Abstract
Expression of tobacco mosaic virus (TMV) coat protein (CP) restricts virus disassembly and alters the accumulation of the movement protein (MP). To characterize the role of structure of transgenic CP in regulating virus disassembly and production of MP, we generated CPs with mutations at residues Glu50 and Asp77, located in the interface between juxtaposed CP subunits. In transgenic Nicotiana tabacum and BY-2 cells, three categories of coat protein-mediated resistance (CP-MR) levels were identified: wild-type CP-MR; elevated CP-MR; and no CP-MR. Mutant CPs that interfered with the accumulation of virus replication complexes conferred very high levels of protection to TMV, except by CPE50D which provided no protection in the systemic host (Xanthi-nn) but high CP-MR in the local lesion host (Xanthi-NN). In transgenic BY-2 cells CPE50D strongly reduced accumulation of MP:GFP. In general, there was a strong correlation between the capacity for CP to assemble to pseudovirions and CP-MR, while there was not strong correlation with packaging viral RNA and CP-MR. The data demonstrate that interference with one or more steps in virus infection and replication by wild type and mutant CP determine the degree of CP-MR.
Keywords: TMV, CP, tobacco, CP-MR, protein interaction, virus assembly, replication, MP
Introduction
The structural proteins of plant viruses have evolved to self-associate into complex macromolecules that are centrally involved in virus biology. The structural and biophysical properties of tobacco mosaic virus (TMV; type member of the genus Tobamovirus) coat protein (CP) have been widely used to study the role of macromolecular assembly in the biology of virus-host interactions, in particular in host resistance and disease development. The TMV genome encodes at least four proteins: the 126-kDa and 183-kDa replicase proteins, the 30-kDa cell-to-cell movement protein (MP), and the 17.5-kDa CP. The MP and CP are encoded by subgenomic RNAs that are co-terminal with the 3' end of virion RNA (Goelet et al., 1982). The CP can elicit specific host resistance in transgenic plants (coat protein mediate resistance; CP-MR). Other studies indicate that the three-dimensional structure of CP is critical to the control of these responses, either directly through specific structural motifs or indirectly via alterations in CP assembly and disassembly processes. [For more details see the review by Culver (2002)].
TMV particles are highly stable in vitro. Upon entry into a cell, however, disassembly of virions proceeds very rapidly via a process that involves virion destabilization that likely results from repulsion between carboxyl-carboxylate groups on amino acids (a.a.) at residues 50 (glutamic acid; Glu, E) and 77 (aspartic acid; Asp, D). These a.a. are located on the interface between adjacent and juxtaposed CP subunits. In the extracellular environment, the negatively charged carboxylate groups are stabilized by cations, such as Ca2+ ions or protons (Caspar and Namba, 1990). Structural studies of the virion revealed inter-subunit carboxylate interactions at three locations within the virus (Namba and Stubbs, 1986), including the interaction of Glu50 from one subunit with Asp77 of the subunit below (Figure 1).
Figure 1.
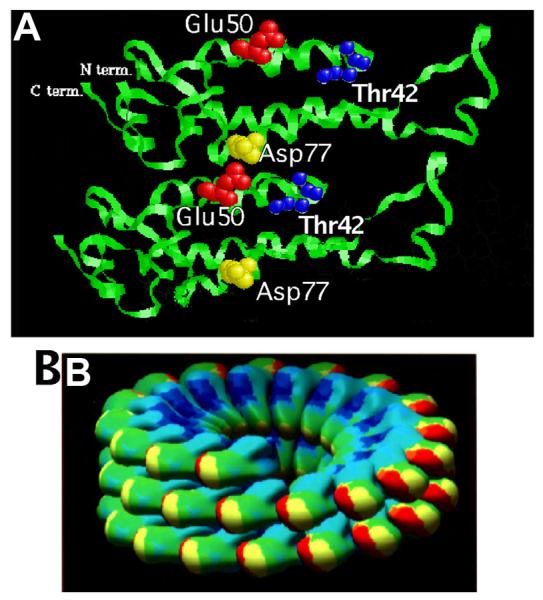
(A) Ribbon representation of the alpha-carbon backbone of two CP subunits belonging to two superimposed helical turns of TMV virion. The N and C termini located at the outer radius if the virus are indicated. The position of the interacting carboxylate residues Glu50 and Asp77 and of the Thr42 residue is shown. (B) Computer graphic representation of two and half virion helical turns of CP subunits. The N-terminus and the C-terminus of the CP molecules lie on the external surface of the assembled subunits.
Coat protein-mediated resistance (CP-MR) refers to the resistance of transgenic plants that produce CP to the virus from which the CP gene is derived (Powell-Abel et al., 1986). Despite extensive studies, the molecular mechanisms that govern CP-MR are not fully understood; furthermore, mechanisms of CP-MR are different for different viruses. In the case of CP-MR against TMV, the CP interferes with virus disassembly (reviewed by Bendahmane and Beachy, 1999). Bendahmane et al. (1997) showed that mutant CPs that lack the ability to aggregate failed to protect against TMV infection, whereas a CP with increased aggregation, eg., Thr42Trp (CPT42W), provided higher levels of protection than did wild type (w.t.) CP. These results established a direct correlation between the capacity for CP self-assembly and CP-MR. These findings were later confirmed by Lu et al., (1998) using similar and also different mutant CPs expressed via a PVX transient expression vector. One of the mutants designed to remove repulsive inter-subunit carboxylate interaction between E50 and D77, namely mutant CPE50Q/D77N, was shown to stabilize CP–CP interactions and to provide significantly higher levels of CP-MR as compared to wild-type CP (Lu et al., 1998). Subsequently, Bendahmane et al. (2002) reported that w.t. CP can have a positive effect on the production of MP and, thereby, on virus replication and cell to cell movement. Other experiments showed that CPT42W interferes with normal function of w.t. CP and has a negative effect on MP accumulation (Bendahmane et al, 2002) that reduces the formation of virus replication complexes (Asurmendi et al, 2004). This in turn restricts cell-cell spread of infection and increases the efficacy of CP-MR. A mutant of CP that did not assemble, CPT28W, did not restrict production of VRCs (Virus Replication Complexes) or provide CP-MR.
In the present study, we applied knowledge of the atomic structure of TMV CP to generate mutant CPs that target the inter-subunit carboxyl-carboxylate interactions between Glu50 and Asp77 residues. The mutant CPs were then tested in virus infection and CP-MR assays. We show that CP-MR to TMV involves at least two independent mechanisms: (i) interference by transgenic CP with disassembly of challenge virus; and (ii) interference of transgenic CP with formation of replication complexes, thus interfering with virus movement. We propose that the degree of regulation of replication by aggregates of CP determines the relative strength of CP-MR that is conferred by w.t. and mutant CP.
RESULTS
Effects of mutants of CP on virus biology
The known atomic structure of the TMV CP (Namba et al., 1989) was used to select for mutation amino acids (a.a.) that are important for CP subunit interactions. Interactions between carboxylate groups between Glu50 and Asp77 (Figure 1; located in RS and RR alpha-helices, respectively; Namba et al., 1989) result in repulsion of axially adjacent subunits. It was proposed that repulsion of these amino acids play an important role in TMV disassembly (Culver, et al., 1995; Lu et al., 1996). The removal or change of inter-subunit interactions is expected to enhance or reduce the stability of aggregates of CP molecules (depending upon the mutation) due to effects on the axially adjacent turns of the helix that forms as virus and virus-like particles (VLPs) are assembled. Ten amino acid substitutions were created to disrupt the specific carboxyl-carboxylate inter-CP subunit interactions between Glu50 and Asp77. The amino acids substitutions that created four types of mutations (see bellow) are expected to interfere with normal quaternary structure of CP subunits but not with its ternary structure.
Effects on virus assembly
Mutant CPs were examined for their ability to form VLPs following infection of tobacco leaves with virus constructs that contain mutant CP (Table 1). All virus mutants produced CP molecules indicating that mutations did not completely abolish virus replication or CP accumulation (data not shown). Electron microscopy studies revealed that all infections produced VLPs (Figure 2) indicating that the mutations did not affect self-assembly of the CP. However, most mutants failed to produce VLPs of the size of w.t. TMV (i.e., ∼300 nm); this indicates that selected mutations affected virion or VLP stability.
Table 1.
Summary showing the effect of mutations in TMV CP at amino acids Thr28, Thr42, Glu50 and Asp77.
|
CP w.t. |
aVLPs Virion |
bVirus systemic movement in Xanthi-nn Yes |
CP-MR against TMV in transgenic | |
|---|---|---|---|---|
|
cXanthi NN (Local lesions) 99% |
dXanthi nn (Fig. 3) W.t. CP-MR |
|||
| CPE50K | VLPs (long rods) |
eYes | 90-96% | Equal to w.t. CP- MR |
| CPE50R | VLPs(long rods) | Delayed | 99-99.4% | Equal to w.t. CP- MR |
| CPD77N | VLPs (short rods) |
Delayed | 99-100% | Equal to w.t. CP- MR |
| fCPT42W | Highly stable VLP |
No | 96-99% | Better than w.t. CP-MR |
| CPD77R | VLPs (long rods) |
Yes | 96-98% | Better than w.t. CP-MR |
| CPE50M CPD77E |
VLPs (W.t.) VLPs (long rods) |
Yes Yes |
99.8-100% 98.6-99.4% |
Better than w.t. CP-MR Better than w.t. CP-MR |
| CPD77A CPE50Q |
VLPs VLPs (long rods) |
Delayed Delayed |
100% 96-99% |
Better than w.t. CP-MR Better than w.t. CP-MR |
| fCPT28W | No ordered aggergates |
No | 0% | No protection |
| CPD77K | VLPs | No | 0% | No protection |
| CPE50D | VLPs (long rods) | No | 90-97% | No protection |
Effect of each mutation on virion assembly was assessed by introducing the mutation in TMV virus (see also Figure 2).
Ability of TMV mutants to move systemically in Xanthi-nn. Virus systemic movement was measured by comparing the rate symptoms development on systemic leaves: Yes = Development of symptoms on the systemic leaves similar to that in wt TMV inoculated plants (about 8 to 10 days post-inoculation); No = inoculated plants never developed symptoms on the systemic leaves; Delayed = inoculated plants developed symptoms on the systemic leaves a least 25 to 30 days post-inoculation.
CP mutated at one of the above amino acids were expressed in transgenic plants and their ability to provide CP-MR against TMV was measured in transgenic Xanthi-NN and in transgenic.
CP mutated at one of the above amino acids were expressed in transgenic plants and their ability to provide CP-MR against TMV was measured in transgenic Xanthi-nn (Figure 3).
Symptoms produced by TMV-CPE50K on systemic leaves of infected N. tabacum Xanthi-nn plants consisted in leaf curling and local lesions formation.
Figure 2.
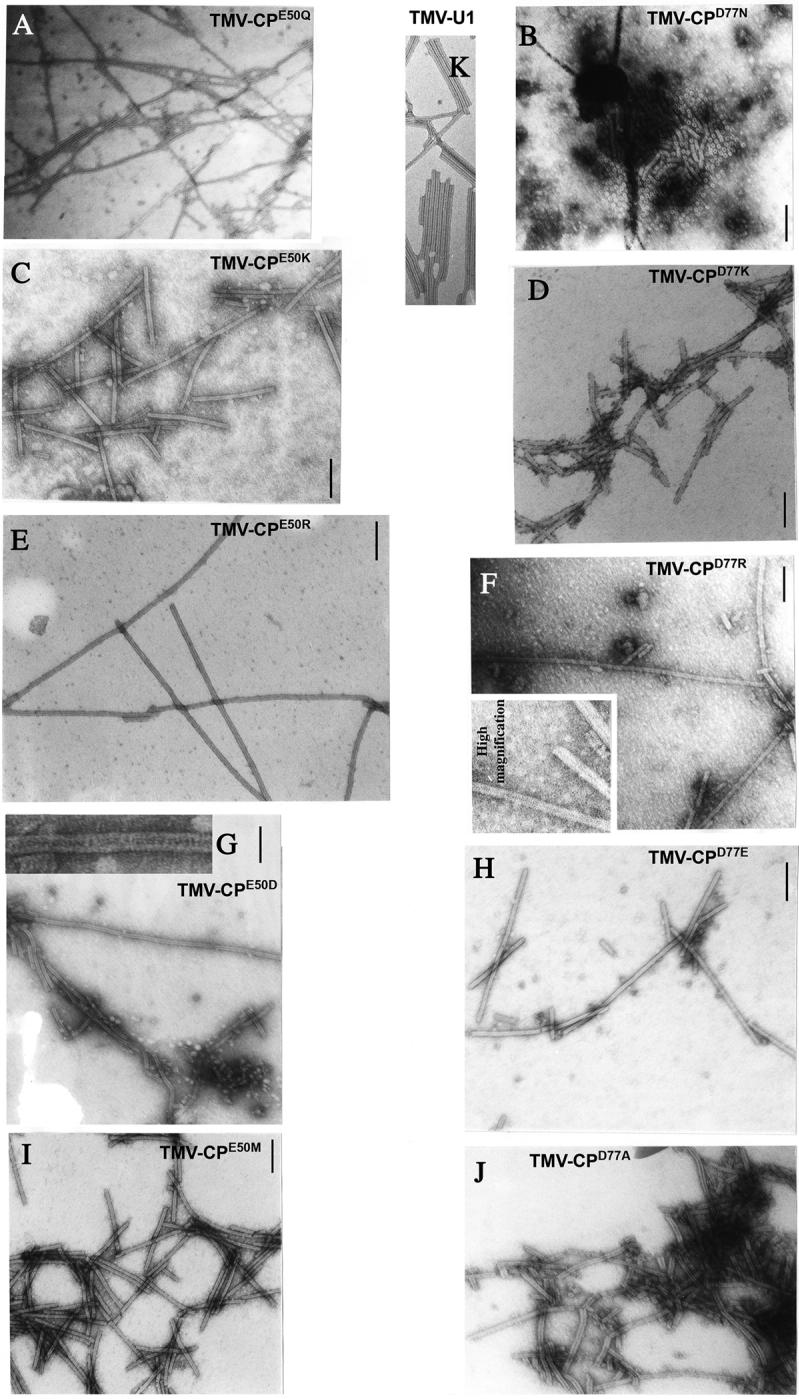
Electron-microscopic analysis of assembled VLPs from N. tabacum plants infected with TMV mutated at amino acid E50 (TMV-CPE50Q, TMV-CPE50K, TMV-CPE50R, TMVCPE50D, TMV-CPE50M) or at amino acid D77 (TMV-CPD77N, TMV-CPD77K, TMV-CPD77R, TMV-CPD77E, TMV-CPD77A) or w.t. (TMV-U1). The samples were negatively stained and analyzed at a magnification of 39,000. The bar represents 100 nm.
Type 1 substitution mutations E50D and D77E were designed to alter hydrogen bonding patterns between side chains of adjacent a.a. while preserving the two negative charges in the axial inter-subunit interface, thus preserving electrostatic repulsion. However, because Glu has two -CH2 groups whereas Asp has one, mutant CPD77E was expected to reduce the distance between side chain residues at 50 and 77, and increase the distance for the mutant CPE50D. Both mutations were predicted to interfere with the packing and stability of VLPs. However, both mutations resulted in the formation of long VLPs, indicating that the protein has greater aggregation potential than w.t. CP, and was not limited to the length of viral RNA (Figure 2, panels G, H).
Type 2 substitution mutants E50Q or D77N were expected to remove the repulsive inter-subunit charges without altering side-chain hydrogen bonding. TMV-CPD77N produced open disks and short stacks of disks in a non-helical structure, while TMV-CPE50Q produced, as expected, long aggregates of CP (i.e., VLPs), (Figure 2, panels A,B).
Type 3 substitution mutants CPE50M and CPD77A are predicted to remove the repulsive inter-subunit charge and alter side-chain hydrogen bonding patterns. EM analysis of leaf extracts from plants infected with TMV-CPE50M showed the presence of virion-like helical rods that are similar to those of the w.t. virus. However, particles formed by CPD77A were flexuous and significantly shorter than those of w.t. TMV (Figure 2, panels I,J).
Type 4 substitution mutants E50R, E50K, D77R or D77K are predicted to alter the electrostatic repulsion site and induce inter-subunit salt-bridges that are expected to stabilize inter-subunit interactions and thus enhance the stability of the CP helical structure in virions and VLPs. Arg substitution mutants, TMV-CPE50R and TMV-CPD77R, as expected, produced very long VLPs (Figure 2, panels E,F), while the Lys mutants TMV-CPE50K and TMV-CPD77K produced VLPs of shorter length than the Arg mutants (Figure 2, panels C,D). The local structural changes introduced by differences in side chains of these basic amino acids may be responsible for differences in the aggregation of the mutant CPs. The side chain of Lys is hydrophobic, due to the four methylene groups and an amino group, whereas the Arg side chain consists of three hydrophobic methylene groups and the strongly basic gamma-guanido group. The gamma-guanido group of Arg and the amino group of Lys possess different ionization properties and positive charge distribution. VLPs formed by CPE50K always co-purified with aggregates of material of unknown nature, presumably as a consequence of the mutation (Figure 2, panel C).
In order to determine whether mutant CPs form infectious virions we determined the ability of TMV carrying mutant CP to cause rapid systemic infection in N. tabacum Xanthi-nn. All mutant viruses, except TMV-CPE50D and TMV-CPD77K, systemically infected N. tabacum Xanthi-nn, at rates similar to, or slower than, w.t. TMV. (Table 1). Plants inoculated with TMV-CPE50K, TMV-CPE50M, TMV-CPD77E or TMV-CPD77R developed systemic symptoms by 8 to 10 dpi, similar to plants inoculated with w.t. TMV. Therefore, the particles formed by these mutant CPs are presumed to assemble with vRNA to provide systemic infection. Plants inoculated with TMV-CPE50R, TMV-CPE50Q, TMV-CPD77A or TMV-CPD77N developed symptoms after 25 dpi. Therefore, the infectious particles formed by these mutant CPs is presumed to be of low efficiency. Alternatively slow systemic spread may indicate lack of virion formation and nonvascular systemic spread. The delay in systemic infection may also be due to reduced cell-to-cell movement of viral RNA as observed with mutant CPT42W (Bendahmane et al., 2002).
Effects of mutations on CP-MP in transgenic plants
Transgenic tobacco plants that express CP genes encoding mutations at E50 or D77 residues were developed. At least five independent transgenic lines were selected for each mutant based on accumulation of CP equivalent to accumulation of w.t. CP in N. tabacum Xanthi-nn line 3646 (Powell Abel et al., 1986), and N. tabacum Xanthi-NN line 748 (Nelson et al, 1987). CP-MR against TMV infection was assayed in homozygous transgenic lines and compared to CP-MR in lines 3646 and 748. TMV was applied at 0.1 μg/ml; this produced a mean of ∼80 local lesions per leaf on non-transgenic N. tabacum Xanthi NN plants. We present the results of experiments with selected lines for each CP mutant; the data were consistent amongst the lines of each CP mutant tested.
CP-MR in Xanthi-nn transgenic lines
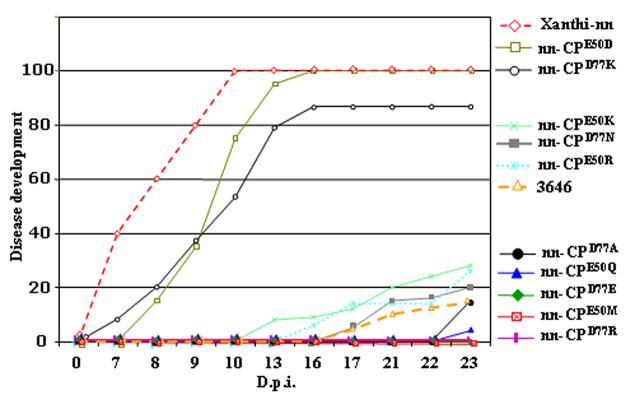
The percentage of Xanthi-nn plants that developed systemic disease symptoms were scored from 7 dpi, averaged, and compared with non-transgenic plants (0% protection) and plant line 3646. As shown in Figure 3, we observed three levels of protection.
Figure 3. Resistance of transgenic plant lines that produce mutant CPs to systemic infection by TMV.
Protection assay for TMV infection in transgenic N. tabacum Xanthi-nn plants expressing wt CP (line 3646) or CP carrying mutations at amino acids Asp77 or Glu50. The plants were inoculated with the TMV-U1 virus (0.1 mg/ml), and disease development was scored as the percentage of plants that exhibit symptoms on upper leaves and accumulation of TMV in the systemic leaves was measured by ELISA using anti-CP antibody. ELISA was performed on samples from 10 plants for each line, as described (Bendahmane et al., 1997).
Transgenic lines accumulating CPE50D or CPD77K (e.g., plant lines e and j, respectively) developed disease symptoms that were not different from non-transgenic plants. In these lines the onset and severity of disease symptoms and virus accumulation in upper systemically infected leaves were similar to non-transgenic plants (as analyzed in ELISA using anti-CP antibody; data not presented).
Transgenic lines accumulating CPE50K, CPE50R or CPD77N (e.g., lines a, b and c, respectively) exhibited levels of CP-MR similar to that provided by w.t. CP in line 3646.
Plant lines accumulating CPE50Q (line a), CPE50M (line d), CPD77A (line a), CPD77E (line h) or CPD77R (line c) did not develop symptoms when inoculated with 0.1 μg/ml of virus, whereas line 3646 showed a delay in symptom appearance. Lines nn-CPE50M, nn-CPD77E and nn-CPD77R did not contain detectable virus in upper leaves at 23 dpi, based on ELISA. Lines nn-CPE50Q and nn-CPD77A showed very mild symptoms of infection and virus accumulation in the systemic leaves at 23 d.p.i., but provided higher protection to TMV infection than line 3646.
CP-MR in Xanthi-NN transgenic lines
Transgenic Xanthi-NN plants which accumulated each of the mutant CPs were also developed, and five lines that accumulated CP levels equivalent to those in plant line 748 (contains w.t. CP) were selected for further study. Plants were inoculated with TMV at an inoculum concentration of 0.1 μg/ml, and the numbers of local necrotic lesions were determined, averaged, and compared with the number of lesions produced on non-transgenic plants (0% protection) and on line 748 plants (Table 1).
Little or no protection was exhibited in NN-CPD77K lines and each plant line carrying this CP was equally as susceptible to infection as non-transgenic Xanthi-NN plants. This result was expected since CPD77K did not provide protection in Xanthi-nn plants. Xanthi-NN plants expressing all other mutant CPs (except for CPE50D) provided high levels of protection to TMV infection. However, there was not strong agreement between levels of protection in Xanthi-NN and Xanthi-nn transgenic lines. Based on these results, we grouped mutants into five classes of protection:
Mutants CPE50R and CPD77N provided protection equivalent to that provided by w.t. CP in Xanthi-nn lines, and provided high levels of protection in Xanthi-NN plants.
Mutants CPE50M, CPE50Q, CPD77A, CPD77E provided equally high levels of protection in Xanthi-nn and Xanthi-NN transgenic lines and levels of protection stronger than provided by w.t. CP. Some plant lines were equally as resistant as plant lines that contain CPT42W (Bendahmane et al., 1997, 2002)
Mutant CPE50K provided protection equivalent to that provided by w.t. CP in Xanthi-nn lines, but provided slightly lower protection in transgenic Xanthi-NN (90% to 96% compared to 99% protection in line 748).
Mutant CPD77R provided stronger protection than w.t. CP in Xanthi-nn plants (equivalent to that in line nn-CPT42W; Bendahmane et al., 1997) and lower protection than w.t. CP in Xanthi-NN.
Mutant CPE50D provided no protection in Xanthi-nn plants but provided a high level of protection in Xanthi-NN.
CP-MR in transgenic BY-2 protoplasts
Earlier studies conducted in BY-2 protoplasts revealed that expression of CPT42W reduced accumulation of MP and consequently reduced the formation of VRCs (virus replication complexes; Bendahmane et al, 2002; Asurmendi et al, 2004). To characterize virus replication in the mutant lines we followed the accumulation of replicase (Rep), MP and CP by ELISA following infection of protoplasts from BY-2 cells lines after inoculation with TMV RNA. The mutant CPs selected for this study represented different structural characteristics represented by the grouping described above. Lines BY-CPT42W and BY-CP (w.t. CP) were included as controls and results are similar to those reported by Bendahmane et al, 2002). Protoplasts of cell lines BY-CPE50D, BY-CPE50R, BY-CPD77K, and BY-CPD77R were infected and aliquots were collected after 11, 16, 24 and 36 hpi: data are presented in Figure 4.
Figure 4. Accumulation of CP (A), MP (B) and replicase (C) proteins in BY-2 protoplasts non-transgenic or expressing wt CP or mutant CPs.

Protoplast were prepared from transgenic BY-2 cells expressing CPT42W, CPD77k, CPD77R, CPE50R or CPE50D, and then infected with TMV RNA. Protoplast samples were harvested at 11, 16, 24, and 36 hpi. The effect of the transgenic CP (mutant or wt) on the accumulation of the CP, the MR and the replicase of the infecting virus was assessed using antibodies directed against each of these viral proteins in ELISA experiments as described (Bendahmane et al., 1997).
Cell lines BY-CPE50R and BY-CPD77K showed a similar pattern of accumulation of rep, MP and CP as the BY-CP line. In these lines the level of MP was significantly greater than in the non-transgenic line; similar results were observed with the level of replicase although the differences relative to the non-transgenic cell line were not as great as with MP. The amount of CP was greater in these cell lines when compared with the non-transgenic line in contrast with the results in BY-CP, which was not significantly different from the non-transgenic cell line.
In contrast infection of protoplasts from BY-CPD77R was similar to infection of cell line BY-CPT42W. These two lines show a marked decrease of all three viral proteins when compared with the non-transgenic line, indicating reduction in virus replication (Bendahmane et al, 2002). The results observed in cell line BY-CPE50D are more difficult to explain, as replication in this line is not as widely different than the non- transgenic line except at 36 hpi. It is worth noting that BY-CPE50D is the most different line within the CPT42W group with regard to accumulation of MP:GFP (see below). The accumulation of MP is indicative of significant virus replication but may indicate that regulation of MP production late in replication does not occur in presence of CPE50D. This is also the only mutant that provides high level of CP-MR in Xanthi NN and low protection on the systemic host (Table 1).
Effect of mutant CPs on MP accumulation
We showed in earlier studies that CPT42W interferes with cell to cell movement by directly affecting MP accumulation and/or function (Bendahmane et al., 2002). Glu50 is located on the RS alpha-helix of the CP, as is Thr42. Furthermore, ionic interactions between Glu50 and Asp77 occur in the same plane as does Thr42, i.e., in the axial interaction between two juxtaposed CP subunits. We therefore analyzed the effect of each mutation at Glu50 and 4 of the 5 mutations at Asp77 on MP:GFP fusion protein accumulation during infection to determine if changes in CP structure will affect accumulation of the fusion protein in infected protoplasts. Protoplasts from transgenic BY2 cells that express mutant or w.t. CP were infected with TMV-MP:GFP and the accumulation of MP:GFP was followed by fluorescence microscopy of each cell line at 10, 16, 24 and 36 hours post-infection (h.p.i). Since infection with TMV RNA overcomes CP-MR (Powell et al., 1986; Register and Beachy, 1988) we achieved a high proportion of infected protoplasts in these studies.
In non-transgenic BY2 cells infected with TMV-MP:GFP, the MP:GFP fusion protein accumulates in unique patterns at different times during the infection cycle (Heinlein et al., 1995). For the present study, the patterns of accumulation of MP:GFP can be grouped in three stages that reflect early, mid, and late stages of infection (Figure 5A). The results of the studies are presented in Figure 5B and, as shown, the progression of accumulation of MP:GFP follows three patterns into which each CP mutant can be placed.
Figure 5. Effect of mutant CPs expressed in transgenic BY2 cells on MP:GFP following TMV-MP:GFP infection.

(A) Patterns of accumulation of MP:GFP in BY-2 protoplasts inoculated with TMV-MP:GFP as described in Heinlein et al. (1995). Protoplasts were inoculated with TMV-MP:GFP RNA and the accumulation of MP:GFP was analyzed by fluorescence microscopy. Pictures were taken at 12, 15, and 24 hpi corresponding to early, mid and late stages of infection by TMV, respectively. MP:GFP patterns found at each stage are presented. (B) Quantification of the presence of each of the six paterns (above) of MP:GFP accumulation during TMV-MP:GFP infection in protoplast expressing mutant CPs at 10, 16, 24 and 36 hpi. The presence of each pattern was normalized for the total number of cells counted per point and expressed as a percent. The three groups of mutants CP with respect to their effect on MP accumulation are presented: group (1) CP mutants with postivie effects on MP accumulation similar to w.t. CP; group (2) CP mutants that lost the positive effect of w.t. CP on MP accumulation patterns; group (3) CP mutants that acquired negative effects on accumulation of MP.
The first group of mutant CPs comprises mutations at residue Glu50, e.g., CPE50K, CPE50M and CPE50R. Expression of these mutant CPs have positive effects on MP:GFP accumulation that are similar to, but not identical with, results previously reported for w.t. CP (Bendahmane et al., 2002). In this group a high percentage of infected cells accumulate large fluorescent bodies that contain MP:GFP (such bodies are known to be virus replication complexes, VRCs; Kawakami et al, 2004) throughout the infection period 0-36 hpi. This result indicates that in these protoplasts VRCs continue to produce and accumulate MP:GFP or that the transition of MP:GFP to microtubules or degradation is negatively affected in these infections than in wild-type protoplasts. We conclude that MP:GFP continues to accumulate throughout infection in these cells; this is in agreement with data in Figure 4.
Group 2 includes cell lines producing CP mutants that do not have an effect on accumulation of MP:GFP; in these cell lines, MP-containing fluorescent structures accumulated as in infected non-transgenic BY2 protoplasts. In this group infection proceeds through the early and mid stages of infection in a now-familiar pattern (described by Bendahmane et al., 2002) and by late stages (36 hpi) the MP:GFP is associated with microtubules and relatively small fluorescent bodies. This group includes CPD77A, CPD77E, CPD77N (all Asp77 mutants except CPD77R). We conclude that these mutants do not affect the production or accumulation of flourescent MP:GFP-containing bodies in a positive or negative manner. Nevertheless, these CPs provide high levels of CP-MR in local lesion and systemic hosts (Table 1, and Figure 3).
The third group of CP mutants includes CPE50D, CPE50Q and CPD77R, each of which has a negative effect on MP:GFP accumulation similar to the effect of CPT42W (Bendahmane et al., 2002). In protoplasts of cell lines that contain these mutants, MP:GFP accumulated in small bodies similar to those produced early in infection in non-transgenic protoplasts. Infections in these cells lines produced very few large VRCs and very few cells proceeded to mid- and late stages of infection. The data are in agreement with results of studies described in Fig. 4 showing that CPT42W, CPE50D and CPD77R restrict production and/or accumulation of MP.
DISCUSSION
Earlier studies have shown that CP-MR against tobamoviruses is provided by accumulation of transgenic CP. Using mesophyll protoplasts from non-transgenic as well as from transgenic plants expressing w.t. CP, previous studies demonstrated inhibition of disassembly of TMV in cells expressing the CP (reviewed by Bendahmane and Beachy, 1999). We also showed a strong positive correlation between the ability of transgenic CP to self-aggregate to form VLPs and CP-MR (Bendahmane et al., 1997). Recently, we provided evidence that protection against CP-MR in this system is the cumulative effect of interference by transgenic CP with virus disassembly and accumulation of MP during virus infection. The latter effect is evident in a CP mutant CPT42W, and reduces cell to cell virus movement of infection; Bendahmane et al., 2002).
To further characterize the mechanism of CP-MR we generated additional mutations in TMV CPs, and selected the site at the electrostatic repulsion site formed by Glu50 and Asp77 residues in adjacent subunits in the quaternary structure. Repulsion of subunits is apparently prevented by binding of divalent cations at this site (Namba and Stubbs, 1986), thus enabling assembly of virions and VLPs. Alterations in virion stability that take place in virus disassembly initiates infection and indicates a stability-switching mechanism that can respond to subtle changes in the surrounding environment. (Caspar 1963; Caspar and Namba, 1990). We generated mutants at each of these amino acid residues in order to interfere with Glu50–Asp77 interactions. Glu50 and Asp77 residues are located on the RS and RR helices within the CP three-dimensional structure, respectively (Figure 1). The objective was to remove this electrostatic repulsion site and increase the stability of interactions between axially adjacent CPs in the quaternary, but not the ternary structure of CP. As predicted, mutations affecting this site of inter-subunits interactions did affect the normal assembly of virions. Most mutant CPs formed long and/or flexuous VLPs, some of which are formed by stacked discs. (Figure 2).
We observed a variety of effects of the mutations on CP-MR in transgenic N. tabacum plants. CPD77K did not provide resistance to TMV infection in transgenic Xanthi-NN or Xanthi-nn. This result was unanticipated since CPD77K can assemble to VLPs. A possible explanation for the lack of CP-MR comes from the electron microscopy analysis of TMV- CPD77K VLP, which shows that the CPD77K is associated with unknown debris in plant extracts. This association may interfere with CP-MR by this mutant CP.
In the local lesion host Xanthi-NN, transgenic plants expressing CPE50D provided 90% to 97% resistance to TMV infection. Resistance to infection per se (reflected in resistance in the local lesion host) was anticipated since CPE50D produces VLPs. In contrast, this mutant conferred no CP-MR in the systemic host. The latter result was unexpected since CPE50D reduced the production of MP and accumulation of MP:GFP in infected protoplasts (Figures 4, 5), conditions that would reduce cell-cell spread. However, a closer analysis of data in Figure 5 (Group 3) revealed that in cell line BY-CPE50D there were higher numbers of large fluorescent bodies (VRCs) than in other cell lines in this group; this may increasing the likelihood of systemic infection. This result could explain the observation that resistance in these plant lines that contain CPE50D gave high levels of CP-MR in some experiments and not in others. This is the only mutant in which variable results were observed.
CPD77N provides protection similar to w.t. CP in Xanthi-nn (line 3646) and higher protection than w.t. CP protection in Xanthi-NN (line 748). The high stability of VLPs produced by TMV-CPD77N indicates that CPD77N interferes with virus disassembly and provides CP-MR equivalent to that provided by w.t. CP. In BY-CPD77N cells the number of VRCs produced during mid-stage of infection is lower than in non-transgenic cells and significantly less than in BY-CP cells following infection with TMV-MP:GFP. Indeed, placing this mutant in either Group 1 or group 3 was not a clear decision. The negative effects of CPD77N on VRC production may be at the origin of the high resistance in the local lesion host NN-CPD77N.
Mutants CPE50Q, CPD77R, CPD77E and CPD77A provided very high levels of protection to TMV in transgenic Xanthi-nn as well as Xanthi-NN plants. Protection levels were similar to protection provided by CPT42W (Bendahmane et al., 1997; 2002). Moreover, these mutant CPs reduced MP:GFP accumulation in infected transgenic BY2 cells. The data are in agreement with results of other mutants (above) and show that the cumulative effect of strong interference with virus disassembly and negative effects on MP accumulation in VRCs are necessary and responsible for high levels of CP-MR to TMV infection.
Mutant CPE50M behaves like w.t. CP in all aspects studied here. Virions formed by CPE50M were similar to those of w.t. TMV and systemic infection in non-transgenic plants was similar. , In transgenic protoplasts that express CPDE50M the accumulation of MP:GFP following infection was similar to similar to protoplasts that produce w.t. CP. However, CPE50M provided higher levels of CP-MR in Xanthi-nn and Xanth-NN plants than did w.t. CP. This indicates that CPE50M may act through combinatorial action that involves interference with disassembly (as does w.t. CP) and a yet to be determined interference with infection by the challenge virus, thus representing another argument for the complexity of CP-MR.
These studies confirm that the role of TMV CP in regulating multiple steps in virus replication and in CP-MR is complex. Studies of mutant CPs such as those reported here are likely to form different conformational forms and intermediates in quaternary structure that are not characterized (see accompanying work by Asurmendi et al., 2007). Our data suggest that some of the transgenic conformers and/or intermediates of w.t. and mutants CPs impact one or more of the functions of CP to regulate virus infection, movement and replication.
Materials and Methods
TMV clones
TMV variants harboring the MP tagged with GFP (TMV-MP:GFP) or the CP tagged with GFP (TMV-CP:GFP) have been described (Heinlein et al., 1995; Bendahmane et al., 2002).
Mutant CPs and virus constructs
The TMV CP amino acids Glu50 and Asp77 were mutated to Asp, Gln, Arg, Lys or Met, and Asp77 was mutated to Glu, Asn, Arg, Lys or Ala using a PCR based site directed mutagenesis and cloned in the vector pKN2 as described (Bendahmane et al., 1997). The resulting intermediate plasmids are referred to as, pCPE50D, pCPE50Q, pCPE50R, pCPE50K, pCPE50M, pCPD77E, pCPD77N, pCPD77R, pCPD77K, and pCPD77A.
A DraI-KpnI fragment containing the mutated CP gene (TMV nucleotide 5708 to 6395; Goelet et al., 1982) from the above plasmids was ligated into an expression cassette that places each cDNA between the 35S promoter and the nos 3' terminator. The expression cassettes were then transferred into the vector pCGN1547 (McBride and Summerfelt, 1990). The resulting plasmids are referred to as pCG-E50D, pCG-E50K, pCG-E50M, pCG-E50Q, pCG-E50R, pCG-D77A, pCG-D77E, pCG-D77K, pCG-D77N or pCG-D77R.
cDNA clones harboring the mutant CPs from which infectious TMV RNA is produced were constructed by replacing the CP gene in the wild type TMV cDNA (U3/12-4; Holt and Beachy, 1991) with the mutant CP as described in Bendahmane et al. (1997). Plasmids thus produced are referred to as pTMV-CPE50D, pTMV-CPE50Q, pTMV-CPE50R, pTMV-CPE50K, pTMV-CPE50M, pTMV-CPD77E, pTMV-CPD77N, pTMV-CPD77R, pTMV-CPD77K, and pTMV-CPD77A.
Construction and analysis of transgenic plants
The plasmids pCG-E50D, pCG-E50K, pCG-E50M, pCG-E50Q, pCG-E50R, pCG-D77A, pCG-D77E, pCG-D77K, pCG-D77N or pCG-D77R were used to produce transgenic N. tabacum Xanthi-NN (a local lesion host of TMV) and Xanthi-nn plants (a systemic host of TMV) via Agrobacterium tumefaciens (Horsch et al., 1985). The regenerated kanamycin resistant plants (kanr) were screened for CP accumulation using the polyclonal rabbit anti-TMV coat protein antibody in western immunoblot assays as described previously (Bendahmane et al., 1997). Plant lines are referred to as NN- or nn-CPE50D, CPE50K, CPE50M, CPE50Q, CPE50R, CPD77A, CPD77E, CPD77K, CPD77N, CPD77R, respectively. Plants that accumulated CP were self-pollinated and R1 plants and homozygous plants were identified and their R2 plant seedlings were used for all studies. Plant lines from amongst more than 15 lines containing each construct were selected for further study on the basis of accumulation of equivalent amounts of CP as determined by quantitative western immunoblot assays
Construction and analysis of transgenic BY2 cell lines
The plasmids pCG-E50D, pCG-E50K, pCG-E50M, pCG-E50Q, pCG-E50R, pCG-D77A, pCG-D77E, pCG-D77K, pCG-D77N or pCG-D77R were used to transform the tobacco BY2 cell line using A. tumefaciens mediated transformation. The regenerated kanamycin resistant cell lines (kanr) were screened for CP accumulation using the polyclonal rabbit anti-TMV coat protein antibody in western immunoblot assay as above. Cell lines with high levels of CP were used for further studies. These lines are referred to as BY-CPE50D, BY-CPE50K, BY-CPE50M, BY-CPE50Q, BY-CPE50R, BY-CPD77A, BY-CPD77E, BY-CPD77K, BY-CPD77N or BY-CPD77R, respectively. Cell lines selected for further study contained equivalent amounts of mutant or wild type CP as determined by western immunoblot assays.
In vitro synthesis of viral RNA and plant and BY2 protoplast inoculation
In vitro transcribed full length viral RNAs were generated from w.t. and mutant TMV cDNA clones and used to inoculate plants as previously described (Bendahmane et al., 1997). Protoplasts were prepared from BY-2 suspension cell cultures, inoculated with in vitro transcribed viral RNA via electroporation, as described (Watanabe et al., 1987, Bendahmane et al., 2002). Infected plants and protoplasts were analyzed by fluorescence and laser scanning confocal microscopy as described (Bendahmane et al., 2002).
Purification and analysis of virus particles from infected plants
Wild type and mutant viruses were purified from infected plant leaves as described previously (Asselin and Zaitlin., 1978; Bendahmane et al., 1997).
Electron microscopy: Purified viruses at concentrations of 10 to 100 μg/ml were applied to carbon coated copper EM grids and then negatively stained with a 2% uranyl acetate solution for 2 min. Grids were allowed to dry prior examination on a Phillips CM100 electron microscope at 39,000 x.
Assays for CP-MR
Transgenic N. tabacum Xanthi-NN and Xanthi-nn plant lines expressing the mutant CPs or wt CP [lines 748 (Nelson et al., 1987) and 3646 (Powell-Abel et al., 1986)] were grown in a greenhouse without supplemental light and with temperatures ranging from 24°C to 26°C during the day and 20°C to 21°C during the night. At 4 weeks after transplantating the plants were inoculated on a single leaf previously dusted with carborundum (320 GRIT, Fisher Scientific). TMV diluted to concentrations of 0.1 μg/ml in 20 mM NaHPO4, pH7, was used as inoculum. On Xanthi-NN transgenic lines the relative levels of protection conferred by the different mutant CPs were scored by comparing the numbers of necrotic lesions on the transgenic lines versus non-transgenic plants. The relative degree of systemic protection was scored by monitoring the appearance of disease symptoms and virus accumulation in the upper non-inoculated leaves. Virus accumulation was quantified using an indirect sandwich ELISA as previously described (Bendahmane et al., 1997).
Acknowledgment
We thank Mike Dyer and Nancy Mathis for assistance with the plants. This work was supported by Grant AI27161 from the National Institutes of Health. Other support was provided by Donald Danforth Plant Science Center and The Scripps Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Asselin A, Zaitlin M. Characterization of a second protein associated with irions of tobacco mosaic virus. Virology. 1978;91:173–81. doi: 10.1016/0042-6822(78)90365-3. [DOI] [PubMed] [Google Scholar]
- Asurmendi S, Berg RH, Koo JC, Beachy RN. Coat protein regulates formation of replication complexes during tobacco mosaic virus infection. Proc Natl Acad Sci U S A. 2004;101:1415–1420. doi: 10.1073/pnas.0307778101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendahmane M, Fitchen JH, Zang GH, Beachy RN. Studies of Coat Protein Mediated Resistance to TMV: Correlation between assembly of mutant coat proteins and resistance. J. Virol. 1997;71:7942–7950. doi: 10.1128/jvi.71.10.7942-7950.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendahmane M, Beachy RN. Control of tobamovirus infections via pathogen derived resistance. Adv. Virus Res. 1999;53:369–386. doi: 10.1016/s0065-3527(08)60357-7. [DOI] [PubMed] [Google Scholar]
- Bendahmane M, Szecsi J, Chen I, Berg, Beachy RN. Characterization of mutant tobacco mosaic virus coat protein that interferes with virus cell-to-cell movement. Proc. Natl. Acad. Sci. USA. 2002;99:3645–3650. doi: 10.1073/pnas.062041499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspar DLD. Assembly and stability of the tobacco mosaic tobamovirus particle. Adv. Protein Chem. 1963;18:37–121. doi: 10.1016/s0065-3233(08)60268-5. [DOI] [PubMed] [Google Scholar]
- Caspar DLD, Namba K. Switching in the self-assembly of tobacco mosaic virus. Adv. Biophys. 1990;26:157–85. doi: 10.1016/0065-227x(90)90011-h. [DOI] [PubMed] [Google Scholar]
- Culver JN, Dawson WO, Plonk K, Stubbs G. Site directed mutagenesis confirms the involvement of carboxylate groups in the disassembly of tobacco mosaic virus. Virology. 1995;206:724–730. doi: 10.1016/s0042-6822(95)80096-4. [DOI] [PubMed] [Google Scholar]
- Culver JN. Tobacco mosaic virus assembly and disassembly: Determinants in pathogenicity and resistance. Annu. Rev. Phytopathol. 2002;40:287–308. doi: 10.1146/annurev.phyto.40.120301.102400. [DOI] [PubMed] [Google Scholar]
- Goelet P, Lomonossoff GP, Butler PJG, Akam ME, Gait MJ, Karn J. Nucleotide sequence of tobacco mosaic virus RNA. Proc. Natl. Acad. Sci. U.S.A. 1982;79:5818–5822. doi: 10.1073/pnas.79.19.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinlein M, Epel BL, Padgett HS, Beachy RN. Interaction of tobamovirus movement proteins with the plant cytoskeleton. Science. 1995;270:1983–1985. doi: 10.1126/science.270.5244.1983. [DOI] [PubMed] [Google Scholar]
- Holt CA, Beachy RN. In vivo complementation of infectious transcripts from mutant tobacco mosaic virus cDNAs in transgenic plants. Virology. 1991;181:109–117. doi: 10.1016/0042-6822(91)90475-q. [DOI] [PubMed] [Google Scholar]
- Horsch RB, Fry JE, Hoffmann NL, Eicholtz D, Rogers SG, Fraley RT. A simple and general method for transferring genes into plants. Science. 1985;227:1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Kawakami S, Watanabe Y, Beachy RN. Tobacco mosaic virus infection spreads cell to cell as intact replication complexes. Proc Natl Acad Sci USA. 2004;101:6291–6296. doi: 10.1073/pnas.0401221101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Stubbs G, Culver JN. Carboxylate interactions involved in the disassembly of tobacco mosaic tobamovirus. Virology. 1996;225:11–20. doi: 10.1006/viro.1996.0570. [DOI] [PubMed] [Google Scholar]
- Lu B, Stubbs G, Culver JN. Coat protein interactions involved in tobacco mosaic tobamovirus cross-protection. Virology. 1998;248:188–198. doi: 10.1006/viro.1998.9280. [DOI] [PubMed] [Google Scholar]
- McBride KE, Summerfelt KR. Improved binary vectors for Agrobacterium-mediated plant transformation. Plant Mol. Biol. 1990;14:269–276. doi: 10.1007/BF00018567. [DOI] [PubMed] [Google Scholar]
- Namba K, Pattanayek R, Stubbs G. Visualization of protein-nucleic acid interactions in a virus. Refined structure of intact tobacco mosaic virus at 2.9 A resolution X-ray fiber diffraction. J. Mol. Biol. 1989;208:307–325. doi: 10.1016/0022-2836(89)90391-4. [DOI] [PubMed] [Google Scholar]
- Namba K, Stubbs G. Structure of tobacco mosaic virus at 3.6 resolution: implications in assembly. Science. 1986;231:1401–1406. doi: 10.1126/science.3952490. [DOI] [PubMed] [Google Scholar]
- Nelson RS, Powell-Abel P, Beachy RN. Lesions and virus accumulation in inoculated transgenic tobacco plants expressing the coat protein gene of tobacco mosaic virus. Virology. 1987;158:126–132. doi: 10.1016/0042-6822(87)90245-5. [DOI] [PubMed] [Google Scholar]
- Powell-Abel P, Nelson RS, De B, Hoffmann N, Rogers SG, Fraley RT, Beachy RN. Delay of disease development in transgenic plants that express the tobacco mosaic virus coat protein gene. Science. 1986;232:738–743. doi: 10.1126/science.3457472. [DOI] [PubMed] [Google Scholar]
- Register JC, III, Beachy RN. Resistance to TMV in transgenic plants results from interference with an early event in infection. Virology. 1988;166:524–532. doi: 10.1016/0042-6822(88)90523-5. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Meshi T, Okada Y. Infection of tobacco protoplasts with in vitro transcribed tobacco mosaic virus RNA using an improved electroporation method. FEBS Lett. 1987;219:65–69. [Google Scholar]