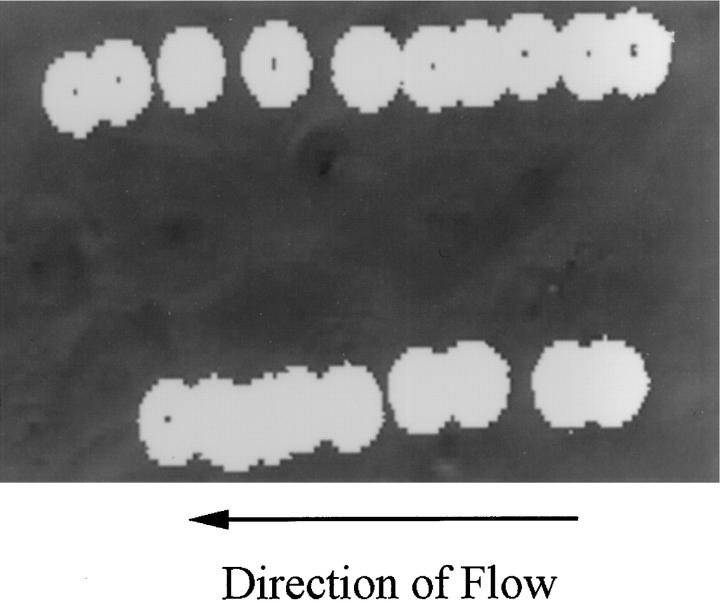
Figure 3.
148.Fc microspheres rolled on TNF-α–stimulated HUVEC. The image shows two 148.Fc microspheres (white spheres) rolling over a TNF-α–activated HUVEC monolayer (gray background). Images were captured, every 0.6 s, from a videotape of the experiment and layered together to give the composite image shown. Note that the 148.Fc microspheres translated in the direction of the flow with a nonconstant velocity. The average velocity of 10 different 148.Fc microspheres was determined and found to be 14 μm/sec, which is <3% of the hydrodynamic velocity of a noninteracting hard sphere translating 50 nm from the surface (13). The length of the image shown is 100 μm. Shear stress = 2 dynes/cm2.
148.Fc Microspheres Attach and Roll on CHO-P and -E Monolayers
While it appears that PSGL-1 is necessary for accumulation of neutrophils on CHO-P monolayers, it is unclear if PSGL-1 is sufficient to mediate attachment and rolling (38). To determine if PSGL-1 is sufficient to mediate attachment and rolling on P-selectin and to further test the hypothesis that PSGL-1 is sufficient to mediate attachment and rolling on E-selectin, we studied the interaction of 148.Fc microspheres with CHO-P and -E monolayers.
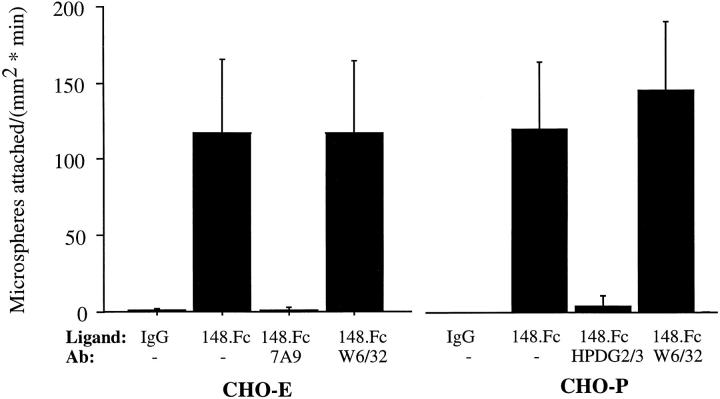
At 2 dynes/cm2, 148.Fc microspheres attached to CHO-E and -P monolayers at a rate of 122 ± 50 microspheres/ mm2/min and 119 ± 43 microspheres/mm2/min, respectively (Fig. 4). Subsequent to attachment, >95% of the attached 148.Fc microspheres rolled on either CHO-E or -P monolayers. Function blocking F(ab′)2 mAbs to E- (7A9) and P-selectin (HPDG2/3) completely inhibited the attachment of 148.Fc microspheres to CHO-E and -P monolayers, respectively (Fig. 4). In contrast, control isotype-matched F(ab′)2 preparation of mAb W6/32 and non–function blocking mAbs to E- (H4/18) and P-selectin (HPDG2/1) (Fig. 4 and data not shown) had no effect on the rate of attachment of the 148.Fc microspheres to CHO-E and -P monolayers, respectively. Under identical conditions, the 148.Fc microspheres did not attach to the parental CHO cell line (n = 2, data not shown), nor did human IgG1 microspheres attach to CHO-P or -E monolayers (Fig. 4). Thus, it appears that the first 148 amino acids of PSGL-1 are sufficient to support attachment and subsequent rolling on CHO cell monolayers expressing E- or P-selectin.
Figure 4.
148.Fc microspheres attached and rolled on CHO-P and -E monolayers. 148.Fc microspheres attached and rolled on CHO-E and -P monolayers while IgG microspheres did not attach to either CHO monolayer. 148.Fc microsphere attachment to CHO-E and -P monolayers was blocked by an mAb to E-selectin (7A9) and an mAb to P-selectin (HPDG2/3), respectively, but was unaffected by an isotype-matched control mAb (W6/32). 148.Fc microspheres did not attach to the parental CHO cell line (data not shown). (Shear stress = 2 dynes/ cm2; n = 3).
Attachment of 148.Fc Microspheres to CHO-E and -P Cell Monolayers Is Eliminated by Pretreatment of 148.Fc Microspheres with OSGE
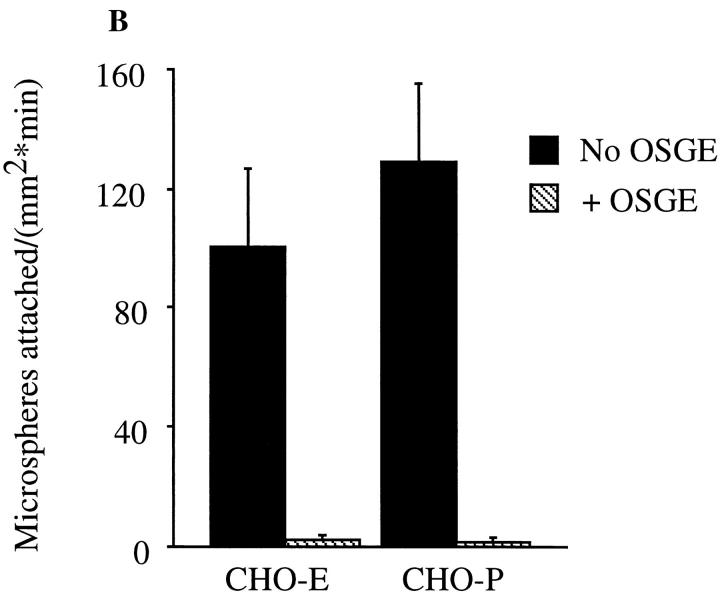
Previous studies have demonstrated that PSGL-1 is cleaved by the metalloprotease OSGE (37). To investigate the specificity of the 148.Fc microsphere attachment events, we treated 148.Fc microspheres with OSGE before use in our flow assay. Treatment of the 148.Fc microspheres with OSGE significantly reduced (>95%) the presence of the epitope recognized by the PSGL-1 mAb PSL-275 (Fig. 5 A). However, the level of human IgG Fc present on the microsphere was unchanged indicating that the PSGL-1 peptide of the 148.Fc chimera was specifically cleaved by OSGE (Fig. 5 A). Treatment of 148.Fc microspheres with OSGE before use in the flow assay eliminated attachment to both CHO-P and -E monolayers (Fig. 5 B). From these data, we conclude that 148.Fc microsphere attachment to CHO-E and -P monolayers is specifically mediated by the PSGL-1 peptide of the 148.Fc chimera.
Figure 5.
OSGE abolishes 148.Fc microsphere attachment to CHO-P or -E monolayers. (A) 148.Fc microspheres were incubated with buffer (a and c) or OSGE (b and d) at 37°C for 30 min. Untreated (a) or OSGE-treated (b) 148.Fc microspheres were incubated with an mAb to PSGL-1 (PSL-275) (open histograms) or an isotype-matched control mAb to ICAM-1 (Hu5/3) (shaded histograms) and subsequently an FITC-labeled secondary antibody. As a control for the specificity of OSGE, the presence of the human Fc region of the 148.Fc chimera was detected on the 148.Fc microspheres. Untreated (c) or OSGE-treated (d) 148.Fc microspheres were incubated with an FITC-labeled polyclonal antibody to human Fc (open histograms) or control, mouse IgG (shaded histograms). Results shown are representative of n = 2–4 separate experiments. (B) Treatment of 148.Fc microspheres with OSGE before use in the in vitro flow assay abolished 148.Fc microsphere attachment to CHO-E and -P monolayers. (Shear stress = 2 dynes/cm2; P < 0.05; n = 3).
PSGL-1 Regions Necessary and/or Sufficient for Attachment and Rolling on CHO-E and -P Monolayers
By using Fc constructs consisting of various extracellular portions of PSGL-1, Sako et al. (44) identified an NH2-terminal anionic polypeptide region within PSGL-1 necessary for recognition of P- but not E-selectin under static conditions. This sequence is rich in acidic amino acids and contains one or more tyrosine sulfate residues (42, 44). To determine whether this region of PSGL-1 is necessary and/or sufficient for attachment and rolling on P- and E-selectin, we used two additional Fc constructs cited in the earlier study (44) (Fig. 6 a). One of these constructs, ΔY.Fc (44), is a mutated form of the 148.Fc chimera with an internal deletion within the anionic polypeptide region, specifically amino acids 5–11. The second construct, 19.ek.Fc, is a modified version of the 19.Fc construct used in the earlier study (44). 19.ek.Fc contains only the first 19 amino acids of PSGL-1, which includes the anionic, sulfated tyrosine region and a critical O-linked glycosylation site at Threonine-16, Thr-16, also present in the 148.Fc. Unique to the 19.ek.Fc construct is a cleavable polypeptide sequence introduced between the PSGL-1 and Fc sequences. This additional sequence contains no potential sites for glycosylation. The ΔY.Fc construct has a similar molecular weight as the 148.Fc (44) and, because of material limitations, was coupled to microspheres at a concentration of 40 μg/ml. For these experiments, 148.Fc microspheres were also prepared at this concentration. Since the molecular weight of the 19.ek.Fc is a little over 1/3 that of the 148.Fc, we prepared the 19.ek.Fc microspheres with 15 μg/ml of the 19.ek.Fc. As shown with a polyclonal antibody to PSGL-1, Rb3026, both mutants were coupled to the microspheres (Table I).
Table I.
Flow Cytometric Analyses of PSGL-1.Fc Constructs on Microspheres
| Mean channel fluorescence | ||||||||
|---|---|---|---|---|---|---|---|---|
| mAb | IgG1 | 148.Fc | ΔY.Fc | 19.ek.Fc | ||||
| NRS* | 243 | 60 | 83 | 167 | ||||
| Rb3026 | 137 | 554 | 520 | 335 | ||||
| Hu5/3* | 16 | 12 | 27 | 42 | ||||
| KPL1 | 12 | 1,595 | 11 | 461 | ||||
| KPL2 | 14 | 7 | 12 | 22 | ||||
Microspheres were coated with indicated PSGL-1 molecules or human IgG and detected with various mAbs as detailed in Methods. Results typical of 2–4 separate experiments.
Negative controls. NRS, normal rabbit serum.
19.ek.Fc microspheres attached to CHO-E and -P monolayers at a rate similar to that observed for 148.Fc microspheres (Fig. 6 b). Subsequent to attachment, >95% of the 19.ek.Fc microspheres were observed to roll on both the CHO-E and -P monolayers. ΔY.Fc microspheres attached to CHO-E monolayers at a rate similar to that observed for 148.Fc microspheres (Fig. 6 b). Subsequent to attachment, >95% of the ΔY.Fc microspheres were observed to roll. In contrast, ΔY.Fc microspheres did not attach to CHO-P monolayers (Fig. 6 b). Neither the 19.ek.Fc nor the ΔY.Fc microspheres attached to the parental CHO cell line (n = 2; data not shown). We next attempted to block 148.Fc and 19.ek.Fc microsphere attachment to CHO-E and -P monolayers with an mAb, KPL1, specific for PSGL-1. KPL1 blocks neutrophil and memory T cell adhesion to CHO-P monolayers (Snapp, K.R., F.W. Luscinskas, R. Warnke, and G.S. Kansas. 1997. J. Allergy Clin. Immunol. 99:s459). As shown in the present study, KPL1 recognition of the 148.Fc microspheres appears to be dependent on the presence of amino acid sequence 5–11 of PSGL-1 (Table I; 148.Fc versus ΔY.Fc). KPL2, a nonblocking isotypematched mAb specific to full-length PSGL-1 (Snapp, K.R., F.W. Luscinskas, R. Warnke, and G.S. Kansas. 1997. J. Allergy Clin. Immunol. 99:s459) was used as a control. KPL2 appears to map to a PSGL-1 sequence COOH-terminal to amino acid 148 as it does not react with any of the Fc constructs by flow cytometric analysis (Table I). For the blocking studies, we used ascites preparations of both KPL1 and KPL2. KPL1 did not block 148.Fc microsphere attachment to CHO-E monolayers but completely blocked attachment to CHO-P monolayers (Fig. 6 c). 148.Fc microsphere attachment to CHO-E monolayers was not inhibited by treatment with a higher concentration of an ascites preparation of KPL1 (1:100 vs. 1:200 dilution) or 80 μg/ml purified KPL1 relative to 148.Fc microspheres treated with control antibodies (data not shown). In contrast, KPL1 blocked 19.ek.Fc microsphere attachment to both CHO-E and -P monolayers (Fig. 6 d). Similar inhibitory effects were obtained with a purified IgG preparation of KPL1. Taken together, these results show that: (a) The first 19 amino acids of PSGL-1 are sufficient to support attachment and rolling on both CHO-P and -E monolayers; (b) the anionic polypeptide segment within the amino terminus of PSGL-1 is necessary for the 148.Fc construct to mediate attachment to CHO-P, but not to CHO-E monolayers; and (c) the 148.Fc construct contains at least two binding sites for E-selectin, one located within the first 19 amino acids and a second (or more) site COOH-terminal to amino acid 19.
An SLex-containing Glycan Is Involved in 19.ek.Fc Microsphere Attachment and Rolling on CHO-P and -E Monolayers
Previous studies have shown that treatment of neutrophils (1), or HL-60 cells (2), with 0.1 U/ml neuraminidase for 30 min inhibits adhesion to P- and E-selectin under flow, suggesting that sialylated structures, presumably SLex and/or related glycans, are involved. However, since both neutrophils and HL-60 cells express multiple surface glycoproteins and glycolipids that may contain SLex-modified glycans, it is difficult to ascribe the role of PSGL-1 glycosylation in attachment and rolling on P- and E-selectin. We attempted to address this issue in studies using 19.ek.Fc-coated microspheres in light of the data presented in the previous section demonstrating that this highly defined construct is sufficient for attachment and rolling on E- and P-selectin. The PSGL-1 peptide region of the 19.ek.Fc construct contains two potential O-linked glycosylation sites, Thr-3 and Thr-16, but no N-linked glycosylation sites. Mass and NMR spectroscopy of the enterokinase-liberated PSGL-1 peptide from 19.ek.Fc indicated that of the two threonines, only Thr-16 is glycosylated, and this glycan contains SLex (data not shown). Preliminary studies with microspheres coated with 15 μg/ml of the 19.ek.Fc construct indicated that a sialylated glycan, presumably SLex, is critically involved in the attachment of 19.ek.Fc microspheres to CHO-E and -P monolayers since pretreatment of 19.ek.Fc microspheres with 0.1 U/ml neuraminidase at 37°C for 30 min diminished the rate of attachment to CHO-E monolayers by >95% and to CHO-P monolayers by >85% (data not shown).
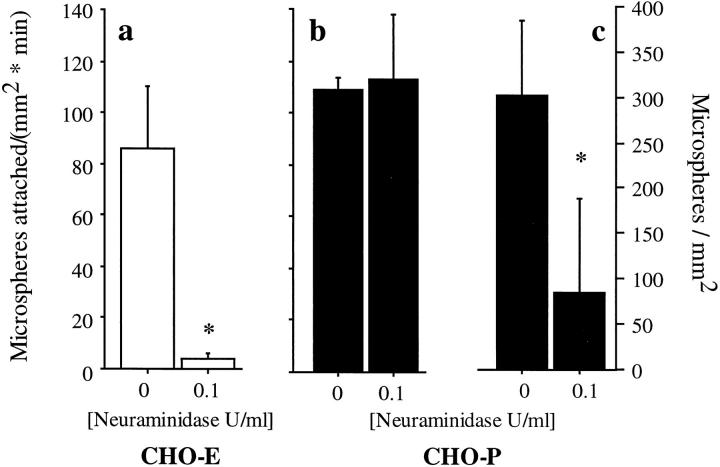
To further investigate the role of glycosylation, we prepared 19.ek.Fc microspheres with 50 μg/ml 19.ek.Fc rather than 15 μg/ml and used the same neuraminidase treatment protocol. Treatment of 50 μg/ml 19.ek.Fc microspheres with neuraminidase significantly diminished, if not eliminated, attachment of 19.ek.Fc microspheres to CHO-E monolayers (Fig. 7 a). In contrast, 19.ek.Fc microspheres treated with neuraminidase attached to CHO-P monolayers at a rate similar to untreated 19.ek.Fc microspheres (Fig. 7 b). This result suggested that neuraminidase digestion of the 50 μg/ml 19.ek.Fc microspheres was incomplete, but this treatment was sufficient to abolish attachment to CHO-E but not CHO-P monolayers. Hence, a higher level of sialylation, presumably the SLex at Thr-16, is required for the 19.ek.Fc microspheres to attach to CHO-E relative to CHO-P monolayers.
Figure 7.
Effect of neuraminidase treatment of 19.ek.Fc microsphere adhesive interactions with CHO-E and -P monolayers. Microspheres were coated with 50 μg/ml 19.ek.Fc, treated with neuraminidase, and perfused over CHO-E (open bars) or CHO-P (filled bars) monolayers. (a) The rate of attachment to CHO-E monolayers was significantly diminished, if not eliminated, by treatment with neuraminidase. (b) In contrast, the rate of attachment to CHO-P monolayers was unaffected by neuraminidase treatment. (c) Accumulation of 19.ek.Fc microspheres on CHO-P monolayers was significantly diminished by treatment with neuraminidase. (Shear stress = 2 dynes/cm2; *P < 0.05; n = 3).
Comparison of the interactions of neuraminidase-treated and -untreated 19.ek.Fc microspheres with CHO-P monolayers also revealed that neuraminidase treatment led to a marked difference in the behavior of the 19.ek.Fc microspheres subsequent to attachment. Typically, neuraminidase-treated 19.ek.Fc microspheres were observed to attach to the CHO-P monolayers, roll and skip for several microsphere diameters, and then detach. The net effect of the increased rate of detachment was a decrease in the accumulation relative to untreated 19.ek.Fc microspheres (Fig. 7 c). The rolling velocities on CHO-P monolayers increased fivefold from 2 μm/sec for untreated 19.ek.Fc microspheres to 10 μm/sec for neuraminidase-treated 19.ek.Fc microspheres (P < 0.05; n = 10 for each condition).
Discussion
The leukocyte–endothelial adhesion cascade is mediated by an overlapping sequence of multiple receptor interactions. Previous studies have used subtractive approaches, such as antibody blocking, enzyme treatment, or activation-induced shedding, to determine if a given receptor is involved in a particular adhesion process. While these approaches can determine if a receptor is necessary for an adhesion event, such approaches are less likely to determine if a receptor is sufficient. Because of the requirement of the presence of fucosyltransferases for generation of functional PSGL-1 (25, 43), the additive transfection approach has the caveat of potential alteration of other cell surface molecules to the extent that they may be able to interact with E- (27, 52) or perhaps P-selectin. Hence, to determine if PSGL-1 is sufficient and to investigate which regions of PSGL-1 are necessary and/or sufficient for attachment and rolling on E- and P-selectin, we developed an in vitro assay that allowed us to study, under flow, the interaction of various regions of extracellular PSGL-1, in isolation, with cellular monolayers expressing E- or P-selectin.
The results demonstrate that PSGL-1 is sufficient to mediate attachment and rolling on both P- and E-selectin, thereby extending previous studies describing PSGL-1 involvement in neutrophil and HL-60 cell adhesion to E- and P-selectin under flow (35, 38) and PSGL-1 recognition of E- and P-selectin in biochemical assays (5, 42, 44). Microspheres coated with a truncated version of extracellular PSGL-1 fused to the Fc region of human IgG1, 148.Fc, attached and rolled on TNF-α–activated HUVEC, which have been shown to express high levels of E-selectin (7), and on CHO-E and -P monolayers. The specificity of this interaction was demonstrated by the following experimental results: (a) The interactions were abolished by function-blocking mAbs to E- and P-selectin but not by control mAbs; (b) microspheres coated with human IgG1 did not attach or roll on these substrates; (c) 148.Fc microspheres did not interact with unstimulated HUVEC or the parental CHO cell monolayers; and (d) 148.Fc microspheres pretreated with OSGE, an enzyme known to cleave PSGL-1 (37), did not attach to CHO-E or -P monolayers.
The results demonstrate that as few as the first 19 amino acids of PSGL-1 are sufficient for attachment and rolling on both P- and E-selectin. The results also show that the NH2-terminal anionic polypeptide segment of PSGL-1 is necessary for PSGL-1–mediated attachment to P- but not E-selectin. This was demonstrated by two sets of experiments. First, microspheres coated with the ΔY.Fc mutant, a mutated form of the 148.Fc chimera with an internal deletion within the anionic polypeptide sequence, specifically amino acids 5–11 (44), did not attach to CHO-P monolayers but attached and rolled on CHO-E monolayers. Secondly, a neutralizing mAb to PSGL-1, KPL1, which requires the same amino acid sequence 5–11 of PSGL-1 for recognition (Table I), eliminated the attachment of 148.Fc microspheres to CHO-P but not to CHO-E monolayers. Previously it was reported that at least one sulfated tyrosine within the NH2-terminal anionic polypeptide segment of PSGL-1 is critical for P-selectin binding under static conditions (42, 44) and that treatment of HL-60 cells with an inhibitor of sulfation attenuates accumulation of HL-60 cells on immobilized P-selectin (42). These earlier observations, combined with the present findings, suggest that at least one sulfated tyrosine within the NH2-terminal anionic polypeptide segment is necessary for PSGL-1–mediated attachment to P-selectin under flow.
The experimental results indicate that SLex plays a critical role in PSGL-1 amino terminus–mediated attachment to P- and E-selectin. Neuraminidase digestion of 19.ek.Fc microspheres, under a relatively low substrate to enzyme ratio, essentially abolished attachment of 19.ek.Fc microspheres to both CHO-P and -E monolayers. Mass and NMR spectroscopy of the enterokinase-liberated PSGL-1 peptide from 19.ek.Fc indicated that of the two potential sites of glycosylation, only Thr-16 is glycosylated, and this glycan contains SLex (data not shown). These observations combined with previous studies describing SLex as a ligand for E- and P-selectin (15, 41, 53) strongly suggest that an SLex-type moiety is necessary for the first 19 amino acids of PSGL-1 to mediate attachment to P- and E-selectin and that this moiety can be provided by the SLex-containing glycan at Thr-16. When the neuraminidase digestion experiment was repeated but with a higher substrate to enzyme ratio, attachment of 19.ek.Fc microspheres to CHO-E monolayers alone was inhibited. This result suggests that incomplete desialylation resulted in 19.ek.Fc microspheres containing residual SLex. This level of SLex, while sufficient to allow attachment of 19.ek.Fc microspheres to the CHO-P monolayer, did not appear to allow attachment to the CHO-E monolayer. Thus, PSGL-1 amino terminus– mediated attachment to the CHO-E monolayer is more sensitive to the quantity of intact SLex presented by Thr16 relative to the sensitivity of attachment to the CHO-P monolayer. This difference in sensitivity may indicate that the NH2-terminal anionic polypeptide region of PSGL-1 plays a significant role in attachment to P-selectin but plays a lesser, if any, role in attachment to E-selectin. These observations are consistent with previous studies demonstrating that: (a) P-selectin has a 50-fold higher affinity for PSGL-1 than does E-selectin (34); (b) prolonged digestion of PSGL-1 with neuraminidase is required to fully eliminate recognition of P-selectin (33); and (c) one or more of the amino-terminal tyrosines within the anionic polypeptide region plays an important role in PSGL-1 amino terminus recognition of P-selectin, but plays a lesser, if any, role in recognition of E-selectin under static conditions (44).
Subsequent to attachment, SLex appears to be critically involved in PSGL-1 amino terminus–mediated stable rolling (relatively low rolling velocities and low rates of detachment) on the P-selectin monolayer. This assertion is suggested by the neuraminidase experiment described above. Under neuraminidase digestion conditions where it appeared that residual SLex still allowed normal attachment of 19.ek.Fc microspheres to the CHO-P monolayer, a significant increase in rolling velocity and rate of detachment relative to undigested 19.ek.Fc microspheres was observed. Since 19.ek.Fc microspheres attach and stably roll on CHO-P monolayers, it appears that the SLex-containing glycan at Thr-16 can provide the SLex-type moiety necessary for stable rolling on P-selectin. In longer forms of PSGL-1 that contain additional sites of glycosylation, a glycan other than that present on Thr-16 could potentially contribute the SLex-type moiety required to achieve stable rolling on P-selectin.
Under flow conditions, PSGL-1 appears to have more than one binding site for E-selectin. As noted above, microspheres coated with 19.ek.Fc, a PSGL-1 construct containing a single site of glycosylation at Thr-16, attach and roll on CHO-E monolayers. When treated with KPL1, an mAb specific to PSGL-1, 19.ek.Fc microspheres no longer attach to CHO-E monolayers. Interestingly, while KPL1 was shown here to require a PSGL-1 epitope located within amino acids 5–11 for recognition, it also appears to disrupt E-selectin recognition of the SLex glycan found at Thr-16, perhaps via steric restrictions. However, KPL1 treatment of microspheres coated with 148.Fc, a construct that also contains at its NH2 terminus the 19–amino acid PSGL-1 sequence found in 19.ek.Fc, did not inhibit attachment to CHO-E monolayers suggesting that sites COOHterminal to amino acid 19 can support attachment. The Fc portions of the PSGL-1.Fc chimeras do not appear to mediate attachment to CHO-E monolayers. This is shown by the observation that 148.Fc microspheres digested with OSGE did not attach to CHO-E (or CHO-P) monolayers (Fig. 5 B) and by the fact that microspheres prepared with 50 μg/ml of human Fc derived from enterokinase-digested 19.ek.Fc did not interact with CHO-E (or CHO-P) monolayers at all shear stresses tested ranging from 0.5 to 2.0 dynes/cm2 (data not shown). Thus, as suggested by Patel et al. (38), PSGL-1 appears to have more than one binding site for E-selectin. The data presented in this study indicate that PSGL-1 contains a binding site for E-selectin located within the first 19 amino acids, presumably the SLex-containing glycan at Thr-16, and one (or more) binding sites located between amino acids 19 through 148, perhaps another SLex-containing glycan. Both of these binding sites appear to mediate attachment and rolling on CHO-E monolayers. These assertions seem plausible in light of the recent findings that PSGL-1 is a highly extended molecule (26) and PSGL-1 has several sites of potential glycosylation between amino acids 19 through 148 (44) (Fig. 6 a), many of which may have glycans that recognize E-selectin (54).
The experimental results presented in this study, as well as a recent report by Brunk et al. (10), give insight into selectin-mediated attachment and rolling. von Andrian et al. (51) recently reported that localization of adhesion molecules on the tips of the microvilli, as opposed to the cell body, enhances the ability of the adhesion molecules to mediate cell attachment under flow. Thus, on neutrophils, localization of PSGL-1 to the microvilli (35) gives PSGL-1 the opportunity to form a bond with E- or P-selectin. Given that PSGL-1 “sees” E- or P-selectin, the following issue arises: Will bonds form between the receptors, and will the formed bonds mediate attachment and rolling? Previously, Alon et al. (3) measured the off rate for the P-selectin–neutrophil counter-receptor bond and attributed the ability of this bond to mediate rolling to its unique kinetic and tensile properties. Mathematical models of neutrophil rolling have also indicated that unique tensile features of the selectin bonds confer the ability of these bonds to support rolling (14, 47). It is unclear if these unique properties are governed primarily by the molecular structure of the receptors or by cellular attributes and functions. We have shown that PSGL-1 immobilized on inert, nondeformable microspheres attach and roll on E- and P-selectin–presenting monolayers. Thus, given that PSGL-1 “sees” E- or P-selectin, the molecular structure of the receptors may be the key factor in determining whether bonds will form between the receptors and whether the formed bonds will give rise to rolling.
Finally, the issue of whether PSGL-1 is sufficient to support adhesive interactions with P- and E-selectin has practical ramifications for potential drug delivery strategies. With the identification and characterization of E-selectin (7, 8), P-selectin (32), PSGL-1 (33, 43), and their synergistic behavior (35, 38), the potential exists for targeting therapeutic agents to sites of inflammation via adhesion molecules selectively expressed on activated endothelium, e.g., E-selectin, and an artificial carrier, presenting PSGL-1. The experimental results presented in this study demonstrate that PSGL-1 is sufficient to mediate selective attachment to activated endothelium under fluid flow, suggesting that such delivery strategies are feasible.
Acknowledgments
The authors wish to thank Dr. Michael Gimbrone, Jr. (Brigham and Women's Hospital, Boston, MA) for helpful discussions; William Atkinson and Kay Case (Brigham and Women's Hospital) for providing cultured HUVEC; Drs. Francis Sullivan, Gray Shaw, and Dianne Sako (Genetics Institute, Cambridge, MA) for cDNAs encoding Fuc-TVII, 148.Fc, and ΔY.Fc; Drs. Debra Brunk (Cornell University, Ithaca, NY) and Daniel Hammer (University of Pennsylvania, Philadelphia, PA) for helpful discussions; and Peter Lopez (Dana Farber Cancer Institute Flow Cytometry Facility, Boston, MA) and Claudia Cabral (Beth Israel Hospital Flow Cytometry Laboratory, Boston, MA) for technical assistance performing flow cytometric analyses.
Footnotes
1. Abbreviations used in this paper: CHO-E, and -P, CHO cell monolayers stably expressing E- or P-selectin; DPBS, Dulbecco's phosphate buffered saline; Fuc-TVII, α(1,3)fucosyltransferase-VII; HUVEC, human umbilical vein endothelial cell(s); OSGE, O-sialoglycoprotein endopeptidase; PSGL-1, P-selectin glycoprotein ligand-1; RT, room temperature; SLex, sialyl Lewis x; TNF-α, tumor necrosis factor-α.
This research was supported by The National Cancer Institute grant F32CA71129, National Institutes of Health (NIH) training grant T32HL07627 (D.J. Goetz), and NIH grants HL36028 and HL53993 (F.W. Luscinskas) and by a grant from the American Cancer Society (G.S. Kansas). G.S. Kansas is an Established Investigator at the American Heart Association. D.M. Greif was supported by the Stanley J. Sarnoff Endowment for Cardiovascular Science, Inc.
Douglas J. Goetz's present address is The University of Memphis, Department of Biomedical Engineering, Engineering Technology Building Room 330, Memphis, TN 38152. Tel.: (901) 678-8675. Fax: (901) 678-5281. E-mail: dgoetz@cc.memphis.edu
Douglas J. Goetz and Daniel M. Greif contributed equally to this work.
References
- 1.Abbassi O, Kishimoto TK, McIntire LV, Anderson DC, Smith CW. E-selectin supports neutrophil rolling in vitro under conditions of flow. J Clin Invest. 1993;92:2719–2730. doi: 10.1172/JCI116889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alon R, Rossitier H, Wang X, Springer TA, Kupper TS. Distinct cell surface ligands mediate T lymphocyte attachment and rolling on P- and E-selectin under physiological flow. J Cell Biol. 1994;127:1485–1495. doi: 10.1083/jcb.127.5.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alon R, Hammer DA, Springer TA. Lifetime of the P-selectin-carbohydrate bond and its response to tensile force in hydrodynamic flow. Nature (Lond) 1995;374:539–542. doi: 10.1038/374539a0. [DOI] [PubMed] [Google Scholar]
- 4.Arbones ML, Ord DC, Ley K, Radech H, Maynard-Curry C, Otten G, Capon DJ, Tedder TF. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin (CD62L) deficient mice. Immunity. 1994;1:247–260. doi: 10.1016/1074-7613(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 5.Asa D, Raycroft L, Ma L, Aeed PA, Kaytes PS, Elhammer AP, Geng J. The P-selectin glycoprotein ligand functions as a common human leukocyte ligand for P- and E-selectins. J Biol Chem. 1995;270:11662–11670. doi: 10.1074/jbc.270.19.11662. [DOI] [PubMed] [Google Scholar]
- 6.Bevilacqua MP. Endothelial-leukocyte adhesion molecules. Annu Rev Immunol. 1993;11:767–804. doi: 10.1146/annurev.iy.11.040193.004003. [DOI] [PubMed] [Google Scholar]
- 7.Bevilacqua MP, Pober JS, Mendrick DL, Cotran RS, Gimbrone MA., Jr Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci USA. 1987;84:9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bevilacqua MP, Stengelin S, Gimbrone MA, Jr, Seed B. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science (Wash DC) 1989;243:1160–1164. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- 9.Bierhuizen MFA, Fukuda M. Expression cloning of a cDNA encoding UDP-GlcNAc:Galβ1-3-GalNAc-R (GlcNAc to GalNAc) β16GlcNAc transferase by gene transfer into CHO cells expressing polyoma large tumor antigen. Proc Natl Acad Sci USA. 1992;89:9326–9330. doi: 10.1073/pnas.89.19.9326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunk DK, Goetz DJ, Hammer DA. Sialyl Lewisx/E-selectin-mediated rolling in a cell-free system. Biophys J. 1996;71:2902–2908. doi: 10.1016/S0006-3495(96)79487-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 12.Goetz DJ, El-Sabban ME, Pauli BU, Hammer DA. The dynamics of neutrophil rolling over stimulated endothelium in vitro. Biophys J. 1994;66:2202–2209. doi: 10.1016/S0006-3495(94)81016-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldman AJ, Cox RG, Brenner H. Slow viscous motion of a sphere parallel to a plane wall. II. Couette flow. Chem Eng Sci. 1967;22:635–660. [Google Scholar]
- 14.Hammer DA, Apte SM. Simulation of cell rolling and adhesion on surfaces in shear flow: general results and analysis of selectinmediated neutrophil adhesion. Biophys J. 1992;62:35–57. doi: 10.1016/S0006-3495(92)81577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Handa K, Neudelman E, Stroud M, Shiozawa T, Hakomori S. Selectin GMP-140 (CD62; PADGEM) binds to sialosyl-Lex and sialosylLea, and sulfated glycans modulate the binding. Biochem Biophys Res Commun. 1991;181:1223–1230. doi: 10.1016/0006-291x(91)92069-v. [DOI] [PubMed] [Google Scholar]
- 16.Jutila MA, Rott L, Berg EL, Butcher EC. Function and regulation of the neutrophil MEL-14 antigen in vivo: comparison with LFA-1 and Mac-1. J Immunol. 1989;143:3318–3324. [PubMed] [Google Scholar]
- 17.Kumar R, Camphausen RT, Sullivan FX, Cumming DA. Core2 β-1,6-N-Acetylglucosaminyltransferase enzyme activity is critical for P-selectin glycoprotein ligand-1 binding to P-selectin. Blood. 1996;88:3872–3879. [PubMed] [Google Scholar]
- 18.Kuo SC, Lauffenburger DA. Relationship between receptor/ ligand binding affinity and adhesion strength. Biophys J. 1993;65:2191–2200. doi: 10.1016/S0006-3495(93)81277-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LaVallie ER, Rehemtulla A, Racie LA, DiBlasio EA, Ferenz C, Grant KL, Light A, McCoy JM. Cloning and functional expression of a cDNA encoding the catalytic subunit of bovine enterokinase. J Biol Chem. 1993;268:23311–23317. [PubMed] [Google Scholar]
- 20.Lawrence MB, Springer TA. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991;65:859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- 21.Lawrence MB, Springer TA. Neutrophils roll on E-selectin. J Immunol. 1993;151:6338–6346. [PubMed] [Google Scholar]
- 22.Lawrence MB, Bainton DF, Springer TA. Neutrophil tethering to and rolling on E-selectin are separable by requirement for L-selectin. Immunity. 1994;1:137–145. doi: 10.1016/1074-7613(94)90107-4. [DOI] [PubMed] [Google Scholar]
- 23.Lewinsohn DM, Bargatze RF, Butcher EC. Leukocyteendothelial cell recognition: evidence of a common molecular mechanism shared by neutrophils, lymphocytes, and other leukocytes. J Immunol. 1986;138:4313–4321. [PubMed] [Google Scholar]
- 24.Ley K, Gaehtgens P, Fennie C, Singer MS, Lasky LA, Rosen SD. Lectin-like cell adhesion molecule 1 mediates leukocyte rolling in mesenteric venules in vivo. Blood. 1991;77:2553–2555. [PubMed] [Google Scholar]
- 25.Li F, Wilkins PP, Crawley S, Weinstein J, Cummings RD, McEver RP. Post-translational modifications of recombinant P-selectin glycoprotein ligand-1 required for binding to P- and E-selectin. J Biol Chem. 1996;271:3255–3264. [PubMed] [Google Scholar]
- 26.Li F, Erickson HP, James JA, Moore KL, Cummings RD, McEver RP. Visualization of P-selectin glycoprotein ligand-1 as a highly extended molecule and mapping of protein epitopes for monoclonal antibodies. J Biol Chem. 1996;271:6342–6348. doi: 10.1074/jbc.271.11.6342. [DOI] [PubMed] [Google Scholar]
- 27.Lowe JB, Stoolman LM, Nair RP, Larsen RD, Berhend TL, Marks RM. ELAM-1-dependent cell adhesion to vascular endothelium determined by a transfected human fucosyltransferase cDNA. J Biol Chem. 1990;63:475–484. doi: 10.1016/0092-8674(90)90444-j. [DOI] [PubMed] [Google Scholar]
- 28.Luscinskas FW, Kansas GS, Ding H, Pizcueta P, Schleiffenbaum BE, Tedder TF, Gimbrone MA., Jr Monocyte rolling, arrest, and spreading on IL-4-activated vascular endothelium under flow is mediated via sequential action of L-selectin, β1-integrins, and β2-integrins. J Cell Biol. 1994;125:1417–1427. doi: 10.1083/jcb.125.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luscinskas FW, Ding H, Lichtman AH. P-selectin and VCAM-1 mediate rolling and arrest of CD4+ T-lymphocytes on TNF-αactivated vascular endothelium under flow. J Exp Med. 1995;181:1179–1186. doi: 10.1084/jem.181.3.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luscinskas FW, Ding H, Tan P, Cumming D, Tedder TF, Gerritsen ME. L- and P-selectins, but not CD49d (VLA-4) integrins, mediate monocyte initial attachment to TNF-α-activated vascular endothelium under flow in vitro. J Immunol. 1996;156:326–335. [PubMed] [Google Scholar]
- 31.Mayadas TN, Johnson RC, Rayburn H, Hynes RO, Wagner DD. Leukocyte rolling and extravasation are severely compromised in P-selectin deficient mice. Cell. 1993;74:541–554. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- 32.McEver RP, Beckstead JH, Moore KL, Marshall-Carlson L, Bainton DF. GMP-140, a platelet α-granule membrane protein, is also synthesized by vascular endothelial cells and is localized in WeibelPalade bodies. J Clin Invest. 1989;84:92–99. doi: 10.1172/JCI114175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore KL, Stults NL, Diaz S, Smith DF, Cummings RD, Varki A, McEver RP. Identification of a specific glycoprotein ligand for P-selectin (CD62) on myeloid cells. J Cell Biol. 1992;118:445–456. doi: 10.1083/jcb.118.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore KL, Eaton SF, Lyons DE, Lichenstein HS, Cummings RD, McEver RP. The P-selectin glycoprotein ligand from human neutrophils displays sialylated, fucosylated, O-linked poly-N-acetyllactosamine. J Biol Chem. 1994;269:23318–23327. [PubMed] [Google Scholar]
- 35.Moore KL, Patel KD, Bruehl RE, Fugang L, Johnson DA, Lichenstein HS, Cummings RD, Bainton DF, McEver RP. P-selectin glycoprotein ligand-1 mediates rolling of human neutrophils on P-selectin. J Cell Biol. 1995;128:661–671. doi: 10.1083/jcb.128.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Natsuka S, Gersten KM, Zentia K, Kannagi R, Lowe JB. Molecular cloning of a cDNA encoding a novel human leukocyte α-1,3fucosyltransferase capable of synthesizing the sialyl Lewis X determinant. J Biol Chem. 1994;269:16789–16794. [PubMed] [Google Scholar]
- 37.Norgard KE, Moore KL, Diaz S, Stults NL, Ushiyama S, McEver RP, Cummings RD, Varki A. Characterization of a specific ligand for P-selectin on myeloid cells. J Biol Chem. 1993;268:12764–12774. [PubMed] [Google Scholar]
- 38.Patel KD, Moore KL, Nollert MU, McEver RP. Neutrophils use both shared and distinct mechanisms to adhere to selectins under static and flow conditions. J Clin Invest. 1995;96:1887–1896. doi: 10.1172/JCI118234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Picker LJ, Warnock RA, Burns AR, Doerschuk CM, Berg EL, Butcher EC. The neutrophil selectin LECAM-1 presents carbohydrate ligands to the vascular selectin ELAM-1 and GMP-140. Cell. 1991;66:921–933. doi: 10.1016/0092-8674(91)90438-5. [DOI] [PubMed] [Google Scholar]
- 40.Pierres A, Tissot O, Malissen B, Bongrand P. Dynamic adhesion of CD8-positive cells to antibody-coated surfaces: the initial step is independent of microfilaments and intracellular domains of cell binding molecules. J Cell Biol. 1994;125:945–953. doi: 10.1083/jcb.125.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Polley MJ, Phillips ML, Wayner E, Nudelman E, Singhal AK, Hakomori S, Paulson JC. CD62 and endothelial cell-leukocyte adhesion molecule 1 (ELAM-1) recognize the same carbohydrate ligand, sialyl-Lewis x. Proc Natl Acad Sci USA. 1991;88:6224–6228. doi: 10.1073/pnas.88.14.6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pouyani T, Seed B. PSGL-1 recognition of P-selectin is controlled by a tyrosine sulfation consensus at the PSGL-1 amino terminus. Cell. 1995;83:333–343. doi: 10.1016/0092-8674(95)90174-4. [DOI] [PubMed] [Google Scholar]
- 43.Sako D, Chang XJ, Barone KM, Vachino G, White HM, Shaw G, Veldman GM, Bean KM, Ahern TJ, Furie B, et al. Expression cloning of a functional glycoprotein ligand for P-selectin. Cell. 1993;75:1179–1186. doi: 10.1016/0092-8674(93)90327-m. [DOI] [PubMed] [Google Scholar]
- 44.Sako D, Comess KM, Barone KM, Camphausen RT, Cumming DA, Shaw GD. A sulfated peptide segment at the amino terminus of PSGL-1 is critical for P-selectin binding. Cell. 1995;83:323–331. doi: 10.1016/0092-8674(95)90173-6. [DOI] [PubMed] [Google Scholar]
- 45.Sasaki K, Kurata K, Funayama K, Nagata M, Watanabe M, Ohta S, Hanai N, Nishi T. Expression cloning of a novel α1,3-fucosyltransferase that is involved in biosynthesis of the sialyl Lewis X carbohydrate determinants in leukocytes. J Biol Chem. 1994;269:14730–14737. [PubMed] [Google Scholar]
- 46.Spertinni O, Luscinskas FW, Kansas GS, Griffin JD, Gimbrone MA, Jr, Tedder TF. Leukocyte adhesion molecule (LAM-1, L-selectin) interacts with an inducible endothelial cell ligand to support leukocyte adhesion. J Immunol. 1991;147:2565–2573. [PubMed] [Google Scholar]
- 47.Tozeren A, Ley K. How do selectins mediate leukocyte rolling in venules? . Biophys J. 1992;63:700–709. doi: 10.1016/S0006-3495(92)81660-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vachino G, Chang X-J, Veldman GM, Kumar R, Fouser LA, Berndt MC, Cumming DA. P-selectin glycoprotein ligand-1 is the major counter-receptor for P-selectin on stimulated T cells and is widely distributed in non-functional form on many lymphocytic cells. J Biol Chem. 1995;270:21966–21974. doi: 10.1074/jbc.270.37.21966. [DOI] [PubMed] [Google Scholar]
- 49.Varki A. Selectin ligands. Proc Natl Acad Sci USA. 1994;91:7390–7397. doi: 10.1073/pnas.91.16.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.von Andrian UH, Chambers JD, McEvoy LM, Bargatze RF, Arfors KE, Butcher EC. Two-step model of leukocyte-endothelial cell interactions in inflammation: distinct roles for LECAM-1 and the leukocyte β2integrins in vivo. Proc Natl Acad Sci USA. 1991;88:7538–7542. doi: 10.1073/pnas.88.17.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.von Andrian UH, Hasslen SR, Nelson RD, Erlandsen SL, Butcher EC. A central role for microvillous receptor presentation in leukocyte adhesion under flow. Cell. 1995;82:989–999. doi: 10.1016/0092-8674(95)90278-3. [DOI] [PubMed] [Google Scholar]
- 52.Wagers AJ, Lowe JB, Kansas GS. An important role for the α1,3 fucosyltransferase, FucT-VII, in leukocyte adhesion to E-selectin. Blood. 1996;88:2125–2132. [PubMed] [Google Scholar]
- 53.Walz G, Aruffo A, Kolanus W, Bevilacqua MP, Seed B. Recognition by ELAM-1 of the sialyl-Lex determinant on myeloid and tumor cells. Science (Wash DC) 1990;250:1132–1135. doi: 10.1126/science.1701275. [DOI] [PubMed] [Google Scholar]
- 54.Wilkins PP, McEver RP, Cummings RD. Structures of the O-glycans on P-selectin glycoprotein ligand-1 from HL-60 cells. J Biol Chem. 1996;271:18732–18742. doi: 10.1074/jbc.271.31.18732. [DOI] [PubMed] [Google Scholar]