Abstract
Laminins, the main components of basement membranes, are heterotrimers consisting of α, β, and γ polypeptide chains linked together by disulfide bonds. Laminins-1 and -2 are both composed of β1 and γ1 chains and differ from each other on their α chain, which is α1 and α2 for laminin-1 and -2, respectively. The present study shows that whereas laminins-1 and -2 are synthesized in the mouse developing lung and in epithelial–mesenchymal cocultures derived from it, epithelial and mesenchymal monocultures lose their ability to synthesize the laminin α1 chain. Synthesis of laminin α1 chain however returns upon re-establishment of epithelial–mesenchymal contact. Cell–cell contact is critical, since laminin α1 chain is not detected in monocultures exposed to coculture-conditioned medium or in epithelial–mesenchymal cocultures in which heterotypic cell–cell contact is prevented by an interposing filter. Immunohistochemical studies on cocultures treated with brefeldin A, an inhibitor of protein secretion, indicated both epithelial and mesenchymal cells synthesize laminin α1 chain upon heterotypic cell– cell contact. In a set of functional studies, embryonic lung explants were cultured in the presence of monoclonal antibodies to laminin α1, α2, and β/γ chains. Lung explants exposed to monoclonal antibodies to laminin α1 chain exhibited alterations in peribronchial cell shape and decreased smooth muscle development, as indicated by low levels of smooth muscle α actin and desmin. Taken together, our studies suggest that laminin α1 chain synthesis is regulated by epithelial–mesenchymal interaction and may play a role in airway smooth muscle development.
Basement membranes (BMs)1 are specialized, sheet-like extracellular matrices that divide tissues into compartments. The BMs function as a dynamic structure in morphogenesis, cell differentiation, and maintenance of the mature cellular structural and functional phenotypes (for review see Kleinman et al., 1993).
Laminins (LMs) are the major constituents of BMs. This complex family of extracellular matrix proteins plays important roles in cell adhesion, growth, morphology, and migration (Timpl and Brown, 1994). LMs consist of three subunit polypeptide chains, classified as α, β, and γ chains. These are linked together by disulfide bonds and associate into a cruciform tertiary structure by a triple-helical coiled coil producing a long, rigid rod-like structure and two to three shorter arms (for review see Engel, 1992). The different LM chains share partial homology, particularly in the globular and rod-like domains containing EGF-like repeats (Engel, 1992) and domains participating in the coiled-coil region (Iivanainen et al., 1995; Miner et al., 1995). Additionally, the α chains contain a large COOH-terminal globular domain with five internal repeat motifs that have been identified as major sites for integrin binding (Hall et al., 1990; Kramer et al., 1990; Elices et al., 1991) and heparin binding (Skubitz et al., 1988).
The first LM reported, isolated from the Engelbreth Holm-Shawm (EHS) tumor (Timpl, 1979), is now referred to as LM-1(Burgeson et al., 1994) and is composed of α1 (400 kD), β1 (210 kD), and γ1 (200 kD) chains. LM-1 is the earliest extracellular matrix molecule produced in mouse embryogenesis (Wu et al., 1983), and during development it can be detected in epithelial BMs of most organs, including the lung (Wan et al., 1983; Klein et al., 1990; Schuger et al., 1992). The presence of LM α1, β1, and γ1 mRNA in the mouse lung epithelial and mesenchymal compartments (Schuger et al., 1992; Thomas and Toziadek, 1994; Lallemand et al., 1995) suggests that both cell populations contribute to its production. However, other studies have shown an exclusively epithelial cell origin (Klein et al., 1990). LM-1 deposits exclusively in epithelial BMs. Vascular BMs and the rest of the extracellular matrix do not contain LM-1 (Klein et al., 1990; Schuger et al., 1992; Thomas and Toziadek, 1994; Lallemand et al., 1995; Virtanen et al., 1996).
LM-1 plays important roles in lung development, more specifically in the processes of branching morphogenesis (Schuger et al., 1990a , 1991), BM assembly (Schuger et al., 1995, 1996), epithelial cell adhesion (Matter and Laurie, 1994; Schuger et al., 1995), and epithelial cell polarization (Schuger et al., 1990b , 1995, 1996). In addition, our recent studies showed that LM-1 polymerization at the epithelial–mesenchymal interface is required for normal arrangement and polarization of bronchial smooth muscle cells (Schuger, L., P. Yurchenco, and Y. Yang, manuscript submitted for publication).
More recently, other LMs have been identified in the developing lung, including LM-2 (Virtanen et al., 1996). However, their functional activity and possible role in morphogenesis remain to be elucidated. LM-2, formerly referred to as merosin (Engvall, 1994), is the main LM in striated muscle and differs from LM-1 only in its α chain (α2, β1, γ1).
Mouse lung development begins on day nine of gestation (Ten Have-Opbroek, 1981; Theiler, 1989) and culminates in the neonatal period giving rise to a complex pattern of branched airways and alveoli (Ten Have-Opbroek, 1981). Cytological markers indicative of smooth muscle differentiation, such as smooth muscle α actin, desmin, and myosin start to appear in mesenchymal cells surrounding the trachea and main bronchi by days 11 to 12, progressing in a proximal to distal fashion (Roman and McDonald, 1992; Yang, Y., and L. Schuger, unpublished observations). LM-1 deposition in the developing lung, although restricted to the epithelial–mesenchymal interface, coincides with the areas of bronchial smooth muscle development. Smooth muscle cells are not detected in the lung vasculature until late development (Roman and McDonald, 1992; Yang, Y., and L. Schuger, unpublished observations).
Here we present evidence suggesting that epithelial– mesenchymal interaction is required for the synthesis of the LM α1 chain, while the LM α2 chain is constitutively synthesized by both cell types. Furthermore, monoclonal antibodies to LM α1 chain but not to LM α2 or β/γ chains altered peribronchial mesenchymal cell shape and inhibited smooth muscle development in lung organ cultures. These results suggest that the LM α1 chain synthesis is stimulated by epithelial–mesenchymal contact and may play a role in airway smooth muscle development.
Materials and Methods
Antibodies
A polyclonal antibody against murine EHS LM was generated as previously described (Palm and Furcht, 1983). The antibody reacts with LM-1 but not fibronectin or type IV collagen (McCarthy et al., 1983). The antibody was further purified on an LM-1 affinity column (McCarthy and Furcht, 1984). Monoclonal antibodies to LM α1 (referred to as AL-4) and β1/γ1 chains (referred to as AL-3) were generated by the immunization of male LOU/MNCr rats against murine EHS LM. The preparation, purification, and characterization of these antibodies has been previously described (Skubitz et al., 1987, 1988). Normal rat IgG was purchased from Cappel (Malvern, PA). A monoclonal antibody to the LM α2 chain (4H8-2) was generated, purified, and characterized as previously described (Schuler and Sorokin, 1995). This antibody does not recognize other LM chains (Schuler and Sorokin, 1995). A rabbit polyclonal antibody to low and high molecular weight cytokeratins was purchased from Dako (Carpinteria, CA). A mouse monoclonal antibody to smooth muscle α actin was obtained from Boehringer Mannheim (Indianapolis, IN). This antibody has been shown to immunoreact with mouse smooth muscle α actin (Roman and McDonald, 1992). A mouse monoclonal antibody to desmin was purchased from Dako. EHS LM was purchased from Collaborative Biomedical (Boston, MA).
Generation of Epithelial and Mesenchymal Monocultures and Cocultures
CD-1 strain (Charles River) mice were mated, and the day of finding a vaginal plug was designated as day zero of embryonic development. Lungs were removed at day 15 of gestation, minced, and placed in PBS containing 0.3% trypsin and 0.1% EDTA for 10 min at 37°C. A single cell suspension was obtained by forcing cell aggregates and pieces of tissue through a micropipet several times. The cells were then filtered through a 100-μm-pore mesh and resuspended in minimal essential medium (MEM; GIBCO BRL, Gaithersburg, MD) with nonessential amino acids (GIBCO BRL), 0.29 mg/ml l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 0.25 mg/ml amphotericin B, and 10% fetal bovine serum (Irvine Scientific, Santa Ana, CA). Monocultures were generated by differential plating as previously described (Schuger et al., 1993). Culture samples were immunostained with an anti-keratin antibody to identify epithelial cells. Only mesenchymal monocultures with <1% keratin-positive cells and epithelial monocultures with 10% or less mesenchymal cell contamination were used to carry out experiments. Homotypic and heterotypic cocultures were generated by plating mixed lung cell populations directly isolated from fetal lungs, by plating together different numbers of epithelial and/or mesenchymal cells obtained from monocultures, or by adding different numbers of one cell type to an established monoculture of the same or the other cell type.
The cultures were established in 24-well plates (GIBCO BRL) or on the upper surface of polycarbonate membrane inserts (Millipore, Bedford, MA). The polycarbonate membrane does not support cell attachment, and under these conditions the cells cluster together at the center of the insert, allowing maximal cell–cell interaction. Approximately 3 to 6 × 105 cells were added to each well or insert. The cells were cultured in complete medium for 2 to 72 h. In some experiments involving epithelial–mesenchymal cocultures, the two cell populations were separated again by differential plating at the end of the coculture period. All of the cultures employed for these studies were at passages 0 to 2.
Treatment of Epithelial–Mesenchymal Cocultures with Brefeldin A
In these studies, organotypic epithelial–mesenchymal cocultures were exposed to the fungal metabolite brefeldin A (Sigma Chemical Co., St. Louis, MO), an inhibitor of protein secretion at the pre-Golgi compartment (Klausner et al., 1992), to determine what cell type synthesizes LM α1 chain. Organotypic cocultures were generated by plating mixed lung cell populations isolated from fetal lungs at high densities (1–5 × 106 cells/ ml; Schuger et al., 1993). When plated at high density, the lung epithelial cells form spheroid clusters and cysts and the mesenchymal cells grow around them as a monolayer (Schuger et al., 1993, 1995). 2-d-old organotypic cocultures were washed and incubated for 3 h in complete medium supplemented with 0, 1, 5, and 10 μg/ml of brefeldin A. At the end of the culture period the cultures were washed and immunostained as described below.
Functional Studies on Lung Organ Cultures
Embryos were collected at day 12 of gestation. Their lungs were then dissected and the lower right lobes were cultured at the air–medium interface on the upper surface of polycarbonate membrane inserts, in a serum free-defined medium, BGJb (GIBCO BRL; Schuger et al., 1996). The lung explants were cultured for 3 d in the presence of monoclonal antibodies to LM α1 chain (monoclonal antibody AL-4), α2 chain (monoclonal antibody 4H8-2), LM β1/γ1 chain (monoclonal antibody AL-3), control IgG, or no treatment. Previous studies demonstrated that these antibodies penetrate into the lung explant and bind to the epithelial BM (Schuger et al., 1991). The immunoglobulins were added to the organ cultures at concentrations of 10, 50, and 100 μg/ml at the beginning of the experiment. These concentrations were not toxic to the cells as indicated by a cytotoxicity assay based on Cr51 release (Schuger et al., 1989; data not shown). Since the embryonic lungs are transparent, the number of terminal airway buds was daily determined as an indicator of branching morphogenesis. After 3 d in culture, the explants were lysed or formalin fixed, and paraffin embedded. 5-μm-thick sections were cut from the latter and stained with hematoxylin-eosin for light microscopy evaluation. These experiments were done in triplicate wells (1 explant/well) and were repeated seven times.
Metabolic Labeling, Immunoprecipitation, and SDS-PAGE
Epithelial and mesenchymal monocultures and cocultures (∼2 × 107 cells each) were incubated for 30 min in methionine-free medium supplemented with 1 mCi/ml of [35S]methionine (NEN Dupont, Boston, MA). After incubation, the cells were washed, cultured in cold medium for an additional 30 min, and then lysed in SDS sample buffer (0.0625 M Tris-HCL, pH 6.8, 10% glycerol, 2% SDS, and 2.5% 2 β-mercaptoethanol; all from BioRad, Richmond, CA). LM-1 was precipitated from the lysates with 10 μg/ml of monoclonal antibody AL-4 (against LM α1 chain) and protein A-Sepharose (Sigma Chemical Co.) as previously described (Schuger et al., 1992). The immunoprecipitates were eluted and fractionated in 4% polyacrylamide gels under reducing conditions. Radioactive bands were visualized by exposing the dried gels to X-ray film (Kodak XAR-2; Eastman Kodak Co., Rochester, NY).
Western Blotting
Cell monocultures, cocultures, and lung organ cultures were lysed by boiling for 10 min in SDS sample buffer under reducing conditions for LM and under nonreducing conditions for smooth muscle α-actin and desmin. Cell supernatants were diluted 1:2 in 2× SDS sample buffer and boiled under reducing conditions. 30 μg of sample was resolved in a 4% acrylamide gel for LM studies and in a 15% gel for smooth muscle α actin and desmin studies. The samples were transferred to nitrocellulose membranes (BioRad) according to the method of Towbin et al. (1979) and blocked with 5% dry nonfat milk in TBS-T (20 mM Tris base, 137 mM sodium chloride, 0.05% Tween-20, pH 7.6; all from BioRad). The membranes were then blotted for 1 h with a 1:100 dilution of polyclonal antibodies to EHS LM that recognize LM α1 and α2 chains or monoclonal antibodies to LM α1 chain (AL-4), LM α2 chain (4H8-2), smooth muscle α actin, and desmin. This was followed by another hour of incubation with a 1:3,000 dilution of the secondary antibody, goat anti–rat, anti–rabbit, or anti–mouse IgG depending on the primary antibody. The bands were detected by chemiluminescence using a commercial kit (Amersham Life Science, Arlington Heights, IL) and following the manufacturer's instructions.
Immunohistochemistry
Immunolocalization of LM α1 chain was examined on organotypic cocultures and on embryonic lungs. Occasionally, double immunostaining (immufluorescence followed by immunoperoxidase) was done for LM-1 and cytokeratins combined. Cocultures and 5-μm-thick lung frozen sections were fixed for 5 min in absolute alcohol, exposed to 5% normal goat serum followed by treatment with a 1:50 dilution of monoclonal antibody to LM α1 chain (AL-4) for 45 min at room temperature. The sections were then washed in PBS and exposed to a 1:50 dilution of the secondary antibody (FITC-conjugated goat anti–rat IgG for LM-1; Cappel) for 30 min at room temperature. To identify epithelial cells, some of the LM-1–stained cocultures were then immunostained with anti-cytokeratin antibodies using a commercial peroxidase–anti-peroxidase kit (Dako) and following the manufacturer's instructions.
ELISA
Epithelial–mesenchymal cocultures and monocultures were washed and incubated for 3 h in serum-free MEM containing 0.02% BSA (MEM-BSA). The culture fluids were then collected and added to quadruplicate wells of a 96-well plate (Falcon Plastics, Oxnard, CA) in aliquots of 0.1 ml/ well. MEM-BSA served as a negative control. Serial dilutions of LM-1 were added to the assay plates to serve as a positive standard. After a 4-h incubation, the fluids were removed and the ELISA was performed as described (Varani et al., 1985) using a monoclonal antibody to the LM α1 chain (AL-4).
Results
LM-1 and LM-2 Are Expressed by the Mouse Fetal Lung
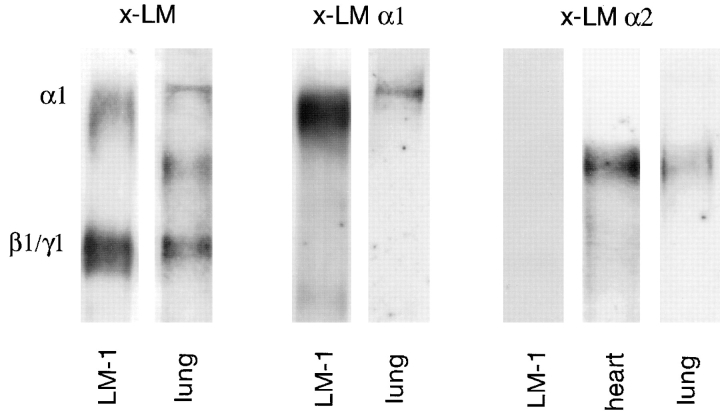
Immunoblots using a polyclonal antibody against EHS LM (Fig. 1, x-LM) that recognizes both α1 and α2 chains, a monoclonal antibody to LM α1 chain (Fig. 1, x-LM α1), and a monoclonal antibody to LM α2 chain (Fig. 1, x-LM α2) demonstrated the presence of LM α1, α2, β1, and γ1 chains in the mouse developing lung. We noticed that β1 and γ1 chains frequently migrated with similar Mr producing a single band in minigels. However, the two bands were resolved in larger gels.
Figure 1.
Immunoblots demonstrating the presence of LM α1, α2, β1, and γ1 chains in the developing lung (day 15 of gestation). A polyclonal antibody against EHS LM that recognizes α1, α2, β1, and γ1 chains (x-LM), a monoclonal antibody to the LM α1 chain (AL-4, x-LM α1), and a monoclonal antibody to LM α2 chain (4H8-2, x-LM α2) were used in these studies. LM-1 from the EHS tumor and embryonic heart (rich in LM-2) served as controls. Immunoblots with normal rabbit IgG or rat IgG did not detect any protein bands.
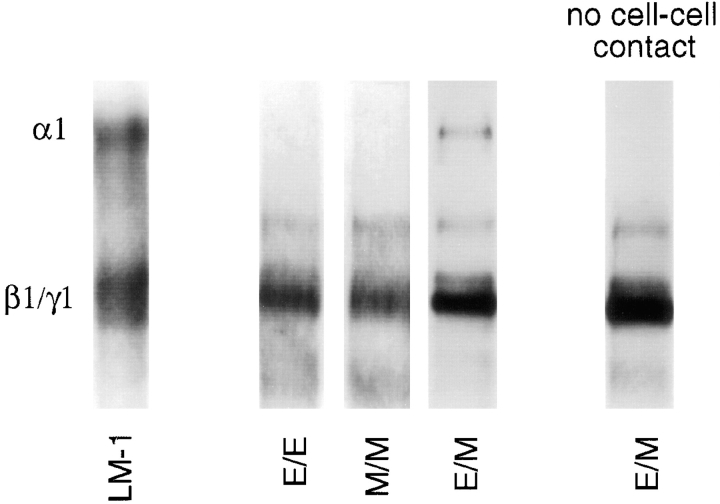
LM α1 Chain is Induced by Epithelial–Mesenchymal Interaction
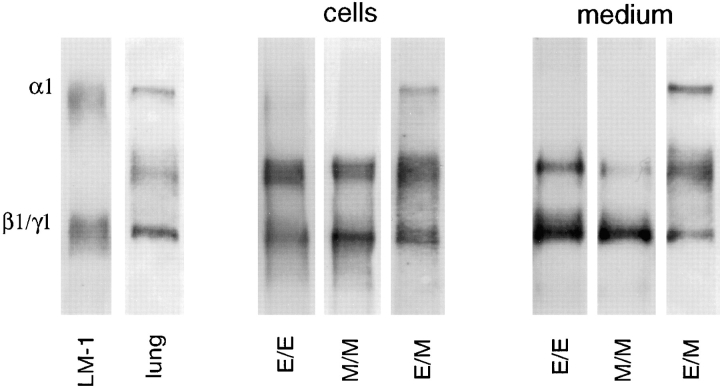
Monocultures of lung epithelial and mesenchymal cells as well as homotypic cocultures (epithelial cells added to an established epithelial monoculture, E/E; or mesenchymal cells added to an established mesenchymal monoculture, M/M) synthesized LM α2, β1, and γ1 but not α1 chain (Fig. 2). Epithelial–mesenchymal cocultures synthesized LM α1 chain in addition to LM α2, β1, and γ1 chains (Fig. 2). There were, however, differences in the amount of LM α1 chain synthesized by the various heterotypic coculture systems. Epithelial–mesenchymal cocultures established by plating epithelial and mesenchymal cell populations directly after trypsinization of lungs (passage 0) synthesized the highest levels of LM α1 chain (Fig. 3, column 1), whereas cocultures established by adding one cell type on top of a heterotypic cell monolayer synthesized the lowest levels (Fig. 3, columns 3 and 4). Cocultures generated by plating a combination of epithelial and mesenchymal cells obtained from monocultures at a ratio of 1:3 to 1:5 synthesized intermediate levels of LM α1 chain (Fig. 3, column 2). In addition, these studies indicated that these cells may require at least 4 h of coculture for LM α1 chain to be synthesized, since LM α1 chains were not detected after 2 to 4 h of coculture by ELISA (not shown).
Figure 2.
LM α1, α2, β1, and γ1 chains produced by epithelial– epithelial (E/E), mesenchymal–mesenchymal (M/M), and epithelial–mesenchymal (E/M) cocultures. A polyclonal antibody against EHS LM that recognizes α1, α2, β1, and γ1 chains was used for immunobloting. LM α1 chain is observed only in epithelial–mesenchymal cocultures. As controls, the first lane contained LM-1 from the EHS tumor and the second lane contained lung extract. Immunoblots with normal rabbit IgG or rat IgG did not detect any protein bands.
Figure 3.
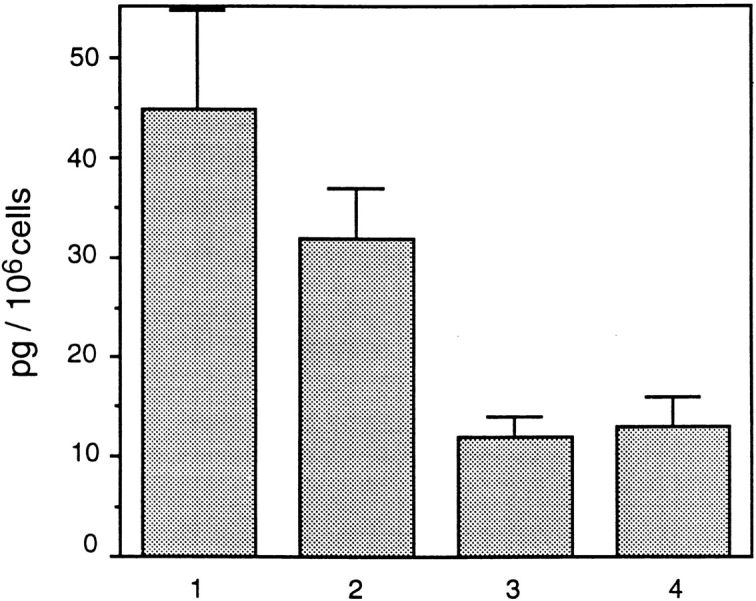
ELISA showing different levels of LM α1 chain synthesis in different cocultures. (Column 1) Epithelial–mesenchymal coculture established from mixed cell populations directly isolated from the lung. (Column 2) Epithelial–mesenchymal coculture established with cells from monocultures mixed in a 1:3 epithelial/mesenchymal ratio. (Column 3) Epithelial–mesenchymal coculture established by adding epithelial cells to a mesenchymal monolayer (both confluent at the time of determining LM-1 production). (Column 4) Epithelial–mesenchymal coculture established by adding mesenchymal cells to an epithelial monolayer (both confluent at the time of determining LM-1 production). The bars represent SD. The means and SD are based on quadruplicate examples in a single experiment. These were repeated three times with similar results.
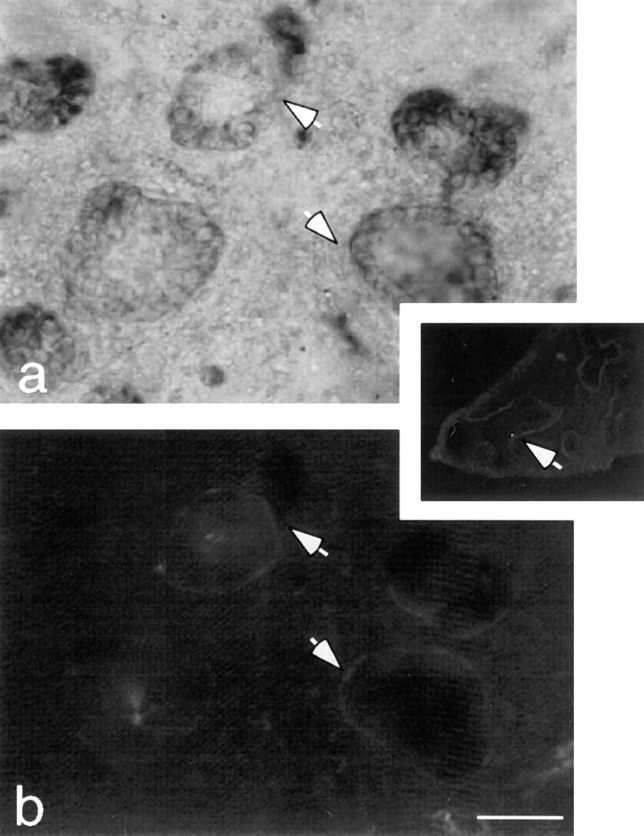
Immunohistochemical studies showed that LM α1 chain was deposited exclusively at the epithelial–mesenchymal interface (Fig. 4). No significant amount of LM α1 chain was detected intracellularly in either cell type. The restricted epithelial–mesenchymal localization of LM α1 chain was also observed in the developing lung. In day 12 lung, LM-1 was found in the BM along the bronchial tree (Fig. 4, inset) where more sustained epithelial–mesenchymal contact occurred, but not at the tips of the growing bronchi, where new epithelial–mesenchymal contacts are continuously established.
Figure 4.
Epithelial–mesenchymal organotypic coculture double-stained with anti-keratin antibodies by immunoperoxidase (a) and with anti-LM α1 chain antibody by immunofluorescence (b). The epithelial cells are recognized because they form round clusters and stain light brown with anti-keratin antibodies (not clearly appreciated in a black and white photograph). LM-1 is exclusively deposited at the epithelial–mesenchymal interface (arrows). (Inset) Day 12 lung stained with anti-LM α1 chain antibody shows LM-1 deposition restricted to the basement membrane alongside the bronchial tree. Bar: (a and b) 20 μm; (inset) 0.2 mm.
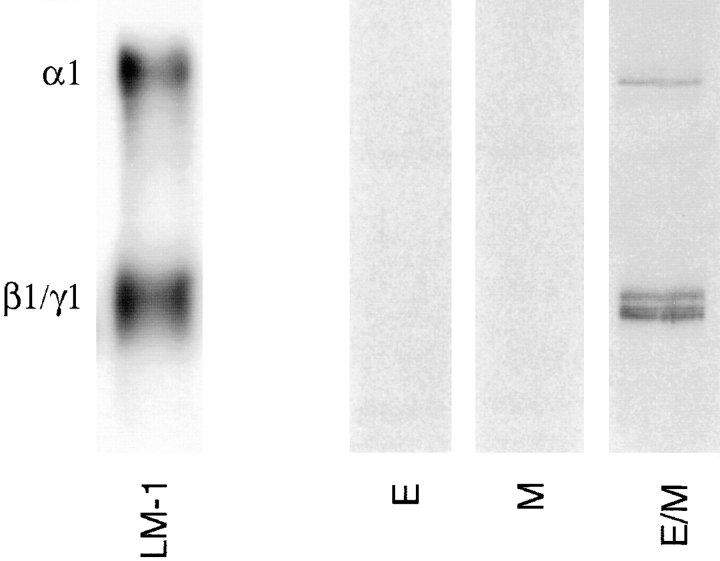
Immunoprecipitation with a monoclonal antibody to the LM α1 chain after metabolic radiolabeling confirmed the synthesis of LM α1 chain by epithelial–mesenchymal cocultures but not by monocultures (Fig. 5). These studies also showed that LM α1 chain is linked to β1 and γ1 chains forming full LM-1 molecules (Fig. 5).
Figure 5.
Immunoprecipitation of 35S-metabolically labeled LM-1 synthesized by epithelial (E) and mesenchymal (M) monocultures and epithelial–mesenchymal cocultures (E/M) using a monoclonal antibody to the LM α1 chain. Only epithelial–mesenchymal cocultures produced detectable levels of LM-1.
Epithelial–mesenchymal cocultures separated by a filter did not synthesize LM α1 chain, however α2, β1, and γ1 expression remained constant (Fig. 6). No Lmα1 chain was produced by culturing monolayers in epithelial-, mesenchymal-, or epithelial–mesenchymal-conditioned medium (not shown).
Figure 6.
Immunoblots using a polyclonal antibody to LM-1 that recognizes α1, α2, β1, and γ1 chains. No LM α1 chain was detected in epithelial–epithelial (E/E) or mesenchymal–mesenchymal (M/M) cocultures, whereas LM α1 chain was seen in epithelial–mesenchymal (E/M) cocultures. LM α1 chain was not detected in the absence of epithelial–mesenchymal contact (monocultures separated by a filter), whereas α2, β1, and γ1 chains remained constant. The first lane is LM-1 from the EHS tumor, used as control.
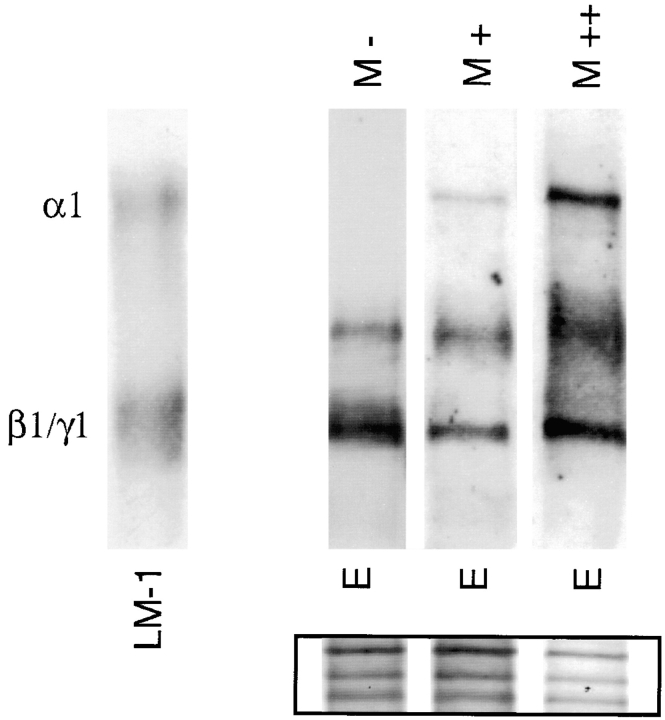
LM α1 chain synthesis seems to be directly proportional to the extent of epithelial–mesenchymal contact. This was suggested by studies in which increasing numbers of mesenchymal cells were added to prestablished (24-h-old) epithelial monocultures (Fig. 7). These ratios were selected based on the normal epithelial/mesenchymal ratio in mixed lung cell populations obtained from day 15 embryonic lungs.
Figure 7.
Addition of mesenchymal cells to epithelial monocultures stimulates LM α1 chain synthesis. (M−) No mesenchymal cells; (M+) 1:2 epithelial/mesenchymal cell ratio; (M++) 1:4 epithelial/mesenchymal cell ratio. The first lane is LM-1 from the EHS tumor, used as control. The inset shows staining of the nitrocellulose membrane with 0.2% amido black after immunobloting to visualize the amount of protein loaded per lane.
LM α1 Chain Is Synthesized by Epithelial and Mesenchymal Cells
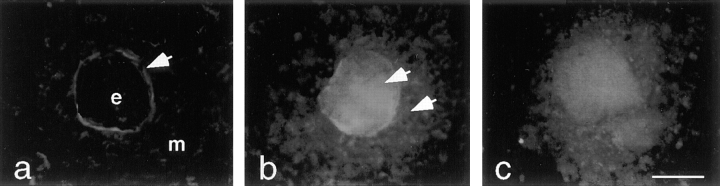
Immunohistochemical studies showed no intracellular LM α1 chain in epithelial–mesenchymal cocultures (Fig. 4). Additionally, we could not determine by immunoblotting or immunoprecipitation which cell population synthesized LM α1 chain, because this chain disappeared very rapidly after interruption of epithelial–mesenchymal contact. However, we occasionally noticed a faint band corresponding to LM α1 chain in both epithelial and mesenchymal cell lysates right after isolation from the lung or from heterotypic cocultures (not shown). To identify the origin of LM α1 chain, we exposed organotypic cocultures to brefeldin A, a fungal metabolite that inhibits protein secretion (Klausner et al., 1992). In organotypic cocultures, the epithelial cells form cysts and the mesenchymal cells surround them as a monolayer (Schuger et al., 1993, 1995); therefore, it is possible to determine what cell type accumulates LM α1 chain. Immunolocalization studies in brefeldin A-treated cultures indicated that LM α1 chain accumulates in both the epithelial cells and in the mesenchymal cells surrounding them (Fig. 8).
Figure 8.
Epithelial–mesenchymal organotypic cocultures exposed to 0 (a), 5 (b), and 10 (c) μg/ml of brefeldin A for 3 h, followed by immunostaining with anti-LM α1 chain antibody. e, Epithelial cell; m, mesenchymal cells. In untreated cocultures (a), LM α1 chain accumulates at the epithelial–mesenchymal interface (arrow). In brefeldin A-treated cocultures (b and c), LM α1 chain can be detected in the epithelial cells as well as in the mesenchymal cells surrounding them (b, arrows). Bar, 20 μm.
Functional Studies Showed Alterations in Peribronchial Cell Shape in Lung Explants Exposed to Anti-LM α1 Antibody
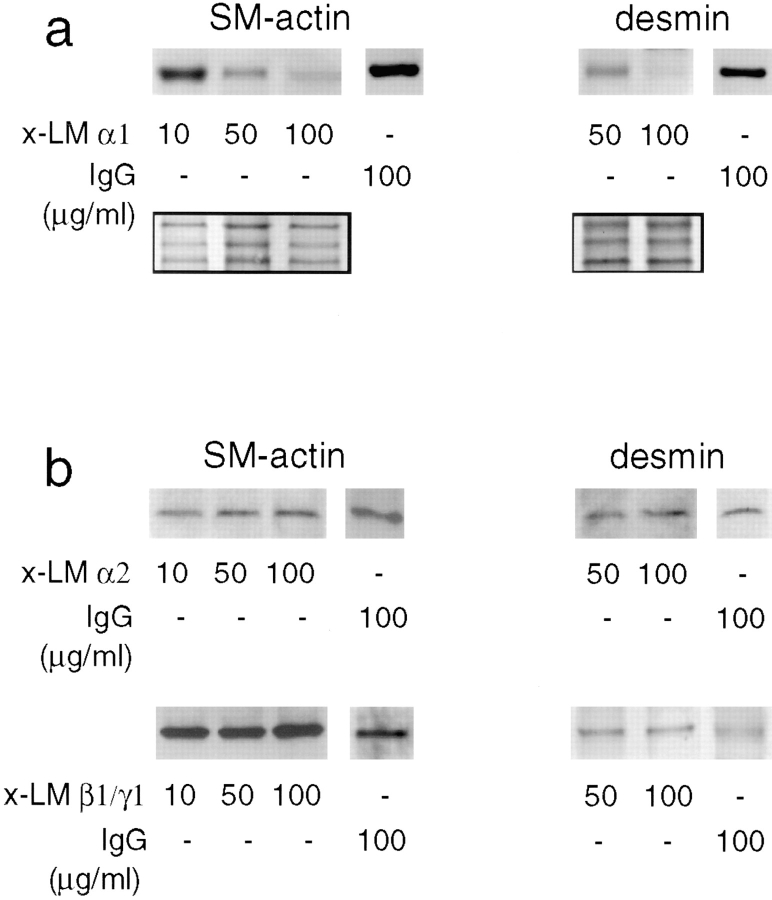
Day 12 lung explants were cultured for 3 d in the presence of monoclonal antibodies to LM chains α1, α2, and β1/γ1, normal mouse IgG, or no immunoglobulin. Since embryonic lungs are transparent, the airway branching activity was daily monitored by counting the number of terminal airway buds per explant and looking for changes in airway tree architecture or lumen caliber. All of the explants presented a similar number of terminal buds regardless of treatment (32 ± 8) and showed no changes in airway architecture (not shown).
Microscopic evaluation of hematoxylin- and eosin-stained sections showed well preserved epithelial and mesenchymal cellular architecture with scattered mitotic figures in both tissue compartments. A normal histological pattern was seen in the mesenchyme of lung explants exposed to antibodies against LM α2 or LM β1/γ1 chains or to normal mouse IgG. In these explants the peribronchial mesenchymal cells were elongated in shape (polarized) and arranged concentrically to the airway (Fig. 9, a, d, and e), whereas the rest of the mesenchymal cells were round in shape (unpolarized). However, in the lung explants exposed to 50 or 100 μg/ml of monoclonal antibody to LM α1 chain, the mesenchymal cells were uniformly round (unpolarized), regardless of their proximity to the bronchial tree (Fig. 9 b). The effect that mAb AL-4 had on mesenchymal cell morphology was corrected by preincubation of AL-4 with LM-1 (Fig. 9 c).
Figure 9.
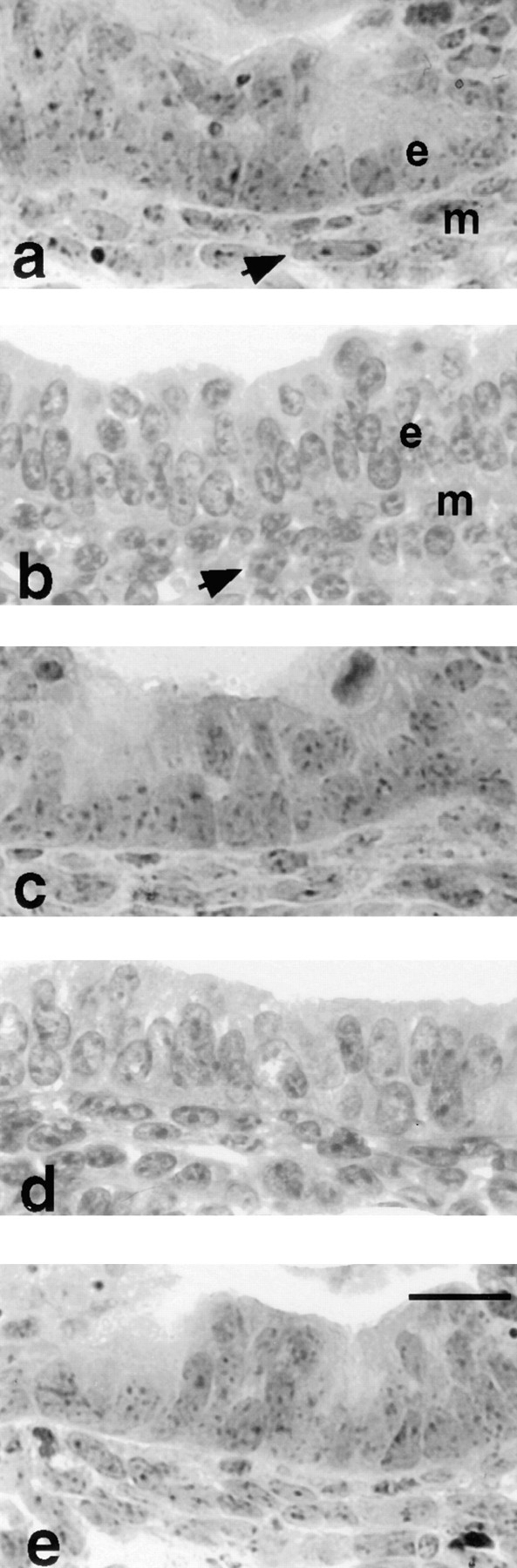
Photomicrographs of a main bronchus including peribronchial mesenchymal cells in lung explants cultured for 3 d in the presence of 100 μg/ml of normal mouse IgG (a), anti-LM α1 chain antibody (b), anti-LM α1 chain antibody preincubated with 200 μg/ml of LM-1 (c), anti-LM α2 antibody (d), and anti-LM β1/γ1 antibody (e). Note that in the control (a) the peribronchial mesenchymal cells (m) are polarized (elongated and oriented concentrically to the bronchus), whereas the peribronchial mesenchymal cells in explants exposed to anti-LM α1 chain antibody (b) are round. This effect was corrected by preincubation of the antibody with LM-1 (c) and was not observed in explants exposed to the other antibodies (d and e). The epithelial cells (e) showed no morphological alterations. Bar, 20 μm.
To assess the magnitude of this effect, longitudinal sections of each explant including the primary and secondary bronchi were projected on a television screen, and the number of mesenchymal cells with elongated (polarized) nuclei and with round (unpolarized) nuclei was determined. Although the main bronchi were surrounded by two to three concentric layers of elongated mesenchymal cells, only the cells directly apposed to the BM were counted.
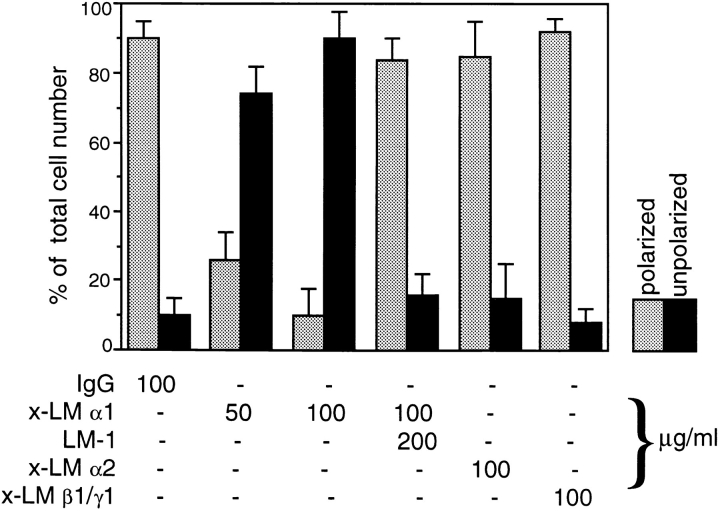
By using this semi-quantitative method, we found a statistically significant difference in the number of elongated versus round peribronchial cells in the explants exposed to 50 or 100 μg/ml of anti-LM α1 chain antibody compared to the other mAbs (Fig. 10). Notice the high percentage of unpolarized peribronchial mesenchymal cells in the lung explants exposed to anti-LM α1 chain antibody (P < 0.005, with Student's t test) and how this is corrected with preincubation of the antibody with LM-1. No significant differences were found in the total number of mesenchymal cells among the explants, including those treated with anti-LM α1 chain (350 ± 45).
Figure 10.
Histogram showing the percentage of polarized (elongated) and unpolarized (round) peribronchial cells in day 12 embryonic lung explants exposed for 3 d to various anti-LM antibodies and control immunoglobulin. The number of polarized (elongated) and unpolarized (round) peribronchial mesenchymal cells was determined on histological sections of the explants. These were cut longitudinally to and including the full main bronchus. Only the cells closest to the BM of the main bronchus and its first order branches were quantitated. The bars represent SD. The means and SD are based on five examples of each treatment.
Functional Studies Suggested That LM α1 May Play a Role in Bronchial Smooth Muscle Development
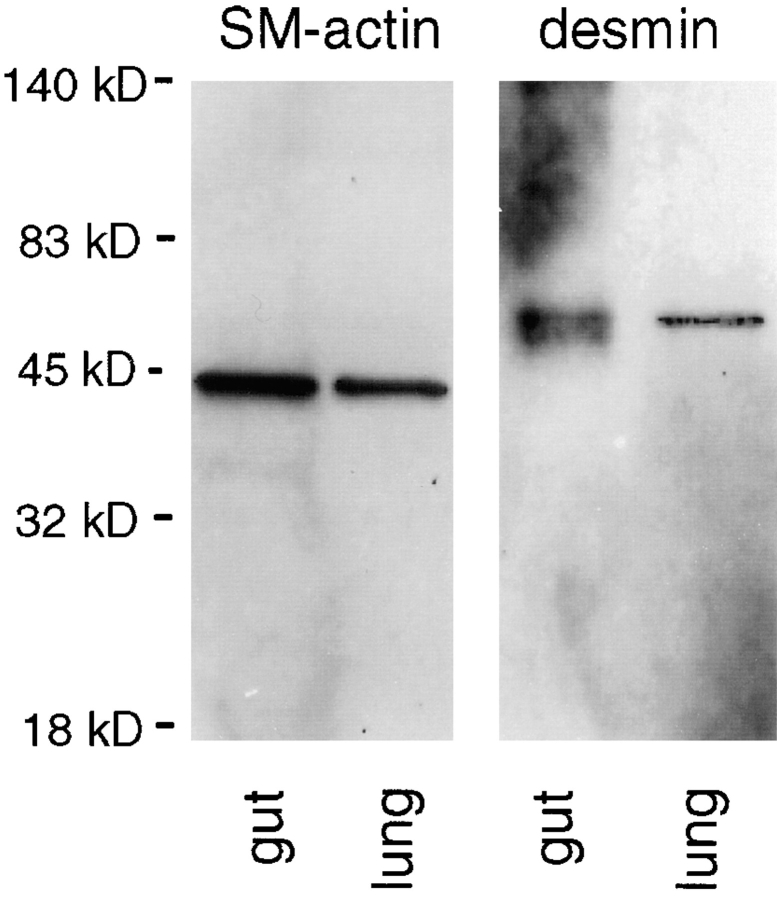
To study the development of bronchial smooth muscle, we used antibodies against two different smooth muscle-specific proteins, smooth muscle α actin and desmin. Desmin is an intermediate filament expressed in all muscle tissue and, when absent, results in smooth muscle hypoplasia and degeneration (Milner et al., 1996). These antibodies recognized the two proteins in immunoblots of mouse adult gut (rich in smooth muscle) and fetal lung tissue lysates (Fig. 11). Lung explant cultured for 3 d in the presence of a monoclonal antibody against LM α1 chain showed a decrease in smooth muscle α actin and desmin proportional to the concentration of antibody (Fig. 12 a). No change in smooth muscle α actin and desmin expression was observed in lung explants cultured for 3 d in the presence of monoclonal antibodies to LM α2 chain or to LM β1/γ1 chains compared with controls (Fig. 12 b).
Figure 11.
Immunoblots using monoclonal antibodies to smooth muscle α actin and desmin on mouse adult gut (rich in smooth muscle) and fetal lung tissue lysates, the latter on day 15 of gestation. A single band migrating at an Mr of ∼42,000, consistent with smooth muscle α actin, and a single band migrating at an Mr of ∼52,000, consistent with desmin, are visualized in both adult gut and fetal lung.
Figure 12.
Detection of smooth muscle α actin (SM-actin) and desmin in lung explants cultured for 3 d in the presence of monoclonal antibodies against LM chains or control IgG. There is a decrease in smooth muscle α actin and desmin proportional to the concentration of anti-α1 chain antibody added to the lung organ cultures (a). The inset shows a portion of the nitrocellulose membrane stained with 0.2% amido black after immunobloting to visualize the amount of protein loaded per lane. No change in smooth muscle α actin or desmin synthesis is observed in lung explants cultured for 3 d in the presence of a mAb to LM β1/γ1 chains, a monoclonal antibody to LM-α2 chain, or normal mouse IgG (b).
Discussion
Epithelial–Mesenchymal Contact Induces Synthesis of LM α1 Chain
Heterotypic cell interactions are long known to be essential for morphogenesis and cell differentiation, however, the molecular mechanisms underlying these processes are poorly understood. Most developing organs, including the lung, are composed of two main cell populations, the epithelium and the mesenchyme, separated by a BM. Among the BM constituents, LM-1 is known to play a significant role in morphogenic epithelial–mesenchymal interactions (for review see Ekblom, 1996). In this study, using a combination of monocultures and cocultures isolated from mouse embryonic lungs, we show that epithelial–mesenchymal interaction induces the synthesis of the LM α1 chain.
LM α1 chain is not the first extracellular matrix protein found to be modulated by epithelial–mesenchymal interactions. In a previous study using tissue recombinants, Vanio et al. (1989) showed that heterotypic interaction between epithelium and mesenchyme stimulates the synthesis of syndecan and tenascin during mouse tooth development. A similar mechanism seems to regulate the expression of certain cytokines in the skin (Reusch et al., 1991; Smola et al., 1993) and in the developing tooth (Mitsiadis et al., 1995).
Homotypic cocultures, epithelial–mesenchymal cocultures separated by a filter, or monocultures exposed to conditioned media, did not produce LM α1 chain, indicating that LM α1 chain synthesis requires heterotypic cell–cell contact. This kind of close cell–cell interaction does not normally occur in the adult but is characteristic of developing organs. In the lung, epithelial and mesenchymal cells are in direct contact at the ends of the bronchial tree, where new airway buds are being formed (Grant et al., 1983; Jaskoll and Slavkin, 1984). Deposition of LM α1 chain begins at these sites, where it is barely detected, and gradually accumulates alongside the bronchial tree, where more prolonged heterotypic cell–cell contact has time to occur.
The need for cellular contact was noticed in a variety of cell–cell interactions (Reichmann et al., 1989; Reusch et al., 1991; Yaeger, et al., 1991; Flaumenhaft et al., 1993; Smola et al., 1993) and underscores the importance of juxtacrine or short range paracrine signals. Such mechanisms have been proposed or demonstrated in many heterotypic cell–cell interactions, including the activation of TGF-β in smooth muscle cell–fibroblast coculture (Flaumenhaft et al., 1993), the transactivation of TGF-α precursor by adjacent cells (Anklesaria et al., 1990), and the production of casein by mammary epithelial cells upon coculture with mammary mesenchymal cells (Reichmann et al., 1989).
The simplest mechanism of cell–cell interaction leading to LM α1 chain synthesis is that a molecule, or molecules, produced by one cell type induces the synthesis of LM α1 by the other. For example, PDGF-A is produced by lung epithelial cells and exerts its effect on the mesenchymal cells surrounding them (Boström et al., 1996). Similarly, scatter factor is produced by mesenchymal cells but acts on epithelial cells (for review see Rosen et al., 1994). Alternatively, cell–cell interaction could involve a regulatory factor with autocrine and paracrine control over LM α1 expression. In such a case, LM α1 chain could be induced in both cell types. Obviously, mediator roles could be played by extracellular matrix components and/or their receptors, instead of cytokines.
Unfortunately, very little is known about the mechanisms regulating LM expression. Retinoic acid has been shown to stimulate LM-1 synthesis by embryonic cells (Carlin et al., 1983) and has been implicated as the mediator of epithelial–mesenchymal interactions in the tooth (Mitsiadis et al., 1995). However, although retinoic acid seems to play a role in lung morphogenesis (Schuger et al., 1993), it should be stressed that it does not affect LM-1 synthesis in lung organ cultures, monocultures, or cocultures (Mitra, R., and L. Schuger, unpublished observations).
LM α1 Chain Is Synthesized by Epithelial and Mesenchymal Cells
There has been some uncertainty in the literature as to what cells synthesize LM α1 chain. Using in situ hybridization, Ekblom et al. (1990) described LM α1 chain mRNA in epithelial cells only; however, we detected LM α1 chain mRNA in both the epithelium and the mesenchyme (Schuger et al., 1992). More recently, LM α1 chain mRNA has been reported in the epithelium and in mesenchymal cells close to it (Thomas and Dziadek, 1994). In the current study, we could not determine by immunobloting or immunoprecipitation which cell type synthesizes the LM α1 chain. Furthermore, since its immunodetection was restricted to the epithelial–mesenchymal interface, immunohistochemistry was not contributory. The origin of LM α1 chain was however elucidated by exposing cocultures to brefeldin A, a fungal metabolite that blocks protein secretion (Klausner et al., 1992). These studies showed that in epithelial–mesenchymal cocultures exposed to brefeldin A, LM α1 chain accumulated in both the epithelial and mesenchymal cells, confirming its origin in both cell types.
Ekblom and coworkers observed that the development of epithelial cell polarity coincides with the expression of LM α1 chain in the developing kidney (Klein et al., 1988; Ekblom et al., 1990). Based on experimental studies, the authors proposed that one of the functions of LM α1 chain is to induce epithelial cell polarization (Klein et al., 1988). Our studies did not test the role of LM α1 chain in epithelial cell polarization but suggested that polarized cells produce more LM α1 chain than their unpolarized counterparts. For example, cocultures established in Millipore inserts, which allow epithelial cells to form spheroids and cysts surrounded by concentric layers of mesenchymal cells, synthesized higher levels of LM α1 chain than surface-anchored bilayer cocultures (as shown in Fig. 3).
Our studies as well as others (Virtanen et al., 1996) confirmed that the developing lung produces LM α2 chain. Immunoprecipitation studies indicated that LM α2 chain is secreted by the cells as part of LM-2 (α2, β1, γ1; He, L., and L. Schuger, unpublished results). In contrast to LM α1 chain, LM α2 chain is not regulated by epithelial–mesenchymal contact and may serve different functions than LM-1. So far, the LM α2 chain has been shown to be essential for myotube stability (Vachon, 1996) in skeletal muscle, however its role in smooth muscle biology remains to be elucidated. Noteworthy, LM-2 deficiency is the underlying defect in several muscular dystrophies in which respiratory problems lead to early death (Mendell et al., 1995). However, at the present time, it is unclear whether these are primary to the lung or secondary to poor ventilation due to muscular dysfunction.
LM α1 Chain May Be Involved in Bronchial Smooth Muscle Development
Lung explants cultured in the presence of antibodies against LM α1 chain exhibited alterations in peribronchial mesenchymal cell shape and synthesized lower levels of smooth muscle α actin and desmin. The anti-LM α1 chain antibody used in these studies reacts with the COOH-terminal G domain of the LM α1 chain, a major adhesion site for several cell types, including myofibroblasts (Engvall, 1994). Therefore, it is likely that the antibody perturbed mesenchymal cell adhesion to LM-1 and this in turn resulted in abnormal smooth muscle cell phenotype.
Interestingly, two previous studies reported the differentiation of mesenchymal cells into smooth muscle upon prolonged contact with the epithelium (Cunha et al., 1992; Duluc et al., 1994). However neither study explored possible mechanisms underlying this process. In light of our results, it may be possible that LM α1 chain was induced by the epithelial–mesenchymal contact, facilitating the differentiation of mesenchymal cells into smooth muscle.
Most mesenchymal cells in the developing lung have a round configuration and obviously do not express smooth muscle markers. Interestingly, stretching forces cause undifferentiated mesenchymal cells to express smooth muscle-specific proteins (Yang, Y., and L. Schuger, unpublished observations). Based on this observation, we propose that in the lung explants exposed to anti-LM α1 antibody, the decreased peribronchial cell attachment to BM LM-1 prevented them from stretching in response to bronchial intraluminal pressure. The deficient stretching capability then resulted in a defective smooth muscle phenotype.
On the other hand, LM-1 has a direct effect on muscle cell shape, unrelated to stretching (Öcalan et al., 1988), and that per se may explain our findings. Furthermore, LM-1 plays roles in proliferation, migration, and differentiation of myoblasts (Goodman et al., 1989; von der Mark and Öcalan, 1989; Kroll et al., 1994; Vachon et al., 1996). Therefore, LM α1 chain may influence smooth muscle differentiation via different mechanisms.
In summary, we have shown that while LMs α2, β1, and γ1 chains are steadily produced by embryonic lung epithelial and mesenchymal cells, the synthesis of LM α1 chain is controlled by epithelial–mesenchymal interactions. Our studies further suggest that LM α1 chain may play a role in the development of bronchial smooth muscle, perhaps by controlling peribronchial mesenchymal cell attachment, shape, and ability to respond to stretching forces. Additional studies focused on the biological activities of LM-1 on smooth muscle cell differentiation will be required to elucidate the mechanism whereby LM-1 affects bronchial smooth muscle development.
Acknowledgments
This work has been supported by National Institutes of Health grants HL48730-01 (L. Schuger) and CA60658 (A.P.N. Skubitz).
Abbreviations used in this paper
- BM
basement membrane
- LM
laminin
Footnotes
Address all correspondence to Lucia Schuger, Wayne State University School of Medicine, Department of Pathology, Gordon H. Scott Hall of Basic Medical Sciences, 540 East Canfield Street, Detroit, MI 48201. Tel.: (313) 577-5651. Fax: (313) 577-0057.
References
- Anklesaria P, Teixidó J, Laiho M, Pierce JH, Greenberger JS, Massagué J. Cell-cell adhesion mediated by binding of membrane-anchored transforming growth factor α to epidermal growth factor receptors promotes cell proliferation. Proc Natl Acad Sci USA. 1990;87:3289–3293. doi: 10.1073/pnas.87.9.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boström H, Willetts K, Pekny M, Leveen P, Lindahl P, Hedstrand H, Pekna M, Hellström M, Gebre-Medhin S, Schalling M, et al. PDGF-A signaling is a critical event in lung alveolar myofibroblast development and alveogenesis. Cell. 1996;85:863–873. doi: 10.1016/s0092-8674(00)81270-2. [DOI] [PubMed] [Google Scholar]
- Burgeson RE, Chiquet M, Deutzmann R, Ekblom P, Engel J, Kleinmann H, Martin GR, Meneguzzi G, Paulsson M, Sanes J, et al. A new nomenclature for the laminins. Matrix Biol. 1994;263:16536–16544. doi: 10.1016/0945-053x(94)90184-8. [DOI] [PubMed] [Google Scholar]
- Carlin BE, Durkin ME, Bender B, Jaffe R, Chung AE. Synthesis of laminin and entactin by F9 cells induced with retinoic acid and dibutyryl cyclic AMP. J Biol Chem. 1983;25:7729–7737. [PubMed] [Google Scholar]
- Cunha GR, Battle E, Young P, Brody J, Donjacour A, Hayashi N, Kinbara H. Role of epithelial-mesenchymal interactions in the differentiation and spatial organization of visceral smooth muscle. Epithelial Cell Biol. 1992;2:76–83. [PubMed] [Google Scholar]
- Duluc I, Freund J-N, Leberquier C, Kedinger M. Fetal endoderm primarily holds the temporal and positional information required for mammalian intestinal development. J Cell Biol. 1994;126:211–221. doi: 10.1083/jcb.126.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblom P. Extracellular matrix and cell adhesion molecules in nephrogenesis. Exp Nephrol. 1996;4:92–96. [PubMed] [Google Scholar]
- Elices MM, Urry LA, Hemler ME. Receptor function for the integrin VLA-3: fibronectin, collagen, and laminin binding are differentially influenced by ARG-GLY-ASP peptide and divalent cations. J Cell Biol. 1991;112:169–181. doi: 10.1083/jcb.112.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J. Laminins and other strange proteins. Biochemistry. 1992;31:10643–10651. doi: 10.1021/bi00159a001. [DOI] [PubMed] [Google Scholar]
- Engvall E. Cell adhesion in muscle. Braz J Med Biol Res. 1994;27:2213–2227. [PubMed] [Google Scholar]
- Flaumenhaft R, Abe M, Sato Y, Miyazono K, Harpel J, Heldin C-H, Rifkin DB. Role of the latent TGF-β binding protein in the activation of latent TGF-β by co-cultures of endothelial and smooth muscle cells. J Cell Biol. 1993;120:995–1002. doi: 10.1083/jcb.120.4.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman SL, Deutzmann R, Nurcombe V. The E8 fragment of laminin promotes locomotion of myoblasts over extracellular matrix. J Cell Biol. 1989;109:799–809. doi: 10.1083/jcb.109.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant MM, Cutts NR, Brody JS. Alterations in lung basement membrane during fetal growth and type-2 cell development. Dev Biol. 1983;97:173–183. doi: 10.1016/0012-1606(83)90074-x. [DOI] [PubMed] [Google Scholar]
- Hall DH, Reichardt LF, Crowley E, Holley B, Moezzi M, Sonnenberg A, Damsky CH. The α6/β1 integrin heterodimers mediate cell attachment to distinct sites on laminin. J Cell Biol. 1990;110:2175–2184. doi: 10.1083/jcb.110.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iivanainen A, Sainio K, Sariola H, Tryggvason K. Primary structure of a novel human laminin α4 chain. FEBS Lett. 1995;365:183–188. doi: 10.1016/0014-5793(95)00462-i. [DOI] [PubMed] [Google Scholar]
- Jaskoll TF, Slavkin HC. Ultrastructural and immunofluorescence studies of basal-lamina alterations during mouse lung morphogenesis. Differentiation. 1984;28:36–48. doi: 10.1111/j.1432-0436.1984.tb00264.x. [DOI] [PubMed] [Google Scholar]
- Klausner RD, Donaldson JG, Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G, Ekblom M, Fecker L, Timpl R, Ekblom P. Differential expression of laminin A and B chains during development of embryonic mouse organs. Development. 1990;110:823–837. doi: 10.1242/dev.110.3.823. [DOI] [PubMed] [Google Scholar]
- Kleinman, H.K., M.C. Kibbey, H.W. Schnaper, M.A. Hadley, M. Dym, and D.S. Grant. 1993. Role of basement membrane in differentiation. In Molecular and Cellular Aspects of Basement Membranes. D.H. Rohrbach, and R. Timpl, editors. Academic Press, San Diego, CA. 309–326.
- Kramer RH, Cheng YF, Clyman RI. Human vascular endothelial cells use β1 and β3 integrin receptor complexes to attach to laminin. J Cell Biol. 1990;110:1233–1243. doi: 10.1083/jcb.111.3.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll TG, Peters BP, Marziasz C, Hustad, Jones PA, Killen PD, Ruddon RW. Expression of laminin chains during myogenic differentiation. J Biol Chem. 1994;269:9270–9277. [PubMed] [Google Scholar]
- Lallemand A, Ruocco SM, Gaillard DA. Synthesis and expression of laminin during human foetal lung development. Anat Rec. 1995;242:233–241. doi: 10.1002/ar.1092420213. [DOI] [PubMed] [Google Scholar]
- Matter ML, Laurie GW. A novel laminin E8 cell adhesion site required for lung alveolar formation in vitro. J Cell Biol. 1994;124:1083–1090. doi: 10.1083/jcb.124.6.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy JB, Furcht LT. Laminin and fibronectin promote the haptotactic migration of B16 mouse melanoma cells in vitro. J Cell Biol. 1984;98:1474–1480. doi: 10.1083/jcb.98.4.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy JB, Palm SL, Furcht LT. Migration by haptotaxis of a Schwann cell tumor line on the basement membrane glycoprotein laminin. J Cell Biol. 1983;97:772–777. doi: 10.1083/jcb.97.3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell JR, Sahenk Z, Prior TW. The childhood muscular dystrophies: diseases sharing a common pathogenesis of membrane instability. J Child Neurol. 1995;10:150–159. doi: 10.1177/088307389501000219. [DOI] [PubMed] [Google Scholar]
- Milner DJ, Weitzer G, Duyen T, Bradley A, Capetanaki Y. Disruption of muscle architecture and myocardial degeneration in mice lacking desmin. J Cell Biol. 1996;134:1255–1270. doi: 10.1083/jcb.134.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JH, Lewis RM, Sanes JR. Molecular cloning of a novel laminin chain, α5, and widespread expression in adult mouse tissues. J Biol Chem. 1995;270:28523–28526. doi: 10.1074/jbc.270.48.28523. [DOI] [PubMed] [Google Scholar]
- Mitsiadis TA, Muramatsu T, Muramatsu H, Thesleff I. Midkine (MK), a heparin-binding/growth differentiation factor, is regulated by retinoic acid and epithelial-mesenchymal interaction in the developing mouse tooth, and affects cell proliferation and morphogenesis. J Cell Biol. 1995;129:267–281. doi: 10.1083/jcb.129.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm SI, Furcht LT. Production of laminin and fibronectin by Schwannoma cells: cell–protein interactions in vitro and protein localization in peripheral nerve in vivo. J Cell Biol. 1983;96:1218–1226. doi: 10.1083/jcb.96.5.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichmann E, Ball R, Groner B, Friis RR. New mammary epithelial and fibroblastic cell clones in coculture form structures competent to differentiate functionally. J Cell Biol. 1989;108:1127–1138. doi: 10.1083/jcb.108.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusch, M.K., V. Mielke, M. Sticherling, and E. Christophers. 1991. Interleukins in epidermo-dermal interactions. Pharmacology of the skin. Immunological Pharmacology Aspects of Atopic and Contact Dermatitis. S. Karger, editor. Basel. 4:39–42.
- Roman J, McDonald JA. Expression of fibronectin, the integrin α5, and α-smooth muscle actin in heart and lung development. Am J Respir Cell Mol Biol. 1992;6:472–480. doi: 10.1165/ajrcmb/6.5.472. [DOI] [PubMed] [Google Scholar]
- Rosen EM, Nigam SK, Goldberg ID. Scatter factor and the c-metreceptor: a paradigm for mesenchymal/epithelial interaction. J Cell Biol. 1994;127:1783–1787. doi: 10.1083/jcb.127.6.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öcalan M, Goodman SL, Kühl U, Hauschka SD, von der Mark Laminin alters cell shape and stimulates motility and proliferation of murine skeletal myoblasts. Dev Biol. 1988;125:1745–1751. doi: 10.1016/0012-1606(88)90068-1. [DOI] [PubMed] [Google Scholar]
- Schuger L, O'Shea S, Rheinheimer J, Varani J. Laminin in lung development: effects of anti-laminin antibody in murine lung morphogenesis. Dev Biol. 1990a;137:26–32. doi: 10.1016/0012-1606(90)90004-3. [DOI] [PubMed] [Google Scholar]
- Schuger L, O'Shea S, Nelson BB, Varani J. Organotypic arrangement of mouse embryonic lung cells on a basement membrane extract: involvement of laminin. Development. 1990b;110:1091–1099. doi: 10.1242/dev.110.4.1091. [DOI] [PubMed] [Google Scholar]
- Schuger L, Skubitz APN, O'Shea KS, Chang JF, Varani J. Identification of laminin domains involved in epithelial branching morphogenesis: effects of anti-laminin monoclonal antibodies on mouse embryonic lung development. Dev Biol. 1991;146:531–541. doi: 10.1016/0012-1606(91)90254-z. [DOI] [PubMed] [Google Scholar]
- Schuger L, Varani J, Killen PD, Skubitz APN, Gilbride K. Laminin expression in the mouse lung increases with development and stimulates spontaneous organotypic rearrangement of mixed lung cells. Dev Dyn. 1992;195:43–54. doi: 10.1002/aja.1001950105. [DOI] [PubMed] [Google Scholar]
- Schuger L, Varani J, Mitra J, Gilbride K. Retinoic acid stimulates mouse lung development by a mechanism involving epithelial-mesenchymal interaction and regulation of epidermal growth factor receptors. Dev Biol. 1993;159:462–473. doi: 10.1006/dbio.1993.1256. [DOI] [PubMed] [Google Scholar]
- Schuger L, Skubitz APN, Morenas A, Gilbride K. Different laminin domains facilitate lung development by independent mechanisms of action. Dev Biol. 1995;169:520–532. doi: 10.1006/dbio.1995.1166. [DOI] [PubMed] [Google Scholar]
- Schuger L, Skubitz APN, Gilbride K, Mandel R, He L. Laminin and heparan sulfate proteoglycan mediate epithelial cell polarization in organotypic cocultures of embryonic lung cells: evidence implicating involvement of the inner globular region of laminin β1 chain and the heparan sulfate groups of heparan sulfate proteoglycan. Dev Biol. 1996;179:264–273. doi: 10.1006/dbio.1996.0256. [DOI] [PubMed] [Google Scholar]
- Schuler F, Sorokin L. Expression of laminin isoforms in myogenic cells in vitro and in vivo. J Cell Sci. 1995;108:3795–3805. doi: 10.1242/jcs.108.12.3795. [DOI] [PubMed] [Google Scholar]
- Skubitz APN, Charonis AS, Tsilibary EC, Furcht LT. Localization of a tumor cell adhesion domain of laminin by a monoclonal antibody. Exp Cell Res. 1987;173:349–360. doi: 10.1016/0014-4827(87)90276-x. [DOI] [PubMed] [Google Scholar]
- Skubitz APN, McCarthy JB, Charonis AS, Furcht LT. Localization of three distinct heparin-binding domains by monoclonal antibodies. J Biol Chem. 1988;263:4861–4868. [PubMed] [Google Scholar]
- Skubitz APN, McCarthy JB, Qi Z, Yi X, Furcht LT. Definition of a sequence, RYVLPR, within laminin peptide F-9 that mediates metastatic fibrosarcoma cell adhesion and spreading. Cancer Res. 1990;50:7612–7622. [PubMed] [Google Scholar]
- Smola H, Thiekötter G, Fusenig NE. Mutual induction of growth factor gene expression by epidermal-dermal cell interaction. J Cell Biol. 1993;122:417–429. doi: 10.1083/jcb.122.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten Have-Opbroek, A.A.W. The development of the lung in mammals: an analysis of concepts and findings. Am J Anat. 1981;162:201–219. doi: 10.1002/aja.1001620303. [DOI] [PubMed] [Google Scholar]
- Theiler, K. 1989. The House Mouse. editor. Springer-Verlag, New York.
- Thomas T, Dziadek M. Expression of collagen α 1 (IV), laminin and nidogen genes in the embryonic mouse lung: implications for branching morphogenesis. Mech Dev. 1994;45:193–201. doi: 10.1016/0925-4773(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Timpl R, Brown JC. The laminins. Matrix Biol. 1994;14:275–281. doi: 10.1016/0945-053x(94)90192-9. [DOI] [PubMed] [Google Scholar]
- Timpl R, Rhode M, Gehron-Robey P, Rennard ST, Foidart JM, Martin GR. Laminin-A glycoprotein from basement membranes. J Biol Chem. 1979;254:9933–9937. [PubMed] [Google Scholar]
- Towbin H, Staehilin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vachon PH, Loechel F, Xu H, Wewer UM, Engvall E. Merosin and laminin in myogenesis; specific requirement for merosin in myotube stability and survival. J Cell Biol. 1996;134:1483–1497. doi: 10.1083/jcb.134.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanio S, Jalkanen M, Thesleff I. Syndecan and tenascin expression is induced by epithelial-mesenchymal interactions in embryonic tooth mesenchyme. J Cell Biol. 1989;108:1945–1954. doi: 10.1083/jcb.108.5.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani J, Fliegel SEG, Till GO, Kunkel RG, Ryan US, Ward PA. Pulmonary endothelial cell killing by human neutrophils: possible involvement of hydroxyl radical. Lab Invest. 1985;53:656–663. [PubMed] [Google Scholar]
- Virtanen I, Laitinen A, Taneli T, Pääkkö P, Laitinen LA, Burgeson RE, Letho V-P. Differential expression of laminins and their integrin receptors in developing and adult human lung. Am J Respir Cell Mol Biol. 1996;15:184–196. doi: 10.1165/ajrcmb.15.2.8703474. [DOI] [PubMed] [Google Scholar]
- von de Mark H, Öcalan M. The differentiation and redifferentiation of myoblasts is triggered by fibronectin and laminin. Differentiation. 1989;40:150–157. doi: 10.1111/j.1432-0436.1989.tb00823.x. [DOI] [PubMed] [Google Scholar]
- Vuolteenaho R, Nissinen M, Sainio K, Byers M, Eddy R, Hirvonen H, Shows TB, Sariola H, Engvall E, Tryggvason K. Human laminin M chain (merosin): complete primary structure, chromosomal assignment, and expression of the M and A chain in human fetal tissues. J Cell Biol. 1994;124:381–394. doi: 10.1083/jcb.124.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan YJ, Wu TC, Chung AE, Damjanov I. Monoclonal antibodies to laminin reveal the heterogeneity of basement membranes in the developing and adult mouse tissues. J Cell Biol. 1984;98:971–979. doi: 10.1083/jcb.98.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TC, Wan YJ, Chung AE, Damajonov I. Immunohistochemical localization of entactin and laminin in mouse embryos and fetuses. Dev Biol. 1983;100:496–505. doi: 10.1016/0012-1606(83)90242-7. [DOI] [PubMed] [Google Scholar]
- Yaeger PC, Stiles CD, Rollins BJ. Human keratinocyte growth-promoting activity on the surface of fibroblasts. J Cell Physiol. 1991;149:110–116. doi: 10.1002/jcp.1041490114. [DOI] [PubMed] [Google Scholar]