Abstract
A microneedle puncture of the fibroblast or sea urchin egg surface rapidly evokes a localized exocytotic reaction that may be required for the rapid resealing that follows this breach in plasma membrane integrity (Steinhardt, R.A,. G. Bi, and J.M. Alderton. 1994. Science (Wash. DC). 263:390–393). How this exocytotic reaction facilitates the resealing process is unknown. We found that starfish oocytes and sea urchin eggs rapidly reseal much larger disruptions than those produced with a microneedle. When an ∼40 by 10 μm surface patch was torn off, entry of fluorescein stachyose (FS; 1,000 mol wt) or fluorescein dextran (FDx; 10,000 mol wt) from extracellular sea water (SW) was not detected by confocal microscopy. Moreover, only a brief (∼5–10 s) rise in cytosolic Ca2+ was detected at the wound site. Several lines of evidence indicate that intracellular membranes are the primary source of the membrane recruited for this massive resealing event. When we injected FS-containing SW deep into the cells, a vesicle formed immediately, entrapping within its confines most of the FS. DiI staining and EM confirmed that the barrier delimiting injected SW was a membrane bilayer. The threshold for vesicle formation was ∼3 mM Ca2+ (SW is ∼10 mM Ca2+). The capacity of intracellular membranes for sealing off SW was further demonstrated by extruding egg cytoplasm from a micropipet into SW. A boundary immediately formed around such cytoplasm, entrapping FDx or FS dissolved in it. This entrapment did not occur in Ca2+-free SW (CFSW). When egg cytoplasm stratified by centrifugation was exposed to SW, only the yolk platelet–rich domain formed a membrane, suggesting that the yolk platelet is a critical element in this response and that the ER is not required. We propose that plasma membrane disruption evokes Ca2+ regulated vesicle–vesicle (including endocytic compartments but possibly excluding ER) fusion reactions. The function in resealing of this cytoplasmic fusion reaction is to form a replacement bilayer patch. This patch is added to the discontinuous surface bilayer by exocytotic fusion events.
Living, nucleated cells respond to microneedle punctures of their plasma membranes by rapidly (within sec) resealing the breach created. The mechanism used is Ca2+ dependent and hypothesized to be an active process governed by specific protein–protein interactions that result in a local exocytotic reaction. As a result of this exocytotic response, new membrane is added locally to the site of cell surface injury (for review see McNeil and Steinhardt, 1997).
An extensive literature documents examples of resealing on a far larger scale than that required for repair of a microneedle prick. For example, we know that transected neurons and skeletal muscle cells reseal after transection, since they are capable of surviving this injury (Yawo and Kuno, 1985; Casademont et al., 1988; Krause et al., 1994). Resealing on this scale requires the replacement of many square micrometers of surface barrier. Unless the exocytotic fusion events induced by disruption are far more numerous than have been detected (Bi et al., 1995; Miyake and McNeil, 1995), it does not seem possible that exocytosis alone could replace the amount of surface membrane requisite for large-scale (many square micrometers) resealing.
The capacity of the echinoderm egg/oocyte for surviving disruptions, large and small, is well established. Heilbrunn and others, for example, described a “surface precipitation reaction” that occurs when large portions of the sea urchin surface are torn off and showed that egg survival, that is, retention of intracellular components, depended on a Ca2+-initiated event (Heilbrunn, 1930a ,b). Indeed, echinoderm eggs are favored subjects of microinjection experiments, not only because they are large, easily obtained, and safely manipulated at room temperature, but also because they are adept at surviving plasma membrane disruptions induced by this experimental manipulation (Chambers and Chambers, 1961).
Here we severely challenge the cytoplasm of starfish oocytes and sea urchin eggs to seal itself off from its normal external environment, sea water (SW).1 We find these cells have a remarkable capacity for rapidly resealing plasma membrane disruptions. We demonstrate that the Ca2+ present in SW induces the rapid fusion of cytoplasmic organelles with one another to form de novo an impermeant, cell surface membrane barrier. This vesicle–vesicle fusion response separates intracellular from extracellular domains, and can occur in the complete absence of plasma membrane involvement. We propose that this intracytoplasmic membrane fusion reaction, coupled with cytoplasmic membrane fusion with the plasma membrane, is essential for resealing large plasma membrane disruptions.
MATERIALS AND METHODS
Oocytes and Eggs
Starfish (Asterina miniata) were obtained from Marinus, Inc. (Venice, CA), and sea urchins (Lytechinus variegatus) were obtained from T. Andacht (Duke University Marine Lab, Beaufort, NC) or S. Decker (independent collector, Davie, FL). They were maintained in running SW at the Marine Biological Laboratory (Woods Hole, MA) or in a SW aquarium at the University of Connecticut (Farmington, CT). Starfish gametes were obtained by using a small sample corer (Fine Science Tools Inc., Foster City, CA). Sea urchin eggs were obtained by injection of a small amount of 0.5 M KCl to spawn eggs from a single gonad at a time (0.15 ml for the North Carolina animals and 0.3 ml for the Florida animals; Fuseler, 1973). Microscope observations were done at room temperature. Starfish oocytes were kept in an incubator at 18–20°C for long-term experiments.
Wounding Procedure
Sea urchin eggs were dejellied with either Ca2+ Mg2+-free SW for 1.5 min (Detering et al., 1977), or by extensive SW washes, resuspended in SW, and attached for 1 min to a polylysine-coated coverslip (500,000 mol wt; coverslips were treated with 1 mg/ml polylysine in water for 5 min followed by several washes in SW; Sigma Chemical Co., St. Louis, MO). The coverslip was used as the top part of a microinjection chamber. A microinjection needle tip was immersed in 1 mg/ml polylysine for 3 min and then mounted in a Narishige SM-20 micromanipulator so that the needle was in the plane of focus of the microscope (Narishige Scientific Instruments Laboratory, Tokyo, Japan). The needle was moved up against the side of an egg for ∼1 min and was then rapidly moved away to generate the wound.
Microscopy
An upright microscope (Axioskop; Carl Zeiss, Inc., Thornwood, NY) was coupled with a scanning confocal microscope (MRC 600; Bio-Rad Laboratories, Cambridge, MA). To make the recordings shown in Figs. 1, 2, 4, 5, 9, and 10, the confocal microscope was set to scan continuously at one or two frames per second, and each frame was recorded on an optical memory disk recorder (OMDR; Panasonic 3038F; Secaucus, NJ). In early experiments, the frames were recorded manually as the scan reached the bottom of the monitor screen, but in later experiments, automatic recording was accomplished by means of a trigger circuit using a sync signal from the confocal microscope (described in detail at http://www.uchc.edu/∼terasaki/trigger.html).
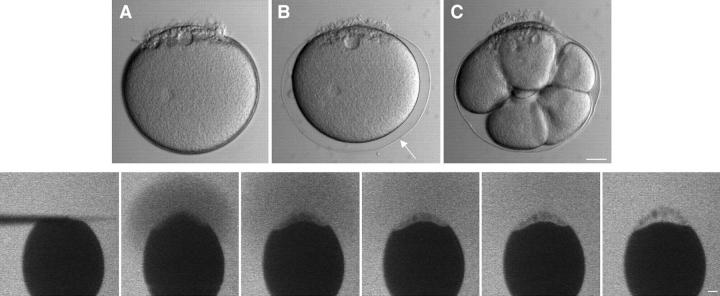
Figure 1.
Survival and rapid healing of a large wound in the plasma membrane. A polylysine-coated microneedle was maneuvered so that it contacted the surface of an immobilized sea urchin egg and then was rapidly moved away from the surface (the movement was in the plane of focus of the microscope). This ripped off the contacted surface and created a wound ∼40 by 10 μm. (Top) (A) an apparent sharp boundary formed immediately (within 1–2 s) at the interface of cytoplasm and SW produced by the rip-off. After addition of sperm, the wounded egg became fertilized (B; arrow denotes fertilization envelope) and underwent several rounds of division (C; shown 2.5 h after fertilization). This shows that the wounded egg healed successfully after the rip-off. (Bottom) A sea urchin egg was immersed in 100 μg/ml FS in SW and observed by confocal microscopy during the rip-off wound procedure. No entry of FS was observed after a rip-off, indicating that the wound had resealed rapidly. Images were obtained at 1-s intervals; consecutive images are shown except the last image, which was 40 s after wounding. Bars: (top) 20 μm; (bottom) 10 μm.
Figure 2.
Cytosolic Ca2+ during the healing of a wound. A sea urchin egg was injected with the fluorescent Ca2+ indicator calcium green dextran (10 kD; 20 μM final concentration). As in Fig. 1, a polylysine-coated microneedle was used to create a wound. There is a local rise in Ca2+ during wounding, but the rise diminishes rapidly. In view of the large gradient of Ca2+ from SW to cytosol, this is further indication of a rapid healing reaction. Images were obtained at 1-s intervals; consecutive images are shown except the last image, which was 15 s after wounding. The small dark circle near the center of the egg is an oil drop resulting from the calcium green dextran injection. Bar, 10 μm.
Figure 4.
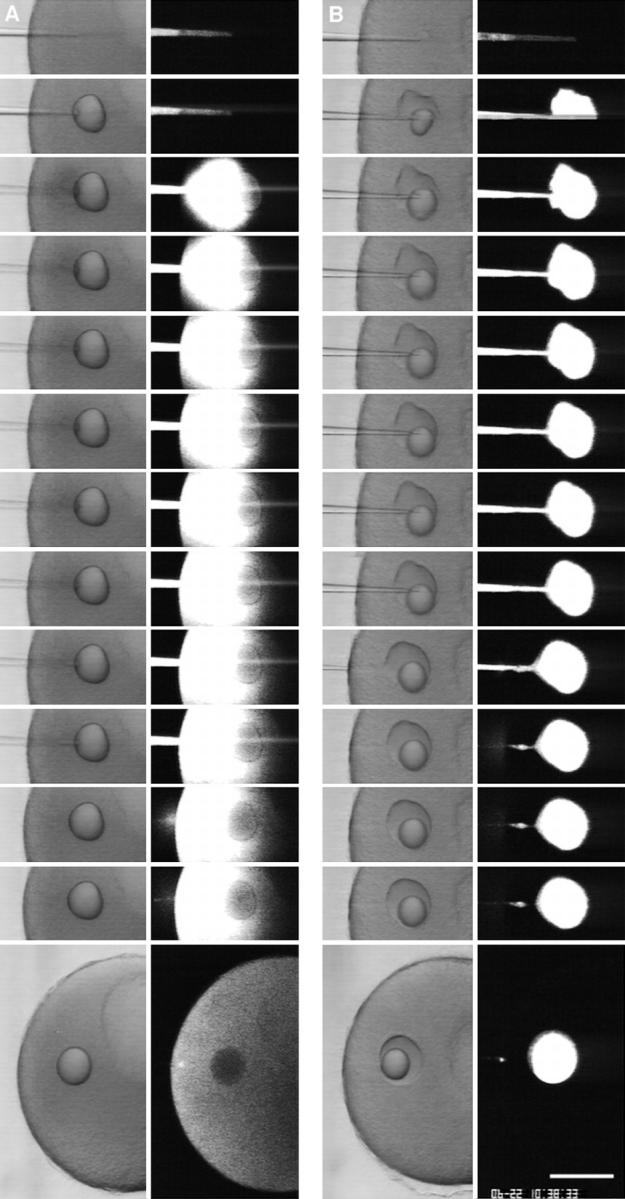
Injected SW is contained at the injection site. FDx (10 mg/ml; 70 kD) dissolved in CFSW or in SW was injected into a starfish oocyte. The micropipet contained an oil cap at the tip to prevent mixing of the injectate with chamber SW. The oil cap comes out first during the injection and forms a spherical droplet in the cytoplasm. The injections were observed by simultaneous scanning transmission and fluorescence imaging. (A) When CFSW containing FDx was injected, the fluorescence spread throughout the cytoplasm. (B) When SW containing FDx was injected, the fluorescence did not spread from the injection site and was contained within a wound vesicle that is visible in the transmitted light image. These results suggest that high Ca2+ causes fusion of intracellular membranes, creating a boundary that prevents spreading of high Ca2+ throughout the cytoplasm. Images were obtained at 1-s intervals; consecutive images are shown except that last image of the CFSW sequence was 125 s after injection, and the last image of the SW sequence was 306 s after injection. Bar, 20 μm.
Figure 5.
Cytosolic Ca2+ during SW injection rises only briefly. A mature starfish egg was injected with the fluorescent Ca2+ indicator calcium green dextran (20 μM final concentration) then was imaged by simultaneous scanning transmission and fluorescence confocal microscopy during an injection of SW. There was a brief rise in fluorescence around the injection site which then decreased rapidly. The oil drop in the first transmitted light image is from the calcium green dextran injection. Images were obtained every 0.5 s; consecutive images are shown except the last frame which is 6 s after the beginning of the sequence. Bar, 20 μm.
Figure 9.
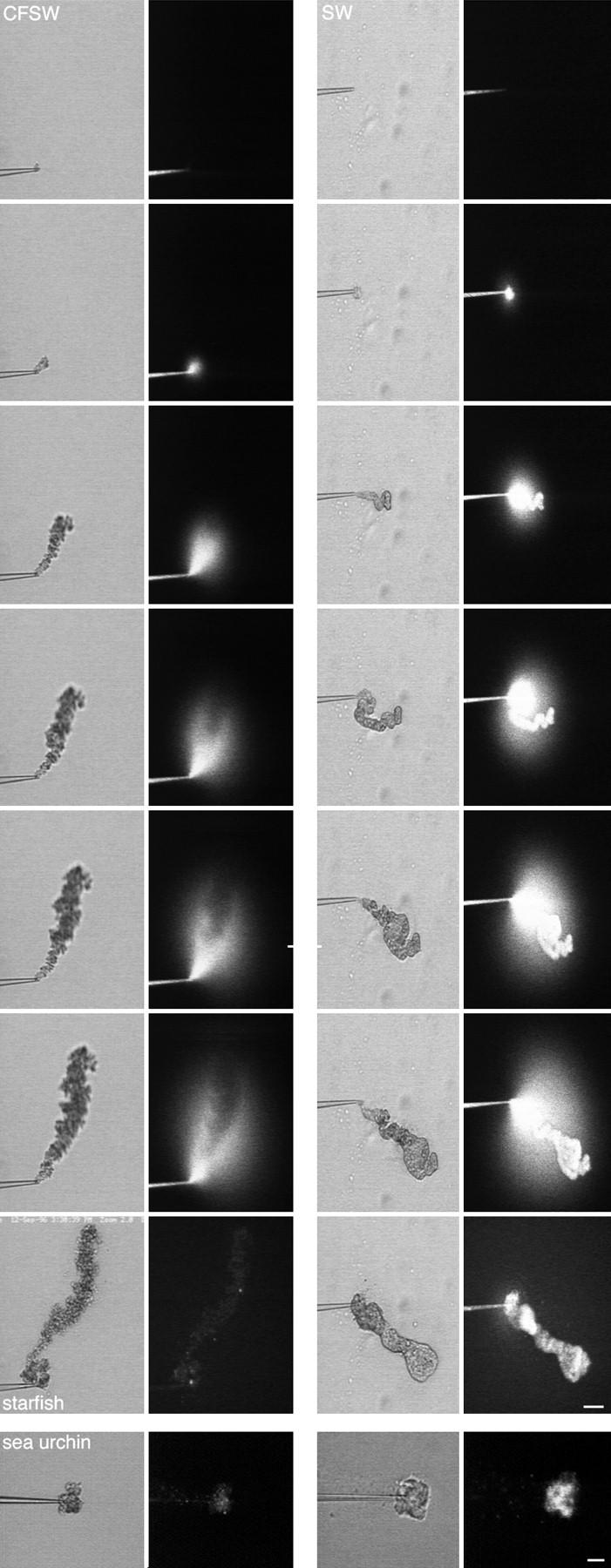
Cytoplasm extruded into SW retains a fluorescent marker in the cytosol. Starfish oocytes were injected with a final concentration of 0.2 mg/ml FS. After allowing the FS to diffuse throughout the oocyte, cytoplasm was removed by micropipet (2% of the oocyte volume). The cytoplasm was then extruded while observing by simultaneous scanning transmission and fluorescence confocal microscopy. When extruded into CFSW (left), the fluorescent marker diffused away, indicating lack of a boundary formation. When extruded into SW (right), the fluorescent marker was retained, indicating that the Ca2+ caused fusion of intracellular membranes, trapping the marker. Images were obtained at 1-s intervals; consecutive images are shown except the last image, which was 30 s after the sequence began. (bottom) Sea urchin eggs were injected as described above with FS and their cytoplasm was then extruded into CFSW (bottom left) or SW (bottom right). As was the case for starfish, when sea urchin egg cytoplasm was extruded into CFSW, the FS diffused away whereas the FS became trapped when cytoplasm was extruded into SW. Both images were taken 45 s after extrusion. Bars, 10 μm.
Figure 10.
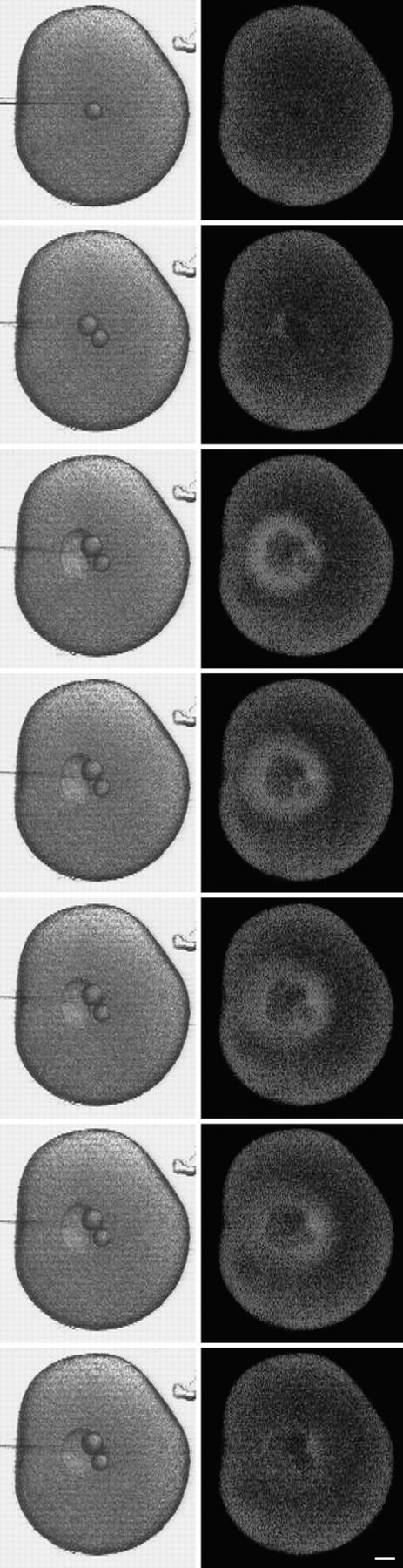
Behavior of extruded cytoplasm from centrifuged eggs. Sea urchin eggs were centrifuged to stratify the cytoplasm (top); the clear region is devoid of yolk granules. Centrifuged eggs were injected with a final concentration of 0.2 mg/ml 70-kD FDx. Cytoplasm was removed by micropipet (2% of the total cytoplasmic volume) and then extruded into SW and observed by simultaneous scanning transmission and fluorescence microscopy. (bottom left) When yolk granule-containing cytoplasm was extruded, the fluorescence was retained. (bottom right) When clear cytoplasm was extruded, the fluorescence diffused away. These results suggest that yolk granule membranes are involved in the Ca2+-dependent fusion of intracellular membranes. Images were obtained at 1-s intervals; consecutive images are shown except for the last images, which were both taken at 40 s. Bar, 10 μm.
Microinjection
The eggs were quantitatively injected using mercury-loaded pipets (Hiramoto, 1962). Briefly, a micropipet was back-loaded with a small drop of mercury, which was pushed to the micropipet tip. The injectate was front-loaded using a microscope with an eyepiece reticle for calibrating the volume injected. A small amount of oil (dimethylpolysiloxane, 100 cps; Sigma Chemical Co.) was drawn up as an oil cap. The injection chamber was as described previously (Kiehart, 1982).
Solutions and Reagents
Solutions requiring SW were made using Marine Biological Laboratory (MBL) artificial SW (9.25 mM Ca2+, 48.4 mM Mg2+, 425 mM Na+, 9.0 mM K+, 25.5 mM SO4 2−, 489 mM Cl−) or CFSW (same formulation without Ca2+). Fluorescent reagents were all obtained from Molecular Probes, Inc. (Eugene, OR). Calcium green-1 dextran (10 kD) was made as a solution of 1 mM in 100 mM potassium glutamate, 10 mM Hepes, pH 7, and injected to a final concentration of 20 μM. Nile red was dissolved at 0.5 mg/ml in acetone and used at 0.5 μg/ml in SW. Intracellular buffer contained 350 mM potassium glutamate, 350 mM glycine, 3 mM MgCl2, 0.57 mM CaCl2, 10 mM EGTA, 20 mM Hepes, pH 6.7. DiI (DiIC16[3])-saturated oil was made using soybean oil (Wesson; Hunt-Wesson, Inc., Fullerton, CA).
Electron Microscopy
Sea urchin eggs attached to polylysine-coated coverslips were injected with SW as described above and then fixed 10–20 min later by immersion in 1% glutaraldehyde, which was made by diluting 8% glutaraldehyde in SW (Electron Microscopy Sciences, Gibbstown, NJ). After ∼1 h, the eggs were changed to SW and then postfixed for 1 h with 1% OsO4 and 0.8% potassium ferricyanide in 0.1 M sodium cacodylate, pH 7.4. The eggs were rinsed thoroughly in distilled water and stained in 0.5% aqueous uranyl acetate for 1 h. They were dehydrated and embedded in Poly/Bed (Polysciences Inc., Warington, PA). Ultra thin sections were cut by using an ultramicrotome (MT6000-XL; Research and Manufacturing Co., Inc., Tucson, AZ) and stained with a fresh mixture of equal parts acetone and saturated uranyl acetate, stained a second time with lead citrate, and then examined in a transmission electron microscope (JEM-1200EX; JEOL LTD., Tokyo, Japan).
Stratification of Sea Urchin Eggs
Approximately 1 ml of dilute egg suspension was layered on top of 50 μl of 18% ficoll (70,000 mol wt; Sigma Chemical Co.) in a 1.5-ml microfuge tube, and was centrifuged at 12,000 g for 15–30 min.
RESULTS
Large Wounds in Sea Urchin Eggs Are Resealed Rapidly
Echinoderm eggs are large (∼100–200 μm), relatively easy to inject, and optically clear, and thus have several experimental advantages for investigating large-scale membrane wound healing. To understand the reaction of eggs to surface disruptions, it is necessary to recall that the surface of unfertilized eggs is poised to undergo large changes at fertilization. Lining the interior of the plasma membrane is a dense monolayer of docked cortical granules (each ∼1 μm in diameter). Exterior to the plasma membrane is the vitelline layer, a thin proteinaceous coat (Chandler and Heuser, 1980). The egg's natural environment is SW, which contains ∼10 mM Ca2+. At fertilization, the sperm initiates a wave of Ca2+ release from the ER, resulting in a rise of cytosolic Ca2+ concentration from ∼0.1 μM to ∼2–3 μM (Steinhardt et al., 1977; Hafner et al., 1988). The elevated Ca2+ triggers exocytosis of the cortical granules, which releases proteases and other enzymes that act on the vitelline layer, resulting in formation of the fertilization envelope (Kay and Shapiro, 1985).
The vitelline layer of unfertilized sea urchin eggs adheres strongly to glass coated with polycationic macromolecules such as protamine sulfate or polylysine (Steinhardt et al., 1971; Mazia et al., 1975). When attached eggs are subjected to a sudden fluid force, they are sheared off, leaving a large circular patch of egg cortex (including the cortical granules) attached to the glass (Vacquier, 1975). To make controlled wounds in the egg surface, we maneuvered a polylysine-coated microneedle so that it lay against the side of an immobilized egg. A quick movement of the microneedle away from the egg ripped off a patch of cortex. The size of this rip-off zone is estimated, based on needle diameter and estimated cell contact length, to be 40 by 10 μm. By transmitted light, an apparent sharp boundary formed rapidly at the wound site (Fig. 1 A). Some cytoplasm always leaked out beyond this apparent boundary and appeared to be transformed into many small spheres of varying sizes. The wounding process usually did not result in artificial activation, i.e., fertilization envelope elevation. Most of the eggs could be fertilized and undergo apparently normal cleavage (Fig. 1, B and C).
We observed this wounding process directly using a confocal microscope. Eggs were immersed in SW containing either fluorescein dextran, 10,000–70,000 mol wt (FDx) or fluorescein stachyose, mol wt 1,146 (FS), both of which are inert, impermeant fluorescent markers. The confocal microscope was set to scan continuously at the rate of either one or two scans per second as the wound was being made. Very little fluorescent marker entered the wounded egg, indicating that the breach in the permeability barrier of the cell surface was repaired rapidly (Fig. 1). In contrast, when eggs were wounded in CFSW (Ca2+-free SW), no sharp boundary formed, and the fluorescent marker entered the egg (not shown). Also, the cytoplasm leaked out steadily, as reported by Heilbrunn (1930a ,b). This confirms that Ca2+ is required for sealing the wound, and the effectiveness of dye exclusion suggested that sealing occurs very rapidly.
Because so little (undetectable amounts) of FDx or FS leaked into the egg during rip-off, we could not determine from this experiment how fast resealing occurred. To further characterize the time course of resealing, eggs were injected with the fluorescent Ca2+ indicator, calcium green dextran, so that we could record the duration of Ca2+ influx into the wounded cell. A local Ca2+ rise was recorded at the wound site, but this local elevation persisted for only a few seconds and did not propagate throughout the cell (Fig. 2). Since intracellular Ca2+ concentration is thought to be ∼0.1 μM, compared to the extracellular SW Ca2+ concentration of 9.3 mM, this shows that very little Ca2+ enters from the outside and suggests resealing of these large disruptions is complete within a few seconds after disruption. The lack of fertilization envelope elevation is a biological indication that Ca2+ entry is restricted.
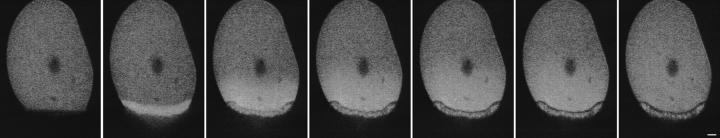
In the original sea urchin egg cortical preparation (Vacquier, 1975), attached eggs are sheared, leaving an ∼50-μm-diam cortical patch on the coverslip. Since this patch comes directly from the egg surface, the sheared eggs must have an equivalently sized wound. We sheared eggs in SW to see if this very large wound can be repaired. By confocal microscopy, the sheared eggs excluded FDx in SW from the cytosol, showing that the wound had been sealed (Fig. 3). Most of the sheared eggs, however, had a fertilization envelope. Most likely, enough of the extracellular Ca2+ had entered into the egg to activate the processes that are normally triggered by Ca2+ at fertilization. In this case, the wound healing was only partially successful, because it did not prevent Ca2+ from activating an inappropriate response for the cell.
Figure 3.
Sea urchin eggs are able to heal very large wounds in their surface. Eggs were attached to a polylysine-coated coverslip and then sheared in SW. This process leaves a ∼50-μm patch of cortex on the coverslip (Vacquier, 1975), and creates an equivalently sized wound in the sheared egg surface. After 3 min, the sheared eggs were transferred to SW containing 0.3 mg/ml 10-kD FDx and then were imaged by scanning transmission and fluorescence confocal microscopy. The fluorescence image shows that this very large wound has healed, since the FDx is excluded from the egg interior. Part of a low fertilization envelope is seen in the transmitted light image (arrow), showing that the wound healing was not able to prevent a partial activation of the egg by Ca2+ entry. Bar, 10 μm.
Since even a small marker such as FS was not permeable to healed eggs, and since influx of Ca2+ is rapidly halted in large wounds, it is very likely that the healing consists of a Ca2+-dependent formation of a membrane barrier rather than a dense precipitation of proteins. Small wounds in unfertilized sea urchin eggs are thought to be healed by exocytosis of cortical granules (Steinhardt et al., 1994; Bi et al., 1995). However, the cortical granules at the wound site are probably removed with the plasma membrane with the type of wounds we have made, so it seemed likely that some of the intracellular membranes had participated in the membrane fusion events that sealed the breach between intra- and extracellular spaces.
Intracellular Injection of Ca2+ Causes Fusion of Intracellular Membranes
When Ca2+ is injected directly into eggs, it can activate them, but only when the injected solution contains millimolar total Ca2+ buffered at free concentrations near 1 μM (Hamaguchi and Hiramoto, 1981). Rapid injection of unbuffered solutions of millimolar Ca2+ concentration does not activate eggs; instead, it causes formation of an inclusion that appears to contain the injectate (Hiramoto, 1965; Kiehart, 1981). For example, it is a very common observation that an inadvertent injection of SW causes formation of an inclusion. We realized that this phenomenon might be relevant for plasma membrane wound healing, so we investigated it more closely.
Injections of CFSW and SW (9.3 mM Ca2+) with fluorescent markers were observed using confocal microscopy. When CFSW was injected, the fluorescent marker spread throughout the cytoplasm (Fig. 4). When SW was injected, a wound vesicle containing apparently all of the injected fluorescence formed rapidly in both starfish oocytes and sea urchin eggs (Fig. 4). Since 1-kD FS as well as FDx is contained in such experiments, it seems very likely that a membrane is formed at the boundary. When SW was injected into an egg containing Ca2+ indicator, there was a transient increase near the wound vesicle that was detectable with this indicator for no longer than 6 s (Fig. 5). As was the case in the large disruption created by a rip-off, a Ca2+ impermeant barrier was rapidly formed when cytoplasm was exposed to SW by injecting it. However, in this case, there could be no involvement of the plasma membrane, suggesting that vesicle–vesicle fusion was responsible.
In transmitted light images, the wound vesicle was often seen to have two boundaries, a sharper inner boundary and finer outer boundary. At other times, there was only one sharp boundary. The presence of one or two boundaries seemed to be related to yolk platelets. Yolk platelets are 1–2-μm-sized organelles that are distributed abundantly throughout the egg cytoplasm. Nile red is a fluorescent dye that stains lipid-rich organelles (Greenspan et al., 1985). It is apparently the active component of Nile blue used for staining yolk platelets in frog eggs (Danilchik and Gerhart, 1987), and we found that it stains yolk platelets in sea urchin eggs as well. In cases where the wound vesicle had two apparent boundaries, Nile red stained individual yolk platelets outside of the fine outer boundary, but it stained the region between the two boundaries more uniformly, as if the yolk platelets had fused together or disintegrated (Fig. 6). Moreover, in the two-boundary vesicle, FS injected with the SW was restricted by the innermost of these two boundaries (Fig. 6). This innermost boundary was stained with DiI, a lipophilic fluorescent dye that stains membrane bilayers (Haugland, 1996), providing strong evidence that the boundary is a membrane (Fig. 6). When the wound vesicle had only one apparent boundary, there was no intermediate zone between the normal appearing yolk platelets and the FS-containing wound vesicle.
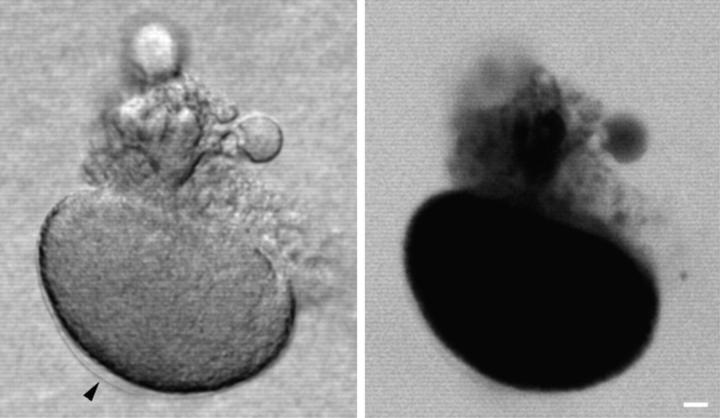
Figure 6.
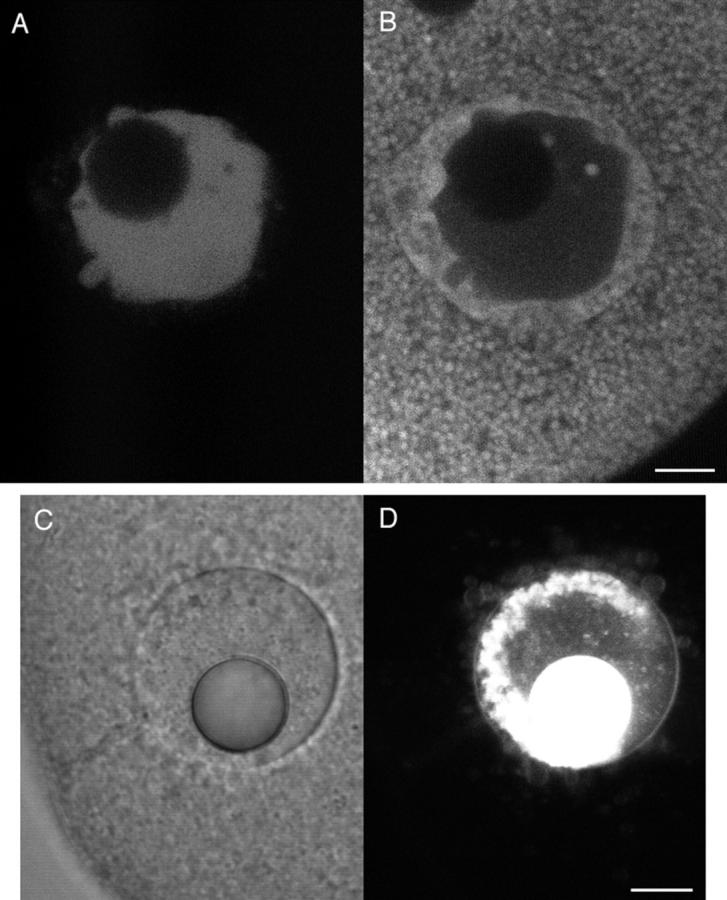
Yolk platelet and membrane staining near the wound vesicle. SW containing 0.5 mg/ml FS was injected into a sea urchin egg whose yolk platelets were stained by Nile red. The egg was imaged using dual channel fluorescence confocal microscopy. (A) Fluorescein channel imaging, showing that the FS had been contained within the wound vesicle. (B) Rhodamine channel imaging showing the fluorescence from Nile red. Unaltered yolk platelets are seen throughout most of the cytoplasm. A more uniformly stained region surrounds the SW injectate that is marked by the FS seen in A. This domain seems to represent yolk platelets that have fused together or disintegrated, and corresponds with the region surrounding the SW in the electron micrographs in Fig. 7. By transmitted light, the wound vesicle often was seen to have a sharp inner boundary and a finer, less distinct outer boundary. The inner boundary corresponds to the sea water injectate interface, and the outer boundary corresponds to the boundary between the disrupted and normal appearing yolk platelets. (C and D) Composition of the wound vesicle boundary. A SW injection was made using DiI-saturated oil for the oil cap in the microinjection needle. DiI spreads in membranes contacted by the oil drop (Terasaki and Jaffe, 1991). 15 min after injection, the wound vesicle was viewed by (C) scanning transmission and (D) fluorescence confocal imaging. The fluorescence image shows that DiI has labeled the wound vesicle boundary, providing strong evidence that the boundary is a membrane. Also labeled are free-floating vesicles that are often observed in the wound vesicle lumen. Bar, 10 μm.
The wound vesicle was examined by thin section EM (Fig. 7). A cross section through the center of a wound vesicle showed that the injected SW was surrounded by a donut-shaped domain corresponding to the more continuously stained Nile red region of a two-boundary wound vesicle (Fig. 6). High magnification revealed that an electron-dense boundary is present continuously around the injected SW (Fig. 7) in a location consistent with the innermost boundary described above (Fig. 6) as limiting FS diffusion and stainable with DiI. This confirms that the wound vesicle is a membrane-bounded inclusion. The donut-shaped domain contains abnormal cytoplasm that may be the result of massive fusion of organelles. Outside of the donut-shaped domain, the cytoplasm appeared normal, except for the presence of large organelles that appeared to be the result of fusion of several yolk platelets. Thus, alterations in normal cytoplasmic structure occur beyond the membrane boundary that excludes the SW.
Figure 7.
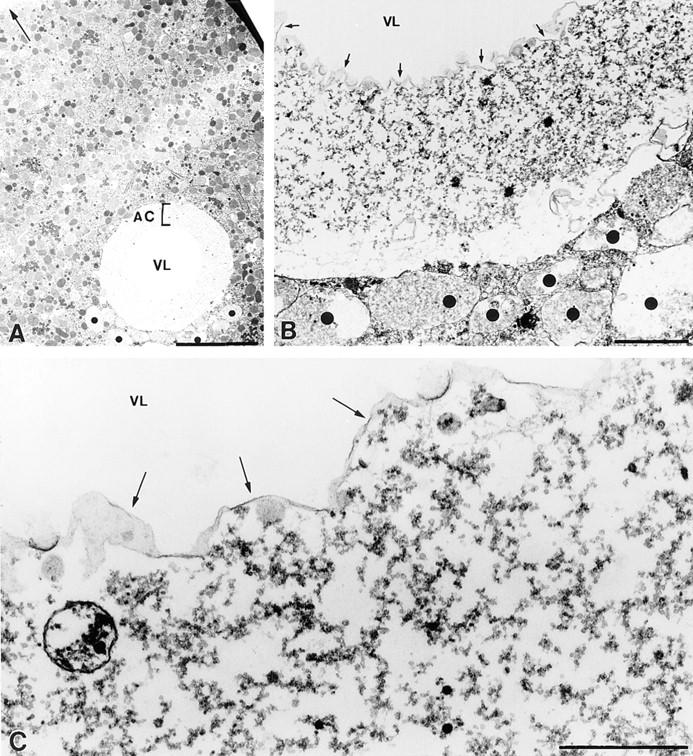
Electron micrographs of the SW injection site. (A) Low magnification view of a section through the SW injection site. An empty central region or vesicle lumen (VL) at the injection site is surrounded by a shell of abnormal cytoplasm (AC) and then, abruptly, by normal appearing cytoplasm (Arrow points to plasma membrane). (B) At higher magnification, the shell of abnormal cytoplasm surrounding the vesicle lumen (VL; arrows indicate boundary to SW) is seen to be devoid of organelles, and appears to consist predominantly of a course granular material. Vesicles (dots) smaller than the wound vesicle but larger than any normally seen in egg cytoplasm, presumably also formed during the SW injection, are common in the immediate vicinity of the injection site. (C) At the interface of the VL with the abnormal cytoplasm is a continuous electron-dense boundary (arrows), suggesting that this is the site of SW vesicle's permeability barrier. These images were from eggs fixed in glutaraldehyde ∼10–20 min after the SW injection. Bars, (A) 10 μm; (B and C) 1 μm.
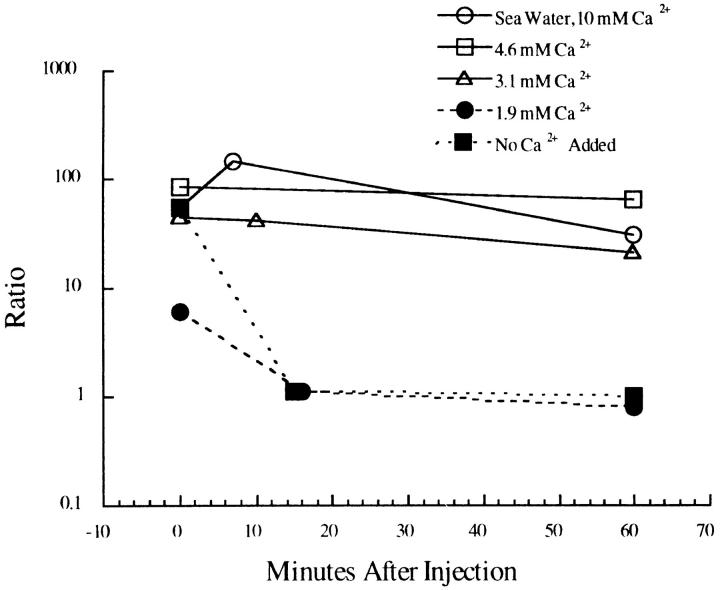
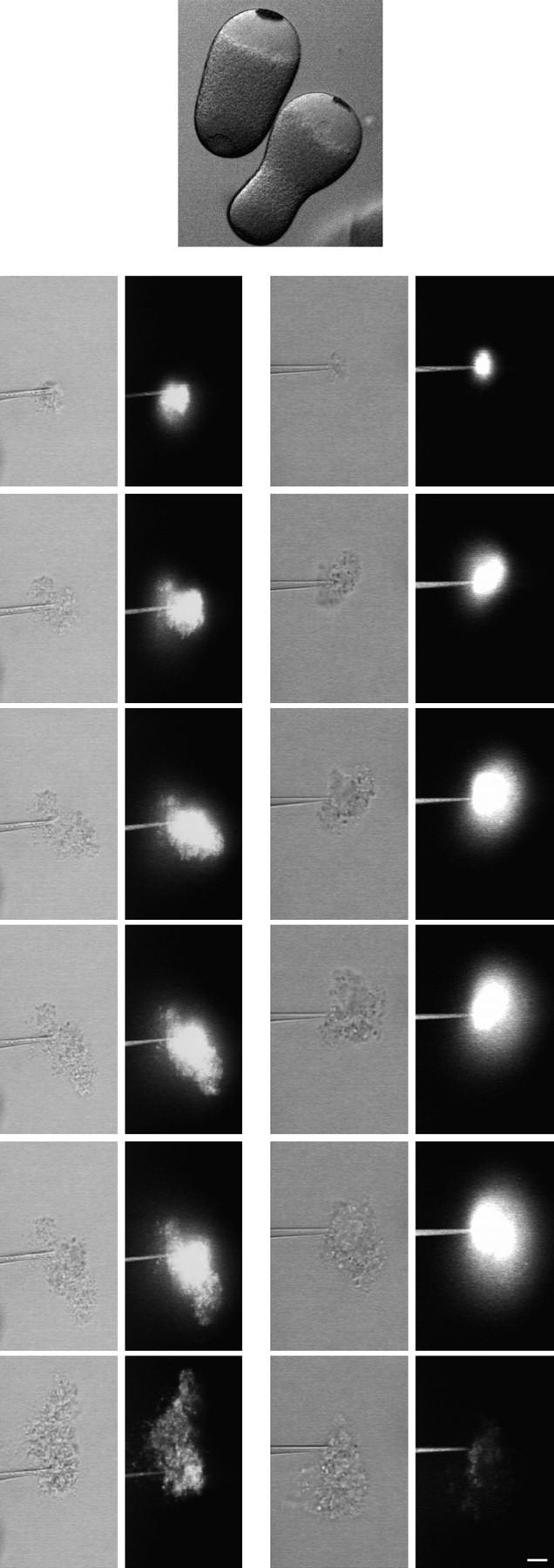
SW of varying Ca2+ concentration was injected to determine the threshold for the formation of the wound vesicle. SW containing 1.9 mM Ca2+ did not form a stable wound vesicle, as indicated by the declining ratio measurement of Fig. 8. Indeed, 1.9 mM Ca2+ yielded a response that was indistinguishable from that elicited by artificial SW to which no Ca2+ was added. SW containing 3 mM Ca2+ formed a more stable vesicle than did 1.9 mM, but one that was quantitatively (Fig. 8) and qualitatively (it could often be observed to break up into numerous smaller vesicles) less stable than those formed with 4.9 mM or normal SW (∼10 mM Ca2+).
Figure 8.
Ca2+ dependence for forming a wound vesicle. SW containing FS and with varying Ca2+ concentration was injected into starfish oocytes and imaged by confocal microscopy. The ratio of fluorescence at the injection site compared with cytoplasmic fluorescence far from the injection site was determined from measurements of the average fluorescence brightness in a small region at these two sites. A high ratio indicates containment of the SW in a wound vesicle whereas a ratio of 1.0 indicates uniform spreading throughout the cytoplasm. Under these conditions, there is a threshold concentration of ∼3 mM Ca2+ for forming stable wound vesicles.
Mg2+, a group II divalent cation, was present at 48.4 mM in the Ca2+-free SW used above, and so we knew this divalent did not induce wound vesicle formation or allow resealing of large disruptions. Strontium and barium are also group II divalent cations, and sometimes have similar physiological effects as Ca2+ (Hille, 1992). When 10 mM SrCl2 added to CFSW was injected, a wound vesicle did not form, but instead the eggs became partially activated as indicated by fertilization envelope elevation near the injection site (three out of three sea urchin eggs; 2% volume injection). Precipitates formed when 10 mM BaCl2 was added to CFSW, so Ba2+ was injected instead in 500 mM NaCl. This did not form a wound vesicle nor cause fertilization envelope elevation (three out of three eggs; as control, 10 mM CaCl2 in 500 mM NaCl formed a wound vesicle in three of three eggs). Therefore, wound vesicle formation appears to be regulated specifically by Ca2+, as opposed to divalent cations in general.
The wound vesicle was generally very stable. In one experiment, eight starfish immature oocytes were injected with a 2% volume of SW. The oocytes were then matured by the hormone 1-methyladenine and fertilized; the wound vesicles remained through the meiotic divisions as well as fertilization and first mitosis in all eight eggs (cleavage was normal in five out of eight eggs). In another experiment, 12 starfish immature oocytes were injected with a 2% volume of SW and cultured for 3 d. Eight out of 12 oocytes survived, and in each, the wound vesicle was still intact.
Extrusion of Cytoplasm into SW Causes Fusion of Intracellular Membranes
We devised another way to test whether Ca2+ causes intracellular membranes to fuse and form boundaries. Mature starfish eggs were first injected with a fluorescent marker (200 μg/ml 70-kD Fl FDx final concentration). After the fluorescent marker had diffused throughout the cytosol, egg cytoplasm was removed by suction using a microneedle. The cytoplasm was then extruded into solutions with or without calcium. When cytoplasm was extruded into CFSW or an intracellular buffer, the fluorescence diffused away; in contrast, when cytoplasm was extruded into SW, a large amount of the fluorescence remained with the extruded cytoplasm (Fig. 9). This is consistent with Ca2+-induced fusion of intracellular membranes which encloses the cytosol as it is extruded. When cytoplasm was extruded into 1 mM Ca2+, significantly less of the dye was retained. This concentration is similar to the threshold for creating wound vesicles, and suggests that this process is similar to what occurs when Ca2+ solutions are injected into the cytoplasm.
Which Intracellular Membranes Are Involved?
The Nile red staining described above suggested that the yolk granules fused with one another around the SW injection site (Fig. 6). To determine whether there is a functional requirement for the yolk platelet compartment in wound vesicle formation, we made use of stratified sea urchin eggs. Centrifugation at 12,000 g causes the yolk platelets to sediment within the egg, leaving a clear area of cytoplasm at the other (centripetal) end of the egg (Fig. 10; Harvey, 1956). The ER is concentrated in the clear area, though there is some ER present among the yolk platelets (Henson et al., 1989). At a time when knowledge of intracellular organelles was fragmentary at best, Heilbrunn (1930b) reported that surface wounds healed better in the yolk platelet end of the egg. We confirmed this (data not shown), which suggests that the yolk platelets are required for healing of large wounds, and that the ER is not sufficient.
FS was injected into stratified eggs, then cytoplasm from either the clear area or the yolk-containing area was removed by micropipet and extruded into SW. There was significantly more dye retained by the cytoplasm containing yolk platelets compared to the clear cytoplasm (Fig. 10), suggesting that yolk platelets are involved in sealing off cytoplasm and that ER membranes by themselves cannot.
SW was injected into fertilized sea urchin eggs because there is evidence that ER membranes become transiently disrupted at fertilization (Jaffe and Terasaki, 1993; Terasaki et al., 1996). When SW was injected at 1–2 min after the fertilization envelope first began to rise, the wound vesicle still formed (three out of three eggs). This also suggests that the ER is not involved in wound vesicle formation.
DISCUSSION
Plasma membrane disruption is a normal and common form of cell injury in many mammalian tissues (McNeil, 1993), and possibly also in other mechanically challenging cellular environments as well. It is an injury that compromises a crucial barrier function, one that is often, but not always, survived by cells in culture and in vivo.
Why are disruptions sometimes lethal? The answer is not known, but one possibility is failure to adequately prevent entry of potentially toxic levels of Ca2+ (Trump and Berezesky, 1995). As Ca2+ is present outside the cell at an ∼104-fold higher concentration than in the cytosol, it is not surprising that whole cell or population measurements reveal a rapid but transient wound-induced rise in cytosolic Ca2+ concentration (McNeil et al., 1985; Steinhardt et al., 1994). Another possibility is that loss of crucial cellular constituents through the disruption is fatal.
What is the mechanism for preventing Ca2+ influx or protein escape? First of all, Ca2+ itself is the signal to repair a disruption. Heilbrunn discovered that healing of large surface wounds does not occur in the absence of extracellular Ca2+ in sea urchin eggs and in many other cells (Heilbrunn, 1930a ,b; summarized in Heilbrunn, 1958). He termed this Ca2+-dependent process the “surface precipitation reaction” and likened it to blood clotting, another process that requires Ca2+. Thus, he envisioned this reaction as involving a “coagulation” of cytoplasmic components—particularly protein filaments—which, once formed, plugged the disruption site, and thereby repaired the surface defect, allowing the cell to survive. Due to uncertainties about the nature of the cell surface, Heilbrunn did not advocate the possibility of Ca2+-induced membrane fusion.
We know of no example of a boundary composed solely of proteins that can exclude small molecules (<10,000 mol wt). Cytosol, even when gelled by elevation of Ca2+ to >5 μM for extended periods, does not significantly restrict the mobility of 10-kD FDx (Luby-Phelps, 1994). Blood clots slow protein diffusion by only ∼20% relative to water alone (Blinc and Francis, 1996). Proteinaceous, Ca2+-induced precipitates of cytosol formed in vitro are readily redissolved by incubation in EGTA, showing that this membrane impermeant molecule can diffuse into such precipitates (Nakajo et al., 1984). Lipid bilayers are the only demonstrated biological barrier to molecule diffusion of the kind described here in the egg response to plasma membrane disruption.
The simplest mechanism of resealing, used by erythrocyte ghosts and liposomes, occurs spontaneously as the energetically favored outcome of disruption-induced exposure of the hydophobic residues of phospholipid molecules to water (for review see McNeil and Steinhardt, 1997). The torn membrane edges must rejoin and then, as a final step at least, a fusion event must occur. Resealing in erythrocytes is slow (minutes to hours) and is apparently a Ca2+-independent process. By contrast, most nucleated cells rapidly reseal small disruptions (within sec) by a Ca2+-dependent mechanism (Steinhardt et al., 1994), suggesting that nucleated and nonnucleated cells lacking internal membranes use fundamentally different mechanisms.
Recently, plasma membrane disruption was found to evoke rapid fusion of internal, vesicular membrane with the plasma membrane (Fig. 11 A) (Steinhardt et al., 1994; Bi et al., 1995). Such fusion events, which constitute an exocytotic response, appear to be required for resealing, but how exactly they facilitate the resealing process has not been addressed experimentally. An alternative but potentially related mechanism that involves rapid resealing was proposed by Wohlfarth-Bottermann and Stockem (1970), who removed large portions of Physarum plasma membrane, and then prepared this giant, unicellular organism for EM at short (1–10 s) intervals thereafter. The electron micrographs indicated that extensive vesicle–vesicle fusion was rapidly induced at the interface of naked cytoplasm with the external medium, and the result was the formation across the disruption gap of a new, continuous plasma membrane sheet from this enlarging vesicle.
Figure 11.
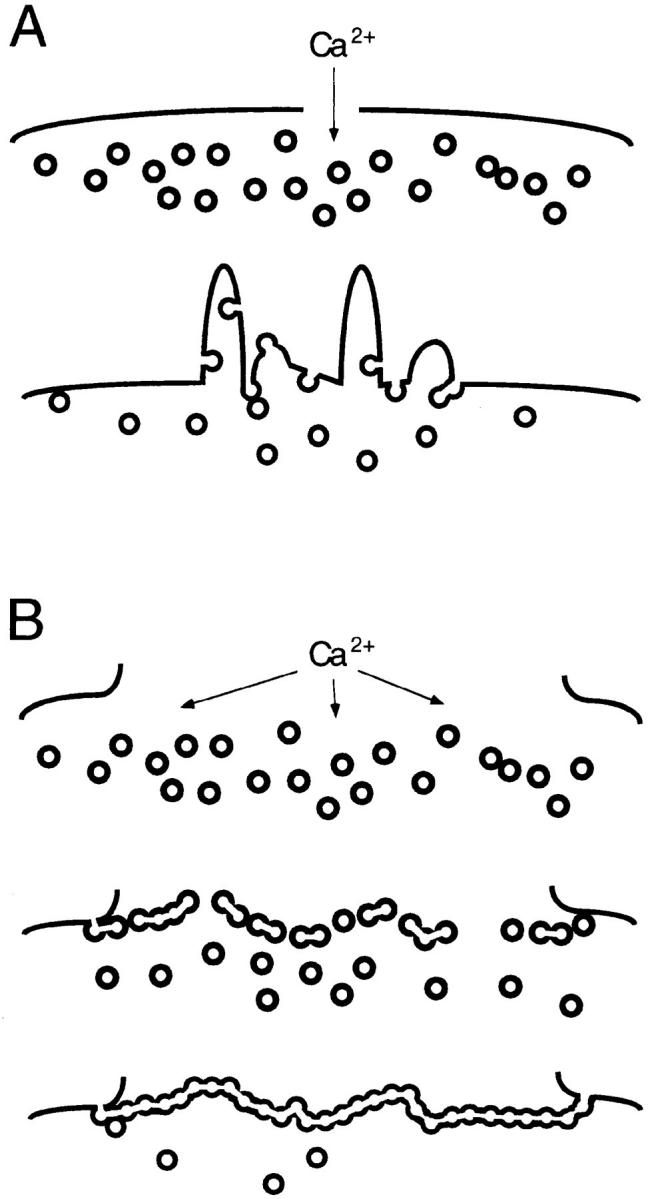
Mechanisms for rapid resealing. (A) Small disruptions evoke vesicle transport to the breached site, followed by an exocytotic reaction at this site; vesicle–plasma membrane fusion predominates. (B) A large plasma membrane disruption evokes the rapid formation of a large membrane sheet across the breach site, followed by exocytotic joining of this sheet with the plasma membrane; vesicle– vesicle fusion predominates. See text for further discussion of this model.
We here show that large disruptions (>10 μm2) can be rapidly resealed on approximately the same time scale as much smaller, microneedle-induced disruptions (Steinhardt et al., 1994). The echinoderm egg or oocyte is able, within 5–10 s after suffering a large disruption (∼40 by 10 μm), to completely prevent the further influx of exogenous molecules, including 10-kD FDx, ∼1-kD FS, and Ca2+, down a steep concentration gradient into cytosol. We suggest that large disruptions such as these cannot be rapidly resealed merely by exocytotic fusion events. We propose instead (Fig. 11 B) that Ca2+ entering through a large disruption initiates vesicle–vesicle fusion. This forms a large vesicular sheet of membrane, as originally envisioned by Wohlfarth-Bottermann and Stockem (1970; see above). Fusion of this sheet with the plasma membrane, that is, vesicle–plasma membrane fusion, then completes resealing.
This second model for rapid resealing (Fig. 11 B) suggests that in addition to exocytosis, organelle–organelle fusion is induced by disruption and is crucial for rapid resealing. If this were the case, then cytoplasm could be separated from the plasma membrane and still form a barrier when exposed to the high Ca2+ in SW. We achieved this separation experimentally in the following two ways: by injecting SW into cytoplasm and by injecting cytoplasm into SW. In both cases, a de novo barrier formed that could restrict for hours the diffusion of FDx, FS, or Ca2+. EM of the SW-induced barrier demonstrated that this boundary displayed the electron-dense morphology expected of a cell membrane. Moreover, this boundary could be stained with DiI, a lipophilic dye that stains membranes.
We propose that exposure of cytoplasm to high Ca2+ caused by a wound results in massive fusion of internal vesicles with each other and with the plasma membrane. These fusion reactions proceed rapidly until a patch of membrane continuous with the plasma membrane has formed, preventing further Ca2+ entry. There are other reactions in response to injury which are likely to be secondary to fusion-mediated resealing, such as alterations in the actin-based cytoskeleton (Jeon and Jeon, 1975; Taylor et al., 1980).
In some cases, the mechanism we propose may not be sufficient to make a rapid seal, whereupon the cell must depend on more slowly acting mechanisms. For instance, transection of large diameter axons (>70 μm) from earthworm or crayfish takes minutes to seal (Krause et al., 1994; Eddleman et al., 1997). These axons are densely packed with cytoskeletal structures and may not support the fusion of enough vesicles to rapidly form a complete membrane boundary. In support of this possibility, an injection of SW is not encapsulated in the squid giant axon (Terasaki, M., unpublished observations). Vesicles (>1 μm) are induced by transection (Fishman et al., 1990), and are found to plug the wound (Krause et al., 1994; Gallant et al., 1995). Vesicles may be derived from glia membranes or from Ca2+ stimulated endocytosis from the axolemma (Eddleman et al., 1997). Constriction of the axonal sheath probably also facilitates wound healing (Gallant, 1988).
In echinoderm eggs, where rapid sealing occurs, there are many new structures in the vicinity of the healed plasma membrane boundary. Outside of the wound created by a rip-off, there are numerous, spherically shaped domains of various sizes (Fig. 3), and on the cytoplasmic side of the SW injection vesicle there are many large membrane-bounded compartments (Fig. 7, A and B, indicated by solid dots). We propose that these are also results of Ca2+-induced fusion events, and that they represent the membranes that did not become incorporated into the plasma membrane. In this view, at least some of the large spherical domains outside of the cell are regions of cytoplasm that are surrounded by a new membrane boundary, whereas the compartments on the cytoplasmic side of the wound are vesicles derived from fusion of smaller compartments.
A wound vesicle formed by vesicle–vesicle fusion in response to a focal source of elevated Ca2+ (e.g., a SW injection) is predicted (Fig. 11) to consist of two major domains: (a) an innermost domain, consisting of the high calcium source (injected SW); and (b) a surrounding domain, consisting of the contents of the vesicle population used (probably the yolk granules, in the model studied here). In fluorescence micrographs of SW-injected eggs stained with Nile red, both of these domains are in fact present: an unstained, SW-filled, innermost domain surrounded by a Nile red–stained outer domain that, in turn, is surrounded by normal appearing cytoplasm. In electron micrographs, too, these two domains are evident. However, a further prediction is that the outer domain, containing the contents of multiple vesicle lumina, would be delimited on each of its boundaries with a continuous membrane. EM performed on eggs fixed 20 min after SW injection confirmed this prediction only partially: a continuous membrane was observed on the SW but not the cytoplasmic side of the outer domain. Its absence may be artifactual in the limited sample examined by EM. Or, if real, its absence suggests that in addition to simple vesicle–vesicle fusion reactions, other membrane-transforming events are also occurring which destabilize or degrade the outer domain's cytoplasmic membrane boundary.
The egg's rapid, de novo formation of a continuous membrane sheet is Ca2+ dependent, but displays an exceptionally high threshold (∼3 mM) in the SW injection experiment. In this it resembles the Ca2+ threshold previously reported for resealing of small plasma membrane disruptions (Steinhardt et al., 1994). Perhaps this high threshold insures that the disruption-induced membrane– membrane fusion mechanism is only activated in case of emergency, when the cytoplasm is exposed to the potentially lethal environment of the outside world. Inadvertent activation would waste organelles and could possibly create abnormal, and hence potentially disruptive internal boundaries.
In echinoderm eggs, the yolk platelet appears to be the organelle principally involved in the fusion events leading to formation of the protective membrane sheet, whereas the ER is probably not involved. In the vicinity of a wound vesicle, the yolk platelets often appear to have fused or disintegrated. Moreover, when the yolk platelet–rich cytoplasm of the centrifuged egg was injected into SW, it was capable, like whole cytoplasm, of trapping dissolved FDx, whereas the ER-rich cytoplasmic domain was not. It is interesting to note that Ca2+ causes isolated sea urchin egg yolk platelets to fuse with each other (Vogel, S.S., unpublished results) whereas Ca2+ does not cause isolated ER microsomes to fuse with each other (Paiement and Bergeron, 1991).
Preliminary evidence (Terasaki, M., and P. McNeil, unpublished results) suggests that the wound vesicle is an acidic compartment as soon as 1 min after its formation. This is an indication that the wound vesicle is at least partially formed from the fusion of endocytic or lysosomal compartments. Such an involvement can be rationalized on the grounds that endocytic membranes are derived from the plasma membrane. There is considerable recent evidence from other systems consistent with an endocytic membrane involvement in wound healing. Fusion of the endosomal/lysosomal compartment vesicles with the plasma membrane, and with one another, is induced by Ca2+ elevation in broken cell preparations (Mayorga et al., 1994), and in intact fibroblast and endothelial cells experiencing plasma membrane disruptions (Miyake and McNeil, 1995) and calcium ionophore treatments (Rodriguez et al., 1997) that elevate cytosolic Ca2+. In axons, endocytosis is apparently induced by transection, and this newly formed vesicle population is suggested to participate in repair (Eddleman et al., 1997). There is much uncertainty about the origin of echinoderm egg yolk platelets (e.g., Smiley, 1990) but it is likely that they are similar to frog egg yolk platelets, which are a derivative of endocytic membranes (Wallace and Jared, 1976) and which have a low pH (Fagotto and Maxfield, 1994). Clearly, it is necessary to find out more about echinoderm yolk platelets, because it will help to determine in what ways these experiments with eggs are applicable to somatic cells.
The molecular components of the disruption-induced exocytotic response (Bi et al., 1995; Miyake and McNeil, 1995), and of vesicle mobilization to wound sites before exocytosis, are now partially characterized. Steinhardt et al. microinjected into fertilized urchin eggs and fibroblasts various toxins known to specifically target membrane docking/fusion proteins, antibodies capable of blocking kinesin function, and peptides that competitively inhibit multifunctional Ca2+/calmodulin kinase activity (Steinhardt et al., 1994). Resealing of microneedle punctures was inhibited by all of these reagents. It was suggested, based on the functional requirement for this set of proteins, that resealing of small disruptions is accomplished by a docking/fusion mechanism similar in its molecular composition to that used in neurotransmitter release at the synapse. In addition to docking and fusion events, disruption-induced exocytosis is postulated to involve active vesicle movement to and accumulation at the disruption site. In fact, an extremely high density of vesicles has been visualized by EM to surround disruption sites (Miyake and McNeil, 1995; Eddleman et al., 1997).
There is, however, very little data to suggest how the vesicle–vesicle fusion induced by disruptions is engineered at the molecular level. In broken cell preparations of the egg, Ca2+-dependent, vesicle–vesicle fusion can be observed but appears to use an unorthodox mechanism. Homotypic fusion of isolated cortical granule vesicles is a trypsin- and N-ethyl maleimide (NEM)-sensitive reaction (Vogel et al., 1992). Surprisingly, however, when only one of two vesicle populations is trypsinized, fusion is still possible (Vogel et al., 1992). Furthermore, in distinction from other described mechanisms, this fusion event does not require ATP or GTP as cofactors. Yolk platelets, too, undergo homotypic fusion in vitro in the presence of Ca2+ and absence of ATP or GTP (Vogel, S.S., unpublished communication).
In summary, we propose that a replacement patch of bilayer is erected in the cytoplasm bordering on a plasma membrane disruption. Ca2+-regulated vesicle–vesicle membrane fusion events are responsible for this rapidly completed event. Exocytotic events, e.g., vesicle–plasma membrane fusions occurring at the same time, join the bilayer patch to the plasma membrane discontinuity. We speculate that disruption-induced vesicle–vesicle and vesicle– plasma membrane fusion events may use a primitive, and relatively indiscriminate molecular mechanism, possessing an exceptionally high threshold for Ca2+ (∼3 mM). The vesicle population mobilized is most likely the endosomal/ lysosomal compartment, but could involve others as well. Vesicle–vesicle fusion-promoting mechanisms may have initially evolved for the purpose of repairing mechanically initiated damage to the cell surface, rather than moving molecules from one compartment to another. If so, resealing may involve a basic or bare bones fusion apparatus, to which numerous additions have been made in evolution as new and more complex functions were assumed. Investigation of the molecular apparatus of resealing may, therefore, provide a better understanding of more complex and difficult-to-study membrane–membrane fusion systems, such as those involved in neurotransmission (Rothman and Sollner, 1994) and intracellular protein trafficking (Rothman and Wieland, 1996), to name just two of many possible examples. New strategies for promoting the survival of plasma membrane disruptions, with the aim, for example, of improving neuronal recovery after traumatic injury or muscle regeneration in a wasting disease such as Duchenne muscular dystrophy, may be possible based on a better understanding of the wound healing process.
Acknowledgments
We thank A. Hand (University of Connecticut Health Center) for help with EM, J. Galbraith (Duke University, Durham, NC) for making the OMDR trigger, D. Serwanski (University of Connecticut Health Center) for technical assistance, and L.A. Jaffe (University of Connecticut Health Center) for loan of equipment. We also thank S. Vogel (Medical College of Georgia), J. Heuser (Washington University Medical School, St. Louis, MO), L.F. Jaffe (Marine Biological Laboratory), D. Kiehart (Duke University, Durham, NC), B. Ehrlich (Yale University, New Haven, CT), and B. Kaminer (Boston University Medical School, Boston, MA) for useful discussions, and L.A. Jaffe (University of Connecticut Health Center) for reading the manuscript. Most of this work was done at the Marine Biological Laboratories; we thank T. Reese (National Institutes of Health) for generously providing laboratory space and facilities.
This work was supported by grants to P.L. McNeil from the Muscular Dystrophy Association and the National Institutes of Health (48091) and by a grant to M. Terasaki from the Patrick and Catherine Weldon Donaghue Medical Research Foundation.
Abbreviations used in this paper
- CFSW
Ca2+-free sea water
- Fdx
fluorescein dextran
- FS
fluorescein stachyose
- SW
sea water
Footnotes
Quicktime movies of most of the figures are available at http://www.uchc.edu/∼terasaki/resealing.html
REFERENCES
- Bi G-Q, Alderton JM, Steinhardt RA. Calcium-regulated exocytosis is required for cell membrane resealing. J Cell Biol. 1995;131:1747–1758. doi: 10.1083/jcb.131.6.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinc A, Francis CE. Transport processes in fibrinolysis and fibrinolytic therapy. Thromb Haemostasis. 1996;76:481–491. [PubMed] [Google Scholar]
- Casademont J, Carpenter S, Karpati G. Vacuolation of muscle fibers near sarcolemmal breaks represents T tubule dilatation secondary to enhanced sodium pump activity. J Neuropathol Exp Neurol. 1988;47:618–628. doi: 10.1097/00005072-198811000-00005. [DOI] [PubMed] [Google Scholar]
- Chambers, R., and E.L. Chambers. 1961. Explorations into the Nature of the Living Cell. Harvard University Press, Cambridge, MA. 352 pp.
- Chandler DE, Heuser J. The vitelline layer of the sea urchin egg and its modification during fertilization. A freeze-fracture study using quick-freezing and deep-etching. J Cell Biol. 1980;84:618–632. doi: 10.1083/jcb.84.3.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilchik MV, Gerhart JC. Differentiation of the animal-vegetal axis in Xenopus laevis oocytes. I. Polarized intracellular translocation of platelets establishes the yolk gradient. Dev Biol. 1987;122:101–112. doi: 10.1016/0012-1606(87)90336-8. [DOI] [PubMed] [Google Scholar]
- Detering NK, Decker GL, Schmell ED, Lennarz WJ. Isolation and characterization of plasma membrane associated cortical granules from sea urchin eggs. J Cell Biol. 1977;75:899–914. doi: 10.1083/jcb.75.3.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddleman CS, Ballinger ML, Smyers ME, Godell CM, Fishman HM, Bittner GD. Repair of plasmalemmal lesions by vesicles. Proc Natl Acad Sci USA. 1997;94:4745–4750. doi: 10.1073/pnas.94.9.4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagotto F, Maxfield FR. Yolk platelets in Xenopus oocytes maintain an acidic internal pH which may be essential for sodium accumulation. J Cell Biol. 1994;125:1047–1056. doi: 10.1083/jcb.125.5.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman HM, Tewari KP, Stein PG. Injury-induced vesiculation and membrane redistribution in squid giant axon. Biochim Biophys Acta. 1990;1023:421–435. doi: 10.1016/0005-2736(90)90135-b. [DOI] [PubMed] [Google Scholar]
- Fuseler JW. Repetitive procurement of mature gametes from individual sea stars and sea urchins. J Cell Biol. 1973;57:879–881. doi: 10.1083/jcb.57.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant PE. Effects of the external ions and metabolic poisoning on the constriction of the squid giant axon after axotomy. J Neurosci. 1988;8:1479–1484. doi: 10.1523/JNEUROSCI.08-05-01479.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant PE, Hammar K, Reese TS. Cytoplasmic constriction and vesiculation after axotomy in the squid giant axon. J Neurocytol. 1995;24:943–954. doi: 10.1007/BF01215644. [DOI] [PubMed] [Google Scholar]
- Greenspan P, Mayer EP, Fowler SD. Nile red: a selective fluorescent stain for intracellular lipid droplets. J Cell Biol. 1985;100:965–973. doi: 10.1083/jcb.100.3.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner M, Petzelt C, Nobiling R, Pawley JB, Kramp D, Schatten G. Wave of free calcium at fertilization in the sea urchin egg visualized with fura-2. Cell Motil Cytoskeleton. 1988;9:271–277. doi: 10.1002/cm.970090309. [DOI] [PubMed] [Google Scholar]
- Hamaguchi Y, Hiramoto Y. Activation of sea urchin eggs by microinjection of calcium buffers. Exp Cell Res. 1981;134:171–179. doi: 10.1016/0014-4827(81)90474-2. [DOI] [PubMed] [Google Scholar]
- Haugland R.P. 1996. Handbook of Fluorescent Probes and Research Chemicals, 6th ed. Molecular Probes, Inc., Eugene, OR. 679 pp.
- Harvey, E.B. 1956. The American Arbacia and Other Sea Urchins. Princeton University Press, Princeton, NJ. 298 pp.
- Heilbrunn LV. The action of various salts on the first stage of the surface precipitation reaction in arbacia egg protoplasm. Protoplasma. 1930a;11:558–573. [Google Scholar]
- Heilbrunn LV. The surface precipitation reaction of living cells. Proc Am Philos Soc. 1930b;419:295–301. [Google Scholar]
- Heilbrunn, L.V. 1958. The Dynamics of Living Protoplasm. Academic Press, New York. 634 pp.
- Henson JH, Begg DA, Beaulieu SM, Fishkind DJ, Bonder EM, Terasaki M, Lebeche D, Kaminer B. A calsequestrin-like protein in the endoplasmic reticulum of the sea urchin: localization and dynamics in the egg and first cell cycle embryo. J Cell Biol. 1989;109:149–161. doi: 10.1083/jcb.109.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille, B. 1992. Ionic Channels of Excitable Membranes. Sinauer Associates, Inc., Sunderland, MA. 607 pp.
- Hiramoto Y. Microinjection of live spermatozoa into sea urchin eggs. Exp Cell Res. 1962;27:416–426. doi: 10.1016/0014-4827(62)90006-x. [DOI] [PubMed] [Google Scholar]
- Hiramoto Y. Further studies on cell division without mitotic apparatus in sea urchin eggs. J Cell Biol. 1965;25:161–167. doi: 10.1083/jcb.25.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe LA, Terasaki M. Structural changes of the endoplasmic reticulum of sea urchin eggs during fertilization. Dev Biol. 1993;156:556–573. doi: 10.1006/dbio.1993.1103. [DOI] [PubMed] [Google Scholar]
- Jeon KW, Jeon MS. Cytoplasmic filaments and cellular wound healing in Amoeba proteus. J Cell Biol. 1975;67:243–249. doi: 10.1083/jcb.67.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay, E., and B.M. Shapiro. 1985. The formation of the fertilization membrane of the sea urchin egg. Biology of Fertilization. Vol. 3. C.B. Metz and A. Monroy, editors. Academic Press, Orlando, FL. 469 pp.
- Kiehart DP. Studies on the in vivo sensitivity of spindle microtubules to calcium ions and evidence for a vesicular calcium-sequestering system. J Cell Biol. 1981;88:604–617. doi: 10.1083/jcb.88.3.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehart DP. Microinjection of echinoderm eggs: apparatus and procedures. Methods Cell Biol. 1982;25:13–31. doi: 10.1016/s0091-679x(08)61418-1. [DOI] [PubMed] [Google Scholar]
- Krause TL, Fishman HM, Ballinger ML, Bittner GD. Extent and mechanism of sealing in transected giant axons of squid and earthworms. J Neurosci. 1994;14:6638–6651. doi: 10.1523/JNEUROSCI.14-11-06638.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby-Phelps K. Physical properties of cytoplasm. Curr Opin Cell Biol. 1994;6:3–9. doi: 10.1016/0955-0674(94)90109-0. [DOI] [PubMed] [Google Scholar]
- Mayorga LS, Beron W, Sarrouf MN, Colombo MI, Creutz C, Stahl PD. Calcium-dependent fusion among endosomes. J Biol Chem. 1994;9:30927–30934. [PubMed] [Google Scholar]
- Mazia D, Schatten G, Sale W. Adhesion of cells to surfaces coated with polylysine. Applications to electron microscopy. J Cell Biol. 1975;66:198–200. doi: 10.1083/jcb.66.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil PL. Cellular and molecular adaptations to injurious mechanical force. Trends Cell Biol. 1993;3:302–307. doi: 10.1016/0962-8924(93)90012-p. [DOI] [PubMed] [Google Scholar]
- McNeil PL, McKenna MP, Taylor DL. A transient rise in cytosolic calcium follows stimulation of quiescent cells with growth factors and is inhibitable with phorbol myristate acetate. J Cell Biol. 1985;101:372–379. doi: 10.1083/jcb.101.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil PL, Steinhardt RA. Loss, restoration and maintenance of plasma membrane integrity. J Cell Biol. 1997;137:1–4. doi: 10.1083/jcb.137.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil PL, Tanasugarn L, Meigs JB, Taylor DL. Acidification of phagosomes is initiated before lysosomal enzyme activity is detected. J Cell Biol. 1983;97:692–702. doi: 10.1083/jcb.97.3.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K, McNeil PL. Vesicle accumulation and exocytosis at sites of plasma membrane disruption. J Cell Biol. 1995;131:1737–1745. doi: 10.1083/jcb.131.6.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajo S, Nakaya K, Yoshida T, Nakamura Y. Characterization of a protein kinase–substrate complex precipitable with Ca2+from the cytosol fraction of AH-66 hepatoma cells. Biochem Biophys Res Commun. 1984;125:251–257. doi: 10.1016/s0006-291x(84)80361-7. [DOI] [PubMed] [Google Scholar]
- Paiement, J.M., and J.J.M. Bergeron. 1991. Specific conditions for fusion of membranes of nuclear envelope, endoplasmic reticulum and Golgi apparatus from vertebrate cells. Membrane Fusion. J. Wilschut and D. Hoekstra, editors. Marcel Dekker Inc., New York. pp. 463–491.
- Rodriguez A, Webster P, Ortego J, Andrews NW. Lysosomes behave as Ca2+-regulated exocytotic vesicles in fibroblasts and epithelial cells. J Cell Biol. 1997;137:93–104. doi: 10.1083/jcb.137.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JE, Wieland FT. Protein sorting by transport vesicles. Science (Wash DC) 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- Smiley S. A review of echinoderm oogenesis. J Electron Microsc. 1990;16:93–114. doi: 10.1002/jemt.1060160203. [DOI] [PubMed] [Google Scholar]
- Sollner T, Rothman JE. Neurotransmission: harnessing fusion machinery at the synapse. TINS (Trends Neurosci) 1994;17:344–348. doi: 10.1016/0166-2236(94)90178-3. [DOI] [PubMed] [Google Scholar]
- Steinhardt RA, Lundin L, Mazia D. Bioelectric responses of the echinoderm egg to fertilization. Proc Natl Acad Sci USA. 1971;68:2426–2430. doi: 10.1073/pnas.68.10.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhardt RA, Zucker R, Schatten G. Intracellular calcium release at fertilization in the sea urchin egg. Dev Biol. 1977;48:185–196. doi: 10.1016/0012-1606(77)90084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhardt RA, Bi G, Alderton JM. Cell membrane resealing by a vesicular mechanism similar to neurotransmitter release. Science (Wash DC) 1994;263:390–393. doi: 10.1126/science.7904084. [DOI] [PubMed] [Google Scholar]
- Taylor DL, Wang YL, Heiple JM. Contractile basis of ameboid movement. VII. the distribution of fluorescently labeled actin in living amebas. J Cell Biol. 1980;86:590–598. doi: 10.1083/jcb.86.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki M, Jaffe LA. Organization of the sea urchin egg endoplasmic reticulum and its reorganization at fertilization. J Cell Biol. 1991;114:929–940. doi: 10.1083/jcb.114.5.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki M, Jaffe LA, Hunnicutt GR, Hammer JA. Structural change of the endoplasmic reticulum during fertilization: evidence for loss of membrane continuity using the green fluorescent protein. Dev Biol. 1996;179:320–328. doi: 10.1006/dbio.1996.0263. [DOI] [PubMed] [Google Scholar]
- Trump BF, Berezesky IK. Calcium-mediated cell injury and cell death. FASEB (Fed Am Soc Exp Biol) J. 1995;9:219–228. doi: 10.1096/fasebj.9.2.7781924. [DOI] [PubMed] [Google Scholar]
- Vacquier VD. The isolation of intact cortical granules from sea urchin eggs: calcium ions trigger granule discharge. Dev Biol. 1975;43:62–74. doi: 10.1016/0012-1606(75)90131-1. [DOI] [PubMed] [Google Scholar]
- Vogel SS, Chernomordik LV, Zimmerberg J. Calcium-triggered fusion of exocytotic granules requires proteins in only one membrane. J Biol Chem. 1992;267:25640–25643. [PubMed] [Google Scholar]
- Wallace RA, Jared DW. Protein incorporation by isolated amphibian oocytes. V. Specificity for vitellogenin incorporation. J Cell Biol. 1976;69:345–351. doi: 10.1083/jcb.69.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfarth-Bottermann KE, Stockem W. Die regeneration des plasmalemms von physarum polycephalum. Wilhelm Roux' Archiv Fuer. 1970;164:321–340. doi: 10.1007/BF00577809. [DOI] [PubMed] [Google Scholar]
- Yawo H, Kuno M. Calcium dependence of membrane sealing at the cut end of the cockroach giant axon. J Neurosci. 1985;5:1626–1632. doi: 10.1523/JNEUROSCI.05-06-01626.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]