Abstract
A null mutation was introduced into the mouse desmin gene by homologous recombination. The desmin knockout mice (Des −/−) develop normally and are fertile. However, defects were observed after birth in skeletal, smooth, and cardiac muscles (Li, Z., E. Colucci-Guyon, M. Pincon-Raymond, M. Mericskay, S. Pournin, D. Paulin, and C. Babinet. 1996. Dev. Biol. 175:362–366; Milner, D.J., G. Weitzer, D. Tran, A. Bradley, and Y. Capetanaki. 1996. J. Cell Biol. 134:1255– 1270). In the present study we have carried out a detailed analysis of somitogenesis, muscle formation, maturation, degeneration, and regeneration in Des −/− mice. Our results demonstrate that all early stages of muscle differentiation and cell fusion occur normally. However, after birth, modifications were observed essentially in weight-bearing muscles such as the soleus or continually used muscles such as the diaphragm and the heart. In the absence of desmin, mice were weaker and fatigued more easily. The lack of desmin renders these fibers more susceptible to damage during contraction. We observed a process of degeneration of myofibers, accompanied by macrophage infiltration, and followed by a process of regeneration. These cycles of degeneration and regeneration resulted in a relative increase in slow myosin heavy chain (MHC) and decrease in fast MHC. Interestingly, this second wave of myofibrillogenesis during regeneration was often aberrant and showed signs of disorganization. Subsarcolemmal accumulation of mitochondria were also observed in these muscles. The lack of desmin was not compensated by an upregulation of vimentin in these mice either during development or regeneration. Absence of desmin filaments within the sarcomere does not interfere with primary muscle formation or regeneration. However, myofibrillogenesis in regenerating fibers is often abortive, indicating that desmin may be implicated in this repair process. The results presented here show that desmin is essential to maintain the structural integrity of highly solicited skeletal muscle.
Desmin, a protein of 52 kD has been identified as the constitutive subunit of the intermediate filaments (IF)1 in skeletal, cardiac, and smooth muscles (Lazarides and Hubbard, 1976; Small and Sobieszek, 1977; Geisler and Weber, 1982; Traub, 1985). During mouse embryogenesis desmin is first detected in the embryo at 8.5 days postcoitum (d.p.c.) in the heart rudiment, and then with increasing intensity in the myocardial cells. From 9 d.p.c., desmin is also found in the myotomes of the somites with a rostro-caudal gradient of expression (Fürst et al., 1989; Herrmann et al., 1989; Schaart et al., 1989; Li et al., l993). The first recognizable step in skeletal muscle myogenesis seems to be the initiation of desmin synthesis in replicating presumptive myoblasts. Other muscle-specific proteins, such as sarcomeric actins, myosins, titin, and myomesin, appear only in the postmitotic mononucleated myoblasts (Grove et al., 1985; Hill et al., 1986; Kaufman and Foster 1988; Babai et al., 1990; Allen et al., 1991; Mayo et al., 1992). At 13 d.p.c., strong desmin synthesis is found in the newly forming primary myotubes in the limbs. Initially these desmin filaments are longitudinally oriented. During subsequent muscle maturation, an extensive change in myofiber architecture occurs whereby Z disks become aligned, nuclei move from central to peripheral locations, and tubules adopt a transverse orientation. The preexisting desmin IF networks shift from a longitudinal to a predominantly transverse orientation, associated with the Z disk. Skeletal muscles are characterized by the precise organization of the contractile proteins into striated myofibrils resulting from repeating units, the sarcomeres arranged in series (for review see Schiaffino and Reggiani, 1996). Associated with the end of the sarcomere, desmin intermediate filaments occupy a strategic position, linking individual myofibrils laterally at their Z disks and interconnecting sarcomeres to the sarcolemma membrane. Morphological data have suggested that desmin filaments are implicated in muscle resistance, and it has been predicted that intermediate filaments may elongate locally if sarcomeres lose the ability to generate and transmit active force. In such a case, desmin may serve as a relay in transmitting tension and preventing a breakdown of force transmission between adjacent sarcomeres (Wang et al., 1993).
Desmin has also been postulated to play a critical role at different early steps of myogenesis both during myogenic commitment and differentiation. Studies carried out with C2C12 cells demonstrated that myotube formation could be blocked by desmin antisense RNA in vitro (Li et al., 1994). The same group, using ES cells with both copies of the desmin gene inactivated, reported an absence of myogenic differentiation in these cultures (Weitzer et al., 1995). However, by using a gene targeting approach in mice, we have demonstrated that desmin does not play such an essential role during myogenesis, since mice lacking desmin develop normal skeletal muscles (Li et al., 1996). Homozygous mutant Des −/− mice have an apparent normal external morphology, but are smaller than control litter mates. We found morphological abnormalities in skeletal, smooth, and cardiac muscle (Li et al., 1996; Thornell et al., 1997). Similar observations were also made with another allele of desmin null mice (Milner et al., 1996). Thus the two different desmin knockouts give rise to mice with the same phenotype.
In the present study we have examined in detail the in vivo consequence of the absence of desmin at different stages of skeletal muscle development to answer the following questions: Is myoblast fusion and myotube formation modified in the absence of desmin? Is the organization and maintenance of myofibrils in register dependent on the transverse desmin distribution? Are biochemical or mechanical properties modified when desmin is absent from muscle fibers?
To answer these questions, we have looked at the different stages of somitogenesis, skeletal muscle formation, maturation, degeneration, and regeneration in Des −/−, −/+, and +/+ mice. To analyze the role of desmin during somitogenesis, we have taken advantage of the replacement of desmin by the LacZ coding sequence. In these desmin knockout mice (Des −/−) we can follow the specific β-galactosidase expression in myogenic cells by their blue coloration and examine whether there are abnormalities or differences either in the chronology or morphology of the formation of myotomes in the mice lacking desmin. We found that early stages of muscle differentiation and cell fusion occurred normally in utero in the absence of desmin. Muscle maturation also occurred on time, and a normal muscle fiber phenotype was seen in all muscles. However, as early as 2 wk after birth, we found that there were ultrastructural modifications in myofibril organization, necrosis, and focal regeneration. These modifications were prominent in muscles that are continually solicited, such as the diaphragm and soleus, where they were eventually accompanied by a modification in muscle phenotype. It is interesting to note that the Des −/− mice fatigued more quickly and had less force than their control littermates. Our results imply that desmin is not required for myogenic commitment, differentiation, and fusion, but is essential to maintain the tensile strength and integrity of muscle fibers.
MATERIALS AND METHODS
Construction of the Targeting Vector, Generation, and Identification of Desmin Knockout Mice
To disrupt the desmin gene, one targeting vector was constructed in which an Escherichia coli LacZ gene was inserted in frame into the first exon of the desmin gene. Details of the construction of the desmin targeting vector, production of targeted ES cell lines, and obtention of desmin knockout mice have been described previously (Li et al., 1996).
Histochemical Staining for β-Galactosidase
Pregnant mice were killed by cervical dislocation; embryos and fetuses at 7–18 d of gestation were isolated, washed in PBS, and fixed for 30–60 min depending on their size in 1% paraformaldehyde plus 0.2% glutaraldehyde in PBS. After washing four times in PBS, embryos were stained in toto for LacZ as described (Li et al., 1993). Photographs were taken using a stereomicroscope (Leitz, Wetzlar, Germany).
Immunofluorescence
Frozen sections (5- and 0.25-μm-thick) of mouse muscles were used for immunofluorescence. For immunostaining, frozen sections were fixed in acetone for 10 min. After four 3-min washes in PBS, tissue sections were treated with 20% goat serum. All antibodies were diluted in 1% BSA in PBS. The primary antibody dilutions were as follows: antidesmin (rabbit polyclonal; Biomakor, Rehouot, Israel), 1:100; antivimentin (goat polyclonal or mouse monoclonal, DAKOPATTS, Copenhagen, Denmark), 1:50; anti–slow, 1:20 and anti–fast myosin heavy chains (MHCs), 1:10 (Novocastra, Newcastle, UK); FITC-conjugated, anti–rabbit and anti–goat IgG antibodies (DAKOPATTS); and anti–mouse IgG antibodies (Biosys, Compiègne, France) were used as the secondary antibodies. Slides were mounted in Mowiol. Immunostaining of semithin cryosections was performed according to the method described by North et al. (l993) with antiactin, anti– α-actinin, antidesmin, and antititin antibodies with the help of J.V. Small (Salzburg, Austria). Fluorescent labeling of semithin cryosections was performed using the biotin streptavidin–FITC system supplied by Amersham Intl. (Little Chalfont, UK). Final mounting was in Gelvatol containing 2.5 mg/ml N-propyl gallate as an antibleaching agent. Microscopy was carried out using a Zeiss Axioskop epifluorescence microscope (Carl Zeiss, Inc., Oberkochen, Germany).
Histological Analysis
For histological analysis, 5-μm-thick paraffin or frozen sections were fixed for 5 min in 2% paraformaldehyde and washed in PBS. Hematoxylin and eosin staining or Gomori's modified trichrome staining were carried out as described (Mallory, 1961).
Myosin Heavy Chain Gel Electrophoresis
Analysis of MHC by gel electrophoresis was carried out according to the method of Talmadge and Roy (1993), with the following modifications (Agbulut et al., 1997); 10 mM β-mercaptoethanol was added to the upper buffer and gels were migrated for 30 h at 72 V.
ATPase Histochemical Analysis
ATPase enzyme histochemistry was carried out as described by Brooke and Kaiser (1970). Muscle samples from diaphragm, soleus, and gastrocnemius were taken from 4 to 12-wk-old mice and frozen sections were analyzed. After preincubation at pH 4.35, ATPase activities were revealed. For each muscle, 10 transverse sections were processed and the number of each fiber type was determined after counting 103 fibers per section. 10 mice were analyzed at each age.
Transmission Electron Microscopy
For electron microscopy, muscles were fixed with 2.5% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4, for 2 h. After postfixation for 1 h at 4°C in 2% osmium tetroxide and acetone dehydration, the samples were embedded in polybed 812 resin. Ultrathin sections were stained with uranyl acetate and lead citrate on a LKB 2168 ultrastainer and observed in a Jeol 1200 electron microscope (JEOL USA, Peabody, MA).
Measurement of Endurance and Force of Mice
The muscular performance was analyzed in 12 5-mo-old animals (Neurofit, Illkirch, France). The endurance was analyzed by measuring the ability of an animal to catch and hold onto a 32-g bar. The mouse was held up by the tail and allowed to catch the bar either with all four limbs or with the two forelimbs. The time that the animal could hold onto the weight was measured. Another series of experiments were carried out by repeating the exercise five times a day during 5 d.
The maximum force was measured using a dynamometer linked to a bar and to an analogical conditioner (Entran MM45A; Neurofit, Illkirch, France). The animal was allowed to catch the bar with either all four limbs or with the two forelimbs. Then the mouse was regularly pulled back by the tail until it released the bar. The maximum force unit was in milliNewtons. All the data were compared by analysis of variance (ANOVA) followed by the Scheffé post hoc test. Fisher tests giving values of P < 0.05 were considered to be statistically significant.
Measurement of the Mechanical Properties of Isolated Muscles
These experiments were carried out as described by Lensel-Corbeil and Goubel (1989). Each muscle was mounted horizontally in a chamber containing Ringer solution (115 mM NaCl, 28 mM NaHCO3, 2.5 mM CaCl2, 3.1 mM MgSO4, 35 mM KCl, 1.2 mM KH2PO4, and 11.1 mM glucose) maintained at 25°C and oxygenated with a gas mixture containing 95% O2 and 5% CO2 (final pH 7.3). The proximal part of the muscle was fixed to a force transducer, and the distal extremity was connected to the moving part of an electromagnetic ergometer described in detail elsewhere (Lensel-Corbeil and Goubel, 1989). Mechanical properties were measured with the help of F. Goubel and M. Almeida-Silveira (Compiègne University, Compiègne, France). The muscular performance was analyzed on isolated soleus muscles of 2- and 5-mo-old mutant and control mice.
Electrophysiological Recording
Mice were deeply anesthetized and normal body temperature was maintained with a heating lamp. The sciatic nerve was stimulated at paraspinal sites. Stimulation consisted of single 0.2-ms, 1-Hz supra maximal pulses through a skin electrode. A ground needle electrode was placed on the back of the mouse. An anode needle was inserted at the base of the tail. A reference needle electrode was placed near the Achilles' tendon. The evoked compound muscle action potentials (CMAP) was recorded from the medial part of gastrocnemius and plantaris muscles with a unipolar needle electrode. The distal latency (DL) of evoked potential was measured in ms. It includes the duration of motor nerve conduction between the stimulating and the recording electrodes, plus the synaptic transmission.
Measure of Motor Coordination
To measure motor coordination, two methods were used. The first method consisted of putting the mice on a rotating apparatus (Rotarod, Neurofit; 1-cm-diam rod at 12 rpm) and measuring the time that mice remained on the bar. The second method measured the time that mice needed to cross a suspended rod (1.5-cm-diam, 40-cm length). Mice were put on the extremity of a rod and walked across the rod to reach a platform.
Regeneration Induced by Cardiotoxin
Muscle regeneration was induced by injection of 500 μl of cardiotoxin (Latoxan, Rosans, France) at 80 μg/ml into the lower hind limb muscles of Des −/− mice. Injected and contralateral muscles were examined 7 and 21 d after injection. Frozen sections were either stained for β-galactosidase or reacted with an antibody specific for neonatal MHC (Ecob-Prince et al., 1986).
RESULTS
During Embryogenesis the Absence of Desmin Is Compatible with Somitogenesis and Myotome Formation
In vertebrates, skeletal muscle is derived from somites, which are formed by segmentation of the paraxial mesoderm. In the mouse, the somites begin to form at 8 d d.p.c. and progress caudally over a period of several days (Tam, 1986). Initially, they appear as epithelial balls of cells with no discernable morphological heterogeneity. Then, somites mature and become compartmentalized, the ventral portions forming the sclerotomes, and the dorsal portions the dermomyotomes. The dermomyotomes also give rise to the dermatome, which contributes to skin and to the myotome; the myotome differentiates into the myotomal muscles and axial musculature (Rugh, 1968).
To analyze the role of desmin during somitogenesis we have taken advantage of the replacement of desmin by the LacZ coding sequence via homologous recombination in ES cells. In the resulting desmin knockout (Des −/−) mice, we can follow the specific β-galactosidase expression in myogenic cells by their blue coloration and examine whether there are abnormalities or differences either in the chronology or morphology of the formation of myotomes in the mice lacking desmin. We have compared the blue staining of the Des −/−, the Des +/− mice obtained after crossing heterozygous mice, and the previously described Des +/+ transgenic mice (Li et al., 1993). As shown in Fig. 1, no morphological differences could be detected at this stage (11 d.p.c.) for Des −/− embryos (Fig. 1 B) compared to desmin-expressing embryos Des +/− (Fig. 1 A), and these mice were indistinguishable from the wild-type counterparts. Somites displayed the same morphology as described previously for the Des +/+ transgenic mice (Li et al., 1993). Sections of somites from Des −/−, Des +/−, and Des +/+ are shown in Fig. 2, A–C. We observed a normal ventral extension of the dermomyotome at the thoracic level.
Figure 1.
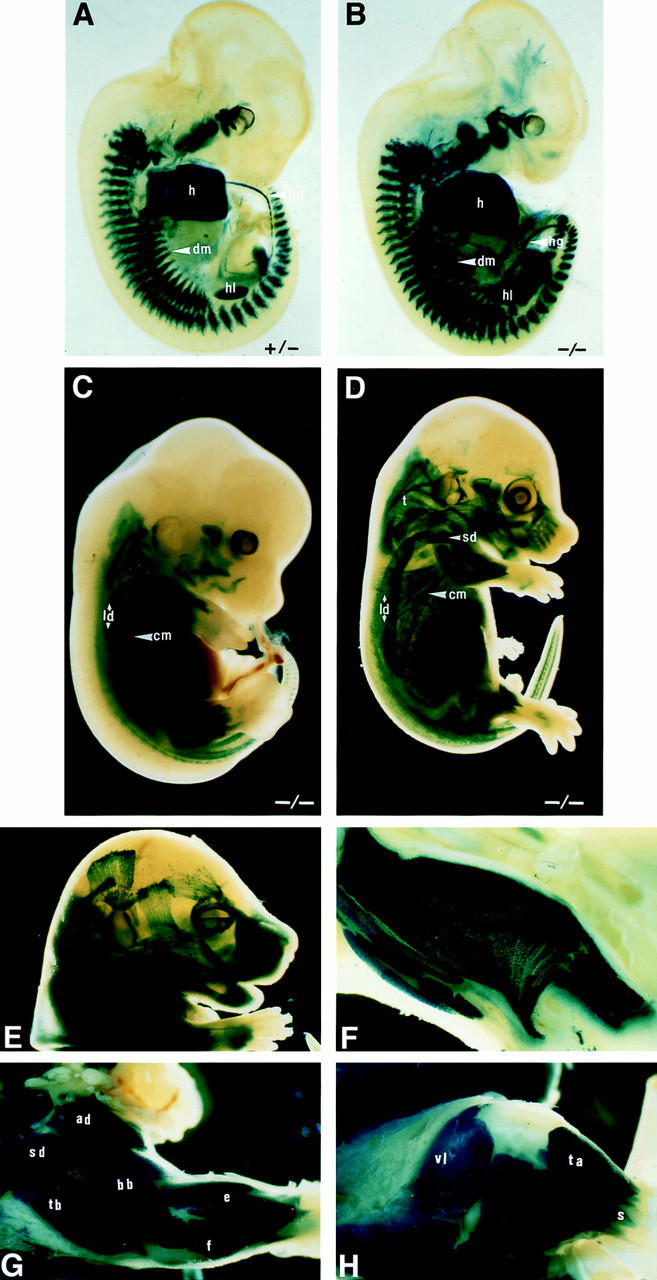
Skeletal muscle formation in mouse embryos. Myotome formation in somites of 11-d.p.c. embryos: Des −/+ (A) and Des −/− (B) embryos were stained for β-galactosidase activity. All myogenic cells appeared blue with a higher intensity in the homozygous (two LacZ copies) than in the heterozygous (one LacZ copy). We observed a normal dermomyotome ventral extension at the thoracic level. h, heart; dm, dermomyotome; hg, hindgut; hl, hindlimb. Muscle growth in 13.5–15-d.p.c embryos in the absence of desmin: Des −/− 13.5-d.p.c. (C) and 15-d.p.c. (D) embryos were stained for β-galactosidase activity. All myofibers are stained blue. We observed a normal morphogenesis and growth of the skeletal muscles. ld, latissimus dorsi; cm, cutaneous maximus; d, deltoidus; t, trapezius. Formation of head and limb musculature at 16 d.p.c. in Des −/− fetuses: β-galactosidase activity in head muscles (E), tongue (F), forelimb (G), and hindlimb musculature (H). bb, biceps brachii; tb, triceps brachii; ad, acromiodeltoidus; sd, spinodeltoidus; e, extensor; f, flexor; vl, vastus lateralis; ta, tibialis anterior; s, soleus.
Figure 2.
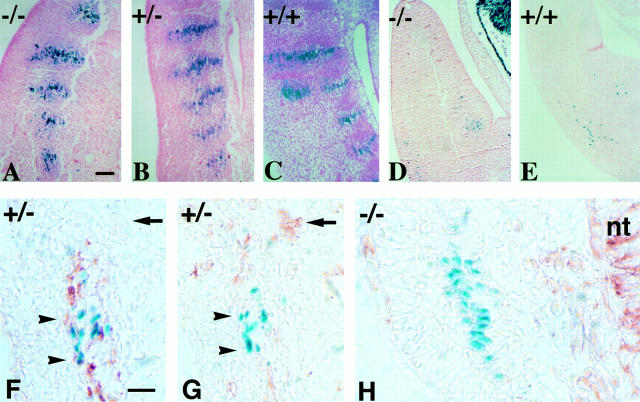
Myotome differentiation and migration of myogenic cells from somites into limbs of 11-d.p.c embryos. Sagittal sections at the somitic level of Des −/− (A), Des +/− (B), and Des +/+ (C) embryos. Myotomes have the same morphology when revealed by the blue staining. Transversal sections of the limb bud of Des −/− (D), Des +/+ (E) embryos, with the presence in both sections of blue mononucleated myogenic cells, which have migrated but not yet fused. Analysis of vimentin and desmin expression during embryonic development in myogenic cells were performed with immunoperoxidase reactions on cryostat sections on 10.5-d.p.c. embryos from Des +/− heterozygous (G), and Des −/− homozygous (H) mice. The nuclear blue staining was used to characterize the myogenic population. In the control, the desmin-positive, mononucleated cells, which are located in the lateral part of the myotome, have blue nuclei. Immunodetection of desmin stained cytoplasm in brown (F). The adjacent section shows same blue cells negative for vimentin (G). The mutant, mononucleated cells located in the lateral part of the Des −/− myotome are labeled with a blue nucleus but are negative for desmin and vimentin (H). The neural tube (nt) is stained with vimentin antibody. Bar, 50 μm.
This stage of somitogenesis is followed by the migration of myogenic progenitor cells from the somites into the limb buds at 11.5 d.p.c. in both mutant and control embryos (Fig. 2, D and E). These results demonstrate that desmin is not needed for the early stages of myogenesis, somite formation, myotome differentiation, and migration of myogenic precursor cells.
Vimentin Does Not Replace Desmin Filaments during Myotomal Differentiation
Previous studies have shown that early in development (8–9 d.p.c.) vimentin is expressed in the presumptive myotomal region and repressed once desmin is expressed. Therefore we asked the question: Would vimentin continue to be expressed after myoblast commitment and fusion to replace desmin? Analysis of vimentin expression during embryonic development in myogenic cells was performed with antibodies against vimentin on cryostat sections on 10.5-d.p.c. embryos from Des +/− heterozygous and Des −/− homozygous mice. The dermomyotome in 10.5-d.p.c. embryos is composed of at least two cell populations, one that will give rise to muscles of the trunk, and the second to epithelial cells of the skin. By morphological criteria, the myotomal cells cannot be distinguished from their neighbors. However in our mice, the nuclear blue staining was used to characterize the myogenic population. At these stages, desmin-positive mononucleated cells located in the lateral part of the Des +/− myotome are labeled with blue nuclei and brown cytoplasm (Fig. 2 F) but are negative for vimentin (Fig. 2 G). Mononucleated cells located in the lateral part of the Des −/− myotome are labeled with a blue nucleus but are negative for desmin and vimentin (Fig. 2 H). We do not find vimentin-positive cells in the dermomyotome in 10.5-d.p.c embryos, only in the neural tube. There was no increase in the amount of vimentin or in the number of positive cells in the Des −/− mice. Preliminary results indicate that in vimentin and desmin null double-mutant mice, all muscles are present and form normally (data not shown).
The Formation of Myofibers Does Not Require Desmin
Muscle histogenesis in mice is initiated by the differentiation of a primary generation of myotubes 13–16 d.p.c. After their formation, myoblasts cluster around the primary cells and use their walls as a cellular scaffold to support the formation of a secondary generation of myotubes. This step begins at 16 d.p.c. in mice. To examine the possibility of abnormalities or differences of timing in the formation of primary and secondary myotubes, we looked at both the enzymatic activity of β-galactosidase and the immunodetection of myosin, to analyze muscle morphogenesis.
The blue staining obtained with the Des −/− mice was compared to Des +/−. The shape and size of the muscles (i.e., latissimus dorsi, spinodeltoideus, trapezius, and cutaneous maximus) in the Des −/− fetuses at 13.5 (Fig. 1 C) and l5 d.p.c. (Fig. 1 D) were indistinguishable from their wild-type littermates. We found that the growth of trunk and limb muscles occurred on time; this was also true for head muscles (Fig. 1, E and F). This was evident for the biceps and triceps brachii, acromio and spinodeltoidus, extensor and flexor muscles of the foreleg (Fig. 1 G), and vastus lateralis, tibialis anterior, and soleus of the hindleg (Fig. 1 H).
Two major waves of muscle formation lead to primary and secondary generation fibers that display different MHC profiles. To determine the stage of maturation of myofibers, MHC profiles were characterized using antibodies against slow and fast MHC on frozen transversal sections of 17.5-d embryos. Fig. 3, A and B, shows the same positive reaction with the slow MHC antibody in the primary fibers of the trapezius muscles in both Des −/− and Des +/+ mice. Fig. 3, C and D, show the same positive reaction in the secondary fibers of muscles in Des −/−, compared to Des +/+ mice. Thus the absence of desmin does not prevent the fusion of myoblasts, nor the formation and further maturation of myofibers. Since we show that skeletal muscles form normally in the Des −/− mice, it is reasonable to propose that in vivo desmin is not essential for myogenic commitment, myoblast fusion, or differentiation.
Figure 3.
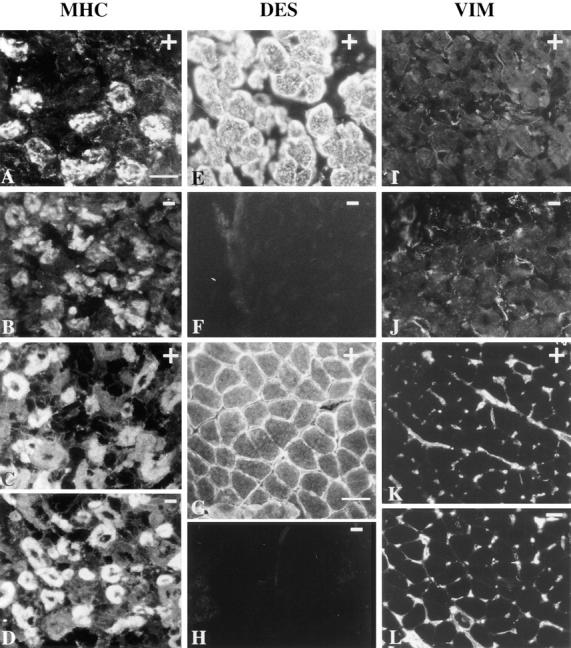
Detection of myosin isoforms, desmin and vimentin, by immunofluorescence in fetuses and in 1-mo-old mice. Mutant Des −/− (B, D, F, H, J, and L) and control Des +/+ (A, C, E, G, I, and K). (A–D) Characterization of primary and secondary generation muscle fibers at 17.5 d.p.c. Immunofluorescent staining of anterior shoulder muscles on transverse sections of the subscapularis muscle with antibody against slow MHC, which labels primary muscle fibers (A and B) and using an antibody against MHC, which labels secondary muscle fibers (C and D). The same pattern was found in Des +/+ and Des −/− fetuses. (E–H) Detection of desmin using a polyclonal antidesmin antibody showed a typical reactivity in Des +/+ in 17.5-d.p.c. fetuses (E) and 1-mo-old mice (G). No reactivity was found in Des −/− fetus (F) or 1-mo-old mice (H). (I–L) Detection of vimentin using polyclonal anti-vimentin antibody performed on 17.5-d.p.c. fetuses (I and J) and 1-mo-old mice (K and L). The same pattern was found in Des +/+ and Des −/− mice. No vimentin reactivity was found inside the myofibers. The vimentin reactivity found around the myofibers corresponded to connective tissue forming the endomysium and the mesenchymal cells of vessels. Bars: (A–F) 25 μm; (I and J) 25 μm; (G, H, K, and L) 50 μm.
At 17.5 d.p.c., myotube formation is committed in muscles of the trunk, limbs, and head. At this stage we confirm that vimentin reactivity is totally absent in multinucleated myotubes for both Des +/+ and Des −/− (Fig. 3, I and J), whereas desmin is found only in Des +/+ normal mice (Fig. 3 E compared to Fig. 3 F).
At birth, heterozygous mice showed no obvious anatomical or behavioral defects, and the homozygous mutant mice had an apparently normal external morphology. The expected Mendelian distribution of wild type (+/+), heterozygous (+/−), and homozygous mutant (−/−) was obtained, indicating that prenatal development can proceed normally without desmin, and that there is no prenatal mortality.
Having demonstrated that development occurs normally without desmin we asked the question: Would vimentin be reexpressed in adult mice? We looked for the presence of desmin and vimentin by immunofluorescence and Western blot analysis in the leg muscles of 1-mo-old mice. Western blot analysis of total extracts prepared from limb muscles confirmed that the desmin immunoreactivity detected in the wild-type was absent in Des −/− mice (not shown). Furthermore, immunocytochemistry performed on the soleus muscles using both monoclonal and polyclonal antidesmin antibodies confirmed the absence of desmin in Des −/− mice. Fig. 3 (K and L) clearly demonstrates that no vimentin reactivity was found inside the myofibers. The vimentin reactivity found around the myofibers corresponds to connective tissue forming the endomysium and to the mesenchymal derivative cells of the vessels. We conclude therefore that vimentin does not replace desmin in the muscle fibers of Des −/− mice.
Myosin Heavy Chain Isoform Switching during Postnatal Development
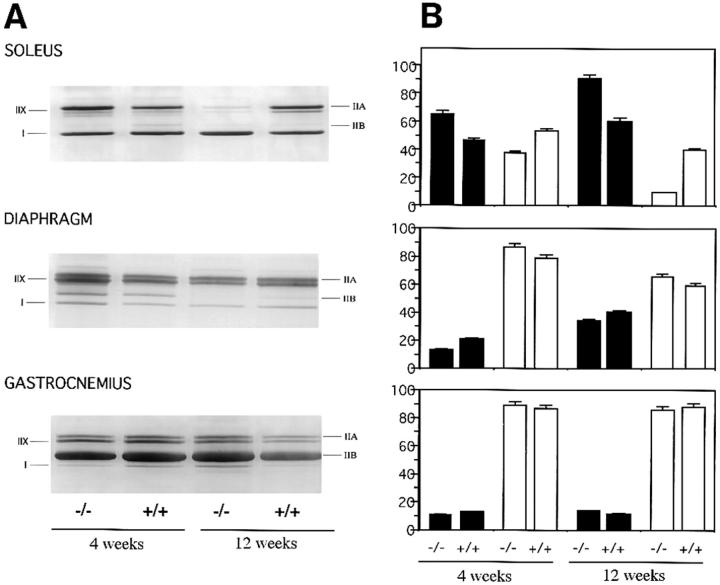
To determine the pattern of muscle fiber type formation we have analyzed the different myosin isoforms by gel electrophoresis. Experiments were carried out on 1- and 3-mo-old mice. Three muscles were chosen: the diaphragm, a continually active muscle even before birth; the soleus, a postural and weight-bearing muscle; and the gastrocnemius, a typical fast-phasic muscle. Electrophoretic separation of MHC isoforms from these muscles is shown Fig. 4 A. Four major isoforms (IIx/D, IIA, IIB, and I) were identified in these muscles. When we compared the Des +/+ and −/− mice, similar patterns of MHC were found at 1 and 3 mo. However, the yield of MHCs per mg of tissue was always less in the Des −/− mice (15–20%).
Figure 4.
MHC isoforms and ATPase activity in 4- and 12-wk-old mice. (A) Electrophoretic separation of MHC isoforms of muscles from soleus, diaphragm, and gastrocnemius. Four major isoforms (IIX/D, IIA, IIB, and I) can be identified. When we compared the desmin +/+, +/−, and −/− mice, similar patterns were found at 4 wk. However, the yield of MHC per mg of tissue was always much less in the Des −/− mice. Note the decreased amount of IIA and IIX MHC in the soleus, and IIB in the diaphragm (at 12 wk). (B) Quantification of the number of slow and fast fibers present in three muscles by ATPase activity at 4 and 12 wk. Slow fibers, such as type I (black), exhibit a high activity, whereas fast fibers, such as type II (white), display a low ATPase activity after acid preincubation. Percentage of fast and slow fibers was measured in the soleus, diaphragm, and gastrocnemius of Des +/+ and −/− mice. In the soleus of Des −/− at 12 wk, note a decrease of fast fibers (white bar) corresponding to the disappearance of the type IIA and X MHC. A relative increase in the percentage of slow fibers (black) was found.
Extracts of soleus muscles showed three bands corresponding to the fast IIA and IIx/D isoforms, and the slow-type isoform I. After three months in the soleus of Des −/− mice, there was a 25–50% decrease in the amount of IIx and IIA MHC. In the diaphragm of these mice, four bands were observed in the extracts corresponding to the three fast isoforms IIA, IIB, IIx/D, and to the slow isoform I. Their proportion differed slightly in the Des −/− mutant, in which there was a slight decrease in the amount of the IIB isoform. For the gastrocnemius muscles, the extracts from the +/+ and −/− mice contained four isoforms with a predominant synthesis of IIB present in the same amount in all three extracts. The change in the ratio of fast to slow MHC for Des −/− soleus and diaphragm could be due to degeneration and regeneration in these highly solicited muscles.
Pattern of Fiber Types in Adult Muscle Characterized by ATPase Activity
To quantify the number of slow and fast fibers present in the three muscles described above we analyzed the ATPase activity. Fast fibers (type II) displayed a low ATPase activity after acid preincubation, whereas slow fibers (type I) exhibited a high activity. The histological differences in the activity of ATPase between type I and type II fibers correspond to differences in their contractile activity. The results for the +/+ and −/− mice are presented in Fig. 4 B. In the gastrocnemius and the diaphragm, the percentage of fibers were the same in all mice: 90% fast and 10% slow in the gastrocnemius; and 86% fast and 14% slow in the diaphragm. In the soleus muscle, a significant difference was seen in the Des −/− mice at 4 wk, where the percentage of fast fibers was 40% compared to 55% in the Des +/+, and at 12 wk the difference is even more pronounced (10% compared to 45%). The decrease observed by gel electrophoresis in the soleus of Des −/− mice was correlated with a decrease in the number of MHC type II fibers.
Sarcomeres Are Formed in the Absence of Desmin
After birth, an extensive shift in myofiber architecture occurs whereby Z disks become aligned, nuclei move from a central to a peripheral location, and tubules adopt a transverse orientation. The preexisting IF networks shift from a longitudinal to a predominantly transverse orientation, and align along the I-Z-I regions. To determine if the absence of desmin influences the organization of individual myofibrillar proteins within the sarcomere, ultrathin sections from different muscles (soleus, diaphragm, gastrocnemius) were stained with antibodies against actin, α-actinin, titin, and desmin. The results (presented in Fig. 5 A) demonstrate the typical regular striated pattern obtained on the soleus muscle from a 2-mo-old Des −/− mutant obtained with the antibodies against actin, α-actinin, and titin. This confirmed that sarcomeric organization occurs normally in the absence of desmin.
Figure 5.
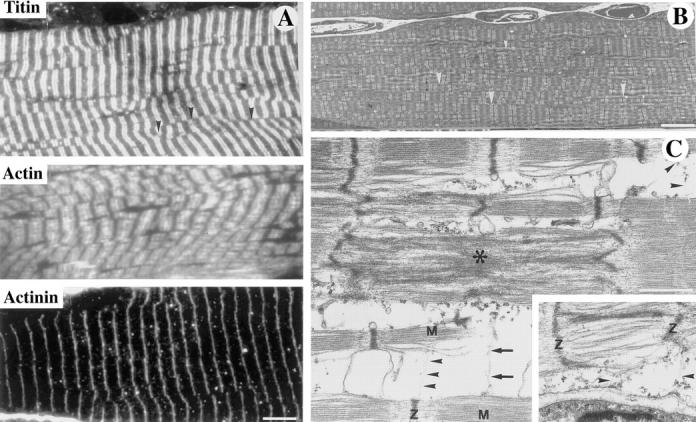
Ultrastructural and immunological characterization of sarcomeres in soleus muscle of Des −/− mice. (A and B) Region of soleus of 8-wk-old Des−/− mice showing sarcomere alignment that is relatively normal or with splitting of the myofibril. (C) In region of soleus of 2-wk-old Des −/− mice with focal alterations. (A) Ultrathin sections stained with antibodies against titin, actin, or α-actinin demonstrate the typical regular striated pattern. However, certain irregularities were observed in the organization of the myofibers that were more easily visualized in the electron microscope. (B) A splitting of the myofibrils can be seen (arrowheads). This splitting is also clearly demonstrated in the ultrathin sections stained with the antibody against titin in A where it can be seen that the Z bands are frequently not in register (arrowheads). (C) Ultrastructure of myofibrillar alterations in the soleus muscle as demonstrated by transmission electron microscopy. On longitudinal sections, filamentous material (arrowheads, top right and bottom center) interlinks the Z disks of one myofibril to the M band region of another myofibril. Another link of filamentous material (arrows) is seen between the M band region of the lower myofibril and the center of two sarcomeres that show Z disk streaming (*). Inset, filamentous material (arrowheads) form myofibril–sarcolemma attachments between the Z disk of a myofibril and dense plaques at the sarcolemma. M, M-line; Z, Z-disk. Bars: (A and B) 5 μm; (C) 1 μm.
However, certain irregularities were observed in the organization of the myofibers in the soleus in the Des −/− mice, such as lack of alignment of myofibrils and insertion of extra sarcomeres giving rise to s.c. nonius periods. Similar findings were observed in the electron microscope (Fig. 5 B). In this figure it can be seen that there is disorganization and splitting of the myofibrils. This splitting is clearly demonstrated in the ultrathin sections stained with the antibody against titin where it can be seen that the Z bands are sometimes not in register.
At higher magnification, examination of the ultrastructure of the soleus shows areas with abnormal sarcomeres with no clear demarcation of I and A bands, as well as disintegrated myofibers with sparse filaments (Fig. 5 C). In longitudinally sectioned myofibers, links between myofibrils in Des −/− mice were observed. These links ran from one myofibril at the M band level to one M band or to the Z disk regions of another myofibril. Links of filamentous material were also observed between myofibrils at the Z and/or M bands levels to small periodic densities at the sarcolemma. This proves that proteins other than desmin form interconnecting links between myofibrils and between myofibrils and the sarcolemma.
However, myofibrillar disorganization is not a prominent finding typical of Des −/− mice and when it occurs it might be a secondary phenomenon due to an abnormal muscle regeneration in the absence of desmin.
Lack of Desmin Results in Focal Degeneration and Regeneration in Highly Solicited Skeletal Muscles
Using the electron microscope, we examined more closely the ultrastructure of the soleus, the weight-bearing muscle, from newborn to 10-wk-old mutant mice. From 2 wk and onwards, Des −/− mice show unquestionable signs of muscle fiber death (Fig. 6). Focal areas with the size of a muscle fiber diameter showed macrophage accumulation and presence of cells having characteristics of activated satellite cells, i.e., light cytoplasm with many ribosomes, nascent myofilaments, and large, centrally placed nuclei. Remnants of a surrounding basement membrane were often seen. In other areas in muscles of the same age profiles concurrent with fiber splitting were observed. In longitudinal sections, muscle fibers having a light cytoplasm contained some organized myofibrils, but mainly disorganized myofibrils were observed in all groups after 2 wk of age.
Figure 6.
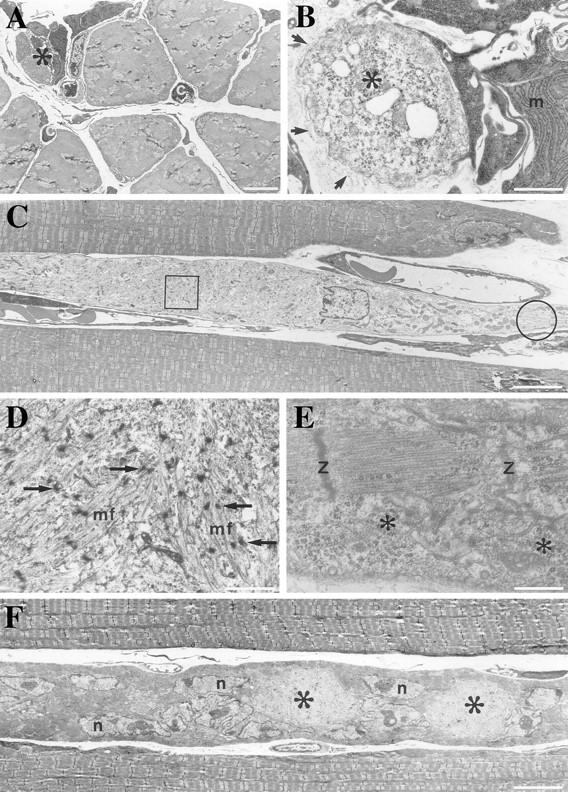
Transmission electron microscopy of myofibers of soleus from Des −/− 2-wk-old mouse. (A) Cross section of 2-wk soleus showing an area of a normal dense myofibrillar pattern and an area containing several small-size cells. c, capillaries. (B) In higher magnification, the small-size cells are identified as macrophages (m) with well-organized rough endoplasmic reticulum. Activated satellite cells having light cytoplasm with dispersed ribosomes (*). All these cells are enclosed by the same basement membrane (arrows). (C) Longitudinal sections of muscle fiber, one with light cytoplasm, runs in parallel with two other well-organized myofibrils. (D) Higher magnification view of the boxed area in C, showing disorganized myofibrils (mf) and the Z bodies (arrows). (E) Higher magnification view of the encircled area in C, showing an organized sarcomere with Z disks and abundant ribosomes (*). (F) Muscle fiber with areas of light cytoplasm (*) and many large nuclei (n) containing prominent nucleoli run parallel to muscle fibers with well-organized myofibrils. Bars: (A, C, and E) 5 μm; (B and D) 1 μm; (E) 0.5 μm.
By 10 wk, a large variability in fiber diameter was observed (Fig. 7 A). Many muscle fibers showed prominent subsarcolemmal accumulations of mitochondria, and extensions and undulations of the sarcolemma (Fig. 7, A and B). Although high concentrations of desmin have been reported to be present at the myotendinous junction (Tidball, 1992), no obvious structural defects were found at the myotendinous junctions of the mutant (data not shown). Empty profiles of basement membranes, some containing interstitial cells, were present in areas of small-sized fibers, which often showed myofibrillar disorganization (Fig. 7, C–E). Thus a typical morphology for muscular dystrophies: degeneration, regeneration, and fibrosis were observed.
Figure 7.
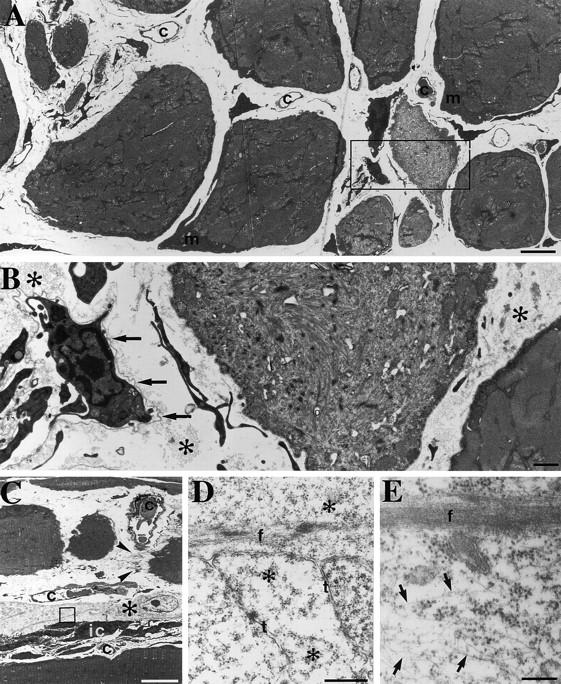
Degeneration and regeneration of soleus from Des −/− 10-wk-old mouse. (A) Transmission electron microscopy of a cross section showing the large variability in fiber diameters seen at 10 wk in the Des −/− soleus. The myofibrillar pattern is well preserved in the largest muscle fibers although abnormal accumulation of mitochondria (m) are present beneath the sarcolemma. Myofibrils are disorganized in some of the small- or intermediate-size fibers. Note that clusters of small fibers occupy the space of a large fiber. The wide interstitial space contains capillaries and cells with slender profiles. (B) Higher magnification of the boxed area in A showing one muscle fiber disorganized myofibrils. An undulating basement membrane (arrows) surrounds interstitial cells with slender processes. Note also the bundles of collagen fibrils (*) in the interstitium. (C) Some muscle fibers show well-organized myofibrils; however, one is divided into three parts, one of which is interrupted by a tendinous junction (arrowheads) after faulty regeneration after fiber damage. Profiles of interstitial cells with slender processes and one cell with a light cytoplasm (*) are seen. (D and E) Higher magnification view of the boxed area in C. In D, strands of myofibrillar material as well as tubules (t) and ribosomes (*) are seen, whereas in E, an array of cytoplasmic filaments are seen beside a myofilamentous strand. Bars: (A and B) 1 μm; (C) 10 μm; (D) 0.5 μm; (E) 0.25 μm.
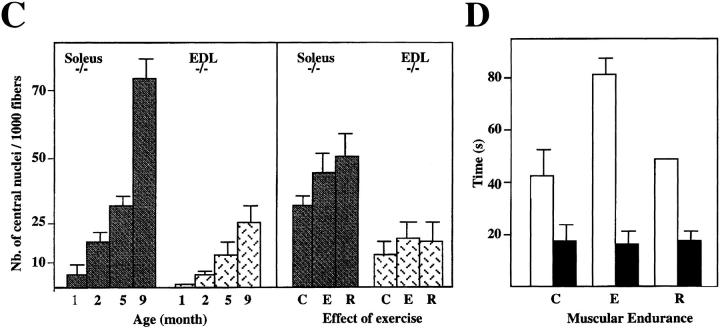
The number of central nuclei in the soleus and extensor digitorum longus (EDL) of Des −/− mice increased with the age: for soleus, 10 central nuclei for 103 fibers for 1-mo-old mice; and 75 central nuclei for 103 fibers for 9-mo-old mice. For EDL the number increased from 2 to 30 central nuclei for 103 fibers (Fig. 8 C), confirming the presence of continual regeneration in Des −/− muscles.
Figure 8.
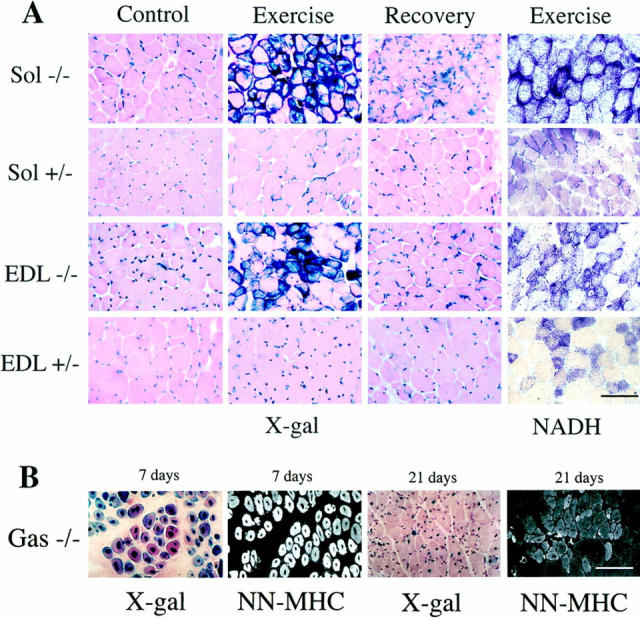
Effect of exercise on muscles. (A) LacZ and NADH activity present on frozen sections of soleus and EDL from 5-mo-old Des +/− and Des −/− mice. Control, non-exercised mice; Exercise, 5 d of exercise. Recovery, exercise plus 5 d recovery. Note the strong expression of β-galactosidase (X-gal), NADH in the soleus (Sol) and EDL of exercise mice. (B) Frozen sections of the gastrocnemius muscles of Des−/− mice (Gas −/−) after 7 and 21 d of regeneration after injection of cardiotoxin. Sections were stained either for β-galactosidase activity (X-gal) or neonatal MHC expression (NN-MHC). (C) Number of central nucleated fibers presented in the soleus and EDL in mice of different ages (1–9 mo) and in the same mice as in A. C, non- exercised mice; E, 5 d of exercise; R, exercise plus 5 d recovery. Note the increase in the number of the central nuclei in different muscles both with age and exercise. (D) Muscular endurance after exercises was analyzed on same group of mice. Des +/+ (white) and Des −/− (black). After 5 d of training, muscular endurance increased in the control group. This was transitory since when the animals were allowed to recuperate for 5 d, muscular endurance returned to the same value as before training. In contrast, in Des −/− mice there was no increase of endurance with exercise. Bars: (A and B) 100 μm.
Muscle Regeneration Induced by Cardiotoxin Injection and Effect of Exercise
Postnatal growth of skeletal muscle fibers is accompanied by the proliferation of muscle satellite cells. Some of these cells remain as undifferentiated myogenic stem cells located between the basal lamina and the plasma membrane of the muscle fibers. After muscle injury, satellite cells proliferate and fuse to form new muscle fibers that express transiently developmental myosin isoforms. To determine whether in the absence of desmin the satellite cells are able to participate to the regeneration process, two types of experiments were performed: (a) injection of myotoxic drug; and (b) intensive exercise.
After injection of cardiotoxin, there was necrosis and degeneration of the muscle fibers. This was followed by a proliferation of the satellite cells, which then fused to form new muscle fibers. Fig. 8 B shows central nuclei with β-galactosidase staining and detection of neonatal MHC by immunofluorescence. At 7 d, the myotubes predominantly express the neonatal MHC. By 21 d, these fibers have increased in size and the neonatal MHC has almost been eliminated to be replaced by the adult myosin isoforms. These results confirm that after injury, skeletal muscle can regenerate successfully in the Des −/− mice.
In the second type of experiment, the Des +/+, Des +/−, and Des −/− mice were submitted to intensive exercise for 5 d, and then animals were divided into two groups. One group was examined after exercise; the other was examined after a subsequent 5-d rest period to permit the recuperation. The following parameters were determined on the soleus and EDL muscles on 10 sections originating from four different animals in each experimental group: NADH activity, X-Gal staining, and the number of central nuclei.
Before exercise, muscle nuclei can be recognized by X-Gal blue staining in peripheral nuclei. After 5 d of exercise, intense X-Gal staining was observed in a subpopulation of fibers, in both the soleus and EDL of Des −/− mice (Fig. 8 A). In the group that had been allowed to recuperate for 5 d after exercise, the intensity and location of the blue staining returned as before exercise. After 5 d of exercise, the number of central nuclei stained with hematoxylin increased slightly in soleus (35–45 for 103 fibers; Fig. 8 C).
To analyze the mitochondrial activity, staining for NADH was performed. After 5 d of exercise, NADH staining was found to be increased in Des −/− EDL and soleus. Large accumulations at the periphery of the fibers was characteristic of soleus fibers of Des −/− mice.
Muscular endurance was measured on the same groups of animals described above. As seen in Fig. 8 D, after 5 d of training, muscular endurance was increased in the control group. However this was only transitory, since when the animals were allowed to recuperate for 5 d, muscular endurance had returned to the same value as before training. In contrast, in Des −/− mice, there was no increase of endurance with exercise.
Mice Without Desmin Show Weakness, and Have No Resistance to Endurance
Muscular performance, endurance and motor coordination were determined on 5-mo-old mice weighing 25 g. Force developed by isolated soleus muscles from 2- and 5-mo-old mice was measured.
Maximum Force.
The first analysis measured the maximum force that could be developed by the Des −/−, Des +/−, and Des +/+ mice (Fig. 9 A). The animals were allowed to catch a bar with either all four limbs or with the two forelimbs. The mice were then pulled out by the tail until they released the bar. The maximum force was significantly reduced by a factor of two in the homozygous Des −/− mice, compared to the wild-type mice (14 mN ± 1 compared to 24 mN ± 1.5 for four limbs, and 4.2 mN ± 0.7 compared to 10). The Fisher test gave a value of P < 0.01 for the four-limb experiment and P < 0.05 for the two-limb experiment. There was no significant difference for the maximum force generated between the heterozygous Des +/− mice and the wild-type mice.
Figure 9.
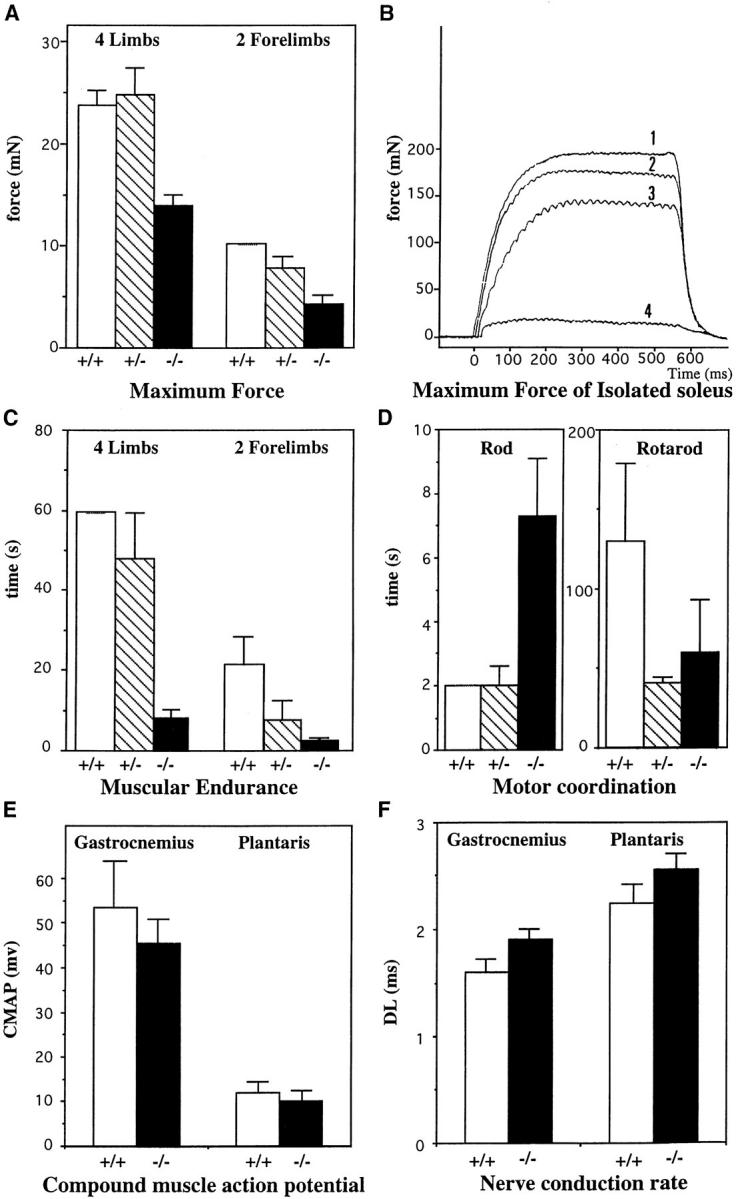
Analyses of muscular performances on control animal Des +/+ (white), heterozygous Des +/− (hatched) and homozygous Des −/− (black). (A) The maximum force developed by the mice was measured by pulling the mouse backwards by the tail until the bar was released. Animals were allowed to hold the bar linked to a dynamometer either with all four limbs or with the two forelimbs. Results are expressed in mN. (B) The maximum force developed by isolated muscle was measured on soleus from 2- (curve 1: Des +/+; curve 3: Des−/−) and 5-mo-old mice (curve 2: Des +/+; and curve 4: Des −/−). (C) The muscular endurance was analyzed by measuring the time that animals could hold onto a 32-g bar, either with all four limbs (4L) or with the two forelimbs (2L). Results are expressed in s. (D) Motor coordination: performances were measured either by measuring the time that mice need to cross a rod or by putting the mice on a rotating apparatus. Capacity of Des −/− mice are considerably modified. (E) CMAP measured in gastrocnemius and plantaris muscles from 5-mo-old mice. Results are expressed in mV. (F) Nerve conduction rate measured in gastrocnemius and plantaris muscles from 5-mo-old mice. The DL is given in ms. Data are the means ± standard errors computed from each set of experiments.
Force of Isolated Soleus.
The force was analyzed on isolated soleus muscles of 2- and 5-mo-old Des −/− mutant and control mice. The Des −/− and Des +/+ groups had been selected to display no significant differences in body weight.
Isolated soleus muscles were maintained at 25°C in a chamber containing Ringer's solution, with one extremity fixed to a force tranducer, and one to an electromagnetic ergometer. Fig. 9 B shows that the maximum force generated during tetanic stimulation was reduced by 25% for the 2-mo-old Des −/− compared to the wild-type. The soleus of the 5-mo-old mutant mice lost almost all the force. By 5 mo, the force had been reduced by 90% in the Des −/−, compared to age-matched controls.
Muscular Endurance.
The muscular endurance was defined by the ability of an animal to catch a bar and hold onto a 32-g weight, and was measured in seconds. These results are presented in Fig. 9 C. All the wild-type mice were able to hold onto the bar with four limbs for at least 60 s. By contrast, the mice lacking desmin showed a severe eightfold decrease in their resistance to endurance, independently of whether they caught the bar with two limbs (2.3 ± 0.4 s, compared to 21.5 ± 6 s) or four limbs (7.7 ± 2 s, compared to 60 s). The Fisher test gave a significance value of P < 0.001.
A significant sixfold reduction in the endurance of the homozygous Des −/− mice, compared to the heterozygous Des +/− mice was also demonstrated for endurance using the four limbs (7.7 ± 2 s, compared to 48.3 ± 11 s). The Fisher test gave a significance value of P < 0.001. It was observed that heterozygous Des +/− mice also have a threefold decreased endurance (7.7 ± 4.7 s, compared to the control mice 21.5 ± 6.5 s) when endurance was measured on the two forelimbs (P < 0.05).
Motor Coordination.
Two methods were used to measure motor coordination. The first method concerns the time that mice stay on a rotating rod and the second method the time the mice needed to cross a suspended rod. Analyses were performed with two groups of 12 5-mo-old animals, weighing 25 g. Performances measured either by putting the mice on a rotating apparatus or measuring the time that mice need to cross a rod shows that the capacity of the Des −/− mice are considerably reduced (Fig. 9 D). On the rotating apparatus the mutant could stay balanced on the rod only half the time that the control (60 s, compared to 130 s). The mutant mice took 7 s to cross the rod; this is more than three times greater than the time for the control mice who crossed the rod in 2 s.
Electrophysiological Recording
CMAP and DL.
Analyses were performed with two groups of Des −/− and Des +/+ mice. The right sciatic nerve of anesthetized mice was stimulated at paraspinal sites. The CMAP were recorded from the medial part of the gastrocnemius and plantaris muscles. Fig. 9, E and F, shows the results obtained for CMAP and for the DL measured in parallel in gastrocnemius and plantaris muscles in both Des −/− or Des +/+. Slight differences were recorded, and these included the duration of motor nerve conduction and the synaptic conduction. The electromyography recordings showed low values of CMAP in gastrocnemius muscles in Des −/− when compared to values of Des +/+, (45 and 53 mV, respectively). The DL increased, suggesting a decrease of the motor nerve conduction velocity (i.e., for gastrocnemius 1.2 ms compared to 0.8 ms at the basal level, and 2 ms compared to 1.5 ms at the upper level). These results could reflect a subtle modification of the neuromuscular junction in muscles of Des −/− mice.
Spontaneous Fibrillation Potentials (SFP).
The presence of SFP is indicative of a denervation of muscle fibers. The potentials were recorded with a needle inserted in several sites of the gastrocnemius muscle. They were taken into account when their amplitude varied between 20 and 300 mV. In the present study no differences in SFP were found with both Des −/− and Des +/+ mice.
DISCUSSION
Desmin is one of the first muscle proteins to be expressed during embryogenesis of the somites and during limb formation. It is found in the mononucleate myoblasts as well as in the differentiated myotubes of mature muscle. It has been suggested that desmin may play critical roles at different steps of myogenesis: during myogenic commitment and differentiation; for myoblast fusion and myotube formation; and to link Z disks together and to the membrane. To determine the functions of desmin in vivo, we have constructed mice lacking desmin. To disrupt the desmin gene, E. coli LacZ gene was inserted in frame into the first exon of the desmin gene. This construct deletes the expression of desmin in cardiac, smooth, and skeletal muscles. In the present study, we have taken advantage of the inserted LacZ gene that allows, after enzymohistochemical processing, a visualization of the muscle tissue. We then analyzed the essential steps involved in muscle formation.
Is Myoblast Commitment, Fusion, and Myotube Formation Modified in the Absence of Desmin?
Muscle formation is a progressive incremental process initiated by the myoblast commitment, differentiation, and fusion to form first primary, and then secondary myotubes. As this occurs, new generations of myoblasts cluster around the primary cells and use their walls as a cellular scaffold to support the formation of secondary myotubes. We found that during embryogenesis, somites and myotomes form normally and mononucleate muscle precursor cells migrate in the absence of desmin. It cannot be excluded that vimentin (present in the earliest stages of development [8–9 d.p.c.]) in presumptive myotomes can partially rescue the desmin null mouse for somitogenesis. However generation of vimentin null mice shows that normal myogenesis occurs without vimentin (Colucci-Guyon et al., 1994). Preliminary results indicate that vimentin and desmin null double-mutant mice are viable and all muscles are present, and apparently normal (Colucci-Guyon, E., personal communication).
After migration, the myoblasts divide to form first primary, and then secondary generation myotubes. This phase of development occurs in an identical manner in Des −/−, Des −/+, and Des +/+ mice demonstrating that desmin is not essential for any of these events. We can thus conclude that desmin is not essential either for the proliferation or for the commitment of these early myoblasts to the myogenic lineage or for the migration, fusion, and subsequent organization of the muscle fiber. Previous reports about the disruption of the intermediate filament network via introduction of mutated desmin in cultured cells (Schultheiss et al., 1991) are in agreement with our conclusion. But these in vivo results contradict in vitro data obtained by the group of Capetanaki, which showed that both myogenic differentiation and myoblast fusion were inhibited in ES cells lacking desmin (Weitzer et al., 1995). Moreover, satellite cell cultures made from Des −/− mice were able to fuse and express MHC (data not shown), reinforcing the fact that desmin is not essential for myoblast commitment and differentiation.
It is interesting to note that there is no lethality with this mutation and the mice appear normal at birth. The lifespan of the mutant is reduced and the oldest Des −/− mice have 1 yr, whereas control mice had a 2-yr lifespan. We noticed that the adult mice are often a little smaller than the control littermate. The reason for this difference in size has not yet been determined.
Is Pattern of Fiber Types Modified in Des −/− Muscle?
After birth the first discernable effects of the lack of desmin were seen on the muscle fiber phenotype. These effects were mainly observed in the soleus and diaphragm muscles where modifications were accompanied by morphological and biochemical changes. These included variability in fiber diameter, the presence of central nuclei, crescent-shaped sarcolemnal masses, and decrease in the amount of type II MHC. Signs of degeneration with fibrosis, macrophage infiltration, and necrosis appear early in the same muscles. It is interesting to note that these effects were most prominent in the diaphragm and the soleus, which have a different morphogenetic pattern when compared to limb muscle (like the EDL). In the anlage of the diaphragm and soleus muscles, primary myotubes are juxtaposed to one another through the entire length and their membranes are extensively coupled by gap junctions; in the anlage of the EDL by contrast, primary myotubes are coupled only at the myotendinous junctions, but are detached and surrounded by mononucleated cells in the belly of the muscle (Kelly and Rubinstein, 1994).
Are the Myofibrillar Proteins Correctly Assembled in the Absence of Desmin?
Skeletal muscle is characterized by the precise organization of the contractile proteins into striated myofibrils resulting from repeating units (the sarcomeres) arranged in series. The sarcomere is formed from an interdigitation of thick myosin and thin actin filaments attached to the Z disk to form a repetitive and elastic structure. The Z disk shows fiber type-specific variation in structure, detectable by electron microscopy, and a decrease in thickness from slow type I to fast type II fibers (Gauthier, 1979; Eisenberg, 1983). Immunohistochemical analysis of ultrathin sections with different antibodies showed that in Des −/− muscles, the sarcomeres and their components, even in the absence of desmin, were normally associated. Thus desmin does not play a critical role in the assembly of myofilaments into sarcomeres and myofibrils. These results are in agreement with those obtained by microinjection of truncated desmin in fertilized Xenopus laevis eggs showing that absence of desmin network does not influence the myofibril assembly (Cary and Klymkowsky, 1995).
Is the Organization of Myofibrils in Register Dependent on the Transverse Desmin Distribution?
Desmin has been postulated to play a critical role in forming the peripheral domain of expanding Z bands, connecting successive Z bands, as well as laterally linking Z bands of adjacent myofibrils to one another and to the sarcolemma. We observed in both the light and electron microscopes that the Z lines were sometimes misaligned and split. This would suggest that when desmin is no longer present to hold the Z disks together and to the membrane, the sarcomere becomes disorganized and eventually disintegrates.
The myofibrillar proteins are all components of the sarcomere and are linked together by a perimyofibrillar cytoskeleton. This cytoskeleton is mainly composed of desmin, but also contains other filamentous proteins such as skelemin, filamin, and synemin (Granger and Lazarides, 1980; Brown and Binder, 1992; Price and Gomer, 1993; Becker et al., 1995). Interestingly, despite the lack of desmin we are able to show interlinking filaments between myofibrils and the sarcolemma, these filaments probably correspond to an additional cytoskeletal network.
Is Desmin Required for Successful Regeneration?
When muscle damage was induced by injection of cardiotoxin into the muscles of Des −/− mice, the process that we observed was very similar to that described in the literature. Satellite cells were activated, proliferated, and then fused to form new myotubes within the existing basal lamina. Therefore the absence of desmin during development seemed to have no effect on these initial events of muscle regeneration.
Focal areas of muscle degeneration and regeneration do, however, occur as early as 5 d after birth in the skeletal muscle of the Des −/− mice. The progressive muscle pathology observed in these Des −/− mice was very similar to that seen in human muscular dystrophies. Degeneration of muscle fibers, followed by regeneration involving satellite cell activation and formation of new fibers and fibrosis were seen. The frequent observation of faulty myofibrillogenesis in regenerating myotubes and fibers from 11 d postnatally up to 12 wk, indicates that the desmin filaments might be needed to obtain a proper myofibrillar assembly during regeneration, whereas they are no longer needed when the muscle forms. This may be due to the additional mechanical stress applied to these muscles after birth as they regenerate, which would not occur in utero.
Are Mechanical Properties Modified in Desmin-defective Muscles?
Des −/− mice were less strong and became fatigued more quickly than either the heterozygous or control littermates. Histological and physiological analyses demonstrate that in the Des −/− mice (with aging or after induced exercises), sarcomeres are stretched and fibers become damaged. Recently, in vitro data has predicted that intermediate filaments may also elongate locally if sarcomeres lose the ability to generate and transmit active force. In such a situation, desmin may serve as a bypass mechanism to transmit tension to prevent a breakdown of force transmission between adjacent sarcomeres (Wang et al., 1993). The desmin filaments of the network that connect parallel myofibrils transversely have been implicated in transmitting tension in the radial direction of the muscle. Measurements of force on isolated soleus muscle differ largely between wild-type and Des −/− mice. At 2 mo, the force of the soleus of the mutant is significantly lower than in the control mice. At 5 mo, the soleus of the mutant mice are unable to respond to the stimulation and generate very little force. It is interesting to note that even though there is an increase in the number of slow fibers and an increase in oxidative enzyme activity, these muscles are able to generate little force and fatigue very quickly suggesting that there is a defect in mitochondrial activity. This is confirmed by large accumulations of mitochondria, which are seen both with NADH staining and ultrastructurally. This could suggest that desmin filaments may be involved in maintaining the mitochondria in position within the muscle fibers.
What Is the Role of Desmin in Skeletal Muscle?
In conclusion, if desmin is not required for myoblast commitment, fusion, and myofibrillar assembly before birth, desmin is essential to maintain the integrity of myofibrils upon stress. The lack of desmin thus disturbs the cytoskeletal network also composed of dystrophin, adhalin, and merosin interlinking the extra cellular matrix with the interior of the muscle fiber. Loss of any of the proteins gives rise to a muscular dystrophy with degeneration, regeneration, and fibrosis. However, only highly solicited muscles such as soleus (a weight-bearing muscle) or diaphragm and tongue (both very active muscles) show pronounced structural defects. Thus the lack of desmin IF linkage between the myofibrils and the sarcolemma seems to be crucial to maintain muscle integrity, and its absence in Des −/− mice leads to disruption of the muscle fibers. This was confirmed by the continual increase in the number of regenerated central nucleated fibers with age observed in the Des −/− mice. It is interesting to note that a similar fragility is seen in mdx (x-linked muscular dystrophy) mice where the absence of dystrophin disturbs the mechanical stability of the plasma membrane. Limbs of dystrophic mice subjected to eccentric contractions experience more fiber necrosis than the contralateral limbs subjected to an equal number of concentric (nonlengthening) contractions (Weller et al., 1990). From our results it is clear that desmin is not only required to maintain structural integrity in mature muscles but is also important for muscle regeneration; this was demonstrated by the aberrant myogenesis observed in Des −/− mice during regeneration. Our results support the hypothesis made originally by Lazarides (1980) that the function of desmin is to distribute the intracellular space to link the Z disks together and to the membrane, and is important to maintain structural integrity of the muscles. In addition rheological studies of Janmey et al. (1991) show that type III IFs play a role as mechanical integrators of space and resist breakage under stresses where other cytoskeletal networks rupture.
The defect provoked by the lack of desmin (i.e., increase in cell fragility and degeneration of muscle fibers) is very similar to what has been described for keratin in epithelial tissue during mechanical stress where a lack of keratin results in a loss in the integrity of the skin (Fuchs, 1994; Fuchs and Weber, 1994; McLean et al., 1994, 1995; McLean and Lane, 1995; Porter et al., 1996) or in liver (Baribault et al., 1993). Epidermolysis bullosa simplex (Bonifas et al., 1991; Coulombe et al., 1991; Lane et al., 1992; Chan et al., 1994), epidermolytic hyperkeratosis (Chipev et al., 1992), and epidermolytic palmoplantar keratoderma (Reis et al., 1994) were the first group of human diseases discovered to be IF disorders and characterized by mechanically induced skin blistering caused by cytolysis within the basal layer of the epidermis.
A similar degenerative process has been observed for astrocytes in the cerebellum of vimentin-deficient mice (Galou et al., 1996), whereas these mice develop and reproduce (Colucci-Guyon et al., 1994) as well as glial fibrillary acidic protein (GFAP)-deficient mice (Gomi et al., 1995; Pekny et al., 1995). However, in older GFAP-deficient mice defects were detected in the central nervous system, such as white matter loss and impairment of blood–brain barrier (Lieddtke at al., 1996). Another class of candidate for IF disorders are those that might involve neurofilament (NF) genes. Neuronal abnormalities, including massive accumulation of neurofilaments in cell bodies and proximal axons, have been described in amyotrophic lateral sclerosis. Similar morphological changes have also been reported in transgenic mice that overexpressed either wild-type or mutant NFs. Thus primary changes in the NF intermediate filament proteins also trigger a neurodegenerative process (Lee and Cleveland, 1994; Collard et al., 1995).
At present, no disease has been found to be directly related to a lack of desmin in humans. However, several myopathies have been described where the desmin filaments are disrupted (Thornell et al., 1980, 1983; Pellissier et al., 1989; Goebel and Borneman, 1993; Ariza et al., 1995; Goebel and Fardeau, 1996; Vicart et al., 1996). Hence although human studies have not yet been conclusive, it seems possible that a myopathy will be found that results from a desmin mutation.
Acknowledgments
We would like to thank V. Small for both helpful advice and the gift of antibodies to analyze our ultrathin sections; F. Goubel and M. Almeida-Silveira for measures on isolated muscles; M. Titeux for help in quantification of the fiber types. We gratefully acknowledge stimulating discussions with D. Daegelen and S. Tajbakhsh.
Abbreviations used in this paper
- CMAP
compound muscular action potentials
- d.p.c.
days postcoitum
- DL
distal latency
- EDL
extensor digitorum longus
- IF
intermediate filaments
- MHC
myosin heavy chain
- NF
neurofilament
Footnotes
Address all correspondence to Denise Paulin, SCME, Institut Pasteur, 25 Rue du Dr Roux, 75015 Paris, France. Tel.: 33-1-45-68-84-93. Fax: 33-1-45-68-86-81. e-mail: dpaulin@pasteur.fr
This work was financed by the Association Française contre les Myopathies, Fondation de France, the Université Paris 7, the CNRS and grants from the Swedish Medical Research Council (12x-3934) and Umëa University (to L.-E. Thornell).
REFERENCES
- Agbulut O, Li Z, Mouly V, Butler-Browne GS. Analysis of skeletal and cardiac muscle from desmin knock-out and normal mice by high resolution separation of MHC isoforms. Biol Cell. 1997;88:131–135. [PubMed] [Google Scholar]
- Allen RE, Rankin LL, Greene EA, Boxhorn LK, Johnson SE, Taylor RG, Pierce PA. Desmin is present in proliferating rat muscle satellite cells but not in bovine muscle satellite cells. J Cell Physiol. 1991;149:525–535. doi: 10.1002/jcp.1041490323. [DOI] [PubMed] [Google Scholar]
- Ariza A, Coll J, Fernandez-Figueras MT, Lopez MD, Mate JL, Garcia O, Fernandez-Vasalo A, Navas-Palacios JJ. Desmin myopathy: a multisystem disorder involving skeletal, cardiac, and smooth muscle. J Hum Pathol. 1995;26:1032–1037. doi: 10.1016/0046-8177(95)90095-0. [DOI] [PubMed] [Google Scholar]
- Babai F, Musevi-Aghdam J, Schurch W, Royal A, Gabbiani G. Coexpression of α-sarcomeric actin, α-smooth muscle actin and desmin during myogenesis in rat and mouse embryos. I. Skeletal muscle. Differentiation. 1990;44:132–142. doi: 10.1111/j.1432-0436.1990.tb00546.x. [DOI] [PubMed] [Google Scholar]
- Baribault H, Price J, Miyai K, Oshima RG. Mid-gestational lethality in mice lacking keratin 8. Genes Dev. 1993;7:1191–1202. doi: 10.1101/gad.7.7a.1191. [DOI] [PubMed] [Google Scholar]
- Becker B, Berllin RM, Sernett SW, Huiatt TW, Robson RM. Synemin contains the rod domain of intermediate filaments. Biochem Biophys Res Commun. 1995;213:796–802. doi: 10.1006/bbrc.1995.2200. [DOI] [PubMed] [Google Scholar]
- Bonifas JM, Rothman AL, Epstein EH. Epidermolysis bullosa simplex: evidence in two families for keratin gene abnormalities. Science (Wash DC) 1991;254:1202–1205. doi: 10.1126/science.1720261. [DOI] [PubMed] [Google Scholar]
- Brooke MH, Kaisser KK. Muscle fiber types: how many and what kind? . Arch Neurol. 1970;23:369–372. doi: 10.1001/archneur.1970.00480280083010. [DOI] [PubMed] [Google Scholar]
- Brown KD, Binder LI. Identification of the intermediate filament-associated protein gyronemin as filamin. J Cell Sci. 1992;102:19–30. doi: 10.1242/jcs.102.1.19. [DOI] [PubMed] [Google Scholar]
- Cary RB, Klymkowsky MW. Disruption of intermediate filament organization leads to structural defects at the intersomite junction in Xenopusmyotomal muscle. Development (Camb) 1995;121:1041–1052. doi: 10.1242/dev.121.4.1041. [DOI] [PubMed] [Google Scholar]
- Chan YM, Yu QC, Christiano A, Uitto J, Kucherlapati RS, LeBlanc J, Straceski, Fuchs E. Mutations in the non-helical linker segment L1-2 of keratin 5 in patients with Weber-Cockayne epidermolysis bullosa simplex. J Cell Sci. 1994;107:765–774. doi: 10.1242/jcs.107.4.765. [DOI] [PubMed] [Google Scholar]
- Chipev CC, Korge BP, Markova N, Bale SJ, DiGiovanna JJ, Compton JG, Steinert PM. A leucine to proline mutation in the H1 subdomain of keratin 1 causes epidermolytic hyperkeratosis. Cell. 1992;70:821–828. doi: 10.1016/0092-8674(92)90315-4. [DOI] [PubMed] [Google Scholar]
- Collard JF, Cote F, Julien JP. Defective axonal transport in a transgenic mouse model of amyotrophic lateral sclerosis. Nature (Lond) 1995;375:61–64. doi: 10.1038/375061a0. [DOI] [PubMed] [Google Scholar]
- Colucci-Guyon E, Portier MM, Dunia I, Paulin D, Pounin S, Babinet C. Mice lacking vimentin develop and reproduce without an obvious phenotype. Cell. 1994;79:679–694. doi: 10.1016/0092-8674(94)90553-3. [DOI] [PubMed] [Google Scholar]
- Coulombe PA, Hutton ME, Letai A, Herbert A, Paller SA, Fuchs E. Point mutations in human keratin 14 genes of epidermolysis bullosa simplex patients: genetic and functional analyses. Cell. 1991;66:1301–1311. doi: 10.1016/0092-8674(91)90051-y. [DOI] [PubMed] [Google Scholar]
- Ecob-Prince M, Jenkinson M, Butler-Browne GS, Whalen RG. Neonatal and adult myosin heavy chain isoforms in a nerve muscle culture system. J Cell Biol. 1986;103:995–1005. doi: 10.1083/jcb.103.3.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg, B. 1983. Quantitative ultrastructure of mammalian skeletal muscle. In Handbook of Physiology. Skeletal Muscle. Chapter 3. American Physiology Society, Bethesda, MD. 73–112.
- Fuchs E. Intermediate filaments and disease: mutations that cripple cell strength. J Cell Biol. 1994;125:511–516. doi: 10.1083/jcb.125.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E, Weber K. Intermediate filaments: structure, dynamics, function and disease. Annu Rev Biochem. 1994;63:345–382. doi: 10.1146/annurev.bi.63.070194.002021. [DOI] [PubMed] [Google Scholar]
- Fürst DO, Osborn M, Weber K. Myogenesis in the mouse embryo: differential onset of expression of myogenic proteins and the involvement of titin in myofibril assembly. J Cell Biol. 1989;109:517–527. doi: 10.1083/jcb.109.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galou M, Colucci-Guyon E, Ensergueix D, Ridet JL, Gimenez-Ribotta M, Privat A, Babinet C, Dupouey P. Disrupted GFAP intermediate filaments in astrocytes from vimentin null mice. J Cell Biol. 1996;133:853–864. doi: 10.1083/jcb.133.4.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier GF. Ultrastructural identification of muscle fiber types by immunocytochemistry. J Cell Biol. 1979;82:391–400. doi: 10.1083/jcb.82.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler N, Weber K. The amino acid sequence of chicken muscle desmin provides a common structural model for intermediate filament proteins. EMBO (Eur Mol Biol Organ) J. 1982;1:1649–1656. doi: 10.1002/j.1460-2075.1982.tb01368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel HH, Fardeau M. Familial desmin-related myopathies and cardiomyopathies—from myopathology to molecular and clinical genetics. Neuromusc Disord. 1996;6:383–388. doi: 10.1016/0960-8966(96)85105-4. [DOI] [PubMed] [Google Scholar]
- Goebel HH, Bornemann A. Desmin pathology in neuromuscular diseases. Virchows Arch B Cell Pathol Incl Mol Pathol. 1993;64:127–135. doi: 10.1007/BF02915105. [DOI] [PubMed] [Google Scholar]
- Gomi H, Yokoyama T, Fujimoto K, Ikeda T, Katoh A, Itohara T. Mice devoid of the glial fibrillary acidic protein develop normally and are susceptible to scrapie prions. Neuron. 1995;14:29–41. doi: 10.1016/0896-6273(95)90238-4. [DOI] [PubMed] [Google Scholar]
- Granger BL, Lazarides E. Synemin: a new high molecular weight protein associated with desmin and vimentin filaments in muscle. Cell. 1980;22:727–738. doi: 10.1016/0092-8674(80)90549-8. [DOI] [PubMed] [Google Scholar]
- Grove BK, Cerny L, Perriard JC, Eppenberger HM. Myomesin and M-protein: expression of two M-band proteins in pectoral muscle and heart during development. J Cell Biol. 1985;101:1413–1421. doi: 10.1083/jcb.101.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann H, Fouquet B, Franke WW. Expression of intermediate filament proteins during development of Xenopus laevis.II. Identification and molecular characterization of desmin. Development (Camb) 1989;105:299–307. doi: 10.1242/dev.105.2.299. [DOI] [PubMed] [Google Scholar]
- Hill CS, Duran S, Lin Z, Weber K, Holtzer H. Titin and myosin, but not desmin, are linked during myofibrillogenesis in postmitotic mononucleated myoblasts. J Cell Biol. 1986;103:2185–2196. doi: 10.1083/jcb.103.6.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janmey P, Euteneuer U, Traub P, Schliwa M. Viscoelastic properties of vimentin compared with other filamentous biopolymer networks. J Cell Biol. 1991;113:155–160. doi: 10.1083/jcb.113.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman SJ, Foster R. Replicating myoblasts express a muscle-specific phenotype. Proc Natl Acad Sci USA. 1988;85:9606–9610. doi: 10.1073/pnas.85.24.9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, A.M., and N.A. Rubinstein. 1994. The diversity of muscle fiber types and its origin during development. In Scientific Basis in Myology. A. Engel, C. Franzini-Amstrong, editors. McGraw-Hill Inc. New York. 110–113.
- Lane EB, Rugg EI, Navsaria HJ, Leigh IM, Heagerty AHM, Ishida-Yamamoto A, Eady RAJ. A mutation in the conserved helix termination peptide of keratin 5 in hereditary skin blistering. Nature (Lond) 1992;356:244–246. doi: 10.1038/356244a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments as mechanical integrator of cellular space. Nature (Lond) 1980;238:249–256. doi: 10.1038/283249a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E, Hubbard BD. Immunological characterization of the subunit of the 100A filaments from muscle cells. Proc Natl Acad Sci USA. 1976;73:4344–4348. doi: 10.1073/pnas.73.12.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MK, Cleveland DW. Neurofilament function and dysfunction: involvement in axonal growth and neuronal disease. Curr Opin Cell Biol. 1994;6:34–40. doi: 10.1016/0955-0674(94)90113-9. [DOI] [PubMed] [Google Scholar]
- Lensel-Corbeil G, Goubel F. Series elasticity in frog sartorius muscle during release and stretch. Arch Int Physiol Biochem. 1989;97:499–509. doi: 10.3109/13813458909075081. [DOI] [PubMed] [Google Scholar]
- Li Z, Paulin D. Different factors interact with myoblast-specific and myotube-specific enhancer regions of the human desmin gene. J Biol Chem. 1993;268:10403–10415. [PubMed] [Google Scholar]
- Li Z, Marchand P, Humbert J, Babinet C, Paulin D. Desmin sequence elements regulating skeletal muscle specific expression in transgenic mice. Development (Camb) 1993;117:947–959. doi: 10.1242/dev.117.3.947. [DOI] [PubMed] [Google Scholar]
- Li H, Choudhary SK, Milner DJ, Munir MI, Kuisk IR, Capetanaki Y. Inhibition of desmin expression blocks myoblast fusion and interferes with the myogenic regulators MyoD and myogenin. J Cell Biol. 1994;124:827–841. doi: 10.1083/jcb.124.5.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Colucci-Guyon E, Pincon-Raymond M, Mericskay M, Pournin S, Paulin D, Babinet C. Cardiovascular lesions and skeletal myopathy in mice lacking desmin. Dev Biol. 1996;175:362–366. doi: 10.1006/dbio.1996.0122. [DOI] [PubMed] [Google Scholar]
- Mallory, F.B. 1961. Pathological Technique. Hafner Publishing Co., New York. 56–60.
- Mayo M, Brinbas M, Santos V, Shum L, Slavkin HC. Desmin expression during early mouse tongue morphogenesis. J Int Dev Biol. 1992;36:255–263. [PubMed] [Google Scholar]
- McLean WHI, Lane B. Intermediate filaments in disease. Curr Opin Cell Biol. 1995;7:118–125. doi: 10.1016/0955-0674(95)80053-0. [DOI] [PubMed] [Google Scholar]
- McLean WHI, Morley SM, Lane EB, Eady RAJ, Griffiths WAD, Paige DG, Harper JI, Higgins C, Leigh IM. Ichthyosis bullosa of Siemens: a disease involving keratin 2e. J Invest Dermatol. 1994;103:277–281. doi: 10.1111/1523-1747.ep12394307. [DOI] [PubMed] [Google Scholar]
- McLean WHI, Rugg EL, Lunny DP, Morley SM, Lane EB, Swensson O, Dopping-Hepenstal PJC, Griffiths WAD, Eady RAJ, Higgins C, et al. Keratin 16 and keratin 17 mutations cause Pachyonychia congenita. . Nat Genet. 1995;9:273–278. doi: 10.1038/ng0395-273. [DOI] [PubMed] [Google Scholar]
- Milner DJ, Weitzer G, Tran D, Bradley A, Capetanaki Y. Disruption of muscle architecture and myocardial degeneration in mice lacking desmin. J Cell Biol. 1996;134:1255–1270. doi: 10.1083/jcb.134.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North AJ, Galazkiewicz B, Byers T, Glenney JR, Jr, Small JV. Complementary distributions of vinculin and dystrophin define two distinct sarcolemma domains in smooth muscle. J Cell Biol. 1993;120:1159–1167. doi: 10.1083/jcb.120.5.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekny M, Leveen P, Pekna M, Eliasson C, Berthold CH, Westermark B, Betsholtz C. Mice lacking glial fibrillary acidic protein display astrocytes devoid of intermediate filaments but develop and reproduce normally. EMBO (Eur Mol Biol Organ) J. 1995;14:1590–1598. doi: 10.1002/j.1460-2075.1995.tb07147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellissier JF, Pouget J, Charpin C, Figarella D. Myopathy associated with desmin type intermediate filaments. J Neurol Sci. 1989;89:49–61. doi: 10.1016/0022-510x(89)90006-3. [DOI] [PubMed] [Google Scholar]
- Porter RM, Leitgeb S, Melton DW, Swensson O, Eady RA, Magin TM. Gene targeting at the mouse cytokeratin 10 locus: severe skin fragility and changes of cytokeratin expression in the epidermis. J Cell Biol. 1996;132:925–936. doi: 10.1083/jcb.132.5.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MG, Gomer RH. Skelemin, a cytoskeletal M-disc periphery protein, contains motifs of adhesion/recognition and intermediate-filament proteins. J Biol Chem. 1993;268:21800–21810. [PubMed] [Google Scholar]
- Reis A, Hennies HC, Langbein L, Digweed M, Mischke D, Drechsler M, Schröck E, Royer-Pokora B, Franke WW, Sperling K, et al. Keratin 9 gene mutations in epidermolytic palmoplantar keratoderma (EPPK) Nat Genet. 1994;6:174–179. doi: 10.1038/ng0294-174. [DOI] [PubMed] [Google Scholar]
- Rugh, R. 1968. The mouse, its reproduction and development. Oxford Science Publications. Oxford. 1–430.
- Schaart G, Viebahn C, Langmann W, Raemakers F. Desmin and titin expression in early post-implantation mouse embryos. Development (Camb) 1989;107:585–596. doi: 10.1242/dev.107.3.585. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol Rev. 1996;76:371–425. doi: 10.1152/physrev.1996.76.2.371. [DOI] [PubMed] [Google Scholar]
- Schultheiss T, Lin Z, Ishikawa H, Zamir I, Stoeckert CJ, Holtzer H. Desmin/vimentin intermediate filaments are dispensable for many aspects of myogenesis. J Cell Biol. 1991;114:953–966. doi: 10.1083/jcb.114.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small JV, Sobieszek A. Studies on the function and composition of the 10-nm (100-Å) filaments of vertebrate smooth muscle. J Cell Sci. 1977;23:243–268. doi: 10.1242/jcs.23.1.243. [DOI] [PubMed] [Google Scholar]
- Talmadge RJ, Roy RR. Electrophoretic separation of rat skeletal muscle myosin heavy-chain isoforms. J Appl Physiol. 1993;75:2337–2340. doi: 10.1152/jappl.1993.75.5.2337. [DOI] [PubMed] [Google Scholar]
- Tam PP. A study on the pattern of prospective somites in the presomitic mesoderm of mouse embryo. J Embryol Exp Morph. 1986;92:269–285. [PubMed] [Google Scholar]
- Thornell LE, Edstrom L, Eriksson A, Henriksson KG, Angquist KA. The distribution of intermediate filament protein (skeletin) in normal and diseased skeletal muscle. An immunohistochemical and electron microscopic study. J Neurol Sci. 1980;47:153–170. doi: 10.1016/0022-510x(80)90001-5. [DOI] [PubMed] [Google Scholar]
- Thornell, L.E., A. Eriksson, and L. Edstrom. 1983. Intermediate filaments in human myopathies. In Cell and Muscle Motility. R.B. Dowben, and J.W. Shay, editors. Plenum Publishing, New York. 85–135. [PubMed]
- Thornell LE, Carlsson L, Li Z, Mericskay M, Paulin D. Null mutation in the desmin gene gives rise to a cardiomyopathy. J Mol Cell Cardiol. 1997;29:2107–2124. doi: 10.1006/jmcc.1997.0446. [DOI] [PubMed] [Google Scholar]
- Tidball JG. Desmin at myotendinous junctions. Exp Cell Res. 1992;199:206–212. doi: 10.1016/0014-4827(92)90425-8. [DOI] [PubMed] [Google Scholar]
- Traub, P. 1985. Intermediate Filaments: a Review. Spinger-Verlag, Berlin. 266 pp.
- Vicart P, Dupret JM, Hazan J, Li Z, Gyapay G, Krishnamoorthy R, Weissenbach J, Fardeau M, Paulin D. Human desmin gene: cDNA sequence, regional localization and exclusion of the locus in a familial desmin-related myopathy. Hum Genet. 1996;98:422–429. doi: 10.1007/s004390050233. [DOI] [PubMed] [Google Scholar]
- Wang K, McCarter R, Wright J, Jennate B, Raminez-Mitchell R. Viscoelasticity of sarcomere matrix of skeletal muscles: the titin myosin composite filament is a dual-stage molecular spring. Biophys J. 1993;64:1161–1177. doi: 10.1016/S0006-3495(93)81482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzer G, Milner D, Kim J, Bradley A, Capetanaki Y. Cytoskeletal control of myogenesis: a desmin null mutation blocks the myogenic pathway during embryonic stem cell differentiation. Dev Biol. 1995;172:422–439. doi: 10.1006/dbio.1995.8070. [DOI] [PubMed] [Google Scholar]
- Weller B, Karpati G, Carpenter S. Dystrophin-deficient mdx muscle fibers are preferentially vulnerable to necrosis induced by experimental lengthening contraction. J Neurol Sci. 1990;100:9–13. doi: 10.1016/0022-510x(90)90005-8. [DOI] [PubMed] [Google Scholar]