Abstract
Adhesion of blood leukocytes to the endothelium involves multiple steps including initial attachment (tethering), rolling, and firm arrest. Presentation of adhesion molecules on leukocyte microvilli can substantially enhance tethering. Localization of L-selectin to microvilli and of CD44 to the planar cell body have been shown to depend upon their transmembrane and cytoplasmic domains. We investigated the role of leukocyte integrin transmembrane and cytoplasmic domains in initiating adhesion under flow and in microvillous localization. Integrins α4β7, αLβ2, and αMβ2 were heterologously expressed in K562 cells. α4β7 initiated adhesion under flow and localized to microvilli, whereas β2 integrins did not initiate adhesion and localized to the cell body. Chimeric integrins were produced by replacing the α4β7 cytoplasmic and/or transmembrane domains with the homologous domains of αLβ2 or αMβ2. Unexpectedly, these chimeras efficiently mediated adhesion to the α4β7 ligand mucosal addressin cell adhesion molecule–1 under flow and localized to microvilli. Therefore, differences between the transmembrane and cytoplasmic domains of α4 and β2 integrins do not account for differences in ability to support attachment under flow or in membrane localization. Integrins α4β1, α5β1, α6Aβ1, αvβ3, and αEβ7 also localized to microvilli. Transmembrane proteins known or suspected to associate with extracellular domains of microvillous integrins, including tetraspans and CD47, were concentrated on microvilli as well. These findings suggest that interactions between the extracellular domains of integrins and associated proteins could direct the assembly of multimolecular complexes on leukocyte microvilli.
Leukocyte recruitment to tissues from blood involves a series of adhesive interactions between leukocytes and the vascular endothelium (Springer, 1994; Butcher and Picker, 1996). In some cases, initial binding of leukocyte adhesion molecules to their endothelial ligands can lead to the transient arrest, or tethering, of the leukocyte followed by leukocyte rolling. Rolling cells can then be “activated” via incompletely understood mechanisms, which lead to an increase in the activity of certain adhesion molecules and the arrest of the leukocyte on the lumenal surface of the endothelium. In other cases, leukocytes may arrest immediately, without rolling. After arrest, leukocytes can extravasate into the underlying tissue. Different leukocyte adhesion molecules are used for different steps in this process (von Andrian et al., 1991). L-selectin and the E- and P-selectin ligands are expressed on some leukocytes and mediate initial adhesion (tethering and rolling), but do not support firm arrest. In contrast, the leukocyte β2 integrins αLβ2 (LFA-1, CD11a/CD18) and αMβ2 (Mac-1, CD11b/CD18) mediate firm arrest but not initial adhesion. Another integrin subfamily, the α4 integrins α4β1 (VLA-4, CD49d/CD29) and α4β7 (LPAM-1), can support both initial adhesion and firm arrest (Sriramarao et al., 1994; Alon et al., 1995; Berlin et al., 1995).
Presentation of certain leukocyte adhesion molecules on microvilli substantially enhances the ability of these molecules to support tethering and rolling on endothelial ligands. The importance of receptor distribution was highlighted by studies of the adhesion molecules L-selectin and CD44 (von Andrian et al., 1995). L-selectin is located primarily on microvilli, whereas CD44 is concentrated on the planar cell body. L-selectin–CD44 chimeras were used to examine the role of cytoplasmic and transmembrane domains in receptor localization and the ability to roll on ligands. A chimera comprising the L-selectin extracellular domain fused to the CD44 transmembrane and cytoplasmic domains (L/CD44) localized to the cell body, and a CD44 extracellular, L-selectin transmembrane and cytoplasmic domain chimera (CD44/L) localized to microvilli. Although replacement of the transmembrane and cytoplasmic domains of L-selectin or CD44 did not alter their ability to adhere under static (no flow) conditions, it affected adhesion under flow. L-selectin (on microvilli) supported initial attachment better than the L/CD44 chimera (cell body), whereas CD44/L (microvilli) supported initial attachment better than CD44 (cell body). These results indicate that the transmembrane and/or cytoplasmic domains account for the differences in localization of L-selectin and CD44, and strongly suggest that microvillous localization is important for optimal initial adhesion under flow.
Available evidence about the distribution of integrins on the leukocyte surface is also consistent with a role for microvillous presentation in initial adhesion under flow. Integrins αLβ2 and αMβ2 are concentrated on the planar cell body and do not support initial adhesion, whereas α4β1 and α4β7 localize primarily to microvilli and do support tethering and rolling (Erlandsen et al., 1993; Berlin et al., 1995). The mechanism underlying the differential topography of these integrins on nonadherent leukocytes is not known. However, studies of other integrins have established a central role for the cytoplasmic domains of integrin β subunits in localization of integrins on membranes of adherent cells (LaFlamme et al., 1992, 1994; Briesewitz et al., 1993; Sastry and Horwitz, 1993; Ylanne et al., 1993; Pasqualini and Hemler, 1994). Interactions with several cytoskeletal proteins such as talin and α-actinin (demonstrated in vitro) are suggestive of links to microfilament fibers that may regulate protein localization. In addition, several transmembrane proteins, such as tetraspan proteins (including CD9, CD53, CD63, CD81, and CD82) (Slupsky et al., 1989; Rubinstein et al., 1994; Berditchevski et al., 1995, 1996, 1997), CD32 (FcγRIIA) (Worth et al., 1996), and CD47 (integrin-associated protein) (Lindberg et al., 1993), have been shown to associate with integrins. These interactions are known or suspected to involve the extracellular domains of these proteins, and their role (if any) in integrin localization is unknown.
We sought to examine the role of leukocyte integrin transmembrane and cytoplasmic domains in microvillous localization and in initial adhesion under flow. Here we report that replacement of α4β7 transmembrane and cytoplasmic domains with the homologous domains of β2 integrins does not alter membrane localization or initiation of adhesion under flow. This unexpected result suggests that differences in localization of leukocyte integrins to microvilli are determined by the extracellular domain.
Materials and Methods
Cell Lines
K562 human erythroleukemia cells (CCL 243; American Type Culture Collection, Rockville, MD) were maintained in growth medium: RPMI 1640 supplemented with 10% FBS, penicillin (50 IU/ml), streptomycin (50 μg/ml), and glutamine (2 mM). Stably transfected K562 lines expressing human integrin α4 (K562-α4β1) and α4β7 (K562-α4β7) were described previously (Tidswell et al., 1997). Additional K562 integrin transfectants were provided by other investigators: K562-αLβ2 and K562-αMβ2 (I. Graham, Washington University, St. Louis, MO) (Graham et al., 1994); K562-α6Aβ1 (A. Sonnenberg, The Netherlands Cancer Institute, Amsterdam, The Netherlands) (Hogervorst et al., 1993); K562-αvβ3 (S. Blystone, Washington University) (Blystone et al., 1994).
cDNAs
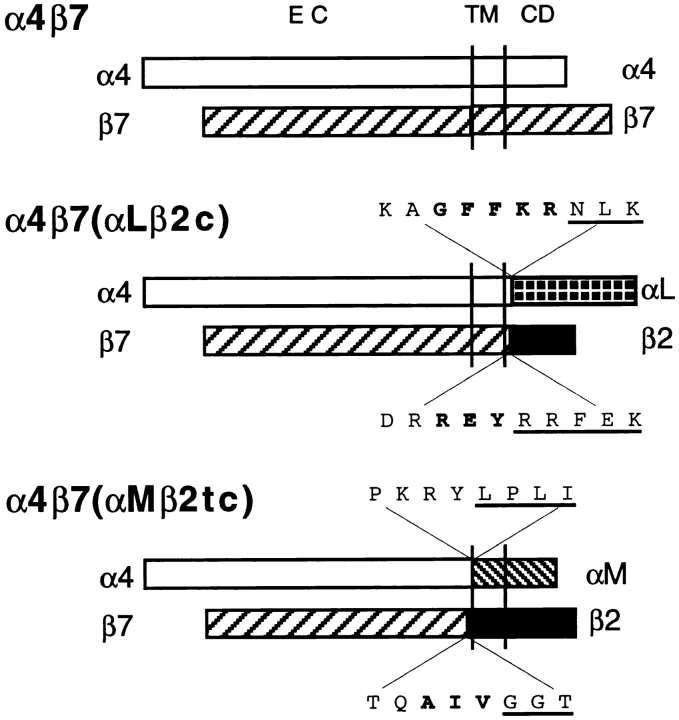
The pCDM8-integrin α4 cDNA plasmid (Kamata et al., 1995) was a gift from Y. Takada (Scripps Research Institute, La Jolla, CA). The cloning of the β7 cDNA has been previously described (Erle et al., 1991). Integrin β2 cDNA (Hickstein et al., 1988) was provided by D. Hickstein (University of Washington, Seattle, WA). Integrin αL (Larson et al., 1989) and αM (Corbi et al., 1988) cDNAs were provided by T. Springer (Center for Blood Research, Boston, MA). αE cDNA (Shaw et al., 1994) was a gift from G. Russell and M. Brenner (Harvard Medical School, Boston, MA). Chimeric integrin subunits were constructed using splice overlap extension PCR (Horton et al., 1989). The amino acid splice sites for each construct are shown in Fig. 1. Chimeric α subunits were subcloned into pCDM8 (Invitrogen, San Diego, CA) and chimeric β subunits were subcloned into pCEP4 (Invitrogen). The integrity of the constructs was confirmed by DNA sequencing.
Figure 1.
Schematic representation of wild-type and chimeric α4β7 integrins. The amino acid sequence at the splice site is shown with conserved regions in bold, and the αLβ2 or αMβ2 sequences underlined.
Transfections and Expression
K562 cells in log phase growth were washed twice in electroporation buffer (HBSS, 20 mM Hepes, 6 mM dextrose). 8 × 106 cells were resuspended in 0.2 ml buffer and transferred to 2 mm electroporation cuvettes (BTX, San Diego, CA). Stable transfections were performed using the pCDM8-α4 and pCEP-β7 constructs (25 μg each) plus 5 μg pBK-neo (Stratagene, La Jolla, CA) at 900 μFarad, 200 V, 13 Ω (Electro Cell Manipulator 600; BTX). Samples were left at room temperature for 10 min before and after electroporation. Cells were then placed in 20 ml growth medium and maintained at 37°C in a 5% CO2 incubator. After 48 h, transfectants were selected using growth medium containing 500 μg/ml each of Hygromycin B (Calbiochem-Novabiochem Corp., La Jolla, CA) and G418 (GIBCO BRL, Gaithersburg, MD). Stably transfected clones were obtained by limiting dilution and analyzed by flow cytometry as previously described (Tidswell et al., 1997). Transfectants were maintained in selection medium.
Antibodies
Fib 504 (anti-integrin β7) was a gift of E.C. Butcher (Stanford University, Palo Alto, CA) (Andrew et al., 1994). HP1/2 was used to detect the integrin α4 subunit (Pulido et al., 1991). 7E4 (anti-β2) and GoH3 (anti-α6) were purchased from Immunotech (Westbrook, ME). L230 (anti-αv) and B11G2 (anti-α5) were provided by D. Sheppard and C. Damsky (University of California, San Francisco, CA). Antibodies against the integrin-associated proteins CD53 (clone HI29; PharMingen, San Diego, CA), CD63 (MAB1787; Chemicon International, Inc., Temecula, CA), and CD32 (clone IV.3; Medarex, East Annandale, NJ) were obtained from commercial sources. The anti-CD47 antibody B6H12 was a gift from E. Brown (Washington University). Hybridoma supernatants or purified IgG preparations were used for flow cytometry analysis and immunoelectron microscopy. Gold-conjugated secondary antibodies (6- or 12-nm particles) were purchased from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA).
Immunoelectron Microscopy
Transfectants were immunolabeled in suspension. Cells were prefixed for 20 min in 0.2% paraformaldehyde/PBS at 4°C. After washing twice with PBS containing 10% goat serum, cells were incubated with primary antibody diluted in PBS containing 10% goat serum. After a 30-min incubation at 4°C and a wash with PBS, gold-conjugated secondary antibody in PBS was added. Cells were incubated for 30 min at 4°C, washed twice with PBS, and fixed in 0.1 M sodium cacodylate buffer, pH 7.4, with 3% glutaraldehyde. Before embedding, cells were rinsed in 0.1 M cacodylate buffer and postfixed in 1% osmium tetroxide/0.1 M cacodylate buffer. After rinsing, cells were dehydrated through a graded series of acetone washes, and infiltrated and embedded in Spurr's epoxy resin (Ted Pella, Inc., Redding, CA). Sections (70-nm thick) were stained with uranyl acetate and lead citrate, and examined with a CM120 Phillips electron microscope (Philips Electron Optics, Inc., Mahwah, NJ). 50–100 cells were examined and representative cells were photographed. Each experiment was repeated at least twice. Colloidal gold distribution on immunolabeled cells was determined by analysis of electron micrographs (×19,500–×40,000). Gold particles associated with cell body or microvilli were counted from 3–11 micrographs that represented different individual cells.
Static Adhesion Assay
Static adhesion assays were performed as previously described (Tidswell et al., 1997). Briefly, 21-well glass slides (Structure Probe, West Chester, PA) were coated with mucosal addressin cell adhesion molecule–1 (MAdCAM–1)1–IgG fusion protein (4 ng/well) or intercellular adhesion molecule–1 (ICAM-1)–Cκ fusion protein (0.23 μg/well) overnight at 4°C. MAdCAM-1–IgG (Tidswell et al., 1997) was a gift of M. Briskin (Leukosite Inc., Boston, MA). ICAM-1–Cκ (Piali et al., 1995) was a gift of B. Imhof (Centre Medicale Universitaire, Geneva, Switzerland). After blocking with 4% BSA for 2 h, 4 × 104 cells were resuspended in 15 μl of 10 mM Hepes, 150 mM NaCl, 1 mM CaCl2, 1 mM MgCl2, with or without 1 mM MnCl2, and added to the wells. Cells were allowed to adhere for 90 min at room temperature. After washing, cells were fixed with 3% glutaraldehyde and stained with 0.5% crystal violet. Adherent cells were counted using a microscope. Assays were performed in triplicate.
Adhesion under Flow
Capillary tubes (100-μl capacity; Drummond, Broomall, PA) were coated at 4°C overnight with 20 μl of solution containing MAdCAM-IgG (0.2 μg/ ml) or ICAM-1–Cκ (0.7 mg/ml) and blocked with 4% BSA for 2 h at 37°C. K562 transfectants were washed with HBSS containing 1 mM EDTA, and resuspended at 5 × 105 cells/ml in HBSS/10 mM Hepes containing 1 mM each of Ca2+ and Mg2+. To measure adhesion under flow, cells were perfused through the coated capillary tube using a syringe pump (74900 series; Cole-Parmer Instrument Co., Vernon Hills, IL) at a flow rate of 0.67 ml/ min. Calculated shear stress was 1.0 dyne/cm2 according to Pousille's law of dynamic shear (Berlin et al., 1995). Results were captured using a TMS microscope (Nikon, Garden City, NJ), CCD video camera (Sony, Park Ridge, NJ), and time lapse SVMS videocassette recorder (Panasonic, Secaucus, NJ). After 2 min of flow, five randomly chosen fields from the coated region were analyzed for 5 s each. All adherent cells (rolling or arrested) were counted. Cells did not adhere to areas coated with 4% BSA alone (control).
Results
Generation of Transfectants Expressing Chimeric and Mutant α4β7 Constructs
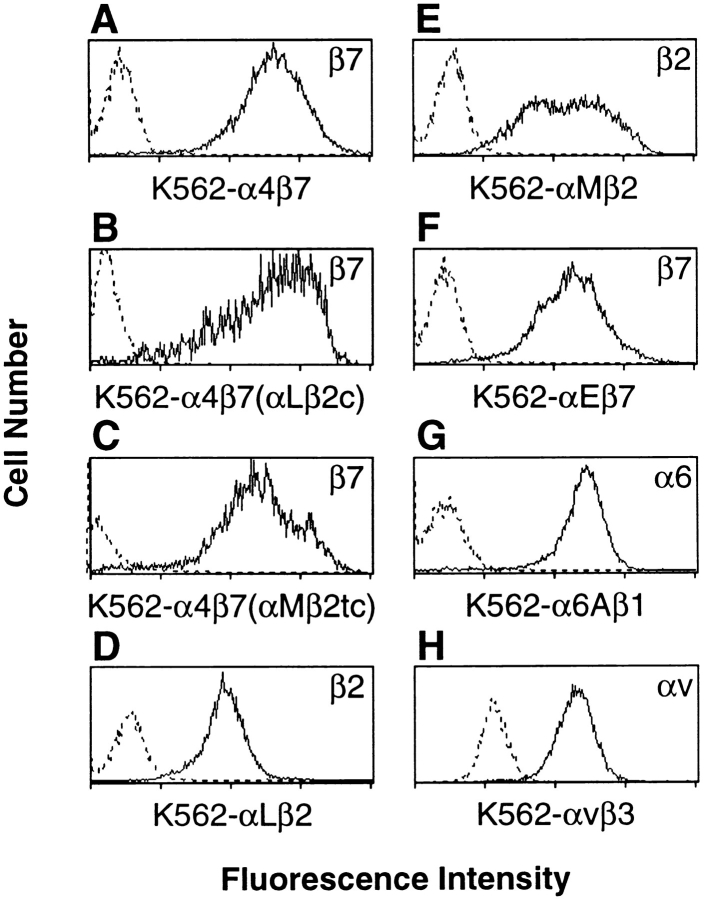
To examine the role of cytoplasmic and transmembrane domains of α4β7 in adhesion and membrane localization, we expressed chimeric integrin heterodimers (Fig. 1). Each construct included the extracellular domains of α4 and β7. In one construct, designated α4β7(αLβ2c), most of the cytoplasmic domains of α4 and β7 were replaced with homologous regions of αL and β2. In a second construct, α4β7(αMβ2tc), all of the transmembrane and cytoplasmic domains of α4 and β7 were replaced with homologous domains of αM and β2. The integrin α and β subunit cDNAs were cotransfected into K562 human erythroleukemia cells, which do not normally express α4, αL, αM, β2, or β7. The levels of protein expression on the transfectants K562-α4β7, K562-α4β7(αLβ2c), and K562-α4β7(αMβ2tc) were determined to be similar by flow cytometry (Fig. 2, A–C). Two truncated cDNAs, α4Δ (truncated after amino acids GFFKR) and β7Δ (truncated after amino acids VLAYR), were also produced. The α4Δ was expressed in combination with β7 on transfected K562 cells (K562-α4Δβ7), although at levels somewhat below those seen with other constructs (data not shown). We were unable to detect expression of β7Δ on cells cotransfected with α4, despite a previous report that the homologous truncation mutant of mouse β7 was expressed on transfected cells (Crowe et al., 1994). We were able to document heterologous expression of other wild-type integrins, including αLβ2, αMβ2, α6Aβ1, αvβ3, and αEβ7, on appropriate K562 transfectants by flow cytometry (Fig. 2, D–H).
Figure 2.
Cell surface expression of wild-type and chimeric integrins. K562-α4β7 (A), K562-α4β7 (αLβ2c) (B), and K562-α4β7 (αMβ2tc) (C) cells were stained with the anti-β7 antibody, Fib 504, as shown. Each of these three transfectants was also recognized by other antibodies specific for the α4 subunit or the α4β7 heterodimer, but were not recognized by anti-αE antibodies (not shown). K562-αLβ2 were recognized by antibodies to β2 (D) and αL (not shown). K562-αMβ2 cells were recognized by antibodies to β2 (E) and αM (not shown). K562-αEβ7 cells stained with antibodies to β7 (F) and αE, but not with anti-α4 antibodies (not shown). K562-α6Aβ1 cells were stained with the anti-α6 antibody GoH3 (G). There was low level expression of αv on nontransfected K562 cells, and higher expression on K562-αvβ3 cells as determined using the anti-αv antibody L230 (H). Fluorescence intensity is shown on a log scale (one log per division). Dotted and solid histograms represent staining with nontransfected and transfected K562 cells, respectively.
Adhesion of Transfectants to MAdCAM-1 under Static Conditions
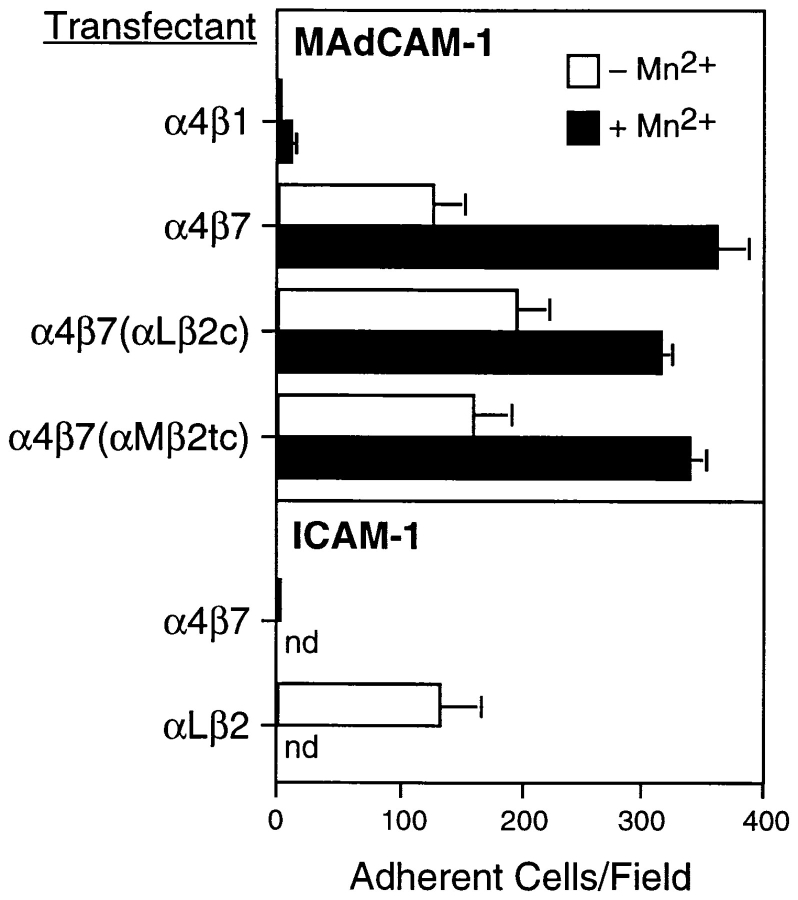
We analyzed the ability of integrin-transfected K562 cells to adhere to MAdCAM-1, an α4β7 ligand, and ICAM-1, an αLβ2 ligand, under static (no flow) conditions (Fig. 3). After transfection with α4 cDNA alone, K562 cells express α4β1 but not α4β7 (Tidswell et al., 1997). These cells failed to adhere to MAdCAM-1. In contrast, cells transfected with both α4 and β7 (K562-α4β7) adhered efficiently to MAdCAM-1. As previously reported, adhesion was increased in the presence of Mn2+. The chimeric transfectants, K562-α4β7(αLβ2c) and K562-α4β7(αMβ2tc), also adhered to MAdCAM-1, and the extent of adhesion was very similar for chimeric and wild-type α4β7 transfectants. As expected, K562-α4β7 cells did not adhere to ICAM-1, whereas K562-αLβ2 cells did.
Figure 3.
Static adhesion of transfectants to immobilized ligands. Adhesion of various integrin transfectants to MAdCAM-1 (top) and ICAM-1 (bottom) was measured in the presence and absence of Mn2+. Bars indicate SEM. nd, not determined.
Adhesion of Transfectants to MAdCAM-1 under Flow
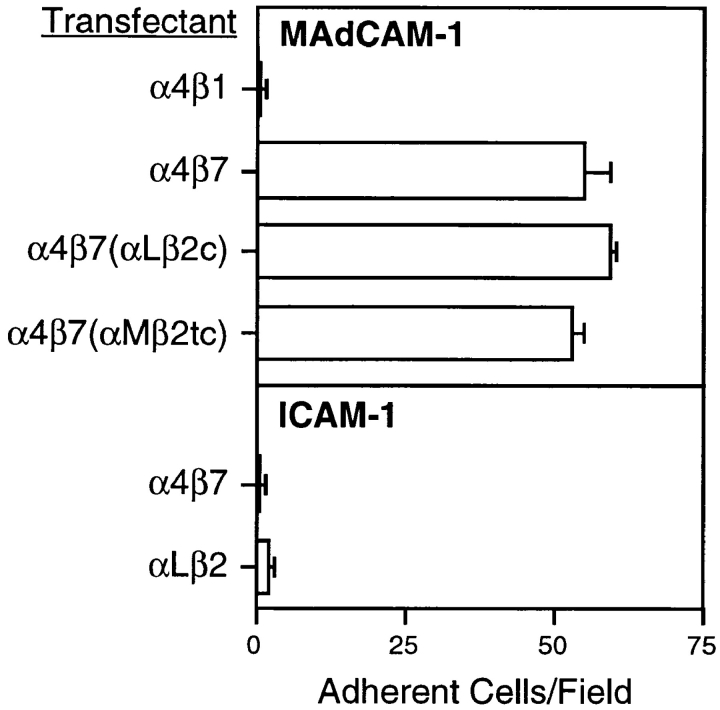
We next examined the ability of the wild-type and chimeric integrins to initiate adhesion under flow (Fig. 4). K562-α4β7 cells adhered to MAdCAM-1 under flow. This adhesion was dependent upon both α4β7 and MAdCAM-1 because K562 cells transfected with α4 alone (K562-α4β1) did not adhere to MAdCAM-1, and K562-α4β7 cells did not adhere to capillary tubes coated with other ligands, such as ICAM-1. The chimeric integrins, α4β7(αLβ2c) and α4β7 (αMβ2tc), both supported adhesion to MAdCAM-1 under flow. Wild-type α4β7 and the chimeric integrins were very similar in their ability to initiate adhesion under flow. We also examined the resistance to detachment from MAdCAM-1 at increasing shear stress conditions (up to 10 dynes/cm2). We did not find differences between wild-type and chimeric transfectants in this assay (data not shown). At a shear stress of 1 dyne/cm2, most cells remained adherent during the time interval of cell counts. Transfectants expressing a truncated α4 subunit (K562-α4Δβ7) also adhered to MAdCAM-1 under flow, although at a somewhat lower rate (perhaps related to lower levels of expression of this construct, data not shown). As expected from previous reports (von Andrian et al., 1991), K562-αLβ2 cells were unable to initiate adhesion to their ligand, ICAM-1, under flow (Fig. 4).
Figure 4.
Adhesion to ligands under flow. Adhesion of various integrin transfectants to MAdCAM-1 (top) and ICAM-1 (bottom) measured at a wall shear stress of 1 dyne/cm2 in the absence of Mn2+, as described in Materials and Methods. Bars indicate SEM.
Membrane Localization of Wild-type and Chimeric α4β7, αLβ2, and αMβ2 Integrins on Transfected K562 Cells
Previous reports demonstrated that integrin α4β7 localizes primarily to microvilli of mouse TK-1 lymphoma cells, whereas integrins αLβ2 and αMβ2 localize primarily to the cell body of TK-1 cells and human neutrophils respectively (Erlandsen et al., 1993; Berlin et al., 1995). We began by using immunoelectron microscopy to determine whether these integrins would localize similarly in transfected K562 cells. K562 cells had numerous microvillous projections. α4β7 was found primarily on microvilli (Fig. 5 A). In contrast, αLβ2 and αMβ2 integrins were located primarily on the cell body (Fig. 5, B–D). Both α4β7(αLβ2c) and α4β7(αMβ2tc) localized to microvillous projections (Fig. 5, E and F). A quantitative analysis of the distributions of these integrins is shown in Table I. The α4Δβ7 construct was also concentrated on microvilli (not shown). These results indicate that the transmembrane and cytoplasmic domains are not responsible for the differential localization of α4β7 versus αLβ2 and αMβ2.
Figure 5.
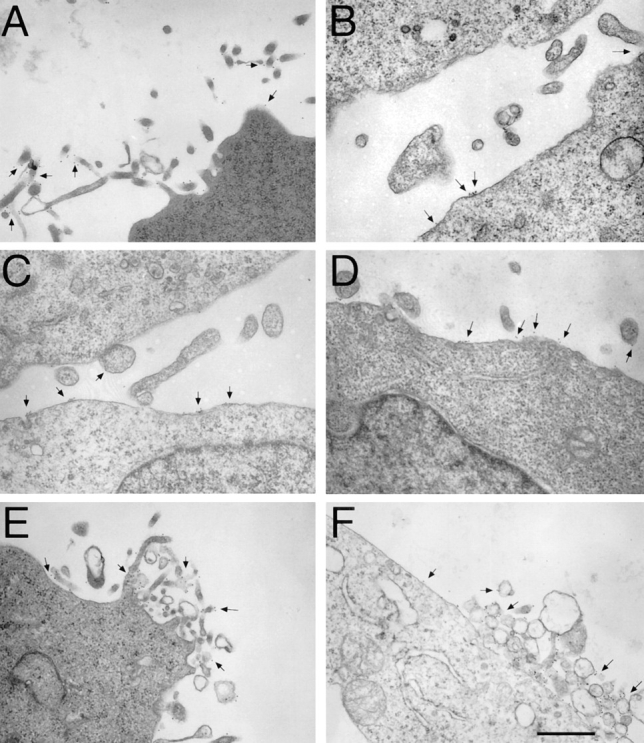
Localization of α4β7, αLβ2, αMβ2, and chimeric integrins by immunoelectron microscopy. K562 transfectants were stained for protein expression using 12-nm gold particles (arrows) as described in Materials and Methods. Wild-type α4β7 (identified using the anti-β7 antibody, Fib 504) was localized predominantly to microvilli of K562-α4β7 tranfectants (A). K562-αLβ2 (B and C) and K562-αMβ2 (D) were stained with antibodies to αL (B) or β2 (C and D), demonstrating that αLβ2 and αMβ2 were expressed mostly on the cell body. K562 cells transfected with the chimeric integrins α4β7(αLβ2c) (E) and α4β7(αMβ2tc) (F) were stained with Fib 504. These chimeric integrins were found predominantly on microvilli. Photomicrographs are representative of integrin distribution on the 50–100 cells examined in each sample. Bar, 0.5 μm.
Table I.
Integrin Distribution on Transfected K562 Cells
| Integrin | Percent on microvilli | Percent on body | Gold particles counted | |||
|---|---|---|---|---|---|---|
| α4β7 | 89 ± 9 | 11 ± 9 | 1,288 | |||
| α4β1 | 81 ± 7 | 19 ± 7 | 348 | |||
| αLβ2 | 31 ± 14 | 69 ± 14 | 99 | |||
| αMβ2 | 22 ± 13 | 78 ± 13 | 236 | |||
| α4β7(αLβ2c) | 90 ± 9 | 10 ± 9 | 260 | |||
| α4β7(αMβ2tc) | 87 ± 6 | 13 ± 6 | 386 | |||
| αEβ7 | 83 ± 6 | 17 ± 6 | 149 | |||
| α6β1 | 77 ± 9 | 23 ± 9 | 401 | |||
| αvβ3 | 80 ± 10 | 20 ± 10 | 2,741 |
Transfected K562 cells expressing various integrins were analyzed by immunoelectron microscopy after staining with appropriate anti-integrin antibodies. For each integrin, all gold particles in at least three photomicrographs were counted. Percentages are presented as mean ± SD.
Presentation of Other Leukocyte Integrins on Microvilli
In addition to α4β7, αLβ2, and αMβ2, leukocytes express other integrins that play roles in adhesion to endothelial cells, to other cells, and to extracellular matrix proteins. One of these, α4β1, can initiate adhesion to vascular cell adhesion molecule–1 (VCAM-1) under flow and has been reported to be expressed on lymphocyte microvilli (Alon et al., 1995; Berlin et al., 1995). We confirmed that α4β1 was also expressed preferentially on microvilli of K562-α4β1 transfectants (Table I). We found that the T cell integrin αEβ7 (Cepek et al., 1994), which can mediate adhesion to epithelium but has no established role in endothelial adhesion, was also localized to microvilli of K562-αEβ7 cells (Fig. 6 A, and Table I). Integrin α6Aβ1, a laminin receptor which is expressed on monocytes and some lymphocytes, was concentrated on microvilli of K562 transfectants (Fig. 6 B, and Table I). The vitronectin receptor, integrin αvβ3, is also a receptor for the endothelial cell ligand platelet/endothelial cell adhesion molecule–1 (Piali et al., 1995) and is expressed on monocytes and other cells. We found that αvβ3 localized predominantly to microvilli (Fig. 6 C, and Table I). The fibronectin receptor, integrin α5β1, is constitutively expressed on K562 cells and was also expressed predominantly on microvilli (not shown).
Figure 6.
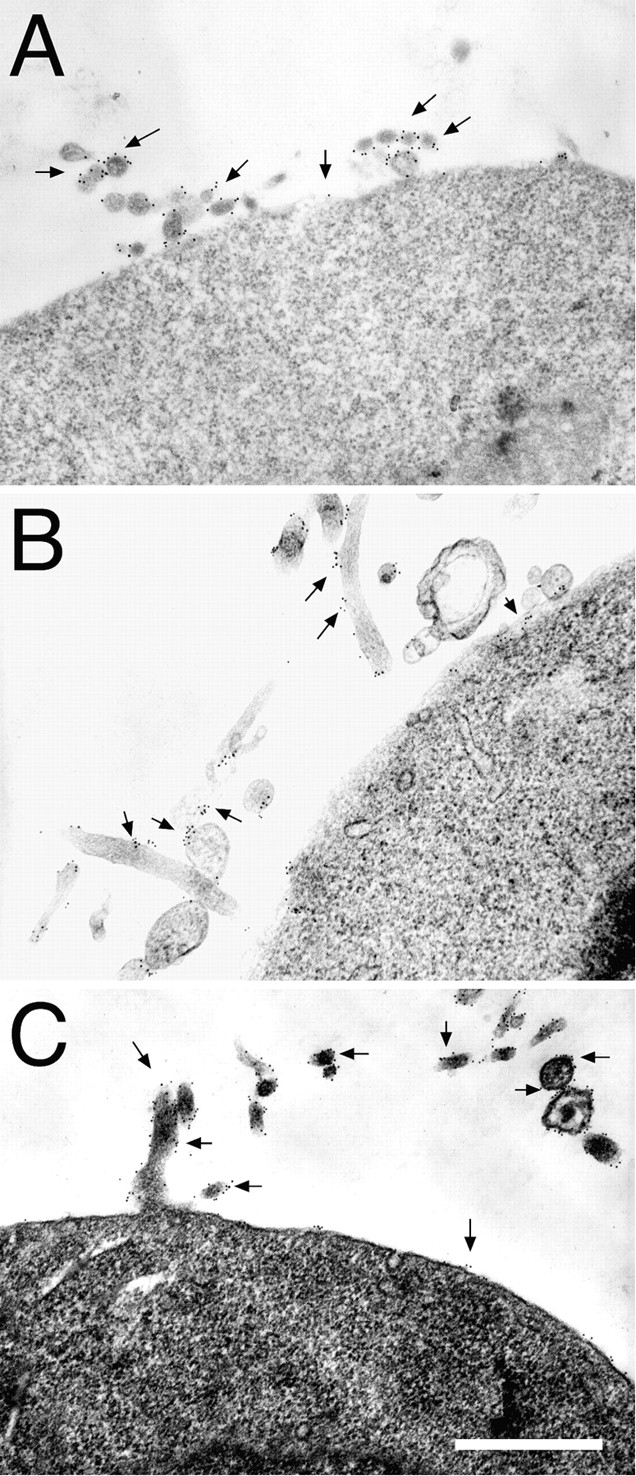
Localization of αEβ7, α6Aβ1, and αvβ3 integrins by immunoelectron microscopy. K562 transfectants were stained for protein expression using 12-nm gold particles (arrows) as described in Materials and Methods. Staining of K562-αEβ7 with Fib 504 (anti-β7, A), K562-α6Aβ1 with anti-α6 (GoH3, B), and K562-αvβ3 with L230 (anti-αv, C) revealed that each of these integrins was found primarily on microvilli. Bar, 0.5 μm.
Localization of Transmembrane Proteins Known to Associate with Integrins
Integrins have been shown to associate with a variety of intracellular and transmembrane proteins. Some of these interactions occur within the cell and are mediated by integrin cytoplasmic domains, whereas others are known or suspected to be extracellular. Since our results suggested that extracellular (and not transmembrane or cytoplasmic) domains determine integrin localization, we performed immunoelectron microscopy to localize several cell surface proteins known or suspected to interact with integrin extracellular domains. Several members of the tetraspan family of transmembrane proteins have been shown to associate with α4β1, α4β7, α6β1, and some other integrins (Berditchevski et al., 1996; Mannion et al., 1996). These associations are likely to involve the extracellular domains of tetraspans and integrins (see Discussion). Several tetraspans are constitutively expressed on K562 cells. CD53 (82 ± 5% on microvilli, Fig. 7 A), CD63 (92 ± 4% on microvilli, Fig. 7 B), and CD81 and CD82 (data not shown) are all localized predominantly to microvilli. Another transmembrane protein, CD47 (integrin-associated protein), has been shown to associate with αvβ3 via its extracellular domain. CD47, like αvβ3, was distributed mostly on microvilli (Fig. 7 C). CD32 is a transmembrane protein that has been reported to associate with αMβ2 (which is on the cell body; Fig. 5 C) and possibly with the tetraspan CD82 (found on microvilli, see above) (Lebel-Binay et al., 1995). CD32 was expressed on both microvilli (62 ± 15%) and the cell body (38 ± 15%) (Fig. 7 D).
Figure 7.
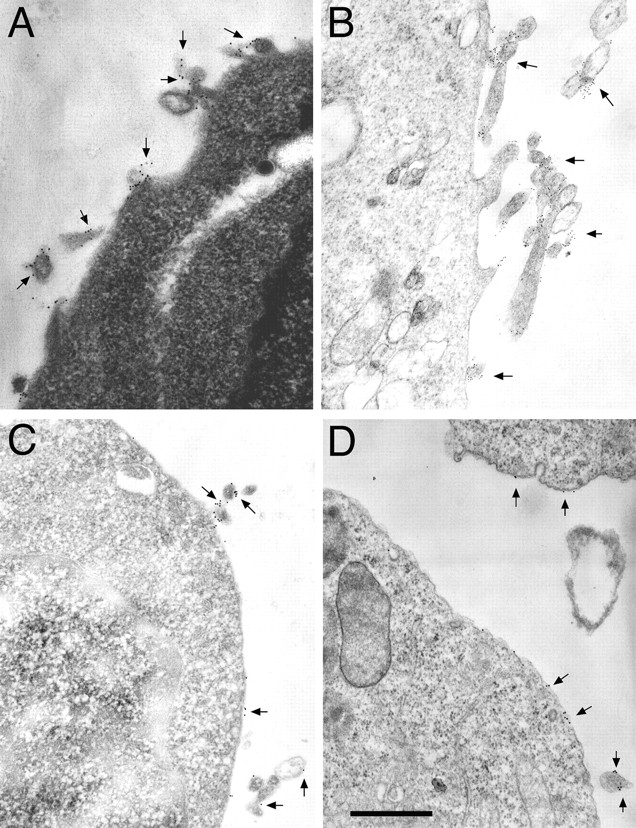
Localization of integrin-associated proteins by immunoelectron microscopy. The tetraspan proteins CD53 (A) and CD63 (B), the αvβ3-associated protein CD47 (C), and CD32 (D) were localized by immunoelectron microscopy using 12-nm (A, C, and D) or 6-nm (B) gold particles (arrows). The tetraspan proteins and CD47 localized primarily to microvilli, whereas CD32 was found in substantial amounts on both the cell body and microvilli. Nontransfected K562 cells (A) and K562-α4β7 (B), K562-αVβ3 (C), and K562-αMβ2 (D) transfectants were used for staining. Bar, 0.5 μm.
Discussion
In this study, we examined the role of leukocyte integrin cytoplasmic and transmembrane domains in adhesion under flow and microvillous localization. We began by confirming that previously described differences in leukocyte integrin adhesive activity and membrane localization were also seen in K562 cell integrin transfectants. As expected, α4β7 was able to initiate adhesion under flow and localized to microvilli, whereas αLβ2 and αMβ2 mediated adhesion only under static conditions and localized to the planar cell body. Two chimeras, α4β7(αLβ2c) and α4β7(αMβ2tc), were expressed to examine the roles of the extracellular, transmembrane, and cytoplasmic domains in adhesion and membrane localization. Both chimeras were as efficient in initiating adhesion under flow as the wild-type integrin α4β7. The chimeras were found predominantly on microvilli, indicating that the extracellular domain (and not the transmembrane or cytoplasmic domains) determined membrane localization of α4β7. We have so far been unable to determine whether β2 integrin localization to the cell body is also independent of the transmembrane and cytoplasmic domains. We attempted to address this issue by expressing a chimeric integrin composed of the extracellular domain of αMβ2 and the transmembrane and cytoplasmic domains of α4β7, but have not yet been successful in these experiments. In addition to α4β7, several other integrins (α4β1, α5β1, α6Aβ1, αvβ3, and αEβ7) were localized to microvilli. Transmembrane proteins known or suspected to interact with integrin extracellular domains, including tetraspan family members and CD47, were also found to be concentrated on microvilli.
The ability of leukocyte adhesion molecules to support initial adhesion under flow is influenced by several factors including affinity, avidity, and accessibility to endothelial ligands. Previous studies of nonintegrin adhesion molecules indicate that cytoplasmic domains can have dramatic effects upon initiating adhesion under flow without altering static adhesion. Experiments involving the use of L-selectin–CD44 chimeras suggest that cytoplasmic domains may influence adhesion under flow by targeting these receptors to the microvillous or cell body (see Introduction). However, it is clear that the cytoplasmic domain of L-selectin also has effects on initiation of adhesion that are independent of receptor positioning. A truncation mutant of L-selectin lacking the 11 COOH-terminal amino acid residues of the cytoplasmic domain was localized to microvilli and retained the ability to bind ligand, but was unable to support rolling on endothelium (Kansas et al., 1993; Pavalko et al., 1995). This mutant lost the ability to associate with the cytoskeletal proteins α-actinin and vinculin, suggesting that interactions between adhesion molecule cytoplasmic domains and cytoskeletal proteins can be important in regulation of initial adhesive interactions under flow. We found that replacement of the cytoplasmic and/or transmembrane domains of α4β7 (which does support adhesion under flow) with the homologous domains of αLβ2 or αMβ2 (which do not) did not affect adhesion to the α4β7 ligand MAdCAM-1 under either static or flow conditions. This indicates that the cytoplasmic domains cannot account for the differences in ability of these integrins to support adhesion under flow. We also found that truncation of the α4 subunit after the conserved GFFKR motif had little if any effect on initiation of α4β7-mediated adhesion to MAdCAM-1. Others have previously shown that the same truncation of α4 did not affect the ability of α4β1 to initiate adhesion to its ligand, VCAM-1 (Kassner et al., 1995). The α4 truncation was reported to decrease the cell's resistance to detachment from VCAM-1 in the face of increasing shear force, suggesting that the α subunit cytoplasmic domain plays a role in strengthening adhesion. We were unable to detect any difference in resistance to detachment between wild-type and chimeric α4β7 integrins, suggesting that α4β7, αLβ2, and αMβ2 integrin cytoplasmic domains are similar in their ability to mediate adhesion strengthening.
Many of the adhesion molecules that initiate adhesion to endothelium under flow are concentrated on leukocyte microvilli.These include L-selectin (Picker et al., 1991; Erlandsen et al., 1993; Pavalko et al., 1995), P-selectin glycoprotein ligand–1 (Moore et al., 1995; Bruehl et al., 1997), and the integrins α4β7 and α4β1 (Berlin et al., 1995; and this report). Other adhesion molecules, including CD44 and the integrins αLβ2 and αMβ2, are found predominantly on the cell body (Erlandsen et al., 1993; Berlin et al., 1995; von Andrian et al., 1995; and this report). Little information is available about the mechanisms that lead to the selective display of certain adhesion molecules on microvilli. It seems likely that interactions between the cytoplasmic domains of adhesion molecules and specific cytoskeletal elements can play an important role. In support of this concept, the localization of L-selectin–CD44 chimeras was shown to be determined by the cytoplasmic and/or transmembrane domains, and not by the extracellular domains (see Introduction). Concentration of integrins in other structures, such as focal adhesions and hemidesmosomes, is known to depend upon the β subunit cytoplasmic domain. We were surprised to find that our analysis of integrin chimeras did not demonstrate a role for the cytoplasmic or transmembrane domains of either the α or β subunit in determining membrane localization. Replacement of the α4β7 cytoplasmic and/or transmembrane domains with homologous domains of αLβ2 or αMβ2 did not interfere with microvillous localization. Put another way, replacement of the extracellular domain of the αLβ2 or αMβ2 integrins with the extracellular domain of α4β7 resulted in a shift from cell body to microvillous localization. These results indicate an important role for the extracellular domain in directing localization of integrins to the microvillous versus the cell body.
Integrin extracellular domains can interact with other cell surface proteins. For example, the transmembrane protein CD47 interacts with αvβ3 and this interaction depends upon the extracellular domain of CD47 (Lindberg et al., 1996). Several members of the tetraspan family of transmembrane proteins, including CD53, CD63, CD81, and CD82, have been shown to coprecipitate with some integrins, including α4β1, α6β1, and α4β7, but not with αLβ2 or some other integrins (Berditchevski et al., 1996; Mannion et al., 1996). These interactions are likely to involve the integrin extracellular domain, since mutations of the α4 subunit extracellular domain substantially reduce association whereas alterations of the α subunit cytoplasmic domain have no effect. We found that CD47, CD53, CD63, CD81, and CD82, all known to associate with integrins that we localized to microvilli, were themselves concentrated on microvilli. Our data are consistent with the hypothesis that interactions with tetraspans and CD47 help target certain integrins to microvilli. The widespread expression of tetraspans and CD47 on leukocytes and other cells makes this hypothesis difficult to test directly. At least one integrin that we localized to microvilli, α5β1, apparently does not associate with tetraspans or CD47, suggesting that other interactions also are important (Berditchevski et al., 1996). This hypothesis assumes that α4β7 and other microvillous integrins are actively concentrated on microvilli, but αLβ2 and αMβ2 are not. An alternative explanation of our results is that microvillous expression is the “default pathway” for integrins, and that the extracellular domains of αLβ2 and αMβ2 prevent these integrins from being displayed on microvilli. This could be mediated by interactions between β2 integrins and associated cell surface proteins. Although αMβ2 has been shown to associate with CD32, the pattern of expression of CD32 (on both cell body and microvilli) suggests that this interaction is not responsible for the concentration of αMβ2 on the cell body.
We found that many integrins and integrin-associated proteins were preferentially expressed on microvilli. Some of these integrins, including α4β7 and α4β1, play important roles in mediating leukocyte adhesion under flow. Other microvillous integrins, such as α5β1 and αEβ7, mediate adhesion to extracellular matrix proteins or epithelial cells, but have no known role in initiating leukocyte– endothelial interactions. This suggests that the assembly of multimolecular complexes containing integrins and integrin-associated proteins on microvilli may have other important roles in adhesion and signaling.
Acknowledgments
We thank K.L. McDonald and P. Sicurello (Robert D. Ogg Electron Microscope Laboratory, University of California, Berkeley, CA) and G. Antipa and G. Lum (San Francisco State University, San Francisco, CA) for their expert advice and courtesy in allowing us to use their electron microscope facilities. We thank S. Wu for optimizing the static adhesion assay protocol and are grateful to R. Pytela and D. Sheppard for critically reviewing this manuscript.
This study was supported by National Institutes of Health grants HL50024 (to D.J. Erle) and HL03230 (to M. Tidswell). M. Abi Abitorabi was supported by National Institutes of Health training grant HL07155 and National Research Service Award 1F32HL09364.
Abbreviations used in this paper
- ICAM-1
intercellular adhesion molecule–1
- MAdCAM-1
mucosal addressin cell adhesion molecule–1
- VCAM-1
vascular cell adhesion molecule–1
Footnotes
Address all correspondence to M. Abi Abitorabi, University of California, San Francisco, Box 0854, San Francisco, CA 94143-0854. Tel.: (415) 206-6649. Fax: (415) 206-4123.
References
- Alon R, Kassner PD, Carr MW, Finger EB, Hemler ME, Springer TA. The integrin VLA-4 supports tethering and rolling in flow on VCAM-1. J Cell Biol. 1995;128:1243–1253. doi: 10.1083/jcb.128.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew DP, Berlin C, Honda S, Yoshino T, Hamann A, Holzmann B, Kilshaw PJ, Butcher EC. Distinct but overlapping epitopes are involved in α4β7-mediated adhesion to vascular cell adhesion molecule-1, mucosal addressin-1, fibronectin, and lymphocyte aggregation. J Immunol. 1994;153:3847–3861. [PubMed] [Google Scholar]
- Berditchevski F, Bazzoni G, Hemler ME. Specific association of CD63 with the VLA-3 and VLA-6 integrins. J Biol Chem. 1995;270:17784–17790. doi: 10.1074/jbc.270.30.17784. [DOI] [PubMed] [Google Scholar]
- Berditchevski F, Zutter MM, Hemler ME. Characterization of novel complexes on the cell surface between integrins and proteins with 4 transmembrane domains (TM4 proteins) Mol Biol Cell. 1996;7:193–207. doi: 10.1091/mbc.7.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berditchevski F, Tolias KF, Wong K, Carpenter CL, Hemler ME. A novel link between integrins, transmembrane-4 superfamily proteins (CD63 and CD81), and phosphatidylinositol 4-kinase. J Biol Chem. 1997;272:2595–2598. doi: 10.1074/jbc.272.5.2595. [DOI] [PubMed] [Google Scholar]
- Berlin C, Bargatze RF, Campbell JJ, von Andrian UH, Szabo MC, Hasslen SR, Nelson RD, Berg EL, Erlandsen SL, Butcher EC. α4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell. 1995;80:413–422. doi: 10.1016/0092-8674(95)90491-3. [DOI] [PubMed] [Google Scholar]
- Blystone SD, Graham IL, Lindberg FP, Brown EJ. Integrin αvβ3 differentially regulates adhesive and phagocytic functions of the fibronectin receptor α5β1. J Cell Biol. 1994;127:1129–1137. doi: 10.1083/jcb.127.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briesewitz R, Kern A, Marcantonio EE. Ligand-dependent and -independent integrin focal contact localization: the role of the α chain cytoplasmic domain. Mol Biol Cell. 1993;4:593–604. doi: 10.1091/mbc.4.6.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruehl RE, Moore KL, Lorant DE, Borregaard N, Zimmerman GA, McEver RP, Bainton DF. Leukocyte activation induces surface redistribution of P-selectin glycoprotein ligand-1. J Leukocyte Biol. 1997;61:489–499. doi: 10.1002/jlb.61.4.489. [DOI] [PubMed] [Google Scholar]
- Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science (Wash DC) 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- Cepek KL, Shaw SK, Parker CM, Russell GJ, Morrow JS, Rimm DL, Brenner MB. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the αEβ7 integrin. Nature (Lond) 1994;372:190–193. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- Corbi AL, Kishimoto TK, Miller LJ, Springer TA. The human leukocyte adhesion glycoprotein Mac-1 (complement receptor type 3, CD11b) α subunit. Cloning, primary structure, and relation to the integrins, von Willebrand factor and factor B. J Biol Chem. 1988;263:12403–12411. [PubMed] [Google Scholar]
- Crowe DT, Chiu H, Fong S, Weissman IL. Regulation of the avidity of integrin α4β7 by the β7 cytoplasmic domain. J Biol Chem. 1994;269:14411–14418. [PubMed] [Google Scholar]
- Erlandsen SL, Hasslen SR, Nelson RD. Detection and spatial distribution of the β2 integrin (Mac-1) and L-selectin (LECAM-1) adherence receptors on human neutrophils by high-resolution field emission SEM. J Histochem Cytochem. 1993;41:327–333. doi: 10.1177/41.3.7679125. [DOI] [PubMed] [Google Scholar]
- Erle DJ, Ruegg C, Sheppard D, Pytela R. Complete amino acid sequence of an integrin β subunit (β7) identified in leukocytes. J Biol Chem. 1991;266:11009–11016. [PubMed] [Google Scholar]
- Graham IL, Anderson DC, Holers VM, Brown EJ. Complement receptor 3 (CR3, Mac-1, integrin αMβ2, CD11b/CD18) is required for tyrosine phosphorylation of paxillin in adherent and nonadherent neutrophils. J Cell Biol. 1994;127:1139–1147. doi: 10.1083/jcb.127.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickstein DD, Howard M, Meuller L, Hickey MJ, Collins SJ. Expression of the β-subunit of the human leukocyte adherence receptor depends upon cell type and stage of differentiation. J Immunol. 1988;141:4313–4317. [PubMed] [Google Scholar]
- Hogervorst F, Kuikman I, Noteboom E, Sonnenberg A. The role of phosphorylation in activation of the α6Aβ1 laminin receptor. J Biol Chem. 1993;268:18427–18430. [PubMed] [Google Scholar]
- Horton RM, Hunt HD, Ho SN, Pullen JK, Pease LR. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene (Amst) 1989;77:61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- Kamata T, Puzon W, Takada Y. Identification of putative ligand-binding sites of the integrin α4β1 (VLA-4, CD49d/CD29) Biochem J. 1995;305:945–951. doi: 10.1042/bj3050945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansas GS, Ley K, Munro JM, Tedder TF. Regulation of leukocyte rolling and adhesion to high endothelial venules through the cytoplasmic domain of L-selectin. J Exp Med. 1993;177:833–838. doi: 10.1084/jem.177.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassner PD, Alon R, Springer TA, Hemler ME. Specialized functional properties of the integrin α4 cytoplasmic domain. Mol Biol Cell. 1995;6:661–674. doi: 10.1091/mbc.6.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFlamme SE, Akiyama SK, Yamada KM. Regulation of fibronectin receptor distribution. J Cell Biol. 1992;117:437–447. doi: 10.1083/jcb.117.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFlamme SE, Thomas LA, Yamada SS, Yamada KM. Single subunit chimeric integrins as mimics and inhibitors of endogenous integrin functions in receptor localization, cell spreading and migration, and matrix assembly. J Cell Biol. 1994;126:1287–1298. doi: 10.1083/jcb.126.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson RS, Corbi AL, Berman L, Springer T. Primary structure of the leukocyte function-associated molecule-1 α subunit: an integrin with an embedded domain defining a protein superfamily. J Cell Biol. 1989;108:703–712. doi: 10.1083/jcb.108.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel-Binay S, Lagaudriere C, Fradelizi D, Conjeaud H. CD82, tetra-span-transmembrane protein, is a regulated transducing molecule on U937 monocytic cell line. J Leukocyte Biol. 1995;57:956–963. doi: 10.1002/jlb.57.6.956. [DOI] [PubMed] [Google Scholar]
- Lindberg FP, Gresham HD, Schwarz E, Brown EJ. Molecular cloning of integrin-associated protein: an immunoglobulin family member with multiple membrane-spanning domains implicated in αvβ3-dependent ligand binding. J Cell Biol. 1993;123:485–496. doi: 10.1083/jcb.123.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg FP, Gresham HD, Reinhold MI, Brown EJ. Integrin-associated protein immunoglobulin domain is necessary for efficient vitronectin bead binding. J Cell Biol. 1996;134:1313–1322. doi: 10.1083/jcb.134.5.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannion BA, Berditchevski F, Kraeft SK, Chen LB, Hemler ME. Transmembrane-4 superfamily proteins CD81 (TAPA-1), CD82, CD63, and CD53 specifically associated with integrin α4β1 (CD49d/CD29) J Immunol. 1996;157:2039–2047. [PubMed] [Google Scholar]
- Moore KL, Patel KD, Bruehl RE, Li F, Johnson DA, Lichenstein HS, Cummings RD, Bainton DF, McEver RP. P-selectin glycoprotein ligand-1 mediates rolling of human neutrophils on P-selectin. J Cell Biol. 1995;128:661–671. doi: 10.1083/jcb.128.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasqualini R, Hemler ME. Contrasting roles for integrin β1 and β5 cytoplasmic domains in subcellular localization, cell proliferation, and cell migration. J Cell Biol. 1994;125:447–460. doi: 10.1083/jcb.125.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavalko FM, Walker DM, Graham L, Goheen M, Doerschuk CM, Kansas GS. The cytoplasmic domain of L-selectin interacts with cytoskeletal proteins via α-actinin: receptor positioning in microvilli does not require interaction with α-actinin. J Cell Biol. 1995;129:1155–1164. doi: 10.1083/jcb.129.4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piali L, Hammel P, Uherek C, Bachmann F, Gisler RH, Dunon D, Imhof BA. CD31/PECAM-1 is a ligand for αvβ3 integrin involved in adhesion of leukocytes to endothelium. J Cell Biol. 1995;130:451–460. doi: 10.1083/jcb.130.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picker LJ, Warnock RA, Burns AR, Doerschuk CM, Berg EL, Butcher EC. The neutrophil selectin LECAM-1 presents carbohydrate ligands to the vascular selectins ELAM-1 and GMP-140. Cell. 1991;66:921–933. doi: 10.1016/0092-8674(91)90438-5. [DOI] [PubMed] [Google Scholar]
- Pulido R, Elices MJ, Campanero MR, Osborn L, Schiffer S, Garcia-Pardo A, Lobb R, Hemler ME, Sanchez-Madrid F. Functional evidence for three distinct and independently inhibitable adhesion activities mediated by the human integrin VLA-4. Correlation with distinct α4 epitopes. J Biol Chem. 1991;266:10241–10245. [PubMed] [Google Scholar]
- Rubinstein E, Le Naour F, Billard M, Prenant M, Boucheix C. CD9 antigen is an accessory subunit of the VLA integrin complexes. Eur J Immunol. 1994;24:3005–3013. doi: 10.1002/eji.1830241213. [DOI] [PubMed] [Google Scholar]
- Sastry SK, Horwitz AF. Integrin cytoplasmic domains: mediators of cytoskeletal linkages and extra- and intracellular initiated transmembrane signaling. Curr Opin Cell Biol. 1993;5:819–931. doi: 10.1016/0955-0674(93)90031-k. [DOI] [PubMed] [Google Scholar]
- Shaw SK, Cepek KL, Murphy EA, Russell GJ, Brenner MB, Parker CM. Molecular cloning of the human mucosal lymphocyte integrin αE subunit. Unusual structure and restricted RNA distribution. J Biol Chem. 1994;269:6016–6025. [PubMed] [Google Scholar]
- Slupsky JR, Seehafer JG, Tang SC, Masellis-Smith A, Shaw AR. Evidence that monoclonal antibodies against CD9 antigen induce specific association between CD9 and the platelet glycoprotein IIb-IIIa complex. J Biol Chem. 1989;264:12289–12293. [PubMed] [Google Scholar]
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Sriramarao P, von Andrian UH, Butcher EC, Bourdon MA, Broide DH. L-selectin and very late antigen-4 integrin promote eosinophil rolling at physiological shear rates in vivo. J Immunol. 1994;153:4238–4246. [PubMed] [Google Scholar]
- Tidswell M, Pachynski R, Wu SW, Qiu S-Q, Dunham E, Cochran N, Briskin MJ, Kilshaw PJ, Lazarovits AI, Andrew DP, et al. Structure-function analysis of the integrin β7 subunit: identification of domains involved in adhesion to MAdCAM-1. J Immunol. 1997;59:1497–1505. [PubMed] [Google Scholar]
- von Andrian UH, Chambers JD, McEvoy LM, Bargatze RF, Arfors KE, Butcher EC. Two-step model of leukocyte-endothelial cell interaction in inflammation: distinct roles for LECAM-1 and the leukocyte β2 integrins in vivo. Proc Natl Acad Sci USA. 1991;88:7538–7542. doi: 10.1073/pnas.88.17.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Andrian UH, Hasslen SR, Nelson RD, Erlandsen SL, Butcher EC. A central role for microvillous receptor presentation in leukocyte adhesion under flow. Cell. 1995;82:989–999. doi: 10.1016/0092-8674(95)90278-3. [DOI] [PubMed] [Google Scholar]
- Worth RG, Mayo-Bond L, van de Winkel JG, Todd RF, 3rd, Petty HR. CR3 (αMβ2; CD11b/CD18) restores IgG-dependent phagocytosis in transfectants expressing a phagocytosis-defective FcγRIIA (CD32) tail-minus mutant. J Immunol. 1996;157:5660–5665. [PubMed] [Google Scholar]
- Ylanne J, Chen Y, O'Toole TE, Loftus JC, Takada Y, Ginsberg MH. Distinct functions of integrin α and β subunit cytoplasmic domains in cell spreading and formation of focal adhesions. J Cell Biol. 1993;122:223–233. doi: 10.1083/jcb.122.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]