Abstract
The endocannabinoid signaling system is composed of the cannabinoid receptors; their endogenous ligands, the endocannabinoids; the enzymes that produce and inactivate the endocannabinoids; and the endocannabinoid transporters. The endocannabinoids are a new family of lipidic signal mediators, which includes amides, esters, and ethers of long-chain polyunsaturated fatty acids. Endocannabinoids signal through the same cell surface receptors that are targeted by Δ9-tetrahydrocannabinol (Δ9THC), the active principles of cannabis sativa preparations like hashish and marijuana. The biosynthetic pathways for the synthesis and release of endocannabinoids are still rather uncertain. Unlike neurotransmitter molecules that are typically held in vesicles before synaptic release, endocannabinoids are synthesized on demand within the plasma membrane. Once released, they travel in a retrograde direction and transiently suppress presynaptic neurotransmitter release through activation of cannabinoid receptors. The endocannabinoid signaling system is being found to be involved in an increasing number of pathological conditions. In the brain, endocannabinoid signaling is mostly inhibitory and suggests a role for cannabinoids as therapeutic agents in central nervous system (CNS) disease. Their ability to modulate synaptic efficacy has a wide range of functional consequences and provides unique therapeutic possibilities. The present review is focused on new information regarding the endocannabinoid signaling system in the brain. First, the structure, anatomical distribution, and signal transduction mechanisms of cannabinoid receptors are described. Second, the synthetic pathways of endocannabinoids are discussed, along with the putative mechanisms of their release, uptake, and degradation. Finally, the role of the endocannabinoid signaling system in the CNS and its potential as a therapeutic target in various CNS disease conditions, including alcoholism, are discussed.
Key Words: Alcoholism, endocannabinoids, CNS, synaptic plasticity, reward, CB1 receptors, alcohol-drinking behavior, therapy
INTRODUCTION
The earliest anthropological evidence of Cannabis use comes from the oldest known Neolithic culture in China where it was used in the production of hemp for ropes and textiles, and also for its psychotropic effects [159]. An 1848 commentary in the British Pharmacopoeia outlined quite accurately the psychotropic effects of Cannabis and pointed out its merits as an antispasmodic and analgesic [59].
The major psychoactive constituent of Cannabis sativa is Δ9- tetrahydrocannabinol (Δ9-THC, dronabinol), which is mainly responsible for the pharmacological effects of the Cannabis plant [79, 147]. Δ9-THC was isolated, stereochemically defined, and synthesized in 1964 [102] and its psychoactive properties were recognized immediately. The hydrophobic nature of ??9-THC delayed experimentation and suggested that the compound might act by influencing membrane fluidity rather than by binding to a specific receptor. The development of new classes of potent and selective Δ9- THC analogues led to the pharmacological identification of cannabinoid- sensitive sites in the brain [77]. Currently Δ9-THC and its analogs are used for the treatment of nausea and vomiting induced by radiotherapy or chemotherapy, and wasting syndrome in AIDS patients. Cannabinoids are also useful for the treatment of pain, aspasticity, glaucoma and other disorders [310]. However, the clinical usefulness of Δ9-THC and its anlogs is greatly hampered by their numerous side effects, including the potential for abuse [133, 241]. Research on the molecular and neurobiological bases of the physiological and neurobehavioral effects of marijuana use was slowed by the lack of specific tools and technology for many decades. Over the last one and half decades, advances in our knowledge of the endocannabinoid signaling system have progressed enormously. This review is focussed on our understanding of the endocannabinoid signaling system in the brain. First, the structure, anatomical distribution, and signal transduction mechanisms of cannabinoid receptors are described. Second, the pathways of endocannabinoid synthesis are discussed, along with the putative mechanisms of endocannabinoid release, uptake, and degradation. Finally, the role of the endocannabinoid signaling system in the central nervous system (CNS) and its potential as a therapeutic target for the treatment of various CNS disease conditions, including alcoholism, are discussed.
THE ENDOCANNABINOID SIGNALING SYSTEM
Cannabinoid Receptors
Evidence for the existence of the marijuana receptor has been accumulating since the 1980s [77, 149]. It has now been shown that cannabinoids have two specific G-protein-coupled cannabinoid receptor subtypes, which have been cloned. These are named CB1 and CB2. Evidence for a third type of G-protein-coupled cannabinoid receptor (“CB3” or “Anandamide receptor”) in brain and in endothelial tissues is mounting [38, 83, 157, 306]. However, the cloning, expression and characterization of CB3 is yet to come.
The CB1 and CB2 receptors belong to the large superfamily of G protein-coupled receptors (GPCR) (for a recent review see [13, 23]). The human CB1 and CB2 receptors share 44% overall amino acid identity (for details see [222]). The CB2 receptors of human and rat share 81% amino acid identity and those of mouse and rat share 81%.
Although significant progress has been achieved into many aspects of the biology of the endocannabinoid system, and our knowledge of cannabinoid genomics and proteomics is increasing, the regulation of cannabinoid receptor genes is poorly understood. cDNA sequences encoding CB1- or CB2-like receptors have been reported for the rat [193], human [106, 208], mouse [1, 52], cow (Wessner, GeneBank submission, 1997), cat [104] (GeneBank submission, 1997), puffer fish [316], leech [268], zebra finch [266], and newt [267].
The CB1 receptor gene is polymorphic, with implications not only for substance abuse but also for other neuropsychiatric disorders. The CB1 receptor gene is intronless and its structure is similar in mouse, rat, and human. The CB2 receptor gene is also intronless, at least in its coding region [222]. Unlike the CB1 receptor gene, which is highly conserved across the human, rat, and mouse species, the CB2 receptor gene is much more divergent [127]. Like other GPCRs, the primary structures of the cannabinoid receptors are characterized by seven hydrophobic stretches of 20–25 amino acids predicted to form transmembrane α helices, which are connected by alternating extracellular and intracellular loops. In both the CB1 and CB2 receptors, no cysteines are found within the second extracellular domain, but the third extracellular domain contains two or more cysteines. Cysteine residues appear to stabilize the tertiary structure of the receptor because of their abililty to form intra-molecular disulfide bridges. In most GPCRs, these cysteines occur in the extracellular domains that lie between hydrophobic domains 2 and 3 and hydrophobic domains 4 and 5 (i.e., the second and third extracellular domains, assuming that the N-terminal domain is also extracellular). One other deviation from most other GPCRs is that the CB1 and CB2 receptors lack a highly conserved proline residue in hydrophobic domain 5 [192]. The structural features of these proteins that are critical for ligand binding and functional properties have been evaluated in vivo and in vitro [2, 3]. The consensus sites for N-glycosylation are mainly concentrated at the N-terminus of the CB1 proteins. Whether any of these potential Nglycosylation sites are naturally glycosylated or even essential for CB1 receptor function is not known.
There are four clusters of potential cAMP-dependent protein kinase and Ca2+-calmodulin-dependent protein kinase sites in CB1 receptors. These clusters are conserved across the human, rat, and mouse receptor proteins. There is a single potential protein kinase C site that is also conserved in these CB1 receptors. There is no such site in CB2 receptors. The biological significance of these sites has been shown in non-neuronal cells. For example, in AtT-20 cells transfected with rat cannabinoid receptor (CB1), the activation of an inwardly rectifying potassium current (Kir current) and depression of P/Q-type Ca2+ channels by cannabinoids were prevented by stimulation of protein kinase C by 100 nM phorbol 12-myristate 13- acetate (PMA) [103]. However, the biological significance of these potential protein phosphorylation sites in these receptor molecules in brain is yet to be determined (for a detailed discussion, see the recent review [23]).
CB1 and CB2 receptor knockout mice have been generated. The CB2 receptor knockout mouse was generated [43] to study the function of CB2 receptors in immune cells and in immunomodulation. Three groups have generated CB1 receptor gene knockout mice independently. The first CB1 knockout mouse were generated on a CD1 background [178], the second knockout mouse was generated on a C57BL background [269, 321], and the third conditional mutant mice that lack expression of the CB1 receptor in principal forebrain neurons but not in adjacent inhibitory interneurons was generated in 2003 [189]. The availability of cannabinoid knockout mice provides an excellent opportunity to study the biological roles of these genes. However, the use of transgenic mouse models to study the effects of over-expression of CB1 or CB2 receptor genes (as opposed to inactivation of the CB1 or CB2 receptor genes as described above) on the regulation and site-specific mechanisms of action of cannabinoids are currently unexplored. Obviously, CB1 or CB2 receptor transgenic animals will provide new in vivo systems for studying genetic regulation, development, normal physiology, and apparent dysfunctions associated with the cannabinoid system.
The genomic location of the human cannabinoid receptor gene (CB1) was mapped using genetic linkage studies and chromosomal in situ hybridization to chromosome 6q14-q15 [145]. The mouse CB1 and CB2 receptor genes are located in the proximal arm of chromosome 4 [222, 272]. The rat CB1 and CB2 receptor genes both map to chromosome 5. The bovine CB1 receptor gene has been mapped to chromosome 9q22 [230]. As the neurobiological effects of marijuana and other cannabinoids suggest the involvement of the cannabinoid receptor genes in mental and neurological disturbances, the mapping of receptor genes and the identification of allelic variants will undoubtedly enhance our understanding of the links between gene (dys) function and cannabinoid abnormalities.
The CB1 receptor is mainly expressed in the brain and spinal cord and thus is often referred to as the “brain cannabinoid receptor”. CB1 receptors are among the most abundant G-proteincoupled receptors in the brain, their densities being similar to the levels of γ-aminobutyric acid (GABA)- and glutamate-gated ion channels [139]. The distribution of CB1 receptors is highly heterogeneous in rats, with the highest densities of receptors present in the basal ganglia, substantia nigra, pars reticulata, and globus pallidus. In addition, very high levels of binding are present in the hippocampus, particularly within the dentate gyrus, and also in the molecular layer of the cerebellum. In contrast, there are few CB1 receptors in the brainstem. There is a similar distribution of CB1 receptors in humans [27, 116]. The highest densities are found in association with limbic cortices, with much lower levels within the primary sensory and motor regions, suggesting an important role in motivational (limbic) and cognitive (associative) information processing. CB1 receptors have been shown to be localized presynaptically on GABAergic interneurons and glutamatergic neurons [131, 132, 162, 163]. This would be consistent with the proposed role of endocannabinoid compounds in modulating neurotransmission.
The CB2 receptor is sometimes referred to as the “peripheral cannabinoid receptor” because it was thought that CB2 receptors were predominantly present in immune cells in the periphery and absent from the brain. Recent studies suggested that CB2 cannabinoid receptors are functionally expressed in neurons in the brain [92, 121, 140, 221, 299]. CB2 is expressed in peripheral tissues, including white blood cells [94, 208]. CB2 receptor mRNA has been found in the spleen, tonsils, and thymus, which are the major tissues of immune cell production and regulation [see [45, 148] for reviews]. CB2 agonists generally suppress the functions of these cells, but both CB1 and CB2 receptors could contribute to these effects [45].
The Signal Transduction Mechanism of Cannabinoid Receptors
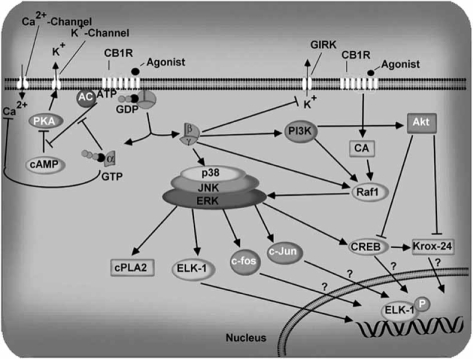
Activation of a cannabinoid receptor promotes its interaction with G proteins, resulting in guanosine diphosphate/ guanosine triphosphate exchange and subsequent dissociation of theαand#x03B2;γ subunits. These subunits regulate the activity of multiple effector proteins to bring about biological functions (Fig. 1). Both CB1 and CB2 are coupled with Gi or Go proteins. However, their affinity for Gi or Go proteins may vary, as reveled by several receptor-ligandstimulated GTPγS binding studies [39, 165]. CB1 receptors differ from many other GPCR proteins in being constitutively active, as they are precoupled with G-proteins in the absence of exogenously added agonists [206]. A schematic diagram of a cannabinoid-mediated signal transduction path-way is shown in Fig. (1).
Fig. (1).
Schematic summary of CB1 receptor signaling. CB1 receptors are 7-transmembrane domain, G-protein-coupled proteins located in the cell membrane. The Ca2+ channels inhibited by CB1 receptors include N-, P/Q- and L-type channels. Actions on Ca2+ channels and adenylyl cyclase (AC) are thought to be mediated by the α subunits of the G-protein, and those on GIRK and PI3K by the αβ subunits. Inhibition of AC and the subsequent decrease in cAMP decreases activation of cAMP-dependent protein kinase A (PKA), which leads to decreased phosphorylation of the K+ channels. Stimulatory effects are shown by a (→) sign and inhibitory effects by a (⊥) sign.
Adenylate cyclase activity was inhibited by anandamide (AEA), R-(+)-methanandamide (MetAEA), and 2-arachi-donylglycerol (2AG) in N18TG2 cells [58, 150, 231]. In some cases, up-regulation of adenylate cyclase activity was reported without Gi/o coupling (pertussis toxin sensitive) probably through activation of Gs proteins [118]. In another study, the in vitro expression of specific isoforms of adenylate cyclase (I, III, V, VI, or VIII) with coexpression of CB1 or CB2 was associated with the inhibition of cyclic AMP accumulation. However, the expression of other adenylate cyclase isoforms (II, IV, or VII) with co-expression of the CB1 or CB2 receptor was associated with stimulation of cAMP accumulation [247]. The questions of whether CB1 receptor coupling to Gs proteins has physiological importance and whether such coupling increases after Gi or Go protein sequestration by co-localized non-cannabinoid Gi/o protein-coupled receptors have yet to be answered.
Inhibition of N-type voltage-gated channels by cannabinoids has been demonstrated in several neuronal cells using a whole-cellvoltage clamp technique and intracellular Ca2+ measurement [50, 2+185, 215, 226]. L-type Ca2+ channels are inhibited by CB1 receptor agonist in cat brain arterial smooth muscle cells, which express mRNA and CB1 receptors [104]. This is blocked by pertussis toxin and SR141716A (rimonabant) [104]. CB1 receptors are also coupled to ion channels through Gi/o proteins, and activate A-type and inwardly rectifying potassium channels. The coupling to A-type and D-type potassium channels is thought to be through adenylate cyclase [205]. These are also stimulated by the inhibition of adenylate cyclase by cannabinoids. Because of the decrease in cAMP accumulation, cAMP-dependent protein kinase (PKA) is inhibited by CB1 receptor activation. Without cannabinoids, PKA phosphorylates the potassium channel protein, resulting in decreased outward potassium current. In the presence of cannabinoids, however, the phosphorylation of the channel by PKA is reduced, which leads to an increased outward potassium current [57]. Cannabinoids inhibit N-type, P/Q-type calcium channels and D-type potassium channels [148, 150]. In addition, cannabinoids can close sodium channels, but whether this effect is receptor-mediated has yet to be proved. Based on these findings, it has been suggested that cannabinoids play a role in regulation of neurotransmitter release. There is also evidence from experiments with rat hippocampal CA1 pyramidal neurons that CB1 receptors are negatively coupled to M-type potassium channels [261]. CB1 receptors may also mobilize arachidonic acid and close 5HT3 receptor ion channels [229], and under certain conditions couple to Gs proteins to activate adenylate cyclase [47] to reduce outward potassium K current, possibly through arachidonic acid-mediated stimulation of protein kinase C [134]. CB1 receptors have also been reported to activate phospholipase C through G proteins in COS-7 cells co-transfected with CB1 receptors and G sub-units [144]. CB1 receptors on cultured cerebellar granule neurons can operate through a phospholipase C-sensitive mechanism to increase N-methyl-D-aspartate (NMDA)-elicited calcium release from inositol 1,4,5-triphosphate-gated intracellular stores [214]. Activation of CB1 receptors by cannabinoid agonists evokes a rapid, transient increase in intracellular free Ca2+ in N18TG2 and NG108-15 cells [274, 275, 277].
One of the most interesting research areas is the regulation of neuritogenesis, axonal growth and synaptogenesis by cannabinoids. The molecular mechanism involved in this process is not yet clear. The regulation of cellular growth has been usually associated with tyrosine kinase receptors. However, recent studies suggest that GPCRs can stimulate the mitogen-activated protein kinase (MAPK) pathway and thereby induce cellular growth. After the first observation of activation of the MAPK cascade by AEA [309], several in vivo and in vitro studies have implicated both the cannabinoids and the endocannabinoids in the MAPK pathway. Activation of two isoforms (p42/p44) of MAPK was observed in non-neuronal U373MG astrocytoma cells and in host cells expressing recombinant CB1 receptors mediated by CB1 receptor and Gi/o protein [37]. Similarly, activation of Gi/o protein by Δ9 -THC and HU-210 via CB1 receptors activated p42/p44 MAPK in C6 glioma and primary astrocytes cultures [130, 257]. In WI-38 fibroblasts, AEA via CB1 receptor and Gi/o proteins promoted tyrosine-phosphorylation of the extracellular signal-regulated kinase 2 (ERK2 or p44) and increased MAPK activity [309]. In some cells, CB1 receptor-mediated activation of MAPK was mediated through the PI3 kinase pathway [37, 309]. AEA, CP,55, 940 and WIN 55,212-2 increased phosphorylation of FAK+ 6,7, a neural isoform of FAK, in hippocampal slices and in cultured neurons [72]. Δ9 -THC, AEA and 2AG stimulated phosphorylation of the Tyr-397 residue of FAK in the hippocampus, which is crucial for FAK activation [73]. Cannabinoids increased phosphorylation of p130-Cas, a protein associated with FAK in the hippocampus. Endocannabinoids increased the association of Fyn, but not Src, with FAK+6,7. These effects were mediated through inhibition of a cAMP pathway. CB1 receptor-stimulated FAK-autophosphorylation was shown to be upstream of the Src family kinases [73]. These new mechanisms for cannabinoid regulation of the MAPK pathway might play a role in endocannabinoid-induced modulation of synaptic plasticity, cell migration and neurite remodeling. Δ9 -THC promoted phosphorylation of Raf-1 and its subsequent translocation to the membrane in cortical astrocytes [257]. CB1 receptor-mediated release of βγ subunits leads to activation of PI3K, resulting in tyrosine phosphorylation and activation of Raf-1 and the phosphorylation of MAPK. Activation of p38 MAPK was observed in CHO cells expressing recombinant CB1 receptors [255] and in human vascular endothelial cells having endogenous CB1 receptors [181]. Δ9 -THC was shown to induce activation of c-Jun N-terminal kinase (JNK1 and JNK2) in CHO cells expressing recombinant CB1 receptors [255]. The pathway for JNK activation involves Gi/o protein, PI3K and Ras [255].
Activation of the Na+/H+ exchanger in CHO cells stably expressing the CB1 receptor [35] was shown to be mediated through MAPK and CB1 receptors. AEA-stimulated activation of MAPK activity was shown to phosphorylate cytoplasmic phospholipase A2 (cPLA2), release of arachidonic acid (AA), and result in the synthesis of prostaglandin E2 in WI-38 cells [309]. MAPK activation by cannabinoids was shown to induce immediate-early gene expression (krox-24) in U373MG human astrocytoma cells [36]. Δ9-THC induced the expression of krox-24, BDNF and c-Fos in mouse hippocampus [74]. CB1- and MEK-ERK-mediated activation of krox-24 is negatively regulated through PI3K-Akt in neuro2a cells 24 is negatively regulated through PI3K-Akt in neuro2a cells [126]. The suppression of prolactin receptor and trk nerve growth factor receptor synthesis by AEA was shown to be associated with a CB1 receptor-mediated decrease in protein kinase A and an increase in MAPK activities [199]. CB1 receptor agonists induced the expression of c-fos and c-Jun in the brain [8, 187, 196]; whether this is mediated by CB1 receptor-activated MAPK is not known. Δ9 THCinduced phosphorylation of the transcription factor Elk-1 is mediated by MAPK/ERK [295]. Intracerebroventricular injection of AEA evoked an increase in c-Fos protein in rat brain with a generally similar distribution to that of CB1 receptors [228]. Δ9 -THC and HU-210 increased glucose metabolism and glycogen synthesis in C6 glioma and astrocytes cultures [130]. The activation of protein kinase B/Akt (isoforms IB) by cannabinoid agonists is mediated by Gi/o and PI3K in U373MG astrocytoma and CHO cells expressing recombinant CB1 receptors [120]. CB1 receptor-mediated gene regulation through the activation of MAPK is an important physiological mechanism by which cannabinoids and endocannabinoids can modulate synaptic plasticity.
Endocannabinoids
Since Δ9- THC was structurally defined and synthesized more than 30 years ago, there has been considerable speculation as to whether endogenous ligands exist, especially as CB1 is the most abundantly expressed neuronal receptor. The identification of endogenous cannabinoid receptors suggested that the brain must produce its own chemicals that interact with the CB1 receptor during normal brain function.
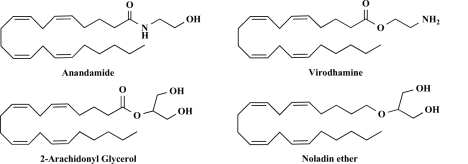
Beginning in 1992, the first endogenous cannabinoid was identified as anandamide (AEA, arachidonylethanolamide). It was named from the Sanskrit ananda, “internal bliss,’’ making reference to its chemical structure (the amide of arachidonic acid and ethanolamine) [78]. Subsequently, another endogenous cannabinoid receptor ligand, 2-arachidonyl-glycerol (2-AG) was discovered and characterized [197, 278] (Fig. 2). The third ether-type endocannabinoid, 2-arachi-donylglycerol ether (noladin ether), was isolated from the CNS and shown to display pharmacological properties similar to AEA [137]. The fourth type of endocannabinoid, virodhamine, in contrast to the previously described endocannabinoids, is a partial agonist with in vivo antagonist activity at the CB1 receptor [239]. The fifth type of endocannabinoid, N-arachidonyldopamine (NADA), not only binds to CB1 receptor but also stimulates vanilloid receptors (VR1) [152]. Previously, the existence of AEA analogs in chocolate had been demonstrated [88]. It is thought that chocolate and cocoa contain N-acylethanolamines (NAEs) and oleamide, but no or very little AEA and no 2-AG [86]. These lipids could mimic cannabinoid ligands either directly by activating cannabinoid receptors or indirectly by increasing AEA levels [40]. These observations indicate that endocannabinoid analogs exist in plants and animals and, further, show the evolutionary conservation of the cannabinoid system in nature.
Fig. (2).
Molecular structure of endocannabinoids. These endocannabinoids share a polyunsaturated fatty acid moiety (arachidonic acid) and a polar head group consisting of ethanolamine or glycerol.
Endocannabinoids are present in peripheral and brain tissues and have recently been found in breast milk [86]. The expression of functional CB1 receptor in the preimplantation embryo and synthesis of AEA in the uterus of pregnant mice suggest that cannabinoid ligand–receptor signaling is operative in the regulation of preimplantation embryo development and implantation [227]. Since the information on noladin ether, virodhamine, and N-arachidonyldopamine is limited, the detailed discussion is limited to AEA and 2-AG.
Anandamide
AEA has been identified and quantitated throughout the human brain and periphery [95]. Signal transduction and ligand binding studies have suggested that it can act at both the CB1 (Ki 61nM), CB2 (Ki 1930nM) and also act as a full agonist at vanilloid TRPV1 receptors (for references see recent review [253]), although it may be more efficacious at CB1 [97]. AEA resembles Δ9 - THC in structural aspects [256, 262]. AEA behaves as an affinity-driven CB1 receptor agonist. Thus its efficacy at CB1 receptors, although higher than that of Δ9 - THC, is often found to be lower than that of other cannabinoid agonists [(+)-WIN55212-2 or CP-55940].
AEA was shown to be widely distributed in the brain and peripheral tissues [95, 96]. AEA levels vary by a factor of 4-6 within different regions of the rat brain, with the highest levels in the striatum and brainstem and the lowest levels in the cerebellum and cortex [28, 317]. AEA was found in regions of both the rat and human brain that contain a high density of CB1 receptors (e.g., hippocampus, cerebellum, and striatum) and in a region that is sparse in CB1 receptors, the thalamus [95]. In the rat, the concentration of AEA in the thalamus was approximately twice the concentration measured in the cerebellum. It is clear from these data that for AEA, the relative regional abundance in the brain does not correlate with the distribution of CB1 receptors. AEA levels in the brain are equivalent to those of other neurotransmitters such as dopamine and serotonin, but at least 10-fold lower than those reported for GABA and glutamate. AEA was also found in peripheral tissues such as human and rat spleen, which express high levels of CB2 receptors, suggesting that AEA is an agonist at both CB1 (Ki = 89± 10 nM) [246] and CB2 receptors (Ki = 371± 102 nM) [246]. Small amounts of AEA were also found in the human heart and thalamus and also in rat skin, whereas only trace quantities were detected in human serum, plasma, and cerebrospinal fluid [95].
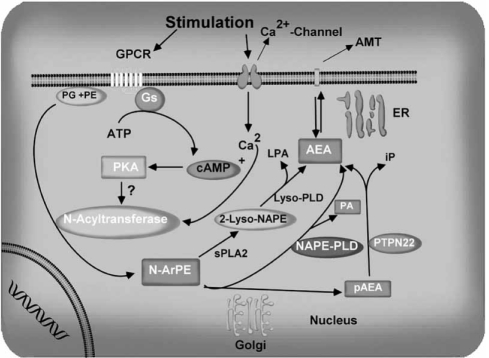
Enzymes responsible for the biosynthesis and degradation of AEA were characterized in various tissues. Unlike classical neurotransmitters and neuropeptides, AEA is not stored in intracellular compartments, but is produced on demand by receptor-stimulated cleavage of lipid precursors [17, 21, 22, 46, 84, 198] and released from neurons immediately afterwards [17, 21, 22, 84, 113, 198]. The postulated biosynthetic pathway for AEA involves the energy-independent condensation of ethanolamine and free arachidonic acid [75, 294]. The biochemical characteristics and inhibitor kinetics observed for the enzyme catalyzing this condensation reaction were essentially identical to those described for the enzyme catalyzing fatty acid amide (FAA) hydrolysis [fatty acid amide hydrolase (FAAH); see discussion below] [66, 75, 294]. Several lines of evidence derived from both physical and biological data argue that the production of AEA in vivo does not occur through the energy-independent condensation of free arachidonic acid and ethanolamine [84, 161, 173]. The AEA precursor is an N-arachidonyl phosphatidylethanolamine (N-ArPE), which is believed to originate from the transfer of arachidonic acid (AA) from the sn-1 position of 1,2-sn-di-arachidonylphos-phatidylcholine to phosphatidylethanolamine, catalyzed by a calcium-dependent N-acyltransferase (NAT) (Fig. 3). N-ArPE is then cleaved by a N-acyl phosphatidylethanolamine (NAPE)-specific phospholipase D (PLD) [84, 212, 260], which releases AEA and phosphatidic acid. It is not clear whether the NAT or the NAPE-PLD controls the rate-limiting step of AEA synthesis [81, 135, 273]. The NAT enzyme has been characterized biochemically in both brain and testis microsomal preparations [46, 84, 279, 280]. The NAT has only recently been purified, cloned, and characterized [220]. AEA biosynthesis was unaffected in NAPE-PLD knockout mice suggesting the involvement of other enzymes [179]. Another pathway which involves the conversion of NAPE into 2-lysol-NAPEs via the action of secretory PLA2 has also been reported. 2-Lysol-NAPEs are then converted into N-acylethanolamides, including AEA, via a selective lyso phospholipase D (lyso-PLD) [283]. A recent study proposed the existence in mouse brain and RAW264.7 macrophages of a parallel pathway through which AEA is generated from NAPE by a two-step process involving the PLC-catalyzed cleavage of NAPE to yield pAEA, which is subsequently dephosphorylated by protein tyrosine phosphatases (PTPN22) [182] (Fig. 3).
Fig. (3).
The potential pathways of anandamide biosynthesis. Stimulation of adenylate cyclase and cAMP-dependent protein kinase potentiate the Nacyltransferase (Ca2+-dependent transacylase, NAT). A fatty arachidonic acid chain is transferred by NAT from the sn-1 position of phospholipids to the primary amine of phosphatidylethanolamine, in a Ca2+-dependent manner, forming an N-arachidonyl phosphatidylethanolamine (N-ArPE). This N-ArPE intermediate is then hydrolyzed by a phospholipase D (PLD)-like enzyme to yield the anandamide (AEA). In addition, possible alternative pathways for AEA formation from N-ArPE are shown. Once synthesized, AEA can transported to the outside of the cell through a process that has not yet been well characterized. AMT, anandamide membrane transporter.
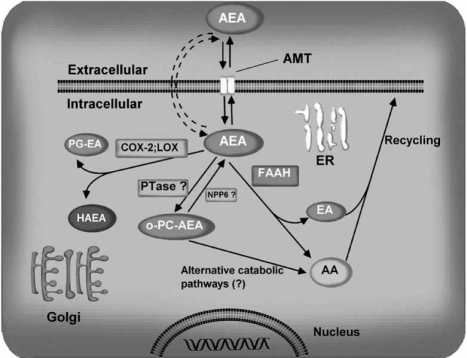
As a putative neuromodulator, AEA that is released into the synaptic cleft is expected to be rapidly inactivated. In general, two mechanisms are known that could remove endocannabinoids from the synaptic cleft to ensure rapid signal inactivation: re-uptake or enzymatic degradation. An enzyme that catalyzes hydrolysis of AEA, forming arachidonic acid and ethanolamine, was detected in rat brain tissue [75] (Fig. 4) (also in recent review [14]) soon after the discovery of AEA and is variously known as anandamide amidase, anandamide hydrolase, anandamide amidohydrolase, or fatty acid amide hydrolase (FAAH). In addition to hydrolysis by FAAH, AEA is metabolized by COX-2, LOX and cytochrome P450. COX-2 has been shown to metabolize AEA in to PGE2-ethanolamide (PGE2-EA) [254]. 12-and 15-LOX, nonheme iron-containing enzymes convert AEA into 12-and 15-hydroxy-AEA (12- and 15HAEA) in vitro, respectively [170, 184]. Cytochrome P450 also metabolizes AEA into several polar lipids [44]. Recently, in the absence of FAAH, exogenously injected AEA has been shown to be converted into o-phosphorylcholine (PC)-AEA in the brain and spinal cord. The choline-specific phosphodiesterase (NPP6) was found to convert PC-NAE into NAE [207] (Fig. 4). Further research is required to elucidate the exact mechanism and enzymes involved in this pathway of AEA metabolism.
Fig. (4).
Scheme illustrating the potential mechanism of anandamide uptake and degradation. Anandamide can be internalized by neurons through a yet unidentified transport mechanism, “the endocannabinoid transporter”. Once inside a neuron, AEA is rapidly cleaved by the hydrolytic enzyme fatty acid amide hydrolase (FAAH), releasing arachidonic acid (AA) and ethanolamine (EA). Alternatively, different lipoxygenases (LOXs) and cyclooxygenase-2 (COX-2) can metabolize AEA, generating hydroxyl derivatives of AEA (HAEAs) and prostaglandins-ethanolamides (PG-EAs), respectively. On the other hand, in FAAH (-/-) mice, AEA is converted into PC-AEA by an unidentified biosynthetic pathway, and PC-AEA can in turn be catabolized to AA by choline-specific phosphodiesterase (NPP6). AMT, anandamide membrane transporter.
The existence of a membrane transporter that mediates the uptake of AEA has also been investigated (see below). Thus, the ability of brain tissue to enzymatically synthesize and metabolize AEA, and the existence of AEA receptors and of carrier-mediated transport mechanisms essential for the termination of AEA signaling, suggest that AEA may represent a member of a new family of fatty acid-derived neuromodulators [6].
2-Arachidonylglycerol
Soon after the discovery of AEA, the existence of a second phospholipid-derived messenger, 2-arachidonylglycerol (2-AG), was shown [197, 278]. 2-AG has been characterized as a unique molecular species of monoacylglycerol isolated from both the canine gut [197] and the rat brain [281], where it presumably functions as an endogenous cannabinoid receptor ligand. According to its chemical structure, this new putative endocannabinoid is an arachidonyl ester rather than an amide (Fig. 2). 2-AG was found to bind both CB1 (Ki 2.4 μM) and CB2 receptors, although its CB1 receptor binding activity is 24-times less potent than that of AEA. 2-AG elicited the typical effects of Δ9 -THC, such as antinociception, immobility, immunomodulation, and inhibition of electrically evoked contractions of the mouse vas deferens [197, 275, 278]. However, further studies are necessary to determine the relative importance of 2-AG in the human body and brain. Information regarding tissue levels of 2-AG is still limited. Brain tissue concentrations of 2-AG are approximately 200-fold higher than those of AEA [28]. The distribution of the two endocannabinoids in the different brain regions is similar. The highest concentrations were found in the brainstem, medulla, limbic forebrain, striatum, and hippocampus and the lowest in the cortex, diencephalons, mesencephalon, hypothalamus, and cerebellum (for review see [273]). However, no correlation was found between 2-AG concentrations and CB1 receptor distribution. 2-AG was also detected in the peripheral nervous system, such as in the sciatic nerve, lumbar spinal cord, and lumbar dorsal root ganglion. 2-AG was also detected in the rat retina and bovine retina (for review see [273]).
Biosynthesis and Metabolism of 2-AG
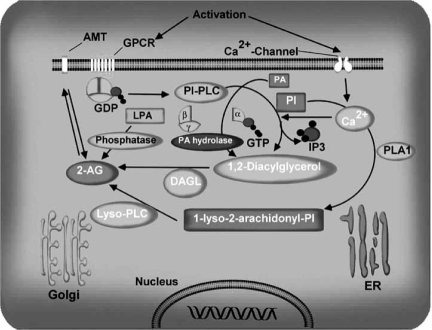
Generation of 2-AG was first described in ionomycinstimulated N18TG2 cells [33], in electrically stimulated rat hippocampal slices, and in ionomycin-stimulated neurons [270]. Rapid generation of 2-AG was also observed in rat brain homogenate during incubation with Ca[167] and in thrombin- or A23187- stimulated human umbilical vein endothelial cells [276]. Intraperitoneal injection of picrotoxin stimulated 2-AG levels in the rat brain [281]. Generation of 2-AG was also observed in cerebellar granular neurons in culture in response to chronic alcohol exposure [21]. Substantial amounts of this stimulus-induced 2-AG were released from the cells [21, 32, 276]. 2-AG biosynthesis occurs by two possible routes in neurons, which are illustrated in Fig. (5) and also in recent review [14]. Phospholipase C (PLC)-mediated hydrolysis of membranephospholipidsmay produce diacylglycerol (DAG), which may be subsequently converted to 2-AG by diacylglycerol lipase (DAGL) activity [240, 278]. The formation of DAG also involves the hydrolysis of phosphatidic acid through Mg and Cadependent PA phsophohydrolase activity [31, 48]. Alternatively, phospholipase A1 (PLA1) may generate a lysophospholipid, which may be hydrolyzed to 2-AG by lyso-PLC activity [278]. Various structurally distinct inhibitors of PLC and DAGL activities prevent 2-AG formation in cultures of cortical neurons, suggesting that the PLC/DAGL pathway may play a primary role 2-AG synthesis [270]. The molecular character of the enzymes involved remains to be identified, although the molecular cloning of DAGLα was reported recently [29]. Both DAGLα and β are associated with the cell membrane and are stimulated by Ca and blocked by DAGL inhibitors. DAGLα appears to be targeted to postsynaptic spines; it is highly enriched at the base of the spine neck and in the adjacent somatodendritic membrane but is excluded from the main body of the spines and the excitatory synapses in cerebellar Purkinje cells. In hippocampal pyramidal cells, DAGLα is distributed at the spine head or neck or at both structures [164, 318]. Under certain conditions, 2-AG can also be synthesized through the conversion of 2 arachidonyl lysophosphatidic acid (LPA) by phosphatase to yield 2AG [211]. Molecular characterization of these potential pathways remains to be accomplished.
Fig. (5).
The biosynthetic pathways of 2-arachidonylglycerol. Intracellular Ca2+ initiates 2-AG biosynthesis by inducing the formation of diacylglycerol (DAG) in the membrane either by stimulating the phosphatidyl-inositol-phospholipase C (PI-PLC) pathway, or the formation of phosphatidic acid (PA), and the subsequent activation of phosphatidic acid hydrolase. 2-AG is the product of DAG-lipase acting on DAG. The second pathway involves hydrolysis of PI by phospholipase A1 (PLA1) and hydrolysis of the resultant lyso-PI by a specific lyso-PLC. In certain conditions, 2-AG can also be synthesized through the conversion of 2-arachidonyl lysophosphatidic acid (LPA) by phosphatase to yield 2-AG. Once synthesized, 2-AG can transported to the outside of the cell through a process that has not yet been characterized. AMT, anandamide membrane transporter.
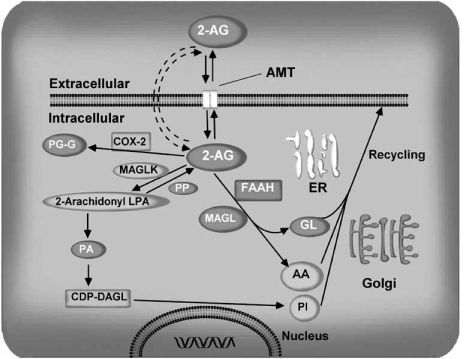
2-AG is inactivated by reuptake [25, 30] via a membrane transport molecule, the ‘AEA membrane transporter’ (AMT) (see below for details) [22, 25, 26, 111, 141, 142, 184], and subsequent intracellular enzymatic degradation [68, 76, 84] by monoacylglycerol (MAGL) lipase (Fig. 6), like other monoacylglycerols [168]. Similarly, 2-AG is metabolized by MAGL lipase from porcine brain cytosol and particulate fractions [125]. FAAH has also been shown to metabolize 2-AG [82, 125]. 2-AG may be degraded by FAAH in addition to monoacylglycerol lipase under some circumstances (Fig. 6). Recently, the gene encoding MAGL lipase was cloned from both mouse adipose tissue and the rat brain [160]. Rat MAGL lipase contains 303 amino acids and is expressed in various regions of the rat brain; the highest levels of expression are in regions where the CB1 receptor is abundant, such as the hippocampus, cortex, and cerebellum. Interestingly, MAGL is expressed in presynaptic terminals [90, 129], suggesting it has a role in terminating retrograde signaling at presynaptic neurons [271]. 2-AG is metabolized to 2-arachidonyl LPA through the action of monoacyl glycerol kinase(s). 2-Arachidonyl LPA is then converted into 1-steroyl-2arachidonyl PA [265]. 1-steroyl-2-arachidonyl PA is further utilized in the “PI cycle” or is used in the de novo synthesis of PC and PE. Furthermore, 2-AG is metabolized by enzymatic oxygenation of 2AG by COX-2 into PGH2 glycerol esters. The biological activity and the role of oxygenated 2-AG are yet to be determined.
Fig. (6).
Schematic summary of 2-AG uptake and degradation. 2-arachidonylglycerol can be internalized by neurons through a yet unidentified transport mechanism, “the endocannabinoid transporter”. Once inside neurons, 2-AG can be hydrolyzed by hydrolytic enzymes, fatty acid amide hydrolase (FAAH) or monoacylglycerol (MAGL) lipase. Alternatively, 2-AG can be metabolized to 2-arachidonyl LPA (2-AG-LPA) through the action of monoacyl glycerol kinase(s) (MAGLK). 2-AG-LPA is then converted into 1-steroyl-2-arachidonyl PA. 1-steroyl-2-arachidonyl PA is further utilized in the de novo synthesis of PC and PE. Furthermore, 2AG is metabolized by enzymatic oxygenation of 2-AG by COX-2 into PGH2 glycerol esters (PG-G). AMT, anandamide membrane transporter.
Endocannabinoid Uptake
AEA appears to be inactivated by a two-step process involving the transport of this lipid into cells [26] (Figs. 4 and 6) followed by intracellular hydrolysis by the integral membrane enzyme FAAH. However, with respect to the inactivation of AEA, only FAAH has been molecularly characterized and structurally studied. Indeed, the actual mechanism of AEA uptake or, more generally, the movement of this fatty acid amide not only through biological membranes but also through aqueous media (extracellular and cytoplasmic) remains an enigmatic and controversial subject. Some authors have suggested that the uptake is due to a facilitated diffusion process [22, 25, 26, 141], whereas others have described it as a passive process driven by the AEA-metabolizing enzyme FAAH [68, 76]. For a current discussion of these two points of view, see the recent articles [114] and [143].
Physiological Roles of Endocannabinoids in the CNS
The discovery of endocannabinoids such as AEA and 2-AG and the widespread localization of CB1 receptors in the brain have stimulated considerable excitement over the previously unrecognized cannabinoid system and questions about the function of this ubiquitous network in the nervous system. There is now overwhelming evidence that AEA and 2-AG interact with CB1 receptors and share some of the biological properties of other cannabinoids, but with significant differences. These significant differential effects involve other non-CB1 receptors and/or postulated CB3 receptors as described. In recent years, the functions of endocannabinoids at the synaptic and network levels have been elucidated. Therefore, in the following, an attempt has been made to provide a comprehensive review of the many pathophysiological roles of endocannabinoids in the CNS, including in alcohol addiction.
ENDOCANNABINOIDS AND SYNAPTIC PLASTICITY
Regulation of Short-Term Synaptic Plasticity
Changes in the strength and number of synaptic connections between neurons are believed to be one of the major mechanisms underlying learning and memory and mediating other physiological functions of the CNS. This phenomenon is called synaptic plasticity. When neuronal cells (postsynaptic neurons and possibly presynaptic terminals as well) are activated, they produce endocannabinoids, which travel in a retrograde direction and transiently (<1 min) suppress presynaptic neurotransmitter release by activating
CB1 receptor-mediated inhibition of voltage-gated Cachannels. Such a negative feedback mechanism should be effective in calming stimulated neurons after excitation.
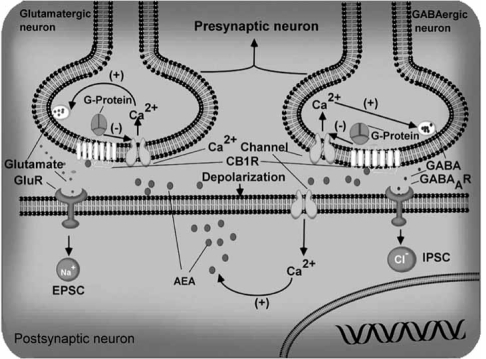
It was reported that brief activation of CA1 pyramidal cells in the hippocampus [4, 5, 218, 235-237] or Purkinje cells in the cerebellum [183, 303-305] (Fig. 7) caused a reduction in the amplitude of GABAergic inhibitory postsynaptic currents (IPSCs). This phenomenon, called depolarization-induced suppression of inhibition (DSI), is initiated postsynaptically by the voltage-dependent influx 2+ of Ca2+into the soma and dendrites of the neuron, but is expressed presynaptically through inhibition of transmitter release from axon terminals of GABA interneurons. This suggests that a chemical messenger generated during depolarization of the pyramidal neurons must travel backwards across the synapse to induce DSI. DSI has been observed in both excitatory and inhibitory neurons in hippocampal cell culture [217], in CA3 pyramidal cells [203], in dentate gyrus granule cells [4], and in neocortical pyramidal cells [320].
Fig. (7).
Schematic diagram to illustrate the role of AEA and CB1 receptors on excitatory and inhibitory neurotransmission. Depolarization of a postsynaptic neuron leads to Ca2+ influx through voltage-gated channels and causes the generation and the release of endocannabinoids such as anandamide (AEA). The mechanism through which Ca2+ promotes endocannabinoid release is not yet known. The released endocannabinoids then activate the CB1 receptors (CB1R) at presynaptic terminals and suppress the release of glutamate (left) or GABA (right) by inhibiting Ca2+ channels [146, 151, 264]. GluR, Glutamate receptors.
The retrograde messenger in DSI remained unknown until recent investigations by Wilson and Nicoll [311-313] and by Ohno-Shosaku et al. [216] indicated that in hippocampal cells the messenger was likely to be an endocannabinoid. Shortly thereafter, cerebellar DSI was also reported to be mediated by an endocannabinoid [89, 171, 319]. Furthermore, it was reported that CB1 receptor agonists selectively reduced IPSCs in both the hippocampus [131, 146, 163] and cerebellum [288]. There is strong evidence that this retrograde signaling process involves an endocannabinoid. (a) CB1 receptor antagonists selectively blocked DSI whereas agonists enhanced it [216, 312]. (b) DSI is absent in CB1 receptor knockout mice [311, 319]. (c) The GABA interneurons that are implicated in DSI express high levels of CB1 receptors, which are localized to their axon terminals [163]. (d) Neuronal activity and Ca entry stimulate the synthesis of 2-AG in hippocampal neurons and AEA and 2-AG in other neuronal cells [17, 21, 22]. Recently, DSI mediated by 2-AG was shown in the mouse substantia nigra pars reticulate and rat cerebellum [286]. It remains to be established that endocannabinoid-mediated DSI is present in other brain regions such as the ventromedial medulla [302], amygdala [163], and striatum [284], in which exogenously applied CB1 receptor agonists are known to suppress IPSCs. These reports convincingly established that endocannabinoids are important mediators of short-term plasticity.
Metabotropic glutamate receptors (mGluRs) are G-proteincoupled receptors distributed throughout the CNS that modulate multiple CNS functions, including neuronal excitability [7, 63] and neurotransmitter release [49]. Recent data from several investigators have begun to uncover an entirely novel signaling mechanism for mGluRs, namely the production and subsequent release of endocannabinoids. Some of the effects (short-and long-term forms of synaptic plasticity) previously attributed directly to mGluR activation are in fact indirectly mediated by signaling through the endocannabinoid system [91]. Recent studies suggest that mGluR/endocannabinoid signaling is a widespread feature of neuronal circuitry [91], given the widespread expression of postsynaptic group I mGluRs throughout the CNS and a similar extensive expression of CB1 receptors. Activation of group I mGluRs can cause the release of endocannabinoids in the cerebellum [186] and hippocampus [300]. Synaptic depression in the cerebellum has been observed at glutamatergic synapses. A similar mechanism operates in the hippocampus. As in the cerebellum, activation of postsynaptic group I mGluRs depresses neurotransmitter release through endocannabinoid-mediated retrograde activation of presynaptic CB1 receptors (Fig. 7). In contrast to the cerebellum, however, synaptic depression in the hippocampus has been observed at GABAergic rather than at glutamatergic synapses.
The neurons in the hippocampus and cerebellum use endocannabinoids to carry out a signaling process that is analogous in mechanism but opposite in sign to DSI, called depolarization-induced suppression of excitation (DSE). Like DSI, DSE is induced by neuronal depolarization; it consists of a transient depression in neurotransmitter release, and it requires a retrograde endocannabinoid messenger. But unlike DSI, DSE targets glutamatergic rather than GABA axon terminals and therefore it reduces the excitatory input to the affected cell [4, 232]. DSE is mimicked and blocked by agonists and antagonists of CB1 receptors respectively [172, 186] and it is absent in the CB1 receptor knockout mouse [219]. CB1 receptor agonists suppress EPSCs in other areas of the brain, evidently through presynaptic actions. For instance, similar DSE was reported in the ventral tegamental area (VTA) as a Ca-dependent phenomenon, blocked by both AM281 and rimonabant, and enhanced by WIN55212-2 [201]. Importantly, DSE was partially blocked by the D2 DA antagonist eticlopride and enhanced by the D2 DA agonist quinpirole without changing the presynaptic cannabinoid activity [201]. These observations indicate that activation of D2 DA receptors in the VTA significantly enhances the depolarization-induced release of endocannabinoids, which are responsible for the inhibition of glutamate transmission in the VTA [201]. The synchronous release of mEPSCs in Sr-substituted extracellular solution was found to be reduced by endocannabinoids in the prefrontal cortex and striatum [10, 107]. Recently, it was shown that 2AG is the retrograde messenger for train-induced suppression of excitation at the VTA-DA synapses [200]. It remains to be demonstrated whether or not DSE is present in the striatum [107], substantia nigra [287], periaqueductal gray [301], and spinal cord [204].
Several studies addressed the issue of how endocannabinoids affect transmitter release from presynaptic neurons [4]. It is clearly established that the βγ-subunits of heterotrimeric G-proteins directly interact with and inhibit the high-voltage-activated Cachannels that mediate transmitter release at most synapses (Fig. 7). CB1 receptors are pertussis-sensitive G-protein-coupled receptors, and their activation reduces N-type Cacurrent [50, 51, 185, 226, 292]. In some cells, it appears that the CB1 receptor could reduce GABA release from at least some nerve terminals through a mechanism that is independent of N-P/Q-type Ca channels [107, 288, 300, 301], perhaps by direct action on the transmitter release machinery.
Regulation of Long-Term Synaptic Plasticity
As in endocannabinoid-mediated short-term plasticity, all the studies to date suggest that endocannabinoid-mediated long-term plasticity takes the form of depression of neurotransmission. It was observed that striatal long-term depression (LTD) was absent in CB1 receptor knockout mice, reduced or eliminated by rimonabant, and enhanced by HU-210 in the striatum. These data suggested that striatal LTD was mediated by an endocannabinoid [109]. As in the striatum, LTD in the nucleus accumbens (NAc) is prevented by CB1 receptor antagonists, enhanced by CB1 receptor agonists, and absent in CB1 receptor knockout mice [249]. Importantly, once NAc LTD was induced, the antagonist did not affect it, demonstrating that the LTD was not maintained by a continual release of endocannabinoids, but rather represented a persistent effect of transient CB1 receptor activation. The endocannabinoid that mediated LTD was evidently released as a retrograde messenger, because LTD was prevented by chelating postsynaptic Ca2+(with 20mM BAPTA) in the recorded cell [249]. CB1 knockout mice showed strongly impaired short-term and long-term extinction in auditory fear-conditioning tests, with unaffected memory acquisition and consolidation. Treatment of wild-type mice with rimonabant mimicked the phenotype of CB1 knockout mice, suggesting that CB1 is required at the moment of memory extinction [190]. Although, the mechanism needs to be elucidated, these studies suggest that endocannabinoid actions are important for the induction, but not the expression, phase of LTD. Activity-dependent modifications of synaptic strength in the dorsal striatum [158, 233] and NAc [213, 251, 290] are thought to underlie neuronal adaptations in the animal behaviors mediated by these structures. CB1 receptors are present on glutamatergic terminals in the prefrontal cortex [138], and activation of the CB1 receptor by agonists suppresses glutamate EPSCs in layer V slices of rat cortex, evidently by acting at a presynaptic site [10]; it remains to be determined whether CB1 receptors mediate LTD in the cortex as well.
Long-term potentiation (LTP) and LTD of CA3-CA1 synaptic transmission are two in vitro models for learning and memory. It has been shown that CB1 receptor activation inhibits both LTP and LTD induction in the hippocampus [270, 282]. Elucidation of the mechanism(s) by which cannabinoids inhibit LTP and LTD may provide clues to the cellular and molecular processes underlying some of the cannabinoid-induced learning and memory impairments. Another important finding is the enhanced LTP found in CB1 receptor knockout mice [34]. These data could not be mimicked by application of CB1 receptor antagonists to wild-type mice [191], suggesting some other factor is involved in the enhancement of LTP. Compensatory up-regulation of other genes in the CB1 receptor knockout mice or altered neurophysiological responses might be responsible. It has been shown that synaptic-driven release of endocannabinoids within the dendritic compartment of CA1 pyramidal cells in a highly localized and efficient process was responsible for facilitating subsequent induction of LTP at nearby excitatory synapses [55, 56]. Recently, it was shown that endocannabinoid-mediated LTD contributes to experience-induced changes in psychomotor behaviors by affecting the information flow in the dorsal striatum and NAc [108]. These early investigations are just beginning to address the effects of endocannabinoids on the neurophysiology of the brain, and further studies are necessary before the roles of endocannabinoids in short-and long-term plasticity are fully elucidated.
The Endocannabinoid Signaling System and Diseases of the CNS
There is mounting evidence that the endocannabinoid signaling system modulates the activity of most neurotransmitters in the CNS. The neurophysiological consequences of the activation of CB1 receptors depend on the localization of these receptors in various brain regions and the excitatory or inhibitory pathways being stimulated. Hence, the clinical potential of cannabinoid drugs in neurological disorders is vast. Reduced levels of endocannabinoids, CB1 receptors, and CB1 receptor mRNA have been reported in Huntington’s disease [71, 117, 119, 176, 177]. While the mechanism and the significance of the loss of endocannabinoid function is not clear at present, these observations may indicate that the endocannabinoid signaling system has a central role in the progression of neurodegeneration in Huntington’s disease, and that cannabinoid agonists could be of significant therapeutic benefit in Huntington’s disease because of their anthyperkinetic and neuroprotective effects [176]. A recent study showed a loss of CB1 receptors in progenitor cells in the adult human brain subependymal layer in Huntington’s disease and suggested the possibility that these cells could be a suitable endogenous source for the replacement of cells lost due to neurodegenerative disease [67].
Increased cannabinoid tone in the globus pallidus has been reported to be responsible for the production of Parkinsonian symptomology [188]. A recent study demonstrated increased 2-AG in the globus pallidus of rats treated with resperpine, which is a rodent model of Parkinson’s disease (PD) [85]. Endocannabinoid signaling was shown to be involved in the pathophysiology of parkinsonism and LID in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)lesioned, non-human primate models of Parkinson’s disease [296]. The deficiency in endocannabinoid transmission may contribute to levodopa-induced dyskinesias; these complications may be alleviated by activation of CB1 receptors [98]. Increased levels of AEA were reported in rat models of PD [85, 128]. Recently, it was found that cannabinoid CB1 receptor binding and the activation of G proteins by cannabinoid agonists were significantly increased in the postmortem basal ganglia of humans affected by PD [175]. The increase in CB1 receptors was also seen in MPTP-treated marmosets, a primate PD model [175]. A recent study found high levels of endocannabinoids in the cerebrospinal fluid of untreated PD patients [234]. Low doses of rimonabant partially attenuated the hypokinesia shown by a rat model of Parkinson’s disease [123]. Further studies to understand the functional interaction between dopamine and the endocannabinoid system should bring new perspectives on the treatment of PD.
Several lines of evidence suggest a role for endocannabinoid signaling in schizophrenia [115]. The highest densities of CB1 receptors are found in regions of the human brain implicated in schizophrenia, including the prefrontal cortex, basal ganglia, hippocampus, and the anterior cingulate cortex [115]. Increased binding of [H]CP-55,940 to CB1 receptors in the dorsolateral prefrontal cortex of schizophrenia patients compared to controls was shown [70]. In addition, Leweke et al. [180] reported significant twofold elevations of AEA levels in the cerebrospinal fluid (CSF) of patients with schizophrenia compared to age-matched controls. Finally, a recent study also indicates that rimonabant reverses ketamine-induced impairment in prepulse inhibition of the acoustic startle reflex, an animal model of the deficient sensorimotor gating observed in schizophrenia [209]. It was recently found that CSF AEA levels are eightfold higher in antipsychotic-naive first-episode paranoid schizophrenics than in healthy controls, dementia patients or affective disorder patients. This alteration is absent in schizophrenics treated with ‘typical’ antipsychotics, which antagonize dopamine D2-like receptors, but not in those treated with ‘atypical’ antipsychotics, which preferentially antagonize 5HT(2A) receptors [112]. Recent data suggest that dysregulated striatal endocannabinoid neurotransmission is associated with a hyperdopaminergic state in dopamine transporter knockout mice [293]. AEA release in the dorsal striatum is stimulated by activation of D2 dopamine receptors [98]. The amounts of AEA were significantly increased in the blood of patients with acute schizophrenia than in healthy volunteers [69].
Several studies have demonstrated the ability of cannabinoids to provide neuroprotection against β-amyloid peptide (Aβ; a key pathological marker of Alzheimer disease) toxicity [156, 202, 243]. Stereotaxic injection of Aβ into the rat cortex, caused a neuronal damage in the hippocampus and increased 2-AG, but not AEA levels. Further, inhibition of endocannabinoid cellular reuptake concomitantly reversed hippocampal damage in rats, and loss of memory retention in the passive avoidance test in mice, but only when administered from the 3rd day after Aβ injection [297]. These observations suggest that pharmacological enhancement of brain endocannabinoid levels through the inhibition of endocannabinoid metabolism or uptake inhibitors might have a therapeutic value in the protection against Aβ-induced neurodegeneration [297]. Blockade of CB1 receptors by rimonabant lessens the amnesia induced by a β-amyloid fragment in mice, suggesting that the endocannabinoid system may be involved in cognitive impairment in Alzheimer’s disease [195]. A recent study provides evidence that Δ9-THC inhibits the enzyme acetylcholinesterase (AchE) as well as prevents AchE-induced Aβ aggregation. Δ9 -THC binds in the peripheral anionic site of AchE, the critical region involved in amyloidgenesis [93].
The compounds such as AEA and and other NAEs present in chocolate [88] may function as a “cannabinoid mimics” [87] in the purported rewarding properties of cocoa [86] suggesting that the endocannabinoid system participate in the control of food intake. Transient inhibition of food intake and reduction in fat mass were observed following treatment of mice and rats with rimonabant [244]. CB1 receptor knockout mice on a high-fat diet were shown to have a lower susceptibility to obesity [245]. To date, data obtained from clinical trials (RIO North America, RIO Europe and RIO Lipid) indicate that rimonabant may have clinical benefits in relation to its anti-obesity properties and as a novel candidate for the treatment of metabolic and cardiovascular disorders associated with overweight and obesity [166, 291]. These studies also suggested that the drug has a reasonable safety profile. Treatment with rimonabant is also associated with favorable changes in serum lipid levels and an improvement in glycemic control in type 2 diabetic patients [105]. Following 1 year of treatment, rimonabant 20 mg/day produced significant increases in the number cigarette smokers who quit smoking compared with a placebo [259]. In another recent clinical study, orlistat, which specifically inhibit the critical enzymes (PLC and DAGL) involved in the biosynthetic pathway of 2-AG [286], reduced weight by 2·7 kg on average and decreased the incidence of type 2 diabetes from 9·0% to 6·2% [225]. In the same study, rimonabant significantly reduced weight by 4·6 kg (95% CI 4·3–5·0), reduced waist circumference, and improved triglyceride and HDL cholesterol profiles [225]. These observations suggest that the CB1 receptor may have a role in both obesity and cessation of smoking. Psychopathological disorders and depression in particular are strongly linked to eating attitude in obese patients. The identification of cannabinoid CB1 receptors (CB1Rs) in areas of the CNS that have been implicated in regulation of mood and food intake suggests that these receptors may mediate such a behavioral link.
In recent years, experimental data overwhelmingly suggested that the endocannabinoid system is a major player among the neurotransmitter systems involved in the regulation of different alcohol-related phenomena, including tolerance, vulnerability, reinforcement, and consumption (for details, see the recent reviews [12, 13, 15]). It was shown that AEA and 2-AG synthesis is increased by chronic alcohol exposure. Chronic alcohol treatment led to a significant increase in the brain levels of AEA and a significant reduction in N-ArPE, an immediate precursor for AEA synthesis [154]. Chronic alcohol exposure of rats caused a decrease in the content of both AEA and 2-AG in the midbrain, while AEA content increased in the limbic forebrain, a key area for the reinforcing properties of habit-forming drugs, including alcohol [122]. It was observed that, in the limbic forebrain, 2-AG content was decreased after 48 h of alcohol deprivation. There was a further decrease in 2AG content when rats were allowed to relapse to alcohol consumption [124]. These observations indicate the involvement of the endocannabinoids in alcohol-induced neuroadaptive changes in the brain and that activation of endocannabinoid-mediated neurotransmission may be responsible for the activation of the reward system by alcohol. It was found that chronic exposure to alcohol leads to an increase in extracellular AEA by inhibiting the uptake of AEA. This effect was independent of the CB1 receptor, since CB1 receptor knockout mice have normal uptake activity [22]. After prolonged exposure to alcohol, cells become tolerant to these effect such that AEA uptake is no longer inhibited by acute alcohol [22]. Chronic alcohol did not show any direct inhibition of FAAH activity in these neurons. These data suggest that alcohol-induced inhibition of AEA uptake may in part be responsible for the alcohol-induced increase in extracellular AEA.
Evidence demonstrating the down-regulation of CB1 receptor numbers and function in chronic alcohol-exposed mouse brain has recently been reported. The results of this study indicate that chronic alcohol exposure decreases the number of CB1 receptors [16]. These observations are consistent with another study which found that forced consumption of high levels of alcohol significantly decreased CB1 receptor gene expression in the caudate putamen (Cpu), ventromedial nucleus of the hypothalamus (VMN), and the CA1 and CA2 fields of the hippocampus [224]. Studies to examine if the chronic alcohol-mediated down-regulation of brain CB1 receptors has any functional effect on CB1 receptor-activated G-proteins revealed that the net binding of [S] GTPγS was reduced significantly without any significant changes in the G-protein affinity in chronic alcohol-exposed mice [18]. These observations support the participation of the endocannabinoid signaling mechanism in mediating some of the pharmacological and behavioral effects of alcohol, and hence the CB1 receptor may constitute an important target for therapeutic intervention in alcohol-related behaviors.
The dopaminergic system that projects from the VTA of the midbrain to the NAc, and to other forebrain sites, including the dorsal striatum, is the major substrate of reward and reinforcement produced by most drugs of abuse, including alcohol [80, 250, 314, 315]. It is well established that cannabinoids activate dopaminergic neurons in the VTA [80, 110, 250, 289, 314, 315], resulting in the release of DA in the NAc [285]. Moreover, the acute alcohol-induced release of DA in the NAc is in fact mediated by CB1 receptors [155]. The acute alcohol-induced increase in DA in NAc dialysates in C57BL/6 mice was completely inhibited by pretreatment with rimonabant or in CB1 receptor knockout mice [155]. These data suggest that activation of the limbic DA system is important for the reinforcing effects of alcohol and further suggest an interaction between the cannabinoidergic and dopaminergic systems in the reinforcing properties of alcohol abuse.
Studies from various laboratories have shown the inhibition of voluntary alcohol intake by rimonabant in rodents. Rimonabant has been shown to decrease voluntary alcohol intake in alcohol-preferring C57BL/6 mice [9], in Sardinian alcohol-preferring (sP) rats [61], in alcohol self-administering Long Evans rats [99], and in alcohol-preferring congenic B6.Cb4i5-β/13C/Vad and B6.Cb4i5β14/Vad mouse strains [154]. The acute administration of rimonabant suppressed alcohol self-administration in chronic alcohol-exposed Wistar rats [252]. A significant increase in alcohol preference (free-choice) was observed when Wistar rats were treated with an acute dose of rimonabant during chronic alcohol treatment [174]. The administration of rimonabant after chronic alcoholization significantly decreased the preference for alcohol. Alcohol withdrawal symptoms were also decreased by administration of rimonabant in these studies. Furthermore, acute administration of the CB1 receptor agonist CP55,940 increased the motivation to consume alcohol in Wistar rats, and this effect was completely prevented by pretreatment with rimonabant [100, 101]. An acute dose of rimonabant completely abolished the alcohol deprivation effect (i.e., the temporary increase in alcohol intake after a period of alcohol withdrawal) in sP rats [263]. Acute administration of CP55,940 or another CB1 receptor agonist, WIN 55,212-2, significantly stimulated voluntary alcohol consumption in alcohol-preferring sP rats and this was completely prevented by rimonabant [62]. The existing evidence taken together suggests that activation of the CB1 receptor by a CB1 receptor agonist may involve the release of DA in the NAc and inactivation of the CB1 receptor by rimonabant may involve the inhibitionofDArelease. This in turn may regulate voluntary alcohol intake in these animals.
The first biochemical evidence to show the participation of the cannabinoidergic system in alcohol-drinking behavior is derived from the observed differences in CB1 receptor function in two genetic strains of alcohol-preferring C57BL/6 and alcohol-avoiding DBA/2 mice. In this study, it was found that C57BL/6 mice have a significantly lower level of CB1 receptor binding sites and higher affinity for [H]CP-55,940 than DBA/2 mice [153]. Interestingly, the significantly higher levels of CB1 receptors found in DBA/2 mice are less coupled to G-proteins (as shown by a GTPγS binding assay) compared to those of C57BL/6 mouse strains [19], suggesting the involvement of these receptors in controlling voluntary alcohol consumption. Another study reported lower regional levels of CB1 receptor function and lower CB1 receptor gene expression in the brain of Fawn Hooded rats (alcohol-preferring) compared to Wistar rats (alcohol-nonpreferring) [223]. These results further strengthen the hypothesis that the inactivation or down-regulation of CB1 receptors blocks voluntary alcohol consumption. It is therefore quite possible that the animals with a genetic predisposition to high alcohol consumption have inherited a greater sensitivity of the endogenous cannabinoidergic system to alcohol. Several investigators using genetically modified CB1 receptor knockout mice further examined this notion. CB1 receptor knockout mice exhibited dramatically reduced voluntary alcohol consumption [155]. There was also a remarkable gender difference, with female wild-type mice consuming significantly more alcohol than male wild-type mice, whereas this gender difference was nonexistent in male and female CB1 knockout mice [155]. It was shown that young wild-type mice exhibited a significantly higher alcohol preference and voluntary alcohol intake compared to their CB1 knockout littermates [308]. Furthermore, rimonabant has been shown to reduce voluntary alcohol intake in wild-type but not in CB1 receptor knockout mice [308]. Similarly, administration of rimonabant significantly reduced alcohol and sucrose intake in C57BL/6x129/Ola mice and had no effect in CB1 receptor knockout C57BL/6x129/Ola mice [238]. Alcohol and sucrose intake were significantly reduced in CB1 receptor knockout C57BL/6x129/Ola mice [238]. The age-dependent decline in the appetite for both alcohol and food was shown using rimonabant and CB1 receptor knockout mice [308].
Another study not only provides evidence for the participation of the CB1 receptor in the regulation of voluntary alcohol consumption, but also for some of the acute intoxicating effects caused by administration of alcohol [210]. Alcohol consumption and preference are decreased, whereas alcohol sensitivity and withdrawal severity are increased, in CB1 receptor knockout mice [210]. CB1 receptor knockout mice showed an increase in alcohol withdrawal-induced convulsions, suggesting that alcohol consumption is also inversely related to alcohol withdrawal severity. In another study, CB1 knockout mice (CD1 background) were more sensitive to the hypothermic and sedative/hypnotic effects of alcohol than wild-type mice [210]. CB1 knockout mice displayed a significant decrease in locomotor activity following injection of alcohol (1-2.5g/kg) [210]. Alcohol self-adminis-trations and alcohol withdrawal severity have been shown to have an inverse genetic relationship [64]. Rodents selectively bred for low alcohol intake showed greater alcohol withdrawal severity than did rodents selectively bred for high alcohol intake [53, 54, 65, 169]. Importantly, analysis of recombinant inbred strains for alcohol withdrawal severity led to identification of a quantitative trait locus on chromosome 4 in close proximity to the CB1 receptor gene [41, 42]. Furthermore, CB1 receptor expression has been shown to be increased in the rat medial frontal cortex after intermittent exposure to alcohol [248]. However, chronic alcohol exposure has been shown to reduce CB1 receptor gene expression in the rat caudate-putamen, ventromedial nucleus of the hypothalamus, and the CA1 and CA2 fields of the hippocampus; conversely, chronic alcohol exposure increases CB1 receptor gene expression in the dentate gyrus [224]. Alcohol withdrawal symptoms observed in CB1 receptor wild-type mice were abolished in CB1 receptor knockout mice [242]. A decreased expression and activity of FAAH was found in the prefrontal cortex (PFC) of alcohol-preferring rats with a compensatory down-regulation of CB1 signaling [136]. Furthermore, intra-PFC injections of the FAAH inhibitor (URB597) increased alcohol self-administration in Wistar rats [136]. Recently, increased alcohol consumption and preference and decreased alcohol sensitivity were observed only in female mice but not in male FAAH knockout mice [24]. These results suggest that impaired FAAH function may present a phenotype of high voluntary alcohol consumption, and identify FAAH both as a regulator of endocannabinoid function and a possible therapeutic target for alcohol-related disorders. These data collectively indicate that the endocannabinoid system could be important for alcohol’s reinforcing effects. These findings are significant for the development of potential therapeutic strategies for the treatment of alcoholism and addiction in general.
Therapeutic Opportunity
Even though the detailed pathophysiology of the endocannabinoid system is not yet fully understood, there is already overwhelming evidence indicating that a pharmacological modulation of the endogenous cannabinoid system could provide new tools for a number of disease states, including drug addiction. In terms of drug development, the CB1 receptor antagonist rimonabant (Fig. 8) has progressed furthest and is in late phase III trials for the treatment of obesity and as an aid for smoking cessation [60, 298]. An NIAAA clinical study of the efficacy of rimonabant to reduce voluntary alcohol drinking is in phase I trials. Pending the results of the clinical trials, rimonabant could become an important addition to the limited arsenal of effective treatments for alcoholism. During disease or drug abuse, including alcohol abuse, there are changes in endocannabinoid levels in various regions of the brain [11, 20, 258, 307]. Therefore, drugs or agents which regulate the level of endocannabinoids by inhibiting their metabolism (FAAH inhibitors such as URB597) or uptake (AM404) or synthesis (orlistat) could locally target sites while limiting the effects on uninvolved cognitive areas, and would thus be expected to have a higher therapeutic value [24, 136]. Recent evidence suggests that the blockade of CB1 receptors with rimonabant might be beneficial to alleviate motor inhibition typical of Parkinson’s disease (PD) [123]. In addition to rimonabant, several specific endocannabinoid transport inhibitors, FAAH and MAGL inhibitors which regulate brain endocannabinoid levels might have a therapeutic value in the protection against Aβ-induced neurodegeneration [297] and memory deficit in rodents [194]. Endocannabinoid interactions with the dopamine system have been offered as a possible mechanism for some of the therapeutic potential of cannabinoid-based drugs in alcoholism. A recent study provides evidence of the ability of rimonabant to mitigate alcohol-withdrawal symptoms and block the formation of physical dependency by inhibiting alcohol intake. Recent data on the role of CB1 receptors in alcohol drinking behavior, including alcohol tolerance, as discussed above collectively suggest that agents such as CB1 receptor antagonists, including rimonabant, will be promising therapeutic agents for the treatment of alcohol abuse.
Fig. (8).
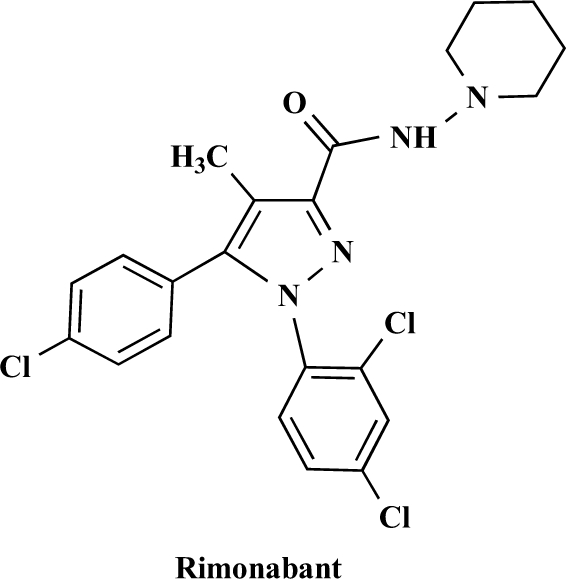
Molecular structure of CB1 receptor-selective antagonist/inverse agonist, rimonabant. Rimonabant (SR141716A) is highly potent and selective CB1 receptor ligand that readily prevents and reverses CB1 mediated effects both in vitro and in vivo [148].
ACKNOWLEDGEMENTS
The author acknowledges support in part by grants from the National Institutes of Health (NIH AA11031) and the New York State Psychiatric Institute.
REFERENCES
- 1.Abood ME, Ditto KE, Noel MA, Showalter VM, Tao Q. Isolation and expression of a mouse CB1 cannabinoid receptor gene. Comparison of binding properties with those of native CB1 receptors in mouse brain and N18TG2 neuroblastoma cells. Biochem Pharmacol. 1997;53:207–214. doi: 10.1016/s0006-2952(96)00727-7. [DOI] [PubMed] [Google Scholar]
- 2.Akinshola BE, Chakrabarti A, Onaivi ES. In-vitro and in-vivo action of cannabinoids. Neurochem Res. 1999;24:1233–1240. doi: 10.1023/a:1020968922151. [DOI] [PubMed] [Google Scholar]
- 3.Akinshola BE, Taylor RE, Ogunseitan AB, Onaivi ES. Anandamide inhibition of recombinant AMPA receptor subunits in Xenopus oocytes is increased by forskolin and 8-bromo-cyclic AMP. Naunyn Schmiedebergs Arch Pharmacol. 1999;360:242–248. doi: 10.1007/s002109900078. [DOI] [PubMed] [Google Scholar]
- 4.Alger BE. Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol. 2002;68:247–286. doi: 10.1016/s0301-0082(02)00080-1. [DOI] [PubMed] [Google Scholar]
- 5.Alger BE, Pitler TA, Wagner JJ, Martin LA, Morishita W, Kirov SA, Lenz RA. Retrograde signalling in depolarization-induced suppression of inhibition in rat hippocampal CA1 cells. J Physiol. 1996;496:197–209. doi: 10.1113/jphysiol.1996.sp021677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ameri A. The effects of cannabinoids on the brain. Prog Neurobiol. 1999;58:315–348. doi: 10.1016/s0301-0082(98)00087-2. [DOI] [PubMed] [Google Scholar]
- 7.Anwyl R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Brain Res Rev. 1999;29:83–120. doi: 10.1016/s0165-0173(98)00050-2. [DOI] [PubMed] [Google Scholar]
- 8.Arnold JC, Topple AN, Mallet PE, Hunt GE, McGregor IS. The distribution of cannabinoid-induced Fos expression in rat brain: differences between the Lewis and Wistar strain. Brain Res. 2001;921:240–255. doi: 10.1016/s0006-8993(01)03127-4. [DOI] [PubMed] [Google Scholar]
- 9.Arnone M, Maruani J, Chaperon F, Thiebot M, Poncelet M, Soubrie P, Le Fur G. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CBI) receptors. Psychopharmacology. 1997;132:104–106. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- 10.Auclair N, Otani S, Soubrie P, Crepel F. Cannabinoids modulate synaptic strength and plasticity at glutamatergic synapses of rat prefrontal cortex pyramidal neurons. J Neurophysiol. 2000;83:3287–3293. doi: 10.1152/jn.2000.83.6.3287. [DOI] [PubMed] [Google Scholar]
- 11.Baker D, Pryce G, Croxford JL, Brown P, Pertwee RG, Makriyannis A, Khanolkar A, Layward L, Fezza F, Bisogno T, Di Marzo V. Endocannabinoids control spasticity in a multiple sclerosis model. FASEB J. 2001;15:300–302. doi: 10.1096/fj.00-0399fje. [DOI] [PubMed] [Google Scholar]
- 12.Basavarajappa BS. Endocannabinoid system in the development of tolerance to alcohol. Klinik Forschung. 2005;11:16–19. doi: 10.1093/alcalc/agh111. [DOI] [PubMed] [Google Scholar]
- 13.Basavarajappa BS. Endocannabinoid Signaling and Alcohol Addiction. In: Baye DR, editor. >New Research on Alcoholism New York: Nova Science Publishers; 2006. [Google Scholar]
- 14.Basavarajappa BS. Critical enzymes involved in endocannabinoid metabolism. Protein Pept Lett. 2007;14:237–246. doi: 10.2174/092986607780090829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basavarajappa BS. The endocannabinoid signaling system: a potential target for next-generation therapeutics for alcoholism. Mini Rev Med Chem. 2007 doi: 10.2174/138955707781387920. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basavarajappa BS, Cooper TB, Hungund BL. Chronic ethanol administration down-regulates cannabinoid receptors in mouse brain synaptic plasma membrane. Brain Res. 1998;793:212–218. doi: 10.1016/s0006-8993(98)00175-9. [DOI] [PubMed] [Google Scholar]
- 17.Basavarajappa BS, Hungund BL. Chronic ethanol increases the cannabinoid receptor agonist, anandamide and its precursor N-arachidonyl phosphatidyl ethanolamine in SK-N-SH cells. J Neurochem. 1999;72:522–528. doi: 10.1046/j.1471-4159.1999.0720522.x. [DOI] [PubMed] [Google Scholar]
- 18.Basavarajappa BS, Hungund BL. Down-regulation of cannabinoid receptor agonist-stimulated [35S] GTPγS binding in synaptic plasma membrane from chronic ethanol exposed mouse. Brain Res. 1999;815:89–97. doi: 10.1016/s0006-8993(98)01072-5. [DOI] [PubMed] [Google Scholar]
- 19.Basavarajappa BS, Hungund BL. Cannabinoid receptor agonist-stimulated [35S]Guanosine TriphosphateγS binding in the brain of C57BL/6 and DBA/2 mice. J Neurosci Res. 2001;64:429–436. doi: 10.1002/jnr.1094. [DOI] [PubMed] [Google Scholar]
- 20.Basavarajappa BS, Hungund BL. Neuromodulatory role of the endocannabinoid signaling system in alcoholism: an overview. Prostaglandins Leukot Essent Fatty Acids. 2002;66:287–299. doi: 10.1054/plef.2001.0352. [DOI] [PubMed] [Google Scholar]
- 21.Basavarajappa BS, Saito M, Cooper TB, Hungund BL. Stimulation of cannabinoid receptor agonist 2-arachidonylglycerol by chronic ethanol and its modulation by specific neuromodulators in cerebellar granule neurons. Biochem Biophys Acta. 2000;1535:78–86. doi: 10.1016/s0925-4439(00)00085-5. [DOI] [PubMed] [Google Scholar]
- 22.Basavarajappa BS, Saito M, Cooper TB, Hungund BL. Chronic ethanol inhibits the anandamide transport and increases extracellular anandamide levels in cerebellar granule neurons. Eur J Pharmacol. 2003;466:73–83. doi: 10.1016/s0014-2999(03)01557-7. [DOI] [PubMed] [Google Scholar]
- 23.Basavarajappa BS, Yalamanchili R, Cooper TB, Hungund BL. Endocannabinoid System. In: Hamon M, Sylvester VE, editors. Handbook of Neurochemistry and Molecular Neurobiology. NY: Springer; 2007. [Google Scholar]
- 24.Basavarajappa BS, Yalamanchili R, Cravatt BF, Cooper TB, Hungund BL. Increased ethanol consumption and preference and decreased ethanol sensitivity in female FAAH knockout mice. Neuropharmacology. 2006;50:834–844. doi: 10.1016/j.neuropharm.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Beltramo M, Piomelli D. Carrier-mediated transport and enzymatic hydrolysis of the endogenous cannabinoid 2-arachidonylglycerol. Neuroreport. 2000;11:1231–1235. doi: 10.1097/00001756-200004270-00018. [DOI] [PubMed] [Google Scholar]
- 26.Beltramo M, Stella N, Calignano A, Lin SY, Makriyannis A, Piomelli D. Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science. 1997;277:1094–1097. doi: 10.1126/science.277.5329.1094. [DOI] [PubMed] [Google Scholar]
- 27.Biegon A, Kerman IA. Autoradiographic study of pre- and postnatal distribution of cannabinoid receptors in human brain. Neuroimage. 2001;14:1463–1468. doi: 10.1006/nimg.2001.0939. [DOI] [PubMed] [Google Scholar]
- 28.Bisogno T, Berrendero F, Ambrosino G, Cebeira M, Ramos JA, Fernandez-Ruiz JJ, Di Marzo V. Brain regional distribution of endocannabinoids: implications for their biosynthesis and biological function. Biochem Biophys Res Commun. 1999;256:377–380. doi: 10.1006/bbrc.1999.0254. [DOI] [PubMed] [Google Scholar]
- 29.Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, Matias I, Schiano-Moriello A, Paul P, Williams EJ, Gangadharan U, Hobbs C, Di Marzo V, Doherty P. Cloning of the first sn1DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163:463–468. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bisogno T, MacCarrone M, De Petrocellis L, Jarrahian A, Finazzi-Agro A, Hillard C, Di Marzo V. The uptake by cells of 2-arachidonoylglycerol, an endogenous agonist of cannabinoid receptors. Eur J Biochem. 2001;268:1982–1989. doi: 10.1046/j.1432-1327.2001.02072.x. [DOI] [PubMed] [Google Scholar]
- 31.Bisogno T, Melck D, De Petrocellis L, Di Marzo V. Phosphatidic acid as the biosynthetic precursor of the endocannabinoid 2arachidonoylglycerol in intact mouse neuroblastoma cells stimulated with ionomycin. J Neurochem. 1999;72:2113–2119. doi: 10.1046/j.1471-4159.1999.0722113.x. [DOI] [PubMed] [Google Scholar]
- 32.Bisogno T, Sepe N, De Petrocellis L, Di Marzo V. Biosynthesis of 2-arachidonoyl-glycerol, a novel cannabimimetic eicosanoid, in mouse neuroblastoma cells. Adv Exp Med Biol. 1997;433:201–204. doi: 10.1007/978-1-4899-1810-9_42. [DOI] [PubMed] [Google Scholar]
- 33.Bisogno T, Sepe N, Melck D, Maurelli S, De Petrocellis L, Di Marzo V. Biosynthesis, release and degradation of the novel endogenous cannabimimetic metabolite 2-arachidonoylglycerol in mouse neuroblastoma cells. Biochem J. 1997;322:671–677. doi: 10.1042/bj3220671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bohme GA, Laville M, Ledent C, Parmentier M, Imperato A. Enhanced long-term potentiation in mice lacking cannabinoid CB1 receptors. Neuroscience. 2000;95:5–7. doi: 10.1016/s0306-4522(99)00483-2. [DOI] [PubMed] [Google Scholar]
- 35.Bouaboula M, Bianchini L, McKenzie FR, Pouyssegur J, Casellas P. Cannabinoid receptor CB1 activates the Na+/H+ exchanger NHE-1 isoform viaGi-mediated mitogen activated protein kinase signaling transduction pathways. FEBS Lett. 1999;449:61–65. doi: 10.1016/s0014-5793(99)00395-6. [DOI] [PubMed] [Google Scholar]
- 36.Bouaboula M, Bourrie B, Rinaldi-Carmona M, Shire D, Le Fur G, Casellas P. Stimulation of cannabinoid receptor CB1 induces krox-24 expression in human astrocytoma cells. J Biol Chem. 1995;270:13973–13980. doi: 10.1074/jbc.270.23.13973. [DOI] [PubMed] [Google Scholar]
- 37.Bouaboula M, Poinot-Chazel C, Bourrie B, Canat X, Calandra B, Rinaldi-Carmona M, Le Fur G, Casellas P. Activation of mitogen-activated protein kinases by stimulation of the central cannabinoid receptor CB1. Biochem J. 1995;312:637–641. doi: 10.1042/bj3120637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Breivogel CS, Griffin G, Di Marzo V, Martin BR. Evidence for a new G protein-coupled cannabinoid receptor in mouse brain. Mol Pharmacol. 2001;60:155–163. [PubMed] [Google Scholar]
- 39.Breivogel CS, Sim LJ, Childers SR. Regional differences in cannabinoid receptor/G-protein coupling in rat brain. J Pharmacol Exp Ther. 1997;282:1632–1642. [PubMed] [Google Scholar]
- 40.Bruinsma K, Taren DL. Chocolate: food or drug? J Am Diet Assoc. 1999;99:1249–1256. doi: 10.1016/S0002-8223(99)00307-7. [DOI] [PubMed] [Google Scholar]
- 41.Buck KJ. Recent progress toward the identification of genes related to risk for alcoholism. Mamm Genome. 1998;9:927–928. doi: 10.1007/s003359900901. [DOI] [PubMed] [Google Scholar]
- 42.Buck KJ, Metten P, Belknap JK, Crabbe JC. Quantitative trait loci involved in genetic predisposition to acute alcohol withdrawal in mice. J Neurosci. 1997;17:3946–3955. doi: 10.1523/JNEUROSCI.17-10-03946.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buckley NE, Mezey E, Bonner T, Zimmer A, Felder CC, Glass M, Zimmer A. Proceedings of the Symposium on the Cannabinoids. Vermont International Cannabinoid Research Society: Berlington; 1997. Development of a CB2 knockout mouse. [Google Scholar]
- 44.Burstein SH, Rossetti RG, Yagen B, Zurier RB. Oxidative metabolism of anandamide. Prostaglandins Other Lipid Mediat. 2000;61:29–41. doi: 10.1016/s0090-6980(00)00053-8. [DOI] [PubMed] [Google Scholar]
- 45.Cabral GA, Dove Pettit DA. Drugs and immunity: cannabinoids and their role in decreased resistance to infectious disease. J Neuroimmunol. 1998;83:116–123. doi: 10.1016/s0165-5728(97)00227-0. [DOI] [PubMed] [Google Scholar]
- 46.Cadas H, di Tomaso E, Piomelli D. Occurrence and biosynthesis of endogenous cannabinoid precursor, N-arachidonoyl phosphatidylethanolamine, in rat brain. J Neurosci. 1997;17:1226–1242. doi: 10.1523/JNEUROSCI.17-04-01226.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Calandra B, Portier M, Kerneis A, Delpech M, Carillon C, Le Fur G, Ferrara P, Shire D. Dual intracellular signaling pathways mediated by the human cannabinoid CB1 receptor. Eur J Pharmacol. 1999;374:445–455. doi: 10.1016/s0014-2999(99)00349-0. [DOI] [PubMed] [Google Scholar]
- 48.Carrier EJ, Kearn CS, Barkmeier AJ, Breese NM, Yang W, Nithipatikom K, Pfister SL, Campbell WB, Hillard CJ. Cultured rat microglial cells synthesize the endocannabinoid 2-arachidonylglycerol, which increases proliferation viaa CB2 receptor-dependent mechanism. Mol Pharmacol. 2004;65:999–1007. doi: 10.1124/mol.65.4.999. [DOI] [PubMed] [Google Scholar]
- 49.Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- 50.Caulfield MP, Brown DA. Cannabinoid receptor agonists inhibit Ca current in NG108-15 neuroblastoma cells viaa pertussis toxin-sensitive mechanism. Br J Pharmacol. 1992;106:231–232. doi: 10.1111/j.1476-5381.1992.tb14321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caulfield MP, Robbins J, Brown DA. Neurotransmitters inhibit the omega-conotoxin-sensitive component of Ca current in neuroblastoma x glioma hybrid (NG 108-15) cells, not the nifedipine-sensitive component. Pflugers Arch. 1992;420:486–492. doi: 10.1007/BF00374623. [DOI] [PubMed] [Google Scholar]
- 52.Chakrabarti A, Onaivi ES, Chaudhuri G. Cloning and sequencing of a cDNA encoding the mouse brain-type cannabinoid receptor protein. DNA Seq. 1995;6:385–388. doi: 10.3109/10425179509020870. [DOI] [PubMed] [Google Scholar]
- 53.Chester JA, Blose AM, Froehlich JC. Further evidence of an inverse genetic relationship between innate differences in alcohol preference and alcohol withdrawal magnitude in multiple selectively bred rat lines. Alcohol Clin Exp Res. 2003;27:377–387. doi: 10.1097/01.ALC.0000056619.98553.50. [DOI] [PubMed] [Google Scholar]
- 54.Chester JA, Risinger FO, Cunningham CL. Ethanol reward and aversion in mice bred for sensitivity to ethanol withdrawal. Alcohol Clin Exp Res. 1998;22:468–473. [PubMed] [Google Scholar]
- 55.Chevaleyre V, Castillo PE. Endocannabinoid-mediated metaplasticity in the hippocampus. Neuron. 2004;43:871–881. doi: 10.1016/j.neuron.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 56.Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-Mediated Synaptic Plasticity in the CNS. Annu Rev Neurosci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- 57.Childers SR, Deadwyler SA. Role of cyclic AMP in the actions of cannabinoid receptors. Biochem Pharmacol. 1996;52:819–827. doi: 10.1016/0006-2952(96)00419-4. [DOI] [PubMed] [Google Scholar]
- 58.Childers SR, Sexton T, Roy MB. Effects of anandamide on cannabinoid receptors in rat brain membranes. Biochem Pharmacol. 1994;47:711–715. doi: 10.1016/0006-2952(94)90134-1. [DOI] [PubMed] [Google Scholar]
- 59.Christison R. Lea and Blancherd: Philidalphia; 1848. In A Dispensatory or Commentary on the Pharmacopoeias of Great Britain (and the United States) pp. 971–974. [Google Scholar]
- 60.Cleland JG, Ghosh J, Freemantle N, Kaye GC, Nasir M, Clark AL, Coletta AP. Clinical trials update and cumulative meta-analyses from the American College of Cardiology: WATCH, SCD-HeFT, DINAMIT, CASINO, INSPIRE, STRATUS-US, RIO-Lipids and cardiac resynchronisation therapy in heart failure. Eur J Heart Fail. 2004;6:501–508. doi: 10.1016/j.ejheart.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 61.Colombo G, Agabio R, Fa M, Guano L, Lobina C, Loche A, Reali R, Gessa G. Reduction of voluntary ethanol intake in ethanol-preferring sP rats by the cannabinoid antagonist SR-141716. Alcohol Alcohol. 1998;33:126–130. doi: 10.1093/oxfordjournals.alcalc.a008368. [DOI] [PubMed] [Google Scholar]
- 62.Colombo G, Serra S, Brunetti G, Gomez R, Melis S, Vacca G, Carai MM, Gessa L. Stimulation of voluntary ethanol intake by cannabinoid receptor agonists in ethanol-preferring sP rats. Psychopharmacology (Berl.) 2002;159:181–187. doi: 10.1007/s002130100887. [DOI] [PubMed] [Google Scholar]
- 63.Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- 64.Crabbe JC, Belknap JK, Metten P, Grisel JE, Buck KJ. Quantitative trait loci: mapping drug and alcohol-related genes. Adv Pharmacol. 1998;42:1033–1037. doi: 10.1016/s1054-3589(08)60923-0. [DOI] [PubMed] [Google Scholar]
- 65.Crabbe JC, Gallaher ES, Phillips TJ, Belknap JK. Genetic determinants of sensitivity to ethanol in inbred mice. Behav Neurosci. 1994;108:186–195. doi: 10.1037//0735-7044.108.1.186. [DOI] [PubMed] [Google Scholar]
- 66.Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- 67.Curtis MA, Faull RL, Glass M. A novel population of progenitor cells expressing cannabinoid receptors in the subependymal layer of the adult normal and Huntington’s disease human brain. J Chem Neuroanat. 2006;31:210–215. doi: 10.1016/j.jchemneu.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 68.Day TA, Rakhshan F, Deutsch DG, Barker EL. Role of fatty acid amide hydrolase in the transport of the endogenous cannabinoid anandamide. Mol Pharmacol. 2001;59:1369–1375. doi: 10.1124/mol.59.6.1369. [DOI] [PubMed] [Google Scholar]
- 69.De Marchi N, De Petrocellis L, Orlando P, Daniele F, Fezza F, Di Marzo V. Endocannabinoid signalling in the blood of patients with schizophrenia. Lipids Health Dis. 2003;2:5. doi: 10.1186/1476-511X-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dean B, Sundram S, Bradbury R, Scarr E, Copolov D. Studies on [3H]CP-55940 binding in the human central nervous system: regional specific changes in density of cannabinoid-1 receptors associated with schizophrenia and cannabis use. Neuroscience. 2001;103:9–15. doi: 10.1016/s0306-4522(00)00552-2. [DOI] [PubMed] [Google Scholar]
- 71.Denovan-Wright EM, Robertson HA. Cannabinoid receptor messenger RNA levels decrease in a subset of neurons of the lateral striatum, cortex and hippocampus of transgenic Huntington’s disease mice. Neuroscience. 2000;98:705–713. doi: 10.1016/s0306-4522(00)00157-3. [DOI] [PubMed] [Google Scholar]
- 72.Derkinderen P, Toutant M, Burgaya F, Le Bert M, Siciliano JC, de Franciscis V, Gelman M, Girault JA. Regulation of a neuronal form of focal adhesion kinase by anandamide. Science. 1996;273:1719–1722. doi: 10.1126/science.273.5282.1719. [DOI] [PubMed] [Google Scholar]
- 73.Derkinderen P, Toutant M, Kadare G, Ledent C, Parmentier M, Girault JA. Dual role of Fyn in the regulation of FAK+6,7 by cannabinoids in hippocampus. J Biol Chem. 2001;276:38289–38296. doi: 10.1074/jbc.M105630200. [DOI] [PubMed] [Google Scholar]
- 74.Derkinderen P, Valjent E, Toutant M, Corvol JC, Enslen H, Ledent C, Trzaskos J, Caboche J, Girault JA. Regulation of extracellular signal-regulated kinase by cannabinoids in hippocampus. J Neurosci. 2003;23:2371–2382. doi: 10.1523/JNEUROSCI.23-06-02371.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Deutsch DG, Chin SA. Enzymatic synthesis and degradation of anandamide, a cannabinoid receptor agonist. Biochem Pharmacol. 1993;46:791–796. doi: 10.1016/0006-2952(93)90486-g. [DOI] [PubMed] [Google Scholar]
- 76.Deutsch DG, Glaser ST, Howell JM, Kunz JS, Puffenbarger RA, Hillard CJ, Abumrad N. The cellular uptake of anandamide is coupled to its breakdown by fatty-acid amide hydrolase. J Biol Chem. 2001;276:6967–6973. doi: 10.1074/jbc.M003161200. [DOI] [PubMed] [Google Scholar]
- 77.Devane WA, Dysarz FAI, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- 78.Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 79.Dewey WL. Cannabinoid pharmacology. Pharmacol Rev. 1986;38:151–178. [PubMed] [Google Scholar]
- 80.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Di Marzo V. ‘Endocannabinoids’ and other fatty acid derivatives with cannabimimetic properties: biochemistry and possible. Biochim Biophys Acta. 1998;1392:153–175. doi: 10.1016/s0005-2760(98)00042-3. [DOI] [PubMed] [Google Scholar]
- 82.Di Marzo V, Bisogno T, Sugiura T, Melck D, De Petrocellis L. The novel endogenous cannabinoid 2-arachidonoylglycerol is inactivated by neuronal- and basophil-like cells: connections with anandamide. Biochem J. 1998;331:15–19. doi: 10.1042/bj3310015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Di Marzo V, Breivogel CS, Tao Q, Bridgen DT, Razdan RK, Zimmer AM, Zimmer A, Martin BR. Levels, metabolism, and pharmacological activity of anandamide in CB(1) cannabinoid receptor knockout mice: evidence for non-CB(1), non-CB(2) receptor-mediated actions of anandamide in mouse brain. J Neurochem. 2000;75:2434–2444. doi: 10.1046/j.1471-4159.2000.0752434.x. [DOI] [PubMed] [Google Scholar]
- 84.Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, Piomelli D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- 85.Di Marzo V, Hill MP, Bisogno T, Crossman AR, Brotchie JM. Enhanced levels of endogenous cannabinoids in the globus pallidus are associated with a reduction in movement in an animal model of Parkinson’s disease. FASEB J. 2000;14:1432–1438. doi: 10.1096/fj.14.10.1432. [DOI] [PubMed] [Google Scholar]
- 86.Di Marzo V, Sepe N, De Petrocellis L, Berger A, Crozier G, Fride E, Mechoulam R. Trick or treat from food endocannabinoids? Nature. 1998;396:636–637. doi: 10.1038/25267. [DOI] [PubMed] [Google Scholar]
- 87.James JS. Marijuana and chocolate. Aids Treat News. 1996;257:3–4. [PubMed] [Google Scholar]
- 88.di Tomaso E, Beltramo M, Piomelli D. Brain cannabinoids in chocolate. Nature. 1996;382:677–678. doi: 10.1038/382677a0. [DOI] [PubMed] [Google Scholar]
- 89.Diana MA, Levenes C, Mackie K, Marty A. Short-term retrograde inhibition of GABAergic synaptic currents in rat Purkinje cells is mediated by endogenous cannabinoids. J Neurosci. 2002;22:200–208. doi: 10.1523/JNEUROSCI.22-01-00200.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci USA. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Doherty J, Dingledine R. Functional interactions between cannabinoid and metabotropic glutamate receptors in the central nervous system. Curr Opin Pharmacol. 2003;3:46–53. doi: 10.1016/s1471-4892(02)00014-0. [DOI] [PubMed] [Google Scholar]
- 92.Ellert-Miklaszewska A, Grajkowska W, Gabrusiewicz K, Kaminska B, Konarska L. Distinctive pattern of cannabinoid receptor type II (CB2) expression in adult and pediatric brain tumors. Brain Res. 2006;1137:161–9. doi: 10.1016/j.brainres.2006.12.060. [DOI] [PubMed] [Google Scholar]
- 93.Eubanks LM, Rogers CJ, Beuscher IV AE, Koob GF, Olson AJ, Dickerson TJ, Janda KD. A molecular link between the active component of Marijuana and Alzheimer’s Disease pathology. Mol Pharm. 2006;3:773–7. doi: 10.1021/mp060066m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Facci L, Dal Toso R, Romanello S, Buriani A, Skaper SD, Leon A. Mast cells express a peripheral cannabinoid receptor with differential sensitivity to anandamide and palmitoylethanolamide. Proc Natl Acad Sci USA. 1995;92:3376–3380. doi: 10.1073/pnas.92.8.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Felder C, Nielsen A, Briley E, Palkovits M, Priller J, Axelrod J, Nguyen D, Richardson J, RM R, Koppel G, Paul S, Becker G. Isolation and measurement of the endogenous cannabinoid receptor agonist, anandamide, in brain and peripheral tissues of human and rat. FEBS Lett. 1996;393:231–235. doi: 10.1016/0014-5793(96)00891-5. [DOI] [PubMed] [Google Scholar]
- 96.Felder CC, Briley EM, Axelrod J, Simpson JT, Mackie K, Devane WA. Anandamide, an endogenous cannabimimetic eicosanoid, binds to the cloned human cannabinoid receptor and stimulates receptor-mediated signal transduction. Proc Natl Acad Sci USA. 1993;90:7656–7660. doi: 10.1073/pnas.90.16.7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Felder CC, Joyce KE, Briley EM, Mansouri J, Mackie K, Blond O, Lai Y, Ma AL, Mitchell RL. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol Pharmacol. 1995;48:443–450. [PubMed] [Google Scholar]
- 98.Ferrer B, Asbrock N, Kathuria S, Piomelli D, Giuffrida A. Effects of levodopa on endocannabinoid levels in rat basal ganglia: implications for the treatment of levodopa-induced dyskinesias. Eur J Neurosci. 2003;18:1607–1614. doi: 10.1046/j.1460-9568.2003.02896.x. [DOI] [PubMed] [Google Scholar]
- 99.Freedland CS, Sharpe AL, Samson HH, Porrino LJ. Effects of SR141716A on ethanol and sucrose self-administration. Alcohol Clin Exp Res. 2001;25:277–282. [PubMed] [Google Scholar]
- 100.Gallate JE, McGregor IS. The motivation for beer in rats: effects of ritanserin, naloxone and SR 141716. Psychopharmacology. 1999;142:302–308. doi: 10.1007/s002130050893. [DOI] [PubMed] [Google Scholar]
- 101.Gallate JE, Saharov T, Mallet PE, McGregor IS. Increased motivation for beer in rats following administration of a cannabinoid CB1 receptor agonist. Eur J Pharmacol. 1999;370:233–240. doi: 10.1016/s0014-2999(99)00170-3. [DOI] [PubMed] [Google Scholar]
- 102.Gaoni Y, Mechoulam R. Isolation, structure and partial synthesis of an active constituent of hashish. J Am Chem Soc. 1964;86:1646–1647. [Google Scholar]
- 103.Garcia DE, Brown S, Hille B, Mackie K. Protein kinase C disrupts cannabinoid actions by phosphorylation of the CB1 cannabinoid receptor. J Neurosci. 1998;18:2834–2841. doi: 10.1523/JNEUROSCI.18-08-02834.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gebremedhin D, Lange AR, Campbell WB, Hillard CJ, Harder DR. Cannabinoid CB1 receptor of cat cerebral arterial muscle functions to inhibit L-type Ca2+ channel current. Am J Physiol. 1999;276:H2085–2093. doi: 10.1152/ajpheart.1999.276.6.H2085. [DOI] [PubMed] [Google Scholar]
- 105.Gelfand EV, Cannon CP. Rimonabant: a selective blocker of the cannabinoid CB1 receptors for the management of obesity, smoking cessation and cardiometabolic risk factors. Expert Opin Investig Drugs. 2006;15:307–315. doi: 10.1517/13543784.15.3.307. [DOI] [PubMed] [Google Scholar]
- 106.Gerard CM, Mollereau C, Vassart G, Parmentier M. Molecular cloning of a human cannabinoid receptor which is also expressed in testis. Biochem J. 1991;279:129–134. doi: 10.1042/bj2790129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gerdeman G, Lovinger DM. CB1 cannabinoid receptor inhibits synaptic release of glutamate in rat dorsolateral striatum. J Neurophysiol. 2001;85:468–471. doi: 10.1152/jn.2001.85.1.468. [DOI] [PubMed] [Google Scholar]
- 108.Gerdeman GL, Partridge JG, Lupica CR, Lovinger DM. It could be habit forming: drugs of abuse and striatal synaptic plasticity. Trends Neurosci. 2003;26:184–192. doi: 10.1016/S0166-2236(03)00065-1. [DOI] [PubMed] [Google Scholar]
- 109.Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- 110.Gessa GL, Melis M, Muntoni AL, Diana M. Cannabinoids activate mesolimbic dopamine neurons by an action on cannabinoid CB1 receptors. Eur J Pharmacol. 1998;341:39–44. doi: 10.1016/s0014-2999(97)01442-8. [DOI] [PubMed] [Google Scholar]
- 111.Giuffrida A, Beltramo M, Piomelli D. Mechanisms of endocannabinoid inactivation: biochemistry and pharmacology. J Pharmacol Exp Ther. 2001;298:7–14. [PubMed] [Google Scholar]
- 112.Giuffrida A, Leweke FM, Gerth CW, Schreiber D, Koethe D, Faulhaber J, Klosterkotter J, Piomelli D. Cerebrospinal anandamide levels are elevated in acute schizophrenia and are inversely correlated with psychotic symptoms. Neuropsychopharmacology. 2004;29:2108–2114. doi: 10.1038/sj.npp.1300558. [DOI] [PubMed] [Google Scholar]
- 113.Giuffrida A, Parsons LH, Kerr TM, Rodriguez de Fonseca , Navarro M, Piomelli D. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci. 1999;2:358–363. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- 114.Glaser ST, Abumrad NA, Fatade , Kaczocha M, Studholme KM, Deutsch DG. Evidence against the presence of an anandamide transporter. Proc Natl Acad Sci USA. 2003;100:4269–4274. doi: 10.1073/pnas.0730816100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Glass M. The role of cannabinoids in neurodegenerative diseases. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:743–765. doi: 10.1016/s0278-5846(01)00162-2. [DOI] [PubMed] [Google Scholar]
- 116.Glass M, Dragunow M, Faull RL. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77:299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- 117.Glass M, Dragunow M, Faull RL. The pattern of neurodegeneration in Huntington’s disease: a comparative study of cannabinoid, dopamine, adenosine and GABA(A) receptor alterations in the human basal ganglia in Huntington’s disease. Neuroscience. 2000;97:505–519. doi: 10.1016/s0306-4522(00)00008-7. [DOI] [PubMed] [Google Scholar]
- 118.Glass M, Felder CC. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: evidence for a Gs linkage to the CB1 receptor. J Neurosci. 1997;17:5327–5333. doi: 10.1523/JNEUROSCI.17-14-05327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Glass M, van Dellen A, Blakemore C, Hannan AJ, Faull RL. Delayed onset of huntington’s disease in mice in an enriched environment correlates with delayed loss of cannabinoid CB1 receptors. Neuroscience. 2004;123:207–212. doi: 10.1016/s0306-4522(03)00595-5. [DOI] [PubMed] [Google Scholar]
- 120.Gomez del Pulgar T, Velasco G, Guzman M. The CB1 cannabinoid receptor is coupled to the activation of protein kinase B/Akt. Biochem J. 2000;347:369–373. doi: 10.1042/0264-6021:3470369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gong JP, Onaivi ES, Ishiguro H, Liu QR, Tagliaferro PA, Brusco A, Uhl GR. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res. 2006;1071:10–23. doi: 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 122.Gonzalez S, Grazia Cascio M, Fernandez-Ruiz J, Fezza F, Di Marzo V, Ramos JA. Changes in endocannabinoid contents in the brain of rats chronically exposed to nicotine, ethanol or cocaine. Brain Res. 2002;954:73. doi: 10.1016/s0006-8993(02)03344-9. [DOI] [PubMed] [Google Scholar]
- 123.Gonzalez S, Scorticati C, Garcia-Arencibia M, de Miguel R, Ramos JA, Fernandez-Ruiz J. Effects of rimonabant, a selective cannabinoid CB1 receptor antagonist, in a rat model of Parkinson’s disease. Brain Res. 2006;1073-1074:209–219. doi: 10.1016/j.brainres.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 124.Gonzalez S, Valenti M, De Miguel R, Fezza F, Fernandez-Ruiz J, Di Marzo V, Ramos JA. Changes in endocannabinoid contents in reward-related brain regions of alcohol-exposed rats, and their possible relevance to alcohol relapse. Br J Pharmacol. 2004;143:455–464. doi: 10.1038/sj.bjp.0705963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Goparaju SK, Ueda N, Yamaguchi H, Yamamoto S. Anandamide amidohydrolase reacting with 2-arachidonoylglycerol, another cannabinoid receptor ligand. FEBS Lett. 1998;422:69–73. doi: 10.1016/s0014-5793(97)01603-7. [DOI] [PubMed] [Google Scholar]
- 126.Graham ES, Ball N, Scotter EL, Narayan P, Dragunow M, Glass M. Induction of KROX-24 by endogenous CB1 receptors in neuro2A cells is mediated by the MEK-ERK MAPK pathway and is suppressed by the PI3K pathway. J Biol Chem. 2006;281:29085–95. doi: 10.1074/jbc.M602516200. [DOI] [PubMed] [Google Scholar]
- 127.Griffin G, Tao Q, Abood ME. Cloning and pharmacological characterization of the rat CB(2) cannabinoid receptor. J Pharmacol Exp Ther. 2000;292:886–894. [PubMed] [Google Scholar]
- 128.Gubellini P, Picconi B, Bari M, Battista N, Calabresi P, Centonze D, Bernardi G, Finazzi-Agro A, Maccarrone M. Experimental parkinsonism alters endocannabinoid degradation: implications for striatal glutamatergic transmission. J Neurosci. 2002;22:6900–6907. doi: 10.1523/JNEUROSCI.22-16-06900.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gulyas AI, Cravatt BF, Bracey MH, Dinh TP, Piomelli D, Boscia F, Freund TF. Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. Eur J Neurosci. 2004;20:441–458. doi: 10.1111/j.1460-9568.2004.03428.x. [DOI] [PubMed] [Google Scholar]
- 130.Guzman M, Sanchez C. Effects of cannabinoids on energy metabolism. Life Sci. 1999;65:657–664. doi: 10.1016/s0024-3205(99)00288-x. [DOI] [PubMed] [Google Scholar]
- 131.Hajos N, Katona I, Naiem SS, MacKie K, Ledent C, Mody I, Freund TF. Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur J Neurosci. 2000;12:3239–3249. doi: 10.1046/j.1460-9568.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- 132.Hajos N, Ledent C, Freund TF. Novel cannabinoid-sensitive receptor mediates inhibition of glutamatergic synaptic transmission in the hippocampus. Neuroscience. 2001;106:1–4. doi: 10.1016/s0306-4522(01)00287-1. [DOI] [PubMed] [Google Scholar]
- 133.Hall W, Solowij N. Adverse effects of cannabis. Lancet. 1998;352:1611–1616. doi: 10.1016/S0140-6736(98)05021-1. [DOI] [PubMed] [Google Scholar]
- 134.Hampson RE, Mu J, Deadwyler SA. Cannabinoid and kappa opioid receptors reduce potassium K current viaactivation of G(s) proteins in cultured hippocampal neurons. J Neurophysiol. 2000;84:2356–2364. doi: 10.1152/jn.2000.84.5.2356. [DOI] [PubMed] [Google Scholar]
- 135.Hansen HH, Hansen SH, Schousboe A, Hansen HS. Determination of the phospholipid precursor of anandamide and other Nacylethanolamine phospholipids before and after sodium azide-induced toxicity in cultured neocortical neurons. J Neurochem. 2000;75:861–871. doi: 10.1046/j.1471-4159.2000.0750861.x. [DOI] [PubMed] [Google Scholar]
- 136.Hansson AC, Bermudez-Silva FJ, Malinen H, Hyytia P, Sanchez-Vera I, Rimondini R, Rodriguez de Fonseca F, Kunos G, Sommer WH, Heilig M. Genetic impairment of frontocortical endocannabinoid degradation and high alcohol preference. Neuropsychopharmacology. 2006;32:117–26. doi: 10.1038/sj.npp.1301034. [DOI] [PubMed] [Google Scholar]
- 137.Hanus L, Abu-Lafi S, Fride E, Breuer A, Vogel Z, Shalev DE, Kustanovich I, Mechoulam R. 2-arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc Natl Acad Sci USA. 2001;98:3662–3665. doi: 10.1073/pnas.061029898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proc Natl Acad Sci USA. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Cost BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;16:8057–8066. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hill EL, Gallopin T, Ferezou I, Cauli B, Rossier J, Schweitzer P, Lambolez B. Functional CB1 receptors are broadly expressed in neocortical GABAergic and glutamatergic neurons. J Neurophysiol. 2007 doi: 10.1152/jn.00603.2006. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 141.Hillard CJ, Edgemond WS, Jarrahian A, Campbell WB. Accumulation of N-arachidonoylethanolamine (anandamide) into cerebellar granule cells occurs via facilitated diffusion. J Neurochem. 1997;69:631–638. doi: 10.1046/j.1471-4159.1997.69020631.x. [DOI] [PubMed] [Google Scholar]
- 142.Hillard CJ, Jarrahian A. The movement of N-arachidonoylethanolamine (anandamide) across cellular membranes. Chem Phys Lipids. 2000;108:123–134. doi: 10.1016/s0009-3084(00)00191-2. [DOI] [PubMed] [Google Scholar]
- 143.Hillard CJ, Jarrahian A. Cellular accumulation of anandamide: consensus and controversy. Br J Pharmacol. 2003;140:802–808. doi: 10.1038/sj.bjp.0705468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ho BY, Uezono Y, Takada S, Takase I, Izumi F. Coupling of the expressed cannabinoid CB1 and CB2 receptors to phospholipase C and G protein-coupled inwardly rectifying K+ channels. Recept Channels. 1999;6:363–374. [PubMed] [Google Scholar]
- 145.Hoehe MR, Caenazzo L, Martinez MM, Hsieh WT, Modi WS, Gershon ES, Bonner TI. Genetic and physical mapping of the human cannabinoid receptor gene to chromosome 6q14-q15. New Biol. 1991;3:880–885. [PubMed] [Google Scholar]
- 146.Hoffman AF, Lupica CR. Mechanisms of cannabinoid inhibition of GABA(A) synaptic transmission in the hippocampus. J Neurosci. 2000;20:2470–2479. doi: 10.1523/JNEUROSCI.20-07-02470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hollister LE. Health aspects of cannabis. Pharmacol Rev. 1986;38:1–20. [PubMed] [Google Scholar]
- 148.Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- 149.Howlett AC, Johnson MR, Melvin LS, Milne GM. Nonclassical cannabinoid analgetics inhibit adenylate cyclase: development of a cannabinoid receptor model. Mol Pharmacol. 1988;33:297–302. [PubMed] [Google Scholar]
- 150.Howlett AC, Mukhopadhyay S. Cellular signal transduction by anandamide and 2-arachidonoylglycerol. Chem Phys Lipids. 2000;108:53–70. doi: 10.1016/s0009-3084(00)00187-0. [DOI] [PubMed] [Google Scholar]
- 151.Huang CC, Lo SW, Hsu KS. Presynaptic mechanisms underlying cannabinoid inhibition of excitatory synaptic transmission in rat striatal neurons. J Physiol. 2001;532(Pt3):731–748. doi: 10.1111/j.1469-7793.2001.0731e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Huang SM, Bisogno T, Trevisani M, Al-Hayani A, De Petrocellis L, Fezza F, Tognetto M, Petros TJ, Krey JF, Chu CJ, Miller JD, Davies SN, Geppetti P, Walker JM, Di Marzo V. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc Natl Acad Sci USA. 2002;99:8400–8405. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hungund BL, Basavarajappa BS. Distinct differences in the cannabinoid receptor binding in the brain of C57BL/6 and DBA/2 mice, selected for their differences in voluntary ethanol consumption. J Neurosci Res. 2000;60:122–128. doi: 10.1002/(SICI)1097-4547(20000401)60:1<122::AID-JNR13>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 154.Hungund BL, Basavarajappa BS, Vadasz C, Kunos G, Rodriguez de Fonseca F, Colombo G, Serra S, Parsons L, Koob GF. Ethanol, endocannabinoids and cannabinoidergic signaling system. Alcoholism Clin. Exp. Res. 2002;26:565–574. [PubMed] [Google Scholar]
- 155.Hungund BL, Szakall I, Adam A, Basavarajappa BS, Vadasz C. Cannabinoid CB1 receptor knockout mice exhibit markedly reduced voluntary alcohol consumption and lack alcohol-induced dopamine release in the nucleus accumbens. J Neurochem. 2003;84:698–704. doi: 10.1046/j.1471-4159.2003.01576.x. [DOI] [PubMed] [Google Scholar]
- 156.Iuvone T, Esposito G, Esposito R, Santamaria R, Di Rosa M, Izzo AA. Neuroprotective effect of cannabidiol, a non-psychoactive component from Cannabis sativa, on beta-amyloid-induced toxicity in PC12 cells. J Neurochem. 2004;89:134–141. doi: 10.1111/j.1471-4159.2003.02327.x. [DOI] [PubMed] [Google Scholar]
- 157.Jarai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, Zimmer AM, Bonner TI, Buckley NE, Mezey E, Razdan RK, Zimmer A, Kunos G. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc Natl Acad Sci USA. 1999;96:14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jog MS, Kubota Y, Connolly CI, Hillegaart V, Graybiel AM. Building neural representations of habits. Science. 1999;286:1745–1749. doi: 10.1126/science.286.5445.1745. [DOI] [PubMed] [Google Scholar]
- 159.Kabelik J, Krejci Z, Santavy F. Cannabis as a Medicant. Bull Narc. 1960;12:5–23. [Google Scholar]
- 160.Karlsson M, Contreras JA, Hellman U, Tornqvist H, Holm C. cDNA cloning, tissue distribution, and identification of the catalytic triad of monoglyceride lipase. Evolutionary relationship to esterases, lysophospholipases, and haloperoxidases. J Biol Chem. 1997;272:27218–27223. doi: 10.1074/jbc.272.43.27218. [DOI] [PubMed] [Google Scholar]
- 161.Katayama K, Ueda N, Katoh I, Yamamoto S. Equilibrium in the hydrolysis and synthesis of cannabimimetic anandamide demonstrated by a purified enzyme. Biochim Biophys Acta. 1999;1440:205–214. doi: 10.1016/s1388-1981(99)00124-9. [DOI] [PubMed] [Google Scholar]
- 162.Katona I, Rancz EA, Acsady L, Ledent C, Mackie K, Hajos N, Freund TF. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J Neurosci. 2001;21:9506–9518. doi: 10.1523/JNEUROSCI.21-23-09506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Katona I, Urban GM, Wallace M, Ledent C, Jung KM, Piomelli D, Mackie K, Freund TF. Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci. 2006;26:5628–5637. doi: 10.1523/JNEUROSCI.0309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kearn CS, Greenberg MJ, DiCamelli R, Kurzawa K, Hillard CJ. Relationships between ligand affinities for the cerebellar cannabinoid receptor CB1 and the induction of GDP/GTP exchange. J Neurochem. 1999;72:2379–2387. doi: 10.1046/j.1471-4159.1999.0722379.x. [DOI] [PubMed] [Google Scholar]
- 166.Kirkham TC, Tucci SA. Endocannabinoids in appetite control and the treatment of obesity. CNS Neurol. Disord. Drug Targets. 2006;5:272–292. doi: 10.2174/187152706777452272. [DOI] [PubMed] [Google Scholar]
- 167.Kondo S, Kondo H, Nakane S, Kodaka T, Tokumura A, Waku K, Sugiura T. 2-Arachidonoylglycerol, an endogenous cannabinoid receptor agonist: identification as one of the major species of monoacylglycerols in various rat tissues, and evidence for its generation through CA2+dependent and -independent mechanisms. FEBS Lett. 1998;429:152–156. doi: 10.1016/s0014-5793(98)00581-x. [DOI] [PubMed] [Google Scholar]
- 168.Konrad RJ, Major CD, Wolf BA. Diacylglycerol hydrolysis to arachidonic acid is necessary for insulin secretion from isolated pancreatic islets: sequential actions of diacylglycerol and monoacylglycerol lipases. Biochemistry. 1994;33:13284–13294. doi: 10.1021/bi00249a015. [DOI] [PubMed] [Google Scholar]
- 169.Kosobud A, Bodor AS, Crabbe JC. Voluntary consumption of ethanol in WSP, WSC and WSR selectively bred mouse lines. Pharmacol Biochem Behav. 1988;29:601–607. doi: 10.1016/0091-3057(88)90026-3. [DOI] [PubMed] [Google Scholar]
- 170.Kozak KR, Crews BC, Morrow JD, Wang LH, Ma YH, Weinander R, Jakobsson PJ, Marnett LJ. Metabolism of the endocannabinoids, 2-arachidonylglycerol and anandamide, into prostaglandin, thromboxane, and prostacyclin glycerol esters and ethanolamides. J Biol Chem. 2002;277:44877–44885. doi: 10.1074/jbc.M206788200. [DOI] [PubMed] [Google Scholar]
- 171.Kreitzer AC, Regehr WG. Cerebellar depolarization-induced suppression of inhibition is mediated by endogenous cannabinoids. J Neurosci. 2001;21:RC174. doi: 10.1523/JNEUROSCI.21-20-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001;29:717–727. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]
- 173.Kurahashi Y, Ueda N, Suzuki H, Suzuki M, Yamamoto S. Reversible hydrolysis and synthesis of anandamide demonstrated by recombinant rat fatty-acid amide hydrolase. Biochem Biophys Res Commun. 1997;237:512–515. doi: 10.1006/bbrc.1997.7180. [DOI] [PubMed] [Google Scholar]
- 174.Lallemand F, Soubrie PH, De Witte PH. Effects of CB1 cannabinoid receptor blockade on ethanol preference after chronic ethanol administration. Alcohol Clin. Exp. Res. 2001;25:1317–1323. [PubMed] [Google Scholar]
- 175.Lastres-Becker I, Cebeira M, de Ceballos ML, Zeng BY, Jenner P, Ramos JA, Fernandez-Ruiz JJ. Increased cannabinoid CB1 receptor binding and activation of GTP-binding proteins in the basal ganglia of patients with Parkinson’s syndrome and of MPTP-treated marmosets. Eur J Neurosci. 2001;14:1827–1832. doi: 10.1046/j.0953-816x.2001.01812.x. [DOI] [PubMed] [Google Scholar]
- 176.Lastres-Becker I, De Miguel R, Fernandez-Ruiz JJ. The endocannabinoid system and Huntington’s disease. Curr Drug Targets CNS Neurol. Disord. 2003;2:335–347. doi: 10.2174/1568007033482751. [DOI] [PubMed] [Google Scholar]
- 177.Lastres-Becker I, Fezza F, Cebeira M, Bisogno T, Ramos JA, Milone A, Fernandez-Ruiz J, Di Marzo V. Changes in endocannabinoid transmission in the basal ganglia in a rat model of Huntington’s disease. Neuroreport. 2001;12:2125–2129. doi: 10.1097/00001756-200107200-00017. [DOI] [PubMed] [Google Scholar]
- 178.Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, Bohme GA, Imperato A, Pedrazzini T, Roques BP, Vassart G, Fratta W, Parmentier M. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- 179.Leung D, Saghatelian A, Simon GM, Cravatt BF. Inactivation of N-acyl phosphatidylethanolamine phospholipase D reveals multiple mechanisms for the biosynthesis of endocannabinoids. Biochemistry. 2006;45:4720–4726. doi: 10.1021/bi060163l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Leweke FM, Schneider U, Thies M, Munte TF, Emrich HM. Effects of synthetic Delta(9)-tetrahydrocannabinol on binocular depth inversion of natural and artificial objects in man. Psychopharmacology. 1999;142:230–235. doi: 10.1007/s002130050884. [DOI] [PubMed] [Google Scholar]
- 181.Liu J, Gao B, Mirshahi F, Sanyal AJ, Khanolkar AD, Makriyannis A, Kunos G. Functional CB1 cannabinoid receptors in human vascular endothelial cells. Biochem J. 2000;346(Pt 3):835–40. [PMC free article] [PubMed] [Google Scholar]
- 182.Liu J, Wang L, Harvey-White J, Osei-Hyiaman D, Razdan R, Gong Q, Chan AC, Zhou Z, Huang BX, Kim HY, Kunos G. A biosynthetic pathway for anandamide. Proc Natl Acad Sci USA. 2006;103:13345–13350. doi: 10.1073/pnas.0601832103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Llano I, Leresche N, Marty A. Calcium entry increases the sensitivity of cerebellar Purkinje cells to applied GABA and decreases inhibitory synaptic currents. Neuron. 1991;6:565–574. doi: 10.1016/0896-6273(91)90059-9. [DOI] [PubMed] [Google Scholar]
- 184.Maccarrone M, van der Stelt M, Rossi A, Veldink GA, Vliegenthart JF, Agro AF. Anandamide hydrolysis by human cells in culture and brain. J Biol Chem. 1998;273:32332–32339. doi: 10.1074/jbc.273.48.32332. [DOI] [PubMed] [Google Scholar]
- 185.Mackie K, Hille B. Cannabinoids inhibit N-type calcium channels in neuroblastoma-glioma cells. Proc Natl Acad Sci USA. 1992;89:3825–3829. doi: 10.1073/pnas.89.9.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Maejima T, Ohno-Shosaku T, Kano M. Endogenous cannabinoid as a retrograde messenger from depolarized postsynaptic neurons to presynaptic terminals. Neurosci Res. 2001;40:205–210. doi: 10.1016/s0168-0102(01)00241-3. [DOI] [PubMed] [Google Scholar]
- 187.Mailleux P, Verslype M, Preud’homme X, Vanderhaeghen JJ. Activation of multiple transcription factor genes by tetrahydrocannabinol in rat forebrain. Neuroreport. 1994;5:1265–1268. doi: 10.1097/00001756-199406020-00028. [DOI] [PubMed] [Google Scholar]
- 188.Maneuf YP, Nash JE, Crossman AR, Brotchie JM. Activation of the cannabinoid receptor by delta 9-tetrahydrocannabinol reduces gamma-aminobutyric acid uptake in the globus pallidus. Eur J Pharmacol. 1996;308:161–164. doi: 10.1016/0014-2999(96)00326-3. [DOI] [PubMed] [Google Scholar]
- 189.Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, Azad SC, Cascio MG, Gutierrez SO, van der Stelt M, Lopez-Rodriguez ML, Casanova E, Schutz G, Zieglgansberger W, Di Marzo V, Behl C, Lutz B. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302:84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- 190.Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- 191.Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- 192.Matsuda LA. Molecular aspects of cannabinoid receptors. Crit Rev Neurobiol. 1997;11:143–166. doi: 10.1615/critrevneurobiol.v11.i2-3.30. [DOI] [PubMed] [Google Scholar]
- 193.Matsuda LA, Bonner TI, Lolait SJ. Localization of cannabinoid receptor mRNA in rat brain. J Comp Neurol. 1993;327:535–550. doi: 10.1002/cne.903270406. [DOI] [PubMed] [Google Scholar]
- 194.Mazzola C, Micale V, Drago Amnesia induced by beta-amyloid fragments is counteracted by cannabinoid CB1 receptor blockade. Eur J Pharmacol. 2003;477:219–225. doi: 10.1016/j.ejphar.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 195.Mazzola C, Micale V, Drago Amnesia induced by beta-amyloid fragments is counteracted by cannabinoid CB1 receptor blockade. Eur J Pharmacol. 2003;477:219–225. doi: 10.1016/j.ejphar.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 196.McGregor IS, Arnold JC, Weber MF, Topple AN, Hunt GE. A comparison of delta 9-THC and anandamide induced c-fos expression in the rat forebrain. Brain Res. 1998;802:19–26. doi: 10.1016/s0006-8993(98)00549-6. [DOI] [PubMed] [Google Scholar]
- 197.Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, Pertwee RG, Griffin G, Bayewitch M, Barg J, Vogel Z. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- 198.Mechoulam R, Fride E, Di Marzo V. Endocannabinoids. Eur J Pharmacol. 1998;359:1–18. doi: 10.1016/s0014-2999(98)00649-9. [DOI] [PubMed] [Google Scholar]
- 199.Melck D, Rueda D, Galve-Roperh I, De Petrocellis L, Guzman M, Di Marzo V. Involvement of the cAMP/protein kinase A pathway and of mitogen-activated protein kinase in the anti-proliferative effects of anandamide in human breast cancer cells. FEBS Lett. 1999;463:235–240. doi: 10.1016/s0014-5793(99)01639-7. [DOI] [PubMed] [Google Scholar]
- 200.Melis M, Perra S, Muntoni AL, Pillolla G, Lutz B, Marsicano G, Di Marzo V, Gessa GL, Pistis M. Prefrontal cortex stimulation induces 2-arachidonoyl-glycerol-mediated suppression of excitation in dopamine neurons. J Neurosci. 2004;24:10707–10715. doi: 10.1523/JNEUROSCI.3502-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Melis M, Pistis M, Perra S, Muntoni AL, Pillolla G, Gessa GL. Endocannabinoids mediate presynaptic inhibition of glutamatergic transmission in rat ventral tegmental area dopamine neurons through activation of CB1 receptors. J Neurosci. 2004;24:53–62. doi: 10.1523/JNEUROSCI.4503-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Milton NG. Anandamide and noladin ether prevent neurotoxicity of the human amyloid-beta peptide. Neurosci Lett. 2002;332:127–130. doi: 10.1016/s0304-3940(02)00936-9. [DOI] [PubMed] [Google Scholar]
- 203.Morishita W, Alger BE. Differential effects of the group II mGluR agonist, DCG-IV, on depolarization-induced suppression of inhibition in hippocampal CA1 and CA3 neurons. Hippocampus. 2000;10:261–268. doi: 10.1002/1098-1063(2000)10:3<261::AID-HIPO6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 204.Morisset V, Urban L. Cannabinoid-induced presynaptic inhibition of glutamatergic EPSCs in substantia gelatinosa neurons of the rat spinal cord. J Neurophysiol. 2001;86:40–48. doi: 10.1152/jn.2001.86.1.40. [DOI] [PubMed] [Google Scholar]
- 205.Mu J, Zhuang SY, Kirby MT, Hampson RE, Deadwyler SA. Cannabinoid receptors differentially modulate potassium A and D currents in hippocampal neurons in culture. J Pharmacol Exp Ther. 1999;291:893–902. [PubMed] [Google Scholar]
- 206.Mukhopadhyay S, McIntosh HH, Houston DB, Howlett AC. The CB(1) cannabinoid receptor juxtamembrane C-terminal peptide confers activation to specific G proteins in brain. Mol Pharmacol. 2000;57:162–170. [PubMed] [Google Scholar]
- 207.Mulder AM, Cravatt BF. Endocannabinoid Metabolism in the absence of Fatty Acid Amide Hydrolase (FAAH): Discovery of phosphorylcholine derivatives of N-Acyl ethanolamines. Biochemistry. 2006;45:11267–11277. doi: 10.1021/bi061122s. [DOI] [PubMed] [Google Scholar]
- 208.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 209.Musty RE, Deyo RA, Baer JL, Darrow SM, Coleman B. Effects of SR141716 on animal models of schizophrenia. Symposium on the Cannabinoids; International Cannabinoid Research Society; Burlington, Vermont. 2000. [Google Scholar]
- 210.Naassila M, Pierrefiche O, Ledent C, Daoust M. Decreased alcohol self-administration and increased alcohol sensitivity and withdrawal in CB1 receptor knockout mice. Neuropharmacology. 2004;46:243–253. doi: 10.1016/j.neuropharm.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 211.Nakane S, Oka S, Arai S, Waku K, Ishima Y, Tokumura A, Sugiura T. 2-Arachidonoyl-sn-glycero-3-phosphate, an arachidonic acid-containing lysophosphatidic acid: occurrence and rapid enzymatic conversion to 2-arachidonoyl-sn-glycerol, a cannabinoid receptor ligand, in rat brain. Arch Biochem Biophys. 2002;402:51–58. doi: 10.1016/S0003-9861(02)00038-3. [DOI] [PubMed] [Google Scholar]
- 212.Natarajan V, Reddy PV, Schmid PC, Schmid HH. On the biosynthesis and metabolism of N-acylethanolamine phospholipids in infarcted dog heart. Biochim Biophys Acta. 1981;664:445–448. doi: 10.1016/0005-2760(81)90067-9. [DOI] [PubMed] [Google Scholar]
- 213.Nestler EJ. Molecular neurobiology of addiction. Am J Addict. 2001;10:201–217. doi: 10.1080/105504901750532094. [DOI] [PubMed] [Google Scholar]
- 214.Netzeband JG, Conroy SM, Parsons KL, Gruol DL. Cannabinoids enhance NMDA-elicited Ca2+ signals in cerebellar granule neurons in culture. J Neurosci. 1999;19:8765–8777. doi: 10.1523/JNEUROSCI.19-20-08765.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Nogueron MI, Porgilsson B, Schneider WE, Stucky CL, Hillard CJ. Cannabinoid receptor agonists inhibit depolarization-induced calcium influx in cerebellar granule neurons. J Neurochem. 2001;79:371–381. doi: 10.1046/j.1471-4159.2001.00567.x. [DOI] [PubMed] [Google Scholar]
- 216.Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminal. Neuron. 2001;29:729–738. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- 217.Ohno-Shosaku T, Sawada S, Kano M. Heterosynaptic expression of depolarization-induced suppression of inhibition (DSI) in rat hippocampal cultures. Neurosci Res. 2000;36:67–71. doi: 10.1016/s0168-0102(99)00110-8. [DOI] [PubMed] [Google Scholar]
- 218.Ohno-Shosaku T, Sawada S, Yamamoto C. Properties of depolarization-induced suppression of inhibitory transmission in cultured rat hippocampal neurons. Pflugers Arch. 1998;435:273–279. doi: 10.1007/s004240050512. [DOI] [PubMed] [Google Scholar]
- 219.Ohno-Shosaku T, Tsubokawa H, Mizushima I, Yoneda N, Zimmer A, Kano, M. Presynaptic cannabinoid sensitivity is a major determinant of depolarization-induced retrograde suppression at hippocampal synapses. J Neurosci. 2002;22:3864–3872. doi: 10.1523/JNEUROSCI.22-10-03864.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Okamoto Y, Morishita J, Tsuboi K, Tonai T, Ueda N. Molecular characterization of a phospholipase D generating anandamide and its congeners. J Biol Chem. 2004;279:5298–5305. doi: 10.1074/jbc.M306642200. [DOI] [PubMed] [Google Scholar]
- 221.Onaivi ES, Ishiguro H, Gong JP, Patel S, Perchuk A, Meozzi PA, Myers L, Mora Z, Tagliaferro P, Gardner E, Brusco A, Akinshola BE, Liu QR, Hope B, Iwasaki S, Arinami T, Teasenfitz L, Uhl GR. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann N Y Acad Sci. 2006;1074:514–536. doi: 10.1196/annals.1369.052. [DOI] [PubMed] [Google Scholar]
- 222.Onaivi ES, Leonard CM, Ishiguro H, Zhang PW, Lin Z, Akinshola BE, Uhl GR. Endocannabinoids and cannabinoid receptor genetics. Prog Neurobiol. 2002;66:307–344. doi: 10.1016/s0301-0082(02)00007-2. [DOI] [PubMed] [Google Scholar]
- 223.Ortiz S, Oliva JM, Perez-Rial S, Palomo T, Manzanares J. Differences in basal cannabinoid CB1 receptor function in selective brain areas and vulnerability to voluntary alcohol consumption in Fawn Hooded and Wistar rats. Alcohol Alcohol. 2004;39:297–302. doi: 10.1093/alcalc/agh063. [DOI] [PubMed] [Google Scholar]
- 224.Ortiz S, Oliva JM, Perez S, Palomo T, Manzanares J. Chronic ethanol consumption regulates cannabinoid CB1 receptor gene expression in selected regions of rat brain. Alcohol Alcohol. 2004;39:88–92. doi: 10.1093/alcalc/agh036. [DOI] [PubMed] [Google Scholar]
- 225.Padwal RS, Majumdar SR. Drug treatments for obesity: orlistat, sibutramine, and rimonabant. Lancet. 2007;369:71–77. doi: 10.1016/S0140-6736(07)60033-6. [DOI] [PubMed] [Google Scholar]
- 226.Pan X, Ikeda SR, Lewis DL. Rat brain cannabinoid receptor modulates N-type Ca2+ channels in a neuronal expression system. Mol Pharmacol. 1996;49:707–714. [PubMed] [Google Scholar]
- 227.Paria BC, Dey SK. Ligand-receptor signaling with endocannabinoids in preimplatation embryo development and implantation. Chem Phys Lipids. 2000;108:211–220. doi: 10.1016/s0009-3084(00)00197-3. [DOI] [PubMed] [Google Scholar]
- 228.Patel NA, Moldow RL, Patel JA, Wu G, Chang SL. Arachidonylethanolamide (AEA) activation of FOS proto-oncogene protein immunoreactivity in the rat brain. Brain Res. 1998;797:225–233. doi: 10.1016/s0006-8993(98)00364-3. [DOI] [PubMed] [Google Scholar]
- 229.Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- 230.Pfister-Genskow M, Weesner GD, Hayes H, Eggen A, Bishop MD. Physical and genetic localization of the bovine cannabinoid receptor (CNR1) gene to bovine chromosome 9. Mamm Genome. 1997;8:301–302. doi: 10.1007/s003359900426. [DOI] [PubMed] [Google Scholar]
- 231.Pinto JC, Potie F, Rice KC, Boring D, Johnson MR, Evans DM, Wilken GH, Cantrell CH, Howlett AC. Cannabinoid receptor binding and agonist activity of amides and esters of arachidonic acid. Mol Pharmacol. 1994;46:516–522. [PubMed] [Google Scholar]
- 232.Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- 233.Pisani A, Centonze D, Bernardi G, Calabresi P. Striatal synaptic plasticity: implications for motor learning and Parkinson’s disease. Mov Disord. 2005;20:395–402. doi: 10.1002/mds.20394. [DOI] [PubMed] [Google Scholar]
- 234.Pisani A, Fezza F, Galati S, Battista N, Napolitano S, Finazzi-Agro A, Bernardi G, Brusa L, Pierantozzi M, Stanzione P, Maccarrone M. High endogenous cannabinoid levels in the cerebrospinal fluid of untreated Parkinson’s disease patients. Ann Neurol. 2005;57:777–779. doi: 10.1002/ana.20462. [DOI] [PubMed] [Google Scholar]
- 235.Pitler TA, Alger BE. Activation of the pharmacologically defined M3 muscarinic receptor depolarizes hippocampal pyramidal cells. Brain Res. 1990;534:257–262. doi: 10.1016/0006-8993(90)90137-z. [DOI] [PubMed] [Google Scholar]
- 236.Pitler TA, Alger BE. Postsynaptic spike firing reduces synaptic GABAA responses in hippocampal pyramidal cells. J Neurosci. 1992;12:4122–4132. doi: 10.1523/JNEUROSCI.12-10-04122.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Pitler TA, Alger BE. Depolarization-induced suppression of GABAergic inhibition in rat hippocampal pyramidal cells: G protein involvement in a presynaptic mechanism. Neuron. 1994;13:1447–1455. doi: 10.1016/0896-6273(94)90430-8. [DOI] [PubMed] [Google Scholar]
- 238.Poncelet M, Maruani J, Calassi R, Soubrie P. Overeating, alcohol and sucrose consumption decrease in CB1 receptor deleted mice. Neurosci Lett. 2003;343:216–218. doi: 10.1016/s0304-3940(03)00397-5. [DOI] [PubMed] [Google Scholar]
- 239.Porter AC, Sauer JM, Knierman MD, Becker GW, Berna MJ, Bao J, Nomikos GG, Carter P, Bymaster FP, Leese AB, Felder CC. Characterization of a novel endocannabinoid, virodhamine, with antagonist activity at the CB1 receptor. J Pharmacol Exp Ther. 2002;301:1020–1024. doi: 10.1124/jpet.301.3.1020. [DOI] [PubMed] [Google Scholar]
- 240.Prescott SM, Majerus PW. Characterization of 1,2-diacylglycerol hydrolysis in human platelets. Demonstration of an arachidonoylmonoacylglycerol intermediate. J Biol Chem. 1983;258:764–769. [PubMed] [Google Scholar]
- 241.Pryce G, Baker, D. Emerging properties of cannabinoid medicines in management of multiple sclerosis. Trends Neurosci. 2005;28:272–276. doi: 10.1016/j.tins.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 242.Racz I, Bilkei-Gorzo A, Toth ZE, Michel K, Palkovits M, Zimmer A. A critical role for the cannabinoid CB1 receptors in alcohol dependence and stress-stimulated ethanol drinking. J Neurosci. 2003;23:2453–2458. doi: 10.1523/JNEUROSCI.23-06-02453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Ramirez BG, Blazquez C, Gomez del Pulgar T, Guzman M, de Ceballos ML. Prevention of Alzheimer’s disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. J Neurosci. 2005;25:1904–1913. doi: 10.1523/JNEUROSCI.4540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Ravinet Trillou C, Arnone M, Delgorge C, Gonalons N, Keane P, Maffrand JP, Soubrie P. Anti-obesity effect of SR141716, a CB1 receptor antagonist, in diet-induced obese mice. Am J Physiol Regul Integr Comp Physiol. 2003;284:R345–353. doi: 10.1152/ajpregu.00545.2002. [DOI] [PubMed] [Google Scholar]
- 245.Ravinet Trillou C, Delgorge C, Menet C, Arnone M, Soubrie P. CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. Int J Obes Relat Metab Disord. 2004;28:640–648. doi: 10.1038/sj.ijo.0802583. [DOI] [PubMed] [Google Scholar]
- 246.Reggio PH. Endocannabinoid structure-activity relationships for interaction at the cannabinoid receptors. Prostaglandins Leukot Essent Fatty Acids. 2002;66:143–160. doi: 10.1054/plef.2001.0343. [DOI] [PubMed] [Google Scholar]
- 247.Rhee MH, Bayewitch M, Avidor-Reiss T, Levy R, Vogel Z. Cannabinoid receptor activation differentially regulates the various adenylyl cyclase isozymes. J Neurochem. 1998;71:1525–1534. doi: 10.1046/j.1471-4159.1998.71041525.x. [DOI] [PubMed] [Google Scholar]
- 248.Rimondini R, Arlinde, C, Sommer W, Heilig M. Long-lasting increase in voluntary ethanol consumption and transcriptional regulation in the rat brain after intermittent exposure to alcohol. FASEB J. 2002;16:27–35. doi: 10.1096/fj.01-0593com. [DOI] [PubMed] [Google Scholar]
- 249.Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci USA. 2002;99:8384–8388. doi: 10.1073/pnas.122149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Robbins TW, Everitt BJ. Neurobehavioural mechanisms of reward and motivation. Curr Opin Neurobiol. 1996;6:228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- 251.Robbins TW, Everitt BJ. Limbic-striatal memory systems and drug addiction. Neurobiol Learn Mem. 2002;78:625–636. doi: 10.1006/nlme.2002.4103. [DOI] [PubMed] [Google Scholar]
- 252.Rodriguez de Fonseca F, Roberts AJ, Bilbao A, Koob GF, Navarro M. Cannabinoid receptor antagonist SR141716A decreases operant ethanol self administration in rats exposed to ethanol-vapor chambers. Acta Pharmacol Sin. 1999;20:1109–1114. [PubMed] [Google Scholar]
- 253.Ross RA. Anandamide and vanilloid TRPV1 receptors. Br J Pharmacol. 2003;140:790–801. doi: 10.1038/sj.bjp.0705467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Ross RA, Craib SJ, Stevenson LA, Pertwee RG, Henderson A, Toole J, Ellington HC. Pharmacological characterization of the anandamide cyclooxygenase metabolite: prostaglandin E2 ethanolamide. J Pharmacol Exp Ther. 2002;301:900–907. doi: 10.1124/jpet.301.3.900. [DOI] [PubMed] [Google Scholar]
- 255.Rueda D, Galve-Roperh I, Haro A, Guzman M. The CB(1) cannabinoid receptor is coupled to the activation of c-Jun N-terminal kinase. Mol Pharmacol. 2000;58:814–820. doi: 10.1124/mol.58.4.814. [DOI] [PubMed] [Google Scholar]
- 256.Ryan WJ, Banner WK, Wiley JL, Martin BR, Razdan RK. Potent anandamide analogs: the effect of changing the length and branching of the end pentyl chain. J Med Chem. 1997;40:3617–3625. doi: 10.1021/jm970212f. [DOI] [PubMed] [Google Scholar]
- 257.Sanchez C, Galve-Roperh I, Rueda D, Guzman M. Involvement of sphingomyelin hydrolysis and the mitogen-activated protein kinase cascade in the Delta9-tetrahydrocannabinol-induced stimulation of glucose metabolism in primary astrocytes. Mol Pharmacol. 1998;54:834–843. doi: 10.1124/mol.54.5.834. [DOI] [PubMed] [Google Scholar]
- 258.Schabitz WR, Giuffrida A, Berger C, Aschoff A, Schwaninger M, Schwab S, Piomelli D. Release of fatty acid amides in a patient with hemispheric stroke: a microdialysis study. Stroke. 2002;33:2112–2114. doi: 10.1161/01.str.0000023491.63693.18. [DOI] [PubMed] [Google Scholar]
- 259.Scheen AJ, Finer N, Hollander P, Jensen MD, Van Gaal LF. Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: a randomised controlled study. Lancet. 2006;368:1660–1672. doi: 10.1016/S0140-6736(06)69571-8. [DOI] [PubMed] [Google Scholar]
- 260.Schmid PC, Reddy PV, Natarajan V, Schmid HH. Metabolism of N-acylethanolamine phospholipids by a mammalian phosphodiesterase of the phospholipase D type. J Biol Chem. 1983;258:9302–9306. [PubMed] [Google Scholar]
- 261.Schweitzer P. Cannabinoids decrease the K(+) M-current in hippocampal CA1 neurons. J Neurosci. 2000;20:51–58. doi: 10.1523/JNEUROSCI.20-01-00051.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Seltzman HH, Fleming DN, Thomas BF, Gilliam AF, McCallion DS, Pertwee RG, Compton DR, Martin BR. Synthesis and pharmacological comparison of dimethylheptyl and pentyl analogs of anandamide. J Med Chem. 1997;40:3626–3634. doi: 10.1021/jm9702950. [DOI] [PubMed] [Google Scholar]
- 263.Serra S, Brunetti G, Pani M, Vacca G, Carai MA, Gessa GL, Colombo G. Blockade by the cannabinoid CB(1) receptor antagonist, SR 141716, of alcohol deprivation effect in alcohol-preferring rats. Eur J Pharmacol. 2002;443:95–97. doi: 10.1016/s0014-2999(02)01594-7. [DOI] [PubMed] [Google Scholar]
- 264.Shen M, Thayer SA. The cannabinoid agonist Win55,212-2 inhibits calcium channels by receptor-mediated and direct pathways in cultured rat hippocampal neurons. Brain Res. 1998;783:77–84. doi: 10.1016/s0006-8993(97)01195-5. [DOI] [PubMed] [Google Scholar]
- 265.Simpson CM, Itabe H, Reynolds CN, King WC, Glomset JA. Swiss 3T3 cells preferentially incorporate sn-2-arachidonoyl monoacylglycerol into sn-1-stearoyl-2-arachidonoyl phosphatidylinositol. J Biol Chem. 1991;266:15902–15909. [PubMed] [Google Scholar]
- 266.Soderstrom K, Johnson, F. CB1 cannabinoid receptor expression in brain regions associated with zebra finch song control. Brain Res. 2000;857:151–157. doi: 10.1016/s0006-8993(99)02393-8. [DOI] [PubMed] [Google Scholar]
- 267.Soderstrom K, Leid M, Moore FL, Murray TF. Behaviroal, pharmacological, and molecular characterization of an amphibian cannabinoid receptor. J Neurochem. 2000;75:413–423. doi: 10.1046/j.1471-4159.2000.0750413.x. [DOI] [PubMed] [Google Scholar]
- 268.Stefano GB, Salzet B, Salzet M. Identification and characterization of the leech CNS cannabinoid receptor: coupling to nitric oxide release. Brain Res. 1997;753:219–224. doi: 10.1016/s0006-8993(96)01484-9. [DOI] [PubMed] [Google Scholar]
- 269.Steiner H, Bonner TI, Zimmer AM, Kitai ST, Zimmer A. Altered gene expression in striatal projection neurons in CB1 cannabinoid receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:5786–5790. doi: 10.1073/pnas.96.10.5786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- 271.Straiker A, Mackie K. Depolarization-induced suppression of excitation in murine autaptic hippocampal neurones. J Physiol. 2005;569:501–517. doi: 10.1113/jphysiol.2005.091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Stubbs L, Chittenden L, Chakrabarti A, Onaivi E. The gene encoding the central cannabinoid receptor is located in proximal mouse Chromosome 4. Mamm Genome. 1996;7:165–166. doi: 10.1007/s003359900043. [DOI] [PubMed] [Google Scholar]
- 273.Sugiura T, Kobayashi Y, Oka S, Waku K. Biosynthesis and degradation of anandamide and 2-arachidonoylglycerol and their possible physiological significance. Prostaglandins Leukot Essent Fatty Acids. 2002;66:173–192. doi: 10.1054/plef.2001.0356. [DOI] [PubMed] [Google Scholar]
- 274.Sugiura T, Kodaka T, Kondo S, Nakane S, Kondo H, Waku K, Ishima Y, Watanabe K, Yamamoto I. Is the cannabinoid CB1 receptor a 2arachidonoylglycerol receptor? Structural requirements for triggering a Ca2+ transient in NG108-15 cells. J Biochem (Tokyo) 1997;122:890–895. doi: 10.1093/oxfordjournals.jbchem.a021838. [DOI] [PubMed] [Google Scholar]
- 275.Sugiura T, Kodaka T, Kondo S, Tonegawa T, Nakane S, Kishimoto S, Yamashita AKW. 2-Arachidonoylglycerol, a putative endogenous cannabinoid receptor ligand, induces rapid, transient elevation of intracellular free Ca2+ in neuroblastoma x glioma hybrid NG108-15 cells. Biochem Biophys Res Commun. 1996;229:58–64. doi: 10.1006/bbrc.1996.1757. [DOI] [PubMed] [Google Scholar]
- 276.Sugiura T, Kodaka T, Nakane S, Kishimoto S, Kondo SS, Waku K. Detection of an endogenous cannabimimetic molecule, 2arachidonoylglycerol, and cannabinoid CB1 receptor mRNA in human vascular cells: is 2-arachidonoylglycerol a possible vasomodulator? . Biochem Biophys Res Commun. 1998;243:838–843. doi: 10.1006/bbrc.1998.8187. [DOI] [PubMed] [Google Scholar]
- 277.Sugiura T, Kodaka T, Nakane S, Miyashita T, Kondo S, Suhara Y, Takayama H, Waku K, Seki C, Baba N, Ishima Y. Evidence that the cannabinoid CB1 receptor is a 2-arachidonylglycerol receptor. J Biol Chem. 1999;274:2794–2801. doi: 10.1074/jbc.274.5.2794. [DOI] [PubMed] [Google Scholar]
- 278.Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- 279.Sugiura T, Kondo S, Sukagawa A, Tonegawa T, Nakane S, Yamashita A, Waku K. Enzymatic synthesis of anandamide, an endogenous cannabinoid receptor ligand, through N-acylphosphatidylethanolamine pathway in testis: involvement of Ca(2+)-dependent transacylase and phosphodiesterase activities. Biochem Biophys Res Commun. 1996;218:113–117. doi: 10.1006/bbrc.1996.0020. [DOI] [PubMed] [Google Scholar]
- 280.Sugiura T, Kondo S, Sukagawa A, Tonegawa T, Nakane S, Yamashita A, Waku K. N-arachidonoylethanolamine (anandamide), an endogenous cannabinoid receptor ligand, and related lipid molecules in the nervous tissues. Lipid Mediat Cell Signal. 1996;14:51–56. doi: 10.1016/0929-7855(96)00508-1. [DOI] [PubMed] [Google Scholar]
- 281.Sugiura T, Yoshinaga N, Kondo S, Waku K, Ishima Y. Generation of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand, in picrotoxinin-administered rat brain. Biochem Biophys Res Commun. 2000;271:654–658. doi: 10.1006/bbrc.2000.2686. [DOI] [PubMed] [Google Scholar]
- 282.Sullivan JM. Cellular and molecular mechanisms underlying learning and memory impairments produced by cannabinoids. Learn Mem. 2000;7:132–139. doi: 10.1101/lm.7.3.132. [DOI] [PubMed] [Google Scholar]
- 283.Sun YX, Tsuboi K, Okamoto Y, Tonai T, Murakami M, Kudo I, Ueda N. Biosynthesis of anandamide and N-palmitoylethanolamine by sequential actions of phospholipase A2 and lysophospholipase D. Biochem J. 2004;380:749–756. doi: 10.1042/BJ20040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Szabo B, Dorner L, Pfreundtner C, Norenberg W, Starke K. Inhibition of GABAergic inhibitory postsynaptic currents by cannabinoids in rat corpus striatum. Neuroscience. 1998;85:395–403. doi: 10.1016/s0306-4522(97)00597-6. [DOI] [PubMed] [Google Scholar]
- 285.Szabo B, Muller T, Koch H. Effects of cannabinoids on dopamine release in the corpus striatum and the nucleus accumbens in vitro. J Neurochem. 1999;73:1084–1089. doi: 10.1046/j.1471-4159.1999.0731084.x. [DOI] [PubMed] [Google Scholar]
- 286.Szabo B, Urbanski MJ, Bisogno T, Di Marzo V, Mendiguren A, Baer, W.U, Freiman I. Depolarization-induced retrograde synaptic inhibition in the mouse cerebellar cortex is mediated by 2-arachidonoylglycerol. J Physiol. 2006;577:263–280. doi: 10.1113/jphysiol.2006.119362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Szabo B, Wallmichrath I, Mathonia P, Pfreundtner C. Cannabinoids inhibit excitatory neurotransmission in the substantia nigra pars reticulata. Neuroscience. 2000;97:89–97. doi: 10.1016/s0306-4522(00)00036-1. [DOI] [PubMed] [Google Scholar]
- 288.Takahashi KA, Linden DJ. Cannabinoid receptor modulation of synapses received by cerebellar Purkinje cells. J Neurophysiol. 2000;83:1167–1180. doi: 10.1152/jn.2000.83.3.1167. [DOI] [PubMed] [Google Scholar]
- 289.Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science. 1997;276:2048–2050. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- 290.Thomas MJ, Malenka RC. Synaptic plasticity in the mesolimbic dopamine system. Philos Trans R Soc Lond B Biol Sci. 2003;358:815–819. doi: 10.1098/rstb.2002.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Tucci SA, Halford JC, Harrold JA, Kirkham TC. Therapeutic potential of targeting the endocannabinoids: implications for the treatment of obesity, metabolic syndrome, drug abuse and smoking cessation. Curr Med Chem. 2006;13:2669–2680. doi: 10.2174/092986706778201512. [DOI] [PubMed] [Google Scholar]
- 292.Twitchell W, Brown S, Mackie K. Cannabinoids inhibit N- and P/Q-type calcium channels in cultured rat hippocampal neurons. J Neurophysiol. 1997;78:43–50. doi: 10.1152/jn.1997.78.1.43. [DOI] [PubMed] [Google Scholar]
- 293.Tzavara ET, Li DL, Moutsimilli L, Bisogno T, Di Marzo V, Phebus LA, Nomikos GG, Giros B. Endocannabinoids activate transient receptor potential vanilloid 1 receptors to reduce hyperdopaminergia-related hyperactivity: therapeutic implications. Biol Psychiatry. 2006;59:508–515. doi: 10.1016/j.biopsych.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 294.Ueda N, Kurahashi Y, Yamamoto S, Tokunaga T. Partial purification and characterization of the porcine brain enzyme hydrolyzing and synthesizing anandamide. J Biol Chem. 1995;270:23823–23827. doi: 10.1074/jbc.270.40.23823. [DOI] [PubMed] [Google Scholar]
- 295.Valjent E, Caboche J, Vanhoutte P. Mitogen-activated protein kinase/extracellular signal-regulated kinase induced gene regulation in brain: a molecular substrate for learning and memory? Mol Neurobiol. 2001;23:83–99. doi: 10.1385/MN:23:2-3:083. [DOI] [PubMed] [Google Scholar]
- 296.van der Stelt M, Fox SH, Hill M, Crossman AR, Petrosino S, Di Marzo V, Brotchie JM. A role for endocannabinoids in the generation of parkinsonism and levodopa-induced dyskinesia in MPTP-lesioned non-human primate models of Parkinson’s disease. FASEB J. 2005;19:1140–1142. doi: 10.1096/fj.04-3010fje. [DOI] [PubMed] [Google Scholar]
- 297.van der Stelt M, Mazzola C, Esposito G, Matias I, Petrosino S, De Filippis D, Micale V, Steardo L, Drago F, Iuvone T, Di Marzo V. Endocannabinoids and beta-amyloid-induced neurotoxicity in vivo: effect of pharmacological elevation of endocannabinoid levels. Cell Mol Life Sci. 2006;63:1410–1424. doi: 10.1007/s00018-006-6037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365:1389–1397. doi: 10.1016/S0140-6736(05)66374-X. [DOI] [PubMed] [Google Scholar]
- 299.Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, Marnett LJ, Di Marzo V, Pittman QJ, Patel KD, Sharkey KA. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- 300.Varma N, Carlson GC, Ledent C, Alger BE. Metabotropic glutamate receptors drive the endocannabinoid system in hippocampus. J Neurosci. 2001;21:RC188. doi: 10.1523/JNEUROSCI.21-24-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Vaughan CW, Connor M, Bagley EE, Christie MJ. Actions of cannabinoids on membrane properties and synaptic transmission in rat periaqueductal gray neurons in vitro. Mol Pharmacol. 2000;57:288–295. [PubMed] [Google Scholar]
- 302.Vaughan CW, McGregor IS, Christie MJ. Cannabinoid receptor activation inhibits GABAergic neurotransmission in rostral ventromedial medulla neurons in vitro. Br J Pharmacol. 1999;127:935–940. doi: 10.1038/sj.bjp.0702636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Vincent P, Armstrong CM, Marty A. Inhibitory synaptic currents in rat cerebellar Purkinje cells: modulation by postsynaptic depolarization. J Physiol. 1992;456:453–471. doi: 10.1113/jphysiol.1992.sp019346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Vincent P, Marty A. Neighboring cerebellar Purkinje cells communicate viaretrograde inhibition of common presynaptic interneurons. Neuron. 1993;11:885–893. doi: 10.1016/0896-6273(93)90118-b. [DOI] [PubMed] [Google Scholar]
- 305.Vincent P, Marty A. Fluctuations of inhibitory postsynaptic currents in Purkinje cells from rat cerebellar slices. J Physiol. 1996;494:(Pt 1), 183–199. doi: 10.1113/jphysiol.1996.sp021484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Wagner JA, Varga K, Jarai Z, Kunos G. Mesenteric vasodilation mediated by endothelial anandamide receptors. Hypertension. 1999;33:429–434. doi: 10.1161/01.hyp.33.1.429. [DOI] [PubMed] [Google Scholar]
- 307.Walker JM, Huang SM. Endocannabinoids in pain modulation. Prostaglandins Leukot Essent Fatty Acids. 2002;66:235–242. doi: 10.1054/plef.2001.0361. [DOI] [PubMed] [Google Scholar]
- 308.Wang L, Liu J, Harvey-white J, Zimmer A, Kunos G. Endocannabinoid signaling viaCB1 receptors is involved in ethanol preference and its age-dependent decline in mice. Proc Natl Acad Sci USA. 2003;100:1393–1398. doi: 10.1073/pnas.0336351100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Wartmann M, Campbell D, Subramanian A, Burstein SH, Davis RJ. The MAP kinase signal transduction pathway is activated by the endogenous cannabinoid anandamide. FEBS Lett. 1995;2-3:133–136. doi: 10.1016/0014-5793(95)00027-7. [DOI] [PubMed] [Google Scholar]
- 310.Watson SJ, Benson JA, Jr, Joy JE. Marijuana and medicine: assessing the science base: a summary of the 1999 Institute of Medicine report. Arch Gen Psychiatry. 2000;57:547–552. doi: 10.1001/archpsyc.57.6.547. [DOI] [PubMed] [Google Scholar]
- 311.Wilson RI, Kunos G, Nicoll RA. Presynaptic specificity of endocannabinoid signaling in the hippocampus. Neuron. 2001;31:453–462. doi: 10.1016/s0896-6273(01)00372-5. [DOI] [PubMed] [Google Scholar]
- 312.Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- 313.Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- 314.Wise RA. Addictive drugs and brain stimulation reward. Annu Rev Neurosci. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- 315.Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- 316.Yamaguchi F, Macrae AD, Brenner S. Molecular cloning of two cannabinoid type 1-like receptor genes from the puffer fish Fugu rubripes. Genomics. 1996;35:603–605. doi: 10.1006/geno.1996.0406. [DOI] [PubMed] [Google Scholar]
- 317.Yang HY, Karoum F, Felder C, Badger H, Wang TC, Markey SP. GC/MS analysis of anandamide and quantification of Narachidonoylphosphatidylethanolamides in various brain regions, spinal cord, testis, and spleen of the rat. J Neurochem. 1999;72:1959–1968. doi: 10.1046/j.1471-4159.1999.0721959.x. [DOI] [PubMed] [Google Scholar]
- 318.Yoshida T, Fukaya M, Uchigashima M, Miura E, Kamiya H, Kano M, Watanabe M. Localization of diacylglycerol lipase-alpha around postsynaptic spine suggests close proximity between production site of an endocannabinoid, 2-arachidonoyl-glycerol, and presynaptic cannabinoid CB1 receptor. J Neurosci. 2006;26:4740–4751. doi: 10.1523/JNEUROSCI.0054-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Yoshida T, Hashimoto K, Zimmer A, Maejima T, Araishi K, Kano M. The cannabinoid CB1 receptor mediates retrograde signals for depolarization-induced suppression of inhibition in cerebellar purkinje cells. J Neurosci. 2002;22:1690–1697. doi: 10.1523/JNEUROSCI.22-05-01690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Zilberter Y. Dendritic release of glutamate suppresses synaptic inhibition of pyramidal neurons in rat neocortex. J Physiol. 2000;528:(Pt 3), 489–496. doi: 10.1111/j.1469-7793.2000.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]