Abstract
The assembly of the vessel wall from its cellular and extracellular matrix components is an essential event in embryogenesis. Recently, we used the descending aorta of the embryonic quail to define the morphological events that initiate the formation of a multilayered vessel wall from a nascent endothelial cell tube (Hungerford, J.E., G.K. Owens, W.S. Argraves, and C.D. Little. 1996. Dev. Biol. 178:375–392). We generated an mAb, 1E12, that specifically labels smooth muscle cells from the early stages of development to adulthood. The goal of our present study was to characterize further the 1E12 antigen using both cytological and biochemical methods. The 1E12 antigen colocalizes with the actin cytoskeleton in smooth muscle cells grown on planar substrates in vitro; in contrast, embryonic vascular smooth muscle cells in situ contain 1E12 antigen that is distributed in threadlike filaments and in cytoplasmic rosette-like patterns. Initial biochemical analysis shows that the 1E12 mAb recognizes a protein, M r = 100,000, in lysates of adult avian gizzard. An additional polypeptide band, M r = 40,000, is also recognized in preparations of lysate, when stronger extraction conditions are used. We have identified the 100-kD polypeptide as smooth muscle α-actinin by tandem mass spectroscopy analysis. The 1E12 antibody is an IgM isotype. To prepare a more convenient 1E12 immunoreagent, we constructed a single chain antibody (sFv) using recombinant protein technology. The sFv recognizes a single 100-kD protein in gizzard lysates. Additionally, the recombinant antibody recognizes purified smooth muscle α-actinin. Our results suggest that the 1E12 antigen is a member of the α-actinin family of cytoskeletal proteins; furthermore, the onset of its expression defines a primordial cell restricted to the smooth muscle lineage.
The proper recruitment and subsequent differentiation of primordial vascular smooth muscle cells (VSMCs)1 is essential for formation of a functionally mature vessel wall. The regulation of these processes is not well understood, due in part to the very nature of the VSMC. Unlike most other cell types, VSMCs do not terminally differentiate into a single phenotype. Instead, VSMCs exist along a continuum of phenotypes during embryogenesis and subsequent maturity. At one extreme is a synthetic, fibroblastic cell, and at the other is a physiologically mature, contractile cell (Chamley-Campbell et al., 1981; Thyberg et al., 1990; Owens, 1995). Therefore, within the developing vessel wall, the VSMC plays both a synthetic and contractile role. The ability of VSMCs to modulate their phenotype is an integral part of the VSMC differentiation program.
Skeletal muscle maturation is perhaps the best example of a paradigm for the regulation of differentiation (for reviews see Olson, 1993a ,b; Ontell et al., 1995). Marker proteins for the various events of skeletal muscle maturation have been defined, and several families of regulatory genes that control the skeletal muscle differentiation program have been identified. In contrast, specific marker proteins for the initial stages of smooth muscle cell (SMC) development have not been identified, despite extensive effort. The smooth muscle α-actin (SMαA) gene that encodes for the major contractile protein isoform found in mature VSMCs (Garrels and Gibson, 1976; Fatigati and Murphy, 1984; Owens and Thompson, 1986) is transiently expressed as an integral part of the differentiation program for both cardiac and skeletal muscle (Ruzicka and Schwartz, 1988; Sawtell and Lessard, 1989; Sugi and Lough, 1992). Expression of the smooth muscle myosin heavy chain gene appears to be tissue specific based on in situ hybridization studies; however, its presence is not detected until after the initial events that establish a multilayered vessel wall (Miano et al., 1994). Similarly, other presumptive “differentiation marker genes,” such as h1calponin and SM22α, are expressed at detectable levels only after a multilayered aortic vessel wall has been established (Duband et al., 1993). Moreover, most of these genes are also transiently expressed as part of the cardiac (calponin and SM22α) and skeletal (SM22α) muscle differentiation programs during early development (Li et al., 1996; Miano and Olson, 1996; Samaha et al., 1996).
Not only has there been a paucity of specific developmental markers, but the molecular mechanisms that regulate smooth muscle cell differentiation remain undefined. Studies from several labs have identified regions within the promoters of smooth muscle genes that account for regulated tissue-specific transcription. These include the SMαA , smooth muscle myosin heavy chain, and SM22α genes, all of which appear to be regulated by distinct transcriptional mechanisms when compared with non–smooth muscle cells (Shimizu et al., 1995; White and Low, 1996; Kallmeier et al., 1996; Li et al., 1996). Several transcription factors have been implicated in the process of SMC differentiation, although there is no indication of which smooth muscle genes are specifically activated by their presence. For example, members of the myocyte enhancer binding factor-2 family of transcription factors have been shown to play a role in the differentiation of skeletal, cardiac, and visceral muscle in Drosophila (Lilly et al., 1995), and thus may be involved in the regulation of a differentiation program common to all muscle lineages (Olson et al., 1995). Two other diverse families of transcription factors, the homeobox genes and GATA genes, may also have members that are important in the regulatory mechanisms of SMC phenotype (Miano et al., 1996; Morrisey et al., 1996). Given these findings, there is to date no clear explanation for how these potential mechanisms are integrated to coordinate the temporal and spacial regulation of smooth muscle–specific genes during SMC differentiation. Clearly, there is a need to define both specific markers and regulators of SMC development and differentiation.
To study the early events of VSMC development and differentiation during the formation of a multilayered vessel wall, we generated mAbs to embryonic vessel wall antigens (Hungerford et al., 1996). Our goal was to identify novel proteins that are expressed as mesodermal cells become committed to the SMC lineage. One mAb, 1E12, specifically labels SMCs in the descending aorta during early avian development (Hungerford et al., 1996). Unlike SMαA, the 1E12 antigen is not expressed as a component of the differentiation program for cardiac and skeletal muscle cells; moreover, expression of the 1E12 antigen has been observed in all smooth muscle tissues within the developing avian embryo (Hungerford, 1995; Hungerford et al., 1996). The 1E12 antigen is first observed 8–12 h after the onset of SMαA expression; furthermore, the 1E12 mAb labels only a subset of the SMαA positive cells, specifically those mesodermal cells most adjacent to the aortic endothelium (stage 17–18). The 1E12-positive cells are presumably more mature than the more peripherally located cells that stain with only the SMαA mAb. In this report we address the hypothesis that mab1E12 defines cells committed to the smooth muscle differentiation program, and also discuss the identity of its cognate tissue-specific antigen.
Materials and Methods
Embryonic Aortic Explants
Dorsal aortae were dissected from 10-d-old embryonic quail, placed in cold Hank's solution, minced, and then transferred to a solution of 1× trypsin-EDTA (GIBCO BRL, Gaithersburg, MD) at 37°C for ∼10 min. DME (GIBCO BRL), supplemented with 10% chicken serum (Sigma Chemical Co., St. Louis, MO), glutamine, penicillin, and streptomycin (Irvine Scientific, Irvine, CA), was added to the aortae/trypsin-EDTA mixture and centrifuged at 1,000 rpm for 10 min. The pellet of small aortic pieces was reconstituted in a small amount of complete media, pipetted onto sterile No. 1 coverslips, and allowed to attach. The resultant explants were cultured ∼3 d (5% CO2 at 37°C). After washing in PBS, cells were fixed and permeabilized in ice-cold methanol for 5 min, followed by dipping in ice cold acetone for several seconds. The fixed, permeabilized cells were then immunolabeled (see below).
Contractile Amniotic Smooth Muscle Cells
Amniotic SMCs were isolated and cultured in serum-free media using a previously described protocol (Bowers and Dahm, 1993). After 5–7 d in culture, the cells were fixed by either the cold methanol/acetone procedure described above or in Omnifix (Zymed Labs, San Franscico, CA), followed by a short (5-s) incubation in ice-cold acetone. Fixed, permeabilized cells were then immunolabeled and analyzed by laser scanning confocal microscopy.
Immunofluorescence Labeling of Coverslips
Fixed and permeabilized aortic smooth muscle cells, amniotic smooth muscle cells, and chicken embryo fibroblasts, grown on glass coverslips, were rehydrated in PBS and blocked (15 min) in 3% BSA/PBS. The cells were then incubated with primary antibody for 30 min to 1 h, washed three times in PBS, and incubated with a fluorochrome-conjugated secondary antibody. Finally, the cells were washed three times in PBS, and the coverslips were mounted on standard slides with Gel Mount (Biomeda Corp., Foster City, CA).
Whole-Mount Immunofluorescent Labeling
Embryos were removed from the yolk, washed in PBS, staged (according to the criteria of Hamburger and Hamilton, 1951), and fixed in methacarn (60% methanol, 30% chloroform, 10% glacial acetic acid). To facilitate diffusion of the antibodies and wash solutions, the fixed tissue was further dissected. Embryos were truncated at the head and tail. Limb buds were removed and a lengthwise cut was made on the ventral surface of the embryonic body wall. Immunolabeling of the embryos was performed as described by Drake et al. (1992), using supernatant from the 1E12 hybridoma and fluorochrome-conjugated (Cy5) secondary antibodies.
Acrylamide Embedding and Vibrotome Sectioning
Whole-mount immunolabeled embryos were postfixed in 3% paraformaldehyde in PBS for 30 min at room temperature, and then embedded in a 15% acrylamide mixture using a modification of Germroth et al. (1995).
Acrylamide-embedded, whole-mount, immunolabeled embryos were sectioned on a Lancer Vibratome (Series 1000; Sherwood Medical, St. Louis, MO) at ∼200 μm thickness. Sections were mounted on clean glass slides in antibleaching medium (5% n-propylgalate, 0.25% 1-4 diaza-bicyclo-(2,2,2)octane, and 0.0025% paraphenylenediamine in glycerol) under a No.1 coverslip.
Immunological Reagents
SMαA mAbs were obtained from Sigma Chemical Co. (clone 1A4) and used diluted (1:200 or 1:400) with PBS. The 1E12 mAb was produced and isotyped as previously described (Hungerford et al., 1996) Undiluted hybridoma culture supernatant was used for immunofluorescent labeling. 1E12 ascites fluid was partially purified on a protamine agarose column (Hudson and Hay, 1980) and concentrated in Aquacide III (CalbiochemNovabiochem Corp., La Jolla, CA) for use in immunoblotting experiments. All secondary antibodies were purchased from Jackson Immunoresearch Laboratories (West Grove, PA) and used at 15 μg/ml. Appropriate controls were used for all of the immunolabeling studies presented in this work. Preimmune (when available) or nonimmune sera were used as a negative control for the primary antibody. Secondary antibody controls entailed labeling of embryos, or cultured cells, with this antibody only.
Confocal Microscopy and Image Analysis
Immunofluorescently labeled acrylamide sections and cultured amniotic SMCs were viewed on an MCR-1000 Bio-RadTM laser scanning confocal microscope (LSCM; Bio Rad Laboratories, Hercules, CA). Sequential optical planes were acquired in 1-μm steps along the z axis through the wall of the descending aorta or through the cultured amniotic cells. The stored graphics files were, in some cases, collapsed to a single virtual image (referred to as a “z-series projection”) using the manufacturer's proprietary software (Bio Rad Laboratories).
Graphics files obtained from confocal microscopy were imported into Adobe PhotoshopTM (Adobe Systems, Inc., Mountain View, CA) for further image processing and pseudocoloration. Biochemical data was scanned on a UMAX Power Look Scanner (UMAX Data Systems, Taiwan, R.D.C.) and imported into Adobe PhotoshopTM for further image processing.
Chicken Gizzard Tissue Extracts
Adult chicken gizzard smooth muscle was dissected from associated connective tissue and fat. Muscle tissue was then minced finely with a sharp razor. Small preparations were made by homogenization of ∼0.25 g of tissue in 1–1.5 ml of TBS with a disposable Kontes (Vineland, NJ) micropestle. Homogenized tissue was stirred overnight at 4°C, and insoluble material was pelleted in a microfuge. The insoluble pellet was reextracted in either TBS or 6 M urea/TBS. Homogenates were stirred overnight at 4°C and repelleted, after which the supernatant was decanted for further use. A standard protease inhibitor cocktail (i.e., EDTA, leupeptin, sodium vanadate, and PMSF) was included in all preparations.
Perfusion Flow Chromatography and ELISA Analysis
Perfusion flow chromatography was performed on a BioCad Sprint system (Perspective Biosystems, Cambridge, MA). Weak anion exchange chromatography, using an HQ column (Perspective Biosystems), was performed on adult chicken gizzard extracts. 50–100 μl of extract was injected for each fractionation. A pH of 8.0 demonstrated the best separation of protein with a salt gradient of 0.0–1.0 M NaCl. Resultant fractions were tested by ELISA for 1E12 mAb immunoreactivity.
ELISA assays were performed by the standard method described by Chapman et al. (1984). The only modification of this process was that the wells were blocked for 1 h at 37°C in 1% BSA/PBS.
Mass Spectrometry Analysis
Samples analyzed by mass spectrometry were subjected to SDS-PAGE (see below) and transferred to polyvinyldifluoride (PVDF; Millipore Corp., Bedford, MA) membranes, using a 10 mM CAPS (3-[cyclohexylamino]-1-propanesulfonic acid) buffer, which included 10% methanol. The membrane was rinsed in distilled water and stained with 0.1% Ponceau S dissolved in 1% acetic acid for 5 min. The putative 1E12-reactive band was cut from the PVDF membrane and digested in situ with trypsin, following the method of Aebersold et al. (1987). The digestion solution was withdrawn and saved, and the band was rinsed twice in 100 μl of fresh digestion buffer. Pooled digestion solutions were acidified (1% acetic acid) and concentrated to 40 μl in a Speed Vac (Savant Institute, Hicksville, NY). Aliquots of the digest were analyzed as previously described (Hunt et al., 1992) on a Finnigan-MAT TSQ-70 triple-quadrupole mass spectrometer, equipped with an APCI source (San Jose, CA). This device was interfaced to a polyimide-coated fused-silica microcapillary HPLC column (i.d. 75 μm; o.d. 200 μm; Polymicro Technologies, Phoenix, AZ), packed with Poros R2H material (Perspective Biosystems). The samples were eluted into the mass spectrometer with an Applied Biosystems 140B Solvent Delivery System (Foster City, CA). In addition to the putative 1E12-reactive band, a blank piece of nitrocellulose and a band containing 2 μg of BSA were also cut from the PVDF membrane and used for internal controls.
The peptide sequences obtained from the above procedures were analyzed for potential matches, using the MOWSE Peptide Mass Fingerprint Database.
Recombinant Antibody Production
A single chain antibody (sFv), consisting of an antibody light chain variable domain (VL) and heavy chain variable domain (VH) connected by a short linker peptide, [(Gly)4Ser]3, was generated from 1E12 hybridoma cells. Briefly, mRNA was isolated from the 1E12 hybridoma cell line and used as a template for synthesis of a single strand cDNA. The first strand cDNA used to generate the light chain product was primed with oligo dT. The mRNA used to generate the VH cDNA was primed with a constant region-specific primer from the Ig-Prime kit (Novagen, Madison, WI), as the heavy chain message is larger and it is essential that the 5′ end of the message encoding the VH is incorporated in the cDNA. The linkered variable region PCR products were generated using the appropriate primers that were fused to a sequence, which, when overlapped with the homologous sequences from the other chain variable region product, encoded the [(Gly)4Ser]3 linker sequence between the two variable domains. The linkered variable domain PCR products were gel purified, annealed with their corresponding partner, and extended in a recombinant PCR reaction for seven cycles in the absence of synthetic primers. A second round of PCR using the 5′- and 3′-most primers results in the production of the intact sFvs.
The 5′- and 3′-most primers incorporated SfiI and NotI sites, respectively, for cloning into a pHEN-1 plasmid (previously described by Hoogenboom et al., 1991). The recombinant sFv, which was cloned between the SfiI and NotI sites, was produced as a fusion with the c-myc epitope tag, which is recognized by the 9E10.2 mAb (submitted to the American Type Culture Collection [Rockville, MD] by J.M. Bishop [G.W. Hooper Research Foundation, University of California, San Francisco] and G.I. Evan [Cell Nucleus Laboratory, Imperial Cancer Research Fund, London, UK]; Evan et al., 1985). It was further fused to the gene III sequences of the filamentous phage in a supE strain of Escherichia coli with constructs that allow secretion of sFvs into the culture media of normal E. coli strains (see Hoogenboom et al. [1991] for details).
To produce soluble 1E12 sFvs, single ampicillin-resistant colonies of infected E. coli HB2151, a non-supE strain, were inoculated into 150 μl of LB-amp-glu in 96-well plates and grown with shaking until an OD600 nm of 0.8–1.0 was reached. Expression of soluble sFv was induced by the addition of isopropyl-β-d-thiogalactopyranoside to a final concentration of 1 mM, as described by Hoogenboom et al. (1991). Cultures were grown overnight at 30°C, and the periplasm containing the soluble 1E12 sFvs was collected and used for immunoblotting experiments.
SDS-PAGE and Immunoblotting
Proteins were separated by SDS-PAGE (Laemmli, 1970), and then transferred to nitrocellulose membrane (MSI, Inc., Westboro, MA) by the method of Towbin et al. (1979). Membranes were blocked, incubated in primary antibody, washed, and then incubated with HRP-conjugated secondary antibodies (Jackson Immunoresearch Laboratories). After washing, the membrane was incubated with chemiluminescent reagents (ECL Western blotting kit; Amersham Life Science, Arlington Heights, IL) for 1 min and immediately exposed on x-ray film. 1E12 ascites were preincubated in 0.5% SDS at 40°C for 3 min before incubation with the membrane. The final concentration of SDS for incubation was 0.0005%.
In experiments using the sFv 1E12 mAb, bacterial periplasm was diluted 1:2 for use as the primary antibody, after the membrane had been blocked as above. The periplasmic solution was incubated with the membrane for 1 h, followed by multiple PBS washes, and then incubated with the 9E10.2 mAb (American Type Culture Collection), which recognizes the c-myc epitope tag on the sFv. After multiple PBS washes, rabbit anti– mouse polyclonal antibodies (1:3,000) were incubated with the membrane, as an amplification step. Again the membrane was washed and incubated with an HRP-conjugated goat anti–rabbit antibody (1:2,000). After final washing in multiple changes of PBS, the membrane was treated with chemiluminescence reagents and exposed to x-ray film. Homogeneously pure chicken gizzard α-actinin was a gift (Dr. Carol Otey, University of Virginia, Charlottesville; purified according to Fermamisco and Burridge, 1980).
Results
The 1E12 Epitope Colocalizes with Actin Filaments in Explanted Aortic Cells Grown on Planar Substrates
To establish the intracellular distribution of the 1E12 antigen with respect to a known component of the cytoskeleton, we compared 1E12 with smooth muscle α-actin distribution in cultured embryonic smooth muscle cells using double immunofluorescence microscopy. Previous low magnification views of early embryonic tissue sections showed that the distribution of the 1E12 antigen is initially restricted to those presumptive VSMCs most adjacent to the aortic endothelium. In addition, these images suggested that the antigen was concentrated in the peripherial cytoplasm of such cells (see Fig. 7 in Hungerford et al., 1996).
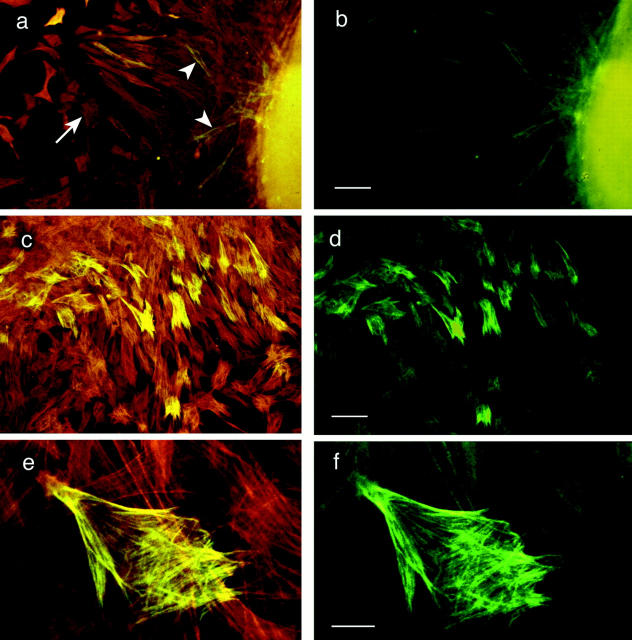
The images in Fig. 1 show double immunofluorescence images of cultured embryonic aortic wall cells. Fig. 1, a and b, shows low magnification views depicting a field of cells that had recently migrated from the explanted aortic tissue. All cells were reactive with the SMαA mab, whereas far fewer cells immunostain with both the SMαA antibody and 1E12. The images in Fig. 1, a, c, and e, show an optical field in which wide-band excitation wavelengths suitable for both fluorescein and rhodamine were used. The redorange fluorescence depicts cells immunoreactive for SMαA, while the yellow staining designates cells immunopositive for both SMαA and 1E12. The green fluorescence in Fig. 1, b, d, and e, designates only those cells immunoreactive with 1E12, when the same optical fields are illuminated with a narrow band-pass fluorescein filter set.
Figure 1.
The 1E12 antigen is located intracellularly and codistributes with SMαA in aortic cells cultured from explants on a planar glass substrate. The cells are double labeled with mAbs to SMαA and 1E12. (a, c, and e) Images of cells examined with simultaneous excitation in both fluorescein and rhodamine wavelengths. (b, d, and f) The same fields of cells under fluorescein only excitation, which denotes those cells that are 1E12 positive. (a and b) Low magnification views of aortic cells migrating from explanted tissue. The majority of cells in these fields are labeled with SMαA (arrowhead, red cells). A subset of cells are double labeled with SMαA and 1E12 (arrow, yellow cells). In this field the majority of cells are motile, spindle-shaped cells and, as such, do not show extensive stress fiber arrays, as shown in c and d. (c and d) The explanted aortic cells have migrated onto the planar glass and have formed extensive microfilament (stress fiber) arrays. As above, all the cells in this field were positive for SMαA, while a subset were positive for both SMαA and 1E12. (e and f) A high magnification view of the embryonic aortic cells shows the 1E12 antigen codistributed with actin microfilament bundles. As shown in f, 1E12 labels the entire length of the actin stress fibers in these cells. The faint staining in the other cells is due to optical bleed-through from the rhodamine channel. Bars: (a and b) 50 μm; (c and d) 50 μm; (e and f) 10 μm.
The cells in Fig. 1, a and b, were motile, spindle-shaped cells, except for the “deepest” cell layer, which was in direct contact with the planar glass surface. The latter cells, depicted in Fig. 1 c, are a population of SMαA- and 1E12labeled cells that have attached to the planar glass surface, flattened, and formed actin microfilament bundles. Every cell is SMαA positive; however, only a subset of the SMαA-positive cells in Fig. 1 c also immunostain with the 1E12 antibody (d). Careful examination of multiple explants suggested that the cells expressing the 1E12 antigen were primarily located at the upper layer of migratory cells that had most recently emigrated from the aortic segment although this impression was not subjected to quantitative analysis.
A high magnification view shows that the 1E12 antigen codistributes with actin microfilament bundles when the embryonic vessel wall cells are in contact with a planar substrate and assume an extended flattened morphology (Fig. 1, e and f). The two fluorescent images (actin and 1E12 antigen) are essentially superimposable (compare e and f). Thus, under nonphysiological conditions where embryonic aortic wall cells assume a conspicuously flattened morphology, the 1E12 antigen codistributes with large actin microfilament bundles (also called “stress fibers”).
The 1E12 Distribution Pattern in Cultures of Contraction-competent Smooth Muscle Cells Is Not Coincident with That of SMαA
Embryonic amniotic SMCs maintain the ability to contract when cultured using defined serum-free conditions (Bowers and Dahm, 1993). As demonstrated by these studies, contractile amniotic smooth muscle cells exhibit a more rounded and plump appearance than cells grown in the presence of serum. To examine the 1E12 antigen under more physiologically relevant conditions, the embryonic amniotic SMCs were simultaneously immunolabeled with SMαA and 1E12 mAbs. When examined with epifluorescence microscopy, all cells expressed both antigens. This is in distinct contrast with the serum-containing aortic cultures described above (Fig. 1 and data not shown).
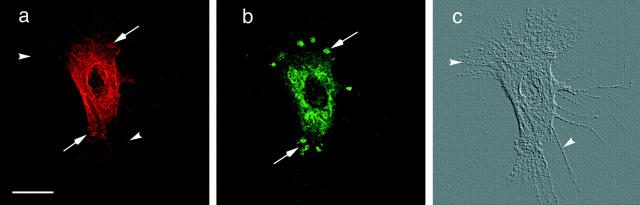
When individual cells from contraction-competent cultures were examined at high magnification using laser scanning confocal microscopy (LSCM), the two fluorescent antigen patterns were not superimposable. This was determined by projecting a series of 10 1-μm optical sections into a single virtual image plane. Instead of microfilament bundles, the SMαA expression pattern resembled fine filamentous whorls surrounding a nonlabeled nuclear compartment (Fig. 2 a). Also, fine retraction fibers extending from the cell periphery, at the level of the glass coverslip, immunostained with the SMαA mAb. These structures are clearly shown by the digitally generated, surface relief image of the cell shown in Fig. 2 c. It is readily apparent from this and similar images that these contraction-competent cells do not form the typical stress fiber morphology of cells grown on planar substrates in the presence of serum.
Figure 2.
This figure shows an amniotic SMC double labeled with SMαA (a) and 1E12 (b). These images were obtained by LSCM. Each image is a series of 10 1-μm optical sections through the long axis of the cell projected into a single image plane. (a) In these contractioncompetent cells, SMαA expression is observed in fine, filamentous “whorls” surrounding the nucleus (arrow). Retraction fibers, which are present at the surface of the coverslip, also are labeled with SMαA (arrowhead). (b) 1E12 expression is distributed in the same area as SMαA (arrow) with the cell. However, 1E12 expression appears in a punctate and granular pattern. Additionally, the 1E12 antigen is distributed to discrete patches of the cell (arrowhead), reminiscent of cell–substrate contact sites. As a means of visualizing the morphology of the amniotic SMC shown in a and b, digital image processing software was used to render the actin immunofluorescence pattern as a surface relief object. From this topographical representation, it is clear that the actin microfilaments extend from the cell body as long processes (arrowheads), which are reminiscent of retraction fibers. 1E12 immunolabeling was not present in these structures. Bar, 10 μm.
Of greater interest is the fact that the 1E12 antigen presents a markedly different immunostaining pattern compared with SMαA. While some fluorescence is present in the perinuclear cytoplasm, the 1E12 immunostaining appears as a punctate and granular pattern, and it is not detected coincidently with the SMαA-positive retraction fibers (compare Fig. 2, a and b). Moreover, and in distinct contrast with SMαA, the 1E12 antigen is distributed in discrete focal patches at the (presumptive) leading and trailing edges of the cell; these structures are reminiscent of cell–substratum contact sites (e.g., see Chen and Singer, 1982).
In Situ Immunostaining of 3-d-old Quail Embryos Demonstrates Embryonic Vessel Wall Structure, As Well As a Unique Expression Pattern for 1E12
To examine the expression of the 1E12 antigen in primordial vascular smooth muscle cells within an intact embryonic vessel wall (i.e., cells that had not been subjected to a tissue-culture environment or physically sectioned), we examined thick (200 μm), sagittal sections of acrylamideembedded, whole-mounted embryos by LSCM. The image in Fig. 3 a is a low magnification projection of 10 1-μm optical sections into one image plane. The tissue, viewed in the sagittal plane, contains undisturbed 1E12-positive cells embedded in the extracellular matrix (ECM) of the developing aortic wall (stage 20 quail embryo). For the purposes of orientation, we have provided a schematic representation of this section in Fig. 3 b.
Figure 3.
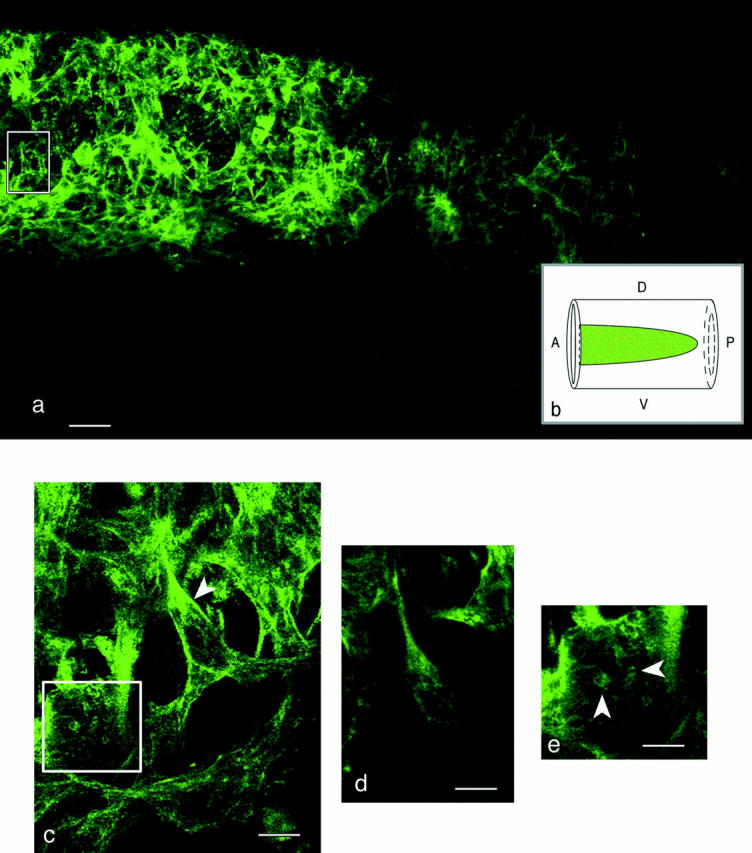
The distribution pattern of 1E12 was examined in primordial VSMCs within an intact embryonic vessel wall by whole-mount immunolabeling. The stage 20 quail embryo shown in this figure was embedded in acrylamide, sectioned, and viewed by LSCM. (a) Low magnification view of a projection of a series of 10 1-μm sagittal sections through the vessel wall. Primordial VSMCs are labeled with 1E12. At this early developmental stage, the VSMCs are loosely arrayed, with open spaces between the cells. The boxed area is magnified in c. (b) For purposes of orientation, a schematic view of the preparation is shown. The drawing depicts a side view of the aorta with the anterior (A) to posterior (P) axis, and dorsal (D) to ventral (V) axis indicated. The area shaded in green is representative of the fluorescent image shown in a. (c) High magnification view of the boxed area in a and a series of 10 1-μm optical sections projected into one image plane. A fortuitous optical section through the main axis of a cell (arrowhead) within the plane of the section shows that 1E12 labeling is concentrated at the distal tips of cellular projections, possible points of attachment. The cell marked with the box is magnified further in e. (d) 1-μm optical section through the cell denoted by the arrowhead in c. 1E12 labeling is seen intracellularly in fine, short, threadlike structures. (e) A high magnification view of the cell denoted by the box in c. Small 1E12-labeled clusters (arrowheads) are similar to adhesive structures termed rosettes or podosomes. Bars: (a) 50 μm; (c and d) 10 μm; (e) 7.5 μm.
The parasagittal section shown in Fig. 3 a passed through the layer of vessel wall cells most adjacent to the endothelium. It is clear from this low magnification image that primordial VSMCs in the embryonic vessel walls are not organized in a regular pattern. The image in Fig. 3 c, corresponding to the boxed area in Fig. 3 a, is a fortuitous optical section that passed parallel to and through the main axis of several adjoining embryonic VSMCs. Other randomly oriented, 1E12-positive cells are also visible in this microscopic field. This image is a series of 10 1-μm optical sections projected into one image plane. One cell within this image plane shows intracellular 1E12 labeling concentrated at the distal tips of cellular projections, possibly sites of cell attachment. Higher magnification of a single 1-μm optical section through this same cell is shown in Fig. 3 d; this image clearly demonstrates discrete intracellular staining in which the 1E12 antigen presents as fine, wisplike structures. The cell within the box in Fig. 3 c is magnified further in Fig. 3 e. This primordial VSMC exhibits several, small, organized foci of 1E12 staining arranged as circular clusters of fluorescence that lie in a single 1-μm plane (Fig. 3 e). These clusters bear a striking resemblance to adhesive structures termed “rosettes” or “podosomes” (David-Pfeuty and Singer, 1980; Tarone et al., 1985; Marchisio et al., 1987; Burridge et al., 1988). Close inspection of multiple optical planes through the embryonic vessel wall revealed that 1E12 rosettes were detected in other smooth muscle cells (data not shown).
Partial Purification of the 1E12 Epitope
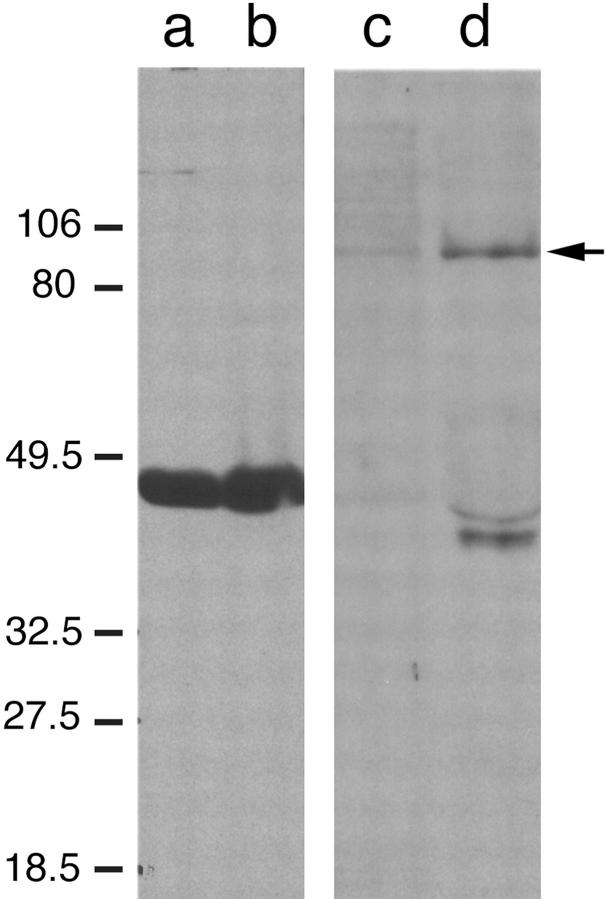
The 1E12 IgM mAb proved to be unsuitable for affinity chromatography and immunoprecipitation. However, 1E12 can be used for immunoblotting if, before incubation with electroblotted antigen, this IgM antibody is pretreated with a buffered solution containing dilute SDS (Hungerford et al., 1996). Using this immunoblotting method, we probed extracts of adult chicken gizzard (an abundant source of smooth muscle antigens) for the 1E12 antigen. When homogenized gizzard tissue was extracted with TBS, a 100-kD band was detected (Fig. 4, lane c). If the resulting pellet was reextracted with the same buffer containing 6 M urea, additional 1E12 antigen was detected (Fig. 4, lane d). This suggested that at least some of the 1E12 antigen remained insoluble at neutral pH and normal ionic strength. Lanes a and b show the results of probing two similar samples with antibodies to SMαA. In addition to the 100-kD polypeptide band recognized by 1E12, a doublet at ∼40 kD was detected in some preparations but not in others. This doublet migrates well ahead of SMαA (compare lanes a and b with c and d). Also, based on the urea extraction data, the 1E12 antigen appears to be less soluble than SMαA.
Figure 4.
Chicken gizzard homogenates were separated by 10% SDS-PAGE, transferred to nitrocellulose, and probed with antibodies to either SMαA (lanes a and b) or the 1E12 antigen (lanes c and d). Gizzard homogenates were prepared by extraction in TBS alone (lanes a and c) or reextraction of the TBS-insoluble pellet in 6 M urea/TBS (lanes b and d). Immunoreactivity was determined by enhanced chemiluminescence techniques. 1E12 ascites were incubated with 0.5% SDS before incubation with the membrane and used at a final dilution of 1:1,000. 1E12 does not immunoreact with actin, but is strongly immunoreactive with a band migrating at M r = 100,000 (arrow). In addition, a doublet migrating ahead of actin on the gel is often observed to be immunoreactive (lane d).
Encouraged by finding the strongly immunoreactive polypeptide band at 100 kD, preparative amounts of chicken gizzards were processed and the resulting extracts were subjected to perfusion–flow ion exchange chromatography. A typical chromatograph showed multiple adsorbance peaks at 280 nm across the entire sodium chloride gradient (data not shown; see Hungerford, 1995). A sample from each absorbance peak was tested for 1E12 immunoreactivity by ELISA. A series of strongly immunoreactive peaks eluted between 0.5 and 1.3 M NaCl (data not shown).
The peak fractions were pooled, concentrated, fractionated using SDS-PAGE under reducing conditions, and transferred to nitrocellulose membranes. The resulting immunoblots showed a strongly reactive polypeptide band at 100 kD similar to the band detected in crude extracts displayed in Fig. 4 (data not shown). Companion immunoblots treated in an identical fashion were prepared, and the nitrocellulose containing the 100-kD band was excised for protein microsequence analysis. Five tryptic fragments from the 100-kD band were successfully sequenced by tandem mass spectrometry analysis, and found to match the sequences reported for the smooth muscle and fibroblastic isotypes of α-actinin (Baron et al., 1987; Arimura et al., 1988, respectively). Table I lists the sequences of the five tryptic fragments (left column). The middle column indicates where the tryptic fragment starts within the sequence of the smooth muscle cell (SMC) and nonmuscle cell (NMC) isoforms of α-actinin. The right column lists the functional domain of α-actinin, in which each fragment is located.
Table I.
1E12 Antigen Amino Acid Sequence Data
| Tryptic peptide | Location in sequence | α-Actinin domain | ||
|---|---|---|---|---|
| ALDFIASK | SMC: 97 | Actin binding domain | ||
| NMC: 60 | ||||
| CQLEINFNTIQTK | SMC: 333 | 1st internal repeat | ||
| NMC: 296 | ||||
| ATLPDADKER | SMC: 557 | 3rd internal repeat | ||
| NMC: 520 | ||||
| GISQEQMNEFR | SMC: 742 | 1st EF-hand | ||
| NMC: 705 | ||||
| ASFNHFDR | SMC: 754 | 1st EF-hand | ||
| NMC: 717 |
The five tryptic peptides generated from the tandem mass spectrometry analysis of the putative 1E12 antigen, M r = 100,000, are listed in the left column. Each of these sequences corresponds to a specific location in the sequence of both the smooth muscle (SMC) and fibroblast or nonmuscle (NMC) isoforms of α-actinin. The middle column indicates where the tryptic fragment starts within each of these sequences. The right column lists the functional domain of α-actinin, in which each fragment is located.
To address the complication that some immunoblots showed the variable doublet at 40 kD, and to obtain an immunoreagent that was easier to use than the parent IgM molecule, we took advantage of recombinant protein technology to produce a single chain antibody (sFv) with 1E12 specificity (see Materials and Methods). Using the sFv immunoreagent, crude chicken gizzard extracts were analyzed by immunoblotting (Fig. 5). Polypeptides in lanes a–d were separated by 10% SDS-PAGE under reducing conditions, transferred to nitrocellulose, and probed with the sFv 1E12. Adult chicken gizzard was extracted in TBS (Fig. 5, lane a) or TBS containing 6 M urea (lane b). A single, strongly immunoreactive band, M r = 100,000, was observed in the crude gizzard homogenates extracted under both mild and stringent conditions. No bands at the 40-kD migration position were detected with the sFv immunoreagent. As shown in lane c, the sFv 1E12 was also immunoreactive with homogeneously pure chicken gizzard α-actinin. In contrast, a control sample containing chicken serum proteins showed no reactive bands (lane d). Also, neither the secondary (anti-myc mAb) nor the tertiary antibodies (goat anti–mouse IgG), used for this immunoblot, exhibited cross-reactivity with the chicken gizzard extracts or the purified α-actinin (data not shown).
Figure 5.
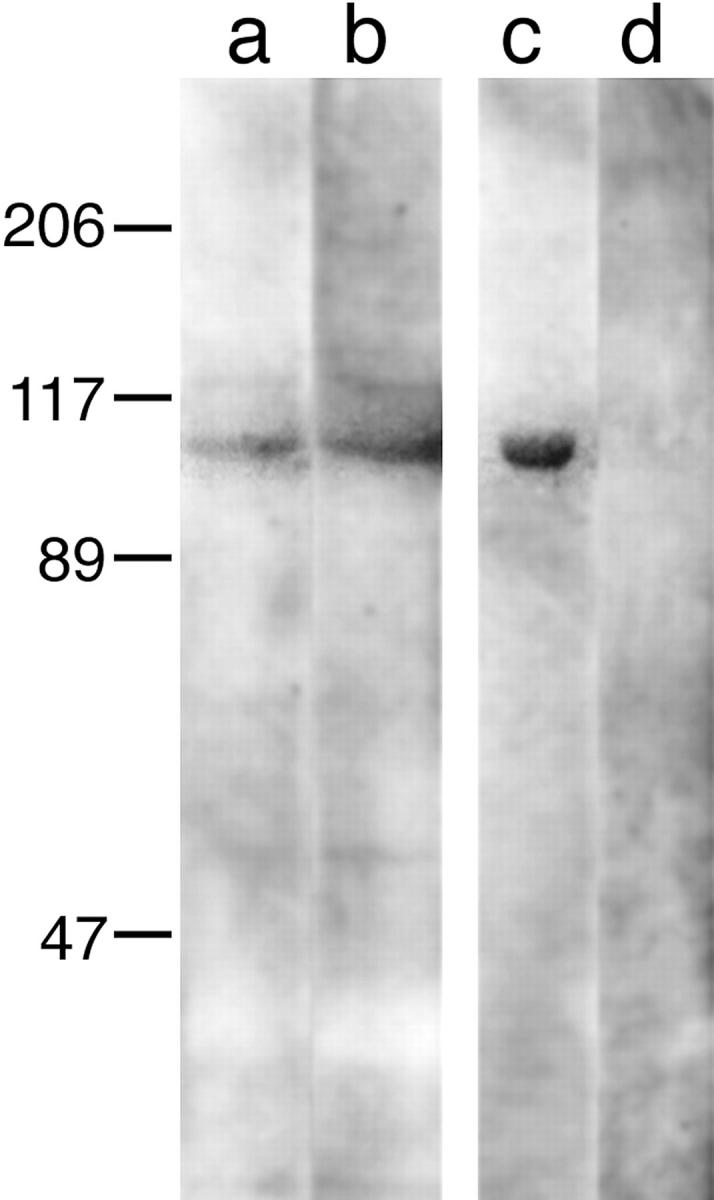
Chicken gizzard homogenates were separated by 10% SDS-PAGE under reducing conditions, transferred to nitrocellulose, and probed with the recombinant 1E12 antibody. Adult chicken gizzard was extracted in TBS alone (lane a) or TBS/6 M urea (lane b). The recombinant 1E12 antibody was reactive with a single band, M r = 100,000, using both mild and stringent conditions. We observed no bands in the 40-kD migration position. In addition, the sFv immunoreagent recognized purified gizzard α-actinin (lane c). A control sample of chicken serum proteins showed no immunoreactive bands (lane d).
Discussion
The goal of this study was to characterize further a novel SMC-specific protein marker, the 1E12 antigen (Hungerford et al., 1996). The 1E12 antigen is expressed very early in the SMC differentiation program; we hypothesized therefore that the protein plays an important role in establishing the cytoskeletal machinery characteristic of the SMC lineage. In this study we have examined the intracellular localization of the 1E12 antigen in three distinctly different embryonic SMC preparations. The use of cell biological, immunological, and biochemical approaches identified the 1E12 antigen as a member of the α-actinin family of cytoskeletal proteins and as an unequivocal marker for specification to a smooth muscle fate.
1E12 Expression in Cultured SMCs
The phenotypic modulation of SMCs in culture has been well documented (Chamley-Cambell et al., 1981; Thyberg et al., 1990; Thyberg, 1996). Indeed, a myriad of studies has shown that the expression and subsequent distribution of smooth muscle proteins in cultured cells is dependent upon many factors, including the contents of the culture media and the type of substratum (for review see Owens, 1995). We decided therefore to examine expression of the 1E12 antigen using two different methods of culturing embryonic SMCs. One method yields a well-spread noncontractile phenotype, and the other method results in more rounded cells that are capable of contractility.
Actin microfilament bundles, also called stress fibers, are formed when animal cells are cultured on highly adhesive planar substrates (see Trinkaus, 1984, for discussion). Consistent with this fact, cells that emigrate from explanted embryonic aortae onto glass, in the presence of serum-derived substrate attachment factors, assume a characteristic stress fiber morphology (Fig. 1 e). The plasma membrane appears to be stretched across the culture substratum in these strongly adherent cells (not shown). In contrast, embryonic amniotic SMCs isolated and maintained by a different method (Dahm and Bowers, 1993) retain the ability to contract in culture, have a more rounded morphology, and do not exhibit exaggerated actin microfilament bundles (Fig. 2 a). Our immunofluorescence analysis of both types of cultured primordial SMCs indicates that the 1E12 epitope is located intracellularly. As expected, the distribution pattern of 1E12, relative to that of SMαA, was different depending on the phenotype/morphological characteristics of the cultured cell. In the highly flattened cells from aortic explant cultures, the 1E12 staining was distributed along the length of the actin-containing microfilament bundles. In contrast, the distributions of 1E12 vs SMαA in rounded, functionally contractile, amniotic SMCs were distinct. Actin was distributed in a whorled pattern surrounding the nucleus and in fine filaments extending from the cell periphery (Fig. 2 a, arrowhead), whereas 1E12 staining appeared as a granular perinuclear stain and as small circular patches at the proximal aspect of lamellapodia-like protrusions (Fig. 2 b). Digital image processing software was used to render the actin immunofluorescence pattern as a surface relief object (Fig. 2 c). This topographical representation demonstrates that actin microfilaments extend within cellular protrusions a considerable distance from the cell body (Fig. 2, arrowheads). The latter structures are reminiscent of retraction fibers created by motile fibroblasts (see Trinkaus, 1984). In addition, broad lamellapodia-like protrusions appear to extend from opposite sides of the cell body (Fig. 2 c). It is noteworthy that neither actin nor 1E12 staining was present in stress fiber–like arrangements in the contraction-competent SMC cultures.
In addition to confirming an intracellular localization for the 1E12 antigen, these in vitro studies strongly support our previous conclusion that 1E12 protein expression is an appropriate marker to define cells committed to the VSMC differentiation program (Hungerford et al., 1996). In the embryonic aortic cell cultures, SMαA and 1E12 doublelabeled cells comprise a minority of the cells present in the culture (all cells are SMαA positive, but only a few are also 1E12 positive). Our hypothesis is that the SMαA and 1E12 double-labeled cells represent primordial VSMCs that were further along their differentiation program relative to cells that express only SMαA. In contrast, all of the contraction-competent, cultured, amniotic cells immunolabeled with both 1E12 and SMαA mAbs. This finding is consistent with other studies showing that SMCs, such as these, grown in serum-free medium often maintain more features of their phenotypic status in vivo than those grown in the presence of serum (Chamley-Cambell et al., 1981; Hedin and Thyberg, 1987; Hedin et al., 1990; Bowers and Dahm, 1993). Of course, we cannot be certain that SMαApositive/1E12-negative cells did not previously express 1E12, but subsequently modulated their smooth muscle phenotype such that 1E12 expression is no longer detectable.
As a biological control, we immunostained chicken embryonic fibroblast cultures with both 1E12 and SMαA mAbs (data not shown). The 1E12 mAb labeled a handful of cells per confluent 100-mm culture dish, while the SMαA mAb labeled the majority of the fibroblasts. This result is not surprising, as it is well documented that many cell types cultured in vitro express SMαA (Skalli et al., 1987, 1989; Rønnov-Jessen et al., 1990; Sappino et al., 1990; Jahoda et al., 1991; Lazard et al., 1993). Thus, 1E12 recognizes only those cells in vitro that manifest a committed smooth muscle phenotype.
1E12 lmmunolabeling In Situ: A Unique Method for Studying Structural Proteins in the Primordial Vessel Wall
LSCM images of 1E12 expression within the aortic wall of a stage 20 quail embryo show a cytoskeletal protein array within a primordial VSMC. Moreover, these cells have not been dissociated from the vessel wall nor subjected to a tissue-culture environment. We know of no other images that depict the cells of the developing vessel wall in this manner. Our previous study showed that the 1E12-positive primordial VSMCs are located in the cell layer(s) most adjacent to the aortic endothelium in stage 20 quail embryos (Hungerford et al., 1996). In the present study we show that the primordial VSMCs are loosely arranged with considerable open space between the cytoskeletal arrays of 1E12 antigen. Since the 1E12 antigen is clearly present in cell processes, this image suggests that the remaining space is largely occupied by ECM.
The somewhat acellular architecture of this early vessel wall also suggests that embryonic VSMCs do not initially form a functionally contractile tissue when recruited to the endothelium, and our unsuccessful attempts to elicit contraction of aortic smooth muscle at early stages of development are in agreement with this hypothesis (Hungerford, J., unpublished observations). Thus, it appears that at early stages VSMCs may be primarily involved in synthetic activity and not contractility. Furthermore, recent collaborative efforts are entirely consistent with the possibility that day 2.5–6 avian VSMCs are engaged in the progressive synthesis of multiple contractile proteins, while at the same time they are in a highly proliferative state (Lee et al., 1996).
The LSCM images of the 1E12 antigen distribution in the intact vessel wall are the first images of this kind, which makes drawing comparisons with previously published work difficult. Standing alone, the data suggest that the 1E12 epitope is involved with an intracellular apparatus that mediates cell adhesion. 1E12 labeling is localized to the tips of cellular extensions, possible sites of attachment (Fig. 3 c). Moreover, the antigen is present as small circular assemblies that resemble rosettes or podosomes, adhesive structures observed in transformed cells, osteoclasts, and cells of the monocytic lineage (David-Pfeuty and Singer, 1980; Tarone et al., 1985; Marchisio et al., 1987; Burridge et al., 1988; Aubin, 1992). These adhesion structures typically contain actin microfilaments in the center, encircled by a rosette containing vinculin, α-actinin, and talin. In cultured, transformed cells, or cells of the monocytic lineage, podosomes are thought to confer increased migratory capabilities to these cell types. From the images in Fig. 3, we cannot determine whether these sites have the same organization as podosomes or perhaps correspond to in vitro cell–ECM adhesion sites (Chen and Singer, 1982). It is reasonable to hypothesize that, within the developing embryo, the adhesions made to the ECM or other cells need to be transitory to allow for the constant rearrangements that occur during morphogenesis. Alternatively, these 1E12-immunolabeled structures may represent the ontogeny of cell–cell junction complexes or the onset of dense body/dense plaque formation. Based on our identification of the 1E12 antigen as a putative α-actinin family member, we believe that these are all valid possibilities (see discussion below).
1E12 mAb Recognition of Smooth Muscle α-Actinin
Initial attempts to immunoblot or immunoprecipitate with 1E12 using standard techniques were unsuccessful. We reasoned that, when concentrated for immunochemical studies, the large multivalent IgM antibody might be aggregating so as to prevent antigen recognition. To address this possibility, 1E12 was incubated with 0.5% SDS before probing the nitrocellulose sheets with the primary antibody solution, the rationale being to promote a more open IgM protein conformation and thereby promote antigen recognition.
This protocol appeared successful, in that a 100-kD band was always strongly recognized by 1E12. There was concern, however, regarding the fact that in some immunoblots bands at 40 kD were variably present when crude extracts of chicken gizzard were probed as seen in Fig. 4. To circumvent problems associated with the the large, pentameric IgM structure, we generated a recombinant, single chain version of 1E12. The recombinant 1E12 recognized a single band, M r = 100,000, in gizzard lysates extracted under both mild and strong (6 M urea) extraction conditions. This result confirmed that the 1E12 antigen displays an apparant molecular mass of 100 kD.
After partial purification on anion exchange chromatography and subsequent fractionation using SDS-PAGE, the bands were transferred to nitrocellulose and the 100-kD band was excised. Tandem mass spectroscopy analysis identified the 100-kD band as the smooth muscle, or fibroblast, isotype of α-actinin. These α-actinins are 98% identical, the greatest diversity being in the second half of the first EF-hand (Blanchard et al., 1989). As shown in Table I, the amino acid sequences of the five tryptic fragments obtained from mass spectrometry match regions of the smooth muscle (Baron et al., 1987) and fibroblastic (Arimura et al., 1988) α-actinin sequences, where the two are identical. The nonmuscle and smooth muscle isoforms of α-actinin are alternative splice products of a single gene (Waites et al., 1992). The alternatively spliced region begins adjacent to the last trypic peptide we obtained in the first EF-hand. Based on our previous study of 1E12 distribution in embryonic tissue sections (Hungerford et al., 1996) and our current study, we conclude that 1E12 recognizes those cells both in vivo and in vitro that possess the phenotype of a committed SMC. Our data suggests that 1E12 distinguishes between smooth muscle and fibroblast (nonmuscle) isoforms of α-actinin. Additionally, the recombinant 1E12 immunoreagent reacts with a preparation of homogenously purified chicken gizzard smooth muscle α-actinin.
The screening criterion that resulted in cloning 1E12 was designed to be an unbiased search for antibodies that marked the early vessel wall (Hungerford, 1995; Hungerford et al., 1996). While we did not anticipate the identity of the 1E12 antigen to be α-actinin, much of the immunolabeling data presented in this study and our previous studies are consistent with this finding. The α-actinins have been implicated as part of the intracellular machinery involved in both cell–cell and cell–matrix adhesion (for reviews see Blanchard et al., 1989; Luna and Hitt, 1992; Hemmings et al., 1995), and the proteins are found in dense bodies and dense plaques of mature SMCs (Draeger et al., 1990; Chou et al., 1994). The distribution of the 1E12 antigen in embryonic tissue and cultured SMCs is highly suggestive of a molecule, such as smooth muscle α-actinin, that is associated with actin organization and involved in cell attachment.
The distribution of α-actinin in cultured cells is variable depending on cell type and shape, type of substratum, and accessibility of the marker antibody to the cell–matrix or cell–cell junctional complex (Lazarides and Burridge, 1975; Pavalko et al., 1995; Crowley and Horwitz, 1995). Typically, α-actinin labeling is observed periodically along the actin stress fibers and in the focal adhesions, located at the ends of the stress fibers; however, the extent to which these structures label can differ, based on the criterion given above. In our hands, the distribution of the 1E12 antigen varies with the preparation of cultured embryonic smooth muscle cells. In explanted, embryonic VSMCs, 1E12 immunostaining is associated with the actin stress fibers, although the characteristic periodicity of α-actinin is not observed. We do not see discrete labeling of the focal adhesions in these cells. In functionally contractile, embryonic, amniotic SMCs, the 1E12 antigen is concentrated in podosome-like structures at the base of broad cellular protrusions. Distinct stress fibers are not present in these cells, but 1E12 is localized to the general area of actin distribution within the cell body.
Although detection of α-actinin in many cell types may be limited because of antibody accessibility (Palvalko et al., 1995), distribution of α-actinin has been studied at the level of the LSCM in embryonic chicken corneal epithelium (Khoory et al., 1993) and by EM in later stages of development in chicken gizzard SMCs (Chou et al., 1994). Localization of α-actinin within the cells of the corneal epithelium of a 6-d-old chicken embryo is similar to our confocal images of the developing VSMCs within the vessel wall. In both cases, short, threadlike staining was observed cytoplasmically, and denser labeling was found in areas of attachment to other cells or the matrix. However, the 1E12-positive, rosette-like structures that we observed in individual cells of the vessel wall are unique to our study. While these structures bear a striking resemblance to the podosomes found on cultured cells, they have not been described in situ previously.
The in vivo equivalents of adhesion structures have not been studied extensively, there are, however, several structures in vivo, including the myotendinous and neuromuscular junctions, intercalated disks in cardiac muscle, and dense bodies and dense plaques of smooth muscle that reflect the diversity of actin organization and the repertoire of proteins found in adhesion structures in vitro. In the future, it will be interesting to see if known markers of in vitro cell–cell and/or cell–matrix adhesion are localized in situ to structures similar to those pictured in this study. Our unpublished observations suggest that, in addition to α-actinin, vinculin is also distributed in analogous circular structures in the developing vessel wall of similarly prepared quail embryos.
While α-actinin has been localized to the dense body/ dense area of mature VSMCs (Draeger et al., 1990), we see no evidence of 1E12 labeling in such discrete structures within the embryonic (stage 20) vessel wall. The formation and protein content of these structures has been examined in chicken gizzard SMCs from embryonic day 10 to posthatchling stages, much later stages than the present studies (Volberg et al., 1986; Chou et al., 1995). There are no equivalent studies of dense body/dense plaque formation at the early stages of vessel wall development. Thus, we do not know to what extent, if any, these structures exist within developing VSMCs at these early stages.
One important issue we are continuing to address concerns reconciling the wide range of α-actinin distribution in vertebrate tissues, with the exquisite specificity 1E12 displays with respect to smooth muscle. There have been no prior reports of such specific α-actinin expression in the developing embryo. Previous developmental studies have focused on α-actinin distribution during differentiation of specific cytoskeletal structures (Endo and Masaki, 1984; Goncharova et al., 1992; Chou et al., 1994), rather than on the onset of expression with respect to the early events of morphogenesis. In contrast, the spacial and temporal distribution pattern of vinculin and talin, two other abundant components of adhesion complexes, has been described in the developing quail embryo (Duband and Thiery, 1990). Interestingly, both of these proteins are expressed at high levels in both the endothelial and smooth muscle components of the vasculature during early developmental stages that correspond to the onset of 1E12 expression. Therefore, it is not surprising that a particular variant of an actin organizing molecule such as α-actinin would be found in the early stages of vessel wall development.
Although the diversity of α-actinin isoforms has been well documented, a better understanding of the mechanisms that generate these variants and the relationship between them is needed. It is clear that two genes encoding for chicken α-actinins (Parr et al., 1992; Waites et al., 1992) and three genes encoding for human α-actinins (Millake et al., 1989; Youssoufian et al., 1990; Beggs et al., 1992) have been identified, and that alternative splicing of these gene products accounts for the major tissue-specific isoforms: nonmuscle, smooth muscle, skeletal muscle, and cardiac α-actinins. However, identification of novel tissuespecific isoforms such as the recently isolated 115-kD, calcium-insensitive, endothelial cell–specific α-actinin (Imamura et al., 1994; Imamura and Masaki, 1994) suggests that either additional alternative spliced isoforms or posttranslational modifications of the known major isoforms also contribute to the repertoire of α-actinins. The differences in calcium sensitivity between variants may also reflect tissue-specific requirements of α-actinin. Considering all of these issues, we may be able to account for 1E12 specificity by considering that there are uncharacterized isoforms or variants of α-actinin that have cell type–specific roles. Interestingly, up to 11 isoelectric variants of smooth muscle α-actinin have been isolated from chicken gizzard (Endo and Masaki, 1982). It is likely that careful developmental studies have been hampered in part by the multitude of α-actinin variants present in various cell types and the lack of specific immunoreagents to target these antigens. Indeed, even a ubiquitously distributed protein such as fibronectin is present as an alternatively spliced vascular form during embryogenesis (Glukhova et al., 1989, 1990; Castellani et al., 1994).
While our data strongly suggest that the identity of the 1E12 antigen is an isoform of smooth muscle α-actinin, we cannot rule out the possibility that 1E12 recognizes an epitope common to another protein that is not abundant in our gizzard preparation. Each of the three structural domains of α-actinin have similarities with other cytoskeletal proteins. In particular, α-actinin belongs to a diverse family that includes the spectrins, fodrins, and dystrophins. There is precedent for antibodies directed aginst the central repeat domains of α-spectrin and -dystrophin to crossreact with α-actinin (Blanchard et al., 1989). In addition, the actin binding and EF-hand motifs of α-actinin are shared with many other proteins.
We have commented previously on the 1E12 antigen with respect to the early avian vessel wall development (Hungerford, 1995; Hungerford et al., 1996). With new and exciting findings in the vascular development field, we feel it is important to comment further on the potential significance of the 1E12 antigen. The molecular basis of vessel wall formation remains enigmatic (Thayer et al., 1995; Folkman and D'Amore, 1996). In particular, the mechanism by which VSMCs gather and differentiate around the nascent endothelium is unknown. Several intriguing reports (Davis et al., 1996; Suri et al., 1996; Vikkula et al., 1996), published during preparation of this manuscript, may provide clues for how a multilayered vessel wall forms. While some data exist to show that a population of VSMCs can transdifferentiate from endothelial cells (DeRuiter et al., 1997), these recent reports suggest a mechanism by which VSMC precursors are recruited from the surrounding mesoderm. In the model proposed by Folkman and D'Amore (1996), angiopoietin-1 activates TIE2 tyrosine kinase receptors on endothelial cells, leading to secretion of a “recruiting signal.” As VSMC precursors contact the endothelium, other signaling factors that induce differentiation of the VSMCs may be secreted. However, at this time, the coordinated regulation of VSMC differentiation is not well understood because there are few known smooth muscle– specific proteins expressed during the earliest stages of vessel wall development. The work presented here indicates that smooth muscle–specific proteins are present in a nascent cytoskeletal apparatus at the time cells appear to become specified to a smooth muscle fate. We have provided evidence that the 1E12 epitope is located on the smooth muscle isoform of α-actinin. Perhaps expression of smooth muscle α-actinin, 1E12 variant, is a critical event in both the coordinated regulation of vessel wall formation and SMC differentiation.
Acknowledgments
We thank Dr. Tom Trusk for assistance with computer imaging; Dr. Carol Otey for providing purified α-actinin and helpful discussions; and Dr. Eleanor Spicer for advice on perfusion flow chromatography. We also thank Dr. Kumar Srikumar for helpful suggestions concerning IgM mAbs.
Footnotes
1. Abbreviations used in this paper: ECM, extracellular matrix; LSCM, laser scanning confocal microscopy; NMC, nonmuscle cell; PVDF, polyvinyldifluoride; SMαA, smooth muscle α-actinin; SMC, smooth muscle cell; VSMC, vascular smooth muscle cell.
Rocco Falchetto was supported by a fellowship from the European Molecular Biology Organization. This work was supported by awards from the National Institutes of Health (NIH) (R01HL45348 and R01HL57645) and the March of Dimes Birth Defects Foundation (FY950453) to C.D. Little. J.E. Hungerford was supported in part by an NIH training grant (T32HL07284-14).
Please address all correspondence to Dr. Charles D. Little, Department of Cell Biology, Medical University of South Carolina, BSB Room 626, 171 Ashley Avenue, Charleston, SC 29425. Tel.: (803) 792-9030. Fax: (803) 792-0664. e-mail: Charles_Little@smtpgw.musc.edu
J.E. Hungerford's current address is Department of Cell Biology, Box 439, University of Virginia, Charlottesville, VA 22908.
References
- Aebersold RH, Leavitt J, Saavedra RA, Hood LE, Kent SBH. Internal amino acid sequence analysis of proteins separated by one- or twodimensional gel electrophoresis after in situprotease digestion on nitrocellulose. Proc Natl Acad Sci USA. 1987;84:6970–6974. doi: 10.1073/pnas.84.20.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubin JE. Osteoclast adhesion and resorption: the role of podosomes. J Bone Miner Res. 1992;7:365–368. doi: 10.1002/jbmr.5650070402. [DOI] [PubMed] [Google Scholar]
- Arimura C, Suzuki T, Yanagisawa M, Imamura M, Hamada Y, Masaki T. Primary structure of chicken skeletal muscle and fibroblast α-actinins deduced from cDNA sequences. Eur J Biochem. 1988;177:649–655. doi: 10.1111/j.1432-1033.1988.tb14419.x. [DOI] [PubMed] [Google Scholar]
- Baron MD, Davison MD, Jones P, Critchley DR. The sequence of chick α-actinin reveals homologies to spectrin and calmodulin. J Biol Chem. 1987;262:17623–17629. [PubMed] [Google Scholar]
- Beggs AH, Byers TJ, Knoll JHM, Boyce FM, Bruns GAP, Kunkel LM. Cloning and characterization of two human skeletal muscle α-actinin genes located on chromosomes 1 and 11. J Biol Chem. 1992;267:9281–9288. [PubMed] [Google Scholar]
- Blanchard A, Ohanian V, Critchley D. The structure and function of α-actinin. J Muscle Res Cell Motil. 1989;10:280–289. doi: 10.1007/BF01758424. [DOI] [PubMed] [Google Scholar]
- Bowers CW, Dahm LA. Maintenance of contractility in dissociated smooth muscle: low density cultures in defined medium. Am J Physiol. 1993;264:C229–C236. doi: 10.1152/ajpcell.1993.264.1.C229. [DOI] [PubMed] [Google Scholar]
- Burridge K, Fath K, Kelly T, Nuckolls G, Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Castellani P, Viale G, Dorcaratto A, Niolo G, Kazmarek J, Querze G, Zardi L. The fibronectin isoform containing the ED-B oncofetal domain: a marker of angiogenesis. Int J Cancer. 1994;59:612–618. doi: 10.1002/ijc.2910590507. [DOI] [PubMed] [Google Scholar]
- Chamley-Campbell JH, Cambell G, Ross R. Phenotype-dependent response of cultured aortic smooth muscle to serum mitogens. J Cell Biol. 1981;89:379–383. doi: 10.1083/jcb.89.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman MD, Sutherland WM, Platts-Mills TAE. Recognition of two Dermatophagoides pteronyssinus-specific epitopes on antigen P1by using monoclonal antibodies: binding to each epitope can be inhibited by serum from dust mite-allergic patients. J Immunol. 1984;133:2488–2495. [PubMed] [Google Scholar]
- Chen W-T, Singer SJ. Immunoelectron microscopic studies of the sites of cell–substratum and cell–cell contacts in cultured fibroblasts. J Cell Biol. 1982;95:205–222. doi: 10.1083/jcb.95.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou R-GR, Stromer MH, Robson RM, Huiatt TW. Substructure of cytoplasmic dense bodies and changes in distribution of desmin and α-actinin in developing smooth muscle cells. Cell Motil Cytoskeleton. 1994;29:204–214. doi: 10.1002/cm.970290303. [DOI] [PubMed] [Google Scholar]
- Crowley E, Horwitz AF. Tyrosine phosphorylation and cytoskeletal tension regulate the release of fibroblast adhesions. J Cell Biol. 1995;131:525–537. doi: 10.1083/jcb.131.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David-Pfeuty T, Singer SJ. Altered distributions of the cytoskeletal proteins vinculin and α-actinin in cultured fibroblasts transformed by Rous sarcoma virus. Proc Natl Acad Sci USA. 1980;77:6687–6691. doi: 10.1073/pnas.77.11.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Vivek J, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–1169. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- DeRuiter, M.C., R.E. Poelmann, J.C. Van Munsteren, V. Mironov, R.R. Markwald, and A.C. Gittenberger-de Groot. 1997. Embryonic endothelial cells transdifferentiate into mesenchymal cells expressing smooth musle actins in vivo and in vitro. Circ. Res. In press. [DOI] [PubMed]
- Draeger A, Amos WB, Ikebe M, Small JV. The cytoskeletal and contractile apparatus of smooth muscle: contraction bands and segmentation of the contractile elements. J Cell Biol. 1990;111:2463–2473. doi: 10.1083/jcb.111.6.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake CJ, Davis LA, Little CD. Antibodies to β1-integrins cause alterations of aortic vasculogenesis, in vivo. Dev Dyn. 1992;193:83–91. doi: 10.1002/aja.1001930111. [DOI] [PubMed] [Google Scholar]
- Duband J-L, Thiery JP. Spacio-temporal distribution of the adherens junction-associated molecules vinculin and talin in the early avian embryo. Cell Differ Dev. 1990;30:55–76. doi: 10.1016/0922-3371(90)90074-7. [DOI] [PubMed] [Google Scholar]
- Duband J-L, Gimona M, Scatena M, Sartore S, Small JV. Calponin and SM 22 as differentiation markers of smooth muscle: spatiotemporal distribution during avian embryonic development. Differentiation. 1993;55:1–11. doi: 10.1111/j.1432-0436.1993.tb00027.x. [DOI] [PubMed] [Google Scholar]
- Endo T, Masaki T. Molecular properties and functions in vitroof chicken smooth-muscle α-actinin in comparison with those of striated-muscle α-actinins. J Biochem (Tokyo) 1982;92:1457–1468. doi: 10.1093/oxfordjournals.jbchem.a134070. [DOI] [PubMed] [Google Scholar]
- Endo T, Masaki T. Differential expression and distribution of chicken skeletal- and smooth-muscle type α-actinins during myogenesis in culture. J Cell Biol. 1984;99:2322–2332. doi: 10.1083/jcb.99.6.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan GI, Lewis GK, Ramsey G, Bishop JM. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatigati V, Murphy RA. Actin and tropomyosin variants in smooth muscles. Dependence on tissue type. J Biol Chem. 1984;259:14383–14388. [PubMed] [Google Scholar]
- Feramisco JR, Burridge K. A rapid purification of α-actinin, filamin, and a 130,000-dalton protein from smooth muscle. J Biol Chem. 1980;255:1194–1199. [PubMed] [Google Scholar]
- Folkman J, D'Amore PA. Blood vessel formation: what is its molecular basis? . Cell. 1996;87:1153–1155. doi: 10.1016/s0092-8674(00)81810-3. [DOI] [PubMed] [Google Scholar]
- Garrels JI, Gibson W. Identification and characterization of multiple forms of actin. Cell. 1976;9:793–805. doi: 10.1016/0092-8674(76)90142-2. [DOI] [PubMed] [Google Scholar]
- Germroth PG, Gurdie RG, Thompson RP. Confocal microscopy of thick sections from acrylamide gel embedded embryos. Microsc Res Tech. 1995;30:513–520. doi: 10.1002/jemt.1070300608. [DOI] [PubMed] [Google Scholar]
- Glukhova MA, Frid MG, Shekhonin BV, Vasilevskaya TD, Grunwald J, Saginati M, Koteliansky VE. Expression of extra domain A fibronectin sequence in vascular smooth muscle cells is phenotype dependent. J Cell Biol. 1989;109:357–366. doi: 10.1083/jcb.109.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glukhova MA, Frid MG, Shekhonin BV, Balabanov YV, Koteliansky VE. Expression of fibronectin variants in vascular and visceral smooth muscle cells in development. Dev Biol. 1990;141:193–202. doi: 10.1016/0012-1606(90)90114-x. [DOI] [PubMed] [Google Scholar]
- Goncharova EJ, Kam Z, Geiger B. The involvement of adherens junction components in myofibrillogenesis in cultured cardiac myocytes. Development (Camb) 1992;114:173–183. doi: 10.1242/dev.114.1.173. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Hedin U, Thyberg J. Plasma fibronectin promotes modulation of arterial smooth-muscle cells from contractile to synthetic phenotype. Differentiation. 1987;33:239–246. doi: 10.1111/j.1432-0436.1987.tb01563.x. [DOI] [PubMed] [Google Scholar]
- Hedin U, Sjolund M, Hultgårdh-Nilsson A, Thyberg J. Changes in expression and organization of smooth-muscle-specific α-actin during fibronectin-mediated modulation of arterial smooth muscle cell phenotype. Differentiation. 1990;44:222–231. doi: 10.1111/j.1432-0436.1990.tb00621.x. [DOI] [PubMed] [Google Scholar]
- Hemmings ST, Barry ST, Critchley DR. Cell-matrix adhesion: structure and regulation. Biochem Soc Trans. 1995;23:619–625. doi: 10.1042/bst0230619. [DOI] [PubMed] [Google Scholar]
- Hoogenboom HR, Griffiths AD, Johnson KS, Chiswell DJ, Hudson P, Winter G. Multi-subunit proteins on the surface of filamentous phage: methodologies for displaying antibody (Fab) heavy and light chains. Nucleic Acids Res. 1991;19:4133–4137. doi: 10.1093/nar/19.15.4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungerford, J.E. 1995. Development of the vessel wall. Dissertation. The University of Virginia, Charlottesville, VA. 244 pp.
- Hungerford JE, Owens GK, Argraves WS, Little CD. Development of the aortic vessel wall as defined by vascular smooth muscle markers and extracellular matrix markers. Dev Biol. 1996;178:375–392. doi: 10.1006/dbio.1996.0225. [DOI] [PubMed] [Google Scholar]
- Hudson, L., and F.C. Hay. 1980. Practical Immunology. Blackwell Science Publications, Oxford, UK. 259 pp.
- Hunt DF, Henderson RA, Shabanowitz J, Sakaguchi K, Michel H, Sevilir N, Cox AL, Appella E, Engelhard VH. Characterization of peptides bound to the class I MHC molecule HLA-A2.1 by mass spectrometry. Science (Wash DC) 1992;255:1261–1263. doi: 10.1126/science.1546328. [DOI] [PubMed] [Google Scholar]
- Imamura M, Masaki T. Identification of a 115-kDa protein from muscle tissues: expression of a novel nonmuscle α-actinin in vascular endothelial cells. Exp Cell Res. 1994;211:380–390. doi: 10.1006/excr.1994.1102. [DOI] [PubMed] [Google Scholar]
- Imamura M, Sakurai T, Ogawa Y, Ishikawa T, Goto K, Masaki T. Molecular cloning of low-Ca2+-sensitive-type nonmuscle α-actinin. Eur J Biochem. 1994;223:395–401. doi: 10.1111/j.1432-1033.1994.tb19006.x. [DOI] [PubMed] [Google Scholar]
- Jahoda CAB, Reynolds AJ, Chaponnier C, Forester JC, Gabbiani G. Smooth muscle α-actin is a marker for hair follicle dermis in vivo and in vitro. J Cell Sci. 1991;99:627–636. doi: 10.1242/jcs.99.3.627. [DOI] [PubMed] [Google Scholar]
- Kallmeier RC, Somasudaram C, Babiji P. A novel smooth musclespecific enhancer regulates transcription of the smooth muscle myosin heavy chain gene in vascular smooth muscle cells. J Biol Chem. 1995;270:30949–30957. doi: 10.1074/jbc.270.52.30949. [DOI] [PubMed] [Google Scholar]
- Khoory W, Wu E, Svoboda KKH. Intracellular relationship between actin and α-actinin in a whole corneal epithelial tissue. J Cell Sci. 1993;106:703–717. doi: 10.1242/jcs.106.3.703. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (Lond) 1970;227:860–865. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazard D, Sastre X, Frid MG, Glukhova MA, Thiery J-P, Koteliansky VE. Expression of smooth muscle specific proteins in myoepithelium and stromal myofibroblasts of normal and malignant human breast tissue. Proc Natl Acad Sci USA. 1993;90:999–1003. doi: 10.1073/pnas.90.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E, Burridge K. α-Actinin: immunofluorescent localization of a muscle structural protein in nonmuscle cells. Cell. 1975;6:289–298. doi: 10.1016/0092-8674(75)90180-4. [DOI] [PubMed] [Google Scholar]
- Lee, S.H., J.E. Hungerford, C.D. Little, and M.L. Iruela-Arispe. 1997. Proliferation and differentiation of smooth muscle cell precursors occurs simultaneously during development of the vessel wall. Dev. Dyn. In press. [DOI] [PubMed]
- Li L, Miano JM, Mercer B, Olson E. Expression of the Sm22 α promoter in transgenic mice provides evidence for distinct transcriptional regulatory programs in vascular and visceral smooth muscle cells. J Cell Biol. 1996;132:849–859. doi: 10.1083/jcb.132.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly B, Zhao B, Ranganayakulu G, Paterson BM, Schulz RA, Olson EN. Requirement of MADS domain transcription factor D-MEF2 for muscle formation in Drosophila. . Science (Wash DC) 1995;267:688–693. doi: 10.1126/science.7839146. [DOI] [PubMed] [Google Scholar]
- Luna EJ, Hitt AL. Cytoskeleton-plasma membrane interactions. Science (Wash DC) 1992;258:955–964. doi: 10.1126/science.1439807. [DOI] [PubMed] [Google Scholar]
- Marchisio PC, Cirillo D, Teti A, Zambonin-Zallone A, Tarone G. Rous Sarcoma virus-transformed fibroblasts and cells of monocytic origin display a peculiar dot-like organization of cytoskeletal proteins involved in microfilament-membrane interactions. Exp Cell Res. 1987;169:202–214. doi: 10.1016/0014-4827(87)90238-2. [DOI] [PubMed] [Google Scholar]
- Miano JM, Olson EN. Expression of the smooth muscle cell calponin gene marks the early cardiac and smooth muscle cell lineages during mouse embryogenesis. J Biol Chem. 1996;271:7095–7103. doi: 10.1074/jbc.271.12.7095. [DOI] [PubMed] [Google Scholar]
- Miano JM, Cserjesi P, Ligon KL, Periasamy M, Olson EN. Smooth muscle myosin heavy chain exclusively marks the smooth muscle lineage during mouse embryogenesis. Circ Res. 1994;75:803–812. doi: 10.1161/01.res.75.5.803. [DOI] [PubMed] [Google Scholar]
- Miano JM, Firulli AB, Olson EN, Hara P, Giachelli CM, Schwartz SM. Restricted expression of homeobox genes distinguishes fetal from adult human smooth muscle cells. Proc Natl Acad Sci USA. 1996;93:900–905. doi: 10.1073/pnas.93.2.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millake DB, Blanchard AD, Patel B, Critchley DR. The cDNA sequence of a human placental α-actinin. Nucleic Acids Res. 1989;17:6725. doi: 10.1093/nar/17.16.6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrisey EE, Ip HS, Lu MM, Parmacek MS. GATA-6: a zinc finger transcription factor that is expressed in multiple cell lineages derived from lateral mesoderm. Dev Biol. 1996;177:309–322. doi: 10.1006/dbio.1996.0165. [DOI] [PubMed] [Google Scholar]
- Olson EN. Regulation of muscle transcription by the MyoD family. Circ Res. 1993a;72:1–6. doi: 10.1161/01.res.72.1.1. [DOI] [PubMed] [Google Scholar]
- Olson EN. Signal transduction pathways that regulate skeletal muscle gene expression. Mol Endocrinol. 1993b;7:1369–1378. doi: 10.1210/mend.7.11.8114752. [DOI] [PubMed] [Google Scholar]
- Olson EN, Perry M, Schulz RA. Regulation of muscle differentiation by the MEF2 family of MADS box transcription factors. Dev Biol. 1995;172:2–14. doi: 10.1006/dbio.1995.0002. [DOI] [PubMed] [Google Scholar]
- Ontell M, Ontell MP, Buckingham M. Muscle-specific gene expression during myogenesis in the mouse. Microsc Res Tech. 1995;30:354–365. doi: 10.1002/jemt.1070300503. [DOI] [PubMed] [Google Scholar]
- Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev. 1995;75:487–517. doi: 10.1152/physrev.1995.75.3.487. [DOI] [PubMed] [Google Scholar]
- Owens GK, Thompson MM. Developmental changes in isoactin expression in rat aortic smooth muscle cells in vivo: relationship between growth and cytodifferentiation. J Biol Chem. 1986;261:13373–13380. [PubMed] [Google Scholar]
- Parr T, Waites GT, Patel B, Millake DB, Critchley DR. A chick skeletal-muscle α-actinin gene gives rise to two alternatively spliced isoforms which differ in the EF-hand Ca2+-binding domain. Eur J Biochem. 1992;210:801–809. doi: 10.1111/j.1432-1033.1992.tb17483.x. [DOI] [PubMed] [Google Scholar]
- Pavalko FM, Schneider G, Burridge K, Lim S-S. Immunodetection of α-actinin in focal adhesions is limited by antibody inaccessibility. Exp Cell Res. 1995;217:534–540. doi: 10.1006/excr.1995.1119. [DOI] [PubMed] [Google Scholar]
- Rønnov-Jessen L, van Deurs B, Celis JE, Petersen OW. Smooth muscle differentiation in cultured human breast gland stromal cells. Lab Invest. 1990;63:532–543. [PubMed] [Google Scholar]
- Ruzicka DL, Schwartz RJ. Sequential activation of α-actin genes during avian cardiogenesis: vascular smooth muscle α-actin gene transcripts mark the onset of cardiomyocyte differentiation. J Cell Biol. 1988;107:2575–2586. doi: 10.1083/jcb.107.6.2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaha FF, Ip HS, Morrisey EE, Seltzer J, Tang Z, Solway J, Parmacek MS. Developmental pattern of expression and genomic organization of the calponin-h1 gene; a contractile smooth muscle cell marker. J Biol Chem. 1996;271:395–403. doi: 10.1074/jbc.271.1.395. [DOI] [PubMed] [Google Scholar]
- Sappino AP, Schürch W, Gabbiani G. Differentiation repertoire of fibroblastic cells: expression of cytoskeletal proteins as marker of phenotypic modulations. Lab Invest. 1990;63:144–161. [PubMed] [Google Scholar]
- Sawtell NM, Lessard JL. Cellular distribution of smooth muscle actins during mammalian embryogenesis: expression of the α-vascular but not the α-enteric isoform in differentiating striated myocytes. J Cell Biol. 1989;109:2929–2937. doi: 10.1083/jcb.109.6.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu RT, Blank RS, Jervis R, Lawrenz-Smith SC, Owens GK. The smooth muscle α-actin gene promoter is differentially regulated in smooth muscle versusnon-smooth muscle cells. J Biol Chem. 1995;270:7631–7643. doi: 10.1074/jbc.270.13.7631. [DOI] [PubMed] [Google Scholar]
- Skalli O, Vandekerckhove J, Gabbiani G. Actin-isoform pattern as a marker of normal or pathological smooth-muscle and fibroblastic tissues. Differentiation. 1987;33:232–238. doi: 10.1111/j.1432-0436.1987.tb01562.x. [DOI] [PubMed] [Google Scholar]
- Skalli O, Pelte M-F, Peclet M-C, Gabbiani G, Gugliotta P, Bussolate G, Ravazzola M, Orci L. α-Smooth muscle actin, a differentiation marker of smooth muscle cells, is present in microfilamentous bundles of pericytes. J Histochem Cytochem. 1989;37:315–321. doi: 10.1177/37.3.2918221. [DOI] [PubMed] [Google Scholar]
- Sugi Y, Lough J. Onset of expression and regional deposition of α-smooth and sarcomeric actin during avian heart development. Dev Dyn. 1992;193:116–124. doi: 10.1002/aja.1001930203. [DOI] [PubMed] [Google Scholar]
- Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- Tarone G, Cirillo D, Giancotti FG, Comoglio PM, Marchisio PC. Rous sarcoma virus-transformed fibroblasts adhere primarily at discrete protrusions of the ventral membrane called podosomes. Exp Cell Res. 1985;159:141–157. doi: 10.1016/s0014-4827(85)80044-6. [DOI] [PubMed] [Google Scholar]
- Thayer JM, Meyers K, Giachelli CM, Schwartz SM. Formation of the arterial media during vascular development. Cell Mol Biol Res. 1995;41:251–262. [PubMed] [Google Scholar]
- Thyberg J. Differentiated properties and proliferation of arterial smooth muscle cells in culture. Int Rev Cytol. 1996;169:183–265. doi: 10.1016/s0074-7696(08)61987-7. [DOI] [PubMed] [Google Scholar]
- Thyberg J, Hedin U, Sjölund M, Palmberg L, Bottger BA. Regulation of differentiated properties and proliferation of arterial smooth muscle cells. Arteriosclerosis. 1990;10:966–990. doi: 10.1161/01.atv.10.6.966. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinkaus, J.P. 1984. Cells into Organs. Second edition. Chapter 7. Prentice-Hall Inc., Englewood Cliffs, NJ. 543 pp.
- Waites GT, Graham IA, Jackson P, Millake DB, Patel B, Blanchard AD, Weller PA, Eperon IC, Critchley DR. Mutually exclusive splicing of calcium-binding domain exons in chick α-actinin. J Biol Chem. 1992;267:6263–6271. [PubMed] [Google Scholar]
- White SL, Low RB. Identification of promoter elements involved in cell-specific regulation of rat smooth muscle myosin heavy chain gene transcription. J Biol Chem. 1996;271:15008–15017. doi: 10.1074/jbc.271.25.15008. [DOI] [PubMed] [Google Scholar]
- Vikkula M, Boon LM, Carraway KL, Calvert JT, Diamonti AJ, Goumnerov B, Pasyk KA, Marchuk DA, Warman ML, Cantley LC, Mulliken JB, Olsen BR. Vascular dysmorphogenesis caused by an activating mutation in the receptor tyrosine kinase TIE2. Cell. 1996;87:1153–1155. doi: 10.1016/s0092-8674(00)81814-0. [DOI] [PubMed] [Google Scholar]
- Volberg T, Sabanay H, Geiger B. Spatial and temporal relationships between vinculin and talin in the developing chicken gizzard smooth muscle. Differentiation. 1986;32:34–43. doi: 10.1111/j.1432-0436.1986.tb00553.x. [DOI] [PubMed] [Google Scholar]
- Youssoufian H, McAfee M, Kwiatkowski DJ. Cloning and chromosomal localization of the human cytoskeletal α-actinin gene reveals linkage to the β-spectrin gene. Am J Hum Genet. 1990;47:62–72. [PMC free article] [PubMed] [Google Scholar]