Abstract
Cosuppression results in the degradation of RNA from host genes and homologous transgenes after transcription in the nucleus. By using grafting experiments, we have shown previously that a systemic signal mediates the propagation of cosuppression of Nia host genes and 35S-Nia2 transgenes from silenced 35S-Nia2 transgenic stocks to nonsilenced 35S-Nia2 transgenic scions but not to wild-type scions. Here, we examined the requirements for triggering and maintenance of cosuppression in various types of scions. Grafting-induced silencing occurred in 35S-Nia2 transgenic lines over-accumulating Nia mRNA whether they are able to spontaneously trigger cosuppression or not and in 35S-Nia2 transgene-free plants over-accumulating host Nia mRNA caused by metabolic derepression. When grafting-induced silenced scions were removed from the silenced stocks and regrafted onto wild-type plants, silencing was not maintained in the 35S-Nia2 transgene-free plants and in the 35S-Nia2 transgenic lines that are not able to trigger cosuppression spontaneously. Conversely, silencing was maintained in the 35S-Nia2 transgenic lines that are able to trigger cosuppression spontaneously. Our results indicate that the presence of a 35S-Nia2 transgene is dispensable for the RNA degradation step of posttranscriptional silencing when host Nia mRNA over-accumulate above the level of wild-type plants. They also suggest that grafting-induced RNA degradation does not result in the production of the systemic silencing signal required for spontaneous triggering and maintenance.
Keywords: Nicotiana tabacum/posttranscriptional silencing/systemic signal/grafting
Posttranscriptional gene silencing (PTGS) was originally described in plants as the coordinated and reciprocal inactivation of host genes and transgenes encoding the same sense RNA. This phenomenon was called cosuppression (1, 2). PTGS results from the degradation of homologous host genes and transgenes RNA transcribed in the nucleus (3–6). PTGS also was reported in the case of transgenes that do not share any homology with the plant genome (7–9). When PTGS affects transgenes sharing homology with an RNA virus, plants become resistant to the infection by this virus. This latter phenomenon was called RNA-mediated virus resistance. It suggests that the RNA degradation step of PTGS occurs in the cytoplasm because RNA viruses replicate in this compartment (10–16).
Several lines of evidence have shown that gene dosage and/or gene product(s) dosage could account for the initiation of PTGS: (i) silencing occurs much more efficiently when the T-DNA is in a homozygous state (7, 9, 17–20); (ii) the frequency of nitrate reductase (NR) cosuppression correlates with the number of both host genes and transgenes per haploid genome (6), (iii) NR cosuppression is abolished when the expression of a 35S-Nia2 transgene is impeded in trans by a 35S-specific transcriptional silencing locus (6); (iv) RNA-mediated resistance against a recombinant potato virus X-β-glucuronidase virus also is abolished when the expression of a 35S-β-glucuronidase transgene is impeded in trans by a 35S-specific transcriptional silencing locus (22); (v) the frequency of chalcone synthase cosuppression correlates with the strength of the promoter driving the ChsA transgene (21); and (vi) in the case of weakly transcribed or promoterless ChsA transgenes, only the transgenic plants carrying a large number of copies of the transgenes show cosuppression (3, 21, 23).
We reported previously that the introduction of a 35S-Nia2 transgene into tobacco can result in cosuppression of Nia host genes and 35S-Nia2 transgenes (20). In each homozygous line, spontaneous triggering occurs at each generation, affecting only a limited but constant fraction (ranging from 0 to 60%) of the population (24). We showed that silencing starts on a single leaf and then propagates to the whole plant as a gradual process (25). This propagation step involves a sequence-specific signal that is transmissible by grafting from transgenic silenced stocks to transgenic nonsilenced scions (26). However, silencing cannot propagate from silenced stocks to wild-type scions nor to transgenic scions in which the expression of the 35S-Nia2 transgene is impeded in trans by a 35S-specific transcriptional silencing locus (6). Therefore, the presence of a transcriptionally active transgene appeared to be required for grafting-induced silencing. To investigate further the respective roles played by the transgene and the level of Nia mRNA accumulation on the triggering and the maintenance of silencing, additional grafting experiments were performed involving plants carrying or not carrying a 35S-Nia2 transgene. Results indicate that the presence of a 35S-Nia2 transgene is dispensable for the RNA-specific degradation step of PTGS when Nia mRNA over-accumulate above the level of wild-type plants, whereas it is absolutely required for spontaneous triggering and maintenance.
MATERIALS AND METHODS
Plant Material.
The transgenic lines 27–8.9, 27–44.3, 27–44.7, 30–18.1, 30–18.2, 30–46.7, 30–91.3, 30–122.6, and 34–19.4 carry the 35S-Nia2 transgene inserted at a single locus. These lines are homozygous for the transgene locus (20, 24). Hemizygous plants were obtained by crossing with the wild-type strain PBD6.
Plant NIA30 is a nontransgenic tobacco mutant that is defective in the production of a functional NR apoenzyme but not of Nia mRNA (27, 28). Plants 271–5.8 and 475–11.5 are transgenic plants that are defective for nitrite reductase (NiR) caused by antisense inhibition and cosuppression of Nii genes respectively (25, 29).
Culture Conditions and Grafting Procedure.
Seeds were sown in a greenhouse and grown with 16-h light/8-h dark photoperiod. Plants were grown for 2–3 mo before grafting. NR and NiR deficient mutants and transgenic plants were sown in vitro on medium B (30) supplemented with 5 mM ammonium succinate and grafted onto wild-type rootstocks to sustain their growth in the greenhouse.
Spontaneously silenced 35S-Nia2 transgenic plants were grafted onto wild-type rootstocks to sustain their growth in the greenhouse. These silenced stocks were beheaded 10 cm below the top of the plant. The outermost cortex of the stock stem was longitudinally cut, thus giving rise to a cortex flap. The terminal apex of the nonsilenced plants used as scions was excised, bevelled, and fastened to the stock between the flap and the main part of the stem by using Parafilm. After 6 wk, the terminal apices of scions were removed and regrafted on wild-type plants.
Plant Analyses.
Silencing was monitored by the appearance of leaf chlorosis and also was confirmed by RNA gel blot analysis by using Nia2 cDNA as described (24).
RESULTS
Transgenic Plants Carrying a Transcriptionally Active 35S-Nia2 Transgene Show Grafting-Induced Silencing.
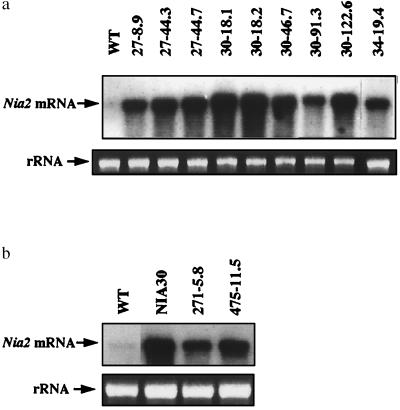
Transgenic lines carrying a transcriptionally active 35S-Nia2 transgene inserted at a single locus were previously characterized (24). In these lines, Nia mRNA accumulation is higher than in wild-type plants (Fig. 1a). These lines belong to two classes. Lines of the first class do not show cosuppression, whether they are homozygous or hemizygous for the transgene locus (class I). Lines of the second class show cosuppression spontaneously at various frequencies, ranging from 5% to 42% (Table 1) when homozygous for the transgene locus and no cosuppression when hemizygous (class II).
Figure 1.
Nia mRNA accumulation in the different nonsilenced scions (a) wild-type plants (WT) and homozygous transgenic plants over-accumulating Nia mRNA caused by the presence of the 35S-Nia2 transgene (classes I and II). (b) Wild-type plants (WT) and NR- or NiR-deficient plants over-accumulating host Nia mRNA caused by metabolic derepression (class III). Ten micrograms of total RNA extracted from leaves were probed with the tobacco Nia2 cDNA. Photographs of the 25S rRNA band in the ethidium-stained gel indicate the amounts of RNA loaded in each lane.
Table 1.
Triggering and maintenance of grafting induced cosuppression
| Scion | Allelic state of the 35S-Nia2 transgene locus | Spontaneous silencing | Grafting-induced silencing | Maintenance of grafting-induced silencing |
|---|---|---|---|---|
| Class I | ||||
| 27-8.9 | Ho | 0/120 | 7/7 | 0/4 |
| 27-44.3 | Ho | 0/120 | 9/9 | 0/9 |
| 30-122.6 | Ho | 0/120 | 5/5 | 0/2 |
| 34-19.4 | Ho | 0/120 | 10/10 | 0/10 |
| 27-8.9 × WT | He | 0/120 | 5/5 | 0/3 |
| 27-44.3 × WT | He | 0/120 | 5/5 | 0/1 |
| 34-19.4 × WT | He | 0/120 | 10/10 | 0/7 |
| Class II | ||||
| 27-44.7 | Ho | 50/120 | 7/7 | 5/5 |
| 30-18.1 | Ho | 18/120 | 5/5 | 3/3 |
| 30-18.2 | Ho | 14/120 | 5/5 | 3/3 |
| 30-46.7 | Ho | 12/120 | 10/10 | 10/10 |
| 30-91.3 | Ho | 10/120 | 7/7 | 5/5 |
| 27-44.7 × WT | He | 0/120 | 10/10 | 0/10 |
| 30-18.1 × WT | He | 0/120 | 3/3 | nd |
| 30-18.2 × WT | He | 0/120 | 3/3 | nd |
| 30-46.7 × WT | He | 0/120 | 10/10 | 0/4 |
| 30-91.3 × WT | He | 0/120 | 1/1 | nd |
| Class III | ||||
| NIA 30 | — | 0/5 | 5/5 | 0/5 |
| 475-11.5 | — | 0/5 | 5/5 | 0/5 |
| 271-5.8 | — | 0/5 | 5/5 | 0/5 |
Nonsilenced scions accumulating Nia mRNA above the level of wild-type plants were grafted onto spontaneously silenced transgenic 35S-Nia2 stocks. Scions of class I and class II carry a transcriptionally active 35S-Nia2 transgene. Only the homozygous scions of class II are able to trigger cosuppression spontaneously. Scions of class III are either nontransgenic NR-deficient mutants or transgenic NiR-deficient plants, which over-accumulate Nia mRNA because of metabolic derepression. Grafting-induced silencing was scored either by the apparition of chlorosis or by RNA gel blot analysis. Six weeks after grafting, the terminal apices of grafting-induced silenced scions were regrafted onto wild-type stocks. Maintenance of silencing was monitored either by the chlorosis or RNA gel blot analysis. Ho, homozygous; He, hemizygous; WT, wild-type; nd, not determined.
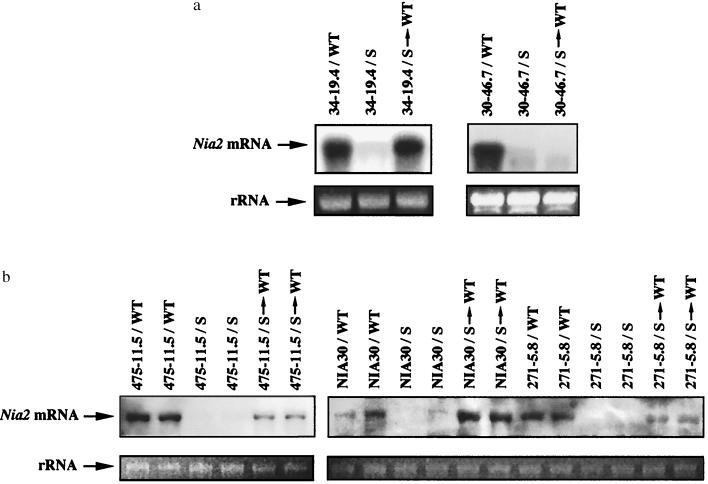
Nonsilenced homozygous or hemizygous plants derived from representative lines of each class were used as scions and grafted onto spontaneously silenced plants of class II used as stocks. As shown previously (26), nonsilenced homozygous plants derived from lines of class II became silenced after grafting (Table 1). In addition, we observed that nonsilenced homozygous plants derived from lines of class I and hemizygous plants derived from lines of both class I and class II became silenced after grafting. Silencing was confirmed by RNA gel blot analysis (Fig. 2a). These results indicate that plants carrying a transcriptionally active 35S-Nia2 transgene can sense the systemic silencing signal provided by the silenced stocks and become silenced with 100% efficiency. Silencing occurs whether the scions derive from transgenic lines that are able to spontaneously trigger Nia cosuppression or not.
Figure 2.
Triggering and maintenance of Nia mRNA degradation. (a) Homozygous transgenic 35S-Nia2 scions of class I (34–19.4) or class II (30–46.7) were grafted onto wild-type (/WT) or spontaneously silenced 35S-Nia2 transgenic stocks of class II (/S). Six weeks later, the terminal apices of grafting-induced silenced scions were removed and regrafted onto wild-type stocks (/S → WT). (b) Scions of class III that do not contain the 35S-Nia2 transgene were grafted as described in a. Two plants of each genotype were analyzed for each conditions. In lane 10 (NIA30 scion grafted onto silenced 35S-Nia2 lines), the spot on the membrane is an artifact, which does not correspond to the Nia mRNA. Its position is above that of the Nia mRNA, and the migration of RNA on the gel was correct as indicated by the picture of the 25S rRNA, which comigrates with the Nia mRNA. Ten micrograms of total RNA extracted from apical leaves were probed with the tobacco Nia2 cDNA. Photographs of the 25S rRNA band in the ethidium-stained gel indicate the amounts of RNA loaded in each lane.
Plants accumulating Nia mRNA Above the Level of Wild-Type Plants in the Absence of a 35S-Nia2 Transgene Show Grafting-Induced Silencing.
Nonsilenced scions accumulating host Nia mRNA above the level of wild-type plants in the absence of a 35S-Nia2 transgene (and defined as plants of class III) also were grafted onto silenced stocks. Plant NIA30 is a nontransgenic tobacco mutant, which is defective in the production of a functional NR apoenzyme but not of Nia mRNA (27, 28). Plants 271–5.8 and 475–11.5 are transgenic plants that are defective for NiR caused by antisense inhibition and cosuppression of Nii genes respectively (25, 28). In wild-type plants, the transcription of both Nia and Nii genes is repressed by reduced N-metabolites resulting from nitrate assimilation. Because of their deficiency in either NR or NiR, plants of class III do not produce reduced N-metabolites (25, 28, 29). They lack transcriptional repression of Nia and Nii genes and accumulate host Nia mRNA above the level of wild-type plants (Fig. 1b).
Scions of class III were already chlorotic before grafting onto spontaneously silenced plants of class II caused by the deficiency in NR (NIA30) or NiR (271–5.8 and 475–11.5). RNA gel blot analysis indicated that, 3 wk after grafting, the accumulation of Nia mRNA was reduced strongly in these scions (Fig. 2b). These results indicate that the presence of a 35S-Nia2 transgene is not required for grafting-induced silencing because it can occur in nontransgenic plants (NIA30) or in transgenic plants that do not carry the 35S-Nia2 transgene (271–5.8 and 475–11.5). However, we showed previously that grafting-induced silencing never occurred in wild-type plants or in transgenic plants carrying a transcriptionally inactive 35S-Nia2 transgene (26). Therefore, the accumulation of Nia mRNA above the level of wild-type plants rather than the presence of a 35S-Nia2 transgene seems to be required for grafting-induced silencing. Because Nia mRNA are produced only by the host Nia genes in plants of class III, our results indicate that the competence of a scion to become silenced after grafting onto silenced stocks relies on a quantitative rather than a qualitative aspect of the accumulation of Nia mRNA.
Grafting-Induced Silencing is Maintained only in Lines Able to Spontaneously Trigger Silencing.
To determine whether grafting-induced silencing can be maintained in the absence of the silenced stock, grafting-induced silenced scions were beheaded and their terminal apices were regrafted onto wild-types stocks. When removed from the source of silencing, only the homozygous scions derived from lines of class II remained completely chlorotic (Table 1). RNA gel blot analysis confirmed that Nia mRNA did not accumulate in these chlorotic scions (Fig. 2a). Conversely, homozygous scions derived from lines of class I and hemizygous scions derived from lines of both class I and class II did not maintained silencing when regrafted onto a wild-type rootstock. The first leaves of the scion remained chlorotic (Fig. 3a). However, as the scion grew, newly developed leaves have a variegated pattern, being chlorotic all along the midvein of the limb and green in the interveinal area (Fig. 3b). Finally, normal green leaves appeared at the top of the scion (Fig. 3c). RNA gel blot analysis indicated that the appearance of green tissues correlated with the accumulation of Nia mRNA (Fig. 2a). The absence of maintenance of grafting-induced silencing was observed with all types of plants that are not able to trigger cosuppression spontaneously (Table 1).
Figure 3.
Absence of maintenance of Nia cosuppression homozygous transgenic 35S-Nia2 scions of class I (34–19.4) were grafted onto spontaneously silenced 35S-Nia2 transgenic stocks of class II. Six weeks later, the terminal apices of grafting-induced silenced scions were removed and regrafted onto wild-type stocks. The first developed leaf was completely chlorotic (a). Then, the upper leaves showed vein-localized chlorosis (b). Finally, chlorosis was absent from the uppermost leaves of the scion (c).
Grafting-induced silenced scions of class III behave as plant of class I. The absence of maintenance was revealed by RNA gel blot experiments (Fig. 2b). These results indicate for the first time that transgenic as well as nontransgenic plants that never trigger Nia cosuppression spontaneously are able to undergo Nia-specific RNA degradation after grafting but cannot maintain the silenced state in the absence of the source of silencing.
DISCUSSION
In this report, we have investigated the different properties that make a plant a target for systemic acquired silencing after grafting onto a silenced stock. In previous analyses, we have shown that wild-type plants or transgenic plants carrying a transcriptionally silenced 35S-Nia2 transgene never displayed spontaneous triggering of Nia cosuppression or grafting-induced silencing. Conversely, nonsilenced plants derived from transgenic lines carrying the same transgene in a transcriptionally active state and showing spontaneous triggering of Nia cosuppression at various frequencies (ranging from 5 to 42%) undergo grafting-induced silencing with 100% efficiency (6, 26). We have suggested that the over-accumulation of Nia mRNA was required for both spontaneous triggering of silencing and grafting-induced silencing. Here, we show that 35S-Nia2 transgenic lines accumulating Nia mRNA above the level of wild-type plants can undergo grafting-induced silencing even if they are not able to spontaneously trigger cosuppression. In addition, transgenic NiR-deficient plants as well as nontransgenic NR-deficient mutants that over-accumulate host Nia mRNA caused by metabolic deregulation also can show grafting-induced silencing. Therefore, the requirements for grafting-induced silencing and for spontaneous triggering of Nia cosuppression are different.
According to the presence or absence of a 35S-Nia2 transgene, to the level of Nia mRNA accumulation, and to their ability to trigger silencing spontaneously or following grafting onto 35S-Nia2 silenced stocks, plants can be classified in three classes (Table 1):
1. Transgenic plants carrying a transcriptionally active 35S-Nia2 transgene in which spontaneous triggering does not occur but showing grafting-induced silencing with 100% efficiency (class I);
2. Transgenic plants carrying a transcriptionally active 35S-Nia2 transgene showing spontaneous triggering with various frequencies (ranging from 5 to 42%) and grafting-induced silencing with 100% efficiency (class II); and
3. Transgenic and nontransgenic plants showing a level of accumulation of host Nia mRNA above the level of wild-type plants caused by metabolic deregulation in the absence of a 35S-Nia2 transgene, in which spontaneous triggering does not occur but showing grafting-induced silencing (class III).
Therefore, the susceptibility to undergo the RNA degradation step of PTGS seems to depend only on the accumulation of Nia mRNA above the level of wild-type plants and not on the presence of a 35S-Nia2 transgene. The fact that Nia mRNA are produced only by the host Nia genes in plants of class III that do not carry a 35S-Nia2 transgene indicates that the competence of a scion to undergo RNA degradation depends on a quantitative rather than a qualitative aspect of the accumulation of Nia mRNA. Conversely, the competence of a plant to trigger Nia cosuppression spontaneously seems to depend on a combination of quantitative and qualitative aspects of Nia mRNA and/or Nia loci. Two lines of evidence are in favor of this hypothesis: (i) the presence of a transcriptionally active 35S-Nia2 transgene is required (6), and (ii) not all combinations of 35S-Nia2 transgene loci trigger cosuppression. Indeed, among 43 transgene loci analyzed (20, 24), only 7 are able to trigger cosuppression spontaneously when they are homozygous but not when they are hemizygous (lines of class II). However, these loci become able to trigger cosuppression spontaneously in a hemizygous state when brought into the presence of each other (24). These results suggest that the frequency of spontaneous triggering of cosuppression reflects the probability to produce sufficient amounts of a particular transgene product (the systemic silencing signal for instance). This probability is increased when the loci that are able to produce this signal are in a homozygous state or when two hemizygous loci are brought together. The fact that each homozygous locus of class II triggers cosuppression with a different frequency and the fact that hybrids carrying two hemizygous loci always trigger cosuppression with a frequency comprised between that of each homozygous parent (24) suggests that each locus produce a different amount of the signal. However, because the frequency of cosuppression in homozygous plants is not twice that in hemizygous, one cannot exclude that ectopic interactions between allelic or ectopic transgene loci increase strongly the production of the signal.
The requirements for spontaneous triggering and for maintenance seem to be similar. Indeed, only the lines showing spontaneous trigger cosuppression (homozygous of class II) maintain a silent state when removed from the source of silencing (silenced stocks). Conversely, plants that are not able to spontaneously trigger cosuppression (hemizygous plants of class II, hemizygous and homozygous plants of class I, and nontransgenic NR-deficient mutants or transgenic NiR-deficient plants of class III) cannot maintain a silent state when removed from the source of silencing. Therefore, the maintenance of grafting-induced silencing seems to depend on the ability of the scions to regenerate the silencing signal provided exogenously by the silenced plants of class II that serve as stocks. When plants of class II trigger silencing spontaneously, the signal propagates from the leaf where it is originally produced to the rest of the plant (25). Propagation occurs because the signal is regenerated in all cells that receive the signal, resulting in the amplification of the phenomenon. Grafting-beheading-regrafting experiments mimic the propagation of silencing in spontaneous conditions. When nonsilenced scions of class II are grafted onto silenced stocks, they receive the signal and thus behave as in the process of spontaneous triggering. Conversely, the maintenance of grafting-induced silencing in plants of class I and class III depends on the permanent production of the Nia-specific degradation signal by the transgenic silenced plants of class II that serve as rootstocks. The absence of maintenance in these lines after beheading and regrafting of their terminal apices onto wild-type stocks suggest that grafting-induced RNA degradation does not result in the production of the systemic silencing signal required for spontaneous triggering and maintenance. Such a signal should therefore be produced only by the transgene loci of class II.
PTGS events that lead to RNA-mediated virus resistance also have been classified in various categories depending on the way plants become resistant to viruses. Transgenic plants can be immune to virus infection if transgene silencing is established before virus infection (12, 14, 15). Alternatively, RNA-mediated virus resistance can be achieved after virus infection, leading to a recovery phenotype in the newly developing leaves (10, 13, 16). These latter plants that are not immune although they carry a transcriptionally active transgene behave as transgenic 35S-Nia2 plants that do not show spontaneous Nia cosuppression although they carry a transcriptionally active 35S-Nia2 transgene. Such plants cannot initiate silencing by themselves because they do not produce the silencing signal or sufficient amounts of it. However, the RNA degradation step of PTGS can be initiated by providing an exogenous source of silencing signal, i. e., by grafting onto silenced stocks in the case of Nia cosuppression or by infecting the plants by the virus in the case of plants showing recovery. Results of grafting-induced silencing and of virus-recovery indicate that the RNA degradation step of PTGS can be uncoupled from the initiation step. Whereas the presence of a transgene is absolutely necessary for the initiation step, it is dispensable for the RNA degradation step. Indeed, we were able to induce RNA degradation in nontransgenic plants that over-accumulated the host Nia RNA after grafting onto silenced transgenic plants. Similarly, it was reported recently that infection of nontransgenic Nicotiania clevelandii plants by nepoviruses or of nontransgenic Brassica oleracea plants by cauliflower mosaic virus induces a resistance mechanism that is similar to the phenomenon of recovery, i. e., it led to the degradation of virus RNA (31, 32). These latter examples confirm that transgenes are not absolutely required to trigger RNA-specific degradation under certain circumstances. In addition, the fact that virus recovery or grafting-induced Nia RNA degradation can occur in nontransgenic plants (31, 32, this work) indicates that only the cellular machinery is involved in the RNA degradation step of PTGS.
To summarize, the cellular machinery involves in the RNA degradation step of PTGS may be activated in a plant cell under (at least) five conditions:
1. when particular transgene loci produce spontaneously a sequence-specific systemic silencing signal that allows the propagation of silencing to the whole plant;
2. when over-accumulated mRNA encoded by host gene and transgene loci that do not allow spontaneous triggering of cosuppression meet the sequence-specific systemic silencing signal provided by spontaneously silenced transgenic stocks;
3. when over-accumulated mRNA encoded by host genes (in the absence of transgenes) meet the sequence-specific systemic silencing signal provided by spontaneously silenced transgenic stocks;
4. when virus infection of nontransgenic plants mimics the spontaneous production of a transgenic sequence-specific systemic silencing signal that activates the cellular RNA degradation pathway; and
5. when mRNA encoded by transgene loci that do not allow spontaneous triggering of RNA-mediated virus resistance meet the sequence-specific systemic silencing signal provided by homologous viruses which infect the plants.
The exact nature of the signal that allows the activation of the cellular RNA degradation pathway remains to be determined. Only some particular transgene loci and some particular viruses seem to be able to produce this signal, suggesting similarities between the RNAs encoded by the transgene loci that trigger silencing spontaneously and the RNAs encoded by viruses that lead to spontaneous recovery in nontransgenic plants.
Acknowledgments
We thank Jean-Marie Pollien for all grafting experiments. We are indebted to Christophe Béclin, Taline Elmayan, Frank Feuerbach, Christian Godon, and Philippe Mourrain for critical reading of the manuscript. This work was partly funded by the European Union-Biotechnology projects on gene silencing (N° CHRXCT940530 and B104-CT96-0253).
ABBREVIATIONS
- NR
nitrate reductase
- NiR
nitrite reductase
- PTGS
posttranscriptional gene silencing
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Napoli C, Lemieux C, Jorgensen R. Plant Cell. 1990;2:279–289. doi: 10.1105/tpc.2.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van der Krol A R, Mur L A, Beld M, Mol J N M, Stuitje A R. Plant Cell. 1990;2:291–299. doi: 10.1105/tpc.2.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Blokland R, Van der Geest N, Mol J N M, Kooter J M. Plant J. 1994;6:861–877. [Google Scholar]
- 4.de Carvalho Niebel F, Fremdo P, Van Montagu M, Cornelissen M. Plant Cell. 1995;7:347–358. doi: 10.1105/tpc.7.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kunz C, Schöb H-P, Stam M, Kooter J M, Meins F J. Plant J. 1996;10:437–450. [Google Scholar]
- 6.Vaucheret H, Nussaume L, Palauqui J-C, Quilleré I, Elmayan T. Plant Cell. 1997;9:1495–1504. doi: 10.1105/tpc.9.8.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dehio C, Schell J. Proc Natl Acad Sci USA. 1994;91:5538–5542. doi: 10.1073/pnas.91.12.5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ingelbrecht I, Van Houdt H, Van Montagu M, Depicker A. Proc Natl Acad Sci USA. 1994;91:10502–10506. doi: 10.1073/pnas.91.22.10502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elmayan T, Vaucheret H. Plant J. 1996;9:787–797. [Google Scholar]
- 10.Lindbo J A, Silva-Rosales L, Proebsting W M, Dougherty W J. Plant Cell. 1993;5:1749–1759. doi: 10.1105/tpc.5.12.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith H A, Swaney S L, Parks T D, Wernsman E A, Dougherty W G. Plant Cell. 1994;6:1441–1453. doi: 10.1105/tpc.6.10.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mueller E, Gilbert J, Davenport G, Brigneti G, Baulcombe D C. Plant J. 1995;7:1001–1013. [Google Scholar]
- 13.Goodwin J, Chapman K, Swaney S, Parks T D, Wernsman E A, Dougherty W G. Plant Cell. 1996;8:95–105. doi: 10.1105/tpc.8.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.English J J, Mueller E, Baulcombe D C. Plant Cell. 1996;8:179–188. doi: 10.1105/tpc.8.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sijen T, Wellink J, Hiriart J B, Van Kammen A. Plant Cell. 1996;8:2277–2294. doi: 10.1105/tpc.8.12.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanzer M M, Thompson W F, Law M D, Wernsman E A, Uknes S. Plant Cell. 1997;9:1411–1423. doi: 10.1105/tpc.9.8.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Carvalho F, Gheysen G, Kushnir S, Van Montagu M, Inzé D, Castresana C. EMBO J. 1992;11:2595–2602. doi: 10.1002/j.1460-2075.1992.tb05324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hart C M, Fischer B, Neuhaus J M, Meins F. Mol Gen Genet. 1992;235:179–188. doi: 10.1007/BF00279359. [DOI] [PubMed] [Google Scholar]
- 19.Angenent G, Franken J, Busscher M, Colombo L, van Tunen A J. Plant J. 1993;4:101–112. doi: 10.1046/j.1365-313x.1993.04010101.x. [DOI] [PubMed] [Google Scholar]
- 20.Dorlhac de Borne F, Vincentz M, Chupeau Y, Vaucheret H. Mol Gen Genet. 1994;243:613–621. doi: 10.1007/BF00279570. [DOI] [PubMed] [Google Scholar]
- 21.Que Q, Wang H-Y, English J J, Jorgensen R A. Plant Cell. 1997;9:1357–1368. doi: 10.1105/tpc.9.8.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.English J J, Davenport G F, Elmayan T, Vaucheret H, Baulcombe D C. Plant J. 1997;12:597–603. [Google Scholar]
- 23.Stam M, de Bruin R, Kenter S, Van der Hoorn R A L, Van Blokland R, Mol J N M, Kooter J. Plant J. 1997;12:63–82. doi: 10.1046/j.1365-313x.2000.00650.x. [DOI] [PubMed] [Google Scholar]
- 24.Palauqui J-C, Vaucheret H. Plant Mol Biol. 1995;29:149–159. doi: 10.1007/BF00019126. [DOI] [PubMed] [Google Scholar]
- 25.Palauqui J-C, Elmayan T, Dorlhac de Borne F, Crété P, Charles C, Vaucheret H. Plant Physiol. 1996;112:1447–1456. doi: 10.1104/pp.112.4.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palauqui J-C, Elmayan T, Pollien J-M, Vaucheret H. EMBO J. 1997;16:4738–4745. doi: 10.1093/emboj/16.15.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Müller A J. Mol Gen Genet. 1983;192:275–281. [Google Scholar]
- 28.Pouteau S, Chérel I, Vaucheret H, Caboche M. Plant Cell. 1989;1:1111–1120. doi: 10.1105/tpc.1.11.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaucheret H, Kronenberger J, Lépingle A, Vilaine F, Boutin J-P, Caboche M. Plant J. 1992;2:559–569. [PubMed] [Google Scholar]
- 30.Bourgin J P, Chupeau Y, Missonier C. Plant Physiol. 1979;45:288–292. [Google Scholar]
- 31.Ratcliff F, Harrison B D, Baulcombe D C. Science. 1997;276:1558–1560. doi: 10.1126/science.276.5318.1558. [DOI] [PubMed] [Google Scholar]
- 32.Covey S N, Al-Kaff N S, Langara A, Turner D S. Nature (London) 1997;387:781–782. [Google Scholar]