Abstract
Previous studies have indicated that newly formed oligodendrocytes are dynamic cells whose production, survival, and differentiation depend upon axonal influences. This study has characterized the appearance and fate of newly formed oligodendrocytes in developing rat brain. Oligodendrocytes appear in predictable locations and radially extend DM-20–positive processes that cover 80-μm domains in the cortex and 40-μm domains in the corpus callosum. These premyelinating oligodendrocytes have one of two fates: they myelinate axons or degenerate. Between 7 and 21 d after birth, ∼20% of premyelinating oligodendrocytes identified in the cerebral cortex were degenerating. Oligodendrocytes that ensheathed axons expressed and selectively targeted proteolipid protein to compact myelin and did not degenerate. These observations support the hypothesis that axonal influences affect oligodendrocyte survival, differentiation, and expression of proteolipid protein gene products.
Oligodendrocytes, the myelin-forming cell in the central nervous system (CNS)1, are neuroepithelial in origin. In vitro studies have identified and characterized a progenitor cell, termed O2A, which gives rise to oligodendrocytes in serum-free medium or to astrocytes in serum-containing medium (Raff et al., 1983). Cells committed to the oligodendrocyte lineage in vitro can respond to a variety of growth factors, and based on morphology and molecular phenotype, they are generally grouped into progenitors, immature oligodendrocytes (premyelinating), and mature oligodendrocytes (myelinating) (Gard and Pfeiffer, 1990; Hardy and Reynolds, 1991; DuboisDalcq and Armstrong, 1992; Miller, 1996). PDGF binds to and stimulates the division of O2A cells in vitro (Richardson et al., 1988; Hart et al., 1989b ), and as O2A cells differentiate into oligodendrocytes, they downregulate PDGFαR and fail to bind or respond to PDGF (Hart et al., 1989a ). The proteoglycan, NG2, colocalizes with PDGFαR in vitro and in vivo (Nishiyama et al., 1996a ), and these molecules may be functionally coupled on the surface of O2A cells (Nishiyama et al., 1996b ). PDGFαR-/NG2-positive cells populate the brain well before myelination begins (Nishiyama et al., 1996a ), and dual-labeling studies indicate that these cells can give rise to galactocerebroside- and myelin basic protein (MBP)-positive oligodendrocytes (Levine et al., 1993; Nishiyama et al., 1996a ).
Studies using retroviral markers (Levison and Goldman, 1993) and immunocytochemical studies using glycolipid and ganglioside antibodies (LeVine and Goldman, 1988; Gard and Pfeiffer, 1990; Hardy and Reynolds, 1991; Warrington and Pfeiffer, 1992) have identified immature or premyelinating oligodendrocytes in developing rodent brain. Myelin protein antibodies have not been used to identify premyelinating oligodendrocytes in vivo. mRNA encoding DM-20, a spliced product of the proteolipid protein (PLP) gene, has been detected in developing brain before myelin formation (Macklin, 1992; Timsit et al., 1995), and mice with PLP gene mutations have phenotypes at stages before active myelin formation (Skoff, 1982; Knapp et al., 1986). These observations suggest that DM-20 is expressed by immature or premyelinating oligodendrocytes.
In vitro studies have established that oligodendrocytes are dynamic cells that can respond to a variety of external influences. Critical to our understanding of oligodendrocyte lineage in vivo is the characterization of factors that regulate their numbers and survival. Recent studies of optic nerve have established that axonal signals regulate oligodendrocyte production and survival (Barres and Raff, 1993, 1994; Barres et al., 1993; Burne et al., 1996) and that as many as 50% of all newly formed oligodendrocytes die by programmed cell death (Barres et al., 1992). Similar to many developing neurons, newly formed oligodendrocytes appear to compete for survival factors, and their overproduction presumably helps assure that all appropriate axons are myelinated. While our understanding of oligodendrocyte lineage in vivo is evolving, further characterization of the appearance, fate, and molecular events that occur during oligodendrocyte differentiation is needed. Therefore, we used antibodies directed against the myelin proteins PLP and DM-20 to characterize oligodendrocyte lineage in vivo. Our results demonstrate that DM-20–positive premyelinating oligodendrocytes are produced in predictable locations during development and suggest that their survival is dependent on myelination of appropriate axons. In addition, we provide evidence that as oligodendrocytes ensheath axons, they express and selectively target PLP to developing myelin internodes.
Materials and Methods
Immunocytochemical Analysis
3-, 7-, 11- 14-, 21-, and 28-d-old and adult Sprague Dawley rats were anesthetized and perfused through the heart with 4% paraformaldehyde and 0.08 M Sorensen's phosphate buffer. The brains were removed, postfixed overnight at 4°C, and cryoprotected in 20% glycerol. Brains were frozen and sectioned on a freezing, sliding microtome (Microm, Heidelberg, Germany) at a thickness of 30 μm. Sections were rinsed in buffer and pretreated with microwave (1 to 2 min), 10% Triton X-100, and 1% H2O2 (30 min). Sections were stained by the avidin/biotin complex procedure by placing the sections in the following solutions: 3% normal serum for 30 min at 22°C, primary antibodies for 5 d at 4°C, biotinylated secondary antibodies (Vector Laboratories, Burlingame, CA) diluted 1:500 for 30 min at 22°C, enzyme (HRP)-linked biotin diluted 1:1,000, and avidin diluted 1:1,000 (Vector Laboratories) in PBS for 1 h, diaminobenzidine/hydrogen peroxide for 8 min, and 0.04% osmium tetroxide for 30 s. After the immunostaining procedure, the sections were rinsed in buffer, placed on glass slides, and covered with 100% glycerol and coverslips. Sections were photographed with a photomicroscope (model Axiophot; Carl Zeiss, Inc., Thornwood, NY).
For double-labeling immunofluorescence and confocal studies, sections were pretreated as described above and incubated in two primary antibodies for 5 d at 4°C. Fluorescence- and Texas red–conjugated secondary antibodies (diluted 1:100) (Jackson Laboratories, West Grove, PA) were applied for 1 h. Sections were rinsed and mounted in a Mowiol-based (Calbiochem, San Diego, CA) mounting medium containing 0.1% paraphenylene-diamine hydrochloride. For combined nuclear chromatin and DM-20/PLP staining, 30-μm-thick sections were immunostained by the avidin/biotin complex procedures as described above, rinsed in buffer, and placed in Hoechst 3325A dye (8 μg/ml in PBS) (Sigma Chemical Co., St. Louis, MO) for 60 min. Sections were rinsed, placed on slides, and covered with glycerol and coverslips. Immunoreactivity was photographed with bright-field optics. Hoechst 3325A staining was photographed under ultraviolet illumination.
Antibodies
Rat monoclonal antibodies directed against the 13 carboxy-terminal amino acids of DM-20 and PLP were purchased from Agmed Inc. (Bedford, MA). Rabbit polyclonal antibodies were generated against the 35 amino acids specific for PLP protein. Western blot analysis confirmed the specificity of the reactivity of these two reagents. Rabbit anti–rat NG2 antibody was kindly provided by Joel Levine (State University of New York at Stony Brook) and has been described (Levine et al., 1993).
Confocal Microscopy
Sections were analyzed on a confocal laser scanning microscope (model Aristoplan; Leica, Inc., Deerfield, IL). Confocal images represent optical sections of ∼1 μm axial resolution. Single images represented an average of 32 line scans of the chosen field. Fluorescence in red and green channels was collected simultaneously. In the red/green merged images, areas of colocalization are yellow. Images were photographed with a focus graphics 35-mm film recorder.
Morphometric and Statistical Analysis
The diameter or area occupied by DM-20/PLP–positive premyelinating oligodendrocytes was measured using a microscope (model Axiophot; Carl Zeiss, Inc.) fitted with a grid-patterned reticule and a 40× objective. In the corpus callosum, the cells were measured perpendicular to the cell cluster and developing axons. Measurements were converted to microns and unpaired Student's t test was used for comparing the diameter of cells in the cortex and corpus callosum.
Results
Characterization of Premyelinating Oligodendrocytes in the Cerebral Cortex and Corpus Callosum
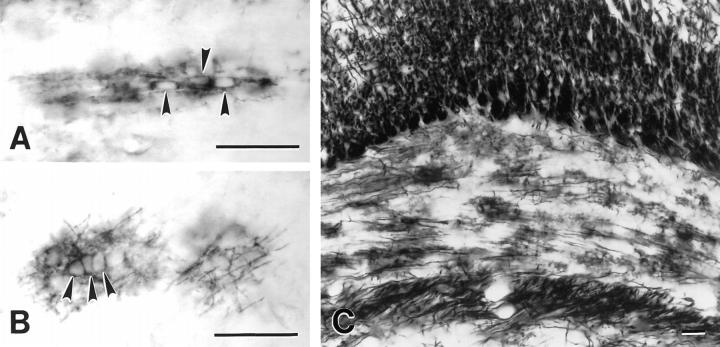
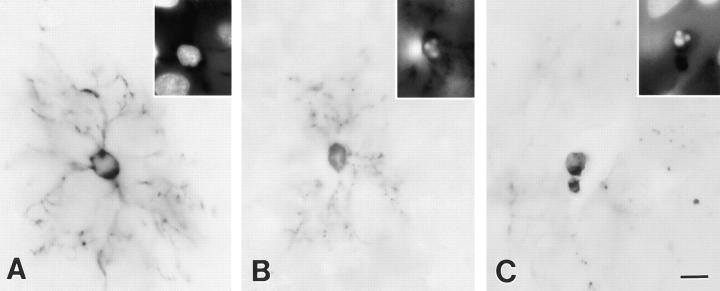
To determine if premyelinating oligodendrocytes could be identified with DM-20/PLP antibodies, sections of developing rat brain were immunostained with a monoclonal antibody directed against the 13 carboxy-terminal amino acids common to both proteins. In the cerebral cortex, myelination occurs in a predictable temporal and spatial sequence during the first three postnatal weeks. Myelination begins rostral to corpus callosum and progresses to the pial surface. Fig. 1 A demonstrates the distribution of DM20/PLP immunoreactivity in 11-d-old rat brain. Vertically oriented DM-20/PLP–positive, myelinated fibers extend from the deep cortical layers toward the pial surface. Patches of DM-20/PLP staining were also present near the leading edge of the myelination front (Fig. 1 A, arrowheads) and in layer I of the cortex. Most brain regions containing patches of DM-20/PLP immunoreactivity at 11 d are myelinated by 21 d (Fig. 1 B). When examined at higher magnification, many of these patches consisted of single cells that symmetrically radiated DM-20/PLP–positive processes (Fig. 1 C, arrowheads). Occasionally, these cells occurred in pairs (Fig. 1 C, asterisk), and processes of these paired cells occupied separate neuropil domains. Other DM-20/PLP–positive cells were connected to developing myelin internodes (Fig 1 C, arrows) and were located in deeper layers of the cortex. Some areas of DM-20/PLP immunoreactivity were fragmented and covered neuropil areas that were similar in size to oligodendrocytes that radially extend multiple processes (Fig. 1 D, arrowheads). These profiles represent dying oligodendrocytes, and they are described in detail below.
Figure 1.
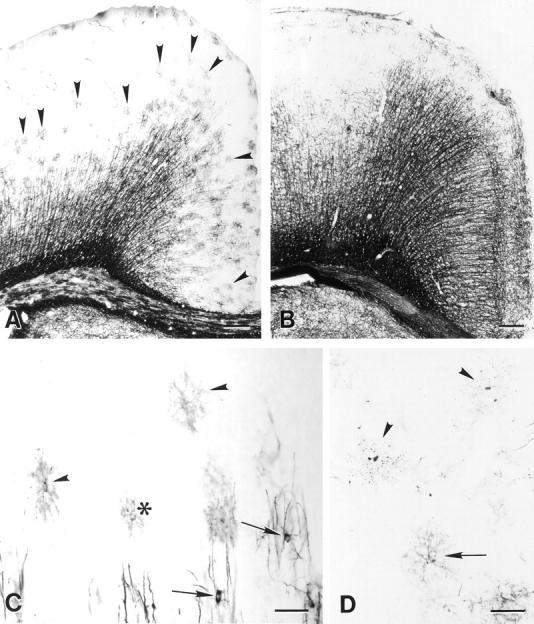
Comparison of the distribution of DM-20/PLP immunoreactivity in sections from 11- (A) and 21-d-old (B) rat brain. The increased distribution of immunoreactivity in B reflects the developmental progression of myelination. Premyelinating oligodendrocytes are concentrated at the leading edge of myelinated fibers projecting toward the pial surface (A, arrowheads) and in layer I of the cortex. Premyelinating oligodendrocytes radially extend multiple processes that cover distinct neuropil domains (C, arrowheads). While most premyelinating oligodendrocytes in the cerebral cortex are separated from each other, occasional pairs are detected (C, asterisk). Processes of adjacent cells rarely overlap even when cells appear in pairs. Myelinating oligodendrocytes (C, arrows) send processes to vertically oriented DM-20/PLP–positive myelin internodes. Other regions of DM-20/PLP immunoreactivity is fragmented (D, arrowheads) and covers approximately the same neuropil area as healthy-appearing premyelinating oligodendrocytes (D, arrow). Bars: (A and B) 200 μm; (C and D) 50 μm.
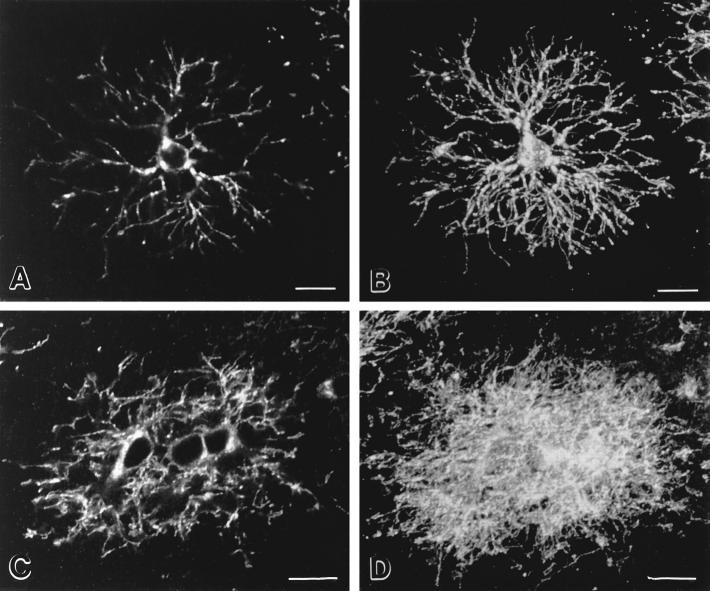
Premyelinating oligodendrocytes detected in the developing corpus callosum were strikingly different from those in the cerebral cortex. DM-20/PLP–positive cell bodies usually occurred in clusters of two to four cells (Fig. 2, A and B, arrowheads) that were oriented parallel to developing axons. These cells extended multiple processes that appeared to overlap extensively. At postnatal day 7, some clusters of DM-20/PLP–positive cells did not include obvious developing myelin internodes (Fig. 2 A; also see Fig. 3, C and D), while others did (Fig. 2 B, arrows). By postnatal day 11, the corpus callosum contained many clusters of DM-20/PLP–positive cells and developing myelin internodes (Fig. 2 C). Since our analysis suggested that premyelinating oligodendrocytes in the cerebral cortex had more orderly arranged processes than those in the corpus callosum, sections stained with DM-20/PLP antibodies were analyzed by confocal microscopy (Fig. 3). Fig. 3 A is a single confocal image (∼1.0-μm-thick) of a premyelinating oligodendrocyte in the developing cerebral cortex. Fig. 3 B is a stack of 20 confocal images (∼20-μm-thick) of the same cell. The relatively symmetrical and nonoverlapping distribution of the DM-20/PLP–positive processes is apparent in both images. When a similar comparison was performed on a cluster of premyelinating oligodendrocytes in the developing corpus callosum (Fig. 3, C and D), their processes arborized and overlapped extensively. Our initial immunocytochemical analysis also suggested that premyelinating oligodendrocytes occupied larger neuropil domains in the cerebral cortex than in the corpus callosum. Therefore, the radius of the area occupied by cell bodies and processes of individual DM-20/PLP–positive premyelinating oligodendrocytes in P7 corpus callosum and cerebral cortex was compared. The radius of premyelinating cells was 78.5 ± 8.2 μm (n = 60) in the cortex and 39.8 ± 9.5 μm (n = 45) in the corpus callosum. Student's t test demonstrated that this difference was statistically significant (P < 0.001).
Figure 2.
Premyelinating oligodendrocytes in the corpus callosum from 7- (A and B) and 11-d-old (C) rats. DM-20/PLP–positive premyelinating oligodendrocytes occur in clusters (A and B, arrowheads) in the corpus callosum. Clustered perikarya appear before evidence of myelin formation (A) and persist during active myelination (B and C). Bars, 50 μm.
Figure 3.
Premyelinating oligodendrocytes in cerebral cortex and corpus callosum have different morphologies. Confocal images of single (A and C) and reconstructions of 20 (B and D) 1-μm-thick optical sections of DM-20/PLP–positive premyelinating oligodendrocytes from the cerebral cortex (A and B) and corpus callosum (C and D). Cortical cells (A and B) extend an orderly array of processes that tend to branch in a Y-shaped manner as they terminate. Premyelinating oligodendrocytes in the corpus callosum (C and D) occur in groups and extend processes in a less orderly and overlapping fashion. Bars, 10 μm.
Premyelinating Oligodendrocytes Differentiate into Myelinating Oligodendrocytes
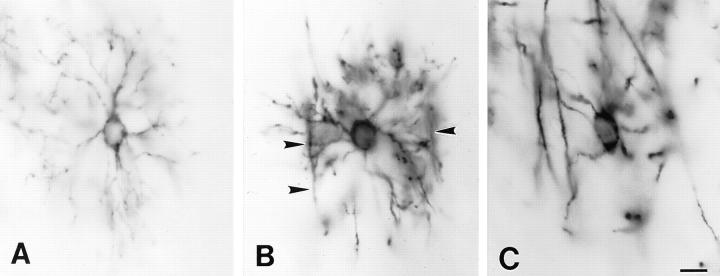
The distribution of DM-20/PLP immunoreactivity indicated that premyelinating oligodendrocytes differentiate into myelinating oligodendrocytes in predictable locations in the cerebral cortex. Fig. 4 depicts three stages in the progression from premyelinating to myelin-forming oligodendrocyte. The premyelinating oligodendrocyte in Fig. 4 A radially extends DM-20/PLP–positive processes that have no obvious extensions along axons. Initial stages of myelination can be identified by the extension of T-shaped processes from the premyelinating oligodendrocytes (Fig. 4 B, arrowheads). The top of the T's are oriented parallel to axonal bundles and represent initial stages of axonal ensheathment. Analysis of cells in more advanced stages of myelination (Fig. 4 C) indicated that only a small percentage of the premyelinating oligodendrocyte processes ensheath axons. Fragmented DM-20/PLP–positive processes were not detected near developing myelin internodes, suggesting that premyelinating oligodendrocyte processes that do not ensheath axons were resorbed.
Figure 4.
Premyelinating oligodendrocytes differentiate into myelinating oligodendrocytes. DM-20/PLP–positive oligodendrocytes before myelination (A), during early stages of axonal ensheathment (B, arrowheads), and during active myelination (C). Bar, 10 μm.
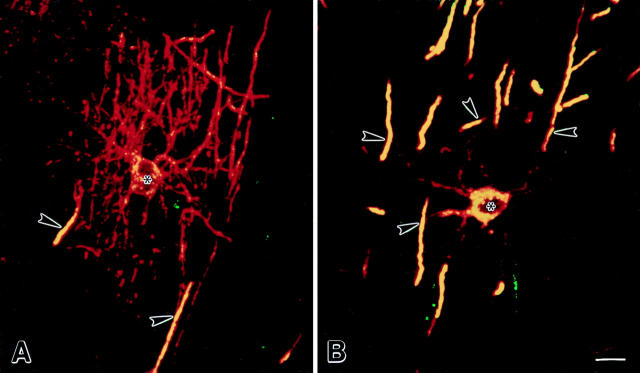
The proteolipid protein gene produces two alternatively spliced proteins (DM-20 and PLP) that differ by 35 additional amino acids (exon 3b) in PLP. Previous studies have indicated that DM-20 is expressed before PLP during brain development (Macklin, 1992; Timsit et al., 1995). To determine if DM-20 and PLP could be detected at different stages of oligodendrocyte differentiation, sections were double-labeled with one antibody specific for DM20/PLP and another specific for PLP. Premyelinating and myelinating oligodendrocytes were identified and examined by confocal microscopy. The PLP-specific antibody did not stain premyelinating oligodendrocytes. During early stages of myelin formation (cells extending multiple DM-20/PLP–positive processes and short DM-20/PLP– positive developing myelin internodes), PLP was detected in oligodendrocyte perinuclear cytoplasm and in some of the developing internodes (Fig. 5 A). At later stages of myelination (Fig. 5 B), all developing myelin internodes were both DM-20/PLP and PLP positive. These observations indicate that PLP is expressed at very low levels in premyelinating oligodendrocytes and during early stages of axonal ensheathment and that PLP is targeted exclusively to myelin internodes. In contrast, DM-20 was detected on all surface membranes of premyelinating and myelinating oligodendrocytes. These observations raise the possibility that axonal influences regulate differential expression of PLP gene products as premyelinating oligodendrocytes differentiate into myelinating oligodendrocytes.
Figure 5.
Comparison of the distribution of DM-20/PLP (red) and PLP (green) in confocal images of oligodendrocytes. Areas of colocalization appear yellow. During early stages of axonal ensheathment, DM-20/PLP immunoreactivity is evenly distributed on oligodendrocyte processes (A, red) and developing myelin internodes (A, red and yellow), while PLP-specific immunoreactivity is restricted to perinuclear cytoplasm and developing myelin internodes (A, yellow). In more mature oligodendrocytes, DM-20/PLP is detected in oligodendrocyte processes and myelin, while PLP is restricted to perinuclear cytoplasm and myelin (B, yellow). Asterisks and arrowheads denote nucleus of cell and myelin internodes. Bars, 10 μm.
Degeneration of Premyelinating Oligodendrocytes
A proportion of DM-20/PLP–positive cells, located in the same brain regions as the premyelinating oligodendrocytes described above, had fragmented DM-20/PLP immunoreactivity. Many such cells retained the shape and size of premyelinating oligodendrocytes, while other regions of DM-20/PLP immunoreactivity consisted of dots that were less orderly but distributed over an 80-μm radius. Cells with fragmented DM-20/PLP immunoreactivity appear to represent premyelinating oligodendrocytes that are degenerating, which is consistent with previous studies demonstrating extensive programmed cell death during early stages of oligodendrocyte differentiation (Barres et al., 1992). A salient morphological feature of cells undergoing programmed cell death is fragmentation and condensation of nuclear chromatin. To determine if premyelinating oligodendrocytes with fragmented PLP/DM-20 staining also contained fragmented nuclear chromatin, sections were stained with DM-20/PLP antibodies and with a DNAbinding dye. Premyelinating oligodendrocytes with evenly distributed DM-20/PLP staining had diffuse and evenly stained nuclear chromatin (Fig. 6 A), while cells with fragmented DM-20/PLP staining had fragmented and condensed nuclear chromatin (Fig. 6, B and C). At the leading edge of myelination in the cerebral cortex, the total number of premyelinating cells was greatest at postnatal days 11 and 14 (Table I), reflecting peak periods of initial stages of myelination. The percentage of total premyelinating cells that were degenerating was ∼20% between postnatal days 7 and 21 and was 37% at day 28 (Table I).
Figure 6.
Comparison of DM-20/PLP immunoreactivity and nuclear chromatin staining in developing rat brain. Premyelinating oligodendrocytes with DM-20/PLP immunoreactivity evenly distributed on their surface (A) have diffuse nuclear chromatin staining (A, inset). Premyelinating oligodendrocytes with fragmented DM-20/PLP staining (B) and DM-20/PLP–positive necrotic-appearing cells (C) have condensed and fragmented nuclear chromatin (B and C, inset). A, B, and C were photographed with bright field optics; insets were photographed with ultraviolet optics. Bar, 10 μm.
Table I.
Percentage of Dying Premyelinating Oligodendrocytes in Developing Cerebral Cortex
| Postnatal age | Total cells counted | Dying cells (percentage of total) | ||
|---|---|---|---|---|
| 7 | 172 | 37 (22.0) | ||
| 11 | 453 | 96 (21.4) | ||
| 14 | 344 | 59 (17.9) | ||
| 21 | 150 | 29 (19.6) | ||
| 28 | 102 | 38 (37.8) |
DM-20/PLP–positive cells were identified and grouped into cells with continuous (healthy) or fragmented (dying) immunoreactivity. Sampling was restricted to the leading edge of myelin in cerebral cortex. Areas of diencephalon were not sampled. Statistical analysis showed no difference in percentage of dying cells at various time points.
Origin of Premyelinating Oligodendrocytes
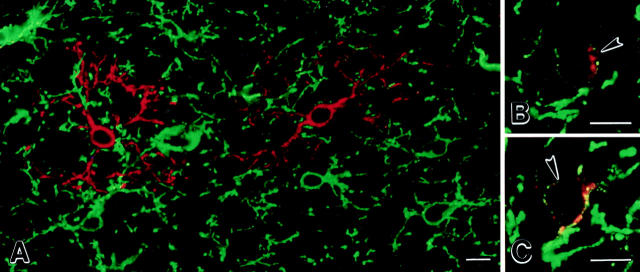
To investigate if DM-20/PLP–positive cells originate from NG2-positive cells or from another cell that is committed to the oligodendrocyte lineage, sections were double-labeled with NG2 and DM-20/PLP antibodies and examined by confocal microscopy. In sections from 3-d-old brain, NG2positive cells were present throughout the cerebral cortex, but few DM-20/PLP-positive cells were detected (data not shown). Sections from 7-d-old brain contained significant numbers of DM-20/PLP–positive premyelinating oligodendrocytes (Fig. 7). While most cells were stained with either NG2 or DM-20/PLP antibodies (Fig. 7 A), a small but consistent population of cells was stained by both antibodies (Fig. 7, B and C). Cells positive for both NG2 and DM-20/PLP were identified by weak DM-20/PLP immunoreactivity in perinuclear cytoplasm and on proximal processes. When both channels were imaged, the intensity of NG2 and DM-20/PLP immunoreactivity in double-labeled cells was much weaker than that detected in surrounding cells, which were either NG2 or DM-20/PLP positive. These observations provide additional support that NG2positive progenitor cells produce oligodendrocytes in vivo (Levine et al., 1993; Nishiyama et al., 1996a ).
Figure 7.
Premyelinating oligodendrocytes are generated from NG2-positive progenitor cells. Confocal images of sections (30-μmthick) from 7-d-old rat cerebral cortices that were immunostained with NG2 (green) and DM-20/PLP (red) antibodies. A network of NG2-positive cells covers the cortical neuropil (A, green). DM-20/PLP–positive premyelinating cells are less abundant (A, red). A small but consistent population of cells are weakly stained by both NG2 and DM-20/PLP antibodies (B and C, arrowheads). NG2 immunoreactivity is punctate on the plasma membrane of these cells, whereas DM-20/PLP appears punctate in perinuclear cytoplasm on proximal cell processes (B and C). Bars, 10 μm.
Discussion
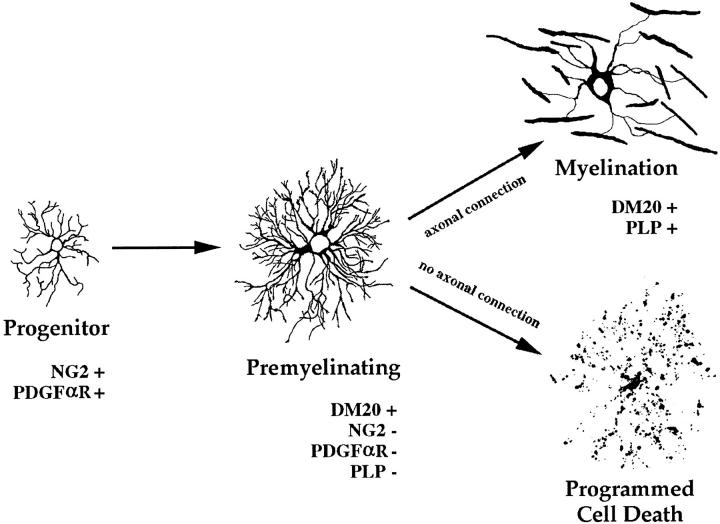
Based on the distribution of PLP and DM-20, this study provides additional insights into the molecular and cellular events that occur during oligodendrocyte differentiation in vivo. Our studies confirm that a significant percentage of newly formed oligodendrocytes degenerate and provide the first description of their location and phenotype. Oligodendrocytes that degenerate express DM-20 and do not ensheath axons or express detectable levels of PLP, the major structural protein of compact myelin. These observations are consistent with the hypothesis that oligodendrocyte cell death occurs before any significant commitment to myelin formation and that axonal ensheathment is essential for oligodendrocyte survival. Fig. 8 schematically summarizes stages and features of oligodendrocyte differentiation in vivo and is based on differential detection of DM-20 and PLP in confocal images. This model supports and extends those proposed previously (Hardy and Reynolds, 1991; Warrington and Pfeiffer, 1992; Ffrench-Constant, 1992; Barres and Raff, 1994) and is based on direct analysis of cells in vivo.
Figure 8.
Schematic summary of oligodendrocyte lineage in vivo. Progenitor cells produce premyelinating oligodendrocytes. Premyelinating oligodendrocytes occupy distinct neuropil domains and depend on axonal signal(s) for survival. Images are two-dimensional reconstructions of confocal images.
Oligodendrocyte Progenitors
PDGFαR-, NG2-positive cells originate from discrete regions of the ventricular zone of the diencephalon (Hart et al., 1989a ; Pringle et al., 1992; Pringle and Richardson, 1993; Nishiyama et al., 1996a ). They migrate, proliferate, and form a network of process-bearing cells that populate the cortex before birth (Nishiyama et al., 1996a ). Each cell establishes a microdomain within the neuropil, and contact inhibition with neighboring NG2 cells may, in part, control their distribution. The location of cells immunostained by both NG2 and DM-20 antibodies (Fig. 7) provides additional evidence that NG2-positive cells give rise to oligodendrocytes (Levine et al., 1993; Nishiyama et al., 1996a ). Our studies indicate that DM-20 immunoreactivity is a reliable marker for newly formed oligodendrocytes, and the detection of the occasional NG2/DM-20–positive cells makes it unlikely that stages of oligodendrocyte lineage exist between NG2- and DM-20–positive cells. NG2-positive cells, however, may represent a heterogeneous population of cells with regard to stage and commitment to oligodendrocyte lineage, and they could have functions unrelated to oligodendrocyte progenitors. The rate of oligodendrocyte lineage cell differentiation can differ significantly in various brain regions. In the hindbrain, oligodendrocytes are produced 2–3 d after the appearance of PDGFαR mRNA– positive cells (Hardy and Friedrich, 1996), whereas a similar transition can take several weeks in the cerebral cortex. In addition, a distinct stage of oligodendrocyte progenitors (04+/R-mAb−) has been described in vitro (Hardy and Reynolds, 1991; Bansal et al., 1992; Gard and Pfeiffer, 1993) and in perinatal forebrain (Pfeiffer et al., 1993) but was not detected in hindbrain (Hardy and Friedrich, 1996). Developmental and regional variations in axonal maturation and in the timing of progenitor cell generation and migration throughout the CNS may be responsible for these differences.
Differentiation of Myelinating Oligodendrocytes
Fig. 4 presents a logical progression in the differentiation of a premyelinating to a myelinating oligodendrocyte. It should be stressed, however, that these are images of different cells and that confirmation of this progression awaits analysis of the development of single cells. The distribution, morphology, and molecular phenotype of premyelinating oligodendrocytes provide new insights into mechanisms that regulate their maturation into myelinforming cells. Premyelinating oligodendrocytes radiate processes in a symmetrical manner and establish spatial domains within the neuropil. In the cerebral cortex, processes of neighboring premyelinating cells do not overlap even when they appear in pairs. In the corpus callosum, premyelinating oligodendrocytes usually occur in groups of two to four cells, their processes overlap extensively, and each cell occupies ∼50% of the neuropil domain that cells in the cortex occupy. Since the number of myelinreceptive axons is much greater in the corpus callosum than in the cerebral cortex, these observations support the hypothesis that axons regulate premyelinating oligodendrocyte numbers and morphology (Barres et al., 1992, 1993; Burne et al., 1996; Hardy and Friedrich, 1996). The spatial separation of premyelinating oligodendrocytes in the cortex suggests that neighboring cells compete less for survival factors than the clusters of premyelinating oligodendrocytes in the corpus callosum. In addition, the symmetrical extension of premyelinating oligodendrocyte processes is consistent with the hypothesis that physical contact with appropriate axons and/or soluble axon-derived factors that work over short distances regulate oligodendrocyte maturation and survival. The hypothesis that axonal contact is needed for oligodendrocyte survival is also supported by studies that demonstrated that neurons, but not neuronal-conditioned medium, can increase the survival of oligodendrocytes maintained in vitro (Barres and Raff, 1994).
Differentiation of oligodendrocytes parallels many aspects of polarized epithelial cell differentiation. These include dependence on cell–cell contact, expression of specific gene products, polarization of cytoskeletal networks, development of specialized transport pathways, and formation of distinct surface membrane domains (Mostov et al., 1992; Rodriguez-Boulan and Powell, 1992). Although myelinating oligodendrocytes do not form the classical apical and basolateral membrane domains or tight junctions seen in typical epithelial cells, they do polarize their surface membranes into several biochemically and morphologically distinct domains (e.g., compact myelin, paranodal membrane, periaxonal membranes, perikaryal surface membrane). Premyelinating oligodendrocytes can be considered as nonpolarized epithelial cells that extend processes in search of appropriate targets. While our studies support the hypothesis that ensheathment of appropriate axons is required for the transformation of the nonpolarized premyelinating oligodendrocyte into a polarized myelinating oligodendrocyte, it remains to be determined if axonal contact is the actual signal.
Our studies indicate that PLP expression and induction of specialized transport pathways occur as oligodendrocytes polarize their surface membranes and form myelin. The immunocytochemical studies in this report indicate that DM-20, but not PLP, is expressed by premyelinating oligodendrocytes. This conclusion is based on the observation that antibodies directed against epitopes shared by DM-20 and PLP stain premyelinating oligodendrocytes, whereas antibodies specific for PLP do not. Although this could represent different binding affinities for the two antibodies, the PLP antibody demonstrates the same intensity and pattern of staining at 1:6,000 and 1:250 dilutions. In addition, DM-20 has been identified as a member of an evolutionarily conserved family of proteins that is expressed by some neurons, fish myelin, choroid plexus, and kidney tubules (Kitagawa et al., 1993; Yan et al., 1993; Yoshida and Colman, 1996). In contrast, PLP evolved as a myelin-specific protein in amphibians and has not been identified in other tissues (Yoshida and Colman, 1996). Our studies raise the possibility that the 35 amino acids specific to PLP contain signals that target PLP exclusively to compact myelin or that prevent PLP from being targeted to nonmyelin domains.
Myelin-associated glycoprotein (MAG) and MBP antibodies also stain premyelinating oligodendrocyte cell bodies and processes (Trapp, B.D., unpublished observation). As premyelinating oligodendrocytes differentiate into myelinating oligodendrocytes, they selectively target MAG to the periaxonal membrane (Trapp et al., 1989), MBP to compact myelin (Sternberger et al., 1978), and MBP mRNA along the myelin internode (Colman et al., 1982; Trapp et al., 1987). Myelinating oligodendrocytes, therefore, develop specialized transport pathways that are not present in premyelinating oligodendrocytes. As described in myelinating Schwann cells (Kidd et al., 1996), axons may induce specialized microtubule networks in myelinating oligodendrocytes that target myelin-specific gene products to distinct regions of the myelin internode. Alternatively spliced forms of the MAG and MBP gene are also produced, and select forms (L-MAG and exon 2–containing MBPs) predominate during the earliest stages of CNS development (Quarles et al., 1973; Barbarese et al., 1978). Differential expression of myelin protein isoforms may be regulated during oligodendrocyte differentiation by alternative splicing, mRNA stabilization, or translational control.
Death of Premyelinating Oligodendrocytes
Programmed cell death is essential to the development of many organ systems and assures appropriate cell numbers and/or appropriate cell–cell contact (Raff et al., 1993). Previous studies that correlated in vivo and in vitro analyses of oligodendrocyte lineage cells estimated that as many as 50% of newly generated oligodendrocytes in the optic nerve undergo programmed cell death (Barres et al., 1992). Our results established that ∼20% of premyelinating oligodendrocytes in sections of the developing cerebral cortex (7–21 d) are degenerating. If oligodendrocyte survival depends upon the availability of myelin-receptive axons, the rate of premyelinating oligodendrocyte death may differ in white matter tracts and cerebral cortex. The incidence of premyelinating oligodendrocyte death in the cerebral cortex or optic nerve cannot be established until the life span of premyelinating oligodendrocytes and the clearance time for degenerating oligodendrocytes are determined.
We did not detect degenerating oligodendrocyte progenitors (NG2+ cells) nor significant numbers of degenerating myelin internodes (PLP-positive). However, hemopoietic and microglia cells with condensed nuclear chromatin were detected in both white and gray matter (Trapp, B.D., unpublished observation). Premyelinating oligodendrocytes were not detected in brain areas after they were myelinated. These observations do not support estimates of a constant rate of oligodendrocyte production and death in rodent optic nerve during the first six postnatal weeks (Barres et al., 1992; Raff et al., 1993) and emphasize the need for phenotyping dying cells identified by nuclear chromatin fragmentation.
Conclusions
This report used PLP/DM-20–specific antibodies to describe several aspects of oligodendrocyte differentiation in vivo. Characterization of oligodendrocyte lineage cells after experimental manipulation of the developing CNS should elucidate the molecular events responsible for production, survival, and maturation of myelin-forming cells and identify potential targets for therapeutic intervention in demyelinating diseases.
Acknowledgments
We thank Dr. Joel Levine for NG2 antibodies, Dr. Judy Drazba for help in confocal analysis, and Lois Becker for typing the manuscript.
This work was supported by grants from the National Institutes of Health (NS-29818 to B.D. Trapp, NS-25304 to W. Macklin) and the National Multiple Sclerosis Society (NMSS RG1901 to W. Macklin).
Abbreviations used in this paper
- CNS
central nervous system
- MAG
myelin-associated glycoprotein
- MBP
myelin basic protein
- PLP
proteolipid protein
Footnotes
Address all correspondence to Dr. Bruce D. Trapp, Department of Neurosciences-NC30, The Cleveland Clinic Foundation, 9500 Euclid Avenue, Cleveland, OH 44195. Tel: (216) 444-7177. Fax: (216) 444-7927.
References
- Bansal R, Stefansson K, Pfeiffer SE. Proligodendroblast antigen (POA), a developmental antigen expressed by A007/04-positive oligodendrocyte progenitors prior to the appearance of sulfatide and galactocerebroside. J Neurochem. 1992;58:2221–2229. doi: 10.1111/j.1471-4159.1992.tb10967.x. [DOI] [PubMed] [Google Scholar]
- Barbarese E, Carson JH, Braun PE. Accumulation of the four myelin basic proteins in mouse brain during development. J Neurochem. 1978;31:779–782. doi: 10.1111/j.1471-4159.1978.tb00110.x. [DOI] [PubMed] [Google Scholar]
- Barres BA, Raff MC. Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature (Lond) 1993;361:258–260. doi: 10.1038/361258a0. [DOI] [PubMed] [Google Scholar]
- Barres BA, Raff MC. Control of oligodendrocyte number in the developing rat optic nerve. Neuron. 1994;12:935–942. doi: 10.1016/0896-6273(94)90305-0. [DOI] [PubMed] [Google Scholar]
- Barres BA, Hart IK, Coles HSR, Burne JF, Voyvodic JT, Richardson WD, Raff MC. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992;70:31–46. doi: 10.1016/0092-8674(92)90531-g. [DOI] [PubMed] [Google Scholar]
- Barres BA, Jacobson MD, Schmid R, Sendtner M, Raff MC. Does oligodendrocyte survival depend on axons? . Curr Biol. 1993;3:489–497. doi: 10.1016/0960-9822(93)90039-q. [DOI] [PubMed] [Google Scholar]
- Burne JF, Staple JK, Raff MC. Glial cells are increased proportionally in transgenic optic nerves with increased numbers of axons. J Neurosci. 1996;16:2064–2073. doi: 10.1523/JNEUROSCI.16-06-02064.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman DR, Kreibich G, Frey AB, Sabatini DD. Synthesis and incorporation of myelin polypeptide into CNS myelin. J Cell Biol. 1982;95:598–608. doi: 10.1083/jcb.95.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois-Dalcq, M., and R.C. Armstrong. 1992. The oligodendrocyte lineage during myelination and remyelination. In Myelin: Biology and Chemistry. R.E. Martenson, editor. CRC Press, Inc., Boca Raton, Florida. 81–122.
- Ffrench-Constant C. Cell death: a division too far. Curr Biol. 1992;2:577–579. doi: 10.1016/0960-9822(92)90150-9. [DOI] [PubMed] [Google Scholar]
- Gard AL, Pfeiffer SE. Two proliferative stages of the oligodendrocyte lineage (A2B5+04- and 04+GalC-) under different mitogenic control. Neuron. 1990;5:615–625. doi: 10.1016/0896-6273(90)90216-3. [DOI] [PubMed] [Google Scholar]
- Gard AL, Pfeiffer SE. Glial cell mitogens bFGF and PDGF differentially regulate development of 04+GalC-oligodendrocyte progenitors. Dev Biol. 1993;159:618–630. doi: 10.1006/dbio.1993.1269. [DOI] [PubMed] [Google Scholar]
- Hardy R, Reynolds R. Proliferation and differentiaion potential of rat forebrain oligodendroglial progenitors both in vitro and in vivo. . Development (Camb) 1991;111:1061–1080. doi: 10.1242/dev.111.4.1061. [DOI] [PubMed] [Google Scholar]
- Hardy RJ, Friedrich VL., Jr Oligodendrocyte progenitors are generated throughout the embryonic mouse brain, but differentiate in restricted foci. Development (Camb) 1996;122:2059–2069. doi: 10.1242/dev.122.7.2059. [DOI] [PubMed] [Google Scholar]
- Hart IK, Richardson WD, Bolsover S, Raff MC. PDGF and intracellular signalling in the timing of oligodendrocyte differentiation. J Cell Biol. 1989a;109:3411–3417. doi: 10.1083/jcb.109.6.3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart IK, Richardson WD, Heldin C-H, Westermark B, Raff MC. PDGF receptors on cells of the oligodendrocyte-type-2 astrocyte (02A) cell lineage. Development (Camb) 1989b;105:595–603. doi: 10.1242/dev.105.3.595. [DOI] [PubMed] [Google Scholar]
- Kidd GJ, Andrews SB, Trapp BD. Axons regulate the distribution of Schwann cell microtubules. J Neurosci. 1996;16:946–954. doi: 10.1523/JNEUROSCI.16-03-00946.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa K, Sinoway MP, Yang C, Gould RM, Colman DR. A proteolipid protein gene family: expression in sharks and rays and possible evolution from an ancestral gene encoding a pore-forming polypeptide. Neuron. 1993;11:433–448. doi: 10.1016/0896-6273(93)90148-k. [DOI] [PubMed] [Google Scholar]
- Knapp PE, Skoff RP, Redsone DW. Oligodendroglial cell death in jimpy mice: an explanation for the myelin deficit. J Neurosci. 1986;6:2813–2822. doi: 10.1523/JNEUROSCI.06-10-02813.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JM, Stincone F, Lee YS. Development and differentiation of glial precursor cells in the rat cerebellum. Glia. 1993;7:307–321. doi: 10.1002/glia.440070406. [DOI] [PubMed] [Google Scholar]
- LeVine SM, Goldman JE. Spatial and temporal patterns of oligodendrocyte differentiation in rat cerebrum and cerebellum. J Comp Neurol. 1988;277:441–455. doi: 10.1002/cne.902770309. [DOI] [PubMed] [Google Scholar]
- Levison SW, Goldman JE. Both oligodendrocytes and astrocytes develop from progenitors in the subventricular zone of postnatal rat forebrain. Neuron. 1993;10:201–212. doi: 10.1016/0896-6273(93)90311-e. [DOI] [PubMed] [Google Scholar]
- Macklin, W.B. 1992. The myelin proteolipid protein gene and its expression. In Myelin: Biology and Chemistry. R.E. Martenson, editor. CRC Press, Inc., Boca Raton, Florida. 257–276.
- Miller RH. Oligodendrocyte origins. Trends Neurosci. 1996;19:92–96. doi: 10.1016/s0166-2236(96)80036-1. [DOI] [PubMed] [Google Scholar]
- Mostov K, Apodaca G, Aroeti B, Okamoto C. Plasma membrane protein sorting in polarized epithelial cells. J Cell Biol. 1992;116:577–583. doi: 10.1083/jcb.116.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A, Lin X-H, Giese N, Heldin C-H, Stallcup WB. Co-localization of NG2 proteoglycan and PDGF α-receptor on 02A progenitor cells in the developing rat brain. J Neurosci Res. 1996a;43:299–314. doi: 10.1002/(SICI)1097-4547(19960201)43:3<299::AID-JNR5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Lin X-H, Giese N, Heldin C-H, Stallcup WB. Interaction between NG2 proteoglycan and PDGF α receptor on 02A progenitor cells is required for optimal response to PDGF. J Neurosci Res. 1996b;43:315–330. doi: 10.1002/(SICI)1097-4547(19960201)43:3<315::AID-JNR6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Pfeiffer SE, Warrington AE, Bansal R. The oligodendrocyte and its many cellular processes. Trends Cell Biol. 1993;3:191–197. doi: 10.1016/0962-8924(93)90213-k. [DOI] [PubMed] [Google Scholar]
- Pringle NP, Richardson WD. A singularity of PDGF α-receptor expression in the dorsoventral axis of the neural tube may define the origin of the oligodendrocyte lineage. Development (Camb) 1993;117:525–533. doi: 10.1242/dev.117.2.525. [DOI] [PubMed] [Google Scholar]
- Pringle NP, Mudhar HS, Collarini EJ, Richardson WD. PDGF receptors in the rat CNS: during late neurogenesis, PDGF α-receptor expression appears to be restricted to glial cells of the oligodendrocyte lineage. Development (Camb) 1992;115:535–551. doi: 10.1242/dev.115.2.535. [DOI] [PubMed] [Google Scholar]
- Quarles RH, Everly JL, Brady RO. Myelin-associated glycoprotein: a developmental change. Brain Res. 1973;58:506–509. doi: 10.1016/0006-8993(73)90022-x. [DOI] [PubMed] [Google Scholar]
- Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitrointo an astrocyte or an oligodendrocyte depending on culture medium. Nature (Lond) 1983;303:390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Raff MC, Barres BA, Burne JF, Coles HS, Ishizaki Y, Jacobson MD. Programmed cell death and the control of cell survival: lessons from the nervous system. Science (Wash DC) 1993;262:695–700. doi: 10.1126/science.8235590. [DOI] [PubMed] [Google Scholar]
- Richardson WD, Pringle N, Mosley MJ, Westermark B, DuboisDalcq M. A role for platelet-derived growth factor in normal gliogenesis in the central nervous system. Cell. 1988;53:309–319. doi: 10.1016/0092-8674(88)90392-3. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Powell SK. Polarity of epithelial and neuronal cells. Annu Rev Cell Biol. 1992;8:395–427. doi: 10.1146/annurev.cb.08.110192.002143. [DOI] [PubMed] [Google Scholar]
- Skoff RP. Increased proliferation of oligodendrocytes in the hypomyelinated mouse mutant-jimpy. Brain Res. 1982;248:19–31. doi: 10.1016/0006-8993(82)91143-x. [DOI] [PubMed] [Google Scholar]
- Sternberger NH, Itoyama Y, Koco MW, Webster H deF. Myelin basic protein demonstrated immunocytochemically in oligodendroglia prior to myelin sheath formation. Proc Natl Acad Sci USA. 1978;75:2521–2524. doi: 10.1073/pnas.75.5.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timsit S, Martinez S, Allinquant B, Peyron B, Puelles L, Zalc B. Oligodendrocytes originate in a restricted zone of the embryonic ventral neural tube defined by DM-20 mRNA expression. J Neurosci. 1995;15:1012–1024. doi: 10.1523/JNEUROSCI.15-02-01012.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp BD, Moench T, Pulley M, Barbosa E, Tennekoon G, Griffin J W. Spatial segregation of mRNA encoding myelin-specific proteins. Proc Natl Acad Sci USA. 1987;84:7773–7777. doi: 10.1073/pnas.84.21.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp BD, Andrews SB, Cootauco C, Quarles RH. The myelinassociated glycoprotein is enriched in multivesicular bodies and periaxonal membranes of actively myelinating oligodendrocytes. J Cell Biol. 1989;109:2417–2426. doi: 10.1083/jcb.109.5.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington AE, Pfeiffer SE. Proliferation and differentiation of O4+oligodendrocytes in postnatal rat cerebellum: analysis in unfixed tissue slices using anti-glycolipid antibodies. J Neurosci Res. 1992;33:338–353. doi: 10.1002/jnr.490330218. [DOI] [PubMed] [Google Scholar]
- Yan Y, Lagenaur C, Narayanan V. Molecular cloning of M6: identification of a PLP/DM20 gene family. Neuron. 1993;11:423–431. doi: 10.1016/0896-6273(93)90147-j. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Colman DR. Parallel evolution and coexpression of the proteolipid proteins and protein zero in vertebrate myelin. Neuron. 1996;16:1115–1126. doi: 10.1016/s0896-6273(00)80138-5. [DOI] [PubMed] [Google Scholar]