Abstract
Polycyclic aromatic hydrocarbons (PAHs) are pollutants created by the incomplete combustion of carbon, and are increasing in the environment largely due to the burning of fossil fuels. PAHs occur as complex mixtures, and some combinations have been shown to cause synergistic developmental toxicity in fish embryos, characterized by pericardial edema and craniofacial malformations. Previous studies have indicated that in the zebrafish model, this toxicity is mediated by the aryl hydrocarbon receptor 2 (AHR2), and enhanced by inhibition of CYP1A activity. In this study, we further examined this interaction of the model PAH and AHR agonist β-naphthoflavone (BNF) with and without the AHR partial agonist/antagonist and CYP1A inhibitor α-naphthoflavone (ANF) to determine 1) whether ANF was acting as an AHR antagonist, 2) what alterations BNF and ANF both alone and in combination had on mRNA expression of the AHR regulated genes cytochrome P450 (cyp) 1a, 1b1, and 1c1, and the AHR repressor (ahrr2) prior to vs. during deformity onset, and 3) compare CYP1A enzyme activity with mRNA induction. Zebrafish embryos were exposed from 24–48 or 24–96 hpf to BNF, 1–100 μg/L, ANF, 1–150 μg/L, a BNF+ANF co-exposure (1 μg/L + 100 μg/L), or a DMSO solvent control. RNA was extracted and examined by quantitative real time PCR. Both BNF and ANF each individually resulted in a dose dependent increase CYP1A, CYP1B1, CYP1C1, and AHRR2 mRNA, confirming their activities as AHR agonists. In the BNF+ANF co-exposures prior to deformity onset, expression of these genes was synergistic, and expression levels of the AHR regulated genes resembled the higher doses of BNF alone. Gene induction during deformities was also significantly increased in the co-exposure, but to a lesser magnitude than prior to deformity onset. EROD measurements of CYP1A activity showed ANF inhibited activity induction by BNF in the co-exposure group; this finding is not predicted by mRNA expression, which is synergistically induced in this treatment. This suggests that inhibition of CYP1A activity may alter metabolism and/or increase the half-life of the AHR agonist(s), allowing for increased AHR activation. This study furthers a mechanistic understanding of interactions underlying PAH synergistic toxicity.
Keywords: quantitative real-time PCR, CYP1A, CYP1B1, CYP1C1, AHRR, zebrafish, polycyclic aromatic hydrocarbons, aryl hydrocarbon receptor
1. Introduction
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous environmental contaminants of both natural and anthropogenic sources, derived from the incomplete combustion of carbon. Unlike many persistent organic pollutants, levels of PAHs are increasing in the environment, largely due to the burning of fossil fuels (Van Metre and Mahler, 2005). In aquatic systems, atmospheric deposition and urban runoff pose significant threats to early life stages of fishes, which are sensitive to toxicity of PAHs (Brinkworth et al., 2003; Incardona et al., 2004; Wassenberg and Di Giulio, 2004; Incardona et al., 2006). Resulting deformities include pericardial edema and craniofacial malformations. Other environmental contaminants, such as chlorinated dioxins and polychlorinated biphenyls (PCBs), cause similar toxicity in developing fish, and this toxicity has been shown to be mediated by the aryl hydrocarbon receptor (Billiard et al., 2006; Carney et al., 2004).
The aryl hydrocarbon receptor is a ligand activated member of the basic helix-loop helix Per-ARNT-Sim family of nuclear receptors. The cytosolic AHR can bind a wide variety of ligands including natural indoles from cruciferous vegetables and tryptophan metabolites (Denison and Heath-Pagliuso, 1998), as well as environmentally relevant pollutants including some chlorinated dioxins, PCBs, and PAHs. The AHR pathway is activated upon ligand binding to the AHR, which then translocates to the nucleus and forms a heterodimer with the aryl hydrocarbon nuclear translocator (ARNT) protein, binds to xenobiotic response elements (XRE) in promoter regions and initiates transcription of numerous genes. The so-called “AHR battery” of genes largely includes Phase I metabolic enzymes and Phase II conjugating enzymes which then in turn metabolize a variety of organic substrates (Nebert et al., 1993).
For the most part, PAHs are excellent substrates for metabolism by the Phase I cytochrome P4501 enzymes (Hawkins et al., 2002; Shimada, 2006). However, within the PAH class, there are varying structures based on the number of rings (2–6), bonding patterns of those rings, and heteroatom substitutions. This variety makes it difficult to ascribe a general biologic mechanism to these contaminants; several PAHs are AHR agonists, while some PAHs can also inhibit the enzymatic activity of the Phase I enzyme cytochrome P450 1A (CYP1A), the fish homolog of the mammalian CYP1A1 and 1A2. Recent studies in our laboratory have shown that co-exposure of several 4- and 5-ring PAH-type AHR agonists with a CYP1A inhibitor results in synergistic toxicity in developing killifish (Fundulus heteroclitus) and zebrafish embryos (Billiard et al., 2006; Wassenberg and Di Giulio, 2004).
To further understand the interactions underlying this AHR mediated synergistic toxicity, in this study we used two well characterized synthetic flavonoids commonly used as surrogate model PAHs, β-naphthoflavone (BNF) as an AHR agonist, and α-naphthoflavone (ANF) which acts as a reversible competitive CYP1A inhibitor that can bind to either the active site or the ferric heme (Goujon et al., 1972; Testa and Jenner, 1981; Miranda et al., 1998). Also, there is evidence that metabolites of ANF may be even more potent in this action than the parent compound (Nesnow and Bergman, 1981; Nesnow et al., 1982). However, ANF has also been described as a partial AHR agonist/antagonist able to displace TCDD from the receptor, resulting in a reduction in CYP1A1 mRNA expression (Merchant et al., 1990; Merchant et al., 1992; Merchant et al., 1993). To complicate matters, other studies have reported that ANF was able to induce CYP1A in its own right, albeit to a lesser extent than the more potent agonist BNF (Aluru et al., 2005).
In our previous studies, we measured CYP1A enzymatic activity using an in vivo ethoxyresorufin-O-deethylase activity assay (EROD) and showed that ANF was able to inhibit activity induction caused by exposure to BNF; this inhibition coincides with a synergistic increase in deformities (Billiard et al., 2006; Wassenberg and Di Giulio, 2004). While our studies suggested ANF was inhibiting CYP1A protein activity, they did not rule out the possibility that the observed EROD inhibition was the result of ANF acting as an AHR antagonist.
In the current study, we examined mRNA expression of ahr2 and four genes known to be upregulated by the AHR pathway, cyp1a, cyp1b1, cyp1c1, and ahrr2 (Hahn, 2001; Andreasen et al., 2002; Evans et al., 2005; Wang et al., 2006; Willett et al., 2006). Zebrafish have three AHR receptors, AHR1A, AHR1B, and AHR2; AHR2 has been shown to be necessary for dioxin and BNF toxicity, and was thus chosen for analysis (Prasch et al., 2003; Dong et al., 2004; Billiard et al., 2006). Our objectives were to determine whether ANF was functioning as an AHR antagonist in the synergistic toxicity, what alterations BNF and ANF both alone and in combination had on mRNA expression of ahr2 and AHR regulated genes, how induction of these genes compare prior to versus during deformity onset, and how mRNA expression of cyp1a compares to the protein’s enzymatic activity.
2. Materials and Methods
2.1 Chemicals
The chemicals α-naphthoflavone (ANF), and β-naphthoflavone (BNF), and 7-ethoxyresorufin were obtained from Sigma Aldrich (Saint Louis, MO), and dimethyl sulfoxide (DMSO) was purchased from Mallinckrodt Baker (Phillipsburg, NJ). All chemicals were dissolved in DMSO and kept protected from light at −20°C and vortexed prior to administration.
2.2 Animals
Zebrafish embryos were collected following mating of AB* strain adults maintained at 28°C on a 14 hr light, 10 hr dark light cycle. Embryos were maintained in 1x Danieau water (Nasevicius and Ekker, 2000) under the same temperature and photoperiod conditions for the duration of experiments.
2.3 Exposure
Chemicals and dosing concentrations were selected based on our earlier observations of synergistic PAH interactions in killifish and zebrafish (Wassenberg and Di Giulio, 2004; Billiard et al., 2006). Model PAHs used in this study were BNF (AHR agonist) and ANF (CYP1A activity inhibitor and potential AHR agonist/antagonist). At 24 hours post fertilization (hpf), embryos were dosed in replicate pools of 5 embryos, with 3 replicates per treatment, in 20 mL glass vials. DMSO concentration was kept at 0.03% in each vial and total volume set at 7.5 mL 1x Danieau water. The dose response for BNF included doses of 0, 1, 10, 50, and 100 μg/L; the dose of 50 μg/L causes a slight increase in pericardial edema, while 100 μg/L embryos appear similar to those in the 1 μg/L BNF+ 100 μg/L ANF co-exposures at 96 hpf (Billiard et al., 2006); no deformities are present at 48 hpf. The dose response for ANF included 0, 1, 10, 50, 100, and 150 μg/L, doses which are not embryotoxic by themselves (Billiard et al., 2006). Dose response experiments were repeated at least twice, and each data point represents an n of at least 6 pools of 5 embryos. Co-exposure experiments consisted of exposures to DMSO, 1 μg/L BNF alone, 100 μg/L ANF alone, and 1 μg/L BNF + 100 μg/L ANF in combination. Co-exposure experiments were repeated at least four times, and each data point represents an n of 12 pools of 5 embryos. For experiments examining in vivo EROD, the 1x Danieau water contained 21 μg/L 7-ethoxyresorufin. Embryos were left in original dosing solutions for the duration of the experiments. At 48 hpf, embryos were rinsed with 1x Danieau water, manually dechorionated, fixed in RNA later (Ambion, Austin, TX) and stored at −80°C. At 96 hpf, larvae were washed and either fixed in RNA later, or imaged and scored blind for measurement of in vivo EROD activity.
Although deformity analysis was not a focus of this paper, our previous work on this matter demonstrated that no deformities are observed with DMSO, or single compound exposures of 1 or 10 μg/L BNF or 1–150 μg/L ANF at 96 hpf; pericardial edema and craniofacial malformations are only observed with the BNF+ANF co-exposure at at the two highest doses of BNF tested, 50 and 100 μg/L (Billiard et al., 2006). These deformities are not yet manifested at 48 hpf.
2.4 In vivo EROD activity
We used a slightly modified in vivo ethoxyresorufin-O-deethylase (EROD) activity assay (Billiard et al., 2006) to measure the CYP1A activity in hatched zebrafish embryos (i.e. larvae) at 96 hpf. At 24 hpf, embryos were dosed in 1x Danieu water containing 21 μg/L 7-ethoxyresorufin (7-ER, in DMSO) as described above until 96 hpf when embryos were rinsed, anesthetized in MS-222, and mounted in a left lateral orientation in 3% methylcellulose. The accumulated fluorescent product of CYP1A metabolism of 7-ER, resorufin, was visualized in the gastro-intestinal tract and imaged using a rhodamine red fluorescent filter set under 50x magnification (Zeiss Axioskop, Thornwood, NY.). Images were then scored blind for EROD activity measured as fluorescence intensity minus background, quantified digitally by IP Lab software (Scanalytics Inc., Fairfax, VA). In vivo EROD values were expressed as a percentage of fluorescent intensity in control embryos.
2.5 Total RNA extraction & reverse transcription
A hand-held homogenizer was sterilized with 70% ethanol, sprayed with RNaseZap (Ambion), and washed three times with DEPC water and sterile dH2O before each 30 second sample homogenization. RNA extractions were carried out according to the RNeasy Micro Kit protocol (Qiagen, Inc; Valencia, CA). RNA quantity and quality were analyzed spectrophotometrically using a NanoDrop ND-1000 (NanoDrop Technologies, Wilmington, DE). The Omniscript cDNA synthesis kit for Reverse Transcription (Qiagen) was used according to the manufacturer’s instructions using 500 ng of RNA, random hexamers, and RNase inhibitor and carried out in a thermocycler for 1 hour at 37°C. cDNA was diluted to a working concentration of 1.88 ng/μl.
2.6 Quantitative real-time PCR
β-actin primers were designed using Light Cycler Probe Design software (Roche, Indianapolis, IN). CYP1A, 1B1, and 1C1 primers were designed using PrimerQuest software (Integrated DNA Technologies, Inc, www.idtdna.com). AHR2 and AHRR2 primers were published previously (Andreasen et al., 2002; Evans et al., 2005). Primer efficiencies were also tested to ensure the housekeeping and target genes amplified at the same rate. Primer sequences and source sequence accession numbers are provided in Table 1.
Table 1.
cDNA target genes, GenBank identification, and primers used for QRT-PCR.
| Gene | GenBank ID | Forward primer (5′–3′)
Reverse primer (5′–3′) |
|---|---|---|
| β-ACTIN | AF057040 | ACATCCGTAAGGACCTG
GGTCGTTCGTTTGAATCTC |
| CYP1A | NM_131879 | AGGACAACATCAGACACATCACCG
GATAGACAACCGCCCAGGACAGAG |
| CYP1B1 | NM_001013267 | CCACCCGAACTCTGAAACTC
AAACACACCATCAGCGACAG |
| CYP1C1 | NM_001020610 | TGGAGGCTGAGTTTGGACTGAAGA
GAGGAAGAAGAGGATGACGAAGGATG |
| AHRR2 | NM_001020610 | AAACAGAAGCTCTGGCCGCAGTGA
CTGTATGCCCATGAAGCGTCCTGAG |
| AHR2 | AF063446 | CTGTATGCCCATGAAGCGTCCTGAG
CTGTATGCCCATGAAGCGTCCTGAG |
A 25 μl reaction contained 200 nm of each primer, 12.5 μl 2x SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA), 9.5 μl dH2O, and 3.75 ng cDNA template (2 μl of 1.88 ng/μl). QRT-PCR was carried out on an ABI PRISM 7300 Real Time PCR System under the following thermal cycle conditions: 10 minutes at 95°C, followed by 40 replicates of 15 seconds at 95°C, then 1 minute at 60°C, followed by a dissociation curve calculation step. All samples were run in duplicate.
QRT-PCR data were analyzed using the ABI PRISM 7000 Sequence Detection System Software, Version 1.1 (Applied Biosystems). The comparative CT (threshold cycle) method was used to determine average fold induction of mRNA by comparing the CT of the target gene to that of the reference gene (β-ACTIN) according to the ABI PRISM users manual (Guide to Performing Relative Quantitation of Gene Expression Using Real-Time Quantitative PCR, Applied Biosystems, https://www2.appliedbiosystems.com/support/apptech/#rt_pcr2). Reference gene expression was not altered by treatment. To find fold induction, the CT of each technical replicate was averaged and the following formulas were used:
Lastly, the fold change obtained for each biological replicate pool of 5 embryos was averaged for treatments; 6–15 replicates were used to obtain final induction averages and standard error per treatment per time point. As this study was designed to examine gene induction and not to compare basal gene expression levels, each gene was analyzed individually and induction determined based on control expression of that gene for each time point.
2.7 Statistical analysis
Data were analyzed with Statview for Windows (version 5.0.1; SAS Institute, Cary, NC). EROD values and mRNA fold changes were analyzed by factorial analysis of variance (ANOVA). When ANOVA yielded significance (p ≤ 0.05), Fisher’s protected least-significant differences (PLSD) was used as a post hoc test. We used significance of the interaction term between factors of BNF and ANF to test our null hypothesis that mixtures tested would yield additive responses. Data are presented as mean ± S.E., and n defined as number of pools of 5 embryos or larvae.
3. Results
3.1 Dose dependent mRNA induction in single compound exposures
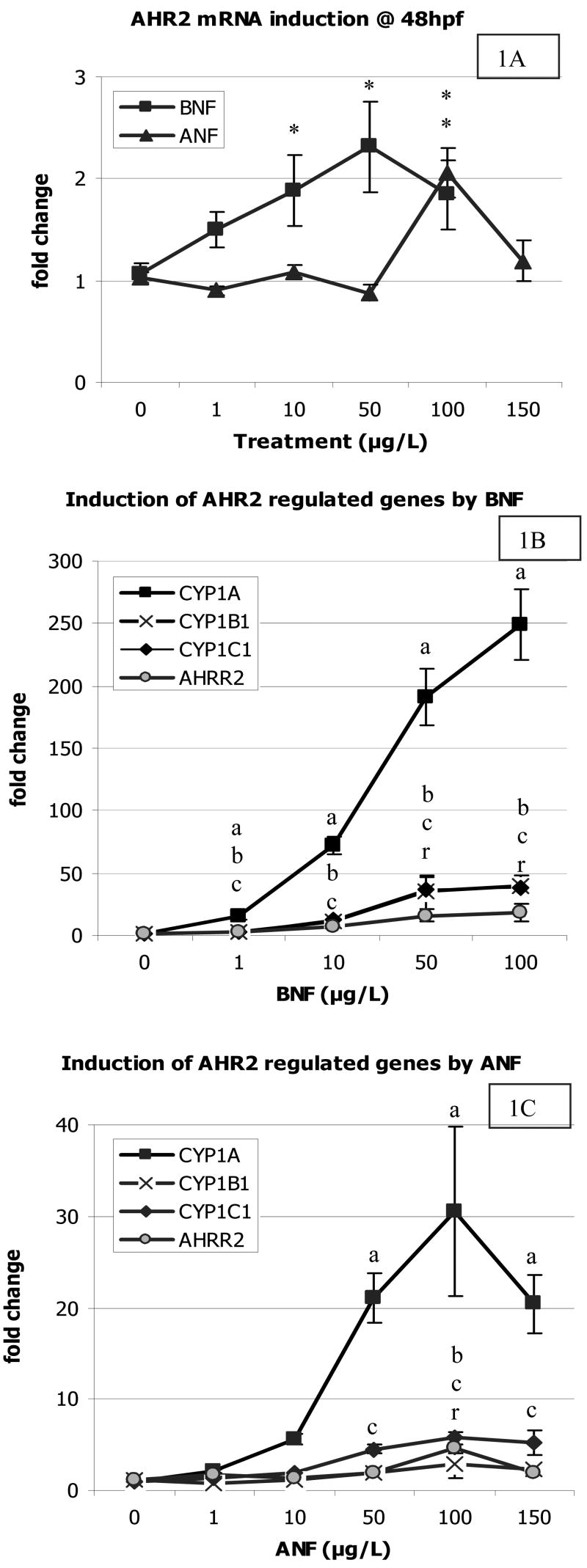
The BNF dose response curve resulted in a dose dependent increase in mRNA expression of ahr2, as well as the AHR regulated genes cyp1a, cyp1b1, cyp1c1, and ahrr2 (Figure 1). AHR2 was only slightly upregulated by BNF (2-fold). CYP1A exhibited the most dramatic increase in expression by BNF, 250-fold at the 100 μg/L dose, while CYP1B1 and CYP1C1 both had the same level of induction, with a maximum mean induction of 40-fold. AHRR2 showed the lowest increase in expression of the AHR2 regulated genes examined, with a maximal mean induction of 18-fold.
Figure 1.
Dose response of BNF and ANF on mRNA induction of AHR2 and AHR2 regulated genes at 48 hpf after exposure for 24 hours. A) Induction of AHR2 by BNF and ANF dose response curves. A “*” indicates a significant difference from control levels. B) BNF induction of CYP1A, 1B1, 1C1, and AHRR2. To differentiate significance between overlapping data points, the letter “a” indicates a significant difference from control levels for CYP1A induction, letter “b” for CYP1B1, letter “c” for CYP1C1, and the letter “r” for AHRR2 expression. C) ANF induction of CYP1A, 1B1, and 1C1, and AHRR2. Significance is indicated by the letters described above. Data presented as mean ± S.E.; n ≥ 6 replicates of pooled embryos; significance defined as p ≤ 0.05.
The ANF dose response curve produced similar mRNA induction patterns as the BNF dose response curve, although at a lower magnitude, consistent with its characterization as a weaker AHR agonist. AHR2 was induced two fold, but only in the 100 μg/L dose, and the induction was not statistically significant. Similar to the BNF gene induction, CYP1A had the highest upregulation, 30-fold compared to BNF’s 250-fold at the same dose of 100 μg/L. CYP1B1 had a maximum induction of nearly 3-fold, while CYP1C1 had a maximum induction of nearly 6-fold, both at the 100 μg/L dose. AHRR2 was induced nearly 5-fold at the same dose. Overall, the AHR2 regulated genes were induced roughly ten times more by BNF than ANF.
3.2 Additive vs. synergistic mRNA induction in co-exposure groups
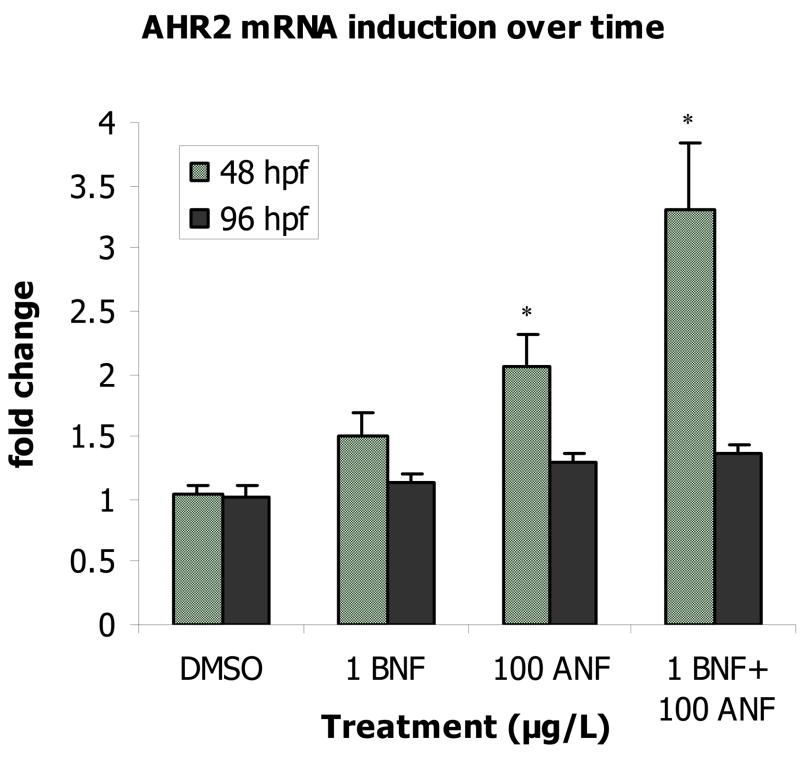
In embryos co-exposed to BNF+ANF, AHR2 was induced 3-fold (p = 0.03) at 48 hpf, but by 96 hpf, this induction had ceased and values returned to control levels (Figure 2). The response observed at 48 hpf was consistent with an additive model of induction (Table 2).
Figure 2.
Induction of AHR2 mRNA at 48 hpf and at 96 hpf. A “*” indicates a significant difference from time-matched controls (p ≤ 0.05). Data presented as mean ± S.E.; 48 hpf time-point data n ≥ 15 replicates of pooled embryos; 96 hpf time-point data n = 6.
Table 2.
Predicted vs. observed gene mRNA induction at 48 hpf. Expression levels of the AHR2 display additive induction while the AHR2 regulated genes in the BNF+ANF co-exposure groups exhibit mRNA induction levels at least three times greater than those predicted by simple additive induction of single compound exposures.
| Gene | BNF (1 μg/L) | ANF (100 μgL) | BNF+ANF Predicted Additive Induction | BNF+ANF Observed Induction |
|---|---|---|---|---|
| AHR2 | 1.5 | 2.1 | 3.6 | 3.3 |
| CYP1A | 16.1 | 31.6 | 47.6 | 190.4 |
| CYP1B1 | 3.3 | 2.9 | 6.2 | 27.3 |
| CYP1C1 | 3.5 | 5.7 | 9.2 | 60.6 |
| AHRR2 | 2.4 | 4.5 | 6.9 | 22.0 |
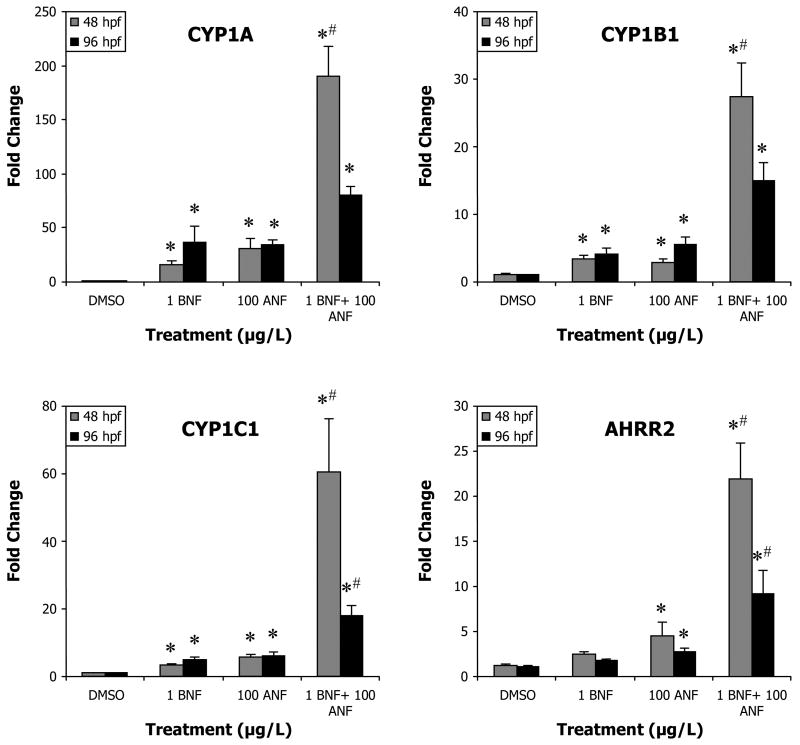
In these same embryos, at 48 hpf a statistically significant effect of BNF, ANF, and a synergistic interaction between the two (p < 0.0001) was observed in all AHR regulated genes. With the exception of AHRR2, 1 μg/L BNF and 100 μg/L ANF single compound exposures significantly induced expression of all four genes (Figure 3). CYP1A exhibited the highest induction, 16.1-fold by BNF (p = 0.0326) and 30.5-fold by ANF (p < 0.0001). CYP1B1 was also significantly induced by BNF and ANF, although to a much lower extent than CYP1A (3.3 and 2.9-fold respectively, p = 0.0002, p = 0.0027). CYP1C1 induced slightly more than CYP1B1, 3.5-fold by BNF and 5.7-fold by ANF (p < 0.0001 for both). AHRR2 induction by BNF alone was not significantly different from DMSO, while ANF caused a 4.5-fold increase (p = 0.0031). In the co-exposures CYP1A showed the highest induction level of 190.5-fold, CYP1C1 showed the second highest induction at 60.5-fold; followed by CYP1B1 at 27.3-fold; and AHRR2 at 22-fold (all p < 0.0001) (Figure 3). These observed inductions were at least three times greater than what would be predicted from an additive model (Table 2).
Figure 3.
Induction of AHR2 regulated genes in the BNF+ANF co-exposure experiments at 48 and 96 hpf. A “#” indicates a synergistic interaction (ANOVA interaction term p ≤ 0.05). A “*” indicates a significant difference from time-matched control (p ≤ 0.05). Data presented as mean ± S.E.; for 48 hpf data n ≥ 12 replicates of pooled embryos; for 96 hpf data n ≥ 6.
At 96 hpf, gene induction in the BNF or ANF single compound treatments either persisted at similar levels or in some cases increased above levels at 48 hpf (Figure 3). BNF and ANF single treatments showed significant effects (ANOVA p ≤ 0.0006). BNF induced CYP1A 36.3-fold (p = 0.0134), more than double the expression level observed at the earlier time-point. ANF caused a 34-fold change (p = 0.0215) which was similar to the expression level at the 48 hpf time-point. CYP1B1 induction levels by BNF were similar to those at 48 hpf, with a 4-fold induction (p = 0.0172) compared to 3.3-fold. ANF was also somewhat higher at 96 hpf at 5.5-fold (p = 0.0018) compared to 2.9-fold at 48 hpf. CYP1C1 expression showed a significant effect of BNF and ANF (p ≤ 0.0002), and a significant interaction between the two factors (p = 0.0441). Expression levels in response to BNF were higher at 96 hpf than at 48 hpf, 5.1-fold (p = 0.0009) compared to 3.5-fold, and remained similar in the ANF treatment, 6.2-fold, (p = 0.0001), compared to 5.7-fold. AHRR2 expression showed a significant effect of BNF (p = 0.0172), ANF (p = 0.0035), and a significant interaction term (p = 0.0481). Induction in the BNF treatment was not different from DMSO control, while induction by ANF was 2.7-fold (p = 0.0016), down from 4.5-fold at 48 hpf.
In the BNF+ANF co-exposure group at 96 hpf, expression of all AHR- regulated genes was about half of the induction observed at 48 hpf, and only CYP1C1 and AHRR2 retained significant interaction terms (p = 0.0441, p = 0.0481). CYP1A was induced 80.4-fold (p < 0.0001) less than half of the 190.5-fold induction at the earlier time-point. CYP1B1 induction levels fell from 27.3-fold at 48 hpf to 15-fold at 96 hpf (p < 0.0001). CYP1C1 induction levels showed the most dramatic decrease, 17.8- fold (p < 0.0001), less than a third of the earlier 60.5-fold. AHRR2 expression was 9.2- fold (p = 0.0374), less than half of the 22- fold observed at 48 hpf.
The expression of all four AHR- responsive genes in the co-exposure group at 96 hpf was much less than levels observed in this treatment at 48 hpf, while single compound exposures either remained steady or increased in expression (Figure 3). This difference could be due to a number of factors such as alternate metabolic clearance mechanisms. Still, the level of AHR- regulated gene induction at 96 hpf is quite substantial in spite of deformities, highlighting the robustness of the AHR pathway response.
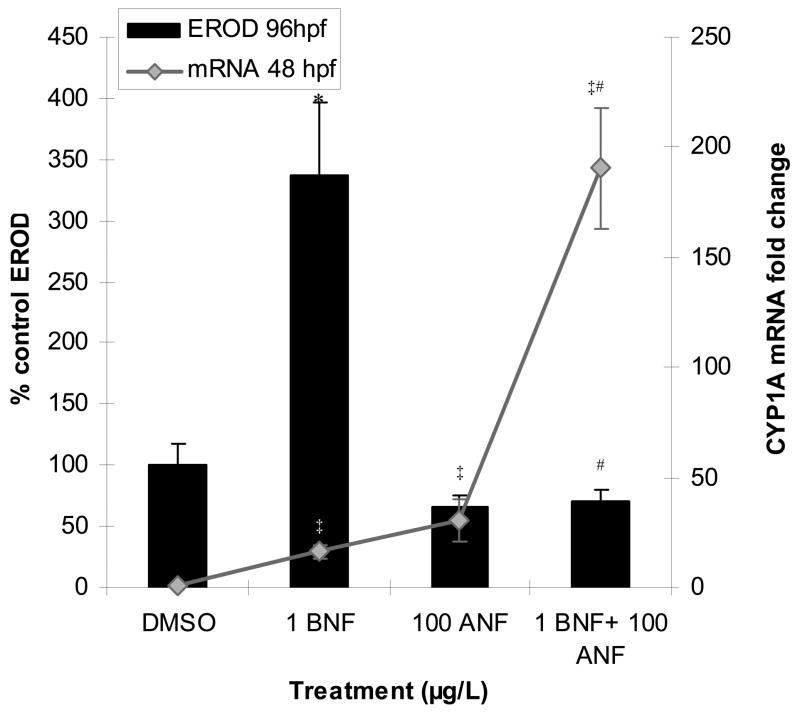
3.3 Gene induction vs. protein activity
Our previous studies have shown that CYP1A enzymatic activity as measured by in vivo ethoxyresorufin-O-deethylase (EROD) activity was inhibited by ANF in the BNF+ANF co-exposures (Wassenberg and Di Giulio, 2004; Billiard et al., 2006). This study repeated those experiments and further show that this enzyme activity inhibition is in contrast to the mRNA expression of CYP1A, which is synergistically increased by this same exposure (Figure 4). In addition, the increase in EROD activity in the BNF but not the ANF single exposures is also not predicted by their similar levels of mRNA induction (Figure 4). These results highlight a disparity between CYP1A mRNA induction and enzyme activity.
Figure 4.
CYP1A EROD activity vs. CYP1A mRNA induction. BNF induction of EROD activity (96 hpf) is inhibited by ANF while mRNA induction (48 hpf) is synergistically enhanced. A “#” indicates a synergistic interaction (ANOVA interaction term p ≤ 0.05). A “*” indicates a significant difference from control among EROD data, and “‡” indicates a significant difference from controls among mRNA data (p ≤ 0.05). Data are presented as mean ± S.E.; EROD n = 3 replicates of pooled embryos and is representative of 75 larvae. mRNA n ≥ 12 replicates of pooled embryos.
4. Discussion
The data presented in this study help clarify the role of ANF in this synergistic toxicity. We observe that ANF acts as a comparatively weak AHR agonist by itself, and in BNF+ ANF co-exposures, it is not acting as an AHR antagonist at the described doses. We would expect that if ANF was acting as an AHR antagonist in the co-exposures, we would have observed a lack of mRNA induction of genes regulated by the AHR. On the contrary, we observed a synergistic induction of mRNA expression of genes regulated by the AHR at 48 hpf, and continued upregulation after deformity onset through 96 hpf. In most instances, the induction observed in the co-exposures at 48 hpf was comparable to induction levels observed at the two highest concentrations of the BNF dose response curve. These two highest doses of BNF also begin to exhibit signs of toxicity by 96 hpf, in great similarity to the co-exposure groups (Billiard et al., 2006). This result coupled with the observed inhibition of CYP1A EROD activity suggests that through ANF’s ability to inhibit CYP1A’s enzymatic activity, metabolism is altered such that the half-life of the AHR agonist(s) is being extended. This would allow for continual activation of the AHR and correlates with the appearance of deformities.
These deformities are similar to those observed by others following zebrafish embryo exposure to 2,3,7,8,-tetrachlorodibenzo-p- dioxin (TCDD) (Teraoka et al., 2003; Carney et al., 2004). It is thus hypothesized that the mechanism of toxicity behind the synergistic developmental toxicity of these model polycyclic aromatic hydrocarbons may, as with TCDD, be due to the continual activation or misregulation of the AHR during critical periods of development. Alternatively, upregulation of additional CYP1s could cause a shift in metabolism and increased production of reactive metabolites.
Our observation of ANF’s interaction with BNF as a weak AHR agonist agrees with a study by Aluru et al. (2005), in which rainbow trout hepatocytes treated with BNF and ANF single treatments both increased mRNA abundance in a dose dependent manner, but that ANF’s maximal induction of AHR and CYP1A protein was about half that of BNF’s. The authors confirmed ANF’s activity as a partial AHR agonist, and not an AHR antagonist. BNF+ANF co-exposures did not decrease BNF’s induction of CYP1A mRNA or protein; instead, similar to our observation of increased mRNA expression in BNF+ANF co-exposures, the authors observed increased hepatocyte CYP1A protein (Aluru et al., 2005).
We propose that it is ANF’s activity as a CYP1A inhibitor that is critical to the synergistic embryo toxicity of BNF+ ANF, not ANF’s activity as a weak AHR agonist. This is supported by previous work in the killifish model (Fundulus heteroclitus) where we observed that treatment with numerous CYP1A inhibitors including ANF, fluoranthene, 2-aminoanthracene, and dibenzothiophene also increased toxicity of BNF and resulted in synergistic embryotoxicity (Wassenberg and Di Giulio, 2004; Wassenberg et al., 2005). We have also observed in our previous zebrafish work, that morpholino knockdown of CYP1A resulted in exacerbation of a nontoxic combination of BNF+ANF, (1 μg/L BNF + 50 μg/L ANF), and also synergized toxicity of a single nontoxic dose of BNF (10 μg/L), such that deformities of both experiments were of similar severity to those observed in the BNF+ANF (1 μg/L BNF + 100 μg/L ANF) synergistic toxicity (Billiard et al, 2006). These studies clearly indicate the importance of CYP1A metabolic activity in protecting from toxicity.
In addition to inhibition of CYP1A activity, it is possible that this toxicity may also be due to the subsequent increased half-life of the AHR agonist(s). We observed a synergistic upregulation of genes regulated by the AHR during co-exposure of BNF and ANF at mRNA expression levels that were similar to those observed with higher doses of BNF alone. While our studies did not include metabolite analysis, our interpretation of these data is supported by microcosm studies examining metabolism of the PAH benzo (a) pyrene (BaP) with co-exposure to the CYP- inhibitor piperonyl butoxide in mosquito fish (Gambusia affinis) which resulted in reduced metabolism of BaP and increased accumulation of the parent compound by 21% in a 3-day aquatic microcosm study and by 12% in a 33-day terrestrial-aquatic study (Lu et al., 1977).
As CYP1A enzyme inhibition appears to result in increased ligand and activation of the AHR, it then follows that we must further examine the importance of this receptor. Not only does the AHR play an important role in toxicant detoxification as an adaptive response, but it also serves critical physiologic functions during normal development; both overactivation as well as inhibition can have severe consequences for normal development, as reviewed in Carney et al (2006a) and Hahn (2003). Our study demonstrated a transient 3-fold upregulation of AHR2 at 48 hpf (24 h post dose administration) in the BNF+ ANF co-exposure group. This is within the range of the 2-4- fold upregulation of AHR2 mRNA observed in in TCDD exposed zebrafish embryos (10–100 nM concentrations administered 1 hpf and analyzed 24 hpf; analyzed by Northern blot) (Tanguay et al., 1999). While only small fold changes were observed with AHR2 mRNA, the genes regulated by the AHR exhibit tremendous responsiveness to the ligand dependent induction by AHR2. It remains unclear whether the upregulation of genes by AHR2 is due to increased abundance of the receptor, increased ligand concentration, or perhaps both, which contribute to the synergistic upregulation of genes by AHR in our experiments.
Three days after treatment administration (96 hpf), BNF and ANF single treatment groups (1 and 100 μg/L) still had elevated induction of CYP1 mRNA. Also notable is that this low level continual activation of the AHR pathway did not lead to deformities, while the higher activation level observed in the co-exposure groups did. One explanation for the lowered mRNA induction observed at the 96 hpf time point compared to the 48 hpf time point in the co-exposure treatment group is that perhaps metabolism by CYP1B1 and CYP1C1 is occurring, and thereby lowering the concentration of AHR ligand.
We examined mRNA expression of four genes upregulated by the AHR pathway: CYP1A, CYP1B1, CYP1C1, and AHRR2. CYP1A has been widely studied in fish. Upregulation of this highly responsive AHR regulated gene by various AHR agonists has been shown in numerous RNA and protein expression studies (Andreasen et al., 2002; Meyer et al., 2003; Teraoka et al., 2003; Chung-Davidson et al., 2004; Volz et al., 2005), and it is often used as a biomarker for exposure to environmental contaminants. CYP1B1 on the other hand, is less well studied in fish. In channel catfish, BaP treatment induced CYP1B1 mRNA in blood, liver, gonad, in cultured gill cells (Willett et al., 2006). In zebrafish, CYP1B1 mRNA has been found to be inducible by BNF in adult gill, and by PCB-126 in heart, liver, kidney, gut, gill, eye, and brain, and by PCB-126 in zebrafish embryos (Jonsson et al., 2007). CYP1B1 has also been shown to be inducible in zebrafish by TCDD through microarray analysis (Handley-Goldstone et al., 2005; Carney et al., 2006).
This study also provides new information about the newly identified CYP1C1. This enzyme was recently identified along with CYP1C2 in scup and zebrafish (Godard et al., 2005) and has also been found in carp and killifish (Itakura et al., 2005; Wang et al., 2006). The zebrafish CYP1C2 cDNA sequence was not available at the start of this study. Homologues to CYP1C1 or the CYP1C2 genes have not been identified in mammals, suggesting this may be a fish specific gene. While only very few studies of CYP1C1 have yet been published, XREs were identified in both cyp1c promoters (Jonsson et al., 2007). Both zebrafish CYP1C1 and CYP1C2 were inducible in embryos exposed to PCB-126, and CYP1C1 was inducible by PCB126 in adult eye, heart, gill, liver, and kidney (Jonsson et al., 2007). CYP1C1 has also been shown to be inducible in the killifish model by the PAH benzo(a)pyrene (Wang et al., 2006) and in zebrafish embryos by TCDD (Carney et al., 2006). Our study contributes a mRNA dose response of CYP1C1 by both BNF and ANF that was quite similar compared to the CYP1B1 dose response; neither was as responsive as CYP1A.
We also examined AHRR2, one of two zebrafish co-orthologs of the mammalian AHRR. While the function of this protein in toxicity is not yet understood, AHRR has been shown to repress the transactivation ability of the AHR/ARNT heterodimer in vitro (Mimura et al., 1999). Additionally, both AHRR1 and AHRR2 mRNA have been shown to be upregulated by TCDD exposure in developing zebrafish embryos (Evans et al., 2005). Here we show that AHRR2 is also responsive to induction by BNF and ANF.
While we have previously shown that AHR2 mediates the synergistic toxicity of BNF+ANF in zebrafish (Billiard et al., 2006), it remains unclear as to whether it is the overactivation of the AHR2 and its downstream interactions with other signaling pathways, or the upregulation of CYP1 metabolic activity altering xenobiotic and endogenous metabolism, that is responsible for toxicity.
The important role of CYP1 metabolic activity in this synergistic interaction is also demonstrated by the work of Hodson et al (2007). In this recent paper, 100 μg/L ANF (the same dose used in our co-exposure experiments) enhanced toxicity of the alkyl-substituted PAH and AHR agonist retene (1-methyl-7-isopropyl phenanthrene) to rainbow trout (Oncorhynchus mykiss) juveniles. Similar to our observations with BNF, higher levels of retene have also been shown to cause blue sac disease and induction of CYP1A (Brinkworth et al., 2003). Metabolite analysis during this retene + ANF (100 μg/L) co-exposure showed decreased concentrations of polar dihydroxylated metabolites, and increased concentrations of less polar monohydroxylated metabolites, suggesting that ANF’s inhibition of CYP1A is causing a shift in metabolism, perhaps to other CYP1 enzymes. Concurrently, protein concentrations of CYP1A increased, indicating increased activation of the AHR pathway. Similar to our previous study in which morpholino knockdown of AHR2 prevented toxicity of BNF+ANF co-exposure, rainbow trout blue sac disease symptoms were substantially lessened when concentrations of ANF (320 μg/L) were high enough to inhibit retene metabolism via AHR antagonism as demonstrated by a decrease in CYP1A protein, increased parent compound concentration and very few retene metabolites (Hodson et al., 2007). Other studies further substantiate the observation for dual modes of action of ANF, and that these modes will differ depending on the dose (Gasiewicz and Rucci, 1991; Santostefano et al., 1993; Aluru et al., 2005).
There is also strong evidence that misregulation of the AHR could be contributing to this toxicity. The AHR has been shown to have a critical endogenous role in normal cardiovascular development in vertebrates (Hahn, 2003). Continual activation of this receptor, or misregulation, is the primary mechanism by which TCDD is thought to cause toxicity, although downstream effects remain unclear. The AHR has been shown to also interact with numerous pathways which could disrupt development including p53, NFkB, steroid receptors, and retinoblastoma protein, and has been the topic of several reviews (Huang and Elferink, 2005; Marlowe and Puga, 2005; Puga et al., 2005). Further studies are necessary to distinguish whether altered metabolism, overactivation of the AHR with subsequent cell signaling disruptions, or both, are involved in developmental toxicity of some PAHs.
5. Conclusions
This study provides additional insight into the mechanisms underlying synergistic developmental toxicity of PAHs. We have confirmed that ANF is not antagonizing the AHR2 in these co-exposures, but is instead acting as a weak AHR agonist. Furthermore, mRNA induction of the AHR regulated genes CYP1A, CYP1B1, CYP1C1, AHRR2 was synergistically upregulated in BNF+ANF co-exposures groups prior to the onset of deformities to levels that resembled the highest doses of BNF alone in the dose-response curve experiments. Induction of CYP1A mRNA was synergistically induced to 190-fold, which starkly contrasts to the CYP1A enzyme activity, which was inhibited in the BNF+ANF co-exposures. These data suggest that through inhibition of the metabolic clearance provided by CYP1A, the half-life of the AHR agonist(s) is being extended, thus allowing for prolonged activation of the AHR. This hypothesis, however, remains to be confirmed.
Acknowledgments
Di Giulio Laboratory, Deena Wassenberg, David Volz, Elwood Linney, Superfund Basic Research Program (P42 ES10356), Integrated Toxicology & Environmental Health Program (T32 ES07031), EPA STAR (to A. T.-L.).
Footnotes
Nomenclature note: Gene names are indicated with lowercase italics; gene products are indicated with uppercase lettering, with mRNA products noted in italics to differentiate from protein names.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aluru N, Vuori K, Vijayan MM. Modulation of Ah receptor and CYP1A1 expression by alpha-naphthoflavone in rainbow trout hepatocytes. Comp Biochem Physiol C Toxicol Pharmacol. 2005;141(1):40–49. doi: 10.1016/j.cca.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Andreasen EA, Spitsbergen JM, Tanguay RL, Stegeman JJ, Heideman W, Peterson RE. Tissue-specific expression of AHR2, ARNT2, and CYP1A in zebrafish embryos and larvae: effects of developmental stage and 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure. Toxicol Sci. 2002;68(2):403–419. doi: 10.1093/toxsci/68.2.403. [DOI] [PubMed] [Google Scholar]
- Billiard SM, Timme-Laragy AR, Wassenberg DM, Cockman C, Di Giulio RT. The role of the aryl hydrocarbon receptor pathway in mediating synergistic developmental toxicity of polycyclic aromatic hydrocarbons to zebrafish. Toxicol Sci. 2006;92(2):526–536. doi: 10.1093/toxsci/kfl011. [DOI] [PubMed] [Google Scholar]
- Brinkworth LC, Hodson PV, Tabash S, Lee P. CYP1A induction and blue sac disease in early developmental stages of rainbow trout (Oncorhynchus mykiss) exposed to retene. J Toxicol Environ Health A. 2003;66(7):627–646. doi: 10.1080/15287390309353771. [DOI] [PubMed] [Google Scholar]
- Carney SA, Peterson RE, Heideman W. 2,3,7,8-Tetrachlorodibenzo-p-dioxin activation of the aryl hydrocarbon receptor/aryl hydrocarbon receptor nuclear translocator pathway causes developmental toxicity through a CYP1A-independent mechanism in zebrafish. Mol Pharmacol. 2004;66(3):512–521. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- Carney SA, Chen J, Burns CG, Xiong KM, Peterson RE, Heideman W. Aryl hydrocarbon receptor activation produces heart-specific transcriptional and toxic responses in developing zebrafish. Mol Pharmacol. 2006;70(2):549–561. doi: 10.1124/mol.106.025304. [DOI] [PubMed] [Google Scholar]
- Chung-Davidson YW, Rees CB, Wu H, Yun SS, Li W. beta-naphthoflavone induction of CYP1A in brain of juvenile lake trout (Salvelinus namaycush Walbaum) J Exp Biol. 2004;207(Pt 9):1533–1542. doi: 10.1242/jeb.00919. [DOI] [PubMed] [Google Scholar]
- Denison MS, Heath-Pagliuso S. The Ah receptor: a regulator of the biochemical and toxicological actions of structurally diverse chemicals. Bull Environ Contam Toxicol. 1998;61(5):557–568. doi: 10.1007/pl00002973. [DOI] [PubMed] [Google Scholar]
- Dong W, Teraoka H, Tsujimoto Y, Stegeman JJ, Hiraga T. Role of aryl hydrocarbon receptor in mesencephalic circulation failure and apoptosis in zebrafish embryos exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci. 2004;77(1):109–116. doi: 10.1093/toxsci/kfh023. [DOI] [PubMed] [Google Scholar]
- Evans BR, Karchner SI, Franks DG, Hahn ME. Duplicate aryl hydrocarbon receptor repressor genes (ahrr1 and ahrr2) in the zebrafish Danio rerio: structure, function, evolution, and AHR-dependent regulation in vivo. Arch Biochem Biophys. 2005;441(2):151–167. doi: 10.1016/j.abb.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Gasiewicz TA, Rucci G. Alpha-naphthoflavone acts as an antagonist of 2,3,7, 8- tetrachlorodibenzo-p-dioxin by forming an inactive complex with the Ah receptor. Mol Pharmacol. 1991;40(5):607–612. [PubMed] [Google Scholar]
- Godard CA, Goldstone JV, Said MR, Dickerson RL, Woodin BR, Stegeman JJ. The new vertebrate CYP1C family: Cloning of new subfamily members and phylogenetic analysis. Biochem Biophys Res Commun. 2005;331(4):1016–1024. doi: 10.1016/j.bbrc.2005.03.231. [DOI] [PubMed] [Google Scholar]
- Goujon FM, Nebert DW, Gielen JE. Genetic expression of aryl hydrocarbon hydroxylase induction. IV. Interaction of various compounds with different forms of cytochrome P-450 and the effect on benzo(a)pyrene metabolism in vitro. Mol Pharmacol. 1972;8(6):667–680. [PubMed] [Google Scholar]
- Hahn ME. Dioxin toxicology and the aryl hydrocarbon receptor: insights from fish and other non-traditional models. Mar Biotechnol (NY) 2001;3(Supplement 1):S224–238. doi: 10.1007/s10126-001-0045-y. [DOI] [PubMed] [Google Scholar]
- Hahn ME. Evolutionary and physiological perspectives on AH receptor function and dioxin toxicity. In: Schecter AGT, editor. Dioxins and health. John Wiley and Sons; Hoboken, NJ: 2003. pp. 559–602. [Google Scholar]
- Handley-Goldstone HM, Grow MW, Stegeman JJ. Cardiovascular gene expression profiles of dioxin exposure in zebrafish embryos. Toxicol Sci. 2005;85(1):683–693. doi: 10.1093/toxsci/kfi116. [DOI] [PubMed] [Google Scholar]
- Hawkins SA, Billiard SM, Tabash SP, Brown RS, Hodson PV. Altering cytochrome P4501A activity affects polycyclic aromatic hydrocarbon metabolism and toxicity in rainbow trout (Oncorhynchus mykiss) Environ Toxicol Chem. 2002;21(9):1845–1853. [PubMed] [Google Scholar]
- Hodson PV, Qureshi K, Noble CA, Akhtar P, Brown RS. Inhibition of CYP1A enzymes by alpha-naphthoflavone causes both synergism and antagonism of retene toxicity to rainbow trout (Oncorhynchus mykiss) Aquat Toxicol. 2007;81(3):275–285. doi: 10.1016/j.aquatox.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Huang G, Elferink CJ. Multiple mechanisms are involved in Ah receptor-mediated cell cycle arrest. Mol Pharmacol. 2005;67(1):88–96. doi: 10.1124/mol.104.002410. [DOI] [PubMed] [Google Scholar]
- Incardona JP, Collier TK, Scholz NL. Defects in cardiac function precede morphological abnormalities in fish embryos exposed to polycyclic aromatic hydrocarbons. Toxicol Appl Pharmacol. 2004;196(2):191–205. doi: 10.1016/j.taap.2003.11.026. [DOI] [PubMed] [Google Scholar]
- Incardona JP, Day HL, Collier TK, Scholz NL. Developmental toxicity of 4-ring polycyclic aromatic hydrocarbons in zebrafish is differentially dependent on AH receptor isoforms and hepatic cytochrome P4501A metabolism. Toxicol Appl Pharmacol. 2006;217(3):308–321. doi: 10.1016/j.taap.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Itakura T, El-Kady M, Mitsuo R, Kaminishi Y. Complementary DNA cloning and constitutive expression of cytochrome P450 1C1 in the gills of carp (Cyprinus carpio) Environ Sci. 2005;12(2):111–120. [PubMed] [Google Scholar]
- Jonsson ME, Orrego R, Woodin BR, Goldstone JV, Stegeman JJ. Basal and 3,3′,4,4′,5-pentachlorobiphenyl-induced expression of cytochrome P450 1A, 1B and 1C genes in zebrafish. Toxicol Appl Pharmacol. 2007;221(1):29–41. doi: 10.1016/j.taap.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu PY, Metcalf RL, Plummer N, Mandel D. The Environmental fate of three carcinogens: benzo-(alpha)-pyrene, benzidine, and vinyl chloride evaluated in laboratory model ecosystems. Arch Environ Contam Toxicol. 1977;6:2–3. 129–142. doi: 10.1007/BF02097756. [DOI] [PubMed] [Google Scholar]
- Marlowe JL, Puga A. Aryl hydrocarbon receptor, cell cycle regulation, toxicity, and tumorigenesis. J Cell Biochem. 2005;96(6):1174–1184. doi: 10.1002/jcb.20656. [DOI] [PubMed] [Google Scholar]
- Merchant M, Arellano L, Safe S. The mechanism of action of alpha-naphthoflavone as an inhibitor of 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced CYP1A1 gene expression. Arch Biochem Biophys. 1990;281(1):84–89. doi: 10.1016/0003-9861(90)90416-v. [DOI] [PubMed] [Google Scholar]
- Merchant M, Krishnan V, Safe S. Mechanism of action of alpha-naphthoflavone as an Ah receptor antagonist in MCF-7 human breast cancer cells. Toxicol Appl Pharmacol. 1993;120(2):179–185. doi: 10.1006/taap.1993.1101. [DOI] [PubMed] [Google Scholar]
- Merchant M, Morrison V, Santostefano M, Safe S. Mechanism of action of aryl hydrocarbon receptor antagonists: Inhibition of 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced CYP1A1 gene expression. Archives of Biochemistry and Biophysics. 1992;298(2):389–394. doi: 10.1016/0003-9861(92)90426-w. [DOI] [PubMed] [Google Scholar]
- Meyer JN, Wassenberg DM, Karchner SI, Hahn ME, Di Giulio RT. Expression and inducibility of aryl hydrocarbon receptor pathway genes in wild-caught killifish (Fundulus heteroclitus) with different contaminant-exposure histories. Environ Toxicol Chem. 2003;22(10):2337–2343. doi: 10.1897/02-495. [DOI] [PubMed] [Google Scholar]
- Mimura J, Ema M, Sogawa K, Fujii-Kuriyama Y. Identification of a novel mechanism of regulation of Ah (dioxin) receptor function. Genes Dev. 1999;13(1):20–25. doi: 10.1101/gad.13.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda CL, Henderson MC, Buhler DR. Evaluation of chemicals as inhibitors of trout cytochrome P450s. Toxicol Appl Pharmacol. 1998;148(2):237–244. doi: 10.1006/taap.1997.8341. [DOI] [PubMed] [Google Scholar]
- Nasevicius A, Ekker SC. Effective targeted gene ‘knockdown’ in zebrafish. Nat Genet. 2000;26(2):216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- Nebert DW, Puga A, Vasiliou V. Role of the Ah receptor and the dioxin-inducible [Ah] gene battery in toxicity, cancer, and signal transduction. Ann N Y Acad Sci. 1993;685:624–640. doi: 10.1111/j.1749-6632.1993.tb35928.x. [DOI] [PubMed] [Google Scholar]
- Nesnow S, Bergman H. Metabolism of alpha-naphthoflavone by rat liver microsomes. Cancer Res. 1981;41(7):2621–2626. [PubMed] [Google Scholar]
- Nesnow S, Easterling R, Bergman H, Roth R. Inhibition of benzo(A)pyrene monooxygenase by [alpha]-naphthoflavone may be partially mediated by the metabolite9-hydroxy-[alpha]-naphthoflavone. Toxicology Letters. 1982;14:1–2. 7–13. doi: 10.1016/0378-4274(82)90003-0. [DOI] [PubMed] [Google Scholar]
- Prasch AL, Teraoka H, Carney SA, Dong W, Hiraga T, Stegeman JJ, Heideman W, Peterson RE. Aryl hydrocarbon receptor 2 mediates 2,3,7,8-tetrachlorodibenzo-p-dioxin developmental toxicity in zebrafish. Toxicol Sci. 2003;76(1):138–150. doi: 10.1093/toxsci/kfg202. [DOI] [PubMed] [Google Scholar]
- Puga A, Tomlinson CR, Xia Y. Ah receptor signals cross-talk with multiple developmental pathways. Biochem Pharmacol. 2005;69(2):199–207. doi: 10.1016/j.bcp.2004.06.043. [DOI] [PubMed] [Google Scholar]
- Santostefano M, Merchant M, Arellano L, Morrison V, Denison MS, Safe S. alpha-Naphthoflavone-induced CYP1A1 gene expression and cytosolic aryl hydrocarbon receptor transformation. Mol Pharmacol. 1993;43(2):200–206. [PubMed] [Google Scholar]
- Shimada T. Xenobiotic-metabolizing enzymes involved in activation and detoxification of carcinogenic polycyclic aromatic hydrocarbons. Drug Metab Pharmacokinet. 2006;21(4):257–276. doi: 10.2133/dmpk.21.257. [DOI] [PubMed] [Google Scholar]
- Tanguay RL, Abnet CC, Heideman W, Peterson RE. Cloning and characterization of the zebrafish (Danio rerio) aryl hydrocarbon receptor. Biochim Biophys Acta. 1999;1444(1):35–48. doi: 10.1016/s0167-4781(98)00252-8. [DOI] [PubMed] [Google Scholar]
- Teraoka H, Dong W, Tsujimoto Y, Iwasa H, Endoh D, Ueno N, Stegeman JJ, Peterson RE, Hiraga T. Induction of cytochrome P450 1A is required for circulation failure and edema by 2,3,7,8-tetrachlorodibenzo-p-dioxin in zebrafish. Biochem Biophys Res Commun. 2003;304(2):223–228. doi: 10.1016/s0006-291x(03)00576-x. [DOI] [PubMed] [Google Scholar]
- Testa B, Jenner P. Inhibitors of Cytochrome P-450s and their mechanism of action. Drug Metab Rev. 1981;12(1):1–117. doi: 10.3109/03602538109011082. [DOI] [PubMed] [Google Scholar]
- Van Metre PC, Mahler BJ. Trends in hydrophobic organic contaminants in urban and reference lake sediments across the United States, 1970–2001. Environ Sci Technol. 2005;39(15):5567–5574. doi: 10.1021/es0503175. [DOI] [PubMed] [Google Scholar]
- Volz DC, Bencic DC, Hinton DE, Law JM, Kullman SW. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) induces organ- specific differential gene expression in male Japanese medaka (Oryzias latipes) Toxicol Sci. 2005;85(1):572–584. doi: 10.1093/toxsci/kfi109. [DOI] [PubMed] [Google Scholar]
- Wang L, Scheffler BE, Willett KL. CYP1C1 Messenger RNA Expression is Inducible by Benzo[a]pyrene in Fundulus heteroclitus Embryos and Adults. Toxicol Sci. 2006;93(2):331–340. doi: 10.1093/toxsci/kfl072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassenberg DM, Di Giulio RT. Synergistic embryotoxicity of polycyclic aromatic hydrocarbon aryl hydrocarbon receptor agonists with cytochrome P4501A inhibitors in Fundulus heteroclitus. Environ Health Perspect. 2004;112(17):1658–1664. doi: 10.1289/ehp.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassenberg DM, Nerlinger AL, Battle LP, Di Giulio RT. Effects of the polycyclic aromatic hydrocarbon heterocycles, carbazole and dibenzothiophene, on in vivo and in vitro CYP1A activity and polycyclic aromatic hydrocarbon-derived embryonic deformities. Environ Toxicol Chem. 2005;24(10):2526–2532. doi: 10.1897/04-440r1.1. [DOI] [PubMed] [Google Scholar]
- Willett KL, Ganesan S, Patel M, Metzger C, Quiniou S, Waldbieser G, Scheffler B. Mar Environ Res. Suppl. Vol. 62. 2006. In vivo and in vitro CYP1B mRNA expression in channel catfish; pp. S332–336. [DOI] [PubMed] [Google Scholar]