Abstract
Many cells coordinate their activities by transmitting rises in intracellular calcium from cell to cell. In nonexcitable cells, there are currently two models for intercellular calcium wave propagation, both of which involve release of inositol trisphosphate (IP3)- sensitive intracellular calcium stores. In one model, IP3 traverses gap junctions and initiates the release of intracellular calcium stores in neighboring cells. Alternatively, calcium waves may be mediated not by gap junctional communication, but rather by autocrine activity of secreted ATP on P2 purinergic receptors. We studied mechanically induced calcium waves in two rat osteosarcoma cell lines that differ in the gap junction proteins they express, in their ability to pass microinjected dye from cell to cell, and in their expression of P2Y2 (P2U) purinergic receptors. ROS 17/2.8 cells, which express the gap junction protein connexin43 (Cx43), are well dye coupled, and lack P2U receptors, transmitted slow gap junction-dependent calcium waves that did not require release of intracellular calcium stores. UMR 106-01 cells predominantly express the gap junction protein connexin 45 (Cx45), are poorly dye coupled, and express P2U receptors; they propagated fast calcium waves that required release of intracellular calcium stores and activation of P2U purinergic receptors, but not gap junctional communication. ROS/P2U transfectants and UMR/Cx43 transfectants expressed both types of calcium waves. Gap junction–independent, ATP-dependent intercellular calcium waves were also seen in hamster tracheal epithelia cells. These studies demonstrate that activation of P2U purinergic receptors can propagate intercellular calcium, and describe a novel Cx43-dependent mechanism for calcium wave propagation that does not require release of intracellular calcium stores by IP3. These studies suggest that gap junction communication mediated by either Cx43 or Cx45 does not allow passage of IP3 well enough to elicit release of intracellular calcium stores in neighboring cells.
Increases in the intracellular calcium concentration that spread from cell to cell provide a mechanism for cells to coordinate many activities, including ciliary beat frequency and insulin secretion. These intercellular calcium waves have been studied in many cell types, including respiratory tract ciliated cells (2, 14), neurons (6), glial cells and cell lines (4, 5, 12, 26), smooth muscle cells (28), osteoblastic cells (27), chondrocytes (9), mast cells (19), insulinoma cells (3), PC12 cells (18), lens cells (8), and hepatocytes (24). In most instances, propagation of intercellular calcium waves induced by mechanical stimulation involves the release of intracellular calcium stores by inositol trisphosphate (IP3).1 In the most extensively characterized mechanism for these waves, mechanical stimulation generates IP3, which diffuses to neighboring cells through gap junction pores, and then triggers calcium release from IP3-sensitive intracellular calcium stores (23). Thus, in rabbit respiratory epithelia, mechanically induced intercellular calcium waves can be elicited in the absence of extracellular calcium (22) and can be inhibited by depleting intracellular calcium stores with thapsigargin (2), by blocking IP3 receptors with heparin (2), by inhibiting phospholipase C activity with U73122 (15), or by blocking gap junctional communication with heptanol, and the waves can be evoked by microinjecting IP3 (22). It is unclear which gap junction proteins are present in these cells, but the same group has demonstrated that transfection of connexin 43 (Cx43) into C6 glioma cells confers upon these cells the ability to propagate intercellular calcium waves (5).
The above studies demonstrate that IP3 releasable calcium stores are required for the propagation of intercellular calcium waves in some cell types, but it is less clear that diffusion of IP3 through gap junction channels is necessarily involved. IP3 can traverse at least some gap junction pores (21), and microinjected IP3 can elicit calcium waves (22), but these observations do not necessarily imply that the quantity of IP3 generated in the stimulated cells is sufficiently great to release calcium stores in neighboring cells after traversing gap junctions. This situation is further complicated by the presence of another propagation mechanism for intercellular calcium waves that requires release of intercellular calcium stores, but that does not occur via gap junctional communication. In this second mechanism, calcium waves appear to be mediated by activation of purinergic receptors, presumably by secreted ATP. This mechanism was first defined in rat mast cells and basophil leukemic cells (19), and has also been reported in hepatocytes (24) and the RINm5f (RIN) insulinoma cell line (3). Studies in these cells suggest that mechanical stimulation results in activation of ATP receptors on neighboring cells. Receptors of the P2Y class are G protein–coupled receptors that activate phospholipase C, resulting in generation of IP3 and release of intracellular calcium stores (16). In particular the P2U (P2Y2) receptor, which is activated by both ATP and UTP, has been implicated in these waves. This receptor is found on many different cell types.
We have recently demonstrated that RIN cells, which are not coupled by gap junctions, propagate intercellular calcium waves, most likely by activation of purinergic receptors (3). RIN cells are “excitable,” and express L-type, voltage-gated calcium channels. Thus when we expressed the gap junction protein Cx43 in these cells, we could identify a second type of intercellular calcium wave in the RIN/ Cx43 transfectants, which required ionic coupling, membrane depolarization, and activation of voltage-gated calcium channels, and also did not require release of intracellular calcium stores. This mechanism is not likely to depend on generation of IP3 and is distinct from the mechanism proposed above for respiratory epithelial cells; it may be an important signaling pathway in many excitable cells, and has been reported in neurons (6).
In the current studies, we investigated intercellular calcium wave propagation in two osteoblastic cell lines that differ in their expression of gap junction proteins and purinergic receptors. In previous studies we found that the rat osteosarcoma cell lines ROS 17/2.8 (ROS) and UMR 106-01 (UMR) differ in the gap junction proteins they express and in their ability to pass negatively charged dyes such as Lucifer yellow, carboxyfluorescein, and calcein. ROS cells express Cx43 on the plasma membrane and are well dye coupled, but UMR predominantly express Cx45 on the cell surface and are poorly dye coupled (25). These and other studies (11) demonstrated that Cx45 gap junction pores are less permeable to negatively charged dyes than are Cx43 pores. In addition, as shown in this study, UMR cells express P2U purinergic receptors, but ROS cells do not. Our results show that P2U receptors and Cx43 play distinct roles in the propagation of intercellular calcium waves. We provide evidence in nonexcitable cells that ATP-mediated calcium waves release intracellular calcium stores, and that propagation of gap junction–mediated calcium waves does not require activation of these stores. We performed further experiments in hamster tracheal epithelia, to more nearly approximate the most thoroughly developed model in which gap junction–mediated calcium waves are felt to be mediated by IP3, and obtained results suggesting that calcium waves in these cells were mediated by purinergic receptors.
Materials and Methods
Cells and Chemicals
The rat osteoblastic cell lines ROS 17/2.8 and UMR 106-01 were grown in MEM (Life Technologies, Grand Island, NY) supplemented with 10% heat-inactivated calf serum, nonessential amino acids, 1 mM sodium pyruvate, 2 mM l-glutamine and penicillin/streptomycin. ROS cells expressing the P2U purinergic receptor (ROS/P2U) were generated by transfecting ROS with a human P2U construct in the pREP9 mammalian expression vector, which was provided by Dr. G. Weisman (University of Missouri, Columbia, MO). Cells were transfected according to the calcium phosphate precipitation method and selected in 400 μg/ml Geneticin. Hamster tracheal epithelia cells (HTE), provided by Dr. W. Goldman (Washington University School of Medicine, St. Louis, MO), were grown in F-12 medium supplemented with 10% heat-inactivated calf serum, 2 mM l-glutamine, and penicillin/streptomycin. All cells were grown in a humidified atmosphere containing 5% CO2. For imaging studies, cells were plated on 25-mm glass coverslips for 2–4 d and used at 70–90% confluence.
The fluorescent calcium indicators fura-2 and fluo-3 were from Molecular Probes, Inc. (Eugene, OR). Thapsigargin, an inhibitor of the calcium-ATPase in the endoplasmic reticulum, was purchased from Calbiochem-Novabiochem Corp. (La Jolla, CA) and used in a concentration of 50 nM. The ATP receptor antagonist suramin (Res. Biochems. Inc., Natick, MA), was used in a final concentration of 100 μM. Other chemicals were from Sigma Chemical Co. (St. Louis, MO). Heptanol, a gap junction inhibitor, was made freshly before experiments as a 1:4, heptanol/ethanol solution and used in final concentrations of 2.0 or 3.5 mM.
Calcium Imaging
Measurement of intracellular calcium concentration [Ca2+]i, was performed using the calcium indicator dyes fura-2 and fluo-3. Cells adherent to 25-mm-diam, No. 1 thickness glass coverslips were incubated at 37°C in PBS with 6 mM glucose and 20 mM sodium bicarbonate (PBSG) containing 5 μM fura-2/AM for 30 min or 5 μM fluo-3/AM for 20 min, and then incubated an additional 20 min in medium without dye. Coverslips were affixed to a Teflon chamber and mounted in a PDMI-2 open perfusion microincubator (Medical Systems Corp., Greenvale, NY) maintained at 37°C with superfused CO2 on a Zeiss Axiovert 35 microscope (Carl Zeiss Inc., Thornwood, NY). Imaging was performed using an IM-4000 system (Georgia Instruments, Roswell, GA). Fura-2 ratio images were acquired using excitation wavelengths of 340 and 380 nm. Relative increase in [Ca2+]i were assessed with fluo-3 using an excitation wavelength of 488 nm. Probenecid (1 mM) was added throughout to prevent dye leakage (10). For fura-2 experiments, ratio images were calibrated using fura- 2–free acid in buffers of known calcium concentration (Molecular Probes Inc.). Background fluorescence was assessed by quenching fura-2 fluorescence with 5 mM Mn2+ in the presence of BrA23187. For fluo-3 experiments, images taken before stimulation, indicating the resting calcium concentration, were subtracted from images taken after stimulation of cells. The subtracted images therefore represent the increase in dye fluorescence after stimulation. Calcium waves were induced by stimulating a single cell with a borosilicate glass pipette affixed to an Eppendorf 5171 micromanipulator (Eppendorf, Inc., Madison, WI), and recording images at intervals. Conduction velocities of calcium waves were estimated by measuring the distance and amount of time required for the wave to spread from the stimulated cell to the cells at the periphery of the video field.
Calcium Measurements by Fluorescence Spectrophotometry
Intracellular calcium was quantitated in cells in suspension as follows. Cells (107 cells/ml were suspended in medium containing 5 μM fura-2 and 1 mM probenecid for 20 min at 37°C, diluted 10-fold, incubated at 37°C for 20 min, washed, and then resuspended in PBSG with probenecid. Cells were kept at room temperature until used for experiments. Cells were placed in cuvettes (106 cells in 2 ml per cuvette) maintained at 37°C and stirred. Ratio pairs (340/380 nm excitation; 510 nm emission) were assessed with a Hitachi F-2000 fluorescence spectrophotometer (Hitachi Instruments, Inc., San Jose, CA).
RNA Blot
RNA blots were performed as previously described (25). Briefly, total cellular RNA was isolated using guanidinium isothiocyanate (7), resolved on formaldehyde-agarose gels, and transferred to nylon membranes. The membranes were hybridized to 32P-labeled probes, the final stringency wash was with 30 mM sodium phosphate and 1% SDS at 55°C, and hybridizing bands were detected by autoradiography.
Results
ROS and UMR Cells Propagate Calcium Waves with Different Kinetics
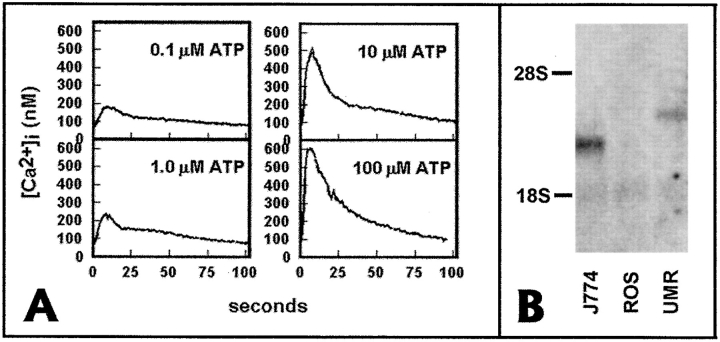
In these studies, we elicited intercellular calcium waves by mechanical stimulation of single cells in monolayers of two rat osteoblastic cell lines, ROS and UMR. We previously demonstrated that ROS cells express Cx43 on the cell surface and are well dye coupled, but that UMR cells express predominantly Cx45 on the plasma membrane and are poorly dye coupled (25). When Lucifer yellow was microinjected into single cells, it diffused into 9.1 cells per injected cell in ROS, and 1.4 cells per injected cell in UMR (25). Because ATP receptors as well as gap junctional communication have been implicated in calcium wave propagation, we measured the calcium response in UMR and ROS after the addition of 1 mM ATP or UTP. Both nucleotides caused a rise in [Ca2+]i in UMR, but not in ROS (not shown). Concentrations of ATP as low as 0.1 μM were able to elicit calcium transients in UMR cells (Fig. 1). Response to both ATP and UTP suggested that UMR cells might express receptors of the P2U (P2Y2) class, which appear to be the most commonly observed receptors of the P2Y class. We therefore performed RNA blots on total RNA from ROS and UMR, using as a probe human P2U cDNA (Fig. 1). UMR, but not ROS, expressed a 3-kbp hybridizing band, consistent with rat P2U (17). The mouse macrophagelike cell line J774, used as a control in these experiments, demonstrated a faster-migrating band of ∼2.4 kbp, consistent with mouse P2U (17). Thus ROS and UMR cells differ both in gap junctional communication and in expression of P2U receptors.
Figure 1.
UMR cells, but not ROS cells express P2U receptor mRNA. (A) Calcium transients elicited by different concentrations of ATP in UMR cells. Fura-2–loaded UMR cells were placed in a stirred cuvette at 37°C, and 340:380 ratios were obtained. (B) Total cell RNA (10 μg) from ROS, UMR, and the mouse macrophage cell line J774 was electrophoresed on agarose-formaldehyde gel, transferred to nylon, and hybridized with a radiolabeled cDNA probe for the human P2U receptor. Both J774 cells and UMR cells expressed a hybridizing band of the appropriate size for mouse and rat P2U, respectively, but no band was detected in ROS cell RNA.
In both ROS and UMR cell lines, mechanical stimulation increased [Ca2+]i in the stimulated cell and subsequently induced a wave of calcium transients spreading to adjacent cells in the monolayer. However, intercellular calcium waves in ROS monolayers were much slower, and propagated to fewer cells, than calcium waves in UMR monolayers. In monolayers of ROS cells, calcium waves spread 5–15 neighboring cells over several minutes (Fig. 2). Although the rate of propagation of these waves showed variability depending on cell density and geometry, these “slow” waves in ROS cells generally propagated at a rate of ∼0.5 μm/s. Frequently there was a time lag of 15–30 s between the calcium rise in one cell and its neighbor. In contrast, the poorly dye-coupled UMR cells transmitted waves to most of the cells in the field of view (30–50 cells) within 15–20 s (Fig. 3), and with little time lag between neighboring cells. The velocity of propagation was ∼10 μm/s in UMR. In some experiments, the stimulated cell was in fact ruptured, as demonstrated by loss of dye from that cell. We detected no differences in the propagated calcium wave between experiments where cells were ruptured and experiments where cells were not ruptured. In either instance, ROS cells demonstrated slower calcium waves and UMR cells demonstrated faster calcium waves.
Figure 2.
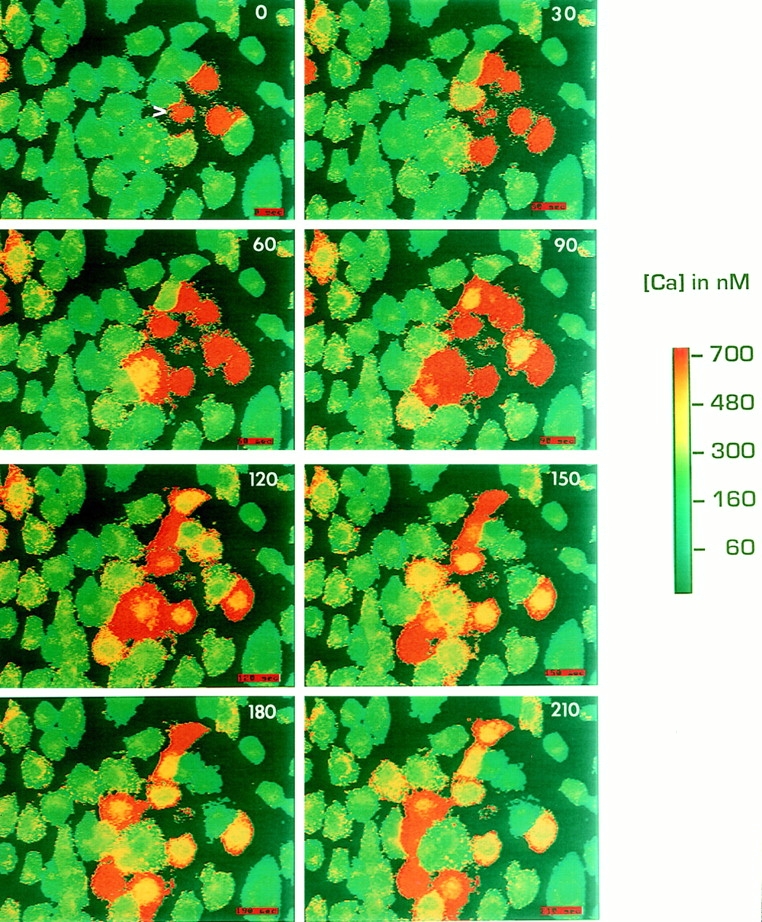
ROS cells propagate slow intercellular calcium waves. Monolayers of ROS cells were loaded with fura-2, and a single cell (arrowhead) was mechanically stimulated during fluorescence ratio imaging. Time after stimulation in seconds is indicated on each panel. The pseudocolor map represents the estimated [Ca2+]i.
Figure 3.
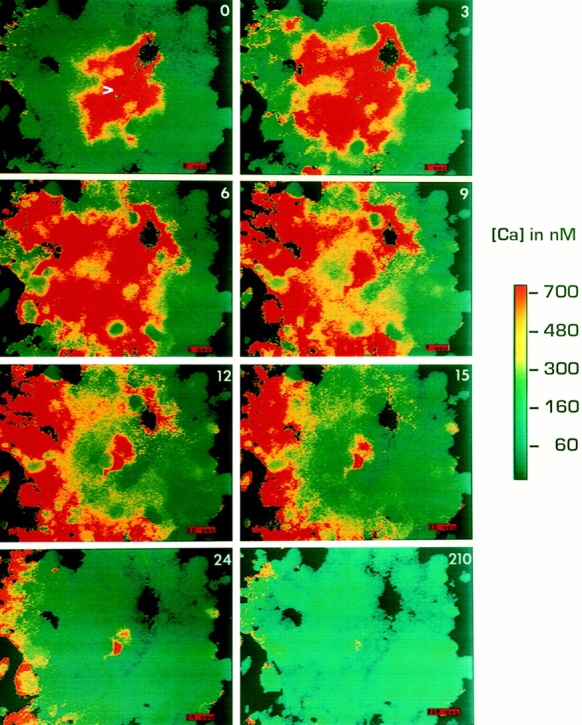
UMR cells propagate fast intercellular calcium waves. Methods as in Fig. 2.
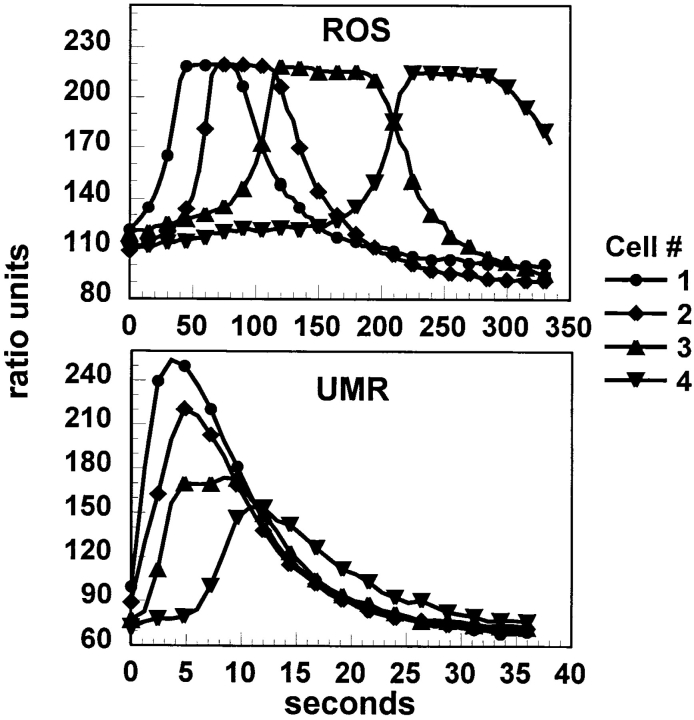
To demonstrate the differences in the kinetics of these calcium waves graphically, we identified four cells in a row extending outward from the mechanically stimulated cell, and measured fura-2 ratios in these cells over time (Fig. 4). These traces confirm the dramatic differences in calcium wave kinetics between the two cell lines. It should be noted that these traces are presented to demonstrate kinetics and not the intensity of the response. Thus they are presented in relative fluorescence ratios, and the ROS cells demonstrate a saturating response. It should also be noted that although UMR cells in these traces demonstrate an attenuating response, frequently the response in these cells does not attenuate significantly, as is seen in Fig. 3.
Figure 4.
Kinetics of calcium wave propagation in ROS and UMR cells. Four cells in a row from the stimulated cells were analyzed from the sequences shown in Figs. 2 and 3. Data are presented as relative fluorescence ratio.
Calcium Waves in UMR, but Not ROS, Require Release of Intracellular Calcium Stores
The differences in kinetics and extent of the calcium waves in ROS and UMR cells suggested that two different mechanisms were responsible for these waves. Because most intercellular calcium waves that have been analyzed require the release of intracellular calcium stores, we asked whether this was true for ROS and UMR calcium waves by depleting intracellular calcium stores with thapsigargin, an inhibitor of the calcium-ATPase of the endoplasmic reticulum (Fig. 5). UMR monolayers were loaded with the calcium indicator dye fluo-3, and single cells were mechanically stimulated to confirm that calcium wave propagation occurred (average 25 cells per stimulated cell, n = 3). Subsequently, 50 nM thapsigargin was added to the bath solution, causing a rapid and prolonged increase in [Ca2+]i as calcium leaked from the intracellular stores. The [Ca2+]i gradually returned nearly to resting levels within 30 min, at which time intracellular calcium stores were depleted, as demonstrated by lack of calcium response to ATP in UMR cells. At this time single cells were again mechanically simulated. The calcium waves in UMR cells were blocked (zero cells per stimulated cell, n = 3) although the stimulated cell still showed an increase in [Ca2+]i. In contrast, intercellular calcium wave propagation in ROS cells were similar before (average six cells per stimulated cell, n = 6) and after (average 5.5 cells per stimulated cell, n = 3) depletion of intracellular calcium stores with 50 nM thapsigargin. Thus UMR calcium waves required release of intracellular calcium stores but ROS waves did not.
Figure 5.
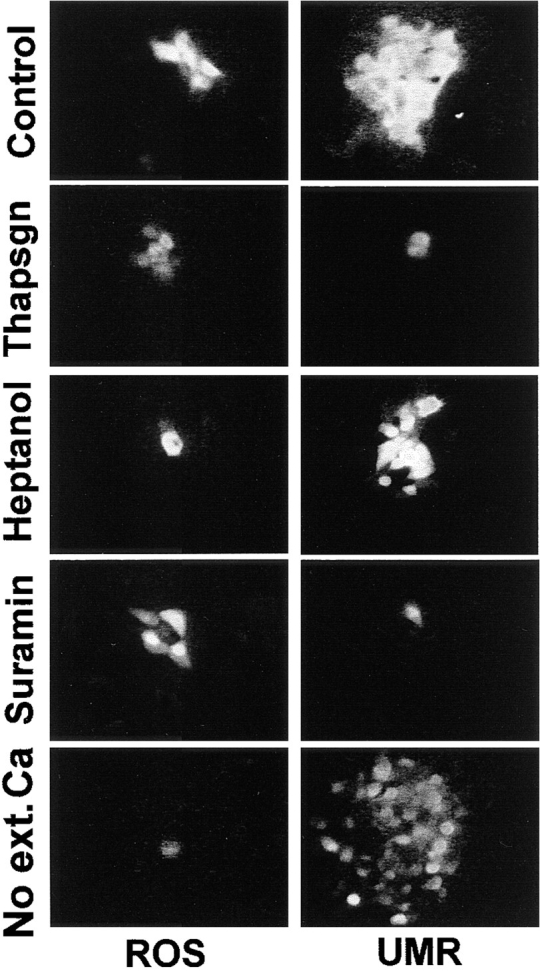
Inhibition of calcium waves in ROS and UMR cells. Adherent cells were loaded with fluo-3, and thapsigargin, heptanol, or suramin was added, or extracellular calcium was removed, as described in the text. Intercellular calcium waves were initiated by mechanical stimulation. Panels were obtained by subtracting a frame taken immediately before mechanical stimulation from a frame taken at the time of maximal wave propagation. Note that, as described in the text, heptanol blocks calcium wave propagation in UMR cells in many cases, and this occurs when heptanol also inhibits ATP-induced calcium transients. The panel shown is the typical response in experiments where ATP-mediated calcium transients remain intact after heptanol treatment.
We then asked whether ROS and UMR could propagate calcium waves in the absence of extracellular calcium. For these experiments, calcium waves were demonstrated, the cells were washed in calcium-free medium, this medium was then added to the monolayer and again wave propagation was assessed (Fig. 5). Calcium waves were not seen after mechanical stimulation of ROS cells under these conditions (n = 4). In contrast, UMR cells continued to propagate calcium waves in the absence of extracellular calcium (>50 cells per stimulated cell, n = 3). It is worth noting that calcium waves in UMR cells appeared to spread farther in the absence of extracellular calcium than in the presence of extracellular calcium, although we have not explored possible reasons for this finding.
UMR Cell Calcium Waves Do Not Require Gap Junctional Communication
We then asked whether calcium waves in these osteoblastic cells required gap junctional communication. Heptanol is a widely used inhibitor of gap junctional communication, and we have previously demonstrated that heptanol blocks gap junctional transfer of microinjected Lucifer yellow in these cells (25). We asked whether heptanol inhibited intercellular calcium waves in UMR cells as follows. Resting calcium images were taken of fluo-3–loaded cells, and calcium waves were elicited before addition of heptanol. Next, the medium was exchanged for medium containing 3.5 mM heptanol, and after 5 min, cells were stimulated mechanically (Fig. 5). Finally, 1 mM ATP was added to the solution to determine whether heptanol affected ATP receptors under these conditions. Using this protocol we found that heptanol did not block intercellular calcium waves in UMR cells (n = 11), and also did not inhibit the calcium transient induced by subsequent addition of ATP. Ethanol alone in the quantities used in these experiments had no effect on intracellular calcium, on calcium wave propagation, or on gap junctional communication (not shown).
When heptanol was added directly to the cell monolayers, calcium wave propagation was inhibited in 11 of 19 attempts. However, whenever heptanol blocked calcium waves in these experiments, there was no subsequent calcium response to ATP, and conversely, where heptanol failed to block calcium waves, there was a subsequent response to ATP. These experiments demonstrate that heptanol must be used with caution in such experiments. ROS cell calcium waves were inhibited by heptanol (Fig. 5), and heptanol consistently blocked gap junctional communication as assessed by dye transfer, with both addition protocols (data not shown).
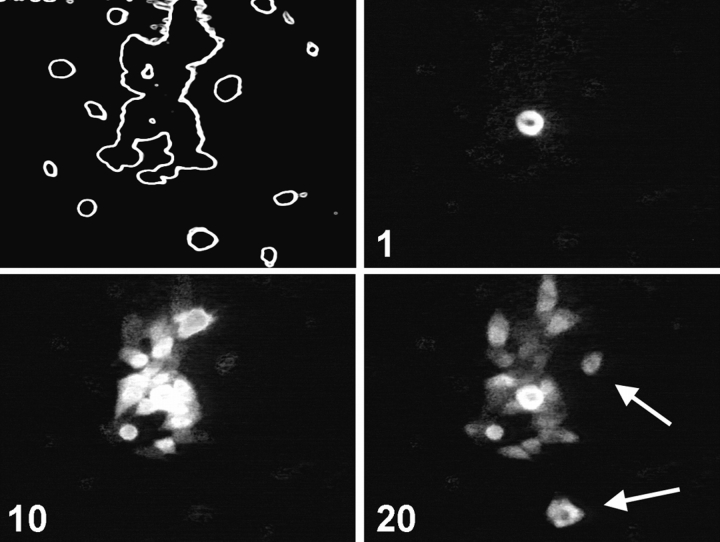
To confirm that calcium waves in UMR cells occur independently of gap junctional communication, we performed experiments using subconfluent cultures in which independent islands of cells could be observed. These islands of cells were not in physical contact as assessed by phase microscopy. We then induced intercellular calcium waves by mechanical stimulation in single cells within these monolayers. Stimulation of single UMR cells resulted in the rapid spread of a calcium wave to all the cells within the small cell islands. In addition, the calcium wave spread with a short time lag to cells in nearby cell islands that were not in physical contact with the stimulated cell (Fig. 6). When we performed similar experiments in cultures of ROS cells, spread of calcium waves between islands of cells was never observed. These experiments confirm that gap junctional communication is not required for UMR calcium waves, and are consistent with a role for gap junctional communication in ROS calcium waves.
Figure 6.
Calcium waves are propagated among islands of UMR cells. Calcium waves were induced in a subconfluent monolayer of fluo-3–loaded UMR cells. The outline of cells in the field of view is shown in the upper left panel. A cell in the middle of the cell island was stimulated, and images were taken at intervals. Cells that propagated the calcium wave but were not in physical contact with the stimulated cell are indicated by arrows. Time after stimulation in s is shown on each panel.
UMR Cell Calcium Waves Require Activation of P2U Receptors
Since UMR cell calcium waves required release of intracellular calcium stores but did not involve gap junctional communication or require cell contact, we asked whether these calcium waves involved activation of P2U receptors. The inhibitor suramin has been widely used to prevent ATP-induced calcium transients, although it is not totally specific for inhibition of purinergic receptors. In preliminary experiments, we found that 100 μM suramin was required to effectively prevent P2U-mediated calcium responses (not shown). This concentration of suramin blocked propagation of mechanically induced calcium waves in UMR cells, even though the stimulated cell showed a calcium rise (Fig. 5). In these experiments, calcium waves spread to an average of 20 cells (n = 8) before suramin, and to an average of one cell (n = 10) after suramin. In contrast, 100 μM suramin did not inhibit calcium wave propagation in ROS cells (Fig. 5; n = 4), nor did it inhibit intercellular transfer of microinjected Lucifer yellow (not shown). Thus, although suramin may affect a number of cellular processes, we were able to use it to distinguish between calcium waves in ROS and UMR cells, and it had no effect on gap junctional communication.
The above experiments suggested that intercellular calcium waves in UMR cells were mediated by activation of purinergic receptors. P2U purinergic receptors, like many other G protein–coupled receptors, undergo prolonged desensitization after stimulation with ligand. After exposure to 10 μM or 1 mM ATP and change of medium, UMR cells failed to respond to subsequent addition of ATP for at least 15 min (not shown). The failure to respond to subsequent challenge with ATP was not due to depletion of intracellular calcium stores, because subsequent challenge with thrombin (20 U/ml), which activates another G protein–coupled receptor in these cells (1), resulted in cytosolic calcium transients. Depletion of intracellular calcium stores with 50 nM thapsigargin for 30 min inhibited thrombin-induced calcium transients (20 U/ml thrombin) in UMR cells, demonstrating that thrombin- induced calcium transients required release of intracellular calcium stores as did ATP-mediated responses (not shown). We therefore assessed the effect of P2U receptor desensitization on calcium waves in UMR cells as follows (n = 4, Fig. 7). Cells were loaded with 5 μM fura-2, and calcium waves were demonstrated. Next, 10 μM ATP was added, which resulted in a calcium transient in all cells. After 2 min, [Ca2+]i returned to resting levels, and single cells were mechanically stimulated. This resulted in a rise in [Ca2+]i in the stimulated cell, but no calcium wave was initiated or propagated in the monolayers. Finally, 20 U/ml thrombin was added, resulting in an increase in [Ca2+]i, demonstrating that there remained releasable calcium stores. The same results were obtained using 1 mM ATP before mechanical stimulation.
Figure 7.
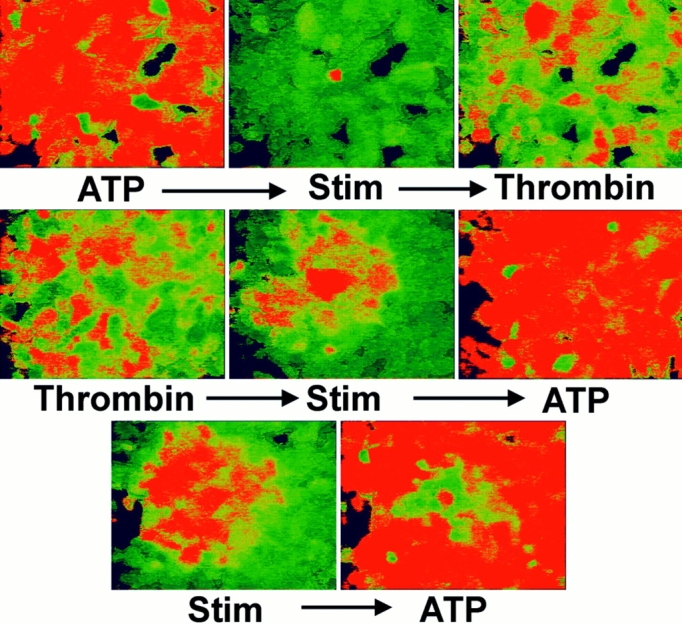
Desensitization of P2U receptors inhibits calcium waves in UMR cells. Ratio imaging was performed on fura-2–loaded UMR cell monolayers. Top panels: left, 1 mM ATP-elicited calcium transients; center, subsequent mechanical stimulation failed to induce intercellular calcium waves; right, subsequent challenge with 20 U/ml thrombin revealed that calcium stores were not depleted. Middle panels: left, thrombin-induced calcium transients; center, subsequent mechanical stimulation induced a calcium wave; right, subsequent addition of ATP induced calcium transients. Bottom panels: propagated calcium waves (left) frequently desensitized a local area to subsequent addition of ATP (right).
To confirm that the inhibition of calcium waves by prior addition of ATP was due to desensitization of the P2U receptor we asked whether stimulation of the thrombin receptor had a similar effect. Adherent UMR cells were stimulated with 20 U/ml thrombin, which elicited a calcium response in the monolayer. After the initial calcium transient elicited by thrombin subsided, single cells were mechanically stimulated, eliciting a calcium wave that was identical to that seen in untreated cells (n = 3, Fig. 7). Addition of 10 μM ATP subsequently elicited a rise in intracellular calcium concentration in almost all cells, although cells in the central area of the propagated calcium wave frequently did not respond to subsequent challenge with ATP, presumably because of desensitization induced by the initial calcium wave (Fig. 7, bottom). Thus activation of signal transduction pathways that resulted in release of intracellular calcium stores was not by itself sufficient to inhibit subsequent mechanically induced intercellular calcium waves; rather, desensitization of the P2U receptor itself was required.
ROS/P2U and UMR/Cx43 Transfectants Propagate Both Gap Junction– and ATP-dependent Calcium Waves
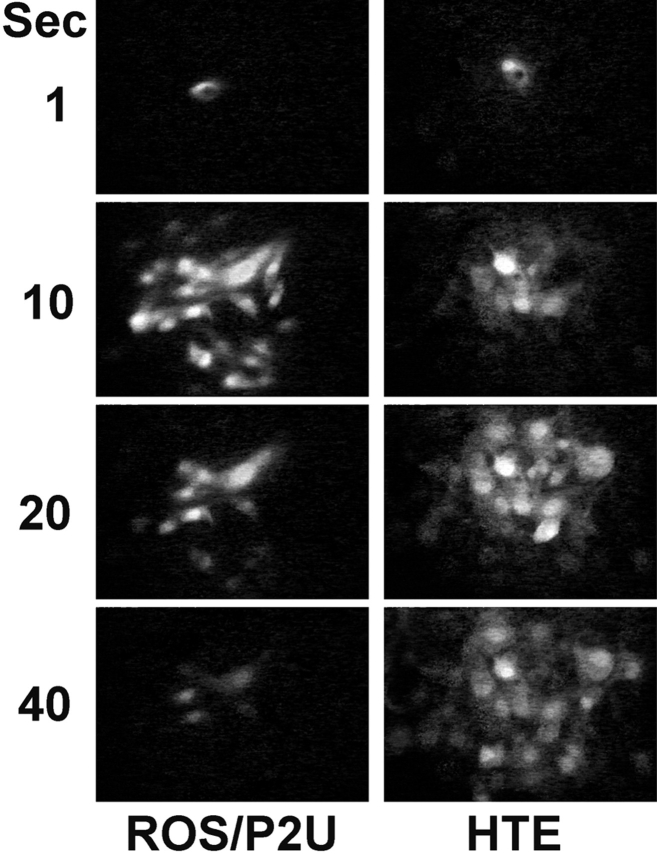
The above studies demonstrated that mechanically induced intercellular calcium waves in the UMR cell line were propagated by activation of P2U purinergic receptors. Because UMR cells expressed P2U receptors but ROS cells did not, we then asked whether expression of P2U in ROS cells was sufficient to allow ROS cells to propagate UMRlike calcium waves. ROS cells were transfected with a construct containing the human P2U receptor cDNA. Unlike the parent ROS cells, ROS/P2U transfectants responded to addition of ATP with a rise in [Ca2+]i (not shown). Mechanical stimulation of ROS/P2U cells (n = 8) elicited intercellular calcium waves that were much faster than the waves in the parent ROS cells and slightly slower than UMR calcium waves (average conduction velocity = 7.4 μm/s), with a maximal extension of the wave to 8–38 cells (Fig. 8; Table I). Maximal extension of the wave was reached after 15–20 s. We confirmed that these fast calcium waves did not involve gap junctional communication by demonstrating that 3.5 mM heptanol had no effect on the speed or extent of propagation (15–44 cells per stimulated cell, n = 4). In contrast, desensitization with 1 mM ATP as described above inhibited the fast UMRlike waves, and uncovered the slow ROS waves with extension to 3–4 cells (n = 4). The combination of 3.5 mM heptanol and 1 mM ATP desensitization resulted in a complete block of wave propagation, where only the stimulated cell showed an increase in intracellular calcium in all experiments (n = 4). These results demonstrated that expression of the P2U receptor in ROS cells was sufficient to allow these cells to propagate gap junction–independent calcium waves via activation of purinergic receptors in addition to the gap junction–dependent calcium waves that these cells normally expressed. These experiments also demonstrated that ROS cells are capable of releasing intracellular calcium via an IP3-dependent mechanism, when such a mechanism is activated.
Figure 8.
Calcium waves in ROS/P2U transfectants and HTE cells. ROS/P2U transfectants (left) and HTE cells (right) were loaded with fluo-3 and images were taken after a mechanical stimulation of a single cell. Panels are images taken at the indicated time after subtraction of the prestimulation fluo-3 image.
Table I.
Extent of Intercellular Calcium Waves in Osteoblast Transfectants and HTE
| ROS/P2U | UMR/Cx43 | HTE | ||||
|---|---|---|---|---|---|---|
| Control | 18 ± 9 (8) | 33 ± 7 (9) | 13 ± 5 (25) | |||
| Heptanol (3.5 mM) | 28 ± 12 (4) | 33 ± 6 (4) | 13 ± 2 (3) | |||
| ATP (1mM) | 3 ± 2 (4) | 4 ± 2 (5) | 0.5 ± 1 (12) | |||
| Thapsigargin (50 nM) | N.D. | 2 ± 1 (9) | N.D. | |||
| Heptanol > ATP | 0 (4) | N.D. | N.D. |
Results are expressed as mean number of cells involved in calcium wave ± SD (number of experiments). N.D., not done.
The above experiments showed that UMR cells and ROS/P2U cells propagated calcium waves via activation of P2U receptors. These waves required release of intracellular calcium stores, and presumably required IP3 generation that is known to occur after ligation of P2U receptors. UMR cells are coupled by gap junctions comprised mostly of Cx45, and are electrically coupled (25), but Cx45 pores are less permeable to negatively charged molecules than are Cx43 pores. Thus it was possible that UMR cells did not propagate gap junction–dependent calcium waves because Cx45 gap junctions did not allow adequate coupling of cells to permit this form of gap junctional communication. For instance, IP3 might be expected to traverse Cx43 gap junction pores more efficiently than Cx45 gap junction pores. We therefore assessed intercellular calcium waves in UMR/Cx43 transfectants, which pass microinjected Lucifer yellow much more efficiently than parent UMR cells, and about half as well as ROS cells (25). After mechanical stimulation the UMR/Cx43 cells propagated calcium waves to 25–50 cells (n = 9) with kinetics similar to parent UMR cells (average conduction velocity = 10 μm/s; see Table I). As with the parent UMR cells, desensitization with 1 mM ATP inhibited the fast calcium waves in UMR/ Cx43 cells, and uncovered a slow ROS-like wave that extended to 2–6 cells (n = 5). The same phenomenon occurred after thapsigargin treatment: addition of 50 nM thapsigargin for 30 min inhibited the fast UMR-like wave, and unmasked a slow wave, which proceeded to as many as four cells with kinetics similar to the ROS calcium waves (n = 10). Heptanol (3.5 mM), but not 1 mM ATP pretreatment, inhibited dye transfer in these cells (not shown). Thus transfection of Cx43 into UMR cells resulted in the expression of a new kind of calcium wave in these cells. This calcium wave, although dependent on Cx43-mediated gap junctional communication, did not require release of IP3-sensitive intracellular stores.
Calcium Waves in HTE Cells
In our experiments with ROS cells, UMR cells, and the above transfectants, as well as in our previous studies with Cx43-transfected rat insulinoma cells (3), we were unable to identify an instance where gap junctional communication allowed the propagation of calcium waves that required release of intracellular calcium stores, even with cells that were well dye coupled and capable of releasing such stores. This model for the propagation of intercellular calcium waves has been defined mostly in experiments using rabbit respiratory epithelial cells (22). Respiratory epithelia also express well-characterized P2U receptors (20), raising the possibility that intercellular calcium waves generated by mechanical stimulation in this model might instead be mediated by the autocrine activity of ATP. We therefore assessed mechanically-stimulated calcium waves in HTE cells (13). These cells propagated calcium waves with an average velocity of 5 μm/s, slower than those seen in UMR cells and in ROS/P2U transfectants, but much faster than those of ROS cells (n = 25; Fig. 8). The maximal extension was reached 30–40 s after the initial mechanical stimulation of a single cell, and the wave propagated to 5–30 cells.
Further experiments, similar to those described above, suggested that the calcium waves in the HTE cells were propagated by activation of purinergic receptors and not by gap junctional communication. Addition of 1 mM ATP to these cells elicited calcium transients, confirming purinergic receptors in these cells (not shown). ATP treatment resulted in a subsequent block of calcium wave propagation with a rise in intracellular calcium in the stimulated cell and sometimes an adjacent cell (n = 12; Table I). Subsequent addition of thrombin elicited a rise in intracellular calcium in most cells, showing again that the inhibition of the waves was not due to emptying of the intracellular IP3-releasable calcium stores (n = 5, not shown). Heptanol did not inhibit the calcium waves in the tracheal epithelia cells (15 cells before heptanol, 13 cells after heptanol, n = 3). The wave still propagated to 11–15 cells, suggesting that the intercellular calcium waves in these cells were not mediated by gap junctional communication, and involved purinergic receptors in the mechanism of propagation.
Discussion
In these studies, summarized in Table II, we found that two osteoblastic cell lines propagated intercellular calcium waves with strikingly different kinetics and by different mechanisms, and have identified the proteins responsible for these differences. In ROS cells, calcium waves were propagated by gap junctional communication and influx of extracellular calcium. These slower calcium waves were reconstituted in UMR cells by transfecting UMR with the gap junction protein Cx43. In UMR cells calcium waves were transmitted from cell to cell by activation of P2U purinergic receptors, which caused release of intracellular calcium stores, presumably by generation of IP3. Introduction of the human P2U receptor into ROS cells was sufficient to elicit calcium waves that mimicked UMR calcium waves.
Table II.
Summary of Calcium Experiments
| Calcium wave condition | ROS | UMR | ROS/P2U | UMR/Cx43 | HTE | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Slow | Fast | Fast | Fast | Fast | |||||
| Depletion of calcium stores | Slow | None | N.D. | Slow | N.D. | |||||
| Heptanol (gap junction inhibitor) | None | Fast | Fast | Fast | Fast | |||||
| Between isolated cell clusters | No | Yes | N.D. | N.D. | N.D. | |||||
| Suramin (P2 inhibitor) | Slow | None | N.D. | N.D. | N.D | |||||
| ATP desensitization | Slow | None | Slow | Slow | None |
“Fast” denotes wave that propagated at a rate ⩾5 μm/s; “slow” wave propagated at a rate ⩽1 μm/s. See text for calculated conduction velocties. N.D., not done.
The slow calcium waves in ROS cells comprise a novel mechanism for the propagation of intercellular calcium waves that required gap junctional communication, but did not require release of intracellular calcium stores. These data imply that ROS calcium waves are generated by the passage of a signal through Cx43 gap junctions that results in activation of a plasma membrane calcium channel either directly or indirectly. We have not identified the calcium channel responsible for calcium influx in these cells, nor have we identified the mechanism by which Cx43 permits activation of these channels. However, this mechanism is unlikely to be similar to the characterized mechanisms for intercellular calcium wave propagation that require gap junctional communication. It is unlikely to be an IP3-mediated mechanism as described in rabbit tracheal epithelia cells because intracellular calcium stores are not required. In RIN/Cx43 transfectants, Cx43 propagates intercellular calcium waves by electrically coupling cells and thereby allowing activation of voltage-dependent calcium channels (3). This mechanism is not responsible for calcium waves in our ROS cells because membrane depolarization does not elicit calcium transients in these cells (data not shown). UMR/Cx43 cells were able to propagate ROS like calcium waves but the parent UMR cells were not. This result may indicate that there is a threshold level of intercellular communication that is required to permit a sufficient amount of the signal to move between cells and activate calcium influx. A more intriguing possibility is that the signal that moves through gap junctions in ROS cells and activates calcium influx in neighboring cells can traverse Cx43 gap junctions more efficiently than Cx45 gap junctions. We have previously shown that Lucifer yellow and calcein pass through Cx43 pores more efficiently than Cx45 pores; thus, negatively charged molecules of a size similar to these dyes would be good candidates for the intercellular messenger molecule in ROS calcium waves.
Calcium waves in UMR cells did require release of intracellular calcium stores, but did not require gap junctional communication. Much of the current literature suggests that mechanically-induced intercellular calcium waves are usually propagated by the passage of IP3 through gap junctions and the subsequent release of intracellular calcium stores in neighboring cells. Although we had initially assumed that transjunctional diffusion of IP3 was a likely mechanism to account for calcium wave propagation in ROS or UMR cells, we were unable to find evidence that this mechanism occurred in either of these cell lines. This was true even though the cells were capable of releasing intracellular calcium stores by mechanisms that are known to involve IP3 generation, and most of the cell types were well coupled via gap junctions. Furthermore, calcium waves in HTE cells were inhibited by desensitization of ATP receptors, but not by heptanol, implying that activation of P2U receptors, and not gap junctional transmission of IP3, was responsible for inducing the release of IP3-sensitive stores. One possible reason for the differences between our data and that of others is that for some reason we are generating less IP3 by mechanical stimulation, either because of technical differences or differences in cells. Another possible explanation is that gap junction proteins other than Cx43 are required to transmit sufficient quantities of IP3 from cell to cell to induce calcium store release. A third possibility is that although IP3 can traverse at least some gap junctional pores, gap junctional communication does not allow sufficient passage of IP3 from cell to cell to propagate intercellular calcium waves under most circumstances. It should be noted that our data do not exclude the possibility that gap junctional communication might play a modulatory role in calcium waves mediated by P2U receptor activation.
Our data define distinct calcium waves that are mediated by gap junctional communication and by activation of P2U receptors. Intercellular calcium waves mediated by Cx43 did not require release of intracellular stores, and must therefore require activation of plasma membrane calcium channels. In contrast, intercellular calcium waves mediated by activation of purinergic receptors did involve release of intracellular calcium stores. P2U receptors have a very wide tissue distribution as do IP3-sensitive calcium stores and it is therefore likely that activation of these receptors is a very common mechanism by which calcium responses can be transmitted among cells that are in close proximity to each other.
Acknowledgments
We thank Ms. A. Johnson and Ms. L. Obermoeller for technical assistance, and Dr. M. Koval for extensive assistance with the manuscript.
This work was supported by grants from the National Institutes of Health (DK46686 and GM45815) to T.H. Steinberg, as well as grant No. 9502125 from the Danish Research Council, and from Løvens Kemiske Fabrik's Forskningsfond and Fhv. Direktør Leo Nielsen og hustru Karen Margrethe Nielsen's Legat for Laegevidenskabelig Grundforskning to N.R. Jørgensen.
Abbreviations used in this paper
- Cx43 and Cx45
connexin 43 and 45
- HTE
hamster tracheal epithelia
- IP3
inositol trisphosphate
Footnotes
Address all correspondence to Thomas H. Steinberg, Washington University School of Medicine, Infectious Diseases, Box 8051, 660 South Euclid Avenue, St. Louis, MO 63110. Tel.: (314) 362-9218. Fax: (314) 362-9218. E-mail: steinber@id.wustl.edu
N.R. Jørgensen's current address is Osteoporosis Research Center, Copenhagen Municipal Hospital, Oster Farimagsgade 5, DK-1399 Copenhagen K, Denmark.
References
- 1.Babich M, Choi H, Johnson RM, King KL, Alford GE, Nissenson RA. Thrombin and parathyroid hormone mobilize intracellular calcium in rat osteosarcoma cells by distinct pathways. Endocrinology. 1991;129:1463–1470. doi: 10.1210/endo-129-3-1463. [DOI] [PubMed] [Google Scholar]
- 2.Boitano S, Dirksen ER, Sanderson MJ. Intercellular propagation of calcium waves mediated by inositol trisphosphate. Science (Wash DC) 1992;258:292–295. doi: 10.1126/science.1411526. [DOI] [PubMed] [Google Scholar]
- 3.Cao D, Lin G, Westphale EM, Beyer EC, Steinberg TH. Mechanisms for the coordination of intercellular calcium signaling in insulin-secreting cells. J Cell Sci. 1997;110:497–504. doi: 10.1242/jcs.110.4.497. [DOI] [PubMed] [Google Scholar]
- 4.Charles AC, Merrill JE, Dirksen ER, Sanderson MJ. Intercellular signaling in glial cells: calcium waves and oscillations in response to mechanical stimulation and glutamate. Neuron. 1991;6:983–992. doi: 10.1016/0896-6273(91)90238-u. [DOI] [PubMed] [Google Scholar]
- 5.Charles AC, Naus CCG, Zhu D, Kidder GM, Dirksen ER, Sanderson MJ. Intercellular calcium signaling via gap junctions in glioma cells. J Cell Biol. 1992;118:195–201. doi: 10.1083/jcb.118.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charles AC, Kodali SK, Tyndale RF. Intercellular calcium waves in neurons. Mol Cell Neurosci. 1996;7:337–353. doi: 10.1006/mcne.1996.0025. [DOI] [PubMed] [Google Scholar]
- 7.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 8.Churchill GC, Atkinson MM, Louis CF. Mechanical stimulation initiates cell-to-cell calcium signaling in ovine lens epithelial cells. J Cell Sci. 1996;109:355–365. doi: 10.1242/jcs.109.2.355. [DOI] [PubMed] [Google Scholar]
- 9.D'Andrea P, Vittur F. Gap junction mediate intercellular calcium signaling in cultured articular chondrocytes. Cell Calcium. 1996;20:389–397. doi: 10.1016/s0143-4160(96)90001-9. [DOI] [PubMed] [Google Scholar]
- 10.Di Virgilio F, Steinberg TH, Swanson JA, Silverstein SC. Fura-2 secretion and sequestration in macrophages. A blocker of organic anion transport reveals that these processes occur via a membrane transport system for organic anions. J Immunol. 1988;140:915–920. [PubMed] [Google Scholar]
- 11.Elfgang C, Eckert R, Lichtenberg-Frate H, Butterweck A, Traub O, Klein RA, Hulser DF, Willecke K. Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa cells. J Cell Biol. 1995;129:805–817. doi: 10.1083/jcb.129.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Enkvist MOK, McCarthy KD. Activation of protein kinase C blocks astroglial gap junction communication and inhibits the spread of calcium waves. J Neurochem. 1992;59:519–526. doi: 10.1111/j.1471-4159.1992.tb09401.x. [DOI] [PubMed] [Google Scholar]
- 13.Goldman WE, Baseman JB. Selective isolation and culture of a proliferating epithelial cell population from the hamster trachea. In Vitro. 1980;16:313–319. doi: 10.1007/BF02618337. [DOI] [PubMed] [Google Scholar]
- 14.Hansen M, Boitano S, Dirksen ER, Sanderson MJ. Intercellular calcium signaling induced by extracellular adenosine 5′-triphosphate and mechanical stimulation in airway epithelial cells. J Cell Sci. 1993;106:995–1004. doi: 10.1242/jcs.106.4.995. [DOI] [PubMed] [Google Scholar]
- 15.Hansen M, Boitano S, Dirksen ER, Sanderson MJ. A role for phospholipase C activity but not ryanodine receptors in the initiation and propagation of intercellular calcium waves. J Cell Sci. 1995;108:2583–2590. doi: 10.1242/jcs.108.7.2583. [DOI] [PubMed] [Google Scholar]
- 16.Harden TK, Boyer JL, Nicholas RA. P2-purinergic receptors: subtype-associated signaling responses and structure. Annu Rev Pharmacol Toxicol. 1995;35:541–579. doi: 10.1146/annurev.pa.35.040195.002545. [DOI] [PubMed] [Google Scholar]
- 17.Lustig KD, Shiau AK, Brake AJ, Julius D. Expression cloning of an ATP receptor from mouse neuroblastoma cells. Proc Natl Acad Sci USA. 1993;90:5113–5117. doi: 10.1073/pnas.90.11.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lynn BD, Marotta CA, Nagy JI. Propagation of intercellular calcium waves in PC12 cells overexpressing a carboxy-terminal fragment of amyloid precursor protein. Neurosci Lett. 1995;199:21–24. doi: 10.1016/0304-3940(95)12028-3. [DOI] [PubMed] [Google Scholar]
- 19.Osipchuk Y, Cahalan M. Cell-to-cell spread of calcium signals mediated by ATP receptors in mast cells. Nature (Lond) 1992;359:241–244. doi: 10.1038/359241a0. [DOI] [PubMed] [Google Scholar]
- 20.Parr CE, Sullivan DM, Paradiso AM, Lazarowski ER, Burch LH, Olsen JC, Erb L, Weisman GA, Boucher RC, Turner JT. Cloning and expression of a human P2U nucleotide receptor, a target for cystic fibrosis pharmacotherapy. Proc Natl Acad Sci USA. 1994;91:3275–3279. doi: 10.1073/pnas.91.8.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saez JC, Connor JA, Spray DC, Bennett MVL. Hepatocyte gap junctions are permeable to the second messenger, inositol 1,4,5-trisphosphate, and to calcium ions. Proc Natl Acad Sci USA. 1989;86:2708–2712. doi: 10.1073/pnas.86.8.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanderson MJ, Charles AC, Dirksen ER. Mechanical stimulation and intercellular communication increases intracellular Ca2+in epithelial cells. Cell Regul. 1992;1:585–596. doi: 10.1091/mbc.1.8.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanderson MJ, Charles AC, Boitano S, Dirksen ER. Mechanisms and function of intercellular calcium signaling. Mol Cell Endrocrinol. 1994;98:173–187. doi: 10.1016/0303-7207(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 24.Schlosser SF, Burgstahler AD, Nathanson MH. Isolated rat hepatocytes can signal to other hepatocytes and bile duct cells by release of nucleotides. Proc Natl Acad Sci USA. 1996;93:9948–9953. doi: 10.1073/pnas.93.18.9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steinberg TH, Civitelli R, Geist ST, Robertson AJ, Veenstra RD, Wang H-Z, Warlow PM, Hick E, Laing JG, Beyer EC. Connexin43 and connexin45 form gap junctions with different molecular permeabilities in osteoblastic cells. EMBO (Eur Mol Biol Organ) J. 1994;13:744–750. doi: 10.1002/j.1460-2075.1994.tb06316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Venance L, Piomelli D, Glowinski J, Giaume C. Inhibition by anandamide of gap junctions and intercellular calcium signalling in striatal astrocytes. Nature (Lond) 1995;376:590–594. doi: 10.1038/376590a0. [DOI] [PubMed] [Google Scholar]
- 27.Xia S-L, Ferrier J. Propagation of a calcium pulse between osteoblastic cells. Biochem Biophys Res Commun. 1992;186:1212–1219. doi: 10.1016/s0006-291x(05)81535-9. [DOI] [PubMed] [Google Scholar]
- 28.Young SH, Ennes HS, Mayer EA. Propagation of calcium waves between colonic smooth muscle cells in culture. Cell Calcium. 1996;20:257–271. doi: 10.1016/s0143-4160(96)90031-7. [DOI] [PubMed] [Google Scholar]