Abstract
In previous studies we have shown that, after stimulation by a receptor ligand such as thrombin, tissue-type plasminogen activator (tPA) and von Willebrand factor (vWf) will be acutely released from human umbilical vein endothelial cells (HUVEC). However, the mechanisms involved in the secretion of these two proteins differ in some respects, suggesting that the two proteins may be stored in different secretory granules.
By density gradient centrifugation of rat lung homogenates, a particle was identified that contained nearly all tPA activity and antigen. This particle had an average density of 1.11–1.12 g/ml, both in Nycodenz density gradients and in sucrose density gradients. A similar density distribution of tPA was found for a rat endothelial cell line and for HUVEC. After thrombin stimulation of HUVEC to induce tPA secretion, the amount of tPA present in high-density fractions decreased, concomitant with the release of tPA into the culture medium and a shift in the density distribution of P-selectin.
vWf, known to be stored in Weibel-Palade bodies, showed an identical distribution to tPA in Nycodenz gradients. In contrast, the distribution in sucrose gradients of vWf from both rat and human lung was very different from that of tPA, suggesting that tPA and vWf were not present in the same particle.
Using double-immunofluorescence staining of HUVEC, tPA- and vWf-containing particles showed a different distribution by confocal microscopy. The distribution of tPA also differed from the distribution of tissue factor pathway inhibitor, endothelin-1, and caveolin. By immunoelectronmicroscopy, immunoreactive tPA could be demonstrated in small vesicles morphologically different from the larger Weibel-Palade bodies. It is concluded that tPA in endothelial cells is stored in a not-previously-described, small and dense (d = 1.11– 1.12 g/ml) vesicle, which is different from a Weibel-Palade body.
Of the physiologically occurring plasminogen activators, tissue-type plasminogen activator (tPA)1 is the most important one in triggering physiological fibrinolysis and thrombolysis. Transgenic mice in which the tPA gene has been functionally disrupted (Carmeliet and Collen, 1996a ,b) and that consequently do not have tPA circulating in their blood, show a reduced thrombolytic potential and an increased thrombogenic tendency. Also, clots from which tPA has been removed (functionally by inactivation, or immunologically by immunoabsorbtion) will not lyse (Wun and Capuano, 1985, 1987). Circulating tPA is derived from vascular endothelial cells, which have been shown to synthesize tPA both in vivo (Levin and del Zoppo, 1994; Lupu et al., 1995b ; Padró et al., 1995; Schneiderman et al., 1995) and in vitro (for review see van Hinsbergh et al., 1991; Emeis et al., 1996; Kooistra and Emeis, 1997). In vivo, tPA can also be demonstrated in vascular endothelial cells by functional and immunocytochemical techniques, although the precise localization of tPA in endothelial cells is still not settled (for review see Emeis et al., 1996). In culture, endothelial cells constitutively secrete tPA into the medium. Regulated secretion from endothelial cells has been demonstrated as well, both in vivo (for review see Emeis, 1988, 1996; Emeis et al., 1996) and recently in vitro (van den Eijnden-Schrauwen et al., 1995, 1997).
A similar situation pertains to von Willebrand factor (vWf), another endothelial cell protein that is synthesized by, and stored in, the endothelium and is secreted from endothelial cells by both constitutive and regulated secretion (for review see Reinders et al., 1988; Wagner, 1990, 1993; Mayadas and Wagner, 1991; Hop and Pannekoek, 1996). In most experimental and clinical situations, tPA and vWf are secreted simultaneously upon stimulation (Tranquille and Emeis, 1990; van den Eijnden-Schrauwen et al., 1995; for review see Emeis, 1996). Recent data, however, suggest that the regulated secretion of tPA and vWf are differently regulated, so that the release of the two proteins can be induced separately (van den Eijnden-Schrauwen et al., 1997). For this to be possible, tPA should be stored in a secretory granule different from the Weibel-Palade body in which vWf is stored in endothelial cells (for review see Reinders et al., 1988; Wagner, 1990, 1993). No storage granule for tPA has yet been described, and the possibility is still open that tPA is also stored in Weibel-Palade bodies. The present study was therefore designed to isolate and describe the endothelial storage particle for tPA and to investigate whether this particle was identical to the Weibel-Palade body, the storage granule for vWf.
MATERIALS AND METHODS
Materials
Nycodenz was obtained from Nycomed AS (Oslo, Norway); sucrose for density gradients from BDH (Poole, UK). Human α-thrombin, BSA, cycloheximide, N-[3-(2-furyl)acryloyl]-l-phenylalanylglycylglycine and kits for the determination of lactate dehydrogenase (LDH: No. LD-L 10) and 5′-nucleotidase (No. 253-3) were purchased from Sigma Chemical Co. (St. Louis, MO). Recombinant human tPA (Activase) was from Genentech (So. San Francisco, CA), 4-chloro-1-naphtol from Merck (Darmstadt, Germany), and sodiumpentobarbital (Nembutal) from Sanofi (Paris, France).
Murine monoclonal antibodies against human tPA (clones ESP-4, ESP-5, ESP-6, PAM-3) and rabbit polyclonal anti-recombinant human tPA IgG (ADI385R) were purchased from American Diagnostica (Greenwich, CT). The murine monoclonal anti-tPA antibodies 8C11 and 2B5 were from Celsus Laboratories (Cincinnati, OH). Rabbit polyclonal anti– mouse tPA was a gift from Dr P. Carmeliet (Leuven, Belgium). Rabbit anti-vWf, murine monoclonal anti-vWf IgG, peroxidase-conjugated swine anti–rabbit Ig, and antibody diluent were from DAKO (Glostrup, Denmark); rabbit anti-caveolin was from Transduction Laboratories (Mamhead Castle, UK). Rabbit anti–human P-selectin (Coughlan et al., 1994) was kindly donated by Dr M.C. Berndt (Prahran, Australia); rabbit anti– human TFPI was a gift from Dr C. Lupu (Thrombosis Research Institute, London, UK); and rabbit anti–human endothelin-1 was a gift from Dr J. Morton (Royal Postgraduate Medical School, London, UK). Texas red- and FITC-conjugated secondary antibodies, as well as Vectashield fluorescence mounting medium were from Vector Laboratories (Peterborough, UK). Goat anti–mouse and goat anti–rabbit IgG labeled with 5- or 10-nm colloidal gold particles were purchased from Nanoprobes, Inc. (Stony Brook, NY). Lowicryl K4M embedding medium was from Polysciences (Eppelheim, Germany). All other materials used were of analytical grade.
Cells and Tissues
Human umbilical vein endothelial cells (HUVEC) were isolated by collagenase digestion (Jaffe et al., 1973) and cultured in Medium 199 supplemented with 10% (vol/vol) heat-inactivated newborn calf serum, 10% (vol/vol) pooled human serum, 100 μg/ml endothelial cell growth factor, 2.5 U/ml heparin, 100 IU/ml penicillin, 100 μg/ml streptomycin and 2 mM l-glutamine, as described (van Hinsbergh et al., 1985). Confluent first passage cells were used throughout. Regulated secretion (acute release) of tPA and vWf from HUVEC was induced in cells that had been preincubated for 30 min in M199 containing 0.3 mg/ml human serum albumin, l-glutamine, and antibiotics, but no serum. To induce regulated secretion, 15 μl human α-thrombin (final concentration 1 NIH U/ml) was added, and the medium was collected after 3 min, as described (van den Eijnden-Schrauwen et al., 1995).
The rat endothelial cell line RHE, kindly provided by Dr C.A. Diglio (Wayne State University School of Medicine, Detroit, MI; Diglio et al., 1988) was cultured in DME containing 10% (vol/vol) fetal calf serum, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine. For experiments, cells were cultured in 6-well plates and were given fresh medium 24 h before an experiment.
Rat lung, mouse lung, and mouse diaphragm were obtained from Nembutal-anaesthetized (60 mg/kg i.p.) animals. Human lung was obtained as anonymous and nontraceable material from lung cancer surgery. Lung specimens for fractionation were washed free of blood and stored in cold saline.
Cell and Tissue Homogenization
Lung tissue was finely divided by a McIlwain tissue chopper and suspended in homogenization buffer (5 mM Tris-HCl, 220 mM sucrose, pH, 7.4; 10 ml buffer/gram of tissue). Confluent HUVEC (60 cm2, ∼6 × 106 cells) were washed once and scraped into 2 ml homogenization buffer. For HUVEC homogenates, which contain only low amounts of protein, this buffer also contained 0.001% Tween-80 to improve tPA recovery (at this concentration, Tween-80 did not lyse organelles). For the experiments on thrombin-induced secretion, 300 cm2 HUVEC cultures were used and scraped into 5 ml buffer. Cells or tissue were homogenized with ten strokes (1500 rpm) in a glass-teflon Potter-Elvehjem tissue homogenizer, and the homogenates were then centrifuged at 800 g for 5 min at 4°C. An aliquot of the supernatant (“total homogenate”) was retained to calculate recoveries, and the remainder was fractionated.
Nycodenz Density Gradient Centrifugation
A Nycodenz solution containing 35% (wt/vol) of Nycodenz was prepared in 5 mM Tris-HCl buffer (pH 7.4). A Nycodenz stock solution of 30% (wt/ vol) was prepared in homogenization buffer. From this stock solution, solutions of Nycodenz of 27.5 to 5% (in increments of 2.5%) were prepared in homogenization buffer. A density gradient was made in 14 ml ultracentrifuge tubes, starting with one ml of 35% Nycodenz, and then in eleven steps of one ml from 30% to 5% Nycodenz, and left to equilibrate overnight at 4°C. Two ml of cell or tissue homogenate was layered on top of the gradient, and the gradient was centrifuged for 150 min at 40,000 rpm (202,000 gav) in a Beckman L7-55 ultracentrifuge equipped with a SW-40 rotor, at 4°C. Subsequently, one ml fractions were collected, starting at the bottom, and stored frozen. The density of the fractions was determined in a Mettler/Paar DMA 602M density measuring cell, and ranged from 1.03-1.17 g/ml (see Results section). For additional protocol see Wanders et al. (1987).
Sucrose Density Gradient Centrifugation
A 46% (wt/vol) stock solution of sucrose (1.375 M) in 5 mM Tris-HCl, pH 7.4, was prepared (for HUVEC, 0.001% Tween 80 was added). From this stock, sucrose solutions of 43 to 10% (in increments of 3%) were prepared in buffer. A sucrose density gradient (46–10% sucrose; 12 steps of 1 ml) was made in 14-ml tubes and left to equilibrate overnight at 4°C. 2 ml of cell or tissue homogenate was layered on top of the gradient, and the gradient was centrifuged exactly as described above for the Nycodenz gradient. 1-ml fractions were collected, and their densities were measured, as described above. The density of the sucrose gradient fractions ranged from 1.03 to 1.16 g/ml.
Assay of Fractions
In the fractions the following parameters were determined, using miniaturized assays in microtiter plates. Where necessary, data were corrected for interference by the Nycodenz or sucrose content of the fractions. Protein concentration was measured spectrophotometrically by the BCA protein assay procedure as prescribed by the manufacturer (Pierce, Rockford, IL). tPA activity (Verheijen et al., 1982) and plasminogen activators inhibitor type-1 (PAI-1) activity (Verheijen et al., 1984) were determined by spectrophotometric assay. Human tPA antigen (Schrauwen et al., 1994), rat tPA antigen (Emeis et al., 1995), PAI-1 antigen (Nieuwenhuizen et al., 1995), vWf antigen (Tranquille and Emeis, 1990), and cellular fibronectin antigen (Friedman et al., 1995) were measured by ELISA.
Acid phosphatase (substrate: p-nitrophenylphosphate at pH 5.0), alkaline phosphatase (substrate: p-nitrophenylphosphate at pH 10.5), neutral esterase (substrate: p-nitrophenylacetate at pH 6.6), 5′-nucleotidase (kit 265-3; Sigma Chemical Co.), glutamate dehydrogenase (using α-ketoglutaric acid and NADH at pH 8.0), LDH (kit LD-L 10; Sigma Chemical Co.), and angiotensin-converting enzyme (ACE; substrate N-[3-(2-furyl) acryloyl]-l-phenylalanylglycylglycine by the method of Buttery and Stuart [1993]) were determined spectrophotometrically, using miniaturized procedures adapted to a microtiterplate reader (Titertek Multiscan MCC/360; Flow Laboratories, Irvine, Scotland).
Western Blotting of P-Selectin
The presence of P-selectin in Nycodenz fractions of HUVEC was determined semi-quantitatively by slot blotting, using a polyclonal rabbit anti– human P-selectin (Coughlan et al., 1994). After blotting 25 μl of the fractions on nitrocellulose paper, the paper was blocked with 10 mM Tris-HCl, pH 7.4, containing 150 mM NaCl, 0.05% Tween 80, and 3% BSA. Subsequently, the paper was incubated with polyclonal rabbit anti–P-selectin (2.3 μg/ml), followed by incubation with peroxidase-conjugated swine anti–rabbit Ig, washing, and staining with 2.8 mM 4-chloro-1-naphtol plus 0.01% H2O2 in 20% methanol/80% 200 mM NaCl plus 50 mM Tris-HCl, pH 7.4. Blots were densitometrically scanned, and the background subtracted. Results will be given as relative OD units per fraction.
Immunocytochemistry
First passage HUVEC were cultured to confluency as described above on glass coverslips coated with glutaraldehyde-crosslinked gelatin. Once confluent, cells were fixed with 2% freshly depolymerized p-formaldehyde in PBS containing 5% sucrose (PBS/sucrose) for 1 h at 20°C. Cells were then washed extensively in PBS/sucrose and briefly permeabilized by treatment with a solution of 0.02% saponin in PBS for 10 min at 18°C. Cells were then washed in PBS, quenched with 100 mM glycine in PBS, blocked by treatment with DAKO antibody diluent supplemented with 1% (vol/ vol) normal goat or horse serum for 30 min, and incubated for 1 h at 18°C with primary antibody diluted in the same blocking solution. As primary antibodies were used, either a cocktail of monoclonal anti-tPA antibodies (IgG 10 μg/ml) for single labeling or the mixture of these anti-tPA monoclonals with polyclonal rabbit anti-vWf IgG (diluted 1:200) for the double immunostaining approach. To confirm the labeling pattern, in double labeling experiments a rabbit polyclonal anti-tPA IgG in combination with monoclonal anti-vWf antibody was used. All specimens were extensively washed with PBS and incubated for 1 h at 18°C with the secondary antibodies. For single staining we used horse anti–mouse IgG-FITC (diluted 1:100 in PBS) and for double staining a mixture of horse anti–mouse IgG-Texas red and goat anti–rabbit IgG-FITC (both diluted 1:100 in PBS). Cells were again extensively washed in PBS, briefly rinsed in distilled water, and mounted in Vectashield. The fluorescent specimens were examined with a confocal laser scanning unit (MRC600; Bio Rad, Hemel Hampstead, UK) attached to a Nikon Diaphot inverted microscope. The light source was a krypton/argon laser with main lines at 488, 568, and 674 nm. For the visualization of FITC and Texas red staining, K1 and K2 filter blocks were used. The recorded images were merged to study colocalization. Some samples were analyzed by serial optical sectioning in the Z-axis of the cells, followed by computer-assisted reconstruction of the images.
Controls to ascertain for the specificity of the binding comprised either replacement of the first antibodies with normal IgG from the same species or incubation of the cells with the conjugates only. Staining for double labeling of tPA and TFPI, endothelin-1, or caveolin, respectively, was performed identically as described for tPA/vWf double labeling, using as antibodies rabbit anti-TFPI, rabbit anti-endothelin-1, and rabbit anti-caveolin, respectively.
Immunogold Electron Microscopy
For immunogold labeling, HUVEC were grown on Thermanox coverslips, washed with serum-free medium, and fixed with 3% (wt/vol) p-formaldehyde (freshly prepared) in PBS for 90 min at room temperature. Mouse lung and diaphragm were fixed by perfusion with 3% p-formaldehyde in PBS for 10 min, followed by immersion fixation at room temperature for a further 2 h. Both the cell monolayers and the mouse tissues were dehydrated in an ascending series of ethanol while the temperature is progressively lowered, following the progressive lowering of temperature protocol (for details see Roth, 1989). Finally, the samples were embedded overnight in 100% Lowicryl K4M at −35°C. Polymerization was carried out by irradiating tissue in gelatin capsules with UV light in a low temperature embedding workstation (MS 5000; Microfield Scientific Ltd., Kingston Bagpuize, UK) for 24 h at −35°C, followed by hardening under UV light at room temperature for 1 to 2 d. This sectioning was carried out on a microtome (Ultracut; Reichert-Jung Optische Werke, Wien, Austria), and thin sections were placed on Formvar-coated 200 mesh nickel grids. For immunogold labeling we used the postembedding staining protocol previously described (Lupu et al., 1993). In brief, grids were floated on droplets of PBS containing 20 mM glycine for 5 min to neutralize any free aldehyde groups, transferred to PBS containing 1% (wt/vol) gelatine for 10 min to neutralize “sticky” sites, and washed for 30 min in PBS containing 5% (vol/vol) nonimmune goat serum (PBS-NGS) to quench nonspecific binding. Subsequently, the sections were incubated for 1 h at room temperature with primary antibodies. For HUVEC, we used a cocktail of murine anti–human tPA monoclonals or a polyclonal rabbit anti–human recombinant tPA IgG, both diluted 1:50 in PBS-NGS, and monoclonal mouse anti–human vWf IgG (1:100). For the mouse tissues we have used a polyclonal rabbit anti–mouse tPA diluted 1:50 in PBS containing 1% NGS. After incubation the grids were washed three times in PBS containing 1% BSA and then incubated for 1 h at room temperature in the same dilution buffer containing the appropriate gold-labeled secondary antibody. Thereafter, grids were washed with PBS and distilled water, stained with uranyl actate and lead citrate, and observed in a transmission electron microscope (EM201; Philips, Eindhoven, The Netherlands).
RESULTS
Density Gradient Centrifugation of Rat Lung on a Nycodenz Gradient
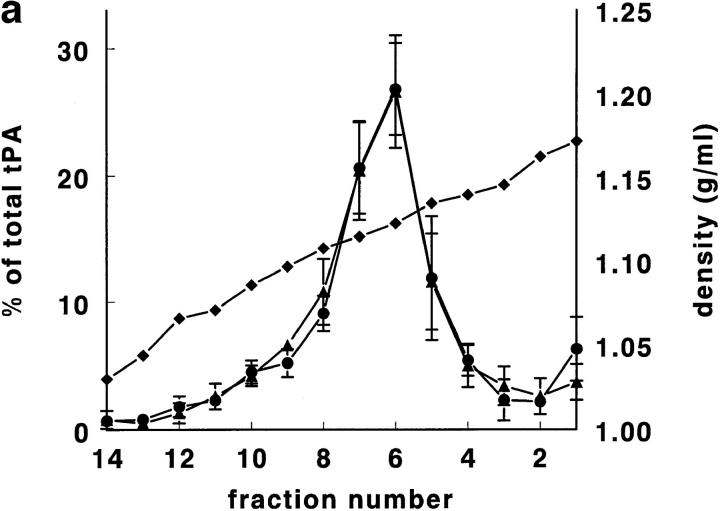
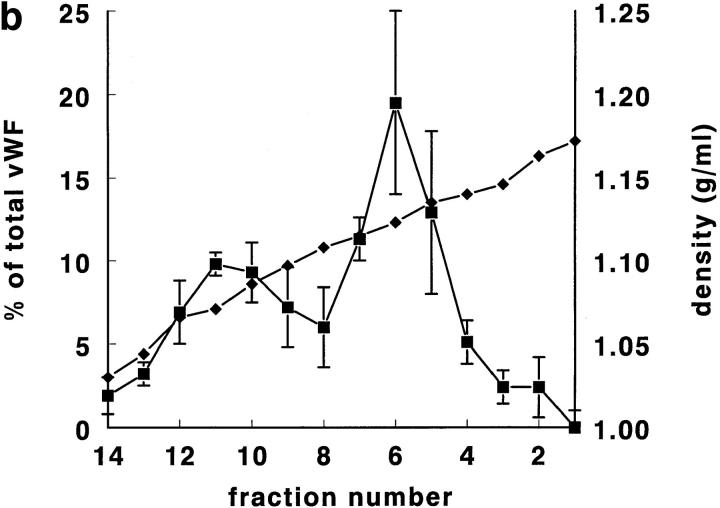
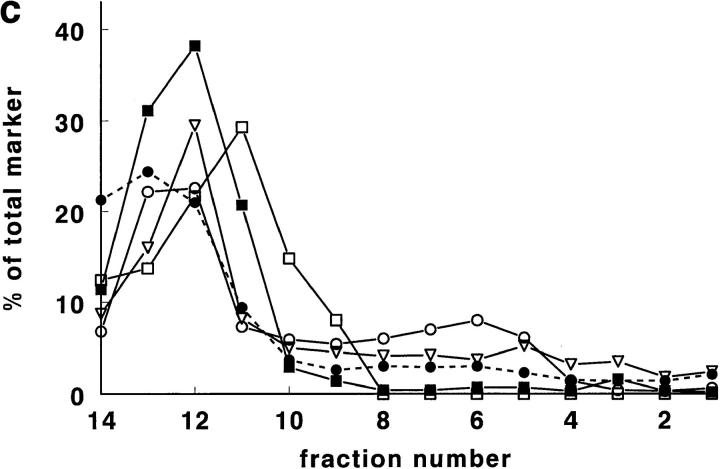
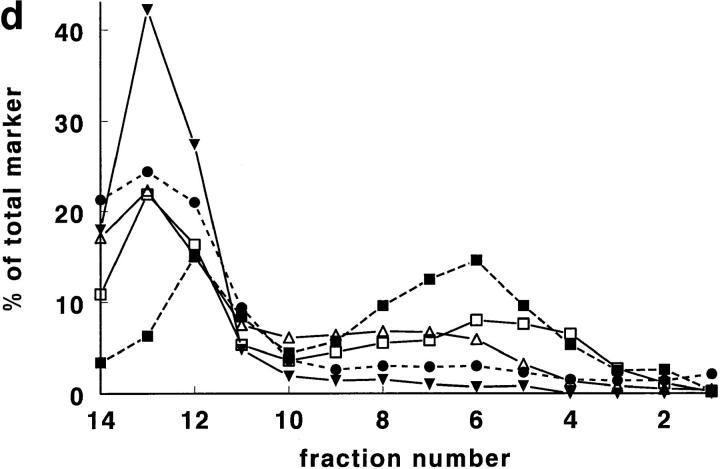
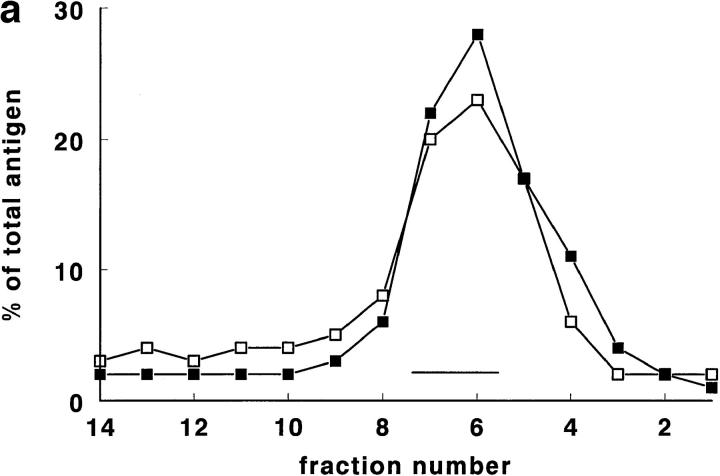
Rat lung was chosen for the first experiments, since it is, of all rat tissues, richest in tPA (Padró et al., 1990). In pilot experiments, using a Nydodenz gradient with density range 1.05–1.27 g/ml, all tPA and vWf was recovered at densities <1.18 g/ml. In subsequent experiments, therefore, a more shallow gradient (density range 1.03 to 1.17 g/ ml) was employed. In such a gradient (Fig. 1, a–d), tPA was found as a single symmetrical peak, with a maximum at density 1.11–1.12 g/ml. Both tPA activity and tPA antigen were found at the same position in the gradient (Fig. 1 a). The correlation between tPA antigen and activity in the 14 fractions was in all four experiments >0.97. In those two fractions that contained maximal amounts of tPA, ∼50% of tPA (Fig. 1 a) and 6–7% of protein (Fig. 1, c and d) was recovered. vWf antigen also peaked at density 1.11–1.12 g/ml, but in addition showed a second, smaller peak at density 1.06–1.07 g/ml (Fig. 1 b). Tissue protein, and most of the marker enzymes measured, showed peak values in the fractions with densities of 1.03 to 1.08 g/ml (Fig. 1, c and d). Typically, >70% of the protein was recovered in the these fractions. Maximal alkaline phosphatase and ACE activity (used as plasma membrane marker enzymes) were found around d = 1.05–1.08 g/ml; acid phosphatase (a marker for lysosomes), neutral esterase, and 5′-nucleotidase (markers for microsomes) around d = 1.05–1.07 g/ml, and LDH (a marker for the cytosol) ∼d = 1.03 g/ml. Only glutamate dehydrogenase activity, a marker enzyme for mitochondria, was recovered at d = 1.11–1.12 g/ml, the density of maximal tPA and vWf concentrations. Cellular fibronectin, a marker of connective tissue contamination, was found at density 1.06 g/ml.
Figure 1.
Gradient centrifugation of rat lung homogenate on a Nycodenz density gradient. In this and in all subsequent figures, percentages refer to percentage per fraction of total activity (or antigen) recovered in the 14 fractions of a gradient. The numbers 1–14 of the fractions are displayed on the X axis. (a) The percentage per fraction of tPA antigen (•) and the percentage per fraction of tPA activity (▴) are shown as mean ± SD (n = 4). The mean density of the fractions is also indicated (♦). (b) The percentage per fraction of vWf antigen (▪) is shown as mean ± SD (n = 4). The mean density of the fractions is given as (♦). (c) The mean (n = 4) percentage per fraction of cell protein (•; dashed line), alkaline phosphatase (○), angiotensin-converting enzyme (□), 5′-nucleotidase (▿), and cellular fibronectin (▪). (d) The mean (n = 4) percentage per fraction of cell protein (•; dashed line), LDH (▾), acid phosphatase (▵), neutral esterase (□), and glutamate dehydrogenase (▪; dashed line).
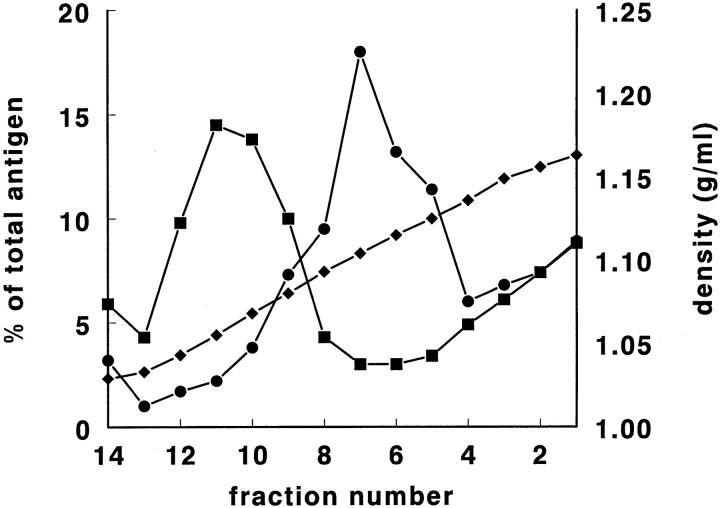
Recentrifugation of the two-peak fractions (d = 1.11– 1.12 g/ml, diluted 1:1 with homogenization buffer to reduce density) on an identical Nycodenz gradient resulted in recovery of tPA and vWf at the density of the original fractions (Fig. 2 a). To decide whether the distribution of tPA in the gradient might have been due to binding of particle-free tPA to a particle of d = 1.11–1.12 g/ml, recombinant human tPA (Activase; final concentration 50 ng/ml) was added to a rat lung homogenate before gradient centrifugation. Subsequently, we separately measured the exogenously added human tPA antigen and the endogenous rat tPA antigen by species-specific tPA ELISA assays. Rat tPA was recovered as a single peak at its normal density, while the human tPA was found distributed diffusely through the gradient (Fig. 2 b). Upon ultracentrifugation of a lung homogenate in homogenization buffer without Nycodenz (d = 1.03 g/ml), >90% of tPA and vWf was recovered at the bottom of the tube. After ultracentrifugation in homogenization buffer without Nycodenz but now containing 1% Triton X-100 to lyse organelles, tPA and vWf were diffusely distributed through the gradient (not shown). From these data it was concluded that almost all tPA was present in rat lung homogenates in sedimentable particles of d = 1.11–1.12 g/ml.
Figure 2.
(a) A rat lung homogenate was centrifuged on a Nycodenz density gradient, and fractions 6 and 7, containing the highest concentrations of tPA and vWf, were recentrifuged after dilution on an identical Nycodenz gradient. Shown are the percentage per fraction of tPA antigen (▪) and vWf antigen (□) in the second density gradient. The fraction numbers 1–14 are shown on the X axis. The position of the original fractions 6 and 7 of the first gradient is indicated by the line. (b) Human recombinant tPA was added to a rat lung homogenate to a final concentration of 50 ng/ml, followed by centrifugation on a Nycodenz density gradient. Shown on the Y axis are the percentage per fraction of endogenous rat tPA antigen (•) and of exogenously added human tPA antigen (▾). The fraction numbers 1–14 are given on the X axis. Rat and human tPA antigen concentrations were determined by species-specific ELISA assays.
To see whether any of the tPA or vWf could be ascribed to tPA or vWf in a pathway of ongoing protein synthesis, rats were pretreated with cycloheximide (2 mg/kg in vivo) at 3 h before being killed. This procedure inhibits rat protein synthesis fully but does not significantly influence the size of the storage pool in rat lung (Tranquille and Emeis, 1989). Cycloheximide treatment did not significantly influence the distribution of tPA or vWf from rat lung homogenate. Also, the amount of vWf present around d = 1.06 g/ml, which might have been located in the protein synthesis pathway, was unchanged (data not shown). As discussed above, no tPA peak is present at this density.
Fractionation of a cell homogenate from a rat heart endothelial cell line (Diglio et al., 1988) on a Nycodenz gradient showed that the bulk of tPA (antigen and activity) was found distributed similarly as in rat lung, though at a slightly lower density of 1.09–1.10 g/ml (not shown). This cell line does not synthetize vWf.
Density Gradient Centrifugation of Rat Lung on a Sucrose Gradient
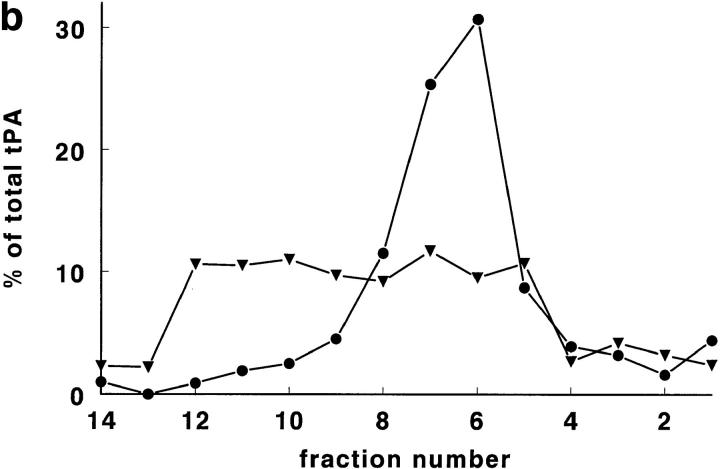
Using a sucrose gradient of d = 1.03–1.16 g/ml, tPA (both antigen and activity) was found at the same density (1.11– 1.12 g/ml) as in Nycodenz gradients (Fig. 3). vWf, however, showed a different distribution than in Nycodenz gradients, as no vWf peak was present in fractions 6 and 7 of sucrose density gradients. This resulted (Fig. 3) in a clear separation of tPA (peak in fractions 6 and 7) and vWf (peak in fractions 10 and 11). Marker enzymes for subcellular fractions, including the mitochondrial marker glutamate dehydrogenase, showed, in sucrose gradients, a similar distribution as in Nycodenz gradients (not shown).
Figure 3.
A rat lung homogenate was centrifuged on a sucrose density gradient. On the Y axis, the percentage per fraction of tPA antigen (•) and of vWf antigen (▪) are shown. Note that the distribution of tPA antigen is practically identical to the distribution on a Nycodenz density gradient (Figs. 1 a and 2). The distribution of vWf on the sucrose gradient is, however, very different, due to an extensive loss of vWf from the density range 1.10–1.12 g/ml (compare Figs. 1 b and 2). The density of the fractions from the sucrose gradient is indicated as well (♦) and is very similar to the density profile of Nycodenz gradients.
Density Gradient Centrifugation of Human Lung
Homogenates of human lung were fractionated on a Nycodenz density gradient and on a sucrose density gradient. The distribution of tPA (antigen and activity) and vWf was more complex in human lung, and less tPA and vWf was present in fractions 7 and 8 of the gradients, compared to rat lung. In sucrose gradients, vWf had practically disappeared from fraction 7 and 8. Of the vWf recovered, 14% ± 3 was found in fractions 7 and 8 in Nycodenz gradients, but only 2% ± 2 in sucrose gradients (mean ± SD, n = 4; P < 0.01). No such difference between Nycodenz and sucrose gradients was found for the tPA content of fractions 7 and 8 (19% ± 4 in Nycodenz gradients versus 16% ± 4 in sucrose gradients, n = 4, P = 0.33).
Density Gradient Centrifugation of HUVEC
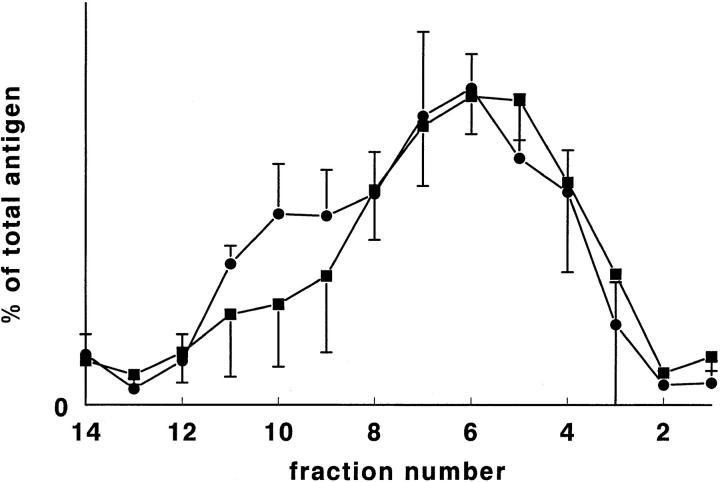
First passage HUVEC (60 cm2/experiment) were fractionated on Nycodenz gradients. In Fig. 4, the averaged distribution of tPA antigen and of vWf is shown for five such fractionations. Slight differences in density distribution, especially for tPA, were found between cultures, but the overall distribution pattern (Fig. 4) resembled that found in rat lung. For tPA, the major peak was found at d = 1.105 g/ml (fraction 6), with a (variably pronounced) shoulder at d = 1.13 g/ml (fraction 4). A second, smaller peak was seen at d = 1.06 g/ml (fraction 10). vWf had a single major peak (d = 1.105–1.115; fractions 5 and 6), and, in three out of five cultures, a small shoulder at d = 1.06 g/ml (fraction 11). No sucrose density gradient centrifugation was performed on HUVEC.
Figure 4.
Percentage per fraction of tPA antigen (•) and of vWf (▪) in Nycodenz gradients after centrifugation of homogenate prepared from 60 cm2 HUVEC cells. Shown are mean ± SD of five homogenates from separate cell cultures.
Thrombin Stimulation of tPA Secretion
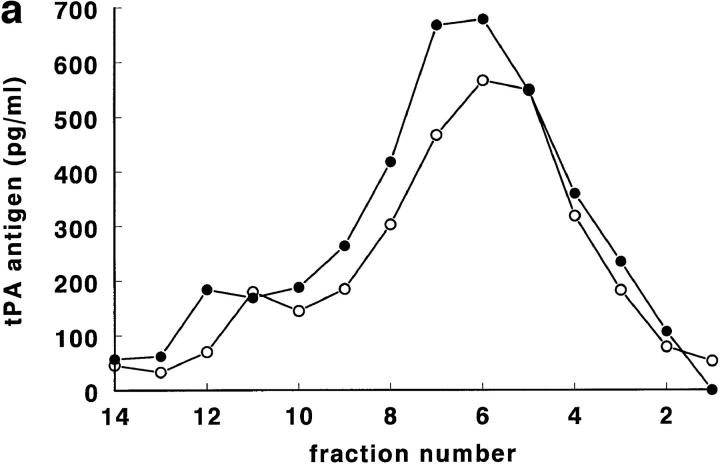
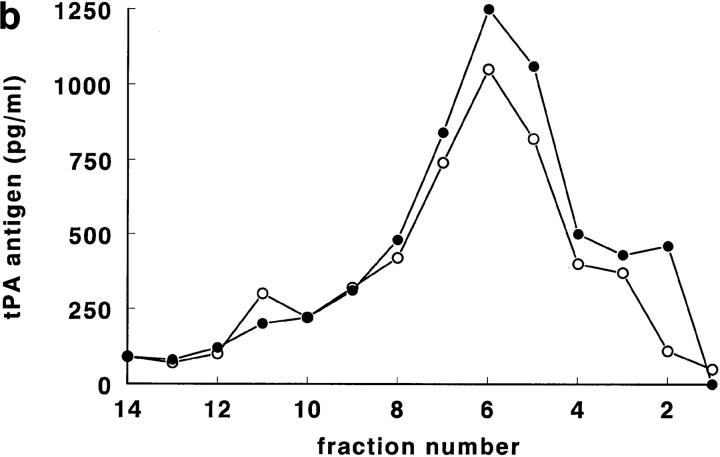
For experiments involving thrombin stimulation of regulated secretion (acute release) of tPA, 300-cm2 HUVEC cultures were used that had been preincubated for 24 h with 1 mM sodiumbutyrate to increase tPA synthesis and storage (Kooistra et al., 1987). HUVEC were stimulated with 1 NIH U/ml of human α-thrombin for 3 min (van den Eijnden-Schrauwen et al., 1995), scraped into cold buffer, and homogenized. Control cultures, not treated with thrombin, were run in parallel. After thrombin, a loss of tPA antigen from the high density range of the gradient (fractions 5–8) was observed, compared to the parallel control cultures. Two typical cultures (out of five) are shown in Fig. 5, a and b. In the experiment shown in Fig. 5 a, tPA disappeared mainly from density range 1.11–1.12 g/ml (fractions 6–8), while in the experiment shown in Fig. 5 b, tPA was reduced in fractions 4–6 (density 1.14 g/ml). The differences between cultures possibly reflect greater experimental variation in cultured HUVEC than in experiments involving rat lungs (note the small SDs in Fig. 1, a and b).
Figure 5.
Distribution of tPA antigen (pg/ml) from a control HUVEC culture (•) and from a culture treated with 1 NIH U/ml of human α-thrombin for 3 min (○). Data from two separate experiments are shown (a and b). HUVEC homogenate was prepared for each condition from a 300-cm2 HUVEC culture (first passage), pretreated for 24 h with 1 mM sodiumbutyrate (see Materials and Methods). Both control and thrombin-treated cells had been obtained from the same primary HUVEC culture. On the X axis the fraction numbers are indicated. The difference in density between corresponding fractions from the thrombin-treated and control Nycodenz gradient fractionations was always <0.004 g/ml. Note that in Fig. 6 a, tPA is lost mainly from fractions 6–10, while in Fig. 6 b, tPA is lost from fractions 4–8. See text for details.
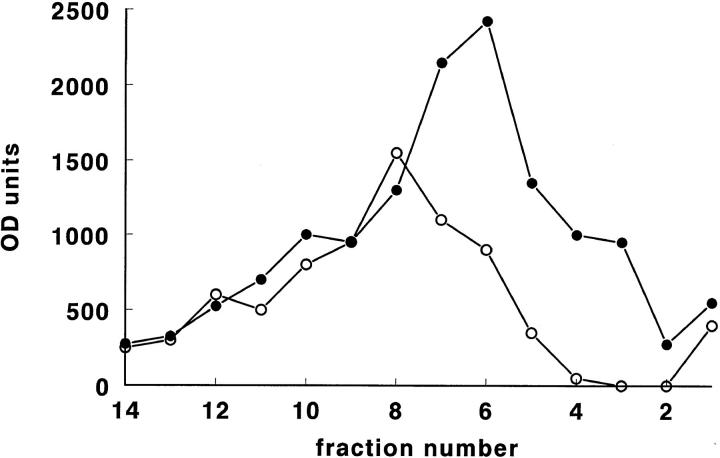
Although the differences in tPA concentration in the fractions induced by thrombin were relatively small, immunocytochemistry of tPA antigen in these cells showed that, after 3 min of thrombin stimulation, the granular tPA staining had disappeared from most cells (Fig. 6), while in other cells a weak granular staining persisted. This loss of staining is in agreement with biochemical data, which showed that, after 1 NIH U/ml thrombin stimulation, no further acute release of tPA could be obtained by ionomycin (van den Eijnden-Schrauwen et al., 1995). By Western blotting, P-selectin was detected (using the 300-cm2 HUVEC cultures) in the density range 1.10–1.15. After thrombin stimulation, the distribution of P-selectin shifted (one to two fractions) to higher densities (Fig. 7) and became more intense.
Figure 6.
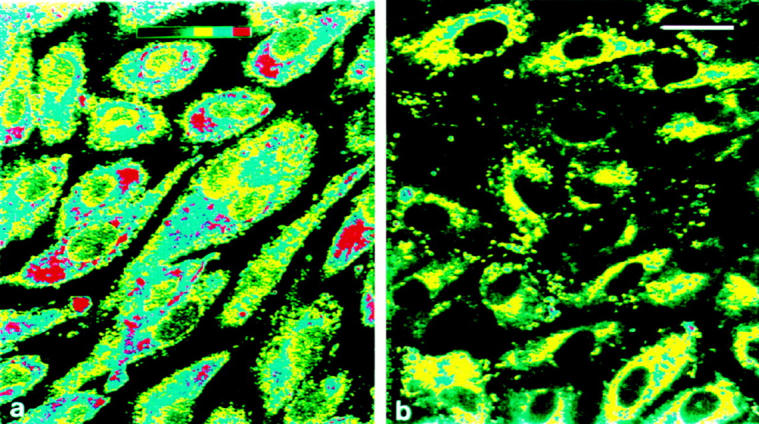
Immunocytochemical staining for tPA in first passage control HUVEC (left) and in HUVEC after induction of regulated secretion by 1 NIH U/ml of human α-thrombin for 3 min (right). Cells were fixed and stained as described in Materials and Methods. The figures show a semiquantitative measurement of fluorescence intensity, using pseudocolor banding. Note that after thrombin treatment, some cells have lost all granular tPA staining, while the other cells show a reduced staining intensity. Bar, 25 μm.
Figure 7.
Distribution of P-selectin–reactive material, as determined by Western blotting and densitometric scanning, in Nycodenz gradient centrifugation fractions of homogenates from control (○) and thrombin-treated (•) HUVEC cultures. Cell culture and preparatory conditions were as in Fig. 5. Note the increase in P-selectin immunoreactive material in fractions 2–8 after thrombin treatment.
Immunohistochemical Localization of tPA and vWf in HUVEC
The cellular localization of tPA in HUVEC was visualized by indirect immunofluorescence labeling and confocal microscopy, as well as at the ultrastructural level by postembedding immunogold labeling and electron microscopy. In formaldehyde-fixed, saponin-permeabilized HUVEC, tPA showed, by immunofluorescence, well defined granular staining in the cytoplasm of the cells (Fig. 8 f; compare with Fig. 6). To enable precise localization of the fluorescence, some specimens were analyzed by serial optical sectioning followed by computer-assisted reconstruction of the image. Serial optical sectioning along the Z-axis revealed that granular tPA staining was localized in the perinuclear, organelle-rich area as well as in the attenuated areas at the cell edges (Fig. 9).
Figure 8.
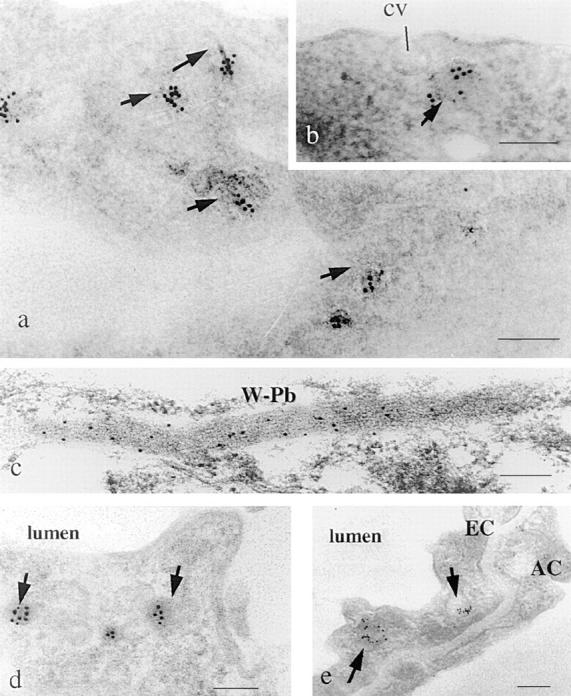
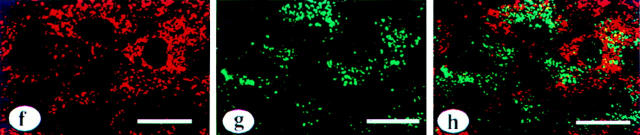
Localization of tPA and vWf in endothelial cells: immunogold labeling in HUVEC of tPA (a and b) and of vWf (c), and immunogold labeling of tPA in murine capillary endothelial cells in lung (d) and diaphragm (e). HUVEC were fixed in 3% p-formaldehyde in PBS, and mouse endothelial cells were perfusion fixed with the same fixative. The samples were dehydrated at progressively lower temperature, embedded in Lowicryl K4M resin at −35°C, and immunolabeled on grid with 10-nm colloidal gold, as described in Materials and Methods. tPA is found, both in situ and in vitro, in vesicular structures with an electron-dense content (a, b, d, and e, arrows). For comparison, a coated vesicle is indicated on the surface of a HUVEC (cv). vWf was detected in structures with the typical characteristics of Weibel-Palade bodies (c, W-Pb): elongated structures containing densely packed fibrilar material. f–h show double immunofluorescent labeling of HUVEC with antibodies specific for tPA (f) and vWf (g). The images collected from both fluorescence channels were superimposed in h. Note that the red and green granules are clearly different, and that in the superimposed figure of h almost no yellow (red over green) grains are observed. Bars: (a–e) 0.2 μm; (f–h) 25 μm.
Figure 9.
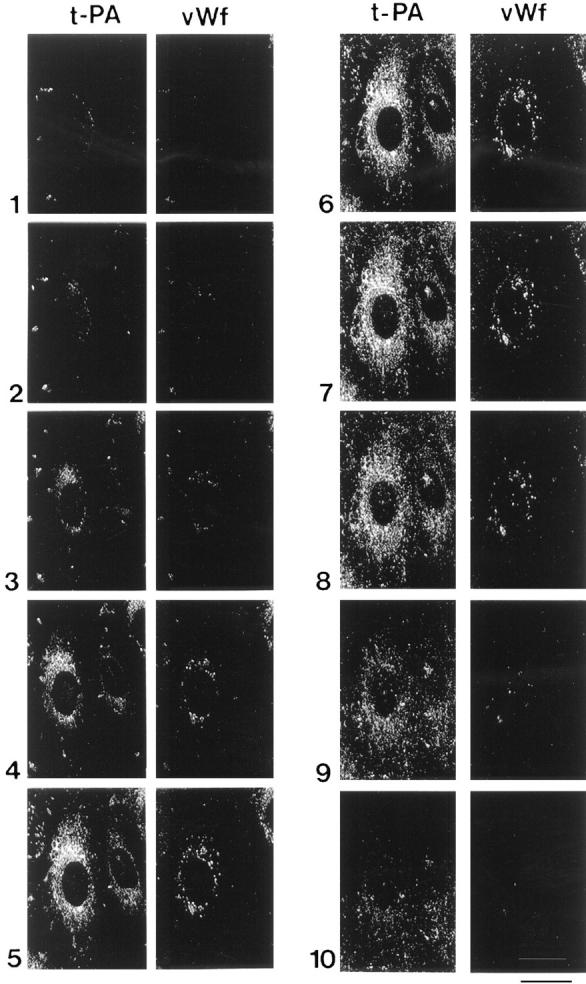
Sequential display of 10 optical sections in the Z-axis through HUVEC. The cells were double stained for tPA and vWf, and the two proteins were simultaneously visualized in serial optical sections from the apical (section 1) to the basal (section 10) side of the cells. tPA is detected throughout the cell, while vWf is distributed mainly in the perinuclear area. Bar, 25 μm.
The granular localization of tPA was confirmed by postembedding immunogold labeling of ultrathin sections of HUVEC (Fig. 8, a and b) and of vascular endothelial cells in mouse lung and diaphragm capillaries (Fig. 8, d and e). The gold particles delineating tPA were present throughout the cells, often in clusters over small, fairly electron-dense vesicles, which in favorable sections could be seen to be surrounded by a membrane (Fig. 8, b, d, and e). The location of the gold particles suggested that they were superimposed on the small vesicles that are abundantly present in endothelial cells after routine fixation and embedding and that are much smaller than Weibel-Palade bodies. The gold particles delineating vWf were detected on much larger, elongated particles, which contained densely packed fibrillar material and had the morphological characteristics compatible with Weibel-Palade bodies (Fig. 8 c, W-Pb).
No Colocalization of tPA with vWf in HUVEC
To further differentiate between tPA- and vWf-containing granules in HUVEC, we performed double immunolabeling for tPA and vWf with Texas red- and FITC-labeled secondary antibodies and simultaneous visualization of the two proteins by confocal microscopy. To detect whether the two proteins were colocalized, the two images were merged; any superimposed green and red areas would then appear in yellow. As illustrated by Fig. 8, f–h (f, tPA; panel g, vWf), no colocalization of the two antigens was observed (h, in which e and f are superimposed, so that colocalization of tPA and vWf would show as yellow). Control experiments gave no labeling in all conditions tested (not shown).
No Colocalization of tPA with endothelin-1, caveolin, or TFPI in HUVEC
Using identical techniques of indirect immunofluorescence and confocal microscopy as described above for vWf, we looked for colocalization of tPA with three other endothelial proteins: endothelin-1 and tissue factor pathway inhibitor, which are both secreted by HUVEC, and caveolin, a component of caveolae (Lisanti et al., 1994). As shown in Fig. 10, no evidence for colocalization of these proteins with tPA in HUVEC was obtained. We show elsewhere (Lupu et al., 1997) that caveolin and TFPI, but not tPA, colocalize in HUVEC in caveolae.
Figure 10.
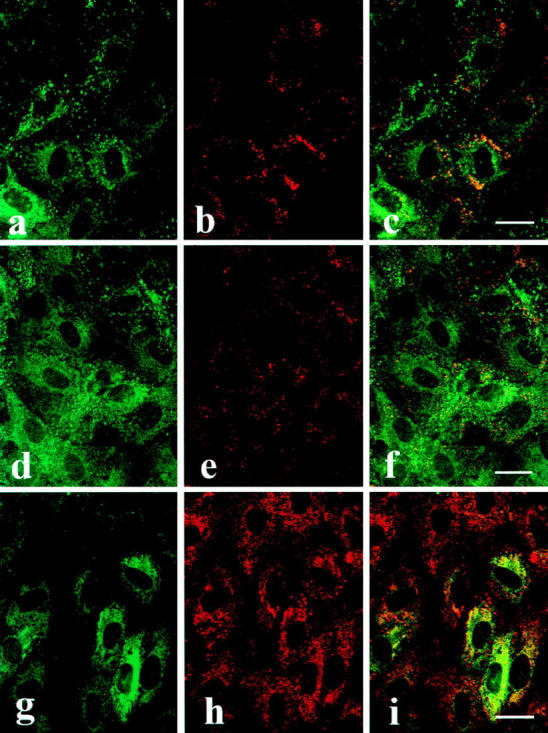
Double staining of first passage HUVEC for tPA (left column, a, d, and g) and either TFPI (b), caveolin (e), or endothelin-1 (h). The panels in the right column represent the superposition of staining for tPA (green) with staining (red) for either TFPI (c), caveolin (f), or endothelin-1 (i). In case of colocalization of two proteins, the resultant superimposed image in the right hand column is yellow. Note that only in the case of tPA and endothelin-1 (i) in some cells is superimposition observed. For technical details, see Materials and Methods. Bar, 25 μm.
DISCUSSION
Remarkably, the first studies on the subcellular fractionation of plasminogen activators (at that time called “fibrinolysokinase activators,” now known to be tPA) from tissue date 1950 (Tagnon and Palade, 1950; Lewis and Ferguson, 1950) and describe that the enzyme activity resides in the “large granule and microsome” fractions of tissue homogenates (Lewis and Ferguson, 1950). Apart from a few similar studies in the mid-sixties (Lack and Ali, 1964; Ali and Lack, 1965; Sugiyama, 1965; Beard et al., 1968), which located tissue PA activity to lysosomes, no major advances in the localization of tPA in tissues were made. Recently, it was shown that, in cells transfected with tPA, tPA is sorted to a granular compartment in the regulated pathway (Harrison et al., 1996).
Meanwhile, the Weibel-Palade body has been firmly established to be the storage particle for vWf. This particle, first described as an endothelial organelle characterized by specific morphological features (Weibel and Palade, 1964), was subsequently shown to be the storage organelle for vWf in endothelial cells (for review see Reinders et al., 1988; Wagner 1990, 1993; Mayadas and Wagner, 1991; Hop and Pannekoek, 1996).
In clinical and in experimental (in vivo and in vitro) studies, the regulated secretion (acute release) of tPA and vWf generally occurs simultaneously (Tranquille and Emeis, 1989, Emeis, 1995, van den Eijnden-Schrauwen et al., 1995). This might be due to the fact that the cellular mechanisms involved in tPA and vWf release from endothelial cells are very similar but also due to colocalization of the proteins in a single particle. The question whether tPA and vWf are colocalized is of practical importance, because colocalization would make it impossible to induce selectively the release of either the procoagulant protein vWf or the anticoagulant protein tPA.
Our data show that tPA and vWf are not located in a single storage granule but in granules that can be separated by sucrose density gradient centrifugation and that can be visualized as separate by light- and electron-microscopical immunocytochemistry. Ultracentrifugation of rat lung homogenate in buffer showed that tPA (and vWf) were present in a sedimentable particle, from which the proteins could be released by Triton treatment. By density gradient ultracentrifugation on a Nycodenz gradient, the density of the particle was determined to be 1.11–1.12 g/ml. The distribution of tPA in the Nycodenz gradient was not due to nonspecific binding of soluble tPA (Fig. 2 b), while the particle was stable upon recentrifugation (Fig. 2 a). In previous experiments, we have shown that tissue stores of tPA, including tPA stores in rat lung, are metabolically highly stable, hardly showing any decrease 3 h after protein synthesis has been inhibited by cycloheximide (Tranquille and Emeis, 1989). Cycloheximide treatment did not cause any change in the density distribution of tPA and vWf either. The large peak of tPA (activity and antigen) found after density gradient fractionation of lung must thus be either active tPA stored in a storage pool, or tPA present in a metabolically inert pool, e.g., extracellular tPA bound to connective tissue. A contribution from extracellular tPA is, however, most unlikely, because connective tissue components (e.g., cellular fibronectin, see Fig. 1 d) equilibrate at a much lower density. The tPA present at d = 1.11–1.12 was, moreover, enzymatically fully active, while extracellular tPA would have been present largely as an inactive complex with its inhibitor PAI-1 (Kooistra et al., 1986; Fearns et al., 1996).
In Nycodenz gradients, the distribution of vWf and tPA was nearly identical, suggesting that the two proteins could be present in a single storage granule (Figs. 1, a and b, and 2 a). The equilibrium density of this putative storage granule was, moreover, similar to the equilibrium density of Weibel-Palade bodies in other types of density gradient, e.g., Percoll gradients (Reinders et al., 1984; Ewenstein et al., 1987).
In sucrose gradients, however, the distribution of vWf differed from that of tPA. tPA showed essentially the same density distribution as in Nycodenz gradients, but vWf had disappeared from the d = 1.11–1.12 g/ml fractions (compare Fig. 3 with Figs. 1, a and b, and 2 a). This observation showed that the two proteins were not stored in the same granule in rat lung. An explanation for the density shift of vWf might be destabilization of the Weibel-Palade body by sucrose, leading to collapse, or dehydration by the hypertonic sucrose solutions, leading to a change in density. The tPA storage particle would then be immune to these forces. Similar to rat lung, sucrose density centrifugation resulted in human lung in a significant loss of vWf antigen from the d = 1.11–1.12 g/ml fractions (from 14% in Nycodenz to 2% in sucrose), without causing a loss of tPA from these fractions (from 19 to 16%).
The distribution of tPA from rat heart endothelial cells was very similar to that from rat lung. In human lung and HUVEC (Figs. 4 and 5) the distribution pattern of tPA and vWf was more complex, although peaks of tPA and vWf could still clearly be identified at d = 1.11–1.12 g/ml. Presumably, the presence of other cells (including blood platelets), more connective tissue in the lung, and more subendothelial matrix in HUVEC contributed to the more complex patterns observed in homogenates from human lung and from HUVEC.
Two other small particles have been isolated from endothelial cells. Caveolae (Lisanti et al., 1994; Schnitzer et al., 1995) are plasmalemmal vesicles characterized by the presence of, among others, GPI-anchored proteins and caveolin. Recently, a storage particle for endothelin-1 has been isolated from bovine aortic endothelial cells (Harrison et al., 1995). It is not likely that tPA storage granules are identical to caveolae, since the former are much denser than caveolae and do not contain plasma membrane markers such as alkaline phosphatase (Fig. 1 c), as do caveolae (Lisanti et al., 1994). In addition, by double immunofluorescent staining, tPA- and caveolin-containing structures were different (Fig. 10). Tissue factor pathay inhibitor (TFPI) has, in HUVEC, also been localized (Lupu et al., 1995a ) by immunocytochemistry in a particle, which contains, in addition, caveolin but not tPA (Lupu et al., 1997), in agreement with Fig. 10. Together, these data suggest that the tPA-containing particle does not contain caveolin, ET-1, and TFPI. We also found no evidence for a colocalization of tPA with ET-1 (Fig. 10 i). The data thus suggest, though they certainly do not prove, that of the proteins mentioned above, those four that are secreted from HUVEC (vWf, tPA, ET-1, and TFPI) may be located in different structures, which opens the possibility that endothelial secretory proteins are packaged in separate secretory pathways.
Stimulation, by thrombin, of acute secretion of tPA and vWf from HUVEC (van den Eijnden-Schrauwen et al., 1995, 1997) resulted in a loss of tPA from the higher-density fractions (d = 1.10–1.14). This loss coincided with the appearance of tPA (and vWf) in the culture medium and thus likely reflects the secretion of tPA (vWf) from its storage pool. The loss was accompanied by a slight shift to higher densities. Stimulation with 1 NIH U of human α-thrombin for 3 min induces maximal release of tPA from HUVEC, since neither an increase in thrombin concentration above 1 U/ml nor subsequent stimulation with the calcium ionophore ionomycin induced any additional tPA secretion (van den Eijnden-Schrauwen et al., 1995). Stimulation with 1 U/ml of thrombin was also accompanied by a complete loss of granular tPA staining in some cells and a severe reduction in tPA staining in the others (Fig. 6). The quantatively, only moderate disappearance of tPA from homogenates of thrombin-stimulated cells was therefore surprising and may reflect the presence, in endothelial cell cultures, of relatively large amounts of tPA outside the secretory pathway.
Thrombin-induced release of tPA was accompanied (Fig. 7) by a shift to slightly higher densities and an increase in immunoreactivity of P-selectin, a component of Weibel-Palade bodies (Wagner, 1993). Whether the tPA storage granule also contained P-selectin could not be decided from the data and is still under investigation. The cell biological substrate of this density shift of P-selectin remains unexplained. It could reflect the fusion of multiple particles, as described by Richardson et al. (1994) in rat endothelial cells after thrombin stimulation in vivo. The remarkable increase in P-selectin immunoreactivity after thrombin (Fig. 8) is also not explained but might be due to an increased accessibility of P-selectin to antibody after thrombin treatment.
The morphological observations on HUVEC, obtained using immunocytochemical labeling procedures with confocal microscopy and immunoelectronmicroscopy on thin sections, showed that tPA was localized throughout the cytoplasm of HUVEC in small vesicular structures, which differed from Weibel-Palade bodies in size and morphology. Particles staining for vWf were larger and contained a more electron-dense and fibrillar core. No significant colocalization of tPA- and vWf-containing particles was observed in double immunocytochemical labeling experiments. In summary, the morphological data support and extend the biochemical data that tPA-containing vesicles and vWf-containing Weibel-Palade bodies are distinct entities.
Recent observations (van den Eijnden-Schrauwen, 1996; van den Eijnden-Schrauwen et al., 1997) on regulated secretion of tPA and vWf from HUVEC have shown that several mechanisms are involved in (thrombin-induced) regulated secretion of tPA and vWf. Regulated secretion of vWf is mainly dependent upon an increase in intracellular calcium and calcium influx, while regulated secretion of tPA requires, in addition, the activation of one or more G proteins (van den Eijnden-Schrauwen et al., 1997). The two secretory pathways also show different sensitivities to actin depolymerization (van den Eijnden-Schrauwen, 1996). Such differences in secretory mechanisms presuppose the existence of two separate storage particles. The present data provide evidence that two such storage particles indeed exist: the Weibel-Palade body for vWf, and a separate, newly described, particle for tPA.
Acknowledgments
This work was supported in part by grants from the Netherlands Heart Foundation (93.126) and the British Heart Foundation (PG/94188).
Abbreviations used in this paper
- HUVEC
human umbilical vein endothelial cells
- TFPI
tissue factor pathway inhibitor
- tPA
tissue-type plasminogen activator
- vWf
von Willebrand factor
Footnotes
Address all correspondence to J.J. Emeis, Gaubius Laboratory TNO-PG, P.O. Box 2215, 2301 CE Leiden, The Netherlands. Tel.: (31) 71-518-1451. Fax: (31) 71-518-1904. e-mail: JJ.Emeis@pg.tno.nl
The assistance, during various stages of this study, of Sachin Apte, Alette van Binsbergen, René Hegeman, Marielle Kroon, and Linda Oudsen is gratefully acknowledged. The authors are indebted to Dr M. Bijsterbosch for introducing them to subcellular fractionation techniques.
REFERENCES
- Ali SY, Lack CH. Studies on the tissue activator of plasminogen. Distribution of activator and proteolytic activity in the subcellular fractions of rabbit kidney. Biochem J. 1965;96:63–74. doi: 10.1042/bj0960063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard EL, Montuori MH, Danos GJ, Busuttil RW. Plasminogen activator activity of rat lysosomes. Proc Soc Exp Biol Med. 1968;129:804–808. doi: 10.3181/00379727-129-33429. [DOI] [PubMed] [Google Scholar]
- Brett JG, Steinberg SF, de Groot PG, Nawroth PP, Stern DM. Norepinephrine down-regulates the activity of protein S on endothelial cells. J Cell Biol. 1988;106:2109–2118. doi: 10.1083/jcb.106.6.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttery JE, Stuart S. Assessment and optimization of kinetic methods for angiotensin-converting enzyme in plasma. Clin Chem. 1993;39:312–316. [PubMed] [Google Scholar]
- Carmeliet P, Collen D. Targeted gene manipulation and transfer of the plasminogen and coagulation systems in mice. Fibrinolysis. 1996a;10:195–213. doi: 10.1055/s-2007-999055. [DOI] [PubMed] [Google Scholar]
- Carmeliet, P., and D. Collen. 1996b Evaluation of the role of the fibrinolytic system in transgenic animals. In Vascular Control of Haemostasis. V.W.M. van Hinsbergh, editor. Gordon and Breach Science Publishers, Victoria, British Columbia. 247–256.
- Coughlan AF, Hau H, Dunlop LC, Berndt MC, Hancock WW. P-selectin and platelet-activating factor mediate initial endotoxin-induced neutropenia. J Exp Med. 1994;179:329–334. doi: 10.1084/jem.179.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diglio CA, Grammas P, Giacomelli F, Wiener J. Rat heart-derived endothelial and smooth muscle cell cultures: isolation, cloning and characterization. Tissue Cell. 1988;20:477–492. doi: 10.1016/0040-8166(88)90051-1. [DOI] [PubMed] [Google Scholar]
- Emeis, J.J. 1988. Mechanisms involved in short-term changes in blood levels of t-PA. In Tissue-type Plasminogen Activator: Physiological and Clinical Aspects, Vol 2. C. Kluft, editor. CRC Press, Boca Raton, FL. 21–35.
- Emeis, J.J. 1996. Normal and abnormal endothelial release of tissue-type plasminogen activator. In Fibrinolysis In Disease, Vol 2. P. Glas-Greenwalt, editor. CRC Press, Boca Raton, FL. 55–64.
- Emeis JJ, Hoekzema R, de Vos AF. Inhibiting interleukin-1 and tumour necrosis factor-α does not reduce the induction of plasminogen activator inhibitor type-1 by endotoxin in rats in vivo. Blood. 1995;85:115–120. [PubMed] [Google Scholar]
- Emeis, J.J., Y. van den Eijnden-Schrauwen, and T. Kooistra. 1996. Tissue-type plasminogen activator and the vessel wall: synthesis, storage and secretion. In Vascular Control of Haemostasis. V.W.M. van Hinsbergh, editor. Gordon and Breach Science Publishers, Victoria, British Columbia. 187–206.
- Ewenstein BM, Warhol MJ, Handin RI, Pober JS. Composition of the von Willebrand factor storage organelle (Weibel-Palade body) isolated from cultured human umbilical vein endothelial cells. J Cell Biol. 1987;104:1423–1433. doi: 10.1083/jcb.104.5.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearns, C., F. Samad, and D.J. Loskutoff. 1996. Synthesis and localization of PAI-1 in the vessel wall. In Vascular Control of Haemostasis. V.W.M. van Hinsbergh, editor. Gordon and Breach Science Publishers, Victoria, Australia. 207–226.
- Friedman SA, Schiff E, Emeis JJ, Dekker GA, Sibai BM. Biochemical corroboration of endothelial involvement in severe preeclampsia. Amer J Obstet Gynecol. 1995;172:202–203. doi: 10.1016/0002-9378(95)90113-2. [DOI] [PubMed] [Google Scholar]
- Harrison VJ, Barnes K, Turner AJ, Wood E, Corder R, Vane JR. Identification of endothelin 1 and big endothelin 1 in secretory vesicles isolated from bovine aortic endothelial cells. Proc Natl Acad Sci USA. 1995;92:6344–9348. doi: 10.1073/pnas.92.14.6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison TM, Chidgey MAJ, Uff S. Novel markers for constitutive secretion used to show that tissue plasminogen activator is sorted to the regulated pathway in transfected PC12 cells. Cell Biol Int. 1996;20:293–300. doi: 10.1006/cbir.1996.0033. [DOI] [PubMed] [Google Scholar]
- Hop, C., and H. Pannekoek. 1996. Properties and biosynthesis of von Willebrand factor: a critical review. In Vascular Control of Haemostasis. V.W.M. van Hinsbergh, editor. Gordon and Breach Science Publishers, Victoria, Australia. 107–125.
- Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical cord veins. Identification by morphological and immunologic criteria. J Clin Invest. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooistra, T., and J.J. Emeis. 1997. Agents which increase synthesis and release of tissue-type plasminogen activator. In Handbook of Experimental Pharmacology. F. Bachmann, editor. Springer Verlag, New York. In press.
- Kooistra T, Sprengers ED, van Hinsbergh VWM. Rapid inactivation of the plasminogen-activator inhibitor upon secretion from cultured human endothelial cells. Biochem J. 1986;239:497–503. doi: 10.1042/bj2390497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooistra, T., J. van den Berg, A. Töns A., G. Platenburg, D.C. Rijken, and E. van den Berg. 1987. Butyrate stimulates tissue-type plasminogen-activator synthesis in cultured human endothelial cells. Biochem. J. 247:605–612. [DOI] [PMC free article] [PubMed]
- Lack CH, Ali SY. Tissue activator of plasminogen. Nature (Lond) 1964;201:1030–1031. doi: 10.1038/2011030a0. [DOI] [PubMed] [Google Scholar]
- Levin EG, del Zoppo GJ. Localization of tissue plasminogen activator in the endothelium of a limited number of vessels. Am J Pathol. 1994;144:855–861. [PMC free article] [PubMed] [Google Scholar]
- Lewis JH, Ferguson JH. Studies on a proteolytic enzyme system of the blood. II. Fibrinolysokinase activators for profibrinolysin. J Clin Invest. 1950;29:1059–1068. doi: 10.1172/JCI102337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanti, M.P., P.E. Scherer, J. Vidugiriene, Z. Tang, A. Hermanowski, Y.H. Tu, R.F. Cook, and M. Sargiacomo. Characterization of caveolin-rich domains isolated from an endothelial-rich source: implications for human disease. J. Cell Biol. 126:111–126. [DOI] [PMC free article] [PubMed]
- Lupu F, Bergonzelli GE, Heim DA, Cousin E, Genton CY, Bachman F, Kruithof EKO. Localization and production of plasminogen activator inhibitor-1 in human healthy and atherosclerotic arteries. Arterioscler Thromb. 1993;13:1090–1100. doi: 10.1161/01.atv.13.7.1090. [DOI] [PubMed] [Google Scholar]
- Lupu C, Lupu F, Dennehy U, Kakkar VV, Scully MF. Thrombin induces the redistribution and acute release of tissue factor pathway inhibitor from specific granules within human endothelial cells in culture. Arterioscler Thromb Vasc Biol. 1995a;15:2055–2062. doi: 10.1161/01.atv.15.11.2055. [DOI] [PubMed] [Google Scholar]
- Lupu F, Heim DA, Bachmann F, Hurni M, Kakkar VV, Kruithof EKO. Plasminogen activator expression in human atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 1995b;15:1444–1455. doi: 10.1161/01.atv.15.9.1444. [DOI] [PubMed] [Google Scholar]
- Lupu, C., C.A. Goodwin, J.J. Emeis, M.F. Scully, V.V. Kakkar, and F. Lupu. 1997. Association of cellular tissue factor pathway inhibitor with caveolae/ glycolipid domains: upregulation of the anticoagulant properties of human endothelial cells. Arterioscler. Thromb. Vasc. Biol. In press. [DOI] [PubMed]
- Mayadas TN, Wagner DD. von Willebrand factor biosynthesis and processing. Ann NY Acad Sci. 1991;614:153–166. doi: 10.1111/j.1749-6632.1991.tb43700.x. [DOI] [PubMed] [Google Scholar]
- Newman GR, Jasani B, Williams ED. A simple post-embedding system for rapid demonstration of tissue antigens under the electron microscope. Histochem J. 1983;15:543–555. doi: 10.1007/BF01954145. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuizen W, Laterveer R, Hoegee-de Nobel E, Bos R. A one-step enzyme immunoassay for total plasminogen activator inhibitor–1 antigen in human plasma. Blood Coagul Fibrinolysis. 1995;6:268–272. doi: 10.1097/00001721-199505000-00011. [DOI] [PubMed] [Google Scholar]
- Padró T, van den Hoogen CM, Emeis JJ. Distribution of tissue-type plasminogen activator (activity and antigen) in rat tissues. Blood Coagul Fibrinolysis. 1990;1:601–608. [PubMed] [Google Scholar]
- Padró T, Emeis JJ, Steins M, Schmid KW, Kienast J. Quantification of plasminogen activators and their inhibitors in aortic vessel wall in relation to the presence and severity of atherosclerotic disease. Arterioscler Thromb Vasc Biol. 1995;15:893–902. doi: 10.1161/01.atv.15.7.893. [DOI] [PubMed] [Google Scholar]
- Reinders JH, de Groot PG, Gonsalves MD, Zandbergen J, Loesberg C, van Mourik JA. Isolation of a storage and secretory organelle containing von Willebrand protein from cultured human endothelial cells. Biochim Biophys Acta. 1984;804:361–369. doi: 10.1016/0167-4889(84)90140-x. [DOI] [PubMed] [Google Scholar]
- Reinders JH, de Groot PG, Sixma JJ, van Mourik JA. Storage and secretion of von Willebrand factor by endothelial cells. Haemostasis. 1988;18:246–261. doi: 10.1159/000215811. [DOI] [PubMed] [Google Scholar]
- Richardson M, Tinlin S, De Reske M, Webster S, Senis Y, Giles AR. Morphological alterations in endothelial cells associated with the release of von Willebrand factor after thrombin generation in vivo. Arterioscler Thromb. 1994;14:990–999. doi: 10.1161/01.atv.14.6.990. [DOI] [PubMed] [Google Scholar]
- Roth J. Post-embedding labeling on Lowicryl K4M tissue sections: detections and modifications of cellular components. Methods Cell Biol. 1989;31:513–550. doi: 10.1016/s0091-679x(08)61625-8. [DOI] [PubMed] [Google Scholar]
- Schneiderman J, Bordin GM, Engelberg I, Adar R, Seiffert D, Thinnes T, Bernstein EF, Dilley RB, Loskutoff DJ. Expression of fibrinolytic genes in atherosclerotic abdominal aortic aneurysm wall. J Clin Invest. 1995;96:639–645. doi: 10.1172/JCI118079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer JE, Liu J, Oh P. Endothelial caveolae have the molecular transport machinery for vesicle budding, docking, and fusion including VAMP, NSF, SNAP, annexins, and GTPases. J Biol Chem. 1995;270:14399–14404. doi: 10.1074/jbc.270.24.14399. [DOI] [PubMed] [Google Scholar]
- Schrauwen Y, Emeis JJ, Kooistra T. A sensitive ELISA for human tissue-type plasminogen activator applicable to the study of acute release from cultured human endothelial cells. Thromb Haemostasis. 1994;71:225–229. [PubMed] [Google Scholar]
- Sugiyama Y. Plasminogen activator in the cytoplasmic granules from different organs of mammals: studies on intracellular localization, release, and molecular size of two kinds of the activators. Kobe J Med Sci. 1965;11:151–167. [PubMed] [Google Scholar]
- Tagnon HJ, Palade GE. Activation of proplasmin by a factor from mammalian tissue. J Clin Invest. 1950;29:317–324. doi: 10.1172/JCI102260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranquille N, Emeis JJ. Protein synthesis inhibition by cycloheximide does not affect the acute release of tissue-type plasminogen activator. Thromb Haemostasis. 1989;61:442–447. [PubMed] [Google Scholar]
- Tranquille N, Emeis JJ. The simultaneous acute release of tissue-type plasminogen activator and von Willebrand factor in the perfused rat hindleg region. Thromb Haemostasis. 1990;63:454–458. [PubMed] [Google Scholar]
- van den Eijnden-Schrauwen, Y. 1996. Acute release of tissue-type plasminogen activator from human endothelial cells. PH.D. thesis State University of Leiden, Leiden, The Netherlands.
- van den Eijnden-Schrauwen Y, Kooistra T, de Vries REM, Emeis JJ. Studies on the acute release of tissue-type plasminogen activator from human endothelial cells in vitro and in rats in vivo: evidence for a dynamic storage pool. Blood. 1995;85:3510–3517. [PubMed] [Google Scholar]
- van den Eijnden-Schrauwen, Y., D.E. Atsma, R.E.M. de Vries, F. Lupu, T. Kooistra, and J.J. Emeis. 1997. On the involvement of calcium and G-proteins in the acute release of tissue-type plasminogen activator and von Willebrand factor from cultured human endothelial cells. Arterioscler. Thromb. Vasc. Biol. In press. [DOI] [PubMed]
- van Hinsbergh VWM, Bertina RM, van Wijngaarden A, van Tilburg NH, Emeis JJ, Haverkate F. Activated protein C decreases plasminogen activator-inhibitor activity in endothelial cell-conditioned medium. Blood. 1985;65:444–451. [PubMed] [Google Scholar]
- van Hinsbergh VWM, Kooistra T, Emeis JJ, Koolwijk P. Regulation of plasminogen activator production by endothelial cells: role in fibrinolysis and local proteolysis. Int J Radiat Biol. 1991;60:261–272. doi: 10.1080/09553009114551981. [DOI] [PubMed] [Google Scholar]
- Verheijen JA, Mullaart E, Chang GTG, Kluft C, Wijngaards G. A simple sensitive spectrophotometric assay for extrinsic (tissue-type) plasminogen activator applicable to measurements in plasma. Thromb Haemostasis. 1982;48:266–269. [PubMed] [Google Scholar]
- Verheijen JH, Chang GTG, Kluft C. Evidence for the occurrence of a fast-acting inhibitor for plasminogen activator in human plasma. Thromb Haemostasis. 1984;51:392–395. [PubMed] [Google Scholar]
- Wagner DD. Cell biology of von Willebrand factor. Annu Rev Cell Biol. 1990;6:217–246. doi: 10.1146/annurev.cb.06.110190.001245. [DOI] [PubMed] [Google Scholar]
- Wagner DD. The Weibel-Palade body: the storage granule for von Willebrand factor and P-selectin. Thromb Haemostasis. 1993;70:105–110. [PubMed] [Google Scholar]
- Wanders RJA, van Roermund CWT, van Wijland MJA. Peroxisomal fatty acid β-oxidation in relation to the accumulation of very long chain fatty acids in cultured skin fibroblasts from patients with Zellweger syndrome and other peroxisomal disorders. J Clin Invest. 1987;80:1778–1783. doi: 10.1172/JCI113271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel ER, Palade GE. New cytoplasmic components in arterial endothelia. J Cell Biol. 1964;23:101–112. doi: 10.1083/jcb.23.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wun T-C, Capuano A. Spontaneous fibrinolysis in whole human plasma. Identification of tissue activator-related protein as the major plasminogen activator causing spontaneous activity in vitro. J Biol Chem. 1985;260:5061–5066. [PubMed] [Google Scholar]
- Wun T-C, Capuano A. Initiation and regulation of fibrinolysis in human plasma at the plasminogen activator level. Blood. 1987;69:1354–1362. [PubMed] [Google Scholar]