Abstract
The transport of the two mannose 6-phosphate receptors (MPRs) from the secretory pathway to the endocytic pathway is mediated by carrier vesicles coated with the AP-1 Golgi-specific assembly protein and clathrin. Using an in vitro assay that reconstitutes the ARF-1–dependent translocation of cytosolic AP-1 onto membranes of the TGN, we have previously reported that the MPRs are key components for the efficient recruitment of AP-1 (Le Borgne, R., G. Griffiths, and B. Hoflack. 1996. J. Biol. Chem. 271:2162–2170). Using a polyclonal antibody against the mouse γ-adaptin, we have now examined the steady state distribution of AP-1 after subcellular fractionation of mouse fibroblasts lacking both MPRs or reexpressing physiological levels of either MPR. We report that the amount of AP-1 bound to membranes and associated with clathrin-coated vesicles depends on the expression level of the MPRs and on the integrity of their cytoplasmic domains. Thus, these results indicate that the concentration of the MPRs, i.e., the major transmembrane proteins sorted toward the endosomes, determines the number of clathrin-coated vesicles formed in the TGN.
In eukaryotic cells, clathrin-coated vesicles mediate the sorting of membrane proteins such as the mannose 6-phosphate receptors (MPRs)1 from the TGN for subsequent transport to endosomal compartments as well as for the endocytosis of plasma membrane receptors (Pearse and Robinson, 1990; Kornfeld and Mellman, 1989). More recently, clathrin-coated buds have also been found associated with membranes of early endosomes (Stoorvogel et al., 1996). While very little is known about these latter structures, the TGN- and plasma membrane– derived clathrin-coated vesicles have been extensively characterized and are now distinguished by the nature of their assembly proteins, AP-1 and AP-2, respectively (Morris et al., 1989; Pearse and Robinson, 1990).
The heterotetrameric structure of these two related assembly proteins suggests that they can interact with multiple components. Both AP-1 and AP-2 promote clathrin cage assembly in vitro via their ∼110-kD β1 and β2 subunits (Ahle et al., 1988; Ahle and Ungewickell, 1989; Gallusser and Kirchhausen, 1993). They also bind in vitro to cytoplasmic domains of membrane receptors (Pearse, 1988; Glickman et al., 1989; Beltzer and Spiess, 1991; Sosa et al., 1993) and interact with their tyrosine- or di-leucine–based sorting motifs (Heilker et al., 1996), known to be important for endocytosis and lysosomal targeting of membrane proteins (Letourneur and Klausner, 1992; for review see Sandoval and Bakke, 1994). In addition, the two-hybrid system in yeast has revealed that both the ∼50-kD μ-1 and μ-2 subunits of AP-1 and AP-2 interact with tyrosinebased endocytosis motifs (Ohno et al., 1995). The function of the ∼100-kD γ and α subunits as well as that of the ∼20-kD σ1 and σ2 subunits of AP-1 or AP-2 complexes remains unknown at present.
Although it is still difficult to understand the molecular basis for the specific interaction of the two related APs with a given membrane, some key cytosolic factors involved in their recruitment have been identified. A number of studies have now illustrated the functional role of the small GTPase ARF-1, a member of the Ras superfamily, in the regulation of the interaction of cytosolic AP-1 with TGN membranes (Stamnes and Rothman, 1993; Traub et al., 1993). This GTPase was first shown to regulate the membrane association of the coatomer, the coat components of COPI-coated vesicles involved in vesicular traffic in the early secretory pathway (Balch et al., 1992; Taylor et al., 1992; Palmer et al., 1993). However, it is still unclear whether ARF-1 acts as a stoichiometric (Serafini et al., 1991) or a catalytic component activating phospholipase D (Ktistakis et al., 1995; Boman and Kahn, 1995) or both. Genetic approaches in yeast have also revealed the importance of the Vps15p/Vps34p complex, a serine/threonine kinase and a phosphatidylinositol (PI) 3-kinase, respectively, in the sorting of vacuolar/lysosomal enzymes from the biosynthetic pathway (Herman et al., 1992). Conversely, the treatment of mammalian cells with wortmannin, a specific inhibitor of PI 3-kinases, results in a drastic missorting of lysosomal enzymes (Brown et al., 1995; Davidson, 1995), suggesting that the AP-1–dependent sorting of their mannose 6-phosphate receptors is impaired.
The fundamental mechanisms by which membranes receptors are specifically segregated into clathrin-coated vesicles has been a difficult question to address. Two distinct models have been proposed. First, receptors can simply be trapped into preexisting coated structures or, second, the receptors actively initiate the formation of coated vesicles. Morphometric analyses performed at the EM level first suggested that the massive overexpression of the human transferrin receptor in mouse cells correlated with a higher number of clathrin-coated pits at the plasma membrane (Iacopetta et al., 1988). Such overexpression in chicken embryonic fibroblasts did not change the number of coated pits but rather increased the number of flat clathrin lattices (Miller et al., 1991). More recent immunofluorescence studies in which FcεRI receptors were relocalized to restricted areas of the plasma membrane could not detect any change in the redistribution of clathrin and assembly protein AP-2 (Santini and Keen, 1996). Thus, whether membrane receptor sorting and clathrin-coated vesicle formation are coupled processes or not has remained controversial so far.
Our previous in vitro studies on fibroblasts genetically devoid of the two MPRs have suggested that these transmembrane proteins are key components for the efficient translocation of cytosolic AP-1 onto membranes of the TGN and possibly on membranes of early endosomes (Le Borgne et al., 1993, 1996). In addition, specific protein motifs in the MPR cytoplasmic domains are important for the high affinity interaction of AP-1 with TGN membranes in vitro (Mauxion et al., 1996). In the present study, we have raised an antibody against the mouse γ-adaptin to directly determine the steady state distribution of AP-1 in mouse fibroblasts lacking MPR expression or reexpressing physiological levels of the MPRs. After cellular fractionation, the results indicate that the expression level of the MPRs not only determines the amount of AP-1 bound to membranes but also determines the number of clathrin-coated vesicles formed in the TGN. We conclude that, in vivo, membrane protein sorting and clathrin-coated vesicle formation in the TGN are coupled processes.
Materials and Methods
Materials
All reagents were of analytical grade. Mouse Apo-Transferrin, Ficoll, and 2H2O were from Sigma Chemical Co. (St. Louis, MO). Ribonuclease A was from Worthington Enzymes Ltd. (Freehold, NJ). 30% (wt/vol) acrylamide/0.8% (wt/vol) bis-acrylamide solution was from National Diagnostics (Atlanta, GA). Protran nitrocellulose membranes (0.45 μm) were from Schleicher and Schuell (Dassel, Germany). Pronase was from Boehringer Mannheim GmbH (Mannheim, Germany). Iodogen was from Pierce Chemical Co. (Rockford, IL). Na-125I and ECL detection system were from Amersham Life Science (Amersham, UK).
Cell Culture
Mouse MPR-negative fibroblasts and MPR-negative fibroblasts stably reexpressing either MPR were generated as previously described (Ludwig et al., 1994; Mauxion et al., 1996; Munier-Lehmann et al., 1996) and grown in DME medium complemented with 10% FCS, 2 mM glutamine, 100 U/ml penicillin, and 100 U/ml streptomycin.
Antibodies
γ-Adaptin and the ubiquitously expressed αC-adaptin (Robinson, 1989) were detected using polyclonal sera prepared by immunizing rabbits with the K-259-274 peptide (K-RNDDDSSEAMNDILAQ) and the K-623-637 peptide (K-STVTDLEETKRERSI), respectively, coupled to keyhole limpet hemocyanin. The crude rabbit sera were further affinity purified by passing them on columns made of the corresponding immunogenic peptides coupled to Affigel-10. The specific Igs were eluted with 4 M MgCl2 and extensively dialyzed against PBS before use.
The antiserum against the αC-adaptin detected a single ∼110-kD band by Western blotting comigrating with a ∼110-kD protein detected with the anti–α-adaptin AP-6 mAb. In indirect immunofluorescence, our polyclonal serum and the AP-6 mAb decorated the same structures (not shown).
The antiserum against the mouse γ-adaptin (the trunk region) recognized two proteins on Western blots. The ∼100-kD protein was identified as the γ-adaptin. This protein is also recognized by a polyclonal antibody directed against the hinge region of the mouse γ-adaptin (a kind gift from M. Robinson, Cambridge, UK; Seaman et al., 1996) and is immunoprecipitated from denatured Hela cells lysates by the monoclonal 100/3 anti–γ-adaptin antibody. The higher molecular mass ∼120-kD protein cross-reacting with our anti-peptide antibody does not cross-react with a polyclonal antibody directed against the hinge region of the mouse γ-adaptin, and therefore is most likely a contaminant. In addition, the in vitro recruitment of this 120-kD protein on membranes is insensitive to the addition of brefeldin A or GTPγS (data not shown). In immunofluorescence, the polyclonal antipeptide antibody against the trunk region of the mouse γ-adaptin decorates the perinuclear region and peripheral structures of mouse fibroblasts and Hela cells as previously observed with the monoclonal 100/3 anti–γ-adaptin antibody. Furthermore, this staining becomes completely cytosolic after a 1–2-min treatment with 5 μg/ml brefeldin A as expected for AP-1.
The rabbit serum against clathrin was as described (Méresse and Hoflack, 1993). The cation-independent (CI)–MPR was detected with a polyclonal antibody directed against a peptide corresponding to the carboxy-terminal domain of the receptor (Méresse and Hoflack, 1993). The mouse transferrin receptor was detected using the H68-4 mAb (Zymed Laboratories, S. San Francisco, CA) directed against the conserved cytosolic domain of human transferrin receptor. β-COP was detected using the E5A3 mAb. Goat anti–mouse and goat anti–rabbit secondary antibodies coupled to HRP were from Dianova-Immunotech GmbH (Hamburg, Germany).
Immunoprecipitations
Confluent Hela cells were washed twice with ice-cold PBS, scraped with a rubber policeman, and spun for 5 min at 2,000 g. A postnuclear supernatant was then prepared in lysis buffer (LB; 50 mM Tris, pH 7.4, 100 mM NaCl, 2 mM EDTA, 1 mM PMSF and benzamidine, 5 μg/ml aprotinin, and 1 μg/ml leupeptin). The sample was then supplemented with 1% Triton X-100 (final) for 30 min on ice (native sample) or boiled in 1% SDS for 5 min (denaturated sample). After diluting the SDS 10 times, the samples were spun for 15 min in an Eppendorf centrifuge. The supernatant was then precleared with protein A–Sepharose for 1 h at 4°C and spun for 15 min. The supernatant was incubated overnight at 4°C on a rotating wheel with either 1 μg of our polyclonal anti–γ-adaptin (1573) or 1 μg of the 100/3 monoclonal anti–γ-adaptin antibody. After spinning down for 15 min, 50 μl of a 50% slurry of protein A–Sepharose (Pharmacia Fine Chemicals Inc., Piscataway, NJ) was added to the supernatant for an additional hour. An aliquot of the supernatant of the immunoprecipitation (10%) was kept for Western blotting analysis. The beads were then washed three times in LB, three times in LB + 500 mM NaCl, and once in LB. After boiling in sample buffer twice for 5 min each, the samples were analyzed by Western blotting.
Immunofluorescence
Cocultures of MPR-positive and MPR-negative fibroblasts were grown on glass coverslips, washed in PBS, and fixed with 3% paraformaldehyde. After an extraction with 0.1% Triton X-100, the cells were incubated with the monoclonal 1D4B anti–mouse Lamp-1 antibody (Developmental Studies Hybridoma Bank, Iowa City, IA) and an affinity-purified rabbit antibody raised against the hinge region of the mouse γ-adaptin (a kind gift from S. Robinson; Seaman et al., 1996). The bound antibodies were detected with FITC- or Texas red–labeled secondary antibodies (DianovaImmunotech GmbH).
Subcellular Fractionation
Mouse fibroblasts were grown in complete DME medium supplemented with 20 mM Hepes. The cells were washed twice with ice-cold PBS and once with vesicle buffer (140 mM sucrose, 75 mM potassium acetate, 10 mM MES, pH 6.6, 1 mM EGTA, 0.5 mM magnesium acetate). The cells were then scraped with a rubber policeman, and DTT (1 mM) and protease inhibitors (1 μg/ml chymostatin, 1 μg/ml pepstatin, 1 mM PMSF, 1 mM benzamidin, 1 μg/ml antipain) were added. After centrifugation for 10 min at 2,000 g, the cells were resuspended in vesicle buffer. An aliquot was directly boiled in Laemmli buffer (total cell extract). Cells were then homogenized by 10–20 passages through a 22G3/4 needle connected to a 1-ml syringe. The postnuclear supernatant obtained after centrifugation at 900 g for 10 min was spun at 100,000 g for 1 h in a TLA 45 rotor (Beckman Instruments, Inc., Palo Alto, CA) at 4°C. The pellet (total membrane fraction) was resuspended in vesicle buffer, and equivalent amounts of proteins (30 μg per lane) from the different cell types were analyzed by SDS-PAGE and Western blotting.
Clathrin-coated vesicles from the different cell types were isolated as described by Woodman and Warren (1991). For each clone, six to eight tissue-culture plates (24.5 × 24.5 cm) were used. A postnuclear supernatant was prepared, treated with 25 μg/ml ribonuclease A for 30 min on ice, and spun for 30 min at 7,000 g to obtain a postmitochondrial supernatant. This supernatant was applied on top of a 10-ml continuous gradient of 10– 90% (wt/vol) 2H2O in vesicle buffer and spun at 45,000 g for 30 min in an SW 40 rotor at 4°C. 1-ml fractions were collected from the top, and fractions 2 to 6 were pooled, diluted threefold in vesicle buffer, and spun for 2 h at 150,000 g in a SW 40 rotor (Beckman Instruments, Inc.) at 4°C. The resulting pellet was resuspended in 2 ml of vesicle buffer, and equivalent amounts of proteins were loaded on top of a 10-ml continuous gradient from 2% (wt/vol) Ficoll/9% (wt/vol) 2H2O to 20% Ficoll/90% 2H2O in vesicle buffer containing 1 mM DTT. This gradient was spun to equilibrium at 4°C in a SW 40 rotor at 23,000 rpm for 16 h. 1-ml fractions were collected from the bottom, and half of each fraction was precipitated with TCA (10% final concentration) for Western blotting analysis.
Electrophoresis, Western Blotting, and Software Analysis
SDS-PAGE was performed using the mini-PROTEAN II electrophoresis system (Bio Rad Laboratories, Hercules, CA). After SDS-PAGE, the proteins were electroblotted onto nitrocellulose for 2 h at 80 V. The membrane was then incubated in blocking buffer (5% defatted milk and 0.05% Tween-20 in PBS) for 2 h and with the primary antibodies diluted in blocking buffer for 2 h at room temperature. After washing, the membranes were incubated with secondary antibodies for 1 hour in blocking buffer. HRP-labeled secondary antibodies were detected using the ECL system (Amersham Life Science). The same blots were sequentially probed with the different antibodies. For this process, between each immunodetection, the membranes were stripped in 62.5 mM Tris-HCl, pH 6.7, 2% SDS, and 100 mM 2-mercaptoethanol for 30 min at 50°C. For quantitations of the signals, different undersaturating exposures of the autoradiograms were scanned using a Microtek scanmaker III scanner at a resolution of 300 dpi (Microtek Electronics Europe, GmbH, Düsseldorf, Germany). The intensities of the signals were quantitated using the NIH Image 1.59/ppc software. Mounting of the figures was performed using Adobe PhotoshopTM 3.0.5 (Adobe Systems, Inc., Mountain View, CA).
Endocytosis of Transferrin
Purified mouse Apo-transferrin (mTf) was first loaded with iron by incubation in a saturation buffer (250 mM Tris-HCl, 10 μM NaHCO3, 2 mM sodium nitrillobicitrate, and 6 mM FeCl3) for 3 h at room temperature. After extensive dialysis against PBS, mTf was iodinated with Iodogen as described in Podbilewicz and Mellman (1990). For internalization studies, triplicate samples of each cell type were grown in 35-mm-diam dishes, washed with PBS, and incubated for 1 h at 37°C in DME medium containing 0.1% BSA and 20 mM Hepes to deplete the cells of bound, bovine Tfn from the culture medium. The cells were then placed on ice and incubated for 1 h with 100 nM mTf (25 nM 125I-mTf + 75 nM cold mTf) in DME medium containing 0.1% BSA and 20 mM Hepes. Cells were then incubated at 37°C for different periods of time (0.5, 1, 2.5, and 5 min). After extensive washing in DME medium, 0.1% BSA, 20 mM Hepes and DME, and 20 mM Hepes, cell surface–bound mTf was removed by pronase treatment (3 mg/ml for 1 h on ice). The cells and the medium were collected and spun down in an Eppendorf centrifuge for 2 min. Both the supernatant (released transferrin) and the cell pellet were quantitated by γ-radiation counting. Endocytosed 125I-Tf was estimated as the residual radioactivity in the cell pellet after pronase treatment and expressed as a percentage of the total cell surface–bound mTf at 4°C.
EM-negative Staining
Material contained in the dense fractions of the last linear density gradient (fractions 8 to 10) was pooled, diluted in MES buffer (0.1 M MES, pH 6.5, 1 mM EGTA, and 0.5 mM MgCl2), and spun at 150,000 g for 1 h. The pellet was carefully resuspended in MES buffer, and the vesicles were adsorbed on a carbon-treated EM grid for 1 min, rapidly washed, and stained with 1% uranyl acetate for 1 min. The dry samples were observed on a transmission electron microscope (Philips Electronic Instruments, Inc., Mahwah, NJ).
Results
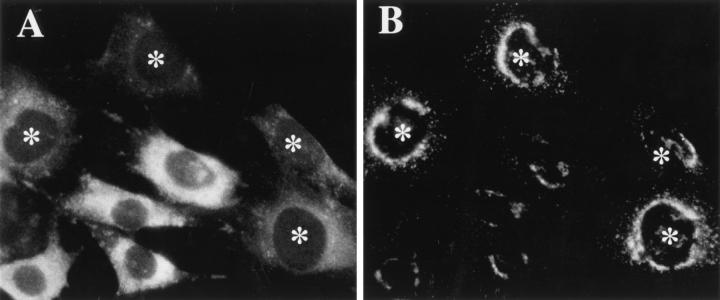
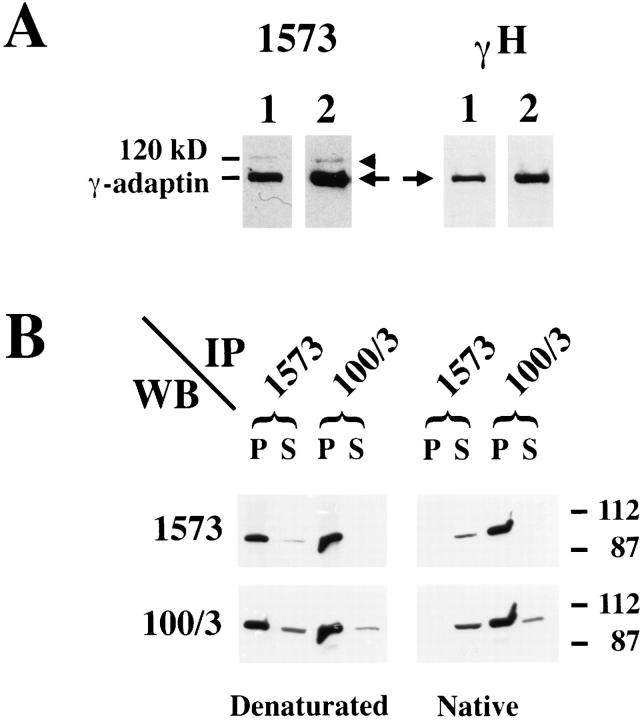
Our previous in vitro studies on MPR-deficient mouse fibroblasts have strongly suggested that the MPRs are key components for the efficient translocation of cytosolic AP-1 onto membranes. These studies were based on an in vitro assay reconstituting the binding of cytosolic bovine AP-1 on membranes of permeabilized mouse fibroblasts. The newly bound AP-1 was detected with the 100/3 anti– γ-adaptin mAb, unable to recognize the endogenous mouse AP-1 (Le Borgne et al., 1993, 1996). When labeled with an antibody against the hinge region of γ-adaptin (Seaman et al., 1996), the mouse fibroblasts expressing the two MPRs exhibit the typical, perinuclear γ-adaptin staining (Fig. 1). In contrast, the fibroblasts devoid of the MPRs, easily identified by the large number of Lamp-1–positive structures as a result of the massive missorting of lysosomal enzymes (Ludwig et al., 1994), show a reduced γ-adaptin staining (Fig. 1). To quantitate by Western blotting the steady state distribution of γ-adaptin in these cells, we have used a polyclonal antibody against a peptide corresponding to a stretch of amino acids (R259–Q274) contained in the trunk domain of the mouse γ-adaptin, also conserved in the human γ-adaptin. Fig. 2 A shows that, in Western blot analysis, this anti–mouse γ-adaptin antibody reacts with two proteins of mouse fibroblasts. The first protein is the ∼100-kD γ-adaptin because it is also recognized by a polyclonal antibody directed against the hinge region of γ-adaptin, while the second, ∼120-kD protein, not recognized by the polyclonal antibody against the hinge region of the mouse γ-adaptin and absent from purified clathrin-coated vesicles (see Fig. 5), most likely represents a contaminant. Furthermore, Fig. 2 B shows that this anti-peptide antibody immunoprecipitates the γ-adaptin from a total, denatured Hela cell lysate as does the 100/3 mAb. However, this antibody does not immunoprecipitate the native protein.
Figure 1.
AP-1 immunostaining in MPR-positive and MPR-negative mouse fibroblasts. MPR-positive and MPR-negative fibroblasts were cocultured on coverslips, fixed, and processed for double immunofluorescence using an mAb against Lamp-1 taken as a lysosomal marker (A) and a polyclonal antibody against the hinge region of the mouse γ-adaptin (B) as indicated in Materials and Methods. The MPR-negative fibroblasts are easily identified by the large number of Lamp-1–positive structures; (stars) MPR-positive fibroblasts.
Figure 2.
Characterization of the polyclonal anti–γ-adaptin antibody. (A) Postnuclear supernatants (lane 1) or total membranes (lane 2) were prepared from mouse fibroblasts. The samples were fractionated by SDS-PAGE, transferred to nitrocellulose, and analyzed by Western blotting using a polyclonal antibody against a peptide corresponding to the trunk region (1573; right) or against the hinge region of the mouse γ-adaptin (γ-H; left). (Arrow) γ-Adaptin; (arrowhead) a 120-kD contaminant. (B) γ-Adaptin was immunoprecipitated from denaturated (left) or native (right) HeLa cell lysates with either the polyclonal antibody (1573) or with the mAb 100/3 as described in Materials and Methods. Both the immunoprecipitated material (P) and a fraction (10%) of the supernatant of the immunoprecipitations (S) were analyzed by Western blotting with either the 1573 or the 100/3 antibodies.
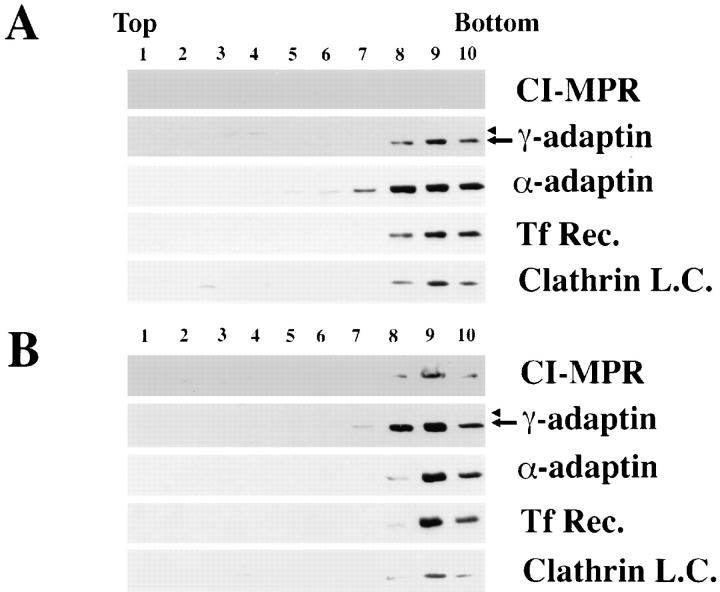
Figure 5.
Distribution of marker proteins on linear Ficoll/2H2O density gradients. Clathrin-coated vesicles were purified from mock-transfected MPR-negative fibroblasts (A) and MPR-negative fibroblasts reexpressing the CI-MPR (clone CI-4) (B) as described in Materials and Methods. Each fraction from the last linear density gradient was analyzed by Western blotting for its content in CI-MPR, γ-adaptin (arrow), α-adaptin, transferrin receptor (Tf Rec.), and clathrin light chains (Clathrin L.C.).
Expression of Protein Markers in MPR-deficient Fibroblasts
We first determined by quantitative Western blotting the level of expression of different markers in MPR-negative fibroblasts and in MPR-negative fibroblasts stably reexpressing physiological levels of either the cation-dependent (CD) MPR or the insulin-like growth factor II/CIMPR (Ludwig et al., 1994; Le Borgne et al., 1996; Mauxion et al., 1996). Fig. 3 shows the relative amounts of γ-adaptin (AP-1), α-adaptin (AP-2), lysosomal marker Lamp-1, or transferrin receptor, a membrane protein recycling between the plasma membrane and the endosomes, as well as that of β-COP, a subunit of the coatomer chosen as a marker of the early secretory pathway in the total cell lysates of the different clones examined. The expression level of each of these markers was nearly identical in all of the different cell types examined. In addition, the ratios of γ-adaptin/α-adaptin (Fig. 3 B), γ-adaptin/transferrin receptor (Fig. 3 C), or γ-adaptin/β-COP (not shown) were very similar in all of the different cell types examined. This indicates that the expression level of these different markers is, as expected, independent of the expression of the MPRs.
Figure 3.
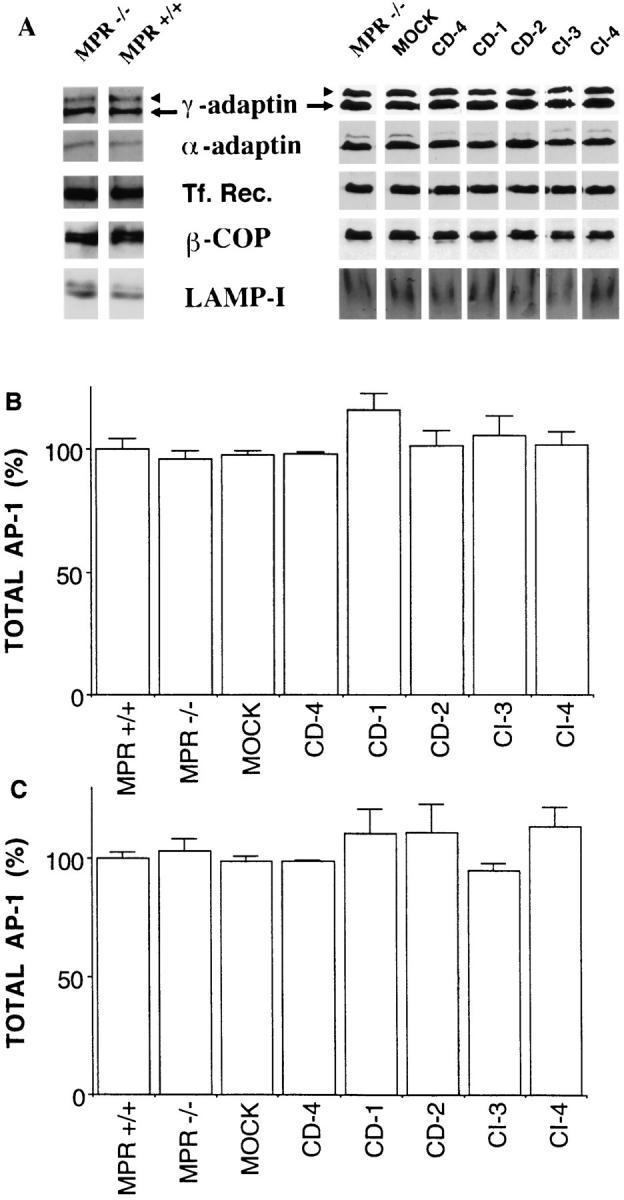
Expression of different marker proteins in MPR-deficient fibroblasts. Total cell extracts of MPR-positive or MPRnegative fibroblasts and MPR-negative fibroblasts reexpressing either MPR were prepared as described in Materials and Methods. Similar amounts of proteins (30 μg) were resolved on SDS-PAGE, transferred onto nitrocellulose, and sequentially analyzed by Western blotting for their content in γ-adaptin (arrow), α-adaptin, transferrin receptor (Tf. Rec.), β-COP (coatomer), and Lamp-1 (A). The γ-adaptin signal was quantitated and then normalized to that of the α-adaptin (B) or transferrin receptor (C) as indicated in Materials and Methods. MPR −/−, MPR-negative fibroblasts; MPR +/+, control fibroblasts expressing the two MPRs; MOCK, mock-transfected MPR-negative cells; CD-4, -1, and -2, MPRnegative fibroblasts reexpressing 1.5-, 3.5-, and 4.4-fold the endogenous level of CD-MPR, respectively; CI-3 and -4, MPR-negative fibroblasts reexpressing one- and fivefold the endogenous level of CI-MPR, respectively. Values correspond to means ± standard error of four independent experiments. When compared with mock-transfected MPR-negative fibroblasts, the sample populations were not found to be significantly different according to the t test.
AP-1 Bound to Membranes of MPR-deficient Fibroblasts
We then prepared microsomal fractions from the different clones and determined by quantitative Western blot analysis the amount of α- and γ-adaptins bound to membranes at steady state. The amount of membrane-bound α-adaptin was very similar in the different clones tested and represented ∼36.7% ± 4.7% of the total α-adaptin. We also determined the internalization rate of the transferrin receptor to follow the dynamics of its AP-2–dependent endocytosis. For this process, the different mouse fibroblasts were first incubated at 4°C with iodinated mouse transferrin, washed to remove the unbound ligand, and subsequently incubated at 37°C for various periods of time. Table I shows that the internalization rate of the transferrin receptor was similar in all of the different clones tested. These results indicate that the dynamics of endocytosis is not significantly affected by the reexpression of physiological levels of MPRs. Furthermore, our previous studies showing that these different clones secrete similar amounts of proteins (Mauxion et al., 1996; Munier-Lehmann et al., 1996) and the fact that the level of β-COP expression is unchanged suggest that the dynamics of the COPI-dependent vesicular traffic in the early secretory pathway is also not affected.
Table I.
Internalization Rate of the Transferrin Receptor
| Cell type | Internalized m-Tf (percentage of prebound) | |
|---|---|---|
| % | ||
| MOCK | 65.6 ± 3.8 | |
| CD-4 | 62.1 ± 3.5 | |
| CD-1 | 60.0 ± 7.9 | |
| CD-2 | 70.6 ± 8.7 | |
| CI-3 | 68.9 ± 7.2 | |
| CI-4 | 67.5 ± 7.2 |
Mock-transfected MPR-negative fibroblasts or MPR-negative fibroblasts reexpressing the CD-MPR (1.4-, 3.5-, and 4.4-fold the endogenous level of CD-MPR for CD-4, -1, and -2, respectively) or the CI-MPR (one- and fivefold the endogenous level for CI-3 and -4, respectively) were incubated at 4°C with iodinated mouse transferrin, washed, and subsequently incubated at 37°C for various periods of time (see Materials and Methods). The indicated values represent the percentage of prebound transferrin internalized during the first minute of uptake. Values correspond to means ± standard error of two independent experiments performed in triplicate.
Since the rate of endocytosis (membrane-bound AP-2 and number of plasma membrane–derived clathrin-coated vesicles formed) is unchanged in these different clones, the amount of membrane-bound γ-adaptin could be normalized to that of α-adaptin or the transferrin receptor. In MPR-positive fibroblasts, 30.5 ± 3.2% of the total AP-1 was typically found associated with membranes. Fig. 4 shows that membranes of MPR-negative fibroblasts contain three times less γ-adaptin than membranes of the corresponding MPR-positive fibroblasts (∼9.6 ± 1.8% of the total AP-1). In addition, the examination of stable clones of MPR-negative fibroblasts reexpressing various amounts of either CDMPR or CI-MPR indicated that the amount of membranebound γ-adaptin increased upon the reexpression of either MPR in MPR-negative fibroblasts. These results show that, in vivo, the amount of γ-adaptin bound to membranes at steady state correlates, to some extent, with physiological levels of expression of the MPRs. It is unlikely that these differences in amounts of bound-AP-1 reflect an indirect effect as a result of the large number of Lamp-1–positive structures in MPR-negative fibroblasts. MPR-negative fibroblasts reexpressing physiological levels of either MPR still contain numerous Lamp-1–positive structures (Munier-Lehmann et al., 1996). However, the amount of bound AP-1 is significantly increased in these cells.
Figure 4.
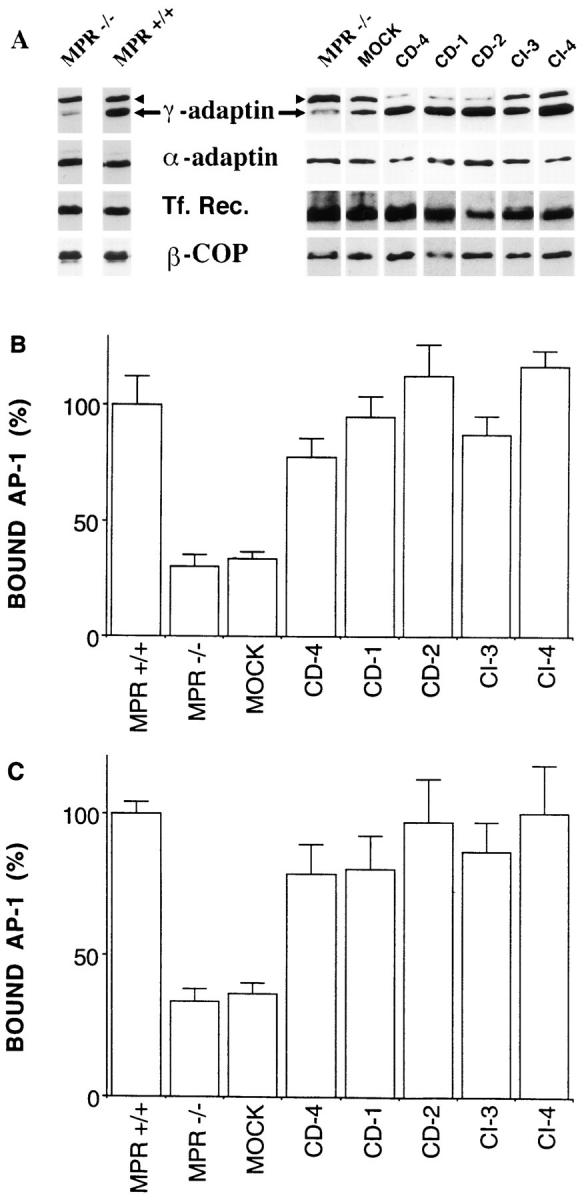
Membrane-bound γ-adaptin and MPR expression. Microsomal membranes of MPR-deficient fibroblasts were prepared as described in Materials and Methods and analyzed by Western blotting for their content in γ-adaptin (arrow), α-adaptin, transferrin receptor, or β-COP (A). The amount of γ-adaptin bound to microsomal membranes was then quantitated and normalized to that of α-adaptin (B) or transferrin receptor (C). (MPR −/−, MPR-negative fibroblasts; MPR +/+, control fibroblasts expressing the two MPRs; MOCK, mock-transfected MPRnegative cells; CD-4, -1, and -2, MPR-negative fibroblasts reexpressing 1.5-, 3.5-, and 4.4-fold the endogenous level of CD-MPR, respectively; CI-3 and -4, MPR-negative fibroblasts reexpressing one- and fivefold the endogenous level of CI-MPR, respectively. Values represent means ± standard error of four independent experiments. When MPR-positive fibroblasts or MPR-negative fibroblasts reexpressing either MPR were compared with the mock-transfected MPR-negative fibroblasts, the confidence limits of the sample populations were found to be >99% in every case based on the t test.
AP-1 Associated with Clathrin-coated Vesicles of MPR-deficient Fibroblasts
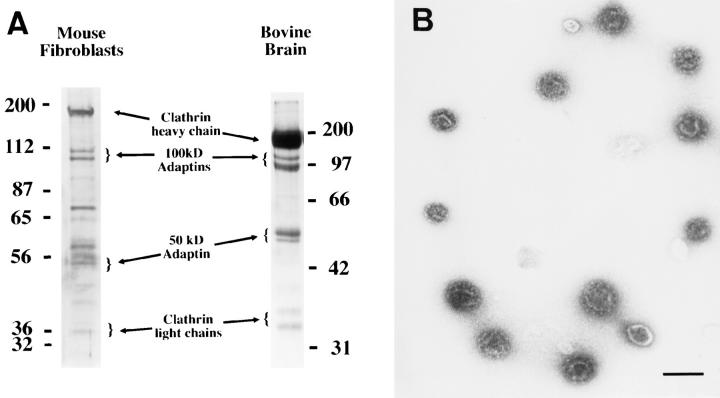
The membrane-bound γ-adaptin represents AP-1 bound to the donor compartment as well as AP-1 present in transport vesicles. Thus, clathrin-coated vesicles were prepared from the different MPR-deficient fibroblasts using the fractionation protocol described previously by Woodman and Warren (1991). Fig. 5 shows a typical example of the distribution of several marker proteins throughout the density gradient of the last purification step after fractionation of MPR-deficient fibroblasts. Typically, the dense fractions contained the clathrin light chain, the γ- and α-adaptins, as well as transmembrane proteins like the transferrin receptor and the CI-MPR, as determined by Western blotting. Very low amounts of β-COP were occasionally detected in the lighter fractions of this gradient (not shown). When analyzed by SDS-PAGE followed by protein staining, the dense fractions exhibited the typical protein profile of purified clathrin-coated vesicles (Fig. 6 A). Beside a few contaminants, the clathrin heavy and light chains as well as the different subunits of the APs were easily detected. At the morphological level, these fractions contained only spherical structures of ∼50–100 nm in diameter with a coat lattice reminiscent of clathrin-coated vesicles (Fig. 6 B).
Figure 6.
Characterization of clathrin-coated vesicles isolated from MPR-deficient fibroblasts. The material contained in fractions 8 to 10 of the density gradients shown in Fig. 5 B were concentrated by centrifugation and analyzed. (A) Protein profile of the vesicles isolated from mouse fibroblasts after SDSPAGE and silver staining (left). Clathrin-coated vesicles from bovine brain were used for comparison (right). (B) Clathrin-coated vesicles purified from mouse fibroblasts observed by negative staining. Bar, 100 nm.
The amount of γ-adaptin present in clathrin-coated vesicles isolated from the different MPR-deficient fibroblasts was analyzed by quantitative Western blotting. Since the dynamics of endocytosis is unchanged in the different cell types, indicating that the number of plasma membrane– derived clathrin-coated vesicles remains unchanged, these values for γ-adaptin could be normalized to the amount of α-adaptin or transferrin receptor present in these fractions. Fig. 7 shows that the clathrin-coated vesicle fractions obtained from MPR-negative fibroblasts contain three times less γ-adaptin than those isolated from MPR-positive fibroblasts. Moreover, the purification of clathrin-coated vesicles from MPR-negative fibroblasts reexpressing the MPRs indicated that the amount of γ-adaptin recovered in clathrin-coated vesicle fractions increased upon the reexpression of physiological levels of either MPR. Since the amount of γ-adaptin found in these fractions reflects a number of vesicles, we conclude from these data that the number of TGN-derived, clathrin-coated vesicles found at steady state in mouse fibroblasts depends on the number of MPR molecules expressed at physiological levels.
Figure 7.
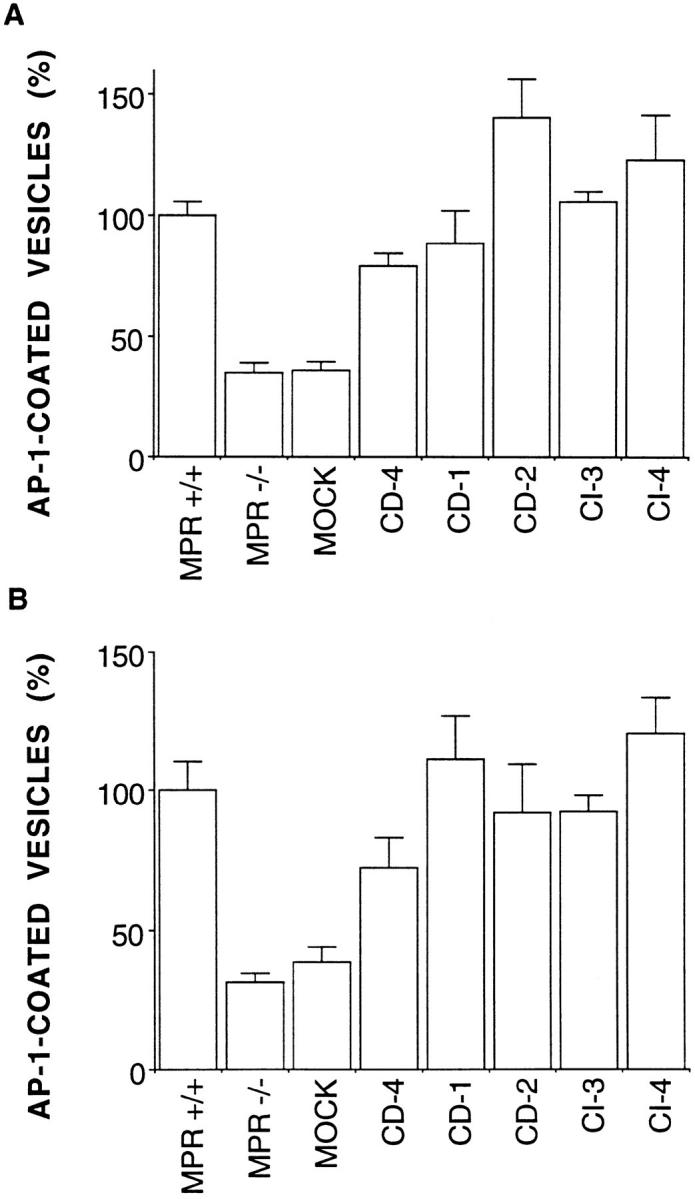
AP-1–coated vesicles and MPR expression. Clathrincoated vesicles were isolated from the different MPR-negative fibroblasts, and their content in γ-adaptin, α-adaptin, and transferrin receptor was analyzed by quantitative Western blotting as shown in Fig. 4. The amount of γ-adaptin was then normalized to that of α-adaptin (A) or transferrin receptor (B). MPR −/−, MPR-negative fibroblasts; MPR +/+, control fibroblasts expressing the two MPRs; MOCK, mock-transfected MPR-negative fibroblasts; CD-4, -1, and -2, MPR-negative fibroblasts reexpressing 1.5-, 3.5-, and 4.4-fold the endogenous level of CD-MPR, respectively; CI-3 and -4, MPR-negative fibroblasts reexpressing one- and fivefold the endogenous level of CI-MPR, respectively. Values correspond to the mean ± standard error of two (CD-1 and CI-3) and three (CD-4, CD-2, and CI-4) independent experiments. When MPRpositive fibroblasts or MPR-negative fibroblasts reexpressing either MPR were compared with the mock-transfected MPR-negative fibroblasts, the confidence limits of the sample populations were found to be >99% in every case based on the t test.
Affinity of AP-1 for Membranes In Vitro and Clathrin-coated Vesicle Formation In Vivo
Our previous in vitro analysis based on the reexpression of CD-MPR mutants in MPR-negative fibroblasts has indicated that the high affinity binding of AP-1 to membranes (K d ∼40 nM) relies on the presence of specific determinants in the CD-MPR cytoplasmic domain (Mauxion et al., 1996). In particular, we observed that mutations introduced in the casein kinase II phosphorylation site present in its carboxyl-terminal domain (mutant A565859 according to Mauxion et al., 1996) significantly reduce the affinity of AP-1 for membranes (K d ∼120 nM) without affecting the number of AP-1 binding sites. This mutant is also drastically, but not completely, impaired in proper transport of lysosomal enzymes in vivo (35% of sorting efficiency). Therefore, these stable clones gave us the opportunity to investigate the relationship between the affinity of AP-1 for its target membrane in vitro and its efficiency in producing TGN-derived vesicles in vivo. Toward this goal, a similar type of analysis, as described above, was performed on these MPR-negative fibroblasts reexpressing a CD-MPR mutated on the casein kinase II phosphorylation site. In these stable clones, the expression level of the different marker proteins (α-adaptin, γ-adaptin, β-COP, and transferrin receptor) was very similar to that of MPR-positive or MPR-negative fibroblasts (not shown). Fig. 8 shows that the amount of γ-adaptin recovered at steady state bound to membranes or recovered in clathrin-coated vesicle fractions was far less abundant in MPR-negative fibroblasts reexpressing this CD-MPR mutant than in those reexpressing similar levels of wild-type CD-MPR. However, the amount of γ-adaptin bound to membranes or recovered associated with purified clathrin-coated vesicles was slightly higher in these cells than in MPR-negative fibroblasts. These data show that this CD-MPR mutant is unable to efficiently recruit AP-1 on membranes and to produce TGN-derived clathrin-coated vesicles. Thus, this CD-MPR mutant must be largely excluded from the AP-1–dependent pathway toward the endosomes. These results further highlight the importance of the casein kinase II phosphorylation site in the AP-1–dependent sorting of the CD-MPR and suggest that only high affinity interactions of AP-1 with its target membrane determine the production of AP-1 and clathrin-coated vesicles in vivo.
Figure 8.
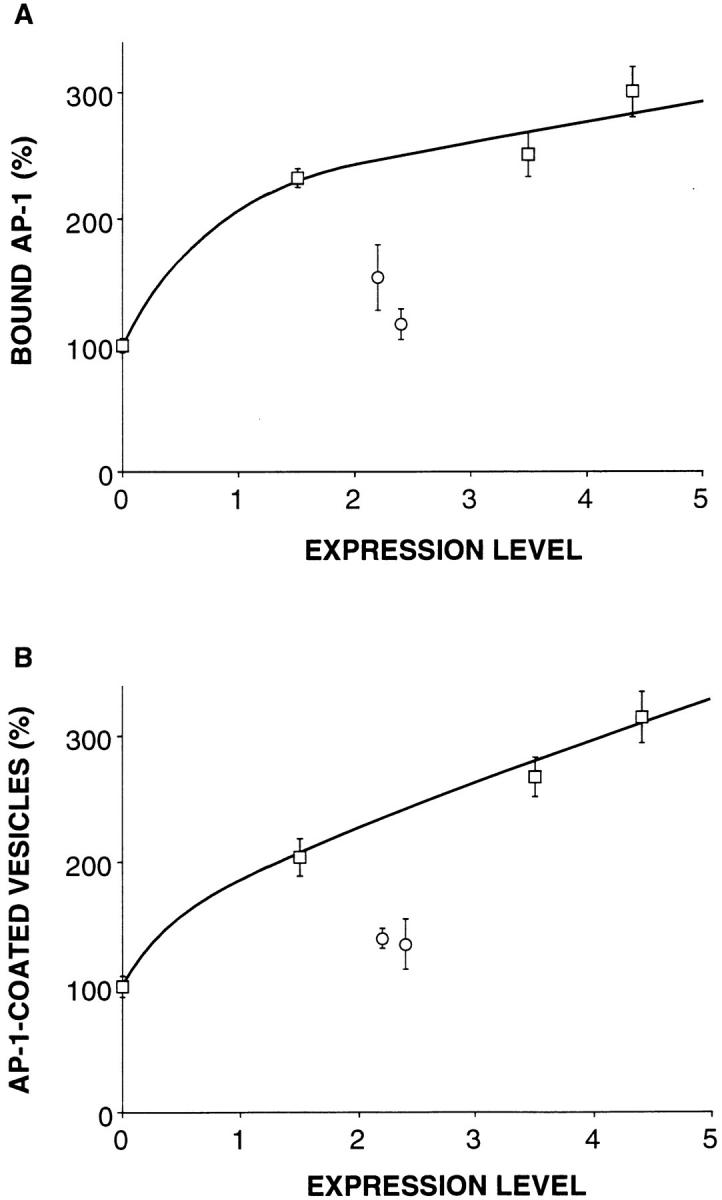
Mutations in the CD-MPR cytoplasmic domain and formation of AP-1–coated vesicles. Microsomal membranes and clathrin-coated vesicles were prepared from MPR-negative fibroblasts reexpressing either the wild-type CD-MPR (squares) or a CD-MPR mutated on the casein kinase II phosphorylation site present in its cytoplasmic domain (circles). The amounts of γ-adaptin bound to membranes (A) and associated with AP-1–coated vesicles (B) were determined by quantitative Western blotting and normalized to the amount of α-adaptin and transferrin receptor. The indicated values represent means ± SEM of three independent experiments. The 100% value corresponds to the amount of γ-adaptin bound to microsomal membranes (A) or associated with clathrincoated vesicles (B) of MPR-negative fibroblasts used as controls.
Discussion
Formation of clathrin-coated vesicles on TGN membranes requires the translocation of cytosolic AP-1 and clathrin. This process leads to the segregation of the MPRs and their bound lysosomal enzymes into a vesicular intermediate. Our biochemical analysis of MPR-negative fibroblasts reexpressing physiological levels of MPRs shows that the amount of AP-1 bound to membranes or recovered into clathrin-coated vesicles depends on the expression of these membrane receptors. While these results further support the notion that the MPRs are key components for the first step of clathrin coat assembly in the TGN, they demonstrate that the number of TGN-derived vesicles is determined, at least in part, by the number of membrane proteins to be sorted.
This biochemical study confirms our former proposals based on in vitro assays (Le Borgne et al., 1996) and demonstrates that, in vivo, membrane protein sorting in the TGN is tightly coupled to the formation of clathrin-coated vesicles on this organelle. If a rather low, physiological level of MPR expression can induce AP-1 recruitment and vesicle formation in cells with an MPR-negative background, it does not apparently affect the number of AP-2–coated vesicles formed at the plasma membrane. The most simple explanation is that, in these cells, the MPRs are the major membrane proteins sorted along the AP-1–dependent pathway as a result of their continuous recycling between this compartment and endosomes, whereas they probably represent a minor population (∼5,000–10,000 molecules of each MPR) among the family of receptors internalized at the plasma membrane via the AP-2–dependent pathway. Thus, the mobilization of AP-2 and clathrin at the plasma membrane would probably require a massive overexpression of the MPRs as previously described for the transferrin receptor (Iacopetta et al, 1988; Miller et al., 1991). It is extremely likely that other membrane proteins sorted along the same AP-1–dependent pathway as the MPRs and sharing similar sorting signals would also contribute to recruit AP-1 on membranes and to generate AP-1–coated vesicles. MPR-negative cells still produce detectable amounts of AP-1 and clathrin-coated vesicles (∼30%). In this respect, it is noteworthy that the overexpression of the major histocompatibility complex class II molecules in Hela cells also increases AP-1 binding in vitro (Salamero et al., 1996).
While this study further stresses the importance of membrane proteins, it remains possible that, in their absence, AP-1 can interact albeit weakly with membranes without producing clathrin-coated vesicles. Indeed, our former in vitro binding studies (Le Borgne et al., 1996) have shown that the membrane insertion of massive amounts of ARF-1 in its GTPγS-bound form would essentially result in the creation of low affinity (K d ∼150 nM) binding sites for AP-1, which, according to the present study using a CDMPR mutant (see below), would not result in the efficient production of vesicles. Thus, it is also possible that the arrival of the MPRs in the TGN would create a favorable context to stabilize AP-1 on the membrane (transition between low and high affinity interactions) to generate a transport intermediate. This model implies that AP-1 interacting with its binding sites containing the cargo membrane proteins are probably scattered in the plane of the membrane and then clustered to form a nascent transport intermediate. This clustering could be mediated either by the APs themselves, which can self-aggregate in vitro (Chang et al., 1993), or alternatively by the polymerization of clathrin triskelions bound to the APs.
Our results argue that the MPRs are rate-limiting components for TGN-derived vesicle formation at low levels of expression. However, it has to be noted that relatively high levels of MPR expression (three to fivefold the physiological level of one MPR) do not necessarily produce a corresponding increase in the amount of membrane-bound AP-1 and in the number of vesicles formed. In other words, there is not a linear relationship between high MPR expression and the number of AP-1–coated vesicles formed. This may first indicate that some steps in the recycling of the MPRs back to the TGN are rate limiting. On the other hand, clathrin-coated vesicle formation is a multistep process involving several regulatory factors. Thus, this observation could alternatively reflect a saturation of other components (or their products) required in the early steps of clathrin coat assembly. Among potential candidates are the GTPase ARF-1, potentially a phospholipase D or the mammalian homologue of the yeast Vps34p, a PI-3 kinase. A GTPase could also regulate the later steps of vesicle formation, like dynamin in the context of the plasma membrane (van der Bliek et al., 1993). This latter possibility could easily explain the differences seen after massive overexpression of the transferrin receptor in mammalian cells, producing a higher number of clathrin-coated pits at the plasma membrane in one cell type (Iacoppetta et al., 1988), while producing a higher number of flat clathrin lattices without modifying the number of clathrin-coated vesicles in another cell type (Miller et al., 1991).
Sorting of membrane proteins and their segregation into specialized transport vesicles is mediated by specific determinants in their cytoplasmic domains. The MPRs contain in their cytoplasmic domains multiple determinants that mediate their sorting along the different steps of their recycling pathway. The endocytosis of the CD-MPR involves a tyrosine-based motif (at position 45) and two phenylalanines (at positions 13 and 18). This receptor as well as the CI-MPR also contain a carboxyl-terminal di-leucine–based motif critical for lysosomal targeting of hydrolases (Johnson and Kornfeld, 1992a ,b; Chen et al., 1993). According to surface plasmon resonance studies performed on immobilized oligopeptides containing either a tyrosine or a dileucine (Heilker et al., 1996), both motifs could potentially interact with both AP-1 and AP-2. Genetic approaches have also revealed that tyrosine-based motifs interact with the μ chains of APs (Ohno et al., 1995). We have previously shown that a CD-MPR containing an endocytosis motif and a di-leucine–based motif but mutated around a casein kinase II phosphorylation site adjacent to this dileucine–based motif provides only low affinity binding sites for AP-1 in vitro and exhibits a reduced capability of transporting lysosomal enzymes in vivo (Mauxion et al., 1996). We show here that the expression of such a mutant in MPRnegative fibroblasts does not result in an efficient production of AP-1–coated vesicles in vivo, indicating that this CD-MPR mutant is largely excluded from the AP-1–dependent pathway. These results further stress the functional importance of this casein kinase II phosphorylation site in regulating the AP-1–dependent sorting of the CD-MPR. It is possible that the phosphorylation of this site could induce conformational changes in the CD-MPR cytoplasmic domain to expose the cryptic tyrosine- and di-leucine– based motifs. A similar casein kinase II phosphorylation site is found at the carboxyl-terminal domain of the other CI-MPR and has also been shown to be implicated in lysosomal enzyme transport in vivo (Chen et al., 1993). It could be anticipated from our results that this phosphorylation site in the CI-MPR also regulates its segregation into AP-1–coated vesicles.
Our former and present studies as well as those performed by others in the context of the plasma membrane (Iacoppetta et al., 1988; Miller et al., 1991) argue that membrane proteins containing specific sorting motifs in their cytoplasmic domains and concentrating soluble ligands via their luminal domains play a critical role in the formation of clathrin-coated vesicles. Could this represent a general rule for the formation of transport intermediates? Some aspects in the assembly of clathrin and non-clathrin coats, namely COPI and COP II functioning in vesicular traffic in the early secretory pathway (Rothman and Wieland, 1996; Schekman and Orci, 1996), argue that they could be related processes. Both COPI (Palmer et al., 1993) and AP-1 (Stamnes and Rothman, 1993; Traub et al., 1993) recruitments onto membranes are regulated by the small GTPase ARF-1, whereas COPII assembly is regulated by the GTPase Sar-1p (Barlowe et al., 1993). The question of whether transmembrane proteins are essential partners in the recruitment of non-clathrin coats is still unresolved. It is interesting to note, however, that both COPI- and COPIIcoated vesicles are enriched in members of the p24 family of transmembrane proteins. The latter have been proposed to function as putative cargo receptors, selecting proteins in COPI and COPII-coated vesicles (Stamnes at al, 1995; Schimmoller et al., 1995). These 16 homologous transmembrane proteins contain in their cytoplasmic domain a mono- or a di-phenylalanine motif that was found to interact in vitro with the F subcomplex of COPI containing β, γ, and ζ COP (Fiedler et al., 1996). A subset of these p24 proteins also contains in their cytoplasmic domains carboxyl-terminal di-lysine motifs known to function in the retrieval of transmembrane proteins from the Golgi complex back to the ER (Nilsson et al., 1989; Jackson et al., 1990). These retrieval motifs, first shown to interact both in vitro and in vivo with COPI (Cosson and Letourneur, 1994; Letourneur et al., 1994), seem to interact in vitro with the B subcomplex of COPI containing α, β′, and ε COP (Fiedler et al., 1996). Thus, these different studies suggest a link between a given type of coat and specific sorting determinants in the cytoplasmic domain of these transmembrane proteins. It would be interesting to know whether these p24 transmembrane proteins also participate in the active recruitment of COPs (or subcomplexes) on their target membranes, as we report here for the MPRs and AP-1.
Acknowledgments
We are grateful to Ulrike Bauer for her excellent technical assistance. We kindly acknowledge Drs. E. Ungewickell for the gift of the mAb 100/3, M. Robinson for the polyclonal antibody against the hinge region of the mouse γ-adaptin, F. Brodsky for the mAb AP-6, and T. Kreis for the mAb E5A3. We also thank Dr. G. Griffiths for his help with EM, and Drs. M. Zerial, A. Alconada, and J. Lanoix for their critical reading of the manuscript.
Footnotes
1. Abbreviations used in this paper: CD, cation dependent; CI, cation independent; MPR, mannose 6-phosphate receptor; mTF, mouse Apo-transferrin; PI, phosphatidylinositol.
This research was supported in part by grants from the European Communities (Bio2-CT93-02205 and HCM ERB-CHRTXCT-940592) and the association “Vaincre les Maladies Lysosomales.”
Please address all correspondence to Bernard Hoflack, Institut de Biologie de Lille, CNRS IFR 3, EP 525, Institut Pasteur de Lille, 1 rue Professeur Calmette, BP 447, 59021 Lille Cedex, France. Fax: (33) 3-20-87-10-19. e-mail: Bernard.Hoflack@Pasteur-Lille.fr
References
- Ahle S, Ungewickell E. Identification of a clathrin binding subunit in the HA2 adaptor protein complex. J Biol Chem. 1989;264:20089–20093. [PubMed] [Google Scholar]
- Ahle S, Mann A, Eichelsbacher U, Ungewickell E. Structural relationships between clathrin assembly proteins from the Golgi and the plasma membrane. EMBO (Eur Mol Biol Organ) J. 1988;4:919–929. doi: 10.1002/j.1460-2075.1988.tb02897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch WE, Kahn R, Schwaninger R. ADP-ribosylation factor is required for vesicular trafficking between the endoplasmic reticulum and the cis-Golgi compartment. J Biol Chem. 1992;267:13053–13061. [PubMed] [Google Scholar]
- Barlowe C, d'Enfert C, Schekman R. Purification and characterization of SAR1p, a small GTP-binding protein required for transport vesicle formation from the endoplasmic reticulum. J Biol Chem. 1993;268:873–879. [PubMed] [Google Scholar]
- Beltzer JP, Spiess MS. In vitro binding of the asialoglycoprotein receptor to the β adaptin of the plasma membrane. EMBO (Eur Mol Biol Organ) J. 1991;12:3735–3742. doi: 10.1002/j.1460-2075.1991.tb04942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman AL, Kahn RA. ARF proteins: the membrane traffic police? . Trends Biochem Sci. 1995;20:147–150. doi: 10.1016/s0968-0004(00)88991-4. [DOI] [PubMed] [Google Scholar]
- Brown WJ, Dewald DB, Emr SD, Plutner H, Balch WE. Role for phosphatidylinositol 3-kinase in the sorting and transport of newly synthesized lysosomal enzymes in mammalian cells. J Cell Biol. 1995;130:781–796. doi: 10.1083/jcb.130.4.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MP, Mallet WG, Mostov KE, Brodsky FM. Adaptor selfaggregation, adaptor-receptor recognition and binding of α-subunits to the plasma membrane contribute to recruitment of adaptor (AP2) components of clathrin-coated pits. EMBO (Eur Mol Biol Organ) J. 1993;12:2169–2180. doi: 10.1002/j.1460-2075.1993.tb05865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HJ, Remmler J, Delaney JC, Messner DJ, Lobel P. Mutational analysis of the cation-independent mannose 6-phosphate/insulin-like growth factor-II receptor. J Biol Chem. 1993;268:22338–22346. [PubMed] [Google Scholar]
- Cosson P, Letourneur F. Coatomer interaction with di-lysine endoplasmic retention motifs. Science (Wash DC) 1994;263:1629–1631. doi: 10.1126/science.8128252. [DOI] [PubMed] [Google Scholar]
- Davidson HW. Wortmannin causes mistargeting of procathepsin D. Evidence for the involvement of a phosphatidylinositol 3-kinase in vesicular transport to lysosomeds. J Cell Biol. 1995;130:797–805. doi: 10.1083/jcb.130.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler K, Veit M, Stamnes M, Rothman JE. Bimodal interaction of coatomer with the p24 family of putative cargo receptors. Science (Wash DC) 1996;273:1396–1399. doi: 10.1126/science.273.5280.1396. [DOI] [PubMed] [Google Scholar]
- Gallusser A, Kirchhausen T. The beta 1 and beta 2 subunits of the AP complexes are the clathrin coat assembly components. EMBO (Eur Mol Biol Organ) J. 1993;12:5237–5244. doi: 10.1002/j.1460-2075.1993.tb06219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman JN, Conibear E, Pearse BMF. Specificity of binding of clathrin adaptors to signals on the mannose 6-phosphate/insulin-like growth factor II receptor. EMBO (Eur Mol Biol Organ) J. 1989;4:1041–1047. doi: 10.1002/j.1460-2075.1989.tb03471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilker R, Manning-Krieg U, Zuber J-F, Spiess M. In vitro binding of clathrin adaptors to sorting signals correlates with endocytosis and basolateral sorting. EMBO (Eur Mol Biol Organ) J. 1996;15:2893–2899. [PMC free article] [PubMed] [Google Scholar]
- Herman PK, Stack JH, Emr SD. An essential role for a protein and lipid kinase complex in secretory protein sorting. Trends Cell Biol. 1992;2:363–368. doi: 10.1016/0962-8924(92)90048-r. [DOI] [PubMed] [Google Scholar]
- Iacopetta BJ, Rothenberger S, Kühn LC. A role for the cytoplasmic domain in transferrin receptor sorting and coated pit formation during endocytosis. Cell. 1988;54:485–489. doi: 10.1016/0092-8674(88)90069-4. [DOI] [PubMed] [Google Scholar]
- Jackson MR, Nilsson T, Peterson PA. Identification of a consensus motif for retention of transmembrane proteins in the endoplasmic reticulum. EMBO (Eur Mol Biol Organ) J. 1990;9:3153–3562. doi: 10.1002/j.1460-2075.1990.tb07513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K, Kornfeld S. A His-Leu-Leu sequence near the carboxyl terminus of the cytoplasmic domain of the cation-dependent mannose 6-phosphate receptor is necessary for the lysosomal enzyme sorting function. J Biol Chem. 1992a;267:17110–17115. [PubMed] [Google Scholar]
- Johnson K, Kornfeld S. The cytoplasmic tail of the mannose 6-phosphate/insulin-like growth factor-II receptor has two signals for lysosomal enzyme sorting in the Golgi. J Cell Biol. 1992b;119:249–257. doi: 10.1083/jcb.119.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld S, Mellman I. The biogenesis of lysosomes. Annu Rev Cell Biol. 1989;5:483–525. doi: 10.1146/annurev.cb.05.110189.002411. [DOI] [PubMed] [Google Scholar]
- Ktistakis NT, Brown HA, Sternweis PC, Roth MG. Phospholipase D is present on Golgi-enriched membranes and its activation by ADP ribosylation factor is sensitive to brefeldin A. Proc Natl Acad Sci USA. 1995;92:4952–4956. doi: 10.1073/pnas.92.11.4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Borgne R, Schmidt A, Mauxion F, Griffiths G, Hoflack B. Binding of AP-1 Golgi adaptors to membranes requires phosphorylated cytoplasmic domains of the mannose 6-phosphate/insulin-like growth factor II recptor. J Biol Chem. 1993;2268:22552–22556. [PubMed] [Google Scholar]
- Le Borgne R, Griffiths G, Hoflack B. Mannose 6-phosphate receptors and ADP-ribosylation factors cooperate for high affinity interaction of the AP-1 Golgi assembly proteins with membranes. J Biol Chem. 1996;271:2162–2170. doi: 10.1074/jbc.271.4.2162. [DOI] [PubMed] [Google Scholar]
- Letourneur F, Klausner RD. A novel di-leucine motif and a tyrosine-based motif independently mediate lysosomal targeting and endocytosis of CD3 chains. Cell. 1992;69:1143–1157. doi: 10.1016/0092-8674(92)90636-q. [DOI] [PubMed] [Google Scholar]
- Letourneur F, Gaynor EC, Hennecke S, Démollière C, Duden R, Emr SD, Riezman H, Cosson P. Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic reticulum. Cell. 1994;79:1199–1207. doi: 10.1016/0092-8674(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Ludwig T, Munier-Lehmann H, Bauer U, Hollinshead M, Ovitt CE, Lobel P, Hoflack B. Differential sorting of lysosomal enzymes in mannose 6-phosphate receptor-deficient mice. EMBO (Eur Mol Biol Organ) J. 1994;13:3430–3437. doi: 10.1002/j.1460-2075.1994.tb06648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauxion F, Le Borgne R, Munier-Lehmann H, Hoflack B. A casein kinase II phosphorylation site in the cytoplasmic domain of the cation-dependent mannose 6-phosphate receptor determines the high affinity interaction of the AP-1 Golgi assembly proteins with membranes. J Biol Chem. 1996;271:2171–2178. doi: 10.1074/jbc.271.4.2171. [DOI] [PubMed] [Google Scholar]
- Méresse S, Hoflack B. Phosphorylation of the cation-independent mannose 6-phosphate receptor is closely associated with its exit from the trans-Golgi network. J Cell Biol. 1993;120:67–75. doi: 10.1083/jcb.120.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K, Shipman M, Trowbridge IS, Hopkins CR. Transferrin receptors promote the formation of clathrin lattices. Cell. 1991;65:621–632. doi: 10.1016/0092-8674(91)90094-f. [DOI] [PubMed] [Google Scholar]
- Morris SA, Ahle S, Ungewickell E. Clathrin-coated vesicles. Curr Opin Cell Biol. 1989;1:684–690. doi: 10.1016/0955-0674(89)90034-3. [DOI] [PubMed] [Google Scholar]
- Munier-Lehmann H, Mauxion F, Bauer U, Lobel P, Hoflack B. Re-expression of the mannose 6-phosphate receptors in receptor-deficient fibroblasts. J Biol Chem. 1996;271:15166–15174. doi: 10.1074/jbc.271.25.15166. [DOI] [PubMed] [Google Scholar]
- Nilsson T, Jackson MR, Peterson PA. Short cytoplasmic sequences serve as retention signals for transmembrane proteins in the endoplasmic reticulum. Cell. 1989;58:707–718. doi: 10.1016/0092-8674(89)90105-0. [DOI] [PubMed] [Google Scholar]
- Ohno H, Stewart J, Fournier MC, Bosshart H, Rhee I, Miyatake S, Saito T, Gallusser A, Kirchhausen T, Bonifacino JS. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science (Wash DC) 1995;269:1872–1875. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- Palmer DJ, Helms JB, Beckers CJM, Orci L, Rothman JE. Binding of coatomer to Golgi membranes requires ADP-ribosylation factor. J Biol Chem. 1993;268:12083–12089. [PubMed] [Google Scholar]
- Pearse BMF. Receptors compete for adaptors found in plasma membrane coated pits. EMBO (Eur Mol Biol Organ) J. 1988;11:3331–3336. doi: 10.1002/j.1460-2075.1988.tb03204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse BMF, Robinson MS. Clathrin, adaptors and sorting. Annu Rev Cell Biol. 1990;6:151–171. doi: 10.1146/annurev.cb.06.110190.001055. [DOI] [PubMed] [Google Scholar]
- Podbilewicz B, Mellman I. ATP and cytosol requirements for transferrin recycling in intact and disrupted MDCK cells. EMBO (Eur Mol Biol Organ) J. 1990;9:3477–3487. doi: 10.1002/j.1460-2075.1990.tb07556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MS. Cloning of cDNAs encoding two related 100-kD coated vesicle proteins (α-adaptins) J Cell Biol. 1989;108:833–842. doi: 10.1083/jcb.108.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JE, Wieland FT. Protein sorting by transport vesicles. Science (Wash DC) 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- Salamero J, Le Borgne R, Saudrais C, Goud B, Hoflack B. Expression of major histocompatibility complex class II molecules in Hela cells promotes the recruitment of AP-1 Golgi-specific assembly protein on Golgi membranes. J Biol Chem. 1996;271:30318–30321. doi: 10.1074/jbc.271.48.30318. [DOI] [PubMed] [Google Scholar]
- Sandoval IV, Bakke O. Targeting of membrane proteins to endosomes and lysosomes. Trends Cell Biol. 1994;4:292–297. doi: 10.1016/0962-8924(94)90220-8. [DOI] [PubMed] [Google Scholar]
- Santini F, Keen JH. Endocytosis of activated receptors and clathrin-coated pit formation: deciphering the chicken or egg relationship. J Cell Biol. 1996;132:1025–1036. doi: 10.1083/jcb.132.6.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schekman R, Orci L. Coat proteins and vesicle budding. Science (Wash DC) 1996;271:1526–1533. doi: 10.1126/science.271.5255.1526. [DOI] [PubMed] [Google Scholar]
- Schimmoller F, Singer-Kruger B, Schroder S, Kruger U, Barlowe C, Riezman H. The absence of Emp24p, a component of ER-derived COPIIcoated vesicles, causes a defect in transport of selected proteins to the Golgi. EMBO (Eur Mol Biol Organ) J. 1995;14:1329–1339. doi: 10.1002/j.1460-2075.1995.tb07119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman MNJ, Sowerby PJ, Robinson MS. Cytosolic and membrane-associated proteins involved in the recruitment of AP-1 adaptors onto the trans-Golgi network. J Biol Chem. 1996;271:25446–25451. doi: 10.1074/jbc.271.41.25446. [DOI] [PubMed] [Google Scholar]
- Serafini T, Orci L, Amherdt M, Brunner M, Kahn RA, Rothman JE. ADP-ribosylation factor (ARF) is a subunit of the coat of Golgiderived COP-coated vesicles: a novel role for a GTP-binding protein. Cell. 1991;67:239–253. doi: 10.1016/0092-8674(91)90176-y. [DOI] [PubMed] [Google Scholar]
- Sosa MA, Schmidt B, von Figura K, Hille-Rehfeld A. In vitro binding of plasma membrane-coated vesicle adaptors to the cytoplasmic domain of lysosomal acid phosphatase. J Biol Chem. 1993;268:12537–12543. [PubMed] [Google Scholar]
- Stamnes MA, Rothman JE. The binding of AP-1 clathrin adaptor particles to Golgi membranes requires ADP-ribosylation factor, a small GTPbinding protein. Cell. 1993;73:999–1005. doi: 10.1016/0092-8674(93)90277-w. [DOI] [PubMed] [Google Scholar]
- Stamnes MA, Craighead MW, Hoe MH, Lampen N, Geromanos S, Tempst P, Rothman JE. An integral membrane component of coatomer-coated transport vesicles defines a family of proteins involved in budding. Proc Natl Acad Sci USA. 1995;92:8011–8015. doi: 10.1073/pnas.92.17.8011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoorvogel W, Oorschot V, Geuze HJ. A novel class of clathrincoated vesicles budding from endosomes. J Cell Biol. 1996;132:21–33. doi: 10.1083/jcb.132.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor TC, Kahn RA, Melançon P. Two distinct members of the ADP-ribosylation factor family of GTP-binding proteins regulate cell-free intra-Golgi transport. Cell. 1992;70:69–79. doi: 10.1016/0092-8674(92)90534-j. [DOI] [PubMed] [Google Scholar]
- Traub LM, Ostrom JA, Kornfeld S. Biochemical dissection of AP-1 recruitment onto Golgi membranes. J Cell Biol. 1993;123:561–573. doi: 10.1083/jcb.123.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bliek AM, Redelmeier TE, Damke H, Tisdale EJ, Meyerowitz EM, Schmiidt SL. Mutations in human dynamin block an intermediate stage in coated vesicle formation. J Cell Biol. 1993;122:553–563. doi: 10.1083/jcb.122.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman PG, Warren G. Isolation of functional, coated, endocytic vesicles. J Cell Biol. 1991;112:1133–1141. doi: 10.1083/jcb.112.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]