Abstract
The sequence of events leading to clathrin-coated pit (CCP) nucleation on the cell surface and to the incorporation of receptors into these endocytic structures is still imperfectly understood. In particular, the question remains as to whether receptor tails initiate the assembly of the coat proteins or whether receptors migrate into preformed CCP. This question was approached through a dissection of the mechanisms implemented by Nef, an early protein of human and simian immunodeficiency virus (HIV and SIV, respectively), to accelerate the endocytosis of cluster of differentiation antigen type 4 (CD4), the major receptor for these viruses. Results collected showed that: (a) Nef promotes CD4 internalization via an increased association of CD4 with CCP; (b) the Nef-mediated increase of CD4 association with CCP is related to a doubling of the plasma membrane area occupied by clathrin-coated structures; (c) this increased CCP number at the plasma membrane has functional consequences preferentially on CD4 uptake and does not significantly affect transferrin receptor internalization or fluid-phase endocytosis; (d) the presence of a CD4 cytoplasmic tail including a critical dileucine motif is required to induce CCP formation via Nef; and (e) when directly anchored to the cytoplasmic side of the plasma membrane, Nef itself can promote CCP formation. Taken together, these observations lead us to propose that CD4 can promote CCP generation via the connector molecule Nef. In this model, Nef interacts on one side with CD4 through a dileucine-based motif present on CD4 cytoplasmic tail and on the other side with components of clathrin-coated surface domain (i.e., adaptins). These Nef-generated complexes would then initiate the nucleation of CCP.
Receptor-mediated endocytosis provides a mechanism through which cells selectively capture nutrients and building blocks from the extracellular medium, transduce extracellular signals, and remove signaling receptors from their surface, thereby modulating their sensitivity to external stimuli (Cohen and Fava, 1985; van Deurs et al., 1989; Rodman et al., 1990; Watts and Marsh, 1992; Carpentier, 1994; Feger et al., 1994). Elucidating the molecular mechanisms governing receptor-mediated endocytosis is, therefore, important to unravel how cell growth and maintenance are controlled. Endocytosis of signaling receptors (i.e., the insulin receptor, the EGF receptor, etc.) occurs in response to ligand binding while endocytosis of transport protein receptors (i.e., the transferrin receptor, the LDL receptor, etc.) is constitutive (Cohen and Fava, 1985; Hanover et al., 1985; Hopkins et al., 1985; Watts, 1985; Carpentier et al., 1992; Sorkin and Carpenter, 1993). In both cases the entry of the receptors inside the cell is mediated by plasma membrane invaginated domains decorated on their cytoplasmic side with clathrin: the clathrin-coated pits (CCP)1 (Goldstein et al., 1985; van Deurs et al., 1989; Rodman et al., 1990; Watts and Marsh, 1992). One single CCP can be responsible for the concomitant uptake of both signaling and transport protein receptors (Maxfield et al., 1978; Carpentier et al., 1982).
The development of cell-free assays, analogous to those which have allowed a breakthrough in our understanding of how transport vesicles bud and fuse along the biosynthetic pathway (Orci et al., 1989; Rothman and Orci, 1992), has recently permitted the dissection of key mechanisms governing and controlling the formation of clathrin-coated vesicles from CCP (Lin et al., 1991; Schmid and Smythe, 1991; Schmid, 1993). At the same time, the combination of mutational, biochemical, and morphological studies has revealed that the association of receptors with CCP relies on a mutual recognition between particular CCP components (adaptins) and specific amino acid sequences present in receptor cytoplasmic tails (Trowbridge, 1991; Paccaud et al., 1993; Ohno et al., 1995). These conceptual advances contrast with the lack of information regarding another key event in receptor-mediated endocytosis, namely the triggering and control of CCP formation. In this respect the question remains open as to whether receptors designed to be internalized induce CCP formation or whether receptors are segregated in preformed CCP. According to one model, CCP components first assemble at the plasma membrane and, in a second step, receptors migrate into these structures to be internalized (Fire et al., 1991; Katzir et al., 1994; Sako and Kusumi, 1994; Lazarovits et al., 1996). This concept is supported by the absence of increase in CCP density in the proximity of stimulated Fc receptors (FcεRI) (Santini and Keen, 1996). By contrast, the second model postulates that the assembly of CCP components is initiated by receptors themselves. Along this line, ligand-induced clustering of IgM receptors was shown to lead to clathrin recruitment at the plasma membrane (Salisbury et al., 1980), and overexpression of transferrin receptors caused an increase in clathrin coating at the plasma membrane (Iacopetta et al., 1988; Miller et al., 1991). Likewise, clathrin-coated vesicle formation at the Golgi membranes was demonstrated to be modulated by expression levels of mannose 6-phosphate receptor as long as the cytoplasmic tail is unaltered (Le Borgne and Hoflack, 1997).
The present study was aimed at exploring the process of CCP formation through the study of Nef, an early protein of primate lentiviruses which downregulates the surface expression of CD4 (Garcia and Miller, 1991; Anderson et al., 1993; Mariani and Skowronski, 1993) by inducing its accelerated internalization followed by lysosomal degradation (Aiken et al., 1994; Rhee and Marsh, 1994). Previous work indicated that 20 membrane-proximal residues of the CD4 cytoplasmic domain are sufficient to confer Nef responsiveness to a heterologous molecule, and that within this region a dileucine-based motif plays a critical role (Aiken et al., 1994; Salghetti et al., 1995). Furthermore, the study of chimeric integral membrane proteins containing Nef as their cytoplasmic domain revealed that, in this context, Nef can act in cis to induce the rapid endocytosis of these fusion molecules via clathrin-coated pits (Mangasarian et al., 1997). Taken together, these data support a model where Nef downregulates CD4 by acting as a connector between this receptor and the endocytic apparatus. Here, the mechanisms of Nef-induced CD4 endocytosis were further investigated. This led to the observation that the viral protein triggers the formation of CD4– enriched CCP. This effect required that Nef be tethered to the plasma membrane either by coexpression with CD4, or as part of a chimeric integral membrane protein. These results strongly suggest that receptors can trigger the formation of CCP at the cell plasma membrane.
MATERIALS AND METHODS
Reagents
Lucifer yellow, holo-transferrin iron-saturated, γ globulins, goat anti– mouse IgG (whole molecule) gold conjugate (10 nm), histidinol, and Hepes were purchased from Sigma Chemical Co. (St Louis, MO). RPMI 1640 culture medium and fetal calf serum (FCS) were purchased from GIBCO BRL (Paisley, Scotland). Purified mouse anti–human CD4 (RPA-T4) was obtained from PharMingen (San Diego, CA); purified mouse anti–human CD4 (Leu3a) and purified mouse anti–human CD71 (anti–human transferrin receptor, clone B3/25) were obtained from Boehringer Mannheim (Mannheim, Germany); and monoclonal mouse anticlathrin (directed against the 180-kD heavy chain) was a gift of R.G.W. Anderson. Iodo-Beads as iodination reagent were purchased from Pierce Chemical Co. (Rockford, IL) and 125I from Amersham International (Little Chalmont, England). Other chemicals were of analytical grade and were obtained from Fluka AG (Buchs, Switzerland) or Sigma Chemical Co. Experiments were performed in PBS or a medium containing (micrometers) 138 NaCl, 6 KCl, 1 MgCl2, 20 glucose, and 20 Hepes, pH 7.4 (medium 1).
DNA Constructions
The HIV1-Nef allele and the retroviral vectors LXSN and LNefSN, used in these experiments, were described previously (Aiken et al., 1994). CD4 mutants and CD4–Nef chimera (44Nef) were created by ligating DNA fragments generated by PCR (Mangasarian et al., 1997). The constructs were verified by DNA sequence analysis. CD4 mutants and 44Nef chimera were expressed from the cytomegalovirus (CMV) immediate early promoter, in the pCMX plasmid vector (Umesono et al., 1991).
Cell Lines, Cultures, and Transfections
CEM and Namalwa cell lines expressing Nef were created using the retroviral vectors LNefSN and LXSN as previously described (Aiken et al., 1994). Stably transfected cells were next cultivated in RPMI 1640 medium supplemented with 10% FCS and selected in the presence of 1 mg/ml G418. The Epstein-Barr virus (EBV)-transformed Namalwa human B cell line was maintained in RPMI 1640 medium supplemented with 10% FCS. For the establishment of cell populations stably producing CD4 and derivatives, Namalwa cells were coelectroporated with a mixture comprising a CMV-based plasmid expressing one of these proteins and pSV2-His, at a 1:10 ratio, using a total of 50 μg of DNA for 5 × 106 cells at 250 V and a capacitance of 960 μF. Cells were then selected in histidine-deficient medium containing 1 mM histidinol (Sigma Chemical Co.) (Mangasarian et al., 1997).
Antibody and Holo-transferrin Iodination
Iodination of purified mouse anti–human CD4 antibodies (RPA-T4) and iron-saturated holo-transferrin was performed using Iodo-Beads according to the manufacturer's instructions (Markwell, 1982). Briefly, Iodo-Beads were washed and preloaded with ∼0.5–1 mCi of Na125I in PBS for 5 min at room temperature. Free-carrier mouse anti–human CD4 antibodies (50 μg, 330 pmol) or holo-transferrin (100 μg, 1.23 nmol) were added to the beads and incubation was continued for 15 min at room temperature. Iodination was stopped by removing the beads from the mixture reaction and free 125I was discarded by passing the solution through a gel filtration sephadex G-25 column (Pharmacia Biotech, Inc., Piscataway, NJ). Iodinated protein was stored at 4°C in PBS + BSA 1%. Specific activity was comprised between 2–3 μCi/μg of protein and 3–4 μCi/μg of protein for 125I–anti-CD4 and 125I–holo-transferrin, respectively.
Acid Wash Assay
Internalization was assayed using the acid wash technique as previously described (Iacopetta et al., 1986; Pelchen Matthews et al., 1989; Pelchen Matthews et al., 1991; Aiken and Trono, 1995). Briefly, cells (10–20 × 106) were washed and incubated for 2 h at 4°C with 125I–RPA-T4 (0.02–0.04 μCi/106 cells, i.e., 7–20 ng/106 cells) or 125I-transferrin (0.01–0.02 μCi/106 cells, i.e., 3–7 ng/106 cells) in 0.5 ml cold PBS/BSA 1%. Cells were next washed twice by centrifugation (200 g, 5 min) to remove unbound radiolabeled molecules and then incubated at 37°C to allow endocytosis. Cells were washed twice in their corresponding buffer at low pH (pH 2) to remove surface-bound radiolabeled antibodies or ligands. Percentage of 125I–anti-CD4/125I-transferrin internalization was expressed as the ratio of acid wash–resistant radioactivity to total radioactivity associated with cells at neutral pH. Counting was performed in a Beckman 5,500 γ counter (Fullerton, CA). The iodinated anti–CD4 antibody binding at 4°C was maximal after 90 min of incubation. The nonspecific binding ranged between 3–5% of the total recovered radioactivity. The sequential washes with buffer at pH 2 detached more than 95% of the surface-bound antibodies after a 2-h incubation at 4°C.
Fluid-Phase Endocytosis Assay
Duplicates of 15 × 106 cells were incubated for different periods of time at 37°C in the presence of Lucifer yellow (1.5 mg/ml in Ca2+- and Mg2+-free medium 1). At the end of each incubation period, the endocytic process was stopped by cooling the cells at 4°C. Cells were next washed four times in ice-cold Ca2+- and Mg2+-free medium and lysed with 0.1% Triton X-100 (Krischer et al., 1993). Cell-associated fluorescence was measured in a Perkin-Elmer LS-3 fluorimeter (excitation 415 nm, emission 535 nm; Perkin-Elmer Corp., Norwalk CT). Finally, fluorescence value was reported to protein content of each sample. Protein determination was performed with the Pierce kit using bicinchonic acid.
Autoradiography
CEM T cells were incubated for 2 h at 4°C with 125I–anti-CD4 (0.2–0.3 μCi/106 cells, i.e., 100 ng/106 cells) in PBS/BSA 1%. After antibody binding, cells were washed twice by centrifugation (200 g, 5 min) to remove antibody excess, and warmed for various periods of time at 37°C to allow endocytosis. Cells were then fixed, dehydrated, and processed for electron microscope autoradiography; labeling was quantitated as previously described (Carpentier et al., 1978, 1981, 1992; Fan et al., 1982). Three experiments were performed. For each incubation time analyzed, three Epon blocks were prepared, and sections were cut from each block. For each time point studied and for each cell line, ∼950–1,150 grains were analyzed from cells judged to be morphologically well preserved. Autoradiographic grains within a distance of ±250 nm from the plasma membrane were considered associated with the cell surface; grains overlying the cytoplasm and >250 nm from the plasma membrane were considered internalized. Grains associated with the plasma membrane were considered associated with CCP if their centers were <250 nm from these surface domains. Indeed, assuming a half distance of 80–100 nm (Salpeter et al., 1977), the radioactive source responsible for each autoradiographic grain has a >80% chance of being contained within this distance of 250 nm around each grain. The center of the grains was determined by overlaying them with a circle of 250-nm radius. This approach has been extensively used and validated in previous studies (Salpeter et al., 1977; Carpentier et al., 1978, 1981, 1992; Fan et al., 1982).
CD4 Immunogold Labeling on EM Thin Sections and Isolated Plasma Membranes
CEM T cells were incubated 2 h at 4°C with anti-CD4 antibodies (Leu3a, 40 ng/106 cells) in cold PBS/BSA 1%. After antibody binding, cells were washed twice by centrifugation (200 g, 5 min) in cold PBS to remove antibody excess and incubated a second time with an anti–mouse IgG coupled with 10-nm colloidal gold particles for 2 h at 4°C. (Antibodies were used at a dilution of 1:15 for a cell suspension of 5 × 107 cell/ml of PBS/BSA 1%.) Unbound secondary antibody was removed by washing twice with cold PBS, and then cells were warmed for various periods of time at 37°C to allow endocytosis. For conventional EM analysis, cells were then fixed, dehydrated, and processed for EM as described previously (Carpentier et al., 1991). Thin sections were examined in an electron microscope (model EM 300; Philips Electron Optics, Eindhoven, The Netherlands), and gold particles were quantitatively analyzed on cells considered well preserved. Gold particles were considered associated with clathrin-coated structures when they were observed immediately adjacent (at a distance <20 nm) to the clathrin coat or totally enclosed in clathrin-coated pits/vesicles. Two experiments were performed. For each incubation time analyzed, two Epon blocks were prepared, and sections were cut from each block. For each time point studied and for each cell line, 700–2,250 gold particles were analyzed from cells judged to be morphologically well preserved. For morphological analysis of isolated plasma membranes, adherent membranes were prepared as described above and gold particles were visualized by transparency. The ratio of gold-labeled CCP/total number of CCP observed was quantitated. A total of 416 and 199 CCP were considered for control and Nef-expressing cells, respectively.
Quantitative Determination of Clathrin-coated Plasma Membrane
Quantitative determination of clathrin-coated plasma membrane CCP and clathrin lattices on the inner face of the plasma membrane were visualized as described by Sanan and Anderson (1991) with minor modifications. Briefly, formvar-coated EM nickel grids were treated with 1 mg/ml of poly-l-lysine for 1 h at 4°C. Cells (106/ml) in cold PBS were then allowed to sediment on the air-dried grids for 1 h at 4°C. Cells adherent to poly-l-lysine–coated grids were next washed with cold PBS, incubated 30 min with cold PBS/BSA 1%, and washed twice again with cold PBS. Adherent isolated plasma membranes were obtained by incubating the grids with the adherent cells in hypotonic (0.65×) PBS for 30 s and then by sonicating the cells at a weak power. This procedure disrupts the cells but allows a large portion of plasma membranes, with conserved internal structures such as clathrin-coated membranes and cytoskeleton elements, to stay adherent to the poly-l-lysine–coated grids. Adherent membranes were next washed with cold PBS and fixed for 15 min at 4°C followed by 15 min at room temperature with 4% glutaraldehyde in PBS. Adherent membranes were subsequently fixed in 2% osmium tetroxide in PBS for 8 min, 30 s at room temperature, washed three times for 5 min with PBS buffer, incubated with 1% aqueous tannic acid for 10 min, washed twice for 5 min in distilled water, incubated with 1% uranyl acetate for 10 min, and finally washed twice for 1 min with distilled water before air-drying. Membranes considered well conserved were then randomly photographed on an electron microscope (model EM 301; Philips Electron Optics). Eventually, the extent of membrane surfaces (%) decorated by clathrin was quantitated on electron micrographs using a Leica Quantimet 500 + (Vienna, Austria).
Concomitant Labeling of CD4 and Transferrin Receptors on EM Thin Sections
Cells in ice-cold PBS/BSA 1% (107/ml) were successively (a) incubated with anti–transferrin receptor antibodies (0.5 μg/106 cells, 90 min at 4°C), (b) washed and incubated a second time with an anti–mouse IgG coupled with 10-nm colloidal gold particles (antibodies diluted 1:15 in 20 × 106 cells/ml, 90 min at 4°C), (c) washed and saturated with nonspecific globulins (100 μg/106cells, 30 min at 4°C), and (d) washed and incubated a third time with 125I–anti-CD4 antibodies (100 ng/106 cells, 90 min at 4°C). Eventually, cells were washed twice with ice-cold PBS and warmed for 5 min at 37°C to allow the recruitment of tagged receptors into CCP and their endocytosis. Cells were then fixed for 30 min at room temperature with 2.5% glutaraldehyde in phosphate buffer, dehydrated, and processed for EM as described previously (Carpentier et al., 1991). Thin sections were examined in an electron microscope (model EM 301; Philips Electron Optics) Finally, association of autoradiographic grains (CD4 labeling) and gold particles (transferrin receptors labeling) with CCP present at the plasma membrane was quantitatively analyzed on cells considered well preserved. Autoradiographic grains located on the plasma membrane were considered associated with CCP if their centers were <250 nm from these surface domains. Gold particles were considered associated with CCP when they were observed immediately adjacent.
RESULTS
Nef Increases CD4 Association with CCP
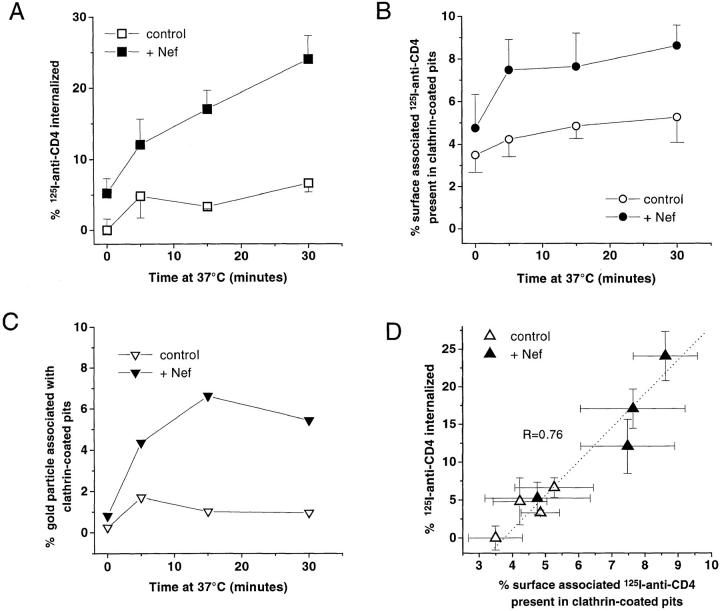
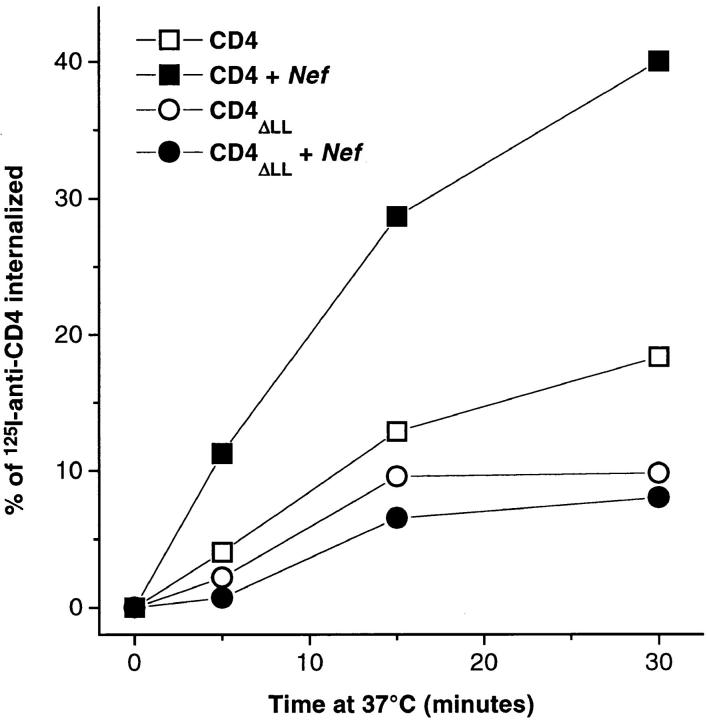
To understand the mechanisms governing Nef-induced CD4 downregulation, CD4 tagged with 125I–anti-CD4 was tracked morphologically by a quantitative EM autoradiographic analysis in CEM T lymphocytes stably expressing the viral protein (Fig. 1). Confirming previous biochemical observations (Aiken et al., 1994; Rhee and Marsh, 1994), the EM analysis showed that at the permissive temperature of 37°C, endocytosis of 125I–anti-CD4 bound to plasma membrane of Nef-expressing CEM T cells was significantly increased as compared to control cells (stably expressing an empty vector) (Fig. 2 A). After a 2-h incubation at 4°C in the presence of 125I–anti-CD4, ∼3% of plasma membrane–bound CD4 was found associated with CCP in control cells. This value slightly increased with time and temperature to reach 5% after 30 min of incubation at 37°C. By contrast, in cells expressing Nef, the association of 125I–anti-CD4 with CCP peaked after 5 min of incubation at 37°C and plateaued at ∼8% (Fig. 2 B). CD4 association with CCP was alternatively determined by EM immunogold labeling visualized on ultrathin sections. This method allows a more precise localization of anti-CD4 on the cell surface and a better definition of its association with specialized membrane domains such as CCP (Fig. 1 C). Similar conclusions to the one reached using 125I–anti-CD4 as a ligand were collected through immunogold labeling: in the presence of Nef at 37°C, anti-CD4 association with CCP increased at each time point studied by a factor between two and three (Fig. 2 C). The amount of cell surface 125I–anti-CD4 internalized, in both control and Nef-expressing cells, was closely related to the propensity of CD4 to associate with CCP, as shown by the linear relationship (R = 0.76) connecting the two events (Fig. 2 D). Thus, in the presence of Nef, the stimulated rate of CD4 internalization was related, at least in part, to an increased association of the receptor with CCP.
Figure 1.
Representative electron micrographs of autoradiographic grains (CD4 radiolabeling, a and b) and colloidal gold particles (CD4 immunogold labeling, c) associated with CCP (arrowheads) in CEM T lymphoid cells. Cells were incubated for 2 h at 4°C with 125I-anti–CD4 antibody or a primary anti-CD4 antibody followed by a secondary colloidal gold–conjugated antibody and endocytosis of the immune complex was then allowed to occur by raising the temperature to 37°C for 5 min.
Figure 2.
Nef-induced CD4 internalization and association with CCP. (A) Kinetics of 125I-antibody–CD4 complex internalization in CEM T lymphoid cells expressing or not expressing Nef. (B) Kinetics of 125I–antibody–CD4 complex association with clathrin-coated structures in CEM T lymphoid cells expressing or not expressing Nef. (C) Kinetics of CD4–gold complex association with clathrin-coated structures in CEM T lymphoid cells expressing or not expressing Nef. (D) CD4 internalization as a function of CD4 association with CCP in EM autoradiography. The relationship between CD4 internalization and CD4 association with CCP, in cells expressing or not expressing Nef, were fitted by a linear regression (R = 0.76). Cells were incubated 2 h at 4°C with 125I– anti-CD4 antibody (or a primary anti-CD4 antibody followed by a secondary gold–conjugated antibody), and endocytosis of the radiolabeled antibody–CD4 complex (or CD4–immunogold complex) was allowed to occur by raising the temperature to 37°C for different periods of time. After cell processing for EM autoradiography or gold detection, quantification was carried out as described previously (Salpeter et al., 1977; Carpentier et al., 1978, 1981, 1991, 1992; Fan et al., 1982). As control, cells harboring an empty plasmid were used. For each time point studied and for each cell line, ∼950–1,150 autoradiographic grains (or 700–2,250 gold particles) were analyzed from cells judged to be morphologically well preserved. Autoradiographic grains within a distance of ±250 nm from the plasma membrane were considered associated with the cell surface; grains overlying the cytoplasm and >250 nm from the plasma membrane were considered internalized. Grains associated with the plasma membrane were considered associated with CCP if their centers were <250 nm from these surface domains. Gold particles were considered associated with clathrin-coated structures when they were observed immediately adjacent (at a distance <20 nm) to the clathrin coat or totally enclosed in clathrin-coated pits/vesicles. Data are mean ± SEM of three experiments.
Nef Triggers the Recruitment and Assembly of CCP Components at the Plasma Membrane
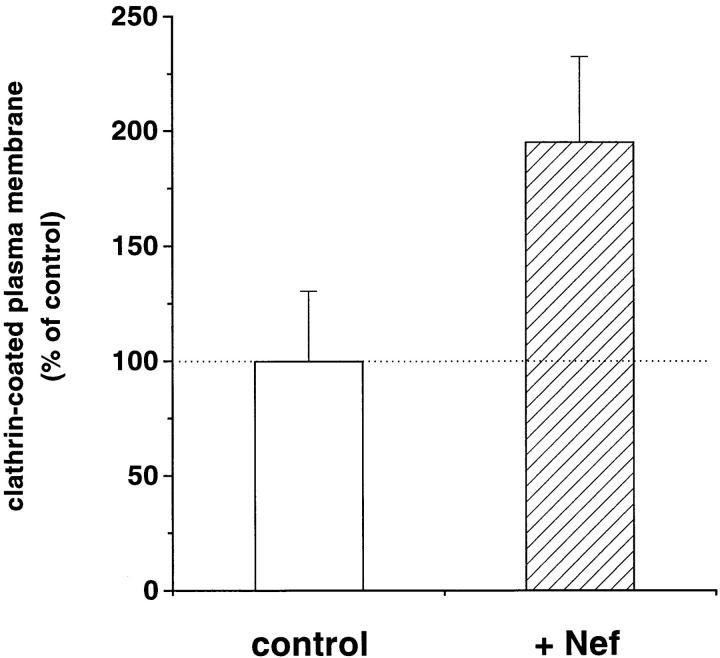
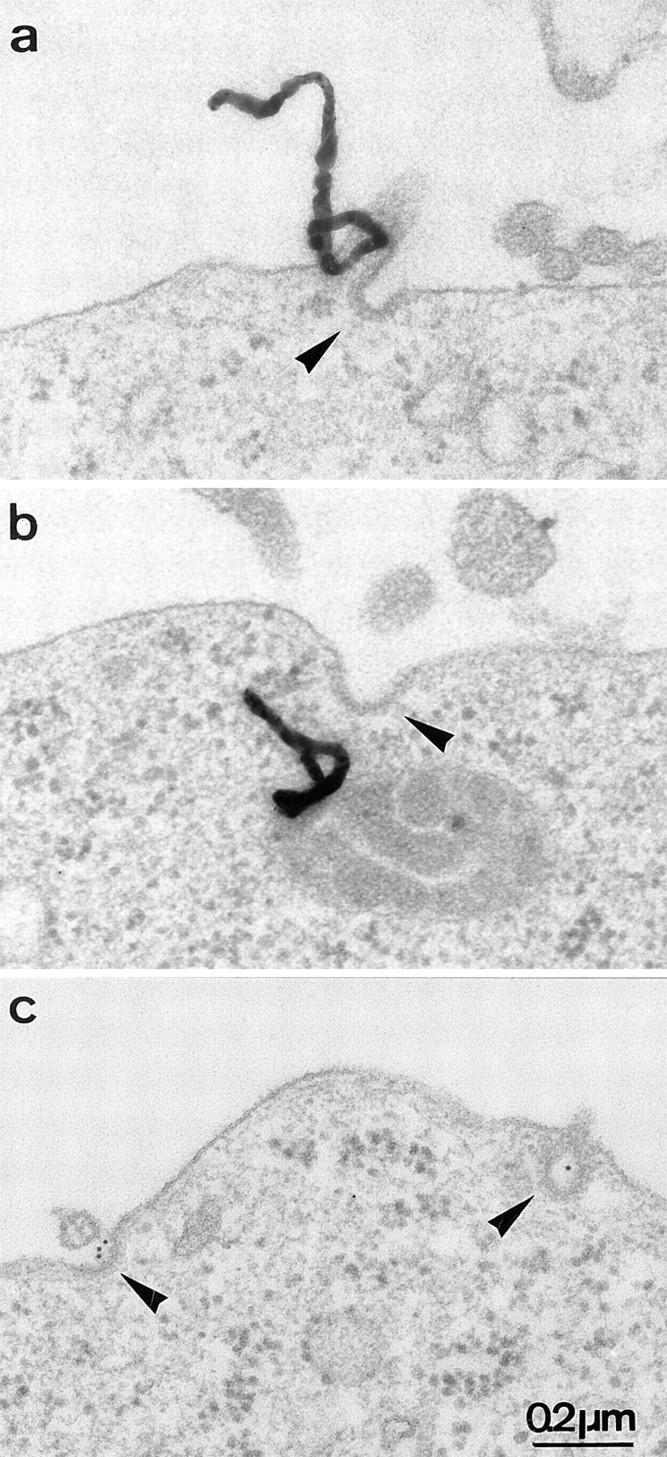
Surprisingly, the Nef-triggered CD4 association with CCP correlated with an increase in the plasma membrane surface coated with clathrin in CEM T cells. As determined on conventional EM thin sections, the plasma membrane surface decorated by a clathrin coat (2.0 ± 0.4% of the plasma membrane in control CEM T lymphocytes, 102 cell sections analyzed) increased by 63% in cells transfected with Nef (data not shown). These initial observations were verified on isolated plasma membranes adherent to EM grids and negatively stained (Sanan and Anderson, 1991). This technique allows: (a) an en face view of large surfaces of the inside of the membrane; (b) a distinction between clathrin-coated flat lattices and clathrin-coated invaginations, both easily identified by their typical honeycomb organization; and (c) an accurate quantification of these respective clathrin-coated structures (Fig. 3). Quantitative analysis confirmed that, in the presence of Nef, surfaces occupied by CCP and flat clathrin lattices on the inner leaflet of the plasma membrane increased by 95.4 ± 37.3% (Fig. 4). Breaking these structures into their two components showed that CCP and flat clathrin lattices were increased 2.2- and 1.7-fold, respectively, by Nef expression (representing two out of three and one out of three, of the total clathrin-coated membrane increase, respectively2) indicating that the increase in clathrin-coated plasma membrane was related primarily to an augmentation of invaginated clathrin-coated structures. Under these conditions, CCP size remained unchanged: 86.1 ± 0.9 and 90.8 ± 2.5 nm in control and Nef-expressing cells, respectively. Clathrin synthesis was also not significantly affected by Nef as determined by SDS-PAGE and quantitative analysis of clathrin-specific immunoblots (data not shown). Taken together, these data demonstrate that Nef-induced CD4 internalization is associated with a stimulation of the recruitment and assembly of clathrin coat constituents at the plasma membrane, giving rise to the production of a majority of invaginated CCP.
Figure 3.
Representative electron micrographs of CCP and clathrin flat lattices on the inner face of CEM plasma membranes. CCP (arrowheads) and flat clathrin lattices (b, arrows) were visualized on adherent plasma membranes as described by Sanan and Anderson (1991) with minor modifications. Briefly, cells were allowed to sediment on poly-l-lysine–coated grids for 1 h at 4°C and adherent plasma membranes were then obtained by incubating the grids in hypotonic medium followed by sonication at weak power. This procedure disrupts the cells but allows a large portion of plasma membranes, with conserved structures such as clathrin-coated membranes and cytoskeleton elements, to stay adherent to the poly-l-lysine–coated grids. Adherent membranes were next fixed and negatively stained for EM. Membranes considered well conserved were randomly photographed and the extent of membrane surfaces coated by clathrin was quantitated on electron micrographs as described in Materials and Methods. In (c) cells were incubated for 2 h at 4°C with anti-CD4 antibodies followed by a 2-h incubation at 4°C in the presence of anti–mouse IgG coupled with 10-nm colloidal gold particles. Cells were next warmed for various periods of time at 37°C to allow endocytosis. Gold particles (localizing CD4 and seen by transparency) are associated with CCP (arrowheads).
Figure 4.
Nef increases the extent of plasma membrane coated by clathrin-lined structures (CCP and clathrin lattices) in CEM lymphoid T cells. Adherent plasma membranes were visualized as described by Sanan and Anderson (1991) with minor modifications. Briefly, cells were allowed to sediment on poly-l-lysine– coated grids for 1 h at 4°C and adherent plasma membranes were then obtained by incubating the whole cells with hypotonic medium followed by a sonication at a weak power. This procedure disrupts the cells but allows a large portion of plasma membranes, with conserved internal structures such as clathrin-coated membranes and cytoskeleton elements, to stay adherent to the poly-l-lysine–coated grids. Adherent membranes were next fixed and negatively stained for EM. Membranes considered well conserved were then randomly photographed and the extent of membrane surfaces coated by clathrin was quantitated on electron micrographs as described in Materials and Methods. Data are means ± SEM of quantitative analysis performed on 110 cells/487.8 μm2 of plasma membrane segments, and 104 cells/447 μm2 of plasma membrane segments for CEM control (transduced with a control retroviral vector) and Nef-expressing cells, respectively.
Nef Specifically Affects CD4 Endocytosis
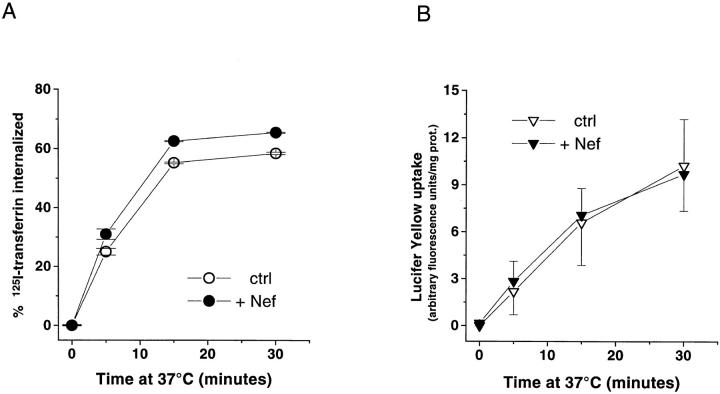
Nef downregulates CD4 and, to a lesser extent, MHCI (Schwartz et al., 1996). In contrast, Nef does not affect the surface expression of a variety of surface receptors including CD8, CD29, CD45RO, interleukin-2 receptor α chain, ICAM-1, CD38, CD69, the transferrin receptor (Tf-R), and a CD4–LDL receptor fusion protein (Benson et al., 1993; Schwartz et al., 1993; Aiken et al., 1994), indicating that the Nef effect on endocytosis is at least partly receptor specific. To verify this point in our experimental conditions, 125I–transferrin uptake was analyzed. The transferrin receptor is a carrier of cargo molecules concentrated in CCP and constitutively internalized through these structures (Hanover et al., 1985; Watts, 1985). Nef expression only moderately affected the uptake of radiolabeled-transferrin, thus confirming the lack of a general effect of Nef on receptor-mediated endocytosis (Fig. 5 A). Similarly, the uptake of a fluid-phase marker (Lucifer yellow) remained unaffected by Nef (Fig. 5 B).
Figure 5.
Nef does not significantly affect receptor-mediated endocytosis in general or the nonspecific fluid-phase endocytosis. (A) Kinetics of 125I-transferrin internalization in CEM T lymphoid cells expressing or not expressing Nef. Cells were incubated 2 h at 4°C with 125I-transferrin and internalization of the 125I–ligand–receptor complex was allowed by raising the temperature to 37°C as previously described (Pelchen Matthews et al., 1991; Aiken et al., 1994). Data are means ± SEM range of two experiments. (B) Kinetics of Lucifer yellow uptake in CEM T lymphoid cells expressing or not expressing Nef. The fluid-phase endocytosis was determined by measuring the nonspecific cell uptake of Lucifer yellow as previously described (Krischer et al., 1993). Data are means ± SEM of three experiments.
Nef-induced CCP Preferentially Incorporate CD4
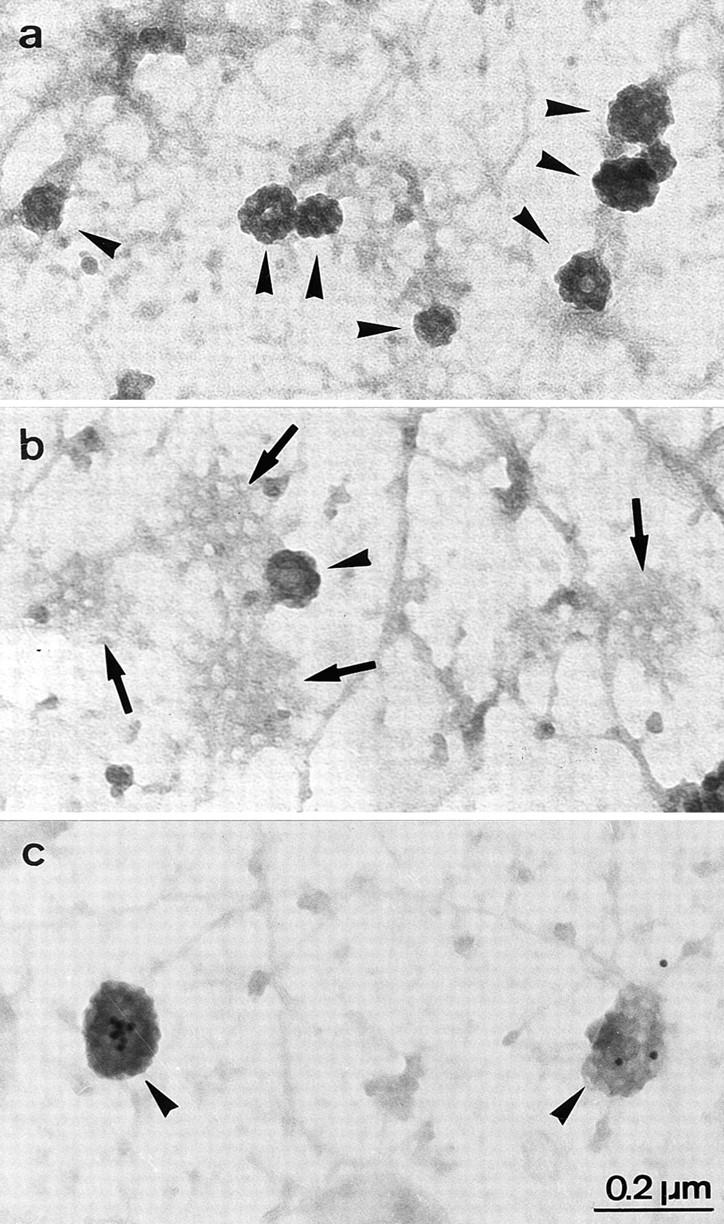
The observation that Nef triggered the formation of cell surface CCP while concomitantly and specifically inducing CD4 endocytosis suggested that the viral protein was mediating the generation of CD4-enriched CCP. This hypothesis was tested by the simultaneous morphological tracking of CD4 and Tf-Rs. Surface CD4 molecules were traced with 125I–anti-CD4 while Tf-Rs were labeled with an anti– Tf-R primary antibody followed by a secondary antibody coupled with colloidal gold (10 nm) (Fig. 6). In the presence of Nef, the per cell number of CCP containing exclusively CD4 tripled, while the corresponding number of CCP incorporating solely the transferrin receptor only increased by ∼7% (Table I). The increase in the number of CCP labeled with both markers showed that additional CCP appearing in Nef-expressing cells do not incorporate exclusively CD4 but may trap, to a low extent, other receptors. This low amount of receptors other than CD4 present in additionally formed CCP is possibly responsible for the small increase in transferrin internalization observed in Nef-expressing cells (Fig. 5 A). The number of unlabeled CCP was also significantly increased by Nef expression. Given that the analysis was performed on thin sections, which does not allow visualization of the entire clathrin-coated pits, and that 125I–anti-CD4 binding was not saturating, results collected led to an underestimation of CD4 potentially present in these structures and to an overestimation of unlabeled CCP. This was verified in the course of an immunogold CD4 labeling detected on adherent plasma membranes preparations (Fig. 3 C). After 5 min of incubation at 37°C of CEM lymphocytes surface- labeled with anti-CD4 immunocomplexes, 13% of the total CCP counted on the adherent membranes incorporated the immunogold probe (data not shown) as compared with the 6.6% observed in the case of 125I–anti-CD4 binding (see above). As compared to the increase in the total number of clathrin-coated pits, these values remain low, which leaves the possibility open that some of the newly formed CCP are not functional, although the preferential Nef- induced formation of CCP versus flat clathrin lattices is in favor of the functionality of the generated structures. Moreover, Nef might generate additional CCP in CEM lymphocytes incorporating some unidentified receptors, the endocytosis of which could also be increased by the viral protein. Of note, in CEM T lymphocytes as well as in Namalwa B lymphocytes, little Nef-induced MHCI downregulation was observed (data not shown). Together, these morphological analyses strongly suggest that at least part of the Nef-induced CCP preferentially contain CD4.
Figure 6.
Representative electron micrographs showing the association of CD4 (a and b, autoradiographic grains) and transferrin receptors (b, gold particles) with CCP (arrowheads) in CEM T lymphoid cells. CD4 and transferrin receptor association with CCP were concomitantly detected in CEM T cells. CD4 was traced with 125I–anti-CD4 with the Tf-R was labeled with an anti– Tf-R primary antibody followed by a secondary antibody coupled with colloidal gold. Endocytosis of the radiolabeled antibody– CD4 complex and gold-conjugated transferrin receptor was the allowed to occur by raising the temperature to 37°C for 5 min. After cell processing for EM autoradiography, quantification was carried out as described previously (Carpentier et al., 1978, 1981, 1992; Fan et al., 1982). Autoradiographic grains located on the plasma membrane were considered associated with CCP if their centers were <250 nm from these surface domains. Gold particles were considered associated with CCP when they were observed immediately adjacent (at a distance <20 nm) to the clathrin coat or totally enclosed in CCP.
Table I.
Nef-induced CCP Incorporate Preferentially CD4
| Cells (Nb of cells) | Total number of CCP | CCP labeled by CD4 solely | CCP labeled by Tf-R solely | CCP labeled by CD4 and Tf-R | Unlabeled CCP | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control (150) | 813 | 15 (1.8%)* | 331 (41.1%)* | 39 (4.8%)* | 429 (52.3%)* | |||||
| Nef (150) | 1,340 | 46 (3.4%)* | 355 (26.5%)* | 58 (4.3%)* | 880 (65.7%)* |
Percentage of total CCP counted.
Generation of New CCP Requires Nef and a Dileucine Motif in CD4 Cytoplasmic Tail
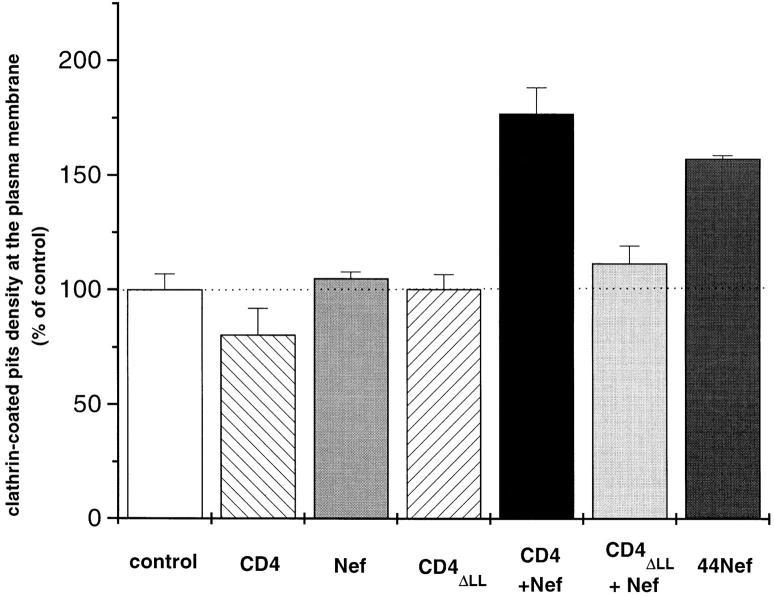
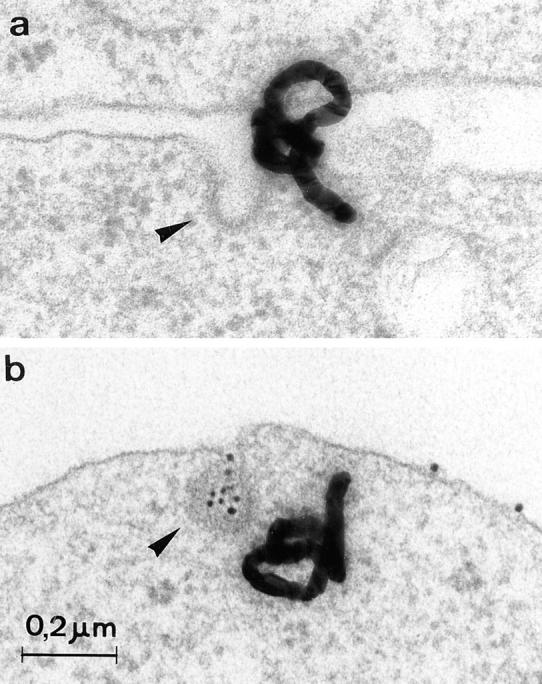
Was CD4 concentrated in Nef-induced preformed CCP or was it the primum movens in the generation of these structures? To answer this question, Namalwa B cells, which do not naturally express CD4, were stably transfected with CD4 and/or Nef (Mangasarian et al., 1997). The presence of either CD4 or Nef alone did not result in any increase of CCP over basal values (control = cells transfected with an empty vector), while the concomitant expression of CD4 and Nef promoted CCP formation (Fig. 7), in agreement with the increased rate of CD4 internalization in these cells (Fig. 8). Thus, CD4 was not segregated in preformed Nef-induced CCP, but instead the receptor participated in CCP formation. A possible implication of the CD4 cytoplasmic dileucine motif in CCP generation was tested by replacing these residues by alanines (CD4ΔLL). As previously described (Aiken et al., 1994), this mutation completely abolished Nef-induced CD4 accelerated endocytosis (Fig. 8). The quantitative morphological analysis of CCP present at the plasma membrane of established cell lines coexpressing Nef together with either wild-type or dileucine-mutated CD4 was consistent with the internalization kinetics of these molecules. The amount of CCP decorating the plasma membrane was thus similar in cells expressing either of these proteins. However, Nef induced the formation of CCP only when coexpressed with wild-type CD4, not with the CD4ΔLL mutant (Fig. 7). Together, these data demonstrate that the generation of new CCP capable of triggering CD4 internalization not only required Nef, but also a receptor cytoplasmic tail capable of a functional interaction with the viral protein.
Figure 7.
Relative amount of CCP decorating the plasma membrane of transfected Namalwa B lymphocytes. Cells were transfected with CD4, Nef, a dileucine mutated form of CD4, (CD4ΔLL), CD4+Nef, CD4ΔLL+Nef, and the chimera 44Nef. As control, cells transfected with an empty plasmid vector were used. CCP on the inner face of the plasma membrane (PM) were visualized as described by Sanan and Anderson (1991) with minor modifications. Briefly, cells were allowed to sediment on poly-l-lysine coated grids for 1 h at 4°C and adherent plasma membranes were then obtained by incubating the whole cells with hypotonic medium followed by a sonication at a weak power. This procedure disrupts the cells but allows a large portion of plasma membranes, with conserved internal structures such as clathrin-coated membranes and cytoskeleton elements, to stay adherent to the poly-l-lysine–coated grids. Adherent membranes were next fixed and negative stained for EM. Membranes considered well conserved were then randomly photographed and the amount of CCP present on plasma membrane segments was quantitated on electron micrographs as described in Materials and Methods. Data are means ± SEM of quantitiatve analysis performed on 155 cells/710.6 μm2, 229 cells/998.0 μm2, 100 cells/ 459.3 μm2, 106 cells/456.4 μm2, 112 cells/445.7 μm2, 158 cells/ 656.3 μm2, and 104 cells/474.6 μm2 of plasma membrane segments from Namalwa transfected with the empty plasmid, CD4, Nef, CD4ΔLL, CD4+Nef, CD4ΔLL+ Nef, and 44Nef, respectively.
Figure 8.
Kinetics of 125I–anti-CD4 antibody internalization in Namalwa B lymphocytic cells transfected with CD4, CD4ΔLL, CD4+Nef, or CD4ΔLL+Nef. Cells were incubated for 2 h at 4°C with an 125I–anti-CD4 antibody and endocytosis of the radiolabeled antibody–CD4 complex was then allowed to occur by raising the temperature to 37°C for different periods of time as previously described (Pelchen Matthews et al., 1991; Aiken et al., 1994).
Nef Directly Mediates CCP Generation
Knowing that both Nef and the CD4 cytoplasmic tail were required to induce the de novo generation of CCP, it remained to be determined which of these two partners was primarily responsible for this process. Namalwa B cells were transfected with a chimeric receptor made of the exoplasmic and transmembrane regions of CD4 coupled with Nef as cytoplasmic domain (44Nef). This chimeric receptor was internalized more efficiently than wild-type CD4 (Mangasarian et al., 1997), and promoted CCP formation to a level similar to the one reached in cells coexpressing CD4 and Nef (Fig. 7). Similar results were obtained when Nef was artificially anchored to the membrane by fusion to the transmembrane and extracellular domains of CD8 (data not shown). Thus, under conditions where Nef is attached to the cytoplasmic side of the plasma membrane as part of an integral membrane protein, the viral protein triggers the de novo formation of CCP, indicating that it contains the determinants responsible for this process.
DISCUSSION
The present work was intended to dissect the mechanisms responsible for the increased CD4 internalization observed in the presence of the HIV Nef protein. We found that Nef promotes CD4 internalization by increasing the association of this receptor with the classical internalization gates, the CCP. Surprisingly, we also noted that the Nef effect correlated with a doubling of the plasma membrane area occupied by clathrin-coated structures. These changes had functional consequences preferentially on CD4 uptake since the internalization of receptors classically internalized via CCP (e.g., transferrin receptors), or the nonspecific uptake of extracellular fluids (e.g., Lucifer yellow), was not significantly affected by Nef expression. This translated into a preferential increase in CD4-containing CCP over ones carrying transferrin receptor. Nef per se could promote CCP formation when artificially anchored to the cytoplasmic leaflet of the plasma membrane as part of a chimeric integral membrane protein. However, under physiological conditions, the Nef effect required the presence of CD4, and was in particular dependent on a dileucine-based motif within the cytoplasmic tail of this receptor. Taken together, these observations lead us to propose that, if properly tethered to the plasma membrane, Nef can induce CCP formation. It is interesting to note, in this regard, that Nef was recently shown to induce the accumulation of endosomes and lysosomes in human T lymphoid cells (Sanfridson et al., 1997).
The CD4 specificity of Nef-induced endocytosis is supported not only by the observation that the uptake of transferrin receptors was only weakly stimulated by Nef, but also by the results of immunocytochemical experiments carried out at the EM level, revealing a clear contrast between the percentage of clathrin-coated pits labeled with CD4 or with transferrin receptor in cells expressing Nef. These observations are in agreement with previously published results showing that the surface expression of receptors including CD8, CD29, CD45RO, interleukin-2 receptor (α chain), ICAM-1, CD38, CD69, HLA-DR, and a CD4-LDL receptor fusion protein are unaffected by Nef (Benson et al., 1993; Schwartz et al., 1993; Aiken et al., 1994). The only known exception is the MHCI molecule, the internalization of which is also stimulated by Nef, although less efficiently than that of CD4 (Schwartz et al., 1996). Nef also did not affect fluid-phase endocytosis in our experimental conditions. This latter observation supports the recent proposal that the total uptake of membrane and fluids is not strictly regulated by the clathrin- dependent endocytic pathway but might involve alternative mechanisms (Hansen et al., 1993; Cupers et al., 1994; Eker et al., 1994; Damke et al., 1995). The receptor-dependent formation of CD4-specific CCP could appear to contradict previous observations showing that CCP are relatively nonspecific structures capable of concentrating receptors of diverse nature (Dickson et al., 1981; Willingham et al., 1981; Carpentier et al., 1982). It is likely, however, that the selectivity of the observed phenomenon reflects a specific interaction between Nef and CD4 (Harris and Neil, 1994; Greenway et al., 1995; Grzesiek et al., 1996; Rossi et al., 1996). As Nef might interact with other receptors such as MHCI, it may appear surprising that the Nef-dependent CCP formation observed here required the presence of CD4, at least if Nef-induced CD4 and MHCI endocytosis proceed through similar mechanisms. However, levels of MHCI were found to be minimally affected by Nef expression in Namalwa cells (data not shown).
The relative receptor specificity of the clathrin-coated structures induced by Nef could be interpreted as evidence for the initiation by receptors of the de novo formation of CCP, or alternatively could mean that CD4 is preferentially segregated in preformed Nef-induced CCP. The second possibility can be ruled out since, except in nonphysiological conditions where Nef is constitutively attached to the plasma membrane as part of a chimeric integral membrane protein, the viral protein alone is not able to induce CCP formation. Concomitant expression of CD4 is indeed necessary for this process; furthermore, there is a strict requirement for the preservation, within the CD4 cytoplasmic tail, of a dileucine-based motif previously identified as critical for CD4 response to Nef (Aiken et al., 1994).
These observations demonstrate that, at least in some circumstances, receptor tails can initiate coat protein assembly. This conclusion contrasts with that of previous studies in which a series of transport protein receptors showed a surface mobility consistent with random movements and occasional encounters with CCP (Fire et al., 1991; Ghosh and Webb, 1994; Katzir et al., 1994; Sako and Kusumi, 1994; Lazarovits et al., 1996). Along the same line, the relocalization and aggregation of FcεRIs induced by multivalent antigens was not found to be accompanied by any change in the distribution of adaptor protein 2 (AP2) or clathrin (Santini and Keen, 1996). However, our findings are in agreement with other studies showing: (a) clathrin recruitment at the plasma membrane subsequent to ligand-induced clustering of IgM receptors in lymphoblastoid cells (Salisbury et al., 1980); and (b) a correlation between the number of surface Tf-Rs and CCP in transfected cells (Iacopetta et al., 1988; Miller et al., 1991). The present work adds another dimension to these results, by providing direct evidence for a causal relationship between the presence of receptors and the formation of CCP.
Since both CD4 and Nef are required for the induction of CCP, what are their respective contributions to this phenomenon? Results presented show that both CD4 and Nef are required for the induction of CCP, and that Nef artificially tethered to the membrane as the cytoplasmic tail of a transmembrane fusion protein is capable of producing the same effect. These observations imply that Nef targeting to the plasma membrane, either through a direct physical interaction with CD4 (or the CD4/p56lck complex), or alternatively through part of a chimeric integral protein, is the primary requirement to induce CCP generation. This targeting may either permit the interaction of the viral protein with other proteins involved in signal transduction pathways leading to CCP generation, or allow a direct participation of the viral protein in CCP formation. Clathrin coat assembly is a multistep process in which a pivotal role is played by AP (Smythe and Warren, 1991; Schmid, 1992; Robinson, 1994). These heterotetrameric proteins participate in at least three crucial stages of clathrin-coated pit formation where Nef might act: (a) they must be recruited onto the right membrane, probably through binding to specific adaptor receptors (Mahaffey et al., 1990); (b) they next are activated to make them competent (possibly as part of complexes with other molecules) to bind clathrin triskelions (Peeler et al., 1993); and (c) they initiate the nucleation process leading to clathrin assembly into lattices (Keen et al., 1991; Gallusser and Kirchhausen, 1993). Several studies have provided evidence for a direct interaction of Nef with signal-transducing molecules such as protein tyrosine kinases of the src family (p56lck, hck) (Saksela et al., 1995; Collette et al., 1996), serine/threonine protein kinases (Sawai et al., 1995), protein kinase C (Bandres et al., 1994), or protein phosphatidylinositol 3-kinase (Graziani et al., 1996), all molecules which may participate in the triggering and/or regulation of endocytotic processes. Via these effects on signal transduction pathways Nef might, therefore, interfere with CD4 endocytosis. However, Nef-induced CD4 downregulation is generally dissociated from the effects of the viral protein on signal transduction pathways (Aiken and Trono, 1995; Chowers et al., 1995; Saksela et al., 1995), suggesting that mechanisms implemented by Nef to induce the formation of CCP are not related to perturbation of signal transduction and might rather involve a more direct participation of the viral protein. Several observations support this alternative: (a) Nef is capable of physical interaction with CD4 and this interaction requires the integrity of a dileucine motif present in CD4 cytoplasmic tail; (b) Nef-induced CD4 endocytosis is specific and is not dependent on CD4 alteration; (c) the chimeric molecule CD4–Nef is internalized efficiently through CCP, suggesting that Nef contains the determinants required to associate with CCP components (Mangasarian et al., 1997); and (d) Nef is capable of physical interactions through different domains with both CD4 and adaptins (Piguet, V., M. Foti, A. Mangasarian, D. Lew, K.-H. Krause, D. Trono, and J.-L. Carpentier, manuscript in preparation). Thus, although we cannot exclude that CCP generation is related to Nef-induced signaling cascades, data collected favor a model where Nef plays the role of a connector between specific recognition signals present in receptor tails (e.g., CD4) and adaptins. They also suggest that the CD4–Nef complex participates together with adaptins in the nucleation process, perhaps via the Nef-mediated activation of adaptins. A third model would be that Nef itself behaves as an adaptin. We do not favor this possibility since it would imply that Nef, which has no structural analogy with adaptins, not only plays all the diverse and complex functions of these molecules, but in addition can generate CCP morphologically identical to those requiring adaptins.
Nef might mimic endogenous molecules capable, in physiological conditions, to link various receptors bearing dileucine-based motifs (or other motifs) to components of the endocytic machinery, and sometimes to induce the formation of CCP. Along this line, if a possible direct interaction of dileucine motifs with adaptins has recently been reported, this concept is not accepted by others (Ohno et al., 1995; Heilker et al., 1996). It is noteworthy that several cellular proteins appear to function as connectors between cell surface receptors and CCP. β-arrestin has thus been demonstrated to act as a clathrin adaptor facilitating the endocytosis of the β2-adrenergic receptor (Ferguson et al., 1996; Goodman et al., 1996), while the Eps15 and Shc proteins are thought to participate in connecting the EGF receptor tyrosine kinase with CCP (Okabayashi et al., 1996; Van Delft et al., 1997). However, no data is available to suggest that these proteins can induce the formation of CCP.
Taken together, the present results demonstrate that CCP nucleation can be receptor driven and support the following ordered sequence of events leading to the nucleation process in these conditions: (1) receptors recruit endogenous helper molecules (here mimicked by Nef) via specific domain(s) of their cytoplasmic tail (e.g., dileucine-based domains); (2) the recruitment of these partner molecules on the cytoplasmic side of the plasma membrane renders possible their interaction with molecules capable of initiating clathrin assembly (i.e., AP2); and (3) after activation of these assembly proteins, the clathrin coat nucleation is generated. According to the model proposed, the internalization capacity of surface receptors might be modulated by factors affecting the expression or the affinity of these hypothetical endogenous connectors for their respective receptor tails. The participation of such connector molecules in the endocytosis of some receptors might be alternative (or complementary) to the recently documented mechanism implicating the direct binding of receptor cytoplasmic tails to AP-2 (Sorkin and Carpenter, 1993; Ohno et al., 1995; Heilker et al., 1996).
Acknowledgments
We thank A. Monod (Hôpital Cantonal Universitaire, Geneva, Switzerland) for cell cultures and G. Porcheron-Berthet (Centre Médical Universitaire, Geveva) for EM samples preparation.
This work was supported by the Fonds National Suisse pour la Recherche Scientifique, grants 31-36063.92 and 31-42409.95, National Institutes of Health (NIH) Fogarty International Center award R03 TW 00531 (to D. Trono and J.-L. Carpentier), a grant from the Fondation Centre de Recherches medicales Carlos et Elsie de Reuter (to J.-L. Carpentier), and NIH award R37 AI34306 (to D. Trono).
Abbreviations used in this paper
- AP
adaptor protein
- CCP
clathrin-coated pit(s)
- CD4
cluster of differentiation antigen type 4
- Tf-R
transferrin receptor
Footnotes
Address all correspondence to Jean-Louis Carpentier, Department of Morphology, Centre Médical Universitaire, 1, rue Michel-Servet, 1211 Geneva 4, Switzerland. Tel.: 41.22.7025201. Fax: 41.22.7025260. E-mail: Jean-LouisCarpentier@medecine.unige.ch
2. These values do not integrate the curvature of CCP which, if taken into account, would have amplified the difference in favor of CCP.
REFERENCES
- Aiken C, Trono D. Nef stimulates human immunodeficiency virus type 1 proviral DNA synthesis. J Virol. 1995;69:5048–5056. doi: 10.1128/jvi.69.8.5048-5056.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiken C, Konner J, Landau NR, Lenburg ME, Trono D. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell. 1994;76:853–864. doi: 10.1016/0092-8674(94)90360-3. [DOI] [PubMed] [Google Scholar]
- Anderson S, Shugars DC, Swanstrom R, Garcia JV. Nef from primary isolates of human immunodeficiency virus type 1 suppresses surface CD4 expression in human and mouse T cells. J Virol. 1993;67:4923–4931. doi: 10.1128/jvi.67.8.4923-4931.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandres JC, Luria S, Ratner L. Regulation of human immunodeficiency virus Nef protein by phosphorylation. Virology. 1994;201:157–161. doi: 10.1006/viro.1994.1278. [DOI] [PubMed] [Google Scholar]
- Benson RE, Sanfridson A, Ottinger JS, Doyle C, Cullen BR. Downregulation of cell-surface CD4 expression by simian immunodeficiency virus Nef prevents viral super infection. J Exp Med. 1993;177:1561–1566. doi: 10.1084/jem.177.6.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier, J.L. 1994. Insulin receptor internalization: molecular mechanisms and physiopathological implications. Diabetologia. 37 (Suppl 2):117s–124s. [DOI] [PubMed]
- Carpentier JL, Gorden P, Amherdt M, Van Obberghen E, Kahn CR, Orci L. 125I-insulin binding to cultured human lymphocytes. Initial localization and fate of hormone determined by quantitative electron microscopic autoradiography. J Clin Invest. 1978;61:1056–1070. doi: 10.1172/JCI109005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier JL, Van Obberghen E, Gorden P, Orci L. Surface redistribution of 125I-insulin in cultured human lymphocytes. J Cell Biol. 1981;91:17–25. doi: 10.1083/jcb.91.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier JL, Gorden P, Anderson RG, Goldstein JL, Brown MS, Cohen S, Orci L. Co-localization of 125I-epidermal growth factor and ferritin-low density lipoprotein in coated pits: a quantitative electron microscopic study in normal and mutant human fibroblasts. J Cell Biol. 1982;95:73–77. doi: 10.1083/jcb.95.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier JL, Lew DP, Paccaud JP, Gil R, Iacopetta B, Kazatchkine M, Stendahl O, Pozzan T. Internalization pathway of C3b receptors in human neutrophils and its transmodulation by chemoattractant receptors stimulation. Cell Regul. 1991;2:41–55. doi: 10.1091/mbc.2.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier JL, Paccaud JP, Gorden P, Rutter WJ, Orci L. Insulin-induced surface redistribution regulates internalization of the insulin receptor and requires its autophosphorylation. Proc Natl Acad Sci USA. 1992;89:162–166. doi: 10.1073/pnas.89.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowers MY, Pandori MW, Spina CA, Richman DD, Guatelli JC. The growth advantage conferred by HIV-1 Nef is determined at the level of viral DNA formation and is independent of CD4 downregulation. Virology. 1995;212:451–457. doi: 10.1006/viro.1995.1502. [DOI] [PubMed] [Google Scholar]
- Cohen S, Fava RA. Internalization of functional epidermal growth factor:receptor/kinase complexes in A-431 cells. J Biol Chem. 1985;260:12351–12358. [PubMed] [Google Scholar]
- Collette Y, Dutartre H, Benziane A, Ramos-Morales F, Benarous R, Harris M, Olive D. Physical and functional interaction of Nef with Lck. J Biol Chem. 1996;271:6333–6341. doi: 10.1074/jbc.271.11.6333. [DOI] [PubMed] [Google Scholar]
- Cupers P, Veithen A, Kiss A, Baudhuin P, Courtoy PJ. Clathrin polymerization is not required for bulk-phase endocytosis in rat fetal fibroblasts. J Cell Biol. 1994;127:725–735. doi: 10.1083/jcb.127.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damke H, Baba T, van der Bliek AM, Schmid SL. Clathrin-independent pinocytosis is induced in cells overexpressing a temperature-sensitive mutant of dynamin. J Cell Biol. 1995;131:69–80. doi: 10.1083/jcb.131.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson RB, Willingham MC, Pastan I. α2-macroglobulin adsorbed to colloidal gold: a new probe in the study of receptor-mediated endocytosis. J Cell Biol. 1981;89:29–34. doi: 10.1083/jcb.89.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elker P, Holm PK, van Deurs B, Sandvig K. Selective regulation of apical endocytosis in polarized Madin-Darby canine kidney cells by mastoparan and cAMP. J Biol Chem. 1994;269:18607–18615. [PubMed] [Google Scholar]
- Fan JY, Carpentier JL, Gorden P, Van Obberghen E, Blackett NM, Grunfeld C, Orci L. Receptor-mediated endocytosis of insulin: role of microvilli, coated pits, and coated vesicles. Proc Natl Acad Sci USA. 1982;79:7788–7791. doi: 10.1073/pnas.79.24.7788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feger J, Gil-Falgon S, Lamaze C. Cell receptors: definition, mechanisms and regulation of receptor-mediated endocytosis. Cell Mol Biol (Oxf) 1994;40:1039–1061. [PubMed] [Google Scholar]
- Ferguson SS, Downey WER, Colapietro AM, Barak LS, Menard L, Caron MG. Role of β-Arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science (Wash DC) 1996;271:363–366. doi: 10.1126/science.271.5247.363. [DOI] [PubMed] [Google Scholar]
- Fire E, Zwart DE, Roth MG, Henis YI. Evidence from lateral mobility studies for dynamic interactions of a mutant influenza hemagglutinin with coated pits. J Cell Biol. 1991;115:1585–1594. doi: 10.1083/jcb.115.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallusser A, Kirchhausen T. The β1 and β2 subunits of the AP complexes are the clathrin coat assembly components. EMBO (Eur Mol Biol Organ) J. 1993;12:5237–5244. doi: 10.1002/j.1460-2075.1993.tb06219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JV, Miller AD. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature (Lond) 1991;350:508–511. doi: 10.1038/350508a0. [DOI] [PubMed] [Google Scholar]
- Ghosh RN, Webb WW. Automated detection and tracking of individual and clustered cell surface low density lipoprotein receptor molecules. Biophys J. 1994;66:1301–1318. doi: 10.1016/S0006-3495(94)80939-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JL, Brown MS, Anderson RG, Russell DW, Schneider WJ. Receptor-mediated endocytosis: concepts emerging from the LDL receptor system. Annu Rev Cell Biol. 1985;1:1–39. doi: 10.1146/annurev.cb.01.110185.000245. [DOI] [PubMed] [Google Scholar]
- Goodman OB, Jr, Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, Keen JH, Benovic JL. β-Arrestin acts as a clathrin adaptor in endocytosis of the β2-adrenergic receptor. Nature (Lond) 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- Graziani A, Galimi F, Medico E, Cottone E, Gramaglia D, Boccaccio C, Comoglio PM. The HIV-1 nef protein interferes with phosphatidylinositol 3-kinase activation 1. J Biol Chem. 1996;271:6590–6593. doi: 10.1074/jbc.271.12.6590. [DOI] [PubMed] [Google Scholar]
- Greenway A, Azad A, McPhee D. Human immunodeficiency virus type 1 Nef protein inhibits activation pathways in peripheral blood mononuclear cells and T cell lines. J Virol. 1995;69:1842–1850. doi: 10.1128/jvi.69.3.1842-1850.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzesiek S, Stahl SJ, Wingfield PT, Bax A. The CD4 determinant for downregulation by HIV-1 nef directly binds to nef. Mapping of the nef binding surface by NMR. Biochemistry. 1996;35:10256–10261. doi: 10.1021/bi9611164. [DOI] [PubMed] [Google Scholar]
- Hanover JA, Beguinot L, Willingham MC, Pastan IH. Transit of receptors for epidermal growth factor and transferrin through clathrin-coated pits. Analysis of the kinetics of receptor entry. J Biol Chem. 1985;260:15938–15945. [PubMed] [Google Scholar]
- Hansen SH, Sandvig K, van Deurs B. Molecules internalized by clathrin-independent endocytosis are delivered to endosomes containing transferrin receptors. J Cell Biol. 1993;123:89–97. doi: 10.1083/jcb.123.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris MP, Neil JC. Myristoylation-dependent binding of HIV-1 Nef to CD4. J Mol Biol. 1994;241:136–142. doi: 10.1006/jmbi.1994.1483. [DOI] [PubMed] [Google Scholar]
- Heilker R, Manning U, Krieg, Zuber JF, Spiess M. In vitro binding of clathrin adaptors to sorting signals correlates with endocytosis and basolateral sorting. EMBO (Eur Mol Biol Organ) J. 1996;15:2893–2899. [PMC free article] [PubMed] [Google Scholar]
- Hopkins, C.R., K. Miller, and J.M. Beardmore. 1985. Receptor-mediated endocytosis of transferrin and epidermal growth factor receptors: a comparison of constitutive and ligand-induced uptake. J. Cell Sci. 3 (Suppl.):173–186. [DOI] [PubMed]
- Iacopetta BJ, Carpentier JL, Pozzan T, Lew DP, Gorden P, Orci L. Role of intracellular calcium and protein kinase C in the endocytosis of transferrin and insulin by HL60 cells. J Cell Biol. 1986;103:851–856. doi: 10.1083/jcb.103.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacopetta BJ, Rothenberger S, Kuhn L C. A role for the cytoplasmic domain in transferrin receptor sorting and coated pit formation during endocytosis. Cell. 1988;54:485–489. doi: 10.1016/0092-8674(88)90069-4. [DOI] [PubMed] [Google Scholar]
- Katzir Z, Nardi N, Geffen I, Fuhrer C, Henis Y I. Dynamic interactions of the asialoglycoprotein receptor subunits with coated pits. enhanced interactions of H2 following association with H1. J Biol Chem. 1994;269:21568–21575. [PubMed] [Google Scholar]
- Keen JH, Beck KA, Kirchhausen T, Jarrett T. Clathrin domains involved in recognition by assembly protein AP-2. J Biol Chem. 1991;266:7950–7956. [PubMed] [Google Scholar]
- Krischer J, Gilbert A, Gorden P, Carpentier JL. Endocytosis is inhibited in hepatocytes from diabetic rats. Diabetes. 1993;42:1303–1309. doi: 10.2337/diab.42.9.1303. [DOI] [PubMed] [Google Scholar]
- Lazarovits J, Naim HY, Rodriguez AC, Wang RH, Fire E, Bird C, Henis YI, Roth MG. Endocytosis of chimeric influenza virus hemagglutinin proteins that lack a cytoplasmic recognition feature for coated pits. J Cell Biol. 1996;134:339–348. doi: 10.1083/jcb.134.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Borgne R, Hoflack B. Mannose 6-phosphate receptors regulate the formation of clathrin-coated vesicles in the TGN. J Cell Biol. 1997;137:335–345. doi: 10.1083/jcb.137.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HC, Moore MS, Sanan DA, Anderson RG. Reconstitution of clathrin-coated pit budding from plasma membranes. J Cell Biol. 1991;114:881–891. doi: 10.1083/jcb.114.5.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaffey DT, Peeler JS, Brodsky FM, Anderson RG. Clathrin-coated pits contain an integral membrane protein that binds the AP-2 subunit with high affinity. J Biol Chem. 1990;265:16514–16520. [PubMed] [Google Scholar]
- Mangasarian A, Foti M, Aiken C, Chin D, Carpentier J-L, Trono D. The HIV-1 Nef protein acts as a connector with sorting pathways in the Golgi and at the plasma membrane. Immunity. 1997;6:67–77. doi: 10.1016/s1074-7613(00)80243-5. [DOI] [PubMed] [Google Scholar]
- Mariani R, Skowronski J. CD4 down-regulation by nef alleles isolated from human immunodeficiency virus type 1-infected individuals. Proc Natl Acad Sci USA. 1993;90:5549–5553. doi: 10.1073/pnas.90.12.5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell MAK. A new solid-state reagent to iodinate proteins. Anal Biochem. 1982;125:427–432. doi: 10.1016/0003-2697(82)90025-2. [DOI] [PubMed] [Google Scholar]
- Maxfield FR, Schlessinger J, Shechter Y, Pastan I, Willingham M C. Collection of insulin, EGF and α2-macroglobulin in the same patches on the surface of cultured fibroblasts and common internalization. Cell. 1978;14:805–810. doi: 10.1016/0092-8674(78)90336-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K, Shipman M, Trowbridge IS, Hopkins CR. Transferrin receptors promote the formation of clathrin lattices. Cell. 1991;65:621–632. doi: 10.1016/0092-8674(91)90094-f. [DOI] [PubMed] [Google Scholar]
- Ohno H, Stewart J, Fournier MC, Bosshart H, Rhee I, Miyatake S, Saito T, Gallusser A, Kirchhausen T, Bonifacino JS. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science (Wash DC) 1995;269:1872–1875. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- Okabayashi Y, Sugimoto Y, Totty NF, Hsuan J, Kido Y, Sakaguchi K, Gout I, Waterfield MD, Kasuga M. Interaction of Shc with adaptor protein adaptins. J Biol Chem. 1996;271:5265–5269. doi: 10.1074/jbc.271.9.5265. [DOI] [PubMed] [Google Scholar]
- Orci L, Malhotra V, Amherdt M, Serafini T, Rothman JE. Dissection of a single round of vesicular transport: sequential intermediates for intercisternal movement in the Golgi stack. Cell. 1989;56:357–368. doi: 10.1016/0092-8674(89)90239-0. [DOI] [PubMed] [Google Scholar]
- Paccaud JP, Reith W, Johansson B, Magnusson KE, Mach B, Carpentier JL. Clathrin-coated pit-mediated receptor internalization. Role of internalization signals and receptor mobility. J Biol Chem. 1993;268:23191–23196. [PubMed] [Google Scholar]
- Peeler JS, Donzell WC, Anderson RG. The appendage domain of the AP-2 subunit is not required for assembly or invagination of clathrin-coated pits. J Cell Biol. 1993;120:47–54. doi: 10.1083/jcb.120.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelchen Matthews, A., J.E. Armes, and M. Marsh. Internalization and recycling of CD4 transfected into HeLa and NIH3T3 cells. EMBO (Eur Mol Biol Organ) J. 1989;8:3641–3649. doi: 10.1002/j.1460-2075.1989.tb08538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelchen Matthews, A., J.E. Armes, G. Griffiths, and M. Marsh. Differential endocytosis of CD4 in lymphocytic and nonlymphocytic cells. J Exp Med. 1991;173:575–587. doi: 10.1084/jem.173.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SS, Marsh JW. Human immunodeficiency virus type 1 Nef-induced down-modulation of CD4 is due to rapid internalization and degradation of surface CD4. J Virol. 1994;68:5156–5163. doi: 10.1128/jvi.68.8.5156-5163.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MS. The role of clathrin, adaptors and dynamin in endocytosis. Curr Opin Cell Biol. 1994;6:538–544. doi: 10.1016/0955-0674(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Rodman JS, Mercer RW, Stahl PD. Endocytosis and transcytosis. Curr Opin Cell Biol. 1990;2:664–672. doi: 10.1016/0955-0674(90)90108-q. [DOI] [PubMed] [Google Scholar]
- Rossi F, Gallina A, Milanesi G. Nef-CD4 physical interaction sensed with the yeast two-hybrid system. Virology. 1996;217:397–403. doi: 10.1006/viro.1996.0130. [DOI] [PubMed] [Google Scholar]
- Rothman JE, Orci L. Molecular dissection of the secretory pathway. Nature (Lond) 1992;355:409–415. doi: 10.1038/355409a0. [DOI] [PubMed] [Google Scholar]
- Sako Y, Kusumi A. Compartmentalized structure of the plasma membrane for receptor movements as revealed by a nanometer-level motion analysis. J Cell Biol. 1994;125:1251–1264. doi: 10.1083/jcb.125.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksela K, Cheng G, Baltimore D. Proline-rich (PxxP) motifs in HIV-1 Nef bind to SH3 domains of a subset of Src kinases and are required for the enhanced growth of Nef+ viruses but not for down-regulation of CD4. EMBO (Eur Mol Biol Organ) J. 1995;14:484–491. doi: 10.1002/j.1460-2075.1995.tb07024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salghetti S, Mariani R, Skowronski J. Human immunodeficiency virus type 1 Nef and p56lck protein-tyrosine kinase interact with a common element in CD4 cytoplasmic tail. Proc Natl Acad Sci USA. 1995;92:349–353. doi: 10.1073/pnas.92.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury JL, Condeelis JS, Satir P. Role of coated vesicles, microfilaments, and calmodulin in receptor-mediated endocytosis by cultured β lymphoblastoid cells. J Cell Biol. 1980;87:132–141. doi: 10.1083/jcb.87.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salpeter MM, Fertuck HC, Salpeter EE. Resolution in electron microscope autoradiography III. Iodine-125, the effect of heavy metal staining and reassessment of critical parameters. J Cell Biol. 1977;72:161–173. doi: 10.1083/jcb.72.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanan DA, Anderson RG. Simultaneous visualization of LDL receptor distribution and clathrin lattices on membranes torn from the upper surface of cultured cells. J Histochem Cytochem. 1991;39:1017–1024. doi: 10.1177/39.8.1906908. [DOI] [PubMed] [Google Scholar]
- Sanfridson A, Hester S, Doyle C. Nef proteins encoded by human and simian immunodeficiency viruses induce the accumulation of endosomes and lysosomes in human T cells. Proc Natl Acad Sci USA. 1997;94:873–878. doi: 10.1073/pnas.94.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini F, Keen JH. Endocytosis of activated receptors and clathrin-coated pit formation: deciphering the chicken or egg relationship. J Cell Biol. 1996;132:1025–1036. doi: 10.1083/jcb.132.6.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai ET, Baur AS, Peterlin BM, Levy JA, Cheng-Mayer C. A conserved domain and membrane targeting of nef from HIV and SIV are required for association with cellular serine kinase activity. J Biol Chem. 1995;270:15307–15314. doi: 10.1074/jbc.270.25.15307. [DOI] [PubMed] [Google Scholar]
- Schmid SL. The mechanism of receptor-mediated endocytosis: more questions than answers. BioEssays. 1992;14:589–596. doi: 10.1002/bies.950140903. [DOI] [PubMed] [Google Scholar]
- Schmid SL. Biochemical requirements for the formation of clathrin- and COP-coated transport vesicles. Curr Opin Cell Biol. 1993;5:621–627. doi: 10.1016/0955-0674(93)90131-9. [DOI] [PubMed] [Google Scholar]
- Schmid SL, Smythe E. Stage-specific assays for coated pit formation and coated vesicle budding in vitro. J Cell Biol. 1991;114:869–880. doi: 10.1083/jcb.114.5.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz O, Riviere Y, Heard JM, Danos O. Reduced cell surface expression of processed human immunodeficiency virus type 1 envelope glycoprotein in the presence of Nef. J Virol. 1993;67:3274–3280. doi: 10.1128/jvi.67.6.3274-3280.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz O, Marechal V, Le Gall S, Lemonnier F, Heard JM. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat Med J India. 1996;2:338–342. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- Smythe E, Warren G. The mechanism of receptor-mediated endocytosis. Eur J Biochem. 1991;202:689–699. doi: 10.1111/j.1432-1033.1991.tb16424.x. [DOI] [PubMed] [Google Scholar]
- Sorkin A, Carpenter G. Interaction of activated EGF receptors with coated pit adaptins. Science (Wash DC) 1993;261:612–615. doi: 10.1126/science.8342026. [DOI] [PubMed] [Google Scholar]
- Trowbridge IS. Endocytosis and signals for internalization. Curr Opin Cell Biol. 1991;3:634–641. doi: 10.1016/0955-0674(91)90034-v. [DOI] [PubMed] [Google Scholar]
- Umesono K, Murakami KK, Thompson CC, Evans RM. Direct repeats as selective response elements for the thyroid hormone, retinoic acid, and vitamin D3 receptors. Cell. 1991;65:1255–1266. doi: 10.1016/0092-8674(91)90020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Delft, S., C. Schumacher, W. Hage, A.J. Verkleij, and P.M.P. van Bergen en Henegouwen. 1997. Association and colocalization of Eps15 with adaptor protein-2 and clathrin. J. Cell Biol. 136:811–821. [DOI] [PMC free article] [PubMed]
- van Deurs B, Petersen OW, Olsnes S, Sandvig K. The ways of endocytosis. Int Rev Cytol. 1989;117:131–177. doi: 10.1016/s0074-7696(08)61336-4. [DOI] [PubMed] [Google Scholar]
- Watts C. Rapid endocytosis of the transferrin receptor in the absence of bound transferrin. J Cell Biol. 1985;100:633–637. doi: 10.1083/jcb.100.2.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts C, Marsh M. Endocytosis: what goes in and how? . J Cell Sci. 1992;103:1–8. doi: 10.1242/jcs.103.1.1a. [DOI] [PubMed] [Google Scholar]
- Willingham MC, Pastan IH, Sahagian GG, Jourdian GW, Neufeld EF. Morphologic study of the internalization of a lysosomal enzyme by the mannose 6-phosphate receptor in cultured Chinese hamster ovary cells. Proc Natl Acad Sci USA. 1981;78:6967–6971. doi: 10.1073/pnas.78.11.6967. [DOI] [PMC free article] [PubMed] [Google Scholar]