Abstract
Angiogenesis is characterized by distinct phenotypic changes in vascular endothelial cells (EC). Evidence is provided that the Hox D3 homeobox gene mediates conversion of endothelium from the resting to the angiogenic/invasive state. Stimulation of EC with basic fibroblast growth factor (bFGF) resulted in increased expression of Hox D3, integrin αvβ3, and the urokinase plasminogen activator (uPA). Hox D3 antisense blocked the ability of bFGF to induce uPA and integrin αvβ3 expression, yet had no effect on EC cell proliferation or bFGF-mediated cyclin D1 expression. Expression of Hox D3, in the absence of bFGF, resulted in enhanced expression of integrin αvβ3 and uPA. In fact, sustained expression of Hox D3 in vivo on the chick chorioallantoic membrane retained EC in this invasive state and prevented vessel maturation leading to vascular malformations and endotheliomas. Therefore, Hox D3 regulates EC gene expression associated with the invasive stage of angiogenesis.
Angiogenesis requires coordinate changes in endothelial cell morphology and gene expression. In the resting vasculature, quiescent capillary endothelial cells are in contact with a laminin-rich basement membrane (BM)1 and are nonmigratory. In response to angiogenic stimuli, including cytokines such as basic fibroblast growth factor (bFGF), tumor necrosis factor α, and vascular endothelial growth factor, vascular endothelial cells (EC) reenter the cell cycle and upregulate proteolytic activity to degrade the existing BM, facilitating their invasion into stromal tissue. These vascular sprouts then synthesize a new BM and undergo morphological reorganization into mature quiescent, lumen-containing capillaries.
Proteolysis of the BM, along with enhanced vascular permeability which accompanies angiogenesis, facilitates an influx of serum proteins including vitronectin, fibronectin, and fibrinogen, creating a provisional extracellular matrix on which EC migrate. Although not typically expressed by quiescent resting EC, angiogenic EC express high levels of integrin αvβ3, which can effectively bind this provisional matrix (Brooks et al., 1994; for review see Cheresh and Mecham, 1994). In fact, both matrix-degrading serine and metalloproteinases and integrin αvβ3 have been shown to play essential roles in new vessel formation, as inhibition of their respective activities will impair tumor- or cytokine-mediated angiogenesis (Mignatti et al., 1989; Brooks et al., 1994; Johnson et al., 1994; Min et al., 1996). The mechanism by which angiogenic cytokines coordinately upregulate expression of proteases and adhesion molecules involved in angiogenesis however is not known.
One class of transcriptional activators that have been linked to extensive tissue remodeling are the homeobox (Hox) genes. In addition to their role in embryonic development, the Hox genes have been shown to play a significant role in differentiation and gene expression in adult tissues (Lawrence and Largman, 1992; Takeshita et al., 1993; Savageau et al., 1995). Inappropriate Hox gene expression has also been linked to tumorigenic tissues (Friedmann et al., 1994; Redline et al., 1994). Hox genes are characterized by a highly conserved 180-bp DNA-binding domain known as the homeodomain, which interacts directly with DNA to activate transcription of genes (Desplan et al., 1985). Putative target genes for Hox DNA-binding proteins include other Hox genes and transcription factors, cell adhesion molecules, extracellular proteins, and growth factors (for reviews see Botas, 1993; and Edelman and Jones, 1993; Immergluck et al., 1990; Goomer et al., 1994; Taniguchi et al., 1995). Interestingly, in the genome, Hox and integrin receptor gene families colocalize in clusters, indicative of parallel, coordinate evolution, further supporting a link between these groups of genes associated with tissue patterning (Wang et al., 1995).
Given the dramatic changes in cell–extracellular matrix interactions, EC morphology, and gene expression that occur during angiogenesis, we investigated expression of Hox genes and their role in endothelial cell behavior.
MATERIALS AND METHODS
Cells and Culture Conditions
Experiments were conducted using primary human umbilical vein endothelial cells (HUVEC; Clonetics, San Diego, CA) or an immortalized HUVEC cell line (line 1998; American Type Culture Collection, Rockville, MD). HUVECs were routinely cultured in M199 plus 20% FCS and ECGS (Upstate Biotechnologies, Lake Placid, NY). The immortalized 1998 cell line was maintained in M199 plus 5% FCS. For experiments in which bFGF was added, HUVECS were maintained in M199 plus 5% FCS, while the 1998 cell line was maintained in M199 with 2% serum. Recombinant human bFGF was purchased from Upstate Biotechnologies, and also kindly supplied by Dr. Judith Abraham (Scios Nova Inc., Mountain View, CA). For experiments using basement membrane, 1 × 106 cells were seeded onto thick layers of Matrigel (Becton Dickinson, Bedford, MA). Chick embryo fibroblast isolation and maintenance of the viral packaging cell line Q4dh was performed as previously described (Stoker and Bissell, 1988).
Transfection of immortalized HUVEC or Q4dh cell lines was performed using calcium phosphate and stable transfectants selected using 800 or 400 μg/ml G418, respectively (Sigma Chemical Co., St. Louis, MO). At least two independently selected pools of stably transfected cells were used for subsequent analysis.
Isolation of HOX Genes Expressed in EC
RT-PCR of HOX genes was performed using total RNA from primary HUVECs cultured in the presence or absence of BM. After reverse transcription with oligo-dT primers (Gene Amp; Perkin Elmer, Norwalk, CT), DNA was amplified using degenerate primers against the conserved homeodomain sequences as described by Friedman et al. (1994): forward primer 5′-GGAATTCCGARCTNGARAARGARTT-3′; and reverse primer 5′-CCCAAGCTTRTTYTGRAACCADATYTT-3′. PCR reaction conditions include initial denaturation for 2 min at 95°C followed by 35 cycles at 95, 55, and 72°C for 1 min each plus a final incubation for 10 min at 72°C. In some instances, samples were reamplified for an additional 25 cycles using similar reaction conditions. Final PCR products were cloned into TA cloning vectors (Invitrogen, San Diego, CA) and positive clones amplified and Hox sequences identified by the dideoxy sequencing method of Sanger et al. (1977).
For quantitative PCR analysis of Hox D3 mRNA, 1 μg of total RNA was reverse transcribed using oligo-dT primers and amplified for 30 cycles at 95, 60, and 72°C with Hox D3 primers spanning a coding region to eliminate amplification of possible contaminating genomic DNA (forward primer [bp 1682–1705] 5′-TGGTCTGAACTCAGAGCAGCAGC-3′ and reverse primer [bp 3962–3938] 5′-TCATGCGCCGGTTCTGGAACCA-3′). This yielded the predicted 415-bp PCR product, which was confirmed by DNA sequencing. To normalize for amounts of starting RNA, equal volumes of reaction mixtures were amplified for 35 cycles at 95, 60, and 72°C for 1 min each using primers for human GAPDH; forward primer (bp 212–236) 5′-CCACCCATGGCAAATTCCATGGCA-3′; and reverse primer (bp 787–811) 5′-TCTAGACGGCAGGTCAGGTCCACC-3′; (Stratagene, La Jolla, CA). PCR products were visualized and isolated by running through 2% agarose gels containing 1 μg/ml ethidium bromide.
Construction of Expression Vectors
Human genomic Hox D3 DNA was a gift from Y. Taniguchi (Tokai School of Medicine, Bohseidai, Isehara, Kanagawu, Japan). A full length Hox D3 cDNA was constructed by inserting the 415-bp PCR product, generated as described above, between the Pst I site (bp 1899) and the EcoRI site (bp 3853) in the genomic Hox D3. Expression vectors were constructed by inserting either the genomic or Hox D3 cDNA between the KpnI and BamHI sites of pcDNA3 mammalian expression vector (Invitrogen). Antisense expression vectors were constructed by inserting the genomic DNA between the BamHI and HindIII sites of the pcDNA3 in the antisense orientation. For controls, cells were transfected with empty pcDNA3 vectors.
Chicken retroviral expression vectors were constructed by inserting either the Hox D3 genomic DNA or cDNA between the ClaI and BamHI sites of the replication-defective retroviral vector CK (a gift from B. Vennstrom, European Molecular Biology Laboratory, Heidelberg, Germany). These vectors were then stably transfected into the packing cell line Q4dh to generate infectious virus (Stoker and Bissell, 1988). Production of infectious retrovirus was monitored by infecting chick embryo fibroblasts with supernatants from cultures of stably transfected packing cells as described by Stoker and Bissell (1988).
RNA Isolation and Northern Blot Analysis
Total RNA was extracted using the Quick-Prep Kit (Qiagen, Santa Clara, CA). For cells cultured on BM, cells were first detached from Matrigel using Matripserse (Becton Dickinson) and RNA isolated as described above. 10 or 20 μg total RNA was run through 1% agarose formaldehyde gels and transferred to Hybond-N membranes (Amersham Intl., Arlington Heights, IL) according to standard procedures. Membranes were hybridized using [32P]dCTP-labeled cDNA probes and exposed to Kodak X-Omat film at −70°C. cDNA probes for human β3 and β5 integrin were from E. Filardo (Brown University, Providence, RI). cDNA probe for human urokinase plasminogen activator (uPA) was kindly provided by Graham Parry (The Scripps Research Institute, La Jolla, CA).
Western Blots
Cultured cells were lysed in buffer containing 1 M NaCl, 10 mM Tris, pH 7.5, 1% Triton X-100 and aprotinin, PMSF, and leupeptin. Total protein concentration was determined using the BCA reagent kit (Pierce, Rockford, IL), and a total of 10 μg was run on 10 or 12% SDS-PAGE under reducing conditions. Gels were transferred to Immobilon nylon membranes (Millipore, Bedford, MA) and blocked in TBS plus 5% milk proteins. Blots were probed using either 1:200 dilution of monoclonal anti–human cyclin D1 (sc-246; Santa Cruz Biotechnologies, Santa Cruz, CA) or 10 μg/ ml polyclonal rabbit antihuman β3 integrin (AP-3) followed by a 1:1,000 dilution of either goat anti–rabbit or goat anti–mouse HRP (Biosource International, Camarillo, CA) and visualized by chemiluminescence (ECL; Amersham Intl.).
Adhesion Assays
Cells were removed by incubation with versene (0.5 M EDTA in PBS, pH 7.4) and resuspended in FBM media (Clonetics Corp., San Diego, CA) supplemented with 0.2 M MnCl2. A total of 5 × 105 cells was seeded into each well of 24-well culture dishes (Costar Corp., Cambridge, MA) that had been previously coated for 1 h at 37°C with either heat-denatured BSA (1%) or 10 μg/ml human fibrinogen (Sigma Chemical Co.). In some cases 25 μg/ml of a function blocking anti–human αvβ3 integrin (mAb LM609) were added along with cells. After extensive washing, cells were fixed in 2% ethanol and stained with 1% crystal violet. The number of adherent cells was quantitated by absorbance at 600 nm.
BrdU Incorporation
To determine the effect of Hox D3 on cell proliferation, the rate of DNA synthesis was established by measuring BrdU incorporation in control and EC transfected with either Hox D3 or Hox D3 antisense expression vectors. After incubation for 4 or 12 h with 10 μM BrdU, cells were fixed with 70% ethanol and stained with an anti-BrdU kit (Boehringer Mannheim, Indianapolis, IN) followed by staining with 0.5 μg/ml DAPI (4,6 diamidino-2-phenylindole; Sigma Chemical Co.). The percentage of BrdU-positive nuclei was determined by counting at least six different fields in each of the cell types tested.
Chick Chorioallantoic Membrane Assay
All assays were performed in 10-d-old pathogen-free embryos (SPAFAS). Briefly, the chorioallantoic membranes (CAMs) were prepared as previously described by Brooks et al. (1994). Q4dh virus-producing cells were trypsinized, and a total of 5 × 106 cells were resuspended in 25 μl serum-free M199 and seeded onto the membranes and embryos incubated at 39°C. After 3 d, CAMs were harvested by washing in PBS and fixed for 30 min in 2% paraformaldehyde followed by washing in 0.1 M glycine/PBS. CAMs were embedded into embedding medium (OCT; Miles, Elkhardt, IN), flash frozen in liquid nitrogen, and stored at −80°C. 5-μm sections were prepared and mounted on slides for immunohistochemistry.
Immunohistochemistry
Sections were briefly fixed in 100% acetone and equilibrated with PBS. After blocking in PBS plus 5% BSA, sections were incubated with either 5 μg/ml monoclonal antihuman αvβ3 (LM609) or a 1:1,000 dilution of polyclonal rabbit anti–human von Willebrand Factor (BioGenex, San Ramon, CA). Goat anti–mouse-rhodamine or goat anti–rabbit-FITC (Biosource International, Camarillo, CA) were used as secondary antibodies. Sections were subsequently mounted using Fluoromount (Southern Biotechnologies, Birmingham, AL).
In Situ Hybridization
Hox D3 sense and antisense digoxigenin-labeled RNA probes were generated by linearizing the vector pCR II (Invitrogen) containing the 415-bp Hox D3 cDNA (generated by RT-PCR described above) and incubating with T7 or SP6 RNA polymerase and digoxigenin-conjugated dUTP (Genius; Boehringer Mannheim). In situ hybridization was performed on 5-μm cryosections mounted on siliconized slides (Sigma Chemical Co.). Sections were fixed in 4% paraformaldehyde for 20 min and dehydrated in 30, 70, and 100% ethanol for 2 min each and hybridized overnight at 45°C with 800 ng/ml of Hox D3 riboprobes in 50% formamide, 10% dextran sulfate, 1% blocking reagent (DIG nucleic acid detection kit; Boehringer Mannheim), 300 μg/ml yeast tRNA (Sigma Chemical Co.), 3 mM NaCl, 10 mM Tris, pH 7.5, and 1 mM EDTA. After washing in 0.2× SSPE at 50°C for 2 h, sections were incubated overnight with a 1:500 dilution of HRP-conjugated antibodies against digoxigenin and color developed using NBT/BCIP (DIG nucleic acid detection Kit; Boehringer Mannheim). Nonspecific hybridization was assessed using Hox D3 sense riboprobes.
RESULTS
Hox Gene Expression and the Angiogenic Phenotype of EC
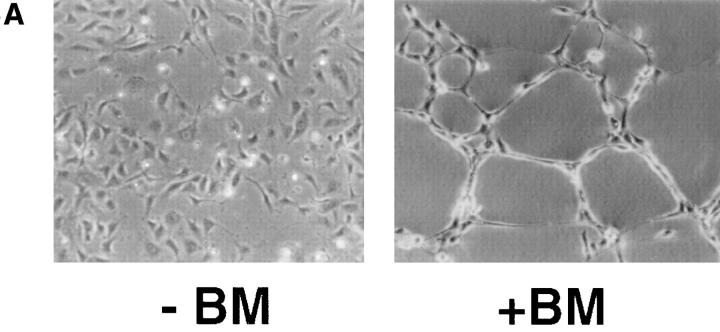
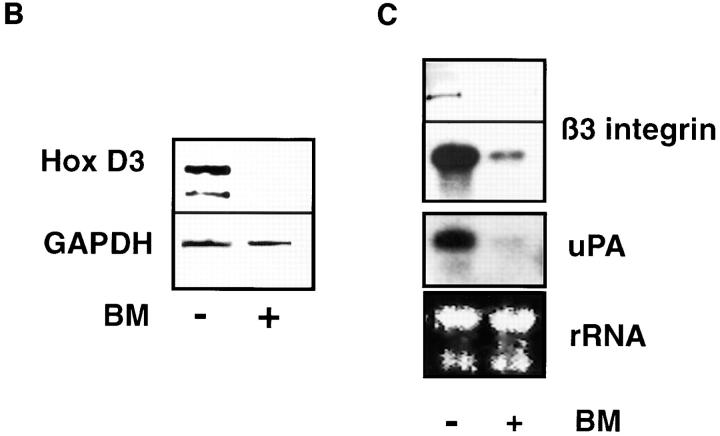
EC cultured on basement membrane adopt a distinct morphology reminiscent of capillaries in vivo (Fig. 1 A, + BM), withdraw from the cell cycle, and cease DNA synthesis within 24 h (Kubota et al., 1988). This “differentiated phenotype” contrasts the characteristic cobblestone morphology associated with proliferating EC cultured in the absence of BM (Fig. 1 A, −BM). RT-PCR of mRNA from cultured EC detected the presence of a number of Hox gene mRNA's including Hox A4, B3, B4, B5, and D3 (data not shown). Previous studies have linked Hox D3 with expression of β3 integrin mRNA in erythroleukemia cells (Tanaguchi et al., 1995). This β3 subunit is common to integrin αvβ3, which is highly expressed by angiogenic EC (Brooks et al., 1994), and prompted us to examine whether Hox D3 expression was related to the expression of αvβ3 on EC. We therefore performed quantitative RT-PCR analysis of EC cultured in the presence (+) or absence (−) of BM for 36 h. As shown in Fig. 1 B, Hox D3 expression was detected in proliferating EC but not in quiescent EC cultured on BM. Northern and Western blot analysis also shows that high levels of both β3 integrin mRNA and protein were preferentially expressed by proliferating EC. Similarly, uPA mRNA was also highly expressed in proliferating but not quiescent EC (Fig. 1 C). Thus, while quiescent EC do not express detectable levels of Hox D3, β3 integrin, or uPA mRNA, EC in the absence of BM express Hox D3 and genes associated with the angiogenic phenotype.
Figure 1.
Expression of Hox D3, β3 integrin, and uPA in quiescent and proliferative EC. (A) Morphology of HUVEC cultured in the presence (+) or absence (−) of reconstituted BM after 24 h in M199 containing 5% FCS. (B) RT-PCR of 1 μg mRNA from HUVEC cultured for 48 h with (+) or without (−) reconstituted BM using primers for Hox D3 or GAPDH. (C) Western (top) and Northern (bottom) blot analysis of integrin β3 expression in HUVEC cultured in the presence (+) or absence (−) of BM for 48 h. Northern blots were reprobed for uPA mRNA expression. The bottom panel shows ethidium bromide staining of total RNA loaded in the corresponding gel.
Hox D3 Induces β3 Integrin and uPA mRNA but Does Not Influence EC Proliferation
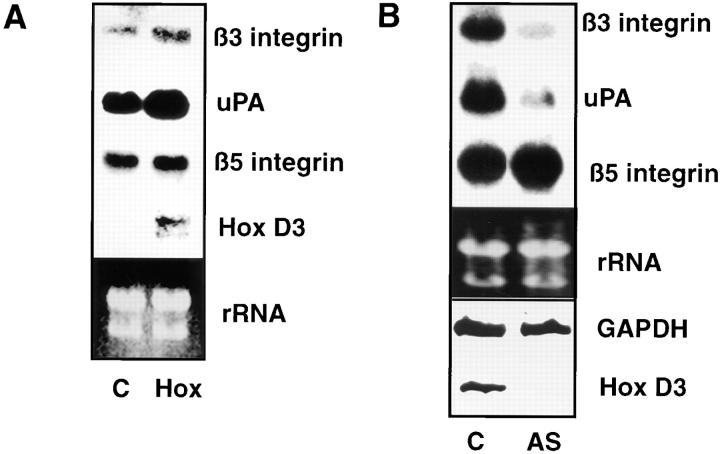
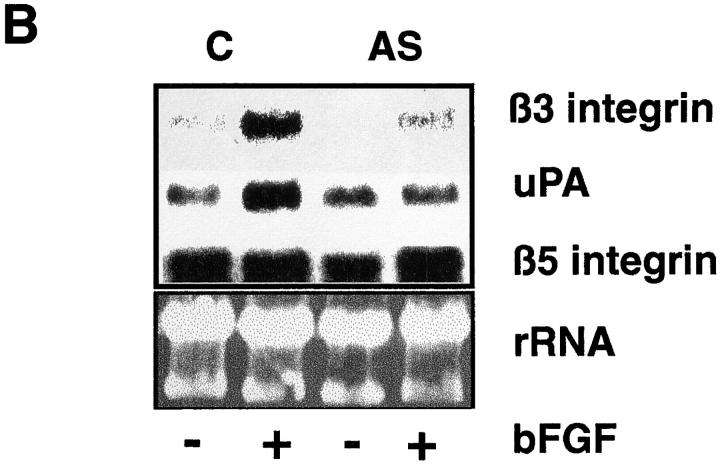
To determine whether the expression of Hox D3 influences expression of genes associated with the angiogenic phenotype of EC, a HUVEC cell line was stably transfected with a Hox D3 expression vector. Northern blot analysis shows that HUVECs overexpressing Hox D3 have significantly higher levels of steady-state β3 integrin and uPA mRNA levels as compared to cells transfected with control vectors. In contrast, mRNA encoding integrin β5, which is not upreglated during angiogenesis, did not differ in control or Hox D3-transfected cells (Fig. 2 A). To establish whether Hox D3 was required for EC expression of β3 integrin and uPA, HUVECs were stably transfected with Hox D3 antisense expression vectors. Compared to control-transfected cells, baseline levels of both β3 integrin and uPA mRNA were significantly reduced in cells expressing Hox D3 antisense. In contrast, levels of β5 integrin mRNA were not influenced by expression of Hox D3 antisense (Fig. 2 B).
Figure 2.
Influence of Hox D3 on β3 integrin and uPA expression in endothelial cells. (A) Northern blot analysis of β3 integrin, β5 integrin, uPA, and Hox D3 mRNA levels from 20 μg total RNA from immortalized HUVEC stably transfected with control (C) or Hox D3 (Hox) expression vectors. The lower panel shows ethidium bromide staining of total RNA loaded for each sample in the corresponding gel. (B) Northern blot analysis of β3 integrin, β5 integrin, and uPA mRNA expression levels from immortalized HUVECS transfected with control (C) or antisense expression vectors for Hox D3 (AS). RNA (20 μg) from each sample was loaded, and total RNA was visualized by ethidium bromide staining of the corresponding gel. The lower box shows RT-PCR of 1 μg total RNA from cells transfected with control (C) or antisense expression vectors against Hox D3 (AS) using primers for Hox D3 or GAPDH.
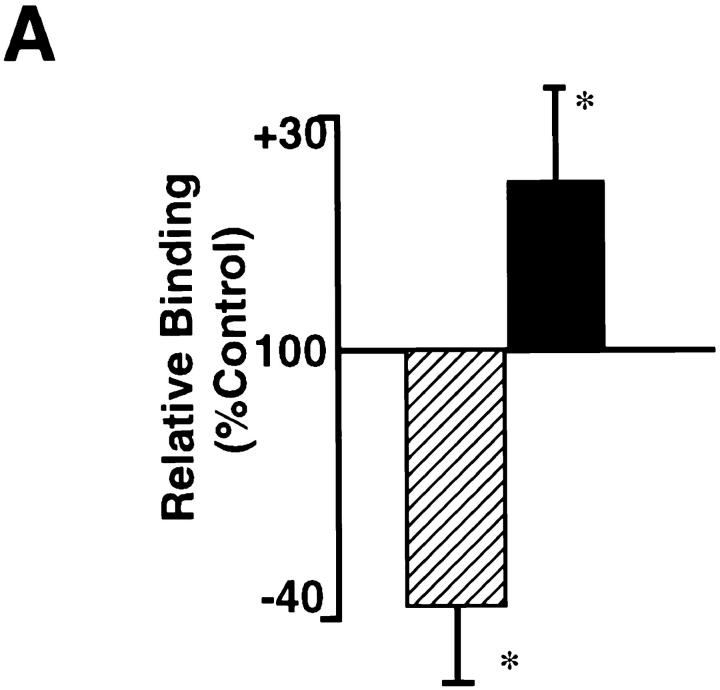
The Hox D3-mediated changes in expression of integrin β3 mRNA also resulted in corresponding changes in surface expression of functional αvβ3 integrin. We compared the ability of EC overexpressing Hox D3 or antisense against Hox D3 to adhere to fibrinogen, a ligand for αvβ3 on endothelial cells (Cheresh et al., 1989). EC expressing antisense against Hox D3 displayed significantly reduced capacity to adhere to fibrinogen, compared to control cells (Fig. 3 A), whereas overexpressing Hox D3 increased EC adhesion to fibrinogen (Fig. 3 A). Treatment of cells with LM609, a function-blocking antibody against αvβ3 (Cheresh, 1987), resulted in a complete inhibition of the fibrinogen-mediated attachment of these cells (data not shown). Thus, Hox D3 regulates the functional expression of αvβ3 on endothelial cells.
Figure 3.
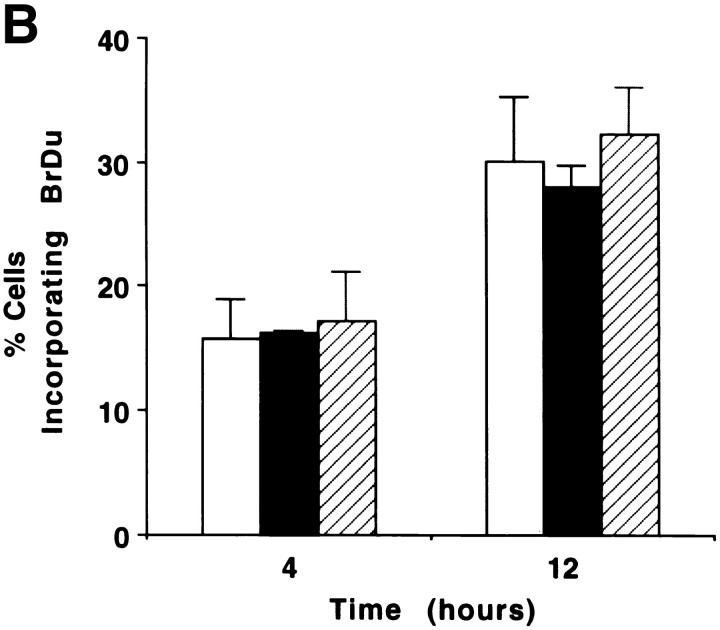
Effects of Hox D3 sense and antisense expression on EC adhesion and proliferation. (A) Adhesion to microtitre wells coated with 10 μg/ml fibrinogen by EC transfected with Hox D3 sense (▪) or antisense (▨ ▨ ▨ ) expression vectors. Adhesion after 30 min was assessed by absorbance at 600 nm and expressed as a percentage of adhesion displayed by control-transfected EC (n = 3). *P < 0.05. (B) BrdU incorporation in control-transfected EC (□), Hox D3-overexpressing cells (▪), or cells expressing antisense against Hox D3 (▨ ▨ ▨ ). Cells were labeled for 4 or 12 h with 10 mM BrdU. The percentage of cells staining positive for BrdU was assessed by counting at least six different fields containing a total of at least 400 cells each.
The fact that angiogenesis is often accompanied by EC proliferation prompted us to examine whether Hox D3 also influenced the rate of EC growth. We therefore compared DNA synthesis, as determined by BrdU incorporation, in control EC and EC transfected with Hox D3 or Hox D3 antisense expression vectors. In contrast to the Hox D3-induced changes in expression of integrin β3 and uPA described above, no differences in DNA synthesis were observed between control EC, EC expressing Hox D3, or antisense against Hox D3 (Fig. 3 B). Therefore, while Hox D3 antisense can influence the expression of integrin αvβ3 and uPA in EC it apparently does not directly influence the proliferation of these cells.
Hox D3 Mediates bFGF-induced Expression of β3 Integrin and uPA but Not Cyclin D1 in EC
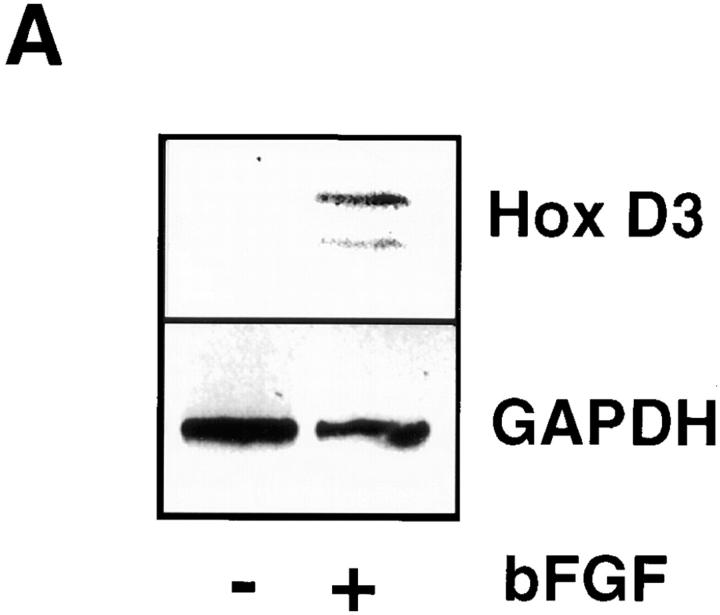
The angiogenic cytokine bFGF leads to expression of both uPA and β3 integrin in EC, which subsequently contribute to the invasive properties of EC during the early stages of angiogenesis (Moscatelli et al., 1985; Enenstein et al., 1992; Brooks et al., 1994; Sepp et al., 1994). To determine whether Hox D3 could influence the bFGF-mediated induction of integrin αvβ3 and uPA, EC were made quiescent by culturing on BM and treating with or without bFGF and analyzed for expression of mRNA encoding these proteins. As shown in Fig. 4, exposure of HUVECs to bFGF led to an increase in Hox D3 expression after 8 h, which was accompanied by increased β3 integrin and uPA steady-state mRNA levels by 24 h (Fig. 4, A and B). In contrast, in EC expressing antisense against Hox D3, the ability of bFGF to induce high levels of expression of β3 integrin and uPA mRNA expression was attenuated. Importantly, integrin β5 mRNA was not altered in either the control or antisense-transfected cells in the presence or absence of bFGF (Fig. 4, A and B).
Figure 4.
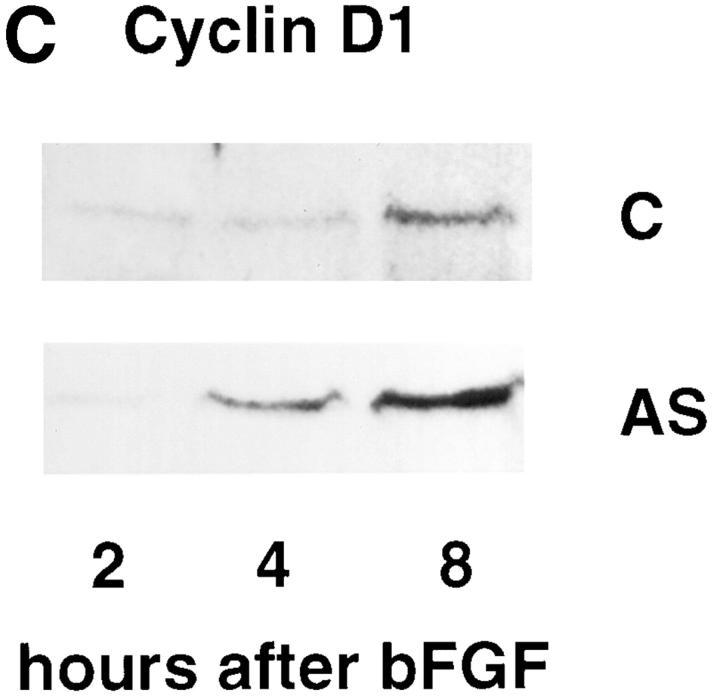
Hox D3 mediates bFGF-induced expression of β3 integrin and uPA. (A) RT-PCR of 1 μg total RNA from HUVEC 24 h after treatment with 20 ng/ml bFGF (+bFGF) using primers for Hox D3 or GAPDH. (B) Northern blot analysis of β3 integrin, β5 integrin, and uPA mRNA levels in HUVEC transfected with control plasmid (C) or antisense against Hox D3 (AS) after 24 h with (+) or without (−) addition of 20 ng/ml bFGF. (C) Western blot of cyclin D1 in immortalized HUVEC transfected with control or antisense against Hox D3. After 24 h in serum-free M199, samples were collected at 2, 4, and 8 h after addition of 20 ng/ml bFGF. 10 μg total cell lysate from each time point was separated on 12% SDS-PAGE, transferred to nylon membranes, and probed with a polyclonal antibody against human cyclin D1.
Although bFGF can also act as an endothelial cell mitogen, expressing Hox D3 antisense had no effect on bFGF's ability to induce cyclin D1 in EC (Fig. 4 C). Thus Hox D3 appears to mediate bFGF-induced expression of β3 integrin and uPA but does not directly influence endothelial cell cycle progression. This is consistent with the observation that Hox D3 antisense had no effect on EC proliferation (Fig. 3 B). These findings indicate that Hox D3 appears to influence EC genes important for invasion but not proliferation.
Sustained Expression of Hox D3 In Vivo Results in Vascular Malformations and Development of Endotheliomas
During the late stages of angiogenesis, integrin αvβ3 and uPA are down regulated as EC reestablish an intact BM and form a lumen, suggesting that Hox D3 may be required only during the initial stages of angiogenesis. Maintaining expression of Hox D3 therefore might be expected to prolong expression of an invasive phenotype and interfere with normal vascular maturation and/or remodelling. To test this possibility, we constructed replication-defective avian retroviral vectors designed to constitutively express human Hox D3 in vivo. Transfection of a transformed viral packaging cell line Q4dh with Hox D3 proviral vectors yielded a retrovirus capable of infecting proliferating embryonic chick cells and driving expression of human Hox D3 in these cells. When grafted onto 10-d chick embryo CAM's, transformed virus-producing Q4dh cells generate solid tumors in these animals. The continued production of infectious virus leads to infection of adjacent, proliferating cells including tumor-associated EC. As shown in Fig. 5 A, maintenance of Hox D3 expression in these tissues caused blood vessel malformations, reminiscent of endotheliomas, which appeared as abnormal capillary-like structures several times greater in diameter than large capillaries and were filled with hematopoietic cells. A majority of the EC and certain hematopoietic cells within these endotheliomas remained positive for αvβ3, providing evidence that Hox D3 was active in these tissues (Fig. 5 B). To confirm the presence of Hox D3 in these vascular structures, we performed in situ hybridization using probes that detect retrovirally produced human Hox D3 but do not detect endogenous chick Hox genes. Retroviral-mediated Hox D3 expression was observed in a wide variety of tissues including epithelium, connective tissue, hematopoietic cells, and endothelial cells (Fig. 5 C). In contrast to the widespread viral infection and expression of Hox D3, αvβ3 integrin was only detected in EC and some hematopoietic cells, suggesting this expression was highly specific.
Figure 5.
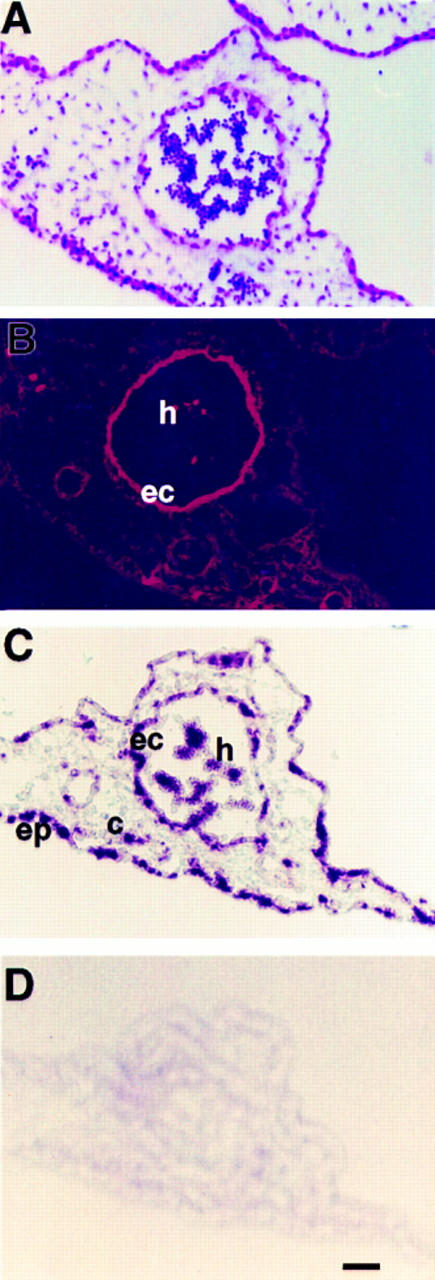
Colocalization of Hox D3 and αvβ3 integrin in endotheliomas in vivo. (A) H&E-stained cross-section of tissue infected by Hox D3-expressing retrovirus shows an abnormally large capillary-like structure filled with erythrocytes. (B) Immunofluorescent staining with LM609 against αvβ3 in the corresponding serial section shows strong positive staining in both the endothelial component (ec) of this vascular structure and in hematopoietic cells (h) contained within. (C) In situ hybridization of retrovirally expressed Hox D3 in corresponding serial section showing widespread expression in epithelium (ep), endothelial cells (ec), connective tissue (c), and hematopoietic cells (h). (D) Control in situ hybridization in a serial section performed with a sense riboprobe for Hox D3. Bar, 20 μm.
These vascular malformations or endotheliomas ultimately resulted in the formation of large hemorrhagic zones within the tumors generated by 15/18 Hox D3-infected embryos, but was not observed in tumors of the control embryos (Fig. 6, A and B). H&E staining of sectioned Hox D3-infected tissue showed large endothelial-lined cysts filled with hematopoietic cells, many of which had hemorrhaged (Fig. 6 D). In contrast, tumors producing control virus showed normal tumor-induced angiogenesis (Fig. 6 C). These findings not only establish a role for Hox D3 in EC function but also emphasize the requirement for tightly regulated expression of Hox D3 during the early events of angiogenesis.
Figure 6.
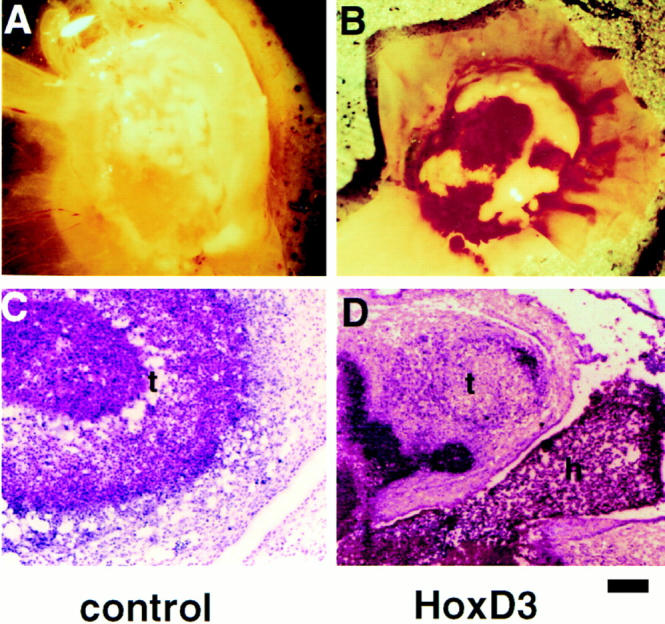
Effect of retroviral-mediated expression of Hox D3 in chick embryos. (A and B) Morphology of tumors generated 3 d after grafting of QT6 cells producing control or Hox D3-expressing retrovirus onto 10 d chick CAMs. (C and D) H&E staining in tissue cross-sections from tumors (t) and vasculature produced by cells shedding control or Hox D3-expressing retrovirus. Note the large hemorrhagic region containing hematopoietic cells (h) adjacent to tumor tissue in the Hox D3-infected tissue. Bar, 50 μm.
DISCUSSION
During embryonic development, normal tissue patterning depends upon temporally and spatially restricted expression of Hox genes. Evidence is presented in this report that patterning of the vasculature during angiogenesis is affected by expression of the Hox D3 homeobox gene in EC. We show that quiescent EC in contact with a BM express minimal levels of Hox D3, integrin αvβ3, and uPA. However, exposure of EC to the angiogenic cytokine bFGF leads to increased levels of Hox D3, resulting in the functional expression of the angiogenic-promoting genes integrin β3 and uPA in these cells.
Our findings that prolonged expression of Hox D3 results in abnormal vascular morphology also emphasizes the requirement for tightly regulated expression of Hox genes in EC. Previous studies have linked sustained expression of angiogenic mediators, including bFGF and uPA with vascular tumors (Takahashi et al., 1994; Hatva et al., 1996; Kraling et al., 1996). In vivo, transgenic mice expressing polyoma middle T oncogene develop endothelial tumors, and in culture, these transformed EC form cystic vascular structures reminiscent of endothelioma, a phenotype that can be reversed by inhibiting uPA activity (Bautch et al., 1987; Montesano et al., 1990). In addition, embryonic chick cells infected with v-src, a downstream effector of FGF, show elevated uPA activity, and v-src infection of chick endothelial cells in vivo results in endotheliomas (Sullivan and Quigley, 1986; Stoker et al., 1990). Thus, while increased uPA activity is involved in normal capillary remodeling, chronic expression driven by Hox D3 would be expected to prevent EC from establishing basement membranes, forming patent vessels with lumens and resuming a quiescent, differentiated phenotype. Furthermore, the sustained expression of integrin avβ3 provides the necessary means for EC to adhere, migrate, and survive in this angiogenic environment and thereby may enhance the activity of matrix-degrading serine and metalloproteinases, which interact with this integrin (Brooks et al., 1996; Strömblad et al., 1996).
Previous studies have linked FGF to the induction of Hox D genes during embryonic limb development, although the precise targets of Hox D gene activity have not been identified (Duprez et al., 1996). In this report it is apparent that Hox D3 induction of integrin αvβ3 is tissue specific, since this integrin was not detected in epithelial tissues expressing human Hox D3. In fact, endogenous Hox D3 is expressed in human skin epithelium, yet this tissue does not express αvβ3 (Detmer et al., 1993; Brooks et al., 1994; Brown et al., 1994; Gailit et al., 1994). We also observed that quiescent vessels in the skin do not express Hox D3 (Boudreau, N., unpublished observations). However, EC and certain hematopoietic cells infected with Hox D3-expressing retrovirus subsequently stained positively for αvβ3. Hox D3 has previously been shown to induce expression of the β3 subunit of the αIIbβ3 platelet adhesion receptor in cultured human erythroleukemia cells which can be induced to differentiate to erythroid cells (Taniguchi et al., 1995). Thus, the ability of Hox D3 to mediate expression of the β3 integrin subunit may be restricted to cells of the hematopoietic/angioblast lineage which arise from common blood islands during embryogenesis (Risau, 1995).
We have also shown that Hox D3 antisense blocks the bFGF-induced expression of integrin β3 and uPA mRNA, yet has no effect on bFGF-induced expression of cyclin D1 in these cells. Furthermore, we have shown that Hox D3 does not directly influence the rate of cell proliferation, and together these results suggest that these aspects of angiogenesis are differentially regulated. These observations are also supported by studies showing Hox D3 overexpression had no effect on cell proliferation (Taniguchi et al., 1995). Therefore in EC, Hox D3 appears to selectively regulate the expression of genes that contribute to extracellular matrix remodeling during angiogenesis.
Although both the integrin β3 and uPA promoters contain several potential Hox-binding consensus sequences, the complex relationship between Hox genes makes it difficult to predict whether Hox D3 acts alone or in combination with sequentially activated Hox genes to induce expression of these angiogenic effectors. For example, studies with compound mutants of Hox D3 and A3, suggests that paralogous Hox genes act in a synergistic manner to influence tissue patterning (Condie and Capecchi, 1994). Similarly, activation or inhibition of the paralogous Hox B3 gene, which is also expressed in EC, modifies expression of several other Hox genes including D3 (Faiella et al., 1994). Nonetheless, in EC, expression of Hox D3 is sufficient to convert quiescent EC to an angiogenic/invasive state. This is supported by the observation that Hox D3 antisense prevents bFGF-mediated expression of both β3 integrin and uPA in these cells. Our findings also reveal that temporally restricted expression of Hox D3 is required for normal angiogenesis since prolonged expression of Hox D3 maintains the angiogenic/invasive EC phenotype, thereby disrupting normal vascular remodeling.
Acknowledgments
The authors wish to thank Drs. Marlene Rabinovitch, Connie Myers, and especially Mina Bissell for critical reading of the manuscript and helpful discussions. We also thank Brian Eliceiri for help with graphics and Dr. Judith Abraham for providing bFGF used in these studies.
This work was supported by National Institutes of Health grant numbers HL54444 to D.A. Cheresh and Department of Energy grant number DEAC03-76-SF00098 to Mina J. Bissell, in whose laboratory this work was initiated. N. Boudreau was supported by a fellowship from the Joseph Drown Foundation. This is manuscript number 10482-IMM from the Scripps Research Institute.
Abbreviations used in this paper
- bFGF
basic fibroblast growth factor
- BM
basement membrane
- CAM
chorioallantoic membrane
- EC
vascular endothelial cells
- HUVEC
human umbilical vein endothelial cells
- uPA
urokinase plasminogen activator
Footnotes
Address all correspondence to Nancy Boudreau, Department of Anatomy, Medical College of Virginia, 1101 E. Marshall Street, Richmond, VA 23298. Tel.: (804) 828-7887. Fax: (804) 828-9477. E-mail: nboudrea @nsc.vcu.edu
REFERENCES
- Bautch VL, Toda S, Hassell JA, Hanahan D. Endothelial cell tumors develop in transgenic mice carrying polyoma middle T oncogene. Cell. 1987;51:529–538. doi: 10.1016/0092-8674(87)90122-x. [DOI] [PubMed] [Google Scholar]
- Botas J. Control of morphogenesis and differentiation by HOM/Hoxgenes. Curr Opin Cell Biol. 1993;5:1015–1022. doi: 10.1016/0955-0674(93)90086-6. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Clark RAF, Cheresh DA. Requirement of vascular integrin αvβ3 for angiogenesis. Science (Wash DC) 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Strömblad S, Sanders L, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP, Cheresh DA. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin αvβ3. Cell. 1996;85:683–693. doi: 10.1016/s0092-8674(00)81235-0. [DOI] [PubMed] [Google Scholar]
- Brown WM, Zhou L, Taylor GR. The nucleotide sequence of the murine Hox D3gene reveals extensive identity with the human protein. Biochim Biophys Acta. 1994;1219:219–222. doi: 10.1016/0167-4781(94)90276-3. [DOI] [PubMed] [Google Scholar]
- Cheresh DA. Human endothelial cells synthesize and express an Arg-Gly-Asp-directed adhesion receptor involved in attachment to fibrinogen and von Willebrand factor. Proc Natl Acad Sci USA. 1987;84:6471–6475. doi: 10.1073/pnas.84.18.6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheresh, D.A., and R.P. Mecham. 1994. Preface. In Integrins: Molecular and Biological Responses to the Extracellular Matrix. D.A. Cheresh and R.P. Mecham, editors. Academic Press, San Diego, CA. xi–xiv.
- Cheresh DA, Smith JW, Cooper HM, Quaranta V. A novel vitronectin receptor integrin (αvβx) is responsible for distinct adhesive properties of carcinoma cells. Cell. 1989;57:59–69. doi: 10.1016/0092-8674(89)90172-4. [DOI] [PubMed] [Google Scholar]
- Condie BG, Capecchi MR. Mice with targeted disruptions in the paralogous genes hoxa-3 and hoxd-3reveal synergistic interactions. Nature (Lond) 1994;370:304–307. doi: 10.1038/370304a0. [DOI] [PubMed] [Google Scholar]
- Desplan C, Theis J, O'Farrel PH. The Drosophila developmental gene engrailedencodes a sequence specific DNA binding protein. Nature (Lond) 1985;318:630–635. doi: 10.1038/318630a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detmer K, Lawrence HJ, Largman C. Expression of class I homeobox genes in fetal and adult murine skin. J Investig Dermatol. 1993;101:517–522. doi: 10.1111/1523-1747.ep12365890. [DOI] [PubMed] [Google Scholar]
- Dubois-Stringfellow N, Kolpack-Martindale L, Bautch VL, Azizkhan R G. Mice with hemangiomas induced by transgenic endothelial cells. Am J Pathol. 1994;144:96–806. [PMC free article] [PubMed] [Google Scholar]
- Duprez DM, Kostakopoulou K, Francis-West PH, Tickle C, Brickell PM. Activation of Fgf-4 and Hox Dgene expression by BMP-2 expressing cells in the developing chick limb. Development. 1996;122:1821–1828. doi: 10.1242/dev.122.6.1821. [DOI] [PubMed] [Google Scholar]
- Edelman GM, Jones FS. Outside and downstream of the homeobox. J Biol Chem. 1993;268:20683–20686. [PubMed] [Google Scholar]
- Enenstein J, Waleh NS, Kramer RH. Basic FGF and TGFβ differentially modulate integrin expression of human microvascular endothelial cells. Exp Cell Res. 1992;203:499–503. doi: 10.1016/0014-4827(92)90028-7. [DOI] [PubMed] [Google Scholar]
- Faiella A, Zappavigna V, Mavilio F, Bonicelli E. Inhibition of retinoic acid-inducible activation of 3′ human HOXB genes by antisense oligonucleotides affects sequential activation of genes located upstream in the four HOXclusters. Proc Natl Acad Sci USA. 1994;91:5335–5339. doi: 10.1073/pnas.91.12.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann Y, Daniel CA, Strickland P, Daniel DW. Hox genes in normal and neoplastic mouse mammary gland. Cancer Res. 1994;54:5981–5985. [PubMed] [Google Scholar]
- Gailit J, Welch MP, Clark RAF. TGF-β 1 stimulates expression of keratinocyte integrins during re-epithelialization of cutaneous wounds. J Investig Dermatol. 1994;103:221–227. doi: 10.1111/1523-1747.ep12393176. [DOI] [PubMed] [Google Scholar]
- Goomer RS, Holst BD, Wood IC, Jones FS, Edelman GM. Regulation in vitro of an L-CAM enhancer by homeobox D9 and HNF-1. Proc Natl Acad Sci USA. 1994;91:7985–7989. doi: 10.1073/pnas.91.17.7985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatva E, Bohling T, Jaaskelainen J, Graziella M, Perisco, Haltia M, Alitalo K. Vascular growth factors and receptors in capillary hemangioblastomas and hemangiopericytomas. Am J Pathol. 1996;148:763–775. [PMC free article] [PubMed] [Google Scholar]
- Immergluck K, Lawrence PA, Bienz M. Induction across germ layers in Drosophilamediated by a genetic cascade. Cell. 1990;62:261–268. doi: 10.1016/0092-8674(90)90364-k. [DOI] [PubMed] [Google Scholar]
- Johnson MD, Kim H-RC, Chesler L, Tsao-Wu G, Bouck N, Polverini PJ. Inhibition of angiogenesis by tissue inhibitor of metalloproteinase. J Cell Physiol. 1994;160:94–202. doi: 10.1002/jcp.1041600122. [DOI] [PubMed] [Google Scholar]
- Kraling BM, Razon MJ, Boon LM, Zurakowski D, Seachord C, Darveau RP, Mulliken JB, Corless CL, Bischoff J. E-selectin is present in proliferating endothelial cells in human hemangiomas. Am J Pathol. 1996;148:1181–1191. [PMC free article] [PubMed] [Google Scholar]
- Kubota Y, Kleinman HK, Martin GR, Lawley TJ. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J Cell Biol. 1988;107:1589–1598. doi: 10.1083/jcb.107.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence HJ, Largman C. Homeobox genes in normal hematopoiesis and leukemias. Blood. 1992;80:2445–2453. [PubMed] [Google Scholar]
- Mignatti P, Tsuboi R, Robbins E, Rifkin DB. In vitro angiogenesis on the human amniotic membrane: requirement for bFGF induced proteinases. J Cell Biol. 1989;108:671–682. doi: 10.1083/jcb.108.2.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min HY, Doyle LV, Vitt CR, Zandonella CL, Stratton-Thomas JR, Shumna MA, Rosenberg S. Urokinase receptor antagonists inhibit angiogenesis and primary tumor growth in syngeneic mice. Cancer Res. 1996;56:2428–2433. [PubMed] [Google Scholar]
- Montesano R, Pepper MS, Mohle-Steinlein U, Risau W, Wagner EF, Orci L. Increased proteolytic activity is responsible for the aberrant morphogenetic behavior of endothelial cells expressing the middle T oncogene. Cell. 1990;62:435–445. doi: 10.1016/0092-8674(90)90009-4. [DOI] [PubMed] [Google Scholar]
- Moscatelli D, Presta M, Rifkin DB. Purification of a factor from human placenta that stimulates capillary endothelial cell protease production, DNA synthesis and migration. Proc Natl Acad Sci USA. 1985;83:2091–2095. doi: 10.1073/pnas.83.7.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redline RW, Hudock P, MacFee M, Patterson P. Expression of AbdB-type homeobox genes in human tumors. Lab Invest. 1994;71:663–670. [PubMed] [Google Scholar]
- Risau W. Differentiation of endothelium. FASEB J. 1995;9:26–933. [PubMed] [Google Scholar]
- Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savageau G, Thorsteinsdottir U, Eaves CJ, Lawrence HJ, Largman C, Lansdrop PM, Humphries KR. Overexpression of HOXB4in hematopoietic cells causes the selective expansion of more primitive populations in vitro and in vivo. Genes Dev. 1995;9:1753–1765. doi: 10.1101/gad.9.14.1753. [DOI] [PubMed] [Google Scholar]
- Sepp NT, Lian-Jie L, Kwang HL, Brown EJ, Caughman SW, Lawley T, Swerlick RA. Basic fibroblast growth factor increases expression of the αvβ3 integrin complex on human microvascular endothelial cells. J Investig Dermatol. 1994;103:295–299. doi: 10.1111/1523-1747.ep12394617. [DOI] [PubMed] [Google Scholar]
- Stoker AW, Bissell MJ. Development of avian sarcoma and leukosis virus-based vector packaging cell lines. J Virol. 1988;62:1008–1015. doi: 10.1128/jvi.62.3.1008-1015.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker AW, Hatier C, Bissell MJ. The embryonic environment strongly attenuates v-src oncogenesis in mesenchymal and epithelial tissues but not in endothelia. J Cell Biol. 1990;111:217–228. doi: 10.1083/jcb.111.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strömblad S, Becker JC, Yebra M, Brooks PC, Cheresh DA. Suppression of p53 activity and p21WAF/CIP1expression by vascular cell integrin αvβ3 during angiogenesis. J Clin Invest. 1996;98:426–433. doi: 10.1172/JCI118808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan LM, Quigley JP. An anticatalytic monoclonal antibody to avian plasminogen activator: its effect on behavior of RSV-transformed chick fibroblasts. Cell. 1986;45:905–915. doi: 10.1016/0092-8674(86)90565-9. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Mulliken JB, Kozakewich HP, Rogers RA, Folkman J, Ezekowitz RA. Cellular markers that distinguish the phases of hemangioma during infancy and childhood. J Clin Invest. 1994;93:2357–2364. doi: 10.1172/JCI117241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita K, Bollekens JA, Hijiya N, Ratajczak M, Ruddle FH, Gerwitz A. A homeobox gene of the Antennapedia class is required for human adult erythropoiesis. Proc Natl Acad Sci USA. 1993;90:3535–3538. doi: 10.1073/pnas.90.8.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi Y, Komatsu N, Moriuchi T. Overexpression of the HOX4A(HOXD3) homeobox gene in human erythroleukemia HEL cells results in altered adhesive properties. Blood. 1995;85:2786–2794. [PubMed] [Google Scholar]
- Wang W, Wei W, Desai T, Ward DC, Kaufmann SJ. Localization of the α7 integrin gene on human chromosome 12q13: clustering of integrin and Hox genes implies parallel evolution of these gene families. Genomics. 1995;26:563–570. doi: 10.1016/0888-7543(95)80176-m. [DOI] [PubMed] [Google Scholar]