Abstract
Previous studies have suggested that salivary amylase and proline-rich protein are sorted differently when expressed in AtT-20 cells (Castle, A.M., L.E. Stahl, and J.D. Castle. 1992. J. Biol. Chem. 267:13093– 13100; Colomer, V., K. Lal, T.C. Hoops, and M.J. Rindler. 1994.EMBO (Eur. Mol. Biol. Organ.) J. 13:3711– 3719). We now show that both exocrine proteins behave similarly and enter the regulated secretory pathway as judged by immunolocalization and secretagogue- dependent stimulation of secretion. Analysis of stimulated secretion of newly synthesized proline-rich protein, amylase, and endogenous hormones indicates that the exogenous proteins enter the granule pool with about the same efficiency as the endogenous hormones. However, in contrast to the endogenous hormones, proline-rich protein and amylase are progressively removed from the granule pool during the process of granule maturation such that only small portions remain in mature granules where they colocalize with the stored hormones. The exogenous proteins that are not stored are recovered from the incubation medium and are presumed to have undergone constitutive-like secretion. These results point to a level of sorting for regulated secretion after entry of proteins into forming granules and indicate that retention is essential for efficient storage. Consequently, the critical role of putative sorting receptors for regulated secretion may be in retention rather than in granule entry.
In cells with a regulated secretory pathway, a subset of soluble proteins undergoing intracellular transport is stored in membrane-bound secretory granules for stimulus-dependent exocytosis, while other proteins are either transported to different organelles or released at the cell surface without stimulation. Widespread efforts to study the secretory sorting process by transfecting cells that have a regulated secretory pathway with cDNAs encoding secretory proteins have suggested that sorting for regulated secretion utilizes a broadly conserved mechanism (Burgess et al., 1985; Ornitz et al., 1985; Fennewald et al., 1988; Stoller and Shields 1989; Sossin et al., 1990; Seethaler et al., 1991; Castle et al., 1992). Associations (aggregation) among secretory proteins that undergo regulated secretion have been detected both in situ and in vitro and are thought to contribute to the sorting process (Chanat and Huttner, 1991; Leblond et al., 1993; Colomer et al., 1994; Kuliawat and Arvan, 1994; Colomer et al., 1996). Much remains to be established, however, regarding how and where proteins that are destined for regulated secretion are segregated from other proteins during intracellular transport.
Two different models have been proposed to explain how and where proteins are sorted for regulated secretion. The active sorting model hypothesizes that proteins destined for regulated secretion bind to a sorting receptor that is concentrated in the TGN and are selectively delivered (either individually or as aggregates) to the immature secretion granule, where they are deposited to complete the sorting operation. This model predicts that signals are required for proteins to enter into the regulated secretory pathway and that proteins lacking appropriate signals are largely denied access and instead follow a TGN-derived constitutive secretory pathway. In contrast, the passive sorting model postulates that entry into the forming granules is not selective, and thus is not contingent on receptor binding. Rather, this model assigns the primary role in sorting to the aggregation of granule proteins as they are concentrated during intracellular transport, especially within the immature granule; thus, “regulated” and “constitutive” proteins alike enter immature granules. As sorting proceeds, regulated proteins are selectively condensed and retained while other proteins are progressively removed. The removal is thought to occur via a constitutive-like secretory pathway initiated by vesicular budding from the maturing granule (for review see Arvan and Castle, 1992). Evidence for the presence of a constitutive-like secretory pathway has been obtained in both endocrine and exocrine cells (von Zastrow and Castle, 1987; Arvan et al., 1991; Grimes and Kelly, 1992; Kuliawat and Arvan, 1992; Milgram et al., 1994).
Very recently, it has been reported that carboxypeptidase E (CPE),1 an enzyme that functions in the proteolytic processing cascade for prohormones, also serves as a universal sorting receptor for the regulated secretory pathway in neuroendocrine cells (Cool et al., 1997). CPE was shown to bind selectively to a number of prohormones and other endocrine proteins that typically undergo regulated secretion, and reduced expression of CPE was correlated with increased unstimulated prohormone secretion. These findings were interpreted to indicate that interaction with CPE was necessary for entry into forming granules and thus were regarded as evidence for the active sorting model. We now present evidence supporting the operation of passive sorting in AtT-20 cells. Using salivary proline-rich protein (PRP) and amylase, which are not expected to interact with CPE because they lack appropriate signals and which show little coaggregation with pituitary granule proteins (Colomer et al., 1994), we show that both exocrine proteins enter immature granules with about the same efficiency as ACTH precursor. However, in contrast to the endogenous hormone, they are substantially excluded from the mature granules.
Materials and Methods
Antibodies
Anti-ACTH antiserum UV16 and the polyclonal antibody against PRPs were described previously (Blair et al., 1991; Castle and Castle, 1993). The antiserum JH93 against the NH2 terminus of ACTH, the antiserum Kathy against mature ACTH and the antiserum JH2 against β-endorphin (gifts from Drs. B. Eipper and R. Mains, Johns Hopkins University, Baltimore, MD) were used as described previously (Schnabel et al., 1989; Zhou et al., 1993). For immunofluorescence and immunoelectron microscopy experiments, affinity-purified anti-ACTH antibody UV16 was biotinylated with biotinamidocaproate-N-hydroxy-sulfo-succinimide ester using the Immunoprobe biotinylation kit from Sigma Chemical Co. (St. Louis, MO). To detect amylase, we used a polyclonal antibody against human amylase (Sigma Chemical Co.) for immunofluorescence, and a polyclonal antibody against rat amylase was utilized for immunoprecipitation. The hybridoma secreting the mAb against mouse transferrin receptor was obtained from American Type Culture Collection (Rockville, MD). mAb against lysosome-associated membrane glycoprotein (LAMP-1) was obtained from the developmental hybridoma bank.
PRP and Amylase Expression Vectors and Transfected Cell Lines
The cDNA coding for the basic rat parotid PRP has been described previously (Castle et al., 1992), and it was cloned into the pRhR1100 expression vector (Stahl et al., 1996). The full-length cDNA coding for rat parotid amylase was a gift from Dr. J. Melvin (University of Rochester, Rochester, NY) and it was cloned into pRhR1100 expression vector.
Mouse pituitary AtT-20 D16v cells were cultured and transfected as described previously (Castle et al., 1992). Levels of expression of each polypeptide were estimated from the total amount of radiolabeled polypeptide (secreted+cell-associated) synthesized during a 1-h labeling period with [3H]lysine. The values were corrected for the number of amino acids in each polypeptide. ACTH-related peptides were quantitated from the same experiments using an anti-ACTH antibody (ACTH-related peptides = pro-opiomelanocortin [POMC] + ACTH biosynthetic intermediate + ACTH + glycosylated ACTH [gACTH]). Two levels of PRP expression were used, one equivalent to 110%, and another equivalent to 6% of ACTH-related peptides. Amylase was expressed at approximately the same level in all clones examined, and its level of expression corresponded to 1.5% of ACTH-related peptides.
Immunofluorescence
Cells were plated on coverslips as described (Castle and Castle, 1993), fixed in 3% depolymerized paraformaldehyde in 120 mM sodium phosphate, pH 7.4, for 1 h, washed in PBS, and then permeabilized and quenched in 0.2% Triton X-100, 1 M glycine in PBS for 20 min. The cells were then either blocked in 16% goat serum in PBS for 30 min, or in 1% milk solids and 1% albumin for amylase immunofluorescence, and subsequently incubated with primary antibodies for either 1–3 h at room temperature, or at 4°C overnight. Cells on coverslips were washed three times with PBS and incubated 1–2 h at room temperature with fluorophore-conjugated secondary antibodies and washed three times with PBS before mounting in Vectashield (Vector Labs, Inc., Burlingame, CA). In experiments using two primary antibodies, the incubations were performed in sequence. When double labeling was performed with a rabbit polyclonal antibody and biotinylated anti-ACTH antibody, the latter was always used as the second primary antibody.
Immunofluorescent images were viewed using a Leitz microscope or analyzed by confocal microscopy using a Zeiss LSM 410 confocal microscope (Carl Zeiss, Inc., Thornwood, NY). The images were collected with the appropriate filters using laser attenuation of 10–30 and scan time of <2 s. Under these conditions, minimal bleaching was detected. In areas used for quantitation the intensity of all pixels was <255.
Immunoelectron Microscopy
Cells grown in 10-cm dishes were fixed with periodate-lysine-paraformaldehyde solution (McLean and Nakane, 1974) for 10 min at room temperature, scraped and pelleted in a microcentrifuge, and then fixed for an additional 2 h at room temperature. The pellets were washed with 5 mM Tris in 3.5% sucrose, 0.1 M sodium phosphate (pH 7.4) three times, and processed for embedding with Lowicryl 4 M (Electron Microscopy Sciences, Fort Washington, PA) according to the manufacturer's procedure. After embedding, immunolabeling was carried out on thin sections mounted on nickel grids. Sections were quenched and blocked by sequential treatment with 0.1 M glycine and blocking buffer (1% ovalbumin, 2% normal goat serum, 0.15 M NaCl, 0.1 M Tris, pH 7.4). PRP antiserum was diluted 1: 3,000 in blocking buffer containing 0.01% Tween 20, and sections were incubated overnight at 4°C, washed in washing buffer (0.1 M Tris, pH 7.4, 0.15 M NaCl), and then incubated 1–2 h with 10 nM gold-conjugated goat anti–rabbit IgG (Electron Microscopy Sciences, Fort Washington, PA). Sections were washed with washing buffer and incubated with biotinylated anti-ACTH antibody diluted to 0.3 μg/ml in blocking buffer for 1 h at room temperature. After washing, the sections were incubated with goat anti-biotin IgG conjugated to 5 nM gold (Electron Microscopy Sciences) for 1 h at room temperature. Finally, the sections were washed with washing buffer, rinsed with distilled water, dried, contrasted by staining with uranyl acetate and lead, and examined in an JEOL electron microscope. Quantitation of immunogold labeling was performed using micrographs from 30 different cells. Gold particles associated with >300 granules were counted.
Metabolic Labeling, Stimulation of Secretion, and Quantitation of Radiolabeled Polypeptides
AtT-20 cells expressing either amylase, PRP, or mock-transfected cells, were plated in 24-well plates with 105 cells per well. Cells were labeled as specified for individual experiments and chased with DME containing trasylol as protease inhibitor (50 kallikrein units/ml). Medium containing secreted proteins was collected, and at the end of the experiment cells were lysed in the presence of protease inhibitors as described previously (Castle et al., 1992). To measure the percentage of stimulated secretion, duplicate wells of cells were labeled and then chased initially in the absence of secretagogue for a specified length of time and subsequently for an additional time period in the absence (basal secretion), or the presence (stimulated secretion) of 5 mM 8-Br-cAMP. Radiolabeled polypeptides were immunoprecipitated and quantitated as follows: PRP, densitometry of fluorographs or scintillation counting of sliced tube gels (Castle et al., 1992); amylase, phosphorimager analysis using ImageQuant software (Molecular Dynamics, Inc., Sunnyvale, CA); POMC-related peptides, scintillation counting of eluted gel slices (Castle et al., 1992) or phosphoimager analysis from Tricine gel profiles (Schagger and von Jagow, 1987). In the latter case, subsets of ACTH-related peptides were immunoprecipitated with antisera UV16 and/or JH93, and β-endorphin–related peptides were immunoprecipitated with JH2 antiserum.
Sucrose Velocity Density Centrifugation
A 10-cm dish of cells was labeled with [3H]proline for 15 h, and chased for 1 or 5 h. The following steps were all performed on ice or at 4°C. The cells were washed twice with a buffer (4.5 mM KCl, 137 mM NaCl, 0.7 mM NaH2PO4, 25 mM Tris, pH 7.4), scraped, pelleted at 600 g, and washed again by resuspending in the same buffer and pelleting. The pellet was resuspended in 0.25 M sucrose, 1 mM MgCl2, 1 mM EDTA, 10 mM Hepes, pH 7.4, containing a protease inhibitor cocktail (50 kallikrein units/ml of aprotinin, 1 mM leupeptin, 10 μM pepstatin, 1 μg/ml antipain, 0.3 mg/ml iodoacetamide, 0.3 mg/ml phenylmethylsulfonyl fluoride), passed five times through a 26-gauge needle and the cells were homogenized by 15 passes through a ball bearing cell cracker (clearance 0.0011 in). Homogenates were spun to remove nuclei and debris (2,000 g for 4 min), and 200-μl aliquots of the postnuclear supernate were loaded on 2-ml discontinuous sucrose density gradients (from the bottom: 100 μl 1.8 M, 200 μl 1.6 M, 300 μl 1.4 M, 350 μl 1.2 M, 350 μl 1.1 M, 350 μl 1.0 M, 300 μl 0.8 M). The gradients were spun at 200,000 g for 2 h in a TLS55 rotor in a table top ultracentrifuge (Beckman Instruments, Inc., Fullerton, CA). The gradients were collected from the top in 180-μl fractions. Radiolabeled PRP was immunoprecipitated from all the fractions, digested with endoglycosidase H, and analyzed by SDS-PAGE and fluorography (Castle et al., 1992). Radiolabeled ACTH was immunoprecipitated from the same fractions and quantitated by scintillation counting of eluted gel slices.
Results
Localization of PRP and Amylase in Transfected AtT-20 Cells
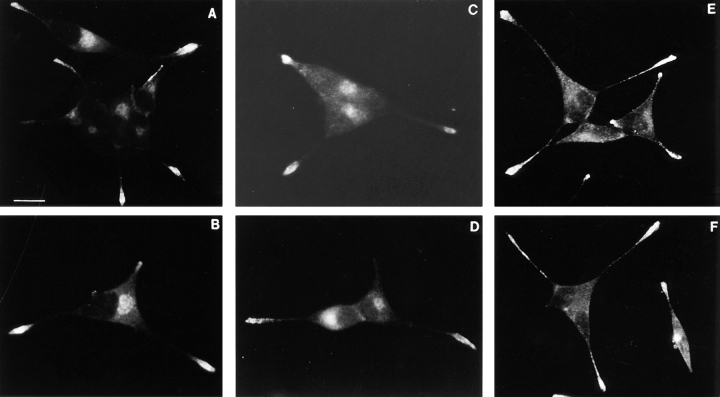
We have previously shown that PRP exhibits stimulus-dependent secretion and is localized within dense-core secretory granules when expressed in AtT-20 cells (Castle et al., 1992). Therefore PRP is expected to show a similar cellular distribution to ACTH, one of the major components of the dense-core granules (Gumbiner and Kelly, 1981). In contrast, we anticipated that salivary amylase would not codistribute with ACTH because it had been reported not to undergo stimulated secretion when expressed in AtT-20 cells (Colomer et al., 1994). As shown in Fig. 1, the distribution of PRP in transfected AtT-20 cells is very similar to that of ACTH, with the greatest concentration of immunofluorescent labeling in the tips of cellular processes where the endogenous granules accumulate. Surprisingly, salivary amylase also exhibited a distribution that is consistent with localization in dense-core granules (Fig. 1, C and D). The labeling in the center of the cells observed with PRP and amylase likely represents the Golgi region. The labeling of this region with the anti-ACTH antibody is less pronounced because this antibody preferentially recognizes processed products of POMC. Nontransfected AtT-20 cells treated with the anti-PRP or anti-amylase antibodies had faint or no staining over the cell body (data not shown), indicating that the immunofluorescent signals are specific. To better evaluate whether the fluorescent signals for the exogenous proteins really coincide with the staining of ACTH within the cellular processes, we have used double labeling and analysis by confocal microscopy.
Figure 1.
Immunocytochemical localization of PRP, amylase, and ACTH in transfected AtT-20 cells. AtT-20 cells expressing PRP were stained with anti-PRP (A and B), or anti-ACTH (E and F); cells expressing amylase were stained with an anti-amylase antibody (C and D). Binding of primary antibodies was detected with Cy3- or Texas red–conjugated goat anti–rabbit IgG. The distribution of ACTH in the amylase expressing cells was highly similar to that shown in (E and F). Bar, 10 μm.
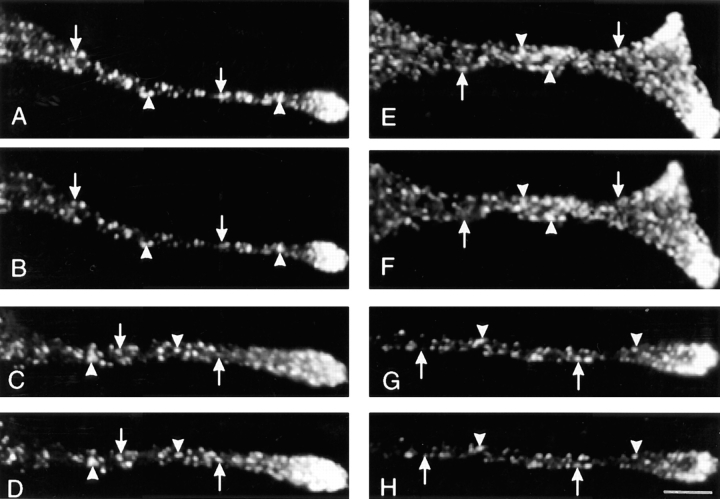
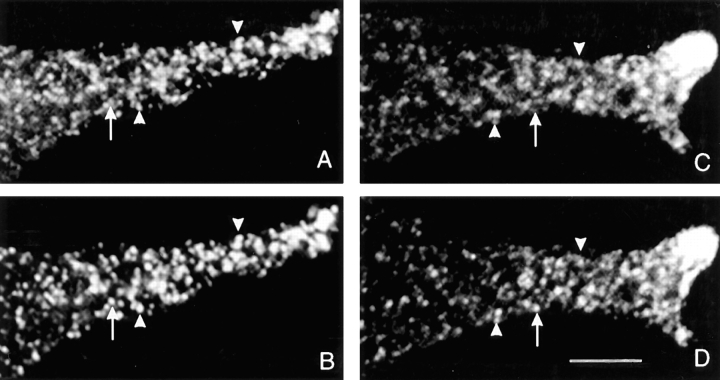
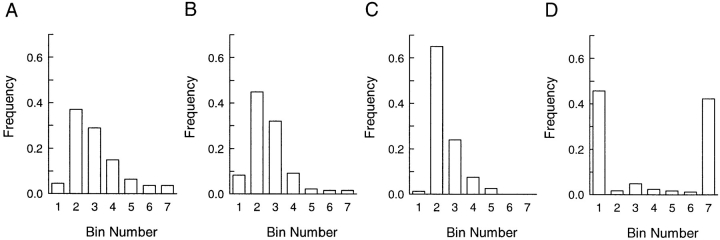
AtT-20 cells expressing either PRP or amylase were successively stained with antibodies against the exogenous proteins and ACTH. In control experiments, we demonstrated that the secondary labeling reagents used did not cross-react with the inappropriate primary antibodies, and there was also no spillover of the fluorescence signals emitted from either Cy3 or Cy5 into the inappropriate recording channel (data not shown). Fig. 2 shows examples of cellular processes after double labeling with anti-PRP and anti-ACTH antibodies. Evidently, there is extensive colocalization of PRP and ACTH, although it is interesting that several of the labeled sites exhibit substantially different relative intensities of staining with some sites having a strong PRP signal and weaker ACTH signal, and vice versa at other sites. The illustrations in Fig. 2, A–D, are from cells expressing comparable levels of PRP and ACTH. Areas of differential distribution of PRP and ACTH within regions of colocalization were also observed with a clone expressing 20 times less PRP (Fig. 2, E–H). When we examined cells expressing amylase we observed very extensive coincidence of amylase and ACTH staining, with a few areas exhibiting different relative intensities of the two antigens (Fig. 3). To gain a better understanding of the distribution of staining of PRP and amylase relative to ACTH, we analyzed the staining intensity ratios of PRP versus ACTH, and amylase versus ACTH (Fig. 4). For the images shown in Figs. 2 and 3, the intensity ratios are concentrated (>65%) in two bins (Fig.4, A and B, bins 2 and 3) indicating extensive overlap. For clones expressing PRP, the distribution favors higher relative intensity ratios as compared to the clone expressing amylase, suggesting that some granules may be enriched in PRP.
Figure 2.
Localization of PRP and ACTH within cellular processes by double label immunofluorescence. Cells expressing PRP were labeled with anti-PRP antibody followed by Cy3-conjugated goat anti–rabbit IgG, and biotinylated anti-ACTH antibody followed by Cy5-conjugated avidin. Shown here are images of cellular processes (PRP in A, C, E, and G; ACTH in B, D, F, and H) obtained by confocal microscopy as a single optical section. The images in A–D were obtained with a clone expressing PRP at the same level as ACTH, and images in E–H were obtained with a clone expressing 1/20 as much PRP as ACTH. The pattern of staining of PRP shows many areas of colocalization with ACTH (arrowheads), but also areas where PRP and ACTH exhibit different relative intensities of staining (arrows). Bar, 5 μm.
Figure 3.
Localization of amylase and ACTH within cellular processes by double label immunofluorescence. Cells expressing amylase were labeled with anti-amylase antibody followed by Cy3-conjugated goat anti– rabbit IgG, and biotinylated anti-ACTH antibody followed by Cy5-conjugated avidin. In single optical sections of cellular processes (amylase in A and C; ACTH in B and D) the arrowheads show examples of the extensive colocalization of amylase and ACTH, while the arrows point to areas with different relative intensities of the two antigens. Bar, 5 μm.
Figure 4.
Fluorescent staining intensity ratios of PRP versus ACTH and amylase versus ACTH. (A–C) Staining intensities for PRP, amylase, and ACTH of fluorescent spots seen in Figs. 2 and 3 were evaluated using the intensity profile function. A section of each paired image (not including the cell body and the tip of the process) was chosen, and the intensities of all individual spots were quantitated. For each spot, the peak intensity of PRP or amylase was divided by the corresponding peak intensity of ACTH. The intensity ratios were then grouped into 7 bins: 1 (0–0.5), 2 (0.6–1), 3 (1.1–1.5), 4 (1.6–2), 5 (2.1–2.5), 6 (2.6–3), and 7 (>3). The frequency in each bin was expressed as a fraction of total spots counted. (A) High-expressing PRP (from Fig. 2, A and B); 110 spots counted. (B) Low-expressing PRP (from Fig. 2, E and F), 132 spots counted. (C) Amylase (from Fig. 3, A and B), 80 spots counted. (D) Staining intensity ratios for PRP versus mouse transferin receptor were evaluated as above (164 spots counted). The last distribution is indicative of two nonoverlapping compartments.
In separate double-labeling experiments, we detected no colocalization of PRP or amylase with markers of the endocytic pathway (transferrin receptor; Fig. 4 D) and lysosomes (LAMP-1) (data not shown). Taken together, these data suggest that PRP and amylase are present within the ACTH-containing, dense-core granules in AtT-20 cells, but PRP exhibits slightly different distribution relative to the endogenous hormone.
PRP Resides in Dense-core Granules
To clarify the possible basis for the partial segregation of exogenous and endogenous proteins, we used immunoelectron microscopy on AtT-20 cells expressing PRP. We focused on PRP because it exhibited the most distinct distribution from ACTH and because in contrast to amylase, we have readily obtained a specific signal for immunoelectron microscopic analysis of PRP.
Previous studies have shown that ACTH is present in essentially all of the granules in AtT-20 cells (Tooze et al., 1987; Schnabel et al., 1989). We have confirmed that this is the case in AtT-20 cells expressing a high level of PRP (Fig. 5 A). In cells double labeled with anti-PRP and biotinylated anti-ACTH, labeling by both antibodies was concentrated mainly over dense-core granules within cell processes. We also observed labeling over cell bodies where much of the concentrated labeling was also present in dense-core granules, or in dilations probably corresponding to the Golgi region. It should be noted that the biotinylated anti-ACTH antibody exhibits a reduced affinity as compared to the native antibody, resulting in an overall weaker labeling of ACTH. Interestingly, the relative labeling for PRP and ACTH within individual granules was variable. Some granules were mainly stained by anti-PRP; others were mainly stained by biotinylated anti-ACTH; and still others were stained by both antibodies (Fig. 5, B–E). Quantitation of the gold particles over labeled granules indicates that 62% exhibit labeling with both antibodies, 22% were labeled with anti-PRP antibody alone, and 18% were labeled with anti-ACTH antibody alone. These results support the conclusion that ACTH and PRP colocalize within the dense-core granules. In addition, the results indicate that the areas of differing intensities of PRP and ACTH observed by immunofluorescence also correspond to dense-core granules.
Figure 5.
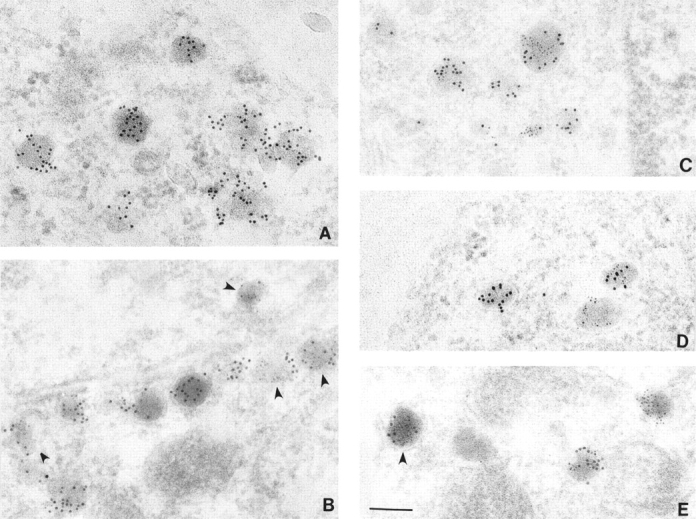
Dense-core granules contain variable amounts of PRP. (A) AtT-20 cells expressing PRP at the same level as ACTH were labeled with affinity-purified anti-ACTH antibody. (B–E) AtT-20 cells expressing PRP at the same level as ACTH double labeled with anti-PRP serum (10 nM gold), and biotinylated anti-ACTH antibody (5 nM gold). Granules labeled with both types of gold are observed near the plasma membrane (B and D) as well as in intracellular locations (C and E). Arrowheads point to a subset of granules which contain significant ACTH staining but where the gold particles are more difficult to visualize. Bar, 150 nm.
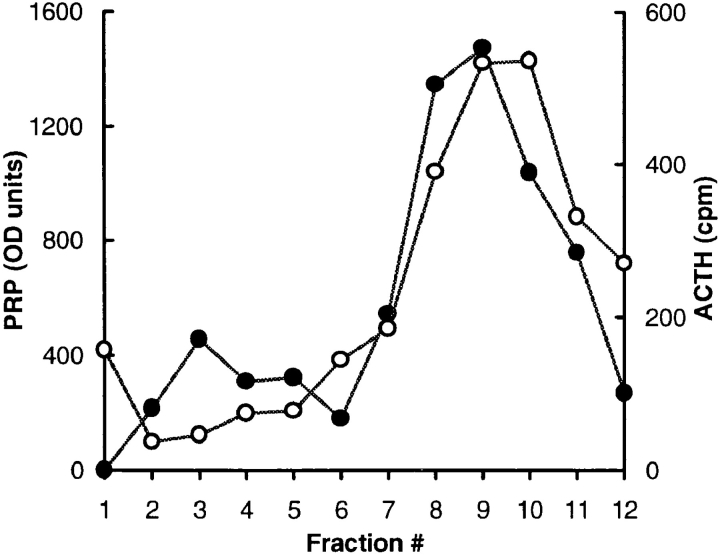
The granules with higher PRP labeling had the same morphological appearance and exhibited no apparent difference in size as compared to those labeled primarily by ACTH antibody. The diameters of immunolabeled dense-core granules were 125 ± 32 nm for granules labeled with ACTH antibody alone, 121 ± 34 nm for granules labeled with PRP antibody alone and 123 ± 32 nm for granules labeled with both antibodies. (The diameters are expressed as mean ± SEM.) To provide independent assessment of the presence of exogenous PRP and endogenous ACTH within granules, we performed sedimentation analysis on sucrose gradients following extended labeling with [3H]proline. As shown in Fig. 6, the majority of the endo H–resistant PRP sediments in dense fractions that also contain the majority of mature ACTH. Quantitation showed that fractions 7–11 contain 75% of total endo H–resistant PRP and 71% of mature ACTH suggesting their presence in a common or closely overlapping pool of granules. Note however, that the peak of endo H–resistant PRP is shifted slightly to less dense fractions suggesting enrichment of PRP in granules of lower density.
Figure 6.
Distribution of endo H–resistant PRP and mature ACTH on a sucrose density gradient. Postnuclear supernates from cells labeled for 15 h (to approach steady state) with 0.4 mCi/ml [3H]proline and chased for 1 h, were subjected to centrifugation on sucrose density gradients. Endo H–resistant PRP (•) and mature ACTH (○) were quantitated from each fraction as described in Materials and Methods. The top and bottom of the gradient are in fractions 1 and 12, respectively. The data come from one of two independent experiments in which nearly identical profiles were obtained.
Secretagogues Stimulate Exocytosis of the Same Fractions of Post-Golgi PRP and ACTH
While it is clear that there is extensive overlap of granule-associated PRP and ACTH, it was important to evaluate whether their discharge was equally responsive to secretagogues. Therefore we compared the fractions of the post-Golgi pools of PRP and ACTH peptides that were released upon treatment of the cells with a secretagogue. Cells expressing PRP were labeled with [3H]proline for 15 h, and chased for 1 h. At this time the post-Golgi pool of PRP was estimated as cell-associated radiolabeled PRP that was endo H resistant and the post-Golgi pool of ACTH was estimated as the sum of cell-associated radiolabeled mature ACTH and gACTH. Table I shows that the post-Golgi pools of PRP and ACTH represented 32 and 74% of the total labeled material, respectively. In separate experiments, cells were labeled and chased as above and subsequently chased for an additional 1 h in the presence or absence of 8-Br-cAMP. Stimulation with 8-Br-cAMP resulted in a net release of 13% of total labeled PRP (Table I). (We have confirmed that all of the secreted PRP was endo H resistant, as reported previously by Castle et al., 1992.) For ACTH, stimulation with 8-Br-cAMP resulted in enhanced secretion of mature ACTH peptides representing 28% of the total labeled material. Based on these results, we have deduced that the fraction of PRP and ACTH discharged from their respective post-Golgi pools was 0.40 and 0.38, respectively (Table I). The close agreement of the fractional stimulations suggests that PRP and ACTH reside in a common stimulatable pool of granules. Comparable fractional stimulation of PRP and ACTH (0.32) was also observed if the labeled cells were first chased for 5 h to insure that most of the labeled polypeptides became associated with mature granules before stimulating discharge with 8-Br-cAMP (Table I). Thus while PRP and ACTH may be somewhat differently distributed within the granule pool, they exhibit equal responsiveness to secretagogues.
Table I.
8-Br-cAMP–dependent Release of ACTH-related Peptides and PRP
| 1-h chase | 5-h chase | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sec | Cell | Post-Golgi pool | 8-Br stim. | Fract. stim. | Sec | Cell | Post-Golgi pool | 8-Br-stim. | Fract. stim. | |||||||||||
| ACTH-related peptides | 21 ± 2 | 78 ± 2 | 74 ± 2 | 28 ± 3* | 0.38 | 35 ± 6 | 65 ± 6 | 65 ± 6 | 21 ± 0* | 0.32 | ||||||||||
| PRP | 43 ± 2 | 57 ± 2 | 32 ± 2 | 13 ± 1 | 0.40 | 87 ± 2 | 13 ± 2 | 10 ± 2 | 3.2 ±0.2 | 0.32 | ||||||||||
Cells expressing PRP were labeled for 15 h with [3H]proline and chased 1 or 5 h in the absence of secretagogues. Secreted (Sec) and cell-associated (Cell) PRP, and ACTH-related peptides (POMC + 23 K intermediate + gACTH + ACTH) were quantitated. Post-Golgi pools of ACTH-related peptides were evaluated as the sum of mature ACTH peptides (ACTH + gACTH). Post-Golgi pools of PRP were estimated as the endo H–resistant PRP in the cell lysate. To evaluate 8-Br-cAMP–dependent stimulation of secretion, cells were chased in the absence (basal) or the presence (stimulated) of 5 mM 8-Br-cAMP. 8-Br stim. represents the net (stimulated minus basal) secretion of radiolabeled PRP or ACTH- related peptides. All values are expressed as percent of total radiolabeled material, and are listed as mean ± SEM (n ⩾ 3). Asterisks indicate the average of two determinations ± range. Fractional stimulation was calculated as 8-Br stim./post-Golgi pool.
Interestingly, the size of the post-Golgi pool of PRP decreases more than threefold between 1 and 5 h of chase, whereas the post-Golgi pool of ACTH decreases only slightly (10%; Table I). Since the majority (>75%) of endo H–resistant PRP is associated with granules (Fig. 6), these data suggest that PRP is removed from the granule pool during granule maturation. Consequently, only a small fraction of total radiolabeled PRP (cells + media) remains in the granules after an extended (5 h) chase incubation. This is consistent with our previous report that the percent stimulated secretion of radiolabeled PRP after a 6-h chase is relatively low during a subsequent 3-h stimulation (Castle et al., 1992). For amylase, the relative lack of stimulated secretion previously reported was observed following a 6-h chase time; but no measurements were made after a shorter chase (Colomer et al., 1994). We have therefore wondered whether both amylase and PRP might enter immature granules and subsequently be removed during the period of granule maturation.
PRP and Amylase Have Similar Rates of Intracellular Transport
To compare the sorting of newly synthesized PRP and amylase, it was necessary to initially evaluate the rate of transit through the early steps of the intracellular transport pathway, i.e., the rate of exit from the ER. Because amylase (unlike PRP) is not glycosylated, its rate of ER exit could not be estimated by monitoring sensitivity to digestion by endo H. Therefore, we examined the rate of unstimulated secretion as a close approximation to the rate of ER exit (Castle et al., 1992). Pulse-labeled amylase and PRP are secreted with similar kinetics (t 1/2 of 1.4 h for amylase, and 1.2 h for PRP), suggesting similar rates of exit from the ER for the two polypeptides. For comparison, the ER exit rate of PRP as measured by following the loss of endo H sensitivity gave a t 1/2 of 1 h, which is in good agreement with the estimates deriving from the t 1/2 of unstimulated secretion.
Stimulus-dependent Secretion of PRP and Amylase Decreases Relative to that of Endogenous Hormone as Granule Maturation Progresses
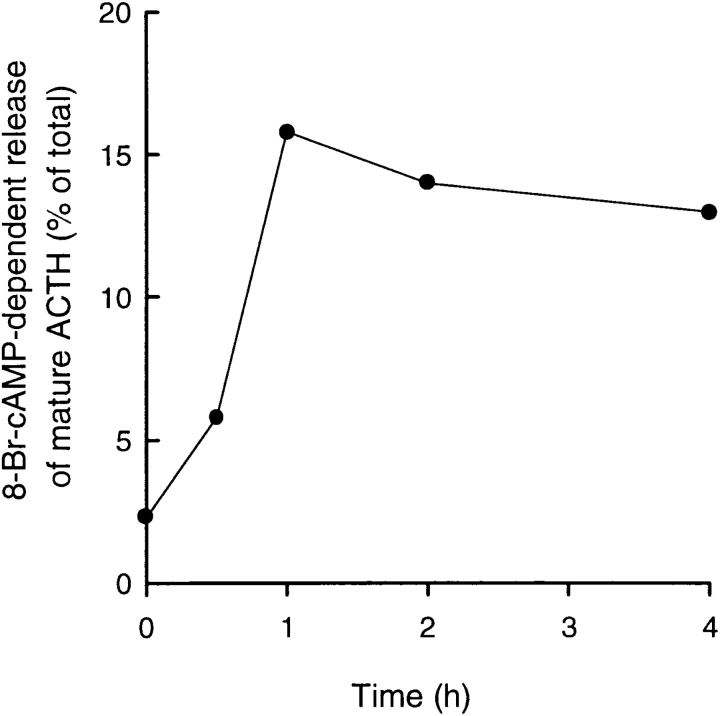
The similar ER exit rates of PRP and amylase facilitated our analysis of the sorting of newly synthesized exogenous proteins. For this purpose, we monitored the entry and subsequent storage of PRP and amylase in the regulated pathway by applying secretagogues early and late after pulse labeling. Since immature and mature granules are able to undergo stimulus-dependent exocytosis (Arvan et al., 1991; Tooze et al., 1991), early stimulation (1-h chase) tests entry into immature granules, whereas later stimulation (5-h chase) evaluates sustained presence in mature storage granules. This strategy was used previously to demonstrate that lysosomal hydrolase precursors initially enter immature granules in endocrine pancreatic β cells before exiting for lysosomes, whereas proinsulin/insulin enters and remains stably associated with the regulated pathway (Kuliawat and Arvan, 1994). As shown in Fig. 7, A and B, the secretion of both PRP and amylase is clearly stimulated. Although the fold stimulation (1.5 ± 0.1 for PRP and 1.4 ± 0.1 for amylase) is rather low, indicating that there is a high background of unstimulated secretion, these findings clearly indicate that both exogenous proteins enter the regulated pathway. Notably, quantitation (Table II) shows that the percentages of total labeled protein undergoing stimulated secretion (Table II, 8-Br stim.) are considerably higher at 1-h chase than at 5-h chase. For PRP, percent stimulated secretion decreases approximately eightfold between 1 and 5 h of chase (from 10.5 to 1.3%); and for amylase, percent stimulated secretion declines approximately fivefold (from 8% to 1.3%) over the same interval. These changes do not reflect protein degradation as all incorporated label has been accounted for at both time points (Table II, legend). In contrast, the stimulated release of ACTH peptides and of β-endorphin (expressed as percent of total labeled POMC and POMC-derived peptides) assayed under the same conditions was essentially unaffected. Because POMC passes through the early secretory pathway more rapidly than amylase and PRP (t 1/2 = 0.5 h; Moore and Kelly, 1985), and thus POMC-related peptides arrive in the immature granule earlier, we also examined stimulability of ACTH peptides using a protocol with better time resolution to confirm that the secretagogue-dependent release is truly constant with time. As shown in Fig. 8, the net stimulated release of ACTH peptides reaches a peak at 1 h of chase and then declines only slightly thereafter. Taken together, these data indicate that both exogenous salivary polypeptides readily enter a stimulatable compartment (immature granules) but are substantially removed during granule maturation. In contrast, POMC-related peptides are largely retained with only minor removal during maturation.
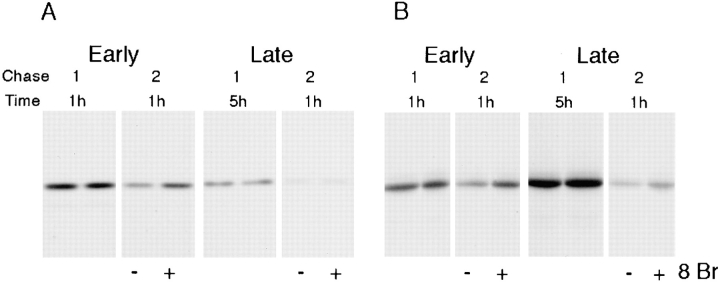
Figure 7.
Stimulation of secretion of newly synthesized PRP and amylase. Cells expressing PRP (A) and amylase (B) were labeled for 1 h with 0.5 mCi/ml of [3H]lysine or 0.15 mCi/ml of Expre35S35S label, respectively. Cells were first chased for 1 or 5 h in the absence of secretagogue, and subsequently for 1 h in the absence (−) or presence (+) of 5 mM 8-Br-cAMP (8 Br). Immunoprecipitated PRP (A) and amylase (B) from the chases are shown. All of each immunoprecipitate was loaded except in A for the 5-h chase, where only 1/3 of the total immunoprecipitate was used.
Table II.
Early and Late Stimulation of PRP, Amylase, ACTH, and β-Endorphin
| Protein | Chase 1 | Chase 2(−) | Chase 2(+) | Cell (−) | Cell (+) | 8-Br stim. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-h Chase | ||||||||||||
| PRP | 50 ± 3 | 20 ± 2 | 30 ± 2 | 26 ± 3 | 18 ± 3 | 10.5 ± 0.5 | ||||||
| Amylase | 36 ± 1 | 23 ± 2 | 32 ± 2 | 40 ± 2 | 34 ± 2 | 8 ± 2 | ||||||
| ACTH | 18 ± 1 | 14 ± 1 | 39 ± 2 | 43 ± 3 | 21 ± 2 | 24 ± 3 | ||||||
| β-Endorphin | 4 ± 1 | 4.5 ± 0.5 | 19 ± 2 | 36 ± 1 | 16 ± 2 | 15 ± 2 | ||||||
| 5-h Chase | ||||||||||||
| PRP | 91 ± 1 | 3 ± 1 | 4 ± 1 | 7 ± 2 | 6 ± 2 | 1.3 ± 0.3 | ||||||
| Amylase | 79 ± 2 | 5.5 ± 0.5 | 7 ± 1 | 12 ± 2 | 11 ± 2 | 1.3 ± 0.6 | ||||||
| ACTH | 23 ± 3 | 5 ± 1 | 25 ± 1 | 31 ± 5 | 14 ± 3 | 21 ± 2 | ||||||
| β-Endorphin | 13 ± 1 | 3.0 ± 0.5 | 18 ± 2 | 38 ± 3 | 21 ± 1 | 15 ± 2 |
AtT-20 cells expressing PRP, amylase, or mock-transfected cells were labeled with 0.5 mCi/ml [3H]lysine (for PRP), 0.25 mCi/ml [35S]methionine (for ACTH and β-endorphin), or 0.15 mCi/ml Expre35S35S label (for amylase). Cells were first chased for 1 or 5 h in the absence of secretagogue (Chase 1), and subsequently for 1 h in the absence (Chase 2 (−)) or presence (Chase 2 (+)) of 5 mM 8-Br-cAMP. Cells (Cells(−) or (+)) were harvested at the end of the experiment. Radiolabeled proteins were immunoprecipitated and quantitated as described in Materials and Methods. For PRP and amylase the values shown represent the radioactivity in each protein expressed as percent of total protein radiolabeled during the 1-h pulse. Recovery of radiolabeled PRP and amylase was 106 ± 5% and 105 ± 9%, respectively. For ACTH, the values shown represent the radioactivity in mature ACTH peptides (ACTH + gACTH + αmelanotropin-sized material, each corrected for the number of methionines) expressed as percent of the total labeled POMC-related peptides (POMC + 23K intermediate + ACTH + gACTH + αmelanotropin-sized material, each corrected for the number of methionines). For β-endorphin, the values shown represent the radioactivity in β-endorphin (corrected for the number of methionines) expressed as percent of the total labeled POMC-related peptides (POMC + β-lipotropin + β-endorphin). Recovery of POMC-related peptides was 92 ± 4% when imunoprecipitating with anti-ACTH antibodies and 101 ± 8% when immunoprecipitating with anti-endorphin antibody. 8-Br stim. is the difference between the stimulated and basal secretion of each protein (Chase 2(+) minus Chase 2(−)) expressed as percent of total labeled material (it was calculated for each experiment and the value presented is an average of all experiments). All data are presented as mean ± SEM from at least three independent experiments.
Figure 8.
Timecourse of stimulation of secretion of ACTH. Mock-transfected AtT-20 cells were labeled with 0.25 mCi/ml of [35S]methionine for 20 min and chased for 0, 0.5, 1, 2, or 4 h. At each timepoint, chase medium was replaced with medium containing or lacking 5 mM 8-Br-cAMP and chased for an additional 30 min. Net stimulated secretion of mature ACTH peptides was determined as described in Table II.
Discussion
The original intent of the present study was to gain insight into the mechanisms of sorting for regulated secretion by comparing the sorting of two salivary proteins, amylase and PRP, to that of the endogenous hormones. Amylase was previously reported to be absent from regulated secretion in AtT-20 cells (Colomer et al., 1994), whereas PRP is stored in the granules of these cells and exhibits increased storage with increased level of expression (Stahl et al., 1996). However, we were surprised by our initial findings on two accounts. First, amylase is clearly present in dense-core granules and thus qualitatively it behaves like a variety of other exogenous proteins (including exocrine proteins) that have been expressed in AtT-20 cells (e.g., Burgess et al., 1985; Fennewald et al., 1988; Seethaler et al., 1991; Castle et al., 1992; Lin et al., 1996). Second, even though we found earlier that the storage of PRP jumps incrementally with increased expression, suggesting involvement of intermolecular associations in sorting for regulated secretion (Stahl et al., 1996), we have now shown that PRP is actually poorly retained relative to endogenous hormone. Thus associations involving exogenous secretory proteins may not necessarily equate with interactions that contribute to the sorting of endogenous proteins.
In reviewing the previous claim that amylase is secreted exclusively by a constitutive pathway in AtT-20 cells, we note that a 1.1-fold stimulation of secretion of newly synthesized amylase after an extended (6 h) chase incubation was reported (Colomer et al., 1994). Our observation that amylase secretion is stimulated 1.4-fold (average of three experiments) under similar conditions (Fig. 7 B) is comparable; yet, we have clearly detected colocalization of amylase with ACTH in mature storage granules by immunocytochemistry (Fig. 3). Thus lowfold stimulation of secretion in newly synthesized exogenous protein is not a definitive criterion for the absence of regulated secretion, especially if the protein in question undergoes intracellular transport more slowly than the endogenous secretory products. On the other hand, our findings indicate that localization to granules in cellular processes does not necessarily translate into extensive storage in the granule pool.
One of the key experimental strategies in analyzing the sorting of salivary amylase and PRP in relation to the endogenous hormones, has been to compare the stimulus-dependent discharge of the proteins at early and late chase times. This approach is based on two assumptions: (a) initial susceptibility to stimulus-dependent secretion reflects arrival in immature granules (the first secretagogue-responsive compartment of the regulated pathway); and (b) subsequent susceptibility to stimulus-dependent secretion reflects the extent of retention within mature granules. An alternative to the first assumption, where early stimulation of the secretion of amylase and PRP might be viewed as enhancement of the rate of constitutive trafficking to the cell surface (Luini and DeMatteis, 1993), seems quite unlikely for the following reason: if stimulation were to reflect acceleration of the constitutive pathway, then unprocessed POMC, which in part follows this route in AtT-20 cells, should also exhibit stimulated secretion. However, several studies indicate that the secretion of POMC is not affected by secretagogues (Natori and Huttner, 1996; Mains and May, 1988; Castle, A.M., unpublished observations). Furthermore, our present data (particularly Table I, which demonstrates comparable fractional stimulation of endo H–resistant PRP and mature ACTH, and Figs. 2–6, which illustrate extensive colocalization of PRP and amylase with ACTH) strongly support both assumptions. That is, they argue that the salivary proteins and endogenous hormone have entered a common regulated pathway.
In using stimulated secretion as an index of entry and subsequent retention in the regulated secretory pathway, storage of 15–25% of newly synthesized endogenous hormones (β-endorphin and ACTH) reported in Table II reflects the capacity of AtT-20 cells to sort proteins for regulated secretion under our experimental conditions. We have found that after 1 h of chase, the percent stimulated secretion of newly synthesized exogenous proteins, PRP and amylase is approximately twofold lower than that of POMC-related peptides. However, since the t 1/2 of ER exit for both salivary proteins is twice as long as that of POMC (>1 h for PRP and amylase, versus 0.5 h for POMC; Moore and Kelly, 1985), a smaller fraction of newly synthesized salivary proteins than that of POMC will reach the regulated pathway at any given time of stimulation. In addition, the departure of the exogenous proteins is likely to commence soon after arrival in the immature granules. When these factors are taken into consideration, our results suggest that exogenous proteins and POMC have comparable access to the regulated secretory pathway.
If exogenous and endogenous proteins are indistinguishable with regard to granule entry, then why is there heterogeneity in the relative distribution of the secretory products among granules? We believe that this reflects differing degrees of intermolecular associations among particular proteins. Earlier studies implicated self-association of PRP during intracellular transport (Castle et al., 1992; Stahl et al., 1996) and aggregation of PRP under different conditions than ACTH (Castle, A.M. and A.Y. Huang, unpublished observation). Regardless of the differences in distribution, the exogenous and endogenous proteins are equally responsive to secretagogues (Table I), suggesting that they reside in a common granule pool.
In contrast to the apparent unrestricted entry, however, the exogenous and endogenous polypeptides differ dramatically in the extent to which they are retained in storage. The fraction of endogenous POMC-related peptides originally entering the granule pool decreases by only 10% (1.1-fold) between 1- and 5-h chase, whereas the fractions of amylase and PRP decrease five- and eightfold, respectively, over the same interval.
In considering the possible pathways followed by the exogenous secretory proteins that are not efficiently retained in granules, we have been able to rule out routes that lead to degradation (e.g., passage into lysosomes) because of our ability to account for all of the originally incorporated radiolabel (Table II). Thus the fate of these proteins is almost certainly secretory discharge originating from the granule pool. As shown by others, and in this work (Fig. 5 A), all granules contain ACTH. However, based on immunocytochemical and cell fractionation evidence, PRP appears to be enriched in granules that are less dense and thus may be either less mature or less completely packaged. We do not believe that these granules tend toward unstimulated secretion because this would result in reduced retention of ACTH in the granule pool. Also, amylase exhibits only small differences in localization from ACTH (Fig. 4), and yet both amylase and PRP are removed from the regulated pathway, suggesting that they are removed from the total granule pool rather than from a subset of granules. Thus, by a process of elimination, we believe that the removal of PRP and amylase occurs via granule-derived vesicles, most likely representing the constitutive-like pathway. Such vesicles would be secreted continuously and thus would not be expected to accumulate within the cell.
By using exogenous proteins that have little tendency to interact with the endogenous secretory products or to aggregate at the acidic pH thought to exist in the TGN and immature granules of AtT-20 cells (Colomer et al., 1994; Castle, A.M., unpublished observations) we have deduced that entry of these proteins into immature granules is apparently unrestricted. Thus, the principal controls on sorting involve retention within or export from the immature granule compartment. Consequently, these observations suggest that CPE is not required for entry into immature granules from the TGN in neuroendocrine cells as recently proposed (Cool et al., 1997). The salivary proteins, particularly PRP, are not proteolytically processed in AtT-20 cells, and they enter immature granules independent of any structural elements implicated in CPE-based sorting (Stahl et al., 1996). Importantly, we are not ruling out the possibility that CPE, through its binding to hormones or their precursors, might aid the granule entry and storage processes, especially because our data do not allow us to distinguish whether POMC actually enters AtT-20 immature granules a little better than the exogenous proteins. Instead, we raise the possibility that proteins such as membrane-associated CPE, or chromogranin B (Pimplikar and Huttner, 1992), may play a complementary and essential role in the passive sorting process by anchoring proteins or protein aggregates within the granule, and thereby guaranteeing their efficient retention. Finally, we emphasize that the relationship between aggregation and passive sorting for regulated secretion is likely to be complex and to involve hierarchical interactions. While PRPs seem to self-associate during intracellular transport even within immature granules (Castle et al., 1992; Stahl et al., 1996), these interactions do not insure efficient storage (Table II).
Acknowledgments
We are grateful to the Electron Microscope facility of the University of Virginia Health Sciences Center with support from the UVA Comprehensive Cancer Center for providing cell sections for immunoEM, and to the members of the Castle laboratory for valuable discussion. We are grateful to the reviewers of this manuscript for their constructive comments. We also thank Dr. J. Melvin (University of Rochester, Rochester, NY) for providing a cDNA encoding salivary amylase, and Drs. B. Eipper and R. Mains (Johns Hopkins University, Baltimore, MD) for anti-ACTH and anti-endorphin antibodies.
These studies were supported by a grant from the National Institutes of Health (DE08941).
Footnotes
1. Abbreviations used in this paper: CPE, carboxypeptidase E; LAMP-1, lysosome-associated membrane protein; POMC, pro-opiomelanocortin; PRP, proline-rich protein.
Please address all correspondence to J. David Castle, Department of Cell Biology, Box 439, University of Virginia Health Sciences Center, Charlottesville, VA 22908. Tel.: (804) 924-1910. Fax: (804) 982-3912.
References
- Arvan P, Castle D. Protein sorting and secretion granule formation in regulated secretory cells. Trends Cell Biol. 1992;2:327–331. doi: 10.1016/0962-8924(92)90181-l. [DOI] [PubMed] [Google Scholar]
- Arvan R, Kuliawat R, Prabakaran D, Zavacki A, Elahi D, Wang S, Pilkey D. Protein discharge from immature secretory granules displays both regulated and constitutive characteristics. J Biol Chem. 1991;266:14171–14174. [PubMed] [Google Scholar]
- Blair EA, Castle AM, Castle JD. Proteoglycan sulfation and storage parallels storage of basic secretory proteins in exocrine cells. Am J Physiol. 1991;261:C897–C905. doi: 10.1152/ajpcell.1991.261.5.C897. [DOI] [PubMed] [Google Scholar]
- Burgess TL, Craik CS, Kelly RB. The exocrine protein trypsinogen is targeted into the secretory granules of an endocrine cell line: studies by gene transfer. J Cell Biol. 1985;101:639–645. doi: 10.1083/jcb.101.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle AM, Castle JD. Novel secretory proline-rich proteoglycans from rat parotid: cloning and characterization by expression in AtT-20 cells. J Biol Chem. 1993;268:20490–20496. [PubMed] [Google Scholar]
- Castle AM, Stahl LE, Castle JD. A 13-amino acid N-terminal domain of a basic proline-rich protein is necessary for storage in secretory granules and facilitates exit from the endoplasmic reticulum. J Biol Chem. 1992;267:13093–13100. [PubMed] [Google Scholar]
- Chanat E, Huttner WB. Milieu-induced, selective aggregation of regulated secretory proteins in the trans-Golgi network. J Cell Biol. 1991;115:1505–1519. doi: 10.1083/jcb.115.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colomer V, Lal K, Hoops TC, Rindler MJ. Exocrine granule specific packaging signals are present in the polypeptide moiety of the pancreatic granule membrane protein GP2 and in amylase: implications for protein targeting to secretory granules. EMBO (Eur Mol Biol Organ) J. 1994;13:3711–3719. doi: 10.1002/j.1460-2075.1994.tb06680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colomer V, Kicska GA, Rindler MJ. Secretory granule content proteins and the luminal domains of granule membrane proteins aggregate in vitro at mildly acidic pH. J Biol Chem. 1996;271:48–55. doi: 10.1074/jbc.271.1.48. [DOI] [PubMed] [Google Scholar]
- Cool DR, Normant E, Shen F, Chen H-C, Pannell L, Zhang Y, Loh YP. Carboxypeptidase E is a regulated secretory pathway sorting receptor: genetic obliteration leads to endocrine disorders in Cpef atmice. Cell. 1997;88:73–83. doi: 10.1016/s0092-8674(00)81860-7. [DOI] [PubMed] [Google Scholar]
- Fennewald SM, Hamilton RL, Gordon JI. Expression of human preproapoA1 and pre(Δpro)apoA1 in a murine pituitary cell line (AtT-20) J Biol Chem. 1988;263:15568–15577. [PubMed] [Google Scholar]
- Grimes M, Kelly RB. Intermediates in the constitutive and regulated secretory pathways released in vitro from semi-intact cells. J Cell Biol. 1992;117:539–549. doi: 10.1083/jcb.117.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner B, Kelly RB. Secretory granules of an anterior pituitary cell line, AtT-20, contain only mature forms of corticotropin and β-lipotropin. Proc Natl Acad Sci USA. 1981;78:318–322. doi: 10.1073/pnas.78.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuliawat R, Arvan P. Protein targeting via the “constitutive-like” secretory pathway in isolated pancreatic islets: passive sorting in the immature granule compartment. J Cell Biol. 1992;118:521–529. doi: 10.1083/jcb.118.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuliawat R, Arvan P. Distinct molecular mechanisms for protein sorting within immature secretory granules of pancreatic β-cells. J Cell Biol. 1994;126:77–86. doi: 10.1083/jcb.126.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblond FA, Viau G, Laine J, Lebel D. Reconstitution in vitro of the pH-dependent aggregation of pancreatic zymogens en route to the secretory granule: implication of GP-2. Biochem J. 1993;291:289–296. doi: 10.1042/bj2910289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Akinbi HT, Breslin JS, Weaver TE. Structural requirements for targeting of surfactant protein B (SP-B) to secretory granules in vitro and in vivo. J Biol Chem. 1996;271:19689–19695. doi: 10.1074/jbc.271.33.19689. [DOI] [PubMed] [Google Scholar]
- Luini A, De Matteis MA. Receptor-mediated regulation of constitutive secretion. Trends Cell Biol. 1993;3:290–292. doi: 10.1016/0962-8924(93)90002-i. [DOI] [PubMed] [Google Scholar]
- Mains R, May V. The role of a low pH intracellular compartment in the processing, storage and secretion of ACTH and endorphin. J Biol Chem. 1988;263:7887–7894. [PubMed] [Google Scholar]
- McLean W, Nakane PF. Periodate-lysine-paraformaldehyde fixative. A new fixative for immunoelectron microscopy. J Histochem Cytochem. 1974;22:1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Milgram SL, Eipper BA, Mains RE. Differential trafficking of soluble and integral membrane secretory granule-asssociated proteins. J Cell Biol. 1994;124:33–41. doi: 10.1083/jcb.124.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore H-PH, Kelly RB. Secretory protein targeting in a pituitary cell line: differential transport of foreign secretory proteins to distinct secretory pathways. J Cell Biol. 1985;101:1773–1781. doi: 10.1083/jcb.101.5.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natori S, Huttner W. Chromogranin B (secretogranin I) promotes sorting to the regulated secretory pathway of processing intermediates derived from a peptide precursor. Proc Natl Acad Sci USA. 1996;93:4431–4436. doi: 10.1073/pnas.93.9.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz DM, Palmiter RD, Hammer RE, Brinster RL, Swift GH, MacDonald RJ. Specific expression of an elastase-human growth hormone fusion gene in pancreatic acinar cells of transgenic mice. Nature (Lond) 1985;313:600–602. doi: 10.1038/313600a0. [DOI] [PubMed] [Google Scholar]
- Pimplikar SW, Huttner WB. Chromogranin B (secretogranin I), a secretory protein of the regulated pathway, is also present in a tightly membrane-associated form in PC12 cells. J Biol Chem. 1992;267:4110–4118. [PubMed] [Google Scholar]
- Schagger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Schnabel E, Mains RE, Farquhar MG. Proteolytic processing of Pro-ACTH/Endorphin begins in the Golgi complex of pituitary corticotropes and AtT-20 cells. Mol Endocrinol. 1989;3:1223–1235. doi: 10.1210/mend-3-8-1223. [DOI] [PubMed] [Google Scholar]
- Seethaler G, Chaminade M, Vlasak R, Ericsson M, Griffiths G, Toffoletto O, Rossier J, Stunnenberg HG, Kreil G. Targeting of frog prodermorphin to the regulated secretory pathway by fusion to proenkephalin. J Cell Biol. 1991;114:1125–1133. doi: 10.1083/jcb.114.6.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sossin WS, Fisher JM, Scheller RH. Sorting within the regulated secretory pathway occurs in the trans-Golgi network. J Cell Biol. 1990;110:1–12. doi: 10.1083/jcb.110.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl LE, Wright RL, Castle JD, Castle AM. The unique proline-rich domain of parotid proline-rich proteins functions in secretory sorting. J Cell Sci. 1996;109:1637–1645. doi: 10.1242/jcs.109.6.1637. [DOI] [PubMed] [Google Scholar]
- Stoller TJ, Shields D. The propeptide of preprosomatostatin mediates intracellular transport and secretion of α-globin from mammalian cells. J Cell Biol. 1989;108:1647–1656. doi: 10.1083/jcb.108.5.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze J, Hollinshead M, Frank R, Burke B. An antibody specific for an endoproteolytic cleavage site provides evidence that pro-opiomelanocortin is packaged into secretory granules in AtT-20 cells before its cleavage. J Cell Biol. 1987;105:155–162. doi: 10.1083/jcb.105.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze SA, Flatmark T, Tooze J, Huttner W. Characterization of the immature secretory granule, an intermediate in granule biogenesis. J Cell Biol. 1991;115:1491–1504. doi: 10.1083/jcb.115.6.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zastrow M, Castle JD. Protein sorting among two distinct export pathways occurs from the content of maturing exocrine storage granules. J Cell Biol. 1987;105:2675–2684. doi: 10.1083/jcb.105.6.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou A, Mains RE. Endoproteolytic processing of proopiomelanocortin and prohormone convertases 1 and 2 in neuroendocrine cells overexpressing prohormone convertases 1 and 2. J Biol Chem. 1994;269:17440–17447. [PubMed] [Google Scholar]