Abstract
Centromere-associated protein E (CENP-E) is a kinesin-related microtubule motor protein that is essential for chromosome congression during mitosis. Using immunoelectron microscopy, CENP-E is shown to be an integral component of the kinetochore corona fibers that tether centromeres to the spindle. Immediately upon nuclear envelope fragmentation, an associated plus end motor trafficks cytoplasmic CENP-E toward chromosomes along astral microtubules that enter the nuclear volume. Before or concurrently with initial lateral attachment of spindle microtubules, CENP-E targets to the outermost region of the developing kinetochores. After stable attachment, throughout chromosome congression, at metaphase, and throughout anaphase A, CENP-E is a constituent of the corona fibers, extending at least 50 nm away from the kinetochore outer plate and intertwining with spindle microtubules. In congressing chromosomes, CENP-E is preferentially associated with (or accessible at) the stretched, leading kinetochore known to provide the primary power for chromosome movement. Taken together, this evidence strongly supports a model in which CENP-E functions in congression to tether kinetochores to the disassembling microtubule plus ends.
Chromosome movements during mitosis are orchestrated by the interaction of spindle microtubules with a specialized chromosome domain located within the centromere. This specialized region, called the kinetochore (Luykx, 1965; Brinkley and Stubblefield, 1966), is the site for spindle microtubule–centromere association. Structurally, the kinetochore is composed of four layers: an innermost plate that apparently consists of a specialized layer of chromatin, an interzone, an outer plate that has been argued to consist of tightly packed fibers (Ris and Witt, 1981; Rattner, 1986), and an outermost fuzzy, fibrous corona that is most clearly seen after microtubule disassembly (e.g., Wordeman et al., 1991). Although kinetochore morphology has been documented in numerous ultrastructural studies (e.g., Brinkley and Stubblefield, 1966; Jokelainen, 1967; Comings and Okada, 1973; Roos, 1973; Rieder, 1982; McEwen et al., 1993), there is little information about kinetochore composition and the respective localization of known kinetochore proteins except for three initially identified as human autoantigens (centromere-associated protein A [CENP-A]1 [attached to centromeric heterochromatin; Palmer et al., 1991; Pluta et al., 1995], CENP-B [underneath the inner plate; Cooke et al., 1990], and CENP-C [a component of the inner plate; Saitoh et al., 1992]).
A generally accepted idea is that microtubule motors located at or near the kinetochore power chromosome movement during mitosis (Nicklas, 1989; Rieder and Alexander, 1990; Hyman and Mitchison, 1991). To date, fluorescence microscopy has been used to localize three microtubule motor proteins to the centromere/kinetochore: cytoplasmic dynein (Pfarr et al., 1990; Steuer et al., 1990), CENP-E (Yen et al., 1992), and MCAK/XKCM1 (Wordeman and Mitchison, 1995; Walzak et al., 1996). Although cytoplasmic dynein has been implicated in transient association with kinetochores (Pfarr et al., 1990; Steuer et al., 1990), microinjection of specific antibodies has resulted instead in spindle collapse (Vaisberg et al., 1993), rather than a direct effect on chromosome attachment to spindles, disruption of chromosome congression, or movement at anaphase. Dynein has also been shown to be involved in aster formation and spindle pole assembly in Xenopus (Verde et al., 1991; Heald et al., 1996; Merdes et al., 1996) and HeLa cell (Gaglio et al., 1996) extracts, while evidence from budding yeast has proven its role in spindle positioning (Eshel et al., 1993; Li et al., 1993) with a possible involvement in anaphase chromosome segregation (Saunders et al., 1995). Echeverri et al. (1996) have localized a proportion of p50, a component of the dynactin complex that can activate cytoplasmic dynein (Steuer et al., 1990), to prometaphase kinetochores followed by release at or after bipolar attachment to spindles. Overexpression of p50 using DNA transfection disrupts spindle assembly and eliminates kinetochore-associated cytoplasmic dynein but does not block microtubule attachment to centromeres. Rather, the aberrant spindles generally display monopolar attachment of chromosomes near microtubule plus ends, findings demonstrating that initial kinetochore attachment to microtubules is mediated, at least in part, by components other than dynein.
For CENP-E, whose cell cycle–dependent accumulation yields a maximum of ∼5,000 molecules per HeLa cell in G2/M, (i.e., about 50 molecules per kinetochore; Brown et al., 1994), there is evidence that altering its action can affect chromosome movements: (a) Antibodies to CENP-E do inhibit poleward chromosome movements powered by microtubule disassembly in vitro (Lombillo et al., 1995a ); (b) antibody injection into cells slows the metaphase- to-anaphase transition (Yen et al., 1992); (c) antibody injection into mouse eggs completely blocks meiosis I at prometaphase/metaphase (Duesbery et al., 1997); and (d) immunodepletion of CENP-E from Xenopus egg extracts blocks chromosome congression but not attachment to spindles assembled in vitro (Wood et al., 1997). The sum of this evidence suggests that CENP-E functions as a kinetochore-associated microtubule motor, but to better understand the exact molecular function of the motor, it is important to know in which of the four layers of the kinetochore CENP-E is located, and whether or how CENP-E distribution changes during the various phases of chromosome movement in mitosis. Using immunoelectron microscopy, we now show that CENP-E binds to the outer surface of the immature kinetochores early in prometaphase, consistent with CENP-E function during the earliest stages of initial microtubule capture. From earliest prometaphase through anaphase A, CENP-E extends from the kinetochore at least 50 nm along spindle microtubules. Thus, CENP-E is one component of the corona fibers that represents the linkers that connect spindle microtubules to kinetochores.
Materials and Methods
Antibodies
Antibodies against a part of the coiled-coil domain of CENP-E (amino acids 955–1571) were raised in rabbits as described by Brown et al. (1996). The rabbit IgG fraction was purified using the affinity beads in which CENP-E955–1571 was immobilized to CNBr-activated Sepharose 4B beads (Sigma Chemical Co., St. Louis, MO). The affinity purification procedure was carried out as described by Harlow and Lane (1988). The IgG fraction was eluted with 0.1 M glycine, pH 2.8, followed by a immediate desalting (Bio-Rad Labs, Hercules, CA) into PBS.
To test the specificity of the CENP-E antibody, mitotic cells were harvested by shake-off followed by sedimentation and solubilization in RIPA buffer (25 mM Tris-HCl, pH 7.5, 5 mM EDTA, 0.5% SDS, and 1% deoxycholate). The extracts were then sonicated and centrifuged to remove the residual insoluble materials. The chromosome scaffolds were prepared as described below. Before electrophoresis, an appropriate amount of extract was diluted with 4× sample buffer and boiled for 2 min. After separation in SDS-PAGE, the proteins were transferred onto a nitrocellulose membrane (Micron Separation Inc., Westborough, MA) and incubated with anti–CENP-E antibody followed by 125I–protein A. Immunoreactive signals were visualized by autoradiography on Kodak BioMAX MS film (Rochester, NY) for 6–8 h at −80°C with an intensifying screen.
Cell Culture
HeLa cells, from American Type Culture Collection (Rockville, MD), were maintained as subconfluent monolayers in RPMI 1640 media (GIBCO BRL; Life Technologies, Gaithersburg, MD) with 10% FCS (Gemini Bio-Products, Inc. Calabasas, CA) and 100 U/ml penicillin plus 100 μg/ml streptomycin (GIBCO BRL; Life Technologies).
Chromosome Scaffold Preparation
Logarithmically growing HeLa cells were treated with 10 ng/ml nocodazole (Sigma Chemical Co.) for 18 h. After arrest, mitotic HeLa cells were harvested by mitotic shake-off and washed with ice-cold PBS. Chromosomes were isolated by the protocol described by Mitchison and Kirschner (1985). Briefly, mitotic HeLa cells were hypotonically swollen for 5 min at room temperature in 10 vol of PEM buffer containing 5 mM Pipes, pH 7.2, 0.5 mM EDTA, 5 mM MgCl2, 5 mM NaCl, and a protease inhibitor cocktail (1 mM PMSF, 2 μg/ml aprotinin, 2 μg/ml pepstatin A, and 2 μg/ml leupeptin). The hypotonically swollen cells were harvested by centrifugation and homogenized in PEM buffer containing 0.1% digitonin (Sigma Chemical Co.). The homogenates were clarified to remove nuclei and the supernatant was loaded onto a stepwise gradient containing 30, 40, 50, and 60% sucrose in PEM buffer and centrifuged (2,500 g for 15 min) at 4°C.
After centrifugation, a visible, flocculent band migrating at the 50–60% sucrose interphase was harvested and suspended in 3 vol of PEM buffer. A subsequent chromosome scaffold preparation was performed according to the protocol described by Lewis and Laemmli (1982).
Immunofluorescence Microscopy
For immunolabeling, cells were trypsinized and seeded onto acid-treated sterile 18-mm coverslips in six-well dishes (Corning Glass Works, Corning, NY). After reaching 75% confluence in ∼36 h, cells were rinsed for 1 min with PHEM buffer (100 mM Pipes, 20 mM Hepes, pH 6.9, 5 mM EGTA, 2 mM MgCl2, and 4 M glycerol) and permeabilized for 1 min with PHEM plus 0.1% Triton X-100 as described (Compton et al., 1992). In some instances, 5 μM Taxol (Sigma Chemical Co.) was included to minimize the depolymerization of microtubules during extraction. Extracted cells were then fixed in 4% freshly made paraformaldehyde (Polysciences, Inc., Warrington, PA) plus 0.05% glutaraldehyde (Tousimis Research Corp., Rockville, MD) in PHEM and rinsed three times in PBS. The coverslips were blocked with 0.05% Tween-20 in PBS (TPBS) containing 1% BSA (Sigma Chemical Co.). The cells were incubated with CENP-E antibody in a humidified chamber for 1 h followed by three washes of TPBS. To visualize microtubules simultaneously, an antitubulin antibody (YL1/2; Kilmartin et al., 1982) was incubated with cells in a humidified chamber for 1 h followed by three washes of TPBS. Visualization of CENP-E location was achieved by rhodamine-conjugated goat anti–rabbit IgG, while labeling of tubulin was achieved by fluorescein and 1.4-nm gold–conjugated goat anti–rat IgG (Nanoprobes, Inc., Stony Brook, NY).
For visualization of cytoplasmic CENP-E distribution in the interphase cells, HeLa cells were fixed in 4% paraformaldehyde plus 0.05% glutaraldehyde before the detergent extraction. After fixation, the cells were permeabilized with 0.2% NP-40 in PBS. The visualization of CENP-B and CENP-E was achieved by using rhodamine-conjugated goat anti–human IgG and FITC-linked goat anti–rabbit IgG, respectively. The slides were examined with a fluorescent microscope (model Axiophot; Carl Zeiss, Inc., Thornwood, NY), and the images were collected and analyzed with MetaMorph software (Universal Imaging Co., West Chester, PA).
Electron Microscopy
After fluorescent examination to verify fixation and antibody binding, CENP-E was visualized by 10-nm colloidal gold by modification of the protocol described by Yao et al. (1996). Coverslips processed as above were rinsed with TPBS (3× 5 min) and fixed with 1% glutaraldehyde (Tousimis) in PBS followed by three washes in PBS. Cells were then postfixed in 2% osmium tetroxide (Electron Microscopy Sciences, Fort Washington, PA), dehydrated in a graded alcohol series followed by 100% acetone, and embedded in Epoxy (Ernest F. Fullam, Inc., Latham, NY). The cells were detached from coverslips using hydrofluoric acid, and the designated areas were excised and glued to blocks. Thin serial sections (silver– gold) were then cut, placed on copper grids, and stained with uranyl acetate and lead citrate. The sections were examined by a JEOL 1200 EM (Peabody, MA). Serial sections were examined to document CENP-E deposition on both sister kinetochores.
In some cases, the immunogold labeling was monitored by using a bifunctional probe, FluoroNanogold, in which rabbit IgG was conjugated with fluorescein and 1.4-nm gold particles (Nanoprobe Inc.). The advantage of using Nanoprobe enabled monitoring the efficiency of immunolabeling and observation of the same specimen with light and electron microscopic analyses. Immunostained coverslips were incubated with a silver enhancement kit for ∼6 min (Ted Pella, Inc., Redding, CA) followed by uranyl acetate staining.
Production of Microtubule-depolymerized Cells Using Nocodazole
To examine CENP-E position in the absence of kinetochore microtubules, nocodazole treatment was used to depolymerize microtubules proximal to kinetochores. Nocodazole treatment (100 ng/ml) was incubated with HeLa cells for 12 h followed by the standard extraction and fixation protocol mentioned above.
Results
Upon Nuclear Envelope Fragmentation in Late Prophase, an Associated Plus End Motor Trafficks CENP-E to Centromeres
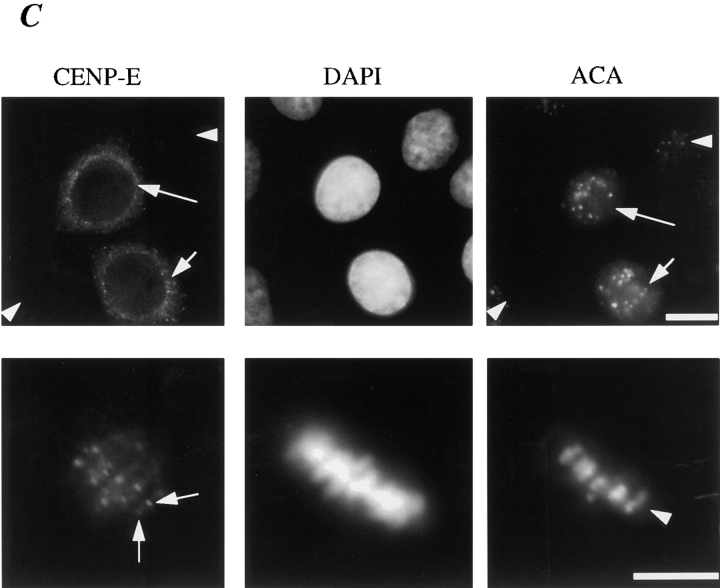
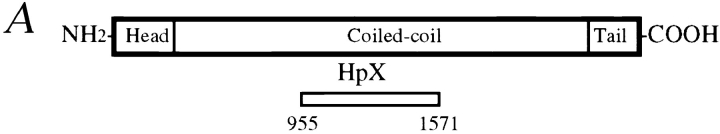
Previous studies revealed cell cycle–regulated distribution of CENP-E and localization of CENP-E near the kinetochore region of mitotic chromosomes. To define more closely the location of CENP-E during kinetochore maturation into a trilaminar structure, a polyclonal antibody against a bacterially expressed portion of the rod domain of CENP-E (amino acids 955–1571, designated as HpX, illustrated in Fig. 1 A) was generated and affinity purified using an antigen-coupled Sepharose matrix. Protein immunoblot analysis revealed that the affinity-purified CENP-E antibody specifically recognized a single protein band of ∼310 kD in whole mitotic HeLa cell extracts and isolated chromosome scaffolds (Fig. 1 B, lanes 2 and 4, respectively). This 310-kD band was not recognized by preimmune serum. To verify further the specificity of this HpX antibody, CENP-E localization was visualized in HeLa cells using HpX antibody and a fluorescein-conjugated goat anti–rabbit secondary antibody (Fig. 1 C, upper left), while a human CREST anticentromere antibody that reacts primarily with CENP-B followed by a rhodamine-conjugated goat anti–human secondary antibody was used to identify the actual centromere (Fig. 1 C, upper right). This revealed that, in accord with previous reports (Yen et al., 1992; Brown et al., 1995), CENP-E accumulates in the cytoplasm of G2 cells (Fig. 1 C, upper left, arrows) but is absent from most interphase cells (arrowheads). At mitosis, CENP-E staining appears as pairs of clearly resolved double dots (lower left, arrows), while CREST centromere antigens are present as pairs of unresolved dots (Fig. 1 C, lower right, arrowhead).
Figure 1.
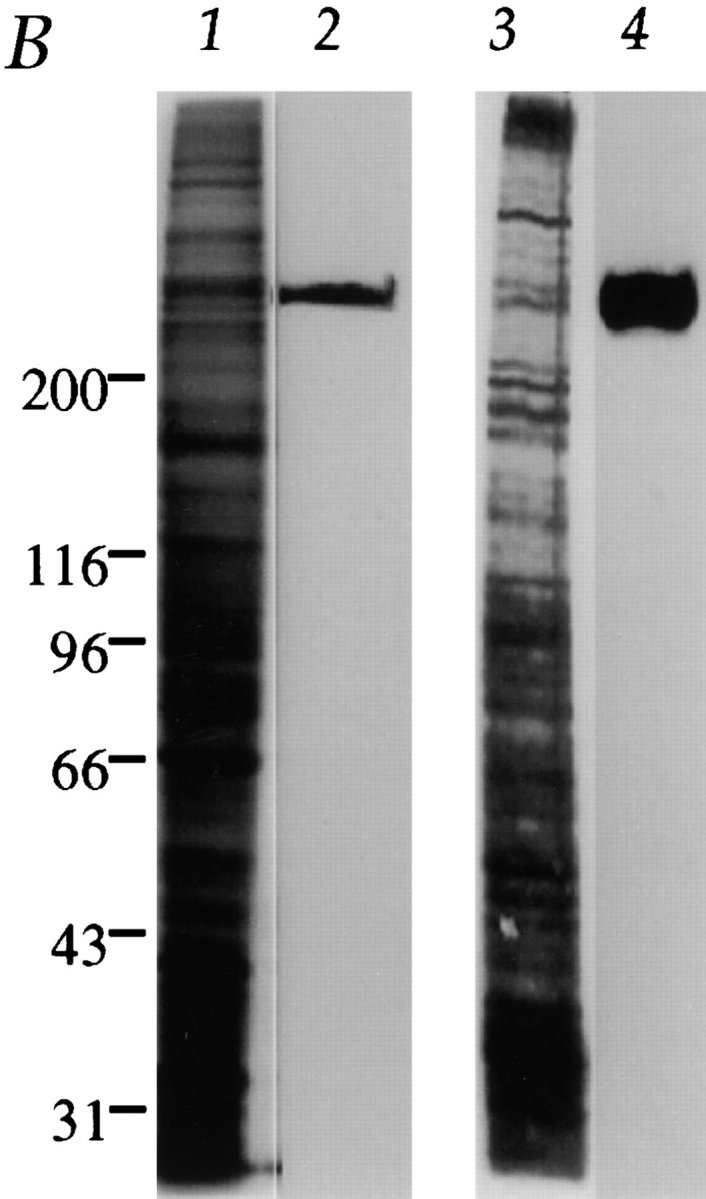
Characterization of the affinity-purified CENP-E antibody HpX. (A) Schematic drawing of CENP-E denoting the region used to generate HpX, a fragment of 70 kD recombinant polypeptide expressed in bacteria. (B) Specificity of affinity-purified HpX antibody. Immunoblots of mitotic whole cell lysates (lane 2, 50 μg) and isolated chromosome scaffold (lane 4, 25 μg). The same materials were separated in SDS-PAGE and stained with Coomassie blue (lanes 1 and 3). (C) Upper panels: CENP-E is accumulated in the cytoplasm just before nuclear envelope breakdown. Indirect immunofluorescence image of HeLa cells stained with HpX antibody (upper left), DAPI (upper middle) and human CREST sera (upper right). CREST sera stained centromeres in both early interphase cell (arrowheads) and late interphase cells (arrows). CENP-E signal appeared only in the late interphase cells (upper left, arrows). Interphase nuclei lack CENP-E staining (arrowheads). Lower panels: CENP-E is located to kinetochores as pairs of clearly resolved double dots (lower left, arrows), while CREST sera mark centromeres as unresolved dots (lower right, arrowhead). Bars: (upper panels) 20 μm; (lower panels) 10 μm.
To examine CENP-E localization as it first associates with chromosomes and/or spindle microtubules, we carried out immunoelectron microscopy on a cell in late prophase/earliest prometaphase, just as the nuclear envelope had started to disassemble (Fig. 2 A). At this point, astral microtubules emanate from centrioles, reach the remaining nuclear envelope (Fig. 2 C, bracket), and in some instances pass though gaps in the envelope, coming in close proximity to newly condensing chromosomes (Fig. 2, D and E). Even at these earliest times, gold particles representing the labeling of CENP-E are found almost exclusively along astral microtubules or at developing kinetochores adjacent to microtubules that have penetrated into the nuclear volume. CENP-E bound to astral microtubules was often closely associated with electron-dense structures (Fig. 2 C, upper left, arrowhead). No similar structures were found in cells before onset of mitosis, suggesting the assembly of a CENP-E–containing complex just at the onset of mitosis. Some CENP-E was found localized to domains of the condensing chromatin (Fig. 2, D and E, arrowhead). Careful examination of serial sections of chromosomes did not reveal any trilaminar kinetochore structures, nor were any microtubules obviously attached laterally or end on. However, in light of CENP-E's association with more mature kinetochores (see below), we infer that these areas of chromosome-bound CENP-E represent the immature kinetochores. All chromosomes with more than one gold particle representing bound CENP-E did have astral microtubules within 200 ± 40 nm (e.g., Fig. 2, D and E, arrowhead). Virtually no gold particles were found on other structures (i.e., vesicular membranes or at other surface regions of the chromosomes) (Fig. 2 D).
Figure 2.
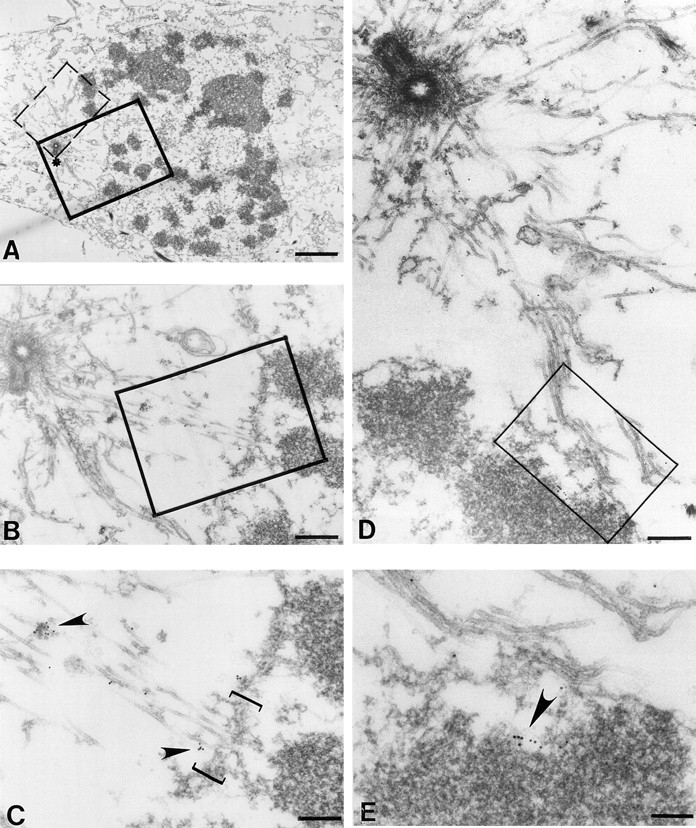
An associated plus end motor activity trafficks CENP-E along newly assembled astral microtubules into the nuclear domain after nuclear envelope fragmentation. HeLa cells were processed as described in Materials and Methods. (A) Low magnification view of a prophase/prometaphase HeLa cell bearing condensed chromosomes and a partially fragmented nuclear envelope. One spindle pole is readily apparent (asterisk). Examination of serial sections did not reveal another pole, consistent with a prophase cell before centriole separation. (B) Magnified view of boxed region in A shows that astral microtubules emanating from the centriole come in close proximity to the nuclear envelope. (C) Higher magnification view reveals that CENP-E is microtubule-associated along astral microtubules adjacent to the remaining nuclear envelope (bracket). Arrowheads point to microtubule-bound gold particles reporting CENP-E location. (D) Magnified view of the dashed box in A and highlighting astral microtubules passing through the fragmented lamina and lying in close proximity to a chromosome. (E) Higher magnification of the area boxed in D, revealing that some CENP-E is found along the microtubules, but additional CENP-E is associated with a localized domain on the chromosome, presumably the developing kinetochore. Note the chromosome is not yet attached to microtubules. Bars: (A) 2 μm; (B) 400 nm; (C) 200 nm; (D) 800 nm; (E) 140 nm.
These findings indicate that even by earliest prometaphase, CENP-E binds to astral microtubules and apparently accumulates at immature kinetochores, before stable chromosome association with microtubules.
Early in Prometaphase CENP-E Binds along the Outermost Surface of Kinetochores as Chromosomes Initially Attach Laterally to Microtubules
Serial micrographs (Fig. 3, A, C, and E) from cells in prometaphase revealed numerous apparently monoorientated chromosomes attached laterally to spindle microtubules. At higher magnification of one telocentric chromosome pair (Fig. 3, B, D, and F), the gold particles marking CENP-E position were seen at the interfaces of the developing kinetochores with their laterally associated spindle microtubules (Fig. 3, B, D, and F), with virtually no gold found on other microtubules or at the surface regions of the chromosome. While we cannot be absolutely certain in this example that all of the microtubules are from the adjacent pole, it is likely that these chromosomes are monooriented. Moreover, it is clear that in this example and in 13 other cells examined, CENP-E is found in a fibrous network extending 30–60 nm from the not fully developed outer kinetochore surfaces of the sister kinetochores. In addition, CENP-E surrounds the semicircular, immature kinetochores, both those with obvious lateral attachment to microtubules and without associated microtubules. These findings demonstrate that at the earliest stages of microtubule–chromosome interaction, CENP-E is highly concentrated at the surface of the centromere as a fibrillar component extending up to 60 nm. Thus, the prometaphase kinetochore outermost surface is surrounded by a collar of CENP-E molecules.
Figure 3.
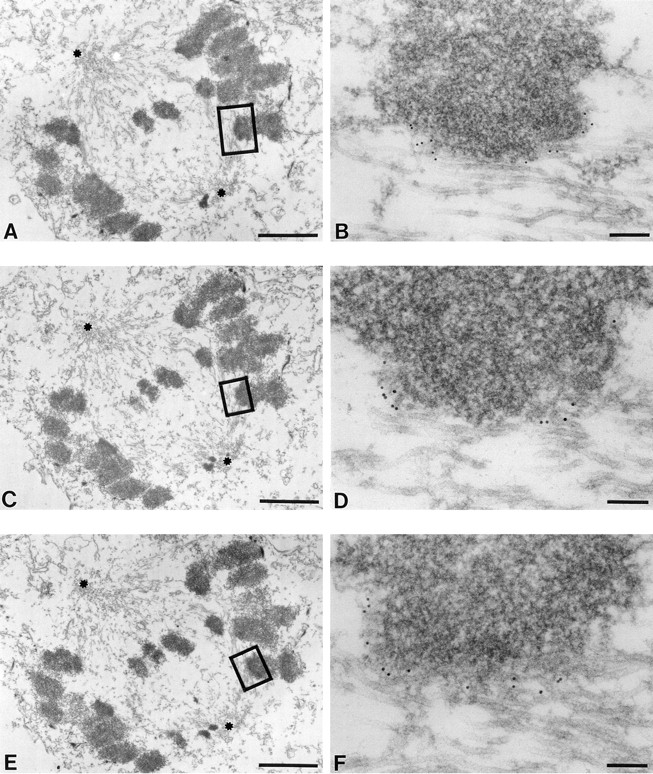
At early prometaphase, CENP-E binds all long the outermost surface of monooriented kinetochores attached laterally to spindle microtubules. HeLa cells grown on coverslips were preextracted and fixed. The visualization of CENP-E was achieved by 10-nm gold–conjugated goat anti–rabbit IgG. (A, C, and E) Low magnification serial sections of an early prometaphase HeLa cell. Asterisks denote the two poles of the developing bipolar spindle. An apparently monooriented chromosome is boxed, and higher power views are shown in B, D, and F. 10-nm gold particles representing CENP-E position decorate the interface between immature kinetochore and the laterally attached spindle microtubules. Note the labeling of CENP-E on the kinetochore appears as a crescent (C) shape. Bars: (A, C, and E) 2 μm; (B) 160 nm; (D and F) 110 nm.
The Earliest Chromosomes to Become Bioriented Always Have CENP-E on the Outer Kinetochore Surface, Although They Are Still Morphologically Immature
To probe for the localization of CENP-E in chromosomes as they congress toward the spindle equator, we examined bioriented chromosomes in prometaphase cells. Two different examples from a single cell are highlighted in Fig. 4, A–C and D–F. At higher magnifications, nine gold particles representing specific labeling of CENP-E can be clearly seen on one leading kinetochore (i.e., defined here to be the one closer to the midzone; Fig. 4 C, arrow), while five particles are found on the other (trailing kinetochore; Fig. 4 C, arrowhead). Again, there was virtually no gold staining on other microtubules or at other surface regions of the chomosome (Fig. 4, B and E). In a second example (Fig. 4, D–F), seven gold particles were associated with the trailing sister kinetochore (Fig. 4 E), while the leading one reveals 14 particles, plus two adjacent clusters of CENP-E associated with a kinetochore microtubule. By examining 23 serial sections from five bioriented chromosomes, we determined that the leading kinetochore always displayed more intense CENP-E reactivity (12 ± 4 gold particles for the leading vs 7 ± 3 for the trailing), demonstrating a difference in abundance, conformation, or accessibility of kinetochore-bound CENP-E during chromosome congression. The increased immunoreactivity on the apparently leading kinetochore was confirmed at the light microscopic level. While CENP-B showed comparable staining on leading and trailing kinetochores of a lagging chromosome pair (Fig. 4 G, arrowhead and arrow, respectively), CENP-E staining was more intense on the kinetochore closest to the midzone (Fig. 4 H, arrowhead). Furthermore, in serial sections, none displayed the clear trilaminar structure seen at metaphase (e.g., see Fig. 5 B), demonstrating that kinetochore assembly is both multistep and incomplete even as late as bipolar microtubule attachment.
Figure 4.
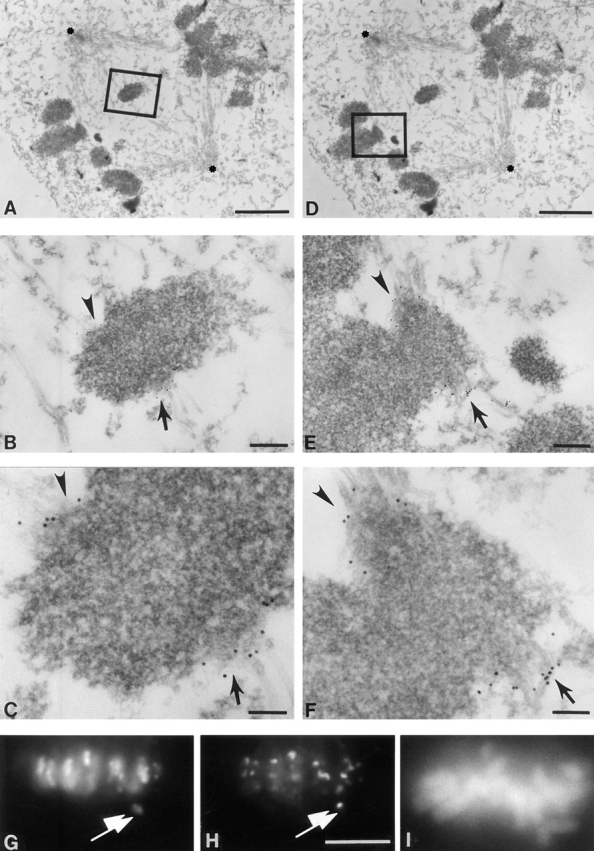
The leading kinetochore of a congressing chromosomes pair has increased level or accessibility of CENP-E. HeLa cells were processed as described in Fig. 2. (A and D) Low magnification views of a prometaphase HeLa cell (poles of the bipolar spindle are labeled with asterisks). (B and E) Intermediate magnification of two examples of a bioriented chromosome. (B) Boxed area of A showing chromosomes pair partially congressed from spindle poles toward the equator of spindle poles, but not yet aligned at the equator. (C and F) High magnification of the two bioriented chromosomes in B and E. 10-nm gold particles representing CENP-E decorate the outer kinetochore surface. A trilaminar structure of the kinetochore is not yet apparent, indicating that these kinetochores are not fully mature. (G–I) Double immunofluorescence demonstrating preferential CENP-E staining on the kinetochore closest to the midzone on a chromosome not yet congressed to the metaphase plate. (G) CENP-B, (H) CENP-E, and (I) DAPI to display chromosome positioning. Bars: (A and D) 2 μm; (B and E) 230 nm; (C) 90 nm; (F) 110 nm; (H–I) 10 μm.
Figure 5.
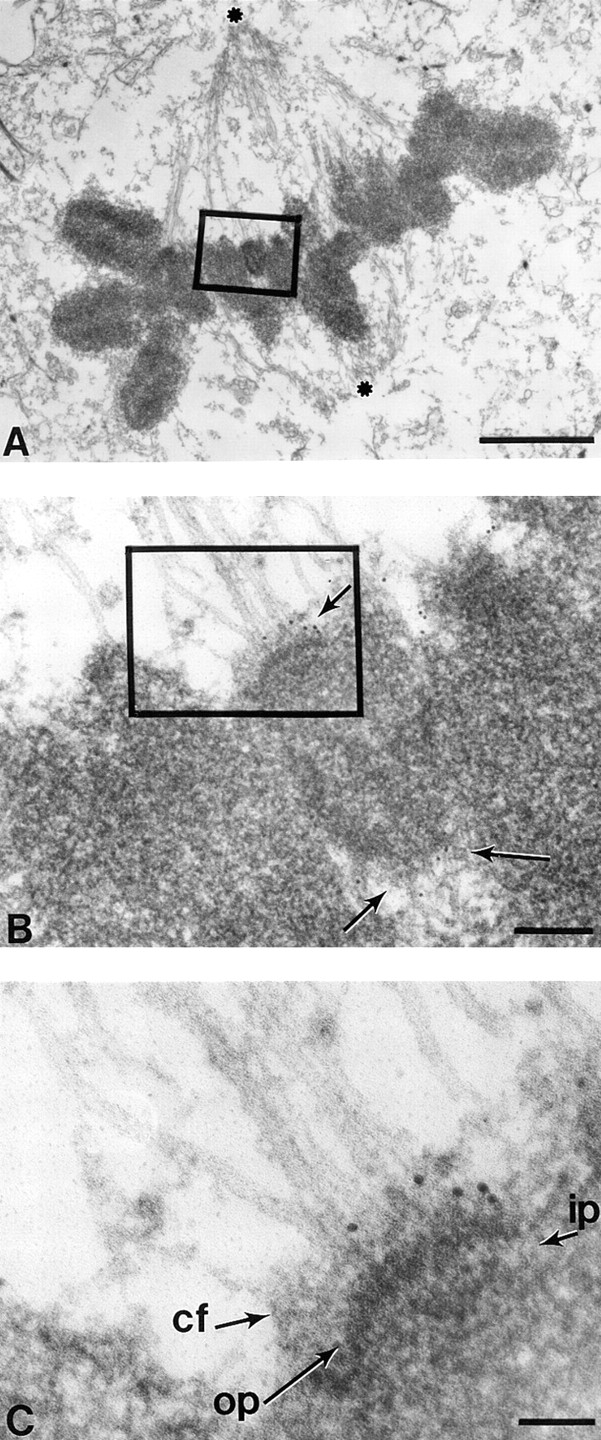
At metaphase CENP-E extends from the kinetochore outer plate at least 50 nm along spindle microtubules. Low magnification view of a metaphase HeLa cell with chromosomes aligned at the equator between the spindle poles (asterisks). (B) Magnified view of one metaphase chromosome showing that spindle microtubules indeed associate with a kinetochore with a trilaminar structure. Five 10-nm gold particles are located to each sister kinetochore (arrows). Five additional gold particles just to the right of the boxed area represent CENP-E associated with the kinetochore of another chromosome (more clearly seen in adjacent sections). (C) Higher magnification view shows that CENP-E is located to the corona fibers of the kinetochore. op, outer plate; ip, inner plate; cf, corona fibers. Bars: (A) 2 μm; (B) 170 nm; (C) 70 nm.
It should be emphasized that since chromosomes oscillate toward and away from the pole during congression, we cannot be certain that the kinetochore closest to the midzone was actually leading movement in any of these examples. Nevertheless, since chromosomes do spend most of their time during congression moving toward the midzone (Skibbens et al., 1993; Waters et al., 1996), this is the most likely case. Since the leading kinetochore produces the pulling force that apparently governs chromosome congression (Khodjakov and Rieder, 1996), the increased labeling of CENP-E on this kinetochore reflects the functional status of congressing kinetochores.
At Metaphase CENP-E Extends from the Mature Kinetochore Outer Plate at Least 50 nm along Spindle Microtubules
HeLa cells with aligned chromosomes were examined (Fig. 5 A) to track CENP-E at bioriented kinetochores that have completed congression but are still under tension exerted by opposing spindle microtubules. Higher magnification views (Fig. 5, B and C) revealed that CENP-E, evidenced by 10-nm gold particles, is readily apparent adjacent to six spindle microtubules that are attached through a fuzzy layer of corona fibers to the electron-dense kinetochore outer plate (Fig. 5 B, top arrow). Serial sections revealed an equivalent level of CENP-E on the sister kinetochores of fully congressed chromosomes. Furthermore, the 10-nm gold particles are uniformly distributed in the fibrous corona and lie an average distance of 50 nm from the outer kinetochore plate (Fig. 5 C). This is in contrast with the previously reported position of CENP-B and CENP-C, localized to heterochromatin and the inner plate, respectively (Cooke et al., 1990; Saitoh et al., 1992). We conclude that CENP-E is located in the fibrous corona connecting kinetochores to spindle microtubules.
CENP-E Is an Integral Component of Kinetochore Corona Fibers Extending 30–60 nm from the Outer Plate
The evidence outlined above implicates CENP-E as a potential linking protein for chromosome attachment to spindle microtubules. To study the nature of the interaction of CENP-E with kinetochore substructures, we examined CENP-E location on condensed prometaphase chromosomes in the absence of microtubules. To this end, nocodazole was used to disassemble microtubules. Immunoelectron microscopy showed that kinetochores appear curved and elongated, becoming three to four times their normal length along the chromosome surface (Fig. 6, A and B), with an easily identifiable trilaminar structure readily apparent in higher magnifications (Fig. 6 B). An electron-translucent zone between the inner and outer plates of the kinetochore was evident, as reported earlier (e.g., Rieder, 1982), indicating significant structural preservation during sample preparation. The outer kinetochore plate appearance was consistent with tightly packed fibers as described earlier (Ris and Witt, 1981; Rattner, 1986). Most interestingly, at the tips of those organized fibers, there are numerous 10-nm gold particles (33 particles in total in the example in Fig. 6 B) denoting the measure of CENP-E evenly dispersed to the outermost region of the outer plate and to corona fibers and lying an average of 90 nm (90 ± 17 nm, n = 33) from the electron-translucent zone. Again, very few gold particles were found in the cell cytoplasm or other regions of chromosomes. The relatively uniform distance of these gold particles from the translucent zone strongly suggests that CENP-E is unidirectionally oriented, extending in a fibrous pattern at least 100 nm away.
Figure 6.
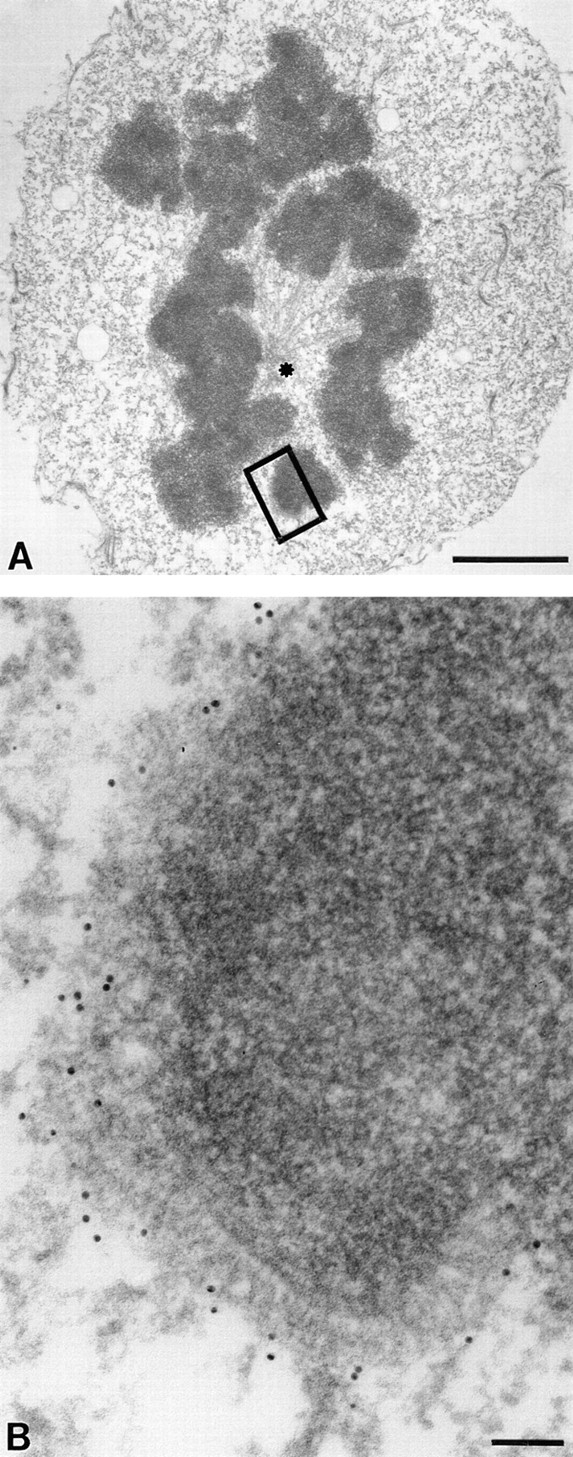
CENP-E is a integral component of kinetochore corona fibers extending 50 nm from the outer plate. HeLa cells were initially treated with a low dose of nocodazole for 12 h to disassemble microtubules and processed as described in Fig. 2. Nocodazole treatment bulges the kinetochore outward from the surface of the chromosome. (A) Low magnification view of a nocodazole-treated prometaphase HeLa cell. The asterisk denotes one spindle pole. Note that not all spindle microtubules are depolymerized. Microtubule-free kinetochores appeared swollen and crescent shaped. (B) Magnified view of boxed area in A shows that 33 gold particles representing CENP-E position are located to the tip of the enlarged kinetochore outer plate, which appears to consist of tightly packed fibers. Bars: (A) 2 μm; (B) 85 nm.
Kinetochore-associated CENP-E Leads as Sister Chromatids Move toward the Poles in Anaphase A, Dissociating Gradually from Corona Fibers and Redistributing to the Midzone in Late Anaphase
To verify if CENP-E is located in the kinetochore corona during anaphase chromosome movement toward the poles, CENP-E positioning was examined in cells early in anaphase (anaphase A; Fig. 7, A and B). The deposition of gold particles demonstrated that CENP-E remains a kinetochore corona component, extending along spindle microtubules (Fig. 7 B, arrow). Later in anaphase, when chromosomes have moved most of the way to the poles and pole separation has been initiated (anaphase B), CENP-E is still localized to the kinetochore outer plate (Fig. 7 C, boxed area, D, arrows), but a significant number of gold particles are now found redistributed to the interzonal microtubules (Fig. 7 C, arrow; E). Examination of 32 anaphase kinetochore sections (from both randomly picked and serial stacks) revealed that the number of gold particles located to kinetochores is reduced by about half compared with metaphase chromosomes (8 ± 3 for metaphase vs 5 ± 2 for anaphase). Again, very few gold particles were found elsewhere in the cytoplasm.
Figure 7.
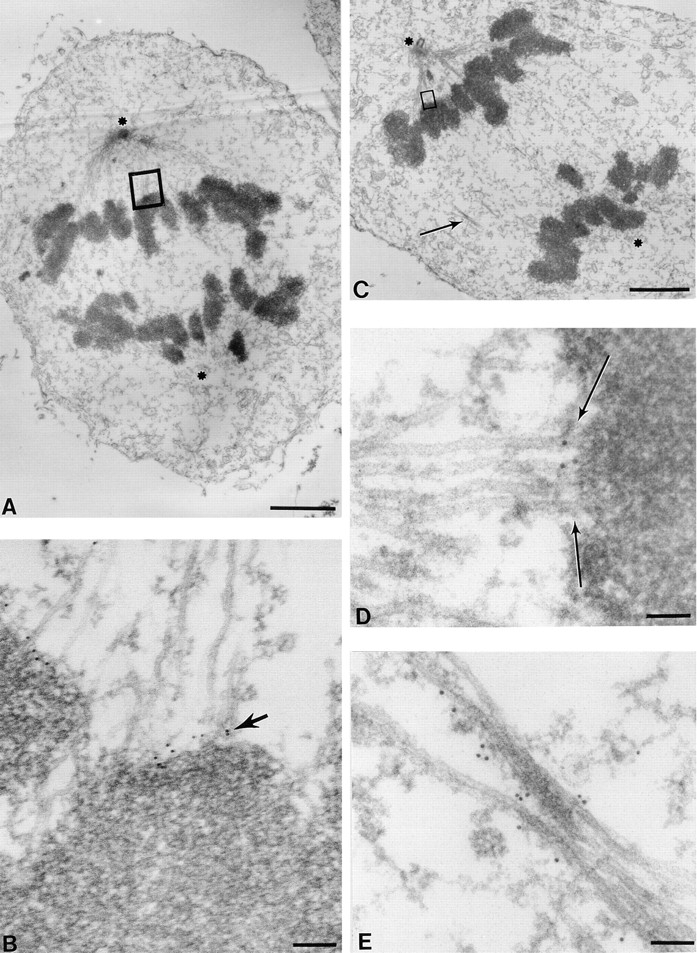
CENP-E remains a component of the kinetochore fibers as sister chromatids move toward the poles in anaphase. HeLa cells were processed as described in Fig. 2. (A) Low magnification view of an early anaphase HeLa cell. The two spindle pole positions are marked with asterisks (one is apparent while another is in a different section). (B) Magnified view of a kinetochore–microtubule interface shows that CENP-E is located between the kinetochore outer plate and the spindle microtubules (arrow). No gold particles are seen in other regions on chromosomes or on microtubules. (C) Low magnification view of a late anaphase HeLa cell bearing elongated spindle poles, labeled with asterisks; one is apparent while another is in a different section. Interzonal microtubules are readily seen (arrow). (D) Magnified view of the upper boxed region in C, showing that CENP-E is located between a kinetochore and its associated spindle microtubules (arrow). Examination of the number of particles revealed a reduction relative to metaphase. (E) Magnified view of area pointed by the arrow in C. Some CENP-E is now localized to the interzonal microtubules. Bars: (A) 2 μm; (B) 120 nm; (C) 2 μm; (D) 70 nm; (E) 90 nm.
CENP-E Cross-Links the Interzonal Microtubules Beginning in anaphase B and Continuing through Telophase
To test if detectable CENP-E remains at telophase centromeres, when the functional kinetochores are disassembled, we examined cells in telophase. Fig. 8 A displays a cell in which chromosomes are decondensing and a nuclear lamina has begun to reform around the DNA. No CENP-E was found chromosome associated. Rather, CENP-E was restricted to bundles of antiparallel microtubules in the midzone. For example (Fig. 8 B), in each of five microtubule bundles formed by antiparallel microtubules, CENP-E was found microtubule associated, but only in the electron dense region of overlapping microtubule plus ends (Fig. 8 C).
Figure 8.
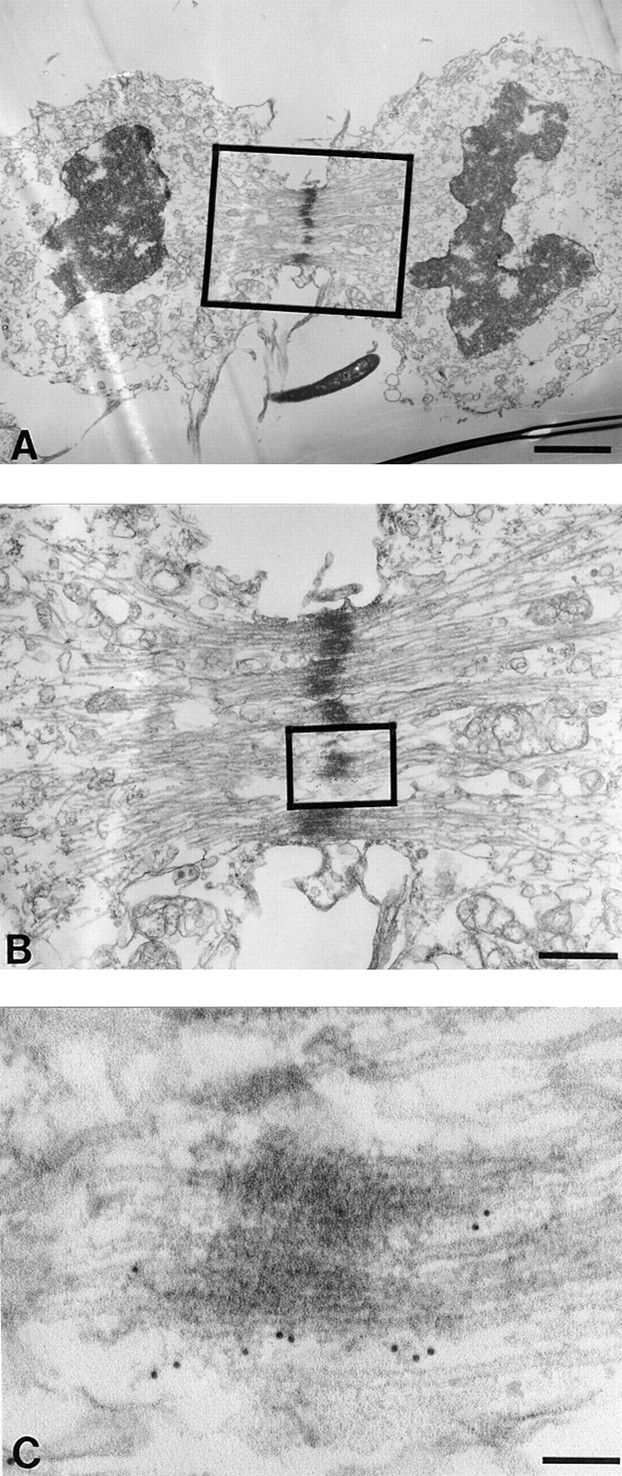
CENP-E cross-links the interzonal microtubules during telophase. HeLa cells were processed as described in Fig. 2. (A) Low magnification view of a late telophase HeLa cell. Lamin deposition to reform nuclei is partially complete. (B) Magnified view of boxed area in A shows that CENP-E is located along and/ or between the interzonal microtubules (boxed). (C) Higher magnification of interzonal microtubules shows that gold particles are primarily located between the microtubule bundles. Bars: (A) 2 μm; (B) 500 nm; (C) 90 nm.
Discussion
CENP-E Links Spindle Microtubules to Kinetochores
From these ultrastructural experiments, we can develop a kinetic picture of CENP-E integration into functional kinetochore assembly (summarized in Fig. 9). The evidence presented here provides proof that CENP-E is localized to developing kinetochores of condensing chromosomes before, or concurrently with, astral microtubule attachment after initial nuclear envelope disassembly (Fig. 2). By prometaphase, CENP-E binds along the outermost surface of monooriented kinetochores that are attached laterally to spindle microtubules (Fig. 3). During congression, CENP-E is asymmetrically localized, with more present (or accessible) at the leading kinetochore (Fig. 4). Before microtubule attachment, during congression, at metaphase, and during anaphase A, CENP-E is a component of kinetochore corona fibers, extending at least 50 nm along spindle microtubules from the mature kinetochore outer plate (Figs. 3–7). Along with CENP-E's structural features (Fig. 1 A), including an amino-terminal kinesin-like motor domain and a 2,069–amino acid coiled-coil domain (Yen et al., 1992) that could extend as much as 300 nm (i.e., 2,069 amino acids × 0.15 nm per residue in an α-helical coiled-coil = 310 nm), the collective evidence strongly implicates CENP-E as a major linker protein (possibly the major linker protein) that mediates centromere–microtubule interactions and is appropriately positioned to power chromosome congression and/or poleward anaphase movement. Moreover, since the epitopes recognized by the antibody we have used lie only about half of the way from the carboxy-terminal end of the helical rod (Fig. 1 A), it is very likely that the remainder of the rod and amino-terminal motor project much further along spindle microtubules (possibly up to 200–300 nm from the kinetochore outer plate).
Figure 9.
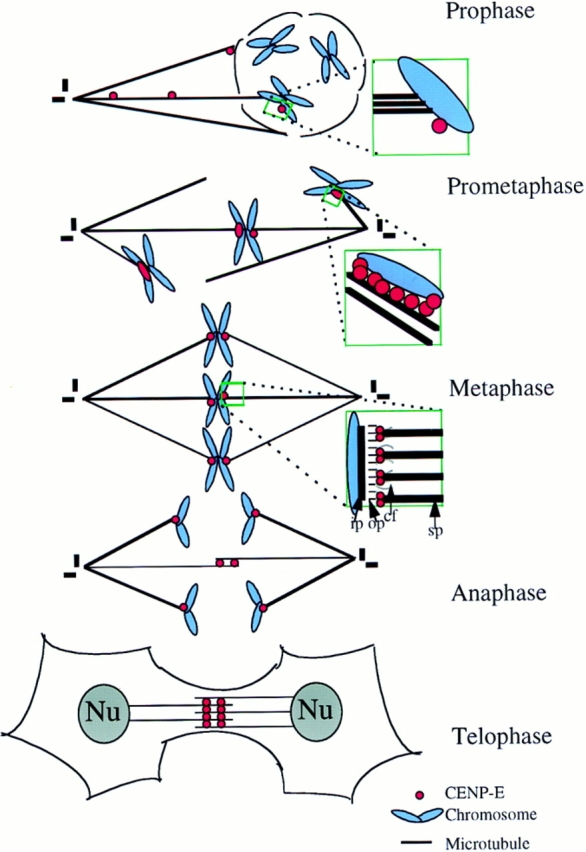
Model for CENP-E function in chromosome movements. CENP-E is recruited to the immature kinetochore as soon as the nuclear envelope disassembles. CENP-E localizes to kinetochores before stable microtubule attachment, apparently by trafficking to the kinetochore by movement over astral microtubules. CENP-E is situated on the outermost surface of the kinetochore during initial lateral microtubule attachment. After biorientation, CENP-E remains on the corona fibers that link kinetochores in an apparent end-on interaction with spindle microtubules. CENP-E abundance, conformation, or accessibility is increased on the leading kinetochore of a congressing pair of chromosomes. Upon the sister chromatid separation, CENP-E remains kinetochore-associated and leads the poleward-moving chromosome. Once the chromosomes have reached the poles, CENP-E releases and redistributes to midzone where it may stabilize interzonal microtubules or power microtubule sliding that leads to pole-to-pole separation. op, outer plate; ip, inner plate; cf, corona fibers; sp, spindle microtubules.
A question not fully settled experimentally is the orientation of CENP-E while kinetochore associated. We had hoped that amino- and carboxy-terminal peptide antibodies to CENP-E would allow us unambiguously to identify which end is extended along microtubules and which is embedded in the kinetochore; however, despite efficient reactivity in immunoblots, both terminal antibodies we produced have failed (not shown) with the immunoelectron microscopic protocols successfully used here for antibody HpX. Nevertheless, with an amino-terminal motor domain, the most pleasing view would be that the carboxy-terminal tail of CENP-E is embedded in the kinetochore, leaving the motor domain flexibly tethered for attachment to, and extension along, spindle microtubules, consistent with the identification of a centromere-targeting domain at the carboxy-terminal tail of CENP-E (Chan, G.K.T., and T.J. Yen. 1996. Mol. Biol. Cell. 7:565a). While it remains formally possible that either or both ends of CENP-E could be exposed for microtubule-binding at the kinetochore, CENP-E's ATP-independent microtubule-binding domain present near the extreme carboxy terminus (Yen et al., 1992; Liao et al., 1994) remains inactive until midanaphase through cdc2 kinase–dependent phosphorylation (Liao et al., 1994). Both this view and the precedent of kinesin itself, a dimer of parallel heavy chains (Hisanaga et al., 1989), makes it seem most likely that CENP-E is oriented with the motor extending away from the kinetochore.
One important question addressed by our studies is the function and nature of the corona fibers. Using real time light microscopy coupled with subsequent electron microscopic analyses, Rieder and Alexander (1990) concluded that corona fibers are the first kinetochore component to interact with spindle microtubules. In addition, they also concluded that the fibers undergo a dynamic size change during the prometaphase–metaphase transition. Given the size of corona fibers, estimated at 250 nm in length (e.g., Brinkley and Stubblefield, 1966) and the calculated length for CENP-E, we hypothesize that CENP-E may be in fact a major component of the corona fibers. This is fully consistent with the notion proposed by Rieder and Alexander (1990) that the motor(s) for prometaphase chromosome movement is on the surface of the kinetochore (i.e., within the corona, but not the plate), or distributed along the surface of kinetochore microtubules, or both.
A Model of CENP-E in Kinetochore Assembly and Chromosome Movement
Our finding that CENP-E extends at least 50 nm from the kinetochore outer surface (modeled in Fig. 9) reinforces several lines of evidence showing that altering CENP-E action can affect chromosome movements: antibodies to CENP-E do inhibit poleward chromosome movements powered by microtubule disassembly in vitro (Lombillo et al., 1995a ), and microinjection of CENP-E antibody efficiently blocks progression past meiotic metaphase I (Duesbery et al., 1997) and slows the metaphase–anaphase transition in mitosis (Yen et al., 1991). Furthermore, immunodepletion of CENP-E from Xenopus egg extracts blocks chromosome congression without affecting spindle assembly. Moreover, a bacterially expressed fragment containing the motor domain has revealed that the CENP-E motor is an ATPase capable of walking toward microtubule plus ends (i.e., away from the spindle poles) at 5 μm/ min (Wood et al., 1997). An earlier demonstration with a partially purified CENP-E complex isolated from HeLa cells arrested in prometaphase showed that CENP-E is also associated with a minus end–directed motor activity whose motility is eliminated by immunodepletion with CENP-E antibodies (Thrower et al., 1995). The initial transit of an apparent CENP-E complex toward the plus ends of astral microtubules at earliest prometaphase, presumably mediated by CENP-E's intrinsic plus end motor activity, adds additional weight to the idea of such a complex. Perhaps even more intriguing for the mechanism of chromosome movement, given that the plus end motor kinesin has been shown to have the capacity to couple cargoes to microtubule depolymerization–driven, minus end–directed movement (Lombillo et al., 1995b ), it is plausible by analogy that kinetochore-bound CENP-E mediates both antipoleward movement (using its ATP-dependent plus end motor) and poleward movement (by coupling movement to energy liberated from disassembly of the microtubule lattice).
Dynamic Kinetochore Assembly and Structure
It has been shown that the kinetochores undergo dramatic structural and morphological changes as mitosis progresses (Rieder, 1982). Jokelainen (1967) found that immature “ball and cup” kinetochore of mammalian prophase chromosomes matures, after nuclear breakdown, into a dense-staining, platelike structure, which appears to be organized, at least in part, from components associated with prophase kinetochores (Roos, 1973). Fully differentiated trilaminar kinetochores are never reported in the prometaphase cells, consistent with our ultrastructural observation in which CENP-E is initially located in the fibrous corona of kinetochores without an apparent trilaminar structure (Figs. 3–5). By metaphase, kinetochores have frequently been observed as trilaminar structures (Jokelainen, 1967; Roos, 1973; Rieder, 1982).
Recent studies from Wilson and his colleagues (Thrower et al., 1996) documented that kinetochore structure, as reported by CENP-E staining, appears as a collarlike shape in early prometaphase chromosomes, becoming a pair of separated dots in later prometaphase or metaphase. Both our electron microscopic and immunofluorescent (not shown) data confirm this and extend it to show that CENP-E is appropriately positioned to mediate lateral kinetochore attachment to spindle microtubules, appearing as a crescent (“C”) shape in early prometaphase (Fig. 3). The C-shaped kinetochores were even more prominent in the absence of microtubule–kinetochore association (Fig. 6), where the kinetochores, with abundant CENP-E, bulge outward from the surface of the centromeres.
Spindle Checkpoints, Tension, and Asymmetric Localization of CENP-E during Congression
That CENP-E antibodies preferentially label the leading kinetochore of a congressing chromosome pair provides direct support for the view that tension exerted on (or generated by) the leading kinetochore triggers either a change in the abundance or conformation of kinetochore-associated CENP-E. Moreover, elimination of spindle microtubules results in increased CENP-E accessibility at the kinetochore (Fig. 6 B), reinforcing the view that tension, or at least microtubule binding, affects CENP-E distribution or epitope availability. As initially proposed by McIntosh (1991), several recent reports have pointed to an intrinsic role of the kinetochore in signaling successful capture and congression of each chromosome as a necessary prelude to triggering the transition to anaphase (Rieder et al., 1994, 1995; Chen et al., 1996; Li and Benezra, 1996; Wells and Murray, 1996; for review see Rudner and Murray, 1996). Manipulation with microneedles has demonstrated that in some meiotic cells, this checkpoint is sensitive to tension on the kinetochore (Li and Nicklas, 1995). Among the changes in kinetochore chemistry is the tension-dependent diminution of one kinetochore phosphoepitope (3F3/2; Nicklas et al., 1995). To these earlier efforts, our finding that the kinetochore-associated, plus end motor CENP-E also displays an asymmetric distribution (or accessibility) concurrent with the release of the spindle assembly checkpoint component MAD2 (Chen et al., 1996; Li and Benezra, 1996) implicates regulation of CENP-E activity as an important contributor to this tension-dependent, kinetochore-mediated mitotic checkpoint.
Acknowledgments
We thank Drs. K. Wood, K. Sullivan, and J. Kilmartin for providing CENP-E antiserum, CREST autosera, and antitubulin antibody, respectively. We also thank Drs. A. Merdes (Ludwig Institute), K. Wood (Ludwig Institute), K. McDonald (University of California, Berkeley, CA), and K. Sullivan (Scripps Research Institute, La Jolla, CA) for critical reading of this manuscript.
This work was supported by a National Institutes of Health grant (GM 29513) to D.W. Cleveland. Salary support for D.W. Cleveland is provided by the Ludwig Institute for Cancer Research. X. Yao was supported by postdoctoral fellowships from the Bank of America Giannini Foundation and from American Cancer Society California Division.
Footnotes
Address all correspondence to Dr. Don W. Cleveland, 3080 CMM-East, University of California, 9500 Gilman Drive, La Jolla, CA 92093-0660. Tel.: (619) 534-7811. Fax: (619) 534-7659.
1. Abbreviation used in this paper: CENP, centromere-associated protein.
References
- Brinkley BR, Stubblefield E. The fine structure of the kinetochore of a mammalian cell in vitro. . Chromosoma (Berl) 1966;19:28–43. doi: 10.1007/BF00332792. [DOI] [PubMed] [Google Scholar]
- Brown KD, Coulson RM, Yen TJ, Cleveland DW. Cyclin-like accumulation and loss of the putative kinetochore motor CENP-E results from coupling continuous synthesis with specific degradation at the end of mitosis. J Cell Biol. 1994;125:1303–1312. doi: 10.1083/jcb.125.6.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KD, Wood KW, Cleveland DW. The kinesin-like protein CENP-E is kinetochore-associated thoughout poleward chromosome segregation during anaphase-A. J Cell Sci. 1996;109:961–969. doi: 10.1242/jcs.109.5.961. [DOI] [PubMed] [Google Scholar]
- Chen R-H, Waters JC, Salmon ED, Murray AW. Association of spindle assembly checkpoint component XMAD2 with unattached kinetochores. Science (Wash DC) 1996;274:242–246. doi: 10.1126/science.274.5285.242. [DOI] [PubMed] [Google Scholar]
- Comings DE, Okada TA. Holocentric chromosomes in oncopettus kinetochore plates are present in mitosis but absent in meiosis. Chromosoma (Berl) 1973;37:177–192. doi: 10.1007/BF00284937. [DOI] [PubMed] [Google Scholar]
- Compton DA, Yen TJ, Cleveland DW. Identification of novel centromere/kinetochore-associated proteins using monoclonal antibodies generated against human mitotic chromosome scaffolds. J Cell Biol. 1992;112:1083–1097. doi: 10.1083/jcb.112.6.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke CA, Bernat RL, Earnshaw WC. CENP-B: a major human centromere protein located beneath the kinetochore. J Cell Biol. 1990;110:1475–1488. doi: 10.1083/jcb.110.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesbery NS, Choi T, Brown KD, Wood KW, Resau J, Fukasawa K, Cleveland DW, Vande GF, Woude CENP-E is an essential kinetochore motor in meiosis and is masked in Mos-dependent, cell cycle arrest at metaphase II. Proc Natl Acad Sci USA. 1997;94:9165–9170. doi: 10.1073/pnas.94.17.9165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverri CJ, Paschal BM, Vaughan KT, Vallee RB. Molecular characterization of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J Cell Biol. 1996;132:617–633. doi: 10.1083/jcb.132.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshel D, Urrestarazu LA, Vissers S, Jauniaux JC, van Vliet-Reedijk JC, Planta RJ, Gibbons IR. Cytoplasmic dynein is required for normal nuclear segregation in yeast. Proc Natl Acad Sci USA. 1993;90:11172–11176. doi: 10.1073/pnas.90.23.11172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaglio T, Saredi A, Bingham JB, Hasbani MJ, Gill SR, Schroer TA, Compton DA. Opposing motor activities are required for the organization of the mammalian mitotic spindle pole. J Cell Biol. 1996;135:399–414. doi: 10.1083/jcb.135.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow, E., and D. Lane. 1988. Antibodies: A Laboratory Manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. 726 pp.
- Hayden JH, Bower SS, Rieder CL. Kinetochores capture astral microtubules during chromosome attachment to the mitotic spindle: direct visualization in live newt lung cells. J Cell Biol. 1990;111:1039–1045. doi: 10.1083/jcb.111.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald R, Tournebize R, Blank T, Sandaltzopoulos R, Becker P, Hyman A, Karsenti E. Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature (Lond) 1996;382:420–425. doi: 10.1038/382420a0. [DOI] [PubMed] [Google Scholar]
- Hisanaga S, Murofushi H, Okuhara K, Sato R, Masuda Y, Sakai H, Hirokawa N. The molecular structure of adrenal medulla kinesin. Cell Motil Cytoskel. 1989;12:264–272. doi: 10.1002/cm.970120407. [DOI] [PubMed] [Google Scholar]
- Hyman AA, Mitchison TJ. Two different microtubule-based motor activities with opposite polarities in kinetochores. Nature (Lond) 1991;351:206–211. doi: 10.1038/351206a0. [DOI] [PubMed] [Google Scholar]
- Jokelainen PT. The ultrastructure and spatial organization of the metaphase kinetochore in mitotic rat cells. J Ultrastruct Res. 1967;19:19–44. doi: 10.1016/s0022-5320(67)80058-3. [DOI] [PubMed] [Google Scholar]
- Khodjakov A, Rieder CL. Kinetochores moving away from their associated pole do not exert a significant pushing force on the chromosome. J Cell Biol. 1996;135:315–327. doi: 10.1083/jcb.135.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmartin JV, Wright B, Milstein C. Rat monoclonal antitubulin antibodies derived by using a new nonsecreting rat cell line. J Cell Biol. 1982;93:576–582. doi: 10.1083/jcb.93.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CD, Laemmli UK. Higher order metaphase chromosome structure:evidence for metal-protein interaction. Cell. 1982;29:171–181. doi: 10.1016/0092-8674(82)90101-5. [DOI] [PubMed] [Google Scholar]
- Li X, Nicklas RB. Mitotic forces control a cell cycle checkpoint. Nature (Lond) 1995;373:630–632. doi: 10.1038/373630a0. [DOI] [PubMed] [Google Scholar]
- Li Y, Benezra R. Identification of a human mitotic checkpoint gene: hsMAD2. Science (Wash DC) 1996;274:246–249. doi: 10.1126/science.274.5285.246. [DOI] [PubMed] [Google Scholar]
- Li YY, Yeh E, Hays T, Bloom K. Disruption of mitotic spindle orientation in a yeast dynein mutant. Proc Natl Acad Sci USA. 1993;90:10096–10100. doi: 10.1073/pnas.90.21.10096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao H, Li G, Yen TJ. Mitotic regulation of microtubule cross-linking activity of CENP-E kinetochore protein. Science (Wash DC) 1994;265:394–398. doi: 10.1126/science.8023161. [DOI] [PubMed] [Google Scholar]
- Lombillo VA, Nislow C, Yen TJ, Gelfand VI, McIntosh R. Antibodies to the kinesin motor domain and CENP-E inhibit microtubule depolymerization-dependent motion of chromosomes in vitro. J Cell Biol. 1995a;128:107–115. doi: 10.1083/jcb.128.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombillo VA, Stewart RJ, McIntosh R. Minus-end-directed motion of kinesin-coated microspheres driven by microtubule depolymerization. Nature (Lond) 1995b;373:161–164. doi: 10.1038/373161a0. [DOI] [PubMed] [Google Scholar]
- Luykx P. The structure of the kinetochore in meiosis and mitosis Urechis eggs. Exp Cell Res. 1965;39:643–657. doi: 10.1016/0014-4827(65)90068-6. [DOI] [PubMed] [Google Scholar]
- McEwen BF, Arena JT, Frank J, Rieder CL. Structure of the colcemid-treated PtK1 kinetochore outer plate as determined by high voltage electron microscopic tomography. J Cell Biol. 1993;120:301–312. doi: 10.1083/jcb.120.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh JR. Structural and mechanical control of mitotic progression. Cold Spring Harbor Symp Quant Biol. 1991;56:613–619. doi: 10.1101/sqb.1991.056.01.070. [DOI] [PubMed] [Google Scholar]
- Merdes A, Ramyar K, Vechio JD, Cleveland DW. A complex of NuMA and cytoplasmic dynein is essential for mitotic spindle assembly. Cell. 1996;87:447–458. doi: 10.1016/s0092-8674(00)81365-3. [DOI] [PubMed] [Google Scholar]
- Mitchison TJ, Kirschner MW. Properties of the kinetochore in vitro. I. Microtubule nucleation and tubulin binding. J Cell Biol. 1985;101:755–765. doi: 10.1083/jcb.101.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklas RB. The motor for poleward chromosome movement in anaphase is in or near the kinetochore. J Cell Biol. 1989;109:2245–2255. doi: 10.1083/jcb.109.5.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklas RB, Ward SC, Gorbsky GJ. Kinetochore chemistry is sensitive to tension and may link mitotic forces to a cell cycle checkpoint. J Cell Biol. 1995;130:929–939. doi: 10.1083/jcb.130.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer DK, O'Day K, Trong HL, Charbonneau H, Margolis RL. Purification of the centromere-specific protein CENP-A and demonstration that it is a distinctive histone. Proc Natl Acad Sci USA. 1991;88:3734–3738. doi: 10.1073/pnas.88.9.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfarr CM, Coue M, Grissom PM, Hays TS, Porter ME, McIntosh JR. Cytoplasmic dynein is localized to kinetochores during mitosis. Nature (Lond) 1990;345:263–265. doi: 10.1038/345263a0. [DOI] [PubMed] [Google Scholar]
- Pluta AF, Mackay AM, Ainsztein AM, Goldberg IG, Earnshaw WC. The centromere: hub of chromosomal activities. Science (Wash DC) 1995;270:1591–1594. doi: 10.1126/science.270.5242.1591. [DOI] [PubMed] [Google Scholar]
- Rattner JB. Organization within the mammalian kinetochore. Chromosoma (Berl) 1986;93:515–520. doi: 10.1007/BF00386793. [DOI] [PubMed] [Google Scholar]
- Rieder CL. The formation, structure and composition of the mammalian kinetochore and kinetochore fiber. Int Rev Cytol. 1982;79:1–58. doi: 10.1016/s0074-7696(08)61672-1. [DOI] [PubMed] [Google Scholar]
- Rieder CL, Alexander SP. Kinetochores are transported poleward along a single astral microtubule during chromosome attachment to the spindle in newt lung cells. J Cell Biol. 1990;110:81–95. doi: 10.1083/jcb.110.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder CL, Schultz A, Cole R, Sluder G. Anaphase onset in vertebrate somatic cells is controlled by a checkpoint that monitors sister kinetochore attachment to the spindle. J Cell Biol. 1994;127:1301–1310. doi: 10.1083/jcb.127.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder CL, Cole RW, Khodjakov A, Sluder G. The checkpoint delaying anaphase in response to chromosome monoorientation is mediated by an inhibitory signal produced by unattached kinetochores. J Cell Biol. 1995;130:941–948. doi: 10.1083/jcb.130.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ris H, Witt PL. Structure of the mammalian kinetochore. Chromosoma (Berl) 1981;82:153–170. doi: 10.1007/BF00286101. [DOI] [PubMed] [Google Scholar]
- Roos U-P. Light and electron microscopy of rat kangaroo cells in mitosis. I. Formation and breakdown of the mitotic apparatus. Chromosoma (Berl) 1973;40:43–82. doi: 10.1007/BF00319836. [DOI] [PubMed] [Google Scholar]
- Rudner AD, Murray AW. The spindle assembly checkpoint. Curr Opin Cell Biol. 1996;8:773–780. doi: 10.1016/s0955-0674(96)80077-9. [DOI] [PubMed] [Google Scholar]
- Saitoh H, Tomkiel J, Cooke CA, Ratrie H, III, Maurer M, Rothfield NF, Earnshaw WC. CENP-C, an autoantigen in scleroderma, is a component of the human inner kinetochore plate. Cell. 1992;70:115–125. doi: 10.1016/0092-8674(92)90538-n. [DOI] [PubMed] [Google Scholar]
- Saunders WS, Koshland D, Eshel D, Gibbons IR, Hoyt MA. Saccharomyces cerevisiaekinesin- and dynein-related proteins required for anaphase chromosome segregation. J Cell Biol. 1995;128:617–624. doi: 10.1083/jcb.128.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibbens RV, Skeen VP, Salmon ED. Directional instability of kinetochore motility during chromosome congression and segregation in mitotic newt lung cells: a push–pull mechanism. J Cell Biol. 1993;122:859–875. doi: 10.1083/jcb.122.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuer ER, Wordeman L, Schroer TA, Sheetz MP. Localization of cytoplasmic dynein to mitotic spindles and kinetochores. Nature (Lond) 1990;345:266–268. doi: 10.1038/345266a0. [DOI] [PubMed] [Google Scholar]
- Thrower DA, Jordan MA, Schaar BR, Yen TJ, Wilson L. Mitotic HeLa cells contain a CENP-E-associated minus end-directed microtubule motor. EMBO (Eur Mol Biol Organ) J. 1995;14:918–926. doi: 10.1002/j.1460-2075.1995.tb07073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrower DA, Jordan MA, Wilson L. Modulation of CENP-E organization at kinetochores by spindle microtubule attachment. Cell Motil Cytoskel. 1996;35:121–133. doi: 10.1002/(SICI)1097-0169(1996)35:2<121::AID-CM5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Vaisberg EA, Koonce MP, McIntosh JR. Cytoplasmic dynein plays a role in mammalian mitotic spindle formation. J Cell Biol. 1993;123:849–858. doi: 10.1083/jcb.123.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verde F, Berrez JM, Antony C, Karsenti E. Taxol-induced microtubule asters in mitotic extracts of Xenopuseggs: requirement for phosphorylated factors and cytoplasmic dynein. J Cell Biol. 1991;112:1177–1187. doi: 10.1083/jcb.112.6.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak CE, Mitchison TJ, Desai A. XKCM1: a Xenopuskinesin-related protein that regulates microtubule dynamics during mitotic spindle assembly. Cell. 1996;84:37–47. doi: 10.1016/s0092-8674(00)80991-5. [DOI] [PubMed] [Google Scholar]
- Waters JC, Skibbens RV, Salmon ED. Oscillating mitotic newt lung cell kinetochores are, on average, under tension and rarely push. J Cell Sci. 1996;109:2823–2831. doi: 10.1242/jcs.109.12.2823. [DOI] [PubMed] [Google Scholar]
- Wells WA, Murray AW. Aberrantly segregating centromeres activate the spindle assembly checkpoint in budding yeast. J Cell Biol. 1996;133:75–84. doi: 10.1083/jcb.133.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, K.W., R. Sakowics, L.S.B. Goldstein, and D.W. Cleveland. 1997. CENP-E is a plus end-directed kinetochore motor required for chromosomes congression. Cell. In press. [DOI] [PubMed]
- Wordeman L, Mitchison TJ. Identification and partial characterization of mitotic centromere-associated kinesin, a kinesin-related protein that associates with centromeres during mitosis. J Cell Biol. 1995;128:95–104. doi: 10.1083/jcb.128.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wordeman L, Steuer ER, Sheetz MP, Mitchison TJ. Chemical subdomains within the kinetochore domain of isolated CHO mitotic chromosomes. J Cell Biol. 1991;114:285–294. doi: 10.1083/jcb.114.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X, Cheng L, Forte JG. Biochemical characterization of ezrin-actin interaction. J Biol Chem. 1996;271:7224–7229. doi: 10.1074/jbc.271.12.7224. [DOI] [PubMed] [Google Scholar]
- Yen TJ, Compton DA, Wise D, Zinkowski RP, Brinkley BR, Earnshaw WC, Cleveland DW. CENP-E, a novel human centromere-associated protein required for progression from metaphase to anaphase. EMBO (Eur Mol Biol Organ) J. 1991;10:1245–1254. doi: 10.1002/j.1460-2075.1991.tb08066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen TJ, Li G, Schaar BT, Szilak I, Cleveland DW. CENP-E is a putative kinetochore motor that accumulates just before mitosis. Nature (Lond) 1992;359:536–539. doi: 10.1038/359536a0. [DOI] [PubMed] [Google Scholar]