Abstract
Megakaryocytes undergo a unique differentiation program, becoming polyploid through repeated cycles of DNA synthesis without concomitant cell division. However, the mechanism underlying this polyploidization remains totally unknown. It has been postulated that polyploidization is due to a skipping of mitosis after each round of DNA replication. We carried out immunohistochemical studies on mouse bone marrow megakaryocytes during thrombopoietin- induced polyploidization and found that during this process megakaryocytes indeed enter mitosis and progress through normal prophase, prometaphase, metaphase, and up to anaphase A, but not to anaphase B, telophase, or cytokinesis. It was clearly observed that multiple spindle poles were formed as the polyploid megakaryocytes entered mitosis; the nuclear membrane broke down during prophase; the sister chromatids were aligned on a multifaced plate, and the centrosomes were symmetrically located on either side of each face of the plate at metaphase; and a set of sister chromatids moved into the multiple centrosomes during anaphase A. We further noted that the pair of spindle poles in anaphase were located in close proximity to each other, probably because of the lack of outward movement of spindle poles during anaphase B. Thus, the reassembling nuclear envelope may enclose all the sister chromatids in a single nucleus at anaphase and then skip telophase and cytokinesis. These observations clearly indicate that polyploidization of megakaryocytes is not simply due to a skipping of mitosis, and that the megakaryocytes must have a unique regulatory mechanism in anaphase, e.g., factors regulating anaphase such as microtubule motor proteins might be involved in this polyploidization process.
Megakaryocytes are unique among mammalian marrow cells in that they leave the diploid (2N) state to differentiate, synthesizing 4–64 times the normal DNA content (Odell et al., 1970) in a single cell. Although this process is initiated after the proliferative phases of development, it precedes development of the earliest morphologically recognizable cell, the megakaryoblast (Long et al., 1982a ,b; Williams and Jackson, 1982). While DNA replication is abnormal in the sense of not having a typical 2N–4N cycle, the process of polyploidization in these cells is tightly regulated. With each replicative event, the entire DNA content is duplicated such that megakaryocytes have multiples of a normal diploid DNA content (i.e., 4N, 8N, 16N, 32N, etc., where 2N is the normal nuclear DNA content of a cell in G0/G1 phase of the cell cycle). In these cells, therefore, the regulation of DNA synthesis is released from its normal cell cycle control, but global control is retained over the total amount of DNA replicated. The nature of polyploidy is the key to understanding the biology of megakaryocytes themselves and their role in platelet production. However, the regulation of this process remains totally unknown.
The process of polyploidization has been thought to be mechanistically divided into three steps: endomitosis, endoreduplication, and nuclear restitution (Therman et al., 1983). Endomitosis is generally defined as reproduction of nuclear elements not followed by chromosome movement or cytoplasmic division and is morphologically characterized as occurring in the presence of an intact nuclear membrane. This definition implies that while there is some degree of mitotic structural change, it occurs within an intact nuclear envelope. The definition has also been cited as the mechanism of polyploidization of megakaryocytes (Ebbe, 1976), although it has not been known whether endomitotic megakaryocytes retain an intact nuclear membrane. Therefore, it has simply been assumed that polyploidization of megakaryocytes is due to the skipping of mitosis after each round of DNA replication. It is well known that the cell division of eukaryotes is regulated by a complex with a maturation promoting factor (MPF or Cdc2–cyclin B complex). The level of Cdc2 is constant throughout the cell cycle, but cyclin B accumulates during the G2 phase and is destroyed by proteasome after anaphase (Draetta et al., 1989; Solomon et al., 1990; Hunt et al., 1992). Therefore, polyploidization of megakaryocytes has been postulated to be caused by either reduction of cyclin B and/or Cdc2 or diminished kinase activity of the complex. It was indeed reported that primary megakaryocytes and the phorbol ester–induced megakaryocytic cell line MegT lack cyclin B (Gu et al., 1993; Wang et al., 1995; Zhang et al., 1996). Inactivation of the Cdc2–cyclin B complex due to the marked reduction of Cdc2 was also described in phorbol ester–induced HEL cells (Datta et al., 1996). It has not been proved, however, that this hypothesis is applicable for thrombopoietin (TPO)1-induced polyploidization of megakaryocytes.
Past studies on megakaryocyte differentiation and platelet production have been hampered because of the rarity of megakaryocytes in bone marrow, the lack of megakaryocyte-specific differentiation factor, TPO, and the lack of a useful polyploidization-inducible megakaryocytic cell line. Some erythroleukemic and/or megakaryocytic cell lines exhibit megakaryocytic markers (Tabilio et al., 1983; Ogura et al., 1985; Sledge et al., 1986; Greenberg et al., 1988; Adachi et al., 1991; Ravid et al., 1993; Datta et al., 1996; Hudson et al., 1996; Takada et al., 1996; Kikuchi et al., 1997) but cannot induce polyploidization or require exposure to substances such as phorbol esters to polyploidize. Polyploidization induced by chemical agents is a widely observed phenomenon, but its mechanism varies depending on the drugs and cell lines. TPO is a recently identified cytokine that specifically regulates proliferation and maturation of megakaryocytes (Bartley et al., 1994; de Sauvage et al., 1994; Kaushansky et al., 1994; Kuter et al., 1994; Wendling et al., 1994) and actually stimulates polyploidization of primary immature megakaryocytes in vitro (Broudy et al., 1995; Debili et al., 1995; Angchaisuksiri et al., 1996). The availability of TPO and its capacity to induce the proliferation and differentiation of megakaryocyte progenitor cells has allowed megakaryocyte numbers to be expanded in vitro. This culture system has allowed us to investigate the cellular and molecular aspects of the polyploidization of these cells.
We therefore attempted to clarify the molecular and cellular mechanism of TPO-induced polyploidization of megakaryocytes in vitro by immunofluorescent microscopic studies. We found that during polyploidization, multiple spindle poles were formed as the polyploid megakaryocytes entered mitosis; the nuclear membrane broke down during prophase; the sister chromatids were aligned on a multifaced plate, and the centrosomes were symmetrically located on either side of each face of the plate at metaphase; and a set of sister chromatids moved into the multiple centrosomes during anaphase A. The two spindle poles in anaphase were located in close proximity during anaphase, so that the reassembling nuclear envelope may enclose all the sister chromatids in a single nucleus at anaphase, followed by the skipping of telophase and cytokinesis. We thus found that polyploidization is not simply caused by skipping mitosis, and here we discuss possible molecular mechanisms of TPO-induced polyploidization in bone marrow megakaryocytes.
Materials and Methods
Antibodies
A monoclonal antibody specific to α-tubulin was purchased from Sigma Chemical Co. (St. Louis, MO). A rabbit polyclonal antibody specific to COOH-terminal peptides of Xenopus γ-tubulin, which recognizes mouse γ-tubulin as well, was provided by Dr. H. Masuda at RIKEN. Autoantibodies against centromere and centriole were identified with indirect immunofluorescence studies with commercial prefixed HEP-2 cell slides (Medical and Biological Laboratories Co., Ltd., Nagoya, Japan) as described (Muro et al., 1990). Anti-RanBP2 antiserum 551 (Yokoyama et al., 1995) was provided by Dr. T. Nishimoto at Kyushu University, Fukuoka, Japan, and a rabbit anti-MCM3 antiserum was provided (Kubota et al., 1994) by Dr. H. Takisawa at Osaka University (Osaka, Japan). The FITC- labeled F(ab′)2 fragment was purchased from Zymed Laboratories (San Francisco, CA) and Cy3-conjugated F(ab′)2 fragment was obtained from Jackson ImmunoResearch Laboratories (West Grove, PA).
Preparation of Megakaryocytes
Bone marrow cells were freshly prepared from BDF1 mice (6- to 8-wk-old females) by flushing marrow cavities with Iscove's modified Dulbecco's medium (IMDM) through 26-gauge needles. Cells (1 × 106 cells/ml) were washed and cultured with bone marrow stromal cells for 2 wk in IMDM containing 10% FCS and the recombinant mouse TPO (50 U/ml) as described previously (Nagahisa et al., 1996). Recombinant mouse TPO was prepared from the supernatants of COS-7 cells transfected with mouse TPO cDNA in expression vector pME18 (Nagata et al., 1995). In 14 d with TPO, various stages of megakaryocytes, which had ploidy between 2N and 128N, were produced in the liquid culture, although few were generated without TPO. Most of the large suspension cells were confirmed to be megakaryocytes by immunostaining with megakaryocyte/platelet-specific antibody Pm-1 (Nagata et al., 1995) and CD61 (PharMingen, San Diego, CA).
Indirect Immunofluorescence Microscopy
Smear samples of cultured megakaryocytes were fixed with 100% methanol for 2 min at room temperature and then washed with PBS for 10 min at room temperature. Cells on coverslips were stained with primary antibodies for 2 h at 37°C. After rinsing with PBS five times, secondary antibodies were applied for 1 h at 37°C. The coverslips were washed with PBS five times and mounted in 90% glycerol/PBS containing 0.1% p-phenylenediamine and 0.2 μg/ml 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI). As a negative control, application of the primary antibodies was omitted from the procedure mentioned above. The cells were observed under a fluorescence microscope (model BX60-34-FLBD1; Olympus Corp., Lake Success, NY) at a final magnification of 1,500, and photographs were taken using Fuji film (ASA 400; Tokyo, Japan).
Results
Multiple Mitotic Spindle Poles Were Formed during Polyploidization of Bone Marrow Megakaryocytes
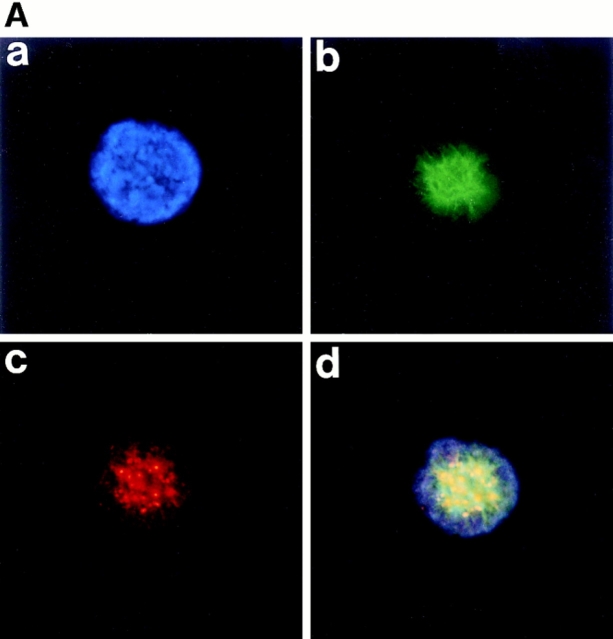
To study the TPO-induced polyploidization process of primary megakaryocytes, we immunostained a number of bone marrow megakaryocytes, which were cultured in the presence of TPO for 2 wk, with antibodies against various kinds of intracellular proteins regulating mitosis. A normal cell division requires a functional bipolar spindle, but how mitotic spindle poles are organized during polyploidization of megakaryocytes has not been characterized. Therefore, first of all, we stained the megakaryocytes with anti– α-tubulin antibody to learn the organization of mitotic spindles. Surprisingly, we found that multiple mitotic spindle poles were formed in all megakaryocytes in mitosis. The photograph in Fig. 1 A shows a megakaryocyte forming 32 spindle poles. The number of spindle poles in a megakaryocyte varies from 4 to 64, or even much more, but we were unable to count the exact number formed when it exceeded 32 because of the abundance.
Figure 1.
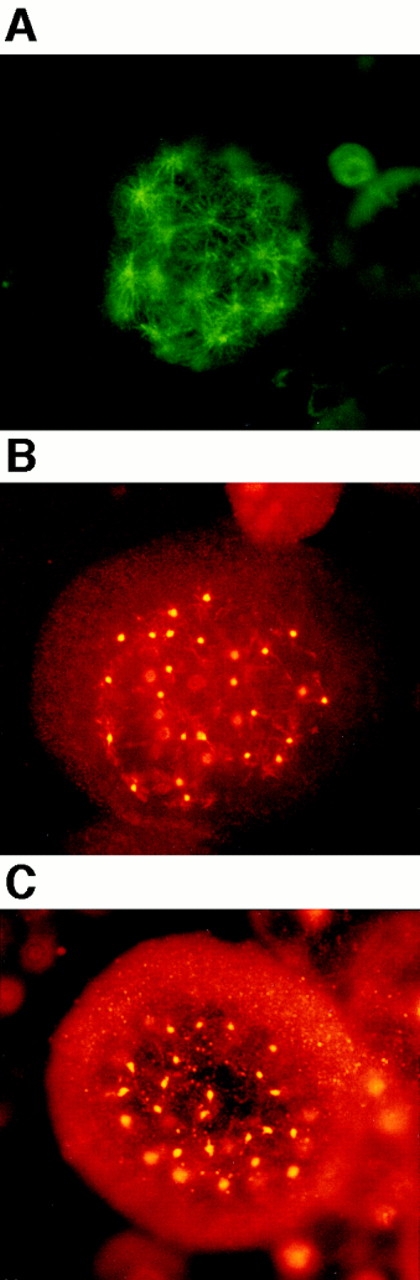
Multiple mitotic spindle poles formation during TPO-induced polyploidization of primary megakaryocytes. Mitotic spindle poles were detected by immunofluorescent light microscopy in TPO-induced primary mouse megakaryocytes. Megakaryocytes cultured with TPO were fixed in methanol for probing with anti–α-tubulin antibody (A), anti–γ-tubulin antibody (B), and anticentriole antibody (C), followed by incubation with an FITC-labeled F(ab′)2 fragment (A) or a Cy3-conjugated F(ab′)2 fragment (B and C).
We next stained the centrosomes with antibody against γ-tubulin, a well-characterized component of the centrosomes (Zheng et al., 1991; Joshi, 1994). Anti–γ-tubulin staining of the megakaryocytes clearly confirmed that multiple centrosomes were formed in megakaryocytes in mitosis (Fig. 1 B). In animal cells, the core of the centrosome has a pair of centrioles. Therefore, we also stained the centrioles with anticentriole antibody (Fig. 1 C) and confirmed that multiple centrosomes were produced in mitotic megakaryocytes. These photographs also show megakaryocytes bearing 32 centrosomes.
In large, matured megakaryocytes that have multilobed nuclei and are just ready to form proplatelets, however, no mitotic spindle pole was recognized by anti–α-tubulin antibody staining (data not shown). The immunostaining of the matured megakaryocytes with antibodies against γ-tubulin and centrioles also confirmed that there were no visible centrosomes (data not shown), indicating that multiple centrosomes disappear as megakaryocytes finally mature for some unknown reason and by an unknown mechanism.
Nuclear Membrane Is Broken Down during Polyploidization
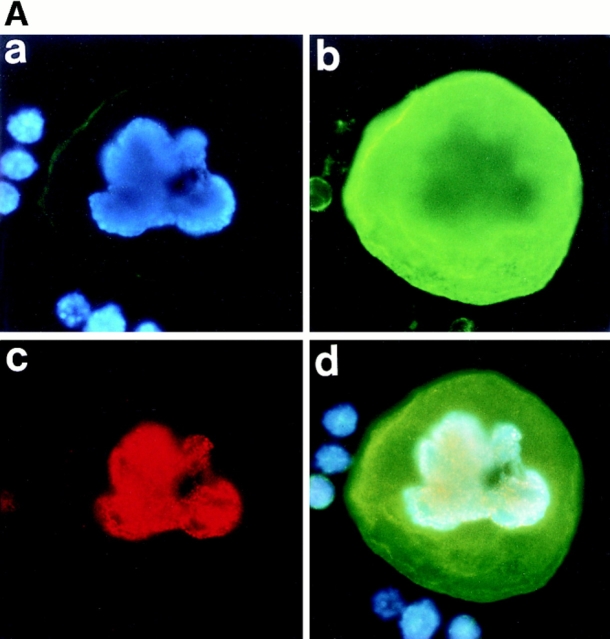
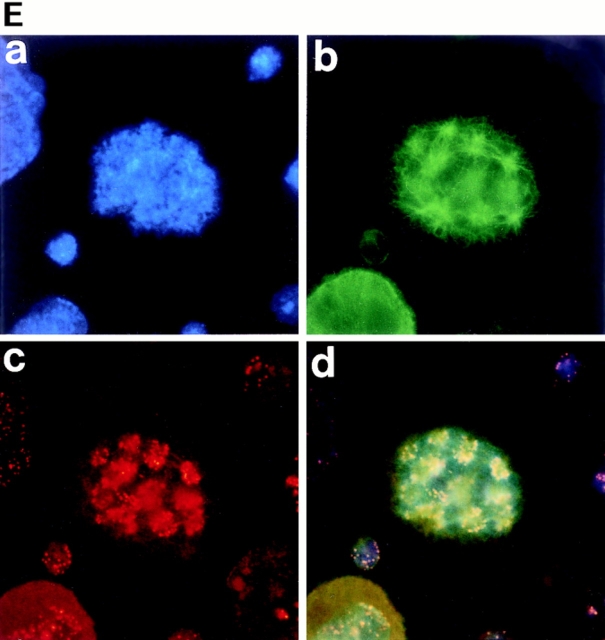
As described above, megakaryocytes were found to enter mitosis. In normal mammalian cells, concomitant with this entry into mitosis, the nuclear envelope breaks down and disappears, and then upon exit from mitosis, the nuclear envelope reassembles to form the nucleus. It has been postulated, however, that polyploidization was caused by the skipping of mitosis after each round of DNA replication (Long, M.W. 1993. 8th Symposium of Molecular Biology of Hematopoiesis. 196; Datta et al., 1996), and thus polyploidization has been thought to occur within an intact nuclear envelope. Whether or not the nuclear membrane breaks down as megakaryocytes polyploidize has never been examined. We therefore stained the nuclear membrane in megakaryocytes with anti-RanBP2 antibody (anti-551). RanBP2 is a nuclear pore complex protein, and thus anti-551 antibody that specifically recognizes RanBP2 can clearly stain the nuclear envelopes as described (Yokoyama et al., 1995). As shown in Fig. 2 A, c, anti-551 antibody staining clearly showed a lobulated nuclear surface of a megakaryocyte in interphase. The whole cell including cytoplasm, however, was stained with anti-551 antibody in a mitotic megakaryocyte forming eight mitotic spindle poles (Fig. 2 B, c), indicating that the nuclear membrane was broken down as the megakaryocyte entered mitosis and was reassembled in interphase. These observations clearly indicate that polyploidization of megakaryocytes is not simply due to the skipping of mitosis and that it does not occur within an intact nuclear envelope.
Figure 2.
Nuclear membrane is broken down during polyploidization of megakaryocytes. Megakaryocytes in interphase (A) or in mitosis (B) were stained with DAPI (a), or probed with anti–α-tubulin antibody (b) or anti-RanBP2 antibody (anti-551) (c), followed by incubation with an FITC-labeled F(ab′)2 fragment (b) or a Cy3-conjugated F(ab′)2 fragment (c). d shows triple stainings of the same cells.
Characterization of Mitosis during Megakaryocyte Polyploidization
We next studied how mitosis progresses during polyploidization of megakaryocytes. Mitosis is classically described as consisting of five major phases: prophase, prometaphase, metaphase, anaphase, and telophase.
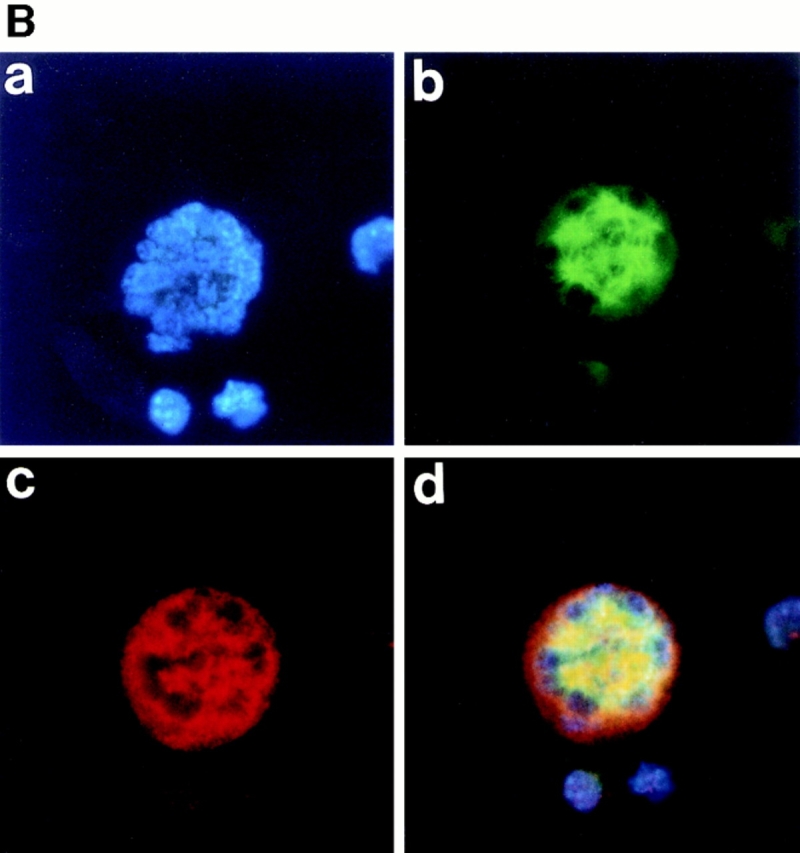
The first sign that a cell is about to enter mitosis is a period called prophase. Fig. 3 A, a, shows that the chromatin, which was diffuse in interphase, slowly condenses into well-defined chromosomes in a polyploidizing megakaryocyte. The cytoplasmic microtubules that were part of the interphase cytoskeleton disassemble, and the main component of the mitotic apparatus, the mitotic spindle, begins to form (Fig. 3 A, b–d). Anticentriole antibody (Fig. 3 A, c) and anti–α-tubulin antibody (Fig. 3 A, b) stainings confirmed that multiple mitotic spindle poles were formed and assembled outside the nucleus in a megakaryocyte in prophase, as described above.
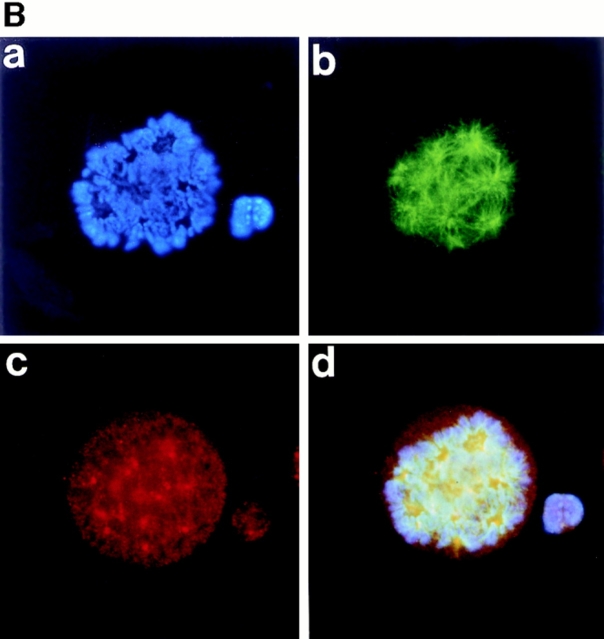
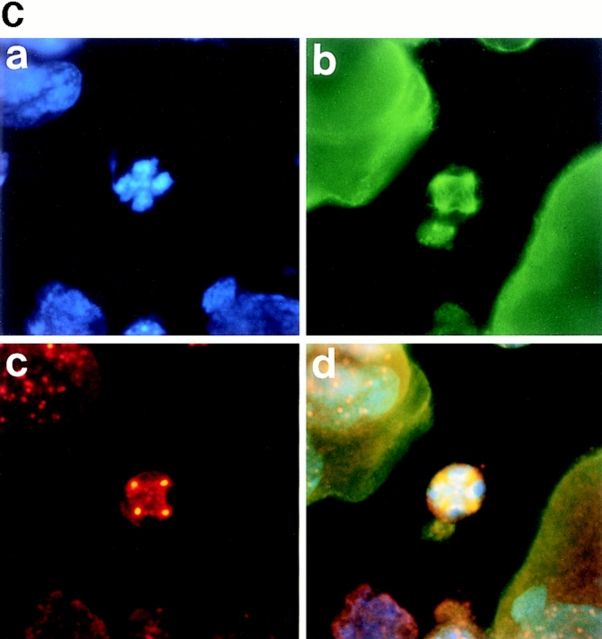
Figure 3.
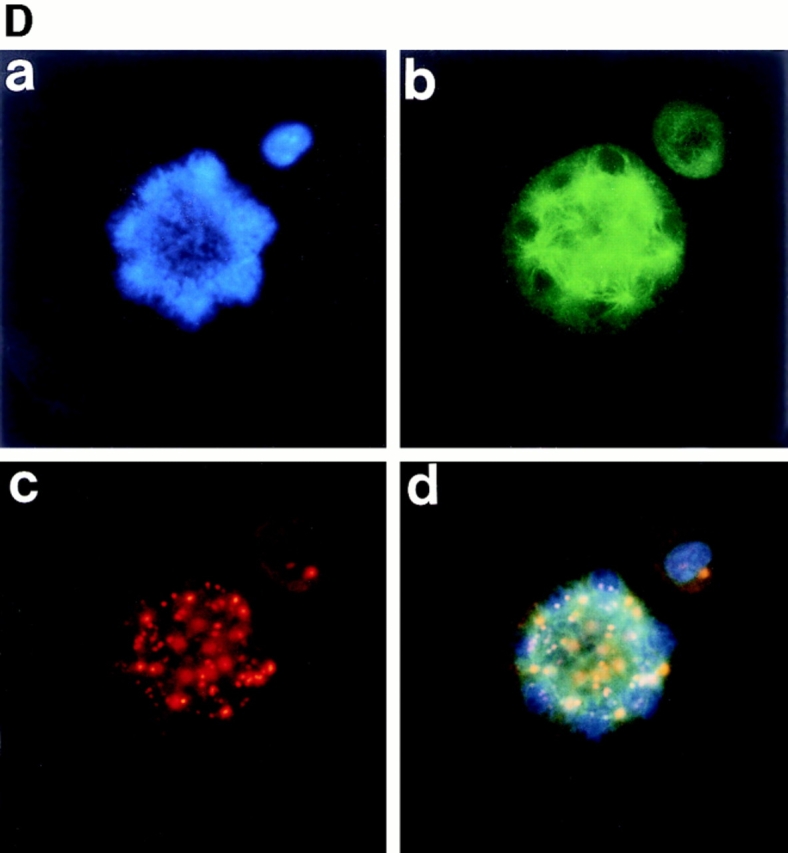
Polyploidizing megakaryocytes in various stages of mitosis. A number of primary megakaryocytes treated with TPO were stained with DAPI, anti–α-tubulin antibody, anti-γ-tubulin antibody, anticentriole antibody, or anticentromere antibody. (A) Megakaryocyte in prophase was stained with DAPI (a), anti–α-tubulin antibody (b), anticentriole antibody (c), or all three (d). (B) Megakaryocyte in prometaphase was probed with DAPI (a), anti–α-tubulin antibody (b), anti–γ-tubulin antibody (c), or all three (d). (C and D) Megakaryocytes in metaphase, with centrosomes numbering four (C) and eight (D), were stained with DAPI (a), anti–α-tubulin antibody (b), anticentriole antibody (c), or all three (d). (E) Megakaryocyte in anaphase A was stained with DAPI (a), anti–α-tubulin antibody (b), anticentromere antibody (c), or all three (d).
Prometaphase starts abruptly with disruption of the nuclear envelope. Stainings of a megakaryocyte in prometaphase with DAPI showed the condensation of the chromosomes (Fig. 3 B, a). Stainings with anti–α-tubulin (Fig. 3 B, b) and anti–γ-tubulin (Fig. 3 B, c) antibodies demonstrated that mitotic spindles entered the nucleus and that the microtubules radiated out from the multiple centrosomes, indicating that the nuclear membrane was broken down as described above.
In metaphase, the chromosomes move to points equidistant from the poles. To learn how the chromosomes align in polyploid megakaryocytes in mitosis, we stained the cells with DAPI (Fig. 3 C and D, a) and anti–α-tubulin antibody (Fig. 3 C and D, b). Here, we show two megakaryocytes forming four (Fig. 3 C) or eight (Fig. 3 D) mitotic spindle poles. As shown in the figures (a–d), we clearly observed that two or four planes of the aligned chromosomes crossed at right angles. Staining with anti–α-tubulin (b) and anticentriole (c) antibodies demonstrated that four or eight pairs of spindle poles were symmetrically located on either side of each face of the plate between the crossed chromosomes (a–d).
Anaphase begins abruptly as the paired kinetochores on each chromosome separate, allowing each chromatid to be pulled toward the spindle pole it faces. Two categories of movement can generally be distinguished. In anaphase A, the sister chromatids separate from each other and move toward the poles. In anaphase B, the polar microtubules elongate and the two poles of the spindle move farther apart. To see the megakaryocytes in anaphase, we stained the separating sister chromatids with anticentromere antibody (Fig. 3 E, c), which recognizes a specific centromere DNA sequence. Anticentromere antibody staining clearly showed that many bundles of the sister chromatids (c) were moving toward multiple centrosomes (b–d), indicating that anaphase A is entirely visible in polyploidizing megakaryocytes. It was observed, however, that the pair of spindle poles in anaphase were located in closer compared with the other cell types and that they remained stationary and did not move farther apart as the other cell types normally behaved during anaphase. In addition, we never observed the megakaryocytes in telophase or cytokinesis.
To see the chromosome movement in more detail, we looked further at the polyploidizing megakaryocytes stained with anticentromere antibody, anti–α-tubulin antibody, and DAPI at all stages of mitosis (Fig. 4). In interphase, the centromeres were dispersed in the entire area of the nucleus (Fig. 4 A). In prophase and prometaphase, the chromatin condensed into well-defined chromosomes, and staining of the centromeres (Fig. 4 B) showed that the chromosomes began to align for metaphase. The multiple mitotic spindle poles were formed at this stage. Fig. 4, C–E, shows a megakaryocyte polyploidizing from ploidy 4N to 8N at the stage just before metaphase, metaphase, and anaphase A, respectively. At the stage just before metaphase, two planes of the aligned chromosomes crossed at right angles, and two pairs of spindle poles were symmetrically located in close proximity to this crossing on either side of each face of the plate (Fig. 4 C). The centromeres were located around the crossing and moving to points equidistant from the poles. In metaphase, two pairs of spindle poles moved outward and stretched the sister chromatids tightly toward the poles; thus, the centromeres were located just in the middle of the crossed chromosome planes between the two poles (Fig. 4 D). In anaphase A, the sister chromatids were pulled toward the spindle poles so that four sets of centromeres were moving toward each pole (Fig. 4 E). No set, however, could be separated far enough to be enclosed by individual nuclear envelopes. Fig. 4 F shows a megakaryocyte polyploidizing from ploidy 8N to 16N in anaphase A. The sets of centromeres were located close to each centrosome, and none of the sets of chromosomes was separated completed. We found no megakaryocytes in telophase or cytokinesis.
Figure 4.
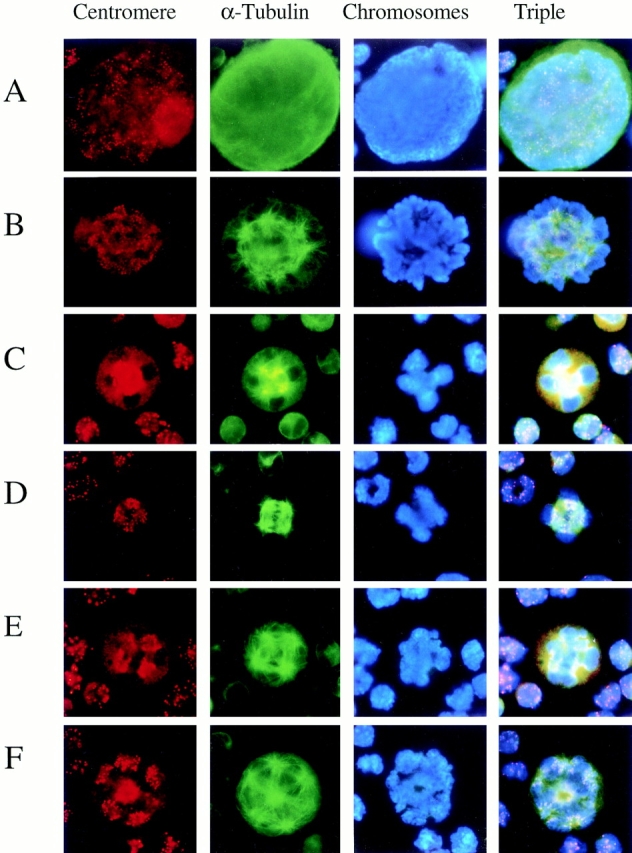
Centromere movement during polyploidizing megakaryocytes. TPO-treated primary megakaryocytes were stained with anticentromere antibody (red, first column), anti–α-tubulin antibody (green, second column), DAPI (blue, third column), and triple staining (fourth column) during mitosis. (A) Megakaryocyte in interphase. (B) Megakaryocytes in prometaphase. (C) Megakaryocyte with ploidy 8N at the stage just before metaphase. (D) Megakaryocyte with ploidy 8N in metaphase. (E) Megakaryocyte with ploidy 8N in anaphase A. (F) Megakaryocyte with ploidy 16N in anaphase A.
These observations indicate that the polyploidizing megakaryocytes actually progress through normal prophase, prometaphase, metaphase, and up to anaphase A, but not to anaphase B, telophase, or cytokinesis, and that the reassembling nuclear envelope may enclose all the sister chromatids in a single nucleus because of the lack of outward movement of the spindle poles during anaphase. It is now obvious that polyploidization of megakaryocytes is not simply due to the skipping of mitosis, abnormal chromosome arrangement, or abnormal number or location of centrosomes.
Discussion
The hypothesis that G1-S-G2-G1 phases continue without entry into mitosis during polyploidization of megakaryocytes has been propounded on the basis of results obtained by chemical-induced polyploidization of megakaryocytic cell lines (Long, M.W. 1993. 8th Symposium of Molecular Biology of Hematopoiesis. 196; Datta et al., 1996). In this study, we examined whether or not this hypothesis is applicable to the TPO-induced polyploidization of primary megakaryocytes. Staining of a number of primary megakaryocytes with various antibodies against intracellular components regulating cell division clearly demonstrated that the formation of multiple mitotic spindle poles (centrosomes) and the rupture of the nuclear envelope are required for the polyploidization process of megakaryocytes. We further showed that megakaryocytes indeed enter mitosis and progress through normal prophase, prometaphase, metaphase, and up to anaphase A, but not to anaphase B, telophase, or cytokinesis. These observations indicate that polyploidization is not simply caused by skipping mitosis, and thus the hypothesis described above was proved to be totally incorrect in naturally occurring polyploidization of megakaryocytes, although it may be true in chemically induced polyploidization of some cell lines. It is also now clear that polyploidization is not caused by either abnormal chromosome arrangement nor abnormal number or location of centrosomes.
Our findings suggest that polyploidizing megakaryocytes have a unique regulatory mechanism in anaphase. One possibility is that factors regulating anaphase B, such as microtubule motor proteins, might be involved in this polyploidization process since we observed that the pair of spindle poles in anaphase were located in close proximity to each other, probably because of the lack of outward movement of spindle poles during anaphase. In normal cells, the polar microtubules elongate much more, and a nuclear reformation occurs by the fusion of vesicles bound to daughter chromosomes and separately encloses each set of daughter chromosomes at telophase. In megakaryocytes, however, sets of daughter chromosomes are not separated far enough to enclose an individual set. Consequently, the reassembling nuclear envelope may enclose all the sister chromatids into a single nucleus at anaphase, thereafter skipping telophase and cytokinesis. Recent work shows that a number of cellular functions are carried out by various types of kinesin and kinesin-related motor proteins, and members of four of the eight kinesin subfamilies play crucial roles in cell division (Moore and Endow, 1996; Walczak and Mitchison, 1996). One of these subfamilies, mitotic kinesin-like protein 1 (MKLP1), causes plus end–directed sliding of microtubules over one another and may mediate anaphase B spindle elongation. MKLP1 is a plus end–directed human kinesein–related motor protein that bundles antiparallel microtubules and slides them past each other at 4 μm/min, a velocity consistent with anaphase B spindle elongation in vivo (Nislow et al., 1992). The association of MKLP1 with the midbody supports its proposed role in separating poles at anaphase B by sliding the antiparallel interdigitating nonkinetochore microtubules past each other (Nislow et al., 1992). Therefore, lack of outward movement of spindle poles during anaphase B in polyploidizing megakaryocytes might be due to the disregulation of MKLP1 in mitotic megakaryocytes. The abnormal regulation of polyploidizing megakaryocytes in anaphase B, especially regulation of MKLP1 activity in anaphase B, remains to be explained.
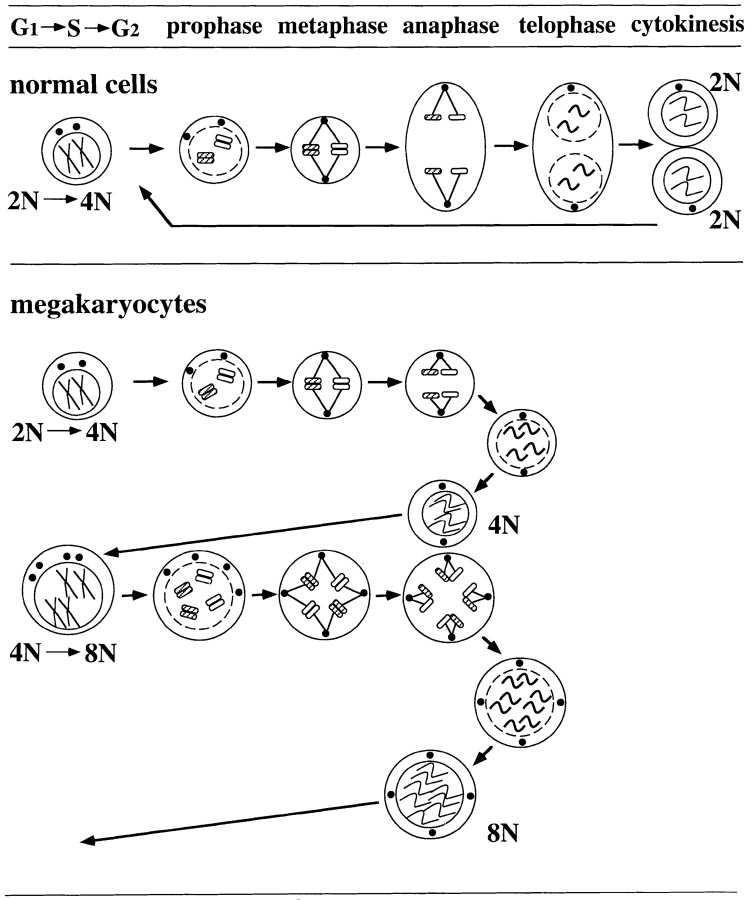
Taken together, a model of the polyploidization process of megakaryocytes is shown in Fig. 5. Here, we describe a megakaryocyte polyploidizing from 4N to 8N as an example (lower panel). Two centrosomes duplicate to form four centrosomes in a cell, and the cell normally enters the first step of mitosis, prophase. The chromatin condenses into chromosomes, and the mitotic spindles are formed. The nuclear envelope is normally disrupted, and mitotic spindles enter the nucleus at prometaphase. At metaphase, two planes of the aligned chromosomes cross at right angles, and four pairs of spindle poles are beautifully aligned between the crossed chromosomes. In anaphase, the sets of sister chromatids separate each other and move toward each pole. However, the pair of spindle poles in this stage is located at a closer distance than normal cells (Fig. 5, upper panel), and the pair of spindle poles stay fixed and do not move farther apart as normal cells do. The reassembling nuclear envelope encloses all the sister chromatids into one nucleus because of the lack of outward movement of the spindle poles during anaphase. Without telophase or cytokinesis, the cell with ploidy 8N goes into another round of cell cycle.
Figure 5.
Schematic drawing of hypothetical mechanism of TPO-induced polyploidization of megakaryocytes. Upper panel shows a normal mitosis, and lower panel shows the mitosis of megakaryocytes during polyploidization.
Replication in eukaryotic cells is precisely regulated so that all the DNA is replicated once during a single S phase (Laskey et al., 1989). A licensing factor minichromosome maintenance 3 (MCM3) has been suggested to regulate once-per-cell-cycle DNA replication (Hennessy et al., 1990; Yan et al., 1993; Kimura et al., 1994). MCM3 is localized in the nucleus throughout the whole interphase and is redistributed in the extrachromosomal region during mitosis. It is also known that MCM3 cannot be detected in the cells that do not divide. We therefore stained polyploid megakaryocytes with anti-MCM3 antibody and found that MCM3 was localized in the nucleus in interphase (data not shown), indicating that these polyploid megakaryocytes are ready to undergo another round of DNA replication. It was found to be localized outside the chromatids at early prophase and dispersed into the cytoplasm during prometaphase and anaphase A (data not shown). These observations suggest that DNA is replicated only once per cell cycle in polyploidizing megakaryocytes and that the megakaryocytes do enter mitosis, and the reassembling nuclear envelope encloses all the sister chromatids in a single nucleus once per cell cycle. We were not able, however, to detect MCM3 either in cytoplasm or in nucleus of the fully matured megakaryocytes (data not shown), indicating that these completely matured megakaryocytes would no longer undergo another round of DNA synthesis.
It has been postulated that polyploidization of megakaryocytes is caused by skipping mitosis either as a result of reduction of cyclin B and/or Cdc2 or by diminished kinase activity of the complex (Gu et al., 1993; Wang et al., 1995; Datta et al., 1996; Zhang et al., 1996). In the TPO-induced polyploidization process, however, megakaryocytes do enter mitosis, and thus the Cdc2–cyclin B complex must be fully active during early mitosis. To confirm this, we examined whether the levels of Cdc2 and cyclin B are normally regulated during polyploidization. Staining with anti-Cdc2 antibody showed that Cdc2 was constantly expressed during all stages of mitosis (data not shown). Staining with cyclin B antibody showed that cyclin B was clearly detected at early mitosis in megakaryocytes, including those with a ploidy >8N, and that the expression of cyclin B decreased in anaphase A (data not shown). While these observations are inconsistent with those described previously, we concluded that polyploidization is apparently not caused by the lack of cyclin B since megakaryocytes driven by active Cdc2–cyclin B complex actually do enter mitosis. On the other hand, however, we could not detect cyclin B in fully matured megakaryocytes that no longer undergo polyploidization and are ready for proplatelet formation (data not shown). These matured megakaryocytes had no centrosomes, nor could we detect any MCM3 anywhere in these megakaryocytes. The phenotypes of the fully matured megakaryocytes thus seem to be completely different from those of actively dividing normal cells and of polyploidizing megakaryocytes. We speculate that the previous controversial reports may describe only fully matured primary megakaryocytes or chemically induced cell lines but not actually polyploidizing primary megakaryocytes. It is still possible that chemically induced polyploidization is caused by the lack of Cdc2–cyclin B complex activity.
Endomitosis is generally defined as reproduction of nuclear elements not followed by chromosome movement or cytoplasmic division and is morphologically characterized as occurring within an intact nuclear envelope, while there is some degree of mitotic structural change. This definition has also been used for a mechanism of polyploidization of megakaryocytes (Ebbe, 1976), although it has not been known whether endomitotic megakaryocytes retain an intact nuclear membrane. Now we have clearly shown that the nuclear membrane does break down as megakaryocytes enter mitosis to polyploidize, and that polyploidizing megakaryocytes progress through mitosis up to anaphase A. Thus, the term endomitosis was found to be no longer applicable to the mechanism of polyploidization of megakaryocytes. Other terminology should be used to define the megakaryocyte polypoidization process.
The regulation of polyploidization of megakaryocytes is totally enigmatic, as is its biological significance. It is reasonable to assume that some evolutionary advantage derives from the ability to make platelet-producing cells in this manner, but what this advantage could be is a still mystery. One might presume that higher-ploidy cells could produce more platelets than lower-ploidy cells, or that actual production and release is more efficient from a single large cell than from several smaller ones, but none of these suppositions has been proven. It is known that megakaryocyte DNA content is related to megakaryocyte cell size and thus to the eventual number of platelets produced. It is also clear that megakaryocytes synthesize increased amounts of DNA before increases in cytoplasmic volume and cytoplasmic maturation. Here, we described the cellular aspects of TPO-induced megakaryocyte polyploidization, and the mechanism of polyploidization can now be investigated at molecular levels. The results shown here will be helpful in understanding the unresolved puzzles regarding polyploidization.
Acknowledgments
We thank Dr. T. Nishimoto for anti-RanBP2 antibody, Dr. H. Takisawa for anti-MCM3 antibody, Dr. H. Masuda for anti–γ-tubulin antibody, and Drs. H. Takisawa, S. Hisanaga, M. Inagaki, K. Matsuzawa, S. Kotani, and T. Nagasawa for valuable discussions.
This work was supported in part by a grant from the Mitsubishi Foundation, a Special Grant for Promotion of Research from RIKEN, and grants from the Ministry of Education, Science, and Culture of Japan.
Abbreviations used in this paper
- DAPI
6′6-diamidino-2-phenylindole
- MKLP1
mitotic kinesin-like protein 1
- TPO
thrombopoietin
Footnotes
Address all correspondence to Kazuo Todokoro, Tsukuba Life Science Center, The Institute of Physical and Chemical Research (RIKEN), 3-1, Koyadai, Tsukuba, Ibaraki 305, Japan. Tel.: 81 298 36 9075. Fax: 81 298 36 9090. e-mail: todokoro@rtc.riken.go.jp
References
- Adachi M, Ryo R, Sato T, Yamaguchi N. Platelet factor 4 gene expression in a human megakaryocytic leukemia cell line (CMK) and its differentiated subclone (CMK11-5) Exp Hematol. 1991;19:923–927. [PubMed] [Google Scholar]
- Angchaisuksiri P, Carlson PL, Dessypris EN. Effects of recombinant human thrombopoietin on megakaryocyte colony formation and megakaryocyte ploidy by human CD34+ cells in a serum-free system. Br J Haematol. 1996;93:13–17. doi: 10.1046/j.1365-2141.1996.4761013.x. [DOI] [PubMed] [Google Scholar]
- Bartley TD, Bogenberger J, Hunt P, Li YS, Lu HS, Martin F, Chang MS, Samal B, Nichol JL, Swift S, et al. Identification and cloning of a megakaryocyte growth and development factor that is a ligand for the cytokine receptor Mpl. Cell. 1994;77:1117–1124. doi: 10.1016/0092-8674(94)90450-2. [DOI] [PubMed] [Google Scholar]
- Broudy VC, Lin NL, Kaushansky K. Thrombopoietin (c-mpl ligand) acts synergistically with erythropoietin, stem cell factor, and interleukin-11 to enhance murine megakaryocyte colony growth and increases megakaryocyte ploidy in vitro. Blood. 1995;85:1719–1726. [PubMed] [Google Scholar]
- Datta NS, Williams JL, Caldwell J, Curry AM, Ashcraft EK, Long MW. Novel alterations in CDK1/cyclin B1 kinase complex formation occur during the acquisition of a polyploid DNA content. Mol Biol Cell. 1996;7:209–223. doi: 10.1091/mbc.7.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debili N, Wendling F, Katz A, Guichard J, Breton-Gorius J, Hunt P, Vainchenker W. The Mpl-ligand or thrombopoietin or megakaryocyte growth and differentiative factor has both direct proliferative and differentiative activities on human megakaryocyte progenitors. Blood. 1995;86:2516–2525. [PubMed] [Google Scholar]
- de Sauvage FJ, Hass PE, Spencer SD, Malloy BE, Gurney AL, Spencer SA, Darbonne WC, Henzel WJ, Wong SC, Kuang WJ, et al. Stimulation of megakaryocytopoiesis and thrombopoiesis by the c-Mpl ligand. Nature (Lond) 1994;369:533–538. doi: 10.1038/369533a0. [DOI] [PubMed] [Google Scholar]
- Draetta G, Luca F, Westendorf J, Brizuela L, Ruderman J, Beach D. Cdc2 protein kinase is complexed with both cyclin A and B: evidence for proteolytic inactivation of MPF. Cell. 1989;56:829–838. doi: 10.1016/0092-8674(89)90687-9. [DOI] [PubMed] [Google Scholar]
- Ebbe S. Biology of megakaryocytes. Prog Hemostasis Thromb. 1976;3:211–229. [PubMed] [Google Scholar]
- Greenberg SM, Rosenthal DS, Greeley TA, Tantravahi R, Handin RI. Characterization of a new megakaryocytic cell line: the Dami cell. Blood. 1988;72:1968–1977. [PubMed] [Google Scholar]
- Gu XF, Allain A, Li L, Cramer EM, Tenza D, Caen JP, Han ZC. Expression of cyclin B in megakaryocytes and cells of other hematopoietic lineages. C R Acad Sci III. 1993;316:1438–1445. [PubMed] [Google Scholar]
- Hennessy KM, Clark CD, Botstein D. Subcellular localization of yeast CDC46 varies with the cell cycle. Genes Dev. 1990;4:2252–2263. doi: 10.1101/gad.4.12b.2252. [DOI] [PubMed] [Google Scholar]
- Hennessy KM, Lee A, Chen E, Botstein D. A group of interacting yeast DNA replication genes. Genes Dev. 1991;5:958–969. doi: 10.1101/gad.5.6.958. [DOI] [PubMed] [Google Scholar]
- Hudson KM, Denko NC, Schwab E, Oswald E, Weiss A, Lieberman MA. Megakaryocytic cell line-specific hyperploidy by cytotoxic necrotizing factor bacterial toxins. Blood. 1996;88:3465–3473. [PubMed] [Google Scholar]
- Hunt T, Luca FC, Ruderman JV. The requirements for protein synthesis and degradation, and the control of destruction of cyclins A and B in the meiotic and mitotic cell cycles of the clam embryo. J Cell Biol. 1992;116:707–724. doi: 10.1083/jcb.116.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi HC. Microtubule organizing centers and γ-tubulin. Curr Opin Cell Biol. 1994;6:54–62. doi: 10.1016/0955-0674(94)90116-3. [DOI] [PubMed] [Google Scholar]
- Kaushansky K, Lok S, Holly RD, Broudy VC, Lin N, Baily MC, Forstorm JW, Buddle MM, Oort PJ, Hagen FS, et al. Promotion of megakaryocyte progenitor expansion and differentiation by the c-Mpl ligand thrombopoietin. Nature (Lond) 1994;369:568–571. doi: 10.1038/369568a0. [DOI] [PubMed] [Google Scholar]
- Kikuchi J, Furukawa Y, Iwase S, Terui Y, Nakamura M, Kitagawa S, Kitagawa M, Komatsu N, Miura Y. Polyploidization and functional maturation are two distinct processes during megakaryocytic differentiation: involvement of cyclin-dependent kinase inhibitor p21 in polyploidization. Blood. 1997;89:3980–3987. [PubMed] [Google Scholar]
- Kimura H, Nozaki N, Sugimoto K. DNA polymerase α associated protein P1, a murine homology of yeast MCM3, changes its intranuclear distribution during the DNA synthetic period. EMBO (Eur Mol Biol Organ) J. 1994;13:4311–4320. doi: 10.1002/j.1460-2075.1994.tb06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y, Mimura S, Nishimoto S, Takisawa H, Nojima H. Identification of the yeast MCM3-related protein as a component of XenopusDNA replication licensing factor. Cell. 1994;81:601–609. doi: 10.1016/0092-8674(95)90081-0. [DOI] [PubMed] [Google Scholar]
- Kuter DJ, Beeler DL, Rosenberg RD. The purification of megapoietin: a physiological regulator of megakaryocyte growth and platelet production. Proc Natl Acad Sci USA. 1994;91:11104–11108. doi: 10.1073/pnas.91.23.11104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey RA, Fairman MP, Blow JJ. S phase of the cell cycle. Science (Wash DC) 1989;246:609–614. doi: 10.1126/science.2683076. [DOI] [PubMed] [Google Scholar]
- Long MW, Williams N, Ebbe S. Immature megakaryocytes in the mouse: physical characteristics, cell cycle status, and in vitro responsiveness to thrombopoietic stimulatory factor. Blood. 1982a;59:569–575. [PubMed] [Google Scholar]
- Long MW, Williams N, McDonald TP. Immature megakaryocytes in the mouse: in vitro relationship to megakaryocyte progenitor cells and mature megakaryocytes. J Cell Physiol. 1982b;112:339–344. doi: 10.1002/jcp.1041120305. [DOI] [PubMed] [Google Scholar]
- Moore JD, Endow SA. Kinesin proteins: a phylum of motors for microtubule-based motility. BioEssays. 1996;18:207–218. doi: 10.1002/bies.950180308. [DOI] [PubMed] [Google Scholar]
- Muro Y, Sugimoto K, Okazaki T, Ohashi M. The heterogeneity of anticentromere antibodies in immunoblotting analysis. J Rheumatol. 1990;17:1042–1047. [PubMed] [Google Scholar]
- Nagahisa H, Nagata Y, Ohnuki T, Osada M, Nagasawa T, Abe T, Todokoro K. Bone marrow stromal cells produce thrombopoietin and stimulate megakaryocyte growth and maturation but suppress proplatelet formation. Blood. 1996;87:1309–1316. [PubMed] [Google Scholar]
- Nagata Y, Nagahisa H, Aida Y, Okutomi K, Nagasawa T, Todokoro K. Thrombopoietin induces megakaryocyte differentiation in hematopoietic progenitor FDC-P2 cells. J Biol Chem. 1995;270:19673–19675. doi: 10.1074/jbc.270.34.19673. [DOI] [PubMed] [Google Scholar]
- Nislow C, Lombillo VA, Kuriyama R, McIntosh JR. A plus-end-directed motor enzyme that moves antiparallel microtubules in vitro localizes to the interzone of mitotic spindles. Nature (Lond) 1992;359:543–547. doi: 10.1038/359543a0. [DOI] [PubMed] [Google Scholar]
- Odell TT, Jackson CW, Friday TJ. Megakaryocytopoiesis in rats with special reference to polyploidy. Blood. 1970;35:775–782. [PubMed] [Google Scholar]
- Ogura M, Morishima Y, Ohno R, Kato Y, Hirabayashi N, Nagura H, Saito H. Establishment of a novel human megakaryoblastic leukemia cell line, MEG-01, with positive Philadelphia chromosome. Blood. 1985;66:1384–1392. [PubMed] [Google Scholar]
- Ravid K, Kuter DJ, Beeler DL, Doi T, Rosenberg RD. Selection of an HEL-derived cell line expressing high levels of platelet factor 4. Blood. 1993;81:2885–2890. [PubMed] [Google Scholar]
- Sledge GW, Glant M, Jansen J, Heerema NA, Roth BJ, Goheen M, Hoffman R. Establishment in long term culture of megakaryocytic leukemia cells (EST-IU) from the marrow of a patient with leukemia and a mediastinal germ cell neoplasm. Cancer Res. 1986;46:2155–2159. [PubMed] [Google Scholar]
- Solomon MJ, Glotzer M, Lee TH, Philippe M, Kirschner MW. Cyclin activation of p34cdc2. Cell. 1990;63:1013–1024. doi: 10.1016/0092-8674(90)90504-8. [DOI] [PubMed] [Google Scholar]
- Tabilio A, Pelicci PG, Vinci G, Mannoni P, Civin CI, Vainchenker W, Testa U, Lipinski M, Rochart H, Breton-Gorius J. Myeloid and megakaryocytic properties of K-562 cell lines. Cancer Res. 1983;43:4569–4573. [PubMed] [Google Scholar]
- Takada M, Morii N, Kumagai S, Ryo R. The involvement of the rho gen product, a small molecular weight GTP-binding protein, in polyploidization of a human megakaryocytic cell line, CMK. Exp Hematol. 1996;24:524–530. [PubMed] [Google Scholar]
- Therman E, Sarto GE, Stubblefield PA. Endomitosis: a reappraisal. Hum Genet. 1983;63:13–18. doi: 10.1007/BF00285390. [DOI] [PubMed] [Google Scholar]
- Walczak CE, Mitchison TJ. Kinesin-related proteins at mitotic spindle poles: function and regulation. Cell. 1996;85:943–946. doi: 10.1016/s0092-8674(00)81295-7. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhang Y, Kamen D, Lee E, Ravid K. Cyclin D3 is essential for megakaryocytopoiesis. Blood. 1995;86:3783–3788. [PubMed] [Google Scholar]
- Wendling F, Maraskovsky E, Debili N, Christina F, Teepe M, Titeux M, Methia N, Breton-Gorius J, Cosman D, Vainchenker W. cMpl ligand is a humoral regulator of megakaryocytopoiesis. Nature (Lond) 1994;369:571–574. doi: 10.1038/369571a0. [DOI] [PubMed] [Google Scholar]
- Williams N, Jackson H. Kinetic analysis of megakaryocyte numbers and ploidy levels in developing colonies from mouse bone marrow cells. Cell Tiss Kinet. 1982;15:483–494. doi: 10.1111/j.1365-2184.1982.tb01571.x. [DOI] [PubMed] [Google Scholar]
- Yan H, Merchant AM, Tye BK. Cell cycle-regulated nuclear localization of MCM2 and MCM3, which are required for the initiation of DNA synthesis at chromosomal replication origins in yeast. Genes Dev. 1993;7:2149–2160. doi: 10.1101/gad.7.11.2149. [DOI] [PubMed] [Google Scholar]
- Yokoyama N, Hayashi N, Seki T, Pante N, Ohba T, Nishii K, Kuma K, Hayashida T, Miyata T, Aebi U, et al. A giant nucleopore protein that binds Ran/TC4. Nature (Lond) 1995;376:184–188. doi: 10.1038/376184a0. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang Z, Ravid K. The cell cycle in polyploid megakaryocytes is associated with reduced activity of cyclin B1-dependent Cdc2 kinase. J Biol Chem. 1996;271:4266–4272. doi: 10.1074/jbc.271.8.4266. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Jung MK, Oakley BR. γ-Tubulin is present in Drosophila melanogaster and Homo sapiensand is associated with the centrosome. Cell. 1991;65:817–823. doi: 10.1016/0092-8674(91)90389-g. [DOI] [PubMed] [Google Scholar]