Abstract
Agrin is a heparan sulfate proteoglycan that is required for the formation and maintenance of neuromuscular junctions. During development, agrin is secreted from motor neurons to trigger the local aggregation of acetylcholine receptors (AChRs) and other proteins in the muscle fiber, which together compose the postsynaptic apparatus. After release from the motor neuron, agrin binds to the developing muscle basal lamina and remains associated with the synaptic portion throughout adulthood. We have recently shown that full-length chick agrin binds to a basement membrane-like preparation called Matrigel™. The first 130 amino acids from the NH2 terminus are necessary for the binding, and they are the reason why, on cultured chick myotubes, AChR clusters induced by full-length agrin are small. In the current report we show that an NH2-terminal fragment of agrin containing these 130 amino acids is sufficient to bind to Matrigel™ and that the binding to this preparation is mediated by laminin-1. The fragment also binds to laminin-2 and -4, the predominant laminin isoforms of the muscle fiber basal lamina. On cultured myotubes, it colocalizes with laminin and is enriched in AChR aggregates. In addition, we show that the effect of full-length agrin on the size of AChR clusters is reversed in the presence of the NH2-terminal agrin fragment. These data strongly suggest that binding of agrin to laminin provides the basis of its localization to synaptic basal lamina and other basement membranes.
Efficient synaptic transmission requires a high local specialization of pre- and postsynaptic cells. At the neuromuscular junction (NMJ),1 these specializations include aggregates of acetylcholine receptors (AChRs) and acetylcholinesterase (AChE) in the muscle cell membrane and accumulated vesicles containing the neurotransmitter acetylcholine in the nerve terminal (for review see Hall and Sanes, 1993). Differentiation of both pre- and postsynaptic cells is directed by molecules that are localized to the synaptic portion of the muscle cell basal lamina (Sanes et al., 1978; Burden et al., 1979). The capability of the synaptic basal lamina to control and maintain synaptic differentiation makes it distinct from the extrasynaptic basal lamina.
Collagen type IV and laminin are the major components of cell basement membranes. Both molecules assemble from three separate chains. Individual chains are encoded by a family of homologous genes giving rise to collagen type IV and laminin isoforms that differ in their chain composition (Timpl, 1996). The collagen type IV molecules and the laminins are thought to form independent networks by self assembly that are linked by entactin/nidogen (Yurchenco and O'Rear, 1994). This scaffold builds the framework with which several other proteins like perlecan, fibulin-1, and fibulin-2 associate (for review see Timpl and Brown, 1996). While perlecan and nidogen are found both at synaptic and extrasynaptic sites of the muscle basal lamina, specific isoforms of collagen type IV and laminin are localized to the NMJ (Sanes, 1995). Other molecules that are concentrated at the NMJ include the neuregulins, AChE, and agrin, some of which have been shown to regulate different aspects of synapse formation (for review see Ruegg, 1996).
Among the best characterized molecules associated with synaptic basal lamina is agrin, a heparan sulfate proteoglycan (HSPG) with an apparent molecular mass on SDSPAGE of 400–600 kD (Denzer et al., 1995; Tsen et al., 1995a ). When added to cultured muscle cells, agrin induces the aggregation of AChRs and several other proteins that are also enriched at the NMJ in vivo (Nitkin et al., 1987). Several lines of evidence have led to the hypothesis that agrin is released from the nerve terminal of motor axons and causes the local accumulation of AChRs and other postsynaptic proteins in muscle fibers (McMahan, 1990). Consistent with this hypothesis, agrin-deficient mutant mice lack AChR clusters, and no functional NMJs are formed (Gautam et al., 1996). In chick agrin, three sites of alternative splicing have been described. One site is located near the NH2 terminus (Denzer et al., 1995; Tsen et al., 1995b ), and two sites, called A and B (y and z in rodents), are located in the COOH-terminal half of the protein. Splicing at site B strongly influences agrin's AChR-aggregating activity. Agrin isoforms with amino acid inserts at the B-site are highly active in inducing myotubes to aggregate AChRs, while agrin isoforms lacking inserts at this site are only marginally or not at all active (Ruegg et al., 1992; Ferns et al., 1993; Gesemann et al., 1995). Transcripts encoding the individual splice variants are differentially distributed. While AChR-aggregating isoforms are selectively expressed by neuronal cells, isoforms lacking inserts at site B are predominantly expressed by nonneuronal cells (for review see McMahan et al., 1992; Bowe and Fallon, 1995).
The local immobilization of motor neuron–derived agrin in the developing muscle basal lamina is thought to be important to maintain postsynaptic structures throughout adulthood (McMahan, 1990). This becomes evident as AChR-aggregating activity and agrin-like immunoreactivity remain localized to synaptic basal lamina for several weeks after degeneration of the cells at the NMJ (Burden et al., 1979; Reist et al., 1987). In addition to the NMJ, agrin-like immunoreactivity is also detected in basement membranes of other nonneuronal tissues, such as blood capillaries, kidney, and lung (Reist et al., 1987; Godfrey et al., 1988a ; Magill-Solc and McMahan, 1988; Rupp et al., 1991). Hence, agrin must strongly bind to components of synaptic basal lamina and other basement membranes.
In a first attempt to characterize the binding of agrin to extracellular matrix (ECM), we have recently shown that full-length chick agrin binds selectively to Matrigel™ (Kleinman et al., 1982), a solubilized basement membrane extracted from the Engelbreth-Holm-Swarm mouse sarcoma. This binding required a 130–amino acid–long region at the NH2-terminal end of agrin (Denzer et al., 1995). AChR clusters on cultured myotubes were also affected by this region as clusters induced by full-length agrin were more numerous but considerably smaller than those induced by any fragment of agrin without this NH2-terminal region (Denzer et al., 1995). We now show that a fragment comprising this region of agrin is sufficient to bind to Matrigel™ and that the binding to this basement membrane preparation is mediated by laminin-1. The fragment also binds to laminin-2 and -4, the laminin isoforms expressed by muscle fibers, and it is concentrated in AChR clusters on cultured chick myotubes. Moreover, an excess of this fragment is sufficient to reverse the effect of full-length chick agrin on the size of AChR clusters. Searches on databases revealed that the NH2-terminal region is highly conserved in mouse and human agrin. Based on these data, the 130 amino acids from the NH2 terminus of agrin define a laminin-binding domain.
Materials and Methods
Cell Culture, Transfection, Protein Labeling, Immunoprecipitation, and Immunoblot
Culturing of primary chick myotubes and transfections of COS-7 (Gluzman, 1981) or HEK 293 cells (Graham et al., 1977) were carried out as described by Gesemann et al. (1995). Iodination of purified protein was performed as described (Gesemann et al., 1996). 35S labeling, immunoprecipitation, and immunoblots were essentially done as described (Denzer et al., 1995). Recombinant cAgrin7 and cΔN15Agrin (see Fig. 1 a) in the supernatant of transiently transfected COS cells were immunoprecipitated with the antiserum raised against cΔN15Agrin (Denzer et al., 1995), whereas cN257Fc and r21Fc (see Fig. 1 a) were directly bound to protein A–Sepharose (Pharmacia, LKB Biotechnology Inc., Piscataway, NJ). 35Slabeled proteins were analyzed by SDS-PAGE on a 3–12% gradient gel followed by fluorography. For immunoblots, 3 μg of purified cAgrin7 or agrin purified from vitreous fluid (VF agrin) and 5 μg of total chick heart laminins (Brandenberger and Chiquet, 1995) was separated by SDSPAGE on a 3–12% gradient gel, transferred to nitrocellulose membrane, and analyzed as described in Denzer et al. (1995).
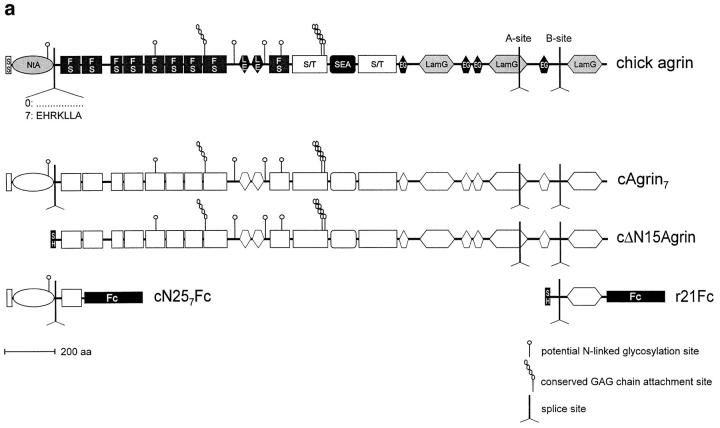
Figure 1.
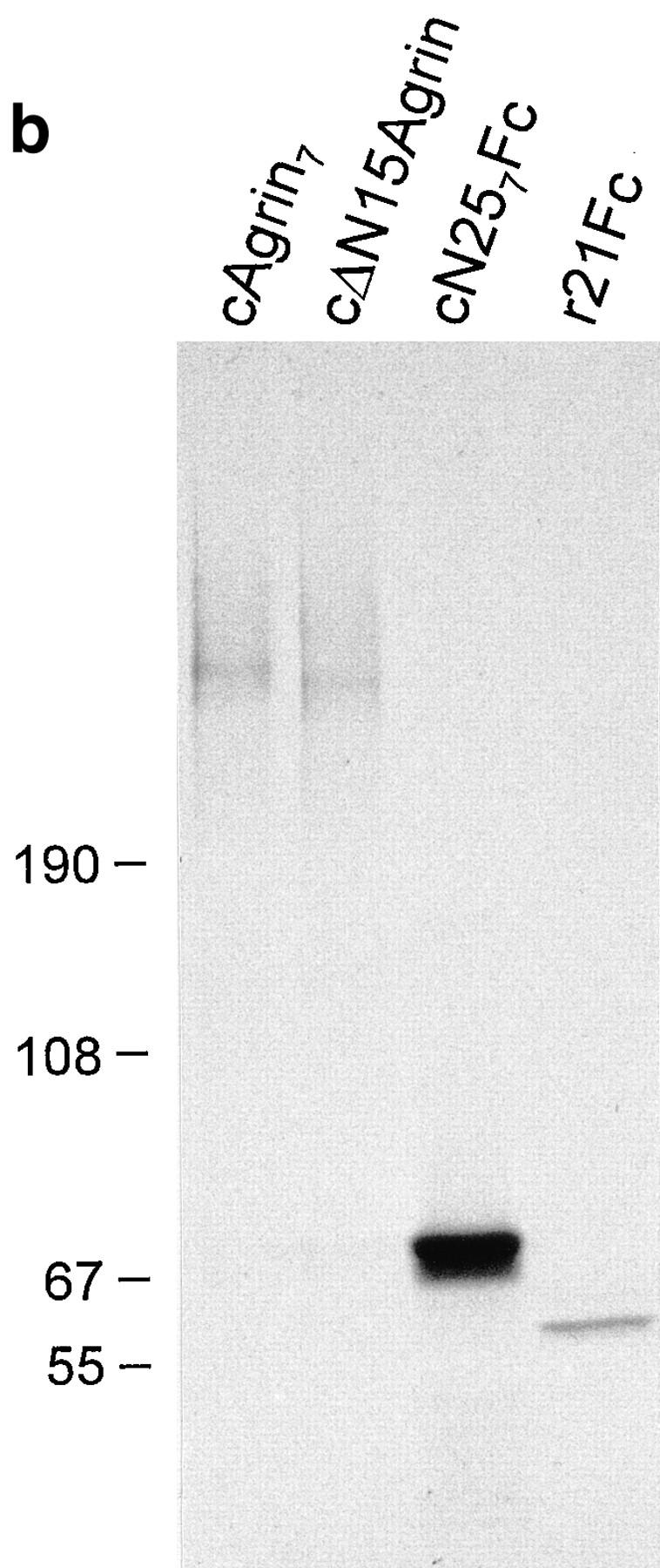
Structure and biochemical analysis of agrin constructs. (a) Structural organization of chick agrin and of fragments used in this study. Symbols and designations of individual domains are according to Bork and Bairoch (1995. Trends Biochem. Sci. 20): FS, follistatin-like module; EG, EGF-like module; LE, laminin EGF-like module; SEA, module first found in sea urchin sperm protein, enterokinase and agrin; LamG, laminin G–like module. Furthermore, the fragment of the constant region of mouse immunoglobulin gamma heavy chain (Fc), the chick agrin signal sequence (SS), the hemagglutinin signal sequence (SH), the serine/threonine rich regions (S/T), potential N-linked glycosylation sites, conserved GAG side chain attachment sites, and the sites of alternative mRNA splicing are indicated. The NH2-terminal region of agrin characterized in this study is named NtA domain. Notes: (1) Inserts at splice sites A and B are not specified. They are mentioned in the text if relevant for the experiment; and (2) the construct cΔN15Agrin was previously called cFull (Denzer et al., 1995; Gesemann et al., 1995) and covers the coding region of chick agrin as described by Tsim et al. (1992). (b) Autoradiogram of the 35S-labeled agrin fragments depicted in a after precipitation from conditioned medium of transiently transfected COS cells. cAgrin7 and cΔN15Agrin were immunoprecipitated with anti-cΔN15Agrin antibodies (Denzer et al., 1995). cN257Fc and r21Fc were precipitated with protein A–Sepharose. Proteins were separated by 3–12% SDS-PAGE. The two protein bands of cN257Fc are probably due to inefficient stop of protein translation at the COOH-terminal end of the construct. Molecular masses in kD of standard proteins are indicated.
Expression Constructs
cDNA constructs pcAgrin and pcΔN15Agrin have been described previously (Denzer et al., 1995). The sequence encoding parts of the constant region of mouse immunoglobulin gamma heavy chain (Fc) were obtained by PCR using the primers sFc_XhoI_BamHI (GGCAGCTCGAGGATCCTCGTGCCCAGGGATTGTGGTTG) and asFc_XbaI (GGCCCTCTAGATCATTTACCAGGAGAGTGGG). As template, the Fc part of mouse immunoglobulin (Bowen et al., 1996) was used. Amplification introduced an XhoI and a BamHI site at the 5′ end and a XbaI site at the 3′ end. The PCR product was digested with XhoI and XbaI and ligated into the expression vector pcDNAI (InVitrogen, San Diego, CA) to yield pFc. To generate pcN257Fc, a second PCR was performed using EcoRI_s-289 (GCATAGAATTCGGCTGCGGGCGATGGG) and as469_BamHI (CACGAGGATCCCCTCTGCACAGGGGTCCTTG) as primers and pcAgrin7A4B8 (Denzer et al., 1995) as template. The product coded for the NH2-terminal domain of chick agrin and contained an EcoRI site at the 5′ end and a BamHI site at the 3′ end. The BamHI site enabled the subsequent in-frame fusion of this PCR fragment to the Fc sequences by digestion with EcoRI and BamHI and ligation into pFc. pr21Fc was similarly constructed. The cDNA part encoding ray agrin was isolated by PCR using the primers EcoRI_s3426 (AGCTTGAATTCAGCCAGTGGAAGTGAATC) and as3967_BamHI (CACGAGGATCCCTTTCTTGGCTGGACAGTG), and r100A4B8 (Gesemann et al., 1995) as template. After digestion of the PCR product with EcoRI and BamHI, it was ligated into pFc. The sequence of pcN257Fc and r21Fc was verified by DNA sequencing.
Antiserum against β and γ Chains of Chick Laminins
Antiserum 648 against the β/γ chains of chick laminin-2 and -4 was generated as follows. 1 mg of chick heart laminin-2 and -4, purified as described (Brandenberger and Chiquet, 1995), was loaded on a preparative 3–15% gradient SDS-PAGE, run under reducing conditions, and blotted onto nitrocellulose (BA-85; Schleicher & Schuell Inc., Keene, NH). The protein band of 200 kD, containing the β and the γ chains, was cut out, suspended in 0.2 ml PBS, and sonicated on ice until homogenization. The sample was mixed with 0.2 ml Freund's complete adjuvant (GIBCO BRL, Gaithersburg, MD) and injected intracutaneously into one rabbit. The rabbit was boosted 1 mo later with the same preparation, suspended in incomplete Freund's adjuvant. Antiserum 648 was obtained 14 d later. In immunoblots, the antiserum recognized the 200-kD band of laminin isoforms from chick heart, chick gizzard, and mouse laminin-1 (Brandenberger and Chiquet, 1995; Perris et al., 1996). As the only chain common to all these laminins is the γ1 chain, antiserum 648 must recognize at least this subunit. As the immunogen also contained the β chain, the antiserum is likely to recognize this subunit as well.
Protein Purification
Recombinant cAgrin7 was obtained from stably transfected HEK 293 cells, whereas VF agrin was derived from eyes of day 14 chick embryos (Ruegg et al., 1989). 100 ml of serum-free conditioned medium or 50 ml of vitreous fluid was passed over a Mono Q–Sepharose (Pharmacia Diagnostics AB, Uppsala, Sweden). The column was washed with 20 mM Tris-HCl, pH 7.2, 500 mM NaCl, and bound proteins were eluted with a linear gradient of NaCl from 500 mM to 2 M. Individual fractions with agrin immunoreactivity, as determined by an ELISA (Gesemann et al., 1995), were analyzed on SDS-PAGE (3–12% gradient) and visualized by silver staining (Morrissey, 1981). Agrin-containing fractions were pooled and dialyzed three times against PBS. Agrin concentration of such a preparation was determined by ELISA. cN257Fc and r21Fc from transiently transfected COS-7 cells were purified with protein A–Sepharose (Pharmacia) according to the manufacturer's advice. Purity of recovered protein was checked by SDS-PAGE and silver staining. The protein concentration was determined as described (Lowry et al., 1951), using the DC Protein assay kit (BioRad Labs, Hercules, CA) and BSA as a standard. Mouse laminin-1, purified from mouse Engelbreth-Holm-Swarm sarcoma as described (Timpl et al., 1979), was provided by Th. Schulthess (Biozentrum, University of Basel, Basel, Switzerland). Mouse nidogen, isolated from conditioned medium of stably transfected HEK 293 cells (Fox et al., 1991), and mouse perlecan (Timpl et al., 1979) were provided by Dr. R. Timpl (Max Planck Institute, Munich, Germany). Human collagen type IV (Weber et al., 1984; Ries et al., 1995) was obtained from Dr. K. Kühn (Max Planck Institute) and chick α-dystroglycan, isolated from skeletal muscle (Brancaccio et al., 1995), was a gift of Dr. A. Brancaccio (Biozentrum, University of Basel). Chick laminin isoforms (laminin-2 and -4) were purified from chick heart as described by Brandenberger and Chiquet (1995). Only laminin preparations functional in a neurite outgrowth assay (Brandenberger and Chiquet, 1995) were used for agrin-binding studies.
Solid-Phase Radioligand Binding Assay
Proteins were diluted to 10 μg/ml with 50 mM sodium bicarbonate, pH 9.6 (coating buffer), and immobilized on 96-well plates (Becton-Dickinson, Bedford, MA) by incubation overnight at 4°C. Remaining binding sites were blocked for 1 h with TBS containing 3% BSA, 1.25 mM CaCl2, and 1 mM MgCl2 (blocking solution). 125I-cAgrin7 or 125I-cN257Fc, diluted in blocking solution, was added and incubated for 3 h. After washing with TBS, 1.25 mM CaCl2, 1 mM MgCl2 four times, bound radioactivity in each well was counted with a gamma counter. Solubilization with SDS sample buffer and subsequent analysis on SDS-PAGE followed by silver staining confirmed that the proteins were indeed coated onto the plastic surface.
Alternatively, mAb 5B1 diluted to 10 μg/ml in coating buffer was first immobilized by overnight incubation at 4°C. Remaining binding sites were saturated with blocking solution for 1 h. Then 3 μg/ml agrin was added. After 1 h of incubation, the plates were washed three times with blocking solution and processed with 125I–laminin-1 as described above.
To calculate half-maximal binding (EC50) of cAgrin7 to laminin-1, individual data points of the dose-response were fit by the following equation: Y = (X/EC50)/(1+X/EC50) × P1. This equation assumes a single class of equivalent and independent binding sites, where Y represents cpm, X represents the concentration of agrin, and P1 represents cpm at saturation.
Immunocytochemistry
Double staining of agrin in COS-7 cells: COS cells, transiently transfected with cDNAs encoding cAgrin7 and cΔN15Agrin, were grown on Matrigel™ (Becton-Dickinson) and stained for agrin as described elsewhere (Denzer et al., 1995). Cells transfected with cDNAs encoding cN257Fc and r21Fc were also stained as described in Denzer et al. (1995), except that fluorescein-conjugated goat anti–mouse IgG (1:200; Cappel, Organon Teknika Corp., West Chester, PA) was used for the extracellular staining and that, before permeabilization and staining with rhodamine-conjugated goat anti–mouse IgG (1:200; Cappel), residual binding sites were blocked with unlabeled goat anti–mouse IgG (1:50; Cappel).
Primary chick myotubes were incubated with 200 nM c21B8 (Gesemann et al., 1995) for 16 h at 37°C. To localize cN257Fc, 20 nM of this fragment was included. Cultures were rinsed with culture medium, and to visualize individual components, they were stained with the following reagents: AChRs with 4 × 10−8 M rhodamine–α-bungarotoxin (Molecular Probes, Eugene, OR); cN257Fc with 5 μg/ml biotinylated goat anti–mouse IgG (Molecular Probes) followed by 3 μg/ml fluorescein-conjugated streptavidin (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA); β/γ subunits of laminin with antiserum 648 (1:1,000) followed by Cy3™-conjugated goat anti–rabbit IgG (1:200; Jackson ImmunoResearch Laboratories, Inc.); and β2 chain of laminin with mAb C4 (10 μg/ml) followed by 5 μg/ml biotinylated goat anti–mouse IgG and 3 μg/ml fluorescein-conjugated streptavidin. The first incubation was done for 1 h at 37°C. Cultures were then washed with culture medium and fixed for 30 min at room temperature with 4% paraformaldehyde, 11% sucrose in 0.1 M potassium phosphate, pH 7.2. After rinsing the cells with PBS and PBS + 20 mM glycine, the cultures were incubated for 1 h with the secondary reagents indicated before, diluted in PBS + 2% normal goat serum (PBSN). After rinsing the cells with PBS, cultures were either dehydrated with 95% ethanol at −20°C or incubated once more with the tertiary reagent diluted in PBSN. Coverslips were mounted with Vectashield™ (Vector Laboratories, Inc., Burlingame, CA), and cultures were examined with a microscope equipped for epifluorescence (Leica Inc., Deerfield, IL).
Competition Experiment and AChR Aggregation
Chick muscle cells were preincubated with 500 nM of cN257Fc for 4 h at 37°C. Conditioned medium from transiently transfected COS cells containing 100 pM cAgrin7A4B8 or cΔN15AgrinA4B8 was added for 12 h at 37°C. AChRs were visualized and analyzed as described in Gesemann et al. (1995). Since cAgrin7A4B8-induced AChR clusters were very small, aggregates with the longer axis of ⩾1 μm were included.
Results
The NH2-terminal End of Agrin Is Sufficient to Bind to ECM
We have recently shown that recombinant full-length chick agrin (cAgrin; Fig. 1 a) binds to a solubilized mixture of ECM molecules, called Matrigel™. In contrast to fulllength agrin, a fragment that lacks the first 130 amino acids from the NH2 terminus (cΔN15Agrin; Fig. 1 a) did not bind to this ECM preparation (Denzer et al., 1995). In this 130–amino acid–long stretch, a site of alternative mRNA splicing is found (Denzer et al., 1995; Tsen et al., 1995b ). Both splice variants are capable of binding to Matrigel™, although with different binding strengths (Denzer et al., 1995). In the current report we have investigated the binding of the splice variant that includes the seven–amino acid–long insert (Fig. 1 a). This splice variant is selectively expressed by embryonic chick motor neurons (Denzer et al., 1995) and is also highly expressed in embryonic chick brain (Tsen et al., 1995b ).
To see whether the NH2-terminal region of agrin alone is sufficient to bind to Matrigel™, we engineered a cDNA construct that encodes an agrin fragment comprising the NH2-terminal region and the first follistatin-like domain. To facilitate detection and purification of the fragment, it was fused to the constant region of a mouse IgG (Fc; Bowen et al., 1996), giving rise to the fragment termed cN257Fc (Fig. 1 a). A chimeric construct of the most COOH-terminal, 21-kD fragment of agrin from the marine ray (Smith et al., 1992) and the Fc part (r21Fc; Fig. 1 a) was used as a control. All recombinant proteins were efficiently synthesized and secreted from COS cells as shown by precipitation of 35S-labeled proteins from conditioned medium of transiently transfected cells (Fig. 1 b). The high apparent molecular mass of 400–600 kD of cAgrin7 and cΔN15Agrin on SDS-PAGE is a result of heparan sulfate glycosaminoglycan (HS-GAG) chains that are attached to the 225-kD core protein (Denzer et al., 1995). The chimeric constructs, cN257Fc and r21Fc, were also secreted from the transfected cells, and they displayed the expected molecular mass of ∼69–65 and 61 kD, respectively (Fig. 1 b). SDSPAGE of the chimeric proteins under nonreducing conditions demonstrated that the intermolecular disulfide bonds of the Fc region dimerize the fragments (data not shown).
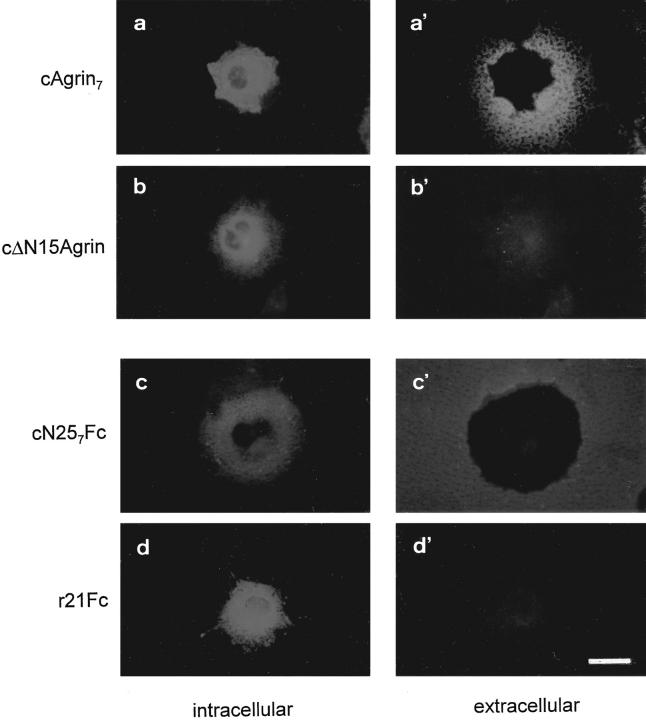
The binding to Matrigel™ was assayed using transiently transfected COS cells that were grown on tissue culture dishes coated with this basement membrane preparation. As previously shown (Denzer et al., 1995), cAgrin7 was deposited on Matrigel™ (Fig. 2, a and a′), and no extracellular agrin-like immunoreactivity was observed with COS cells expressing cΔN15Agrin (Fig. 2, b and b′). Extracellular deposits of the recombinant proteins, cN257Fc and r21Fc, were visualized with fluorescein-conjugated goat anti–mouse IgG antibodies added to nonfixed, nonpermeabilized cells. The cells were subsequently permeabilized and stained with rhodamine-conjugated goat anti–mouse IgG antibodies to identify transfected cells. As shown in Fig. 2, c and c′, cN257Fc was deposited on Matrigel™ around the transfected COS cells, which demonstrates that the NH2-terminal region is sufficient to bind to this ECM mixture. The binding is not caused by the Fc part, as no deposits were seen with r21Fc-transfected cells (Fig. 2, d and d′).
Figure 2.
The NH2-terminal end of agrin is sufficient to bind to Matrigel™. COS-7 cells, which had been transiently transfected with cDNAs encoding the agrin constructs indicated on the left of each row, were grown on Matrigel™. Deposition of recombinant protein onto Matrigel™ (right column) was monitored by staining living cells with the appropriate antibodies. To identify agrin-expressing cells, antibodies were added after permeabilizing the cells (left). To detect cAgrin7 and cΔN15Agrin deposits, antiagrin antiserum followed by a fluorescein-conjugated secondary antibody was used (a′ and b′). cN257Fc and r21Fc deposits were visualized with a fluorescein-conjugated goat anti–mouse IgG (c′ and d′). Permeabilized cells were incubated with antiagrin mAb 5B1 followed by a rhodamine-conjugated secondary antibody (a and b) or with rhodamine-conjugated goat anti–mouse antibody (c and d). Only cells synthesizing cAgrin7 and cN257Fc show extracellular deposits of recombinant protein (a′ and c′). Bar, 40 μm.
Laminin-1 Is a Binding Partner for Agrin
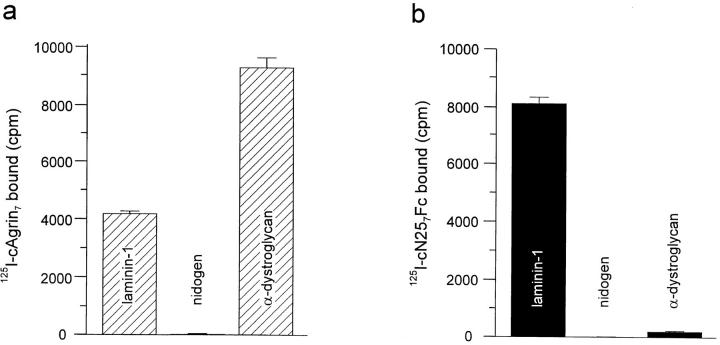
Matrigel™ consists of a mixture of several ECM molecules, such as collagen type IV, laminin-1, nidogen/entactin, and perlecan (Kleinman et al., 1982). To identify the components to which agrin binds, a solid-phase radioligand binding assay was used (Gesemann et al., 1996). Laminin-1 and perlecan were purified from the Engelbreth-HolmSwarm mouse tumor (Timpl et al., 1979), and collagen type IV was isolated from human placenta (Weber et al., 1984; Ries et al., 1995). After coating the purified proteins onto the plastic surface of a microtiter plate, wells were incubated with 5 nM 125I-cAgrin7 or 125I-cN257Fc and bound radioactivity was measured. Both agrin constructs bound to laminin-1 (Fig. 3), while no or only little binding was seen to perlecan and collagen type IV (data not shown). Since laminins bind to nidogen with high affinity (K d ∼1 nM; Fox et al., 1991), most laminin preparations also contain nidogen (Paulsson et al., 1987). To test whether the binding of agrin to laminin-1 was due to nidogen, we also measured binding of cAgrin7 and cN257Fc to recombinant mouse nidogen, expressed in stably transfected HEK 293 cells (Fox et al., 1991). No binding was observed for both agrin constructs (Fig. 3). Hence, the binding of agrin to the laminin–nidogen complex is based on a direct interaction with laminin-1. As expected from previous experiments (Bowe et al., 1994; Campanelli et al., 1994; Gee et al., 1994; Sugiyama et al., 1994; Gesemann et al., 1996), cAgrin7 also bound to α-dystroglycan, purified from chick skeletal muscle (Brancaccio et al., 1995; Fig. 3 a). In contrast, the NH2terminal fragment, cN257Fc, did not bind (Fig. 3 b). This is consistent with the notion that the binding site to this peripheral membrane protein resides in its COOH-terminal half of agrin (Bowe et al., 1994; Campanelli et al., 1994; Gee et al., 1994; Sugiyama et al., 1994; Gesemann et al., 1996). Accordingly, only the laminin-binding site is common to cN257Fc and cAgrin7. In addition, no binding of 125I-r21Fc was observed to any of the components tested (data not shown), which excludes a contribution of the Fc part in the binding of cN257Fc to laminin-1.
Figure 3.
Agrin binds to laminin-1. Solid-phase radioligand binding assay with purified proteins. Mouse laminin-1, nidogen, and chick α-dystroglycan were immobilized in microtiter plates and subsequently incubated with 125I-cAgrin7 or 125I-cN257Fc. Each value represents binding after subtraction of the background (BSA-coated wells). The values given are the mean ± SD from one representative experiment with three independent measurements in the case of 125IcN257Fc and two measurements for 125I-cAgrin7. (a) 5 nM of 125I-cAgrin7 bound to α-dystroglycan and to laminin-1, but not to nidogen. Background counts were 89 ± 15 cpm. (b) 5 nM 125IcN257Fc bound to laminin-1 but not to α-dystroglycan and nidogen. Background counts were 65 ± 3 cpm. These experiments show that the binding of agrin to laminin is mediated by the NH2-terminal end of agrin. Counts measured in a and b cannot be compared because cAgrin7 and cN257Fc are iodinated to different extents.
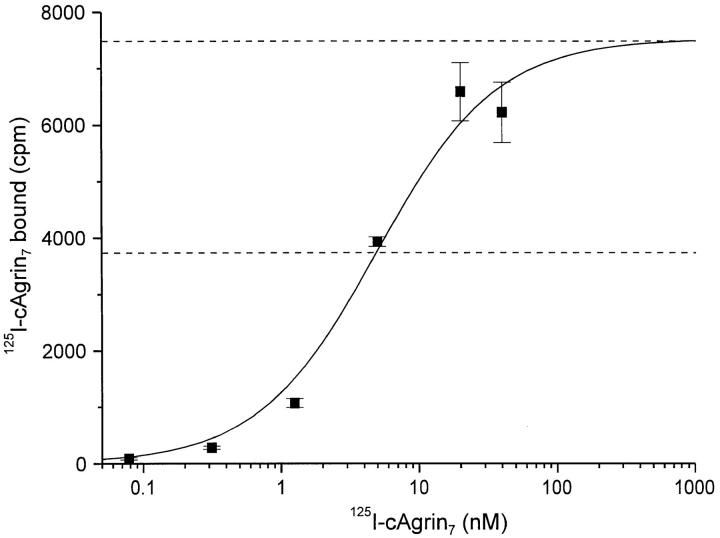
The strength of the binding of agrin to laminin-1 was measured by dose-response curves using 125I-cAgrin7. On immobilized laminin-1, half-maximal binding (EC50) was reached at ∼5 nM (Fig. 4), suggesting a rather strong interaction of agrin with laminin-1. This binding is of similar strength as the binding of laminin-1 to nidogen (K d ∼1 nM; Fox et al., 1991). At 4 nM 125I-cAgrin7, 90.3 ± 0.5% (mean ± SEM; n = 3) of the binding was competed with 100 nM unlabeled cN257Fc, indicating that the majority of the binding of full-length agrin to laminin-1 is mediated by the NH2-terminal end. Since agrin is an HSPG (Denzer et al., 1995; Tsen et al., 1995a ) and laminin-1 is known to bind to heparin (Ott et al., 1982; Yurchenco et al., 1993), the residual binding of cAgrin7 to laminin-1 could be due to the binding of the HS-GAG side chains of agrin to the heparin-binding site of laminin-1. Indeed, only the HS-GAG side chain-carrying construct cAgrin7, but not cN257Fc, binds to the elastase fragment E3, which contains the major heparin-binding site of laminin-1 (Ott et al., 1982; data not shown). In summary, our data show that laminin-1, the laminin isoform present in Matrigel™, is a basement membrane binding partner for agrin. Furthermore, they show that the interaction is mediated by the NH2-terminal end of agrin, and we therefore propose that this part of agrin forms a structural unit (see also Discussion).
Figure 4.
Binding of agrin to laminin-1 is of high affinity. Doseresponse curve of the binding of 125I-cAgrin7 to laminin-1. Halfmaximal binding was reached at ∼5 nM, suggesting a rather strong binding of agrin to laminin-1. The binding curve shown results from one representative experiment. Background values (BSA-coated wells) are subtracted from each data point. Values shown are the mean ± SEM from three measurements.
Agrin Expressed in Developing Chick Retina Binds to Laminin-1
The results presented so far show that recombinant chick agrin, expressed in mammalian cells, binds to laminin-1. If this binding were important for agrin's function in vivo, agrin purified from tissue should have the same binding properties. To test this, agrin was isolated by anion exchange chromatography from the vitreous fluid of embryonic day 14 chick eyes, a rich source for agrin and other molecules secreted from retinal cells (e.g., Ruegg et al., 1989; Halfter, 1993; Denzer et al., 1995). As a control, cAgrin7 from conditioned medium of stably transfected HEK 293 cells was purified by the same procedure. When agrin-containing fractions were assayed by Western blot, agrin-like protein from vitreous fluid and cAgrin7 had a high apparent molecular mass (Fig. 5 a, left), which is consistent with previous results (Denzer et al., 1995). The bands with an apparent molecular mass of ∼115 kD are probably due to proteolytic degradation similar to what has been reported elsewhere (e.g., Nitkin et al., 1987; Godfrey, 1991; Rupp et al., 1991; Denzer et al., 1995). The same samples were also probed with antiserum 245, raised against native chick heart laminin (Brubacher et al., 1991). No laminin-like immunoreactivity was observed in the samples containing cAgrin7, derived from stably transfected HEK 293 cells, whereas immunoreactive bands were visible in the sample containing agrin from the vitreous fluid (Fig. 5 a, right). The strong immunoreactive band with the apparent molecular mass of 200 kD most likely corresponds to the β/γ subunits of laminin isoforms, while the faint bands with the molecular masses of 400 and 150 kD could represent α subunits and nidogen, respectively (Brubacher et al., 1991). The copurification of chick laminin isoforms from the vitreous fluid with agrin by a procedure that is selective for highly charged anions is unexpected and may reflect the binding of laminin to agrin. Consistent with this idea, Halfter (1993) reported copurification of laminin with a chick HSPG, which later was shown to be agrin (Tsen et al., 1995a ).
Figure 5.
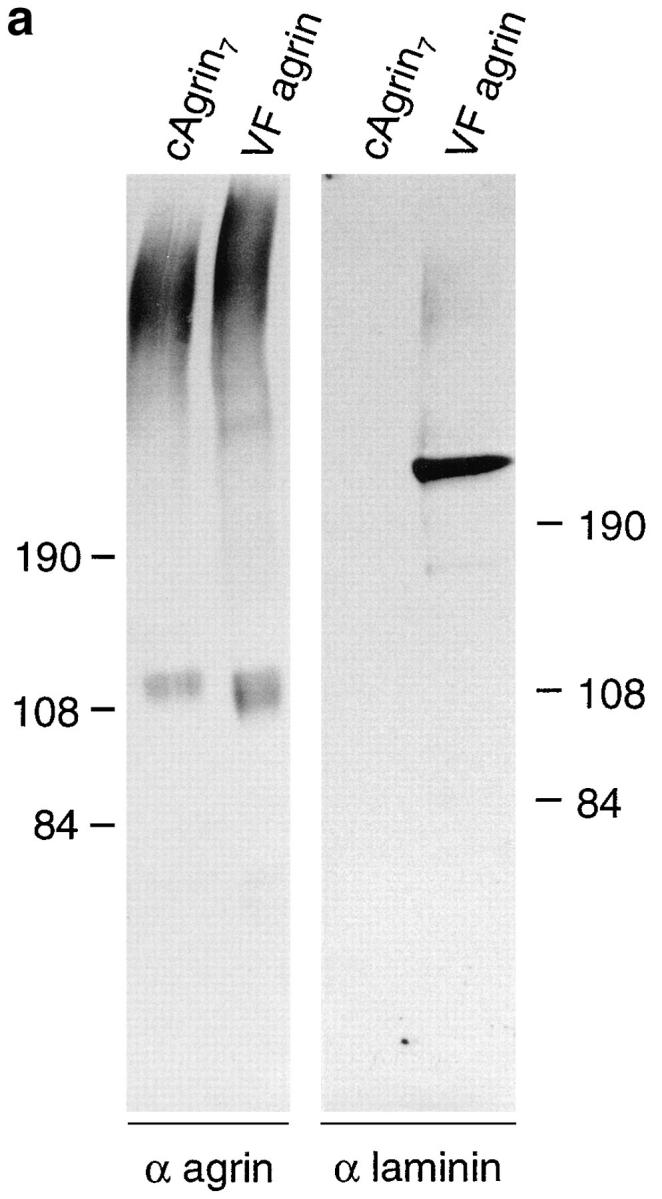
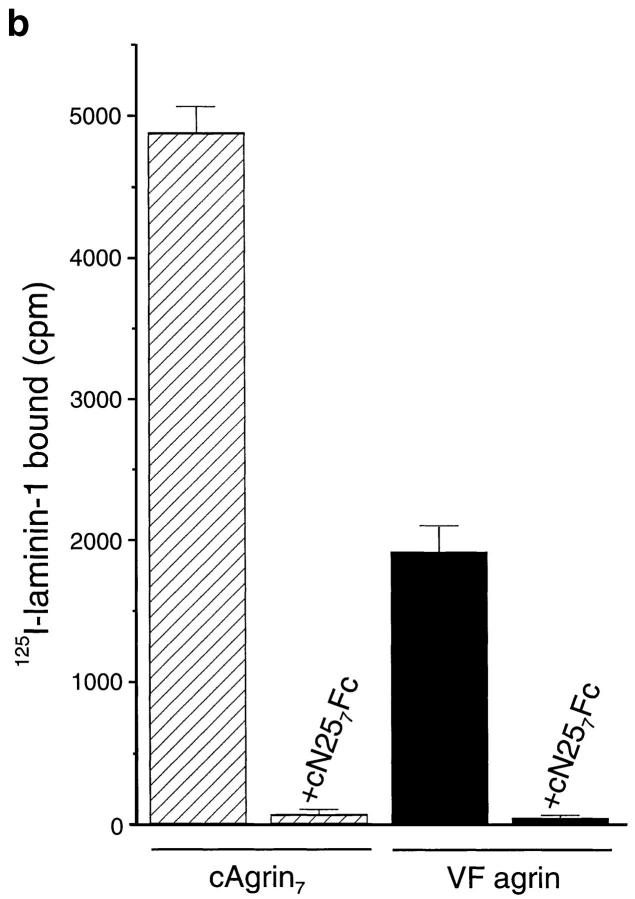
Chick agrin synthesized in vivo contains the laminin-binding domain. (a) Western blot of recombinant cAgrin7 and agrin purified from vitreous fluid (VF agrin). Transferred protein were stained with antiagrin antibodies (α agrin) or antilaminin antibodies (α laminin). Agrin purified from vitreous fluid has approximately the same apparent molecular mass as recombinant full-length agrin. The band detected at ∼115 kD most likely results from proteolytic degradation. No laminin-like immunoreactivity is associated with recombinant agrin (right). In contrast to this, the agrin-containing fractions from vitreous fluid are positive for laminin-like protein. Based on their apparent molecular mass and the specificity of the antiserum used (Brubacher et al., 1991), they may represent nidogen (∼150 kD), the β and γ chain (200 kD), and α chain (400 kD). Molecular masses of standard proteins are given in kD. (b) cAgrin7 and agrin from vitreous fluid were bound to immobilized antiagrin mAb 5B1 and incubated with 125I– laminin-1. The data result from one representative experiment and are the mean ± SD of three measurements. Background counts (no agrin added; 132 ± 15 cpm) was subtracted. 100 nM cN257Fc (+ cN257Fc) inhibits binding of 125I–laminin-1 to cAgrin7 and VF agrin by more than 97%, indicating that the NH2-terminal domain is responsible for the binding.
To test directly whether VF agrin is capable of binding to laminin-1, we performed a modified solid-phase radioligand binding assay. In this assay, purified cAgrin7 or VF agrin was immobilized on microtiter plates that had been first coated with the antiagrin mAb 5B1 (Reist et al., 1987). To these wells, 5 nM of 125I–laminin-1 was added and bound radioactivity was measured. As shown in Fig. 5 b, 125I–laminin-1 bound to cAgrin7 and VF agrin. In both cases, binding was competed by more than 97% by including 100 nM of unlabeled cN257Fc during the incubation with 125I–laminin-1 (Fig. 5 b). These experiments show that agrin synthesized and secreted by cells of the chick retina can bind to laminin-1 and that this binding is also mediated by the NH2-terminal domain of agrin. Although the same amount of agrin was added to the 5B1-coated microtiter wells, the amount of 125I–laminin-1 bound to VF agrin was only half of that bound to cAgrin7. The most likely explanation for this difference is that some of the binding sites in VF agrin may already be occupied by laminin isoforms from the vitreous fluid that copurified with VF agrin (see Fig. 5 a). In summary, chick agrin synthesized in vivo has the same laminin-binding properties as recombinant agrin, and its binding is also mediated by the NH2-terminal domain.
The Laminin-binding Domain Is Highly Conserved in Mouse and Human Agrin
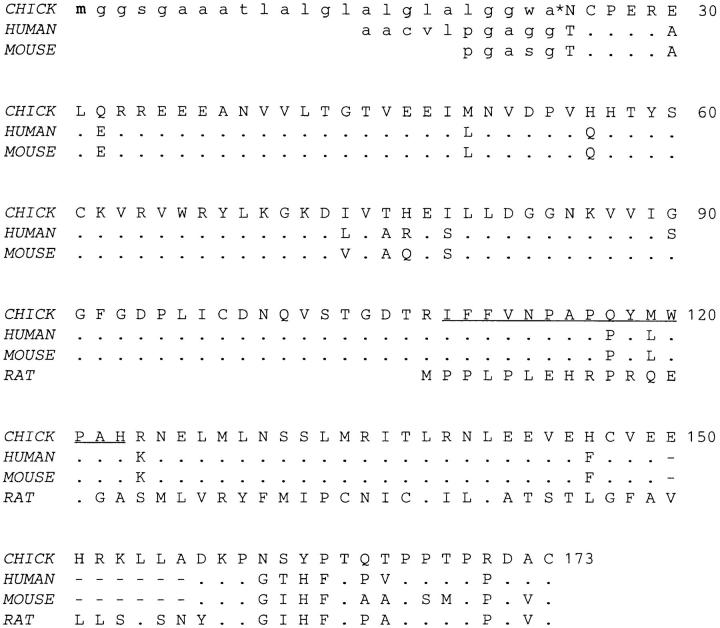
The NH2-terminal region required for the binding of agrin to laminin-1 has so far only been described in chick (Denzer et al., 1995). Full-length cDNA encoding rat agrin lacks this region and instead, the first follistatin-like domain is preceded by a sequence that has been proposed to serve as signal sequence (Rupp et al., 1991). To see whether the NH2-terminal region of chick agrin is found in other species, we searched for homologous sequences using the BLAST algorithm (Altschul et al., 1990). Four expressed sequence tags, isolated from different tissues in mouse and in human (Lennon et al., 1996), closely matched this amino acid sequence. Sequencing of the clones confirmed that the deduced amino acid sequences of mouse and human agrin are highly homologous to each other and to chick agrin (Fig. 6). The homology starts at residue 26 of chick agrin, which corresponds to the predicted cleavage site for the signal sequence (Denzer et al., 1995). In the stretch from residue 26 to 149, 96% of the amino acids are identical between mouse and human, and 90% are identical between chick and the mammalian sequences (Fig. 6). This is by far the most highly conserved region in agrin (see also Tsim et al., 1992), suggesting that this domain may also confer binding to laminin in mammals.
Figure 6.
The laminin-binding domain is present in mouse and human agrin. Deduced amino acid sequences of the expressed sequence tags (Lennon et al., 1996) from mouse and human agrin were aligned to the first 173 amino acids of chick agrin. In addition, the first 67 amino acids of rat agrin are shown. Amino acids identical to chick agrin are denoted with dots. The small letters in the chick sequence represent the proposed signal sequence (Denzer et al., 1995) with the initiator methionine (bold) and the proposed signal sequence cleavage site (*). Underlined amino acids show the position of the tryptic peptide derived from a HSPG of bovine kidney (Hagen et al., 1993). Homology between chick and human starts after the signal sequence cleavage site of chick (Cys 26). Amino acids between Cys 26 and Glu 149 are almost 90% identical between human, mouse, and chick. Note that the amino acid sequences of mouse and human agrin that precede the proposed signal peptide cleavage site (*) are not homologous to chick and consist mainly of hydrophobic amino acids. Thus, as in chick these stretches might be part of a signal sequence (lowercase letters). The homology between chick and rat begins at Asp 157 immediately after the boundary where chick is alternatively spliced. The human and mouse sequences lack the seven–amino acid–long insert at this splice site (dashes). These sequence data are available from GenBank/EMBL/DDBJ under accession number U84406 (human agrin) and U84407 (mouse agrin).
Agrin Binds to Laminin Isoforms Expressed at the NMJ
The data presented so far show that agrin binds to mouse laminin-1. The laminins are a family of heterotrimeric glycoproteins composed of α, β, and γ chains (Burgeson et al., 1994; Timpl, 1996). Each laminin is characterized by its chain composition, for example laminin-1 is a trimer with the α1, the β1, and the γ1 chains. Recent data suggest that laminin-1 is not expressed in muscle basal lamina but instead is replaced by laminin-2 and -4 (Schuler and Sorokin, 1995). Laminin-2 (α2, β1, γ1) is present early in development throughout the extracellular matrix of muscle fibers. In adult muscle, it is expressed in the extrasynaptic region of the basal lamina. Laminin-4 (also called s-laminin; Hunter et al., 1989), in which the β1 chain is replaced by the β2 chain, is expressed later in development and localizes to the synaptic portion of the muscle cell basal lamina (Sanes et al., 1990).
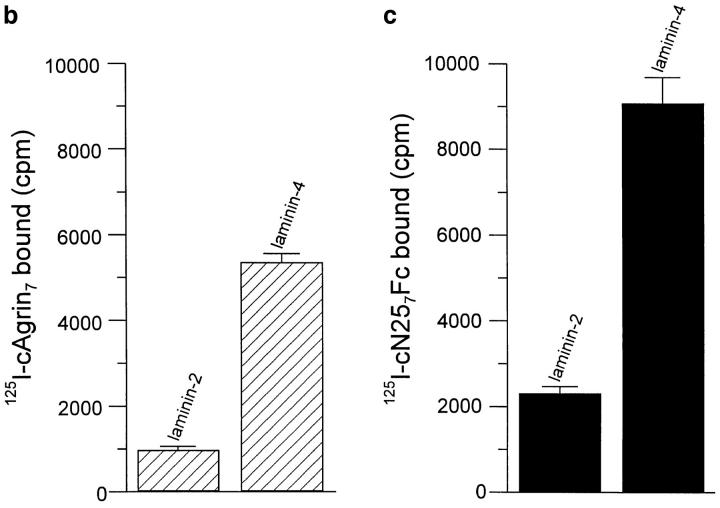
Since we were interested in whether agrin's NH2-terminal domain would also mediate the tethering to muscle cell basal lamina, we measured binding of cAgrin7 and cN257Fc to laminin-2 and -4. The source for laminin-2 and -4 was a laminin preparation isolated from adult chick heart after EDTA extraction and sequential purification by wheat germ agglutinin Sepharose and immunoaffinity chromatography (Brandenberger and Chiquet, 1995). As shown by Western blot analysis using antiagrin antibodies, this laminin preparation contained substantial amounts of agrinlike protein (Fig. 7 a). As this laminin preparation did not contain α-dystroglycan, detected by a transfer overlay assay using iodinated chick agrin (data not shown), we conclude that the copurification of agrin with laminin isoforms from cardiac muscle is most likely a consequence of the association of agrin with the laminins. To test directly whether laminin-2 and -4 are binding proteins for agrin, we performed a solid-phase radioligand binding assay using purified laminin isoforms. With the same amount of laminin-2 and -4 coated on the microtiter wells, 5 nM of 125I-cAgrin7 gave a clear signal on laminin-2 and a fivefold stronger signal on laminin-4 (Fig. 7 b). Like cAgrin7, the NH2-terminal fragment, cN257Fc, also bound approximately four times more strongly to laminin-4 than to laminin-2 (Fig. 7 c). The weaker signal on laminin-2 was not due to a contamination with laminin-4, since this isoform represents less than 5% in the laminin-2 preparation (Brandenberger et al., 1996). A difference in the coating efficiency between laminin-2 and -4 was also excluded because equal amounts were immobilized when tested with an mAb specific for the α2 chain (Brandenberger et al., 1996). In summary, full-length chick agrin binds to both laminin-2 and -4, but at this particular concentration the interaction with the extrasynaptic laminin-2 is of lower apparent affinity.
Figure 7.
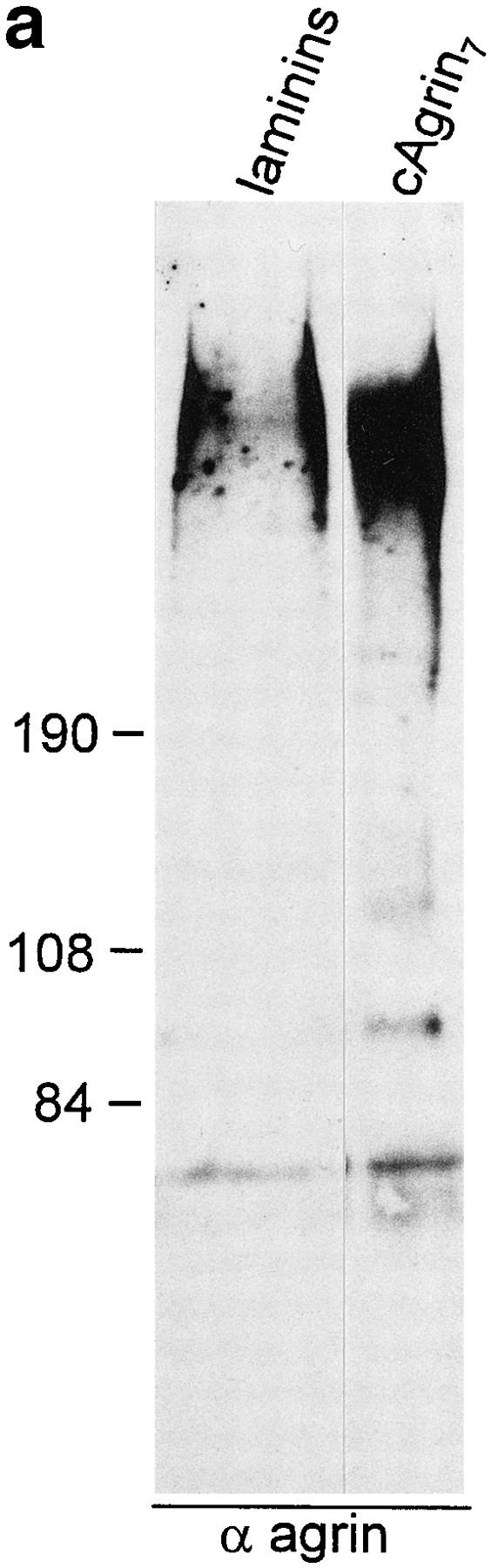
Agrin binds to laminin isoforms expressed in muscle. (a) Immunoblot using antiagrin antibodies of laminin isoforms purified from EDTA extracts of adult chick heart by immunoaffinity column with the anti-γ1 subunit–specific mAb 11B7 (Brandenberger and Chiquet, 1995). Agrin-like protein with the same apparent molecular mass as cAgrin7 is detected in the laminin preparation. Since no α-dystroglycan was detected in the same laminin preparation (data not shown), the copurification of agrin suggests that laminins are associated with agrin in muscle tissue. (b and c) Agrin binds directly to purified chick laminin-2 and -4. Equal amounts of the two laminins were immobilized on microtiter plates and incubated either with 5 nM of iodinated cAgrin7 (b) or cN257Fc (c). Values given are the result of one representative experiment and represent the mean ± SD of three measurements where the background (BSAcoated wells) has been subtracted. Background values were 163 ± 24 cpm in b and 75 ± 11 cpm in c. The binding of cAgrin7 to laminin-4 is fivefold stronger than to laminin-2 (b) and a similar difference in the binding (fourfold) is observed with cN257Fc (c). Note that the difference in the counts measured with 125I-cAgrin7 and 125I-cN257Fc is due to different iodination efficiencies.
Many proteins that are highly concentrated at the NMJ in vivo are also found in AChR clusters in vitro (for review see Bowe and Fallon, 1995). These include proteins of the ECM, such as HSPG (Wallace, 1989) and laminin (Nitkin and Rothschild, 1990). Consequently, the laminin-binding fragment, cN257Fc, should colocalize with AChR clusters. To test this, AChR aggregation was induced on cultured chick myotubes with 200 nM c21B8, the minimal COOHterminal agrin fragment required for AChR aggregation (Gesemann et al., 1995), and 20 nM of cN257Fc was included. After 16 h, AChR clusters were visualized with rhodamine–α-bungarotoxin and myotube-bound cN257Fc was stained with biotinylated goat anti–mouse IgG followed by fluorescein-conjugated streptavidin. As shown in Fig. 8 (first row), cN257Fc localized to the agrin-induced AChR clusters. cN257Fc also displayed a more widespread distribution, often along the edge of the myotubes (Fig. 8, arrowhead). In myotubes, where AChR aggregation had been induced with c21B8, but no cN257Fc was included, no specific staining was seen (Fig. 8, second row). These results illustrate that binding sites for the NH2-terminal fragment of agrin are concentrated in AChR clusters but are also found on the remaining surface of the myotube.
Figure 8.
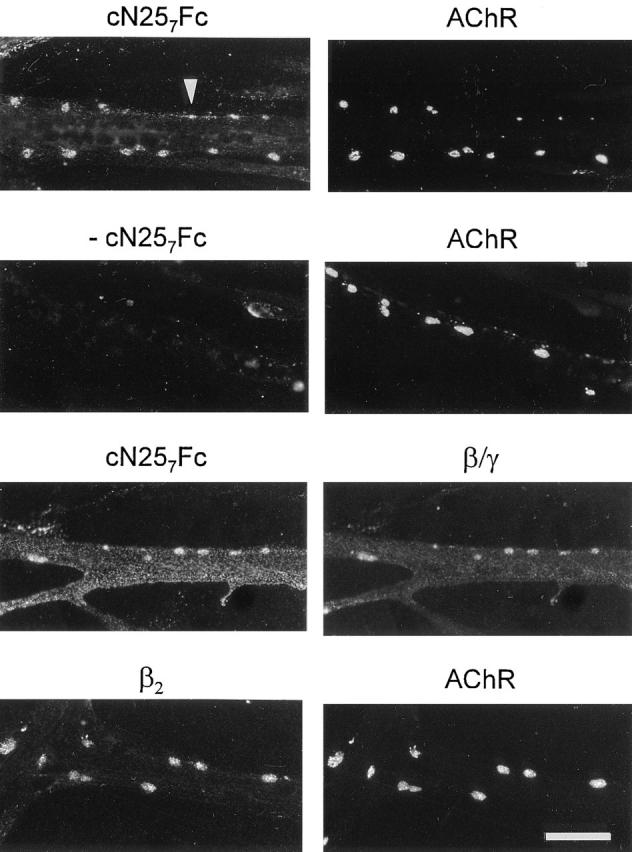
Binding sites for agrin on cultured chick myotubes colocalize with AChRs and laminin. Cultured chick myotubes were induced to form AChR clusters with 200 nM c21B8. Simultaneously, 20 nM cN257Fc were included. AChR aggregates were stained by rhodamine–α-bungarotoxin, and cN257Fc bound to the myotubes was visualized with biotinylated goat anti–mouse IgG followed by fluorescein-conjugated streptavidin. cN257Fc is concentrated in AChR clusters and is distributed along the edges of the myotubes (arrowhead). No staining is seen in the absence of this fragment (−cN257Fc). Consistent with the idea that cN257Fc binds to laminin, the distribution of myotube-bound cN257Fc resembles the staining pattern obtained with anti-β/γ-specific antiserum 648 (β/ γ). The β2 chain of laminin, also called s-laminin, is concentrated in AChR clusters. In light of the colocalization of cN257Fc and AChR clusters, laminin-4 (α2, β2, γ1) is a binding partner of agrin in these clusters. Bar, 40 μm.
To look at the relationship between the localization of cN257Fc and laminin, antiserum 648, directed against the β and γ chains of laminin-2 and -4, was used. Examination of many myotubes showed that the distribution of myotubebound cN257Fc matched laminin-like immunoreactivity (Fig. 8, third row). In addition, staining with the mAb 11B7, directed against the γ1 chain of chick laminins (Brandenberger and Chiquet, 1995), gave the same staining pattern (data not shown). Since these antibodies do not distinguish between laminin-2 and -4, we also applied mAb C4, recognizing the β2 chain, to localize the synapse-specific laminin isoform on myotubes (Hunter et al., 1989). In Fig. 8 (fourth row), staining with the β2-specific mAb C4 is shown. As reported for spontaneous AChR clusters on mouse C2 myotubes (Martin et al., 1995), the β2 chain was concentrated in AChR clusters on chick myotubes. Interestingly, no or only little laminin β2-like immunoreactivity was detected outside of the clusters. This differs from the pattern obtained with mAbs specific for the γ1 or the α2 chain of laminin, where immunoreactivity was also seen along the edge of myotubes (data not shown). In summary, these experiments demonstrate that the binding sites for agrin coincide well with the distribution of laminin on cultured myotubes, and they suggest that laminin-4 mediates binding of agrin to AChR clusters.
Binding of Agrin to Laminin Alters the Size of the AChR Aggregates
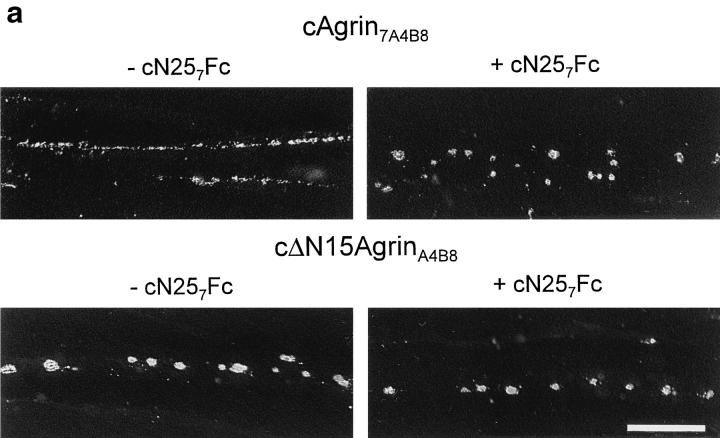
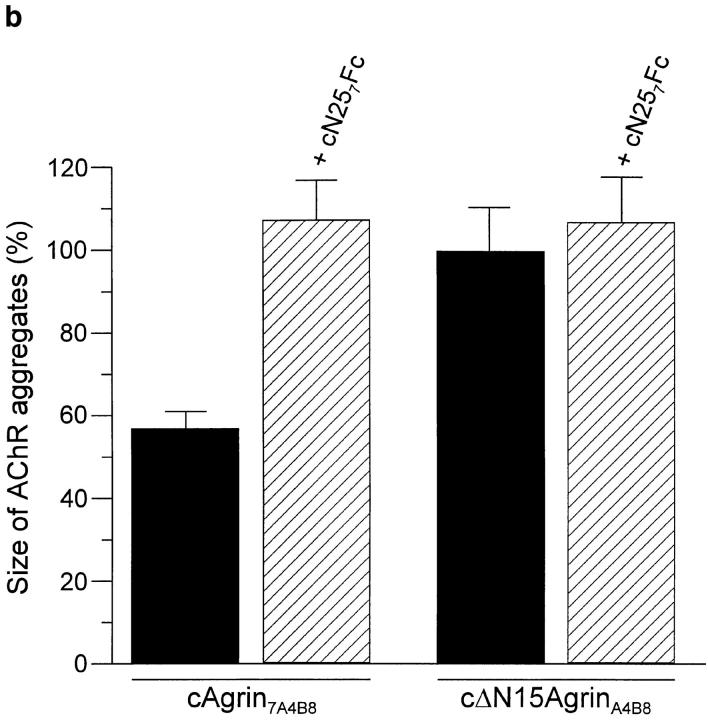
AChR aggregates induced by the active full-length splice variant cAgrin7A4B8 are more than twofold smaller than those induced by the fragment cΔN15AgrinA4B8 lacking the first 130 NH2-terminal amino acids (Denzer et al., 1995). Aggregation of AChRs induced by agrin is mainly based on the lateral migration of diffusely distributed molecules (Godfrey et al., 1984). We have speculated that the binding of cAgrin7A4B8 to ECM influences this process by immobilizing agrin in the ECM. If this were so, inhibition of the binding of cAgrin7A4B8 to muscle laminins should result in AChR clusters with the same size as AChR clusters induced by cΔN15AgrinA4B8. Myotubes incubated with cAgrin7A4B8 had considerably smaller AChR aggregates than those incubated with cΔN15AgrinA4B8 (Fig. 9 a). When available laminin-binding sites for full-length agrin were blocked by 500 nM cN257Fc, cAgrin7A4B8-induced AChR clusters became indistinguishable from those induced by cΔN15AgrinA4B8 (Fig. 9 a). In contrast to this, 500 nM of cN257Fc did not alter the shape of AChR aggregates induced by cΔN15AgrinA4B8. Quantification of this effect with a computerized image analysis system confirmed that the presence of cN257Fc increased the average size of AChR aggregates induced by cAgrin7A4B8 approximately twofold, while the average size of cΔN15AgrinA4B8induced AChR clusters was not affected (Fig. 9 b). These experiments provide evidence that the smaller size of the AChR clusters induced by full-length agrin is mainly based on its binding to laminin isoforms expressed by the myotubes.
Figure 9.
The NH2-terminal, laminin-binding domain of agrin causes AChR clusters to be small. (a) Fluorescence micrographs of cultured chick myotubes labeled with rhodamine–α-bungarotoxin. Myotubes were grown for 12 h in the presence of conditioned medium of transiently transfected COS cells containing either 100 pM cAgrin7A4B8 or cΔN15AgrinA4B8. AChR clusters induced by cAgrin7A4B8 are considerably smaller than those induced by cΔN15AgrinA4B8. In the presence of cN257Fc, the size of cAgrin7A4B8-induced AChR clusters increases. (b) Quantification of the effect on the size of the AChR aggregates. The presence of 500 nM cN257Fc (+ cN257Fc) increases the size of the AChR clusters induced by cAgrin7A4B8 twofold and makes them indistinguishable from AChR clusters induced by cΔN15AgrinA4B8. In contrast, addition of 500 nM cN257Fc does not alter the size of cΔN15AgrinA4B8-induced AChR aggregates. The result (mean ± SEM) of one representative experiment is shown where the size of AChR clusters in 20 myotube segments was determined. Bar, 50 μm.
Discussion
Agrin is a large, multidomain protein that is associated with basement membranes in many tissues (for review see Denzer et al., 1996). In the current study, we investigated the molecular basis of agrin's binding to basement membrane. We found that agrin binds with high affinity to laminin isoforms, including those that are concentrated at the NMJ, and we have mapped the main laminin-binding site to the NH2-terminal end of agrin. Agrin has been shown to be a key regulator of synaptic differentiation at the NMJ (McMahan, 1990; Gautam et al., 1996), and thus we will mainly discuss the significance of the binding of agrin to laminin in this process.
The Laminin-binding Domain of Agrin Defines a Novel Module
At the outset of the current work, it was known that the binding of recombinant chick agrin to Matrigel™ requires the first 130 amino acids at the NH2 terminus of full-length agrin (Denzer et al., 1995). We now find that laminin-1, one component of Matrigel™, serves as a binding partner for chick agrin. The binding of recombinant cAgrin7 (Fig. 1 a) to laminin-1 is of high affinity (EC50 of ∼5 nM; Fig. 4) and is mainly carried by the NH2-terminal end of agrin as an excess of the NH2-terminal fragment, cN257Fc, competes the binding of full-length agrin by more than 90%.
Although cN257Fc includes the first follistatin-like domain, this domain is not necessary for the binding to laminin-1 (Denzer, A.J., and M.A. Ruegg, unpublished observation). The stretch preceding the follistatin-like domain is the most highly conserved region of agrin (Fig. 6). This high homology and the fact that this region in chick agrin mediates binding to all laminin isoforms so far tested (i.e., laminin-1, -2, and -4) strongly suggest it being important for the integration of agrin into the scaffold of ECM molecules formed by the self-assembled laminins and collagen type IV. Since no homology to any so far defined modules was found using BLAST or FASTA algorithms (Altschul et al., 1990; Pearson and Lipman, 1988), we propose to call this novel module NtA domain (for NH2-terminal domain in agrin; see also Fig. 1 a).
The binding of the NtA domain of agrin to laminin-1 is confined to a particular region in the upper part of the triple coiled-coil domain of laminin-1, as shown by the binding of cN257Fc to proteolytic fragments of laminin-1 and visualization of this interaction by electron microscopy after rotary shadowing (Denzer, A.J., T. Schulthess, C. Fauser, B. Schumacher, J. Engel, and M.A. Ruegg, manuscript in preparation). These results show that the binding of agrin to laminin-1 is mediated by specific domains in both molecules and they support the result of this study (Fig. 4) that the agrin–laminin interaction is of high affinity.
Binding of Agrin to Laminin and Its Role in AChR Aggregation
Induction and maintenance of postsynaptic differentiation at nerve–muscle contacts in vivo is triggered by neural agrin in a restricted area of muscle fibers (McMahan, 1990). The simplest explanation for this local action of neural agrin is that it becomes trapped in the extracellular matrix near its release site, the tip of the motor neuron's axon. Although we cannot exclude that other molecules are also involved in this process, we propose that the local immobilization of neural agrin is mainly mediated by its binding to laminin isoforms expressed by muscle fibers. Consistent with this view, the fragment cN257Fc binds to purified laminin-2 and -4 (Fig. 7), the two main laminin isoforms expressed in developing muscle fibers (Chiu and Sanes, 1984; Sanes et al., 1990). In addition, agrin-like protein copurifies with laminin isoforms (Fig. 7 a), and cN257Fc colocalizes with laminin and AChR clusters on cultured myotubes (Fig. 8). Similarly, laminin-like immunoreactivity colocalizes with agrin-like protein in chick embryo hindlimb muscle in vivo and in vitro, and both proteins are enriched in AChR clusters (Godfrey et al., 1988b ; Nitkin and Rothschild, 1990). The binding of neural agrin to laminin would also explain earlier observations that motor neuron–derived agrin associates with the earliest AChR clusters in frog nerve–muscle cocultures (Cohen and Godfrey, 1992) and that it is deposited onto culture substrates containing laminin (Cohen et al., 1994).
We have shown that the size of agrin-induced AChR aggregates is affected by the NtA domain of agrin (Denzer et al., 1995; Fig. 9). On cultured chick myotubes, the size of cΔN15AgrinA4B8-induced AChR clusters is indistinguishable from the size of the clusters induced by c21B8, the minimal fragment required for AChR aggregation that does not bind to α-dystroglycan (see Figs. 8 and 9; Gesemann et al., 1996). Hence, unlike the binding of agrin to α-dystroglycan, the NtA domain has a clear effect on the size of the AChR clusters. One explanation for this phenomenon may be that agrin becomes immobilized on the muscle cell surface by its binding to laminin and thereby prevents small AChR clusters from fusing (see also Discussion in Denzer et al., 1995).
We also think that the binding to laminin is the molecular basis that, after degeneration of the nerve terminals and the muscle fibers, AChR-aggregating activity and agrinlike immunoreactivity is maintained at former synaptic sites for several weeks (Burden et al., 1979; Reist et al., 1987). At least in vitro, the tight association of agrin with muscle cell basal lamina requires the NtA domain; cultured chick myotubes that are incubated for only 30 min with AChR-aggregating full-length agrin (cAgrin7A4B8), subsequently washed and further incubated for 15 h in agrin-free culture medium, show agrin-induced AChR aggregates. In contrast to this, no AChR clusters are induced with the same paradigm using cΔN15AgrinA4B8 or any other active, COOH-terminal agrin fragment (Denzer, A.J., and M.A. Ruegg, unpublished data). Our observation that cΔN15AgrinA4B8 is not capable of inducing AChR clusters by short-term incubation is similar to results of Wallace (1988), who showed that maintenance of AChR clusters in vitro needs the continuous presence of agrin. Since his agrin preparation mainly contained proteolytic fragments of the COOH-terminal half of agrin (Nitkin et al., 1987), these fragments also lack the NtA domain.
Binding of Agrin to Laminin Isoforms and Neuromuscular Junction Development In Vivo
Upon formation of primary muscle fibers, β1-containing laminin isoforms are expressed throughout the muscle cell basal lamina (Chiu and Sanes, 1984; Schuler and Sorokin, 1995). At later stages of synaptogenesis, the β2 chain of laminin (s-laminin) accumulates in synaptic basal lamina, and the β1 chain is displaced from this region and continues to be expressed extrasynaptically (Chiu and Sanes, 1984; Hunter et al., 1989; Sanes et al., 1990). In contrast to the β chains, the α2 and γ1 chains are found throughout the muscle fiber basal lamina (Sanes et al., 1990). These results strongly suggest that laminin-2 (α2, β1, γ1) and laminin-4 (α2, β2, γ1) are the laminin isoforms in the extrasynaptic and synaptic basal laminae, respectively. We find that, at one particular concentration, laminin-4 binds more strongly to agrin than laminin-2 (Fig. 7). Hence, agrin may preferentially bind to laminin-4, and this may be the basis of the tight association of neural agrin with synaptic basal lamina throughout adulthood.
Similarly, the phenotype of mice that are deficient of the β2 chain of laminin may, at least partially, be based on alterations of agrin. In these mice, NMJs are formed on schedule, but maturation of pre- and postsynaptic specializations is impaired, and terminal Schwann cells penetrate partially the synaptic cleft (Noakes et al., 1995). Although the loss of the β2 chain is compensated by continued expression of the β1 chain at the NMJ (Martin et al., 1996), this may not be sufficient to tether agrin to the NMJ. Since agrin has been shown to influence the formation of presynaptic specializations (Campagna et al., 1995; Gautam et al., 1996), alterations in the binding of agrin to the NMJ may also explain the abnormalities in presynaptic specialization in the β2-deficient mice.
For the current studies, we used agrin isoforms that include a 7–amino acid insert at the NH2-terminal splice site. Motor neurons in the developing chick spinal cord contain agrin transcripts encoding this agrin variant, whereas the majority of agrin mRNA in nonneuronal cells codes for agrin without the insert (Denzer et al., 1995; Tsen et al., 1995b ). Interestingly, the cDNA clones encoding mouse and human agrin, which are derived from nonneuronal tissue, all encode the splice variant lacking the insert (Fig. 6). Preliminary results show that both splice variants bind to laminin-1 (Denzer, A.J., and M.A. Ruegg, unpublished observation). Since there appears to be a difference in the binding of the two splice variants to Matrigel™ (Denzer et al., 1995), it will be interesting to see whether splicing at this site influences binding of agrin to different laminin isoforms.
Little is known about the function of agrin isoforms without AChR-aggregating activity. Since these inactive isoforms bind more strongly to α-dystroglycan than AChRaggregating isoforms and since they also bind to laminin, the inactive isoforms might have a structural role to link the basement membrane with the underlying cytoskeleton via the dystrophin–glycoprotein complex (for reviews see Campbell, 1995; Worton, 1995). We have now generated the tools that will enable studies on the physiological significance of the binding of agrin to laminin isoforms and to the different binding proteins expressed on the muscle cell membrane, like α-dystroglycan.
Acknowledgments
We thank Beat Schumacher for excellent technical assistance and Angelo Marangi for determining the sequence of the mouse cDNAs. We thank Drs. J.L. Bixby (Miami University School of Medicine, Miami, FL) for providing the construct encoding the Fc part of mouse IgG and P. Sonderegger (University of Zurich, Switzerland) for the vitreous fluid. We are indebted to Dr. A. Brancaccio, Dr. K. Kühn, T. Schulthess, and Dr. R. Timpl for providing us with purified proteins. In addition, we are grateful to Dr. T. Meier for his comments on the manuscript.
This work was supported by grants to M.A. Ruegg and M. Chiquet from the Swiss National Science Foundation, and grants to M.A. Ruegg from the Swiss Foundation for Research on Muscle Diseases and the Rentenanstalt/Swiss Life.
Abbreviations used in this paper
- AChE
acetylcholinesterase
- AChR
acetylcholine receptor
- ECM
extracellular marix
- HS-GAG
heparan sulfate glycosaminoglycan
- HSPG
heparan sulfate proteoglycan
- NMJ
neuromuscular junction
- NtA
NH2-terminal domain in agrin
- VF
vitreous fluid
Footnotes
Address all correspondence to Markus A. Ruegg, Department of Pharmacology, Biozentrum, University of Basel, Klingelbergstrasse 70, CH-4056 Basel, Switzerland. Tel.: 41 61 267 2246 or 2213. Fax: 41 61 267 2208. E-mail: rueegg@ubaclu.unibas.ch
Ralph Brandenberger's current address is the Department of Physiology, University of California San Francisco School of Medicine, San Francisco, CA 94143-0724.
Matthias Chiquet's current address is Maurice E. Müller-Institute for Biomechanics, P.O. Box 30, CH-3010 Bern, Switzerland.
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bowe MA, Fallon JR. The role of agrin in synapse formation. Annu Rev Neurosci. 1995;18:443–462. doi: 10.1146/annurev.ne.18.030195.002303. [DOI] [PubMed] [Google Scholar]
- Bowe MA, Deyst KA, Leszyk JD, Fallon JR. Identification and purification of an agrin receptor from Torpedo postsynaptic membranes: a heteromeric complex related to the dystroglycans. Neuron. 1994;12:1173–1180. doi: 10.1016/0896-6273(94)90324-7. [DOI] [PubMed] [Google Scholar]
- Bowen MA, Lee RK, Miragliotta G, Nam SY, Podack ER. Structure and expression of murine CD30 and its role in cytokine production. J Immunol. 1996;156:442–449. [PubMed] [Google Scholar]
- Brancaccio A, Schulthess T, Gesemann M, Engel J. Electron microscopic evidence for a mucin-like region in chick muscle α-dystroglycan. FEBS (Fed Eur Biol Soc) Lett. 1995;368:139–142. doi: 10.1016/0014-5793(95)00628-m. [DOI] [PubMed] [Google Scholar]
- Brandenberger R, Chiquet M. Distinct heparin-binding and neurite-promoting properties of laminin isoforms isolated from chick heart. J Cell Sci. 1995;108:3099–3108. doi: 10.1242/jcs.108.9.3099. [DOI] [PubMed] [Google Scholar]
- Brandenberger R, Kammerer RA, Engel J, Chiquet M. Native chick laminin-4 containing the β2 chain (s-laminin) promotes motor axon growth. J Cell Biol. 1996;135:1583–1592. doi: 10.1083/jcb.135.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubacher D, Wehrle-Haller B, Chiquet M. Chick laminin: isolation by monoclonal antibodies and differential distribution of variants in the embryo. Exp Cell Res. 1991;197:290–299. doi: 10.1016/0014-4827(91)90435-w. [DOI] [PubMed] [Google Scholar]
- Burden SJ, Sargent PB, McMahan UJ. Acetylcholine receptors in regenerating muscle accumulate at original synaptic sites in the absence of the nerve. J Cell Biol. 1979;82:412–425. doi: 10.1083/jcb.82.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgeson RE, Chiquet M, Deutzmann R, Ekblom P, Engel J, Kleinman H, Martin GR, Meneguzzi G, Paulsson M, Sanes J, et al. A new nomenclature for the laminins. Matrix Biol. 1994;14:209–211. doi: 10.1016/0945-053x(94)90184-8. [DOI] [PubMed] [Google Scholar]
- Campagna JA, Rüegg MA, Bixby JL. Agrin is a differentiationinducing “stop signal” for motoneurons in vitro. Neuron. 1995;15:1365–1374. doi: 10.1016/0896-6273(95)90014-4. [DOI] [PubMed] [Google Scholar]
- Campanelli JT, Roberds SL, Campbell KP, Scheller RH. A role for dystrophin-associated glycoproteins and utrophin in agrin-induced AChR clustering. Cell. 1994;77:663–674. doi: 10.1016/0092-8674(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Campbell KP. Three muscular dystrophies: loss of cytoskeleton-extracellular matrix linkage. Cell. 1995;80:675–679. doi: 10.1016/0092-8674(95)90344-5. [DOI] [PubMed] [Google Scholar]
- Chiu AY, Sanes JR. Development of basal lamina in synaptic and extrasynaptic portions of embryonic rat muscle. Dev Biol. 1984;103:456–467. doi: 10.1016/0012-1606(84)90333-6. [DOI] [PubMed] [Google Scholar]
- Cohen MW, Godfrey EW. Early appearance of and neuronal contribution to agrin-like molecules at embryonic frog nerve-muscle synapses formed in culture. J Neurosci. 1992;12:2982–2992. doi: 10.1523/JNEUROSCI.12-08-02982.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MW, Moody CF, Godfrey EW. Neuritic deposition of agrin on culture substrate: implications for nerve-muscle synaptogenesis. J Neurosci. 1994;14:3293–3303. doi: 10.1523/JNEUROSCI.14-05-03293.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denzer AJ, Gesemann M, Schumacher B, Ruegg MA. An aminoterminal extension is required for the secretion of chick agrin and its binding to extracellular matrix. J Cell Biol. 1995;131:1547–1560. doi: 10.1083/jcb.131.6.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denzer AJ, Gesemann M, Ruegg MA. Diverse functions of the extracellular matrix molecule agrin. Semin Neurosci. 1996;8:357–366. [Google Scholar]
- Ferns MJ, Campanelli JT, Hoch W, Scheller RH, Hall Z. The ability of agrin to cluster AChRs depends on alternative splicing and on cell surface proteoglycans. Neuron. 1993;11:491–502. doi: 10.1016/0896-6273(93)90153-i. [DOI] [PubMed] [Google Scholar]
- Fox JW, Mayer U, Nischt R, Aumailley M, Reinhardt D, Wiedemann H, Mann K, Timpl R, Krieg T, Engel J, Chu M-L. Recombinant nidogen consists of three globular domains and mediates binding of laminin to collagen type IV. EMBO (Eur Mol Biol Organ) J. 1991;10:3137–3146. doi: 10.1002/j.1460-2075.1991.tb04875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam M, Noakes PG, Moscoso L, Rupp F, Scheller RH, Merlie JP, Sanes JR. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell. 1996;85:525–535. doi: 10.1016/s0092-8674(00)81253-2. [DOI] [PubMed] [Google Scholar]
- Gee SH, Montanaro F, Lindenbaum MH, Carbonetto S. Dystroglycan-α, a dystrophin-associated glycoprotein, is a functional agrin receptor. Cell. 1994;77:675–686. doi: 10.1016/0092-8674(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Gesemann M, Denzer AJ, Ruegg MA. Acetylcholine receptoraggregating activity of agrin isoforms and mapping of the active site. J Cell Biol. 1995;128:625–636. doi: 10.1083/jcb.128.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesemann M, Cavalli V, Denzer AJ, Brancaccio A, Schumacher B, Ruegg MA. Alternative splicing of agrin alters its binding to heparin, dystroglycan, and the putative agrin receptor. Neuron. 1996;16:755–767. doi: 10.1016/s0896-6273(00)80096-3. [DOI] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981;23:175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Godfrey EW. Comparison of agrin-like proteins from the extracellular matrix of chicken kidney and muscle with neural agrin, a synapse organizing protein. Exp Cell Res. 1991;195:99–109. doi: 10.1016/0014-4827(91)90504-n. [DOI] [PubMed] [Google Scholar]
- Godfrey EW, Nitkin RM, Wallace BG, Rubin LL, McMahan UJ. Components of Torpedo electric organ and muscle that cause aggregation of acetylcholine receptors on cultured muscle cells. J Cell Biol. 1984;99:615–627. doi: 10.1083/jcb.99.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey EW, Dietz ME, Morstad AL, Wallskog PA, Yorde DE. Acetylcholine receptor-aggregating proteins are associated with the extracellular matrix of many tissues in Torpedo. . J Cell Biol. 1988a;106:1263–1272. doi: 10.1083/jcb.106.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey EW, Siebenlist RE, Wallskog PA, Walters LM, Bolender DL, Yorde DE. Basal lamina components are concentrated in premuscle masses and at early acetylcholine receptor clusters in chick embryo hindlimb muscles. Dev Biol. 1988b;130:471–486. doi: 10.1016/0012-1606(88)90343-0. [DOI] [PubMed] [Google Scholar]
- Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Hagen SG, Michael AF, Butkowski RJ. Immunochemical and biochemical evidence for distinct basement membrane heparan sulfate proteoglycans. J Biol Chem. 1993;268:7261–7269. [PubMed] [Google Scholar]
- Halfter W. A heparan sulfate proteoglycan in developing avian axonal tracts. J Neurosci. 1993;13:2863–2873. doi: 10.1523/JNEUROSCI.13-07-02863.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall ZW, Sanes JR. Synaptic structure and development: the neuromuscular junction. Cell. 1993;72:99–121. doi: 10.1016/s0092-8674(05)80031-5. [DOI] [PubMed] [Google Scholar]
- Hunter DD, Shah V, Merlie JP, Sanes JR. A laminin-like adhesive protein concentrated in the synaptic cleft of the neuromuscular junction. Nature (Lond) 1989;338:229–234. doi: 10.1038/338229a0. [DOI] [PubMed] [Google Scholar]
- Kleinman HK, McGarvey ML, Liotta LA, Robey PG, Tryggvason K, Martin GR. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982;21:6188–6193. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- Lennon G, Auffray C, Polymeropoulos M, Soares MB. The I.M.A.G.E. Consortium: an integrated molecular analysis of genomes and their expression. Genomics. 1996;33:151–152. doi: 10.1006/geno.1996.0177. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Magill-Solc C, McMahan UJ. Motor neurons contain agrin-like molecules. J Cell Biol. 1988;107:1825–1833. doi: 10.1083/jcb.107.5.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PT, Ettinger AJ, Sanes JR. A synaptic localization domain in the synaptic cleft protein laminin β2 (s-laminin) Science (Wash DC) 1995;269:413–416. doi: 10.1126/science.7618109. [DOI] [PubMed] [Google Scholar]
- Martin PT, Kaufman SJ, Kramer RH, Sanes JR. Synaptic integrins in developing, adult, and mutant muscle: selective association of α1, α7A, and α7B integrins with the neuromuscular junction. Dev Biol. 1996;174:125–139. doi: 10.1006/dbio.1996.0057. [DOI] [PubMed] [Google Scholar]
- McMahan UJ. The agrin hypothesis. Cold Spring Harbor Symp Quant Biol. 1990;55:407–418. doi: 10.1101/sqb.1990.055.01.041. [DOI] [PubMed] [Google Scholar]
- McMahan UJ, Horton SE, Werle MJ, Honig LS, Kroger S, Ruegg MA, Escher G. Agrin isoforms and their role in synaptogenesis. Curr Opin Cell Biol. 1992;4:869–874. doi: 10.1016/0955-0674(92)90113-q. [DOI] [PubMed] [Google Scholar]
- Morrissey JH. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981;117:307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Nitkin RM, Rothschild TC. Agrin-induced reorganization of extracellular matrix components on cultured myotubes: relationship to AChR aggregation. J Cell Biol. 1990;111:1161–1170. doi: 10.1083/jcb.111.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitkin RM, Smith MA, Magill C, Fallon JR, Yao Y-MM, Wallace BG, McMahan UJ. Identification of agrin, a synaptic organizing protein from Torpedo electric organ. J Cell Biol. 1987;105:2471–2478. doi: 10.1083/jcb.105.6.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noakes PG, Gautam M, Mudd J, Sanes JR, Merlie JP. Aberrant differentiation of neuromuscular junctions in mice lacking s-laminin/laminin β2. Nature (Lond) 1995;374:258–262. doi: 10.1038/374258a0. [DOI] [PubMed] [Google Scholar]
- Ott U, Odermatt E, Engel J, Furthmayr H, Timpl R. Protease resistance and conformation of laminin. Eur J Biochem. 1982;123:63–72. doi: 10.1111/j.1432-1033.1982.tb06499.x. [DOI] [PubMed] [Google Scholar]
- Paulsson M, Aumailley M, Deutzmann R, Timpl R, Beck K, Engel J. Laminin-nidogen complex. Extraction with chelating agents and structural characterization. Eur J Biochem. 1987;166:11–19. doi: 10.1111/j.1432-1033.1987.tb13476.x. [DOI] [PubMed] [Google Scholar]
- Pearson WR, Lipman DJ. Improved tools for biological sequence comparison. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perris R, Brandenberger R, Chiquet M. Differential neural crest cell attachment and migration on avian laminin isoforms. Int J Dev Neurosci. 1996;14:297–314. doi: 10.1016/0736-5748(96)00015-9. [DOI] [PubMed] [Google Scholar]
- Reist NE, Magill C, McMahan UJ. Agrin-like molecules at synaptic sites in normal, denervated, and damaged skeletal muscles. J Cell Biol. 1987;105:2457–2469. doi: 10.1083/jcb.105.6.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries A, Engel J, Lustig A, Kuhn K. The function of the NC1 domains in type IV collagen. J Biol Chem. 1995;270:23790–23794. doi: 10.1074/jbc.270.40.23790. [DOI] [PubMed] [Google Scholar]
- Ruegg MA. Agrin, laminin β2 (s-laminin) and ARIA: their role in neuromuscular development. Curr Opin Neurobiol. 1996;6:97–103. doi: 10.1016/s0959-4388(96)80014-6. [DOI] [PubMed] [Google Scholar]
- Ruegg MA, Stoeckli ET, Kuhn TB, Heller M, Zuellig R, Sonderegger P. Purification of axonin-1, a protein that is secreted from axons during neurogenesis. EMBO (Eur Mol Biol Organ) J. 1989;8:55–63. doi: 10.1002/j.1460-2075.1989.tb03348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegg MA, Tsim KW, Horton SE, Kröger S, Escher G, Gensch EM, McMahan UJ. The agrin gene codes for a family of basal lamina proteins that differ in function and distribution. Neuron. 1992;8:691–699. doi: 10.1016/0896-6273(92)90090-z. [DOI] [PubMed] [Google Scholar]
- Rupp F, Payan DG, Magill-Solc C, Cowan DM, Scheller RH. Structure and expression of a rat agrin. Neuron. 1991;6:811–823. doi: 10.1016/0896-6273(91)90177-2. [DOI] [PubMed] [Google Scholar]
- Sanes JR. The synaptic cleft of the neuromuscular junction. Semin Dev Biol. 1995;6:163–173. [Google Scholar]
- Sanes JR, Marshall LM, McMahan UJ. Reinnervation of muscle fiber basal lamina after removal of myofibers. Differentiation of regenerating axons at original synaptic sites. J Cell Biol. 1978;78:176–198. doi: 10.1083/jcb.78.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR, Engvall E, Butkowski R, Hunter DD. Molecular heterogeneity of basal laminae: isoforms of laminin and collagen IV at the neuromuscular junction and elsewhere. J Cell Biol. 1990;111:1685–1699. doi: 10.1083/jcb.111.4.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler F, Sorokin LM. Expression of laminin isoforms in mouse myogenic cells in vitro and in vivo. J Cell Sci. 1995;108:3795–3805. doi: 10.1242/jcs.108.12.3795. [DOI] [PubMed] [Google Scholar]
- Smith MA, Magill-Solc C, Rupp F, Yao YM, Schilling JW, Snow P, McMahan UJ. Isolation and characterization of an agrin homologue in the marine ray. Mol Cell Neurosci. 1992;3:406–417. doi: 10.1016/1044-7431(92)90052-4. [DOI] [PubMed] [Google Scholar]
- Sugiyama J, Bowen DC, Hall ZW. Dystroglycan binds nerve and muscle agrin. Neuron. 1994;13:103–115. doi: 10.1016/0896-6273(94)90462-6. [DOI] [PubMed] [Google Scholar]
- Timpl R. Macromolecular organization of basement membranes. Curr Opin Cell Biol. 1996;8:618–624. doi: 10.1016/s0955-0674(96)80102-5. [DOI] [PubMed] [Google Scholar]
- Timpl R, Brown JC. Supramolecular assembly of basement membranes. Bioessays. 1996;18:123–131. doi: 10.1002/bies.950180208. [DOI] [PubMed] [Google Scholar]
- Timpl R, Rohde H, Robey PG, Rennard SI, Foidart JM, Martin GR. Laminin—a glycoprotein from basement membranes. J Biol Chem. 1979;254:9933–9937. [PubMed] [Google Scholar]
- Tsen G, Halfter W, Kroger S, Cole GJ. Agrin is a heparan sulfate proteoglycan. J Biol Chem. 1995a;270:3392–3399. doi: 10.1074/jbc.270.7.3392. [DOI] [PubMed] [Google Scholar]
- Tsen G, Napier A, Halfter W, Cole GJ. Identification of a novel alternatively spliced agrin mRNA that is preferentially expressed in nonneuronal cells. J Biol Chem. 1995b;270:15934–15937. doi: 10.1074/jbc.270.27.15934. [DOI] [PubMed] [Google Scholar]
- Tsim KWK, Ruegg MA, Escher G, Kröger S, McMahan UJ. cDNA that encodes active agrin. Neuron. 1992;8:677–689. doi: 10.1016/0896-6273(92)90089-v. [DOI] [PubMed] [Google Scholar]
- Wallace BG. Regulation of agrin-induced acetylcholine receptor aggregation by Ca++and phorbol ester. J Cell Biol. 1988;107:267–278. doi: 10.1083/jcb.107.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace BG. Agrin-induced specializations contain cytoplasmic, membrane, and extracellular matrix-associated components of the postsynaptic apparatus. J Neurosci. 1989;9:1294–1302. doi: 10.1523/JNEUROSCI.09-04-01294.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber S, Engel J, Wiedemann H, Glanville RW, Timpl R. Subunit structure and assembly of the globular domain of basement-membrane collagen type IV. Eur J Biochem. 1984;139:401–410. doi: 10.1111/j.1432-1033.1984.tb08019.x. [DOI] [PubMed] [Google Scholar]
- Worton R. Muscular dystrophies: diseases of the dystrophin-glycoprotein complex. Science (Wash DC) 1995;270:755–756. doi: 10.1126/science.270.5237.755. [DOI] [PubMed] [Google Scholar]
- Yurchenco PD, O'Rear JJ. Basal lamina assembly. Curr Opin Cell Biol. 1994;6:674–681. doi: 10.1016/0955-0674(94)90093-0. [DOI] [PubMed] [Google Scholar]
- Yurchenco PD, Sung U, Ward MD, Yamada Y, O'Rear JJ. Recombinant laminin G domain mediates myoblast adhesion and heparin binding. J Biol Chem. 1993;268:8356–8365. [PubMed] [Google Scholar]