Abstract
Evidence for an important link between sensitization of midbrain dopamine (DA) neuron reactivity and enhanced self-administration of amphetamine and cocaine has been reported. To the extent that exposure to nicotine also sensitizes nucleus accumbens DA reactivity, it is likely that it will also impact subsequent drug taking. It is thus necessary to gain an understanding of the long-term effects of exposure to nicotine on nicotinic acetylcholine receptors (nAChRs), neuronal excitability and behavior. A review of the literature is presented in which different regimens of nicotine exposure are assessed for their effects on upregulation of nAChRs, induction of LTP in interconnected midbrain nuclei and development of long-lasting locomotor and DA sensitization. Exposure to nicotine upregulates nAChRs and nAChR currents and produces LTP of excitatory inputs to midbrain DA neurons. These effects appear in the hours to days following exposure. Exposure to nicotine also leads to long-lasting sensitization of nicotine’s nucleus accumbens DA and locomotor activating effects. These effects appear days to weeks after drug exposure. A model is proposed in which nicotine exposure regimens that produce transient nAChR upregulation and LTP consequently produce long-lasting sensitization of midbrain DA neuron reactivity and nicotine-induced behaviors. These neuroadaptations are proposed to constitute critical components of the mechanisms underlying the initiation, maintenance and escalation of drug use.
Keywords: Dopamine, Nicotinic acetylcholine receptors, nAChR upregulation, LTP, Ventral tegmental area, Nucleus accumbens, Drug-abuse liability, Drug self-administration, Withdrawal, Locomotion
1. Introduction
Many abused drugs, including amphetamine, cocaine, opiates and nicotine, activate brain dopamine (DA) neurotransmission, increase locomotor activity and support self-administration in humans and laboratory animals. When repeatedly administered, these effects are enhanced so that re-exposure to the drug, weeks to months later, produces greater dopaminergic and behavioral activation than seen initially. This long-term enhancement in the ability of such drugs to activate DA neurotransmission and elicit appetitive behaviors is termed sensitization and may have relevance to the initiation, maintenance and escalation of drug use that is characteristic of the transition from casual experimentation with drugs to drug craving and abuse in humans. Sensitization may also contribute to the reinstatement of drug taking in individuals after prolonged abstinence. Understanding the mechanisms that underlie the induction and expression of sensitization may thus help elucidate the neural events and neuroadaptations underlying the development, maintenance and reinstatement of drug abuse and indicate how they may be prevented.
Among these drugs, nicotine has received far less attention and little is known about its long-term effects or their impact on the pursuit and self-administration of the drug. Considering that rats, like humans, will work to obtain this drug, and that its use is most often characterized by habitual, repeated intake over long periods of time, it is clear that the potential is present for the development of long-term effects with harmful consequences for the individual. This is underscored by the findings of epidemiological studies strongly suggesting that previous exposure to nicotine places individuals at risk for future nicotine use despite widespread appreciation of the dangers associated with continued exposure to tobacco.
This review focuses on sensitization as a potential adaptation that impacts the pursuit and self-administration of nicotine. As such, it is not meant to provide a comprehensive review of all adaptations that have been reported following exposure to the drug. Indeed, other adaptations like tolerance have received far more attention (Collins et al., 1988; Stolerman, 1999; Perkins, 2002). The contribution of other effects of nicotine, such as its ability to interact associatively and non-associatively with contextual stimuli, has been addressed by others (Caggiula et al., 2002; Donny et al., 2003) and will not be discussed here (for a discussion of conditioning-sensitization interactions, see Stewart and Vezina, 1988, 1991; Anagnostaras and Robinson, 1996). Rather, this review concentrates on describing the background and rationale for a model outlining how nicotine exposure can lead to long-lasting sensitization of midbrain DA neuron reactivity and nicotine-related behaviors. The neuroadaptations leading to this form of plasticity are proposed to constitute critical components of the mechanisms underlying the initiation, maintenance and escalation of drug use.
2. Exposure to nicotine and the subsequent pursuit of the drug
Tobacco use in humans is the leading cause of preventable, premature death in the United States (US Department of Human and Health Services, 1988). This 1988 report of the Surgeon General concluded that nicotine is the drug in tobacco that causes addiction and it has been reported that almost all smokers meet diagnostic criteria for dependence. The enormous negative impact of tobacco use on the health of the individual and society as a whole has created an urgent need to identify the neuronal mechanisms underlying the generation of nicotine seeking behaviors as well as those factors that place individuals at risk for such behaviors. Many social and family environmental risk factors have been identified that promote tobacco use. In addition, the contribution to smoking of identified nonnicotine factors including sensory cues, components of tar that reduce nicotine irritation, and MAO inhibitors contained in tobacco smoke must not be overlooked (Rose, 2006). However, such factors do not negate the primary reinforcing role of nicotine. Indeed, a number of studies point to the harmful impact exposure to nicotine, in and of itself, may have on the subsequent display of nicotine seeking behavior in the adult individual.
Several epidemiological studies have now shown that exposure to nicotine in utero (Kandel et al., 1994; Hofvendahl et al., 1997; Isohanni et al., 1991; cf, Fergusson et al., 1998), during childhood (Osler et al., 1995) or adolescence (Kandel, 1975; Kandel et al., 1992) can lead to a greater predisposition to use nicotine and other drugs in adulthood. Considering that the use of nicotine is most often characterized by habitual, repeated intake over long periods of time, it is likely that short- and long-term drug effects are produced that would serve to perpetuate the pursuit of the drug in adults. It has been suggested, for example, that exposure to nicotine during development or in adulthood may induce modifications in the mesoaccumbens DA system that increase individuals’ predisposition to initiate and maintain tobacco use (Kandel et al., 1994). Curiously, there have been few animal studies of the effect of nicotine exposure on the subsequent pursuit of the drug and consequently few attempts made to investigate such a possibility. In one study, adult rats were exposed to seven daily injections of nicotine and tested for self-administration of the drug starting the day following the last exposure injection. Slight but significant enhancements in the acquisition of nicotine self-administration were found in drug compared to saline exposed animals (Shoaib et al., 1997). In another study, again conducted in the adult rat, it was shown that previous exposure to nicotine enhanced this drug’s ability to support conditioned place preference (Shoaib et al., 1994). These limited findings, suggesting that the pursuit of nicotine is enhanced by prior exposure to the drug, are reminiscent of the effects of other psychomotor stimulant drugs. These are reviewed below.
3. Mesoaccumbens DA, sensitization and drug seeking
Psychomotor stimulant drugs produce their locomotor effects and support self-administration behavior at least in part through their action on the mesoaccumbens DA system. VTA DA neurons that project to the NAcc are a central component of recent theories of drug taking and are a focus for many investigations of underlying mechanisms.
Wise and Bozarth (1987) argued, for example, that a wide range of addictive substances, including nicotine, have in common the ability to elicit approach behaviors by virtue of their actions on this system. For the psychomotor stimulants, there is considerable evidence to support this view (Deminiere et al., 1989; Bozarth, 1991), both for self-administration of (Roberts et al., 1980; Pettit et al., 1984; Woolverton and Virus, 1989; Caine and Koob, 1994) and locomotor responding to these drugs (Clarke at al., 1988b; McCreary and Marsden, 1993; Meyer et al., 1993; Vezina, 1996).
Robinson and Berridge (1993) have proposed that addictive drugs and associated environmental stimuli elicit excessive incentive salience and craving due to their ability to sensitize mesoaccumbens DA neuron reactivity, an effect known to accompany long-term locomotor sensitization to these drugs. Repeated exposure to amphetamine and cocaine is well known to lead to long-term enhancements in NAcc DA overflow and locomotor responding to these drugs (for references, see Vanderschuren and Kalivas, 2000; Vezina, 2004). In a manner paralleling this sensitization, previous exposure to these drugs not only promotes self-administration, but also enhances the acquisition and expression of conditioned place preference (Lett, 1989; Shippenberg and Heidbreder, 1995). Prenatal exposure to cocaine leads to enhanced self-administration of the drug in adult rats (Keller et al., 1996; cf, Hecht et al., 1998). Exposing adult rats to a regimen of amphetamine or cocaine injections known to produce locomotor and dopaminergic sensitization leads to long lasting enhancements in the subsequent self-administration of both low doses (Woolverton et al., 1984; Piazza et al., 1989, 1991; Horger et al., 1990, 1992; Valadez and Schenk, 1994; Pierre and Vezina, 1997, 1998) and high doses of these drugs (Lorrain et al., 2000; Mendrek et al., 1998; Vezina et al., 2002; for review, see Vezina, 2004). In addition, enhanced amphetamine self-administration is accompanied by enhanced extracellular DA levels in the NAcc (Lorrain et al., 2000; Vezina et al., 2002). Importantly, manipulations known to block the induction of locomotor and DA sensitization by amphetamine, such as preceding pre-exposure injections of this drug with antagonists for D1 DA receptors, also block the facilitation of drug self-administration (Pierre and Vezina, 1998; Suto et al., 2002). Similarly, antagonists of AMPA, NMDA and mGlu receptors also block the facilitation of cocaine self-administration (Suto et al., 2003; for a review, see Vezina and Suto, 2003). Finally, previous exposure to drugs like Δ9-THC, that fails to sensitize NAcc DA overflow in response to amphetamine, does not enhance self-administration of amphetamine (Vezina et al., 2003). Taken together, these findings strongly support an important link between the sensitization of midbrain dopamine neuron reactivity and the facilitation of self-administration of psychomotor stimulant drugs. To the extent that exposure to nicotine produces sensitization of both its locomotor and NAcc DA activating effects, it is likely that it will also impact subsequent drug taking. These critical experiments with nicotine remain to be conducted and carefully evaluated.
4. Mesoaccumbens DA, sensitization and nicotine-induced behavior
In the rat, acute systemic administration of nicotine produces locomotor activation. This activation may sometimes be preceded by a period of locomotor depression depending on the dose administered and whether or not animals were first habituated to the test environment (Clarke and Kumar, 1983a,b; Ksir et al., 1985). When injections are repeated, tolerance develops to the depressant effect (see Collins et al., 1988) and sensitization of the stimulant effect of the drug is observed (Clarke and Kumar, 1983a; Ksir et al., 1985). It has also been demonstrated in several laboratories that intravenous nicotine can maintain stable levels of self-administration in the rat (Corrigall and Coen, 1989; Donny et al., 1995; Tessari et al., 1995; Shoaib et al., 1996, 1997; Shaham et al., 1997; cf, Dworkin et al., 1993).
4.a. Acute responding to nicotine
There are several lines of evidence indicating that nicotine, in a manner similar to the above stimulants, produces these behavioral effects via actions on the mesoaccumbens DA system (Pich et al., 1997; for a review, see Rose and Corrigall, 1997). Different subtypes of nAChRs are expressed both on the perikarya and the terminals of neurons in this system (Clarke and Pert, 1985; Pidoplichko et al., 1997) as well as by non-DA cells and afferent terminals in the VTA (Schilstrom et al., 1998a,b; Clarke et al., 1985; Dominguez et al., 1994; see below). Systemically or iontophoretically applied nicotine increases the rate of firing and the prevalence of burst firing in mesoaccumbens DA neurons (Lichtensteiger et al., 1982; Grenhoff et al., 1986; Mereu et al., 1987). Studies using a variety of techniques have also shown that locomotion-inducing systemic or local applications of nicotine increase DA utilization (Grenhoff and Svensson, 1988; Vezina et al., 1992) and release (Imperato et al., 1986; Mifsud et al., 1989; Rahman et al., 2003). These, as well as the locomotor effects of nicotine, are blocked by centrally but not peripherally acting nicotine receptor antagonists (Clarke and Kumar, 1983a). Nicotine preferentially stimulates activity in and release from DA neurons in the mesoaccumbens relative to the nigrostriatal system (Mereu et al., 1987; Imperato et al., 1986; Benwell and Balfour, 1997). Within the NAcc, acute nicotine-induced DA overflow is observed preferentially in the shell relative to the core (Pontieri et al., 1996). nAChR blockade in the VTA (but not in the NAcc) reduces the ability of systemically administered nicotine to increase NAcc DA release (Nisell et al., 1994a) and locomotion (Corrigall and Coen, 1994) and to support self-administration (Corrigall et al., 1994). Infusion of nicotinic agonists into this site produces locomotor activation (Reavill and Stolerman, 1990; Leikola-Pelho and Jackson, 1992; Museo and Wise, 1995; Panagis et al., 1996) and increases NAcc DA release (Nisell et al., 1994a,b). Finally, DA receptor blockade (Corrigall and Coen, 1991; O’Neill et al., 1991; Museo and Wise, 1995) and selective lesions of the mesoaccumbens DA system (Clarke et al., 1988a; Louis and Clarke, 1998; Corrigall et al., 1992) have each been reported to prevent the ability of nicotine to produce locomotor activation and support its self-administration. Thus, it is clear from these findings that the mesoaccumbens DA system, and particularly the VTA, plays a large and critical role in the ability of nicotine to impact behavior.
4.b. Sensitized responding to nicotine
Based on the findings outlined above, it is reasonable to expect that repeated exposure to nicotine would produce sensitization of its NAcc DA activating effect. Initial studies produced mixed results. Although some reported sensitized NAcc DA overflow in response to nicotine (Benwell and Balfour, 1992; Balfour et al., 1998), others observed either no change (DOPA accumulation: Mitchell et al., 1989; in vitro [3H]DA release: Harsing et al., 1992; in vivo microdialysis: Damsma et al., 1989; Nisell et al., 1996) or decreased responding to the drug (DA utilization: Clarke et al., 1988a; Vezina et al., 1992; Lapin et al., 1989). Following exposure to nicotine and other drugs, enhanced DA overflow has also been reported by some to occur in the core rather than the shell of the NAcc (Cadoni and Di Chiara, 1999, 2000; Cadoni et al., 2000; Iyaniwura et al., 2001), although the opposite has been reported by others (Pierce and Kalivas, 1995). While attempts have been made to incorporate these findings into comprehensive models of drug addiction that attribute different specific functions to the NAcc subnuclei (e.g., Balfour et al., 2000; Di Chiara, 2002), it is important to note that, with the exception of the experiments by Pierce and Kalivas (1995), testing of NAcc DA responsivity in the above studies was conducted at short withdrawal times (usually one day) following the repeated nicotine injections. This point may be critical to the interpretation of these results. Enhanced NAcc DA overflow in response to amphetamine or cocaine is not observed one to two days but rather has been reported 10 days to three months following exposure to these drugs (Hamamura et al., 1991; Hurd et al., 1989; Segal and Kuczenski, 1992a,b; Paulson and Robinson, 1995; for reviews, see Vanderschuren and Kalivas, 2000; Vezina, 2004), indicating that it may be associated with the persistence of behavioral sensitization. Consistent with these findings, one recent report showed that previous exposure to nicotine three weeks earlier led to enhanced electrically evoked [3H]DA release from NAcc slice (Schoffelmeer et al., 2002). Thus, it is conceivable that enhanced NAcc DA responding to nicotine is preferentially expressed after long withdrawal periods, similar to amphetamine and cocaine. Interestingly, sensitized locomotor responding to nicotine in the absence of enhanced NAcc DA overflow at early withdrawal times may be mediated, as proposed for amphetamine and cocaine (Wolf et al., 1994; Kim et al., 2001; De Vries et al., 2002), by functional upregulation of DA receptors in the NAcc (Suemaru et al., 1993; Birrell and Balfour, 1998; Le Foll et al., 2003).
Another factor that influences NAcc DA responding to nicotine is the intensity of the drug exposure regimen. One day following discontinuation of exposure to high drug concentrations delivered continuously over several days (as with an osmotic pump), rats show a decreased NAcc DA response to a drug challenge injection. Similar findings have been reported for several drugs of abuse including amphetamine (Wise and Munn, 1995), cocaine (Markou and Koob, 1991), heroin (Leri et al., 2003) and nicotine (Benwell et al., 1995). For nicotine, such regimens lead to expression, in the days following exposure, of a nicotine withdrawal syndrome characterized by somatic signs of withdrawal and elevated thresholds for intracranial self-stimulation (ICSS) that is proposed to motivate increased intake of the drug (Epping-Jordan et al., 1998). The mechanisms underlying these effects remain unknown but appear to involve alterations in both peripheral (somatic signs: Watkins et al., 2000) and central glutamate and ACh systems (ICSS thresholds and somatic signs: Watkins et al., 2000; Kenny et al., 2003). Increasing the dose and duration of exposure to nicotine increases the duration and magnitude of the withdrawal syndrome observed (Skjei and Markou, 2003; O’Dell et al., 2007). Conversely, ICSS thresholds are lowered (Kokkinidis and McCarter, 1990; Lin et al., 2000), locomotor (Leri et al., 2003) and NAcc DA (Benwell et al., 1995; Schoffelmeer et al., 2002) responses become elevated, and drug seeking increases (Sorge and Stewart, 2005) when sufficient time elapses between exposure to the drug and testing.
Taken together, these results suggest that tolerance to the appetitive effects of a drug occurs during and immediately following periods of intense exposure whereas sensitization requires time to develop, and that the expression of either is dependent on the drug dose and exposure regimen. This is consistent with the view that sensitization of dopaminergic reactivity can exert long-lasting effects that promote the pursuit and self-administration of nicotine and other drugs (Stewart, 2003, 2004; Vezina, 2004; cf, Di Chiara, 2000; Laviolette and van der Kooy, 2004). It remains, however, that while many nicotine dose and exposure regimens have been tested for their effect on a number of measures, there has not yet been a systematic assessment of their impact on the induction and expression of behavioral and dopaminergic sensitization by nicotine at early and late withdrawal times.
5. nAChRs, mesoaccumbens DA and sensitization of nicotine-induced behavior
The neuronal nAChR is a pentameric ion channel that is permeable to Na+, K+ and Ca2+ (McGehee and Role, 1995). The nine nAChR subunit genes that are expressed in the mammalian nervous system include α2-α7 and β2-β4 (Le Novere and Changeux, 1995; Dani and Bertrand, 2007). In the CNS, high-affinity heteromeric receptors have been proposed to contain 3 α subunits and 2 non-α subunits (Anand et al., 1991), whereas low-affinity homomeric receptors contain α7 subunits and exhibit high Ca2+ permeability (Seguela et al., 1993). Current evidence indicates that mesoaccumbens DA neurons express both high-affinity α3, 4, 5, 6 and β2 and 3 subunit containing nAChRs, as well as low-affinity nAChRs consisting of the α7 subunit and that these receptors are expressed somatodendritically and on axon terminals (Marks and Collins, 1982; Clarke, 1993; Pidoplichko et al., 1997; Klink et al., 2001; Champtiaux et al., 2003; Cui et al., 2003). Both low- and high-affinity nAChRs are also expressed on afferent inputs to the VTA as well as by non-DA interneurons in this site (Dominguez et al., 1994; Schilstrom et al., 1998a,b; Mansvelder and McGehee, 2000; Mansvelder et al., 2002). It has been proposed that nicotine acts preferentially in the VTA to increase NAcc DA release, enhance locomotor behavior and promote self-administration by directly or indirectly activating mesoaccumbens DA neurons (Pidoplichko et al., 1997; see also Sorenson et al., 1998; Schilstrom et al., 1998a,b; Mansvelder et al., 2002). Indirect mechanisms of activation of VTA DA neurons include modulation of glutamatergic and GABAergic inputs to these cells to favor excitation (Mansvelder and McGehee, 2000; Mansvelder et al., 2002). Clearly, nAChRs in the VTA and perhaps other sites are well positioned to regulate the activity of mesoaccumbens DA neurons and to initiate long-term changes in their reactivity.
A number of phenomena have been associated with nAChRs including short-term activation and desensitization. Results from early radioligand binding studies both in vitro and in vivo suggested that nicotine exposure also increases the levels of [3H]-acetylcholine and [3H]-nicotine binding in several brain areas. This phenomenon, referred to as nAChR upregulation, was initially hypothesized to underlie both tolerance to this drug’s locomotor depressant effect (Marks and Collins, 1985) as well as sensitization to its locomotor activating effect (Ksir et al., 1985, 1987). Subsequent studies showed, however, that a correlation does not always exist between increased binding and these behaviors. Rats, for example, have been shown to maintain tolerance to the locomotor depressant effect of nicotine for periods far exceeding those during which increases in binding are observed (Clarke and Kumar, 1983a; Collins et al., 1988, 1990; Stolerman et al., 1973). In addition, increased binding has been observed in the absence of enhanced locomotor responding to nicotine (Ksir et al., 1987).
5.a. Transient upregulation of nAChRs, LTP and the induction of sensitization
The lack of correlation between sensitized behaviors and nAChR upregulation excludes this phenomenon as the primary cause but it is possible that the transient upregulation of nAChRs observed following nicotine exposure represents an early step in a sequence of neuronal events that lead ultimately to sensitized responding. Chronic exposure to nicotine in vitro was recently shown to produce an upregulation of ACh-evoked currents in rat and human α4β2 nAChRs expressed in human kidney epithelial cells (Buisson et al., 2000; Buisson and Bertrand, 2001; Vallejo et al., 2005) or cultured rat midbrain neurons (Nashmi et al., 2003). Similarly, exposing rat pups to nicotine in vivo was found to enhance ACh-evoked currents in α4β2- and α4β3-like nAChRs in hippocampal slices (Alkondon and Albuquerque, 2005). The importance of transient receptor desensitization in the upregulation of nAChRs remains to be determined (Pidoplichko et al., 1997). Within the VTA, receptor desensitization is important in the acute nicotine-induced decrease in GABAergic drive to DA neurons (Mansvelder et al., 2002). How this phenomenon is altered by chronic nicotine exposure and whether it is impacted by nAChR upregulation are questions that remain to be answered. It follows that upregulating nAChR responses on VTA DA neurons would be expected to increase the excitability of these cells directly and would favor the induction of drug-induced LTP of the excitatory inputs they receive. These processes could contribute to the induction of NMDA-dependent sensitization. DA neuron activation increases somatodendritic DA release creating a positive feedback on local glutamate release (Kalivas and Duffy, 1995; Wolf and Xue, 1998). The potentiation of excitatory inputs onto VTA DA neurons by drugs like cocaine and nicotine has been proposed as a possible component of sensitization (Ungless et al., 2001; Saal et al., 2003). Both are prevented by NMDA receptor blockade during drug exposure (Shoaib and Stolerman, 1992; Shoaib et al., 1994; Vezina and Queen, 2000; Ungless et al., 2001; Saal et al., 2003). However, drug-induced LTP persists for less than 10 days following drug exposure, independent of the number of exposure injections (Ungless et al., 2001; Borgland et al., 2004). In combination with the observation that sensitized DA release is seen in NAcc slices that contain DA neuron terminals but not their cell bodies (Schoffelmeer et al., 2002; for references, see Vezina, 1996), these findings indicate that LTP of excitatory inputs to VTA DA neurons may be important for the induction rather than the expression of sensitization.
5.b. Localization of relevant nAChRs necessary for the induction of sensitization
Activation of nAChRs is necessary for the induction of locomotor and dopaminergic sensitization not only by nicotine but also by other drugs such as amphetamine and cocaine (Schoffelmeer et al., 2002). These findings indicate a central role for nAChRs in drug sensitization. Upregulation of nAChRs is observed in a number of brain regions following exposure to nicotine (see Table 1) and appears to occur in receptors containing the α3, 4, 6 and β2 subunits (Ryan and Loiacono, 2001; Mugnaini et al., 2002; Parker et al., 2004). Results from some groups suggest that upregulation of the high-affinity nAChRs occurs through modifications in receptor assembly and trafficking, leading to a greater number of cell surface receptors (Peng et al., 1994; Harkness and Millar, 2002; Nashmi et al., 2003; Sallette et al., 2005). Alternatively, nAChR upregulation may involve a conformational change that hypersensitizes the receptor by increasing both its response and sensitivity to agonist (Vallejo et al., 2005; see also Buisson et al., 2000; Buisson and Bertrand, 2001; Alkondon and Albuquerque, 2005). Notably, either model would lead to increased reactivity in the cells expressing upregulated receptors.
Table 1.
Exposure to nicotine leads to upregulation of brain nAChRs as indexed by increased ligand binding. These examples in rat brain show that nAChR upregulation is produced in a manner dependent on the intensity of the nicotine exposure regimen: low doses administered intermittently (1) or continuously (via osmotic minipump; 4) produce no effect while increasingly higher doses administered via either route produce upregulation (1, 4) that is observed in a greater number of brain regions (2, 3, 4, 5, 6) and persists for a longer period of time following exposure (2, 3). Note that enhanced locomotor responding to nicotine is observed in the period soon (1) and late (7) after intermittent exposure to moderate doses but not in the period immediately following exposure to a more intense regimen of nicotine injections (1). Exposure to a moderate intermittent nicotine injection regimen that leads to long-term locomotor sensitization produces transient nAChR upregulation in the VTA but not in the NAcc. Blockade of nAChRs in the VTA, but not the NAcc, during exposure prevents the development of locomotor sensitization by nicotine (7). These results are consistent with an important role for the transient upregulation of nAChRs in the VTA (and possibly other sites sending direct and indirect projections to this site) in the development of behavioral sensitization by nicotine. See text for additional references. All doses are expressed as base. Abbreviations: AuditC, primary auditory cortex; BLA, basolateral amygdaloid nucleus; CeAmyg, central amygdaloid nucleus; CingC, cingulate cortex; Cort, cortex; CPu, caudate putamen; Crb, cerrebellar cortex; Hb, hindbrain; Hip, hippocampus; Hyp, hypothalamus; LimbC, prelimbic and infralimbic cortex; Mid, midbrain; MotC, primary motor cortex; NAcc-C, nucleus accumbens core; NAcc-S, nucleus accumbens shell; NST, nucleus of the solitary tract; ParC, parietal cortex; PFC, prefrontal cortex; PirC, piriform/entorhinal cortex; SensC, primary sensory cortex; SNc, substantia nigra pars compacta; Str, striatum; Thal-c, central thalamic nucleus; Thal-pv, paraventricular thalamic nucleus; VTA, ventral tegmental nucleus. ↑, significant increase; →, no change; →↑, non-significant trend.
| Nicotine Exposure Regimen | nAChR | Withdrawal | Locomotion | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|
| [3H]ACh - Cortex | 1 day
0.2 nic at test |
loco-10min | 1. Ksir et al., 1987 | ||||||
| 5 days sc - 1 X 0.01 | → | → | |||||||
| 0.03 | ↑ | ↑ | |||||||
| 0.10 | ↑ | ↑ | |||||||
| 0.30 mg/kg/day | ↑ | ↑ | |||||||
| 10 days sc - 2 X 1.6 mg/kg/day | ↑ | 1 day
0.2 nic at test |
→
cf saline exp |
||||||
|
| |||||||||
| [3H]Nic | 2. Collins et al., 1988 | ||||||||
| Str | Cort | Hip | Mid | Hyp | Hp | ||||
| 7 days - 2 X 1.6 mg/kg/day | → | ↑ | → | ↑ | → | ↑ | 0 day | ||
| → | ↑ | → | ↑ | → | ↑ | 1 day | |||
| → | ↑ | → | ↑ | → | ↑ | 2 day | |||
| → | ↑ | → | ↑ | → | ↑ | 3 day | |||
| → | → | → | → | → | → | 7 day | |||
|
| |||||||||
| [3H]Nic | 3. Collins et al., 1990 | ||||||||
| Str | Cort | Hip | Mid | Hyp | Hb | ||||
| 7 days sc - continuous 0.8/mg/kg/hr | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | 2 hr | ||
| ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | 1 day | |||
| ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | 2 days | |||
| ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | 4 days | |||
| → | ↑ | ↑ | → | → | → | 8 days | |||
| → | → | → | → | → | → | 21 days | |||
|
| |||||||||
| [3H]cytosine | < 1 day | 4. Rowell & Li, 1997 | |||||||
| Striatum | PFC | Hip | |||||||
| 10 days sc - continuous 0.6 | → | → | → | ||||||
| 1.2 | → | ↑ | → | ||||||
| 2.4 | ↑ | ↑ | ↑ | ||||||
| 4.8 mg/kg/day | ↑ | ↑ | ↑ | ||||||
| - 2 X 0.3 | → | → | ↑ | ||||||
| 0.6 | → | ↑ | ↑ | ||||||
| 1.2 | ↑ | ↑ | ↑ | ||||||
| 2.4 mg/kg/day | ↑ | ↑ | ↑ | ||||||
| -4 X 0.6 mg/kg/day | → | ↑ | ↑ | ||||||
| - 8 X 0.3 mg/kg/day | → | → | → | ||||||
|
| |||||||||
| [3H]nicotine | < 1 day | 5. Mugnaini et al., 2002 | |||||||
| 15 days sc - continuous 3.0 mg/kg/day | AuditC ↑↑↑ | NAcc-C ↑↑ | |||||||
| LimbC ↑↑ | NAcc-S ↑↑ | ||||||||
| MotC ↑↑ | CeAmyg ↑ | ||||||||
| BLA ↑↑ | VTA ↑ | ||||||||
| SensC ↑↑ | SNc →↑ | ||||||||
| CingC ↑↑ | Thal-c →↑ | ||||||||
| CPu ↑↑ | Thal-pv →↑ | ||||||||
|
| |||||||||
| [125I]epibatidine | < 1 day | 6. Parker et al., 2004 | |||||||
| i.v. self-admin (5 days acq. + 13 days maint.)
13 days 1.5 mg/kg/day |
NST ↑↑↑ | Amyg ↑↑ | |||||||
| VTA/SN ↑ ↑ ↑ | PirC ↑ | ||||||||
| NAcc ↑↑↑ | CPu ↑ | ||||||||
| Thal ↑↑ | Hip ↑ | ||||||||
| ParC ↑↑ | Crb →↑ | ||||||||
| Hyp ↑↑ | |||||||||
|
| |||||||||
| [125I]epibatidine | loco-2hr | 7. Baker et al., 2005 | |||||||
| 5 days ip - 1 X 0.4 every other day | VTA | ↑ | 3 hrs | ||||||
| NAcc | → | ||||||||
| VTA | → | 3 days - 3 weeks | |||||||
| NAcc | → | ||||||||
| 5 days ip - 1 X 0.4 every other day | 3 weeks
0.4 nic at test |
↑ | |||||||
| + VTA mecamylamine | → | ||||||||
| + NAcc mecamylamine | ↑
cf saline exp |
||||||||
As outlined above, there are several reasons to suspect that nAChRs in the VTA play a critical role in the induction of sensitization by nicotine. These receptors have been implicated in the ability of nicotine to increase locomotion and NAcc DA overflow as well as to support self-administration. They have also been implicated in cocaine place preference (Zachariou et al., 2001), the self-administration of methamphetamine and morphine (Glick et al., 2002) and, to some extent, in brain stimulation reward (Yeomans and Baptista, 1997). Nicotine is also known to upregulate these receptors and to enhance the synaptic strength and produce LTP of excitatory synapses onto VTA DA neurons (Mansvelder and McGehee, 2000; Saal et al., 2003). Consistent with these findings, it was recently shown that blocking nAChRs in the VTA, but not in the NAcc, during exposure to a moderate intermittent nicotine injection regimen blocks the induction of long-term locomotor sensitization observed three weeks later. Importantly, the same nicotine exposure regimen also produced a transient upregulation of nAChRs in the VTA observed 3 hours but not 3 days or 3 weeks following exposure. No upregulation of nAChRs was observed in the NAcc at any time tested (Baker et al., 2005). These findings indicate that nAChRs in the VTA, but not the NAcc (in a manner reminiscent of the effects of amphetamine; Vanderschuren and Kalivas, 2000; Vezina, 2004), are necessary for the induction of sensitization by nicotine and that transient upregulation of nAChRs specifically in sites important for nicotine sensitization may be associated with this effect.
It should be noted that, unlike more intense exposure regimens that produce nAChR upregulation in several brain regions (see Table 1), the sensitizing regimen of nicotine injections used by Baker et al., (2005) above was relatively moderate and may be particularly well suited for characterizing the selective regulation of nAChRs in different brain areas and their participation in the induction of behavioral sensitization by nicotine. That said, the significance of nAChR upregulation observed in other sites must be assessed. For example, upregulation of nAChRs in the NAcc may enhance the ability of nicotine to amplify phasic relative to tonic firing induced DA release in this site (Rice and Cragg, 2004; Zhang and Sulzer, 2004). Both the PFC and amygdala are involved in the induction of sensitization by amphetamine (Wolf et al., 1995; Cador et al., 1999), although their contribution to nicotine sensitization remains unknown. Other sites include the pedunculopontine (PPT) and laterodorsal (LDT) tegmental nuclei. Upregulation of nAChRs following exposure to nicotine does not appear to have been assessed in either nucleus. However, both sites have been heavily implicated in the effects of nicotine. For example, pharmacological inhibition, selective cholinergic lesions of the PPT or blockade of nAChRs in this site reduce nicotine self-administration (Lanca et al., 2000a; Corrigall et al., 2001, 2002), whereas non-selective lesions of the PPT block VTA nicotine induced conditioned place preference (Laviolette et al., 2002). Although most efferents from the PPT project to the substantia nigra (SN), this nucleus also sends some ACh, GABA and glutamate efferents to the VTA that could contribute to these effects (Oakman et al., 1995; Charara et al., 1996). The LDT on the other hand, sends major ACh projections to the VTA (Charara et al., 1996; Omelchenko and Sesack, 2005) that, together with glutamate inputs, modulate NAcc DA release (Blaha et al., 1996; Forster and Blaha, 2000; Forster et al., 2001). Nicotine and ICSS increase Fos immunoreactivity in both sites mostly in non-cholinergic cells (Lanca et al., 2000b; Nakahara et al., 2001). The latter has also been shown to increase ACh efflux in the VTA, an effect that may depend on inter-connectivity between the LDT and PPT (Semba and Fibiger, 1992; Forster et al., 2002; Miller et al., 2002).
The findings obtained thus far for nicotine sensitization are consistent with and extend current circuit models of sensitization proposed for other psychostimulants (e.g., Vanderschuren and Kalivas, 2000). In these models, descending glutamatergic projections from the infralimbic prefrontal cortex to the VTA are argued to play a critical role in the induction of sensitization by drugs such as amphetamine and cocaine. However, the fact that these projections do not form synapses with mesoaccumbens cells in the VTA (Carr and Sesack, 2000) has made it difficult to propose a direct role for these cortical afferents in the sensitization process. On the other hand, both the PPT and LDT receive afferents from the prefrontal cortex (Cornwall et al., 1990; Semba and Fibiger, 1992) and in turn send ACh, GABA and glutamate projections to the VTA (Charara et al., 1996; Forster and Blaha, 2000; Omelchenko and Sesack, 2005), positioning them well to play an important role in the induction of sensitization by nicotine. The circuit diagram in Figure 1 illustrates the interconnectivity between these and other nuclei that may be important for sensitization by nicotine.
Figure 1.
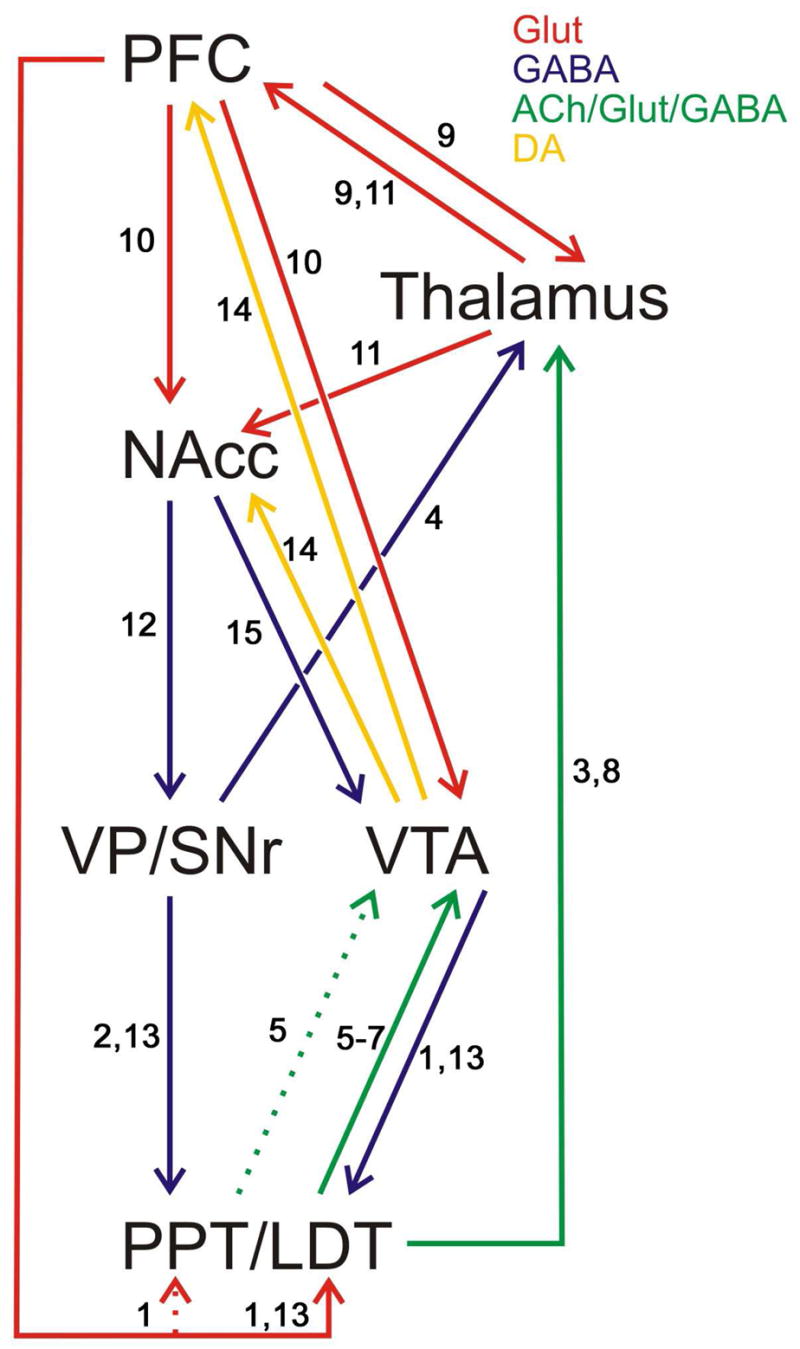
Illustration of interconnectivity between nuclei and neurotransmitters in a thalamo-cortico-pontine-VTA-NAcc circuit proposed to be important for locomotor sensitization by nicotine. Data already support a role for part of this circuit (excluding the LDT/PPT) in sensitization by psychostimulants (Pierce and Kalivas, 1997; Vanderschuren and Kalivas, 2000). To date, findings indicate that activation of nAChRs in the VTA, but not the NAcc, is necessary for the induction of sensitization by nicotine. Exposure to a sensitizing regimen of nicotine injections also produces transient upregulation of nAChRs in the VTA but not the NAcc (Baker et al. 2005; see Section 4b). Dotted lines indicate weaker projections. 1. Semba and Fibiger, 1992. 2. Beckstead et al., 1979. 3. Cornwall and Phillipson, 1988, 4. Inglis et al., 1994. 5. Charara et al., 1996. 6. Omelchenko and Sesack, 2005. 7. Forster and Blaha, 2000. 8. Woolf and Butcher, 1989. 9. Krettek and Price, 1977. 10. Sesack and Pickel, 1992. 11. Bubser and Deutch, 1998. 12. Jones and Mogenson, 1980. 13. Cornwall et al., 1990. 14. Swanson, 1982. 15. Zahm et al., 2001.
6. Nicotine exposure and sensitization of nicotine-induced behaviors
Taken together, the findings reviewed above are consistent with the model of the impact of nicotine exposure proposed and outlined in the flowchart below. In this model, nicotine exposure first produces transient upregulation of nAChR function, leading to enhanced excitation of midbrain DA neurons and the induction of LTP. These successive events lead ultimately to long-lasting sensitization of midbrain DA neuron reactivity and to behavioral manifestations of sensitized responding to the drug.
As reviewed earlier, two factors interact to influence responding to nicotine after exposure to the drug: the intensity of the drug exposure regimen (as determined by the number of injections and the drug dose) and the time of testing following exposure to the drug. As illustrated in Figure 2, increases in the intensity of the drug exposure regimen would be expected to lead to temporally different outcomes in subsequent responding to nicotine. Moderate exposure regimens (Figure 2B) are known to lead to a progressive long-lasting increase in nicotine’s locomotor effects and later enhancement of its DA activating effects (Clarke and Kumar, 1983a; Ksir et al., 1985, 1987; Schoffelmeer et al., 2002; Baker et al., 2005), whereas intense exposure regimens (Figure 2C) are not associated with enhanced locomotor or DA responding (Ksir et al., 1987; Benwell et al., 1995) but rather with expression of somatic signs of withdrawal and elevated ICSS thresholds in the period soon after exposure (Epping-Jordan et al., 1998). Long-lasting increases in locomotor and DA responding to nicotine are likely to emerge later after the negative withdrawal effects have dissipated.
Figure 2.
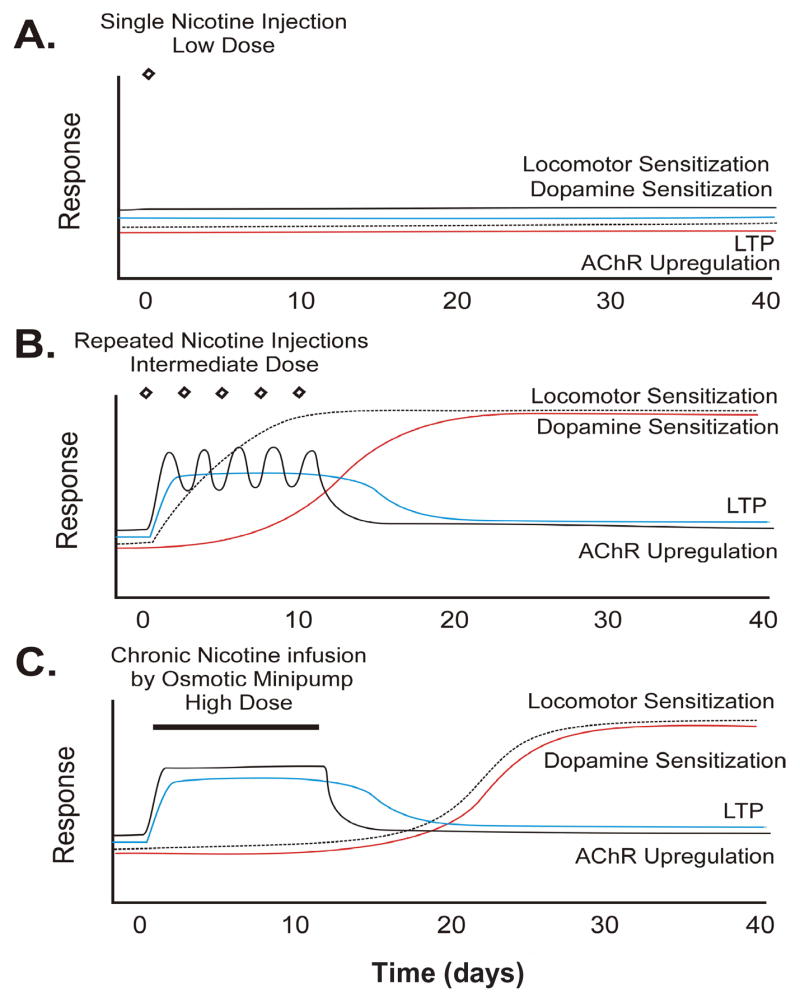
Proposed model of the effect of increasingly intense exposure regimens on the induction and long-lasting expression of sensitization by nicotine. Plots show predictions by the model of effects produced by three examples of exposure regimens ranging from weak to intermediate to intense. A. Weak. Exposure to an insufficient number of injections or subthreshold doses of nicotine is without effect on any of the measures. B. Intermediate. Moderate exposure to nicotine (e.g., 5 injections of 0.4 mg/kg, i.p., one injection every other day) leads to the progressive enhancement of the drug’s locomotor and later enhancement of the drug’s DA activating effects. C. Intense. Prolonged and continuous exposure to nicotine (e.g., 15 days of continuous 3.0/mg/kg/day, s.c., or repeated injections of high doses) does not enhance responding in the days following termination of drug exposure. Enhanced responding is predicted to appear at later withdrawal times. Both intermediate and intense exposure regimens have been associated with transient nAChR upregulation. Transient expression of LTP has been reported following repeated injections of cocaine and is predicted to occur transiently following both nicotine exposure regimens. Both effects are proposed to be the initiating neuroadaptations leading to long-lasting sensitization by nicotine. See text for supporting references.
A critical element of this model is the role played by nAChR upregulation and LTP in the initiation of the long-lasting neuroadaptations underlying sensitization. Both moderate and intense nicotine exposure regimens are associated with transient upregulation of nicotine binding sites (see Table 1). Although less is known about functional nAChR upregulation and LTP following different nicotine exposure regimens, based on reports with cocaine (Borgland et al., 2004), it is likely that these effects are also transient. Thus, exposure regimens that produce nAChR upregulation and LTP would be expected to sensitize responding to and for nicotine (Vezina, 2004). With more intense drug exposure regimens, such as continuous exposure to high concentrations with an osmotic minipump, the sensitized phenotype would likely be established via the same mechanisms, but the expression of DA and behavioral sensitization would only be measurable after recovery from the drug withdrawal effects.
Findings obtained with psychomotor stimulants such as amphetamine support an important link between the long-lasting sensitization of midbrain DA neuron reactivity and the enhanced self-administration of these drugs (Vezina, 2004). To the extent that nicotine acutely increases locomotion and NAcc DA release, and that repeated exposure to the drug leads to sensitization of both effects, it is likely in light of these findings, that exposure to nicotine can also have long lasting effects on subsequent drug taking. This possibility has not yet been directly assessed. Interestingly, rats characterized as showing enhanced nAChR function in the VTA (High vs Low Responders to novelty; Fagen et al., in revision), also show enhanced drug-induced NAcc DA responses (Bradberry et al., 1991; Hooks et al., 1991) and enhanced nicotine self-administration (Suto et al., 2001). The potentiated excitatory input onto VTA DA neurons observed in High Responder rats does not, in and of itself, provide a good model for the long-lasting expression of sensitization as it is observed for only a short period after drug exposure in sensitization experiments (e.g., 5 days; Ungless et al., 2001). Nonetheless, conditions that enhance excitation of VTA DA neurons are conducive to the induction of sensitization (Vezina, 2004) and High Responder rats are more susceptible to develop sensitization (Pierre and Vezina, 1997). Thus, together, the above findings are consistent with the hypothesis that drug exposure regimens that lead to nAChR upregulation and potentiation of excitatory synapses onto VTA DA neurons (or the presence of these conditions in High Responder rats) could lead to long lasting locomotor and dopaminergic sensitization that promotes self-administration of the drug. For a number of cultural and political reasons, along with the limited number of neurobiological investigations, nicotine addiction has been somewhat underestimated. Despite this history, it is clear that nicotine has profound long-term effects on behavior and it is likely that this drug induces lasting changes in neurochemistry analogous to those reported for cocaine and amphetamine.
Long-term neuroadaptations produced by previous exposure to nicotine or psychostimulants could thus exert critical impact on liability to initiate or resume excessive drug use (see also Koob and Le Moal, 1997). It has been argued, however, that the escalation of drug intake observed in rats given long (6-hr) rather than short (1-hr) access to cocaine in a self-administration paradigm (Ahmed and Koob, 1998) reflects tolerance (Ahmed et al., 2002) rather than sensitization of the appetitive (locomotor and DA activating) properties of the drug. This view is based in part on the lack of evidence for sensitized responding in tests conducted during or soon after the escalating phase of the experiments (Ahmed et al., 2003; Ahmed and Cador, 2006). Importantly, the absence of evidence for sensitization in these experiments is entirely consistent with the idea that sensitization develops gradually and is observed at later time points following drug exposure. If sensitization is not expressed in these animals, the escalation of drug intake observed could very well have been due to the incremental recruitment of opponent processes (Koob and Le Moal, 1997) or tolerance to the drug’s aversive or suppressive effects (Robinson and Berridge, 1993, 2004; Zernig et al., 2004). It is argued here that sensitization may be a consequence of drug exposure in all of these cases, but that the timing of the behavioral and biochemical assays is critical for its assessment. According to the current model, sensitization could exert its impact on behavior during self-administration testing in rats previously exposed to the drug (e.g., Vezina et al., 2002), as well as long after drug exposure in these sessions. Interestingly, unlike cocaine, escalation of intake does not occur in rats given long (12-hr) access to nicotine (Kenny and Markou, 2006), which may reflect differences between the two drugs in the opponent processes or aversive effects each is associated with. It is important to note, however, that the total daily intake in this study (0.38mg/kg/1-hr and 1.36mg/kg/12-hr) was within the range previously observed to produce nAChR upregulation (see Table 1) and thus would be expected, according to the current model, also to lead to long-lasting sensitization in responding to nicotine.
Acknowledgments
This review was made possible by grants (DA09397, PV; DA15918, DSM; DA13602, WNG; DA19695, all authors) from the National Institutes of Health and the Brain Research Foundation (PV). The authors are grateful to Dr. Susan R. Sesack for assistance delineating the anatomical circuitry underlying the proposed model.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SH, Cador M. Dissociation of psychomotor sensitization from compulsive cocaine consumption. Neuropsychopharmacology. 2006;31:563–71. doi: 10.1038/sj.npp.1300834. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nature Neurosci. 2002;5:625–6. doi: 10.1038/nn872. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: Change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Lin D, Koob GF, Parsons LH. Escalation of cocaine self-administration does not depend on altered cocaine-induced accumbens dopamine levels. J Neurochem. 2003;86:102–13. doi: 10.1046/j.1471-4159.2003.01833.x. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX. Nicotine receptor subtypes in rat hippocampal slices are differentially sensitive to desensitization and early in vivo functional up-regulation by nicotine and to block by bupropion. J Pharmacol Exp Ther. 2005;313:740–50. doi: 10.1124/jpet.104.081232. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Robinson TE. Sensitization to the psychostimulant effects of amphetamine: Modulation by associative learning. Behav Neurosci. 1996;110:1397–414. doi: 10.1037//0735-7044.110.6.1397. [DOI] [PubMed] [Google Scholar]
- Anand R, Conroy WG, Schoepfer R, Whiting P, Lindstrom J. Neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes have a pentameric quaternary structure. J Biol Chem. 1991;266:11192–8. [PubMed] [Google Scholar]
- Balfour DJK, Benwell MEM, Birrell CE, Kelly RJ, Al-Aloul M. Sensitization of the mesoaccumbens dopamine response to nicotine. Pharmacol Biochem Beh. 1998;59:1021–30. doi: 10.1016/s0091-3057(97)00537-6. [DOI] [PubMed] [Google Scholar]
- Balfour DJK, Wright AE, Benwell MEM, Birrell CE. The putative role of extra-synaptic mesolimbic dopamine in the neurobiology of nicotine dependence. Beh Brain Res. 2000;113:73–83. doi: 10.1016/s0166-4328(00)00202-3. [DOI] [PubMed] [Google Scholar]
- Baker LK, Alexander JK, Vallejo YF, Chi H, Green WN, Vezina P. Nicotine upregulates VTA nAChRs and requires these receptors to induce locomotor sensitization. Soc Neurosci Abst. 2005;31:1027.17. [Google Scholar]
- Beckstead RM, Domesick VB, Nauta WJ. Efferent connections of the substantia nigra and ventral tegmental area in the rat. Brain Res. 1979;175:191–217. doi: 10.1016/0006-8993(79)91001-1. [DOI] [PubMed] [Google Scholar]
- Benwell MEM, Balfour DJK. The effects of acute and repeated nicotine treatment on nucleus accumbens dopamine and locomotor activity. Br J Pharmacol. 1992;105:849–56. doi: 10.1111/j.1476-5381.1992.tb09067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ, Birrell CE. Desensitization of the nicotine-induced mesolimbic dopamine responses during constant infusion with nicotine. Br J Pharmacol. 1995;114:454–60. doi: 10.1111/j.1476-5381.1995.tb13248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ. Regional variation in the effects of nicotine on catecholamine overflow in the rat brain. Eur J Pharmacol. 1997;325:13–20. doi: 10.1016/s0014-2999(97)00101-5. [DOI] [PubMed] [Google Scholar]
- Birrell CE, Balfour DJ. The influence of nicotine pretreatment on mesoaccumbens dopamine overflow and locomotor responses to d-amphetamine. Psychopharmacology. 1998;140:142–9. doi: 10.1007/s002130050751. [DOI] [PubMed] [Google Scholar]
- Blaha CD, Allen LF, Inglis DS, Latimer MP, Vincent SR, Winn P. Modulation of dopamine efflux in the nucleus accumbens after cholinergic stimulation of the ventral tegmental area in intact, pedunculopontine tegmental nucleus-lesioned, and laterodorsal tegmental nucleus-lesioned rats. J Neurosci. 1996;16:714–22. doi: 10.1523/JNEUROSCI.16-02-00714.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Malenka RC, Bonci A. Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: Electrophysiological and behavioral correlates in individual rats. J Neurosci. 2004;24:7482–90. doi: 10.1523/JNEUROSCI.1312-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozarth MA. The mesolimbic dopamine system as a model reward system. In: Willner P, Scheel-Kruger J, editors. The mesolimbic dopamine system: From motivation to action. New York: Wiley; 1991. pp. 301–30. [Google Scholar]
- Bradberry CW, Green RJ, Berridge CW, Roth RH. Individual differences in behavioral measures: Correlations with nucleus accumbens dopamine measured with microdialysis. Pharmacol Biochem Behav. 1991;39:887–92. doi: 10.1016/0091-3057(91)90047-6. [DOI] [PubMed] [Google Scholar]
- Bubser M, Deutch AY. Thalamic paraventricular nucleus neurons collateralize to innervate the prefrontal cortex and nucleus accumbens. Brain Res. 1998;787:304–10. doi: 10.1016/s0006-8993(97)01373-5. [DOI] [PubMed] [Google Scholar]
- Buisson B, Bertrand D. Chronic exposure to nicotine upregulates the human a4b2 nicotinic acetylcholine receptor function. J Neurosci. 2001;21:1819–29. doi: 10.1523/JNEUROSCI.21-06-01819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisson B, Vallejo YF, Green WN, Bertrand D. The unusual nature of epibatidine responses at the a4b2 nicotinic acetylcholine receptor. Neuropharmacology. 2000;39:2561–9. doi: 10.1016/s0028-3908(00)00158-1. [DOI] [PubMed] [Google Scholar]
- Cadoni C, Di Chiara G. Reciprocal changes in dopamine responsiveness in the nucleus accumbens shell and core and in the dorsal caudate-putamen in rats sensitized to morphine. Neuroscience. 1999;90:447–55. doi: 10.1016/s0306-4522(98)00466-7. [DOI] [PubMed] [Google Scholar]
- Cadoni C, Di Chiara G. Differential changes in accumbens shell and core dopamine in behavioral sensitization to nicotine. Eur J Pharmacol. 2000;387:R23–R25. doi: 10.1016/s0014-2999(99)00843-2. [DOI] [PubMed] [Google Scholar]
- Cadoni C, Solinas M, Di Chiara G. Psychostimulant sensitization: Differential changes in accumbens shell and core dopamine. Eur J Pharmacol. 2000;388:69–76. doi: 10.1016/s0014-2999(99)00824-9. [DOI] [PubMed] [Google Scholar]
- Cador M, Bjijou Y, Cailhol S, Stinus L. D-amphetamine-induced behavioral sensitization: Implication of a glutamatergic medial prefrontal cortex-ventral tegmental area innervation. Neuroscience. 1999;94:705–21. doi: 10.1016/s0306-4522(99)00361-9. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Chaudhri N, Perkins KA, Evans-Martin FF, Sved AF. Importance of nonpharmacological factors in nicotine self-administration. Physiol Beh. 2002;77:683–7. doi: 10.1016/s0031-9384(02)00918-6. [DOI] [PubMed] [Google Scholar]
- Caine SB, Koob GF. Effects of dopamine D1 and D2 antagonists on cocaine self-administration under different schedules of reinforcement in the rat. J Pharmacol Exp Ther. 1994;270:209–18. [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: Target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci. 2000;20:3864–73. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przbylksi C, Lena C, Clementi F, Moretti M, Rossi FM, Le Novere N, McIntosh JM, Gardier AM, Changeux JP. Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J Neurosci. 2003;23:7820–9. doi: 10.1523/JNEUROSCI.23-21-07820.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charara A, Smith Y, Parent A. Glutamatergic inputs from the pedunculopontine nucleus to midbrain dopaminergic neurons in primates: Phaseolus vulgaris-leucoagglutinin anterograde labeling combined with postembedding glutamate and GABA immunohistochemistry. J Comp Neurol. 1996;364:254–66. doi: 10.1002/(SICI)1096-9861(19960108)364:2<254::AID-CNE5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Clarke PBS. Nicotinic receptors in mammalian brain: Localization and relation to cholinergic innervation. Prog Brain Res. 1993;98:77–83. doi: 10.1016/s0079-6123(08)62383-3. [DOI] [PubMed] [Google Scholar]
- Clarke PBS, Kumar R. Characterization of the locomotor stimulant action of nicotine in tolerant rats. Br J Pharmac. 1983a;80:587–94. doi: 10.1111/j.1476-5381.1983.tb10733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PBS, Kumar R. The effects of nicotine on locomotor activity in non-tolerant and tolerant rats. Br J Pharmac. 1983b;78:329–37. doi: 10.1111/j.1476-5381.1983.tb09398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PBS, Pert A. Autoradiographic evidence for nicotine receptors on nigrostriatal and mesolimbic dopaminergic neurons. Brain Res. 1985;348:355–8. doi: 10.1016/0006-8993(85)90456-1. [DOI] [PubMed] [Google Scholar]
- Clarke PBS, Fu DS, Jakubovic A, Fibiger HC. Evidence that mesolimbic dopaminergic activation underlies the locomotor stimulant action of nicotine in rats. J Pharmacol Exp Ther. 1988a;246:701–7. [PubMed] [Google Scholar]
- Clarke PBS, Jakubovic A, Fibiger HC. Anatomical analysis of the involvement of mesolimbocortical dopamine in the locomotor stimulant actions of d-amphetamine and apomorphine. Psychopharmacology. 1988b;96:511–20. doi: 10.1007/BF02180033. [DOI] [PubMed] [Google Scholar]
- Clarke PBS, Schwartz RD, Paul SM, Pert CB, Pert A. Nicotinic binding in rat brain: autoradiographic comparison of [3H]acetylcholine, [3H]nicotine, and [125I]-bungarotoxin. J Neurosci. 1985;5:1307–15. doi: 10.1523/JNEUROSCI.05-05-01307.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AC, Romm E, Wehner JM. Nicotine tolerance: an analysis of the time course of its development and loss in the rat. Psychopharmacology. 1988;96:7–14. doi: 10.1007/BF02431526. [DOI] [PubMed] [Google Scholar]
- Collins AC, Romm E, Wehner JM. Dissociation of the apparent relationship between nicotine tolerance and up-regulation of nicotinic receptors. Brain Res Bull. 1990;25:373–9. doi: 10.1016/0361-9230(90)90222-l. [DOI] [PubMed] [Google Scholar]
- Cornwall J, Cooper JD, Phillipson OT. Afferent and efferent connections of the laterodorsal tegmental nucleus in the rat. Brain Res Bull. 1990;25:271–84. doi: 10.1016/0361-9230(90)90072-8. [DOI] [PubMed] [Google Scholar]
- Cornwall J, Phillipson OT. Afferent projections to the dorsal thalamus of the rat as shown by retrograde lectin transport. II. The midline nuclei. Brain Res Bull. 1988;21:147–61. doi: 10.1016/0361-9230(88)90227-4. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology. 1989;99:473–8. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Selective dopamine antagonists reduce nicotine self-administration. Psychopharmacology. 1991;104:171–6. doi: 10.1007/BF02244174. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Dopamine mechanisms play at best a small role in the nicotine discriminative stimulus. Pharmacol Biochem Beh. 1994;48:817–20. doi: 10.1016/0091-3057(94)90353-0. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 1994;653:278–84. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Zhang J, Adamson KL. GABA mechanisms in the pedunculopontine tegmental nucleus influence particular aspects of nicotine self-administration selectively in the rat. Psychopharmacology. 2001;158:190–7. doi: 10.1007/s002130100869. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Zhang J, Adamson KL. Pharmacological manipulations of the pedunculopontine tegmental nucleus in the rat reduce self-administration of both nicotine and cocaine. Psychopharmacology. 2002;160:198–205. doi: 10.1007/s00213-001-0965-2. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Franklin KB, Coen KM, Clarke PBS. The mesolimbic dopamine system is implicated in the reinforcing effects of nicotine. Psychopharmacology. 1992;107:285–9. doi: 10.1007/BF02245149. [DOI] [PubMed] [Google Scholar]
- Cui C, Booker TK, Allen RS, Grady SR, Whiteaker P, Marks MJ, Salminen O, Tritto T, Butt CM, Allen WR, Stitzel JA, McIntosh JM, Boulter J, Collins AC, Heinemann SF. The beta3 nicotinic receptor subunit: A component of alpha-conotoxin MII-binding nicotinic acetylcholine receptors that modulate dopamine release and related behaviors. J Neurosci. 2003;23:11045–53. doi: 10.1523/JNEUROSCI.23-35-11045.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsma G, Day J, Fibiger HC. Lack of tolerance to nicotine-induced dopamine release in the nucleus accumbens. Eur J Pharmacol. 1989;168:363–8. doi: 10.1016/0014-2999(89)90798-x. [DOI] [PubMed] [Google Scholar]
- Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- Deminiere J, Piazza P, Le Moal M, Simon H. Experimental approach to individual vulnerability to psychostimulant addiction. Neurosci Biobehav Rev. 1989;13:141–7. doi: 10.1016/s0149-7634(89)80023-5. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer ANM, Binnekade R, Raaso H, Vanderschuren LJMJ. Relapse to cocaine- and heroin-seeking behavior mediated by dopamine D2 receptors is time-dependent and associated with behavioral sensitization. Neuropsychopharmacology. 2002;26:18–26. doi: 10.1016/S0893-133X(01)00293-7. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Role of dopamine in the behavioral actions of nicotine related to addiction. Eur J Pharmacol. 2000;393:295–314. doi: 10.1016/s0014-2999(00)00122-9. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Nucleus accumbens shell and core dopamine: Differential role in behavior and addiction. Beh Brain Res. 2002;137:75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- Dominguez del TE, Juiz JM, Peng X, Lindstrom J, Criado M. Immunocytochemical localization of the a7 subunit of the nicotine acetylcholine receptor in the rat central nervous system. J Comp Neurology. 1994;349:325–42. doi: 10.1002/cne.903490302. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Knopf S, Brown C. Nicotine self-administration in rats. Psychopharmacology. 1995;122:390–4. doi: 10.1007/BF02246272. [DOI] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: Implications for nicotine self-administration and reinforcement. Psychopharmacology. 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Dworkin SI, Varna SL, Broadbent J, Robinson JH. Comparing the reinforcing effects of nicotine, caffeine, methylphenildate and cocaine. Med Chem Res. 1993;2:593–602. [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Fagen ZM, Mitchum R, Vezina P, McGehee DS. Enhanced nicotinic receptor function and drug abuse vulnerability. doi: 10.1523/JNEUROSCI.2017-06.2007. In revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, Woodward LJ, Horwood LJ. Maternal smoking during pregnancy and psychiatric adjustment in late adolescence. Arch Gen Psychiatry. 1998;55:721–7. doi: 10.1001/archpsyc.55.8.721. [DOI] [PubMed] [Google Scholar]
- Forster GL, Blaha CD. Laterodorsal tegmental stimulation elicits dopamine efflux in the rat nucleus accumbens by activation of acetylcholine and glutamate receptors in the ventral tegmental area. Eur J Neurosci. 2000;12:3596–604. doi: 10.1046/j.1460-9568.2000.00250.x. [DOI] [PubMed] [Google Scholar]
- Forster GL, Falcon AD, Miller GA, Heruc GA, Blaha CD. Effects of laterodorsal tegmentum excitotoxic lesions on behavioral and dopamine responses evoked by morphine and d-amphetamine. Neuroscience. 2002;114:817–23. doi: 10.1016/s0306-4522(02)00365-2. [DOI] [PubMed] [Google Scholar]
- Forster GL, Yeomans JS, Takeuchi J, Blaha CD. M5 muscarinic receptors are required for prolonged accumbal dopamine release after electrical stimulation of the pons in mice. J Neurosci. 2001;21:RC190. doi: 10.1523/JNEUROSCI.22-01-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Kitchen BA, Fleck MW. Antagonism of a3β4 nicotinic receptors as a strategy to reduce opioid and stimulant self-administration. Eur J Phramacol. 2001;438:99–105. doi: 10.1016/s0014-2999(02)01284-0. [DOI] [PubMed] [Google Scholar]
- Grenhoff J, Aston-Jones G, Svenssom TH. Nicotine effects on the firing pattern of midbrain dopamine neurons. Acta Physiol Scand. 1986;128:351–8. doi: 10.1111/j.1748-1716.1986.tb07988.x. [DOI] [PubMed] [Google Scholar]
- Grenhoff J, Svenssom TH. Selective stimulation of limbic dopamine activity by nicotine. Acta Physiol Scand. 1988;133:595–6. doi: 10.1111/j.1748-1716.1988.tb08450.x. [DOI] [PubMed] [Google Scholar]
- Hamamura T, Akiyama K, Akimoto K, Kashihara K, Okumura K, Ujike H, Otsuki S. Co-administration of either a selective D1 or D2 dopamine antagonist with methamphetamine prevents methamphetamine-induced behavioral sensitization and neurochemical change, studied by in vivo microdialysis. Brain Res. 1991;546:40–6. doi: 10.1016/0006-8993(91)91156-u. [DOI] [PubMed] [Google Scholar]
- Harkness PC, Millar NS. Changes in conformation and subcellular distribution of alpha4 beta2 nicotinic acetylcholine receptors revealed by chronic nicotine treatment and expression of subunit chimeras. J Neurosci. 2002;22:10172–81. doi: 10.1523/JNEUROSCI.22-23-10172.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsing LG, Sershen H, Lajtha A. Dopamine efflux from striatum after chronic nicotine: evidence for autoreceptor desensitization. J Neurochem. 1992;59:48–54. doi: 10.1111/j.1471-4159.1992.tb08874.x. [DOI] [PubMed] [Google Scholar]
- Hecht GS, Spear NE, Spear LP. Alterations in the reinforcing efficacy of cocaine in adult rats following prenatal exposure to cocaine. Beh Neurosci. 1998;112:410–8. doi: 10.1037//0735-7044.112.2.410. [DOI] [PubMed] [Google Scholar]
- Hofvendahl E, Hofvendahl S, Wallander B. Is smoking in pregnancy interrelated between generations? Acta Pædiatr. 1997;86:1224–8. doi: 10.1111/j.1651-2227.1997.tb14851.x. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Smith AD, Neill DB, Justice JB., Jr Response to novelty predicts the locomotor and nucleus accumbens dopamine response to cocaine. Synapse. 1991;9:121–8. doi: 10.1002/syn.890090206. [DOI] [PubMed] [Google Scholar]
- Horger BA, Giles MK, Schenk S. Preexposure to amphetamine and nicotine predisposes rats to self- administer a low dose of cocaine. Psychopharmacology. 1992;107:271–6. doi: 10.1007/BF02245147. [DOI] [PubMed] [Google Scholar]
- Horger BA, Shelton K, Schenk S. Preexposure sensitizes rats to the rewarding effects of cocaine. Pharmacol Biochem Behav. 1990;37:707–11. doi: 10.1016/0091-3057(90)90552-s. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Weiss F, Koob GF, Anden NE, Ungerstedt U. Cocaine reinforcement and extracellular dopamine overflow in rat nucleus accumbens: An in vivo microdialysis study. Brain Res. 1989;498:199–203. doi: 10.1016/0006-8993(89)90422-8. [DOI] [PubMed] [Google Scholar]
- Imperato A, Mulas A, Di Chiara G. Nicotine preferentially stimulates dopamine release in the limbic system of freely moving rats. Eur J Pharmacol. 1986;132:337–8. doi: 10.1016/0014-2999(86)90629-1. [DOI] [PubMed] [Google Scholar]
- Inglis WL, Dunbar JS, Winn P. Outflow from the nucleus accumbens to the pedunculopontine tegmental nucleus: a dissociation between locomotor activity and the acquisition of responding for conditioned reinforcement stimulated by d-amphetamine. Neuroscience. 1994;62:51–64. doi: 10.1016/0306-4522(94)90314-x. [DOI] [PubMed] [Google Scholar]
- Isohanni M, Moilanen I, Rantakallio P. Determinants of teenage smoking, with special reference to non-standard family background. Br J Addict. 1991;86:391–8. doi: 10.1111/j.1360-0443.1991.tb03416.x. [DOI] [PubMed] [Google Scholar]
- Iyaniwura TT, Wright AE, Balfour DJK. Evidence that mesoaccumbens dopamine and locomotor responses to nicotine in the rat are influenced by pretreatment dose and strain. Psychopharmacology. 2001;158:73–9. doi: 10.1007/s002130100852. [DOI] [PubMed] [Google Scholar]
- Jones DL, Mogenson GJ. Nucleus accumbens to globus pallidus GABA projection: electrophysiological and iontophoretic investigations. Brain Res. 1980;188:93–105. doi: 10.1016/0006-8993(80)90559-4. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. D1 receptors modulate glutamate transmission in the ventral tegmental area. J Neurosci. 1995;15:5379–88. doi: 10.1523/JNEUROSCI.15-07-05379.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel D. Stages in adolescent involvement in drug use. Science. 1975;190:912–4. doi: 10.1126/science.1188374. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Wu P, Davies M. Maternal smoking during pregnancy and smoking by adolescent daughters. Am J Pub Health. 1994;84:1407–13. doi: 10.2105/ajph.84.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel D, Yamaguchi K, Chen K. Stages of progression in drug involvement from adolescence to adulthood: Further evidence for the gateway theory. J Stud Alcohol. 1992;53:447–57. doi: 10.15288/jsa.1992.53.447. [DOI] [PubMed] [Google Scholar]
- Keller RW, Jr, LeFevre R, Raucci J, Carlson JN, Glick SD. Enhanced cocaine self-administration in adult rats prenatally exposed to cocaine. Neurosci Lett. 1996;205:153–6. doi: 10.1016/0304-3940(96)12409-5. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Gasparini F, Markou A. Group II metabotropic and a-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA)/kainate glutamate receptors regulate the deficit in brain reward function associated with nicotine withdrawal in rats. J Pharmacol Exp Ther. 2003;306:1068–76. doi: 10.1124/jpet.103.052027. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacology. 2006;31:1203–11. doi: 10.1038/sj.npp.1300905. [DOI] [PubMed] [Google Scholar]
- Kim J-H, Perugini M, Austin JD, Vezina P. Previous exposure to amphetamine enhances the subsequent locomotor response to a D1 dopamine receptor agonist when glutamate reuptake is inhibited. J Neurosci. 2001;21:RC133. doi: 10.1523/JNEUROSCI.21-05-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klink R, de Kerchove d’Exaerde A, Zoli M, Changeux JP. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci. 2001;21:1452–63. doi: 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokkinidis L, McCarter BD. Post cocaine depression and sensitization of brain stimulation reward: Analysis of reinforcement and performance effects. Pharmacol Biochem Behav. 1990;36:463–71. doi: 10.1016/0091-3057(90)90242-a. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: Hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. The cortical projections of the mediodorsal nucleus and adjacent thalamic nuclei in the rat. J Comp Neurol. 1977;171:157–91. doi: 10.1002/cne.901710204. [DOI] [PubMed] [Google Scholar]
- Ksir C, Hakan R, Hall DP, Jr, Kellar KJ. Exposure to nicotine enhances the behavioral stimulant effect of nicotine and increases binding of [3H]acetylcholine to nicotine receptors. Neuropharmacology. 1985;24:527–31. doi: 10.1016/0028-3908(85)90058-9. [DOI] [PubMed] [Google Scholar]
- Ksir C, Hakan RL, Kellar KJ. Chronic nicotine and locomotor activity: influences of exposure dose and test dose. Psychopharmacology. 1987;92:25–9. doi: 10.1007/BF00215474. [DOI] [PubMed] [Google Scholar]
- Lanca AJ, Adamson KL, Coen KM, Chow BLC, Corrigall WA. The pedunculopontine mesencephalic tegmental nucleus and the role of cholinergic neurons in nicotine self-administration in the rat: A correlative neuroanatomical and behavioral study. Neuroscience. 2000a;96:735–42. doi: 10.1016/s0306-4522(99)00607-7. [DOI] [PubMed] [Google Scholar]
- Lanca AJ, Sanelli TR, Corrigall WA. Nicotine-induced Fos expression in the pedunculopontine mesencephalic tegmentum in the rat. Neuropharmacology. 2000b;39:2808–17. doi: 10.1016/s0028-3908(00)00129-5. [DOI] [PubMed] [Google Scholar]
- Lapin EP, Maker HS, Sershen H, Lajtha A. Action of nicotine on accumbens dopamine and attenuation with repeated administration. Eur J Pharmacol. 1989;160:53–9. doi: 10.1016/0014-2999(89)90653-5. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Alexson TO, van der Kooy D. Lesions of the tegmental pedunculopontine nucleus block the rewarding effects and reveal the aversive effects of nicotine in the ventral tegmental area. J Neurosci. 2002;22:8653–60. doi: 10.1523/JNEUROSCI.22-19-08653.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, van der Kooy D. The neurobiology of nicotine addiction: Bridging the gap from molecules to behaviour. Nature Rev Neurosci. 2004;5:55–65. doi: 10.1038/nrn1298. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Diaz J, Sokoloff P. Increased dopamine D3 receptor expression accompanying behavioral sensitization to nicotine in rats. Synapse. 2003;47:176–83. doi: 10.1002/syn.10170. [DOI] [PubMed] [Google Scholar]
- Le Novere N, Changeux J. Molecular evolution of the nicotinic acetylcholine receptor: An example of multigene family in excitable cells. J Mol Evol. 1995;40:155–72. doi: 10.1007/BF00167110. [DOI] [PubMed] [Google Scholar]
- Leikola-Pelho T, Jackson DM. Preferential stimulation of locomotor activity by ventral tegmental microinjections of (-)-nicotine. Pharmacol Toxicol. 1992;70:50–2. doi: 10.1111/j.1600-0773.1992.tb00425.x. [DOI] [PubMed] [Google Scholar]
- Leri F, Flores J, Rajabi H, Stewart J. Effects of cocaine in rats exposed to heroin. Neuropsychopharmacology. 2003;28:2102–16. doi: 10.1038/sj.npp.1300284. [DOI] [PubMed] [Google Scholar]
- Lett RT. Repeated exposures intensify rather that diminish the rewarding effects of amphetamine, morphine, and cocaine. Psychopharmacology. 1989;98:357–62. doi: 10.1007/BF00451687. [DOI] [PubMed] [Google Scholar]
- Lichtensteiger W, Hefti F, Felix D, Huwyler T, Malamed E, Schlumpf M. Stimulation of nigrostriatal dopamine neurons by nicotine. Neuropharmacology. 1982;21:963–8. doi: 10.1016/0028-3908(82)90107-1. [DOI] [PubMed] [Google Scholar]
- Lin D, Koob GF, Markou A. Time-dependent alterations in ICSS thresholds associated with repeated amphetamine administrations. Pharmacol Biochem Behav. 2000;65:407–17. doi: 10.1016/s0091-3057(99)00213-0. [DOI] [PubMed] [Google Scholar]
- Lorrain DS, Arnold GM, Vezina P. Previous exposure to amphetamine increases incentive to obtain the drug: Long-lasting effects revealed by the progressive ratio schedule. Beh Brain Res. 2000;107:9–19. doi: 10.1016/s0166-4328(99)00109-6. [DOI] [PubMed] [Google Scholar]
- Louis M, Clarke PB. Effect of ventral tegmental 6-hydroxydopamine lesions on the locomotor stimulant action of nicotine in rats. Neuropharmacology. 1998;37:1503–13. doi: 10.1016/s0028-3908(98)00151-8. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, Keath DS, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905–19. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron. 2000;27:349–57. doi: 10.1016/s0896-6273(00)00042-8. [DOI] [PubMed] [Google Scholar]
- Markou A, Koob GF. Post cocaine anhedonia: An animal model of cocaine withdrawal. Neuropsychopharmacology. 1991;4:17–26. [PubMed] [Google Scholar]
- Marks MJ, Collins AC. Characterization of nicotine binding in mouse brain and comparison with the binding of a-bungarotoxin and quinuclidinyl benzilate. Mol Pharmacol. 1982;22:554–64. [PubMed] [Google Scholar]
- Marks MJ, Collins AC. Tolerance, cross-tolerance, and receptors after chronic nicotine or oxotremorine. Pharmacol Biochem Beh. 1985;22:283–91. doi: 10.1016/0091-3057(85)90392-2. [DOI] [PubMed] [Google Scholar]
- McCreary AC, Marsden CA. Dopamine D1 receptor antagonism by SCH23390 prevents expression of conditioned sensitization following repeated administration of cocaine. Neuropharmacology. 1993;32:387–91. doi: 10.1016/0028-3908(93)90161-u. [DOI] [PubMed] [Google Scholar]
- McGehee DS, Role LW. Physiological diversity of nicotinic acetycholine receptors expressed by vertebrate neurons. Ann Rev Physiol. 1995;57:521–46. doi: 10.1146/annurev.ph.57.030195.002513. [DOI] [PubMed] [Google Scholar]
- Mendrek A, Blaha C, Phillips AG. Preexposure to amphetamine sensitizes rats to its rewarding properties as measured by a progressive ratio schedule. Psychopharmacology. 1998;135:416–22. doi: 10.1007/s002130050530. [DOI] [PubMed] [Google Scholar]
- Mereu G, Yoon K-WP, Boi V, Gessa GL, Naes L, Westfall TC. Preferential stimulation of ventral tegmental area dopaminergic neurons by nicotine. Eur J Pharmacol. 1987;141:395–9. doi: 10.1016/0014-2999(87)90556-5. [DOI] [PubMed] [Google Scholar]
- Meyer ME, Cottrell GA, Van Hartesveldt C, Potter TJ. Effects of dopamine D1 antagonists SCH23390 and SKF83566 on locomotor activities in rats. Pharmacol Biochem Beh. 1993;44:429–32. doi: 10.1016/0091-3057(93)90486-d. [DOI] [PubMed] [Google Scholar]
- Mifsud J-C, Hernandez L, Hoebel BG. Nicotine infused into the nucleus accumbens increases synaptic dopamine as measured by in vivo microdialysis. Brain Res. 1989;478:365–7. doi: 10.1016/0006-8993(89)91518-7. [DOI] [PubMed] [Google Scholar]
- Mitchell SN, Brazell MP, Joseph MH, Alavijeh MS, Gray JA. Regionally specific effects of acute and chronic nicotine on rates of catecholamine and 5-hydroxytryptamine synthesis in rat brain. Eur J Pharmacol. 1989;167:311–22. doi: 10.1016/0014-2999(89)90440-8. [DOI] [PubMed] [Google Scholar]
- Miller AD, Forster GL, Metcalf KM, Blaha CD. Excitotoxic lesions of the pedunculopontine differentially mediate morphine- and d-amphetamine-evoked striatal dopamine efflux and behaviors. Neuroscience. 2002;111:351–62. doi: 10.1016/s0306-4522(01)00595-4. [DOI] [PubMed] [Google Scholar]
- Mugnaini M, Tessari M, Tarter G, Pich EM, Chiamulera C, Bunnemann B. Upregulation of [3H]methyllycaconitine binding sites following continuous infusion of nicotine, without changes of a7 or a6 subunit mRNA: An autoradiography and in situ hybridization study in rat brain. Eur J Neurosci. 2002;16:1633–46. doi: 10.1046/j.1460-9568.2002.02220.x. [DOI] [PubMed] [Google Scholar]
- Museo E, Wise RA. Cytisine-induced behavioral activation: Delineation of neuroanatomical locus of action. Brain Res. 1995;670:257–63. doi: 10.1016/0006-8993(94)01286-q. [DOI] [PubMed] [Google Scholar]
- Nakahara D, Ishida Y, Nakamura M, Furuno N, Nishimori T. Intracranial self-stimulation induces Fos expression in GABAergic neurons in the rat mesopontine tegmentum. Neuroscience. 2001;106:633–41. doi: 10.1016/s0306-4522(01)00298-6. [DOI] [PubMed] [Google Scholar]
- Nashmi R, Dickinson ME, McKinney S, Jareb M, Labarca C, Fraser SE, Lester HA. Assembly of a4β2 nicotinic acetylcholine receptors assessed with functional fluorescently labeled subunits: Effects of localization, trafficking, and nicotine-induced upregulation in clonal mammalian cells and in cultured midbrain neurons. J Neurosci. 2003;23:11554–67. doi: 10.1523/JNEUROSCI.23-37-11554.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisell M, Nomikos GG, Svensson TH. Systemic nicotine-induced dopamine release in the rat nucleus accumbens is regulated by nicotinic receptors in the ventral tegmental area. Synapse. 1994a;16:36–44. doi: 10.1002/syn.890160105. [DOI] [PubMed] [Google Scholar]
- Nisell M, Nomikos G, Svensson TH. Infuson of nicotine in the ventral tegmental area or the nucleus accumbens of the rat differentially affects accumbal dopamine release. Pharmacol Toxicol. 1994b;75:348–52. doi: 10.1111/j.1600-0773.1994.tb00373.x. [DOI] [PubMed] [Google Scholar]
- Nisell M, Nomikos GG, Hertel P, Panagis G, Svenssom TH. Condition-independent sensitization of locomotor stimulation and mesocortical dopamine release following chronic nicotine treatment in the rat. Synapse. 1996;22:369–81. doi: 10.1002/(SICI)1098-2396(199604)22:4<369::AID-SYN8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Oakman SA, Faris PL, Kerr PE, Cozzari C, Hartman BK. Distribution of pontomesencephalic cholinergic neurons projecting to substantia nigra differs significantly from those projecting to ventral tegmental area. J Neurosci. 1995;15:5859–69. doi: 10.1523/JNEUROSCI.15-09-05859.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Laterodorsal tegmental projections to identified cell populations in the rat ventral tegmental area. J Comp Neurol. 2005;483:217–35. doi: 10.1002/cne.20417. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Chen SA, Smith RT, Specio SE, Balster RL, Paterson NE, Markou A, Zorrilla EP, Koob GF. Extended access to nicotine self-administration leads to dependence: Circadian measures, withdrawal measures, and extinction behavior in rats. J Pharmacol Exp Ther. 2007;320:180–93. doi: 10.1124/jpet.106.105270. [DOI] [PubMed] [Google Scholar]
- O’Neill MF, Dourish CT, Iversen SD. Evidence for an involvement of D1 and D2 dopamine receptors in mediating nicotine-induced hyperactivity in rats. Psychopharmacology. 1991;104:343–50. doi: 10.1007/BF02246034. [DOI] [PubMed] [Google Scholar]
- Osler M, Clausen J, Ibsen KK, Jensen G. Maternal smoking during childhood and increased risk of smoking in young adulthood. Int J Epid. 1995;24:710–4. doi: 10.1093/ije/24.4.710. [DOI] [PubMed] [Google Scholar]
- Panagis G, Nisell M, Nomikos GG, Chergui K, Svensson TH. Nicotine injections into the ventral tegmental area increase locomotion and Fos-like immunoreactivity in the nucleus accumbens of the rat. Brain Res. 1996;730:133–42. doi: 10.1016/0006-8993(96)00432-5. [DOI] [PubMed] [Google Scholar]
- Parker SL, Fu Y, McAllen K, Luo J, McIntosh M, Lindstrom JM, Sharp BM. Up-regulation of brain nicotinic acetylcholine receptors in the rat during long-term self-administration of nicotine: Disproportionate increase of the a6 subunit. Mol Pharmacology. 2004;65:611–22. doi: 10.1124/mol.65.3.611. [DOI] [PubMed] [Google Scholar]
- Paulson PE, Robinson TE. Amphetamine-induced time-dependent sensitization or dopamine neurotransmission in the dorsal an ventral striatum: A microdialysis study in behaving rats. Synapse. 1995;19:56–65. doi: 10.1002/syn.890190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Gerzanich V, Anand R, Whiting PJ, Lindstrom J. Nicotine-induced increase in neuronal nicotinic receptors results from a decrease in the rate of receptor turnover. Mol Pharmacol. 1994;46:523–30. [PubMed] [Google Scholar]
- Perkins KA. Chronic tolerance to nicotine in humans and its relationship to tobacco dependence. Nicotine Tob Res. 2002;4:405–22. doi: 10.1080/1462220021000018425. [DOI] [PubMed] [Google Scholar]
- Pettit HO, Ettenberg A, Bloom FE, Koob GF. Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration in rats. Psychopharmacology. 1984;84:167–73. doi: 10.1007/BF00427441. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere J-M, Le Moal M, Simon H. Factors that predict vulerability to amphetamine self-administration. Science. 1989;245:1511–3. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Maccari S, Deminiere J-M, Le Moal M, Mormede P, Simon H. Corticosterone levels determine individual vulnerability to amphetamine self-administration. PNAS. 1991;88:2088–92. doi: 10.1073/pnas.88.6.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pich EM, Pagliusi SR, Tessari M, Talabot-Ayer D, Van Huijsduijnen RH, Chiamulera C. Common neural substrates for the addictive properties of nicotine and cocaine. Science. 1997;275:83–6. doi: 10.1126/science.275.5296.83. [DOI] [PubMed] [Google Scholar]
- Pidoplichko VI, DeBiasi M, Williams JT, Dani JA. Nicotine activates and desensitizes midbrain dopamine neurons. Nature. 1997;390:401–4. doi: 10.1038/37120. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. Amphetamine produces sensitized increases in locomotion and extracellular dopamine preferentially in the nucleus accumbens shell of rats administered repeated cocaine. J Pharmacol Exp Ther. 1995;275:1019–29. [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Pierre PJ, Vezina P. Predisposition to self-administer amphetamine: the contribution of response to novelty and prior exposure to the drug. Psychopharmacology. 1997;129:277–84. doi: 10.1007/s002130050191. [DOI] [PubMed] [Google Scholar]
- Pierre PJ, Vezina P. D1 dopamine receptor blockade prevents the facilitation of amphetamine self-administration induced by prior exposure to the drug. Psychopharmacology. 1998;138:159–66. doi: 10.1007/s002130050658. [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Orzi F, Di Chiara G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996;382:255–7. doi: 10.1038/382255a0. [DOI] [PubMed] [Google Scholar]
- Rahman S, Zhang J, Corrigall WA. Effects of acute and chronic nicotine on somatodendritic dopamine release of the rat ventral tegmental area: In vivo microdialysis study. Neurosci Lett. 2003;348:61–4. doi: 10.1016/s0304-3940(03)00723-7. [DOI] [PubMed] [Google Scholar]
- Reavill C, Stolerman IP. Locomotor activity in rats after administration of nicotinic agonists intracerebrally. Br J Pharmacol. 1990;99:273–8. doi: 10.1111/j.1476-5381.1990.tb14693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nature Neurosci. 2004;7:583–4. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- Roberts DCS, Koob GF, Klonoff P, Fibiger HC. Extinction and recovery of cocaine self-administration following 6-OHDA lesions of the nucleus accumbens. Pharmacol Biochem Beh. 1980;12:781–7. doi: 10.1016/0091-3057(80)90166-5. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and drug ‘wanting’. Psychopharmacology. 2004;171:352–3. [Google Scholar]
- Rose JE. Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacology. 2006;184:274–85. doi: 10.1007/s00213-005-0250-x. [DOI] [PubMed] [Google Scholar]
- Rose JE, Corrigall WA. Nicotine self-administration in animals and humans: Similarities and differences. Psychopharmacology. 1997;130:28–40. doi: 10.1007/s002130050209. [DOI] [PubMed] [Google Scholar]
- Rowell PP, Li M. Dose-response relationship for nicotine-induced up-regulation of rat brain nicotinic receptors. J Neurochem. 1997;68:1982–9. doi: 10.1046/j.1471-4159.1997.68051982.x. [DOI] [PubMed] [Google Scholar]
- Ryan RE, Loiacono RE. Nicotine regulates a7 nicotinic receptor subunit mRNA: Implications for nicotine dependence. NeuroReport. 2001;12:569–72. doi: 10.1097/00001756-200103050-00027. [DOI] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–82. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Sallette J, Pons S, Devillers-Thiery A, Soudant M, Prado de Carvalho L, Changeux JP, Corringer PJ. Nicotine upregulates its own receptors through enhanced intracellular maturation. Neuron. 2005;46:595–607. doi: 10.1016/j.neuron.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Schilström B, Nomikos GG, Nisell M, Hertel P, Svensson TH. N-methyl-D-aspartate receptor antagonism in the ventral tegmental area diminishes the systemic nicotine-induced dopamine release in the nucleus accumbens. Neuroscience. 1998a;82:781–9. doi: 10.1016/s0306-4522(97)00243-1. [DOI] [PubMed] [Google Scholar]
- Schilström B, Svensson HM, Svensson TH, Nomikos GG. Nicotine and food induced dopamine release in the nucleus accumbens of the rat: Putative role of a7 nicotinic receptors in the ventral tegmental area. Neuroscience. 1998b;85:1005–9. doi: 10.1016/s0306-4522(98)00114-6. [DOI] [PubMed] [Google Scholar]
- Schoffelmeer ANM, De Vries TJ, Wardeh G, van de Ven HWM, Vanderschuren LJMJ. Psychostimulant-induced behavioral sensitization depends on nicotinic receptor activation. J Neurosci. 2002;22:3269–76. doi: 10.1523/JNEUROSCI.22-08-03269.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R. In vivo microdialysis reveals a diminished amphetamine-induced DA response corresponding to behavioral sensitization produced by repeated amphetamine pretreatment. Brain Res. 1992a;571:330–7. doi: 10.1016/0006-8993(92)90672-v. [DOI] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R. Repeated cocaine administration induces behavioral sensitization and corresponding decreased extracellular dopamine responses in caudate and accumbens. Brain Res. 1992b;577:351–5. doi: 10.1016/0006-8993(92)90297-m. [DOI] [PubMed] [Google Scholar]
- Seguela P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba K, Fibiger HC. Afferent connections of the laterodorsal and the pedunculopontine tegmental nuclei in the rat: a retro- and antero-grade transport and immunohistochemical study. J Comp Neurol. 1992;323:387–410. doi: 10.1002/cne.903230307. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Pickel VM. Prefrontal cortical efferents in the rat synapse on unlabeled neuronal targets of catecholamine terminals in the nucleus accumbens septi and on dopamine neurons in the ventral tegmental area. J Comp Neurol. 1992;320:145–60. doi: 10.1002/cne.903200202. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Adamson KL, Grocki S, Corrigall W. Reinstatement and spontaneous recovery of nicotine seeking in rats. Psychopharmacology. 1997;130:396–403. doi: 10.1007/s002130050256. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Heidbreder CA. Sensitization to the conditioned rewarding effects of cocaine: Pharmacological and temporal characteristics. J Pharmacol Exp Ther. 1995;273:808–15. [PubMed] [Google Scholar]
- Shoaib M, Benwell ME, Akbar MT, Stolerman IP, Balfour DJ. Behavioral and neurochemical adaptations to nicotine in rats: influence of NMDA antagonists. Br J Pharmacol. 1994;111:1073–80. doi: 10.1111/j.1476-5381.1994.tb14854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoaib M, Schindler CW, Goldberg SR. Nicotine self-administration in rats: Strain and nicotine pre-exposure effects on acquisition. Psychopharmacology. 1997;129:35–43. doi: 10.1007/s002130050159. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Stolerman IP. MK801 attenuates behavioural adaptation to chronic nicotine administration in rats. Br J Pharmacol. 1992;105:514–5. doi: 10.1111/j.1476-5381.1992.tb09010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoaib M, Swanner LS, Prada J, Yasar S, Goldberg SR. Caffeine potentiates both acquisition and maintenance of intravenous nicotine self-administration. Behav Pharmacol. 1996;7:103. [Google Scholar]
- Skjei KL, Markou A. Effects of repeated withdrawal episodes, nicotine dose, and duration of nicotine exposure on the severity and duration of nicotine withdrawal in rats. Psychopharmacology. 2003;168:280–92. doi: 10.1007/s00213-003-1414-1. [DOI] [PubMed] [Google Scholar]
- Sorenson EM, Shiroyama T, Kitai ST. Postsynaptic nicotinic receptors on dopaminergic neurons in the substantia nigra pars compacta of the rat. Neuroscience. 1998;87:659–73. doi: 10.1016/s0306-4522(98)00064-5. [DOI] [PubMed] [Google Scholar]
- Sorge RE, Stewart J. The contribution of drug history and time since termination of drug taking to footshock stress-induced cocaine seeking in rats. Psychopharmacology. 2005;183:210–7. doi: 10.1007/s00213-005-0160-y. [DOI] [PubMed] [Google Scholar]
- Stewart J. Stress and relapse to drug seeking: Studies in laboratory animals shed light on mechanisms and sources of long-term vulnerability. Amer J Addict. 2003;12:1–17. [PubMed] [Google Scholar]
- Stewart J. Pathways to relapse: Factors controlling the reinitiation of drug seeking after abstinence. Neb Symp Motiv. 2004;50:197–234. [PubMed] [Google Scholar]
- Stewart J, Vezina P. Conditioning and behavioral sensitization. In: Kalivas PW, Barnes CD, editors. Sensitization in the nervous system. Caldwell NJ: Telford Press; 1988. pp. 207–24. [Google Scholar]
- Stewart J, Vezina P. Extinction procedures abolish conditioned stimulus control but spare sensitized responding to amphetamine. Beh Pharmacol. 1991;2:65–71. [PubMed] [Google Scholar]
- Stolerman IP. Inter-species consistency in the behavioural pharmacology of nicotine dependence. Beh Pharmacol. 1999;10:559–80. doi: 10.1097/00008877-199911000-00002. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Fink R, Jarvik ME. Acute and chronic tolerance to nicotine measured by activity in rats. Psychopharmacologia. 1973;30:329–42. doi: 10.1007/BF00429192. [DOI] [PubMed] [Google Scholar]
- Suemaru K, Gomita Y, Furuno K, Araki Y. Chronic nicotine treatment potentiates behavioral responses to dopaminergic drugs in rats. Pharmacol Biochem Behav. 1993;46:135–9. doi: 10.1016/0091-3057(93)90329-r. [DOI] [PubMed] [Google Scholar]
- Suto N, Austin JD, Vezina P. Locomotor response to novelty predicts a rat’s propensity to self-administer nicotine. Psychopharmacology. 2001;158:175–80. doi: 10.1007/s002130100867. [DOI] [PubMed] [Google Scholar]
- Suto N, Austin JD, Tanabe LM, Kramer MK, Wright DA, Vezina P. Previous exposure to VTA amphetamine enhances cocaine self-administration under a progressive ratio schedule in a D1 dopamine receptor dependent manner. Neuropsychopharmacology. 2002;27:970–9. doi: 10.1016/S0893-133X(02)00379-2. [DOI] [PubMed] [Google Scholar]
- Suto N, Tanabe LM, Austin JD, Creekmore E, Vezina P. Previous exposure to VTA amphetamine enhances cocaine self-administration under a progressive ratio schedule in an NMDA, AMPA/kainate, and metabotropic glutamate receptor-dependent manner. Neuropsychopharmacology. 2003;28:629–39. doi: 10.1038/sj.npp.1300075. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–53. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Tessari M, Valerio E, Chiamulera C, Beardsley PM. Nicotine reinforcement in rats with histories of cocaine self- administration. Psychopharmacology. 1995;121:282–3. doi: 10.1007/BF02245640. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–7. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services. The health consequences of smoking: Nicotine addiction. A report of the surgeon general. US Department of Health and Human Services, Public Health Service, Center for Disease Control, Office on Smoking and Health; Rockville, MA: 1988. [Google Scholar]
- Valadez A, Schenk S. Persistence of the ability of amphetamine pre-exposure to facilitate acquisition of cocaine self-administration. Pharmacol Biochem Beh. 1994;47:203–5. doi: 10.1016/0091-3057(94)90132-5. [DOI] [PubMed] [Google Scholar]
- Vallejo YF, Buisson B, Bertrand D, Green WN. Chronic nicotine exposure upregulates nicotinic receptors by a novel mechanism. J Neurosci. 2005;25:5563–72. doi: 10.1523/JNEUROSCI.5240-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Vezina P. D1 dopamine receptor activation is necessary for the induction of sensitization to amphetamine in the ventral tegmental area. J Neurosci. 1996;16:2411–20. doi: 10.1523/JNEUROSCI.16-07-02411.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev. 2004;27:827–39. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Vezina P, Austin JD, Lorrain DS, Beeler JA, Tang WJ. Previous exposure to 39-THC does not promote the pursuit of amphetamine. Soc Neurosci Abst. 2003;29:111.1. [Google Scholar]
- Vezina P, Blanc G, Glowinski J, Tassin J-P. Nicotine and morphine differentially activate brain dopamine in prefrontocortical and subcortical terminal fields: Effects of acute and repeated injections. J Pharmacol Exp Ther. 1992;261:484–90. [PubMed] [Google Scholar]
- Vezina P, Lorrain DS, Arnold GM, Austin JD, Suto N. Sensitization of midbrain dopamine neuron reactivity promotes the pursuit of amphetamine. J Neurosci. 2002;22:4654–62. doi: 10.1523/JNEUROSCI.22-11-04654.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P, Queen AL. Induction of locomotor sensitization by amphetamine requires the activation of NMDA receptors in the rat ventral tegmental area. Psychopharmacology. 2000;151:184–91. doi: 10.1007/s002130000463. [DOI] [PubMed] [Google Scholar]
- Vezina P, Suto N. Glutamate and the self-administration of psychomotor-stimulant drugs. In: Herman BH, editor. Glutamate and Addiction. Totowa, NJ: Humana Press; 2003. pp. 183–220. [Google Scholar]
- Watkins SS, Stinus L, Koob GF, Markou A. Reward and somatic changes during precipitated nicotine withdrawal in rats: Centrally and peripherally mediated effects. J Pharmacol Exp Ther. 2000;292:1053–64. [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psych Rev. 1987;94:469–92. [PubMed] [Google Scholar]
- Wise RA, Munn E. Withdrawal from chronic amphetamine elevates baseline intracranial self-stimulation thresholds. Psychopharmaocology. 1995;117:130–6. doi: 10.1007/BF02245178. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Dahlin SL, Hu XT, Xue CJ, White FJ. Effects of lesions of prefrontal cortex, amygdala or fornix on behavioral sensitization to amphetamine: Comparisons with N-methyl-D-aspartate antagonists. Neuroscience. 1995;69:417–39. doi: 10.1016/0306-4522(95)00248-h. [DOI] [PubMed] [Google Scholar]
- Wolf ME, White FJ, Hu XT. MK-801 prevents alterations in the mesoaccumbens dopamine system associated with behavioral sensitization to amphetamine. J Neurosci. 1994;14:1735–45. doi: 10.1523/JNEUROSCI.14-03-01735.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, Xue CJ. Amphetamine and D1 dopamine receptor agonists produce biphasic effects on glutamate efflux in rat ventral tegmental area: Modification by repeated amphetamine administration. J Neurochem. 1998;70:198–209. doi: 10.1046/j.1471-4159.1998.70010198.x. [DOI] [PubMed] [Google Scholar]
- Woolf NJ, Butcher LL. Cholinergic systems in the rat brain: III. Projections from the pontomesencephalic tegmentum to the thalamus, tectum, basal ganglia and basal forebrain. Brain Res Bull. 1989;16:603–37. doi: 10.1016/0361-9230(86)90134-6. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Cervo L, Johanson CE. Effects of repeated methamphetamine administration on methamphetamine self-administration in rhesus monkeys. Pharmacol Biochem Beh. 1984;21:737–41. doi: 10.1016/s0091-3057(84)80012-x. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Virus RM. The effects of D1 and D2 dopamine antagonists on behavior maintained by cocaine or food. Pharmacol Biochem Beh. 1989;32:691–7. doi: 10.1016/0091-3057(89)90019-1. [DOI] [PubMed] [Google Scholar]
- Yeomans J, Baptista M. Both nicotinic and muscarinic receptors in ventral tegmental area contribute to brain-stimulation reward. Pharmacol Biochem Behav. 1997;57:915–21. doi: 10.1016/s0091-3057(96)00467-4. [DOI] [PubMed] [Google Scholar]
- Zachariou V, Caldarone BJ, Weathers-Lowin A, George TP, Elsworth JD, Roth RH, Changeux JP, Picciotto MR. Nicotine receptor inactivation decreases sensitivity to cocaine. Neuropsychopharmacology. 2001;24:576–89. doi: 10.1016/S0893-133X(00)00224-4. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Grosu S, Williams EA, Qin S, Berod A. Neurons of origin of the neurotensinergic plexus enmeshing the ventral tegmental area in rat: retrograde labeling and in situ hybridization combined. Neuroscience. 2001;104:841–51. doi: 10.1016/s0306-4522(01)00118-x. [DOI] [PubMed] [Google Scholar]
- Zernig G, Wakonigg G, Madlung E, Haring C, Saria A. Do vertical shifts in dose-response rate-relationships in operant conditioning procedures indicate “sensitization” to “drug wanting”? Psychopharmacology. 2004;171:349–51. doi: 10.1007/s00213-003-1601-0. [DOI] [PubMed] [Google Scholar]
- Zhang H, Sulzer D. Frequency-dependent modulation of dopamine release by nicotine. Nature Neurosci. 2004;7:581–2. doi: 10.1038/nn1243. [DOI] [PubMed] [Google Scholar]


