Abstract
The osteoclast is distinguished from other macrophage polykaryons by its polarization, a feature induced by substrate recognition. The most striking component of the polarized osteoclast is its ruffled membrane, probably reflecting insertion of intracellular vesicles into the bone apposed plasmalemma. The failure of osteoclasts in c-src−/− osteopetrotic mice to form ruffled membranes indicates pp60c-src (c-src) is essential to osteoclast polarization. Interestingly, c-src itself is a vesicular protein that targets the ruffled membrane. This being the case, we hypothesized that matrix recognition by osteoclasts, and their precursors, induces c-src to associate with microtubules that traffic proteins to the cell surface. We find abundant c-src associates with tubulin immunoprecipitated from avian marrow macrophages (osteoclast precursors) maintained in the adherent, but not nonadherent, state. Since the two proteins colocalize only within adherent avian osteoclast-like cells examined by double antibody immunoconfocal microscopy, c-src/tubulin association reflects an authentic intracellular event. C-src/tubulin association is evident within 90 min of cell-substrate recognition, and the event does not reflect increased expression of either protein. In vitro kinase assay demonstrates tubulin-associated c-src is enzymatically active, phosphorylating itself as well as exogenous substrate. The increase in microtubule-associated kinase activity attending adhesion mirrors tubulin-bound c-src and does not reflect enhanced specific activity. The fact that microtubule-dissociating drugs, as well as cold, prevent adherence-induced c-src/tubulin association indicates the protooncogene complexes primarily, if not exclusively, with polymerized tubulin. Association of the two proteins does not depend upon protein tyrosine phosphorylation and is substrate specific, as it is induced by vitronectin and fibronectin but not type 1 collagen. Finally, consistent with cotransport of c-src and the osteoclast vacuolar proton pump to the polarized plasmalemma, the H+-ATPase decorates microtubules in a manner similar to the protooncogene, specifically coimmunoprecipitates with c-src from the osteoclast light Golgi membrane fraction, and is present, with c-src, in preparations enriched with acidifying vesicles reconstituted from the osteoclast ruffled membrane.
The osteoclast, a member of the monocyte/macrophage family, is the principal, if not exclusive resorptive cell of bone (50). While ontogenetically related to other macrophage polykaryons, such as those foreign body derived, the osteoclast is distinguished by its striking polarization. Upon matrix recognition, the osteoclast's resorptive molecules migrate towards the bone surface. Many of these bone-degrading proteins, such as the cell's vacuolar H+-ATPase (proton pump) (7), are likely confined to vesicles that insert into the polarized plasmalemma, greatly enhancing its surface extent. The highly convoluted resultant structure, known as the ruffled membrane, is unique to the osteoclast and composes its resorptive apparatus (50).
The molecular mechanisms regulating ruffled membrane formation are not yet defined but appear to involve reorganization of cytoskeletal proteins, including tubulin (36). The fact that resorption is blunted by microtubuledissociating drugs (40) and the osteoclast-inhibiting hormone, calcitonin, has the capacity to disrupt the cell's microtubular network (53) suggest tubulin polymerization is essential to the resorptive process. Given the role microtubules play in polarized vesicular movement in other cells (3, 15, 16), it seems likely these filaments participate in transport of vesicles containing the osteoclast's resorptive molecules to the nascent ruffled membrane.
pp60c-src (c-src) is a widely expressed, nonreceptor tyrosine kinase particularly abundant in platelets, neural tissues (2, 13, 15, 27, 33), and osteoclasts (25, 49). It is therefore surprising that the unique phenotypic abnormality of the c-src gene–disrupted mouse is osteopetrosis (47), a family of sclerotic skeletal diseases caused by osteoclast dysfunction. Interestingly, while incapable of bone resorption, the c-src knockout mouse contains abundant osteoclasts. These cells exhibit many features of normal osteoclasts, such as tartrate-resistant acid phosphatase activity, but fail to form a polarized ruffled membrane (10). Rescue of c-src−/− mice by marrow transplantation restores the osteoclast's resorptive capacity and endows it with the ability to develop a ruffled membrane (35). Given the above, a reasonable hypothesis holds that both tubulin and c-src participate in osteoclast polarization. Just how these entities relate to each other in the polarization process is unknown, but their distribution in osteoclasts is modified by matrix recognition. Specifically, c-src preferentially localizes to the ruffled membrane (25, 49), which only appears upon cell–bone contact, and tubulin polymerizes in the same circumstance (infra vide). These observations suggest that a physical relationship, modulated by matrix-derived signals, exists between tubulin and c-src. In fact, we show c-src and tubulin associate in avian osteoclast precursors and this association is regulated by specific matrix components. These data suggest matrix recognition by osteoclast progenitors induces c-src to associate with tubulin in the form of microtubules, an event that may mediate trafficking resorptive proteins to the polarized plasma membrane.
Materials and Methods
Reagents
Monoclonal antibody 327 (34), directed against the c-src protein, and enolase, a substrate for the kinase, were a gift of Dr. A. Shaw (Department of Pathology, Washington University). Polyclonal anti-src antibody was purchased from Upstate Biotechnology Inc. (Lake Placid, NY). Monoclonal anti–β tubulin isotype I + II and polyclonal rabbit anti–tubulin antibodies were purchased from Sigma Chemical Co. (St. Louis, MO). The polyclonal anti–H+-ATPase raised against the 70-kD subunit was generated as described previously (48). Digitonin with high solubility in water was purchased from Wako Chemicals (Richmond, VA). Herbimycin-A, colchicine, and nocodazole were purchased from Calbiochem (San Diego, CA). Enhanced chemiluminescence (ECL)1 kit, fluorescein-labeled donkey anti– rabbit antibodies, and Texas red–labeled goat anti–mouse antibodies were obtained from Amersham Corp. (Arlington Heights, IL). Fibronectin, vitronectin, and collagen-I were from Collaborative Biomedicals (Bedford, MA). All other chemicals were obtained from Sigma Chemical Co.
Cell Culture
Avian bone marrow macrophage precursors that differentiate into osteoclasts were isolated from calcium-deprived laying hens (1). The chicken whole bone marrow preparation was layered on a Ficoll-Hypaque gradient, and the cells at the gradient interface were collected and incubated in α-MEM, supplemented with 5% FBS and 5% chicken charcoal-stripped serum, at 39°C in 5% CO2. After 24 h in culture, nonadherent cells were collected and resuspended in fresh medium at 5 × 106 cells/ml. Cells were then maintained in the same α-MEM indicated above and plated according to each experimental condition.
Immunoprecipitation
Adherent cells were scraped from the dish in the presence of digitonin lysis buffer (1% digitonin, 10 mM triethanolamine, 150 mM NaCl, 10 mM iodoacetoamide, 1 mM EDTA, 10 μg/ml aprotinin, 1 mM 4-(2-Aminoethyl)benzenesulfonylfluoride (AEBSF), pH 7.8) (28) and incubated at 37°C with gentle rocking for 30 min. The cells were then passed through a 25-gauge needle and spun at 10,000 rpm for 10 min in a microfuge. Lysates were precleared with excess of protein A–Sepharose (Sigma Chemical Co.) and protein G–Sepharose (Pharmacia LKB Biotechnology Inc., Piscataway, NJ). Cleared lysates were incubated with various antibodies followed by protein A or G beads, as indicated in each experiment; the beads containing immune complexes were washed extensively with lysis buffer.
Immunoblotting
Immunoprecipitated proteins were boiled in the presence of SDS sample buffer (0.5 M Tris-HCl, pH 6.8, 10% [wt/vol] SDS, 10% glycerol, 0.05% [wt/vol] bromophenol blue, distilled water) for 5 min and subjected to electrophoresis on 7.5–10% SDS-PAGE (31). Proteins were transferred to nitrocellulose membranes using a semi-dry blotter (BioRad Labs, Richmond, CA) and incubated in blocking solution (10% skim milk prepared in PBS containing 0.05% Tween-20) to reduce nonspecific binding. Membranes were washed with PBS/Tween buffer and exposed to primary antibodies, washed again four times, and incubated with secondary goat anti– mouse HRP-conjugated antibody. Membranes were washed extensively, and an ECL detection assay was performed following manufacturer's directions. Image quantitation of relative band densities on autoradiograms was performed using ISS SepraScan 2001 (Integrated Separation Systems, Hyde Park, MA).
In Vitro Kinase Assay
The method described by Clark and Brugge (13) was followed, with slight modifications. Beads containing immunoprecipitated c-src were washed with kinase lysis buffer (1.5% NP-40, 150 mM NaCl, 25 mM Tris, pH 8.0, 25 mM NaF, 100 μM NaVO3) and then preincubated with 15 μl of kinase reaction buffer (25 mM Hepes, pH 7.4, 150 mM NaCl, 5 mM MnCl2, 5 mM MgCl2, 100 μM NaVO3) for 10 min at 22°C. The kinase reaction was started by addition of 5 μCi of [32P]ATP (6,000 Ci/mmol; Amersham Corp.), 5 μM ATP, 1 μg enolase (all final concentrations). The reaction was terminated after 2 min by the addition of an equal volume of 2× SDS sample buffer and heating at 65°C for 5 min. Samples were subjected to electrophoresis on SDS-PAGE gel, dried, and exposed to film.
Immunofluorescence Confocal Microscopy
We followed published methods with slight modifications (3, 16). Cells were dipped briefly in K-Pipes buffer (80 mM K-Pipes, 5 mM EGTA, 2 mM MgCl2, pH 6.8) prewarmed to 37°C and fixed in 3% paraformaldehyde– K-Pipes buffer, pH 6.5, for 5 min at room temperature, followed by removal of the fixative and incubation for 10 min in 3% paraformaldehyde– 100 mM NaB4O7, pH 11. The reaction was then quenched by incubating the cells for 10 min in 1 mg/ml NaBH4 freshly dissolved in PBS, pH 8 (to reduce background fluorescence). Cells were then washed three times with PBS, pH 7.4, and permeabilized with 0.1% Triton X-100 in PBS for 5 min. Cells were washed twice with PBS-gelatin solution (0.2% fish skin gelatin, 0.1% Triton X-100, 0.01% NaN3 in PBS) and incubated (for 45 min) with primary antibody in the same solution in a humidified chamber at 37°C. Cells were then washed four times with PBS-gelatin solution followed by another four washes with Triton X-100 and NaN3-containing PBS and once again washed with PBS-gelatin solution. Cells were incubated (for 45 min) with secondary antibody, Texas red–labeled goat anti– mouse IgG (identifying c-src) and FITC-labeled donkey anti–rabbit IgG (identifying tubulin), and washed as described for the first antibody. Cells were postfixed for 30 min in 4% paraformaldehyde in 50 mM Na-cacodylate, pH 7.5, quenched for 45 min with 50 mM NH4CI/PBS, both at room temperature, and then washed in PBS, three times for 5 min each. To examine the labeled cells, coverslips were mounted with 50% glycerol-PBS containing DABCO (an antibleaching agent) and visualized with a confocal microscope (model Axioplan, Carl Zeiss, Inc., Oberkochen, Germany).
Preparation of Microtubules from Cell Lysates In Vitro
Cell extracts were cooled to depolymerize microtubules and centrifuged to remove large debris. Cleared lysates were warmed to 37°C in the presence of 0.5 mM MgSO4, 1 mM EGTA, 1 mM GTP. Polymerization of microtubules was initiated by adding 8% of dimethylsulfoxide. After 30 min at 37°C, microtubules were stabilized by adding taxol to the final concentration of 2 μg/ml. Assay mixture (microtubule-containing lysate) was layered on 1.2 M sucrose and centrifuged at 50,000 rpm for 60 min in a rotor (model TLS 55; Beckman Instrs., Fullerton, CA). Microtubules were collected, bound to coverslips, and fixed with 2% glutaraldehyde.
Sucrose Density Gradient Fractionation
Avian osteoclasts were homogenized in buffer A (150 mM NaCl, 1 mM EGTA, 1 mM MgCl2, 10 mM Hepes, pH 7.4) supplemented with a cocktail of protease inhibitors (leupeptin, pepstatin, aprotinin, and chymostatin [all 10 ng/ml], AEBSF [1 mM], O-phenanthroline [1 μg/ml], and benzamidine [1 μM]), and spun at 1,000 g for 5 min. Nuclei and large cell debris (P1) were discarded and supernatant (S0) was collected and spun at 27,000 g for 35 min. Supernatant from the last step (S1) was spun at 127,000 g for 1.5 h. Pellets (P3) enriched with Golgi and small intracellular membranes were collected, adjusted to 1.22 M sucrose, and loaded at the bottom of a discontinuous sucrose gradient (26, 46). The gradient was spun for 3 h at 100,000 g, and interface bands were collected and pelleted. Pellets were lysed in sample buffer and analyzed by immunoblot.
Preparation of Osteoclast Membrane Vesicles
Osteoclasts were isolated from the long bones of calcium-deprived laying hens (5). Membrane vesicles from freshly isolated cells were prepared using the method of Gluck and Al-Awqati (21) with slight modifications (37). In brief, cell fragmentation was achieved via nitrogen activation in 250 mM sucrose, 1 mM EGTA, 1 mM DTT, and 10 mM Tris, pH 7.0 (lysis buffer). Disruption of 20 × 106 cells in 20 ml of lysis buffer, contained in a 50-ml centrifuge tube placed within the cavitation bomb chamber, was initiated by pressurization to 40 atm N2 for 30 min at 4°C. Decompression occurred over 2 s through a 5-mm-diam orifice. For some experiments, sucrose was isosomatically replaced with 140 mM KCl. Nuclei and large cell fragments were removed by centrifugation at 1,000 g for 5 min, a mitochondrial fraction by centrifugation at 4,700 g for 10 min, and the vesicular fraction by centrifugation at 49,000 g for 40 min. The last pellet was frozen at −80°C at ∼900 μg total protein per aliquot in lysis buffer.
Results
Cell Adherence Increases C-src Association with Tubulin
The osteoclast ruffled membrane is rich in c-src (49), and as such, a reasonable hypothesis holds that microtubules mediate transport of the protooncogene to the cell surface. Thus, we asked if c-src and tubulin associate in avian osteoclast progenitors. As seen in Fig. 1, lysate derived from marrow macrophages contains proteins comigrating with c-src and tubulin (lane 5). Furthermore, while numerous other proteins are also evident, bands of tubulin and c-src are present in lysate immunoprecipitated with either anti– c-src (lane 2) or antitubulin (lane 3) antibodies. In this and all subsequent figures, immunoblots were performed in the linear range of the ECL detection system (Fig. 2).
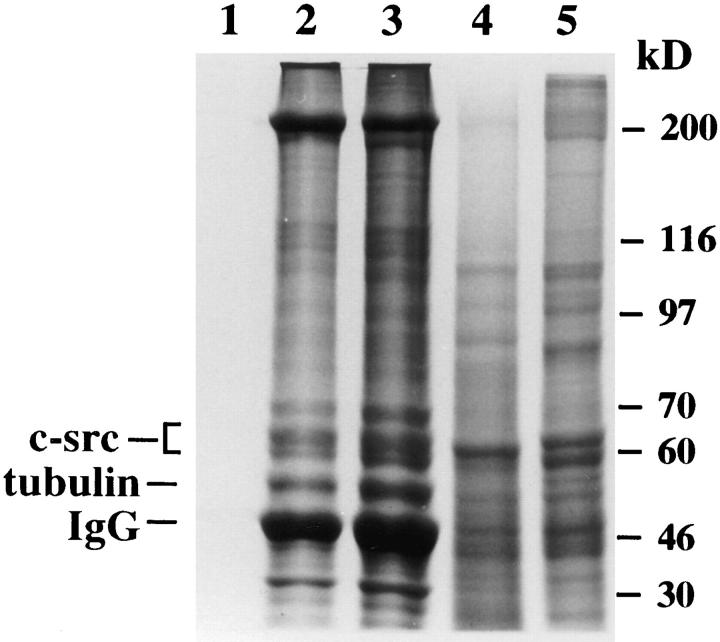
Figure 1.
Tubulin and c-src proteins coimmunoprecipitate in avian marrow macrophages. Adherent macrophages were either lysed directly in sample buffer (lane 5) or in lysis buffer and immunoprecipitated with either anti–c-src (lane 2) or antitubulin antibody (lane 3). Lysate and immunoprecipitates were subjected to SDS-PAGE, and the gel was stained with Coomassie blue. Lane 1 contains sample buffer, and lane 4 contains a positive control of enriched c-src protein extract.
Figure 2.
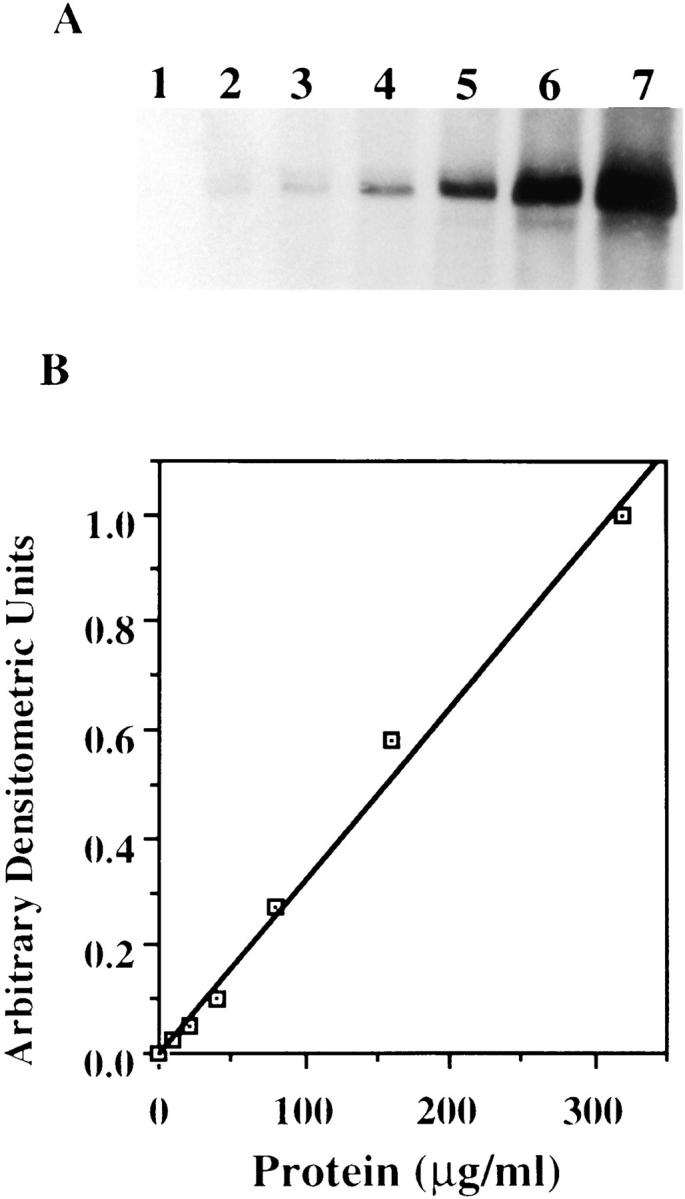
Immunoblot analysis of a wide range of cellular c-src. (A) Cell lysates containing 0, 10, 20, 40, 80, 160, and 320 μg of protein were loaded in lanes 1, 2, 3, 4, 5, and 6, respectively. The gels were electrophoresed and probed with anti–c-src antibody. (B) Densitometric analysis of A plotted in arbitrary units as a function of protein.
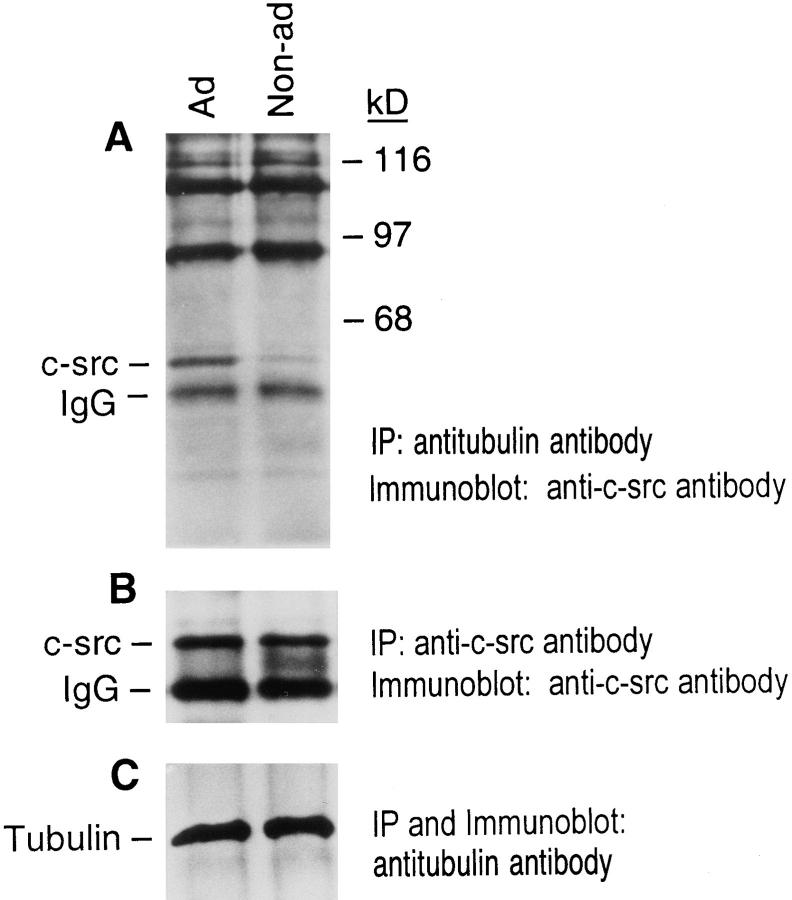
To further characterize the putative tubulin and c-src proteins present in marrow macrophage lysate, and to determine if they associate in a cell-matrix–dependent manner, we performed c-src immunoblots on immunoprecipitated tubulin. To this end, chicken marrow macrophages were maintained, nonadherent, for 2 d, after which they were transferred to serum-free medium. One half the cells were kept nonadherent, and the other half were plated on tissue culture plastic, to which they rapidly attach. 90 min later both samples were lysed with digitonin in conditions known to preserve microtubule architecture (51). The lysate was immunoprecipitated with an antitubulin antibody and the immunoprecipitate c-src content assessed by immunoblot. Fig. 3 A shows minimal c-src precipitates with tubulin in nonadherent cells, which typically exhibit little cytoskeletal organization. In contrast, abundant c-src associates with tubulin precipitated from macrophages adherent for 90 min, a period sufficient to permit microtubule formation (infra vide). Importantly, while a number of slower migrating bands are present in both immunoprecipitates, only the quantity of c-src is regulated by substrate adherence.
Figure 3.
C-src associates with tubulin in a substrate adhesion– dependent manner. Avian marrow macrophages were maintained, nonadherent, in Teflon beakers for 2 d. The cells were then plated on tissue culture plastic (adherent) or retained in Teflon beakers (nonadherent) for an additional 90 min. Adherent or nonadherent cells were lysed and immunoprecipitated with anti– c-src or antitubulin antibodies. The immunoprecipitates were separated by nonreducing 8% SDS-PAGE. The proteins were transferred to a nitrocellulose membrane and probed with anti–csrc or antitubulin antibodies, followed by ECL detection, using HRP-conjugated goat anti–mouse IgG antibody. A represents the entire gel.
Although unlikely, considering the short duration of substrate attachment, the differences in c-src/tubulin association between adherent and nonadherent macrophages might reflect adhesion-induced expression of either protein. Thus, we compared the quantities of both proteins in suspended and plated cells by immunoprecipitating cell lysates with excess anti–c-src or antitubulin and immunoblotting the respective product with the same antibody. As seen in Fig. 3, B and C, nonadherent and adherent macrophages contain the same amounts of both proteins. For reasons unknown, we consistently find c-src migrates slower when immunoprecipitated with antitubulin antibody as compared to its direct immunoprecipitation (IP) with anti–c-src antibody (e.g., see Figs. 4 and 14). In the conditions of these experiments, c-src immunoblots are within the linear range of analysis (see Fig. 2).
Figure 4.
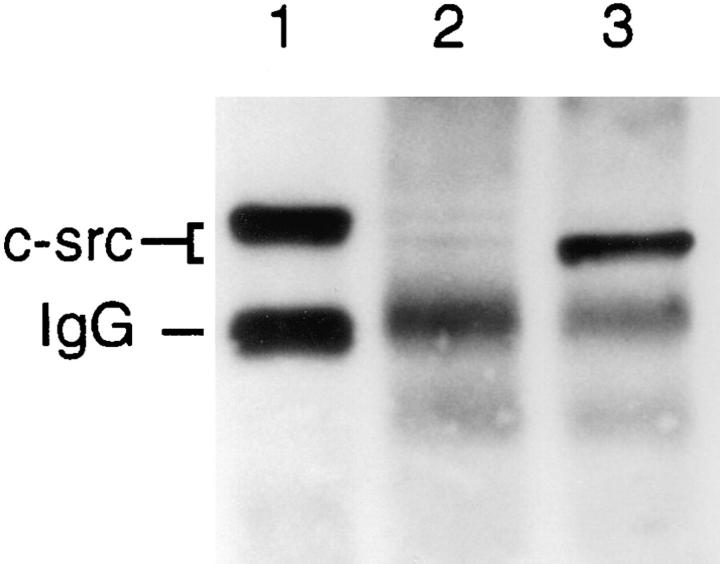
Tubulin-bound and nonbound c-src in adherent cells. The lysate of adherent avian marrow macrophages was immunoprecipitated with antitubulin antibody (lane 1), and the supernatant was reimmunoprecipitated with the same antibody (lane 2). The supernatant, now depleted of tubulin-associated c-src, was immunoprecipitated with anti–c-src antibody (lane 3). All lanes were probed with anti–c-src antibody.
Figure 14.
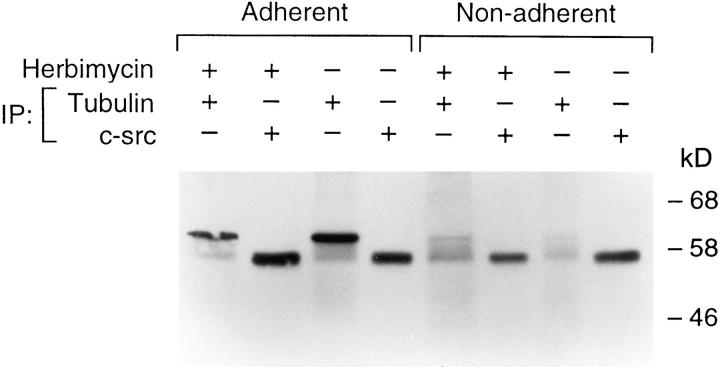
Effect of protein tyrosine kinase inhibitor on c-src/tubulin association. Avian marrow macrophages maintained in suspension for 2 d were exposed for 2 h to the tyrosine kinase inhibitor (herbimycin-A 4 μM). Cells, in the presence of the drug, were kept suspended or plated on tissue culture plastic for 90 min. They were then lysed and immunoprecipitated with anti–c-src or -tubulin antibodies. Proteins were separated by SDSPAGE, transferred to nitrocellulose membranes, and probed with the anti–c-src antibody.
We next determined the proportion of total c-src that is tubulin associated in adherent macrophages. To this end, we removed tubulin-bound c-src by repeatedly immunoprecipitating adherent cell lysate with antitubulin antibody. Fig. 4 shows all detectable tubulin-associated c-src is removed by this exercise (lanes 1 and 2), documenting this and all antitubulin immunoprecipitations are performed with excess antibody. Immunoprecipitation of the supernatant, now devoid of tubulin-bound c-src, with anti–c-src antibody and probing with the same antibody (lane 3), documents the detectable amount of c-src that is non–tubulinbound is ∼32% less, as measured densitometrically, than c-src that is tubulin associated (compare lanes 1 and 3).
Adherence Rapidly Induces C-src/Tubulin Association in Avian Marrow Macrophages
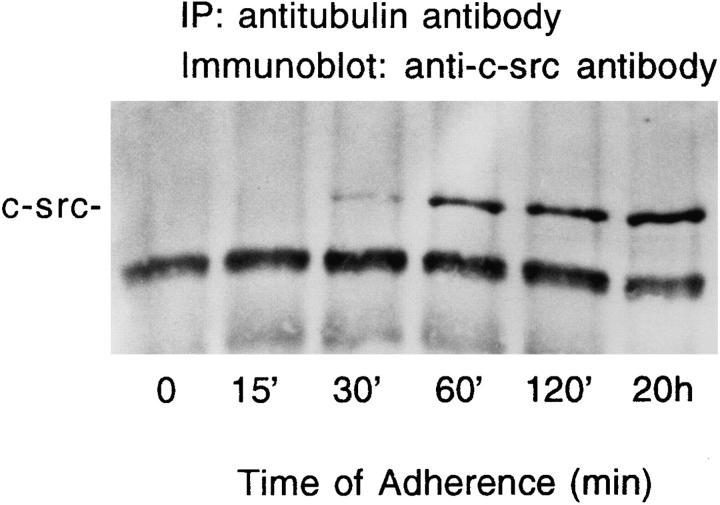
Immunoprecipitation with antitubulin antibody, followed by anti–c-src immunoblotting, demonstrates that the proteins complex after 30 min of adherence and association peaks by 1–2 h (Fig. 5). Phase contrast microscopy indicates the cells attach to substrate and are in early stages of spreading within this time (data not shown).
Figure 5.
Time course of c-src/tubulin association. Avian marrow macrophages, maintained in suspension for 2 d, were plated on tissue culture plastic for the indicated times. Cells were lysed, the lysate was immunoprecipitated with antitubulin antibody, and c-src content of the immunoprecipitate was assessed by immunoblotting, as described in Fig. 3.
C-src Colocalizes with Intracellular Microtubules
C-src/tubulin association documented by techniques described thus far might reflect a postlysis phenomenon. To address this possibility, Triton-permeabilized cells were incubated with anti–c-src and antitubulin antibodies followed by fluorescent-tagged secondary antibodies (Texas red for antimurine IgG[c-src], fluorescein for anti–rabbit IgG[tubulin]) and subjected to confocal microscopy (Fig. 6). As expected, antitubulin staining reveals a characteristic network of microtubules. A pattern similar to that obtained with antitubulin is also observed with anti–c-src. Confirmation that c-src decorates the microtubular network comes with excitation of both fluorochromes, resulting in a characteristic yellow color. We point out that as seen in Fig. 6, C and D, a subset of c-src remains in a punctate (non–filament-associated) form (arrows).
Figure 6.
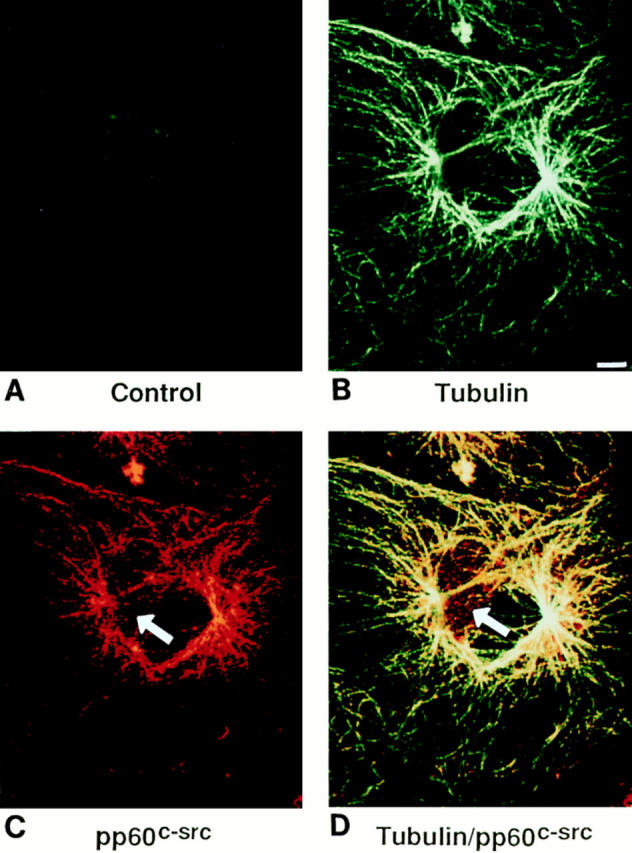
Intracellular localization of c-src and tubulin by immunoconfocal microscopy. Avian marrow macrophages, plated on coverslips, were differentiated into osteoclast-like cells as previously described (1). The cells were fixed, Triton permeabilized, incubated with both antitubulin and anti–c-src antibodies followed by fluorescent-labeled secondary antibodies, Texas red (for src) and FITC (for tubulin), and examined by confocal microscopy. B–D represent differential excitation of the same cell. B represents excitation of FITC (tubulin), and C represents Texas red (c-src). D represents excitation of both fluorochromes and thus, colocalization of the antibodies. The control experiment (A) represents an osteoclast-like cell incubated with irrelevant primary murine and rabbit antibodies and FITC and Texas red and subjected to excitation of both fluorochromes. Bar, 5 μm.
Tubulin-associated C-src Is Kinetically Active
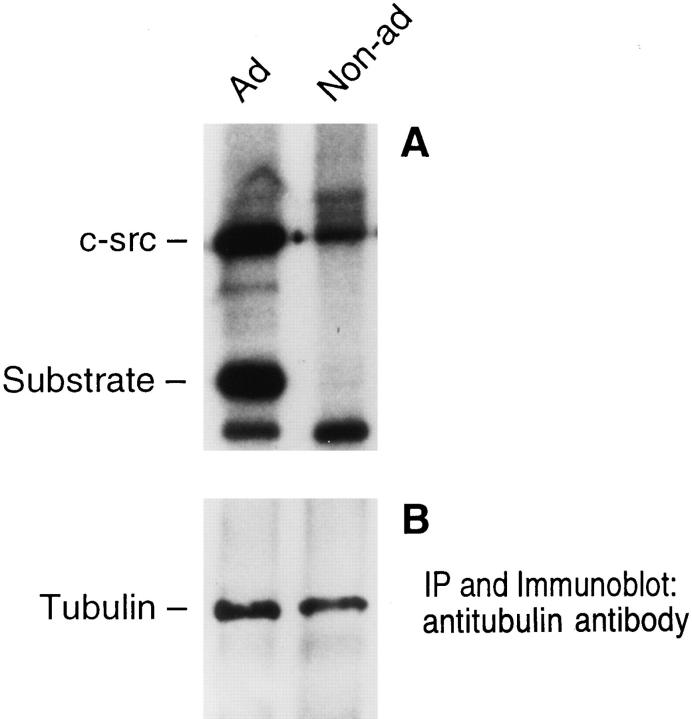
To confirm the species immunoprecipitating with tubulin is, in fact, active c-src, we performed in vitro kinase assays on antitubulin immunoprecipitates, using as exogenous substrate, enolase, known to be specifically phosphorylated by the tyrosine kinase. Reflecting the abundance of c-src we find associated with tubulin in adherent osteoclast precursors, Fig. 7 A shows intense autophosphorylation of c-src and phosphorylation of exogenous substrate. In contrast to adherent cells, the magnitude of c-src autophosphorylation is much less pronounced in nonadherent macrophages (18% of the value in adherent cells normalized to protein levels), and phosphorylation of enolase is virtually undetectable. These differences in protein phosphorylation do not reflect altered tubulin abundance in adherent as compared to nonadherent cells (Fig. 7 B). The observed c-src kinase activity parallels, however, the amount of tubulin-associated c-src protein in adherent and nonadherent cells, respectively (Fig. 3 A).
Figure 7.
Tyrosine kinase activity of tubulin-associated c-src. One half of avian marrow macrophages maintained nonadherent for 2 d were plated on tissue culture plastic while the other half continued in suspension. After 90 min, the cells were lysed, and the lysate was immunoprecipitated with antitubulin antibody. One half the immunoprecipitate (A) was assayed for kinase activity by measuring 32P incorporation into c-src and enolase (an exogenous substrate). Tubulin content of the remaining immunoprecipitate was determined by immunoblot (B).
Inhibitors of Microtubule Polymerization Prevent C-src/Tubulin Association
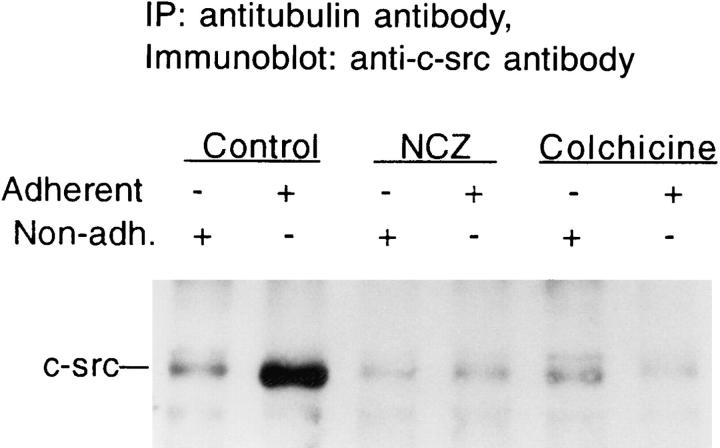
Substrate adherence enhances microtubule assembly (Fig. 6) and c-src/tubulin association (Fig. 3). To determine if these events are functionally related, we asked if the two proteins coimmunoprecipitate from adherent cells in circumstances in which microtubule formation is inhibited. In the first instance, we used the microtubule-disrupting agents, nocodazole and colchicine. Fig. 8 demonstrates that treatment of adherent marrow macrophages with either agent diminishes the quantity of c-src immunoprecipitating with tubulin to levels indistinguishable from those derived from nonadherent cells.
Figure 8.
Effect of microtubule-disrupting drugs on c-src/tubulin association. Avian marrow macrophages, grown in Teflon beakers for 2 d, were treated with 10 μM nocodazole, 1 μM colchicine or vehicle for 2 h, followed by incubation for 90 min (in the presence of the drugs) in suspension or on tissue culture plastic. The cells were lysed and immunoprecipitated with antitubulin antibody, and immunoprecipitate c-src content was assessed by immunoblot as described in Fig. 3.
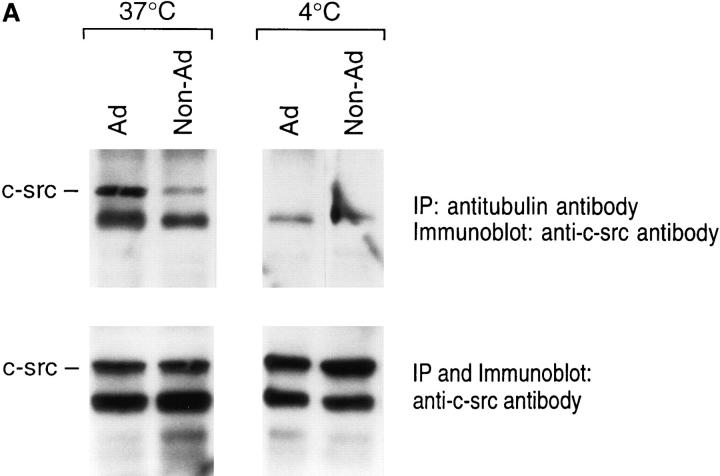
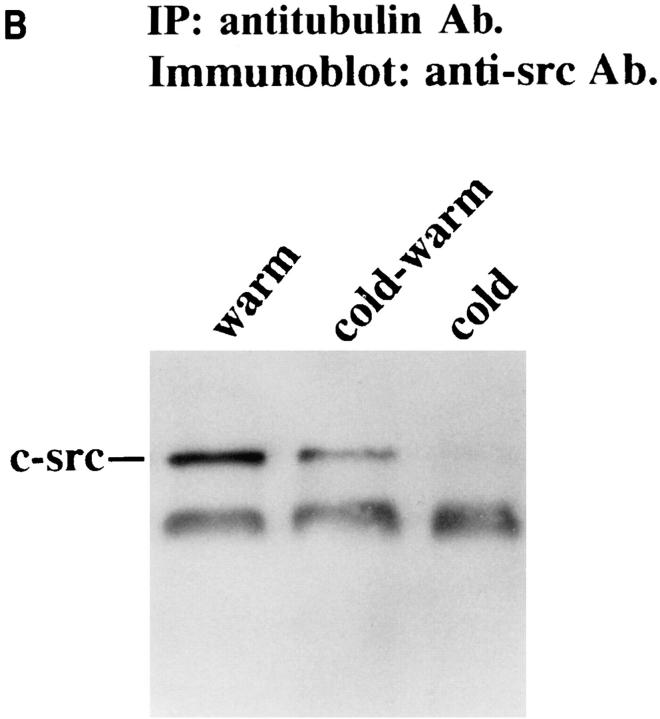
To further confirm the role of microtubule formation in c-src/tubulin association, we exposed macrophages to cold, also known to inhibit tubulin polymerization within solution or cells (9, 29, 54). Thus, adherent or nonadherent cells were lysed and immunoprecipitated either at 4 or 37°C. While the protein complex is maintained in adherent cells at 37°C, little detectable c-src associates (<5% compared to c-src in adherent cells at 37°C) with tubulin at 4°C (Fig. 9 A). To determine if this phenomenon may be biologically significant, we asked if loss of c-src/tubulin association, induced by cold, is reversible by rewarming, a circumstance that repolymerizes tubulin (14). As seen in Fig. 9 B, coimmunoprecipitation of the two proteins is reestablished upon warming 4°C-exposed cells.
Figure 9.
Effect of temperature on tubulin/c-src association. Avian marrow macrophages, incubated in suspension for 2 d, were retained nonadherent or plated on tissue culture plastic for 90 min. (A) Cells were lysed with either ice-cold or warm (37°C) lysis buffer and the lysate immunoprecipitated with antitubulin or anti–c-src antibodies at the same respective temperatures. In part B, ice-cold prepared lysates were divided equally; one half was rewarmed and maintained at 37°C in the presence of GTP for 30 min, and the other half was kept at 4°C. Lysates were immunoprecipitated and c-src content of the immunoprecipitates was assessed by immunoblot as described in Fig. 3.
C-src Associates with Microtubules Reconstituted In Vitro
Although our lysis conditions are known to preserve cytoskeletal architecture (51), the possibility exists that microtubules depolymerize in vitro. To assure, as in vivo, c-src associates in vitro with microtubules, we reconstituted these structures from cell lysates, separated them on a sucrose gradient from “free” c-src, and examined them using double antibody immunofluorescent confocal microscopy. As seen in Fig. 10, c-src decorates reconstituted microtubules.
Figure 10.
C-src associates with microtubules generated in vitro. Isolated microtubules were generated from cell lysates and isolated. The microtubules were fixed and stained with both antitubulin and anti–c-src antibodies followed by fluorescent-labeled secondary antibodies, Texas red (for c-src) and FITC (for tubulin), and examined by confocal microscopy. b represents excitation of FITC (tubulin), c represents Texas red (c-src), and d represents both fluorochromes and thus, colocalization of the antibodies. a represents incubation with irrelevant primary murine and rabbit antibodies and FITC and Texas red and excitation of both fluorochromes. Bar, 10 μm.
Microtubule Disassembly Does Not Alter C-src Kinase Activity
We have shown c-src kinase activity preferentially associates with tubulin derived from adherent rather than nonadherent cells (Fig. 7). To determine if the low level of tubulinbound c-src activity in nonadherent macrophages reflects enzymatic repression by globular tubulin, we assessed specific activity of the tyrosine kinase in lysates derived from adherent cells maintained at 37 or 4°C, respectively. Thus, lysates of cells cultured at either temperature were immunoprecipitated with antitubulin and then anti–c-src antibodies. Equal amounts of protein were subjected to in vitro kinase assay or subjected to immunoblot with anti–c-src antibody. Once again, c-src activity is tubulin associated at 37 but not at 4°C (Fig. 11). Establishing the absence of microtubules does not repress the tyrosine kinase, non–tubulin-bound c-src–specific activity is similar in lysates derived from macrophages cultured at either temperature (compare lanes 2 and 4).
Figure 11.
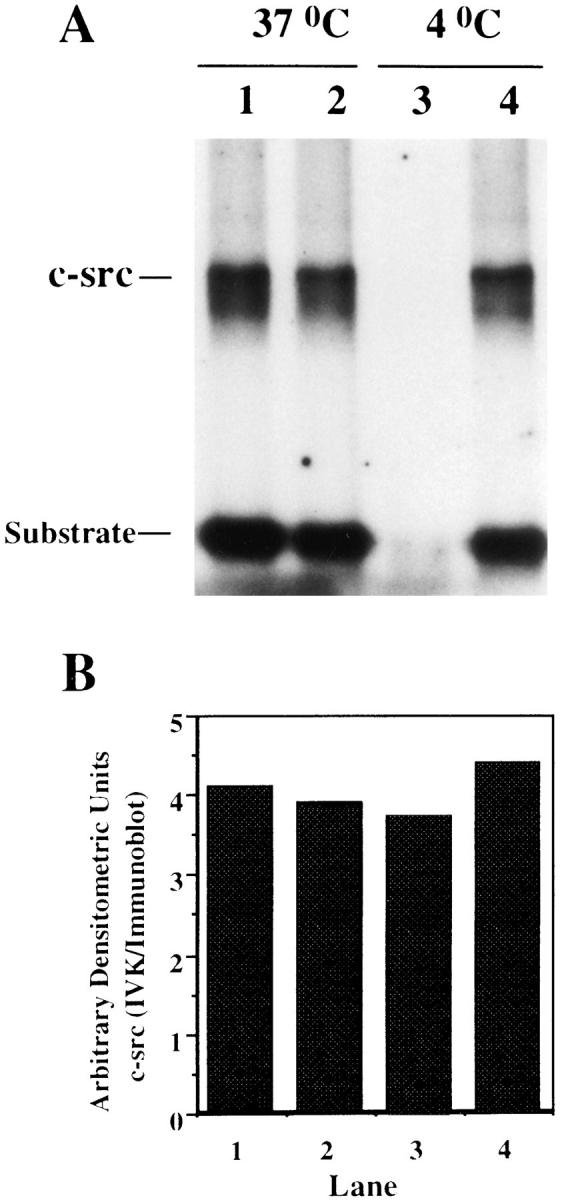
Effect of microtubule association/dissociation on c-src kinase activity. Adherent macrophages were lysed at 37°C (to maintain intact microtubules) or at 4°C. Lysates were immunoprecipitated with antitubulin antibody (lanes 1 and 3) followed by immunoprecipitation with anti–c-src antibody (lanes 2 and 4, respectively). Immunoprecipitates were divided equally. One half was subjected to in vitro kinase assay (A) and the other half to Western immunoblot analysis with anti–c-src antibody. B represents the specific activity determined from densitometric analysis of the ratio between c-src kinase activity and c-src protein in each condition.
C-src Associates with Tubulin upon Adherence to Specific Extracellular Matrices
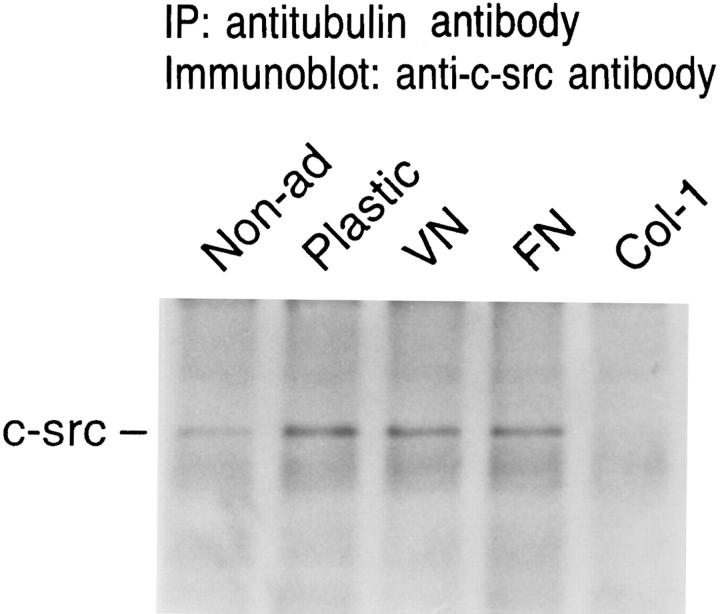
The substrate binding experiments described thus far were performed in serum-free medium devoid of potential extracellular attachment molecules, a nonphysiological circumstance. Thus, we asked if c-src/tubulin association is prompted by specific extracellular matrix proteins. To this end, cells were maintained, serum free, for 90 min in suspension or plastic dishes, the latter either untreated or coated with fibronectin, vitronectin, or type I collagen. The macrophages were harvested in lysis buffer and lysates immunoprecipitated with antitubulin antibody. Fig. 12 shows that when analyzed by immunoblot, antitubulin immunoprecipitates, derived from cells adherent to fibronectin or vitronectin, contain as much c-src as do those plated on untreated plastic. In contrast, plating on type I collagen yields no detectable c-src/tubulin association.
Figure 12.
Effect of specific extracellular matrix proteins on c-src/ tubulin association. Avian marrow macrophages, incubated in Teflon beakers for 2 d, were plated on untreated tissue culture plastic or that coated with indicated extracellular matrix proteins. After 90 min, the cells were lysed, and the lysate was immunoprecipitated with antitubulin antibody. C-src content of immunoprecipitates was assessed by immunoblot as described in Fig. 3.
Intracellular C-src/Microtubule Association Is Substrate Specific
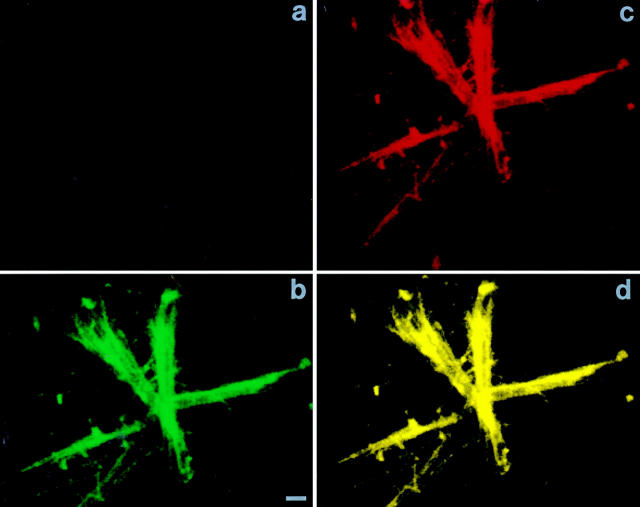
To determine if the specific matrix induction of c-src/tubulin association, demonstrated, by immunoprecipitation (Fig. 12), is an intracellular event, we once again turned to immunoconfocal microscopy (Fig. 13). Macrophages were permeabilized and incubated with anti–c-src and antitubulin antibodies as well as fluoresceinated secondary antibodies as described in Fig. 4. Whereas c-src/microtubule colocalization is extant in cells plated on vitronectin (Fig. 13, A–C), microtubules are not evident in those on collagen (Fig. 13, D–F), which are indistinguishable from their nonadherent counterparts (Fig. 13, G–I), wherein the two molecules fail to associate.
Figure 13.
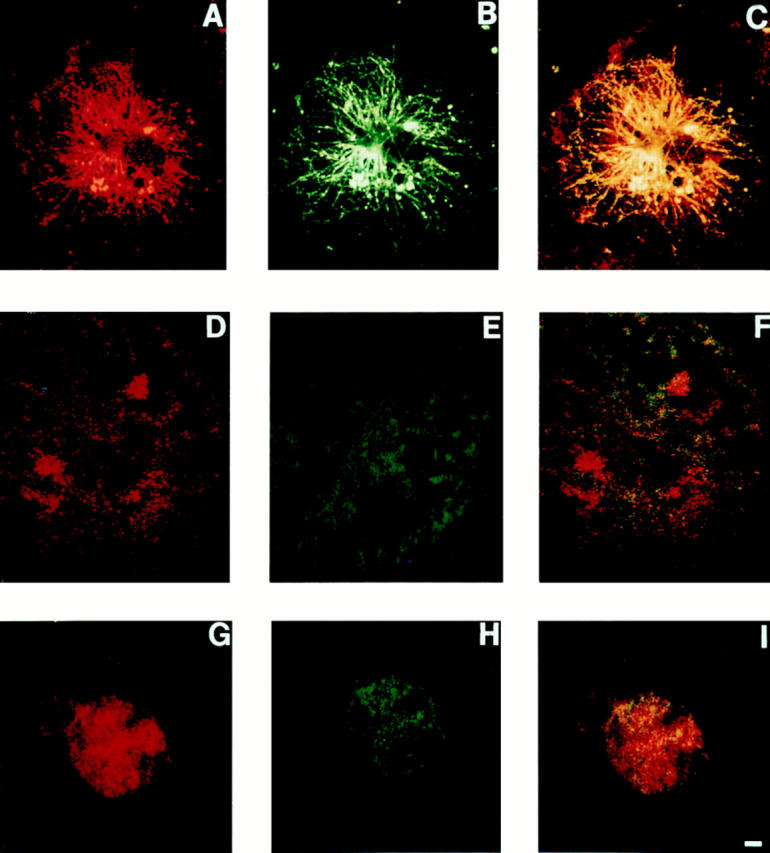
Matrix-dependent intracellular association of c-src/tubulin association by confocal microscopy. Avian marrow macrophages were kept in Teflon-coated beakers or plated on coverslips. Adherent cells were differentiated into osteoclast-like cells as previously described (1). Adherent and nonadherent cells were fixed, Triton permeabilized, incubated with both antitubulin and anti–c-src antibodies followed by fluorescent-labeled secondary antibodies, Texas red (for src) and FITC (for tubulin), and examined by confocal microscopy. A–C represent cells plated on vitronectin-coated plates. D–F represent cells plated on collagen type-I, and G–I represent cells kept nonadherent. Each group represents excitation of the same cell. A, D, and G represent excitation of Texas red (c-src), and B, E, and H represent FITC (tubulin). C, F, and I represent excitation of both fluorochromes and thus, colocalization of the antibodies. Similar results were reproduced when using anti–c-src polyclonal and antitubulin monoclonal antibodies, followed by the relevant secondary-labeled antibodies (data not shown).
Protein Tyrosine Kinase Inhibitor Does Not Alter Tubulin/C-src Association
To determine if induction of c-src/tubulin association is tyrosine kinase dependent, adherent or nonadherent macrophages were exposed for 2 h to the tyrosine kinase inhibitor, herbimycin-A, at a concentration 20-fold greater than that known to block osteoclast tyrosine kinase activity (55). Cells were lysed, the lysate was immunoprecipitated with anti–c-src or antitubulin antibodies, and the immunoprecipitate was probed with an anti–c-src antibody. As seen in Fig. 14, the quantity of c-src immunoprecipitating with tubulin from adherent cells is unaltered by the tyrosine kinase inhibitor (87% of control; compare lanes 1 and 3). Similar results were obtained in nonadherent cells (lanes 5 and 7). C-src immunoprecipitated directly from adherent or nonadherent cells is also not affected by herbimycin-A (114% of control; compare lanes 2 and 4).
Vacuolar H+-ATPase Colocalizes with Intracellular Microtubules
The osteoclast vacuolar proton pump (H+-ATPase) probably polarizes towards the bone-apposed plasma membrane after matrix-derived signals, raising the possibility of a microtubule-based transport mechanism. In fact, immunoconfocal microscopy demonstrates the osteoclast antivacuolar H+-ATPase mAb (Texas red–labeled secondary antibody), in a manner similar to c-src, decorates microtubules (fluorescein-labeled secondary antibody) (Fig. 15; compare to Figs. 6, 10, and 13).
Figure 15.
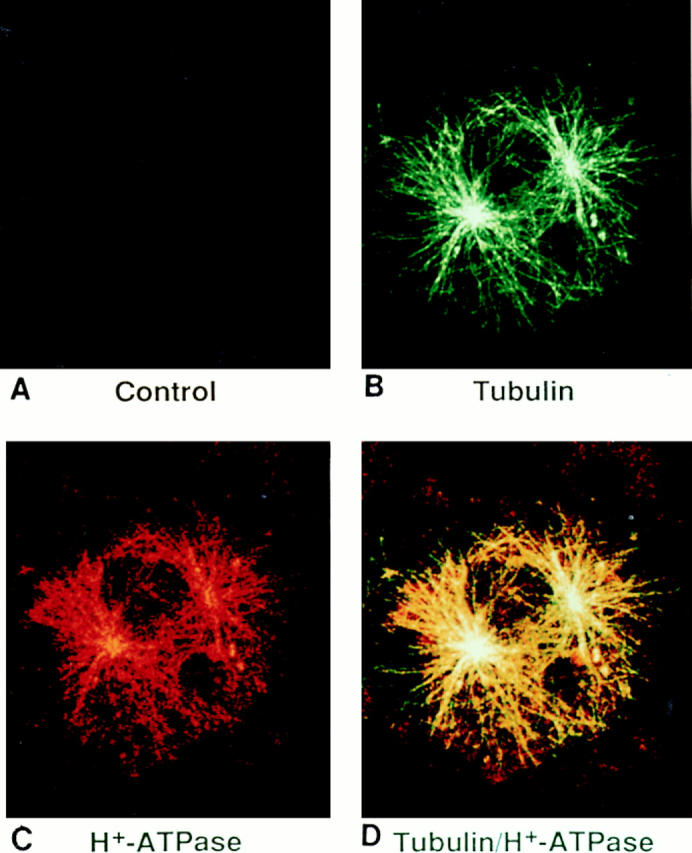
Intracellular localization of vacuolar H+-ATPase with microtubules. Cells were treated as described for Fig. 6. The panels indicate immunostaining with antitubulin (B) or anti-vacuolar proton pump antibodies (C). D represents colocalization of both antibodies.
Vacuolar H+-ATPase Specifically Colocalizes with C-src
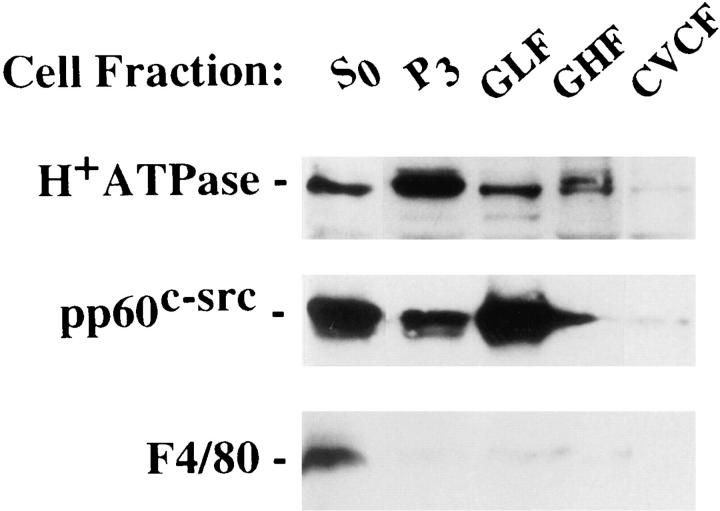
To determine if the osteoclast H+-ATPase and c-src colocalize, we analyzed density gradient–separated membrane fractions derived from generated avian osteoclasts. Fig. 16 shows that in contrast to the Golgi heavy fraction, which contains H+-ATPase but not c-src, both molecules are present in the Golgi light fraction. Documenting specificity, F4/80, a cell surface marker of mature macrophages, is present in total cell lysate but not in intracellular fractions.
Figure 16.
Subcellular colocalization of vacuolar H+-ATPase and c-src. Cells maintained on tissue culture plates for 3 d were lysed (S 0), and the lysate was centrifuged at 1,000 g to remove nuclei and large cell debris. Supernatant was centrifuged at 27,000 g and the membrane pellet (P 3) was fractionated on a sucrose density gradient. An equal amount of protein from each gradient fraction was subjected to immunoblot using antibodies to c-src, H+-ATPase, and F4/80. GLF, Golgi light; GHF, Golgi heavy; CVCF, crude vesicle fractions.
Osteoclast Acidifying Vesicles Contain C-src
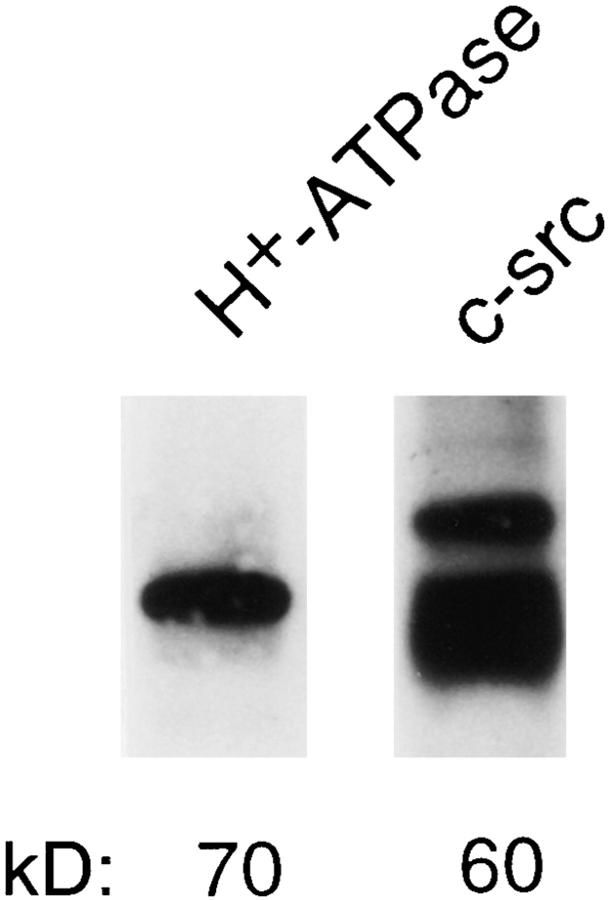
We previously reported techniques of isolating protontransporting vesicles, the majority being ruffled membranederived, from mature chicken–isolated osteoclasts (8). To determine if these membranes, known to contain the vacuolar proton pump (8), also accommodate c-src, the fraction was lysed, and the lysate was subjected to immunoblot with anti–c-src and H+-ATPase antibodies. As seen in Fig. 17, this membrane fraction, rich in authentic acidifying vesicles, contains both proteins.
Figure 17.
C-src localization with acidifying vesicle preparation from authentic osteoclasts. Acidifying membrane vesicles were isolated from authentic osteoclasts and lysed, and the lysate was probed, by immunoblot, with anti– c-src and H+-ATPase antibodies.
Discussion
Reflecting development of numerous in vitro models, recent years have witnessed major insights into the mechanisms by which osteoclasts degrade bone (50). The cell is established as a member of the monocyte/macrophage family, and a variety of molecules mediating the resorptive process are in hand. Identification and localization of these bone-degrading proteins brought realization that polarization is a major event in development of the anatomical and functional osteoclast phenotype. Specifically, entities such as the vacuolar proton pump (H+-ATPase) (7), mannose6-phosphate receptor (4, 6, 27), and lysosomal enzymes (22), all of which appear to mediate skeletal degradation, target to the bone-apposed plasma membrane. The molecules governing osteoclast polarization remained enigmatic until generation of the c-src−/− mouse, whose osteoclasts lack ruffled membranes (47). Thus, the protooncogene must play a pivotal role in targeted protein trafficking in these cells. This conclusion is in keeping with the observation that c-src modulates vacuolar exocytosis of chromaffin granules from the adrenal medulla (23), a process not dissimilar to formation of the ruffled membrane by vesicular insertion. The fact that c-src localizes with the mannose6-phosphate receptor (27), which plays a role in osteoclast endosomal recycling (6), suggests this protooncogene not only governs formation of the ruffled membrane but is also transported to the bone-apposed cell surface in vesicles containing molecules involved in the resorptive process. Indeed, we find c-src localizes, in a specific intracellular fraction containing Golgi-derived membranes, with the osteoclast vacuolar H+-ATPase. This fraction also contains exocytic Rab 3 but not endocytic Rab 4 proteins (AbuAmer, Y., unpublished data). These observations suggest that the osteoclast proton pump and the protooncogene may be packaged within sub-Golgi vesicles for delivery to the ruffled membrane. Supporting this hypothesis is the fact c-src and the H+-ATPase both decorate microtubules in a similar fashion and are found in acidifying vesicles derived primarily from the ruffled membrane.
Antero- and retrograde movement of vesicles often occurs by their association with microtubules (32, 38, 41, 43). The role of microtubules in polarized vesicular transport has been studied in epithelial and neuronal cells (11, 17, 18, 45). Given the necessity for targeted vesicular transport in bone resorption, microtubules are likely to also play a role in osteoclast polarization.
Osteoclast differentiation involves attachment of precursors to bone where they polarize (50). With this in mind, and the role microtubules play in the polarization process, we hypothesized c-src, a protein transported to the ruffled membrane, associates in osteoclast progenitors with tubulin. We also suspected this association is regulated by matrix recognition, a process fundamental to generating the osteoclast phenotype and one that prompts microtubule polymerization (12, 16, 29).
We find that the majority of total c-src immunoprecipitates with tubulin when osteoclast precursors are in contact with matrix. Protooncogene/tubulin association occurs rapidly, being evident within the first hour of cell-matrix recognition. Furthermore, tubulin-bound c-src is enzymatically active as manifest by its capacity to autophosphorylate and to phosphorylate an exogenous substrate. Most importantly, double antibody confocal microscopy documents c-src/tubulin association in situ, obviating concerns that our immunoprecipitation data may reflect a postlysis artifact. The predominantly filamentous distribution of c-src within osteoclast precursors, as well as the inhibitory impact of nocodazole and colchicine on c-src/tubulin association, indicates the protooncogene complexes largely with polymerized tubulin. This contention is supported by the fact c-src/tubulin association is disrupted by cold, an established depolymerizing event (14), and most importantly, is reestablished by rewarming. Furthermore, tubulin polymerized from cell lysates in vitro is decorated by c-src, indicating our immunoprecipitation experiments reflect, in fact, association of the protooncogene with microtubules. On the other hand, c-src unassociated with microtubules is also apparent in adherent osteoclast-like cells, a finding consistent with the protooncogene's localization in membrane bound vesicles (27).
The matrix-derived signals prompting c-src/tubulin association are, as yet, undefined, although the event is ligand specific, being induced by vitronectin. This protein contains the Arg-Gly-Asp (RGD) motif recognized by the functional osteoclast integrin αvβ3, which transmits matrixderived signals across the plasma membrane (39). These data, taken with the fact that translocation of c-src from the soluble to insoluble fraction of platelets depends upon expression of the related integrin, αIIbβ3 (13), by these cells, suggest αvβ3 occupancy may initiate osteoclast polarization.
Our study raises two important and related issues, namely the molecular mechanisms by which c-src localizes with microtubules and the means by which the protooncogene mediates osteoclast polarization. While the tyrosine kinase associates with the detergent-insoluble fraction containing polymerized tubulin in other cells (19, 20, 24, 28, 44), it is unknown if the protooncogene binds directly to tubulin or if such binding is effected by other, intermediary proteins. Given lysis conditions were designed to maintain protein–protein interaction, various entities other than c-src, none of which are regulated by substrate adherence, also coimmunoprecipitate with tubulin. Thus, while c-src, per se, might interact with microtubules or molecules such as kinectin (30) and dynactin (52), which anchor molecular motors to intracellular membranes, any of a number of coimmunoprecipitating proteins may modulate association of the protooncogene with tubulin.
Our data indicate that c-src kinase activity is not accelerated by c-src/microtubule binding. This observation is in keeping with the fact that c-src and cytoskeletal proteins associate in absence of the kinase catalytic domain (42). The most compelling evidence that c-src enzymatic activity is also not essential to osteoclast polarization is the rescue of c-src−/− osteopetrotic mice by a c-src kinase inactive transgene (Schwartzberg, P., L. King, C.A. Lowell, E. Lee, L. Garrett, S. Reddy, B.D. Roodman, B. Boyce, and H.E. Varmus. 1996. J. Bone Miner. Res. 11:S135a).
Collectively, these data suggest other components of the c-src protein, such as src homology (SH) domains, may mediate osteoclast polarization and perhaps microtubule binding. In this regard, the c-src SH2 domain promotes interaction with detergent-insoluble components through a phosphotyrosine-independent event (20). While our data do not address the possibility that SH domains participate in c-src/microtubule association, they are consonant with the hypothesis that c-src and microtubules both participate in osteoclast polarization and do so through a physical interaction mediated by cell-matrix adherence.
Acknowledgments
Supported in part by National Institutes of Health grant Nos. DE05413 and AR32788 (S.L. Teitelbaum), AR42404 (F.P. Ross), and AR 44089 (M.M. Tondravi) and a grant from the Shriners Hospital for Crippled Children, St. Louis Unit (S.L. Teitelbaum).
Abbreviations used in this paper
- ECL
enhanced chemiluminescence
- IP
immunoprecipitation
- SH
src homology
Footnotes
Address all correspondence to Steven L. Teitelbaum, M.D., Department of Pathology, Washington University School of Medicine, 216 South Kingshighway, St. Louis, MO 63110. Tel.: (314) 454-8463. Fax: (314) 454-5505. E-mail: Teitelbs@medicine.wustl.edu
References
- 1.Alvarez JI, Teitelbaum SL, Blair HC, Greenfield EM, Athanasou NA, Ross FP. Generation of avian cells resembling osteoclasts from mononuclear phagocytes. Endocrinology. 1991;128:2324–2335. doi: 10.1210/endo-128-5-2324. [DOI] [PubMed] [Google Scholar]
- 2.Anand R, Wilkinson JM, Kellie S. Localization of pp60c-srcto the surface membrane of human platelets. Oncogene. 1993;8:3013–3020. [PubMed] [Google Scholar]
- 3.Bacallao R, Antony C, Dotti C, Karsenti E, Stelzer EHK, Simons K. The subcellular organization of Madin-Darby canine kidney cells during the formation of a polarized epithelium. J Cell Biol. 1989;109:2817–2832. doi: 10.1083/jcb.109.6.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barengolts E, Buschmann R, Shevrin DH, Abramson EC, Kukreja SC. Effects of hypercalcemia-producing tumor extract and parathyroid hormone on osteoclast ultrastructure. Acta Anat. 1991;137:160–164. doi: 10.1159/000146877. [DOI] [PubMed] [Google Scholar]
- 5.Blair HC, Kahn AJ, Crouch EC, Jeffrey JJ, Teitelbaum SL. Isolated osteoclasts resorb the organic and inorganic components of bone. J Cell Biol. 1986;102:1164–1172. doi: 10.1083/jcb.102.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blair HC, Teitelbaum SL, Schimke PA, Konsek JD, Koziol CM, Schlesinger PH. Receptor-mediated uptake of mannose-6-phosphate bearing glycoprotein by isolated chicken osteoclasts. J Cell Physiol. 1988;137:476–482. doi: 10.1002/jcp.1041370312. [DOI] [PubMed] [Google Scholar]
- 7.Blair HC, Teitelbaum SL, Ghiselli R, Gluck S. Osteoclastic bone resorption by a polarized vacuolar proton pump. Science (Wash DC) 1989;245:855–857. doi: 10.1126/science.2528207. [DOI] [PubMed] [Google Scholar]
- 8.Blair HC, Teitelbaum SL, Tan H, Koziol CM, Schlesinger PH. Passive chloride permeability charge coupled to H+-ATPase of avian osteoclast ruffled membrane. Am J Physiol. 1991;260:C1315–C1324. doi: 10.1152/ajpcell.1991.260.6.C1315. [DOI] [PubMed] [Google Scholar]
- 9.Boll W, Partin JS, Katz AI, Caplan MJ, Jamieson JD. Distinct pathways for basolateral targeting of membrane and secretory proteins in polarized epithelial cells. Proc Natl Acad Sci USA. 1991;88:8592–8596. doi: 10.1073/pnas.88.19.8592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyce BF, Yoneda T, Lowe C, Soriano P, Mundy GR. Requirement of pp60c-srcexpression for osteoclasts to form ruffled borders and resorb bone in mice. J Clin Invest. 1992;90:1622–1627. doi: 10.1172/JCI116032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown D, Sabolic I. Endosomal pathways for water channel and proton pump recycling in kidney epithelial cells. J Cell Sci. 1993;17:49–59. doi: 10.1242/jcs.1993.supplement_17.8. [DOI] [PubMed] [Google Scholar]
- 12.Burridge K, Turner CE, Romer LH. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J Cell Biol. 1992;119:893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark EA, Brugge JS. Redistribution of activated pp60c-srcto integrin-dependent cytoskeletal complexes in thrombin-stimulated platelets. Mol Cell Biol. 1993;13:1863–1871. doi: 10.1128/mcb.13.3.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conrad PA, Smart EJ, Ying Y, Anderson RGW, Bloom GS. Caveolin cycles between plasma membrane caveolae and the Golgi complex by microtubule-dependent and microtubule-independent steps. J Cell Biol. 1995;131:1421–1433. doi: 10.1083/jcb.131.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.David-Pfeuty T, Nouvian-Dooghe Y. Immunolocalization of the cellular src protein in interphase and mitotic NIH c-srcoverexpresser cells. J Cell Biol. 1990;111:3097–3116. doi: 10.1083/jcb.111.6.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eilers U, Klumperman J, Hauri H. Nocodazole, a microtubuleactive drug, interferes with apical protein delivery in cultured intestinal epithelial cells (Caco-2) J Cell Biol. 1989;108:13–22. doi: 10.1083/jcb.108.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elferink LA, Scheller RH. Synaptic vesicle proteins and regulated exocytosis. J Cell Sci. 1993;17:75–79. doi: 10.1242/jcs.1993.supplement_17.11. [DOI] [PubMed] [Google Scholar]
- 18.Fath, K.R., S.N. Mamajiwalla, and D.R. Burgess. 1993. The cytoskeleton in development of epithelial cell polarity. J. Cell Sci. 17(Suppl.):65–73. [DOI] [PubMed]
- 19.Ferrell JE, Jr, Noble JA, Martin GS, Jacques YV, Bainton DF. Intracellular localization of pp60c-srcin human platelets. Oncogene. 1990;5:1033–1036. [PubMed] [Google Scholar]
- 20.Fukui Y, O'Brien MC, Hanafusa H. Deletions in the SH2 domain of p60v-srcprevent association with the detergent-insoluble cellular matrix. Mol Cell Biol. 1991;11:1207–1213. doi: 10.1128/mcb.11.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gluck S, Al-Awqati Q. An electrogenic proton-translocating adenosine triphosphatase from bovine kidney medulla. J Clin Invest. 1984;73:1704–1710. doi: 10.1172/JCI111378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goto T, Kiyoshima T, Moroi R, Tsukuba T, Nishimura Y, Himeno M, Yamamoto K, Tanaka T. Localization of cathepsins B, D, and L in the rat osteoclast by immuno-light and -electron microscopy. Histochemistry. 1994;101:33–40. doi: 10.1007/BF00315829. [DOI] [PubMed] [Google Scholar]
- 23.Grandori C, Hanafusa H. pp60c-srcis complexed with a cellular protein in subcellular compartments involved in exocytosis. J Cell Biol. 1988;107:2125–2135. doi: 10.1083/jcb.107.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamaguchi M, Hanafusa H. Association of p60srcwith Triton X-100-resistant cellular structure correlates with morphological transformation. Proc Natl Acad Sci USA. 1987;84:2312–2316. doi: 10.1073/pnas.84.8.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horne WC, Neff L, Chatterjee D, Lomri A, Levy JB, Baron R. Osteoclasts express high levels of pp60c-srcin association with intracellular membranes. J Cell Biol. 1992;119:1003–1013. doi: 10.1083/jcb.119.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howell KE, Palade GE. Hepatic Golgi fractions resolved into membrane and content subfractions. J Cell Biol. 1982;92:822–832. doi: 10.1083/jcb.92.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaplan KB, Swedlow JR, Varmus HE, Morgan DO. Association of p60c-srcwith endosomal membranes in mammalian fibroblasts. J Cell Biol. 1992;118:321–333. doi: 10.1083/jcb.118.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katagiri K, Katagiri T, Kajiyama K, Yamamoto T, Yoshida T. Tyrosine-phosphorylation of tubulin during monocytic differentiation of HL-60 cells. J Immunol. 1993;150:585–593. [PubMed] [Google Scholar]
- 29.Kelly RB. Microtubules, membrane traffic, and cell organization. Cell. 1990;61:5. doi: 10.1016/0092-8674(90)90206-t. [DOI] [PubMed] [Google Scholar]
- 30.Kumar J, Yu H, Sheetz MP. Kinectin, an essential anchor for kinesin-driven vesicle motility. Science (Wash DC) 1995;267:1834–1837. doi: 10.1126/science.7892610. [DOI] [PubMed] [Google Scholar]
- 31.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (Lond) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 32.Lafont F, Burkhardt JK, Simons K. Involvement of microtubule motors in basolateral and apical transport in kidney cells. Nature (Lond) 1994;372:801. doi: 10.1038/372801a0. [DOI] [PubMed] [Google Scholar]
- 33.Linstedt AD, Vetter ML, Bishop JM, Kelly RB. Specific association of the proto-oncogene product pp60c-srcwith an intracellular organelle, the PC12 synaptic vesicle. J Cell Biol. 1992;117:1077–1084. doi: 10.1083/jcb.117.5.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lipsich LA, Lewis AJ, Brugge JS. Isolation of monoclonal antibodies that recognize the transforming proteins of avian sarcoma viruses. J Virol. 1983;48:352–360. doi: 10.1128/jvi.48.2.352-360.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lowe C, Yoneda T, Boyce BF, Chen H, Mundy GR, Soriano P. Osteopetrosis in src-deficient mice is due to an autonomous defect of osteoclasts. Proc Natl Acad Sci USA. 1993;90:4485–4489. doi: 10.1073/pnas.90.10.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marchisio PC, Cirillo D, Naldini L, Primavera MV, Teti A, Zambonin-Zallone A. Cell-substratum interaction of cultured avian osteoclasts is mediated by specific adhesion structures. J Cell Biol. 1984;99:1696–1705. doi: 10.1083/jcb.99.5.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mattsson JP, Schlesinger PH, Keeling DJ, Teitelbaum SL, Stone DK, Xie X. Isolation and reconstitution of a vacuolar-type proton pump of osteoclast membranes. J Biol Chem. 1994;269:24979–24982. [PubMed] [Google Scholar]
- 38.Mellman I, Yamamoto E, Whitney JA, Kim M, Hunziker W, Matter K. Molecular sorting in polarized and non-polarized cells: common problems, common solutions. J Cell Sci. 1993;17:1–7. doi: 10.1242/jcs.1993.supplement_17.1. [DOI] [PubMed] [Google Scholar]
- 39.Miyauchi A, Alvarez J, Greenfield EM, Teti A, Grano M, Colucci S, Zambonin-Zallone A, Ross FP, Teitelbaum SL, Cheresh D. Recognition of osteopontin and related peptides by an αvβ3integrin stimulates immediate cell signals in osteoclasts. J Biol Chem. 1991;266:20369–20374. [PubMed] [Google Scholar]
- 40.Ohya K, Ogura H. The effects of colchicine or vinblastine on the blood calcium level in rats. Eur J Pharmacol. 1993;248:111–119. doi: 10.1016/0926-6917(93)90032-l. [DOI] [PubMed] [Google Scholar]
- 41.Ojakian GK, Schwimmer R. Antimicrotubule drugs inhibit the polarized insertion of an intracellular glycoprotein pool into the apical membrane of Madin-Darby canine kidney (MDCK) cells. J Cell Sci. 1992;103:677–687. doi: 10.1242/jcs.103.3.677. [DOI] [PubMed] [Google Scholar]
- 42.Okamura H, Resh MD. Differential binding of pp60c-src and pp60v-srcto cytoskeleton is mediated by SH2 and catalytic domains. Oncogene. 1994;9:2293–2303. [PubMed] [Google Scholar]
- 43.Raff, E.C. 1994. The role of multiple tubulin isoforms in cellular microtubule function. In Microtubules. J.S. Hyams and C.W. Lloyd, editors. Wiley-Liss, Inc., New York. 85–109.
- 44.Rodgers W, Crise B, Rose JK. Signals determining protein tyrosine kinase and glycosyl-phosphatidylinositol-anchored protein targeting to a glycolipid-enriched membrane fraction. Mol Cell Biol. 1994;14:5384–5391. doi: 10.1128/mcb.14.8.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodriguez-Boulan E, Powell SK. Polarity of epithelial and neuronal cells. Annu Rev Cell Biol. 1992;8:395–427. doi: 10.1146/annurev.cb.08.110192.002143. [DOI] [PubMed] [Google Scholar]
- 46.Saucan L, Palade GE. Membrane and secretory proteins are transported from the Golgi complex to the sinusoidal plasmalemma of hepatocytes by distinct vesicular carriers. J Cell Biol. 1994;125:733–741. doi: 10.1083/jcb.125.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soriano P, Montgomery C, Geske R, Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- 48.Sudhof TC, Fried VA, Stone DK, Johnston PA, Xie X. Human endomembrane H+pump strongly resembles the ATP-synthetase of Archaebacteria. Proc Natl Acad Sci USA. 1989;86:6067–6071. doi: 10.1073/pnas.86.16.6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tanaka S, Takahashi N, Udagawa N, Sasaki T, Fukui Y, Kurokawa T, Suda T. Osteoclasts express high levels of pp60c-src, preferentially on ruffled border membranes. FEBS (Fed Eur Biochem Soc) Lett. 1992;313:85–89. doi: 10.1016/0014-5793(92)81190-w. [DOI] [PubMed] [Google Scholar]
- 50.Teitelbaum SL, Abu-Amer Y, Ross FP. Molecular mechanisms of bone resorption. J Cell Biochem. 1995;59:1–10. doi: 10.1002/jcb.240590102. [DOI] [PubMed] [Google Scholar]
- 51.Temkin RK, So DY, Lea PJ. Advantages of digitonin extraction to reveal the intracellular structure of rat glomerular podocytes for high-resolution scanning electron microscopy. Micro Res Tech. 1993;26:260–271. doi: 10.1002/jemt.1070260308. [DOI] [PubMed] [Google Scholar]
- 52.Vallee RB, Sheetz MP. Targeting of motor proteins. Science (Wash DC) 1996;271:1539–1544. doi: 10.1126/science.271.5255.1539. [DOI] [PubMed] [Google Scholar]
- 53.Warshafsky B, Aubin JE, Heersche JNM. Cytoskeleton rearrangements during calcitonin-induced changes in osteoclast motility. Bone (NY) 1985;6:179–185. doi: 10.1016/8756-3282(85)90051-1. [DOI] [PubMed] [Google Scholar]
- 54.Wordeman, L., and T.J. Mitchison. 1994. Dynamics of microtubule assembly in vivo. In Microtubules. J.S. Hyams and C.W. Lloyd, editors. WileyLiss, Inc., New York. 287–301.
- 55.Yoneda T, Lowe C, Lee C, Gutierrez G, Niewolna M, Williams PJ, Izbicka E, Uehara Y, Mundy GR. Herbimycin A, a pp60c-srctyrosine kinase inhibitor, inhibits osteoclastic bone resorption in vitro and hypercalcemia in vivo. J Clin Invest. 1993;91:2791–2795. doi: 10.1172/JCI116521. [DOI] [PMC free article] [PubMed] [Google Scholar]