Abstract
Among the nuclear proteins associated with mRNAs before their export to the cytoplasm are the abundant heterogeneous nuclear (hn) RNPs. Several of these contain the M9 signal that, in the case of hnRNP A1, has been shown to be sufficient to signal both nuclear export and nuclear import in cultured somatic cells. Kinetic competition experiments are used here to demonstrate that M9-directed nuclear import in Xenopus oocytes is a saturable process. Saturating levels of M9 have, however, no effect on the import of either U snRNPs or proteins carrying a classical basic NLS. Previous work demonstrated the existence of nuclear export factors specific for particular classes of RNA. Injection of hnRNP A1 but not of a mutant protein lacking the M9 domain inhibited export of mRNA but not of other classes of RNA. This suggests that hnRNP A1 or other proteins containing an M9 domain play a role in mRNA export from the nucleus. However, the requirement for M9 function in mRNA export is not identical to that in hnRNP A1 protein transport.
The transport of macromolecules between the nucleus and cytoplasm is a bi-directional process. The best understood aspect is the import of nuclear proteins that carry a basic nuclear localization signal (NLS)1 like the simple NLS found in SV-40 T antigen or the bipartite NLS found in nucleoplasmin (Dingwall and Laskey, 1991). Proteins of this class are recognized by the heterodimeric importin receptor, composed of importin α and importin β (for review see Powers and Forbes, 1994; Melchior and Gerace, 1995; Görlich and Mattaj, 1996). The NLS binds directly to the importin α subunit. The importin NLS protein complex docks at the cytoplasmic face of the nuclear pore complex in an energy-independent manner (Newmeyer and Forbes, 1988; Richardson et al., 1988). Subsequently, the small GTPase Ran/TC4 (Melchior et al., 1993; Moore and Blobel, 1993) and a protein of unknown function named variously pp15, p10, or NTF2 (Moore and Blobel, 1994; Paschal and Gerace, 1995) are required for translocation of the NLS-containing complex through the nuclear pore complex.
A second major class of imported macromolecules are the uracil rich small nuclear (U sn) RNPs. They do not have a basic NLS but instead have a bipartite nuclear targeting signal. This is composed of an essential signal formed when the Sm core proteins bind to the U snRNA and an additional signal, the trimethyl-guanosine (m3G) cap, which depending on the cell type or the U snRNA is either essential or required for optimal U snRNP import efficiency (Fischer and Lührmann, 1990; Hamm et al., 1990; Fischer et al., 1993). Kinetic competition experiments have supported the conclusion that U snRNPs require different limiting factors than do NLS-containing proteins for their import and that U snRNPs do not bind to importin α (Fischer et al., 1991, 1993; Michaud and Goldfarb, 1991; van Zee et al., 1993). There is also preliminary evidence that additional different receptors may be required for the nuclear uptake of other RNA species (Michaud and Goldfarb, 1992).
Similarly, RNA export from the nucleus relies on recognition of the RNA or RNP export substrates by saturable factors (Zasloff, 1983; Bataillé et al., 1990; Jarmolowski et al., 1994). As for import, evidence for the existence of RNA class-specific export receptors has been obtained from kinetic competition experiments (Jarmolowski et al., 1994). Two RNA-binding proteins have been directly shown to function in RNA export, a nuclear cap binding protein complex in the case of U snRNAs (Izaurralde et al., 1995a ) and the HIV-1 Rev protein in the case of RNAs containing a rev response element (Fischer et al., 1994, 1995). In the case of mRNAs, the best candidates for export mediators are the heterogeneous nuclear (hn) RNP proteins (for review see Piñol-Roma and Dreyfuss, 1993; Izaurralde and Mattaj, 1995).
About 20 different hnRNP proteins have been characterized in vertebrate cells (for review see Dreyfuss et al., 1993). The association of hnRNP proteins with mRNA in the nucleus and the cytoplasm suggests that they may regulate and/or facilitate different aspects of gene expression. The possibility that hnRNP proteins might be directly involved in the nucleocytoplasmic trafficking of mRNA molecules was suggested by the observation that several hnRNP proteins, including A1, A2, D, E, I, and K shuttle continuously and rapidly between the nucleus and the cytoplasm and are associated with mRNA in both compartments (Piñol-Roma and Dreyfuss 1992, 1993; Michael et al., 1995a , b). Of these, the best studied example is hnRNP A1. An A1-like hnRNP protein has been shown by immunoelectron microscopy to be associated with a specific mRNA in transit to the cytoplasm through the nuclear pore complex in the insect Chironomus tentans (Visa et al., 1996a ). In mammalian cells, the amount of A1 which is in constant flux between nucleus and cytoplasm is striking. It has been estimated that at least 120,000 molecules of A1 are exported to the cytoplasm per minute but then rapidly reimported such that the steady state localization of A1 is nuclear (Michael et al., 1995a ). Taken together, these results suggest that A1 and other shuttling hnRNP proteins such as A2, D, E, I, and K could play a significant role in the transport of mRNA from the nucleus to the cytoplasm.
One key in understanding how hnRNPs may facilitate mRNA export is to determine the signals that mediate their shuttling, i.e., their import into and exit from the nucleus. The nucleocytoplasmic transport of A1 has been recently studied in detail, and the signals that mediate shuttling have been identified (Michael et al., 1995b ; Siomi and Dreyfuss, 1995; Weighardt et al., 1995). Nuclear import of A1 is determined by a 38-amino acid sequence, termed M9, located near the COOH terminus of the protein between amino acids 268 and 305. Its fusion to cytoplasmic reporter proteins such as pyruvate kinase resulted in rapid import of the fusion protein into the nucleus (Siomi and Dreyfuss, 1995). However, the A1 NLS has no sequence similarity to classical protein NLSs such as that of SV-40 large T antigen or nucleoplasmin (Siomi and Dreyfuss, 1995).
Surprisingly, M9 also acts as a nuclear export signal (NES). In heterokaryon shuttling assays this domain is necessary and sufficient to allow the export of heterologous proteins, such as the nucleoplasmin core domain (NPLc), which are normally retained in the nucleus (Michael et al., 1995b ). Thus, M9 alone can account for the shuttling of A1. Other hnRNPs such as A2 and B1 bear sequences with striking similarities to M9 (Siomi and Dreyfuss, 1995). Mutagenesis experiments indicate that the NES and NLS activities of M9 are either identical or overlapping as mutants which block M9 NLS activity also abolish NES activity (Michael et al., 1995b ). It is therefore possible that M9 is recognized in the nucleus and the cytoplasm by the same receptor.
The second category of NES described was first found in the HIV-1 Rev protein and the inhibitor of protein kinase A (Fischer et al., 1995; Wen et al., 1995; Bogerd et al., 1996; for review see Gerace, 1995). These short, leucine-rich NES sequences bear no relationship to the primary sequence of M9. Furthermore, saturation of the export factor recognized by the Rev NES has no effect on mRNA export (Fischer et al., 1995). A model for mRNA export has been postulated on the basis of the hnRNP data described above. In this model, NES/NLS containing hnRNPs bind in the nucleus to mRNA molecules and deliver them, via the export pathway they access, to the cytoplasm. In the cytoplasm these hnRNP proteins dissociate from the mRNA and return to the nucleus. To further test this model we have analyzed the transport of hnRNP A1 and mRNA in Xenopus laevis oocytes. The oocyte offers a unique opportunity to manipulate specific import or export pathways, like that accessed by M9, and examine the effect on mRNA nuclear export. By using this approach we show here that M9 is, as in somatic cells, a functional NLS in oocytes. Moreover, competition studies indicate that M9 defines a novel class of NLS, since saturation of the M9- mediated import pathway does not interfere with the two previously identified import pathways used by classical NLS-bearing proteins or m3G-capped-spliceosomal U snRNPs. Injection of an excess of hnRNP A1 but not of a mutant form of the protein lacking the M9 domain, resulted in a specific inhibition of mRNA export, demonstrating that the M9 domain is recognized by a saturable component of the mRNA export machinery. The export of other cellular RNAs such as U snRNAs and tRNA was, in contrast, not affected. Further analysis of mutant hnRNP A1 proteins provides evidence that M9 recognition during mRNA export differs from its recognition during protein transport.
Materials and Methods
All enzymes used for DNA manipulations were purchased from New England Biolabs Inc. (Beverly, MA). T7 RNA polymerase and RNasin were from Stratagene (La Jolla, CA). AmpliTaq DNA polymerase was from Perkin-Elmer Cetus (Norwalk, CT). The cap analogue m7GpppG and the modified nucleotide γ-mGTP used to prime the synthesis of DHFR mRNA, U1ΔSm, U5ΔSm, and U6Δss, respectively, were a kind gift of E. Darzynkiewicz (Institute of Experimental Physiology, Warsaw, Poland). Labeled nucleoside triphosphates and [35S]methionine were from Amersham Corp. (Arlington Heights, IL). Sequences were determined using the dideoxynucleotide chain termination method (Sanger et al., 1977) and T7 DNA polymerase (Pharmacia LKB, Freiburg, Germany). Isolation of plasmid DNA, cloning, transformation of Escherichia coli, and gel analysis of recombinant plasmids were performed as described by Sambrook et al. (1989). Reverse transcription and PCR were performed as described (Frohman, 1990). Peptide synthesis and coupling to BSA was performed as described (Fischer et al., 1995). The sequence of the NLS peptide was as follows: CGGGPKKKRKVED and that of the NLSrev: CGGGDEVKRKKKP.
Recombinant Protein Expression and Plasmid DNAs
The recombinant IBB domain, amino acids 1–55 of Xenopus importin α, fused to the IgG binding z domain of protein A and the truncated importin β binding (IBB) fusion corresponding to amino acids 1–43 of Xenopus importin α were a kind gift of D. Görlich (Zentrum für Molekulare Biologie, Heidelberg, Germany). For expression in E. coli and in vitro translation, cDNAs encoding the full length human hnRNP A1, the hnRNP A1 M9mu (Gly 274 mutated to Ala), or amino acids 1–236 (hnRNP A1ΔM9) were introduced by PCR between the BamHI and HindIII sites of either the pRSETA or pRSETB vectors (Invitrogen Corp., San Diego, CA). The pRSETB constructs used in Fig. 7 C have an EcoRI site (Glu–Phe) inserted between amino acids 267 and 268 of the hnRNP A1 sequence. The nucleoplasmin core–M9 fusion construct used in Fig. 1 was obtained by cloning cDNAs encoding amino acids 1–149 of Xenopus nucleoplasmin and amino acids 255–320 of human hnRNP A1 (including the M9 domain) between the NheI–BamHI and BamHI–HindIII sites of pRSETA vector, respectively. For in vitro transcription the DNA was linearized with HindIII and transcribed with T7 RNA polymerase, and the resulting RNA was translated in vitro.
Figure 7.
The M9 domain is required to specifically saturate the mRNA export pathway. (A) Recombinant hnRNP A1 protein at 2 mg/ml was injected into oocyte cytoplasm. Control oocytes received a PBS injection. After 1 h incubation, the oocytes received a nuclear injection of the same mixture of radioactively labeled RNAs described in Fig. 6. Dissection was performed after 150 min in lanes 4–9 or immediately after injection in lanes 1–3. (B) Recombinant hnRNP A1 or hnRNP A1ΔM9 were injected into oocyte nuclei as indicated above the lanes. After 1 h incubation, a second microinjection was performed into the oocyte nuclei with the same mixture of the radioactively labeled RNAs described in Fig. 6. In lanes 4–18 RNA was extracted 150 min after injection; in lanes 1–3, RNA was extracted immediately after injection. The concentrations of the recombinant proteins in the injected samples were as indicated above the lanes. (C) Recombinant hnRNP A1 or hnRNP A1-M9mu (Gly 274 → Ala) was injected into oocyte nuclei 1 h before a mixture of radioactively labeled RNAs (Fig. 6). In lanes 1–3, RNA was extracted immediately after injection; in lanes 4–12 dissection and RNA extraction was 160 min after injection. The concentration of recombinant proteins in the injected samples was 3 mg/ml.
Figure 1.
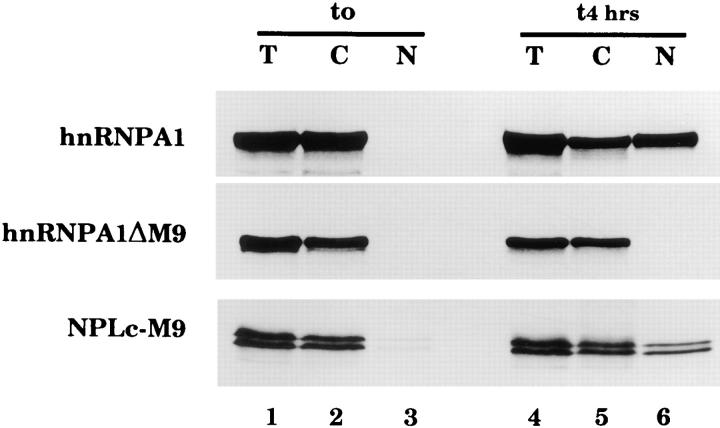
The hnRNP A1 M9 domain mediates nuclear import in Xenopus oocytes. Xenopus laevis oocytes were injected into the cytoplasm with the following in vitro translated 35S-labeled proteins: full length human hnRNP A1 (aa1-320), hnRNP A1ΔM9, a truncated form of A1 lacking the M9 domain (aa1-236), and NPLc-M9, a nucleoplasmin core–M9 fusion (A1 aa255-320), as indicated. In lanes 1–3, proteins were extracted immediately after injection and in lanes 4–6, 4 h after injection. T, C, and N indicate proteins extracted from total oocytes or after dissection from cytoplasmic or nuclear fractions, respectively. Proteins were analyzed by SDS-PAGE followed by fluorography.
HnRNP A1, hnRNP A1ΔM9, hnRNP A1 M9mu, and NPLc-M9 were expressed from the pRSET constructs described above and purified by their 6 X His tag on Ni-NTA agarose beads (Qiagen, Chatsworth, CA) according to the instructions of the manufacturer. Elution of recombinant proteins was performed in PBS buffer containing 0.5 M imidazole (Sigma Chemical Co., St. Louis, MO). Recombinant proteins were concentrated and desalted using microconcentrator devices (Pall Filtron Technology Corporation, Northborough, MA).
The hnRNP A1 construct used in Fig. 2 and the plasmids PK-M9 and PK-M9mu, have been described previously (Michael et al., 1995b ; Siomi and Dreyfuss, 1995). In these PK-M9 constructs, the M9 domain (amino acids 268–305 of hnRNP A1) was cloned at the KpnI site corresponding to codon 443 of chicken muscle pyruvate kinase and was functional, however we have observed that when amino acids 255–320 of hnRNP A1 were inserted at the COOH terminus of pyruvate kinase, the fusion was no longer functional. The plasmid encoding glutathione-S-transferase (GSTM9) was made by inserting the EcoRI–XhoI fragment of PK-M9 into the pGEX-5X-1 vector (Pharmacia Fine Chemicals, Piscataway, NJ). The GSTM9mu plasmid, which contains an amino acid substitution Gly 274 to Ala in the M9 domain (Michael et al., 1995b ), was constructed by ligation of the EcoRI–XhoI fragment of PK-M9mu (Gly to Ala) into pGEX-5X-1. All GST fusion proteins were expressed under standard conditions as described by the manufacturers (Pharmacia Fine Chemicals). All constructs obtained by PCR were fully sequenced.
Figure 2.
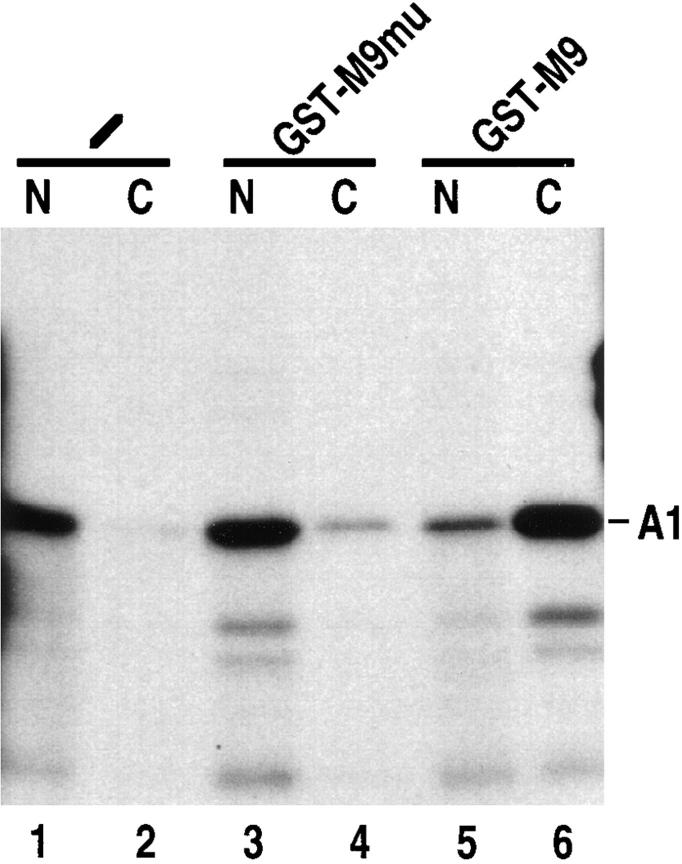
M9-mediated nuclear import is saturable. In vitro translated 35S-labeled hnRNP A1 (A1) was injected into Xenopus laevis oocyte cytoplasm either without competitor (lanes 1 and 2) or with bacterially expressed GST-M9 mutant fusion (A1 aa268-305, Gly 274 mutated to Ala; GST-M9mu; lanes 3 and 4) or GST-M9 fusion (A1 aa268-305; lanes 5 and 6). The concentration of recombinant proteins in the injected samples was 10 mg/ml, and the final concentration in the oocyte is 5–10% of this value. Transport was analyzed after 5 h incubation as described in Fig. 1.
In Vitro Transcription and Translation
For generation of 35S-labeled in vitro translated fusion proteins, the combined in vitro transcription/translation (TNT) kit from Promega Biotech. (Madison, WI) was used. In brief, 1 μg of plasmid DNA containing the gene to be transcribed and translated was incubated in a total volume of 50 μl containing 25 μl reticulocyte lysate, 2 μl TNT-polymerase, 2 μl reaction buffer, 2 μl amino acid mix lacking methionine, 40 μCi [35S]methionine (Amersham Corp.) and 1 μl (10 U) RNasin (Promega Biotech.). The reaction was carried out at 30°C for 2 h. Translation was checked by SDSPAGE and subsequent fluorography using intensify solutions from DuPont (Wilmington, DE). In vitro translated proteins were injected directly into oocytes without further purification.
3′ End Labeling of Gel-purified U snRNAs
1 μg of gel purified U snRNA was assayed for 3′ end labeling as described previously (Fischer et al., 1993) in a buffer containing 50 mM HepesKOH, pH 7.9, 18 mM MgCl2, 3 mM DTE, 10 μg/ml BSA, 1 U/μl T4 RNA ligase (GIBCO BRL, Gaithersburg, MD) and 60 μCi [32P]pCp (3,000 Ci/ mMol; Amersham Corp.). The assay mixture was incubated for 18 h at 4°C. The RNA was ethanol precipitated, resuspended in water, and used for injection.
Oocyte Injections
Oocyte injections and analysis of microinjected RNA by denaturing gel electrophoresis, autoradiography, or phosphoimager analysis were performed as described (Fischer et al., 1993; Jarmolowski et al., 1994). The concentration of the recombinant proteins in the injected samples is indicated in the figure legends. Microinjection of in vitro translated protein into oocytes, isolation of protein from oocytes, and SDS-PAGE analysis were all carried out as described (Kambach and Mattaj, 1992; Fischer et al., 1993). The mutant RNAs used (U1ΔSm, U5ΔSm, U6Δss) lack protein binding sites required for the nuclear import of these RNAs (Hamm et al., 1990; Jarmolowski and Mattaj, 1993) and thus remain in the cytoplasm after export from the nucleus.
Results
The M9 Domain of hnRNP A1 Serves as a Nuclear Import Signal in Oocytes
Nucleocytoplasmic transport of hnRNPs has so far only been examined in somatic cells. To test whether the M9 domain of hnRNP A1 is able to mediate nuclear import in oocytes, in vitro translated 35S-labeled, full length human hnRNP A1 protein or a truncated form (aa1–236) lacking the M9 domain (termed hnRNP A1ΔM9) was injected into the cytoplasm of Xenopus leavis oocytes. After 4 h, oocytes were dissected into nuclear and cytoplasmic fractions and analyzed by SDS-PAGE followed by fluorography. Approximately 50% of the injected hnRNP A1 was imported into the nucleus, while the A1ΔM9 mutant remained entirely in the cytoplasm (Fig. 1, lanes 4–6). The M9 domain is therefore essential for the nuclear uptake of A1.
Next we tested whether the M9 domain is also sufficient to target an otherwise cytoplasmic protein to the nucleus. For this purpose, hnRNP A1 amino acids 255–320, including the M9 domain, were fused to the NPLc. The NPLc is able to form multimers larger than the exclusion size limit for free diffusion through nuclear pore complexes. Thus, only in the presence of an appropriate signal can NPLc be transported across the nuclear envelope (Dingwall et al., 1982). The fusion protein (NPLc-M9) was produced by translation in vitro and injected into the oocyte cytoplasm. NPLc-M9 was transported to the nucleus with an efficiency similar to that of full length hnRNP A1 (Fig. 1, lanes 4–6). Similar results were obtained in experiments carried out with fusions of M9 (A1 aa268–305) to either the cytoplasmic protein pyruvate kinase (PK-M9; see Fig. 3) or to the bacterial protein GST-M9 (data not shown). Thus, M9 is sufficient to mediate nuclear import of a protein in both somatic cells and Xenopus oocytes.
Figure 3.
Saturation of the M9 import pathway does not interfere with NLS-mediated import. In vitro translated 35S-labeled PKNLS and PK-M9 (A1 aa268-305) were injected into the cytoplasm of Xenopus laevis oocytes either without competitor (lanes 1 and 2 and 7 and 8) or with bacterially expressed recombinant GST-M9 (lanes 5 and 6 and 11 and 12) or GST-M9mu (lanes 3 and 4 and 9 and 10). Lanes 13 and 14 show in vitro translated PK-NLS and PK-M9, respectively. The concentration of recombinant proteins in the injected samples was 10 mg/ml. Transport was analyzed 5 h after injection as described in Fig. 1.
To determine whether M9-mediated protein import is saturable and hence a receptor-mediated process, 35S- labeled, in vitro translated hnRNP A1 was injected into the cytoplasm of oocytes either without a competitor (Fig. 2, lanes 1 and 2) or along with either bacterially expressed GST-M9 (A1 aa268–305) fusion (Fig. 2, lanes 5 and 6) or GST fused to an inactive mutant of M9 (termed GSTM9mu in which Gly 274 has been mutated to Ala; Fig. 2, lanes 3 and 4). Transport was analyzed 5 h later. While GST-M9mu did not interfere with A1 import (Fig. 2, compare lanes 1 and 2 and 3 and 4), GST-M9 strongly inhibited the import of A1 (Fig. 2, lanes 5 and 6). The inhibition was dependent on the concentration of GST-M9 used and was overcome after prolonged incubation (data not shown), indicating that GST-M9 is a competitive inhibitor of hnRNP A1 import. Thus, M9 accesses a saturable and therefore receptor-mediated import pathway in oocytes.
M9 Does Not Use the Classical NLS-mediated Import Pathway
The saturability of the M9-mediated transport pathway allowed us to ask whether M9 accesses the two previously described import pathways used by either proteins containing a classical NLS (the importin pathway) or by spliceosomal m3G-capped U snRNAs, respectively. To address this issue we first tested the effect of a large excess of unlabeled GST-M9 on the import kinetics of pyruvate kinase fused to a classical bipartite basic NLS derived from the hnRNP protein K (PK-NLS; Michael et al., 1995b ). PKNLS was translated in vitro and injected into the oocyte cytoplasm either without competitor or with a large excess of GST-M9 or GST-M9mu (Fig. 3, lanes 1–6). Nuclear import was analyzed 5 h later. In a parallel experiment the effect of the competitor on the import of labeled PK-M9 was tested (Fig. 3, lanes 7–12). As shown in Fig. 3, GST-M9 efficiently blocked PK-M9 import, but the nuclear uptake of PK-NLS was not affected. These results suggest that the factor titrated by the excess of GST-M9 is not essential for NLS-mediated import but do not unequivocally exclude the possibility that the NLS is recognized by the same receptor as M9, but with a higher affinity. We therefore investigated whether M9-mediated import is affected by inhibitors of the NLS pathway. The inhibitors were coinjected into the oocyte cytoplasm along with two 35S-labeled proteins, the 80-kD subunit of the cap binding complex (CBP80), and hnRNP A1. CBP80 has a canonical bipartite NLS (Izaurralde et al., 1995b ) and therefore was used as an indicator of NLS-dependent import. hnRNP A1 contains the M9 domain and is imported by virtue of this signal. First, NLS peptides crosslinked to BSA (BSA–NLS), a well characterized competitive inhibitor of NLS-dependent import (Goldfarb et al., 1986), was tested. As expected, the import of CBP80 was specifically inhibited when BSA– NLS was injected along with the 35S-labeled proteins into the cytoplasm of oocytes. In contrast, the import of A1 was not affected (Fig. 4, lanes 16–18). BSA coupled to peptides whose sequence is the reverse of the NLS (BSANLSrev), failed to inhibit the import of CBP80 (Fig. 4, lanes 13–15).
Figure 4.
M9-mediated import is importin α independent. Xenopus laevis oocytes were injected into the cytoplasm with a mixture of labeled CBP80 and hnRNP A1 as indicated. In lanes 1–6, labeled proteins were diluted in PBS; in lanes 7–18 the labeled proteins were coinjected with the inhibitors indicated above the lanes. Bacterially expressed recombinant IBB (lanes 10–12) or truncated IBB (IBB trunc; lanes 7–9) were injected at a concentration of 20 mg/ml. The concentration of the BSA-NLS peptide conjugate (lanes 16–18) or of the BSA crosslinked to the reverse NLS peptide (BSA-NLSrev; lanes 13–15) was 20 mg/ml in the injection mixtures. Protein samples from total oocytes (T) or cytoplasmic (C) and nuclear (N) fractions were collected 5 h after injection in lanes 4–18 or immediately after injection in lanes 1–3. Proteins were analyzed as described in Fig. 1.
The NLS import receptor is a heterodimer of the importin α and β proteins. Importin α binds directly to the NLS and interacts with importin β via its NH2-terminal or IBB domain (Görlich et al., 1996a ; Weis et al., 1996). Polypeptides corresponding to the IBB domain served as efficient inhibitors of NLS-mediated import by preventing interaction between the subunits. Coinjection of IBB together with CBP80 and hnRNP A1 efficiently and specifically inhibited the import of CBP80 but not of A1 (Fig. 4, lanes 10–12). As a control, a truncated form of the IBB domain (IBB trunc) which is unable to interact with importin β, did not affect the nuclear accumulation of either CBP80 or A1 (Fig. 4, lanes 7–9). Taken together, these data show that M9 accesses an import pathway that is different from that utilized by proteins containing a classical, basic NLS, and is independent of importin α.
Saturation of M9-mediated Transport Does Not Interfere with the Import of Spliceosomal U snRNAs
We wished next to analyze whether M9 accesses a second import pathway that has been described, namely that of m3G-capped spliceosomal U snRNAs. The spliceosomal snRNAs U1, U2, U4, and U5 were 3′ end labeled and injected into the cytoplasm of oocytes either without competitor or with an excess of GST-M9mu or GST-M9. Import was analyzed 3 and 12 h later. The four U snRNAs were imported with approximately the same efficiency (Fig. 5, lanes 1–5), and their import kinetics were not affected by either GST-M9 or GST-M9mu (Fig. 5, lanes 6– 10 and 11–15, respectively). We conclude that the spliceosomal U snRNA import pathway and the M9-mediated import pathway require different limiting transport factors.
Figure 5.
Saturation of M9mediated import does not interfere with nuclear uptake of U snRNAs. 32pCp-labeled U1, U2, U4, and U5 snRNAs were injected into the cytoplasm of oocytes either without competitor (lanes 1–5) or together with 10 mg/ml of recombinant GST-M9mu (lanes 6–10) or GST-M9 (lanes 11–15). Oocytes were dissected 3 or 12 h after injection as indicated, and RNA was analyzed on denaturing polyacrylamide gels. Lanes 1, 6, and 11 were loaded with RNA extracted from total oocytes immediately after injection.
M9-mediated Transport Is Directly Linked to mRNA Export
Available data provide strong evidence for the existence of a specific, mRNA nuclear export pathway (see Introduction). If the transport pathway that is accessed by M9containing proteins is important for mRNA export one would predict that specific interference with hnRNP A1 transport should also interfere with mRNA export but not with the export of other RNAs. Conversely, inhibitors of the importin α/β pathway, as shown in Fig. 4, that do not interfere with M9-mediated import should not affect mRNA export.
It has previously been shown that inhibitors of importinmediated nuclear protein import inhibit U snRNA export (Görlich et al., 1996b ). To test whether they also affect mRNA export, the inhibitors described in Fig. 4 were preinjected into Xenopus oocyte cytoplasm. 1 h later the oocytes received a nuclear injection of a mixture of 32P-labeled DHFR mRNA, U1ΔSm, U5ΔSm, tRNA, and U6Δss. U6Δss serves as a control for nuclear injection, as this RNA is not exported to the cytoplasm, while the other RNAs are exported via specific pathways. After 150 min incubation the oocytes were dissected into nuclear and cytoplasmic fractions and the RNAs analyzed on denaturing polyacrylamide gels.
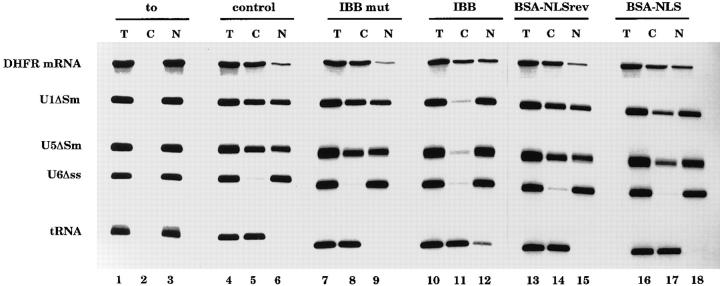
As expected, U snRNA export was inhibited by cytoplasmic injection of the IBB domain (Görlich et al., 1996b ). At the concentration at which the IBB domain completely abolishes U snRNA export (Fig. 6, lanes 10–12) and also NLS-mediated import (see Fig. 4), mRNA export is only slightly affected (Fig. 6, lanes 10–12). In this experiment, IBB injection also partially inhibited tRNA export. In other experiments, where lower amounts of IBB were injected, U snRNA export was inhibited while DHFR mRNA and tRNA export were not affected (Görlich et al., 1996b and data not shown). Saturating amounts of BSA-NLS peptide conjugate (Fig. 4) did not affect tRNA export but reduced U snRNA export by 55% and mRNA export by 20% (Fig. 6, lanes 16–18). In summary, the different sensitivities of mRNA and U snRNA export to the presence of the IBB domain or to BSA-NLS conjugate are consistent with previously published data that demonstrated the existence of a specific mRNA export pathway. The minor effect on mRNA export of inhibitors of the classical NLSmediated protein import pathway indicate that such proteins play only a minor kinetic role in mRNA export.
Figure 6.
Inhibitors of basic NLS import differentially affect U snRNA and mRNA export. Xenopus leavis oocytes were injected into the cytoplasm with the inhibitors indicated above the lanes. As a control, oocytes were injected with PBS alone (lanes 1–6). After 1 h incubation a second microinjection was performed into the oocyte nuclei with a mixture of the following radioactively labeled RNAs: DHFR mRNA, U1ΔSm, U5ΔSm, U6Δss, and human initiator methionyl tRNA. U6Δss does not leave the nucleus and is an internal control for nuclear integrity. Synthesis of DHFR, U1ΔSm, and U5ΔSm RNAs was primed with the m7GpppG cap dinucleotide, whereas synthesis of U6Δss RNA was primed with γ-mGTP. In lanes 4–18 RNA was extracted 150 min after injection; in lanes 1–3, RNA was extracted immediately after injection. The concentration of the inhibitors in the injected samples was as indicated in Fig. 4.
To investigate if proteins carrying an M9 domain play a role in mRNA export, we injected full length hnRNP A1 into the cytoplasm and analyzed its effect on RNA export. 1 h after injection of hnRNP A1 in the cytoplasm, a mixture of DHFR mRNA, U1ΔSm, U5ΔSm, U6Δss, and tRNA were injected to the nucleus, and export was analyzed 150 min later. hnRNP A1 injection specifically inhibited mRNA export (Fig. 7 A, compare lanes 4–6 with 7–9). In separate experiments we determined that cytoplasmic injection of A1ΔM9, which lacks the M9 domain but is fully functional in nonspecific RNA binding, has no effect on mRNA export (data not shown).
Analysis of M9-mediated protein import in the presence of the amount of hnRNP A1 used in Fig. 7 A revealed only a partial block (data not shown) suggesting that the inhibitory effect may be directly on mRNA export rather than an indirect consequence of inhibiting M9-mediated import. To examine this question further, increasing amounts of recombinant hnRNP A1 or, as control, A1ΔM9 were injected into oocyte nuclei 1 h before injection of the labeled RNAs. After an additional 150 min, RNA export was analyzed. In control oocytes that were preinjected with PBS, 90% of the DHFR mRNA and 70% of the two U snRNAs moved to the cytoplasm. tRNA export was complete (Fig. 7 B, lanes 16–18). Nuclear preinjection of A1ΔM9 did not interfere with the export of any of these RNAs (Fig. 7 B, lanes 7–9 and 13–15). However, preinjection of hnRNP A1 very efficiently inhibited nuclear export of mRNA (Fig. 7 B, lanes 4–6 and 10–12). The inhibition was specific for mRNA since neither tRNA nor U snRNA export was affected. The effect was not specific to DHFR mRNA, since export of other microinjected mRNAs was similarly inhibited by hnRNP A1 injection (data not shown). The comparison of the full length and truncated A1 proteins provides strong evidence that the M9 domain is required for interaction with the factor(s) saturated by hnRNP A1 injection. Surprisingly, however, we found that injection of either a GST–M9 fusion protein (A1 aa268-305) or a nucleoplasmin–M9 fusion protein (A1 aa255-320) into either the nucleus or cytoplasm did not result in inhibition of mRNA export, even at a concentration sufficient to completely block M9-mediated protein import (data not shown). This suggested that the factor(s) required for mRNA export that are saturated by hnRNP A1 injection recognize the A1 protein via a more extensive interaction than is conferred by the M9 domain alone.
To further investigate the recognition of hnRNP A1 by export factors, we first tested a second A1 truncation mutant (A1 aa1-267) that removed the M9 domain but no amino acids NH2-terminal to it. This protein, like hnRNP A1ΔM9, had no effect on mRNA export (data not shown), providing strong support for the hypothesis that M9 recognition is required but not sufficient to saturate mRNA export. These results suggest that hnRNP A1 recognition during mRNA export is not identical to that which occurs during protein import and export, for which the M9 signal is sufficient (Michael et al., 1995b ; Siomi et al., 1995). To provide additional evidence for this conclusion we tested the effect of the Gly 274 to Ala point mutation (hnRNP A1-M9mu), which abolishes the function of M9 in import (Siomi et al., 1995) and hnRNP A1 shuttling (Michael et al., 1995b ). Like hnRNP A1 itself (Fig. 7 C, compare lanes 4–6 with 7–9), microinjection of the hnRNP A1-M9mu protein into the nucleus reproducibly caused a strong, specific inhibition of mRNA export (Fig. 7 C, lanes 10–12).
Discussion
In this study we analyzed the hnRNP A1 transport pathway in oocytes. Consistent with previous results obtained in somatic cells, we found that the M9 domain of A1 is necessary and sufficient to mediate nuclear import. Moreover, it was possible to show that the M9-mediated import pathway is saturable and hence a receptor-mediated process. Competition studies allowed comparison of M9- mediated import with previously characterized import pathways. Interestingly, the limiting factors that are involved in the nuclear import of proteins with the well studied basic types of NLS appear to not be required for M9mediated import. In particular, we failed to saturate the import mediated by a bipartite (nucleoplasmin-type) NLS by coinjection of GST-M9 while under the same conditions M9-mediated import was completely blocked. Conversely, we did not observe any inhibition of the M9-mediated import pathway by a BSA-NLS conjugate under conditions where the conjugate saturates the basic NLSmediated import pathway. Finally, IBB, the importin β binding domain of importin α, which has recently been shown to block the interaction between importin α and β and therefore serves as a potent inhibitor of the classical NLS-mediated import pathway (Görlich et al., 1996a ; Weis et al., 1996) did not interfere with M9 import. These data were obtained in parallel with those of Pollard et al. (1996). By analysis of protein import into the nuclei of permeabilized mammalian cells in vitro these investigators showed that, unlike classical NLS-mediated import, M9mediated import does not require either importin α or β. They identified a protein, transportin, that interacts with the M9 domain and mediates import of M9-containing proteins into the nucleus (Pollard et al., 1996).
The import of spliceosomal m3G-capped U snRNPs has previously been shown not to be dependent on saturable factors required for the classical NLS-mediated import pathway (Fischer et al., 1991, 1993; Michaud and Goldfarb, 1991; van Zee et al., 1993). Injection of saturating amounts of a GST-M9 fusion protein failed to inhibit the import of a mixture of four U snRNAs, indicating that M9 does not interact with limiting factors required for U snRNP import. We conclude from the two sets of data on specific crosscompetition and on the basis of the data in Pollard et al. (1996) that the M9 domain represents a new class of NLS that accesses a novel import pathway. Given the fact that other hnRNP proteins are imported into the nucleus but appear to lack either a classical NLS or an M9type NLS it is to be expected that additional signal receptor systems for nuclear import may exist.
The M9 Domain and mRNA Export
Study of RNA export has led to the understanding that protein import into the nucleus and RNA export from the nucleus are intimately related processes. The first indication that this is the case came from the observation that proteins proposed to serve in RNA export are not permanently nuclear but rather shuttle continuously between the nucleus and cytoplasm. Examples are some hnRNP proteins (Piñol-Roma and Dreyfuss, 1991, 1992, 1993; Michael et al., 1996a) or the HIV-1 Rev protein (Kalland et al., 1994; Meyer and Malim, 1994). More recently, it was observed that inhibition of the basic NLS-mediated protein import pathway inhibits specifically the export of U snRNAs from the nucleus (Görlich et al., 1996b ). This inhibition is not a direct effect on export but rather appears to reflect depletion of the nuclear pool of an NLS-bearing protein required for U snRNA export. The data presented here show that inhibitors of basic NLS-mediated import have only a minor kinetic effect on mRNA export. This indicates that basic NLS-bearing proteins likely play only a minor role in mRNA export. Previous data have shown that the mRNA cap structure has a similar minor effect on the mRNA export rate (Jarmolowski et al., 1994), suggesting that the observed effect might be due to the nuclear cap-binding complex, which has a bipartite basic NLS (Izaurralde et al., 1994, 1995b; Kataoka et al., 1994) and accompanies the mRNA to the cytoplasm (Visa et al., 1996b ).
A much greater effect on mRNA export was obtained when hnRNP A1 protein was injected. Injection of full length hnRNP A1 into either the nucleus or the cytoplasm resulted in mRNA export inhibition, whereas injection into either location of hnRNP A1 truncation mutants lacking the M9 domain had no effect. This suggests that recognition of M9 is involved in the interaction between hnRNP A1 and the mRNA export mediator which is saturated by A1 injection. However, since an M9-containing fusion protein was unable to inhibit mRNA export, even at a concentration which blocked M9-mediated protein import, M9 recognition alone is insufficient to explain the effect of hnRNP A1 on mRNA export. Furthermore, a point mutation in the M9 domain (Gly 274 to Ala) that is sufficient to inactivate the M9 signal when either protein import or protein shuttling (nuclear export followed by reimport) was measured (Michael et al., 1995b ; Siomi et al., 1995) did not prevent saturation of the mRNA export pathway. These data, in sum, suggest that the recognition of hnRNP A1 during mRNA export is not identical to its recognition during protein transport.
The nuclear import of hnRNP A1 is mediated by transportin (Pollard et al., 1996). Our data show that recognition of the M9 domain is required but not sufficient to saturate the mRNA export pathway. We can suggest two possible model explanations for this. The first invokes a factor required for mRNA export distinct from transportin that would bind cooperatively to hnRNP A1 with transportin and stabilize its binding such that the Gly to Ala mutation is insufficient to prevent recognition. The second would propose that an mRNA export factor distinct from transportin would recognize hnRNP A1 in a way that requires the M9 domain but also additional amino acids lying NH2-terminal to the M9 domain. Since M9 is sufficient to direct protein export from the nucleus (Michael et al., 1995b ) the first model seems more plausible, but further study is required to distinguish the possibilities.
HnRNP A1 injection specifically inhibited mRNA export and had no effect on either U snRNA or tRNA export, while inhibitors of the importin α/β pathway specifically block U snRNA export (Görlich et al., 1996b and this investigation). These results confirm and extend the conclusion reached by microinjection of different RNAs (Jarmolowski et al., 1994) that different specific factors mediate the export of different classes of RNAs from the nucleus. The results from Jarmolowski et al. (1994) could have been explained by the existence of factors that had different RNA binding specificity but interacted with a common export mediator. However, the observation that conjugates of Rev NES peptides and BSA specifically inhibited U snRNA export without affecting mRNA export (Fischer et al., 1995) together with results reported by Görlich et al. (1996b) and this investigation make it obvious that the differences in the factors involved in RNA class-specific export are more extensive. Our present knowledge allows us to distinguish at least two independent transport pathways: one defined by importin α/β that is somehow linked to U snRNA and Rev NES-mediated export and a second defined by the M9 domain, which is linked to mRNA export.
Is Npl3p/Nop3p the Functional Homologue of hnRNP A1?
It is of interest to compare transport behavior of the hnRNP A1 protein and its effect on mRNA export with the data on the yeast Npl3p/Nop3p protein (Flach et al., 1994; Wilson, et al., 1994; Russell and Tollervey, 1995; Singleton et al., 1995). This protein is related in sequence to hnRNP A1, and mutation of the protein affects the export of polyadenylated (m)RNA from the nucleus of yeast cells (Russell and Tollervey, 1995; Singleton et al., 1995). Mutation of Npl3p/Nop3p also affects protein import into the yeast nucleus (Bossie et al., 1992). However there are critical differences between the two proteins. The first is that mutations in Npl3p/Nop3p affect the import of classical NLSbearing proteins in yeast (Bossie et al., 1992) whereas we show here that the M9 domain saturates an import pathway that is apparently distinct from that used by proteins with a classical basic NLS. Secondly, the export of Npl3p/ Nop3p from the nucleus requires continuous transcription of RNA, consistent with the idea that Npl3p/Nop3p leaves the nucleus only in association with RNA (Lee et al., 1996). hnRNP A1, on the other hand, leaves the nucleus independent of ongoing transcription, but surprisingly, its nuclear import is transcription dependent (Piñol-Roma and Dreyfuss, 1991, 1992, 1993). These data suggest that although the hnRNP A1 and Npl3p/Nop3p proteins may well have analogous functions in vertebrates and yeast, respectively, the detailed mechanism by which they act and through which they exercise their functions are likely to exhibit considerable differences.
In conclusion, we provide evidence for the existence of a novel saturable factor involved in the nuclear import of a specific subclass of nuclear proteins, namely hnRNP A1 and other proteins carrying the M9 domain. Moreover, our data suggest a direct link between recognition of M9 in the context of the hnRNP A1 protein and mRNA export, consistent with previous suggestions that hnRNP A1 may interact with factors required for the export of mRNA from the nucleus.
Acknowledgments
We wish to thank Drs. D. Görlich, R. Lührmann, and E. Darzynkiewicz for the kind gift of recombinant IBB fusion proteins, isolated U snRNAs, and cap analogues, respectively; P. Fortes, I. Palacios, and M. Ohno for comments on the manuscript; the European Molecular Biology Laboratory peptide synthesis service for providing the NLS and NLS-rev peptides and the photolab for help preparing the manuscript.
Footnotes
1.Abbreviations used in this paper: CBC, cap binding complex; hn, heterogenous nuclear; IBB, importin β binding; m3G, methyl2,2,7guanosine; NES, nuclear export signal; NLS, nuclear localization signal; NPLc, nucleoplasmin core domain; U sn, uracil rich small nuclear.
E. Izaurralde and U. Fischer were recipients of fellowships from the Human Frontier Science Program Organization and the Deutsches Krebsforschungszentrum (AIDS-Stipendienprogramm), respectively. A. Jarmolowski was supported by the Polish Research Committee (grant KBN 6P04A03511). G. Dreyfuss is an investigator of the Howard Hughes Medical Institute.
Please address all correspondence to Iain Mattaj, European Molecular Biology Laboratory, D-69117 Heidelberg, Germany. Tel.: 49-6221-387-393; Fax: 49-6221-387-518; E-mail: Mattaj@EMBL-Heidelberg.DE
Elisa Izaurralde's present address is University of Geneva, Department of Molecular Biology, 30 Quai Ernest-Ansermet, CH-1211 Geneva 4, Switzerland.
Artur Jarmolowski's present address is Institute of Molecular Biology and Biotechnology, Department of Biopolymer Biochemistry, 60371 Poznan, Poland.
References
- Bataillé N, Helser T, Fried HM. Cytoplasmic transport of ribosomal subunits microinjected into the Xenopus laevisoocyte nucleus: a generalized, facilitated process. J Cell Biol. 1990;111:1571–1582. doi: 10.1083/jcb.111.4.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogerd HP, Fridell RA, Benson RE, Hua J, Cullen BR. Protein sequence requirements for function of the human T-cell leukemia virus type I Rex nuclear export signal delineated by a novel in vivorandomization– selection assay. Mol Cell Biol. 1996;16:4207–4214. doi: 10.1128/mcb.16.8.4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossie MA, DeHoratius C, Barcelo G, Silver PA. A mutant nuclear protein with similarity to RNA-binding proteins interferes with nuclear import in yeast. Mol Biol Cell. 1992;3:875–893. doi: 10.1091/mbc.3.8.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingwall C, Laskey RA. Nuclear targeting sequences: a consensus? . Trends Biochem Sci. 1991;16:478–481. doi: 10.1016/0968-0004(91)90184-w. [DOI] [PubMed] [Google Scholar]
- Dingwall C, Sharnick S, Laskey RA. A polypeptide domain that specifies migration of nucleoplasmin into the nucleus. Cell. 1982;30:449–458. doi: 10.1016/0092-8674(82)90242-2. [DOI] [PubMed] [Google Scholar]
- Dreyfuss G, Matunis MJ, Piñol-Roma S, Burd CG. hnRNP proteins and the biogenesis of mRNA. Annu Rev Biochem. 1993;62:289–321. doi: 10.1146/annurev.bi.62.070193.001445. [DOI] [PubMed] [Google Scholar]
- Fischer U, Lührmann R. An essential signaling role for the m3G cap in the transport of U1 snRNP to the nucleus. Science (Wash DC) 1990;249:786–790. doi: 10.1126/science.2143847. [DOI] [PubMed] [Google Scholar]
- Fischer U, Darzynkiewicz E, Tahara SM, Dathan NA, Luhrmann R, Mattaj IW. Diversity in signals required for nuclear accumulation of U snRNPs and variety in the pathways of nuclear transport. J Cell Biol. 1991;113:705–714. doi: 10.1083/jcb.113.4.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer U, Sumpter V, Sekine M, Satoh T, Luhrmann R. Nucleocytoplasmic transport of U snRNPs: definition of a nuclear location signal in the Sm core domain that binds a transport receptor independently of the m3G-cap. EMBO (Eur Mol Biol Organ) J. 1993;12:573–583. doi: 10.1002/j.1460-2075.1993.tb05689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer U, Meyer S, Teufel M, Heckel C, Lührmann R, Rautmann G. Evidence that HIV-1 Rev directly promotes the nuclear export of unspliced RNA. EMBO (Eur Mol Biol Organ) J. 1994;13:4105–4112. doi: 10.1002/j.1460-2075.1994.tb06728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer U, Huber J, Boelens WC, Mattaj IW, Lührmann R. The HIV-1 Rev activation domain is a nuclear export signal that accesses an export pathway used by specific cellular RNAs. Cell. 1995;82:475–483. doi: 10.1016/0092-8674(95)90436-0. [DOI] [PubMed] [Google Scholar]
- Flach J, Bossie M, Vogel J, Corbett A, Jinks T, Willins DA, Silver PA. A yeast RNA-binding protein shuttles between nucleus and cytoplasm. Mol Cell Biol. 1994;14:8399–8407. doi: 10.1128/mcb.14.12.8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohman, M.A. 1990. In PCR Protocols. A Guide to Methods and Applications. M.A. Innis, D.H. Gelfand, J.J. Sninsky, and T.J. White, editors. Academic Press, Inc., San Diego, CA. 28–39.
- Gerace L. Nuclear export signals and the fast track to the cytoplasm. Cell. 1995;82:341–344. doi: 10.1016/0092-8674(95)90420-4. [DOI] [PubMed] [Google Scholar]
- Goldfarb DS, Gariepy J, Schoolnik G, Kornberg RD. Synthetic peptides as nuclear localization signals. Nature (Lond) 1986;322:641–644. doi: 10.1038/322641a0. [DOI] [PubMed] [Google Scholar]
- Görlich D, Mattaj IW. Nucleo-cytoplasmic transport. Science (Wash DC) 1996;271:1513–1518. doi: 10.1126/science.271.5255.1513. [DOI] [PubMed] [Google Scholar]
- Görlich D, Henklein P, Laskey RA, Hartmann E. A 41 amino acid motif in importin alpha confers binding to importin beta and hence transit into the nucleus. EMBO (Eur Mol Biol Organ) J. 1996a;15:1810–1817. [PMC free article] [PubMed] [Google Scholar]
- Görlich D, Kraft R, Kostka S, Vogel F, Hartmann E, Laskey RA, Mattaj IW, Izaurralde E. Importin provides a link between nuclear protein import and U snRNA export. Cell. 1996b;87:21–32. doi: 10.1016/s0092-8674(00)81319-7. [DOI] [PubMed] [Google Scholar]
- Hamm J, Darzynkiewicz E, Tahara SM, Mattaj IW. The trimethylguanosine cap structure of U1 snRNA is a component of a bipartite nuclear targeting signal. Cell. 1990;62:569–577. doi: 10.1016/0092-8674(90)90021-6. [DOI] [PubMed] [Google Scholar]
- Izaurralde E, Mattaj IW. RNA export. Cell. 1995;81:153–159. doi: 10.1016/0092-8674(95)90323-2. [DOI] [PubMed] [Google Scholar]
- Izaurralde E, Lewis J, McGuigan C, Jankowska M, Darzynkiewicz E, Mattaj IW. A nuclear cap binding protein complex involved in premRNA splicing. Cell. 1994;78:657–668. doi: 10.1016/0092-8674(94)90530-4. [DOI] [PubMed] [Google Scholar]
- Izaurralde E, Lewis J, Gamberi C, Jarmolowski A, McGuigan C, Mattaj IW. A cap-binding protein complex mediating U snRNA export. Nature (Lond) 1995a;376:709–712. doi: 10.1038/376709a0. [DOI] [PubMed] [Google Scholar]
- Izaurralde, E., C. McGuigan, and I.W. Mattaj. 1995b. Nuclear localization of a Cap-binding Protein Complex. Cold Spring Harbor Symp. Quant. Biol. LX: 669–675. [DOI] [PubMed]
- Jarmolowski A, Mattaj IW. The determinants for Sm protein binding to XenopusU1 and U5 snRNAs are complex and nonidentical. EMBO (Eur Mol Biol Organ) J. 1993;12:223–232. doi: 10.1002/j.1460-2075.1993.tb05648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarmolowski A, Boelens WC, Izaurralde E, Mattaj IW. Nuclear export of different classes of RNA is mediated by specific factors. J Cell Biol. 1994;124:627–635. doi: 10.1083/jcb.124.5.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalland K-H, Szilvay AM, Brokstad KA, Saetrevik W, Haukenes G. The human immunodeficiency virus type 1 Rev protein shuttles between the cytoplasm and nuclear compartments. Mol Cell Biol. 1994;14:7436–7444. doi: 10.1128/mcb.14.11.7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambach C, Mattaj IW. Intracellular distribution of the U1A protein depends on active transport and nuclear binding to U1 snRNA. J Cell Biol. 1992;118:11–21. doi: 10.1083/jcb.118.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka N, Ohno M, Kangawa K, Tokoro Y, Shimura Y. Cloning of a complementary DNA encoding an 80 kilodalton nuclear cap binding protein. Nucleic Acids Res. 1994;22:3861–3865. doi: 10.1093/nar/22.19.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MS, Henry M, Silver PA. A protein that shuttles between the nucleus and the cytoplasm is an important mediator of RNA export. Genes Dev. 1996;10:1233–1246. doi: 10.1101/gad.10.10.1233. [DOI] [PubMed] [Google Scholar]
- Melchior F, Gerace L. Mechanisms of nuclear protein import. Curr Opin Cell Biol. 1995;7:310–318. doi: 10.1016/0955-0674(95)80084-0. [DOI] [PubMed] [Google Scholar]
- Melchior F, Paschal B, Evans J, Gerace L. Inhibition of nuclear protein import by nonhydrolyzable analogs of GTP and identification of the small GTPase Ran/TC4 as an essential transport factor. J Cell Biol. 1993;123:1649–1659. doi: 10.1083/jcb.123.6.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer B, Malim MH. The HIV-1 rev trans-activator shuttles between the nucleus and the cytoplasm. Genes Dev. 1994;8:1538–1547. doi: 10.1101/gad.8.13.1538. [DOI] [PubMed] [Google Scholar]
- Michael, W.M., H. Siomi, M. Choi, S. Piñol-Roma, S. Nakielny, Q. Liu, and G. Dreyfuss. 1995a. Signal sequences that target nuclear import and nuclear export of pre-mRNA binding proteins. Cold Spring Harbor Symp. Quant. Biol. LX: 663–668. [DOI] [PubMed]
- Michael WM, Choi M, Dreyfuss G. A nuclear export signal in hnRNP A1: a signal-mediated, temperature-dependent nuclear protein export pathway. Cell. 1995b;83:415–422. doi: 10.1016/0092-8674(95)90119-1. [DOI] [PubMed] [Google Scholar]
- Michaud N, Goldfarb D. Multiple pathways in nuclear transport: the import of U2 snRNP occurs by a novel kinetic pathway. J Cell Biol. 1991;112:215–223. doi: 10.1083/jcb.112.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud N, Goldfarb D. Microinjected U snRNAs are imported to oocyte nuclei via the nuclear pore complex by three distinguishable targeting pathways. J Cell Biol. 1992;116:851–861. doi: 10.1083/jcb.116.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MS, Blobel G. The GTP-binding protein Ran/TC4 is required for protein import into the nucleus. Nature (Lond) 1993;365:661–663. doi: 10.1038/365661a0. [DOI] [PubMed] [Google Scholar]
- Moore MS, Blobel G. Purification of a Ran-interacting protein that is required for protein import into the nucleus. Proc Natl Acad Sci USA. 1994;91:10212–10216. doi: 10.1073/pnas.91.21.10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newmeyer DD, Forbes DJ. Nuclear import can be separated into distinct steps in vitro: nuclear pore binding and translocation. Cell. 1988;52:641–653. doi: 10.1016/0092-8674(88)90402-3. [DOI] [PubMed] [Google Scholar]
- Paschal B, Gerace L. Identification of NTF2, a cytosolic factor for nuclear import that interacts with nuclear pore protein p62. J Cell Biol. 1995;129:925–937. doi: 10.1083/jcb.129.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piñol-Roma S, Dreyfuss G. Transcription-dependent and transcription-independent nuclear transport of hnRNP proteins. Science (Wash DC) 1991;253:312–314. doi: 10.1126/science.1857966. [DOI] [PubMed] [Google Scholar]
- Piñol-Roma S, Dreyfuss G. Shuttling of pre-mRNA binding proteins between nucleus and cytoplasm. Nature (Lond) 1992;355:730–732. doi: 10.1038/355730a0. [DOI] [PubMed] [Google Scholar]
- Piñol-Roma S, Dreyfuss G. hnRNP proteins: localization and transport between the nucleus and the cytoplasm. Trends Cell Biol. 1993;3:151–155. doi: 10.1016/0962-8924(93)90135-n. [DOI] [PubMed] [Google Scholar]
- Pollard VW, Michael WM, Nakielny S, Siomi MC, Wang F, Dreyfuss G. A novel receptor-mediated nuclear protein import pathway. Cell. 1996;86:985–994. doi: 10.1016/s0092-8674(00)80173-7. [DOI] [PubMed] [Google Scholar]
- Powers MA, Forbes DJ. Cytosolic factors in nuclear transport: what's importin? . Cell. 1994;79:931–934. doi: 10.1016/0092-8674(94)90024-8. [DOI] [PubMed] [Google Scholar]
- Richardson WD, Mills AD, Dilwoth SM, Laskey RA, Dingwall C. Nuclear protein migration involves two steps: rapid binding at the nuclear envelope followed by slower translocation through the nuclear pores. Cell. 1988;52:655–664. doi: 10.1016/0092-8674(88)90403-5. [DOI] [PubMed] [Google Scholar]
- Russell I, Tollervey D. Yeast Nop3p has structural and functional similarities to mammalian pre-mRNA binding proteins. Eur J Cell Biol. 1995;66:293–301. [PubMed] [Google Scholar]
- Sambrook, J., E. Fritsch, and T. Maniatis. 1989. Molecular Cloning: A Laboratory Manual. Second ed. Cold Spring Harbor Laboratory. Cold Spring Harbor, NY.
- Sanger F, Nicklen S, Coulson AR. DNA sequencing with chainterminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton DR, Shaoping C, Hitomi C, Kumagai C, Tartakoff A. A yeast protein that bidirectionally affects nucleocytoplasmic transport. J Cell Sci. 1995;108:265–272. doi: 10.1242/jcs.108.1.265. [DOI] [PubMed] [Google Scholar]
- Siomi H, Dreyfuss G. A nuclear localization domain in the hnRNP A1 protein. J Cell Biol. 1995;129:551–560. doi: 10.1083/jcb.129.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zee K, Dickmanns A, Fischer U, Luhrmann R, Fanning E. A cytoplasmically anchored nuclear protein interferes specifically with the import of nuclear proteins but not U1 snRNA. J Cell Biol. 1993;121:229–240. doi: 10.1083/jcb.121.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visa N, Alzhanove-Ericsson AT, Sun X, Kiseleva E, Bjorkroth B, Wurtz T, Daneholt B. A pre-mRNA-binding protein accompanies the RNA from the gene through the nuclear pores and into polysomes. Cell. 1996a;84:253–264. doi: 10.1016/s0092-8674(00)80980-0. [DOI] [PubMed] [Google Scholar]
- Visa N, Izaurralde E, Ferreira J, Daneholt B, Mattaj IW. A nuclear cap-binding complex binds balbiani ring pre-mRNA co-transcriptionally and accompanies the ribonucleoprotein particle during nuclear export. J Cell Biol. 1996b;133:4–14. doi: 10.1083/jcb.133.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weighardt F, Biamonti G, Riva S. Nucleo-cytoplamic distribution of human hnRNP proteins: search for the targeting domains in hnRNP A1. J Cell Sci. 1995;108:545–555. doi: 10.1242/jcs.108.2.545. [DOI] [PubMed] [Google Scholar]
- Weis K, Ryder U, Lamond AI. The conserved amino terminal domain of hSRP1a is essential for nuclear protein import. EMBO (Eur Mol Biol Organ) J. 1996;15:1818–1825. [PMC free article] [PubMed] [Google Scholar]
- Wen W, Meinkoth JL, Tsien RY, Taylor SS. Identification of a signal for rapid export of proteins from the nucleus. Cell. 1995;82:463–473. doi: 10.1016/0092-8674(95)90435-2. [DOI] [PubMed] [Google Scholar]
- Wilson S M, Datar KV, Paddy MR, Swedlow JR, Swanson M. Characterization of nuclear polyadenylated RNA-binding proteins in Saccharomyces cerevisiae. J Cell Biol. 1994;127:1173–1184. doi: 10.1083/jcb.127.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff M. tRNA transport from the nucleus in a eukaryotic cell: carriermediated translocation process. Proc Natl Acad Sci USA. 1983;80:6436–6440. doi: 10.1073/pnas.80.21.6436. [DOI] [PMC free article] [PubMed] [Google Scholar]