Abstract
In transplantation, activation of complement has largely been equated to antibody-mediated rejection, but complement is also important in recognition of apoptotic and necrotic cells as well as in modifying antigen presentation to T cells and B cells. As a part of the innate immune system, complement is one of the first responses to injury, and it can determine the direction and magnitude of the subsequent responses. Consequently, the effects of complement in allorecognition and graft rejection are increased when organs are procured from cadaver donors because these organs sustain a series of stresses from brain death, prolonged life support, ischemia and finally reperfusion that initiate proinflammatory processes and tissue injury. In addition, these organs are transplanted to patients, who frequently have been sensitized to histocompatibility antigens as the result of transfusions, pregnancies or transplants.
Complement activation generates a series of biologically active effector molecules that can modulate graft rejection by directly binding to the graft or by modifying the response of macrophages, T and B cells of the recipient. However, complement is regulated and the process of regulation produces split products that can decrease as well as increase immune responses. Small animal models have been developed to test these variables. The guide for evaluating results from these models remains clinical findings because there are significant differences between the rodent and human complement systems.
INTRODUCTION
The potential for complement to function as an effector in graft rejection has been realized since the early experiences with clinical xenografts and allografts. Hyperacute rejection was a frequent occurrence in allografts before tests were devised to screen potential recipients for circulating antibodies to the prospective donor (1). This type of rejection occurs immediately upon perfusion of the transplants with the blood of the recipient. Antibodies in the blood of the recipient bind to the vascular endothelium of the transplant and activate complement, which results in neutrophil infiltration, vascular disruption, hemorrhage, fibrin deposition and platelet aggregation. Tests for donor specific antibodies have almost eliminated hyperacute rejection. The possibility that antibodies and complement contributed to acute or chronic forms of rejection was not widely appreciated until more sophisticated reagents were developed to demonstrate complement deposition in diagnostic biopsies from organ transplants (2–4). The use of monoclonal antibodies to certain complement split products has demonstrated that complement is activated and deposited on the vascular endothelium in a significant number of acute rejections. These rejections are categorized as antibody-mediated rejection when the biopsies contain marginated neutrophils or monocytes, and donor-specific antibodies are detected in the circulation. The incidence of antibody-mediated rejection varies from around 2% to more than 50% depending on the proportion of sensitized patients in the study (5–8). The contribution of antibody and complement to more chronic forms of graft failure is an area of active investigation (9–11). The recent upsurge in interest in antibody-mediated rejection has stimulated the development of in vivo and in vitro experimental models to study antibody and complement in acute and chronic rejection.
Surprisingly little is known about the impact of complement on allorecognition. As part of the innate immune system, there is universal agreement that complement is critical for the rapid recognition of pathogens. Complement is also essential for macrophages to remove ischemic, apoptotic or necrotic cells. Responses to auotantigens on apoptotic or necrotic cells are controlled by different complement components modulating cytokine production by macrophages and dendritic cells towards pro- or anti-inflammatory paths. Similar mechanisms would be expected to modulate the initial recognition of allogeneic tissues that experience significant ischemia and even necrosis at the time of transplantation.
Beyond the antigen presentation phase, complement is now known to modify the response of T and B cells of the adaptive immune system. Generally, complement alters the compartmentalization and localization of lymphocytes by increasing vascular permeability and upregulating the expression of adhesion molecules. At the level of individual cells, complement can alter the interaction between antigen presenting cells and T cells or B cells.
Although complement is categorized in the innate immune system, the complement cascade is very adaptable because it encompasses not only a series of effector molecules, but also regulatory molecules and receptors, which can stimulate or inhibit responses of the adaptive immune system. Moreover, the regulators of complement that are membrane anchored both regulate activation of complement, and regulate the function of the cell through signal transduction. This review will consider complement from the perspective of allorecognition and graft rejection.
Critical interactions between complement and antigen presenting cells
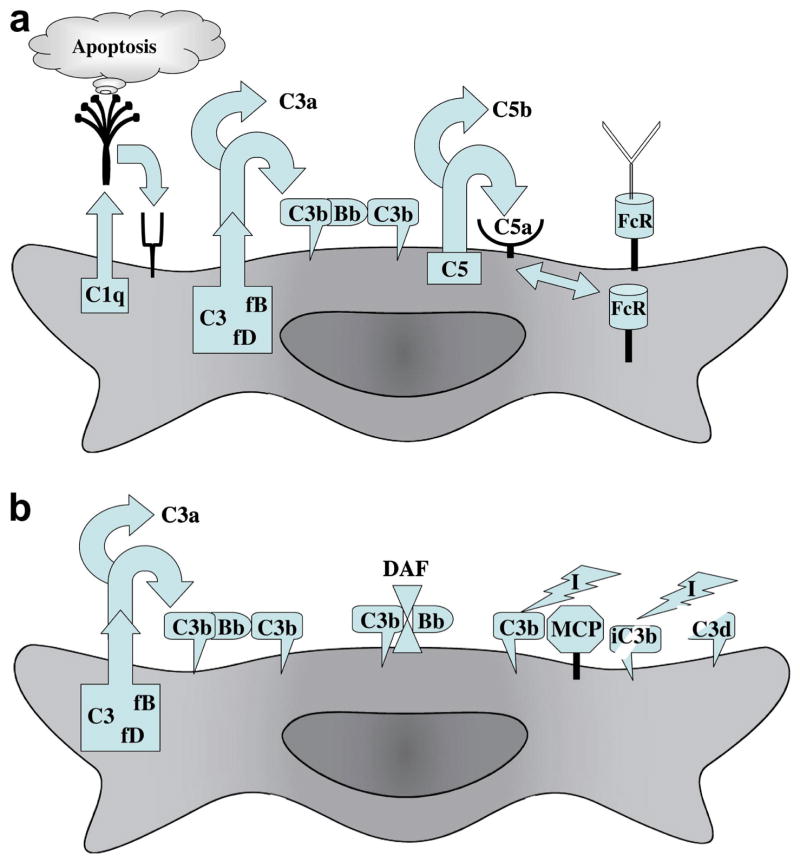
Allorecognition starts with antigen presentation by cells of donor or recipient origin. Macrophages, dendritic cells, B cells, endothelial cells and other cells capable of presenting alloantigens have dynamic interactions with the complement system. Macrophages and dendritic cells, which are the most potent antigen presenting cells, have receptors for the initiating components of each of the 3 activation pathways for complement: The classical (C1), lectin (mannose binding lectin; MBL) and alternative (C3) pathways. C3, the component that is the central hub of all 3 activation pathways, best exemplifies the intricate relationship between complement and antigen presenting cells. C3 was the first component to evolve and it is produced by a wide range of cells including macrophages and dendritic cells. In fact, macrophages produce most of the components of the complement system. This allows for the local production and activation of complement at sites of inflammation. Macrophages and dendritic cells also express receptors and regulators for biologically active split products of C3 (Figure 1). Moreover, the production and expression of all of these effectors, receptors and regulators are responsive to autocrine and paracrine feedback.
Figure 1.
Critical interactions between complement and antigen presenting cells. Macrophages and immature dendritic cells produce all of the components of the classical and alternative pathways of complement activation. In addition, they express receptors for C1q, C3b, iC3b, C3a, C5a and the Fc segment of antibodies. (A) C1q is critical to the recognition and clearance of apoptotic cells by macrophages and dendritic cells. The production of C3, factor B and factor D can result in deposition of C3bBb on the cell surface, which can cleave more C3b to form C3bBbC3b, the C5 convertase of the alternative pathway. This C5 convertase or other serine proteases produced by macrophages can cleave C5 into C5a and C5b. A feedback loop between C5a and FcR exists, in which C5 synthesis and cleavage is upregulated by antibodies engaging FcγRIII. Binding of the resultant C5a to receptors on macrophages in turn upregulates FcγRIII and downregulates the inhibitory FcγRIIB. (B) Complement regulatory proteins on macrophages and dendritic cells modulate interactions with T and B cells. Decay accelerating factor (DAF; CD55) is a glycosylphosphatidylinositol-anchored membrane regulator that accelerates the dissociation of Bb from C3b. This limits the amount of C3b bound to the surface of antigen presenting cells. Membrane co-factor protein (MCP; CD46) is a type 1 transmembrane glycoprotein that regulates complement by acting as a co-factor for factor I to cleave C3b into enzymatically inactive iC3b and then C3d. DAF and MCP are also expressed by T cells and modulate their responses. As the result of alternative splicing, MCP is expressed with two different cytoplasmic tails that modulate T cell responses divergently.
Findings about complement in autoantigen presentation of relevance to alloantigen presentation
The effects of complement on antigen presentation have been explored more fully for autoantigens than alloantigens because of the association of complement deficiencies with autoimmunity. In an effort to understand the association of C1 deficiency with autoimmunity, Korb and Ahearn (12) found that the C1q facilitated the clearance of apoptotic cells by macrophages. Later, the isolated globular heads of C1q were demonstrated to bind directly to the blebs on apoptotic cells and C1q deposition was shown to increase as the blebs matured (13). After binding to apoptotic blebs, C1 has been shown to activate C4, C2 and C3 (14).
Other molecules that are capable of activation complement, such as MBL, ficolin and C-reactive protein (CRP), have also been found to bind to and facilitate the clearance of apoptotic cells (15–17). In the lung, surfactant proteins (surfactant protein A and D; SP-A and SP-D) that bind to and clear apoptotic cells. Many of these same molecules can augment the uptake of apoptotic blebs by immature dendritic cells (17). The ligands that C1q and MBL bind on apoptotic and necrotic cells are not fully defined, but include phospholipids, nucleic acids and cardiolipins (18, 19). Likewise, the receptors for C1q and MBL on antigen presenting cells are only partly identified and include calreticulin (20, 21).
Complement not only opsonizes apoptotic cells for uptake by antigen presenting cells, but also modulates the consequent production of cytokines. The relative effects on cytokine secretion are dependent on the amount and range of complement components bound to the apoptotic cells. In vitro experiments with purified C1q or MBL and apoptotic blebs in the absence of necrotic cells usually result in the upregulation of IL-6 and IL-10 and downregulation of IL-1 and IL-12 (16, 22). C3b deposited on apoptotic cells is regulated rapidly into iC3b (23), which is a ligand for complement receptors 3 (CR3; CD11b/CD18) and CR4 (CD11c/CD18). Stimulation of immature dendritic cells through CR3 causes down-regulation of MHC class II, CD86, and CC chemokine receptor (CCR)2 and CCR5, and up-regulation of CCR7 (24). These changes generally inhibit inflammation and adaptive immune responses.
The anti-inflammatory effects of complement in vitro are often counterbalanced by pro-inflammatory effects in vivo. For example, C1q deficiency is associated with decreased clearance of apoptotic blebs and increased incidence of severe autoimmune diseases in humans and mice, but this phenotype is dependent on other background genes in mice (25) and C1q does not prevent the inflammatory response when normal tissue homeostasis is altered by interventions that cause ischemia-reperfusion. Furthermore, MBL increases tissue injury initiated by ischemia-reperfusion (26, 27). The pathway of complement that is activated in vivo is dependent on the extent of injury, fundamental properties of the tissue and other variables. For cardiac and skeletal muscle, experiments using complement inhibitors or complement deficient animals have documented a critical contribution of MBL, C3 and C6 to the inflammation and injury related to ischemia and reperfusion (26–29). MBL can bind endothelial cells, possibly through cytokeratin 1, and activate complement after hypoxia in vitro and ischemia-reperfusion in vivo (27, 30). Factor B of the alternative pathway and C1 of the classical pathway are not necessary in these models. However, Carroll and colleagues (31) have presented some evidence that C1 may be responsible for the edema that develops locally in the ischemic skeletal muscle and the inflammation caused by C5a in distant tissues, such as the lung.
Renal ischemia-reperfusion also elicits less complement activation and renal dysfunction in MBL knockout mice than in normal mice (32). These findings in knockout mice confirm earlier immmunohistological studies that detected deposition of MBL, C3, C6 and C9 in mouse and human kidneys following ischemic insults (33). In addition, factor B and decreased expression of complement regulatory molecules augment renal injury from ischemia-reperfusion (34). Besides initiating the alternative pathway of complement, factor B triggers an autocrine amplification loop by binding to C3b on the surface of cells regardless of the pathway responsible for the first C3b. Therefore, deposition of C3b initiated by MBL or C1 can result in binding of factor B to C3b, the formation of C3bBb and amplification through cleavage of more C3 (35, 36). This autocrine amplification loop often is responsible for generating the majority of C3 split products (37). C3a and C3b as well as the subsequent split products of C3b, can contribute to leukocyte infiltrates in kidneys. C3a stimulates renal epithelial cells through C3a receptors (C3aR) to produce chemokines that attract neutrophils and monocytes (38). The tissue-bound C3b would serve as a ligand for CR1 (CD35) on neutrophils and monocytes that are chemoattracted by the renal epithelium.
The finding that expression of complement regulatory proteins is decreased in ischemic or necrotic tissues in vivo (34) is another distinction to apoptotic cells in vitro. Decreased regulation of C3 would result in more cleavage of C5 into C5a and C5b. These two split products of C5 have many pro-inflammatory effects. C5a is a powerful chemoattractant and would contribute to accumulation of leukocytes following ischemia (39). Furthermore, C5b is the first component of the membrane attack complex (MAC) that has been detected in ischemic tissues (28, 33, 40). Experiments with C6 deficient mice confirmed the importance of MAC to tissue injury caused by ischemia-reperfusion (28, 33, 40).
Two groups have now reported that IgM antibodies can activate MBL (29, 41, 42). This mechanism of MBL activation is noteworthy because Carroll and colleagues have identified “natural” IgM antibodies to autoantigens that are exposed on ischemic tissues (42). One of the autoantigens identified is nonmuscle myosin heavy chain type II A and C (43). Recent studies suggest that IgM may also augment the binding of C4 and C3 to apoptotic and necrotic cells (44, 45).
The greater proinflammatory responses initiated in vivo by MBL compared with C1 are surprising in view of the many overlapping functions of these similar pattern recognition proteins. However, two recent studies revealed potentially relevant differences. One study demonstrated that MBL could activate C3 and form the alternative pathway C3 convertase (C3bBb) in the absence of C4 or C2 (35). This pathway would also bypass regulation directed at C4b and could lead to greater amplification of C3 activation. Extracellular matrix is another important component of tissues that is not included in many in vitro models. Human C1q and MBL both bind to the extracellular matrix proteoglycans decorin and biglycan, but the consequences are different. Decorin and biglycan bind the collagen and globular domains C1q. This inhibits activation of the classical complement cascade, and the cellular interactions that lead to production of proinflammatory cytokines. Although decorin and biglycan also bind MBL, only biglycan inhibits activation of the lectin pathway (46). Therefore, when MBL penetrates into the extra cellular matrix of ischemic tissues, it retains more activity than C1q.
Given the extensive attention to complement in ischemia, remarkably few experiments have probed the contribution of complement to allorecognition of ischemic or necrotic tissues. In one immunohistological study, de Vries et al (33) observed that MBL deposition in kidneys procured from non-heart beating donors correlated with the amount of warm ischemia and the degree of initial graft dysfunction. A second study by Berger et al (47) analyzed genetic variants that determine MBL serum levels as a risk factor for graft failure in renal transplant recipients. They found that patients with high MBL levels had an increased risk of graft rejection that did not respond to therapy. This finding from a single center deserves to be pursued. The availability of MBL knockout mice would provide useful experimental options.
Experimentally, the immunogenicity of necrotic cells has been examined in the context of largely undefined “danger” signals or endogenous adjuvants. In this context Matzinger and colleagues demonstrated that dendritic cells can be activated by endogenous signals delivered by stressed or necrotic cells (48). Rock and co-workers have extended this finding to apoptotic cells (49). Neither of these groups has investigated the possible involvement of complement in the recognition of antigen from injured tissues by antigen presenting cells.
Transplantation favors a pro-inflammatory environment
The pro-inflammatory functions of complement are favored in the setting of transplantation because the ischemia necessitated by organ procurement is preceded by the stresses of brain death and prolonged life support. Organs harvested from cadaver donors are known to produce pro-inflammatory cytokines and contain leukocyte infiltrates at the time of transplantation (50–52). Pathological manifestations of overt tissue injury associated with clinical transplantation of organs from cadaver donors has been described most thoroughly in hearts, which are at particular risk for the effects of catecholamines released by brain death. Histological features of contraction band necrosis or coagulative myocyte necrosis are commonly found in protocol endomyocardial biopsies taken within the first 6 weeks after transplantation. The inflammatory response to this necrotic tissue “may contain mixed inflammatory infiltrates, including neutrophils as well as lymphocytes, macrophages and eosinophils, and it is at this point that confusion with acute rejection may occur” according to the international committee that revised the grading system for cardiac allograft biopsies in 2004 (53). The committee further warned that injury from the peri-transplantation period can have overlapping features with antibody-mediated rejection.
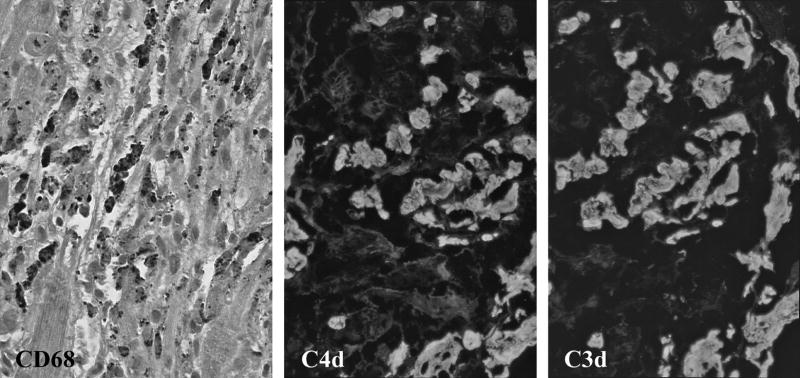
Fyfe and colleagues (54) reviewed tissue sections from 140 consecutive cardiac transplant recipients and found that some degree of myocyte necrosis was evident in early protocol biopsies from 89% of the transplants. This study was performed before criteria for antibody-mediated rejection were included in the grading system. One of us (M.H.) has prospectively graded all of the biopsies from adults who received cardiac transplants in the last 2 years at Johns Hopkins Hospital and carefully excluded biopsies from patients with any evidence of antibody-mediated rejection. Moderate to marked peri-transplantation tissue injury was observed in at least one of the first 4 protocol biopsies from 56% (22/39) of the patients. These histological manifestations were most frequent 10 to 28 days after transplantation. In addition to inflammatory infiltrates that were primarily composed of macrophages, the injured myocytes contained deposits of C4 and C3 split products (Figure 2).
Figure 2.
Peri-transplantation tissue injury in routine protocol endomyocardial biopsies. Inflammatory infiltrates composed of macrophages demonstrated by immunoperoxidase stain for CD68 (dark cells) surround necrotic myocytes. Deposits of C4d and C3d (white) on necrotic myocytes demonstrated by immunofluorescence in frozen sections.
Tilney and colleagues (51) demonstrated in a rat model that kidneys grafted from brain dead donors to isogeneic recipients had deposits of C3 on vascular endothelial cells 1 and 6 hours after transplantation. The C3 deposition was associated with leukocyte infiltrates. C3 was not found in kidneys transplanted from control living donors. These findings in rats mirror observations in humans that neutrophil infiltrates and activated platelets are more frequently found in kidneys recovered from cadaver than living donors (50). In this study, platelet activation in donor kidneys was demonstrated by double staining for P-selectin. This is of relevance because of a recent report that P-selectin on activated platelets binds C3b efficiently (55).
Even without brain death of the donor, transplantation stimulates local production of complement. Within 6 hours after transplantation, renal allografts in rats express high levels of mRNA for C1 and C3 (56). C1 and C3 peak at one day, and C1 remains elevated for 14 days, but C3 returns to baseline at day 3. Both C1 and C3 are synthesized by macrophages, dendritic cells, endothelial cells and renal tubular epithelial cells (57, 58). The first peak of C3 synthesis at 24 hours is primarily attributable to donor cells, but a subsequent increase in C3 synthesis coincides with increasing infiltrates of recipient mononuclear cells (59).
The effects of complement may be altered in clinical transplants when cyclosporine A (CsA) is used for immunosuppression. CsA inhibits at least one regulatory mechanism, namely the complement regulatory protein decay accelerating factor (DAF; CD55). DAF is a membrane anchored protein that disassociates Bb from C3b (Figure 1B). Many mediators of acute inflammation, such as, thrombin, TNFα and IFNγ, increase DAF expression by endothelial cells in vitro (60, 61). CsA inhibits upregulation of DAF to certain stimuli (62). As a result, treatment with CsA may allow more proinflamatory C3 and C5 split products and MAC to be generated. CsA also inhibits some effects of complement. For example, CsA inhibits IL-1α production by endothelial cells in response to MAC (63). The potential effects of CsA or other drugs commonly used in transplant recipients on the expression of complement regulatory proteins or the secretion of cytokines in response to complement have not been studied. The disruption of the protective mechanisms to complement activation could have wide ranging effects in transplant patients.
Complement production by antigen presenting cells modulates alloreactivity of T cells
There are several lines of evidence that complement produced by antigen presenting cells modulates responses of T cells to alloantigens. Sacks and co-workers (64, 65) found that macrophages or dendritic cells from C3 deficient donors are less stimulatory to T cells in mixed leukocyte reactions in vitro and less sensitizing when administered in vivo. The mechanism underlying these observations has not been completely defined, but the macrophages and dendritic cells from C3 deficient donors were found to express less MHC class II antigens. This coincided with decreased responses of CD4+ T cells as measured by production of IL-2, IL-12 and IFNγ. Conversely, dendritic cells from C3 deficient donors stimulated increased IL-4 and IL-10 production by allogeneic T cells.
The simplest mechanism to explain this effect of C3 on the interaction of antigen presenting cells and T cells would involve C3 produced by antigen presenting cells interacting with C3 receptors on T cells. Sachs and colleagues demonstrated low levels of C3 production by macrophages and dendritic cells, but addition of complement to cultures with antigen presenting cells from C3 deficient donors did not reconstitute normal stimulation of T cells. Nor did these investigators detect C3 on the surface of antigen presenting cells. However, other groups have found C3 on the surface of peritoneal macrophages (66). Heeger and co-workers (67) detected C3 at the interface between macrophages and T cells by immunoelectron microscopy, and further demonstrated that expression of DAF on T cells diminished their response to alloantigen presented by macrophages. The mechanism proposed for this finding is that DAF limited the formation of the alternative C3 convertase (C3bBb) on the surface of T cells. In support of this hypothesis, both antigen presenting cells and T cells were shown to produce C3, factor B and factor D within hours of stimulation by alloantigen in vitro.
Membrane cofactor protein (MCP; CD46) is another complement regulatory protein on T cells that interacts with C3b. MCP has also been demonstrated to alter T cell responses (68). As its name implies, MCP regulates complement by acting as a cofactor for factor I to cleave C4b or C3b deposited on cell membranes. Factor I cleaves C4b or C3b into enzymatically inactive products: first iC4b or iC3b and then C4d or C3d (Figure 1B). MCP is a type 1 transmembrane glycoprotein that is expressed with two different cytoplasmic tails as the result of alternative splicing of mRNA. The two resulting isoforms (CD46-1 and CD46-2) in humans modulate T cell responses divergently (69). Stimulation with solid-phase antibodies to CD3 and CD46 resulted in decreased production of IL-2 by CD4+ T cells from CD46-1 transgenic mice, but not by CD4+ T cells from CD46-2 transgenic mice. The reverse pattern was found for IL-10. Proliferative responses of purified CD4+ T cells were also divergently modulated by CD46 isoforms: co-stimulation with antibodies to CD3 and either C3b or antibodies to CD46 increased proliferation of CD46-1 expressing T cells, but decreased the proliferative response of CD46-2 T cells.
The relevance of C3 to allorecognition and transplant rejection has been shown with C3 knockout mice. Sacks and colleagues reported that kidneys from C3 deficient donors survived longer than normal kidneys in allogeneic recipients (70). This is consistent with their findings with C3 deficient antigen presenting cells. C3 deficient recipients were found to have decreased cellular and antibody responses to skin grafts that were associated with slightly longer graft survival (71). Finally, in parallel with their in vitro findings for DAF, Heeger et al (67) found that skin grafts were rejected more acutely by DAF knockout mice than by normal recipients.
Complement modulates antibody production and possible antigen presenting function of B cells
B cells are relevant to both allorecognition and graft rejection because they can function as antigen presenting cells as well as antibody producing cells. Complement modulates both of these functions. Complement is activated when antigen is bound by either secreted antibodies or membrane immunoglobulins that serve as antigen receptors on B cells (72). The deposition of biologically active C3 split products on the surface of B cells is linked with optimal antibody responses. C3d, the final end product of regulation of C3b by factor I, is the ligand for CR2 (CD21) on B cells. In humans and mice, cross-linking of CR2 and its signaling partner, CD19, with membrane IgM rapidly up-regulates the co-stimulatory molecules CD86 and CD80. This is associated with heightened B cell activation of resting allogeneic T cells (73).
C3d also modulates the production of antibodies by B cells. Fearon and colleagues (74) demonstrated that antigens bearing two and three C3d molecules are 1,000 to 10,000-fold more immunogenic than antigen alone. The effects of C3d are dependent on several variables including the number or density of C3d bound to the antigen. With soluble antigens, C3d can down-regulate antibody production to certain antigens (75). The effects of C3d on antibody responses to alloantigens needs to be better defined.
In transplant recipients, complexes of C3d and antigen may be presented to B cells either in secondary lymphoid tissues or within the transplant. Several groups have recently reported that B cells in transplants are organized in tertiary lymphoid structures surrounded by a T cell compartment that contains high endothelial venules. In experimental models, tertiary lymphoid nodules have been described in two models of transplantation (76, 77). Lakkis and colleagues (76) described organized nodules of lymphocytes with T and B cell compartments in heterotopic mouse hearts undergoing chronic rejection. Thaunat and co-workers (77) used segmental aortic grafts in rats to demonstrate that lymphoid nodules formed adjacent to these large arteries were associated with the production of antibodies to histocompatibility antigens. Clinically, Steinmetz and co-workers (78) described 4 renal biopsies obtained in the first 4 to 9 days after transplantation, which contained nodules of B cells that stained with CXCL13 and the corresponding CXCR5 receptor. We have found well developed tertiary nodules in the adventitia of coronary arteries of cardiac transplants with severe graft vasculopathy (11). Much remains to be learned about the function of these organized lymphoid structures. For example, B cells may function as antibody producing cells or antigen presenting cells to the surrounding T cells in tertiary lymphoid nodules.
Pro-inflammatory effects of complement induced by antibodies
As in the allorecognition phase complement has been found in both pro- and anti-inflammatory contexts during episodes of rejection in experimental models and clinical biopsies. The use of antibodies to C4 split products including C4d revealed that complement is activated during rejection more frequently than appreciated previously (5–8). The pro-inflammatory features of complement activation in transplants include activation of platelets, neutrophils and macrophages.
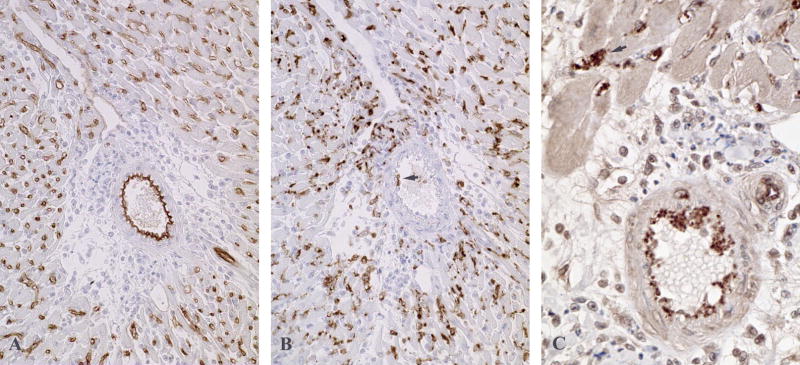
Animal models have provided insights into the variables determining the character and locations of inflammation influenced by complement. Intense pro-inflammatory responses are elicited by high titers of antibodies to MHC antigens. Such antibody responses are elicited by transplants to recipients that are treated with suboptimal immunosuppression or that are presensitized by previous blood transfusions, pregnancies or transplants. As illustrated in figure 3, split products of C4 are detectable as thick linear deposits on endothelial cells of arteries, capillaries and veins in transplants to recipients with high titers of circulating antibodies to MHC antigens (79, 80). The deposition of complement on arteries (Figure 3A) is significant because the high flow rate of blood through arteries requires high avidity interactions to allow binding of antibodies and complement. When large amounts of complement are deposited on vascular endothelium, regulatory mechanisms are overwhelmed and the entire complement cascade is activated. The most conspicuous features of this type of rejection are the released von Willebrand factor and platelet aggregates. Platelets enmeshed in von Willebrand factor line the arteries and plug capillaries (Figure 3C). This process is promoted by formation of MAC because it is greatly decreased in C6 deficient animals (81).
Figure 3.
Immunoperoxidase stained sections from cardiac transplants to rats that produced high levels of antibodies to donor MHC antigens. (A) In cardiac transplants to C6 deficient recipients vascular integrity is maintained and split products of C4 are detectable as thick linear deposits on endothelial cells of arteries, capillaries and veins. (B) A serial section from this heart contains large numbers of macrophages stained for CD68 in the capillaries and a few macrophages (arrow) that are attached to the arterial endothelium. (C) In cardiac transplants to C6 sufficient rats platelets enmeshed in von Willebrand factor line the arteries and plug capillaries (arrow).
In vitro experiments with purified C5b-C9 and endothelial cell monolayers have delineated several mechanisms, by which MAC makes endothelial cells become pro-adhesive and pro-coagulant. The two most immediate effects of MAC on endothelial cells are the retraction of the plasma membrane to expose underlying extracellular matrix and the release of preformed adhesion molecules from Weibel-Palade storage granules (82, 83). Weibel-Palade storage granules contain both von Willebrand factor and P-selectin. The multimeric von Willebrand factor that is released from the Weibel-Palade granules is effective at aggregating platelets even in the high shear stress conditions of arteries. In contrast to von Willebrand factor, P-selectin has low affinity for ligands on leukocytes and platelets that results in transient interactions in the low flow conditions of capillaries and venules. In the high flow environment of arteries, P-selectin may act as a signaling molecule between endothelial cells and platelets or monocytes enmeshed in von Willebrand factor (84–86).
In unpublished studies using DNA microarrays (87), we have established gene expression profiles of cardiac allografts undergoing antibody-mediated rejection. We identified 62 genes that had significantly increased expression (> 2 fold) and 17 were known to be related to macrophages. The following cluster of genes that are related to activation of M1-type macrophages had more than 4-fold increased in expression: CD68, IL-1β, IL-18, macrophage inflammatory protein-1 (MIP-1α; CCL3), MIP-1β (CCL4), RANTES (CCL5), CCR5, CCR2, monocyte chemotattractant protein-1 (MCP-1; CCL2), FcγR I, IIb and III. Upregulation of IL-18 and FcγR IIb were confirmed by real time PCR.
Complement and platelets elicit a series of more delayed responses from endothelial cells that probably are more relevant in the low flow conditions of capillaries and veins (88–90). These include MAC-induced production of IL-8 (CXCL8), MCP-1, P-selectin (CD62P), E-selectin (CD62E), and intracellular adhesion molecule-1 (ICAM-1; CD54) that would increase recruitment and attachment of leukocytes in low flow conditions. Activated platelets augment recruitment of leukocytes by both secreting chemokines and inducing endothelial cells to secrete chemokines. Platelets secrete IL-8, RANTES, MIP-1α and MCP-3 (CCL7) that recruit leukocytes. Endothelial cells stimulated by platelets secrete MCP-1 and IL-8. Activated platelets stimulate more changes in endothelial cells including expression of tissue factor activity and E-selectin (91). These changes are induced in part by IL-1 on the surface of the platelets. Activated platelets also express P-selectin, which can bind C3b and propagate the complement cascade. Complement activation by platelets provides more routes for recruitment and activation of leukocytes. In addition, platelets are a major source of soluble CD154, which is the ligand for CD40 on B lymphocytes, macrophages, dendritic cells and endothelial cells (92). Platelet-derived CD154 has been demonstrated to initiate rejection of hearts transplanted to CD154 deficient mice (93). Finally, platelets secrete a variety of growth factors, such as platelet derived growth factor, epidermal growth factor and fibroblast growth factor, that promote proliferation of endothelial cells, smooth muscle cells and fibroblasts (94).
Platelet activation is most apparent in transplants exposed to high titers of antibodies, but macrophages can be stimulated by lower titers of antibodies. Lower titers of antibodies that do not activate enough complement to overcome all of the regulatory controls that prevent eventual formation of MAC are also associated with less deposition of C4 split products on arterial endothelium. In the absence of MAC formation, alloantibodies still cause deposition of complement split products on capillary endothelium associated with intra- and peri-vascular accumulations of macrophages (81). Macrophage infiltrates can be extensive in transplants to C6 deficient rats that are incapable of forming MAC (Figure 1B).
C5a is the strongest complement-mediated signal for macrophage recruitment and activation. Activation of monocytes through their C5aR causes upregulation of CR1 and CR3, and production of proinflammatory cytokines. Upregulation of CR1 and CR3 on neutrophils and monocytes allows tissue bound C3b and its subsequent cleavage product iC3b to serve as accessory adhesion molecules that strengthen the contact between cells in the graft and responding cells. In addition, human vascular endothelial cells express C5aR in vivo (95). C5a stimulation causes a rapid expression of the adhesion molecule P-selectin and secretion of von Willebrand factor on endothelial cells (96, 97).
Anti-inflammatory effects of complement induced by antibodies
Complement activation by antibodies is not always pro-inflammatory. Platt and colleagues (98) described the phenomenon of accommodation in blood group incompatible transplants. As with antibodies to MHC, hyperacute rejection of A or B blood group incompatible transplants can result when high titers of antibodies to these antigens are present at the time of transplantation. Removal of these antibodies by immunoadsorption or plasmapheresis before and during the first weeks after transplantation permits successful transplantation (99). Discontinuance of immunoadsorption or plasmapheresis and the return of antibodies to the A or B blood group is often associated with the deposition of C4 split products on the vascular endothelium of the graft. However, these products of complement activation are usually not associated with evidence of inflammation or graft dysfunction (100).
This fascinating finding has fostered many hypotheses and experiments that have been reviewed recently (101). The fact that complement is activated in the transplants indicates that antibodies do bind to target antigens in the graft, and suggests that the graft is more resistant to injury caused by complement. Of relevance to this review, are the findings that complement regulatory proteins are upregulated in accommodated transplants. Basal levels complement regulatory proteins vary for different cells, and as already discussed, these levels can be modulated by inflammation and tissue injury. CD59, a membrane anchored regulator of MAC formation, is one regulatory protein that is upregulated in accommodation (101).
Antibodies modulate local complement production
Antibodies not only activate complement, but they also provide powerful feedback through Fc receptors (FcR) to increase complement production. Likewise, in addition to stimulating production of antibodies by B cells, complement split products can modulate the expression and function of FcR for antibodies (Figure 1A).
There is a large array of FcR, but FcγR for IgG antibodies are of the most relevance to rejection of transplants. Macrophages express the high affinity FcγRI (CD64) that is capable of binding monomeric IgG as well as the low affinity FcγRIIA (CD32), FcγRIII (CD16) and FcγRIV that are engaged by clusters of IgG on antigen (102). These FcγR signal through immunoreceptor tyrosine-based activation motifs (ITAMs). Activation of macrophages through FcγR upregulates many proinflammatory functions, including the production MCP-1 and IL-8, and promotes survival of macrophages at sites of inflammation (103, 104). FcγRIII is expressed by NK cells as well as macrophages and neutrophils. This receptor signals through ITAM to initiate antibody-dependent cell-mediated cytotoxicity (ADCC) by NK cells.
Activating Fcγ receptors are counterbalanced by FcγRIIB on macrophages that functions through an immunoreceptor tyrosine-based inhibitory motif (ITIM). FcγRIIB is also expressed by B lymphocytes and functions as a feed back inhibitor of antibody production. The balance of activating and inhibitory FcγR expression by macrophages is controlled by different mediators including cytokines and complement split products.
Macrophage production of C3 and C5 is increased through both the Fc (105, 106) and C1q receptors (107). Gessner and colleagues (105, 106) have found that production and cleavage of C5 by macrophages is regulated by FcγR. These researchers have demonstrated a feedback loop between FcγR and C5a in several models of autoimmunity. In these models, C5 synthesis and cleavage is upregulated by antibodies engaging FcγRIII. Binding of the resultant C5a to receptors on macrophages in turn upregulates the stimulatory FcγRIII and downregulates the inhibitory FcγRIIB. This feedback is independent of the early components of complement because it occurs in C3 knock out mice. In the absence of C3, C5 is cleaved by an as yet uncharacterized enzyme produced by macrophages. It is likely that this mechanism is even more significant in normal individuals because macrophages can synthesize all of the complement components, and therefore, macrophages could generate more C5a through multiple pathways including the alternative pathway. The large numbers of macrophages infiltrating transplants would provide a rich environment for macrophages to propagate inflammation through complement. We have demonstrated that macrophages can be a critical source of complement in acute rejection of cardiac allografts (108).
The interactions of complement and FcR have largely been studied in models other than transplantation. However, the availability of monoclonal antibodies to C5 and small molecule inhibitors of C5a provide methods that can be applied to transplantation. Antibodies to C5 have already been shown to decrease macrophage infiltrates and inhibit acute rejection of cardiac allografts in mice (109).
A question of memory
Excellent survival of transplants is much more easily achieved in animals than in humans. Often inhibition of a subset of lymphocytes, a single receptor or one mediator is sufficient to achieve very extended graft survival in mice. In contrast, protocols encompassing multiple immunosuppressive drugs are required to prevent acute rejection and inhibit chronic graft dysfunction in humans. Animal models ordinarily use young, healthy recipients that have not experienced previous blood transfusion, pregnancies or transplants. In humans, transfusions are critical components in the management of transplant patients. Prior to cardiac transplantation, for example, many patients receive transfusions when they undergo surgical procedures to alleviate cardiac dysfunction. These procedures include repairs of congenital abnormalities, replacements of valves, application of coronary bypass grafts, and insertion of ventricular assist devices. In multivariant analyses, platelet transfusions are frequently found to be associated with the induction of antibodies to HLA in these patients (110). In addition, many adult female patients have been pregnant prior to transplantation. Approximately 15–30% of women produce alloantibodies directed against the paternal MHC antigens of the fetus following pregnancy (111). Pregnancy can also act as an added risk factor in sensitization caused by blood transfusion. Patients with previous pregnancies develop anti-HLA antibodies after blood transfusion at a much higher rate than nulliparous female and male patients (112).
Presensitization has a significant impact on transplants because, in spite of the extreme polymorphism of HLA, the high frequency of a few antigens increases the chance that patients will harbor memory cells for a random organ donor after sensitization by prior transplantation, transfusion or pregnancy. HLA-A2, for example, is expressed by about half of the Caucasian population. This means that an HLA-A2 negative patient has about a 50% chance that a prior random donor of a transfusion or transplant was HLA-A2 positive.
In order to test the relative risk of re-exposure to specific antigens in a real population, Halushka and colleagues (113) examined the incidence of repeat HLA-A and B mismatches in the 25 patients who received a second heart transplant at Johns Hopkins. We chose this population because the allocation of hearts for transplantation is not skewed by prospective HLA matching, which is not performed routinely for unsensitized recipients. Repeat mismatches of serologically identified HLA class I antigens were expressed on 13 of 25 (52%) second heart transplants. If cross-reactive group antigens (CREG) are considered, all but 3 of the patients had repeat mismatches of HLA class I antigens. The risk of repeat exposure to HLA would be the same for blood transfusions and pregnancy.
Presensitization is a major risk factor for antibody-mediated rejection as evidenced by deposition of complement split products in diagnostic biopsies. In a retrospective study of renal biopsies, pregnancy was found to be a risk factor for deposits of C4 split products (114). As already stated, the incidence of antibody-mediated rejection varies from around 2% to more than 50% depending on the proportion of sensitized patients in the study (5–8). Likewise, presensitization augments complement deposition in transplants to rats (79, 80).
In summary, the widespread use of more sensitive reagents for detection of complement split products in clinical biopsies of transplants has led to an increased awareness of complement as an effector mechanism in graft rejection. The frequency of antibody-mediated rejection in clinical transplants is increased by the large number of patients, who have been exposed to previous transfusions, pregnancy or transplants. To better understand antibody-mediated rejection, there are increasing numbers of experiments investigating effector mechanisms of complement in animal models. The potential contributions of complement to allorecognition have been relatively neglected. However, observations in the areas of autoimmunity, ischemia-reperfusion and brain death indicate that complement could be a significant factor in allorecognition.
Acknowledgments
The authors wish to thank Karen Fox-Talbot for her expert help with the immunohistochemistry preparations. The authors are supported by NIH grants R01AI42387, P01 HL65608 and P01 HL56091. B.A.W. is also supported by grant ID#508303540 from the Roche Foundation (ROTRF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Patel R, Terasaki PI. Significance of the positive crossmatch test in kidney transplantation. N Engl J Med. 1969;280:735–739. doi: 10.1056/NEJM196904032801401. [DOI] [PubMed] [Google Scholar]
- 2.Feucht HE, Felber E, Gokel MJ, Hillebrand G, Nattermann U, Brockmeyer C, et al. Vascular deposition of complement-split products in kidney allografts with cell-mediated rejection. Clin Exp Immunol. 1991;86:464–470. doi: 10.1111/j.1365-2249.1991.tb02954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lederer SR, Schneeberger H, Albert E, Johnson JP, Gruber R, Land W, et al. Early renal graft dysfunction. The role of preformed antibodies to DR-typed lymphoblastoid cell lines. Transplantation. 1996;61:313–319. doi: 10.1097/00007890-199601270-00025. [DOI] [PubMed] [Google Scholar]
- 4.Baldwin WM, III, Pruitt SK, Brauer RB, Daha MR, Sanfilippo F. Complement in organ transplantation: contribution to inflammation, injury and rejection. Transplantation. 1995;59:797–808. [PubMed] [Google Scholar]
- 5.Mauiyyedi S, Colvin RB. Humoral rejection in kidney transplantation: new concepts in diagnosis and treatment. Curr Opin Nephrol Hypertens. 2002;11(6):609–618. doi: 10.1097/00041552-200211000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Michaels PJ, Espejo ML, Kobashigawa J, Alejos JC, Burch C, Takemoto S, et al. Humoral rejection in cardiac transplantation: risk factors, hemodynamic consequences and relationship to transplant coronary artery disease. J Heart Lung Transplant. 2003;22(1):58–69. doi: 10.1016/s1053-2498(02)00472-2. [DOI] [PubMed] [Google Scholar]
- 7.Mengel M, Bogers J, Bosmans JL, Seron D, Moreso F, Carrera M, et al. Incidence of C4d stain in protocol biopsies from renal allografts: results from a multicenter trial. Am J Transplant. 2005;5(5):1050–1056. doi: 10.1111/j.1600-6143.2005.00788.x. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez ER, Skojec DV, Tan CD, Zachary AA, Kasper EK, Conte JV, et al. Antibody-mediated rejection in human cardiac allografts: evaluation of immunoglobulins and complement activation products C4d and C3d as markers. Am J Transplant. 2005;5(11):2778–2785. doi: 10.1111/j.1600-6143.2005.01074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith RN, Kawai T, Boskovic S, Nadazdin O, Sachs DH, Cosimi AB, et al. Chronic antibody mediated rejection of renal allografts: pathological, serological and immunologic features in nonhuman primates. Am J Transplant. 2006;6(8):1790–1798. doi: 10.1111/j.1600-6143.2006.01351.x. [DOI] [PubMed] [Google Scholar]
- 10.Uehara S, Chase C, Cornell L, Madsen J, Russell P, Colvin R. Chronic cardiac transplant arteriopathy in mice: Relationship of alloantibody, C4d deposition and neointimal fibrosis. Am J Transpl. 2007;7:57–65. doi: 10.1111/j.1600-6143.2006.01599.x. [DOI] [PubMed] [Google Scholar]
- 11.Wehner J, Morrell CN, Reynolds T, Rodriguez ER, Baldwin WM., III Antibody and complement in transplant vasculopathy. Circ Res. 2007 doi: 10.1161/01.RES.0000255032.33661.88. in press. [DOI] [PubMed] [Google Scholar]
- 12.Korb LC, Ahearn JM. C1q binds directly and specifically to surface blebs of apoptotic human keratinocytes: complement deficiency and systemic lupus erythematosus revisited. J Immunol. 1997;158(10):4525–4528. [PubMed] [Google Scholar]
- 13.Navratil JS, Watkins SC, Wisnieski JJ, Ahearn JM. The globular heads of C1q specifically recognize surface blebs of apoptotic vascular endothelial cells. J Immunol. 2001;166(5):3231–3239. doi: 10.4049/jimmunol.166.5.3231. [DOI] [PubMed] [Google Scholar]
- 14.Nauta AJ, Trouw LA, Daha MR, Tijsma O, Nieuwland R, Schwaeble WJ, et al. Direct binding of C1q to apoptotic cells and cell blebs induces complement activation. Eur J Immunol. 2002;32(6):1726–1736. doi: 10.1002/1521-4141(200206)32:6<1726::AID-IMMU1726>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 15.Gershov G, Kim S, Brot N, Elkon KB. C-Reactive Protein binds to apoptotic cells, protects the cells from assembly of the terminal complement components, and sustains an antiinflammatory innate immune response: implications for systemic autoimmunity. J Exp Med. 2000;192:1353–1363. doi: 10.1084/jem.192.9.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nauta AJ, Castellano G, Xu W, Woltman AM, Borrias MC, Daha MR, et al. Opsonization with C1q and mannose-binding lectin targets apoptotic cells to dendritic cells. J Immunol. 2004;173(5):3044–3050. doi: 10.4049/jimmunol.173.5.3044. [DOI] [PubMed] [Google Scholar]
- 17.Kuraya M, Ming Z, Liu X, Matsushita M, Fujita T. Specific binding of L-ficolin and H-ficolin to apoptotic cells leads to complement activation. Immunobiology. 2005;209(9):689–697. doi: 10.1016/j.imbio.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Gardai SJ, Bratton DL, Ogden CA, Henson PM. Recognition ligands on apoptotic cells: a perspective. J Leukoc Biol. 2006;79(5):896–903. doi: 10.1189/jlb.1005550. [DOI] [PubMed] [Google Scholar]
- 19.Elward K, Griffiths M, Mizuno M, Harris CL, Neal JW, Morgan BP, et al. CD46 plays a key role in tailoring innate immune recognition of apoptotic and necrotic cells. J Biol Chem. 2005;280(43):36342–36354. doi: 10.1074/jbc.M506579200. [DOI] [PubMed] [Google Scholar]
- 20.Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123(2):321–334. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 21.Bohlson SS, Fraser DA, Tenner AJ. Complement proteins C1q and MBL are pattern recognition molecules that signal immediate and long-term protective immune functions. Mol Immunol. 2007;44(1–3):33–43. doi: 10.1016/j.molimm.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 22.Fraser DA, Bohlson SS, Jasinskiene N, Rawal N, Palmarini G, Ruiz S, et al. C1q and MBL, components of the innate immune system, influence monocyte cytokine expression. J Leukoc Biol. 2006;80(1):107–116. doi: 10.1189/jlb.1105683. [DOI] [PubMed] [Google Scholar]
- 23.Mevorach D, Mascarenhas JO, Gershov D, Elkon KB. Complement-dependent clearance of apoptotic cells by human macrophages. J Exp Med. 1998;188(12):2313–2320. doi: 10.1084/jem.188.12.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verbovetski I, Bychkov H, Trahtemberg U, Shapira I, Hareuveni M, Ben-Tal O, et al. Opsonization of apoptotic cells by autologous iC3b facilitates clearance by immature dendritic cells, down-regulates DR and CD86, and up-regulates CC chemokine receptor 7. J Exp Med. 2002;196(12):1553–1561. doi: 10.1084/jem.20020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchell DA, Pickering MC, Warren J, Fossati-Jimack L, Cortes-Hernandez J, Cook HT, et al. C1q deficiency and autoimmunity: the effects of genetic background on disease expression. J Immunol. 2002;168(5):2538–2543. doi: 10.4049/jimmunol.168.5.2538. [DOI] [PubMed] [Google Scholar]
- 26.Jordan JE, Montalto MC, Stahl GL. Inhibition of mannose-binding lectin reduces postischemic myocardial reperfusion injury. Circulation. 2001;104(12):1413–1418. doi: 10.1161/hc3601.095578. [DOI] [PubMed] [Google Scholar]
- 27.Walsh MC, Bourcier T, Takahashi K, Shi L, Busche MN, Rother RP, et al. Mannose-binding lectin is a regulator of inflammation that accompanies myocardial ischemia and reperfusion injury. J Immunol. 2005;175(1):541–546. doi: 10.4049/jimmunol.175.1.541. [DOI] [PubMed] [Google Scholar]
- 28.Weisman HF, Bartow T, Leppo MK, Marsh HC, Jr, Carson GR, Concino MF, et al. Soluble human complement receptor type 1: In vivo inhibitor of complement suppressing post-ischemic myocardial inflammation and necrosis. Science. 1990;249:146–151. doi: 10.1126/science.2371562. [DOI] [PubMed] [Google Scholar]
- 29.Zhang M, Takahashi K, Alicot EM, Vorup-Jensen T, Kessler B, Thiel S, et al. Activation of the lectin pathway by natural IgM in a model of ischemia/reperfusion injury. J Immunol. 2006;177(7):4727–4734. doi: 10.4049/jimmunol.177.7.4727. [DOI] [PubMed] [Google Scholar]
- 30.Collard CD, Vakeva A, Morrissey MA, Agah A, Rollins SA, Reenstra WR, et al. Complement activation after oxidative stress: role of the lectin complement pathway. Am J Pathol. 2000;156:1549–1556. doi: 10.1016/S0002-9440(10)65026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan RK, Ibrahim SI, Takahashi K, Kwon E, McCormack M, Ezekowitz A, et al. The differing roles of the classical and mannose-binding lectin complement pathways in the events following skeletal muscle ischemia-reperfusion. J Immunol. 2006;177(11):8080–8085. doi: 10.4049/jimmunol.177.11.8080. [DOI] [PubMed] [Google Scholar]
- 32.Moller-Kristensen M, Wang W, Ruseva M, Thiel S, Nielsen S, Takahashi K, et al. Mannan-binding lectin recognizes structures on ischaemic reperfused mouse kidneys and is implicated in tissue injury. Scand J Immunol. 2005;61(5):426–434. doi: 10.1111/j.1365-3083.2005.01591.x. [DOI] [PubMed] [Google Scholar]
- 33.de Vries B, Walter SJ, Peutz-Kootstra CJ, Wolfs TG, van Heurn LW, Buurman WA. The mannose-binding lectin-pathway is involved in complement activation in the course of renal ischemia-reperfusion injury. Am J Pathol. 2004;165(5):1677–1688. doi: 10.1016/S0002-9440(10)63424-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thurman JM, Ljubanovic D, Royer PA, Kraus DM, Molina H, Barry NP, et al. Altered renal tubular expression of the complement inhibitor Crry permits complement activation after ischemia/reperfusion. J Clin Invest. 2006;116(2):357–368. doi: 10.1172/JCI24521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selander B, Martensson U, Weintraub A, Holmstrom E, Matsushita M, Thiel S, et al. Mannan-binding lectin activates C3 and the alternative complement pathway without involvement of C2. J Clin Invest. 2006;116(5):1425–1434. doi: 10.1172/JCI25982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harboe M, Garred P, Borgen MS, Stahl GL, Roos A, Mollnes TE. Design of a complement mannose-binding lectin pathway-specific activation system applicable at low serum dilutions. Clin Exp Immunol. 2006;144(3):512–520. doi: 10.1111/j.1365-2249.2006.03072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harboe M, Ulvund G, Vien L, Fung M, Mollnes TE. The quantitative role of alternative pathway amplification in classical pathway induced terminal complement activation. Clin Exp Immunol. 2004;138(3):439–446. doi: 10.1111/j.1365-2249.2004.02627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thurman JM, Lenderink AM, Royer PA, Coleman KE, Zhou J, Lambris JD, et al. C3a is required for the production of cxc chemokines by tubular epithelial cells after renal ishemia/reperfusion. J Immunol. 2007;178:1819–1828. doi: 10.4049/jimmunol.178.3.1819. [DOI] [PubMed] [Google Scholar]
- 39.Arumugam TV, Shiels IA, Strachan AJ, Abbenante G, Fairlie DP, Taylor SM. A small molecule C5a receptor antagonist protects kidneys from ischemia/reperfusion injury in rats. Kidney Int. 2003;63(1):134–142. doi: 10.1046/j.1523-1755.2003.00737.x. [DOI] [PubMed] [Google Scholar]
- 40.Zhou W, Farrar CA, Abe K, Pratt JR, Marsh JE, Wang Y, et al. Predominant role for C5b-9 in renal ischemia/reperfusion injury. J Clin Invest. 2000;105(10):1363–1371. doi: 10.1172/JCI8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weiser MR, Williams JP, Moore FD, Jr, Kobzik L, Ma M, Hechtman HB, et al. Reperfusion injury of ischemic skeletal muscle is mediated by natural antibody and complement. J Exp Med. 1996;183:2343–2348. doi: 10.1084/jem.183.5.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McMullen ME, Hart ML, Walsh MC, Buras J, Takahashi K, Stahl GL. Mannose-binding lectin binds IgM to activate the lectin complement pathway in vitro and in vivo. Immunobiology. 2006;211(10):759–766. doi: 10.1016/j.imbio.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 43.Zhang M, Alicot EM, Chiu I, Li J, Verna N, Vorup-Jensen T, et al. Identification of the target self-antigens in reperfusion injury. J Exp Med. 2006;203(1):141–152. doi: 10.1084/jem.20050390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ciurana CL, Zwart B, van Mierlo G, Hack CE. Complement activation by necrotic cells in normal plasma environment compares to that by late apoptotic cells and involves predominantly IgM. Eur J Immunol. 2004;34(9):2609–2619. doi: 10.1002/eji.200425045. [DOI] [PubMed] [Google Scholar]
- 45.Ogden CA, Kowalewski R, Peng Y, Montenegro V, Elkon KB. IGM is required for efficient complement mediated phagocytosis of apoptotic cells in vivo. Autoimmunity. 2005;38(4):259–264. doi: 10.1080/08916930500124452. [DOI] [PubMed] [Google Scholar]
- 46.Groeneveld TW, Oroszlan M, Owens RT, Faber-Krol MC, Bakker AC, Arlaud GJ, et al. Interactions of the extracellular matrix proteoglycans decorin and biglycan with C1q and collectins. J Immunol. 2005;175(7):4715–4723. doi: 10.4049/jimmunol.175.7.4715. [DOI] [PubMed] [Google Scholar]
- 47.Berger SP, Roos A, Mallat MJ, Fujita T, de Fijter JW, Daha MR. Association between mannose-binding lectin levels and graft survival in kidney transplantation. Am J Transplant. 2005;5(6):1361–1366. doi: 10.1111/j.1600-6143.2005.00841.x. [DOI] [PubMed] [Google Scholar]
- 48.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5(11):1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 49.Shi Y, Zheng W, Rock KL. Cell injury releases endogenous adjuvants that stimulate cytotoxic T cell responses. Proc Natl Acad Sci U S A. 2000;97(26):14590–14595. doi: 10.1073/pnas.260497597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koo DD, Welsh KI, Roake JA, Morris PJ, Fuggle SV. Ischemia/reperfusion injury in human kidney transplantation: an immunohistochemical analysis of changes after reperfusion. Am J Pathol. 1998;153(2):557–566. doi: 10.1016/S0002-9440(10)65598-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kusaka M, Pratschke J, Wilhelm MJ, Ziai F, Zandi-Nejad K, Mackenzie HS, et al. Activation of inflammatory mediators in rat renal isografts by donor brain death. Transplantation. 2000;69(3):405–410. doi: 10.1097/00007890-200002150-00017. [DOI] [PubMed] [Google Scholar]
- 52.Schuurs TA, Gerbens F, van der Hoeven JA, Ottens PJ, Kooi KA, Leuvenink HG, et al. Distinct transcriptional changes in donor kidneys upon brain death induction in rats: insights in the processes of brain death. Am J Transplant. 2004;4(12):1972–1981. doi: 10.1111/j.1600-6143.2004.00607.x. [DOI] [PubMed] [Google Scholar]
- 53.Stewart S, Winters GL, Fishbein MC, Tazelaar HD, Kobashigawa J, Abrams J, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24(11):1710–1720. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 54.Fyfe B, Loh E, Winters GL, Couper GS, Kartashov AI, Schoen FJ. Heart transplantation-associated perioperative ischemic myocardial injury. Morphological features and clinical significance. Circulation. 1996;93(6):1133–1140. doi: 10.1161/01.cir.93.6.1133. [DOI] [PubMed] [Google Scholar]
- 55.Del Conde I, Cruz MA, Zhang H, Lopez JA, Afshar-Kharghan V. Platelet activation leads to activation and propagation of the complement system. J Exp Med. 2005;201(6):871–879. doi: 10.1084/jem.20041497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nagano H, Nadeau KC, Takada M, Kusaka M, Tilney NL. Sequential cellular and molecular kinetics in acutely rejecting renal allografts in rats. Transplantation. 1997;63(8):1101–1108. doi: 10.1097/00007890-199704270-00009. [DOI] [PubMed] [Google Scholar]
- 57.Castellano G, Woltman AM, Nauta AJ, Roos A, Trouw LA, Seelen MA, et al. Maturation of dendritic cells abrogates C1q production in vivo and in vitro. Blood. 2004;103(10):3813–3820. doi: 10.1182/blood-2003-09-3046. [DOI] [PubMed] [Google Scholar]
- 58.Farrar CA, Zhou W, Lin T, Sacks SH. Local extravascular pool of C3 is a determinant of postischemic acute renal failure. Faseb J. 2006;20(2):217–226. doi: 10.1096/fj.05-4747com. [DOI] [PubMed] [Google Scholar]
- 59.Pratt JR, Abe K, Miyazaki M, Zhou W, Sacks SH. In situ localization of C3 synthesis in experimental acute renal allograft rejection. Amer J Pathol. 2000;157:825–831. doi: 10.1016/S0002-9440(10)64596-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mason JC, Yarwood H, Sugars K, Morgan BP, Davies KA, Haskard DO. Induction of decay-accelerating factor by cytokines or the membrane-attack complex protects vascular endothelial cells against complement deposition. Blood. 1999;94(5):1673–1682. [PubMed] [Google Scholar]
- 61.Lidington EA, Haskard DO, Mason JC. Induction of decay-accelerating factor by thrombin through a protease-activated receptor 1 and protein kinase C-dependent pathway protects vascular endothelial cells from complement-mediated injury. Blood. 2000;96(8):2784–2792. [PubMed] [Google Scholar]
- 62.Mason JC, Steinberg R, Lidington EA, Kinderlerer AR, Ohba M, Haskard DO. Decay-accelerating factor induction on vascular endothelium by vascular endothelial growth factor (VEGF) is mediated via a VEGF receptor-2 (VEGF-R2)- and protein kinase C-alpha/epsilon (PKCalpha/epsilon)-dependent cytoprotective signaling pathway and is inhibited by cyclosporin A. J Biol Chem. 2004;279(40):41611–41618. doi: 10.1074/jbc.M407981200. [DOI] [PubMed] [Google Scholar]
- 63.Brunn GJ, Saadi S, Platt JL. Differential regulation of endothelial cell activation by complement and interleukin 1alpha. Circ Res. 2006;98(6):793–800. doi: 10.1161/01.RES.0000216071.87981.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou W, Patel H, Li K, Peng Q, Villiers MB, Sacks SH. Macrophages from C3-deficient mice have impaired potency to stimulate alloreactive T cells. Blood. 2006;107(6):2461–2469. doi: 10.1182/blood-2005-08-3144. [DOI] [PubMed] [Google Scholar]
- 65.Peng Q, Li K, Patel H, Sacks SH, Zhou W. Dendritic cell synthesis of C3 is required for full T cell activation and development of a Th1 phenotype. J Immunol. 2006;176(6):3330–3341. doi: 10.4049/jimmunol.176.6.3330. [DOI] [PubMed] [Google Scholar]
- 66.Kerekes K, Prechl J, Bajtay Z, Jozsi M, Erdei A. A further link between innate and adaptive immunity: C3 deposition on antigen-presenting cells enhances the proliferation of antigen-specific T cells. Int Immunol. 1998;10(12):1923–1930. doi: 10.1093/intimm/10.12.1923. [DOI] [PubMed] [Google Scholar]
- 67.Heeger PS, Lalli PN, Lin F, Valujskikh A, Liu J, Muqim N, et al. Decay-accelerating factor modulates induction of T cell immunity. J Exp Med. 2005;201(10):1523–1530. doi: 10.1084/jem.20041967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Longhi MP, Harris CL, Morgan BP, Gallimore A. Holding T cells in check--a new role for complement regulators? Trends Immunol. 2006;27(2):102–108. doi: 10.1016/j.it.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 69.Marie JC, Astier AL, Rivailler P, Rabourdin-Combe C, Wild TF, Horvat B. Linking innate and acquired immunity: divergent role of CD46 cytoplasmic domains in T cell induced inflammation. Nat Immunol. 2002;3(7):659–666. doi: 10.1038/ni810. [DOI] [PubMed] [Google Scholar]
- 70.Pratt JR, Basheer SA, Sacks SH. Local synthesis of complement component C3 regulates acute renal transplant rejection. Nat Med. 2002;8(6):582–587. doi: 10.1038/nm0602-582. [DOI] [PubMed] [Google Scholar]
- 71.Marsh JE, Farmer CK, Jurcevic S, Wang Y, Carroll MC, Sacks SH. The allogeneic T and B cell response is strongly dependent on complement components C3 and C4. Transplantation. 2001;72:1310–1318. doi: 10.1097/00007890-200110150-00022. [DOI] [PubMed] [Google Scholar]
- 72.Rossbacher J, Shlomchik MJ. The B cell receptor itself can activate complement to provide the complement receptor 1/2 ligand required to enhance B cell immune responses in vivo. J Exp Med. 2003;198(4):591–602. doi: 10.1084/jem.20022042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mongini PK, Tolani S, Fattah RJ, Inman JK. Antigen receptor triggered upregulation of CD86 and CD80 in human B cells: augmenting role of the CD21/CD19 co-stimulatory complex and IL-4. Cell Immunol. 2002;216(1–2):50–64. doi: 10.1016/s0008-8749(02)00512-9. [DOI] [PubMed] [Google Scholar]
- 74.Dempsey PW, Allison MED, Akkaraju S, Goodnow CC, Fearon DT. C3d of complement as a molecular adjuvant: Bridging innate and acquired immunity. Science. 1996;271:348–350. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- 75.Lee Y, Haas KM, Gor DO, Ding X, Karp DR, Greenspan NS, et al. Complement component C3d-antigen complexes can either augment or inhibit B lymphocyte activation and humoral immunity in mice depending on the degree of CD21/CD19 complex engagement. J Immunol. 2005;175(12):8011–8023. doi: 10.4049/jimmunol.175.12.8011. [DOI] [PubMed] [Google Scholar]
- 76.Baddoura FK, Nasr IW, Wrobel B, Li Q, Ruddle NH, Lakkis FG. Lymphoid neogenesis in murine cardiac allografts undergoing chronic rejection. Am J Transplant. 2005;5(3):510–516. doi: 10.1111/j.1600-6143.2004.00714.x. [DOI] [PubMed] [Google Scholar]
- 77.Thaunat O, Field AC, Dai J, Louedec L, Patey N, Bloch MF, et al. Lymphoid neogenesis in chronic rejection: evidence for a local humoral alloimmune response. Proc Natl Acad Sci U S A. 2005;102(41):14723–14728. doi: 10.1073/pnas.0507223102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Steinmetz OM, Panzer U, Kneissler U, Harendza S, Lipp M, Helmchen U, et al. BCA-1/CXCL13 expression is associated with CXCR5-positive B-cell cluster formation in acute renal transplant rejection. Kidney Int. 2005;67(4):1616–1621. doi: 10.1111/j.1523-1755.2005.00244.x. [DOI] [PubMed] [Google Scholar]
- 79.Minami K, Murata K, Lee C-Y, Fox-Talbot K, Wasowska B, Pescovitz M, et al. C4d deposition and clearance in cardiac transplants correlates with alloantibody levels and rejection in rats. Am J Transplant. 2006;6(5):923–932. doi: 10.1111/j.1600-6143.2006.01281.x. [DOI] [PubMed] [Google Scholar]
- 80.Qian Z, Lee CY, Murata K, Liu J, Fox-Talbot K, Wasowska BA, et al. Antibody and complement mediated injury in transplants following sensitization by allogeneic blood transfusion. Transplantation. 2006;82(7):857–864. doi: 10.1097/01.tp.0000232335.06792.35. [DOI] [PubMed] [Google Scholar]
- 81.Ota H, Fox-Talbot K, Hu W, Qian Z, Sanfilippo F, Hruban RH, et al. Terminal complement components mediate release of von Willebrand factor and adhesion of platelets in arteries of allografts. Transplantation. 2005;79(3):276–281. doi: 10.1097/01.tp.0000146195.76904.d3. [DOI] [PubMed] [Google Scholar]
- 82.Hattori R, Hamilton KK, McEver RP, Sims PJ. Complement proteins C5b-C9 induce secretion of high molecular weight multimers of endothelial von Willebrand factor and translocation of granule membrane protein GMP-140 to the cell surface. J Biol Chem. 1989;264:9053–9060. [PubMed] [Google Scholar]
- 83.Saadi S, Holzknecht RA, Patte CP, Stern DM, Platt JL. Complement-mediated regulation of tissue factor activity in endothelium. J Exp Med. 1995;182:1807–1814. doi: 10.1084/jem.182.6.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Celi A, Pellegrini G, Lorenzet R, De Blasi A, Ready N, Furie BC, et al. P-selectin induces the expression of tissue factor on monocytes. Proc Natl Acad Sci U S A. 1994;91:8767–8771. doi: 10.1073/pnas.91.19.8767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weyrich AS, McIntyre TM, McEver RP, Prescott SM, Zimmerman GA. Monocyte tethering by P-selectin regulates monocyte chemotactic protein-1 and tumor necrosis factor-alpha secretion. Signal integration and NF-kappa B translocation. J Clin Invest. 1995;95:2297–2303. doi: 10.1172/JCI117921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weyrich AS, Elstad MR, McEver RP, McIntyre TM, Moore KL, Morrissey JH, et al. Activated platelets signal chemokine synthesis by human monocytes. J Clin Invest. 1996;97:1525–1534. doi: 10.1172/JCI118575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee C-Y, Asano H, Fox-Talbot K, Reynolds M, Wasowska BA, Baldwin WM., III Expression profiles of cardiac allografts transplanted to sensitized recipients reveal upregulated genes related to macrophages. Am J Transpl. 2006 Abstract (2079) [Google Scholar]
- 88.Kilgore KS, Shen JP, Miller BF, Ward PA, Warren JS. Enhancement by the complement membrane attack complex of tumor necrosis factor-alpha-induced endothelial cell expression of E-selectin and ICAM-1. J Immunol. 1995;155:1434–1441. [PubMed] [Google Scholar]
- 89.Selvan RS, Kapadia HB, Platt JL. Complement-induced expression of chemokine genes in endothelium: regulation by IL-1-dependent and -independent mechanisms. J Immunol. 1998;161(8):4388–4395. [PubMed] [Google Scholar]
- 90.Saadi S, Holzknecht RA, Patte CP, Platt JL. Endothelial cell activation by pore-forming structures: pivotal role for interleukin-1alpha. Circulation. 2000;101(15):1867–1873. doi: 10.1161/01.cir.101.15.1867. [DOI] [PubMed] [Google Scholar]
- 91.Bustos M, Saadi S, Platt JL. Platelet-mediated activation of endothelial cells: implications for the pathogenesis of transplant rejection1. Transplantation. 2001;72(3):509–515. doi: 10.1097/00007890-200108150-00025. [DOI] [PubMed] [Google Scholar]
- 92.Elzey BD, Tian J, Jensen RJ, Swanson AK, Lees JR, Lentz SR, et al. Platelet-mediated modulation of adaptive immunity. A communication link between innate and adaptive immune compartments. Immunity. 2003;19(1):9–19. doi: 10.1016/s1074-7613(03)00177-8. [DOI] [PubMed] [Google Scholar]
- 93.Xu H, Zhang X, Mannon RB, Kirk AD. Platelet-derived or soluble CD154 induces vascularized allograft rejection independent of cell-bound CD154. J Clin Invest. 2006;116(3):769–774. doi: 10.1172/JCI27155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Coppinger JA, Cagney G, Toomey S, Kislinger T, Belton O, McRedmond JP, et al. Characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in human atherosclerotic lesions. Blood. 2004;103(6):2096–2104. doi: 10.1182/blood-2003-08-2804. [DOI] [PubMed] [Google Scholar]
- 95.Haviland DL, McCoy RL, Whitehead WT, Akama H, Molmenti EP, Brown A, et al. Cellular expression of the C5a anaphylatoxin receptor (C5aR): demonstration of C5aR on nonmyeloid cells of the liver and lung. J Immunol. 1995;154:1861–1869. [PubMed] [Google Scholar]
- 96.Foreman KE, Vaporciyan AA, Bonish BK, Jones ML, Johnson KJ, Glovsky MM, et al. C5a-induced expression of P-selectin in endothelial cells. J Clin Invest. 1994;94:1147–1155. doi: 10.1172/JCI117430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yamakuchi M, Kirkiles-Smith NC, Ferlito M, Cameron SJ, Bao C, Fox-Talbot K, et al. Antibody to human leukocyte antigen triggers endothelial exocytosis. Proc Natl Acad Sci U S A. 2007 doi: 10.1073/pnas.0602035104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chopek MW, Simmons RL, Platt JL. ABO-incompatible kidney transplantation: initial immunopathologic evaluation. Transplant Proc. 1987;19(6):4553–4557. [PubMed] [Google Scholar]
- 99.Alexandre GP, Squifflet JP, De Bruyere M, Latinne D, Reding R, Gianello P, et al. Present experiences in a series of 26 ABO-incompatible living donor renal allografts. Transplant Proc. 1987;19(6):4538–4542. [PubMed] [Google Scholar]
- 100.Haas M, Rahman MH, Racusen LC, Kraus ES, Bagnasco SM, Segev DL, et al. C4d and C3d staining in biopsies of ABO- and HLA-incompatible renal allografts: correlation with histologic findings. Am J Transplant. 2006;6(8):1829–1840. doi: 10.1111/j.1600-6143.2006.01356.x. [DOI] [PubMed] [Google Scholar]
- 101.Koch CA, Khalpey ZI, Platt JL. Accommodation: preventing injury in transplantation and disease. J Immunol. 2004;172(9):5143–5148. doi: 10.4049/jimmunol.172.9.5143. [DOI] [PubMed] [Google Scholar]
- 102.Nimmerjahn F, Ravetch JV. Fcgamma receptors: old friends and new family members. Immunity. 2006;24(1):19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 103.Marsh CB, Wewers MD, Tan LC, Rovin BH. Fc(gamma) receptor cross-linking induces peripheral blood mononuclear cell monocyte chemoattractant protein-1 expression: role of lymphocyte Fc(gamma)RIII. J Immunol. 1997;158(3):1078–1084. [PubMed] [Google Scholar]
- 104.Marsh CB, Pomerantz RP, Parker JM, Winnard AV, Mazzaferri EL, Jr, Moldovan N, et al. Regulation of monocyte survival in vitro by deposited IgG: role of macrophage colony-stimulating factor. J Immunol. 1999;162(10):6217–6225. [PubMed] [Google Scholar]
- 105.Shushakova N, Skokowa J, Schulman J, Baumann U, Zwirner J, Schmidt RE, et al. C5a anaphylatoxin is a major regulator of activating versus inhibitory FcgammaRs in immune complex-induced lung disease. J Clin Invest. 2002;110(12):1823–1830. doi: 10.1172/JCI200216577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kumar V, Ali SR, Konrad S, Zwirner J, Verbeek JS, Schmidt RE, et al. Cell-derived anaphylatoxins as key mediators of antibody-dependent type II autoimmunity in mice. J Clin Invest. 2006;116(2):512–520. doi: 10.1172/JCI25536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bajtay Z, Jozsi M, Banki Z, Thiel S, Thielens N, Erdei A. Mannan-binding lectin and C1q bind to distinct structures and exert differential effects on macrophages. Eur J Immunol. 2000;30:1706–1713. doi: 10.1002/1521-4141(200006)30:6<1706::AID-IMMU1706>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 108.Qian Z, Wasowska BA, Behrens E, Brody JR, Kadkol SS, Cangello DL, et al. C6 produced by macrophages contributes to cardiac allograft rejection. Amer J Pathol. 1999;155:1293–1302. doi: 10.1016/S0002-9440(10)65231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang H, Jiang J, Liu W, Kubelik D, Chen G, Gies D, et al. Prevention of acute vascular rejection by a functionally blocking anti-C5 monoclonal antibody combined with cyclosporine. Transplantation. 2005;79(9):1121–1127. doi: 10.1097/01.tp.0000161218.58276.9a. [DOI] [PubMed] [Google Scholar]
- 110.Moazami N, Itescu S, Williams MR, Argenziano M, Weinberg A, Oz MC. Platelet transfusions are associated with the development of anti-major histocompatibility complex class I antibodies in patients with left ventricular assist support. J Heart Lung Transplant. 1998;17(9):876–880. [PubMed] [Google Scholar]
- 111.Pollack MS, Trimarchi HM, Riley DJ, Casperson PR, Manyari LE, Suki WN. Shared cadaver donor-husband HLA class I mismatches as a risk factor for renal graft rejection in previously pregnant women. Hum Immunol. 1999;60(11):1150–1155. doi: 10.1016/s0198-8859(99)00104-4. [DOI] [PubMed] [Google Scholar]
- 112.Rebibou JM, Chabod J, Alcalay D, Coussediere MC, Deteix P, Touchard G, et al. Flow cytometric evaluation of pregnancy-induced anti-HLA immunization and blood transfusion-induced reactivation. Transplantation. 2002;74(4):537–540. doi: 10.1097/00007890-200208270-00018. [DOI] [PubMed] [Google Scholar]
- 113.Halushka MK, Zachary AA, Borja MCSDR, Baldwin WM., III Risk of repeat HLA mismatches in second cardiac transplants. Am J Transpl. 2006 Abstract [876] [Google Scholar]
- 114.Herzenberg AM, Gill JS, Djurdjev O, Magil AB. C4d deposition in acute rejection: an independent long-term prognostic factor. J Am Soc Nephrol. 2002;13(1):234–241. doi: 10.1681/ASN.V131234. [DOI] [PubMed] [Google Scholar]