Abstract
In cells specialized for secretory granule exocytosis, lysosomal hydrolases may enter the regulated secretory pathway. Using mouse pancreatic islets and the INS-1 β-cell line as models, we have compared the itineraries of procathepsins L and B, two closely related members of the papain superfamily known to exhibit low and high affinity for mannose-6-phosphate receptors (MPRs), respectively. Interestingly, shortly after pulse labeling INS cells, a substantial fraction of both proenzymes exhibit regulated exocytosis. After several hours, much procathepsin L remains as precursor in a compartment that persists in its ability to undergo regulated exocytosis in parallel with insulin, while procathepsin B is efficiently converted to the mature form and can no longer be secreted. However, in islets from transgenic mice devoid of cation-dependent MPRs, the modest fraction of procathepsin B normally remaining within mature secretory granules is increased approximately fourfold. In normal mouse islets, immunoelectron microscopy established that both cathepsins are present in immature β-granules, while immunolabeling for cathepsin L, but not B, persists in mature β-granules. By contrast, in islets from normal male SpragueDawley rats, much of the proenzyme sorting appears to occur earlier, significantly diminishing the stimulusdependent release of procathepsin B. Evidently, in the context of different systems, MPR-mediated sorting of lysosomal proenzymes occurs to a variable extent within the trans-Golgi network and is continued, as needed, within immature secretory granules. Lysosomal proenzymes that fail to be sorted at both sites remain as residents of mature secretory granules.
Lysosomes are digestive organelles found in all eukaryotic cells (Kornfeld and Mellman, 1989), whereas secretory granules are apparent only in a subgroup of cell types specialized for regulated secretion of certain proteins that are stored at high concentration (Kelly, 1985; Burgess and Kelly, 1987). In recent years, studies of specialized cell types have reported structural and functional overlap between lysosomes and secretory granules (Taugner and Hackenthal, 1988; Bonifacino et al., 1989; Burkhardt et al., 1989, 1990), including the idea that a fraction of lysosomal enzymes/proenzymes may exist in the regulated secretory pathway of endocrine and exocrine tissues under physiological conditions (Brands et al., 1982; Docherty et al., 1984; Tooze et al., 1991; Hirano et al., 1992).
To date, the trafficking of lysosomal prohydrolases has been well studied only in cells that produce few or no secretory granules. From these studies, the basic schema of posttranslational carbohydrate modification and recognition by mannose-6-phosphate receptors (MPRs)1 is now generally accepted (von Figura and Hasilik, 1986; von Figura, 1991). By contrast, two substantially different kinds of hypotheses, which are not mutually exclusive, have prevailed concerning the sorting of regulated secretory proteins. The “sorting for entry” theories include the possibility of one or more conserved receptors whose primary function would be to bind lumenal content proteins in the TGN and “usher” them (Kelly, 1987) or “zipper” them (Kelly, 1985; Kelly, 1991; Tooze and Stinchcombe, 1992) into forming granules. Thus, it has been envisaged in sorting for entry models that at the time of entry into immature granules (IGs), regulated secretory proteins are either bound to receptors (Moore et al., 1989) or bound to other regulated secretory proteins that are bound to receptors (Palmer and Christie, 1992; Pimplikar and Huttner, 1992; Leblond et al., 1993; Yoo, 1993; Natori and Huttner, 1996; Cool et al., 1997), while nongranule secretory proteins remain soluble and are excluded from entry into IGs (Bauerfeind and Huttner, 1993), being shunted to the constitutive secretory pathway by default (Moore et al., 1983; Kelly, 1985). Alternatively, the “sorting by retention” hypothesis argues that proteins need not enter IGs in an aggregated state but may be soluble; subsequently, much of the condensation (insolubility) of regulated secretory proteins is promoted within IGs (Garreau de Loubresse et al., 1994; Gautier et al., 1994; Kuliawat and Arvan, 1994; Huang and Arvan, 1994, 1995). Condensation limits the ability of regulated secretory proteins to escape from maturing granules by constitutive-like vesicle budding (Arvan and Castle, 1987; Arvan and Chang, 1987; vonZastrow and Castle, 1987; Arvan et al., 1991; Grimes and Kelly, 1992; Kuliawat and Arvan, 1992), while other lumenal proteins may be removed by these constitutive-like vesicles (Arvan and Castle, 1992).
In the β-cells of pancreatic islets, 99% of newly synthesized proinsulin may enter IGs (Rhodes and Halban, 1987), which are the major if not exclusive site of proinsulin endoproteolytic conversion to C-peptide and insulin (Orci et al., 1985, 1994; Rhodes et al., 1987; Steiner et al., 1987; Huang and Arvan, 1994; for review see Halban, 1994). Under unstimulated conditions, the release of newly synthesized C-peptide in molar excess of newly synthesized insulin can be accounted for by the constitutive-like secretory pathway (Arvan et al., 1991), which is hypothesized to be initiated by clathrin-coated vesicle budding from IGs (Kuliawat and Arvan, 1992). Indeed, clathrin patches and buds can be observed on IG membranes but are no longer present on mature granules (Tooze and Tooze, 1986; Orci et al., 1987b ), while treatments that inhibit the loss of clathrin from maturing granules (Orci et al., 1984a ) largely block initiation of the constitutive-like secretory pathway (Kuliawat and Arvan, 1992). Presumably, the immediate target of these IG-derived clathrin-coated vesicles is endosomes, compartments from which some newly synthesized proteins can be relocated to the cell surface (Fishman and Fine, 1987; Futter et al., 1995).
The presence of clathrin coats on IGs implicates the presence of transmembrane receptors that cluster and bud from this compartment, during which C-peptide may be conveyed nonspecifically as a fluid-phase marker. Interestingly, recent work has indicated that the AP-1 clathrinassociated protein complex interacts with the cytosolic tails of MPRs (Glickman et al., 1989; Mauxion et al., 1996), and further, the AP-1 adaptors can associate with IGs (Dittie et al., 1996). If, in regulated secretory cells, MPRs can include the IG compartment as well as the TGN in their trafficking itinerary, this could account for the presence of lysosomal enzymes in secretory granules. Specifically, it has been proposed that in large part, the specialized lysosomal enzyme precursors of cytotoxic lymphocytes are targeted to secretory granules via MPRs (Griffiths and Isaaz, 1993). By contrast, we have reported that in pancreatic islets treated with tunicamycin, the entry of unglycosylated procathepsin L into IGs appears unimpaired, implying that MPRs facilitate a step other than proenzyme entry into this compartment (Kuliawat and Arvan, 1994).
These considerations have raised several important questions. First, because tunicamycin affects all newly synthesized glycoproteins, including putative sorting receptors, what is the ability to interpret the fate of procathepsin L after generalized inhibition of N-linked glycosylation? Second, knowing that there are several cell types in pancreatic islets, can β-cells account for the stimulated release of prohydrolases from pancreatic islets? Third, given reports of stimulated externalization of lumenal contents from the endosomal system (Bomsel and Mostov, 1993; Hansen and Casanova, 1994) and the fact that lysosomal proenzymes normally traffic through this system (Ludwig et al., 1991), do immature secretory granules really contain hydrolase precursors, or does stimulated proenzyme discharge derive from externalization of endosomal contents? Fourth, could procathepsin L targeting to lysosomes in β-cells be mediated largely by a non–MPR-dependent sorting mechanism? To explore these questions, we have compared the targeting in β-cells of two close relatives in the papain superfamily of thiol proteases (Barrett and Kirschke, 1981; Takio et al., 1983; Koga et al., 1990) with distinct affinity for MPRs: procathepsin B (ProB) exhibits a high affinity (Hanewinkel et al., 1987), while procathepsin L (ProL) exhibits low-affinity MPR binding (Dong et al., 1989; Dong and Sahagian, 1990; Lazzarino and Gabel, 1990; Stearns et al., 1990). We report that from studies of the INS-1 β-cell line and mouse islets, a significant fraction of both proenzymes enters IGs, after which ProB is more efficiently removed from maturing granules. Moreover, a small but definite decrease in the efficiency of ProB removal from IGs is observed in the islets of transgenic mice lacking one of the two types of MPR, the cation-dependent MPR. On the other hand, lysosomal proenzyme sorting in the TGN and IGs is a dynamic process, underscored by new observations that in islets isolated from male SpragueDawley rats, newly synthesized ProB can reach lysosomes without appearing in large quantity in the stimulus-dependent secretory pathway. These data support a view of IGs as a functional continuation of the TGN (Arvan and Castle, 1992). Thus, to the degree that lysosomal enzyme precursors enter IGs, the efficiency of their subsequent sorting appears related to MPR recognition, leading to a clathrin-coated vesicle escape route from maturing secretory granules.
Materials and Methods
Antibodies and Other Materials
Immunoprecipitating antisera against rat ProB (Rowan et al., 1992) and cathepsin L were graciously provided by Drs. J. Mort (Shriners Hospital, Montreal, Canada) and G. Sahagian (Tufts University, Boston, MA), respectively. For immunoelectron microscopy, an mAb directed against a proinsulin cleavage site (Orci et al., 1986), a polyclonal antibody against rat cathepsin B, and anticlathrin (Hille et al., 1992) were obtained from Drs. O Madsen (Hagedorn Research Institute, Copenhagen, Denmark), A. Saluja (Beth Israel Hospital, Boston, MA), and F. Brodsky (University of California, San Francisco), respectively. Guinea pig antiinsulin was from Linco (St. Louis, MO). Horseradish peroxidase–conjugated anti–rabbit serum (Cappel/Worthington) was used as a secondary for Western blotting (enhanced chemiluminescence; Amersham Corp., Arlington Heights, IL). Collagenase P was from Boehringer Mannheim (Indianapolis, IN); hypaque, human serum albumin, BSA, soybean trypsin inhibitor, other protease inhibitors, tunicamycin, DTT, and iodoacetamide were from Sigma Chemical Co. (St. Louis, MO); calf serum and antibiotic-antimycotic solution were from GIBCO-BRL (Gaithersburg, MD); [35S]methionine/cysteine (Expre35S35S) was from New England Nuclear (New Bedford, MA); zysorbin–protein A was from Zymed Labs (So. San Francisco, CA).
Preparation and Labeling of Cell Lines and Islets
The INS-1 cell line (generously provided Dr. P. Halban, Institut Jeantet, University of Geneva) was cultured as described (Neerman-Arbez and Halban, 1993). INS cells were washed twice with methionine-free, cysteinefree DME before pulse labeling for 30 min at 37°C in the same medium containing ∼100 μCi of [35S]methionine and cysteine per two million cells. At the conclusion of the pulse labeling, the cells were washed and chased in complete DME containing 1.67 mM glucose. For studies of nonglycosylated lysosomal enzymes, cells were pretreated with 20 μg/ml tunicamycin or mock-treated for 5 h. Rat islets (Arvan et al., 1991) and mouse islets (Kuliawat and Arvan, 1994) were prepared, pulse labeled, and chased exactly as described previously. All labeling and chase media also contained 0.5 μg/ml human serum albumin (plus 0.005% soybean trypsin inhibitor for islets). When comparing islets from adult male and female MPR-deficient mice (Ludwig et al., 1993) vs. control males and females matched for age and parentage (C57B/129), identical protocols were followed. All rat islets were prepared from 275-g male Sprague-Dawleys supplied by Charles River Labs, Inc. (Wilmington, MA). Granule exocytosis was stimulated in DME with 0.5 mg/ml RIA grade BSA plus a combination secretagogue including glucose (10 mM for INS cells, 22 mM for islets), 1 μM phorbol 12-myristate 13-acetate, 1 mM isobutyl-methylxanthine, and 1 mM tolbutamide (and 10 mM leucine and glutamine for INS cells).
Islets or cells were lysed in boiling 1% SDS. Samples were then diluted 1:10 in immunoprecipitation buffer yielding final concentrations of: 100 mM NaCl, 1% Triton X-100, 0.2% Na deoxycholate, 0.1% SDS, 10 mM EDTA, 0.7 mM DTT, 10 mM iodoacetamide, and 25 mM Tris, pH 7.4. An antiprotease cocktail of aprotinin (1 mU/ml), leupeptin (0.1 mM), pepstatin (10 mM), EDTA (5 mM), and diisopropylfluorophosphate (1 mM) was added to the islet lysates and collected media. Both cells and media were precleared by a brief incubation with Zysorbin followed by spinning in a microfuge before further analysis.
SDS-PAGE, Fluorography, and Phosphorimaging
Immunoprecipitated lysosomal enzymes were resolved by SDS 12%PAGE for cathepsins B and L, while immunoprecipitated insulin was analyzed by SDS 15%-PAGE plus urea using a Tricine buffer system (Schagger and vonJagow, 1987). The products of the two insulin genes expressed in rodents were not distinguished from one another. The insulin gels were fixed initially in 20% TCA (20 min), then in 12.5% TCA plus 50% methanol (15 min), then bathed in water (5 min), and finally incubated with 1 M Na salicylate for 15 min. Dried gels were exposed to XAR film at −70°C. Scanned x-ray films were finally analyzed using the ImageQuant program (Molecular Dynamics, Sunnyvale, CA).
Ultrathin Cryosectioning of Mouse Pancreatic Islets
Mouse islets prepared as above were fixed for 2 h at room temperature in a mixture of 0.5% glutaraldehyde and 2% formaldehyde, freshly prepared from paraformaldehyde, in 100 mM Na-phosphate buffer, pH 7.4. The fixed islets were embedded in 10% gelatin in PBS and cut into 1-mm3 blocks. These blocks were impregnated with 2.3 M sucrose and further prepared for cryosectioning as described (Slot et al., 1988). Multiple immunogold labeling was performed as described (Slot et al., 1991), after which the grids were analyzed in an electron microscope (model 1010; JEOL U.S.A., Inc., Peabody, MA). Quantitation of mean gold particle density per granule was determined from secretory granules encountered at random by counting the number of gold particles representing either cathepsin B or cathepsin L in sections that were double immunolabeled for proinsulin. Granule profiles that contained proinsulin label were marked as IGs. By videoplanometer, mean surface area measured in the same sections was not significantly different for proinsulin-immunopositive and proinsulin-immunonegative granules. In total, cathepsin B labeling was counted in 45 IGs and 60 mature granules, while 55 IGs and 59 mature granules were analyzed for cathepsin L.
Results
A Significant Fraction of ProB Enters and Then Exits the Regulated Secretory Pathway of INS Cells
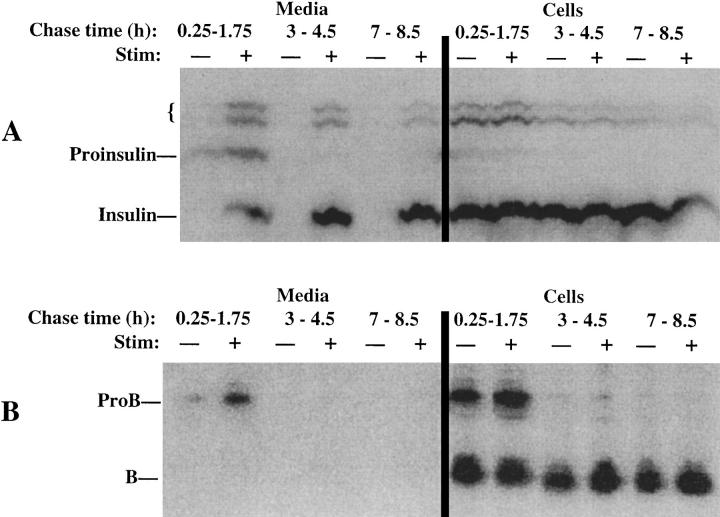
The trafficking of ProB, a high-affinity ligand for MPRs, was examined in the well-differentiated β-cell line, INS-1 (Asfari et al., 1992), which exhibits regulated exocytosis of insulin in a nearly linear fashion during prolonged (4 h) stimulation (Neerman-Arbez and Halban, 1993). After a 30-min pulse labeling with 35S–amino acids, stimulated or unstimulated secretions from labeled INS cells were sampled at different 90-min chase intervals during an 8.5-h time course. At all chase times, secretagogue-induced exocytosis of newly synthesized insulin (or proinsulin) was obtained (Fig. 1 A, left). During the interval from 15–105 min of chase, stimulus-dependent secretion of ProB could be elicited (Fig. 1 B, left). By quantitative scanning densitometry, the stimulus-dependent release of ProB represented 17% of all immunoprecipitable B obtained at the 105-min chase time and 37.3% of immunoprecipitable ProB obtained at this chase time, while stimulus-dependent secretion of immunoprecipitable insulin–containing peptides was 32% during the same interval. These data indicate that a significant fraction of ProB entered the regulated secretory pathway. Of course, ProB delivery to lysosomes was also ongoing during this chase period, as judged by processing in the cell lysates to the mature 31-kD cathepsin B. Indeed, at times >3 h of chase, labeled ProB was essentially absent from INS cell lysates (Fig. 1 B, right, and data not shown), indicating that its arrival in lysosomes was complete. Consequently, in response to secretagogue addition after 3 h of chase, despite continued regulated exocytosis of labeled insulin (Fig. 1 A, left), INS cells did not release any form of cathepsin B into the medium (Fig. 1 B, left), indicating that neither ProB nor mature cathepsin B remained to any significant extent in the secretory pathway.
Figure 1.
Stimulus-dependent release from INS cells of peptides immunoprecipitable with antiinsulin (A) or anti– cathepsin B (B). The cells, pulse labeled as described in Materials and Methods, were chased for up to 8.5 h. During different 90-min chase intervals, either stimulated (+) or unstimulated (−) secretion was collected (left), and the cells were then lysed. Equal fractions of media and cells were immunoprecipitated using anti-ProB, and one-tenth of these volumes were taken for precipitation with antiinsulin. The positions of proinsulin, insulin, presumptive proinsulin conversion intermediates (small bracket), ProB, and mature cathepsin B (B) are shown. Scanning densitometry of these gels led to the numerical data described in the text for this representative experiment (of three).
Normal Entry but Deficient Exit of ProL in the Regulated Secretory Pathway of INS Cells
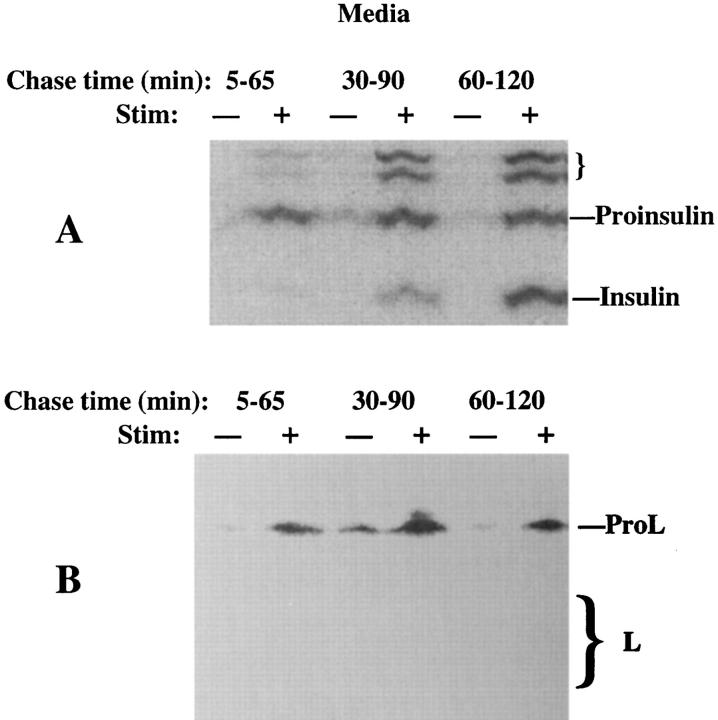
We next used INS cells to examine the trafficking of ProL, a low-affinity ligand for MPRs (Dong and Sahagian, 1990; Lazzarino and Gabel, 1990). Because of the possibility of rapid intracellular transport of ProL (Dong et al., 1989; Kane, 1993) and long stimulation times required to elicit granule exocytosis from INS cells (Neerman-Arbez and Halban, 1993), we initially screened for ProL secretion beginning 5 min after a 30-min pulse labeling (Fig. 2). As shown during the first 2 h of chase, while proinsulin to insulin conversion was clearly ongoing (Fig. 2 A), secretagogue-induced secretion of ProL was also observed (Fig. 2 B). Evidently, like ProB, newly synthesized ProL could also enter a stimulus-dependent secretory pathway in INS cells. However, unlike ProB, at chase times up to 6 h, only ∼20% of ProL ever reached lysosomes, as judged by conversion in the cell lysates to the bands comprising processed forms of mature L (Fig. 3). A 3-h secretagogue exposure elicited secretion of ⩾50% of granule insulin from INS cells, in agreement with previous reports (NeermanArbez and Halban, 1993). Importantly, the residual fraction of labeled ProL (Fig. 3, right) exhibited comparable stimulated secretion (Fig. 3, left).
Figure 2.
Stimulus-dependent release from INS cells of peptides immunoprecipitable with antiinsulin (A) or anti–cathepsin L (B). The cells were pulse labeled and chased as in Fig. 1, except that the stimulated (+) or unstimulated (−) medium collections began at 5 min of chase, were 60 min in duration, and were terminated at 2 h of chase. Only the medium is shown. While stimulated secretion of ProL is seen at all chase times, note the progression of processing from proinsulin to insulin in the regulated secretory pathway. Measurement of stimulus-dependent secretion during a 1-h period for L-containing peptides (∼16%) was in a similar range to that of insulin-containing peptides (∼21%) in this experiment. The positions of proinsulin, insulin, presumptive proinsulin conversion intermediates (small bracket), ProL, and bands comprising mature cathepsin L (M r ∼32,000 and ∼27,000, respectively, large bracket) are shown.
Figure 3.
Inefficient arrival of newly synthesized ProL in lysosomes of INS cells. Cells pulse labeled as in Fig. 1 were chased to 3 h before a further 3-h collection of stimulated (+) or unstimulated (−) secretion was obtained and the cells analyzed by immunoprecipitation for lysosomal arrival of cathepsin L. Stimulus-dependent secretion contained ∼50% of all immunoprecipitable L-containing peptides found in the 3 h of collected medium plus cell lysate, while insulin secretion as measured by RIA averaged 53% during this period. The positions of ProL and bands comprising mature cathepsin L (large bracket) are shown.
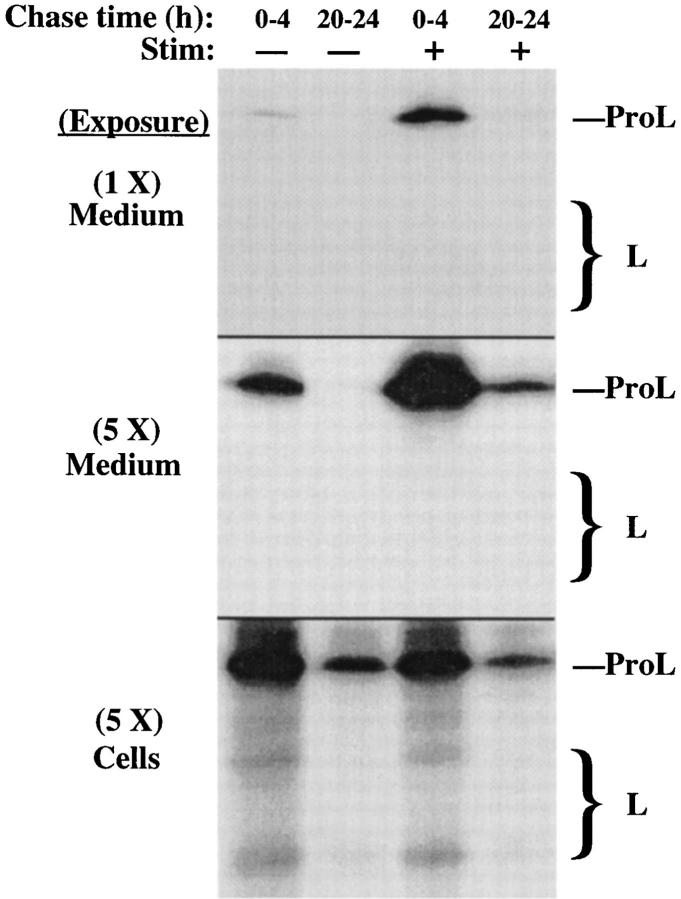
To characterize the ultimate fate of intracellular cathepsin L, we compared early chase times to those prolonged up to 1 d to allow maximum opportunity for newly synthesized ProL molecules to reach their proper final targets. Unstimulated and stimulated secretions from INS cells were collected from either 0–4 h of chase or 20–24 h of chase. A short exposure of the media (Fig. 4, top) demonstrated a fivefold stimulated release of ProL during the early chase period (Fig. 4, first and third lanes). In the cell lysates, the arrival of labeled cathepsin L in lysosomes was modest, based on the appearance of the processed mature forms (Fig. 4, bottom, first and third lanes). Remarkably, by 24 h of chase, the newly synthesized mature lysosomal cathepsin L had almost entirely turned over, while intracellular ProL was still detected in a pool that appeared longer lived (Fig. 4, bottom, second and fourth lanes). When the INS cells were exposed to secretagogue during the last 4 h of the experiment, the signal was diminished in proportion to the amount of labeled ProL remaining, but an approximately fivefold stimulated release of ProL was still obtained (Fig. 4, middle), and the fraction of the remaining ProL that exhibited stimulus-dependent exocytosis was not diminished from that seen at the earlier chase time. Moreover, the disappearance of mature L forms, with the sustained presence of ProL, suggested that ProL was contained in a compartment from which delivery to lysosomes was not actively ongoing. Taken together, these findings indicated that a considerable fraction of ProL persisted in secretory granules.
Figure 4.
A significant fraction of ProL in INS cells becomes entrapped in long-term storage in the regulated secretory pathway. The stimulated (+) and unstimulated (−) secretions from pulselabeled INS cells were collected either during the first 4 h after labeling or after a chase of 20 h to first allow all newly synthesized ProL molecules to reach their final targets. At the conclusion of each set, both media and cell lysates were analyzed by immunoprecipitation with anti–cathepsin L. Two exposures of the media, differing fivefold, are shown in the upper two panels. Note the disappearance of labeled forms of mature L at later chase times, and the persistent stimulus-dependent secretion of the precursor. The positions of ProL and bands comprising mature cathepsin L (large bracket) are shown.
Is the Correctly Delivered Fraction of ProL to Lysosomes Conveyed by a Non-MPR Mechanism?
Although only a minority of ProL ever arrives in lysosomes in INS cells, we sought to determine whether this modest fraction, some of which apparently traverses the IG compartment, uses MPRs to escape from the regulated secretory pathway. This issue seemed especially important since there is evidence that the propeptide of ProL can interact with a putative receptor in intracellular membranes that can assist in sorting ProL from prelysosomes to lysosomes (McIntyre and Erickson, 1991), and this interaction occurs even for unglycosylated ProL synthesized after tunicamycin treatment (McIntyre and Erickson, 1993; McIntyre et al., 1994). For this reason, we examined the effects of tunicamycin to block N-linked glycosylation and thereby prevent formation of the mannose-6-phosphate (M6P) moiety on ProL. It has previously been shown that such inhibition does not prevent the entry of ProL into the stimulus-dependent secretory pathway in mouse β-cells (Kuliawat and Arvan, 1994); however, whether unglycosylated ProL can escape from maturing granules has never previously been examined.
In the experiment shown in Fig. 5, INS cells were pulse labeled and chased for 3.5 h before a 1-h period of stimulus-dependent secretion was analyzed. The left panels show the results from control cells that are similar to Fig. 3, insofar as partial delivery to lysosomes was detected. The right panels were performed in parallel from INS cells treated with tunicamycin. In this case, samples were intentionally double loaded to enhance the sensitivity of detection of mature cathepsin L forms. Nevertheless, it was clear that the arrival of unglycosylated L in lysosomes was profoundly inhibited, while there was a commensurate increase in the amount of intracellular ProL. Evidently, in these cells, despite the low affinity of ProL for MPRs, that portion of ProL which is successfully targeted to lysosomes still uses a carbohydrate-dependent sorting mechanism. Moreover, the increase in missorted ProL after tunicamycin treatment resulted in a proportionate increase in the pool of unglycosylated precursor in the regulated secretory pathway, as demonstrated by markedly increased stimulus-dependent secretion (Fig. 5, right).
Figure 5.
Stimulus-dependent release from INS cells treated with tunicamycin. Untreated INS cells (left) or those pretreated with tunicamycin (right) were pulse labeled and chased for 3.5 h before 60-min collections of stimulated (+) or unstimulated (−) secretion, or the resulting cell lysates were analyzed by immunoprecipitation with antiinsulin (A) or anti– cathepsin L (B). For INS cells treated with tunicamycin, samples were intentionally double loaded to enhance the sensitivity of detection of mature cathepsin L forms. Note that after tunicamycin treatment, the amount of intracellular precursor increased disproportionately, as did the amount of stimulus-dependent secretion of ProL. The positions of proinsulin, insulin, presumptive proinsulin conversion intermediates (small bracket), glycosylated and unglycosylated ProL, and bands comprising glycosylated and unglycosylated mature cathepsin L (large brackets) are shown.
Immunoelectron Microscopy of ProB and ProL in Mouse Islet β-Cells
Our data obtained in INS cells as well as mouse pancreatic islets (Kuliawat and Arvan, 1994) seemed consistent with the hypothesis that a fraction of prohydrolases, unsorted at the level of the TGN, may enter IGs and thereby acquire the property of regulated secretion. Subsequently, ProB is efficiently removed from IGs, while most intragranular ProL fails to be removed. To further explore this hypothesis, we examined ultrathin cryosections of mouse pancreatic islet tissue to directly localize these antigens in β-cells by immunoelectron microscopy.
In studying the distribution of immunoreactive cathepsins in pancreatic β-cells, one theoretical concern was the ability to discriminate between insulin-containing lysosomes (Orci et al., 1984b ; Halban et al., 1987) and true secretory granules. In fact, lysosomes, tubular lysosomes, and crinophagic lysosomes were all unmistakably distinguishable from secretory granules by immunoelectron microscopy, as well as by conventional morphological criteria (Fig. 6, A–C). Further, to reliably differentiate IGs from mature granules, we capitalized on the existence of a monoclonal antibody to proinsulin, whose immunoreactivity is lost upon processing to insulin (Orci et al., 1986). Thus, when examining β-cells of mouse islets, mature granules that have little residual proinsulin remain unlabeled, while IGs of different ages contain varying degrees of labeling (Fig. 7). When double or triple labeling with this mAb was performed along with anti–cathepsin B, cathepsin immunoreactivity was clearly evident in early granules (Figs. 7 and 8, A and B). In addition, proinsulin-positive IGs not infrequently exhibited electron-dense coated membrane buds similar to coated vesicles forming in the TGN. Using an antibody to clathrin, coated buds on both IGs and the TGN were specifically decorated (Fig. 9). However, not only did such buds appear absent from mature granules, but cathepsin B immunolabeling also appeared far less abundant over mature granules (Figs. 7 and 8, A and B).
Figure 6.
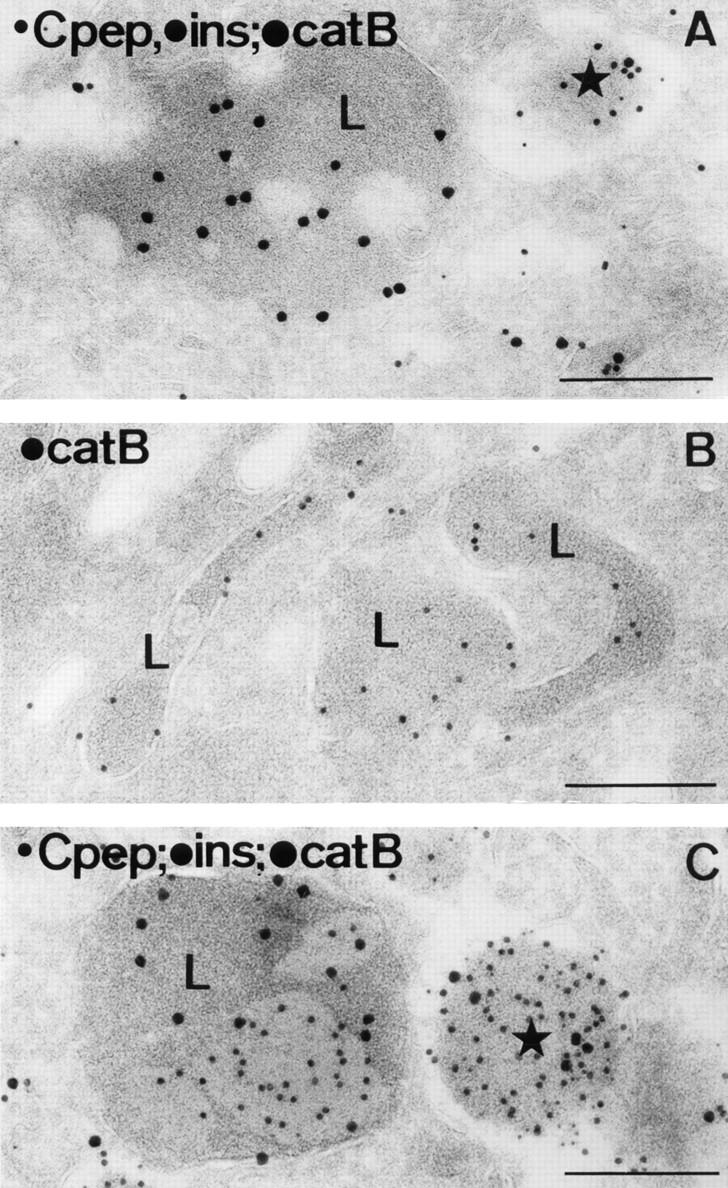
In β-cells of mouse pancreatic islets, lysosomes are morphologically distinct from secretory granules. (A) A typical lysosome (L) juxtaposed next to a mature secretory granule (star). The section was triple immunolabeled for C-peptide (5-nm gold), insulin (10-nm gold) and cathepsin B (15-nm gold). (B) Tubular lysosomes (L) immunopositive for cathepsin B (10-nm gold). (C) A crinophagic vacuole (L) juxtaposed next to a mature secretory granule (star). Triple immunolabeling was as in A. Consistent with previous reports (Orci et al., 1984b ; Halban et al., 1987), C-peptide labeling is absent from lysosomes, although insulin labeling may be present. Bars, 200 nm.
Figure 7.
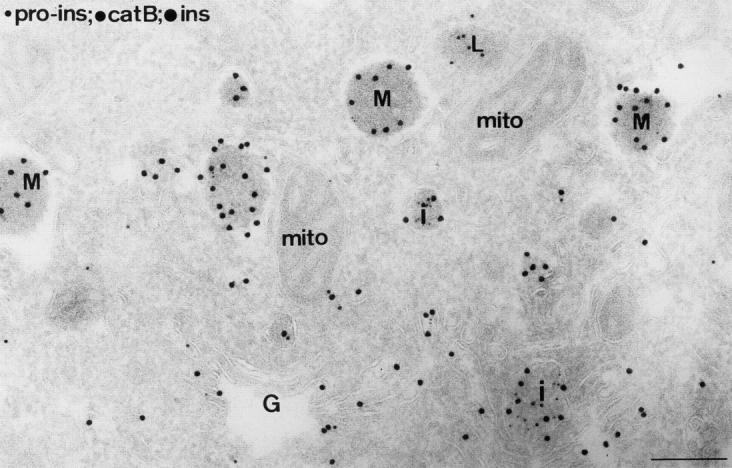
Ultrathin cryosection of the Golgi (G) region of a mouse islet β-cell, triple immunolabeled for proinsulin (5-nm gold), cathepsin B (10-nm gold), and insulin (15-nm gold). Insulin label was found over all compartments of the secretory pathway. Granule profiles with the presence of proinsulin label were used to define IGs (I), while those with insulin labeling only and a variable-sized halo between the content and surrounding membrane were considered mature granules (M). A lysosome (L) is seen to be labeled exclusively for cathepsin B, although additional cathepsin B label can be seen in IGs. mito, mitochondrion. Bar, 200 nm.
Figure 8.
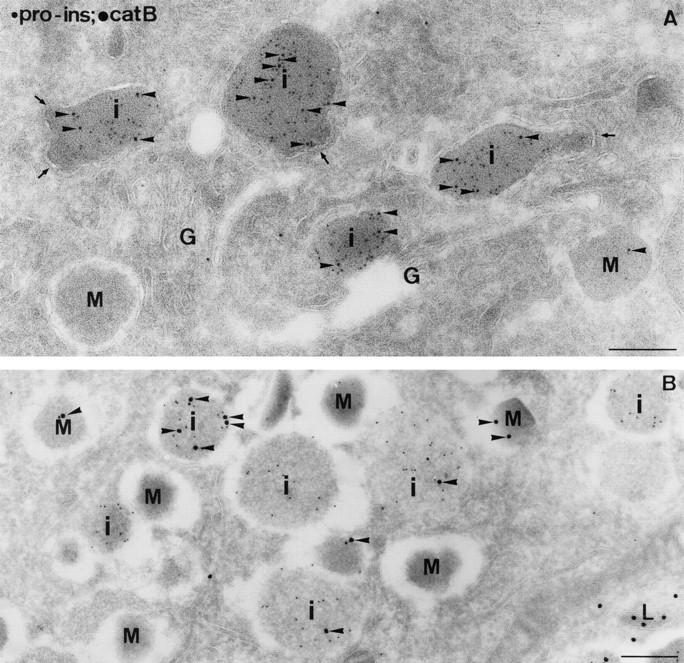
The occurrence of cathepsin B in IGs (i) of mouse islet β-cells. Ultrathin cryosections were double immunolabeled for proinsulin and cathepsin B. Cathepsin B label (arrowheads) was clearly present over proinsulin-positive IGs (i), whereas mature granules (M) were almost devoid of label. (A) Proinsulin (5-nm gold); cathepsin B (10-nm gold). Coated buds (arrows) were often observed on IGs, especially in the Golgi (G) region. Immunoreactive cathepsins are occasionally found directly in the buds, but, similar to reports from nonregulated secretory cells (Geuze et al., 1984, 1985; Ludwig et al., 1991), immunogold detection of hydrolase precursors tends to be impaired directly in the bud region. (B) Proinsulin (5-nm gold); cathepsin B (15-nm gold). L, lysosome. Bars, 200 nm.
Figure 9.
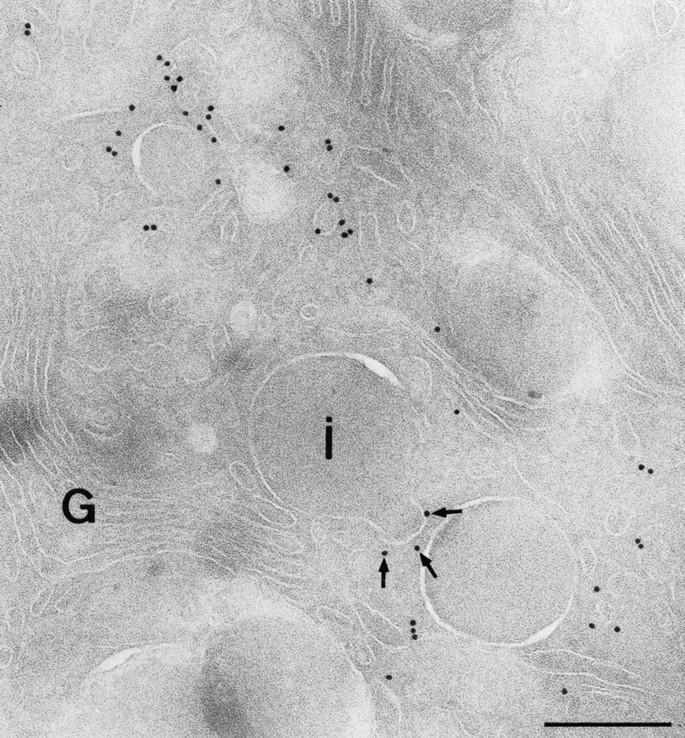
Coated buds that form on IGs contain clathrin. Ultrathin cryosections were immunolabeled with an antibody to clathrin heavy chain. Labeling is present on numerous vesicular profiles at the trans-side of the Golgi complex (G) and on a membrane bud of an IG (i), marked with arrows. Bar, 200 nm.
Similar double labelings confirmed the presence of cathepsin L immunoreactivity in IGs, while substantial persistence of this labeling was observed in many granules that no longer contained proinsulin immunoreactivity (Fig. 10). Indeed, mature β-granules (identified with antiinsulin) were strongly, albeit heterogeneously, immunolabeled for cathepsin L (Fig. 11, A and B). Moreover, in triple immunolabeling, mature β-granules that could be strongly labeled for cathepsin L contained cathepsin B labeling at only low levels (Fig. 11 C). A quantitative analysis of β-cells immunolabeled with antiproinsulin was then performed to estimate the relative concentration of cathepsin B between IGs and mature granules. The mean labeling density fell an order of magnitude from 2.3 ± 0.35 (SEM) over IGs to 0.23 ± 0.06 over mature granules, and this decline was statistically significant (P < 0.005). In similar double labelings with anti–cathepsin L, the mean labeling density over IGs (2.5 ± 0.25) was not significantly different from that over mature granules (2.5 ± 0.68). These data clearly indicate that in mouse islets, both proenzymes can enter β-cell IGs, but ProL tends to remain to a much higher degree than ProB in mature storage granules.
Figure 10.
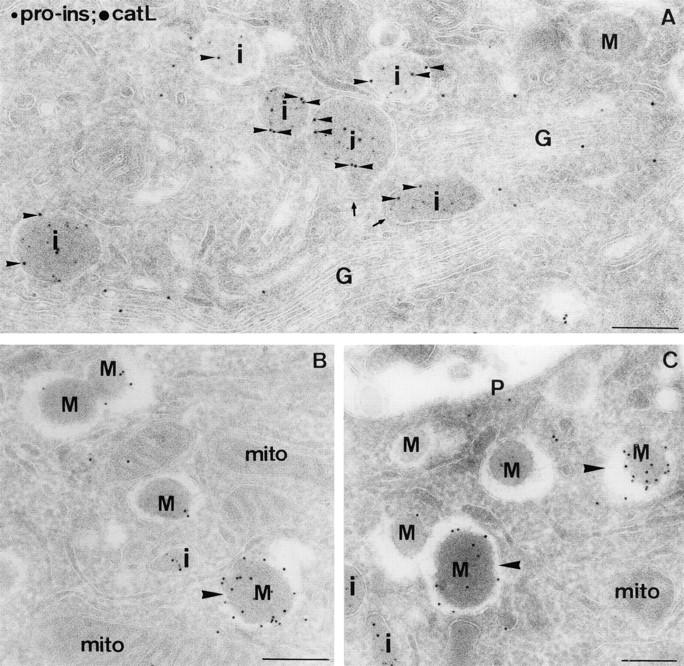
The occurence of cathepsin L in IGs and mature granules of mouse islet β-cells. Ultrathin cryosections were double immunolabeled for proinsulin (5-nm gold) and cathepsin L (10-nm gold). (A) Overview of the Golgi (G) region. Cathepsin L labeling (small arrowheads) was clearly seen over proinsulin-positive IGs (i). The arrows point to coated buds on IGs. (B and C) Cathepsin L label was abundantly observed over both immature (i) and mature granule profiles (M), although some granules were more extensively labeled (large arrowheads) than others. mito, mitochondrion; P, plasmalemma. Bars, 200 nm.
Figure 11.

Immunolabeling of cathepsins L and B in mature granules of mouse islet β-cells. (A and B) Ultrathin cryosections were immunolabeled for cathepsin L (10-nm gold) and insulin (15-nm gold), demonstrating the presence of cathepsin L labeling in mature β-granules. In B, two exocytotic profiles at the plasma membrane (P) are seen. (C) Triple labeling for insulin (5-nm gold), cathepsin L, (10-nm gold), and cathepsin B (15-nm gold) demonstrates a mature granule immunopositive for cathepsin L and immunonegative for cathepsin B (arrow). Occasional immunogold labeling of cathepsin B is indicated by arrowheads. Bars, 200 nm.
Stimulated Secretion of ProB from the Islets of Transgenic Mice Which Lack the Cation-dependent MPR
Most lysosomal proenzymes use MPRs to traffic from the biosynthetic pathway to endosomes, for ultimate delivery to lysosomes (Kornfeld and Mellman, 1989). While M6Pdependent sorting of procathepsin D is thought to be mediated almost exclusively by cation-independent MPRs (CI- MPRs), for most other M6P-containing ligands, CI-MPRs and cation-dependent MPRs (CD-MPRs) are used in combination, each to a greater or lesser degree (Ludwig et al., 1994; Kasper et al., 1996). Because in INS cells and the β-cells of mouse islets, ProB, a high-affinity ligand for MPRs, is efficiently sorted to lysosomes, while a significant fraction of ProL, a low-affinity ligand, remains in secretory granules, the foregoing data imply that prohydrolase exit from maturing granules involves an MPRdependent mechanism. We therefore investigated the trafficking of ProB in the islets of adult mice with targeted disruption of both alleles encoding the CD-MPR (Ludwig et al., 1993) or age-matched controls from genetically similar parentage.
Because CI-MPRs can compensate to a large degree for the absence of CD-MPRs (Ludwig et al., 1994; Kasper et al., 1996), it was not unexpected that in control experiments, fibroblasts from the knockout mice yielded steady-state levels of mature lysosomal cathepsin B that were only slightly altered, and the sorting efficiency measured by pulse-chase was reproducibly diminished by a modest (⩽10%) fraction (data not shown). Nevertheless, because in normal mouse islets at later chase times, the stimulusdependent release of ProB from mature β-granules (i.e., “noise”) is vanishingly small (Kuliawat and Arvan, 1994), it seemed plausible to detect changes in “signal” from missorted ProB stranded in mature β-granules of CD-MPR knockout mice.
Therefore, islets from CD-MPR–deficient or control mice were pulse labeled and chased in the absence of secretagogues for a sufficient period to allow the labeled proinsulin and ProB to chase into mature granules and lysosomes, respectively. Upon subsequent analysis of stimulus-dependent secretion, MPR-deficient mice exhibited a clear increase in stimulated discharge of ProB along with insulin from mature β-granules (Fig. 12). In age-matched control mice, ProB was barely detectable in the stimulusdependent secretion from mature β-granules, representing only ∼3% of all immunoprecipitable forms of cathepsin B,2 whereas in the islets from CD-MPR–deficient mice, this value was ∼11%, i.e., increasing the missorted fraction of ProB nearly fourfold.
Figure 12.
Stimulus-dependent exocytosis of mature β-granules releases an increased fraction of ProB from the islets of transgenic MPRdeficient mice. Islets from CD-MPR–deficient mice were pulse labeled and chased overnight in the absence of secretagogues to allow the labeled proinsulin and ProB to chase to their final destinations, before collecting sequential 30-min periods of unstimulated (−) and stimulated (+) secretion. See text for details. Secretion of labeled insulin and immunoprecipitable ProB were analyzed by SDS-PAGE and fluorography.
Newly Synthesized Prohydrolases Can Exit the Biosynthetic Pathway at Either the TGN or IGs in Pancreatic β-Cells
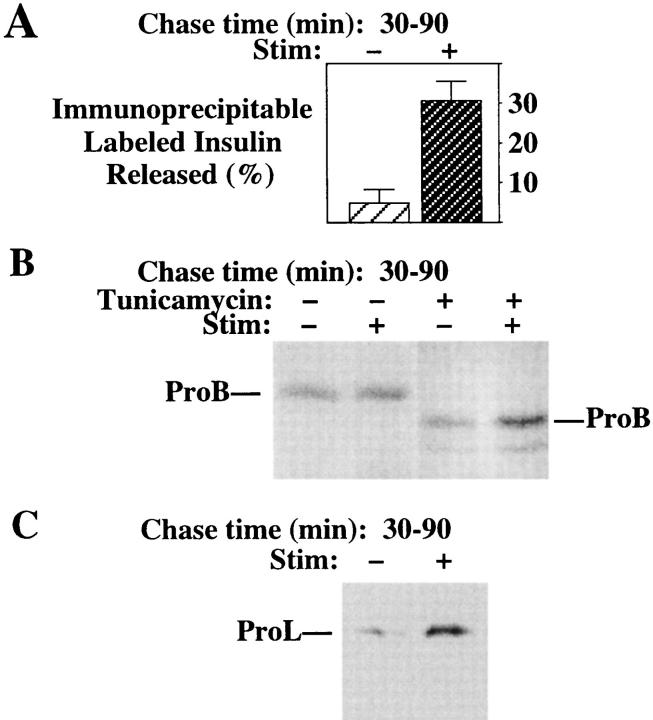
Regulated secretory tissues and cell lines do have a bona fide TGN compartment (Tooze and Huttner, 1990) through which newly synthesized proteins travel before their arrival in post-Golgi compartments (Kuliawat and Arvan, 1992; Miller et al., 1992; Rosa et al., 1992; Xu and Shields, 1993; Huang and Arvan, 1994). Moreover, the potential for clathrin-coated vesicle budding is shared by both the TGN and IGs (Fig. 9). These facts raise an important question (considered further in the Discussion): Specifically, does the sorting of lysosomal hydrolase precursors from TGN to endosomes always have the same efficiency in regulated secretory cells generally, and in β-cells in particular? With this question in mind, we have been interested to explore the extent of prohydrolase entry into β-cell IGs from islets prepared from different animal models. We therefore proceeded to examine the islets of normal male Sprague-Dawley rats. Surprisingly, in spite of the brisk stimulated release of labeled proinsulin and insulin (Fig. 13 A), even at early chase times the presence of newly synthesized ProB in IGs (as detected by stimulus-dependent secretion; Fig. 13 B, left) was obviously diminished from that observed in INS cells (Fig. 1) or the islets of normal mice (Kuliawat and Arvan, 1994). Similar “negative” results were obtained upon examination of a second high-affinity MPR ligand, the ∼75-kD β-glucuronidase precursor (data not shown). By contrast, after tunicamycin treatment such that newly synthesized ProB was unglycosylated, ProB entrance into the regulated secretory pathway resumed (Fig. 13 B, right). Moreover, in the case of ProL, even without tunicamycin treatment, stimulus-dependent secretion could be detected in the islets of normal male Sprague Dawley rats (Fig. 13 C). These data indicate that although lysosomal proenzymes may enter IGs in abundance, this entrance can vary considerably. Thus, IGs may functionally extend the TGN but do not serve as a mandatory intermediate in the trafficking of hydrolase precursors to lysosomes in all cases in regulated secretory cells.
Figure 13.
Unstimulated and stimulated secretion of newly synthesized insulin (A), ProB (B), and ProL (C) from pancreatic islets of normal male Sprague-Dawley rats. The islets, untreated or pretreated with tunicamycin, were pulse labeled for 30 min. During 30–90 min of chase, the islets were exposed either to unstimulated (−) or stimulated (+) conditions. Secretion of proinsulin + insulin was analyzed by immunoprecipitation as described (Arvan et al., 1991). Immunoprecipitable ProB and ProL were analyzed by SDS-PAGE and fluorography. After tunicamycin treatment, ProB was secreted as the unglycosylated form.
Discussion
In this report, we have tried to examine the dynamic nature of lysosomal proenzyme sorting in pancreatic β-cells. These studies evolved from earlier work using tunicamycin treatment to investigate the trafficking of lysosomal proenzymes, which led to the hypothesis that M6P recognition is likely to be used for prohydrolase escape from maturing granules (Kuliawat and Arvan, 1994). However, because of theoretical concerns about the use of tunicamycin (see introduction), and because of recent reports of regulated exocytosis that is not mediated by secretory granules (Chavez et al., 1996), herein we have taken several independent approaches, focusing especially on a comparative analysis of the trafficking of two homologous proenzymes, ProL and ProB, that exhibit low and high affinity for MPRs, respectively (Hanewinkel et al., 1987; Dong et al., 1989; Dong and Sahagian, 1990; Lazzarino and Gabel, 1990; Stearns et al., 1990).
Despite differences in MPR affinity, a significant fraction of both prohydrolases initially gain access to the regulated secretory pathway in INS cells (Figs. 1–3). However, with further time, the regulated secretion of ProL persists in parallel with insulin (Fig. 4), while ProB is selectively chased out of compartments exhibiting stimulus-dependent exocytosis (Fig. 1), just as has been observed in normal mouse pancreatic islets (Kuliawat and Arvan, 1994). Importantly, using mouse islets, we have directly visualized immunoreactive cathepsins L and B in β-cell IGs by immunoelectron microscopy (Figs. 8 and 10), and we have further confirmed the significant persistence of immunolabeled cathepsin L in fully mature β-granules (Figs. 10 and 11). These data help to exclude the possibility that the regulated exocytosis of prohydrolases derives from alternative stimulus-dependent discharge pathways (Bomsel and Mostov, 1993; Hansen and Casanova, 1994; Chavez et al., 1996)3. Instead, our findings strongly suggest that lysosomal proenzymes can enter IGs, and, while not directing this entry, MPR association facilitates prohydrolase exit from maturing β-granules.
From a balance sheet of the ultimate fate of ProL in INS cells beginning from the zero chase time, we estimate that of 100 newly synthesized molecules, ∼50 molecules of ProL are lost by secretion throughout a 20-h unstimulated chase period, ∼20 molecules are converted to mature L and subsequently turn over intralysosomally, while ∼30 molecules are still present as intracellular ProL, such that when ∼50% of insulin undergoes stimulus-dependent exocytosis during the last 4 h of the experiment, approximately half of these 30 molecules of labeled ProL are released in parallel. For ProB in INS cells, the major difference appears to be that after a 20-h chase, ∼50 molecules remain intracellularly as mature lysosomal cathepsin B, whereas <1 molecule remains in secretory granules for exocytosis. Mature β-granules in isolated mouse islets also contain essentially no radiolabeled ProB (Kuliawat and Arvan, 1994). Thus, the biochemical and morphological analyses presented in this report indicate that, because of a low affinity for MPRs, the sorting efficiency of ProL during exit from maturing secretory granules is impaired approximately one order of magnitude compared to ProB. Nevertheless, nearly all of the ProL that does successfully reach lysosomes requires carbohydrate-dependent sorting,4 and when this sorting is abrogated, even more ProL accumulates in secretory granules (Fig. 5). Furthermore, in the case of ProB, despite that CI-MPRs can direct the trafficking of most but not the entire fraction that becomes available when both CD-MPR alleles are disrupted (these results being consistent with independent reports [Kasper et al., 1996]), a distinctly increased fraction of ProB is stranded in mature β-granules in the islets of transgenic mice that are normal except for the absence of CD-MPRs (Fig. 12).
All of these results point to the idea that in pancreatic β-cells, although lysosomal prohydrolases may be recognized by MPRs in the TGN and from there conveyed to endosomes, should they fail to be sorted at the TGN, lysosomal proenzymes may then enter IGs. Nevertheless, this does not automatically mean missorting because MPR recognition can still be used for escape from the regulated secretory pathway. Only the “doubly missorted” prohydrolases (i.e., those that fail to be sorted at both the TGN and IGs) appear to become stranded as residents of mature secretory granules.
Active debate continues about different protein sorting mechanisms involved in the formation of mature secretory granules and the fidelity of sorting in different model systems. Our studies of the clonally derived INS cell line indicate that (a) secretory granules in the β-cell can account for the stimulated release of procathepsins previously observed in mouse islets and (b) by replicating the features of lysosomal proenzyme trafficking, INS cells serve as a valuable model system, in which biochemical analyses tend to be easier to execute than in primary islet preparations. However, because post-Golgi trafficking in professional secretory cells is physiologically regulated, no model system can quantitatively represent all aspects of postGolgi sorting that at different times takes place under a diverse range of conditions.
Prohydrolase sorting at the TGN is a complex process based on the use of predominantly previously synthesized MPRs for the sorting of newly synthesized lysosomal enzymes (Kornfeld and Mellman, 1989). Aside from consideration of ligand affinities, the efficiency of TGN sorting from moment to moment is based on dynamic changes in the concentration of available ligands and MPRs fluxing through the biosynthetic pathway. Notably, in β-cells, lysosomal biogenesis is modulated largely by the same factors that alternately stimulate either granule exocytosis or crinophagy (Borg and Schnell, 1986; Landstrom et al., 1988, 1991; Schnell et al., 1988; Borg et al., 1995). Most importantly, protein flow rate through the secretory pathway, which can be extraordinarily high in regulated secretory cells, influences the “lumenal dwell time” of the ligands and receptors. This dwell time is much shorter in the TGN than is the case for IGs (which can serve as a functional extension of TGN [Arvan and Castle, 1992]). Thus, in certain systems and under certain conditions, substantial fractions of lysosomal precursors may escape the TGN and enter IGs; by contrast, in other systems prohydrolase entry into IGs can be greatly attenuated (Fig. 13 B). However, even in the latter case, entry of lysosomal precursors into IGs resumes under conditions when TGN sorting by MPRs is incomplete (Fig. 13, B and C).
Because prohydrolase sorting efficiency in the TGN of different regulated secretory cells can vary, investigations need to be extended to exocrine and other regulated secretory cell types to determine the extent to which protein sorting during granule maturation is generally applicable or limited to selective systems. However, we note that constitutive-like secretion has been observed in exocrine tissues (Arvan and Castle, 1987; Arvan and Chang, 1987; vonZastrow and Castle, 1987; Arvan and Lee, 1991) in which there is evidence for progressive condensation of regulated secretory proteins in conjunction with progressive loss of membrane by vesicular budding from IGs (Sesso, A., B. Kachar, S.M. Carneiro, and I. Zylberman. 1990. J. Cell Biol. 111:312a), an increase in dry mass concentration due to volume reduction during granule maturation (Wong et al., 1991), and, during size reduction, a decrease in the granule membrane of the number of intramembranous particles, which appear to be removed by the budding of small vesicles heavily studded with such particles (Sesso et al., 1980). However, an important distinction may need to be considered between granule maturation in endocrine and exocrine cell types. Specifically, in endocrine cell types, as granules mature, they become more acidic (Orci et al., 1994), and as the pH continues to drop towards pH 5, MPRs can no longer interact with lysosomal proenzymes. Thus, even if MPRs remained available for clathrin-coated vesicle budding late in granule maturation, they would not efficiently remove lysosomal proenzymes, which would then be missorted. Moreover, such acidity has the potential to autoactivate missorted hydrolases within granules, leading to the possibility of digestion of intragranular contents (Neerman-Arbez and Halban, 1993; Conlon et al., 1995). By contrast, mature exocrine granules do not appreciably acidify (Arvan et al., 1984; Arvan et al., 1985; Arvan and Castle, 1986; Orci et al., 1987a ). Thus, the only factors that could cause lysosomal proenzyme missorting to mature granules in these cells (Tooze et al., 1991) would be a limited availability of MPRs, the budding off of which is thought to be a process kinetically limited to the period of granule maturation (Arvan and Castle, 1992). Clarification of these issues will clearly require further investigation.
Acknowledgments
This work was supported in part by National Institutes of Health grant DK48280 and a Research Grant from the American Diabetes Association to P. Arvan.
Footnotes
1. Abbreviations used in this paper: CD- and CI-MPR, cation-dependent and -independent mannose-6-phosphate receptors; IG, immature granules; M6P, mannose-6-phosphate; MPR, mannose-6-phosphate receptor; ProB and L, procathepsin B and L.
4. Notably, our data in pancreatic β-cells do not specifically support the function of a non–carbohydrate-dependent lysosomal proenzyme receptor (McIntyre and Erickson, 1991, 1993; McIntyre et al., 1994) in sorting out of the biosynthetic pathway, although whether such a receptor might be involved in trafficking steps that occur within the endocytic pathway remains an open question.
2. The quantitative data from transgenic and control animals were obtained from film scanning of two independent experiments, in which all individual values were within 30% of the mean. Because there is conservation of 14 cysteines and four methionines between ProB and the mature form, no correction factor to account for isotope recovery was applied to our calculations.
The authors are greatly indebted to Drs. P. Halban and M. NeermanArbez for providing and instructing us on the use of INS cells, as well as important suggestions and discussions during the course of this work. We are grateful to Drs. J. Mort, O. Madsen, G. Sahagian, A. Saluja, and F. Brodsky for generously providing the antibodies used in these studies. We thank T. Broers-Vendrig and J. Griffiths for technical assistance, as well as R. Scriwanek and M. Niekerk (Free University, Amsterdam) for preparation of the electron micrographs. We also thank members of the Arvan lab and Professor H.J. Geuze (University of Utrecht, The Netherlands) for advice and support.
Address all correspondence to Peter Arvan, Diabetes Research Center and Division of Endocrinology, Albert Einstein College of Medicine, Bronx, NY 10461. Tel.: (718) 430-8685. Fax: (718) 430-8557.
3. MPRs are involved in proenzyme delivery to endosomes (Griffiths et al., 1988). If stimulated prohydrolase secretion were to derive from β-cell endosomes, the reduced affinity of ProL for MPRs should have decreased ProL delivery from the biosynthetic pathway to the stimulatable compartment, which in turn would diminish apparent regulated secretion, whereas in fact, just the opposite was observed (Figs. 3 and 5). Further, long-term storage of ProL in endosomes would not be an expected consequence of a low affinity for MPRs (Kornfeld and Mellman, 1989).
References
- Arvan P, Castle JD. Isolated secretion granules from parotid glands of chronically stimulated rats possess an alkaline internal pH and inward-directed H+pump activity. J Cell Biol. 1986;103:1257–1267. doi: 10.1083/jcb.103.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvan P, Castle JD. Phasic release of newly synthesized secretory proteins in the unstimulated rat exocrine pancreas. J Cell Biol. 1987;104:243–252. doi: 10.1083/jcb.104.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvan P, Castle JD. Protein sorting and secretion granule formation in regulated secretory cells. Trends Cell Biol. 1992;2:327–331. doi: 10.1016/0962-8924(92)90181-l. [DOI] [PubMed] [Google Scholar]
- Arvan P, Chang A. Constitutive protein secretion from the exocrine pancreas of fetal rats. J Biol Chem. 1987;262:3886–3890. [PubMed] [Google Scholar]
- Arvan P, Lee J. Regulated and constitutive protein targeting can be distinguished by secretory polarity in thyroid epithelial cells. J Cell Biol. 1991;112:365–376. doi: 10.1083/jcb.112.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvan P, Rudnick G, Castle JD. Osmotic properties and internal pH of isolated rat parotid secretory granules. J Biol Chem. 1984;259:13567–13572. [PubMed] [Google Scholar]
- Arvan P, Rudnick G, Castle JD. Relative lack of ATP-driven H+ translocase activity in isolated parotid secretory granules. J Biol Chem. 1985;260:14945–14952. [PubMed] [Google Scholar]
- Arvan P, Kuliawat R, Prabakaran D, Zavacki AM, Elahi D, Wang S, Pilkey D. Protein discharge from immature secretory granules displays both regulated and constitutive characteristics. J Biol Chem. 1991;266:14171–14174. [PubMed] [Google Scholar]
- Asfari M, Janjic D, Meda P, Li G, Halban PA, Wollheim CB. Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology. 1992;130:167–178. doi: 10.1210/endo.130.1.1370150. [DOI] [PubMed] [Google Scholar]
- Barrett, A.J., and H. Kirschke. 1981. Cathepsin B, cathepsin H, and cathepsin L. Methods Enzymol. 80(Pt C):535–561. [DOI] [PubMed]
- Bauerfeind R, Huttner WB. Biogenesis of constitutive secretory vesicles, secretory granules and synaptic vesicles. Curr Opin Cell Biol. 1993;5:628–635. doi: 10.1016/0955-0674(93)90132-a. [DOI] [PubMed] [Google Scholar]
- Bomsel M, Mostov KE. Possible role of both the α and βγ subunits of the heterotrimeric G protein, Gs, in transcytosis of the polymeric immunoglobulin receptor. J Biol Chem. 1993;258:25824–25835. [PubMed] [Google Scholar]
- Bonifacino JS, Yuan L, Sandoval IV. Internalization and recycling to serotonin-containing granules of the 80K integral membrane protein exposed on the surface of secreting rat basophilic leukaemia cells. J Cell Sci. 1989;92:701–712. doi: 10.1242/jcs.92.4.701. [DOI] [PubMed] [Google Scholar]
- Borg LA, Schnell AH. Lysosomes and pancreatic islet function: intracellular insulin degradation and lysosomal transformations. Diabetes Res. 1986;3:277–285. [PubMed] [Google Scholar]
- Borg LA, Dahl N, Swenne I. Age-dependent differences in insulin secretion and intracellular handling of insulin in isolated pancreatic islets of the rat. Diabetes Metab Rev. 1995;21:408–414. [PubMed] [Google Scholar]
- Brands R, Slot JW, Geuze HJ. Immunocytochemical localization of β-glucuronidase in the rat preputial gland. Eur J Cell Biol. 1982;27:213–220. [PubMed] [Google Scholar]
- Burgess TL, Kelly RB. Constitutive and regulated secretion of proteins. Annu Rev Cell Biol. 1987;3:243–293. doi: 10.1146/annurev.cb.03.110187.001331. [DOI] [PubMed] [Google Scholar]
- Burkhardt JK, Hester S, Argon Y. Two proteins targeted to the same lytic granule compartment undergo very different posttranslational processing. Proc Natl Acad Sci USA. 1989;86:7128–7132. doi: 10.1073/pnas.86.18.7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt JK, Hester S, Lapham CK, Argon Y. The lytic granules of natural killer cells are dual-function organelles combining secretory and pre-lysosomal compartments. J Cell Biol. 1990;111:2327–2340. doi: 10.1083/jcb.111.6.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez RA, Miller SG, Moore H-PH. A biosynthetic regulated secretory pathway in constitutive secretory cells. J Cell Biol. 1996;133:1177–1191. doi: 10.1083/jcb.133.6.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon JM, Hoog A, Grimelius L. Intracellular degradation of the C-peptide of proinsulin, in a human insulinoma: identification of sites of cleavage and evidence for a role for cathepsin B. Pancreas. 1995;10:167–172. doi: 10.1097/00006676-199503000-00010. [DOI] [PubMed] [Google Scholar]
- Cool DR, Normant E, Shen F-s, Chen H-C, Pannel L, Zhang Y, Loh YP. Carboxypeptidase E is a regulated secretory pathway sorting receptor: genetic obliteration leads to endocrine disorders in Cpe(fat) mice. Cell. 1997;88:73–83. doi: 10.1016/s0092-8674(00)81860-7. [DOI] [PubMed] [Google Scholar]
- Dittie AS, Hajibagheri N, Tooze SA. The AP-1 adaptor complex binds to immature secretory granules from PC12 cells, and is regulated by ADP-ribosylation factor. J Cell Biol. 1996;132:523–536. doi: 10.1083/jcb.132.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty K, Hutton JC, Steiner DF. Cathepsin B-related proteases in the insulin secretory granule. J Biol Chem. 1984;259:6041–6044. [PubMed] [Google Scholar]
- Dong J, Sahagian GG. Basis for low affinity binding of a lysosomal cysteine protease to the cation-independent mannose 6-phosphate receptor. J Biol Chem. 1990;265:4210–4217. [PubMed] [Google Scholar]
- Dong J, Prence EM, Sahagian GG. Mechanism for selective secretion of a lysosomal protease by transformed mouse fibroblasts. J Biol Chem. 1989;264:7377–7383. [PubMed] [Google Scholar]
- Fishman JB, Fine R. A trans Golgi-derived exocytic coated vesicle can contain both newly synthesized cholinesterase and internalized transferrin. Cell. 1987;48:157–164. doi: 10.1016/0092-8674(87)90366-7. [DOI] [PubMed] [Google Scholar]
- Futter CE, Connolly CN, Cutler DF, Hopkins CR. Newly synthesized transferrin receptors can be detected in the endosome before they appear on the cell surface. J Biol Chem. 1995;270:10999–11003. doi: 10.1074/jbc.270.18.10999. [DOI] [PubMed] [Google Scholar]
- Garreau de Loubresse N, Gautier M-C, Sperling L. Immature secretory granules are not acidic in Paramecium: implications for sorting to the regulated pathway. Biol Cell. 1994;82:139–147. [Google Scholar]
- Gautier M-C, Garreau de Loubresse N, Madeddu L, Sperling L. Evidence for defects in membrane traffic in Parameciumsecretory mutants unable to produce functional storage granules. J Cell Biol. 1994;124:893–902. doi: 10.1083/jcb.124.6.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze HJ, Slot JW, Strous GJAM, Hasilik A, von Figura K. Ultrastructural localization of the mannose 6-phosphate receptor in rat liver. J Cell Biol. 1984;98:2047–2054. doi: 10.1083/jcb.98.6.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze HJ, Slot JW, Strous GJAM, Hasilik A, von Figura K. Possible pathways of lysosomal enzyme delivery. J Cell Biol. 1985;101:2253–2262. doi: 10.1083/jcb.101.6.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman JN, Conibear E, Pearse BMF. Specificity of binding of clathrin adaptors to signals on the mannose-6-phosphate/insulin-like growth factor II receptor. EMBO (Eur Mol Biol Organ) J. 1989;8:1041–1047. doi: 10.1002/j.1460-2075.1989.tb03471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G, Hoflack B, Simons K, Mellman I, Kornfeld S. The mannose 6-phosphate receptor and the biogenesis of lysosomes. Cell. 1988;52:329–341. doi: 10.1016/s0092-8674(88)80026-6. [DOI] [PubMed] [Google Scholar]
- Griffiths GM, Isaaz S. Granzymes A and B are targeted to the lytic granules of lymphocytes by the mannose-6-phosphate receptor. J Cell Biol. 1993;120:885–896. doi: 10.1083/jcb.120.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes M, Kelly RB. Intermediates in the constitutive and regulated secretory pathways released in vitro from semi-intact cells. J Cell Biol. 1992;117:539–550. doi: 10.1083/jcb.117.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halban PA. Proinsulin processing in the regulated and the constitutive secretory pathway. Diabetologia. 1994;37:S65–S72. doi: 10.1007/BF00400828. [DOI] [PubMed] [Google Scholar]
- Halban PA, Mutkoski R, Dodson G, Orci L. Resistance of the insulin crystal to lysosomal proteases: implications for pancreatic β-cell crinophagy. Diabetologia. 1987;30:348–353. doi: 10.1007/BF00299029. [DOI] [PubMed] [Google Scholar]
- Hanewinkel H, Glossl J, Kresse H. Biosynthesis of cathepsin B in cultured normal and I-cell fibroblasts. J Biol Chem. 1987;262:12351–12355. [PubMed] [Google Scholar]
- Hansen SH, Casanova JE. Gsa stimulates transcytosis and apical secretion in MDCK cells through cAMP and protein kinase A. J Cell Biol. 1994;126:677–687. doi: 10.1083/jcb.126.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille A, Klumperman J, Geuze HJ, Peter C, Brodsky FM, von Figura K. Lysosomal acid phosphatase is internalized via clathrin-coated pits. Eur J Cell Biol. 1992;59:106–115. [PubMed] [Google Scholar]
- Hirano T, Manabe T, Saluja AK, Steer ML. Pancreatic secretion of lysosomal enzymes stimulated by intraduodenal instillation of a liquid meal in rabbits. Clin Sci (Colch) 1992;83:277–280. doi: 10.1042/cs0830277. [DOI] [PubMed] [Google Scholar]
- Huang XF, Arvan P. Formation of the insulin-containing secretory granule core occurs within immature β-granules. J Biol Chem. 1994;269:20838–20844. [PubMed] [Google Scholar]
- Huang XF, Arvan P. Intracellular transport of proinsulin in pancreatic β-cells: structural maturation probed by disulfide accessibility. J Biol Chem. 1995;270:20417–20423. doi: 10.1074/jbc.270.35.20417. [DOI] [PubMed] [Google Scholar]
- Kane SE. Mouse procathepsin L lacking a functional glycosylation site is properly folded, stable, and secreted by NIH 3T3 cells. J Biol Chem. 1993;268:11456–11462. [PubMed] [Google Scholar]
- Kasper D, Dittmer F, von Figura K, Pohlmann R. Neither type of mannose 6-phosphate receptor is sufficient for targeting of lysosomal enzymes along intracellular routes. J Cell Biol. 1996;134:615–623. doi: 10.1083/jcb.134.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RB. Pathways of protein secretion in eukaryotes. Science (Wash DC) 1985;230:25–32. doi: 10.1126/science.2994224. [DOI] [PubMed] [Google Scholar]
- Kelly RB. From organelle to organelle. Nature (Lond) 1987;326:14–15. doi: 10.1038/326014a0. [DOI] [PubMed] [Google Scholar]
- Kelly R. Secretory granule and synaptic vesicle formation. Curr Opin Cell Biol. 1991;3:654–660. doi: 10.1016/0955-0674(91)90037-y. [DOI] [PubMed] [Google Scholar]
- Koga H, Yamada H, Nishimura Y, Kato K, Imoto T. Comparative study on specificities of rat cathepsin L and papain: amino acid differences at substrate-binding sites are involved in their specificities. J Biochem. 1990;108:976–982. doi: 10.1093/oxfordjournals.jbchem.a123324. [DOI] [PubMed] [Google Scholar]
- Kornfeld S, Mellman I. The biogenesis of lysosomes. Annu Rev Cell Biol. 1989;5:483–525. doi: 10.1146/annurev.cb.05.110189.002411. [DOI] [PubMed] [Google Scholar]
- Kuliawat R, Arvan P. Protein targeting via the “constitutive-like” secretory pathway in isolated pancreatic islets: passive sorting in the immature granule compartment. J Cell Biol. 1992;118:521–529. doi: 10.1083/jcb.118.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuliawat R, Arvan P. Distinct molecular mechanisms for protein sorting within immature secretory granules of pancreatic β-cells. J Cell Biol. 1994;126:77–86. doi: 10.1083/jcb.126.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landstrom AHS, Westman J, Borg LAH. Lysosomes and pancreatic islet function. Diabetes. 1988;37:309–316. doi: 10.2337/diab.37.3.309. [DOI] [PubMed] [Google Scholar]
- Landstrom AHS, Andersson A, Borg LA. Lysosomes and pancreatic islet function: adaptation of beta-cell lysosomes to various metabolic demands. Metabolism. 1991;40:399–405. doi: 10.1016/0026-0495(91)90151-l. [DOI] [PubMed] [Google Scholar]
- Lazzarino D, Gabel CA. Protein determinants impair recognition of procathepsin L phosphorylated oligosaccharides by the cation-independent mannose 6-phosphate receptor. J Biol Chem. 1990;265:11864–11871. [PubMed] [Google Scholar]
- Leblond FA, Viau G, Laine J, Lebel D. Reconstitution in vitro of the pH-dependent aggregation of pancreatic zymogens en route to the secretory granule: implication of GP-2. Biochem J. 1993;291:289–296. doi: 10.1042/bj2910289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig T, Griffiths G, Hoflack B. Distribution of newly synthesized lysosomal enzymes in the endocytic pathway of normal rat kidney cells. J Cell Biol. 1991;115:1561–1572. doi: 10.1083/jcb.115.6.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig T, Ovitt CE, Bauer U, Hollinshead M, Remmler J, Lobel P, Ruther U, Hoflack B. Targeted disruption of the mouse cation-dependent mannose 6-phosphate receptor results in partial missorting of multiple lysosomal enzymes. EMBO (Eur Mol Biol Organ) J. 1993;12:5225–5235. doi: 10.1002/j.1460-2075.1993.tb06218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig T, Munier-Lehmann H, Bauer U, Hollinshead M, Ovitt C, Lobel P, Hoflack B. Differential sorting of lysosomal enzymes in mannose6-phosphate receptor-deficient fibroblasts. EMBO (Eur Mol Biol Organ) J. 1994;13:3430–3437. doi: 10.1002/j.1460-2075.1994.tb06648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauxion F, Le Borgne R, Munier-Lehmann H, Hoflack B. A casein kinase II phosphorylation site in the cytoplasmic domain of the cation-dependent mannose 6-phosphate receptor determines the high affinity interaction of the AP-1 Golgi assembly proteins with membranes. J Biol Chem. 1996;271:2171–2178. doi: 10.1074/jbc.271.4.2171. [DOI] [PubMed] [Google Scholar]
- McIntyre GF, Erickson AH. Procathepsins L and D are membrane-bound in acidic microsomal vesicles. J Biol Chem. 1991;266:15438–15445. [PubMed] [Google Scholar]
- McIntyre GF, Erickson AH. The lysosomal proenzyme receptor that binds procathepsin L to microsomal membranes at pH 5 is a 43-kDa integral membrane protein. Proc Natl Acad Sci USA. 1993;90:10588–10592. doi: 10.1073/pnas.90.22.10588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre GF, Godbold GD, Erickson AH. The pH-dependent membrane association of procathepsin L is mediated by a 9-residue sequence within the propeptide. J Biol Chem. 1994;269:567–572. [PubMed] [Google Scholar]
- Miller SG, Carnell L, Moore H-PH. Post-Golgi membrane traffic: brefeldin A inhibits export from distal Golgi compartments to the cell surface but not recycling. J Cell Biol. 1992;118:267–285. doi: 10.1083/jcb.118.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore HPH, Walker MD, Lee F, Kelly RB. Expressing a human proinsulin cDNA in a mouse ACTH-secreting cell. Intracellular storage, proteolytic processing, and secretion on stimulation. Cell. 1983;35:531–538. doi: 10.1016/0092-8674(83)90187-3. [DOI] [PubMed] [Google Scholar]
- Moore, H.-P.H., C. Brion, K.-N. Chung, L. Lehmicke, R. Rivas, and D. Quinn. 1989. Protein secretion by constitutive and regulated pathways. In Secretion and Its Control. G.S. Oxford and C.M. Armstrong, editors. Rockefeller University Press, New York. 189–201. [PubMed]
- Natori S, Huttner WB. Chromogranin B (secretogranin I) promotes sorting to the regulated secretory pathway of processing intermediates derived from a peptide hormone precursor. Proc Natl Acad Sci USA. 1996;93:4431–4436. doi: 10.1073/pnas.93.9.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neerman-Arbez M, Halban PA. Novel, non-crinophagic, degradation of connecting peptide (C-peptide) in transformed pancreatic beta cells. J Biol Chem. 1993;268:16248–16252. [PubMed] [Google Scholar]
- Orci L, Halban P, Amherdt M, Ravazzola M, Vassalli JD, Perrelet A. Nonconverted, amino acid analog-modified proinsulin stays in a Golgi-derived clathrin-coated membrane compartment. J Cell Biol. 1984a;99:2187–2192. doi: 10.1083/jcb.99.6.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L, Ravazzola M, Amherdt M, Yanaihara C, Yanaihara N, Halban P, Renold AE, Perrelet A. Insulin, not C-peptide (proinsulin), is present in crinophagic bodies of the pancreatic β-cell. J Cell Biol. 1984b;98:222–228. doi: 10.1083/jcb.98.1.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L, Ravazzola M, Amherdt M, Madsen O, Vassalli JD. Direct identification of prohormone conversion site in insulin-secreting cells. Cell. 1985;42:671–681. doi: 10.1016/0092-8674(85)90124-2. [DOI] [PubMed] [Google Scholar]
- Orci L, Ravazzola M, Amherdt M, Madsen O, Perrelet A, Vassalli J-D, Anderson RGW. Conversion of proinsulin to insulin occurs coordinately with acidification of maturing secretory vesicles. J Cell Biol. 1986;103:2273–2281. doi: 10.1083/jcb.103.6.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L, Ravazzola M, Anderson RGW. The condensing vacuole of exocrine cells is more acidic than the mature secretory vesicle. Nature (Lond) 1987a;326:77–79. doi: 10.1038/326077a0. [DOI] [PubMed] [Google Scholar]
- Orci L, Ravazzola M, Storch MJ, Anderson RGW, Vassalli JD, Perrelet A. Proteolytic maturation of insulin is a post-Golgi event which occurs in acidifying clathrin-coated secretory vesicles. Cell. 1987b;49:865–868. doi: 10.1016/0092-8674(87)90624-6. [DOI] [PubMed] [Google Scholar]
- Orci L, Halban P, Perrelet A, Amherdt M, Ravazzola M, Anderson RGW. pH-independent and -dependent cleavage of proinsulin in the same secretory vesicle. J Cell Biol. 1994;126:1149–1156. doi: 10.1083/jcb.126.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer DJ, Christie DL. Identification of molecular aggregates containing glycoproteins III, J, K (carboxypeptidase H), and H (Kex2-related proteases) in the soluble and membrane fractions of adrenal medullary chromaffin granules. J Biol Chem. 1992;267:19806–19812. [PubMed] [Google Scholar]
- Pimplikar SW, Huttner WB. Chromogranin B (secretogranin I), a secretory protein of the regulated pathway, is also present in a tightly membrane-associated form in PC12 cells. J Biol Chem. 1992;267:4110–4118. [PubMed] [Google Scholar]
- Rhodes CJ, Halban PA. Newly synthesized proinsulin/insulin and stored insulin are released from pancreatic B cells predominantly via a regulated, rather than a constitutive, pathway. J Cell Biol. 1987;105:145–153. doi: 10.1083/jcb.105.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes CJ, Lucas CA, Mutkoski RL, Orci L, Halban PA. Stimulation by ATP of proinsulin to insulin conversion in isolated rat pancreatic islet secretory granules. J Biol Chem. 1987;262:10712–10717. [PubMed] [Google Scholar]
- Rosa P, Barr FA, Stinchcombe JC, Binacchi C, Huttner WB. Brefeldin A inhibits the formation of constitutive secretory vesicles and immature secretory granules from the trans-Golgi network. Eur J Cell Biol. 1992;59:265–274. [PubMed] [Google Scholar]
- Rowan AD, Mach L, Mort JS. Antibodies to rat procathepsin B recognize the active mature enzyme. Biol Chem Hoppe-Seyler. 1992;373:427–432. doi: 10.1515/bchm3.1992.373.2.427. [DOI] [PubMed] [Google Scholar]
- Schagger H, vonJagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Schnell AH, Swenne I, Borg LAH. Lysosomes and pancreatic islet function. A quantitative estimation of crinophagy in the mouse pancreatic β-cell. Cell Tiss Res. 1988;252:9–15. doi: 10.1007/BF00213820. [DOI] [PubMed] [Google Scholar]
- Sesso A, Assis JE, Kuwajima VY, Kachar B. Freeze-fracture and thin-section study of condensing vacuoles in rat pancreatic acinar cells. Acta Anat. 1980;108:521–539. doi: 10.1159/000145351. [DOI] [PubMed] [Google Scholar]
- Slot JW, Geuze HJ, Weerkamp AJ. Localization of macromolecular components by application of the immunogold technique on cryosectioned bacteria. Methods Microbiol. 1988;20:211–236. [Google Scholar]
- Slot JW, Geuze HJ, Gigengack S, Lienhard GE, James DE. Immunolocalization of the insulin regulatable glucose transporter in brown adipose tissue of the rat. J Cell Biol. 1991;113:123–135. doi: 10.1083/jcb.113.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns NA, Dong JM, Pan JX, Brenner DA, Sahagian GG. Comparison of cathepsin L synthesized by normal and transformed cells at the gene, message, protein, and oligosaccharide levels. Arch Biochem Biophys. 1990;283:447–457. doi: 10.1016/0003-9861(90)90666-m. [DOI] [PubMed] [Google Scholar]
- Steiner DF, Michael J, Houghten R, Mathieu M, Gardner PR, Ravazzola M, Orci L. Use of a synthetic peptide antigen to generate antisera reactive with a proteolytic processing site in native human proinsulin: demonstration of cleavage within clathrin-coated (pro)secretory vesicles. Proc Natl Acad Sci USA. 1987;84:6184–6188. doi: 10.1073/pnas.84.17.6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takio K, Towatari T, Katunuma N, Teller DC, Titani K. Homology of amino acid sequences of rat liver cathepsins B and H with that of papain. Proc Natl Acad Sci USA. 1983;80:3666–3670. doi: 10.1073/pnas.80.12.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taugner R, Hackenthal E. On the character of the secretory granules in juxtaglomerular epithelioid cells. Int Rev Cytol. 1988;110:93–131. doi: 10.1016/s0074-7696(08)61848-3. [DOI] [PubMed] [Google Scholar]
- Tooze J, Tooze SA. Clathrin-coated vesicular transport of secretory proteins during the formation of ACTH-containing secretory granules in AtT20 cells. J Cell Biol. 1986;103:839–850. doi: 10.1083/jcb.103.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze J, Hollinshead M, Hensel G, Kern HF, Hoflack B. Regulated secretion of mature cathepsin B from rat exocrine pancreatic cells. Eur J Cell Biol. 1991;56:187–200. [PubMed] [Google Scholar]
- Tooze SA, Huttner WB. Cell-free protein sorting to the regulated and constitutive secretory pathways. Cell. 1990;60:837–847. doi: 10.1016/0092-8674(90)90097-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze SA, Stinchcombe JC. Biogenesis of secretory granules. Semin Cell Biol. 1992;3:357–366. doi: 10.1016/1043-4682(92)90021-m. [DOI] [PubMed] [Google Scholar]
- von Figura K. Molecular recognition and targeting of lysosomal proteins. Curr Opin Cell Biol. 1991;3:642–646. doi: 10.1016/0955-0674(91)90035-w. [DOI] [PubMed] [Google Scholar]
- von Figura K, Hasilik A. Lysosomal enzymes and their receptors. Annu Rev Biochem. 1986;55:167–193. doi: 10.1146/annurev.bi.55.070186.001123. [DOI] [PubMed] [Google Scholar]
- vonZastrow M, Castle JD. Protein sorting among two distinct export pathways occurs from the content of maturing exocrine storage granules. J Cell Biol. 1987;105:2675–2684. doi: 10.1083/jcb.105.6.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JG, Izutsu KT, Robinovitch MR, Iversen JM, Cantino ME, Johnson DE. Microprobe analysis of maturation-related elemental changes in rat parotid secretory granules. Am J Physiol. 1991;261:C1033–1041. doi: 10.1152/ajpcell.1991.261.6.C1033. [DOI] [PubMed] [Google Scholar]
- Xu H, Shields D. Prohormone processing in the trans-Golgi network: endoproteolytic cleavage of prosomatostatin and formation of nascent secretory vesicles in permeabilized cells. J Cell Biol. 1993;122:1169–1184. doi: 10.1083/jcb.122.6.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SH. pH-dependent association of chromogranin A with secretory vesicle membrane and a putative membrane binding region of chromogranin A. Biochemistry. 1993;32:8213–8219. doi: 10.1021/bi00083a023. [DOI] [PubMed] [Google Scholar]