Abstract
An 85-kD cytosolic complex (p62cplx), consisting of a 62-kD phosphoprotein (p62) and a 25-kD GTPase, has been shown to be essential for the cell-free reconstitution of polymeric IgA receptor (pIgA-R)-containing exocytic transport vesicle formation from the TGN (Jones, S.M., J.R. Crosby, J. Salamero, and K.E. Howell. 1993. J. Cell Biol. 122:775–788). Here the p62cplx is identified as a regulatory subunit of a novel phosphatidylinositol 3–kinase (PI3-kinase). This p62cplx-associated PI3-kinase activity is stimulated by activation of the p62cplx-associated GTPase, and is specific for phosphatidylinositol (PI) as substrate, and is sensitive to wortmannin at micromolar concentrations. The direct role of this p62cplx-associated PI3-kinase activity in TGN-derived vesicle formation is indicated by the finding that both lipid kinase activity and the formation of pIgA-R–containing exocytic vesicles from the TGN are inhibited by wortmannin with similar dose-response curves and 50% inhibitory concentrations (3.5 μM). These findings indicate that phosphatidylinositol-3-phosphate (PI[3]P) is required for the formation of TGN-derived exocytic transport vesicles, and that the p62cplx-associated PI3-kinase and an activated GTPase are the essential molecules that drive production of this PI(3)P.
In a series of unanticipated developments, it is now appreciated that molecules involved in growth factor receptor–mediated signaling cascades leading to mitogenic responses are also required for membrane trafficking reactions. One such class of molecules is the phosphatidylinositol 3–kinases (PI3-kinases)1 (for review see Liscovitch and Cantley, 1995; De Camilli et al., 1996; Shepherd et al., 1996). An essential role for a PI3-kinase in a specific membrane trafficking step has been demonstrated by the identification of the protein product of the yeast VPS34 gene as a homologue of the catalytic subunit of the mammalian PI3-kinases (Schu et al., 1993). The Vps34 protein forms a functional complex with the VPS15 gene product, a serine/threonine protein kinase (Stack et al., 1993, 1995b ), and the Vps34p/Vps15p complex is required for the efficient sorting of soluble vacuolar hydrolases at the TGN (Banta et al., 1988).
Recent data suggest that the involvement of PI3-kinases in membrane trafficking reactions also extend to mammalian systems. The challenge of mammalian cells with PI3-kinase inhibitors such as wortmannin or the quercetin analogue, LY294002, results in lysosomal enzyme missorting to the cell surface (Brown et al., 1995; Davidson, 1995; Matsuoka et al., 1995; Reaves et al., 1996). Moreover, Volinia et al. (1995) have characterized human homologues of Vps34p and Vps15p, which together form a functional PI3-kinase in vivo. Other mammalian catalytic subunits have been identified by Stephens et al. (1994) and Virbasius et al. (1996). Neither of these catalytic subunits is activated by growth factor receptors and both are more biochemically similar to Vps34p than to the human Vps34 homologue identified by Volinia et al. (1995).
Previously we identified an 85-kD cytosolic complex (p62cplx), composed of p62, a 62-kD phosphoprotein associated with a small GTPase, and showed that this 85-kD complex cycles on and off the cytoplasmic domain of TGN38 in a phosphorylation-dependent manner (Jones et al., 1993). TGN38 is an abundant transmembrane protein that is predominately localized to the TGN, but undergoes a rapid recycling between the TGN and the plasma membrane (Ladinsky and Howell, 1992; Bos et al., 1993; Humphrey et al., 1993; Reaves et al., 1993). Using a cell-free assay developed by Salamero et al. (1990), the recruitment of the p62cplx to TGN membranes was demonstrated to be essential for the formation of plasma membrane–directed exocytic transport vesicles, which contain the mature form of the polymeric IgA receptor (pIgA-R). The requirement for the p62cplx in vesicle formation, coupled with the phosphorylation/dephosphorylation cycle that controls p62cplx association with the TGN membrane, raises the question of how the p62cplx acts to stimulate vesicle formation. To address this question, we have undertaken a biochemical characterization of the function of the p62cplx.
In this paper, the p62 molecule is shown to share primary sequence homology with the p85α regulatory subunit of a PI3-kinase and, consistent with the homology data, the membrane-associated p62cplx regulates phosphatidylinositol (PI)-specific, and wortmannin-inhibitable PI3-kinase activity. From these data we conclude that a critical pool of phosphatidylinositol-3-phosphate (PI[3]P) is required for the formation of TGN-derived exocytic transport vesicles, and that the p62cplx-associated PI3-kinase and an activated GTPase are the essential molecules that drive production of this PI(3)P pool.
Materials and Methods
Chemical Reagents
Unless otherwise indicated, all chemicals were obtained from Sigma Chemical Co. (St. Louis, MO) or Boehringer Mannheim Biochemicals (Indianapolis, IN). 10 mM wortmannin in DMSO was stored in aliquots at −70°C and each aliquot was used only once. LysC was purchased from Waco Chemicals (Waco, TX). Phosphatidlyinositol was purchased from Avanti Polar Lipids (Alabaster, AL).
Antibodies
Production of specific antibodies against p62, TGN38, and the pIgA-R has been described (Sztul et al., 1985; Luzio et al., 1990; Jones et al., 1993). p62cplx antibodies (rabbit 950) were raised against the 85-kD cytosolic complex, and were isolated using immunoaffinity chromatography. Monoclonal antibodies against TGN38 were from hybridoma 2F7.1, provided by G. Banting (University of Bristol, Bristol, U.K.) (Horn and Banting, 1994). Preimmune sera used in all experiments is from rabbit 950. Antibodies against p85α and p110 α/β were purchased from Transduction Laboratories (Lexington, KY) and Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Antibodies against wortmannin were the gift of Dr. R. Abraham (The Mayo Foundation, Rochester, MN) (Brunn et al., 1996).
Subcellular Fractionation Procedures
Stacked Golgi fractions (SGFs) were isolated from rat liver using modifications of the method of Leelavathi et al. (1970) (Taylor et al., 1997a ). Livers were removed, finely minced and resuspended at 6 g per 10 ml 0.5 M sucrose in 100 mM KPO4, pH 6.8, 5 mM MgCl2 and 1 μg/ml each of a mixture of proteolytic inhibitors: chymostatin, leupeptin, antipain, and pepstatin. All sucrose solutions contained the same buffer and proteolytic inhibitors. Homogenization was with a single pass of a Polytron probe (Brinkmann Instruments, Inc., Westbury, NY) running low speed, followed by low speed centrifugation (1,500 g for 10 min) to pellet unbroken cells, cell debris, and nuclei. This pellet contained at least 50% of the cell protein. The resulting postnuclear supernatant (PNS) was loaded in the middle of a sucrose step gradient in an SW28 tube: steps of 1.3 M and 0.86 M sucrose were overlaid with the PNS (0.5 M), followed by a 0.25 M layer, and then were centrifuged for 1 h at 100,000 g (Beckman Instruments, Fullerton, CA). The 0.5 M sucrose-soluble fraction was collected and used for the preparation of cytosol. The SII fraction (0.5/0.86 M interface) was adjusted to 1.15 M sucrose with 2 M sucrose using a refractometer (Bausch & Lomb, Boston, MA). The adjusted SII was loaded into the bottom of an SW28 tube, overlaid with equal volumes of 1.0, 0.86, and 0.25 M sucrose, and then centrifuged for 3 h at 76,000 g. The resulting SGF floated to the 0.25/0.86 M sucrose interface. Characterization of this fraction shows an ∼400-fold enrichment over the PNS of three Golgi membrane proteins, p28 (Subramanian et al., 1995), mg160 (Gonatas et al., 1989), and TGN38 (Luzio et al., 1990). The fractionation protocol and characterization of the resulting fractions are described (Taylor et al., 1997a , b ). Cytosol was prepared from the soluble 0.5 M sucrose fraction of the first gradient, which was adjusted to 0.25 M sucrose with 100 mM KPO4, pH 6.8, 5 mM MgCl2, and centrifuged for 30 min at 100,000 g to remove any pelletable material. The resulting supernatant was concentrated using an Amicon fitted with a PM10 membrane to ∼40 mg/ml (Amicon Corp., Danvers, MA). Protein assays (DC Protein Assay; Bio Rad Laboratories, Hercules, CA) were carried out on all fractions. Aliquots of these fractions were frozen in liquid nitrogen and stored at −70°C.
Immunopurification of p62 Complexes
Antisera against p62 was purified on a protein A–Sepharose column and the IgG was covalently coupled to CNBr Sepharose following the manufacturer's instructions (Pharmacia Fine Chemicals, Piscataway, NJ). The p62cplx was purified from both cytosol and CHAPS-solubilized SGF. The solubilization procedure involved suspension of the SGF in CHAPS buffer (20 mM CHAPS, 20 mM Hepes-KOH, pH 6.8, 100 mM KCl, 0.3 M sucrose, containing the cocktail of proteolytic inhibitors), incubation on ice for 1 h, and then centrifugation (200,000 g × 20 min) to remove insoluble material. Cytosol or CHAPS-solubilized SGF was circulated through the immunoaffinity column overnight at 4°C. The nonbound fraction was eluted and the column washed with PBS until no further protein eluted (flowthrough). The p62cplx was eluted from the column with 0.2 M glycine-HCl, pH 2.8, neutralized, and concentrated to their original volumes for use in the kinase assays. The flowthrough was concentrated with an Amicon and used as depleted cytosol in cell-free assays. Both the flowthrough and purified p62cplx were characterized by SDS-PAGE and immunoblot analysis to confirm depletion and enrichment of p62cplx.
Phosphorylation Assays
Immunopurified p62cplx (10 ng isolated from the SGF) was incubated in a 50-μl reaction volume with 2 mM Pipes, pH 7.0, 0–1.0 μM CaCl2, 10 mM MgCl2, and ∼5 μCi γ32P-ATP for 15 min at 30°C. The samples were solubilized in SDS-PAGE sample buffer, resolved, the gel dried, and exposed to film for autoradiography.
Gel Electrophoresis and Immunoblotting
SDS-PAGE was carried out using a 5–15% acrylamide gradient and the buffer system of Maizel (1971). SDS-PAGE molecular weight standards were from Bio Rad. For immunoblots, nitrocellulose filters (Schleicher & Schuell, Inc., Keene, NH) were blocked for 1 h in 5% defatted milk/PBS/ 0.02% sodium azide. The filters were incubated overnight in primary antibody and washed. When using a mouse primary antibody, the filters were incubated with rabbit antibodies against mouse IgG for 2 h before the blots were visualized using 125I-protein A (ICN Biomedicals, Inc., Costa Mesa, CA). Autoradiography and/or PhosphorImager analysis was carried out either on the dried gels or immunoblots (Molecular Dynamics, Inc., Sunnyvale, CA).
Immunoprecipitation of PI3-kinase Activity
1 mg SGF was solubilized in 1 ml E buffer (10 mM Tris, pH 7.6, 1% Triton X-100, 50 mM NaCl, 5 mM EDTA, 50 mM NaF, 2 mM Na vanadate, plus the above proteolytic inhibitors) for 1 h on ice with vortexing. Samples were centrifuged for 30 min at 14,000 g in a microfuge and the soluble material was placed in a new tube. Specific antisera (10 μl) was added to the samples and they were rotated at 4°C for 2 h. Immune complexes were incubated for 1 h at 4°C with sheep antibodies against the Fc domain of rabbit IgG, which were covalently coupled to fibrous cellulose (Luzio, 1977). Immune complexes were pelleted and washed seven times: once with E buffer; twice with RIPA (10 mM Na phosphate, pH 7.0, 1% NP-40, 1% Na deoxycholate, 0.1% SDS, 2 mM EDTA, 20 mM NaF, 2 mM Na vanadate), twice with PAN–NP-40 (20 mM Pipes, pH 7.4, 100 mM NaCl, 1% NP-40), and then twice with PAN (the proteolytic inhibitors were included in all washing buffers). The immunoprecipitates were resuspended in 50 μl PAN and frozen in 5-μl aliquots for PI3-kinase assays.
PI3-kinase Assays
PI3-kinase assays were as described by Kazlauskas and Cooper (1990). Immunoprecipitates or isolated complexes (5 μl) in PAN, were resuspended in a reaction mixture containing 20 mM Hepes, pH 7.4, 5 mM MgCl2, 0.45 mM EGTA, 10 μM ATP (∼5 μCi [γ32P]ATP) and 0.2 mg/ml phosphatidylinositol (PI) in a final reaction volume of 10 μl, and then incubated 0–20 min at 30°C. After incubation, the reaction was stopped with 100 μl 1 M HCl and the lipids extracted with 200 μl CHCl3/MeOH (1:1), followed by 80 μl 1 M HCl/MeOH (1:1) and dried in a speed vac (Savant Instruments Inc., Holbrook, NY). The samples were resuspended in 10 μl CHCl3/MeOH (1:1) and spotted onto Silica Gel 60 TLC plates (JT Baker Chromatography, Union City, CA). The TLC plates were pretreated with 60 mM EDTA, 2% Na tartrate, and 50% EtOH, and then were dried in a 100°C oven overnight. Development of the TLC plates was in CHCl3/ MeOH/4 N NH4OH (9:7:2) for ∼2 h. The TLC plates were dried and exposed to film for autoradiography and phosphorimager analysis. Positive and negative controls were immunoprecipitates of stimulated and nonstimulated PDGF receptors, provided by A. Kazlauskas (Schepens Eye Institute, Boston, MA).
Gel Filtration Chromatography
Gel filtration chromatography was carried out using Sephacryl S500 (Pharmacia Fine Chemicals). Native SGF (2.0 mg, not high pH washed) was solubilized in CHAPS buffer, loaded onto the column, and eluted with 20 mM Hepes-KOH, pH 6.8, 100 mM KCl, 0.3 M sucrose, 20 mM CHAPS containing the cocktail of proteolytic inhibitors. 3.0 ml fractions were collected, and half were immunoprecipitated for PI3-kinase assays and the other half TCA precipitated for SDS-PAGE and immunoblot analysis. The column was calibrated with molecular weight standards: 669-kD thyroglobulin; 443-kD apoferritin; 232-kD β-amylase; and 66-kD BSA.
Cell-free Assay of pIgA-R–Containing Exocytic Vesicle Formation from the TGN
The cell-free assay of budding from an immobilized SGF was carried out as described by Salamero et al. (1990) and Howell et al. (1994). Each assay contains 2.5 mg magnetic core and shell beads with ∼50 μg SGF immobilized. The immobilized fraction is characterized in Jones et al. (1997). For the budding reaction the immobilized fraction was incubated in 2.5 ml, containing 0.70 mg/ml cytosol, 25 mM Hepes, pH 6.7, 25 mM KCl, 1.5 mM Mg acetate, 1.0 mM ATP, an ATP regenerating system (8.0 mM creatine phosphate, 0.043 μg/ml creatine phosphokinase, and 5 mg/ml BSA [final concentrations]). After 10 min at 37°C, the Golgi fraction remaining on the beads was retrieved with a magnet and the budded vesicles remained in the supernatant. The budded fraction was pelleted through a 0.25 M sucrose cushion (for 1 h at 100,000 g), to reduce the large amounts of cytosolic protein and 5 mg/ml BSA present in the budding reaction. The high concentration of soluble protein made it impractical to carry out gel analysis on the total budded fraction. The pellet was resuspended in gel sample buffer and resolved by SDS-PAGE. The amount of exocytic vesicle budding was determined by quantitative immunoblotting using the mature, sialylated pIgA-R (116 kD) as the marker. The pIgA-R is a plasma membrane receptor synthesized in relatively high amounts in rat liver (Sztul et al., 1985) and is used to define a specific population of exocytic vesicles (Salamero et al., 1990). Budding of this marker in the presence of the complete cell-free system is ∼70% efficient. When the ATP regenerating system and cytosol are omitted, the background budding is ∼5%.
Results
p62 Has Sequence Identity to the p85 Regulatory Subunits of PI3-kinases
To further characterize the p62 molecule, amino acid sequence data were obtained. p62 was coimmunoprecipitated with antibodies against TGN38 from high pH washed, rat liver SGF as previously described (Jones et al., 1993). The p62 band was excised from an SDS gel and digested with lysC as described (Matsudaira, 1993). Peptides were separated by reverse phase liquid chromatography; and two peptides were identified that yielded unambiguous sequence information (Macromolecular Resources, Ft. Collins, CO). An almost identical amino acid sequence was obtained from these two peptides (Table I). The most striking difference was that the threonine at position 7 in peptide No. 2 could not be identified in peptide No. 1, suggesting that the threonine residue was posttranslationally modified in peptide No. 1. A BLAST search of the NCBI database (Altschultz et al., 1990) revealed that the 24–amino acid sequence determined for peptide No. 2 was identical to amino acids 82–105 of the mouse PI3-kinase regulatory subunit, p85α (Escobedo et al., 1991) (Table I). Both the human and bovine sequences are >94% identical to the mouse and p62 sequence in this region. The 24–amino acid sequence of p62 lies within the bcr domain of p85α, a domain that Liscovich and Cantley (1995) suggest binds small GTPases.
Table I.
p62 Has Sequence Identity to the p85 Regulatory Subunits of PI3-kinases
| Sequence Comparison of p62 | ||||||
|---|---|---|---|---|---|---|
| P62_peptide1 | 1 | KXISPPXPKPRPPRPLPVAPG | 21 | |||
| P62_peptide2 | 2 | KRISPPTPKPRPPRPLPVAPGSSK | 24 | |||
| P85A_MOUSE | 82 | KRISPPTPKPRPPRPLPVAPGSSK | 105 | |||
| P85A_HUMAN | 82 | KKISPPTPKPRPPRPLPVAPGSSK | 105 | |||
| P85A_BOVIN | 82 | KKISPPTPKPRPPRPLPVAPGPSK | 105 | |||
| * ******************* ** | ||||||
Alignment of two different peptides generated from p62 (21 and 24–amino acids long) with amino acids 82–105 of the p85α subunit of PI3-kinases from mouse, human, and bovine. Asterisks at the bottom of the sequence indicate identical amino acids between p62-peptide2 and the p85α subunits. Noted in bold in the p62-peptide sequences is a threonine residue in p62-peptide2 which could not be determined in p62-peptide1.
These data suggest that p62 may represent a novel regulatory subunit of a PI3-kinase that shares some identity with p85α. Other possibilities are that it might be a shorter form of a p85 regulatory subunit, a proteolytic fragment of p85α, or a protein that shares a common bcr domain (or GTPase binding domain) with p85.
p62 Immunoprecipitates Have PI3-kinase Activity
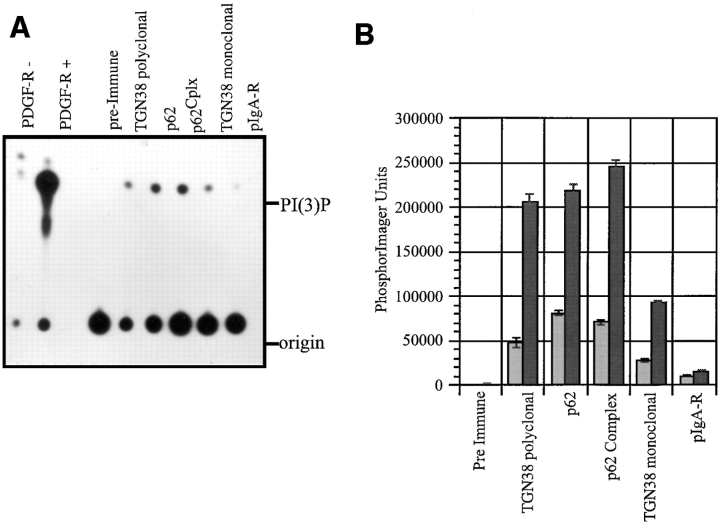
Enzymatic assays were carried out to directly determine whether PI3-kinase activity is associated with the p62cplx. Since the assay for PI3-kinase associated with PDGF receptors was established using immunoprecipitates of the activated receptor (Kazlauskas and Cooper, 1990), we used immunoprecipitates of activated and nonactivated PDGF receptor as controls. Activated PDGF receptors generate PI(3)P from PI, therefore, the mobility of the PI(3)P serves as a standard on the TLC plates. For the assay, immunoprecipitates were prepared from CHAPS-solubilized native SGF using antibodies raised against characterized components of the membrane-associated p62cplx: TGN38 (luminal domain, polyclonal); p62; p62cplx; and TGN38 (luminal domain, monoclonal). As shown in Fig. 1, A and B, PI3-kinase activity was readily detected in all immunoprecipitates containing the membrane-associated p62cplx. The level of activity measured was at least as robust as that obtained for the unstimulated PDGF receptor. This comparison is germaine because the p62cplx activity most likely represents the unstimulated endogenous activity. Controls for the specificity of the assays included immunoprecipitates derived from the same fraction using either preimmune sera or antibodies directed against the pIgA-R, an abundant transmembrane protein of SGF. The control immunoprecipitates had minimal kinase activity. The amount of p62cplx-associated PI3-kinase activity obtained with the immunoprecipitates correlated with the amount of p62 precipitated by the different antibodies (p62cplx > p62 alone > TGN38 polyclonal > TGN38 monoclonal [data not shown]).
Figure 1.
p62 immunoprecipitates have PI3-kinase activity. (A) PI3-kinase enzymatic assays were carried out on immunoprecipitates as described in Materials and Methods. The autoradiogram of the TLC plate is shown: lane 1, nonstimulated PDGF receptor; lane 2, stimulated PDGF receptor; lane 3, preimmune sera to p62; lane 4, TGN38 polyclonal; lane 5, p62; lane 6, p62cplx; lane 7, TGN38 monoclonal; and lane 8, pIgA-R. Immunoprecipitates of stimulated PDGF receptor (i.e., ligand bound) are known to generate PI(3)P and together with the nonstimulated receptor, provide controls for the mobility of PI(3)P on the TLC plate. The amount of PI(3)P formed by each immunoprecipitate was quantitated by PhosphorImager analysis and the data plotted in B (light bars). The small GTPase-dependent stimulation of the PI3- kinase activity of the same set of immunoprecipitates was assayed in the presence of GTPγS (1 μm) (dark bars) and plotted with the nonstimulated values. The immunoprecipitated antigens are labeled at the bottom of the bar graph.
Members of the Rac, Rho, and Ras families of small GTPases have been demonstrated to associate with and stimulate PI3-kinases (Zhang et al., 1993; Kodaki et al.,1994; Zheng et al., 1994; Tolias et al., 1995; Bokoch et al., 1996). In the presence GTPγS the PI3-kinase activity associated with each of the specific immunoprecipitates was activated three- to fourfold (Fig. 1 B), although the activity attained was still below that of the activated PDGF receptor. Significantly, the activities associated with the control immunoprecipitates remained at background levels. These data indicate a stimulatory role for the p62 associated GTPases in the generation of PI(3)P.
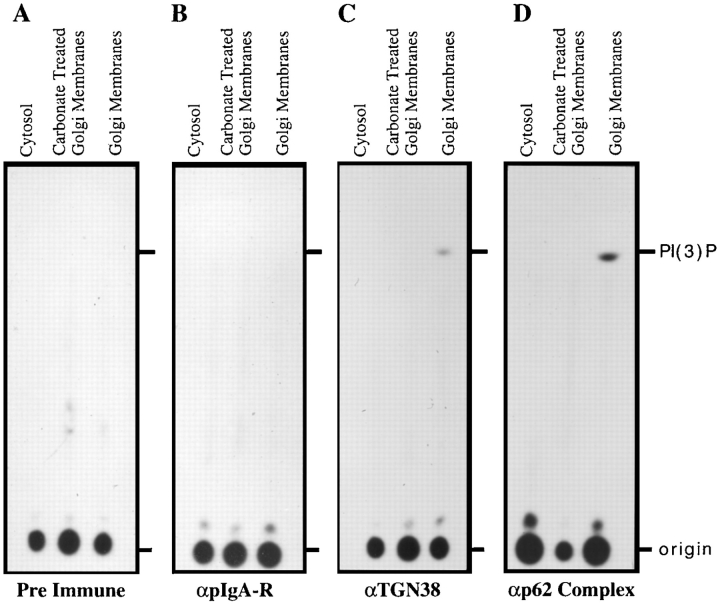
High pH Treatment of SGF Dissociates the PI3-kinase Activity
Previous experiments indicated a molecular mass for the membrane bound p62cplx of ∼250 kD. The complex consisted of dimeric TGN38 (160 kD) and the p62cplx (85 kD); and therefore could not accommodate an ∼100-kD, PI3-kinase catalytic subunit. To gain additional information on how the regulatory and catalytic subunits functionally interact, immunoprecipitates were analyzed from cytosol as well as high pH–treated and native SGF using preimmune sera or antibodies against pIgA-R, TGN38, or p62cplx (Fig. 2). Both preimmune sera and antibodies against the pIgA-R failed to precipitate PI3-kinase activity from any fraction. Antibodies directed against TGN38 and p62cplx precipitated PI3-kinase activity only from native SGF. No measurable activity was precipitated from cytosol or high pH–treated SGF. Therefore, neither the 85-kD p62cplx from cytosol nor the high pH–treated, membrane-associated p62cplx was associated with a PI3-kinase catalytic subunit. Only the membrane-associated p62cplx recovered from native SGF retained PI3-kinase activity, indicating that the catalytic subunit of the PI3-kinase is dissociated from the membrane complex by high pH treatment. These collective data imply that the p62cplx and the PI3-kinase catalytic subunit are not associated with each other in cytosol, and that the regulatory and catalytic subunits of the enzyme are assembled into an active complex on the Golgi membrane.
Figure 2.
Carbonate treatment dissociates the p62cplx-associated, PI3-kinase activity. PI3-kinase enzymatic activity was carried out as described in Materials and Methods; the autoradiogram of the TLC plate is shown. Immunoprecipitation was carried out on the following rat liver fractions: cytosol; carbonate-treated SGF; and native SGF (without carbonate treatment) (as labeled on top of each panel). Antibodies used for the immunoprecipitates are labeled at the bottom of each panel. The origin and mobility of PI(3)P is labeled on the right of D.
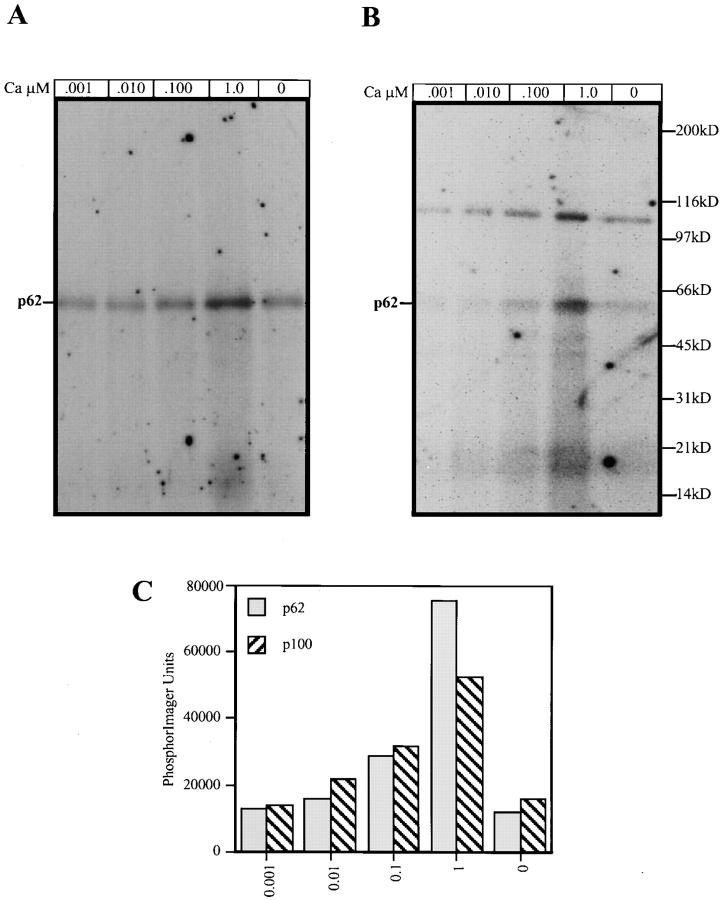
Phosphorylation Identifies a 100-kD Protein in the p62cplx Purified from Detergent-solubilized Native SGF
Since in the yeast system the PI3-kinase regulatory subunit (Vps 15p) is a protein kinase that has been postulated to phosphorylate the PI3-kinase catalytic subunit (Vps34p) (Stack et al., 1995a ) and it has been shown that the mammalian p110γ subunit can autophosphorylate (Vanhaesebroeck et al., 1997), in vitro phosphorylation reactions were performed in an attempt to identify the p62cplx-associated PI3-kinase catalytic subunit. The p62cplx was purified from detergent-solubilized, high pH washed, and native membranes using an immunoaffinity column prepared by covalently attaching an IgG fraction of p62 antisera. Kinase assays demonstrated that a single band at 62 kD was phosphorylated when the p62cplx was purified from detergent-solubilized, high pH–washed SGF and the phosphorylation was stimulated at increasing concentrations of Ca2+ (Fig. 3 A). Moreover, when the p62cplx was purified from detergent-solubilized native SGF, which has PI3-kinase activity (data not shown), an additional ∼100-kD phosphorylated subunit was present and the phosphorylation of this subunit exhibited the same calcium activation profile as the p62 subunit (Fig. 3, B and C). From these data we conclude that the membrane-associated p62cplx contains an additional 100-kD subunit that is detected only when the SGF is not high pH washed before detergent solubilization. Both the 62- and 100-kD subunits are likely to be phosphorylated by the same kinase because their phosphorylation displays the same calcium activation curve.
Figure 3.
Phosphorylation of p62cplx purified from detergent-solubilized native SGF reveals a 100-kD subunit not present if the SGF is high pH–washed before solubilization. p62cplx purified from detergent-solubilized, high pH–washed SGF (A) and native SGF (B) (10 ng) was incubated in an in vitro phosphorylation reaction containing 10 μCi [γ32P]ATP at increasing concentration of calcium from 0 to 1.0 μM. The calcium concentrations are noted above each lane and the molecular weight markers at the right of B. The mobility of p62 is noted at the left of A and B. Quantitation of the phosphorylation is plotted in PhosphorImager units (C).
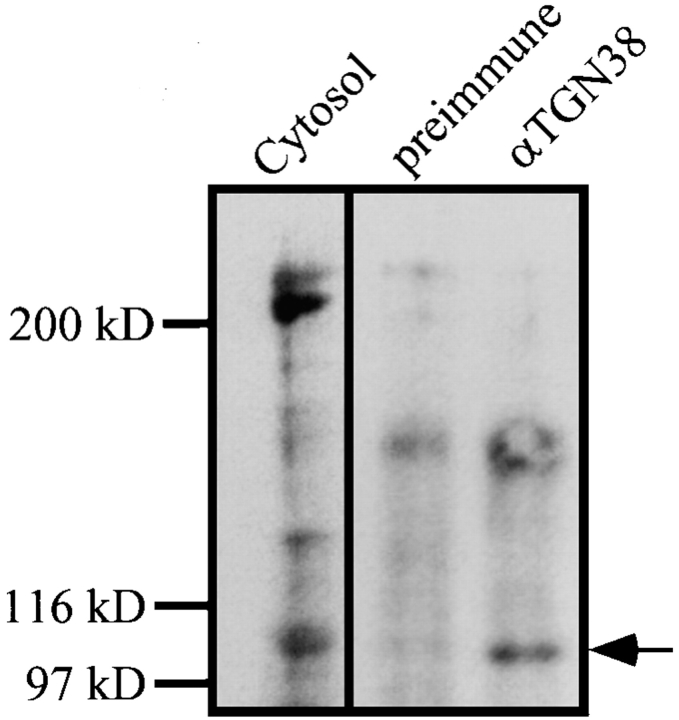
Wortmannin Binding Identifies a 100-kD Protein in the p62cplx Purified from Detergent-solubilized Native SGF as the PI3-kinase Catalytic Subunit
Wortmannin inhibits PI3-kinases by covalently coupling to the active site of the catalytic subunit and this interaction is stable after resolution by SDS-PAGE (Wymann et al., 1996). The bound wortmannin can be identified by using either [3H]wortmannin and autoradiography or an antibody against wortmannin and immunoblotting. Brunn et al. (1996) (using rat brain extracts and in vitro incubations) showed wortmannin covalently coupled to a very broad band of proteins that span 100–110 kD, as well as several proteins at ∼200 kD. To identify the catalytic subunit associated with the membrane-bound, p62cplx-associated PI3-kinase, fractions were incubated with wortmannin, resolved by SDS-PAGE, and then immunoblotted with antibodies against wortmannin. In rat liver, cytosol bands of 100, ∼130, and ∼200 kD bind wortmannin. Immunoprecipitates of the membrane-bound, p62cplx-associated PI3-kinase (the same as those used for the PI3-kinase assays) contains only the 100-kD wortmannin binding band and this band is not precipitated by the preimmune sera (Fig. 4). The findings that the immunoprecipitates have PI3-kinase activity and a 100-kD wortmannin binding protein, support the identification of the 100-kD band as the PI3-kinase catalytic subunit.
Figure 4.
Wortmannin binding identified a 100-kD protein in the p62cplx as the PI3-kinase catalytic subunit. Total cytosol (100 μg, cytosol) or immunoprecipitates from SGF using preimmune sera (preimmune), or antibodies against TGN38 (αTGN38) were incubated with 1 μM wortmannin at 30°C for 10 min and prepared for SDS-PAGE. The gels were transferred to nitrocellulose and blotted with affinity-purified antibodies against wortmannin, detected with 125I-protein A and autoradiography. SDS-PAGE molecular weight markers are shown at the left and the mobility of the 100-kD protein is noted at the right of the panel.
Neither the 62- Nor the 100-kD Subunits Are Antigenically Related to p85α or p110α and p110β
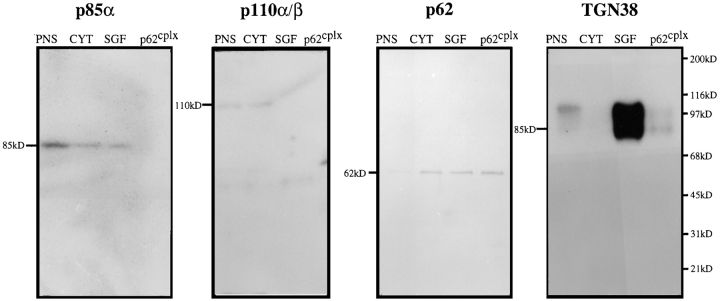
The antigenic relationship of p62 and p85α and the 100-kD subunit and p110α and p110β was tested using available antibodies. Immunoblot analysis was carried out on rat liver fractions: PNS, cytosol (Cyt), SGF, and the p62cplx purified from native SGF (the complex contains in addition to p62, the 25-kD GTPase, TGN38, and the 100-kD wortmannin-binding subunit). Antibodies directed against the p85α, p110α, and p110β subunits of the growth factor– associated PI3-kinase identified their cognate subunits in PNS and Cyt (Fig. 5). Little or no reactivity with these antibodies was observed in SGF and p62cplx fractions. Using antibodies directed against p62, an immunoreactive 62-kD band was detected in all of the fractions (albeit only weakly in PNS), and the electrophoretic mobility of both p62 and p85 bands were clearly differentiated in these same fractions. The demonstration that p62 exhibited a consistent molecular mass in all fractions tested, including PNS, suggested that p62 was unlikely to represent a proteolytic fragment of p85α. Antibodies directed against TGN38 confirmed the presence of this transmembrane protein in PNS, SGF, and the p62cplx isolated from membranes. However, we were unable to immunologically confirm the presence of a known PI3-kinase catalytic subunit in the p62 complex as none of the available monoclonal antibodies were able to recognize any subunit of the immunopurified membrane form of p62cplx.
Figure 5.
p62 is not antigenically related to the PI3-kinase p85α subunit. Cross-reactivity of antibodies against PI3-kinase subunits and p62 was tested by immunoblot analysis of subcellular fractions and the purified p62cplx. PNS, Cyt, and SGF were isolated from rat liver as described in Materials and Methods. The purified p62cplx was isolated from CHAPS-solubilized SGF using the immunoaffinity column. Fractions were resolved by SDS-PAGE and transferred to nitrocellulose: 200 μg PNS; 100 μg Cyt; 25 μg SGF, and 12.5 μg p62cplx. Immunoblots using antibodies against the growth factor–associated PI3-kinase p85α, p110α/β, and p62 and TGN38, members of the membrane-associated p62cplx, labeled at the top of each panel. Antibodies against p85β and p85γ were also tested and were negative. SDS-PAGE molecular weight markers are shown at the right of the TGN38 panel and the molecular weight of each antigen noted at the left of its respective panel. Note that the intense signal for TGN38 in SGF correlates with the >400-fold enrichment over PNS on Golgi transmembrane proteins in SGF.
Identification of PI3-kinase Activity in p62cplx Purified from Detergent Extracts of Native SGF
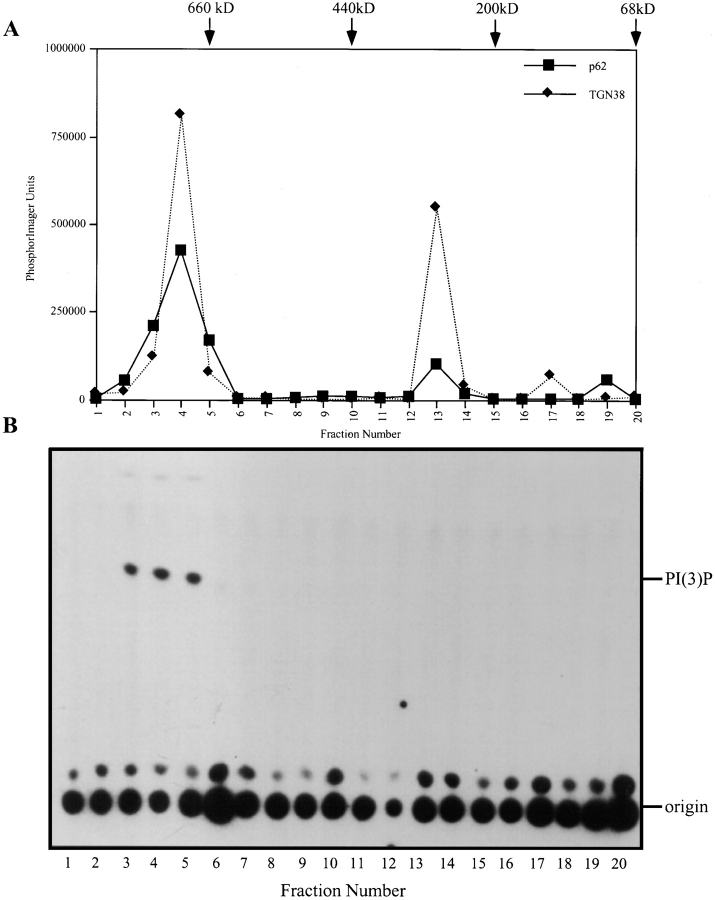
Sephacryl S500 gel filtration chromatography of CHAPS-solubilized native SGF was used to identify a membrane-bound, p62cplx-associated PI3-kinase activity (Fig 6). Two major peaks of TGN38 and p62 immunoreactive materials were eluted at ∼250 and ∼700 kD. Only immunoprecipitates of p62 from the ∼700-kD peak contained PI3-kinase activity. These data indicate that a PI3-kinase catalytic subunit (100-kD protein) associates with TGN38 and p62 to form a significantly larger complex than the p62cplx/ TGN38-containing complex recovered from high pH– treated Golgi membranes, and that this larger complex contains PI3-kinase activity.
Figure 6.
The catalytically active p62cplx-associated PI3-kinase associated with the Golgi membrane elutes as ∼700 kD complex by gel filtration. CHAPS-solubilized SGF (2 mg) was resolved on a Sephacryl S500 column. Fractions (3.0 ml) were collected and half were used for immunoprecipitation with antibodies against p62 and the other half resolved by SDS-PAGE (after TCA precipitation) for immunoblot analysis. Immunoblots were detected with 125I-protein A and quantitated using the PhosphorImager. The PhosphorImager units are plotted for both TGN38 (♦) and p62 (▪) (A). PI3-kinase assays were carried out on the p62 immunoprecipitates and the TLC plate exposed to film for autoradiography (B). The mobility of PI(3)P is noted at the right of B. The column was calibrated with molecular weight standards: thyroglobulin, 660 kD; apoferritin, 440 kD; β-amylase, 200 kD; BSA, 66 kD, shown at the top of A.
Properties of the p62cplx-associated PI3-kinase Activity
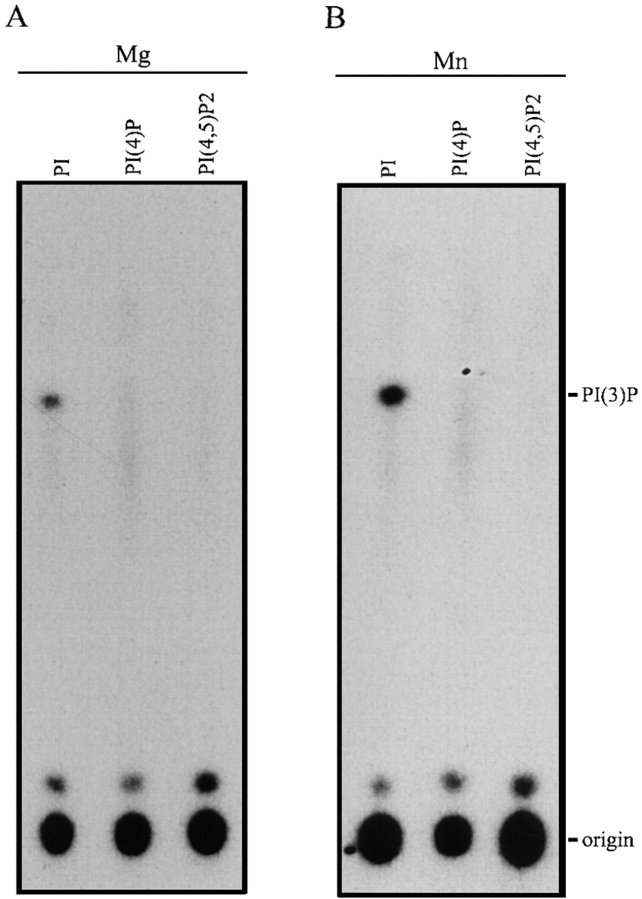
The biochemical properties of the p62cplx-associated PI3-kinase activity were determined and compared with those of several other PI3-kinases, including the growth factor– associated p110, Vps34p, and the human Vps34p homologue (Stack et al., 1995a ; Volinia et al., 1995) (Figs. 7 and 8; and Table II). The 50% inhibitory concentration (IC50) of the p62cplx-associated PI3-kinase activity with wortmannin was ∼3.5 μM. This value is similar to that determined for Vps34p (∼3 μM) and is three orders of magnitude greater than the IC50s of the p110 PI3-kinase and of the mammalian Vps34p homologue (2–3 nM). The p62cplx-associated PI3-kinase activity was also stimulated by low concentrations of non-ionic detergent (0.1% NP-40), similar to p110 and Vps34p, whereas all these PI3-kinases were inhibited by NP-40 at higher concentrations. p62cplx-associated PI3-kinase and all other PI3-kinase activities were insensitive to high (mM) concentrations of adenosine (data not shown). These properties are used to distinguish PI3-kinases (activation by low concentration of detergent and insensitivity to adenosine) from phosphatidylinositol 4–kinases, which are not detergent activated and are sensitive to adenosine.
Figure 7.
Properties of the p62cplx-associated PI3-kinase. PI3-kinase assays were carried out as described in Materials and Methods. Either Mg2+ (A) or Mn2+ (B) were used as the cation in the presence of multiple substrates: PI, PI(4)P, and PI(4,5)P2, as listed at the top of each lane. The mobility of PI(3)P is noted at the right of B.
Figure 8.
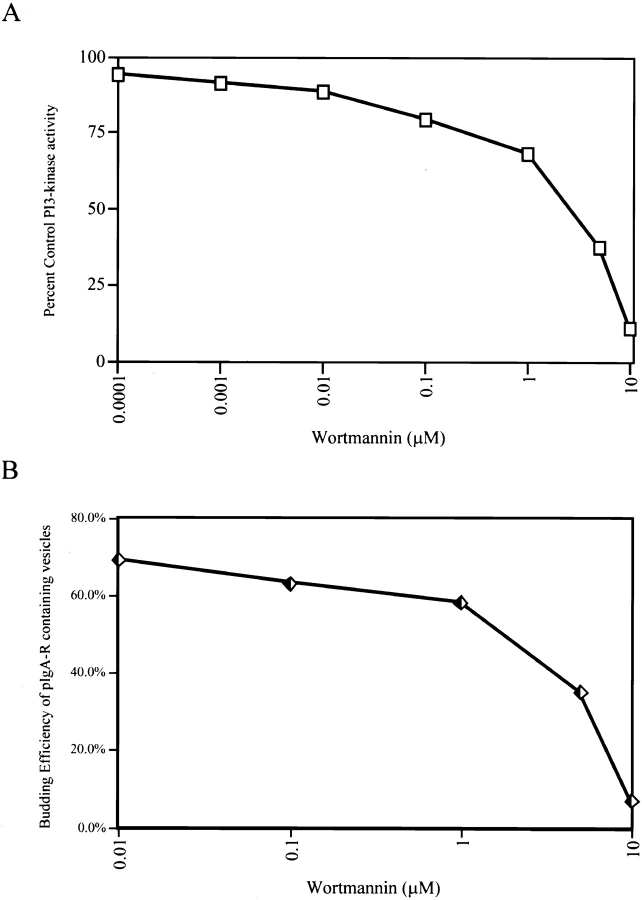
The p62cplx-associated PI3-kinase activity and the formation of pIgA-R–containing vesicles from the TGN are sensitive to the same concentrations of wortmannin. PI3-kinase assays were carried out as described in Materials and Methods at increasing concentrations of wortmannin (0.001–10 μM). The amount of PI(3)P formed was determined by PhosphorImager quantitation of the TLC plates. The activity expressed as percent of control (no wortmannin) is plotted versus wortmannin concentration (A). The cell-free assay was carried out as described in Materials and Methods at increasing concentrations of wortmannin (0.01–10 μM). The efficiency of formation of pIgA-R–containing vesicles was determined and is plotted versus wortmannin concentration (B).
Table II.
Comparison of the Properties of the p62cplx-associated PI3-kinase with Other Described PI3-kinases
| p62cplx-associated | Vps34p‡ | Human PI3-kinase* (VPS34 homologue) | PI3-kinase* (p110) | |||||
|---|---|---|---|---|---|---|---|---|
| Adaptor molecule | p62 | Vps15p | p150 | p85 | ||||
| Wortmannin (IC50) | 3.5 μM | 3 μM | 2.5 nM | 3 nM | ||||
| Affect of low NP-40 (0.1–0.5%) | Stimulation | Stimulation | Inhibition | Stimulation | ||||
| NP-40 (IC50) | 0.35% | 1.0% | 0.01% | 0.1% | ||||
| Adenosine sensitivity | No | No | No | No | ||||
| PI (4)P as substrate | No | No | No | Yes | ||||
| Cation preference | Mn2 > Mg2+ | Mn2+ = Mg2+ | Mn2+ | Mg2+ = Mn2+ |
Our data for the p62cplx-associated activity is summarized. Data for the other PI3-kinases is from the references noted. Only the well-characterized PI3-kinases have been included in this table.
Data from Volinia et al., 1995.
Data from Stack and Emr, 1995.
The substrate specificity of the p62cplx-associated activity was assayed, in the absence or presence Mg2+or Mn2+, with PI, phosphatidylinositol-4-phosphatate (PI[4]P), phosphatidylinsositol-4,5-diphosphate (PI[4,5]P2) as substrates. The p62cplx-associated activity showed a preference for Mn2+ and a specificity for PI (Fig. 7). Although the full set of data on the properties of each PI3-kinase is not available, the biochemical properties of the p62cplx-associated PI3- kinase were most similar to those of Vps34p. In summary, the lipid kinase activity associated with the p62cplx is a PI-specific, Mn2+-activated PI3-kinase that is sensitive to wortmannin at micromolar concentrations.
Correlation between the Inhibition of p62–PI3-kinase Activity and the Formation of Exocytic Vesicles In Vitro
The p62cplx has been shown to be essential for cell-free formation of pIgA-R–containing exocytic vesicles from the TGN (Jones et al., 1993). In this cell-free assay, an SGF is immobilized on magnetic beads, and in the presence of cytosol, ATP, and an ATP regenerating system at 37°C a mixed population of vesicles bud (Salamero et al., 1990). The amount of the mature sialylated pIgA-R (116 kD) present in the budded fraction is quantitated and compared with that present in the starting Golgi fraction to determine budding efficiency of this population of vesicles.
If PI3-kinase activity is essential for vesicle formation, then the vesicle budding reaction should be wortmannin inhibitable, and the pharmacology of this inhibition should closely resemble that of the p62cplx-associated PI3-kinase activity. The data shown in Fig. 8, A and B were consistent with both predictions. Increasing concentrations of wortmannin inhibited both the formation of pIgA-R–containing vesicles and the PI3-kinase activity with similar dose-response curves and IC50s of ∼3–5 μM.
Discussion
The cytosolic p62cplx is required for the formation of pIgA-R–containing exocytic vesicles from the TGN in a cell-free assay system (Jones et al., 1993). A biochemical characterization of this complex is presented here and we discuss the function of the p62cplx in the process of vesicle formation from the TGN.
p62cplx Regulates a Novel PI3-kinase Activity
Sequence analysis of peptides derived from the p62 subunit of the p62cplx revealed a region of homology within this protein to the regulatory subunit, p85α of a PI3-kinase (Table I). The 24–amino acid sequence of p62 was identical to amino acids 82–105 in the brc domain of mouse p85α. No other homology with any other protein was found indicating that the homology was not to a generalized domain that binds small GTPases and found in many proteins. The stronger data supporting that p62 is a regulator subunit of a PI3-kinase comes from the biochemical characterization. Enzyme assays of immunopurified p62cplx from an SGF confirmed p62 as a regulatory subunit of a PI3-kinase (Figs. 1–3, and 6–8). Maximal production of PI(3)P by the p62cplx-associated PI3-kinase required the activation of the p62-associated GTPase. The p62cplx-associated PI3-kinase activity showed a substrate specificity for PI, and an IC50 for wortmannin of 3.5 μM (Table II, and Fig. 8). Inhibition of the formation of the pIgA-R–containing exocytic vesicles in vitro, by wortmannin had the same dose-response curve as the 62cplx-associated PI3-kinase activity (Fig. 8). These data implicate a role for PI(3)P in the multitude of steps that are required to sort molecules and form vesicles at the TGN.
Only the membrane-associated p62cplx isolated from non-high pH–treated SGF had PI3-kinase activity, and an additional 100-kD phosphoprotein subunit was detected in the active complex. The phosphorylation of p62 and p100 followed the same Ca2+ activation profile, suggesting that both subunits are phosphorylated by the same protein kinase (Fig. 3). Although it cannot be ruled out that the p100 subunit can autophosphorylate, as has been shown for p110γ (Vanhaesebroeck et al., 1997). Since the immunopurified p62cplx from detergent-solubilized, high pH–washed SGF (containing TGN38, p62, and a 25-kD GTPase) is shown to have protein kinase activity, p62 itself is likely to be the protein kinase. However, the possibility that the immunopurified material is contaminated with a small amount of a protein kinase cannot be ruled out. Confirmation of the protein kinase activity of p62 will require cloning and sequencing of the molecule. Importantly, a 100-kD wortmannin binding protein was identified in the enzymatically active p62cplx identifying the p100 as the catalytic subunit of p62cplx-associated PI3-kinase. A number of genes encoding PI3-kinase catalytic subunit isoforms have been cloned, and the products found to be ∼100 kD, therefore this data does not identify the specific isoform associated with the p62cplx (for review see Liscovitch and Cantley, 1994). Consistent with the biochemical data, the potential relationship of p100 as a substrate for the p62 protein kinase recapitulates the predicted situation in yeast vacuolar protein sorting where the Vps15p serine/threonine kinase is thought to phosphorylate the associated Vps34p catalytic subunit of PI3-kinase (Stack and Emr, 1994; Stack et al., 1995a ).
The molecular interactions between components of p62cplx and its associated PI3-kinase catalytic subunit are quite stable as evidenced by having withstood the multiple detergents used in the immunoprecipitations. In gel filtration experiments, the enzymatically active complex resolved with an apparent molecular size of 700 kD. Since the p62cplx isolated from high pH–treated Golgi membranes was 250 kD (Jones et al., 1993), and the catalytic subunit is 100 kD, the molecular mass of the complex suggests either that the complex is dimeric or other signaling molecules are associated with the isolated complex.
The properties of the p62cplx-associated PI3-kinase differ from those of the activated growth factor receptor–associated PI3-kinases. The latter PI3-kinases phosphorylate PI in vitro, but their in vivo substrate is PI(4,5)P2, and these exhibit an IC50 for wortmannin in the low nM range (for reviews see Panayotou et al., 1993; Liscovitch and Cantley, 1994, 1995; Carpenter and Cantley, 1996). The regulatory (p85) and catalytic (p110) subunits of these PI3-kinases are associated with each other in cytosol and the p85 subunits are not protein kinases. Upon ligand binding to (and activation of) a receptor tyrosine kinase, the activated growth factor receptor–associated PI3-kinases bind phosphorylated tyrosines in the receptor cytoplasmic domain via an SH2 domain in the regulatory subunit. Thus, the PI3-kinase assembles en bloc with the activated receptor (for review see Kazlauskas, 1994). The TGN membrane receptor for the p62cplx-associated PI3-kinase is dimeric TGN38, but the interaction motif remains undefined. It probably does not involve a phosphotyrosine signal. The sole tyrosine in the cytoplasmic domain of TGN38 does not reside in a proper context for phosphorylation, nor is it phosphorylated under the conditions we have used to study the reversible phosphorylation of p62.
The p62cplx-associated PI3-kinase activity is more comparable biochemically to the trafficking PI3-kinase, Vps15p/ Vps34p, characterized in Saccharomyces cerevisiae (for review see Stack et al., 1995b ). Both are PI-specific PI3- kinases with IC50s for wortmannin of ∼3 μM. In addition, Vps15p-protein kinase is required to recruit Vps34p to the membrane and activate the production of PI(3)P (Stack et al., 1995a ). The behavior of p62 is consistent with the Vps15p/Vps34p paradigm in that p62 is likely to be the kinase that phosphorylates the 100-kD protein, a wortmannin-binding protein, and presumably the catalytic subunit of the p62cplx-associated PI3-kinase. However, we have no direct evidence that this phosphorylation results in activation of the PI3-kinase leading to increased production of PI(3)P.
Recently it has been proposed that PI3-kinase can be divided into three different classes (Domin and Waterfield, 1997). The first class is made up of the classical signaling PI3-kinase, which includes the components of the p85/ p110 heterodimeric complexes. These lipid kinases have substrate specificity in vivo for PI(4,5)P2 and are stimulated upon growth factor signaling. The class II kinases are made up of large molecular weight catalytic subunits that have no known regulatory subunits and substrate specificity for PI(4)P. These proteins contain C2 domains which convey Ca2+ sensitivity to PKC. The mechanism of activation of the class II kinases is not understood. The class III kinases are made up of Vps15p/Vps34p and its human homologues. This class has substrate specificity for PI exclusively and is likely to be involved in membrane trafficking events. Our data suggest that p62 and its associated PI3-kinase catalytic subunit will fit into this last class of PI3-kinases.
Finally, we emphasize that, although both the p62cplx-associated PI3-kinase and the Vps15p/Vps34p complex are involved in protein traffic from the TGN, the major function of the former is the sorting/regulation of a stage in the constitutive secretory pathway, while the latter has no involvement with constitutive secretion. Rather, the Vps15p/ Vps34p complex controls the sorting and trafficking of vacuolar enzymes.
A Model for p62cplx Involvement in Budding of Constitutive Secretory Vesicles from the TGN
Our current data are summarized as follows. In cytosol, the p62cplx (85 kD) consists of a 62-kD phosphoprotein and a 25-kD GTPase. The cytosolic p62cplx does not have PI3-kinase catalytic activity, reflecting the finding that the p62cplx is not associated with the catalytic subunit in cytosol. Upon some unknown signal, p62 is dephosphorylated, leading to the assembly of the regulatory p62cplx and PI3-kinase catalytic subunits with the cytoplasmic domain of TGN38. This “receptor” (TGN38) is a dimeric, transmembrane protein, predominately localized to the TGN. The membrane-associated PI3-kinase complex is stimulated by activation of its small GTPase resulting in formation of the required amounts of PI(3)P to drive the formation of the pIgA-R vesicles from the TGN.
Potential Effectors of PI(3)P in Membrane Traffic
In vivo levels of PI(3)P in mammalian cells are relatively low, reasonably constant, and do not change upon growth factor stimulation (De Camilli et al., 1996; Shpetner et al., 1996). These data suggest that in signaling PI(3)P acts locally, and through interaction with different effectors, regulates different and even diverse functions.
One hypothesis for the role of PI(3)P in vesicle budding is that its presence in the outer leaflet of the bilayer drives outward curvature of the membrane, thereby facilitating bud formation (Stack et al., 1995b ). This hypothesis is a direct derivation of the bilayer-couple model of Sheetz and Singer (1974) that relates the headgroup size and charge of phospholipids exhibiting significant local bilayer asymmetries with membrane curvature. Other hypotheses that account for the rather constant amounts of PI(3)P in cells are equally tenable at this time. The PI(3)P may be bound to a carrier molecule, like phosphatidlyinositol transfer protein, rather than being incorporated into the lipid bilayer (Bankaitis et al., 1990). This scenario would implicate PI(3)P as a second messenger.
Many phosphatidyl- and phosphoinositide-sensitive proteins with diverse functions have been identified: (a) isoforms of protein kinase C (Singh et al., 1993; Toker et al., 1994; Akimoto et al., 1996); (b) dynamin GTPase activity (Tuma, 1993); (c) calcium channels (Nori et al., 1993); (d) actin binding proteins (Weeds and Maciver, 1993; Sohn and Goldschmidt-Clermont, 1994); and (e) unconventional, nontransmembrane channels formed by vesicle coat proteins (e.g., coatomer and the AP-2 and AP-3 clathrin adapter complexes) (Timerman et al., 1990; Kijima et al., 1993; Fleischer et al., 1994). All of these PI/IPX-sensitive molecules play roles in membrane traffic, albeit diverse roles. It now remains for us to identify the PI(3)P effector molecule/s that regulate membrane traffic from the TGN.
Acknowledgments
We are grateful to J. Sherman for doing the wortmannin binding experiment. S.M. Jones would like to thank his thesis committee, P. Melançon, M.-F. Pfenninger, J. Caldwell, and J. Hutton for their continued support and contributions to this work. We would like to thank J. Ugelstad and R. Schmid (SINTEF, University of Trondheim, Trondheim, Norway) for the shell and core magnetic beads used in the cell-free assay. V. Bankaitis (University of Alabama at Birmingham, Birminghan, AL) was especially helpful in critical discussion of the data.
This work was supported by National Institutes of Health grant GM 42629 (to K.E. Howell) and additional support from the Cell Biology Core of the Hepatobiliary Center, grant P30 DK34914, and the Monoclonal Core of the Cancer Center, grant P30 CA-46934.
Abbreviations used in this paper
- Cyt
cytosol
- GTPγS
guanosine-5′-O- (3-thiophosphate)
- IC50
50% inhibitory concentration
- PI
phosphatidylinositol
- PI(3)P
phosphatatidylinositol-3-phosphate
- PI(4)P
phosphatidylinositol-4-phosphatate
- PI(4,5)P2
phosphatidylinositol-4,5-diphosphate
- pIgA-R
polymeric IgA receptor
- PI3-kinase
phosphatidylinositol 3-kinase
- PNS
postnuclear supernatant
- SGF
stacked Golgi fraction
Footnotes
Address all correspondence to K.E. Howell, Department of Cellular and Structural Biology, University of Colorado School of Medicine, Denver, CO 80262. Tel.: (303) 315-5153. Fax: (303) 315-4729. E-mail: kathryn.howell@uchsc.edu
References
- Akimoto K, Takahashi R, Moriya S, Nishioka N, Takayanagi J, Kimura K, Fukui Y, Si, Osada, Mizuno K, Si, Hirai, et al. EGF or PDGF receptors activate atypical PKC lambda through phosphatidylinositol 3-kinase. EMBO (Eur Mol Biol Organ) J. 1996;15:788–798. [PMC free article] [PubMed] [Google Scholar]
- Altschulz SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bankaitis VA, Aitken JR, Cleves AE, Dowhan W. An essential role for a phospholipid transfer protein in yeast Golgi function. Nature (Lond) 1990;347:561–562. doi: 10.1038/347561a0. [DOI] [PubMed] [Google Scholar]
- Banta LM, Robinson JS, Kliosky DJ, Emr SD. Organelle assembly in yeast: characterization of yeast mutants defective in vacuolar biogenesis and protein sorting. J Cell Biol. 1988;107:1369–1383. doi: 10.1083/jcb.107.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokoch GM, Vlahos CJ, Wang Y, Knaus UG, Traynor-Kaplan AE. Rac GTPase interacts specifically with phosphatidylinositol 3-kinase. Biochem J. 1996;315:775–779. doi: 10.1042/bj3150775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos K, Wraight C, Stanley KK. TGN38 is maintained in the trans-Golgi network by a tyrosine-containing motif in the cytoplasmic domain. EMBO (Eur Mol Biol Organ) J. 1993;12:2219–2228. doi: 10.1002/j.1460-2075.1993.tb05870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WJ, DeWald DB, Emr SD, Plutner H, Balch WE. Role for phosphatidylinositol 3-kinase in the sorting and transport of newly synthesized lysosomal enzymes in mammalian cells. J Cell Biol. 1995;130:781–790. doi: 10.1083/jcb.130.4.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunn GJ, Williams J, Sabers C, Wiederrecht G, Lawrence JC, Jr, Abraham RT. Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin and LY294002. EMBO (Eur Mol Biol Organ) J. 1996;15:5256–5267. [PMC free article] [PubMed] [Google Scholar]
- Carpenter CL, Cantley LC. Phosphoinositide 3-kinase and the regulation of cell growth. Biochim Biophys Acta. 1996;1288:11–16. doi: 10.1016/0304-419x(96)00018-2. [DOI] [PubMed] [Google Scholar]
- Davidson H. Wortmannin causes mistargeting of procathepsin D. Evidence for the involvement of a phosphatidylinositol 3-kinase in vesicular transport to lysosomes. J Cell Biol. 1995;130:797–805. doi: 10.1083/jcb.130.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Camilli P, Emr SD, McPherson PS, Novick P. Phosphoinositides as regulators of membrane traffic. Science (Wash DC) 1996;271:1533–1539. doi: 10.1126/science.271.5255.1533. [DOI] [PubMed] [Google Scholar]
- Domin J, Waterfield MD. Using structure to define the function of phosphoinositide 3 kinase family members. FEBS (Fed Eur Biochem Soc) Lett. 1997;410:91–95. doi: 10.1016/s0014-5793(97)00617-0. [DOI] [PubMed] [Google Scholar]
- Escobedo JA, Navankasattusas S, Kavanaugh WM, Milfay D, Fried VA, Williams LT. cDNA cloning of a novel 85 kD protein that has SH2 domains and regulates binding of PI3-kinase to the PDGF β-receptor. Cell. 1991;65:75–82. doi: 10.1016/0092-8674(91)90409-r. [DOI] [PubMed] [Google Scholar]
- Fleischer B, Xie J, Mayrleitner M, Shears SB, Palmer DJ, Fleischer S. Golgi coatomer binds, and forms K(+)-selective channels gated by, inositol polyphosphates. J Biol Chem. 1994;269:17826–17832. [PubMed] [Google Scholar]
- Gonatas JO, Mezitis SG, Stieber A, Fleischer B, Gonatas NK. MG-160. A novel sialoglycoprotein of the medial cisternae of the Golgi apparatus. J Biol Chem. 1989;264:646–653. [PubMed] [Google Scholar]
- Horn M, Banting G. Okadaic acid treatment leads to a fragmentation of the trans-Golgi network and an increase in expression of TGN38 at the cell surface. Biochem J. 1994;301:68–73. doi: 10.1042/bj3010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell, K.E., J.R. Crosby, M.S. Ladinsky, S.M. Jones, R. Schmid, and J. Ugelstad. 1994. Magnetic solid supports for cell-free analysis of vesicular transport. In Advances in Biomagnetic Separation. M. Uhlén, E. Hornes, and Ø. Olsvik, editors. Eaton Publishing, Natick, MA. 195–204.
- Humphrey JS, Peters PJ, Yuan LC, Bonifacino JS. Localization of TGN38 to the trans-Golgi network: involvement of a cytoplasmic tryosine-containing sequence. J Cell Biol. 1993;120:1123–1135. doi: 10.1083/jcb.120.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SM, Crosby JR, Salamero J, Howell KE. A cytoplasmic complex of p62 and rab6 associates with TGN38/41 and is involved in budding of exocytic vesicles from the trans-Golgi network. J Cell Biol. 1993;122:775–788. doi: 10.1083/jcb.122.4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, S.M., R.H. Dahl, J. Ugelstad, and K.E. Howell. 1997. Immunoisolation of Organelles using Magnetic Solid Supports in Cell Biology, a Laboratory Handbook. 2nd Edition. Vol. II. J. Celis, editor. Academic Press, San Diego, CA. 12–25.
- Kazlauskas A. Receptor tyrosine kinases and their targets. Curr Opin Genet Dev. 1994;4:5–14. doi: 10.1016/0959-437x(94)90085-x. [DOI] [PubMed] [Google Scholar]
- Kazlauskas A, Cooper JA. Phosphorylation of the PDGF receptor β subunit creates a tight binding site for phosphatidylinositol 3 kinase. EMBO (Eur Mol Biol Organ) J. 1990;9:3279–3286. doi: 10.1002/j.1460-2075.1990.tb07527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kijima Y, Mayrleitner M, Timerman AP, Saito A, Schindler H, Fleischer S. A cardiac clathrin assembly protein forms a potassium channel in planar lipid bilayers. J Biol Chem. 1993;268:16253–16258. [PubMed] [Google Scholar]
- Kodaki T, Woscholski R, Hallber B, Rodriguez-Viciano P, Downard J, Parker PJ. The activation of phosphatidylinositol 3-kinase by Ras. Curr Biol. 1994;4:798–806. doi: 10.1016/s0960-9822(00)00177-9. [DOI] [PubMed] [Google Scholar]
- Ladinsky MS, Howell KE. The trans-Golgi network can be dissected structurally and functionally from the cisternae of the Golgi complex by brefeldin A. Eur J Cell Biol. 1992;59:92–105. [PubMed] [Google Scholar]
- Leelavathi DE, Estes LW, Feingold DS, Lombardi B. Isolation of a Golgi-rich fraction from rat liver. Biochim Biophys Acta. 1970;211:124–138. [Google Scholar]
- Liscovitch M, Cantley LC. Lipid second messengers. Cell. 1994;77:329–334. doi: 10.1016/0092-8674(94)90148-1. [DOI] [PubMed] [Google Scholar]
- Liscovitch M, Cantley LC. Signal transduction and membrane traffic: the PITP/phosphoinositide connection. Cell. 1995;81:659–662. doi: 10.1016/0092-8674(95)90525-1. [DOI] [PubMed] [Google Scholar]
- Luzio JP. Immunological approaches to the study of membrane features in adipocytes. Methodol Surv Biochem. 1977;6:131–142. [Google Scholar]
- Luzio PJ, Brake B, Banting G, Howell KE, Braghetta P, Stanley KK. Identification, sequencing and expression of an integral membrane protein of the trans-Golgi network (TGN38) Biochem J. 1990;270:97–102. doi: 10.1042/bj2700097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizel JV. Polyacrylamide electrophoresis of viral proteins. Methods Virol. 1971;5:179–246. [Google Scholar]
- Matsudaira, P. 1993. A practical guide to protein and peptide purification for microsequencing, 2nd ed. Academic Press, San Diego, CA. 1–184.
- Matsuoka K, Bassham DC, Raikhel NV, Nakamura K. Different sensitivity to wortmannin of two vacuolar sorting signals indicates the presence of distinct sorting machineries in tobacco cells. J Cell Biol. 1995;130:1307–1318. doi: 10.1083/jcb.130.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nori A, Villa A, Podini P, Witcher DR, Volpe P. Intracellular Ca2+stores of rat cerebellum: heterogeneity within and distinction from endoplasmic reticulum. Biochem J. 1993;291:199–204. doi: 10.1042/bj2910199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panayotou G, Waterfield MD. The assembly of signalling complexes by receptor tyrosine kinases. Bioessays. 1993;15:171–177. doi: 10.1002/bies.950150305. [DOI] [PubMed] [Google Scholar]
- Reaves B, Horn M, Banting G. TGN38/41 recycles between the cell surface and the TGN: brefeldin A affects its rate of return to the TGN. Mol Biol Cell. 1993;4:93–105. doi: 10.1091/mbc.4.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaves BJ, Bright NA, Mullock BM, Luzio JP. The effect of wortmannin on the localisation of lysosomal type 1 integral membrane glycoprotein suggests a role for phosphoinositide 3-kinase activity in regulating membrane traffic late in the endocytic pathway. J Cell Sci. 1996;109:749–762. doi: 10.1242/jcs.109.4.749. [DOI] [PubMed] [Google Scholar]
- Salamero J, Sztul ES, Howell KE. Exocytic transport vesicles generated in vitro from the trans-Golgi network carry secretory and plasma membane proteins. Proc Natl Acad Sci USA. 1990;87:7717–7721. doi: 10.1073/pnas.87.19.7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schu PV, Takegawa K, Fry MJ, Stack JH, Waterfield MD, Emr SD. Phosphatidylinositol 3-kinase encoded by yeast VPS34 gene is essential for protein sorting. Science (Wash DC) 1993;260:88–91. doi: 10.1126/science.8385367. [DOI] [PubMed] [Google Scholar]
- Sheetz M, Singer S. Biological membranes as bilayer couples. A molecular mechanism of drug-erythrocyte interactions. Proc Natl Acad Sci USA. 1974;71:4457–4461. doi: 10.1073/pnas.71.11.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd PR, Reaves BJ, Davidson HW. Phosphoinositide 3-kinase and membrane traffic. Trends Cell Biol. 1996;6:92–97. doi: 10.1016/0962-8924(96)80998-6. [DOI] [PubMed] [Google Scholar]
- Shpetner H, Joly M, Hartley D, Corvera S. Potential sites of PI-3 kinase function in the endocytic pathway revealed by the PI–3 kinase inhibitor, wortmannin. J Cell Biol. 1996;132:595–605. doi: 10.1083/jcb.132.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SS, Chaunan A, Bockerhoff H, Chauhan VP. Activation of protein kinase C by phosphatidylinositol 3,4,5 trisphosphate. Biochem Biophys Res Commun. 1993;195:104–112. doi: 10.1006/bbrc.1993.2016. [DOI] [PubMed] [Google Scholar]
- Sohn RH, Goldschmidt-Clermont PJ. Profilin: at the crossroads of signal transduction and the actin cytoskeleton. Bioessays. 1994;16:465–472. doi: 10.1002/bies.950160705. [DOI] [PubMed] [Google Scholar]
- Stack JH, Emr SD. Vps34 required for yeast vacuolar protein sorting is a multiple specificity kinase that exhibits both protein kinase and phosphatidylinositol-specific PI3-kinase activities. J Biol Chem. 1994;269:31552–31562. [PubMed] [Google Scholar]
- Stack JH, Herman PK, Schu PV, Emr SD. A membrane-associated complex containing the Vps25 protein kinase and the Vps34 PI3-kinase is essential for protein sorting to the yeast lysosome like vacuole. EMBO (Eur Mol Biol Organ) J. 1993;12:2195–2204. doi: 10.1002/j.1460-2075.1993.tb05867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack JH, Wald D, Takegawa K, Emr SD. Vesicle-mediated protein transport: regulatory interactions between the Vps15 protein kinase and the Vps34 PtdIns 3-kinase essential for protein sorting to the vacuole in yeast. J Cell Biol. 1995a;129:321–334. doi: 10.1083/jcb.129.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack JH, Horazdovsky B, Emr SD. Receptor-mediated protein sorting to the vacuole in yeast: roles for protein kinase, a lipid kinase and GTP-binding proteins. Annu Rev Cell Dev Biol. 1995b;11:1–33. doi: 10.1146/annurev.cb.11.110195.000245. [DOI] [PubMed] [Google Scholar]
- Stephens L, Cooke FT, Walters R, Jackson T, Volinia S, Gout I, Waterfield MD, Hawkins PT. A phosphatidylinositol-specific PI3-K in mammalian cells. Curr Biol. 1994;4:203–214. doi: 10.1016/s0960-9822(00)00049-x. [DOI] [PubMed] [Google Scholar]
- Subramaniam VN, Krijnse-Locker J, Tang BL, Ericsson M, Yusoff AR, Griffiths G, Hong W. Monoclonal antibody HFD9 identifies a novel 28 kDa integral membrane protein on the cis-Golgi. J Cell Sci. 1995;108:2405–2414. doi: 10.1242/jcs.108.6.2405. [DOI] [PubMed] [Google Scholar]
- Sztul ES, Howell KE, Palade GE. Biogenesis of the polymeric IgA-R in rat hepatocytes. J Cell Biol. 1985;100:1248–1254. doi: 10.1083/jcb.100.4.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RS, Jones SM, Dahl RH, Nordeen M, Howell KE. Characterization of the Golgi complex cleared of proteins in transit and examination of calcium uptake activities. Mol Biol Cell. 1997a;8:1911–1931. doi: 10.1091/mbc.8.10.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, R.S., I. Fialka, S.M. Jones, L.A. Huber, and K.E. Howell. 1997b. 2D mapping of the endogenous proteins of the rat hepatocyte Golgi complex clear of proteins in transit. Electrophoresis. In press. [DOI] [PubMed]
- Timerman AP, Mayrleitner M, Lukas TJ, Chadwick CC, Saito A, Watterson DM, Schindler H, Fleischer S. Inositol polyphosphate receptor and clathrin assembly protein AP-2 are related proteins that form potassium-selective ion channels in planar lipid bilayers. Proc Natl Acad Sci USA. 1992;89:8976–8980. doi: 10.1073/pnas.89.19.8976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toker A, Meyer M, Reddy KK, Falck JR, Aneja R, Aneja S, Parra A, Burns DJ, Ballas LM, Cantley LC. Activation of protein kinase C family members by the novel polyphosphoinositides PtdIns-3,4-P2 and PtdIns-3,4,5-P3. J Biol Chem. 1994;269:32358–32367. [PubMed] [Google Scholar]
- Tolias KF, Cantley LC, Carpenter CL. Rho family GTPases bind to phosphoinositide kinases. J Biol Chem. 1995;270:17656–17659. doi: 10.1074/jbc.270.30.17656. [DOI] [PubMed] [Google Scholar]
- Tuma PL, Stachniak MC, Collins CA. Activation of dynamin GTPase by acidic phospholipids and endogenous rat brain vesicles. J Biol Chem. 1993;268:17240–17246. [PubMed] [Google Scholar]
- Vanhaesebroeck B, Welham MJ, Kotani K, Stein R, Warne PH, Zvelebil MG, Higashi K, Volinia S, Downward J, Waterfield MD. P110δ, a novel phosphoinositide 3-kinase in leukocytes. Proc Natl Acad Sci USA. 1997;94:4330–4335. doi: 10.1073/pnas.94.9.4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virbasius JV, Guilherme A, Czech MP. Mouse p170 is a novel phosphoinositide 3-kinase containing a C2 domain. J Biol Chem. 1996;271:13304–13307. doi: 10.1074/jbc.271.23.13304. [DOI] [PubMed] [Google Scholar]
- Volinia S, Dhand R, Vanhaesenbroek B, MacDougal LK, Stein R, Zvelebil MJ, Domin J, Panaretou C, Waterield MD. A human phosphatidylinositol 3-kinase complex related to the yeast Vps34p-Vps15p protein sorting system. EMBO (Eur Mol Biol Organ) J. 1995;14:3339–3348. doi: 10.1002/j.1460-2075.1995.tb07340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeds A, Maciver S. F-actin capping proteins. Curr Opin Cell Biol. 1993;5:63–69. doi: 10.1016/s0955-0674(05)80009-2. [DOI] [PubMed] [Google Scholar]
- Wymann MP, Bulgarella-Leva G, Zvelebil MJ, Pirola L, Vanhaesenbroek B, Waterfield MD, Panayotou G. Wortmannin inactivates phosphoinositide 3-kinase by covalent modification of lys-802, a residue involved in the phosphate transfer reaction. Mol Cell Biol. 1996;16:1722–1733. doi: 10.1128/mcb.16.4.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, King WG, Dillon S, Hall A, Feig L, Rittenhouse SE. Activation of platelet phosphatidylinositide 3-kinase requires the small GTP-binding protein Rho. J Biol Chem. 1993;268:22251–22254. [PubMed] [Google Scholar]
- Zheng Y, Bagrodia S, Cerione R. Activation of phosphoinositide 3-kinase activity by Cdc42Hs binding to p85. J Biol Chem. 1994;269:18727–18730. [PubMed] [Google Scholar]