Abstract
Previous studies have implicated the heat shock cognate (hsc) protein of 73 kD (hsc73) in stimulating a lysosomal pathway of proteolysis that is selective for particular cytosolic proteins. This pathway is activated by serum deprivation in confluent cultured human fibroblasts. We now show, using indirect immunofluorescence and laser scanning confocal microscopy, that a heat shock protein (hsp) of the 70-kD family (hsp70) is associated with lysosomes (ly-hsc73). An mAb designated 13D3 specifically recognizes hsc73, and this antibody colocalizes with an antibody to lgp120, a lysosomal marker protein. Most, but not all, lysosomes contain ly-hsc73, and the morphological appearance of these organelles dramatically changes in response to serum withdrawal; the punctate lysosomes fuse to form tubules.
Based on susceptibility to digestion by trypsin and by immunoblot analysis after two-dimensional electrophoresis of isolated lysosomes and isolated lysosomal membranes, most ly-hsc73 is within the lysosomal lumen. We determined the functional importance of the ly-hsc73 by radiolabeling cellular proteins with [3H]leucine and then allowing cells to endocytose excess mAb 13D3 before measuring protein degradation in the presence and absence of serum. The increased protein degradation in response to serum deprivation was completely inhibited by endocytosed mAb 13D3, while protein degradation in cells maintained in the presence of serum was unaffected. The intralysosomal digestion of endocytosed [3H]RNase A was not affected by the endocytosed mAb 13D3. These results suggest that ly-hsc73 is required for a step in the degradative pathway before protein digestion within lysosomes, most likely for the import of substrate proteins.
Stress proteins, also known as heat shock proteins (hsps)1, constitute several families of proteins that were originally discovered because they were highly inducible upon heat shock (Morimoto et al., 1994). In addition, several heat shock proteins are constitutively expressed or are regulated by factors other than stress. Heat shock proteins of 70 kD (hsp70s) have been the subject of considerable study (Craig et al., 1994). These molecular chaperones are localized to different cellular compartments where protein folding has to be achieved, altered, or stabilized (Hendrick and Hartl, 1993; Hartl, 1996). One of the members of this family of proteins, the constitutively expressed heat shock cognate (hsc) protein of 73 kD (hsc73), has been implicated in the mediation of clathrin uncoating from endosomes (Rothman and Schmid, 1986); regulation of cytoskeletal interactions (Green and Liem, 1989); transient association with nascent polypeptides (Beckman et al., 1990); and facilitation of the import of polypeptides into mitochondria (Deshaies et al., 1988; Murakami et al., 1988; Sheffield et al., 1990), the ER (Chirico et al., 1988; Deshaies et al., 1988), the nucleus (Shi and Thomas, 1992), and peroxisomes (Walton et al., 1994).
Genetic and biochemical analyses indicate that organellar hsp70s are required for import of substrate proteins into the ER (Vogel et al., 1990; Nicchitta and Blobel, 1993; Panzner et al., 1995) and mitochondria (Kang et al., 1990; Rassow et al., 1994). In these cases, a distinct hsp70 family member resides within the ER (mammalian glucose-regulated protein of 78 kD [grp78]; yeast karyogamy gene 2 product) and mitochondria (mammalian glucose-regulated protein of 75 kD; yeast stress 70 subgroup C gene product).
When cultured cells are deprived of serum, rates of intracellular proteolysis increase (Amenta and Brocher, 1981; Hendil et al., 1990). Our laboratory has previously reported that serum deprivation of confluent human fibroblasts activates a selective pathway of lysosomal proteolysis (Chiang and Dice, 1988) and that cytosolic hsc73 stimulates this pathway of protein degradation (Chiang et al., 1989). This selective pathway of lysosomal proteolysis, which we will hereafter refer to as the hsc73-stimulated pathway, is also activated in liver, kidney, and heart of starved rats (Chiang and Dice, 1988; Wing et al., 1991; Cuervo et al., 1995).
RNase A was originally studied as a substrate for the hsc73-stimulated pathway of lysosomal proteolysis (Neff et al., 1981; Backer et al., 1983). Residues 7–11 of RNase A, KFERQ, are required for entry of RNase A into this proteolytic pathway (Dice et al., 1986). Furthermore, the KFERQ pentapeptide is an important element in the binding of RNase A and RNase S-peptide (amino acids 1–20 of RNase A) by hsc73 (Terlecky et al., 1992). Approximately 30% of cytosolic proteins contain peptide sequences immunologically related to KFERQ, and such proteins are also targeted to the hsc73-stimulated pathway of lysosomal proteolysis (Chiang and Dice, 1988; Wing et al., 1991).
We have been able to reproduce the hsc73-stimulated pathway of proteolysis using isolated lysosomes (Chiang et al., 1989; Terlecky et al., 1992; Terlecky and Dice, 1993; Cuervo et al., 1994, 1995). Uptake of substrate proteins is saturable, and specific binding to a lysosomal membrane protein occurs before uptake. Lysosomal uptake and degradation of substrate polypeptides is stimulated by hsc73 and Mg2+-ATP, and it is activated by withdrawal of serum from cells before isolation of lysosomes. This pathway of proteolysis is inhibited by low temperatures, NH4Cl, and leupeptin.
Aniento et al. (1993) have shown that glyceraldehyde-3phosphate dehydrogenase is selectively taken up by rat liver lysosomes in vitro. The process appears to be similar to that for RNase A by fibroblast lysosomes since it is also stimulated by Mg2+-ATP and hsc73. Furthermore, uptake of glyceraldehyde-3-phosphate dehydrogenase can be competed with RNase A and vice versa (Cuervo et al., 1994). The pathway in rat liver lysosomes is activated by prolonged starvation (Cuervo et al., 1995). Our laboratory has recently identified the lysosomal glycoprotein (lgp) of 96 kD (lgp96) as the lysosomal receptor for this proteolytic pathway (Cuervo and Dice, 1996).
We now report colocalization of an hsp70 with the lysosomal marker protein lgp120 (Green et al., 1987). The lysosomal hsp70 (ly-hsc73) appears to be primarily within the lysosomal lumen. An mAb that recognizes hsc73 and lyhsc73 when delivered to lysosomes by endocytosis completely blocks this pathway of proteolysis. Thus, ly-hsc73 appears to play a critical role in the operation of this proteolytic pathway.
Materials and Methods
Cell Culture and Cell Fractionation
IMR-90 diploid human lung fibroblasts were maintained (Neff et al., 1981; Terlecky and Dice, 1993), and cells were fractionated into organelles and cytosol (Chiang and Dice, 1988) as previously described. The membrane fraction was the pellet from the centrifugation at 100,000 g for 1 h, and the supernatant from this centrifugation contained cytosolic proteins. Lysosomes were purified from the postnuclear supernatant as previously reported (Terlecky and Dice, 1993). For immunolocalization, IMR-90 human diploid fibroblasts were cultured at low density in six-well plates (Costar, Cambridge, MA) on glass coverslips. Half of the plates were serum deprived for 18 h, and the rest remained in serum-supplemented culture medium before fixation.
Proteins, Antibodies, and Protein Assays
hsc73 purification from bovine brain cytosol was carried out according to Welch and Feramisco (1985). The peptide binding protein (PBP) of 74 kD (PBP74) was a gift from Drs. Diane DeNagel and Susan Pierce (Northwestern University, Evanston, IL). The stress 70 subgroup A1 protein (SSA1p) was a gift of Drs. Bruce Koch and Randy Schekman (University of California, Berkeley). Affinity-purified mouse mAb 13D3 against hsc73 (Maekawa et al., 1989) was kindly provided by Joseph Chandler (Maine Biotechnology Services Inc., Portland, ME). A polyclonal rabbit antibody directed against rat lgp120 was a generous gift of Dr. Ira Mellman (Yale University, New Haven, CT). A polyclonal rabbit antibody raised against amino acids 1–16 of PBP74 (antiPBP74-1-16; Domanico et al., 1993) was kindly provided by Drs. Diane DeNagel and Susan Pierce. Mouse mAb P32 was a gift from Dr. Henry Wortis (Tufts University, Boston, MA). This IgM recognizes α-1,3-dextran (Schilling et al., 1980). Mouse mAb 7.10 was purchased from Affinity Bioreagents (Neshanic Station, NJ). Fluorescein-labeled secondary anti–mouse IgM, μ chain specific, and Texas red–labeled anti–rabbit antibodies were purchased from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA). Protein determinations were performed using either the BioRad protein assay reagent (Bio Rad Laboratories, Hercules, CA) or the Lowry assay (Lowry et al., 1951).
One- and Two-dimensional Gel Electrophoresis
One-dimensional SDS-PAGE was carried out as previously described (Maekawa et al., 1989; Terlecky et al., 1992). Two-dimensional electrophoresis was performed essentially as described (O'Farrell, 1975). IEF with pH 5.0–6.0 ampholines (Sigma Chemical Co., St. Louis, MO) was carried out in 20-cm-long gels at 1.5 kV for 18 h.
Immunoblotting
Proteins were electrotransferred onto either nitrocellulose (Schleicher & Schuell, Keene, NH) or Immobilon-P (Millipore Corp., Bedford, MA) membranes. Immunoblotting with mAb 13D3 and mAb 7.10 was carried out as described (Maekawa et al., 1989; Terlecky et al., 1992). Immunoblotting with antiPBP74-1-16 was performed either at 37°C for 3 h or overnight at 4°C at a 1:300 dilution.
After transfer of the two-dimensional gels to Immobilon-P, mAb 13D3 bound was visualized by incubation for 1 h with HRP-conjugated affinitypurified goat anti–mouse IgM, μ chain specific (Jackson ImmunoResearch Laboratories, Inc.) at a 1:10,000 dilution. The peroxidase was visualized by chemiluminescence using reagents from the ECL detection system (Amersham Life Science, Inc., Arlington Heights, IL) and exposure to either X-OMAT AR film (Eastman Kodak Co., Rochester, NY) or Hyperfilm-ECL (Amersham Life Science, Inc.).
Immunoprecipitation
The mAb 13D3 or an irrelevant IgM was preincubated at 25°C with a mixture of short peptides to eliminate nonspecific binding. This mixture consisted of the dipeptides, MK, RE, and KF, and the hexapeptide, LTMRFA, each at 3 mg/ml. Purified hsc73 or PBP74 (1 μg) was incubated with 8 μg of mAb 13D3 at 37°C for 3 h followed by the addition of another 8 μg of mAb 13D3 and an overnight incubation at 4°C. The samples were centrifuged for 15 min at 13,600 g max at 25°C. Pellets were washed three times with 2× PBS containing 0.2% Tween-20 and 0.02% NaN3, and then once with PBS, containing 0.1% Tween-20 and 0.02% NaN3. The pellets were then resuspended in gel loading buffer and run in 8% polyacrylamide gels.
Indirect Immunofluorescence and Confocal Microscopy
The cells grown on glass coverslips were washed five times with serumfree culture medium, and then fixed in −20°C methanol for 1 min and stored at 4°C in PBS containing 0.02% NaN3. For fluorescent double labeling of ly-hsc73 and lgp120, all incubations and washes were at 25°C. Primary antibodies were diluted 1:50, and secondary antibodies were diluted 1:100 in PBS containing 0.1% BSA and 0.02% NaN3. Cells were incubated for 1 h with a mixture of primary antibodies, washed 10 times in PBS, and incubated for 1 h with each of the corresponding secondary antibodies. Coverslips were mounted in mounting media containing an antibleaching agent (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, MD). Images were collected on a laser scanning confocal microscope, either an MRC 600 (Bio Rad Laboratories) or an ODYSSEY XL (NORAN Instruments, Middleton, WI). The image analysis software used was INTERVISION (NORAN Instruments). Pictures were taken with Kodachrome 64 and TMAX400 film (Eastman Kodak Co.). Fig. 3 C was generated using Adobe Photoshop 3.0 software (Adobe Systems Inc., Mountain View, CA). No fluorescence was associated with cells after incubation with secondary antibodies alone (data not shown).
Figure 3.
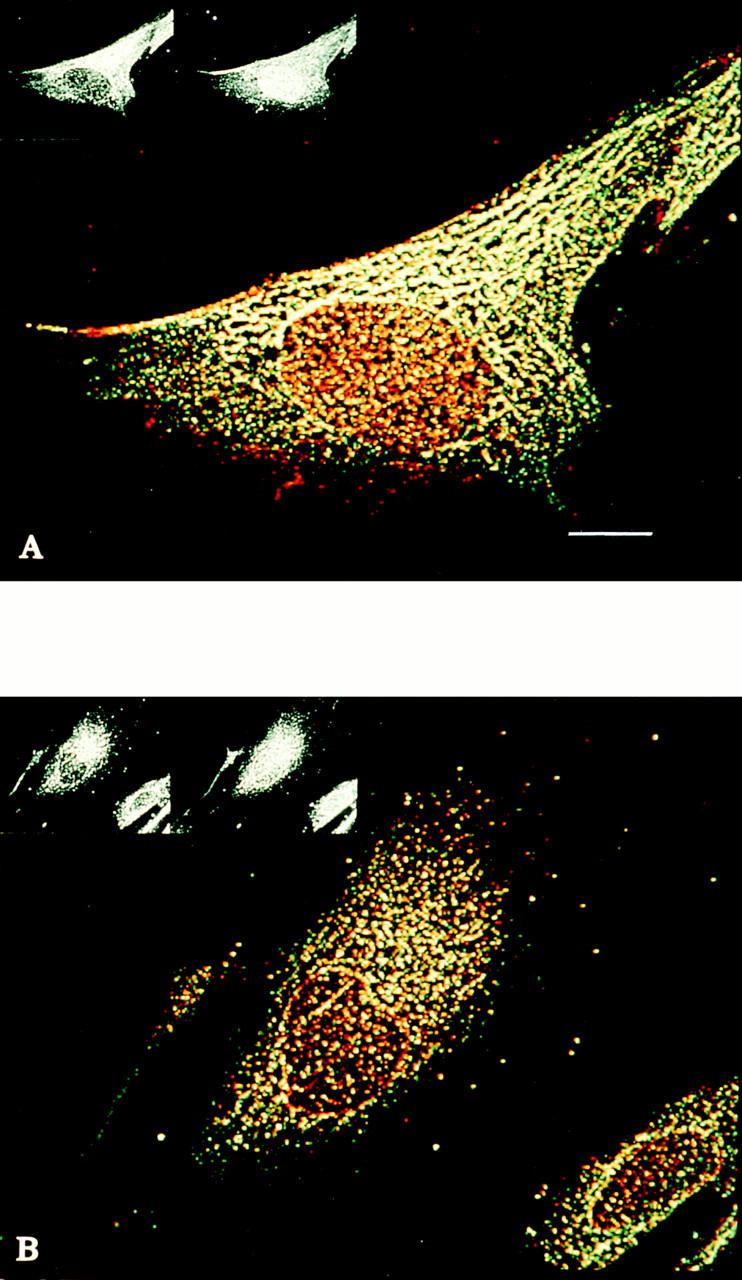
Distribution of ly-hsc73 and lgp120 in serum-deprived and serumsupplemented fibroblasts using a thick optical section. Methanol-fixed fibroblasts were stained with primary antibodies to hsc73 (green) and lgp120 (red) as described in the legend to Fig. 2. The merged color images using an optical section of 4 μm show: (A) serum-deprived fibroblast and (B) serum-supplemented fibroblast. Note that lysosomes appear to fuse, forming a tubular network when cells are serum deprived (A). Bar, 10 μm.
Trypsinization of Hsc73 and Ly-hsc73
Purified bovine brain hsc73 (12.5 μg/ml) was incubated with 0, 200, 400, or 800 μg/ml trypsin in a final vol of 100 μl in the presence or absence of 1% Triton X-100 at 4°C for 20 min. Soybean trypsin inhibitor was added at 1.375-fold the concentration of trypsin, and lysosomal proteins were precipitated with 12% TCA containing 0.1% sodium deoxycholate. The resultant pellet was resuspended in gel loading buffer, and the pH was neutralized with 25% NH4OH.
Lysosomes from 2 × 106 cells were pelleted as described (Terlecky and Dice, 1993), resuspended in a final vol of 100 μl of PBS with or without 800 μg/ml trypsin and 1% Triton X-100, and incubated at 4°C. After 20 min, soybean trypsin inhibitor (1.1 mg) was added and the samples were treated as described above.
Antibody Endocytosis and Intracellular Protein Degradation
Confluent IMR-90 fibroblasts in six-well plates were radiolabeled with 25 μCi/ml [3H]l-leucine (131 Ci/mmol; ICN Biomedicals, Inc., Irvine, CA) for 2 d. Cell monolayers were washed four times with HBSS (GIBCO BRL, Gaithersburg, MD) and incubated overnight at 37°C in 1 ml of fresh medium containing 10% newborn calf serum (NCS) with or without the following: (a) 10 μg mAb 13D3, (b) 10 μg mAb P32, or (c) 10 μg mAb 13D3 plus 12 μg hsc73 (preincubated overnight at 4°C). Cell monolayers were washed four times with HBSS and chased for 1 h with 6 ml of fresh medium containing 10% NCS. Cell monolayers were then washed four times with HBSS and cultured with fresh medium with or without 10% NCS. Aliquots of media were taken over a time course, and protein degradation followed by measuring the radioactivity released into the medium that was soluble in 3.5% phosphotungstate (PTA) and 5% HCl. Protein degradation was calculated as described previously (Backer et al., 1983) and plotted as the exponential loss of radioactivity associated with the monolayer at increasing times. The calculated best fit lines and the halflives were obtained by Fig.P software (Biosoft, Cambridge, UK).
Antibody Endocytosis and Degradation of Endocytosed [3H]RNase A
Confluent fibroblasts in six-well plates were incubated overnight at 37°C in medium plus 10% NCS containing 8 × 107 dpm of [3H]RNase A (2.96 × 104 dpm/pmol) and either 10 μg/ml of mAb 13D3, 100 μg/ml of mAb 13D3, 10 μg/ml of nonspecific IgM, 100 μg/ml of nonspecific IgM, or no antibody. Cells were washed four times with HBSS containing 5% NCS and three times with HBSS. Cells were returned to medium containing 10% NCS and, after 1 h, the medium was changed. Aliquots of media were taken and the appearance of PTA/HCl-soluble radioactivity was determined.
Stability of Endocytosed Proteins
RNase A, mAb 13D3, and hsc73 were radiolabeled by reductive methylation using NaB3H4 as described (Backer et al., 1983). Confluent cultures of IMR-90 fibroblasts were labeled for 2 d with 12 μCi/ml of [35S]l-methionine (422 Ci/mmol; ICN Biomedicals, Inc.), and cytosolic proteins were prepared. Radiolabeled proteins were added to cell monolayers in six-well plates in medium containing 10% NCS. The amount of radioactivity added per well and the specific radioactivities of the proteins were as follows: mAb 13D3: 1.75 × 106 dpm, 5 × 106 dpm/nmol; RNase A: 3 × 107 dpm, 2.4 × 105 dpm/nmol; hsc73: 2.5 × 107 dpm, 1.4 × 108 dpm/nmol; repurified hsc73: 5 × 105 dpm, 1.4 × 108 dpm/nmol; cytosolic proteins: 4 × 106 dpm, 2.1 × 104 dpm/nmol (assuming an average molecular mass of 40 kD). After an overnight incubation, cells were washed four times with HBSS containing 5% NCS and three times with HBSS alone. Cells were chased in medium containing 10% NCS for 6 h, and the medium was then changed. Monolayers were washed and harvested in PBS containing 1% Triton X-100, and the PTA/HCl-precipitable radioactivity was determined.
Counting of Radioactivity
Radioactivity was determined in a Beckman LS 1801 liquid scintillation spectrophotometer using Ready Safe Scintillation Cocktail (Beckman Instruments, Inc., Fullerton, CA). Quenching was corrected by using an external radioactive standard.
Results
Characterization of mAb 13D3
Previous studies have shown that mAb 13D3 recognizes hsc73 but not the major heat-inducible hsp70 of human fibroblasts in immunoblots (Terlecky et al., 1992). mAb 13D3 also recognized hsc73, or another as yet unidentified hsp70, in a cellular membrane fraction (see Materials and Methods; Fig. 1 A, lane 1) but did not recognize what we presume to be grp78 (Fig. 1 A, lane 2, upper band). Both hsc73 and grp78 could be visualized by mAb 7.10, which is known to recognize several members of the hsp70 family (Kurtz et al., 1986; Fig. 1 A, lane 2). In addition, mAb 13D3 did not recognize the yeast hsp70, SSA1p (Fig. 1 B, lane 1), which is recognized by mAb 7.10 (Fig. 1 B, lane 2).
Figure 1.
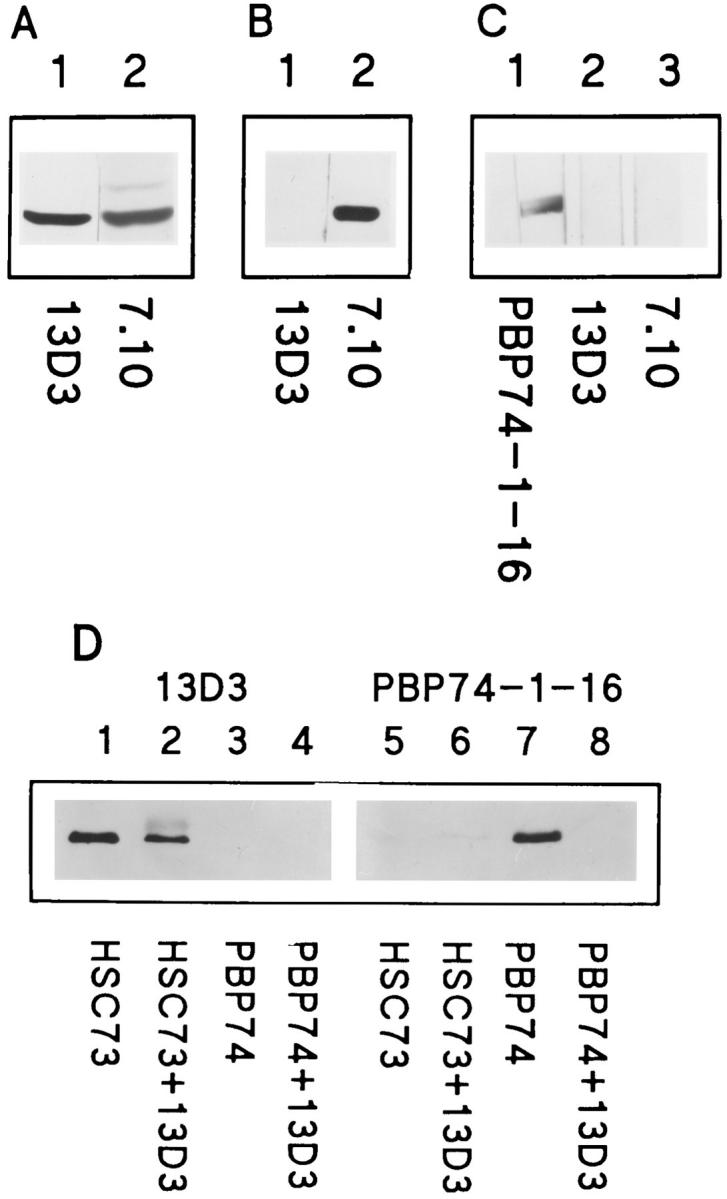
Immunoblot and immunoprecipitation analysis of mAb 13D3 specificity. 25 μg of fibroblast membranes (A), 1.5 μg of SSA1p (B), or 4.5 μg of PBP74 (C) were separated by SDSPAGE and transferred to nitrocellulose membranes. Lanes were probed with mAb 13D3, mAb 7.10, or antiPBP74-1-16 as indicated. Immunoprecipitations (D) were with mAb 13D3 and hsc73 or PBP74. (Lanes 1–8) Immunoblots of the purified proteins and the immunoprecipitates. (Lanes 1 and 5) 0.2 μg hsc73; (lanes 2 and 6) hsc73 immunoprecipitated with mAb 13D3; (lanes 3 and 7) 0.2 μg PBP74; (lanes 4 and 8) PBP74 incubated with mAb 13D3. Lanes were probed with mAb 13D3 or antiPBP74-1-16 as indicated.
Domanico et al. (1993) identified a new member of the hsp70 family, PBP74, and showed that it was localized to mitochondria (Dahlseid et al., 1994). Fig. 1 C shows an immunoblot analysis of purified PBP74. A wide single lane of an SDS-PAGE gel was transferred to a membrane, the membrane was cut in thirds longitudinally, and each strip was incubated with a different antibody. Lane 1 was incubated with antiPBP74-1-16, lane 2 with mAb 13D3, and lane 3 with mAb 7.10. AntiPBP74-1-16 recognized purified PBP74, but neither mAb 13D3 nor mAb 7.10 recognized PBP74 (Fig. 1 C, lanes 2 and 3, respectively).
We next determined whether or not mAb 13D3 could immunoprecipitate hsc73. Purified hsc73 and PBP74 were directly immunoprecipitated with mAb 13D3 as described in Materials and Methods. Immunoprecipitates and purified hsp70s were analyzed by immunoblots reacting either with mAb 13D3 (Fig. 1 D, lanes 1–4) or antiPBP74-1-16 (Fig. 1 D, lanes 5–8). mAb 13D3 immunoprecipitates hsc73 (Fig. 1 D, lane 2) but does not immunoprecipitate PBP74 (Fig. 1 D, lane 8). The higher molecular weight band in lane 2 is due to altered mobility of a portion of the hsc73 as a result of the high concentration of antibody required for the immunoprecipitation. Controls using a nonspecific IgM showed no detectable precipitation of hsc73 (data not shown). Furthermore, mAb 13D3 was able to recognize native hsc73 in immunoblots performed at the expected intralysosomal pH of 5.5 (data not shown).
Localization of ly-hcs73 by Confocal Microscopy
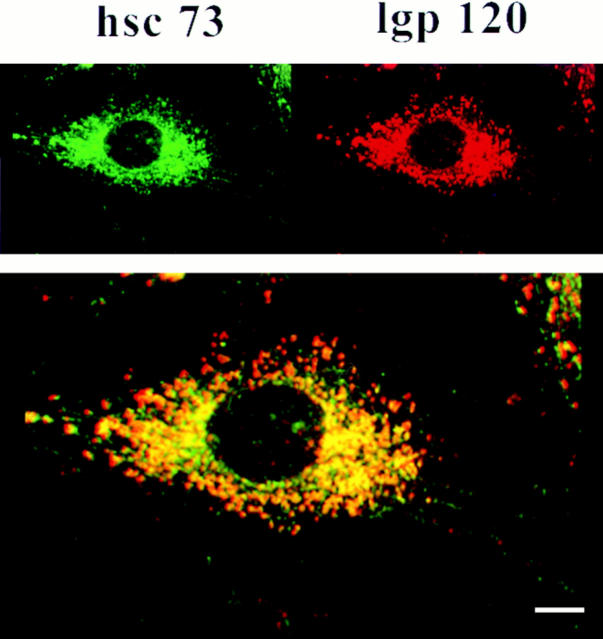
We examined and quantitated the lysosomal localization of ly-hsc73 further by confocal microscopy. Double fluorescence staining of fibroblasts was carried out with mAb 13D3 and anti-lgp120 antibodies (Figs. 2 and 3). lgp120 is the major glycoprotein in lysosomal membranes (Green et al., 1987). As previously described, hsc73 is an abundant cytosolic protein (Giebel et al., 1988). To avoid the interference of cytosolic hsc73 during immunolabeling with mAb 13D3, cells were fixed in cold methanol, which did not fix the cytosolic fraction of hsc73 and allowed us to examine organelle-associated hsp70s that are recognized by mAb 13D3. The mAb 13D3 (green signal) largely colocalized with the anti-lgp120 antibodies (red signal). A thin optical section (0.09 μm) shows that both proteins are confined to the cytoplasmic compartment (Fig. 2). Colocalization in the merged images (Figs. 2 and 3) registers as orange/yellow.
Figure 2.
Distribution of ly-hsc73 and lgp120 by confocal microscopy using a narrow optical section. Fibroblasts were serum deprived overnight, methanol fixed, and then incubated with mAb 13D3 and anti-lgp120 simultaneously followed by Texas red– and fluorescein-conjugated second antibodies to reveal ly-hsc73 (green) and lgp120 (red). The thickness of the optical section analyzed was 0.09 μm. Colocalization of both proteins registers as yellow/ orange. (Insets) Left panel, fluorescein channel; right panel, Texas red channel. Bar, 10 μm.
To quantitate Fig. 2 and similar images, we scored 1,000 immunofluorescent vesicles. More than 90% of the lysosomes contained ly-hsc73, and >80% of the vesicular hsp70 was lysosomal. However, some lysosomes did not contain detectable levels of ly-hsc73, visible as a pure red signal. In addition, some nonlysosomal vesicular structures contain an immunoreactive hsp70, visible as a pure green signal. These organelles may be clathrin-coated vesicles and/or peroxisomes since hsc73 is known to associate with these structures (Rothman and Schmidt, 1986; Walton et al., 1994).
Fig. 3 compares cells grown in the presence (Fig. 3 B) and absence (Fig. 3 A) of serum using a thick optical section (4 μm) that includes cytoplasm containing lysosomes above the nucleus. This analysis shows a remarkable change in the lysosomal morphology in response to serum withdrawal. Lysosomes in serum-deprived fibroblasts appear to fuse, adopting a tubular shape (defined as structures with a length greater than four times their diameter). These tubular lysosomes were not evident using the thin optical section (Fig. 2) probably because the tubules repeatedly traverse the optical section. Tubular lysosomes have been previously described in activated macrophages (Swanton et al., 1987; Heuser, 1989; Knapp and Swanson, 1990), smooth muscle cells (Robinson et al., 1986), endothelial cells (Kuijpers et al., 1994), and hepatocytes (Novikoff and Shin, 1978). We scored 1,600 immunofluorescent vesicular structures or equivalent areas within tubules in cells deprived of serum. Once again, >90% of the lysosomes contained ly-hsc73, and >80% of the vesicular or tubular hsp70 was lysosomal.
The Majority of ly-hsc73 Is in the Lumen
We characterized the amount of trypsin required to completely digest purified bovine brain hsc73 in 20 min at 4°C. Fig. 4 A is an immunoblot using mAb 13D3 that shows that 1% Triton X-100 does not alter the ability of mAb 13D3 to recognize hsc73 (Fig. 4 A, lanes 1 and 2), and that 200 μg/ml of trypsin is sufficient to completely digest hsc73 in the absence (lane 3) but not in the presence (lane 4) of 1% Triton X-100. To digest all of the hsc73 in the presence of 1% Triton X-100, 800 μg/ml of trypsin was required (Fig. 4 A, lane 9). The presence of 1% Triton X-100 did not affect trypsin's activity against radiolabeled RNase A or BSA (data not shown), suggesting that the stabilization was due to the detergent interacting with hsc73.
Figure 4.
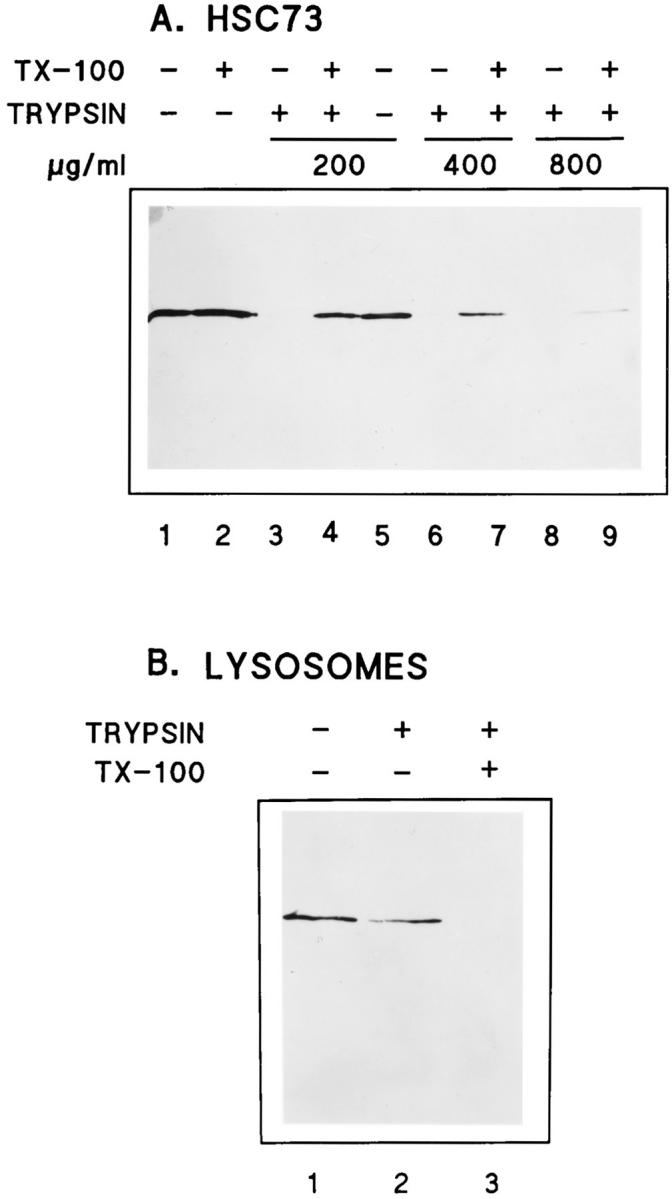
Protease digestion of purified hsc73 and ly-hsc73. Purified bovine brain hsc73 (A) and lysosomal proteins (B) were separated by SDS-PAGE, transferred to nitrocellulose, and immunoblotted with mAb 13D3. hsc73 (A) or lysosomes purified from serum-deprived fibroblasts (B) were treated with buffer alone, buffer plus trypsin, or buffer plus trypsin and 1% Triton X-100 as indicated and described in Materials and Methods.
When isolated lysosomes were incubated with 800 μg/ml trypsin and analyzed by immunoblotting with mAb 13D3, only 25% of the signal was lost (Fig. 4 B, lanes 1 and 2). However, when the lysosomes were permeabilized with 1% Triton X-100 before the incubation with trypsin, lyhsc73 was completely digested (Fig. 4 B, lane 3).
This luminal localization of the majority of ly-hsc73 is further supported by our two-dimensional electrophoresis analyses followed by immunoblotting with mAb 13D3. Fig. 5 A shows the multiple isoforms of fibroblast cytosolic hsc73. Fig. 5 B represents mixed ly-hsc73 and bovine brain hsc73 purified as described previously (Welsh and Feramisco, 1985). Fibroblast cytosol and bovine brain cytosol showed similar isoforms of hsc73 (data not shown). Fig. 5 C shows that the isoelectric point of the ly-hsc73 corresponds to the most acidic isoform present in the cytosol. A longer exposure of the immunoblot allowed us to visualize the presence of a small amount of the most abundant cytoplasmic hsc73 isoform (data not shown). This hsc73 isoform was localized to the lysosomal membrane (Fig. 5 D). The cause of these charge variants is not known, but such isoforms have also been reported for other cell types (Watowich and Morimoto, 1988; Bhattacharyya et al., 1995).
Figure 5.
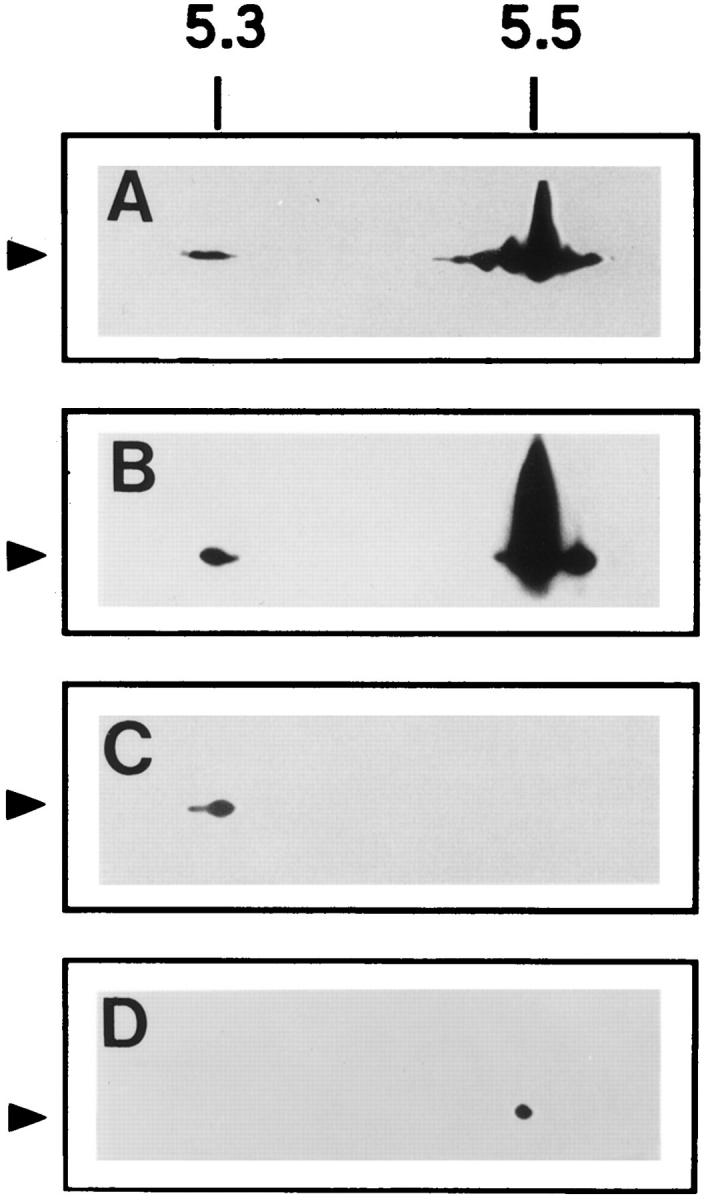
Two-dimensional gel electrophoresis of hsc73 and lyhsc73. Fibroblast cytosol (150 μg protein; A), a mixture of fibroblast lysosomes (200 μg protein) and purified bovine brain hsc73 (1 μg protein; B), fibroblast lysosomes (200 μg protein), and fibroblast lysosomal membranes (100 μg protein; C) were separated as described in Materials and Methods. The membranes were immunoblotted with mAb 13D3 and developed with the ECL system. (Arrows) hsc73.
Role of ly-hsc73 in the hsc73-Stimulated Lysosomal Proteolytic Pathway
We studied the possible role of ly-hsc73 in the selective lysosomal protein degradation pathway during serum withdrawal by attempting to block the ly-hsc73 with endocytosed mAb 13D3. To determine how much mAb 13D3 would be needed to neutralize ly-hsc73, we radiolabeled mAb 13D3 with NaB3H4 and determined the amount of [3H]mAb 13D3 that could be endocytosed by fibroblasts and its half-life after endocytosis. These studies indicated that mAb 13D3 was internalized by absorptive endocytosis at 12 times the rate of [3H]sucrose, a fluid-phase marker (Gurley and Dice, 1988), and it had a half-life within lysosomes of 45 h (data not shown). The previous quantitation of the amount of lyhsc73 (Terlecky and Dice, 1993) allowed us to calculate that overnight endocytosis of 10 μg/ml of mAb 13D3 should result in an eightfold molar excess of mAb 13D3 over lyhsc73.
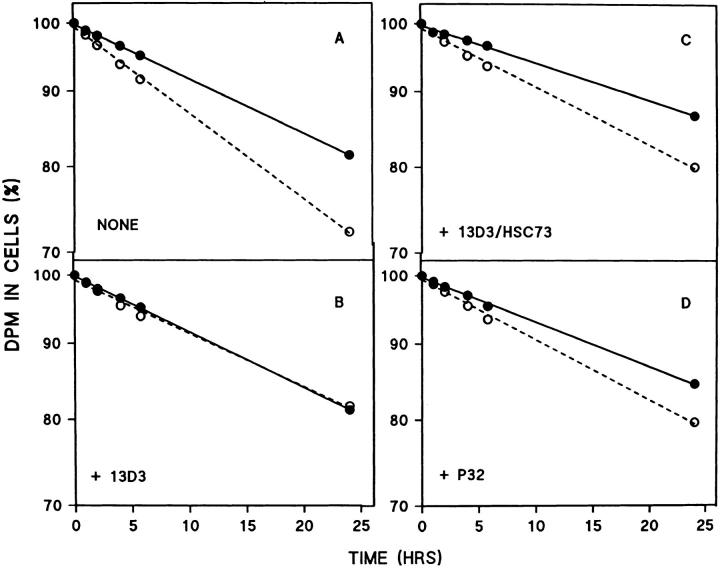
Chiang and Dice (1988) showed that the enhanced degradation of bulk cellular proteins within confluent fibroblasts in response to serum withdrawal was entirely due to activation of the hsc73-stimulated pathway of lysosomal proteolysis. Therefore, we radiolabeled confluent cultures of fibroblasts for 2 d with [3H]leucine. These fibroblasts were then incubated overnight in media containing 10% NCS and either no antibody, mAb 13D3, mAb 13D3 preincubated with hsc73, or an irrelevant IgM, P32. Protein degradation during the overnight endocytosis period was the same for all groups of cells (data not shown). This result makes it unlikely that endocytosis of any of the antibodies was generally toxic to cells.
In contrast, the enhanced protein degradation in response to serum withdrawal (Fig. 6 A) was blocked when the mAb was endocytosed alone (Fig. 6 B). Endocytosis of mAb 13D3 previously neutralized with hsc73 (Fig. 6 C), or the control antibody (P32; Fig. 6 D), had little effect. These results were obtained in two additional experiments except that the slightly slower protein degradation observed for the P32-treated cells (Fig. 6 D) was not a consistent finding.
Figure 6.
Effect of mAb 13D3 on enhanced degradation of cellular proteins in response to serum deprivation. Fibroblasts were labeled with [3H]leucine for 2 d, and then incubated overnight in medium containing 10% NCS and either no addition (NONE), or mAb 13D3 (13D3), mAb 13D3 preincubated with hsc73 (13D3/HSC73), or mAb p32 (P32). The cells were chased with fresh media containing 10% NCS for 1 h, and then changed to serumsupplemented (solid lines) or serumdeprived (dotted lines) media. Acidsoluble radioactivity in the media was followed as a measure of proteolysis. Results shown are the mean for n = 6 (NONE), or n = 4 (13D3, 13D3/ HSC73, and P32). The half-lives (in h) in the presence and absence of serum, respectively, are: (A) 80 and 52; (B) 81 and 84; (C) 100 and 74; and (D) 120 and 77.
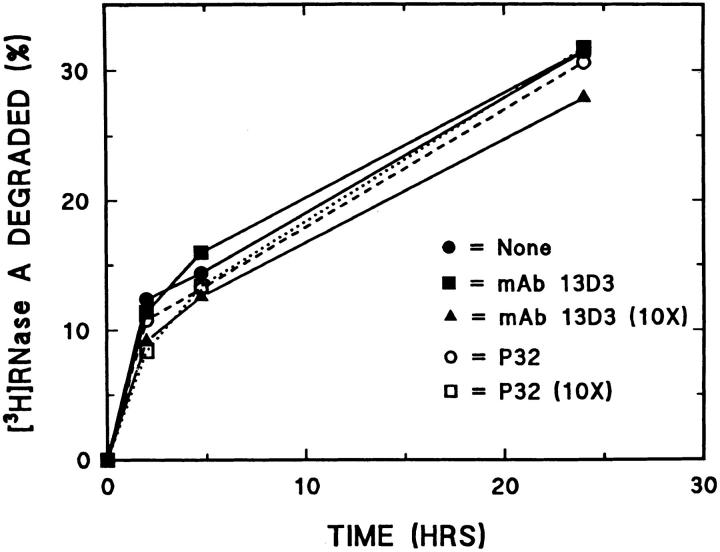
To determine whether endocytosis of mAb 13D3 inhibited degradation of proteins once they had entered lysosomes, we incubated fibroblasts with [3H]RNase A as a substrate for degradation together with mAb 13D3 or mAb P32. Fig. 7 shows that mAb 13D3 had no effect on lysosomal degradation of endocytosed [3H]RNase A even at a 10-fold higher concentration than that which inhibits the enhanced intracellular protein degradation during serum withdrawal.
Figure 7.
Effect of endocytosed antibodies on intralysosomal degradation of endocytosed [3H]RNase A. Fibroblasts were incubated overnight in media containing 10% NCS and [3H]RNase A alone (filled circles) or in combination with 10 μg/ml mAb 13D3 (filled squares), 100 μg/ml mAb 13D3 (triangles), 10 μg/ml of an irrelevant IgM (P32; open circles), or 100 μg/ml of P32 (open squares), and then chased 1 h with fresh media containing 10% NCS. After the chase, fresh media containing 10% NCS was added to each well, and acid-soluble radioactivity in the media was determined at the indicated times. Results shown are the mean for n = 4.
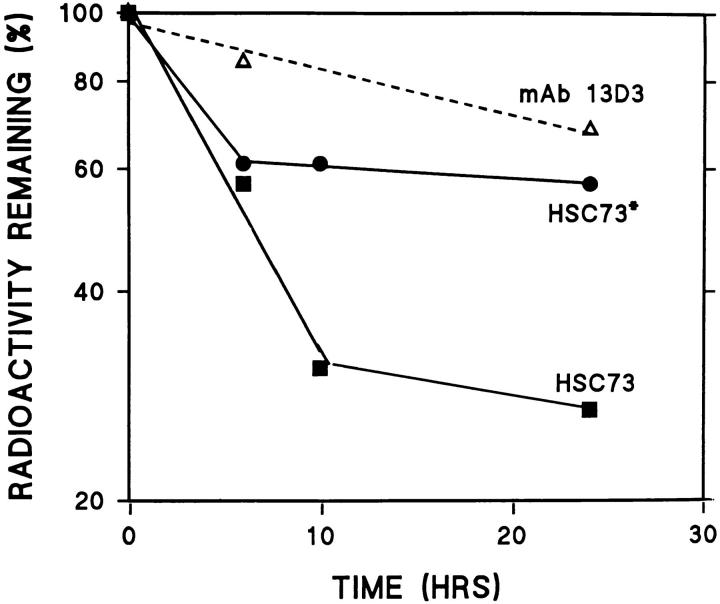
The ly-hsc73 appears to be cytosolic hsc73 that enters lysosomes through macroautophagy or other processes and is slightly modified to become more acidic. If ly-hsc73 is hsc73, we reasoned that hsc73 might be particularly resistant to intralysosomal digestion. We measured the degradation rates of RNase A, a mixture of cytosolic proteins, and two different preparations of hsc73 after endocytosis (Fig. 8). RNase A was degraded with a half-life of 47 h, while the half-lives of cytosolic proteins were heterogeneous in the 20–60-h range (data not shown). Radiolabeled hsc73 that was repurified by ATP-affinity chromatography before endocytosis was remarkably stable (t1/2 = 170 h). Degradation of total reductively methylated hsc73, most of which could not bind to ATP, was biphasic with rapid (t1/2 = 5.5 h) and longer-lived (t1/2 = 26 h) components. These results are consistent with most of the [3H]hsc73 being denatured by the reductive methylation and short-lived after endocytosis, while the native [3H]hsc73 is very stable within lysosomes.
Figure 8.
Stability of endocytosed proteins. Fibroblasts were incubated overnight in media containing: 35S-labeled cytosolic proteins from IMR-90 fibroblasts (cytosol), [3H]RNase A, total [3H]hsc73 (HSC73), or [3H]hsc73 that had been repurified by ATP-affinity chromatography (HSC73*). Cultures were chased for 6 h in media containing 10% NCS, and then degradation was followed by measuring acid-soluble radioactivity appearing in the medium. Results are the mean for n = 3–6.
Discussion
The experiments described here were aimed at further characterizing ly-hsc73 and directly testing the role(s) of ly-hsc73 in the hsc73-stimulated pathway of lysosomal proteolysis. We identified mAb 13D3 as a reagent that recognizes both the denatured and native forms of hsc73 without cross-reacting with other known hsp70 family members (Fig. 1).
We analyzed the subcellular distribution of ly-hsc73 in methanol-fixed cells by laser scanning confocal microscopy (Figs. 2 and 3). ly-hsp73 is localized to most, but not all, lysosomes; to a lesser extent, ly-hsc73, hsc73, or another hsp70 that is recognized by mAb 13D3 is also associated with other organelles.
Images generated using a thick optical section unexpectedly showed that lysosomes in fibroblasts have the capacity to fuse into a tubular network when cells are serum deprived. Tubular lysosomes have been described before in other cell types (Novikoff and Shin, 1978; Robinson et al., 1986; Swanson et al., 1987; Heuser, 1989; Knapp and Swanson, 1990; Kuijpers et al., 1994) but not, to our knowledge, in fibroblasts. It is possible that fusion of lysosomes favors a more efficient degradation process by correcting heterogeneities among separate lysosomal vesicles. For example, if certain lysosomes contain abundant levels of the putative receptor, lgp96 (Cuervo and Dice, 1996), or import channels for substrate proteins but low levels of lysosomal proteases, fusion into tubules might correct such imbalances.
The morphological change in lysosomes could also be unrelated to their degradative capacity. For example, certain organelles vesiculate before cell division presumably to allow efficient partitioning into the two daughter cells (Birky, 1983; Koning et al., 1993). Indeed, serum-supplemented nonconfluent cells are rapidly dividing, while serum-deprived cells are not.
Trypsin treatment of purified lysosomes (Fig. 4) indicated that ∼25% of the lysosomal hsc73 is membrane bound, while 75% is within the lysosomal lumen. These results are consistent with immunogold EM analysis of lyhsc73 distribution in rat liver lysosomes (Cuervo et al., 1995). In these studies, 83% of the ly-hsc73 was localized in the lysosomal lumen and 17% on the lysosomal membrane.
Our two-dimensional gel analyses of hsc73 and ly-hsc73 (Fig. 5) indicate that most of the ly-hsc73 corresponds to the most acidic isoform of cytosolic hsc73. Whether this isoform is preferentially taken up by lysosomes or whether other isoforms are converted within lysosomes to more acidic molecules remains to be established. A minor ly-hsc73 is also of the same isoelectric point as the major cytosolic hsc73 species and is confined to the lysosomal membrane (Fig. 5 D). Additional experiments using purified rat liver lysosomes indicate that cytosolic hsc73 can be internalized by lysosomes and is functional in stimulating the uptake of substrate proteins (Cuervo et al., 1997).
Hsc73 may enter lysosomes by a variety of mechanisms including the hsc73-mediated pathway (Cuervo et al., 1997). Whatever its mode of entry, Fig. 8 shows that [3H]hsc73, if it is first purified by ATP-affinity chromatography, is very stable to intralysosomal hydrolysis. The biochemical features of hsc73 that render it resistant to lysosomal proteolysis are not known, but this protein stability must contribute to its steady state localization within lysosomes.
Our preliminary experiments concerning the endocytic uptake and degradation of mAb 13D3 indicated that we could deliver excess mAb 13D3 to lysosomes and that mAb 13D3 could recognize hsc73 under the acidic conditions within lysosomes. The fact that the endocytosed mAb 13D3 completely blocked the enhanced proteolysis in response to serum deprivation (Fig. 6) shows that the endocytosed mAb 13D3 interfered with the function of lyhsc73.
An alternative interpretation of these results is that the endocytosed mAb 13D3 somehow leaked from endosomes/ lysosomes into the cytosol and blocked the selective lysosomal degradation pathway by neutralizing cytosolic hsc73. This possibility seems unlikely since, by indirect immunofluorescence in formaldehyde-fixed fibroblasts, we were able to localize the majority of endocytosed mAb 13D3 to lysosomes (data not shown). In addition, Okada and Rechsteiner (1982) showed that only 20% of endocytosed HRP was found in a postlysosomal supernatant after cell fractionation on sucrose gradients. This result includes the fraction of the endocytosed HRP released by ruptured endosomes and lysosomes during cell fractionation. It should be noted that even if 20% of the mAb 13D3 did escape from the vacuolar apparatus into the cytosol, it would block only ∼4% of the cytosolic hsc73, which is an abundant cytosolic protein (Giebel et al., 1988; Terlecky and Dice, 1993).
There are several ways in which ly-hsc73 might be required for the hsc73-stimulated pathway of lysosomal proteolysis. ly-hsp73 might be required for the import of substrate proteins by mechanisms similar to those previously proposed for other hsp70 family members in mitochondria (Kang et al., 1990) and the ER (Vogel et al., 1990; Nicchitta and Blobel, 1993; Panzner et al., 1995). In addition, ly-hsc73 could aid in the digestion of proteins once they have entered lysosomes, since other molecular chaperones can facilitate degradation of substrate proteins (Sherman and Goldberg, 1992). Degradation of endocytosed [3H]RNase A was not affected by the presence of large amounts of endocytosed mAb 13D3 (Fig. 6). Nevertheless, it remains possible that the digestion of proteins is facilitated by hsc73 only if the proteins enter the lysosome from the cytosol.
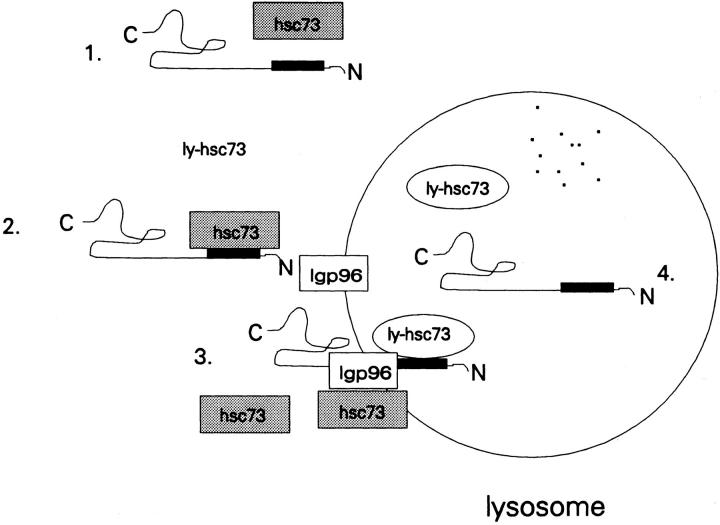
These results together with previously defined features of the hsc73-stimulated pathway of lysosomal proteolysis lead us to the working model shown in Fig. 9. Cytosolic hsc73, acting as a molecular chaperone, recognizes specific sequences (KFERQ and related peptides) in proteins that are targeted for lysosomal degradation in response to serum withdrawal. These sequences may be exposed by changes in the conformation of the substrate proteins or by alterations in protein–protein interactions. Alternatively, cytosolic hsc73 may be altered by serum withdrawal in some way so that it now acts in the lysosomal degradative pathway rather than in organelle synthetic pathways or in the uncoating of clathrin-coated vesicles.
Figure 9.
Model representing the roles of hsc73 and ly-hsc73 in the selective lysosomal protein degradation pathway. The protein substrate depicted is RNase A, and the black rectangle represents the KFERQ sequence. Steps 1–4 are as described in the text.
The substrate protein is then recognized by the lysosomal membrane receptor protein lgp96 (Cuervo and Dice, 1996). In addition, the fraction of hsc73 associated with the lysosomal membrane (Fig. 5 D) might participate in this step. For RNase A, there is a kinetic intermediate in the import process in which 2 kD of the carboxyl terminus remains outside of the lysosome (Cuervo et al., 1994).
The translocation of the substrate protein across the lysosomal membrane may require ly-hsc73, in an analogous way to the roles of other intraorganellar hsp70s in the complete import of proteins into mitochondria and the lumen of the ER (Kang et al., 1990; Vogel et al., 1990; Nicchitta and Blobel, 1993; Panzner et al., 1995). In this case, the action of the ly-hsc73 might require repeated rounds of peptide binding and ATP hydrolysis. Whether or not sufficient intralysosomal ATP exists for such an action remains to be established. Alternatively, intralysosomal hsc73 may facilitate protein import in some fashion not dependent on ATP hydrolysis. For example, hsc73 and substrate proteins might enter lysosomes as a complex. Finally, the substrate protein is released inside the lysosome and degraded. It remains possible that ly-hsc73 also facilitates this process, but such a role could not be demonstrated using endocytosed [3H]RNase A.
Two major models have been proposed to explain involvement of intraorganellar hsp70s in the protein translocation process across membranes. The Brownian ratchet model predicts that Brownian motion drives the translocation of a polypeptide across membranes, and that luminal hsp70s act simply by binding to their polypeptide substrates, conferring vectorial transport (Simon et al., 1992). The second model predicts that intraorganellar hsp70s act as translocation motors (Glick, 1995). In this case, the hsc70s must be attached to the organellar membrane, and, through conformational changes driven by cycles of ATP hydrolysis, the polypeptide is simultaneously unfolded and pulled inside the organelle. Further studies are required to distinguish between these models and to elucidate additional features of this selective lysosomal proteolytic pathway.
Acknowledgments
We thank Dr. Joseph Chandler for his generous gifts of mAb 13D3; Dr. David Albertini, Dr. Joan King, Mr. Marc Shaffel, and Mr. Rob Wilson for technical advice regarding the confocal microscopy; and Dr. Laura Liscum and Ms. Marilyn Negron for critical review of the manuscript.
This work was supported by grant AG06116 from the National Institutes of Health.
Footnotes
1. Abbreviations used in this paper: hsc, heat shock cognate; hsp, heat shock protein; lgp, lysosomal glycoprotein; NCS, newborn calf serum; PBP, peptide binding protein; PTA, phosphotungstate; SSA1p, stress 70 subgroup A gene 1 product.
Please address all correspondence to J. Fred Dice, Sackler School of Graduate Biomedical Sciences, Department of Physiology, Tufts University School of Medicine, 136 Harrison Avenue, Boston, MA 02111. Tel.: (617) 636-6707. Fax: (617) 636-0445.
S.R. Terlecky's current address is Department of Biology, University of California at San Diego, La Jolla, CA 92093.
References
- Amenta J, Brocher S. Mechanism of protein turnover in cultured cells. Life Sci. 1981;28:1195–1208. doi: 10.1016/0024-3205(81)90444-6. [DOI] [PubMed] [Google Scholar]
- Aniento F, Roche E, Cuervo AM, Knecht E. Uptake and degradation of glyceraldehyde-3-phosphate dehydrogenase by rat liver lysosomes. J Biol Chem. 1993;268:10463–10470. [PubMed] [Google Scholar]
- Backer JM, Bourret L, Dice JF. Regulation of catabolism of microinjected ribonuclease A requires the amino-terminal 20 amino acids. Proc Natl Acad Sci USA. 1983;80:2166–2170. doi: 10.1073/pnas.80.8.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya T, Karnezis AN, Murphy SP, Hoang T, Freeman BC, Phillips B, Morimoto RI. Cloning and subcellular localization of human mitochondrial hsp70. J Biol Chem. 1995;270:1705–1710. doi: 10.1074/jbc.270.4.1705. [DOI] [PubMed] [Google Scholar]
- Beckman RP, Mizzen LA, Welch WJ. Interaction of Hsp 70 with newly synthesized proteins: implications for protein folding and assembly. Science (Wash DC) 1990;248:850–854. doi: 10.1126/science.2188360. [DOI] [PubMed] [Google Scholar]
- Birky, C.W., Jr. 1983. The partitioning of cytoplasmic organelles at cell division. Int. Rev. Cytol. (Suppl.). 15:49–89. [DOI] [PubMed]
- Chiang H-L, Dice JF. Peptide sequences that target proteins for enhanced degradation during serum withdrawal. J Biol Chem. 1988;263:6797–6805. [PubMed] [Google Scholar]
- Chiang H-L, Terlecky SR, Plant CP, Dice JF. A role for a 70- kilodalton heat shock protein in lysosomal degradation of intracellular proteins. Science (Wash DC) 1989;246:382–385. doi: 10.1126/science.2799391. [DOI] [PubMed] [Google Scholar]
- Chirico WJ, Waters MG, Blobel G. 70K heat shock related proteins stimulate protein translocation into microsomes. Nature (Lond) 1988;332:805–810. doi: 10.1038/332805a0. [DOI] [PubMed] [Google Scholar]
- Craig, E.A., B.K. Baxter, J. Becker, J. Halladay, and T. Ziegelhoffer. 1994. Cytosolic hsp70s of Sacchaormyces cerevisiae: roles in protein synthesis, protein translocation, proteolysis, and regulation. In The Biology of Heat Shock Proteins and Molecular Chaperones. R.I. Morimoto, A. Tissieres, and C. Georgopolous, editors. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. 31–52.
- Cuervo AM, Dice JF. A receptor for the selective uptake and degradation of proteins by lysosomes. Science (Wash DC) 1996;273:501–503. doi: 10.1126/science.273.5274.501. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Terlecky SR, Dice JF, Knecht E. Selective binding and uptake of ribonuclease A and glyceraldehyde-3-phosphate dehydrogenase by isolated rat liver lysosomes. J Biol Chem. 1994;269:26374–26380. [PubMed] [Google Scholar]
- Cuervo AM, Terlecky SR, Knecht E, Dice JF. Activation of a selective pathway of lysosomal proteolysis in rat liver by prolonged starvation. Am J Physiol. 1995;269:C1200–C1208. doi: 10.1152/ajpcell.1995.269.5.C1200. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Dice JF, Knecht E. A population of rat liver lysosomes responsible for the selective uptake and degradation of cytosolic proteins. J Biol Chem. 1997;272:5606–5615. doi: 10.1074/jbc.272.9.5606. [DOI] [PubMed] [Google Scholar]
- Dahlseid JN, Lill R, Green JM, Xu X, Qiu Y, Pierce SK. PBP74, a new member of the mammalian 70-kDa heat shock protein family, is a mitochondrial protein. Mol Biol Cell. 1994;5:1265–1275. doi: 10.1091/mbc.5.11.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshaies RJ, Koch BD, Werner-Washburne M, Craig EA, Schekman R. A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature (Lond) 1988;332:800–805. doi: 10.1038/332800a0. [DOI] [PubMed] [Google Scholar]
- Dice JF, Chiang H-L, Spencer EP, Backer JM. Regulation of catabolism of microinjected ribonuclease A. J Biol Chem. 1986;261:6853–6859. [PubMed] [Google Scholar]
- Domanico SZ, DeNagel DC, Dahlseid JN, Green JM, Pierce SK. Cloning of the gene encoding Peptide-Binding Protein 74 shows that it is a new member of the heat shock protein 70 family. Mol Cell Biol. 1993;13:3598–3610. doi: 10.1128/mcb.13.6.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebel LB, Dworniczak BP, Bautz EKF. Developmental regulation of a constitutively expressed mouse mRNA encoding a 72 kDa heat shock-like protein. Dev Biol. 1988;125:200–207. doi: 10.1016/0012-1606(88)90073-5. [DOI] [PubMed] [Google Scholar]
- Glick BS. Can Hsp70 proteins act as force-generating motors? . Cell. 1995;80:11–14. doi: 10.1016/0092-8674(95)90444-1. [DOI] [PubMed] [Google Scholar]
- Green LAD, Liem RKH. β-Internexin is a microtubule-associated protein identical to the 70-kDa heat-shock cognate protein and the clathrin uncoating ATPase. J Biol Chem. 1989;264:15210–15215. [PubMed] [Google Scholar]
- Green SA, Zimmer K-P, Griffiths A, Mellman I. Kinetics of intracellular transport and sorting of lysosomal membrane and plasma membrane proteins. J Cell Biol. 1987;105:1227–1240. doi: 10.1083/jcb.105.3.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley R, Dice JF. Degradation of endocytosed proteins is unaltered in senescent human fibroblasts. Cell Biol Int Rep. 1988;12:885–893. doi: 10.1016/0309-1651(88)90052-5. [DOI] [PubMed] [Google Scholar]
- Hartl FU. Molecular chaperones in cellular protein folding. Nature (Lond) 1996;381:571–580. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- Hendil KB, Lauridsen A-MB, Seglen PO. Both endocytic and endogenous protein degradation in fibroblasts is stimulated by serum/amino acid deprivation and inhibited by 3-methyladenine. Biochem J. 1990;272:577–581. doi: 10.1042/bj2720577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrick JP, Hartl F-U. Molecular chaperone functions of heatshock proteins. Annu Rev Biochem. 1993;62:349–384. doi: 10.1146/annurev.bi.62.070193.002025. [DOI] [PubMed] [Google Scholar]
- Heuser J. Changes in lysosome shape and distribution correlated with changes in cytoplasmic pH. J Cell Biol. 1989;108:855–864. doi: 10.1083/jcb.108.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang P-J, Ostermann J, Neupert W, Craig EA, Pfanner N. Requirement for hsp70 in the mitochondrial matrix for translocation and folding of precursor proteins. Nature (Lond) 1990;348:137–143. doi: 10.1038/348137a0. [DOI] [PubMed] [Google Scholar]
- Knapp PE, Swanson JA. Plasticity of the tubular lysosomal compartment in macrophages. J Cell Sci. 1990;95:433–439. doi: 10.1242/jcs.95.3.433. [DOI] [PubMed] [Google Scholar]
- Koning AJ, Lum PY, Williams JM, Wright R. DiOC6staining reveals organelle structure and dynamics in living yeast cells. Cell Motil Cytoskeleton. 1993;25:111–128. doi: 10.1002/cm.970250202. [DOI] [PubMed] [Google Scholar]
- Kuijpers TW, Raleigh M, Kavanagh T, Janssen H, Calafat J, Roos D, Harlan JM. Cytokine-activated endothelial cells internalize E-selectin into a lysosomal compartment of vesiculotubular shape. A tubulin-driven process. J Immunol. 1994;152:5060–5069. [PubMed] [Google Scholar]
- Kurtz S, Rossi J, Petko L, Lindquist S. An ancient developmental induction: heat shock proteins induced in sporulation and oogenesis. Science (Wash DC) 1986;231:1154–1157. doi: 10.1126/science.3511530. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Maekawa M, O'Brien D, Allen RL, Eddy EM. Heat-shock cognate protein (hsc71) and related proteins in mouse spermatogenic cells. Biol Reprod. 1989;40:843–852. doi: 10.1095/biolreprod40.4.843. [DOI] [PubMed] [Google Scholar]
- Morimoto, R.I., A. Tissieres, and C. Georgopolous. 1994. Progress and perspectives on the biology of heat shock proteins and molecular chaperones. In The Biology of Heat Shock Proteins and Molecular Chaperones. R.I. Morimoto, A. Tissieres, and C. Georgopolous, editors. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. 1–30.
- Murakami H, Pain D, Blobel G. 70-kD heat shock–related protein is one of at least two distinct cytosolic factors stimulating protein import into mitochondria. J Cell Biol. 1988;107:2051–2057. doi: 10.1083/jcb.107.6.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff NT, Bourret L, Miao P, Dice JF. Degradation of proteins microinjected into IMR-90 human diploid fibroblasts. J Cell Biol. 1981;91:184–194. doi: 10.1083/jcb.91.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicchitta CV, Blobel G. Lumenal proteins of the mammalian endoplasmic reticulum are required to complete protein translocation. Cell. 1993;73:989–998. doi: 10.1016/0092-8674(93)90276-v. [DOI] [PubMed] [Google Scholar]
- Novikoff AB, Shin WY. Endoplasmic reticulum and autophagy in rat hepatocytes. Proc Natl Acad Sci USA. 1978;75:5039–5042. doi: 10.1073/pnas.75.10.5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell PH. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Okada CY, Rechsteiner M. Introduction of macromolecules into cultured cells by osmotic lysis of pinosomes. Cell. 1982;29:33–41. doi: 10.1016/0092-8674(82)90087-3. [DOI] [PubMed] [Google Scholar]
- Panzner S, Dreier L, Hartmann E, Kostka S, Rapoport TA. Posttranslational protein transport in yeast reconstituted with a purified complex of Sec proteins and Kar2p. Cell. 1995;81:561–570. doi: 10.1016/0092-8674(95)90077-2. [DOI] [PubMed] [Google Scholar]
- Rassow J, Maarse AC, Krainer E, Kubrich M, Muller H, Meijer M, Craig EA, Pfanner N. Mitochondrial protein imort: biochemical and genetic evidence for interaction of matrix hsp70 and the inner membrane protein MIM44. J Cell Biol. 1994;127:1547–1556. doi: 10.1083/jcb.127.6.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JM, Okada T, Castellot JJ, Karnovsky MJ. Unusual lysosomes in aortic smooth muscle cells: presence in living and rapidly frozen cells. J Cell Biol. 1986;102:1615–1622. doi: 10.1083/jcb.102.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JE, Schmid SL. Enzymatic recycling of clathrin from coated vesicles. Cell. 1986;46:5–9. doi: 10.1016/0092-8674(86)90852-4. [DOI] [PubMed] [Google Scholar]
- Schilling JB, Clevinger JB, Davie JM, Hood L. Amino acid sequence of homogeneous antibodies to dextran and DNA rearrangements in heavy chain V region gene segments. Nature (Lond) 1980;283:35–40. doi: 10.1038/283035a0. [DOI] [PubMed] [Google Scholar]
- Sheffield WP, Shore GC, Randall SK. Mitochondrial precursor protein. Effect of 70-kilodalton heat shock protein on polypeptide folding, aggregation, and import competence. J Biol Chem. 1990;265:11069–11076. [PubMed] [Google Scholar]
- Sherman MY, Goldberg AL. Involvement of the chaperonin dnaK in the rapid degradation of a mutant protein in Escherichia coli. . EMBO (Eur Mol Biol Organ) J. 1992;11:71–77. doi: 10.1002/j.1460-2075.1992.tb05029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Thomas JO. The transport of proteins into the nucleus requires the 70-Kilodalton heat shock protein or its cytosolic cognate. Mol Cell Biol. 1992;12:2186–2192. doi: 10.1128/mcb.12.5.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SM, Peskin CS, Oster GF. What drives the translocation of proteins? . Proc Natl Acad Sci USA. 1992;89:3770–3774. doi: 10.1073/pnas.89.9.3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J, Burke E, Silverstein SC. Tubular lysosomes accompany stimulated pinocytosis in macrophages. J Cell Biol. 1987;104:1217–1222. doi: 10.1083/jcb.104.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terlecky SR, Dice JF. Polypeptide import and degradation by isolated lysosomes. J Biol Chem. 1993;268:23490–23495. [PubMed] [Google Scholar]
- Terlecky SR, Chiang H-L, Olson TS, Dice JF. Protein and peptide binding and stimulation of in vitro lysosomal proteolysis by the 73-kDa heat shock cognate protein. J Biol Chem. 1992;267:9202–9209. [PubMed] [Google Scholar]
- Vogel JP, Misra LM, Rose MD. Loss of BiP/GRP78 function blocks translocation of secretory proteins in yeast. J Cell Biol. 1990;110:1855–1895. doi: 10.1083/jcb.110.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton PA, Wendland M, Subramani S, Rachubinski RA, Welch WJ. Involvement of 70-kD heat-shock proteins in peroxisomal import. J Cell Biol. 1994;125:1037–1046. doi: 10.1083/jcb.125.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watowich SS, Morimoto RI. Complex regulation of heat shock- and glucose-responsive genes in human cells. Mol Cell Biol. 1988;8:393–405. doi: 10.1128/mcb.8.1.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch WJ, Feramisco JR. Rapid purification of mammalian 70,000-dalton stress proteins: affinity of the proteins for nucleotides. Mol Cell Biol. 1985;5:1229–1237. doi: 10.1128/mcb.5.6.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing SS, Chiang H-L, Goldberg AL, Dice JF. Proteins containing peptide sequences related to Lys-Phe-Glu-Arg-Gln are selectively depleted in liver and heart, but not skeletal muscle, of fasted rats. Biochem J. 1991;275:165–169. doi: 10.1042/bj2750165. [DOI] [PMC free article] [PubMed] [Google Scholar]