Abstract
Calcineurin (CaN) is a Ca2+- and calmodulin-dependent protein phosphatase (PP2B) that, in yeast, is an integral intermediate of a salt-stress signal transduction pathway that effects NaCl tolerance through the regulation of Na+ influx and efflux. A truncated form of the catalytic subunit and the regulatory subunit of yeast CaN were coexpressed in transgenic tobacco plants to reconstitute a constitutively activated phosphatase in vivo. Several different transgenic lines that expressed activated CaN also exhibited substantial NaCl tolerance, and this trait was linked to the genetic inheritance of the CaN transgenes. Enhanced capacity of plants expressing CaN to survive NaCl shock was similar when evaluation was conducted on seedlings in tissue culture raft vessels or plants in hydroponic culture that were transpiring actively. Root growth was less perturbed than shoot growth by NaCl in plants expressing CaN. Also, NaCl stress survival of control shoots was enhanced substantially when grafted onto roots of plants expressing CaN, further implicating a significant function of the phosphatase in the preservation of root integrity during salt shock. Together, these results indicate that in plants, like in yeast, a Ca2+- and calmodulin-dependent CaN signal pathway regulates determinants of salt tolerance required for stress adaptation. Furthermore, modulation of this pathway by expression of an activated regulatory intermediate substantially enhanced salt tolerance.
Salinity is a major environmental stress that is a substantial constraint to crop production both for dry-land and irrigated agriculture (1). The detrimental impact of this stress is perpetuated and exacerbated by management practices used to facilitate high-output crop production. The maladies caused by salt stress arise from the disruption of cellular aqueous and ionic equilibria, so tolerance determinants include effectors that function to restore cellular homeostasis. Recently, results from metabolic engineering experiments have confirmed functional roles for compatible osmolytes, such as mannitol, proline, and betaine, in salt adaptation of higher plants (2–5). These compounds are presumed to function both in osmoprotection and osmotic adjustment (5, 6) but, individually, seem to contribute minutely to salt tolerance of plants. This is not unexpected because salt tolerance is mediated by multiple determinants, such as those that are intrinsically cellular and function to restrict Na+ uptake across the plasma membrane, facilitate Na+ and Cl− sequestration into the vacuole, and mediate compatible osmolyte and osmoprotectant production and accumulation (7). Consequently, it is predicted that enhanced capacity of one effector is insufficient to elicit a substantial degree of tolerance. The capacity to effect coordinate control of several effectors through the modulation of signal-regulatory cascades or transcriptional activation of multiple genes may have the greatest impact on salt tolerance resulting from metabolic engineering (8).
Experimental evidence has implicated the likely involvement of cytosolic Ca2+ (9) and phosphoinositide (PI) turnover (10) in a salt-stress signaling cascade. By analogy with animal and fungal systems, this evidence suggests that Ca2+ and PI are components of a protein phosphorylation/dephosphorylation cascade(s) that couple stress perception and signal transduction to physiological mechanisms of adaptation in plants (8). However, little is known about the determinants that are involved. Only recently, Sheen (11) has shown that two related Ca2+-dependent protein kinases (CDPKs) induced a stress- and ABA-responsive HVA1 promoter. Activation of these CDPKs substituted for stress signals and was antagonized by a constitutively active protein phosphatase 2C (PP2C) that is capable of abolishing ABA responses. Molecular characterization of salt-stress responses also implicates mitogen-activated protein kinase (MAPK) cascades and transcription factors as being involved in coordinate control of tolerance determinants (8). However, to date, no evidence has linked CDPKs, MAPKs, or other regulatory elements directly to a phenotype of salt tolerance in plants.
The PP2B phosphatase calcineurin (CaN) is a focal component of a Ca2+-dependent signal transduction pathway that mediates Na+, Li+, and Mn2+ tolerances of Saccharomyces cerevisiae (12–14). CaN functions to limit intracellular Na+ accumulation by regulating processes that restrict influx and enhance efflux of this cation across the plasma membrane (12, 13). CaN modulates the K+ and Na+ uptake system to have higher affinity for K+, restricting uptake of Na+. This CaN function depends on TRK1, a putative high-affinity K+ transporter. In addition, CaN induces transcription of ENA1 (12, 13), a gene that encodes a P-type ATPase that is located in the plasma membrane and is primarily responsible for Na+ efflux from S. cerevisiae cells. CaN also participates in cytosolic Ca2+ homeostasis through the positive regulation of Golgi apparatus and vacuolar membrane-localized P-type ion pumps (PMR1 and PMC1, respectively) and negative control of a vacuolar H+/Ca2+ exchanger (VCX1). PMR1 also is required for Mn2+ tolerance (14, 15).
Functional CaN is a heterodimer composed of catalytic (CNA) and regulatory (CNB) subunits. The topology of CNA consists of an amino-terminal catalytic core followed sequentially by domains that function in CNB and immunophilin–immunosuppressant interactions, calmodulin binding, and autoinhibition (16). The catalytic core contains an iron–zinc active center whereas the other domains are involved in CNB binding and Ca2+- and calmodulin-dependent activation of CaN (16, 17). Deletion of the calmodulin binding and the autoinhibitory domains from CNA eliminated calmodulin but not Ca2+ dependency for phosphatase activity (16, 18). However, functional sufficiency for Na+ tolerance in yeast required coexpression of the truncated CNA subunit and CNB, indicating that the regulatory subunit is essential for CaN activation in vivo (13). Activated CaN mediated Na+ tolerance through the coordinate induction of high-affinity K+ transport (requiring TRK1) and ENA1 expression. ENA1 expression was both constitutively induced as well as potentiated for induction by salt stress (13).
Pharmacological and biochemical evidence for the existence of a plant CaN that functions to regulate ion transport (19, 20) as well as isolation of plant cDNAs that complement a salt-sensitive phenotype by suppressing CaN null mutations (ref. 21 and T.M., J.M.P., R.A.B., and P.M.H., unpublished data) led us to postulate that the yeast protein could activate a Ca2+- and calmodulin-dependent salt-stress cascade in plants, possibly resulting in greater salt tolerance. We show here that activated yeast CaN functions in apparent conjunction with a salt-stress signaling pathway present in plants to mediate salt tolerance.
MATERIALS AND METHODS
Plasmid Construction and Plant Transformation.
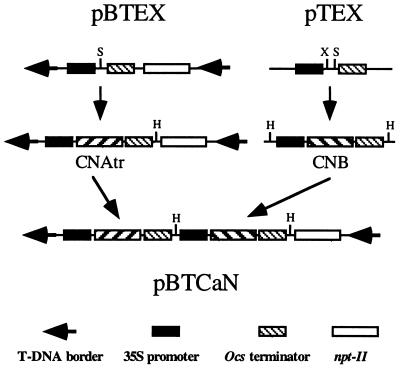
Yeast strains used have been described elsewhere (13). Their relevant genotypes are DBY746 (CNA1 CNA2 CNB1), MCY100 (Δcna1 Δcna2), and YP9 (Δcnb1). Plasmid pCAtrB allows the expression of activated CaN in yeast and has been described previously (13). A truncated yeast CNA2 gene, denoted CNAtr henceforth, was amplified by PCR with primers that annealed to the start codon and introduced a stop codon after Thr-459 as described by Mendoza et al. (13). Because the yeast CNB1 gene includes a 76-bp intron, a CNB1 cDNA was produced by reverse transcriptase–PCR (RT-PCR) amplification using as template mRNA from yeast cells overexpressing CNB1 (13), sense primer 5′-TCTAGACATGGGTGCTGCTCCTTCC-3′ (annealed to the start codon), and antisense primer 5′-GTCGACTTACACATCGTATTGCAA-3′ (annealed to the stop codon). CNAtr and CNB1 cDNA PCR products were confirmed by sequence analysis and functionally tested for complementation of the Na+/Li+ sensitivity of Δcna1,2 (MCY100) and Δcnb1 (YP9) mutants (13). For plant transformation, CNAtr was inserted into the pBI121 derivative pBTEX, between the cauliflower mosaic virus 35S promoter and octopine synthase (Ocs) terminator (Fig. 1). CNB1 cDNA was inserted into a similar expression cassette in pTEX, a pUC19 derivative, and transferred to pBTEX containing CNAtr. The resulting plasmid, pBTCaN, was mobilized to Agrobacterium tumefaciens strain EHA105 for transformation of tobacco (Nicotiana tabacum L. var. Wisconsin 38) leaf disks by using 200 mg/liter kanamycin for selection (22).
Figure 1.
Agrobacterium tumefaciens binary vector used for coexpression of catalytic (CNA) and regulatory (CNB) subunits for reconstitution of activated yeast calcineurin (CaN) in transgenic tobacco. The gene encoding a truncated form of the yeast CNA2 subunit deleted after Thr-459 (CNAtr) was inserted into the SalI site of pBTEX. The marker gene in this vector encodes for neomycin phosphotransferase (npt-II) for kanamycin selection driven by the nopaline synthase gene promoter and terminator (not shown). The CNB1 cDNA was inserted between the cauliflower mosaic virus 35S promoter and Ocs terminator in plasmid pTEX by using the XbaI and SalI sites. Subsequently, the entire cassette was cloned into the HindIII site of pBTEX containing CNAtr to obtain the binary plasmid pBTCaN. Cloning sites are indicated as follows: HindIII (H), SalI (S), and XbaI (X).
Immunodetection of CNB.
The RT-PCR CNB1 product was inserted into the XbaI site in pGEX-KG (23) to produce a glutathione S-transferase (GST) fusion peptide. Recombinant GST-CNB was expressed in Escherichia coli DH5α after isopropyl β-d-thiogalactoside induction and purified by glutathione agarose affinity chromatography (23). The CNB peptide was separated from GST by digestion with thrombin, purified by SDS/PAGE, transferred to poly(vinylidene difluoride) membrane, and N-terminally sequenced to confirm the identity of CNB. Chicken IgY antibodies were prepared against purified recombinant protein and used for immunodetection of CNB. Rabbit anti-chicken IgG conjugated to alkaline phosphatase was used as secondary antibody.
Plant Materials, Growth, and Evaluation of Salt Tolerance.
Salt tolerance of plants was measured as the capacity to withstand and recover from salt shock based on experiments with seedlings grown in tissue culture raft vessels and plants grown in hydroponic culture. Progeny of first self-pollinated generation (T1) or second self-pollinated generation (T2) were evaluated for salt tolerance. For experiments in tissue culture raft vessels, tobacco seeds of the T1 generation were germinated aseptically on filter paper moistened with 1/4× Murashige and Skoog (MS) nutrient solution (Sigma, M-5524). Seven-day-old seedlings were transferred onto the rafts in culture vessels (Lifeline System, Osmotek, Rehovot, Israel) containing the same nutrient solution. After 7 days in the raft vessels (14 days total since imbibition), seedlings were administered a salt shock by transferring the raft to a new vessel containing 1/4× MS solution with 250 mM NaCl for 4 days. Seedlings then were transferred to 1/4× MS solution without NaCl, and after 10–12 days, survival was determined. For experiments in hydroponic culture, seeds were germinated in a greenhouse potting mixture and grown for a period of 45 days. Soil was rinsed gently from the roots with water and plants were transferred to hydroponic culture (1/4× MS solution) in a growth chamber (25°C, 300 μEm−2 ⋅ s−1 PAR, 16-hr light/8-hr dark photoperiod). Plants were allowed to acclimate to hydroponic culture for 10 days before salt treatment. The salt-shock treatment was 200 mM NaCl for 7 days. Plants were transferred to nutrient solution without salt for 10 days, and then the number of surviving plants and growth parameters were determined. For evaluation of resistance to kanamycin, seedlings were transferred to agar-solidified medium containing 1/4× MS and 200 μg/ml kanamycin. The presence of the npt-II gene was further assessed in plants surviving antibiotic media by direct measurement of NPT-II activity in leaf disks (24).
Reciprocal Shoot and Root Grafting.
T2 seeds of a line homozygous for Kanr (but not expressing CaN) and a line homozygous for CaN expression were germinated and plants were grown in the greenhouse as indicated above for hydroponic culture. These 45-day-old plants were the sources of scions and root stocks for wedge grafts. The grafted plants were maintained under high humidity for 7–8 days and then under normal greenhouse conditions for 7–8 additional days, during which time the rubber strip covering the graft union was removed. Actively growing plants then were transferred to hydroponic culture and evaluated for salt tolerance as described above.
RESULTS
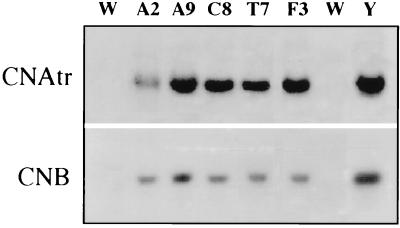
The gene encoding a truncated yeast CNA2 subunit deleted of the calmodulin-binding and autoinhibitory domains (CNAtr) and a cDNA-encoding CNB were constructed into separate expression cassettes in an Agrobacterium binary vector for coexpression of the subunits and reconstitution of activated CaN in planta (Fig. 1). The binary vector contained the neomycin phosphotransferase (nptII) gene as a selectable marker. Leaf disks were cocultivated with Agrobacterium and plants were regenerated in the presence of kanamycin selection. Plants (T0) from different transformation events were obtained that expressed both CNAtr and CNB transcripts (Fig. 2).
Figure 2.
Expression of yeast CNAtr and CNB1 transcripts in transgenic tobacco plants. Total RNA was isolated from leaves of seed-derived untransformed W38 plants (W) and T0 plants regenerated after transformation with plasmid pBTCaN and kanamycin selection (A2, A9, C8, T7, and F3) and from cells of yeast cna1 cna2 double-disputant MCY100 transformed with plasmid pCAtrB, also containing CNAtr and CNB1 (Y) (2). Ten micrograms of RNA was loaded into each lane, transferred to nitrocellulose filters, and hybridized with CNAtr (Upper) or CNB1 (Lower) insert. Approximate transcript sizes of CNAtr and CNB1 are 1.4 and 0.5 kb, respectively.
Two-week-old seedlings of an untransformed tobacco line (W38) and transgenic progeny populations from self-pollinations (T1) of a line expressing the nptII gene only (PKY, transformed with the binary vector without CNAtr and CNB) or of five independent lines expressing CaN (A2, A9, C8, F3, and T7) were transferred directly to nutrient solution containing 250 mM NaCl in tissue culture raft vessels (Table 1). Seedlings derived from the five CaN expressing lines exhibited increased NaCl survival compared with the those from PKY and W38 control lines (Table 1). T1 seedling populations of PKY and T7 both segregated 3:1 for NPT-II activity based on kanamycin resistance/susceptibility (Knr:Kns) and enzyme-activity determinations. The T7 seedling population that survived NaCl stress was enriched with Knr segregants [i.e., depleted of transgene null (azygous) seedlings] as indicated by the increase in the ratio of Knr:Kns genotypes from 3:1 for seedlings grown without salt to 9.4:1 for seedlings after NaCl treatment. Conversely, the Knr:Kns genotype ratio of 2.5:1 for PKY seedling progeny that survived NaCl treatment indicates that salt shock did not enrich this control population for Knr seedlings. The lack of enrichment for Knr seedlings in the PKY population negates the possibility that salt survival depends on nptII expression. Cosegregation of Knr with NaCl survival in the T7 population indicates that the stress-tolerance phenotype is linked to the CaN transgenes and is not the consequence of somaclonal variation or other epigenetic phenomena in this line. Furthermore, because T1 progeny of four other independent CaN-expressing lines exhibited salt-stress survival comparable to those of the T7 line, then the salt tolerance phenotype did not result from insertional mutagenesis.
Table 1.
NaCl tolerance of transformed tobacco plants genetically cosegregates with the yeast CaN transgenes
| Line | Plants
|
Knr:Kns segregation ratio
|
||
|---|---|---|---|---|
| Initial, n | Survival, % | Before NaCl shock | After NaCl shock | |
| Experiment 1 | ||||
| PKY | 107 | 36.4 (27–45)* | 3:1 | 2.5:1 |
| W38 | 54 | 37.0 (24–50) | — | — |
| A2 | 49 | 74.0 (61–85) | 15:1 | ND |
| A9 | 36 | 69.4 (54–84) | 15:1 | ND |
| C8 | 51 | 82.4 (71–93) | 3:1 | ND |
| F3 | 56 | 75.0 (64–86) | 3:1 | ND |
| T7 | 81 | 64.2 (54–74) | 3:1 | 9.4:1† |
| Experiment 2 | ||||
| PKY | 120 | 24.1 (16–32) | 3:1 | ND |
| A9 | 36 | 77.7 (64–92) | 15:1 | ND |
| T7 | 115 | 53.4 (44–62) | 3:1 | 11.2:1‡ |
Seedlings of an untransformed tobacco line (W38), and first-generation progeny from self-pollinations (T1) of a line expressing only the neomycin phosphotransferase-selectable marker gene npt-II (PKY), effecting kanamycin resistance (Knr), and lines expressing CNAtr and CNB in addition to NPT-II (A2, A9, C8, F3, and T7) were evaluated. Experiment 1: Seedlings grow in plant tissue culture raft vessels. T1 tobacco seedlings were transferred to mineral nutrient solution containing 250 mM NaCl. After 4 days of salt shock, seedlings were transferred to nutrient solution without NaCl and left to recover for 10 days. Seedlings remaining viable after this period were evaluated for sensitivity (Kns) or resistance (Knr) to kanamycin and NPT-II activity. Presented are the percentages, of each population, surviving NaCl treatment (Survival) and the Knr:Kns ratio of the population before and after NaCl shock treatment. Experiment 2: Plants grown in hydroponic culture in a plant growth chamber. Hydroponically grown T1 progeny of lines PKY, A9, and T7 were transferred to fresh mineral nutrient (1/4× MS) solution containing 200 mM NaCl. After 7 days, the plants were transferred to solution without NaCl. Ten days subsequently, the number of surviving plants was determined. Knr:Kns of the T7-derived plants, after NaCl shock, was assessed by using the NPT-II assay. ND, not determined.
Confidence intervals (95%) for a binomial distribution/proportion.
Different from the expected 3:1 values at 95% level.
Different from the expected 3:1 values at 99% level.
In a subsequent experiment, T1 progeny were grown in hydroponic culture inside of a standard plant growth chamber, rather than in tissue culture raft vessels (Table 1). Again, progeny of CaN-expressing lines survived salt stress substantially better than those of PKY. Capacity to survive 200 mM NaCl also resulted in enrichment of the T7-derived population for Knr segregants, indicating that salt tolerance was linked genetically to the CaN transgenes. Plants grown in hydroponic culture in a plant growth chamber have higher stomatal conductances and transpirational fluxes, which should result in greater photoassimilate production and shoot ion accumulation, than those grown under the low-light intensity of the culture growth room and high humidity within the raft culture vessel. It thus is apparent that CaN mediates salt tolerance through mechanisms that are operative in photosynthetically active plants.
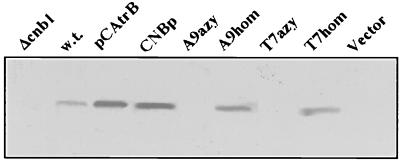
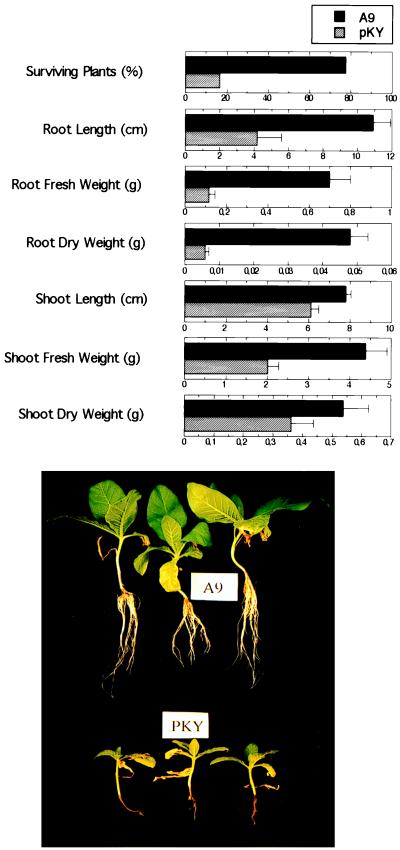
Second-generation progeny from self-pollination (T2) of lines PKY, T7, and A9 homozygous for Knr and the corresponding T7 and A9 azygous lines were obtained. Homozygous T2 of lines T7 and A9 expressed yeast CaN, as determined by immunodetection of recombinant CNB in plant protein extracts (Fig. 3) and expression of CNAtr and CNB1 transcripts (data not shown). Southern analyses indicated that homozygosity for Knr in the progeny of line A9 was attributable to a single transgene allele (not shown). T2 plants of lines PKY and A9 homozygous for Knr, growing in hydroponic culture, were shocked with 200 mM NaCl in 1/4× MS salt solution for 7 days and then transferred to fresh solution without NaCl for an additional 12 days. Plants expressing CaN exhibited substantially greater salt tolerance, manifested not only in higher survival frequency but also in greater fresh and dry weight accumulation of surviving seedlings when compared with PKY progeny (Fig. 4). These results indicate that CaN enhances survival of plants to salt shock but do not establish whether or not expression of the phosphatase enhances growth of plants continuously exposed to salt. In fact, during the period of salt exposure, no appreciable growth was detected. Homozygous CaN expressing T2 progeny from the C8 line (kanamycin segregation ratio of T1 progeny was Knr:Kns = 3:1) also exhibited greater NaCl tolerance than progeny of control lines in hydroponic experiments (not shown).
Figure 3.
Calcineurin expression based on immunodetection of CNB. Illustrated is the immunoblot of 15 μg protein extracted from the Δcnb1 yeast disruptant YP9 (Δcnb1), wild-type yeast DBY746 (w.t.), or DBY746 transformed with plasmid pCAtrB for overproduction of CNB (pCAtrB), 0.5 μg purified recombinant CNB protein (CNBp), and 45 μg protein isolated from leaves of T2 plants homozygous for npt-II (A9hom, T7hom), the complementary azygous (A9azy, T7azy), or homozygous T2 plants transformed with an empty plasmid (vector). Proteins were separated on a 15% SDS-polyacrylamide gel and reacted with chicken anti-CNB antibodies.
Figure 4.
Activated yeast CaN mediates NaCl tolerance of tobacco plants. Homozygous T2 plants from lines PKY (expressing only npt-II) and A9 (expressing CNAtr and CNB1 as well as npt-II) were treated with 200 mM NaCl. After 7 days of salt shock, plants were transferred to fresh nutrient solution without NaCl and left to recover for 12 days. (Upper) Percentage of surviving seedlings of 36 progeny from each line (significantly different at the 95% level) and growth parameters of all plants surviving the NaCl treatment ± SE (6 and 28 plants from lines PKY and A9, respectively). Average fresh weights of PKY and A9 plants before the NaCl treatment were 1.63 ± 0.11 g and 1.76 ± 0.10 g, respectively. (Lower) Three representative plants from lines A9 (top row) and PKY (bottom row) that survived NaCl shock and after the recovery period.
Plants expressing CaN sustained less root injury during stress that was manifested as greater root development after stress recovery (Fig. 4). Maintenance of root integrity during salt stress by CaN-expressing plants was most likely the main contributory factor to the relative vigor of the plants (compared with control plants) after transfer into nutrient solution without salt. Furthermore, NaCl shock survival of PKY (control) shoots was increased substantially when these shoots were grafted onto root stocks from A9 CaN homozygous plants (Table 2). Conversely, A9 shoots grafted onto PKY roots exhibited a small increase in NaCl tolerance. Presumably, activated CaN functions mainly in roots to regulate the movement of ions from the apoplast to symplast, which controls the ion content of the xylem sap, which, in turn, is transported to the shoots by the strength of the transpirational sink (7).
Table 2.
NaCl tolerance of plants expressing CaN is based on root function
| Scion/root stock | Plants
|
|
|---|---|---|
| Initial, n | Survival, % | |
| PKY/PKY | 35 | 8.8 (0–17.6)* |
| A9/PKY | 24 | 33.3 (14.5–52.1) |
| PKY/A9 | 24 | 66.7 (47.9–85.5) |
| A9/A9 | 33 | 69.7 (54–85.4) |
T2 progeny of lines homozygous for Knr (vector control, PKY) and CaN expression (A9) were the resource for scions and root stocks for wedge-grafted plants evaluated for NaCl tolerance. PKY scion onto PKY root stock (PKY/PKY). PKY scion onto A9 root stock (PKY/A9), A9 scion onto PKY root stock (A9/PKY), and A9 scion onto A9 root stock (A9/A9).
Confidence intervals (95%) for a binomial distribution/proportion.
DISCUSSION
Functional reconstitution of activated yeast CaN in tobacco cells results in substantial salt tolerance of transgenic plants. Consequently, the essential components of a Ca2+- and calmodulin-dependent CaN salt-stress signal pathway appear to be present in plants and can function in conjunction with the yeast protein to facilitate salt adaptation. Recently, techniques of map-based cloning have been used to determine that the salt-sensitive phenotype of the Arabidopsis sos3 mutant (25) is a result of a defect in a gene that encodes a protein with sequence homology to the regulatory subunit of calcineurin (26).
Salt adaptation in plants has been shown to involve numerous transport processes, including those required for ion homeostasis, that can be modulated by exposure to varying concentrations of salt (1, 7). Furthermore, the expression of genes encoding several transport proteins (e.g., H+- or Ca2+-ATPases) are known to be responsive to NaCl stress, and the responsiveness of these genes often is more pronounced in halophytes than glycophytes (7). In yeast, activated CaN mediated salt tolerance through the regulation of the major K+ uptake system to restrict Na+ influx and potentiation of ENA1 expression to enhance Na+ efflux. Therefore, the salt-adaptation regulatory cascade in plants that is activated by fungal CaN may, as it does in yeast, exert control over biochemical and physiological processes that function coordinately to regulate, most probably, intracellular K+/Na+ transport and homeostasis. Recently, an Arabidopsis cDNA (AtNHX) has been isolated (F. J. Quintero and J.M.P., unpublished data) that encodes a homologue of the yeast vacuolar membrane Na+/H+ exchanger, NHX1 (27). Genetic complementation of the salt-sensitive phenotype of the yeast mutant nhx1 by AtNHX was CaN-dependent, indicating that the phosphatase facilitates the activity of the plant transporter in yeast (unpublished data).
The expression of both the vacuolar- and Golgi-localized Ca2+-ATPase genes (PMC1 and PMR1) and the activity of the vacuolar H+/Ca2+ antiporter (VCX1) in S. cerevisiae are regulated through CaN-dependent mechanisms (14). These transporters facilitate tolerances to Ca2+, apparently by regulating Ca2+ homeostasis and signaling. Suppression of the Ca2+-sensitive phenotype of the yeast double-mutant pmc1 vcx1 by either plant H+/Ca2+ antiporter gene (CAX1 or CAX2) (28) required CaN (K. Hirschi, personal communication), indicating that the yeast phosphatase can regulate the plant antiporters as it does the yeast transporter VCX1 (14). A current model links increases in cytoplasmic Ca2+ activity with salinity stress signaling (8, 9, 11). Thus, facilitation of plant salt tolerance by yeast CaN also may involve the activity of transporters functioning in Ca2+ homeostasis, which, in turn, may affect Na+ tolerance determinants. Recently, Ca2+-mediated signal transduction in plants has been implicated in the regulation of osmotic stress-induced promoter activity (11). Two transiently expressed, activated, Ca2+-dependent protein kinases from Arabidopsis thaliana were able to activate the stress-induced HVA1 promoter fused to green fluorescent protein reporter gene. The results we present not only confirm the existence of a Ca2+-dependent stress signal cascade but establish that this pathway is functionally involved in salt adaptation.
Our results provide experimental evidence that modulation of a signal pathway in plants can impart a stress tolerance phenotype and, at least in this instance, with no apparent negative impact on plant growth and development. Salt tolerance of the CaN-expressing plants may be the result of the activated phosphatase either preadapting plants or potentiating the response of plants to the stress (13). Both possibilities could be envisaged as useful strategies to enhance survival of plants to high salinity, although preadaptation might impose yield costs. Preliminary results indicate that yeast CaN does not promote constitutive expression of the salt-induced PR-5 protein osmotin, suggesting that the fungal phosphatase may specifically regulate salt-tolerance determinants (as yet unidentified) rather than to elicit a generalized plant-stress response. If this is the case, then identifying the genes differentially regulated by CaN might be a very effective strategy for determining effectors of salt tolerance.
As indicated, analogy with yeast predicts involvement of cellular mechanisms as the basis for plant salt adaptation resulting from activated CaN. This implies that salt tolerance of a multicellular organism is highly dependent on processes that are intrinsically cellular, particularly if the capacity to withstand salt shock is the measure of salt tolerance. Data from pharmacological experiments indicate that guard cell behavior is controlled by CaN, which functions in the modulation of ion channels (19, 20). The results from the grafting experiments presented here make it apparent that CaN also has a pivotal effect on salt tolerance of roots, where adaptive mechanisms independent of guard cells must function. Presumably, some of these are responsible for maintenance of ion homeostasis in the xylem and regulation of salt flux to shoot that, based on thermodynamic requirements, would involve energy-dependent ion transporters or pumps (7). Taken together, these observations implicate CaN as a pivotal intermediate in a salt-adaptation signal cascade that is common to cells both in the shoot and root but must regulate different physiological events in these organs. The ability of a single signal intermediate to control responses to salt stress that are so differentially manifested in the physiology of separate organs may reflect the very specific and unique response of individual cell types (e.g., root and guard cells) to the same signal.
Acknowledgments
This is journal paper 15,705 of the Purdue University Agricultural Experiment Station. Research was supported by the U.S. Department of Agriculture National Research Initiative Competitive Grant 97-00558 (to R.A.B. and P.M.H.) and by Grant BI097-0629 from Comision Interministerial de Ciencia y Tecnologia (to J.M.P.).
ABBREVIATIONS
- CaN
calcineurin
- MS
Murashige and Skoog
References
- 1.Epstein E, Norlyn J D, Rush D W, Kingsbury R W, Kelley D B, Cunningham G A, Wrona A F. Science. 1980;210:399–404. doi: 10.1126/science.210.4468.399. [DOI] [PubMed] [Google Scholar]
- 2.Tarczynski M C, Jensen R G, Bohnert H J. Science. 1993;259:508–510. doi: 10.1126/science.259.5094.508. [DOI] [PubMed] [Google Scholar]
- 3.Kishor P B K, Hong Z, Miao G H, Hu C A A, Verma D P S. Plant Physiol. 1995;108:1387–1394. doi: 10.1104/pp.108.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lilius G, Holmberg N, Bülow L. Bio/Technology. 1996;14:177–180. [Google Scholar]
- 5.Hayashi H, Alia, Mustardy L, Deshnium P, Ida M, Murata N. Plant J. 1997;12:133–142. doi: 10.1046/j.1365-313x.1997.12010133.x. [DOI] [PubMed] [Google Scholar]
- 6.Shen B, Jensen R G, Bohnert H J. Plant Physiol. 1997;115:527–532. doi: 10.1104/pp.115.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niu X, Bressan R A, Hasegawa P M, Pardo J M. Plant Physiol. 1995;109:735–742. doi: 10.1104/pp.109.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shinozaki K, Shinozaki-Yamaguchi K. Plant Physiol. 1997;115:327–334. doi: 10.1104/pp.115.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lynch J, Polito V S, Lauchli A. Plant Physiol. 1989;90:1271–1274. doi: 10.1104/pp.90.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirayama T, Ohto C, Mizoguchi T, Shinozaki K. Proc Natl Acad Sci USA. 1995;92:3903–3907. doi: 10.1073/pnas.92.9.3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheen J. Science. 1996;274:1900–1902. doi: 10.1126/science.274.5294.1900. [DOI] [PubMed] [Google Scholar]
- 12.Mendoza I, Rubio F, Rodriguez-Navarro A, Pardo J M. J Biol Chem. 1994;269:8792–8796. [PubMed] [Google Scholar]
- 13.Mendoza I, Quintero F J, Bressan R A, Hasegawa P M, Pardo J M. J Biol Chem. 1996;271:23061–23067. doi: 10.1074/jbc.271.38.23061. [DOI] [PubMed] [Google Scholar]
- 14.Cunningham K W, Fink G R. Mol Cell Biol. 1996;16:2226–2237. doi: 10.1128/mcb.16.5.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lapinkas P J, Cunningham K W, Liu X F, Fink G R, Culotta V C. Mol Cell Biol. 1995;15:1382–1388. doi: 10.1128/mcb.15.3.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parsons J N, Wiederrecht G J, Salowe S, Burbaum J J, Rokosz L L, Kincaid R L, O’Keefe S J. J Biol Chem. 1994;269:19610–19616. [PubMed] [Google Scholar]
- 17.Wang X, Culotta V C, Klee C B. Nature (London) 1996;383:434–437. doi: 10.1038/383434a0. [DOI] [PubMed] [Google Scholar]
- 18.Stemmer P M, Klee C B. Biochemistry. 1994;33:6859–6866. doi: 10.1021/bi00188a015. [DOI] [PubMed] [Google Scholar]
- 19.Luan L S, Li W, Rusnak F, Assmann S M, Schreider S L. Proc Natl Acad Sci USA. 1993;90:2202–2206. doi: 10.1073/pnas.90.6.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allen G J, Sanders D. Plant Cell. 1995;7:1473–1483. doi: 10.1105/tpc.7.9.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lippuner V, Cyert M S, Gasser C S. J Biol Chem. 1996;271:12859–12866. doi: 10.1074/jbc.271.22.12859. [DOI] [PubMed] [Google Scholar]
- 22.Liu D, Raghothama K G, Hasegawa P M, Bressan R A. Proc Natl Acad Sci USA. 1994;91:1888–1892. doi: 10.1073/pnas.91.5.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guan K L, Dixon J E. Anal Biochem. 1991;192:262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- 24.Peng J, Wen F, Hodges T K. Plant Mol Biol Rep. 1993;11:38–47. [Google Scholar]
- 25.Liu J, Zhu J-K. Proc Natl Acad Sci USA. 1997;94:14960–14964. doi: 10.1073/pnas.94.26.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J, Zhu J-K. Science. 1998;280:1943–1945. doi: 10.1126/science.280.5371.1943. [DOI] [PubMed] [Google Scholar]
- 27.Nass R, Cunningham K W, Rao R. J Biol Chem. 1997;272:26145–26152. doi: 10.1074/jbc.272.42.26145. [DOI] [PubMed] [Google Scholar]
- 28.Hirschi K D, Zhen R-G, Cunningham K W, Rea P A, Fink G R. Proc Natl Acad Sci USA. 1996;93:8782–8786. doi: 10.1073/pnas.93.16.8782. [DOI] [PMC free article] [PubMed] [Google Scholar]