Abstract
The spindle assembly checkpoint prevents cells whose spindles are defective or chromosomes are misaligned from initiating anaphase and leaving mitosis. Studies of Xenopus egg extracts have implicated the Erk2 mitogen-activated protein kinase (MAP kinase) in this checkpoint. Other studies have suggested that MAP kinases might be important for normal mitotic progression. Here we have investigated whether MAP kinase function is required for mitotic progression or the spindle assembly checkpoint in vivo in Xenopus tadpole cells (XTC). We determined that Erk1 and/or Erk2 are present in the mitotic spindle during prometaphase and metaphase, consistent with the idea that MAP kinase might regulate or monitor the status of the spindle. Next, we microinjected purified recombinant XCL100, a Xenopus MAP kinase phosphatase, into XTC cells in various stages of mitosis to interfere with MAP kinase activation. We found that mitotic progression was unaffected by the phosphatase. However, XCL100 rendered the cells unable to remain arrested in mitosis after treatment with nocodazole. Cells injected with phosphatase at prometaphase or metaphase exited mitosis in the presence of nocodazole—the chromosomes decondensed and the nuclear envelope re-formed—whereas cells injected with buffer or a catalytically inactive XCL100 mutant protein remained arrested in mitosis. Coinjection of constitutively active MAP kinase kinase-1, which opposes XCL100's effects on MAP kinase, antagonized the effects of XCL100. Since the only known targets of MAP kinase kinase-1 are Erk1 and Erk2, these findings argue that MAP kinase function is required for the spindle assembly checkpoint in XTC cells.
Inhibitors of microtubule polymerization arrest most cells in a prometaphase-like stage, indicating that a checkpoint monitors chromosome alignment and/or the status of the spindle and regulates the metaphase to anaphase transition. This safeguard helps to ensure accurate transmission of genetic information to the daughter cells. Defects in cell cycle checkpoints increase the frequency of chromosome loss and gene amplification and may play a role in tumor progression (Hartwell et al., 1994; Hartwell and Weinert, 1989; Murray, 1994, 1995).
In the budding yeast Saccharomyces cerevisiae, spindle disruption arrest depends on the products of three MAD (mitotic arrest defective) genes (Hardwick and Murray, 1995; Hardwick et al., 1996; Li et al., 1994), three BUB (budding uninhibited by benzimidazole) genes (Hoyt et al., 1991; Roberts et al., 1994), and MPS1 (multipolar spindle; Lauze et al., 1995; Weiss and Winey, 1996). Vertebrate homologs of one of these genes, MAD2, have recently been identified and found to be important for the spindle assembly checkpoint, indicating that the mechanisms underlying this checkpoint are likely to be well conserved evolutionarily (Chen et al., 1996; Li and Benezra, 1996).
In a wide variety of organisms, phosphoprotein dephosphorylation is essential for progression from metaphase to anaphase. Certain fission yeast and Drosophila mutants with altered phosphatase activity exhibit metaphase arrest phenotypes (Kinoshita et al., 1991; Mayer-Jaekel et al., 1993; Ohkura et al., 1989). Studies with phosphoepitopespecific antibodies have also implicated dephosphorylation in progression to anaphase (Campbell and Gorbsky, 1995; Gorbsky and Ricketts, 1993; Nicklas et al., 1995). Inactivation of phosphatases that are necessary for anaphase, or activation of kinases that oppose progression into anaphase, could be the mechanism through which defective spindles and misaligned chromosomes bring about mitotic arrest.
Minshull et al. (1994) implicated the Erk2 or p42 mitogen-activated protein kinase (MAP kinase)1 protein in the spindle assembly checkpoint through studies of cytoplasmic extracts of Xenopus eggs, a system that offers great biochemical manipulability. Previous work had shown that even though Xenopus eggs and early embryos lack the DNA synthesis checkpoint—DNA synthesis inhibitors do not affect blastomere cleavage—this checkpoint can be reconstituted by the addition of sperm nuclei to a concentration of ∼250 per μl (higher than the concentration of nuclei in an early embryo, but lower than the concentration of ∼250,000 nuclei per μl for a 4 pl somatic cell; Dasso and Newport, 1990). Minshull et al. (1994) examined whether the spindle assembly checkpoint could also be reconstituted by addition of sperm nuclei to the extracts. They found that it could: extracts supplemented with ∼9,000 nuclei per μl arrested in a mitotic state in the presence of nocodazole, and destruction of cyclins B1 and B2 (but not cyclin A1) was suppressed. They also noted that p42 MAP kinase underwent sustained activation in the nocodazoletreated, arrested extracts. Addition of a MAP kinase phosphatase (mouse Mkp-1) that can dephosphorylate and inactivate p42 (Erk2), p44 (Erk1), and p38 (Hog1) MAP kinases, but is minimally active towards other phosphoproteins (Alessi et al., 1993; Groom et al., 1996; Keyse and Emslie, 1992; Sun et al., 1993), resulted in inactivation of the p42 MAP kinase, destruction of cyclins B1 and B2, and release of the extracts from mitotic arrest. These studies showed that MAP kinase, or some closely related protein, is essential for the spindle assembly checkpoint in this reconstituted in vitro system.
Although MAP kinases are probably best known for their transient activation when quiescent cells are prompted to reenter the cell cycle, they are also activated during meiotic maturation in oocytes (Ferrell et al., 1991; Haccard et al., 1990; Jessus et al., 1991; Posada et al., 1991). MAP kinase activation triggers or facilitates the activation of Cdc2 (Gotoh et al., 1995; Haccard et al., 1995; Huang and Ferrell, 1996a ; Kosako et al., 1994), although the mechanism through which MAP kinase regulates Cdc2 activity remains to be elucidated. Given the similarities between meiotic and mitotic M-phase regulation, these findings raise the possibility that MAP kinase might participate in the activation of Cdc2 at the G2-M transition in somatic cells.
MAP kinase may contribute to M phase in other ways as well. Gotoh et al. (1991b) observed that MAP kinase is capable of increasing the dynamic instability of microtubules in Xenopus egg extracts, which raises the possibility that MAP kinase activity may be necessary for microtubule reorganization at the onset of M phase.
In this study we set out to evaluate the hypotheses that p42 or p44 MAP kinase function is important for entry into mitosis in somatic cells, as suggested by studies of meiotic maturation; for establishing a mitotic spindle, as suggested by studies of microtubule dynamics in cytoplasmic Xenopus egg extracts; or for spindle assembly checkpoint control, as suggested by studies of nocodazole-treated, sperm nucleus–supplemented Xenopus egg extracts. We chose to carry out our studies in the Xenopus tadpole cell line (XTC), both because of the availability of good quality homologous reagents for perturbing MAP kinase function, and because XTC cells remain relatively flat during mitosis, facilitating microscopic examination of mitotic cells. Here we demonstrate that MAP kinase is present in the spindle during specific stages of M phase, consistent with the possibility that MAP kinase regulates the spindle or monitors the status of the spindle. Microinjection of a specific MAP kinase–inactivating enzyme, Xenopus MAP kinase phosphatase-1 or XCL100, has no obvious effect on normal mitotic progression but renders cells unable to maintain a mitotically arrested state when mitosis is disrupted by nocodazole treatment. Our results provide in vivo support for the hypothesis that p42 and/or p44 MAP kinase function are required for the spindle assembly checkpoint.
Materials and Methods
Cell Culture
The Xenopus tadpole cell line (XTC) was obtained from Dr. Tim Stearns (Stanford University) and grown at room temperature in 70% L-15 medium supplemented with 10% FBS. Cells to be used for microinjection were plated onto coverslips with photoetched locator grids (Bellco Glass, Inc., Vineland, NJ) and grown for at least 2 d.
Antibodies
The rabbit MAP kinase antiserum X15 was raised against a Xenopus p42 MAP kinase COOH-terminal peptide (Hsiao et al., 1994) and affinity purified on a peptide column. Affinity-purified X15 was tested for its specificity by immunoblotting XTC cell lysates. An Erk1 antibody (C-16) was obtained from Santa Cruz Biochemicals (Santa Cruz, CA). MKK-1 antiserum 662, raised against a Xenopus MKK-1/Mek-1 NH2-terminal peptide, has been described previously (Hsiao et al., 1994). An antibody that recognizes active, but not inactive, Erks 1 and 2 was purchased from Promega (Madison, WI). XCL100 antiserum was raised and provided by M.L. Sohaskey (Stanford University).
Preparation of Cell Lysates
XTC cells were grown to near confluence. Cells were trypsinized and collected by centrifugation. SDS sample buffer was added to the pelleted cells.
Lysates from Xenopus oocytes or electrically activated eggs, containing nonphosphorylated p42 MAP kinase, and from progesterone-treated oocytes or nonactivated eggs, containing phosphorylated p42 MAP kinase, were prepared as described (Ferrell et al., 1991; Murray, 1991).
Immunoblotting
Oocyte, egg, and XTC cell lysates were separated by SDS 10% polyacrylamide gel electrophoresis, transferred to an Immobilon-P membrane (Millipore Corp., Bedford, MA), and probed with the affinity-purified X15 antibody at 1 μg/ml followed by affinity-purified alkaline phosphatase–conjugated goat anti–rabbit IgG at 1:3,000 (Sigma Chemical Co., St. Louis, MO).
Construction of an XCL100 Expression Plasmid
The wild-type XCL100 cDNA was inserted into pGEX-4T for expression in bacteria as follows: gel-purified 1.6-kb BamHI–XhoI fragment, which contained all but the NH2-terminal 130 bp of the XCL100 coding region plus 0.66 kb of the 3′-untranslated region, was ligated into pGEX-4T to yield pGEX-ΔN. The NH2-terminal 130-bp fragment was obtained by PCR, with the 5′ primer consisting of a synthetic BamHI site fused to the codon for the initiator methionine and the 3′ primer spanning the natural XCL100 BamHI site. The fidelity of the PCR product was verified by sequencing. The PCR product was digested with BamHI, gel purified, and ligated into the BamHI site of pGEX-ΔN to yield pGEX-XCL100.
A cDNA for the catalytically inactive phosphatase XCL100 C260S, which has a serine residue in place of the essential active site cysteine residue, was generated by PCR by M.L. Sohaskey. An EcoRI–NheI fragment spanning the mutagenized codon was excised and ligated into pGEX-XCL100 to yield pGEX-XCL100 C260S. The protein products of the wild-type and mutant forms of pGEX-XCL100 are fused to the COOH terminus of glutathione-S-transferase (GST).
Expression and Purification of XCL100
Overnight cultures of Escherichia coli transformed with pGEX-XCL100 or pGEX-XCL100 C260S were grown and lysed essentially as described (Wang et al., 1996). The clarified cell extracts were recycled over a 2.5-ml column of glutathione agarose (Sigma Chemical Co.) for 5 h at 4°C. The column was washed with 10 vol of PBS, and fusion proteins were eluted with 50 mM Tris, pH 8.0, containing 15 mM glutathione. Fractions were assessed for MAP kinase phosphatase activity, as described below, and peak fractions were pooled. As has been previously reported, XCL100 and XCL100 C260S manifested substantial proteolytic degradation; much of the glutathione agarose–purified protein was digested back to the GST moiety (see Fig. 4 A; see also Sun et al., 1993). However, we were able to obtain purified full-length XCL100 or XCL100 C260S proteins by anion exchange chromatography. Peak fractions from the glutathione agarose column were applied to a Mono-Q column (Pharmacia Fine Chemicals, Piscataway, NJ) equilibrated with 20 mM Tris, pH 7.8, 20 mM NaCl, and 2 mM DTT. Proteins were eluted from the column with a linear gradient of 20 mM–1 M NaCl. Activity was recovered in the fractions containing 640–660 mM NaCl, pooled, and dialyzed against the microinjection buffer (130 mM KCl, 10 mM NaPipes, pH 7.0, and 1 mM MgCl2 ). Material obtained after the glutathione agarose purification step and after the Mono-Q step exhibited indistinguishable biochemical and biological activities.
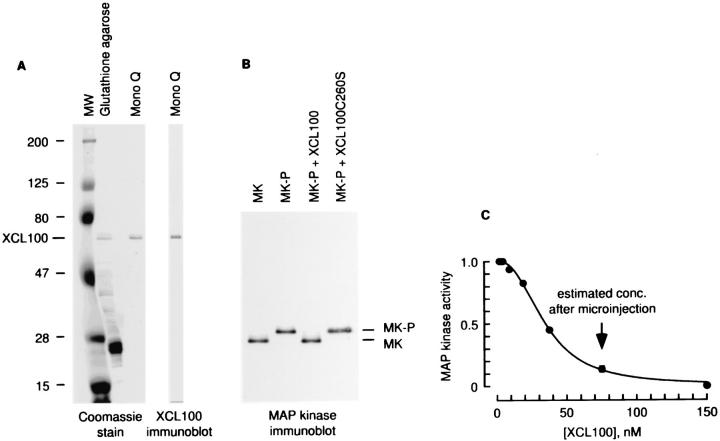
Figure 4.
Purification and characterization of the GST–XCL100 fusion protein. (A) Purification of bacterially expressed GST-XCL100. Full-length GST-XCL100 migrates at ∼67 kD. The prominent band at ∼27 kD in the glutathione agarose purified sample is GST. After Mono-Q purification, full-length GST-XCL100 was the main band detectable by Coomassie staining, and a single band was detectable by XCL100 immunoblotting. (B) Activity of GST-XCL100. This panel shows a MAP kinase immunoblot of recombinant nonphosphorylated rat Erk2 (MK), and phosphorylated rat Erk2 (MK-P) incubated with no phosphatase, with GST-XCL100, or with the inactive GST-XCL100 C260S mutant. (C) In vitro titration of GST-XCL100 activity. Various concentrations of GST-XCL100 were mixed with 250 nM MKK-1* and 1 μM Xenopus Erk2, and the system was allowed to reach steady state. MAP kinase activity was determined by a myelin basic protein phosphorylation assay.
We found that the biochemical activity of XCL100 did not survive multiple freeze-thaw cycles or prolonged storage at 4°C. Therefore, for studies described below, we used XCL100 and XCL100 C260S that had been stored at 4°C for no more than 48 h, or, in some experiments, used XCL100 that had been frozen in 10% glycerol, stored at −70°C, and thawed just before its use.
Other Recombinant Proteins
A pRSET-based plasmid for a constitutively active, His6-tagged mutant of human MKK-1/Mek-1 (with Ser 218 replaced by Glu, Ser 222 replaced by Asp, and a deletion of amino acids 32–51, hereafter denoted MKK-1*) was provided by Natalie Ahn (University of Colorado, Boulder). An NheI–HindIII fragment containing the MKK-1* coding region was subcloned into pFastBac1 for generation of baculoviruses. MKK-1* was expressed in insect cells and purified to homogeneity by nickel-chelate chromatography by Ramesh Bhatt (Stanford University).
Hexahistidine-tagged, kinase-minus (K52R) rat Erk2 was expressed in E. coli and purified by Chi-Ying Huang (Stanford University; Huang and Ferrell, 1996a ) from a plasmid provided by Melanie Cobb and Tom Geppert (University of Texas Southwestern Medical Center) (Robbins et al., 1993). A pT7-7–based plasmid for expression of hexahistidine-tagged Xenopus Erk2 was engineered from a Xenopus Erk2 cDNA provided by Jonathan Cooper and Jim Posada (Fred Hutchinson Cancer Research Center, Seattle, WA), and it was expressed in E. coli and purified by Ramesh Bhatt.
In Vitro Phosphatase Activity Assay
To prepare phosphorylated Erk2 for use as a substrate in an XCL100 phosphatase assay, we incubated recombinant kinase-minus rat Erk2 with active MKK-1 that had been obtained from Xenopus egg lysates by immunoprecipitation with antibody 662 (Hsiao et al., 1994), and MgATP. The XCL100 phosphatase assay was performed by incubating phosphorylated MAP kinase with purified XCL100 fusion proteins in phosphatase reaction buffer (50 mM Hepes, pH 7.5, 1 mg/ml BSA, 2 mM DTT, and 2 mM EDTA) in a final reaction vol of 60 μl. After incubation for 30 min at 30°C, the reaction was stopped by adding SDS sample buffer, and the sample was boiled. Proteins were separated by SDS 10% polyacrylamide gel electrophoresis and immunoblotted as described above. Dephosphorylation of Erk2 was detected as a shift of the blotted Erk2 band to a lower apparent molecular weight.
MAP Kinase Assay
MAP kinase activity was assessed by a myelin basic protein phosphorylation assay essentially as described (Huang and Ferrell, 1996a ). Reaction mixtures were analyzed by SDS 12.5% polyacrylamide gel electrophoresis, proteins were transferred to an Immobilon P membrane, and incorporation of 32P into myelin basic protein was quantified using a phosphorimager (Molecular Dynamics, Sunnyvale, CA).
Microinjection and Nocodazole Treatment
Micropipettes were pulled on a horizontal pipette puller (Sutter Instrument Co., Novato, CA). Proteins were microinjected with a Narishiga manual microinjector (Greenvale, NY). The needle concentration of XCL100 protein (both wild-type and mutant forms) was 1.5 μM, and MKK1* was 10 μM when MKK-1* was injected alone, and 5 μM when MKK-1* was coinjected with XCL100.
For checkpoint control experiments, we first microinjected the appropriate protein(s), and then aspirated the medium and added fresh F-15 medium containing nocodazole at 50 ng/ml. Cells were viewed with a ×40 phase-contrast objective (NA 0.75) on an inverted microscope (Carl Zeiss, Inc., Thornwood, NY) equipped with a CCD camera and recorded with an optical disk recorder (Panasonic, Secaucus, NJ) for up to 4 h after injection or treatment with the drug.
Immunofluorescence
XTC cells were grown on coverslips, fixed with methanol (−20°C) for 6 min, and then treated with a microtubule stabilizing buffer (25 mM imidazole, pH 7.0, 10 mM KCl, 1 mM MgSO4, 10 mM EGTA, and 20% glycerol) containing 0.1% Triton X-100 (Pierce Chemical Co., Rockford, IL), followed by washing with PBS. XTC cells were blocked in 3% milk, and then stained with affinity-purified X15 at 8 μg/ml followed by affinitypurified FITC-conjugated goat anti–rabbit IgG at 1:100 (Kirkegaard & Perry Laboratories Inc., Gaithersburg, MD). For triple labeling, the cells were incubated sequentially with X15 and then mouse monoclonal anti– β-tubulin antibody (Amersham Corp., Arlington Heights, IL) at 1:100. FITC-conjugated anti–rabbit IgG (1:100) and affinity-purified Texas red– conjugated goat anti–mouse IgG (1:200; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) were mixed and applied. Finally, chromosomes were stained with 4′,6-diamidino-2-phenylindole (DAPI) at 1 μg/ ml for 30 s. Each step was followed by four 10-min rinses with PBS.
Images were obtained using either a ×100, 1.3 NA objective on a Zeiss microscope equipped with a cooled CCD camera or a ×40, 0.9 NA objective on a Zeiss microscope equipped with a conventional 35-mm camera.
Results
Erk2/p42 MAP Kinase Localizes at the Mitotic Spindle after Nuclear Envelope Breakdown and Leaves the Spindle before Anaphase
The localization of some cell cycle regulators provides a clue to their function. Accordingly, we examined the localization of MAP kinase during M phase in a tissue-culture cell line, XTC. We determined the most suitable of several antisera for immunolocalization studies to be X15, an antipeptide antiserum raised against a Xenopus Erk2 (p42) MAP kinase COOH-terminal peptide (Hsiao et al., 1994). X15 recognizes active and inactive forms of Xenopus Erk2 equally well, both in immunoprecipitations (nondenaturing conditions) and in immunoblots (denaturing conditions; Hsiao et al., 1994; Fig. 1).
Figure 1.
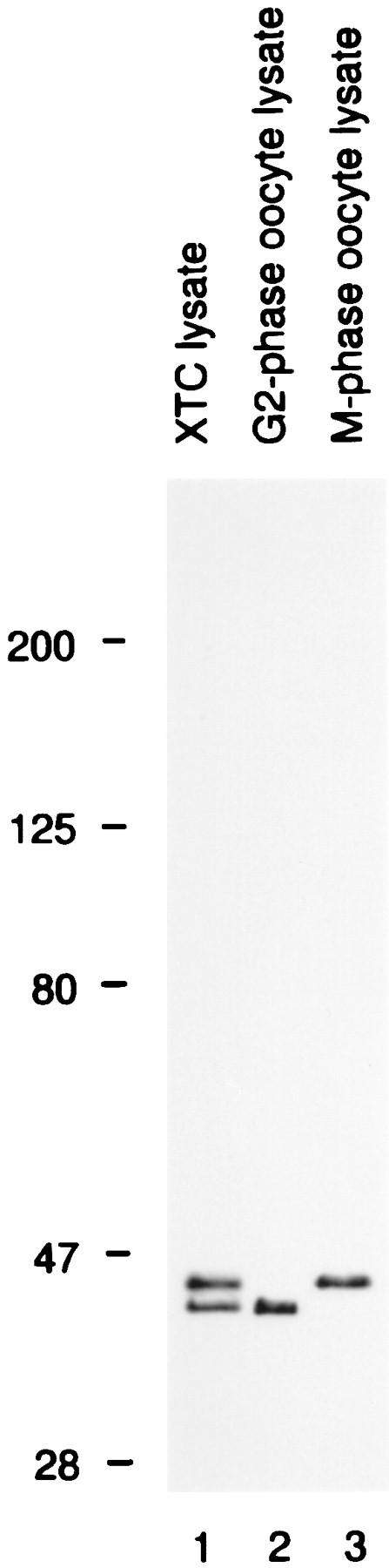
The specificity of antibody X15. Immunoblots of lysates from XTC cells (lane 1), G2-phase oocytes (lane 2), and M-phase oocytes (lane 3) were probed with X15. XTC cells exhibited two bands: a lower band that comigrated with nonphosphorylated Erk2, and an upper band that comigrated with both Erk1 and phosphorylated Erk2. The upper band was identified as Erk1, rather than phosphorylated Erk2, based on its cross-reactivities with other Erk1 and Erk2 antisera, and on the fact that it did not shift to a lower apparent molecular weight when lysates were treated with XCL100 (not shown).
We affinity purified X15 antibody on a peptide column and characterized the affinity-purified X15 for its specificity in total XTC lysates. Affinity-purified X15 recognized two bands on immunoblots (Fig. 1), corresponding to Xenopus Erk1 and Erk2.
We then carried out immunofluorescence studies of XTC cells using affinity purified X15. Fig. 2 shows the results of triple label staining with X15 (fluorescein staining), antitubulin antiserum (Texas red), and DAPI for DNA staining. In interphase, MAP kinase was diffusely distributed through the cytoplasm (Fig. 2 A). There was no obvious concentration of MAP kinase in the interphase microtubules.
Figure 2.
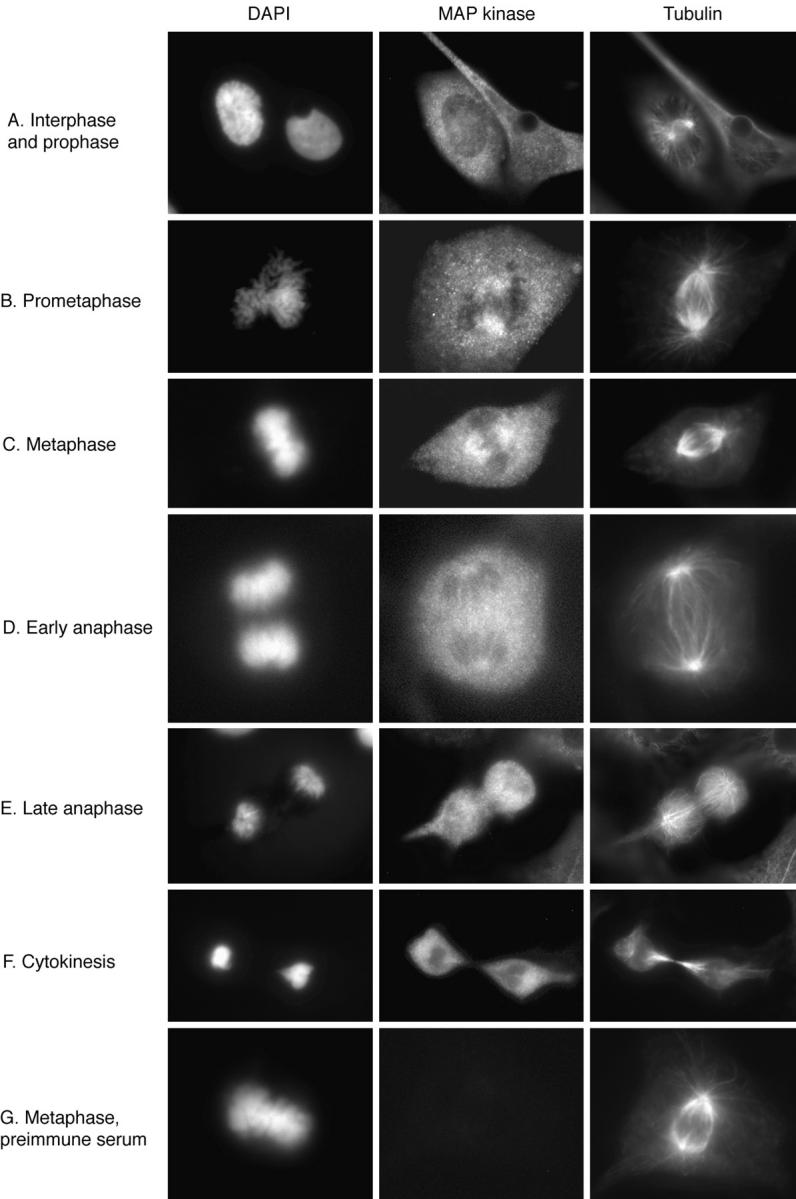
Immunolocalization of MAP kinase at different mitotic stages. XTC cells were fixed, permeabilized, and subjected to triple label staining with DAPI, X15, and a β-tubulin antibody (A–F), or with DAPI, X15 preimmune serum, and the tubulin antibody (G).
When cells entered prometaphase, much of the MAP kinase staining remained diffusely cytoplasmic, but some of the staining became concentrated on the spindle (Fig. 2 B). MAP kinase was detectable on the spindle during early prometaphase just after nuclear envelope breakdown, and the staining increased during metaphase (Fig. 2, B–C). During anaphase, the intensity of the MAP kinase staining of the spindle decreased to about the level of intensity of general cytoplasmic staining (Fig. 2 D). Thus, the spindlelike “fingers” of MAP kinase staining seen in Fig. 2 D could include some spindle-associated MAP kinase, but could also simply represent fingers of cytoplasm between MAP kinase–free masses of chromosomes. By late anaphase, the intensity of MAP kinase staining around the spindle was lower than that in other regions of the cell (Fig. 2 E). MAP kinase was not detectable in the midbody, the prominent microtubule-containing structure that forms between the two daughter cells during cytokinesis (Fig. 2 F). Thus, MAP kinase was found to be associated with the spindle during prometaphase and metaphase and to dissociate from the spindle and midbody, or to become inaccessible to antibody staining during anaphase and cytokinesis.
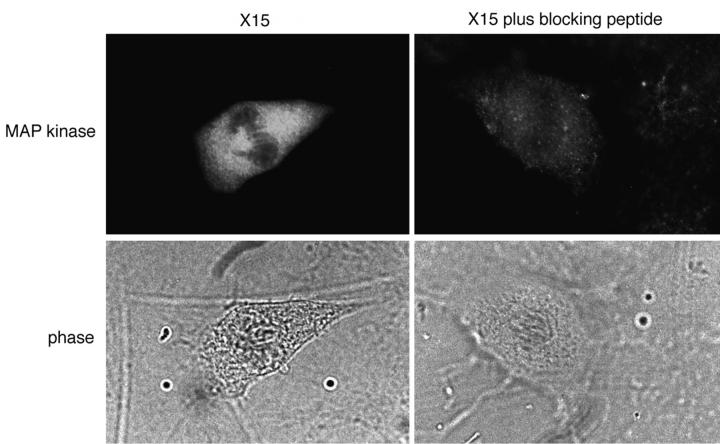
Preimmune serum yielded no spindle staining (Fig. 2 G), indicating that the spindle staining observed with affinitypurified X15 was specific. Preincubation of X15 with the peptide against which it was raised markedly decreased the intensity of both spindle and cytoplasmic staining (Fig. 3). Finally, single label immunofluorescence studies with affinity-purified X15 showed spindle staining, confirming that the results seen in the triple label studies were not due to cross-reactivity between secondary antibodies or leakage between the fluorescein and Texas red filters (Fig. 3).
Figure 3.
Single label immunolocalization of MAP kinase. XTC cells were fixed, permeabilized, stained with DAPI (not shown) and X15 or X15 plus blocking peptide (35 μM), and examined by fluorescence and phase microscopy. The cell shown is in metaphase.
These findings demonstrate that Erk1 or Erk2 is present in the spindle during specific subphases of mitosis. This places MAP kinase in an appropriate position to be involved in either regulating the spindle or relaying information about the status of the spindle.
Purified Bacterially Expressed GST-XCL100 Dephosphorylates Erk2 MAP Kinase In Vitro
We set out to determine whether, in intact XTC cells, MAP kinase activation might be necessary to trigger or maintain Cdc2 activation, as appears to be the case during meiotic maturation (Gotoh et al., 1995; Haccard et al., 1995; Huang and Ferrell, 1996a ; Kosako et al., 1994b ); MAP kinase might be needed to maintain the dynamic properties of the M-phase microtubules, as suggested by studies of interphase Xenopus egg extracts (Gotoh et al., 1991b ); or MAP kinase activity might be required for the spindle assembly checkpoint, as suggested by studies of nocodazole-treated, nucleus-supplemented cycling Xenopus egg extracts (Minshull et al., 1994). The approach chosen was to microinject a Xenopus MAP kinase phosphatase, XCL100, which could prevent or reverse MAP kinase activation, and determine the consequences by time-lapse video microscopy. XCL100 and its homologs CL100 (human) and Mkp-1 (mouse) are dual-specificity phosphatases that dephosphorylate the phosphothreonine and phosphotyrosine regulatory sites of MAP kinase, thereby inactivating MAP kinase (Alessi et al., 1993; Lewis et al., 1995; Sun et al., 1993). CL100/Mkp-1 proteins are highly specific for the p42 Erk2, p44 Erk1, and p38 Hog1 MAP kinases in vitro (Alessi et al., 1993; Groom et al., 1996; Keyse and Emslie, 1992; Sun et al., 1993). Transient expression of Mkp-1 in COS cells leads to selective dephosphorylation of MAP kinase from the spectrum of phosphotyrosine-containing proteins (Sun et al., 1993), indicating that XCL100/Mkp-1 proteins are highly specific in vivo as well.
We expressed wild-type XCL100 and an engineered catalytically inactive version of XCL100, with Cys 260 mutagenized to Ser (XCL100 C260S), as GST fusion proteins in bacteria. The proteins were purified to homogeneity by glutathione affinity chromatography and Mono-Q anion exchange chromatography (Fig. 4 A). The purified wildtype XCL100 protein potently dephosphorylated p42 MAP kinase (Fig. 4 B) but not Mos, Mek, or Rsk (Huang, C.Y.-F., unpublished data), other enzymes in the MAP kinase pathway. The mutant XCL100 C260S protein did not dephosphorylate MAP kinase (Fig. 4 B).
We then carried out in vitro studies to determine whether it was likely that we could achieve a high enough concentration of XCL100 by microinjection to interfere with Erk1 and Erk2 activation. By microinjection, we should be able to increase the cell's XCL100 concentration by ∼75 nM (assuming we can inject 50 fl into a 1 pl cell, and given that the needle concentration of XCL100 was 1.5 μM). The abundance of MKK-1 and Erk1/2 in several cell lines has been estimated to be on the order of 1 μM (Ferrell, 1996b ). In Xenopus oocyte extracts, activation of ∼25% of the MKK-1 results in activation of ∼50% of the Erk2 (Huang and Ferrell, 1996b ). We added various dilutions of XCL100 to a mixture of recombinant Xenopus Erk2 (1 μM) and MKK-1* (250 nM), in the presence of MgATP, and let the system reach steady state. We found that 38 nM XCL100 was sufficient to half-maximally inhibit Erk2 activation, 75 nM XCL100 inhibited Erk2 activation by 87%, and 150 nM XCL100 inhibited Erk2 activation completely (Fig. 4 C). Based on these in vitro titrations, it appears reasonable to expect XCL100 microinjection to have an effect on MAP kinase activation in vivo, provided the microinjected phosphatase is not rapidly degraded or inactivated.
Microinjection of MAP Kinase Phosphatase Does Not Affect Mitotic Progression
To test whether interfering with MAP kinase activation by XCL100 microinjection would affect the mitotic progression, we microinjected XTC cells at different stages of mitosis— prophase, prometaphase, metaphase, and anaphase—with purified XCL100 protein, and then followed the injected cells by time-lapse video microscopy. We found that 20 out of 20 cells injected with XCL100 (needle concentration 1.5 μM, estimated intracellular concentration 75 nM) progressed to metaphase, initiated anaphase, and cleaved, all with normal kinetics (Table I). These findings indicate that either a high level of MAP kinase activity is not required for normal mitotic progression, or the intracellular concentration of XCL100 achieved by microinjection was not sufficient to substantially interfere with MAP kinase activation. The fact that some of the same preparations of XCL100 were found to interfere with checkpoint control, as described below, argues for the first possibility.
Table I.
The Effect of Inhibiting MAP Kinase Activation on the Spindle Assembly Checkpoint: Summary of Microinjection Data
| Treatment | Outcome | |||||
|---|---|---|---|---|---|---|
| Remained arrested in mitosis through 4 h | Mitosis completed within 4 h | Progressed to interphase within 4 h without completing mitosis | ||||
| Nocodazole plus buffer injection | 10/10 | 0/10 | 0/10 | |||
| XCL100 injection | 0/20 | 20/20 | 0/20 | |||
| Nocodazole plus XCL100‡ | 0/15‡ | 0/15‡ | 15/15‡ | |||
| Nocodazole plus XCL100 C260S | 17/17 | 0/17 | 0/17 | |||
| MKK-1* injection | 0/19 | 19/19 | 0/19 | |||
| Nocodazole plus XCL100 plus MKK-1* | 8/8 | 0/8 | 0/8 | |||
The data tabulated here are taken from four independent microinjection experiments using four separate preparations of XCL100. Three other preparations of XCL100 showed no effect (cells remained arrested in mitosis for 4 h after nocodazole treatment) when microinjected into a total of about a dozen mitotic cells.
Microinjection of MAP Kinase Phosphatase Causes Mitotically Arrested Cells to Exit Mitosis without Sister Chromatid Segregation, Karyokinesis, or Cytokinesis
Next, we tested the hypothesis that MAP kinase function is required for the spindle assembly checkpoint, as suggested by experiments in nucleus-supplemented Xenopus egg extracts (Minshull et al., 1994). Treatment of noninjected, mitotic XTC cells with nocodazole (0.05 μg/ml) resulted in rapid disappearance of the spindle (generally within 3 min), including the kinetochore microtubules (data not shown). Cells then remained blocked in a pseudo-prometaphase stage with scattered chromosomes for up to 12 h.
Our initial approach to determining whether XCL100 microinjection might interfere with this arrest was to arrest XTC cells in a pseudo-prometaphase stage by treatment with nocodazole, then inject XCL100 protein, and finally follow the cells to see whether they would exit mitosis. However, the nocodazole-treated XTC cells proved too fragile to microinject; they often swelled and died (as indicated by cessation of cytoplasmic streaming) immediately after injection.
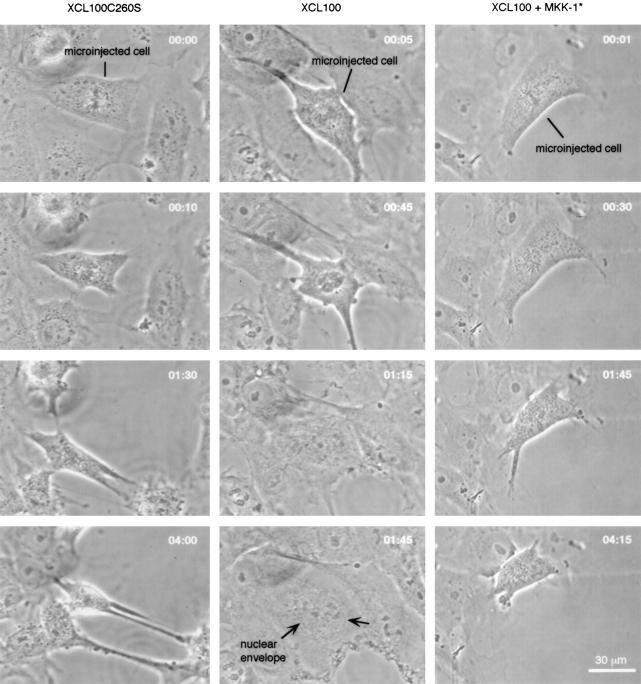
Therefore, we chose to identify and microinject mitotic cells first, and then immediately thereafter treat them with nocodazole. We found that cells injected with wild-type XCL100 protein during prometaphase or metaphase gradually began to exit mitosis ∼45 min to 1 h after nocodazole treatment, without re-forming spindles or carrying out sister chromatid segregation, karyokinesis, or cytokinesis (Table I). Initially, the chromosomes became clustered and then decondensed, and finally the nuclear envelope reformed and nucleoli appeared (Fig. 5). The resulting cells resembled typical, flat interphase cells, except that the cells and their nuclei were unusually large (Fig. 5). When cells were injected during prophase (before nuclear envelope breakdown) and then treated with nocodazole, they did not exit mitosis.
Figure 5.
Effects of perturbing MAP kinase function on the spindle assembly checkpoint. Mitotic cells were microinjected with the inactive XCL100 C260S mutant (left), wild-type XCL100 (middle), or XCL100 plus MKK-1* (right), and then treated with nocodazole. The morphological consequences were assessed by time-lapse video microscopy. Four images are shown from each video series. Numbers in each panel denote the time (in h and min) after nocodazole treatment.
We carried out a variety of experiments to assess the specificity of the effects of XCL100 microinjection on metaphase arrest. First, the possibility that cells escaped the checkpoint control because of microinjection trauma was assessed by injection of buffer alone. All cells (10/10) injected with buffer behaved the same as noninjected ones: they remained mitotically arrested for at least 4 h after nocodazole treatment, indicating no detectable effect of microinjection per se (Table I).
Next, we determined whether the effect of XCL100 on mitotic arrest was dependent on its phosphatase activity. We injected cells with purified recombinant XCL100 C260S protein, the inactive mutant form, at a needle concentration of 1.5 μM. All cells (17/17) injected with XCL100 C260S protein maintained normal mitotic arrest for at least 4 h when treated with nocodazole (Fig. 5; Table I). This result indicates that the effect of XCL100 on the spindle assembly checkpoint is dependent on its phosphatase activity.
We also set out to determine whether any active Erk1 or Erk2 was present in nocodazole-arrested XTC cells, as should be the case if the effects of XCL100 were due to inactivation of one of these MAP kinases. We prepared lysates from asynchronous XTC cells, from cells incubated for various lengths of time (15 min through 7 h) with nocodazole, and from cells shaken off of a plate of cells that had been treated with nocodazole for 16 h. We subjected these samples to immunoblotting with an antiserum that recognizes active Erk1 and Erk2 (Promega). Small amounts of active Erk2 were detected in all of the nocodazoletreated samples (∼⩽10% of the total Erk2), and no active Erk1 was detected (data not shown). The presence of some active Erk2 in the nocodazole-treated cells is compatible with the hypothesis that XCL100's effects on mitotic arrest were due to inactivation of Erk2. However, similar amounts of active Erk2 were detected in untreated, asynchronous cells (data not shown). In contrast, Minshull et al. (1994) found marked differences in the activity of Erk2 in interphase vs mitotically arrested cycling extracts. Thus, mitotic activation of MAP kinase is either more subtle or more difficult to detect in intact XTC cells than it is in extracts.
XCL100 C260S can bind avidly to phosphorylated Erk2, and therefore could potentially interfere with MAP kinase function by preventing MAP kinase from interacting with its substrates (Sun et al., 1993). However, it seems unlikely that this type of inhibition would occur to any significant extent at the concentration of XCL100 C260S we have injected in our studies. Sun et al. (1993) found that expression of the C258S mutant of mouse Mkp-1 (the mouse homolog of XCL100) in COS cells at levels substantially greater than the levels of Erk2 resulted in the coprecipitation of ∼30% of the cells' Erk2 with the Mkp-1 C258S. However, in our case, the concentration of microinjected XCL100 C260S was ∼20-fold lower than the concentration of Erk2 (75 nM vs 1–2 μM). Thus, we would expect our injected phosphatases to be able to inhibit MAP kinase function only by dephosphorylation, not by sequestration. This is consistent with our finding here that XCL100 interfered with mitotic arrest, and XCL100 C260S did not. Similarly, we have found that microinjection of wild-type XCL100 mRNA inhibits progesterone-induced oocyte maturation, whereas XCL100 C260S does not (Sohaskey, M.L., unpublished data).
The Effect of XCL100 on Mitotic Arrest Is Due to Its Effects on p42 or p44 MAP Kinase
We set out to determine whether the effect of XCL100 on mitotic arrest was due to its effect on p42/p44 MAP kinase, p38 Hog1, or some other as yet unidentified target. Our approach was to coinject XCL100 with a constitutively active MAP kinase kinase (MKK-1*) (Mansour et al., 1994). MKK-1* has no direct effect on XCL100 but exerts an effect on MAP kinase that is opposite to the effect of XCL100: it phosphorylates the same residues that XCL100 dephosphorylates. MKK-1 is a highly specific protein kinase. It does not phosphorylate p38 Hog1, and biochemical and genetic studies have thus far uncovered no evidence for the existence of substrates of MKK-1 other than p42 and p44 MAP kinase (Cano and Mahadevan, 1995; Ferrell, 1996a ; Lin et al., 1995; Rouse et al., 1994). Thus, if MKK-1* were found to antagonize the effect of XCL100 on mitotic arrest, this would be strong evidence that XCL100's effects on mitotic arrest were due to its effect on p42 or p44 MAP kinase.
We coinjected XCL100 (needle concentration 1.5 μM) with MKK-1* (needle concentration 5 μM; based on in vitro assays [Fig. 4 C], this concentration should substantially counteract the effects of the coinjected XCL100) into XTC cells at metaphase or prometaphase, and then immediately treated the cells with nocodazole. We found that the injected cells (8/8) did not exit mitosis; they remained blocked at pseudo-prometaphase stage with scattered chromosomes for at least 4 h (Fig. 5; Table I). These findings indicate that MKK-1* inhibited or overcame the effects of XCL100 on mitotic arrest. This supports the hypothesis that the effects of XCL100 on mitotic arrest are due to its effects on p42 or p44 MAP kinase, rather than to some other possible target.
Finally, we assessed whether MAP kinase activation was sufficient to bring about metaphase arrest. We addressed this question by injecting MKK-1* alone (needle concentration 10 μM) into mitotic XTC cells. We found that microinjection of this protein did not bring about a mitotic arrest. This finding suggests either that MAP kinase activation is not sufficient for mitotic arrest, or that we were unable to achieve a high enough level of MAP kinase activation to bring about mitotic arrest.
Discussion
Spindle Localization of MAP Kinase Provides a Clue to Its Role in Mitosis
Several workers have previously examined the localization of the Erk1 and Erk2 MAP kinases in interphase tissueculture cells. Erk1/Erk2 have been reported to be distributed in a diffuse (Dikic et al., 1994; Gonzalez et al., 1993; Lenormand et al., 1993; Traverse et al., 1994) or diffusely speckled (Chen et al., 1992) pattern in a variety of quiescent cells and to translocate to the nucleus when the cells are treated with mitogens. However, recent evidence indicates that, in at least some cell types, MAP kinase may concentrate on or around microtubules (Morishima-Kawashima and Kosik, 1996; Reszka et al., 1995). Here we found that, in interphase XTC cells, the Erk1/Erk2 distribution is diffuse; we saw no examples of microtubule-like staining in these cells.
When XTC cells entered M phase, some MAP kinase immunofluorescence was found to concentrate in the spindle. This could represent MAP kinase translocating to the spindle or, possibly, microtubule-associated MAP kinase undergoing some change in association that made it more accessible to antibody staining. MAP kinase spindle staining was strongest during metaphase and decreased by anaphase, and MAP kinase staining was absent from the cytokinesis midbody.
In some ways, the changes in MAP kinase localization during mitotic M phase reported here are similar to those reported by Verlhac et al. (1993) and Choi et al. (1996) for meiotic M phase. Verlhac et al. (1993) found MAP kinase to concentrate at the spindle poles in mouse oocytes in meiotic M phase and to become more diffuse in anaphase. This is similar to our finding, except that we found MAP kinase to be present throughout the spindle rather than just at the spindle poles. Choi et al. (1996) found MAP kinase to concentrate in the miniasters that form near the chromosomes in mouse oocytes in early meiotic M phase, again reminiscent of the spindle localization we found.
The presence of MAP kinase in the mitotic spindle puts it in a position either to monitor the status of the spindle, or to regulate spindle-associated proteins, or both. It also places MAP kinase in the proximity of key cell cycle regulators. In particular, cyclin B1/Cdc2 complexes appear to be largely spindle associated during M phase, and a fraction of the cyclin B2 is as well (Jackman et al., 1995; Ookata et al., 1992, 1993, 1995; Pines and Hunter, 1991; Rattner et al., 1990).
Microinjection of MAP Kinase Phosphatase Has No Apparent Effect on Mitotic Progression
Erk2 becomes activated concomitantly with Cdc2 during oocyte maturation, and manipulations that inhibit the activation of MAP kinase (Gotoh et al., 1995; Huang and Ferrell, 1996a ; Kosako et al., 1994b ) or its upstream activators (Freeman et al., 1990; Sagata et al., 1988) inhibit Cdc2 activation. In addition, microinjection of oocytes with constitutively active MAP kinase (Haccard et al., 1995), constitutively active Mek-1 (a MAP kinase kinase; Huang et al., 1995), constitutively active Raf-1 (a MAP kinase kinase kinase; Muslin et al., 1993), or Mos (a MAP kinase kinase kinase; Yew et al., 1992) brings about Cdc2 activation. These findings place Erk2 upstream of Cdc2 during meiosis and raise the possibility that MAP kinases may be important for Cdc2 activation at the onset of mitosis as well. It has also been shown that purified Erk2 can cause centrosomenucleated microtubules to change from interphase-like stability to M-phase–like instability (Gotoh et al., 1991b ). Thus, MAP kinase could be involved in the changes in microtubule dynamics that occur during both meiosis and mitosis.
However, there is conflicting evidence on whether Erk1 or Erk2 is activated during mitotic M phase (Edelmann et al., 1996; Heider et al., 1994; Tamemoto et al., 1992), and the question of whether Erk1 or Erk2 function is required for entry into or progression through mitotic M phase has not been previously addressed. In the present study we detected small amounts of active Erk2 in M-phase cells but found no indication that MAP kinase function was important for normal mitotic progression; microinjection of the MAP kinase phosphatase XCL100 had no obvious effect. We cannot rule out the possibility that microinjection of a higher concentration of XCL100, or microinjection of XCL100 before prophase, might alter mitotic progression.
MAP Kinase Function Is Required for the Spindle Assembly Checkpoint
Previous work demonstrated that Xenopus egg extracts supplemented with nuclei arrest in a mitotic state when treated with nocodazole, and that addition of mouse MAP kinase phosphatase (Mkp-1) to these extracts releases them from mitotic arrest (Minshull et al., 1994). These findings suggest a role for MAP kinase in the spindle assembly checkpoint, provided the reconstituted extract system faithfully recapitulates the checkpoint controls found in intact cells.
Here we present strong evidence that MAP kinase function is required for the spindle assembly checkpoint in intact XTC cells. Microinjection of active XCL100 during prometaphase or metaphase (but not prophase) abrogates the normal spindle assembly checkpoint, whereas microinjection of saline or an engineered inactive XCL100 mutant protein does not. Coinjection of MKK-1*, a constitutively active MAP kinase kinase, abolishes the effect of XCL100 on the spindle assembly checkpoint. This finding argues that the relevant target of XCL100 is p42 or p44 MAP kinase, rather than p38 Hog1 or some adventitious target. It is also possible that the relevant target is some as yet unidentified protein that can be regulated by both XCL100 and MKK-1*.
Previous work has also shown that MAP kinase function is required for the metaphase arrest that occurs naturally during the maturation of frog oocytes (Haccard et al., 1993; Kosako et al., 1994a ; Minshull et al., 1994); i.e., MAP kinase is an essential mediator of “cytostatic factor” function. Taken together with Minshull et al.'s studies of the spindle assembly checkpoint in extracts and the present studies of the spindle assembly checkpoint in intact cells, it appears that M-phase arrest in a variety of biological contexts depends upon MAP kinase function. It is therefore possible that studies of any one of these systems might yield insights into all of them.
At present, the mechanism through which MAP kinase mediates the spindle assembly checkpoint is unknown. The possibilities can be organized into three general classes of mechanism. (a) MAP kinase could regulate the machinery that detects spindle misassembly or chromosome misalignment. (b) MAP kinase could regulate the cyclin destruction machinery. For example, MAP kinase could regulate whatever specific ubiquitin-conjugating enzymes are responsible for ubiquitinating cyclins B1 and B2. (c) MAP kinase could convert cyclins B1 and B2 to phosphorylated, nondestructible forms. This possibility is supported by the observation that cyclin B1 is a good substrate for p42 MAP kinase (Izumi and Maller, 1991). Cyclin B2 is not a good substrate for Erks 1 or 2, but Minshull et al. have demonstrated the existence of one or more cyclin B2 kinase activities that appear to be MAP kinase dependent (Minshull et al., 1994).
Likewise, it is not understood what lies upstream of MAP kinase in the spindle assembly checkpoint. It is unclear whether the checkpoint makes use of known MAP kinase kinases (MKK-1/Mek-1, MKK-2/Mek-2) and known MAP kinase kinase kinases (Mos, Raf-1, A-Raf, and B-Raf), or whether there are unknown MAP kinase cascade components used specifically for this function. Our hope is that through a combination of in vivo analysis, like that described here, and examination of in vitro systems, as described previously, it may be possible to delineate the signaling pathways used in the spindle assembly checkpoint in detail.
Acknowledgments
We thank Mike Sohaskey, Chi-Ying Huang, Natalie Ahn, Melanie Cobb, Jon Cooper, Jim Posada, and Tom Geppert for providing plasmids and recombinant proteins, and for helpful discussions; Tim Stearns for XTC cells, helpful discussions, and the use of his immunofluorescence microscope; and Andrew Murray for helpful discussions. We especially thank Tim Mitchison, Claire Walczak, Louise Cramer, and members of the Mitchison laboratory for their hospitality and assistance in the microinjection studies.
This work was supported by a grant from the National Institutes of Health (GM46383) and a Howard Hughes Junior Faculty Scholars award (to J.E. Ferrell, Jr.), and by a postdoctoral fellowship from Cancer Biology training grant CA09302 to X.M. Wang.
Footnotes
1. Abbreviations used in this paper: DAPI, 4′,6-diamidino-2-phenylindole; GST, glutathione-S-transferase; MAP kinase, mitogen-activated protein kinase; XTC, Xenopus tadpole cell line.
Please address all correspondence to James E. Ferrell, Jr., Department of Molecular Pharmacology, Stanford University School of Medicine, Stanford, CA 94305-5332. Tel.: (415) 725-0765. Fax: (415) 725-2952. e-mail: ferrell@cmgm.stanford.edu
References
- Alessi DR, Smythe C, Keyse SM. The human CL100 gene encodes a Tyr/Thr-protein phosphatase which potently and specifically inactivates MAP kinase and suppresses its activation by oncogenic ras in Xenopus oocyte extracts. Oncogene. 1993;8:2015–2020. [PubMed] [Google Scholar]
- Campbell MS, Gorbsky GJ. Microinjection of mitotic cells with the 3F3/2 anti-phosphoepitope antibody delays the onset of anaphase. J Cell Biol. 1995;129:1195–1204. doi: 10.1083/jcb.129.5.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano E, Mahadevan LC. Parallel signal processing among mammalian MAPKs. Trends Biochem Sci. 1995;20:117–122. doi: 10.1016/s0968-0004(00)88978-1. [DOI] [PubMed] [Google Scholar]
- Chen RH, Sarnecki C, Blenis J. Nuclear localization and regulation of erk- and rsk-encoded protein kinases. Mol Cell Biol. 1992;12:915–927. doi: 10.1128/mcb.12.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RH, Waters JC, Salmon ED, Murray AW. Association of spindle assembly checkpoint component XMAD2 with unattached kinetochores. Science (Wash DC) 1996;274:242–246. doi: 10.1126/science.274.5285.242. [DOI] [PubMed] [Google Scholar]
- Choi T, Rulong S, Resau J, Fukasawa K, Matten W, Kuriyama R, Mansour S, Ahn N, Vande GF, Woude Mos/mitogen-activated protein kinase can induce early meiotic phenotypes in the absence of maturationpromoting factor: a novel system for analyzing spindle formation during meiosis I. Proc Natl Acad Sci USA. 1996;93:4730–4735. doi: 10.1073/pnas.93.10.4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasso M, Newport JW. Completion of DNA replication is monitored by a feedback system that controls the initiation of mitosis in vitro: studies in Xenopus. Cell. 1990;61:811–823. doi: 10.1016/0092-8674(90)90191-g. [DOI] [PubMed] [Google Scholar]
- Dikic I, Schlessinger J, Lax I. PC12 cells overexpressing the insulin receptor undergo insulin-dependent neuronal differentiation. Curr Biol. 1994;4:702–708. doi: 10.1016/s0960-9822(00)00155-x. [DOI] [PubMed] [Google Scholar]
- Edelmann HM, Kuhne C, Petritsch C, Ballou LM. Cell cycle regulation of p70 S6 kinase and p42/p44 mitogen-activated protein kinases in Swiss mouse 3T3 fibroblasts. J Biol Chem. 1996;271:963–971. doi: 10.1074/jbc.271.2.963. [DOI] [PubMed] [Google Scholar]
- Ferrell JE., Jr MAP kinases in mitogenesis and development. Curr Top Dev Biol. 1996a;33:1–60. doi: 10.1016/s0070-2153(08)60336-1. [DOI] [PubMed] [Google Scholar]
- Ferrell JE., Jr Tripping the switch fantastic: how a protein kinase cascade can convert graded inputs into switch-like outputs. Trends Biochem Sci. 1996b;21:460–466. doi: 10.1016/s0968-0004(96)20026-x. [DOI] [PubMed] [Google Scholar]
- Ferrell JE, Jr, Wu M, Gerhart JC, Martin GS. Cell cycle tyrosine phosphorylation of p34cdc2 and a microtubule-associated protein kinase homolog in Xenopusoocytes and eggs. Mol Cell Biol. 1991;11:1965–1971. doi: 10.1128/mcb.11.4.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman RS, Kanki JP, Ballantyne SM, Pickham KM, Donoghue DJ. Effects of the v-mos oncogene on Xenopus development: meiotic induction in oocytes and mitotic arrest in cleaving embryos. J Cell Biol. 1990;111:533–541. doi: 10.1083/jcb.111.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez FA, Seth A, Raden DL, Bowman DS, Fay FS, Davis RJ. Serum-induced translocation of mitogen-activated protein kinase to the cell surface ruffling membrane and the nucleus. J Cell Biol. 1993;122:1089–1101. doi: 10.1083/jcb.122.5.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbsky GJ, Ricketts WA. Differential expression of a phosphoepitope at the kinetochores of moving chromosomes. J Cell Biol. 1993;122:1311–1321. doi: 10.1083/jcb.122.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh Y, Moriyama K, Matsuda S, Okumura E, Kishimoto T, Kawasaki H, Suzuki K, Yahara I, Sakai H, Nishida E. Xenopus M phase MAP kinase: isolation of its cDNA and activation by MPF. EMBO (Eur Mol Biol Organ) J. 1991a;10:2661–2668. doi: 10.1002/j.1460-2075.1991.tb07809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh Y, Nishida E, Matsuda S, Shiina N, Kosako H, Shiokawa K, Aikyama T, Ohta K, Sakai H. In vitro effects on microtubule dynamics of purified Xenopus M phase activated MAP kinase. Nature (Lond) 1991b;349:251–254. doi: 10.1038/349251a0. [DOI] [PubMed] [Google Scholar]
- Gotoh Y, Masuyama N, Dell K, Shirakabe K, Nishida E. Initiation of Xenopus oocyte maturation by activation of the mitogen-activated protein kinase cascade. J Biol Chem. 1995;270:25898–258904. doi: 10.1074/jbc.270.43.25898. [DOI] [PubMed] [Google Scholar]
- Groom LA, Sneddon AA, Alessi DR, Dowd S, Keyse SM. Differential regulation of the MAP, SAP, and RK/p38 kinases by Pyst1, a novel cytosolic dual-specificity phosphatase. EMBO (Eur Mol Biol Organ) J. 1996;15:3621–3632. [PMC free article] [PubMed] [Google Scholar]
- Haccard O, Jessus C, Cayla X, Goris J, Merlevede W, Ozon R. In vivo activation of a microtubule-associated protein kinase during meiotic maturation of the Xenopus oocyte. Eur J Biochem. 1990;192:633–642. doi: 10.1111/j.1432-1033.1990.tb19270.x. [DOI] [PubMed] [Google Scholar]
- Haccard O, Sarcevic B, Lewellyn A, Hartley R, Roy L, Izumi T, Erikson E, Maller JL. Induction of metaphase arrest in cleaving Xenopus embryos by MAP kinase. Science (Wash DC) 1993;262:1262–1265. doi: 10.1126/science.8235656. [DOI] [PubMed] [Google Scholar]
- Haccard O, Lewellyn A, Hartley RS, Erikson E, Maller JL. Induction of Xenopus oocyte meiotic maturation by MAP kinase. Dev Biol. 1995;168:677–682. doi: 10.1006/dbio.1995.1112. [DOI] [PubMed] [Google Scholar]
- Hardwick KG, Murray AW. Mad1p, a phosphoprotein component of the spindle assembly checkpoint in budding yeast. J Cell Biol. 1995;131:709–720. doi: 10.1083/jcb.131.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick KG, Weiss E, Luca FC, Winey M, Murray AW. Activation of the budding yeast spindle assembly checkpoint without mitotic spindle disruption. Science (Wash DC) 1996;273:953–956. doi: 10.1126/science.273.5277.953. [DOI] [PubMed] [Google Scholar]
- Hartwell LH, Weinert TA. Checkpoints: controls that ensure the order of cell cycle events. Science (Wash DC) 1989;246:629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- Hartwell L, Weinert T, Kadyk L, Garvik B. Cell cycle checkpoints, genomic integrity, and cancer. Cold Spring Harbor Symp Quant Biol. 1994;59:259–263. doi: 10.1101/sqb.1994.059.01.030. [DOI] [PubMed] [Google Scholar]
- Heider H, Hug C, Lucocq JM. A 40-kDa myelin basic protein kinase, distinct from erk1 and erk2, is activated in mitotic HeLa cells. Eur J Biochem. 1994;219:513–520. doi: 10.1111/j.1432-1033.1994.tb19966.x. [DOI] [PubMed] [Google Scholar]
- Hoyt MA, Totis L, Roberts BT. S. cerevisiaegenes required for cell cycle arrest in response to loss of microtubule function. Cell. 1991;66:507–517. doi: 10.1016/0092-8674(81)90014-3. [DOI] [PubMed] [Google Scholar]
- Hsiao K-M, Chou S-y, Shih S-J, Ferrell JE., Jr Evidence that inactive p42 mitogen-activated protein kinase and inactive Rsk exist as a heterodimer in vivo. Proc Natl Acad Sci USA. 1994;91:5480–5484. doi: 10.1073/pnas.91.12.5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C-YF, Ferrell JE., Jr Dependence of Mos-induced Cdc2 activation on MAP kinase function in a cell-free system. EMBO (Eur Mol Biol Organ) J. 1996a;15:2169–2173. [PMC free article] [PubMed] [Google Scholar]
- Huang C-YF, Ferrell JE., Jr Ultrasensitivity in the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1996b;93:10078–10083. doi: 10.1073/pnas.93.19.10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Kessler DS, Erikson RL. Biochemical and biological analysis of Mek1 phosphorylation site mutants. Mol Biol Cell. 1995;6:237–245. doi: 10.1091/mbc.6.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi T, Maller J. Phosphorylation of Xenopus cyclins B1 and B2 is not required for cell cycle transitions. Mol Cell Biol. 1991;11:3860–3867. doi: 10.1128/mcb.11.8.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman M, Firth M, Pines J. Human cyclins B1 and B2 are localized to strikingly different structures: B1 to microtubules, B2 primarily to the Golgi apparatus. EMBO (Eur Mol Biol Organ) J. 1995;14:1646–1654. doi: 10.1002/j.1460-2075.1995.tb07153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessus C, Rime H, Haccard O, Van Lint J, Goris J, Merlevede W, Ozon R. Tyrosine phosphorylation of p34cdc2 and p42 during meiotic maturation of Xenopus oocyte. Antagonistic action of okadaic acid and 6-DMAP. Development (Camb) 1991;111:813–820. doi: 10.1242/dev.111.3.813. [DOI] [PubMed] [Google Scholar]
- Keyse SM, Emslie EA. Oxidative stress and heat shock induce a human gene encoding a protein-tyrosine phosphatase. Nature (Lond) 1992;359:644–647. doi: 10.1038/359644a0. [DOI] [PubMed] [Google Scholar]
- Kinoshita N, Yamano H, Le Bouffant-Sladeczek F, Kurooka H, Ohkura H, Stone EM, Takeuchi M, Toda T, Yoshida T, Yanagida M. Sister-chromatid separation and protein dephosphorylation in mitosis. Cold Spring Harbor Symp Quant Biol. 1991;56:621–628. doi: 10.1101/sqb.1991.056.01.071. [DOI] [PubMed] [Google Scholar]
- Kosako H, Gotoh Y, Nishida E. Mitogen-activated protein kinase kinase is required for the mos-induced metaphase arrest. J Biol Chem. 1994a;269:28354–28358. [PubMed] [Google Scholar]
- Kosako H, Gotoh Y, Nishida E. Requirement for the MAP kinase kinase/MAP kinase cascade in Xenopusoocyte maturation. EMBO (Eur Mol Biol Organ) J. 1994b;13:2131–2138. doi: 10.1002/j.1460-2075.1994.tb06489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauze E, Stoelcker B, Luca FC, Weiss E, Schutz AR, Winey M. Yeast spindle pole body duplication gene MPS1 encodes an essential dual specificity protein kinase. EMBO (Eur Mol Biol Organ) J. 1995;14:1655–1663. doi: 10.1002/j.1460-2075.1995.tb07154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenormand P, Sardet C, Pages G, L'Allemain G, Brunet A, Pouyssegur J. Growth factors induce nuclear translocation of MAP kinases (p42mapk and p44mapk) but not of their activator MAP kinase kinase (p45mapkk) in fibroblasts. J Cell Biol. 1993;122:1079–1088. doi: 10.1083/jcb.122.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis T, Groom LA, Sneddon AA, Smythe C, Keyse SM. XCL100, an inducible nuclear MAP kinase phosphatase from Xenopus laevis: its role in MAP kinase inactivation in differentiated cells and its expression during early development. J Cell Sci. 1995;108:2885–2896. doi: 10.1242/jcs.108.8.2885. [DOI] [PubMed] [Google Scholar]
- Li R, Havel C, Watson JA, Murray AW. The mitotic feedback control gene MAD2 encodes the alpha-subunit of a prenyltransferase [correction] Nature (Lond) 1994;371:438. doi: 10.1038/366082a0. [DOI] [PubMed] [Google Scholar]
- Li Y, Benezra R. Identification of a human mitotic checkpoint gene: hsMAD2. Science (Wash DC) 1996;274:246–248. doi: 10.1126/science.274.5285.246. [DOI] [PubMed] [Google Scholar]
- Lin A, Minden A, Martinetto H, Claret FX, Lange-Carter C, Mercurio F, Johnson GL, Karin M. Identification of a dual specificity kinase that activates the Jun kinases and p38-Mpk2. Science (Wash DC) 1995;268:286–290. doi: 10.1126/science.7716521. [DOI] [PubMed] [Google Scholar]
- Mansour SJ, Matten WT, Hermann AS, Candia JM, Rong S, Fukasawa K, Vande GF, Woude, Ahn NG. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science (Wash DC) 1994;265:966–970. doi: 10.1126/science.8052857. [DOI] [PubMed] [Google Scholar]
- Mayer-Jaekel RE, Ohkura H, Gomes R, Sunkel CE, Baumgartner S, Hemmings BA, Glover DM. The 55 kd regulatory subunit of Drosophilaprotein phosphatase 2A is required for anaphase. Cell. 1993;72:621–633. doi: 10.1016/0092-8674(93)90080-a. [DOI] [PubMed] [Google Scholar]
- Minshull J, Sun H, Tonks NK, Murray AW. A MAP kinase-dependent spindle assembly checkpoint in Xenopusegg extracts. Cell. 1994;79:475–486. doi: 10.1016/0092-8674(94)90256-9. [DOI] [PubMed] [Google Scholar]
- Morishima-Kawashima M, Kosik KS. The pool of MAP kinase associated with microtubules is small but constitutively active. Mol Biol Cell. 1996;7:893–905. doi: 10.1091/mbc.7.6.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray AW. Cell cycle extracts. Methods Cell Biol. 1991;36:581–605. [PubMed] [Google Scholar]
- Murray A. Cell cycle checkpoints. Curr Opin Cell Biol. 1994;6:872–876. doi: 10.1016/0955-0674(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Murray AW. The genetics of cell cycle checkpoints. Curr Opin Genet Dev. 1995;5:5–11. doi: 10.1016/s0959-437x(95)90046-2. [DOI] [PubMed] [Google Scholar]
- Muslin AJ, MacNicol AM, Williams LT. Raf-1 protein kinase is important for progesterone-induced Xenopus oocyte maturation and acts downstream of mos. Mol Cell Biol. 1993;13:4197–4202. doi: 10.1128/mcb.13.7.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklas RB, Ward SC, Gorbsky GJ. Kinetochore chemistry is sensitive to tension and may link mitotic forces to a cell cycle checkpoint. J Cell Biol. 1995;130:929–939. doi: 10.1083/jcb.130.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkura H, Kinoshita N, Miyatani S, Toda T, Yanagida M. The fission yeast dis2+ gene required for chromosome disjoining encodes one of two putative type 1 protein phosphatases. Cell. 1989;57:997–1007. doi: 10.1016/0092-8674(89)90338-3. [DOI] [PubMed] [Google Scholar]
- Ookata K, Hisanaga S, Okano T, Tachibana K, Kishimoto T. Relocation and distinct subcellular localization of p34cdc2-cyclin B complex at meiosis reinitiation in starfish oocytes. EMBO (Eur Mol Biol Organ) J. 1992;11:1763–1772. doi: 10.1002/j.1460-2075.1992.tb05228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ookata K, Hisanaga S, Okumura E, Kishimoto T. Association of p34cdc2/cyclin B complex with microtubules in starfish oocytes. J Cell Sci. 1993;105:873–881. doi: 10.1242/jcs.105.4.873. [DOI] [PubMed] [Google Scholar]
- Ookata K, Hisanaga S, Bulinski JC, Murofushi H, Aizawa H, Itoh TJ, Hotani H, Okumura E, Tachibana K, Kishimoto T. Cyclin B interaction with microtubule-associated protein 4 (MAP4) targets p34cdc2 kinase to microtubules and is a potential regulator of M-phase microtubule dynamics. J Cell Biol. 1995;128:849–862. doi: 10.1083/jcb.128.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines J, Hunter T. Human cyclins A and B1 are differentially located in the cell and undergo cell cycle-dependent nuclear transport. J Cell Biol. 1991;115:1–17. doi: 10.1083/jcb.115.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posada J, Sanghera J, Pelech S, Aebersold R, Cooper JA. Tyrosine phosphorylation and activation of homologous protein kinases during oocyte maturation and mitogenic activation of fibroblasts. Mol Cell Biol. 1991;11:2517–2528. doi: 10.1128/mcb.11.5.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattner JB, Lew J, Wang JH. p34cdc2 kinase is localized to distinct domains within the mitotic apparatus. Cell Motil Cytoskeleton. 1990;17:227–235. doi: 10.1002/cm.970170309. [DOI] [PubMed] [Google Scholar]
- Reszka AA, Seger R, Diltz CD, Krebs EG, Fischer EH. Association of mitogen-activated protein kinase with the microtubule cytoskeleton. Proc Natl Acad Sci USA. 1995;92:8881–8885. doi: 10.1073/pnas.92.19.8881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins DJ, Zhen E, Owaki H, Vanderbilt CA, Ebert D, Geppert TD, Cobb MH. Regulation and properties of extracellular signal-regulated protein kinases 1 and 2 in vitro. J Biol Chem. 1993;268:5097–5106. [PubMed] [Google Scholar]
- Roberts BT, Farr KA, Hoyt MA. The Saccharomyces cerevisiaecheckpoint gene BUB1 encodes a novel protein kinase. Mol Cell Biol. 1994;14:8282–8291. doi: 10.1128/mcb.14.12.8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, Hunt T, Nebreda AR. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78:1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- Sagata N, Oskarsson M, Copeland T, Brumbaugh J, Vande GF, Woude Function of c-mos proto-oncogene product in meiotic maturation in Xenopusoocytes. Nature (Lond) 1988;335:519–525. doi: 10.1038/335519a0. [DOI] [PubMed] [Google Scholar]
- Sun H, Charles CH, Lau LF, Tonks NK. MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell. 1993;75:487–493. doi: 10.1016/0092-8674(93)90383-2. [DOI] [PubMed] [Google Scholar]
- Tamemoto H, Kadowaki T, Tobe K, Ueki K, Izumi T, Chatani Y, Kohno M, Kasuga M, Yazaki Y, Akanuma Y. Biphasic activation of two mitogen-activated protein kinases during the cell cycle in mammalian cells. J Biol Chem. 1992;267:20293–20297. [PubMed] [Google Scholar]
- Traverse S, Seedorf K, Paterson H, Marshall CJ, Cohen P, Ullrich A. EGF triggers neuronal differentiation of PC12 cells that overexpress the EGF receptor. Curr Biol. 1994;4:694–701. doi: 10.1016/s0960-9822(00)00154-8. [DOI] [PubMed] [Google Scholar]
- Verlhac MH, de Pennart H, Maro B, Cobb MH, Clarke HJ. MAP kinase becomes stably activated at metaphase and is associated with microtubule-organizing centers during meiotic maturation of mouse oocytes. Dev Biol. 1993;158:330–340. doi: 10.1006/dbio.1993.1192. [DOI] [PubMed] [Google Scholar]
- Wang XM, Peloquin JG, Zhai Y, Bulinski JC, Borisy GG. Removal of MAP4 from microtubules in vivo produces no observable phenotype at the cellular level. J Cell Biol. 1996;132:345–357. doi: 10.1083/jcb.132.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E, Winey M. The Saccharomyces cerevisiae spindle pole body duplication gene MPS1is part of a mitotic checkpoint. J Cell Biol. 1996;132:111–123. doi: 10.1083/jcb.132.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yew N, Mellini ML, Vande GF, Woude Meiotic initiation by the mos protein in Xenopus. . Nature (Lond) 1992;355:649–652. doi: 10.1038/355649a0. [DOI] [PubMed] [Google Scholar]