Abstract
In Xenopus laevis development, β-catenin plays an important role in the Wnt-signaling pathway by establishing the Nieuwkoop center, which in turn leads to specification of the dorsoventral axis. Cadherins are essential for embryonic morphogenesis since they mediate calcium-dependent cell–cell adhesion and can modulate β-catenin signaling. α-catenin links β-catenin to the actin-based cytoskeleton. To study the role of endogenous α-catenin in early development, we have made deletion mutants of αN-catenin. The binding domain of β-catenin has been mapped to the NH2-terminal 210 amino acids of αN-catenin. Overexpression of mutants lacking the COOH-terminal 230 amino acids causes severe developmental defects that reflect impaired calcium-dependent blastomere adhesion. Lack of normal adhesive interactions results in a loss of the blastocoel in early embryos and ripping of the ectodermal layer during gastrulation. The phenotypes of the dominant-negative mutants can be rescued by coexpressing full-length αN-catenin or a mutant of β-catenin that lacks the internal armadillo repeats.
We next show that coexpression of αN-catenin antagonizes the dorsalizing effects of β-catenin and Xwnt-8. This can be seen phenotypically, or by studying the effects of expression on the downstream homeobox gene Siamois. Thus, α-catenin is essential for proper morphogenesis of the embryo and may act as a regulator of the intracellular β-catenin signaling pathway in vivo.
Cells in a developing organism depend on various forms of adhesion for their proper morphogenetic movements. Several types of adhesion molecules have been described during the early development of the frog Xenopus laevis. Widely studied are the cadherins, which are transmembrane glycoproteins that mediate calcium-dependent cell–cell adhesion (for review see Huber et al., 1996a ). The cadherins are in turn linked through their cytoplasmic domains to several cytoplasmic proteins called catenins. β-catenin (and its homologue plakoglobin) has been shown to bind directly to the cadherin cytoplasmic domain, and α-catenin has been shown to bind to the NH2 terminus of β-catenin (and plakoglobin) (Hülsken et al., 1994; Funayama et al., 1995; Jou et al., 1995; Aberle et al., 1996). In turn, α-catenin appears to bind to α-actinin and actin, establishing a link between the cadherins and the cytoskeleton (Knudsen et al., 1995; Rimm et al., 1995). The regulation of interactions mediated by these molecules is crucial for proper embryogenesis.
α-catenin is a 102-kD protein with homology to vinculin (Herrenknecht et al., 1991; Nagafuchi et al., 1991). Two genes encoding isoforms of this protein have been described with αN-catenin having 81.6% identity to the originally described α-catenin (Hirano et al., 1992). αN-catenin has similar properties to α-catenin: both bind to the cadherin complex, but αN-catenin is more prevalent in the nervous system (Hirano et al., 1992). PC9 cells that normally lack α-catenin exhibit cadherin-dependent aggregation upon introduction of α-catenin, identifying the latter as a molecule that promotes cadherin-dependent adhesion (Hirano et al., 1992). In addition, reintroduction of α-catenin or αN-catenin into the same cell line has been shown to induce a polarized phenotype typical of epithelial cells and has also been shown to alter the growth rate (Watabe et al., 1994). Expression of fusion proteins between E-cadherin and the COOH terminus of α-catenin circumvents the requirement of β-catenin for cell adhesion but reduces cell migration, suggesting that β-catenin functions in the cadherin–catenin complex as a regulatable linker between the cytoplasmic domain of cadherins and α-catenin (Nagafuchi et al., 1994). Recently, a gene trap screen in mice identified a fusion between the NH2-terminal 632 amino acids of α-catenin and β-geo reporter. Embryos homozygous for this mutant allele were shown to exhibit deficits in cell adhesion resulting in embryonic lethality (Torres et al., 1997). Thus, α-catenin appears to be essential both for cadherin-based adhesion in vitro and for embryonic development in vivo.
In the early development of Xenopus, α-catenin is initially supplied maternally and is expressed zygotically after the midblastula transition (Schneider et al., 1993). The protein is found in a large intracellular pool but localizes to membranes in the late blastula to early gastrula, eventually accumulating in the presumptive ectoderm and blastopore lip regions (Schneider et al., 1993). The localization to the ectoderm layer after gastrulation correlates with the expression of E-cadherin, which is essential for proper ectoderm formation (Levine et al., 1994). Other studies involving cadherins have shown that overexpression of the cytoplasmic domain of N-cadherin, the primary neuronal cadherin, results in the dissociation of ectodermal cells in midgastrulation, presumably by competing with the endogenous cadherins for catenin binding (Kintner, 1992). Expression of a similar extracellular truncation of XB-cadherin, which is normally expressed earlier in development, results in perturbations in anterior structures that are different than those observed after expression of an N-cadherin dominant-negative mutant (Dufour et al., 1994). Overexpression of C-cadherin (also called EP-cadherin) or E-cadherin cytoplasmic domains has also been shown to have differing effects. These results suggest that different cadherin cytoplasmic domains transmit individual as well as shared signals (Dufour et al., 1994; Broders and Thiery, 1995).
Recently, cadherins have been shown to be involved in the regulation of the Wnt-signaling pathway (Fagotto et al., 1996). The Wnt-signaling pathway is required for normal body-axis formation of Xenopus embryos (see Gumbiner, 1995). Components in this pathway include members of the Wnt family of secreted proteins; Wnt receptors of the D-frizzled family; an inhibitory Wnt-binding protein Xfrzb-1; several cytoplasmic proteins, specifically Xdishevelled, glycogen synthase kinase-3β (GSK-3), β-catenin, and APC; and the transcription factors Lef-1 and Tcf-1 of the high mobility group family and Siamois, a homeobox-containing protein (for review see Peifer, 1997; Huber et al., 1996a ; Miller and Moon, 1996; Gumbiner, 1995). The dual role of β-catenin in adhesion and signaling leads to questions regarding its coordinate regulation. In early Xenopus embryos, β-catenin has been shown to be essential in establishing the Nieuwkoop center and is required for dorsal mesoderm induction, but it is not required for blastomere adhesion, perhaps because a homologous catenin, plakoglobin, is also expressed (DeMarais and Moon, 1992; Heasman et al., 1994; Karnovsky and Klymkowsky, 1995). Xwnt-8, when expressed in the early Xenopus blastula, is thought to induce ectopic β-catenin–dependent signaling, which leads to embryo dorsalization (Smith and Harland, 1991; Fagotto et al., 1997). Adenomatous polyposis coli tumor suppressor protein (APC)1 negatively regulates β-catenin, possibly by targeting it for degradation (for review see Peifer, 1997; Munemitsu et al., 1995). Paradoxically, APC can also induce an ectopic dorsoanterior axis in Xenopus, suggesting that it is an active component in the Wnt-signaling pathway (Vleminckx et al., 1997). Since APC has been shown to form cytosolic complexes with α- and β-catenin (or plakoglobin), regulation of levels of these complexes may be important in β-catenin signaling (Hülsken et al., 1994). GSK-3 also regulates the levels of β-catenin and its association with APC (Dominguez et al., 1995; Rubinfeld et al., 1996; Yost et al., 1996). Recently, β-catenin has been shown to also bind to the high mobility group transcription factor Lef-1 and to be translocated with it to the nucleus, where the complex activates transcription (Behrens et al., 1996; Molenaar et al., 1996; Huber et al., 1996b ; Brunner et al., 1997; van de Wetering et al., 1997; Riese et al., 1997).
In addition to affecting β-catenin's signaling capacity, proteins in the Wnt-signaling pathway can also influence intercellular adhesion. Members of the Wnt-5A class of molecules disrupt calcium-dependent adhesion and also antagonize the signaling effects of the Wnt-1 family (of which Xwnt-8 is a member) (Torres et al., 1996). Moreover, in cell lines, Wnt-1 stabilizes the free cytosolic pool of β-catenin and of APC–catenin complexes (Papkoff et al., 1996; Papkoff, 1997). This can affect cell–cell adhesion by stabilizing β-catenin or plakoglobin binding to cadherins (Bradley et al., 1993; Hinck et al., 1994b ). Finally, overexpression of C-cadherin in Xenopus embryos has been shown to inhibit β-catenin signaling (Fagotto et al., 1996). Therefore, cadherin-based adhesion and the Wnt-signaling pathway are interdependent.
In the present study, we have used deletion mutants of αN-catenin to ascertain the endogenous functions of α-catenin in early Xenopus development. We first map the binding site of β-catenin to the NH2 terminus of α-catenin. We then show that mutants lacking the COOH-terminal third of α-catenin, when overexpressed in Xenopus embryos, cause severe defects in gastrulation that can be explained by severely impaired calcium-dependent cell adhesion. Finally, we find that overexpression of α-catenin can affect the dorsalizing properties of Xwnt-8 or β-catenin when these proteins are introduced early into ventral blastomeres; it can also perturb normal dorsoanterior axis formation when injected into dorsal blastomeres. We therefore propose that the COOH terminus of α-catenin is essential for the proper function of this molecule, that α-catenin plays an instrumental role in the adhesion of Xenopus blastomeres and their subsequent morphogenesis, and that α-catenin may also be a modulator of β-catenin signaling.
Materials and Methods
Plasmid Construction
The construct named GFPαNcatCterm was made by fusing green fluorescent protein (GFP) to the NH2 terminus of αN-catenin. GFP was obtained in the vector pCDNA-1 (Invitrogen, Carlsbad, CA) from Dr. C.M. Fan (University of California, San Francisco, CA), and chick αN-catenin in pBS SK+ (Stratagene, La Jolla, CA) was obtained from Dr. Masatoshi Takeichi (Hirano et al., 1992). GFP was amplified using PCR. The primers were upstream, GGTCGACCCATGAGTAAAGGAGAAGAACTTTTCACTGG, and downstream, CCAAGCTTATTTGTATAGTTCATCCATGCC. This product was ligated into pCR3 (Invitrogen). The resulting vector (pCR3-GFPa) was cut with XhoI. An XhoI fragment containing αN-catenin was excised from pBS SK+. This results in a deletion of the first NH2-terminal 750 bases of the full-length clone. This fragment was ligated into the pCR3-GFPa vector.
αNtermGFP was made using PCR, amplifying GFP and fusing it to a COOH-terminal deletion mutant of αN-catenin. The upstream primer was, CCCGGGGATGAGTAAAGGAGAAGAACTTTTCACTGG, and downstream, CCAAGCTTATTTGTATAGTTCATCCATGCC. The resulting product was ligated into pCR3. αN-catenin in pBS SK+ was cut with BamHI and BalI and fused into the BamHI-SmaI sites in the pCR3-GFPb construct. BalI, which cuts at position 2121, removes the COOH-terminal 724 bases of αN-catenin. The constructs described above were all sequenced through junctional regions.
The constructs GFPαNcatCterm and αNcatNtermGFP were subcloned from pCR3 into pCS2+ for expression in Xenopus embryos using the BamHI and XbaI sites. pCS2+ (Rupp et al., 1994) was a gift from Dr. Monica Vetter (University of Utah, Salt Lake City, UT).
GFP was subcloned from pCDNA-1 into pCS2+ using the sites BamHI-XbaI. ΔArm is described as ΔR in Kypta et al. (1996) and was excised from the expression vector p6R using the sites SalI and XbaI. It was subcloned into pCS2+ using XhoI and XbaI.
The construct GFPαNcat was made using pEGFP-C1 (CLONTECH Laboratories, Inc., Palo Alto, CA). αN-catenin was cut from pBS SK+ using EcoRI and NsiI, and subcloned into pEGFP-C1 using EcoRI and PstI. The resultant clone was cut with AgeI, and Klenow was used to create a blunt 5′ end. The insert was cut out with XbaI and subcloned into the StuI-XbaI sites of pCS2+. To remove intervening 5′-untranslated sequence that contains a stop codon, site-directed mutagenesis was performed using the Quick-Change mutagenesis kit (Stratagene, La Jolla, CA). The primers were CCCCACCCACCGAGATCTGGGAGTATGACTTC and its reverse complement. This introduced a BglII site in the intervening sequence at the site of the stop codon, so that BglII from pEGFP-C1 could be used to delete this sequence.
The construct GFPαNcatNterm was made by introducing a stop codon at position 2121 of αN-catenin in GFPαFL. The primers used for site- directed mutagenesis with the Stratagene kit were GACCAGCTTATTGCTGGCTAGAGTGCAAGGGC and its reverse complement.
Treatment of Embryos and mRNA Injections
All synthetic mRNAs were prepared using the SP6 Message Machine capped mRNA kit (Ambion, Inc., Austin, TX). GFP, GFPαNcatCterm, and αNcatNtermGFP in pCS2+ were all linearized using Asp718. NotI was used to linearize ΔArm, Xwnt-8, GFPαNcat, and GFPαNcatNterm. Xwnt-8 in pGEM5Zf(−)/RI was provided by Dr. Richard Harland (University of California, Berkeley, CA) (Smith and Harland, 1991). Dr. Barry Gumbiner provided the T1 HA-tagged β-catenin construct in the vector pSP64, which was linearized with EcoRI (Funayama et al., 1995).
Xenopus laevis frogs were obtained from Nasco (Fort Atkinson, WI). Sperm was added to eggs in 1× MMR: 100 mM NaCl, 5 mM, Hepes-Na, pH 7.4, 2 mM KCl, 1 mM MgCl2, and 2 mM CaCl2. After 5 min, 0.1× MMR was added to dilute the sperm and initiate fertilization. Embryos were dejellied after 10 min with 2.5% cysteine hydrochloride, pH 8.0, and allowed to develop in 0.1× MMR. 10 nl of a synthetic mRNA solution was injected either at the two- or four-cell stage using a glass micropipette.
Embryos were allowed to develop to various stages (as described by Nieuwkoop and Faber, 1967). Animals were visualized for green fluorescence using a 10× objective on a fluorescent microscope (model Microphot FXA; Nikon, Inc., Melville, NY). All photography of embryos was done using a Leica M420 macrophot microscope (Deerfield, IL). For experiments determining levels of dorsalization, embryos were injected at the four-cell stage in one ventral blastomere and scored at tadpole stages using the Dorsoanterior index scale (Kao and Elinson, 1988). For experiments leading to ventralized embryos, both dorsal blastomeres were injected at the four-cell stage.
Immunoblots and Immunoprecipitations
For experiments determining the association of β-catenin with the various αN-catenin constructs, 1.5 ng mRNA was injected into one cell of two-cell embryos. Embryos developed until stage 9–10, when 10 embryos were lysed in 1 ml of NP-40 LB (20 mM Tris-Cl, pH 8.0, 150 mM NaCl, 50 mM NaF, 10 mM DTT, 1 mM EDTA, 10 mg/ml aprotinin, 10 mg/ml leupeptin, 1% NP-40). Samples were spun at 8,169 g for 15 min. For immunoblots, reducing SDS sample buffer was added to 40 μl of extracts, boiled at 100°C, and loaded on 7.5% SDS–polyacrylamide gels. Immunoprecipitations were performed as described in Kypta et al. (1996). 2.5 μg of an anti– β-catenin monoclonal antibody (Transduction Labs, Lexington, KY) were used for immune precipitations. The anti-GFP polyclonal antibody serum was developed in the lab by Kuanhong Wang and Dr. Ulrich Müller (University of California, San Francisco, CA) and was used at a concentration of 1:500. For experiments using ΔArm to immunoprecipitate αN-catenin constructs, the same immunoprecipitation protocol was used with 10 μl (1 μg) of the monoclonal anti-myc tag antibody 9E10 (Santa Cruz Biotech Inc., Santa Cruz, CA). Cell fractionation experiments were performed exactly as described by Fagotto et al. (1996) but without acetone precipitations. 40 μl of the soluble fraction were boiled in SDS reducing sample buffer and loaded directly on 7.5% SDS–polyacrylamide gels. For experiments determining the levels of endogenous Xenopus α-catenin bound to immune complexes of cadherins, 10 embryos were lysed in NP-40 lysis buffer and immunoprecipitated using the monoclonal C-cadherin antibody 6B6 (Brieher and Gumbiner, 1994) as described above. The polyclonal antisera to αN-catenin (CME) was raised in the lab by Dr. Cindy-Murphy Erdosh. It was raised against a peptide corresponding to the COOH-terminal peptide of 21 amino acids (SQKKHISPVQALSEFKAMDSF) of αN-catenin and was used at a dilution of 1:1,000 for Western blots. Equal amounts of precipitates were loaded in each lane of SDS-PAGE gels.
Histology
For paraplast sectioning and staining, stage 11–12 embryos were fixed overnight in MEMFA (0.1 M MOPS, pH 7.4, 2 mM EGTA, 1 mM MgSO4, 3.7% formaldehyde). Embryos were then dehydrated through an ethanol series (50, 70, 90, 95, 100, and 100%) and placed in 100% butanol for 1.5 h. Embryos were carefully transferred to paraplast (Oxford Labware, St. Louis, MO) at 55–60°C for several hours and then positioned and embedded in paraplast. Sectioning was done at 7 μM using a rotary microtome. Sections were mounted and stained with eosin-hematoxylin using standard protocols. For plastic sections, embryos were fixed in MEMFA, dehydrated through an ethanol series, and embedded in glycol-methacrylate (Historesin, Leica, Heidelberg, Germany). 5-μM sections were stained with 0.5% toluidine blue.
Animal Cap Aggregation Assays
Both blastomeres of two-cell embryos were injected with 1.5 ng of mRNA. Animal caps were explanted at stage 9 in 1× MMR. To observe involution and healing, they were placed in 1× MMR in six-well tissue culture plates coated with 1% agarose for 1 h. Calcium-dependent aggregation assays were performed on either five or six animal caps. Explants were immediately dissociated in CMF medium (20 mM Na-Hepes, pH 7.2–7.4, 88 mM NaCl, 1 mM KCl) using a Pasteur pipette. Then CaCl2 was added back to 2 mM, and blastomeres were allowed to reaggregate on a rotating table for 1 h.
RNAse Protection Assays
For experiments involving animal cap explants, mRNAs were coinjected into the animal hemisphere of one cell of two-cell embryos. At stage 9, animal caps were removed from 10–15 embryos and allowed to develop until same stage embryos were stage 10.5. For experiments testing levels of endogenous Siamois expression, whole embryos injected in both dorsal blastomeres at the four-cell stage were taken at stage 10. Total RNA was extracted using RNAzol B according to the manufacturer's methods (Tel-Test, Inc., Friendswood, TX). RNAse protection assays were performed using the RPA II and MAXIscript T7 kits from Ambion. The EF1α probe construct was a gift from Dr. Tom Musci (University of California, San Francisco) (Cornell et al., 1995), and the Siamois (pXSia BglII 350) probe was a gift from Dr. Patrick Lemaire (Cambridge University, Cambridge, UK) (Carnac et al., 1996). Both constructs were linearized with HindIII and transcribed using T7 polymerase. 10 μg of total RNA was protected with 5 × 105 cpm of probe. Protected fragments were run on 5% denaturing polyacrylamide gels. Experiments were performed three times.
Results
The NH2-terminal Domain of αN-Catenin Is Essential for β-Catenin Binding
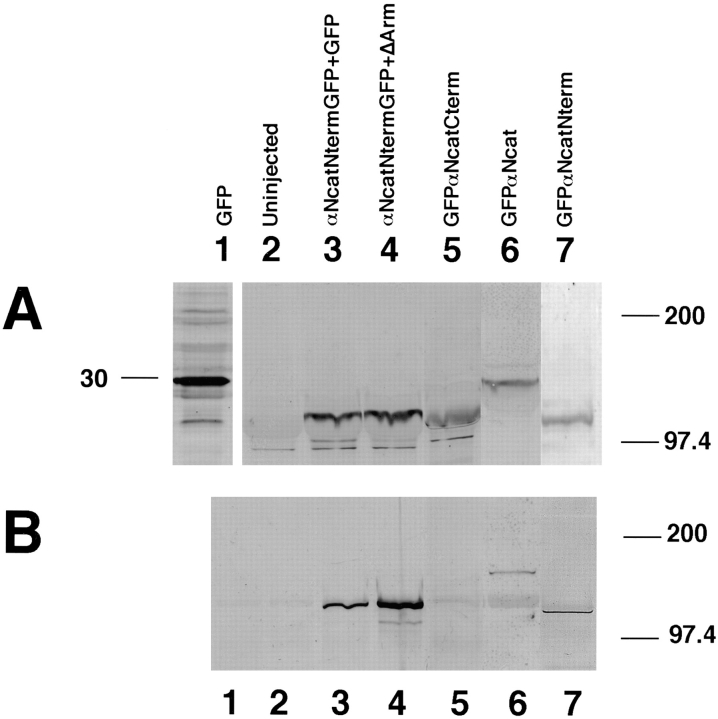
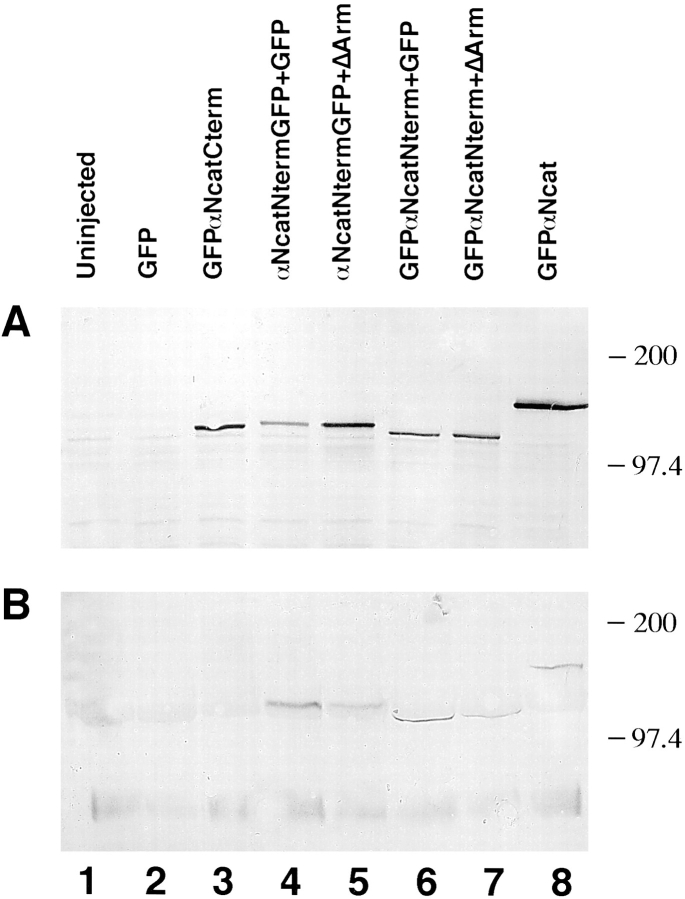
GFP was fused to various constructs of αN-catenin to permit assays of expression and localization. Fig. 1 illustrates the domains of αN-catenin and β-catenin present in each of the constructs used in these studies. mRNA prepared using each construct was injected into one cell of two-cell Xenopus embryos. To quantitate expression of different αN-catenin protein fragments, immunoblots were performed using a GFP polyclonal antibody for detection. Results in Fig. 2 A show that each of the proteins was expressed efficiently, a result confirmed by fluorescence detection of GFP. When a monoclonal antibody against β-catenin was used to immunoprecipate β-catenin and associated proteins, all αN-catenin constructs except GFPαNcatCterm, which lacks the NH2-terminal 210 amino acids catenin (Fig. 2 B, lane 5), were shown to bind to β-catenin. Thus, this NH2-terminal domain is essential for αN-catenin binding to β-catenin.
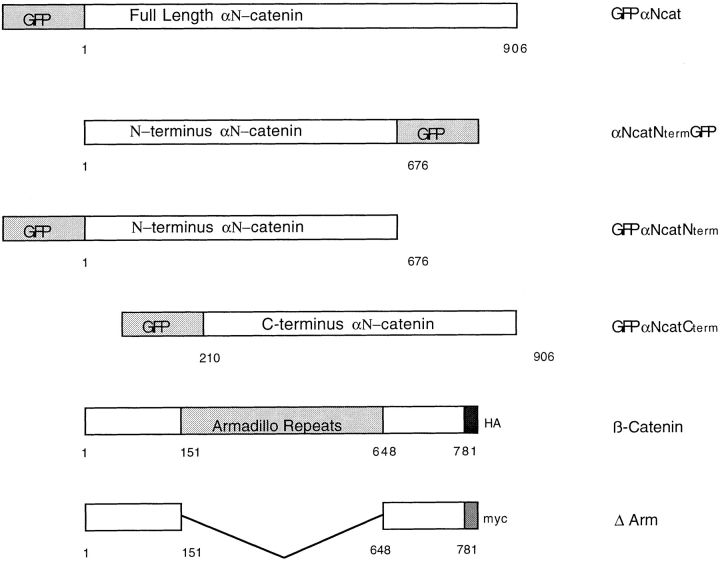
Figure 1.
α-catenin–GFP and β-catenin constructs used in this work. GFP was added to either the NH2 terminus of full-length, or to the NH2 or COOH termini of deletion mutants of chick αN-catenin. 1 represents the start methionine of αN-catenin, and 906 is the terminal amino acid residue. See Materials and Methods for details on the construction of these mutants. Full-length β-catenin has a COOH-terminal hemagglutinin (HA) tag, and ΔArm has a COOH-terminal myc tag.
Figure 2.
Expression of α-catenin–GFP fusion proteins in Xenopus embryos and the localization of the β-catenin binding site. (A) Expression of proteins detected by anti-GFP. (B) After immunoprecipitations with anti–β-catenin, associated α-catenin was detected using anti-GFP. In both A and B, the numbering is as follows: lane 1, GFP injected; lane 2, uninjected; lane 3, αNcatNtermGFP + GFP; lane 4, αNcatNtermGFP + ΔArm; lane 5, GFPαNcatCterm; lane 6, GFPαNcat; lane 7, GFPαNcatNterm. Note that GFPαNcatCterm, the protein lacking the NH2 terminus of αN-catenin, does not bind to β-catenin (B, lane 5). Results show that the presence of GFP does not prevent α-catenin binding to β-catenin, provided that the NH2-terminal binding domain is present. In addition, coexpression of ΔArm does not affect expression levels. In each case, 1.5 ng total RNA was injected into one blastomere at the two-cell stage.
Mutants that Lack the COOH Terminus of αN-Catenin Cause Severe Defects in Gastrulation
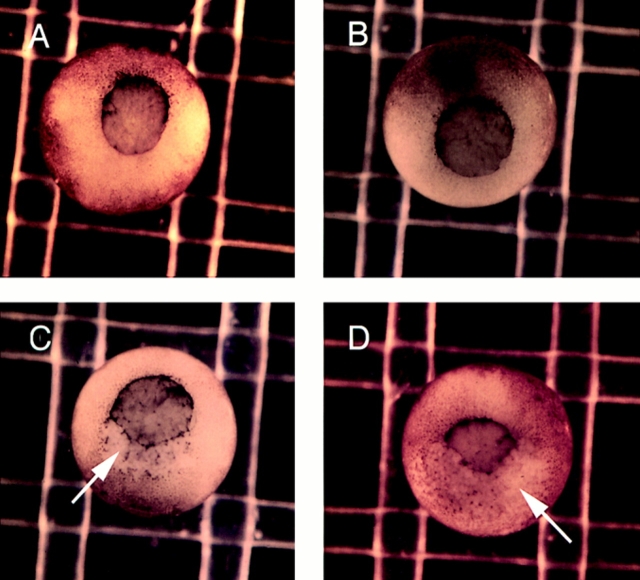
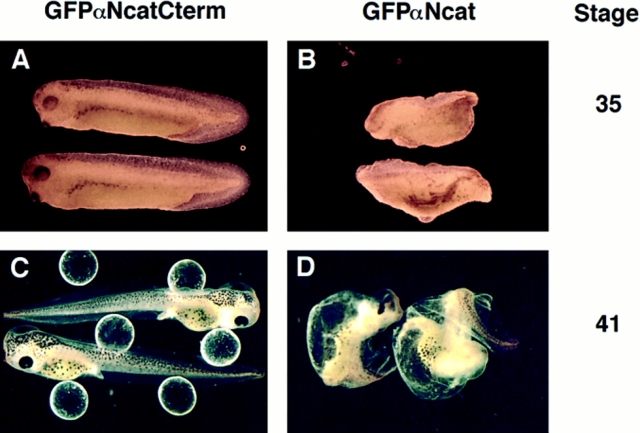
The injection of mRNA encoding αN-catenin with COOH-terminal domain deletions (αNcatNtermGFP or GFPαNcatNterm) caused severe defects in embryogenesis, noticeable first at the onset of gastrulation. The observed phenotype was a marked ripping of the outer ectodermal layer of the embryo, similar to what has been observed after the overexpression of the extracellular domain of E-cadherin (Levine et al., 1994) or the cytoplasmic domain of N-cadherin (Kintner, 1992), but on a more global scale, with the embryos succumbing to the defects at stage 11, unable to develop further. Fig. 3 shows the onset of this ripping as seen in embryos expressing GFPαNcatNterm or αNcatNtermGFP (Fig. 3, C and D). In these photos, the noticeable effects are observed at the dorsal involuting lip of the blastopore. Embryos injected with GFPαNcat or GFPαNcatCterm mRNA developed normally (Fig. 3, A and B) and expressed high levels of the respective proteins as visualized by immunoblots (Fig. 2) or GFP fluorescence (data not shown). Table I shows that the COOH-terminal deletion mutations are very potent, with 99–100% of the embryos injected with these mRNAs developing this phenotype. GFP-injected controls, or embryos injected with GFPαNcat or GFPαNcatCterm mRNA, rarely developed gastrulation defects (6% in the case of GFPαNcatCterm).
Figure 3.
Mutants lacking the COOH terminus of αN-catenin result in severe gastrulation defects. (A) GFPαNcat-injected embryos develop normally. (B) GFPαNcatCterm-expressing embryos develop normally. (C and D) Both αNcatNtermGFP- and GFPαNcatNterm-injected mRNAs induce defects beginning at stage 10, here shown by rips (arrows) occurring at the dorsal lips of the embryos. Embryos are shown with the ventral side toward the top. mRNA was injected into the animal pole of one cell of two-cell embryos.
Table I.
Frequency of Gastrulation Defects by α-Catenin–GFP Fusion Proteins
| RNA injected | No. of embryos | No. of gastrulation mutants | Percent mutant | |||
|---|---|---|---|---|---|---|
| noninjected | 460 | 4 | <1 | |||
| GFP | 274 | 9 | 3 | |||
| GFPαNcatCterm | 164 | 10 | 6 | |||
| αNcatNtermGFP | 344 | 341 | 99 | |||
| GFPαNcatNterm | 89 | 89 | 100 | |||
| GFPαNcat | 240 | 7 | 3 |
Numbers shown are sums taken from 10 separate experiments, except for GFPαNcatNterm, which were taken from three experiments. In each case, 1.5 ng of mRNA was injected into one cell of a two-cell embryo.
When single blastomeres of later-staged embryos were injected with αNcatNtermGFP, i.e., one blastomere of a four- or eight-cell embryo, embryos developed further but had incomplete morphogenesis with open areas shedding cells (data not shown). These data point to the COOH-terminal 230 amino acid residues of αN-catenin as essential for its proper function of the protein since overexpression of mutants lacking this domain caused severe deficiencies in morphogenesis.
ΔArm and GFPαNcat Rescue the Defects Caused by αN-Catenin Mutants
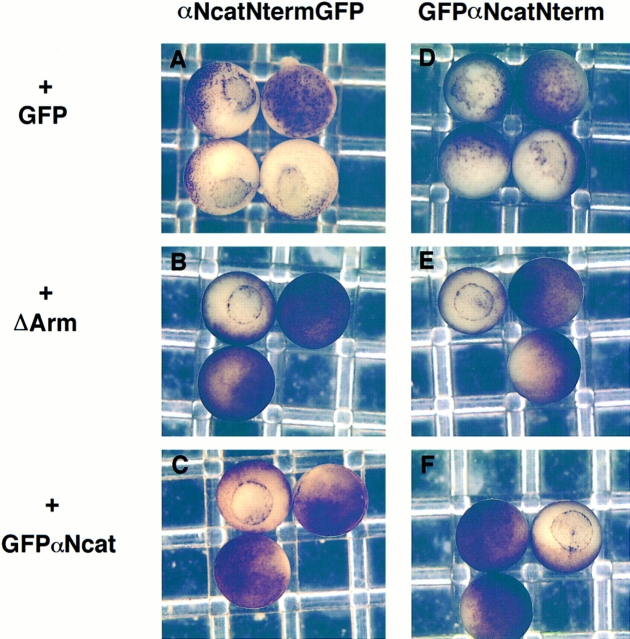
To ensure that the effects due to the mRNA injections were specific for α-catenin, we performed coinjection experiments. The first mRNA coinjected was a β-catenin construct lacking the internal armadillo repeats (ΔArm), which was described previously as ΔR in Kypta et al. (1996). This protein has been shown to retain binding to α-catenin but does not bind to cadherins or APC (Funayama et al., 1995). Fig. 4 shows that αNcatNtermGFP bound to immunoprecipitates of ΔArm in Xenopus extracts. Coexpression of ΔArm with either αNcatNtermGFP or GFPαNcatNterm resulted in a complete rescue of the phenotype, when the ratio of rescuing mRNA to αN-catenin truncation mRNA was 4:1 (Fig. 5, B and E, and Table II). Coinjecting the same amount of GFP mRNA as a control did not rescue the phenotype (Fig. 5, A and D, and Table II).
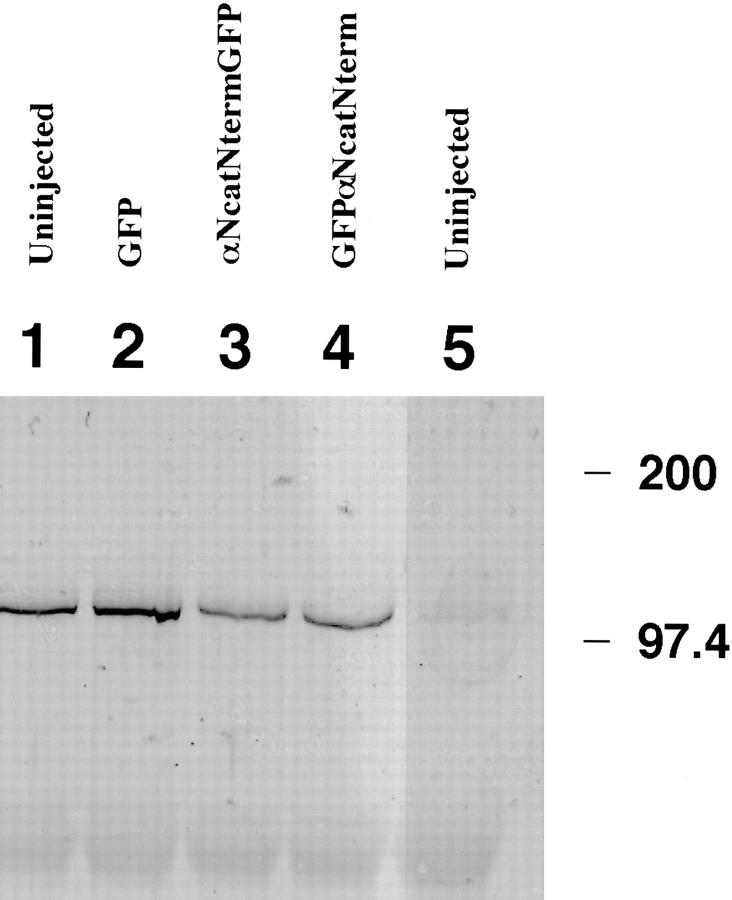
Figure 4.
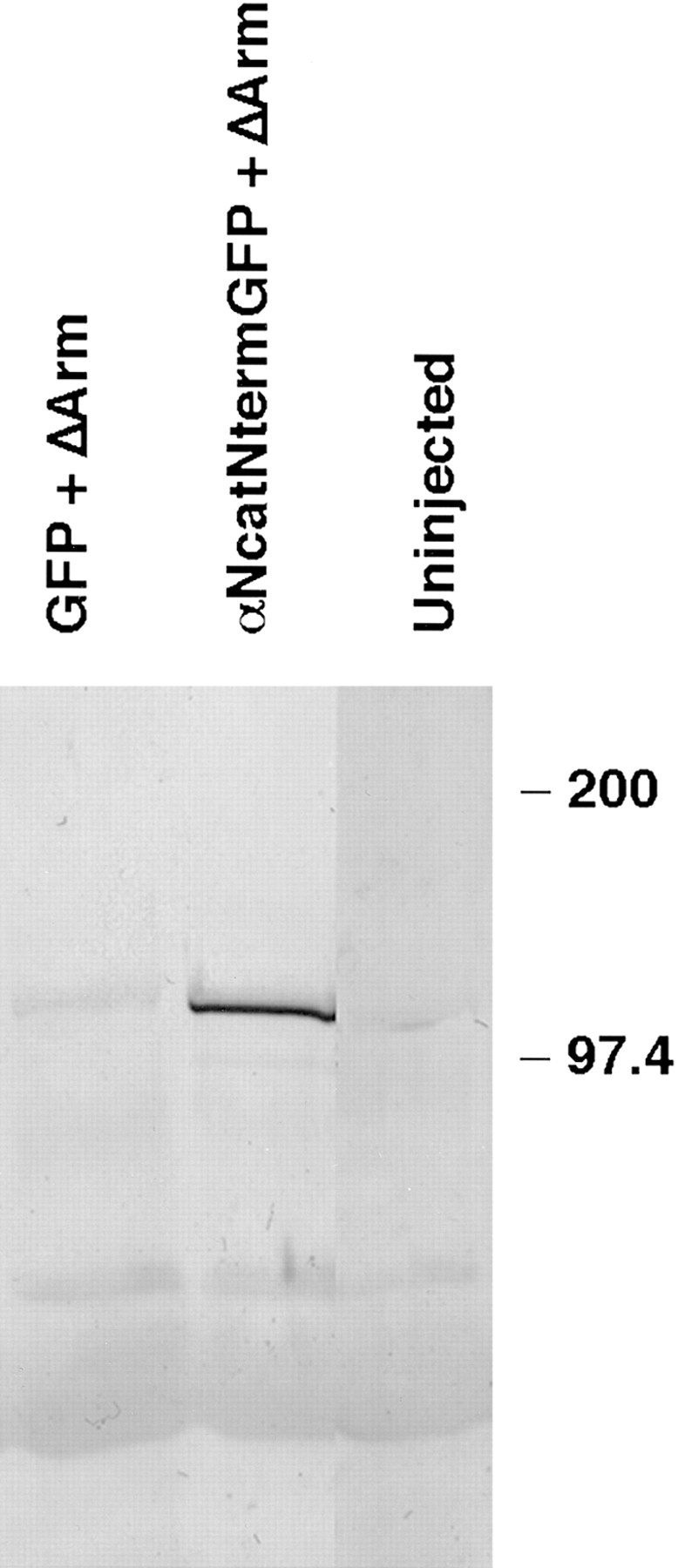
ΔArm binds α-catenin– GFP fusion proteins. The 9E10 monoclonal antibody that recognizes the myc tag on ΔArm was used to immunoprecipitate, and anti-GFP was used with subsequent immunoblotting to detect α-catenin–GFP fusion proteins in the precipitates. Embryos were coinjected with 0.3 ng of the first mRNA and 1.2 ng of the second. First lane, GFP + ΔArm; second lane, αNcatNtermGFP + ΔArm; third lane, uninjected.
Figure 5.
ΔArm and GFPαNcat rescue the defects caused by dominant-negative α-catenin mutants. (A, B, and C) αNcatNtermGFP + GFP, ΔArm, or GFPαNcat, respectively. (D–F) GFPαNcatNterm + GFP, ΔArm, or GFPαNcat. In all cases, 0.3 ng of αNcatNtermGFP or GFPαNcatNterm mRNA was coinjected with 1.2 ng of the second mRNA. Mutants in A and D do not develop further than gastrulation. Rescued mutants gastrulate (B and C, and E and F) and continue to develop normally. mRNA was injected into the animal pole of one cell of two-cell embryos.
Table II.
ΔArm and GFPαNcat Rescue Defects Caused by αNcatNtermGFP and GFPαNcatNterm
| RNA injected | No. of embryos | No. of gastrulation mutants | Percent mutant | |||
|---|---|---|---|---|---|---|
| Noninjected | 591 | 6 | 1 | |||
| GFP + ΔArm | 372 | 7 | 2 | |||
| GFP + GFPαNcat | 166 | 1 | <1 | |||
| αNcatNtermGFP + GFP | 483 | 465 | 96 | |||
| αNcatNtermGFP + ΔArm | 417 | 69 | 17 | |||
| αNcatCtermGFP + GFPαNcat | 62 | 0 | 0 | |||
| GFPαNcatNterm + GFP | 98 | 74 | 76 | |||
| GFPαNcatNterm + ΔArm | 100 | 0 | 0 | |||
| GFPαNcatNterm + GPFαNcat | 91 | 4 | 4 |
Numbers shown are sums taken from 15 separate experiments, except for experiments with GFPαNcatNterm, which were taken from three experiments. Also, the rescue of αNcatNtermGFP with GPFαNcat is the sum of three experiments. In each case, 0.3 ng of the first mRNA was coninjected with 1.2 ng of the second into one cell of two-cell embryos.
Similarly, the construct encoding full-length αN-catenin named GFPαNcat completely rescued the gastrulation-defective phenotypes induced by overexpression of the COOH-terminal deletion mutants of αN-catenin. This full-length αN-catenin construct with GFP fused to the NH2 terminus caused no observable phenotype when high protein expression was achieved by injecting mRNA encoding this protein into one cell of two-cell embryos. Again, when this mRNA was coinjected with GFPαNcatNterm, the embryos developed normally with no apparent defects (Fig. 5, C and F). A summary of these results is presented in Table II. Coinjection of GFPαNcat mRNA rescued consistently and completely embryos injected with the COOH-terminal truncation mutants of αN-catenin. In most cases, ΔArm completely rescued the defects caused by αNcatNtermGFP, but there was some variability in batches of mRNA, so the final average rescue was incomplete with 17% of embryos expressing a mutant phenotype.
To further characterize the variant phenotype, stage 10.5–11 embryos were sectioned and examined histologically (Fig. 6). At this stage, normal embryos have well- developed germ layers surrounding a central blastocoel (Fig. 6 A). In contrast, embryos expressing GFPαNcatNterm or αNcatNtermGFP protein showed a complete loss of the blastocoel and complete disorganization of the interior of the embryo (Fig. 6, C and D). In addition, defects in the integrity of the outer ectodermal layer are apparent in these embryos. This disorganization is in stark contrast to embryos injected with GFPαNcatCterm (Fig. 6 B), which develop normally. Further magnification of embryos shows the disorganized nature of cells in the ectodermal layer of an embryo expressing αNcatNtermGFP (Fig. 6 F). Cells have lost adhesive contacts and are round compared to cells of an embryo expressing GFPαNcatCterm (Fig. 6 E).
Figure 6.
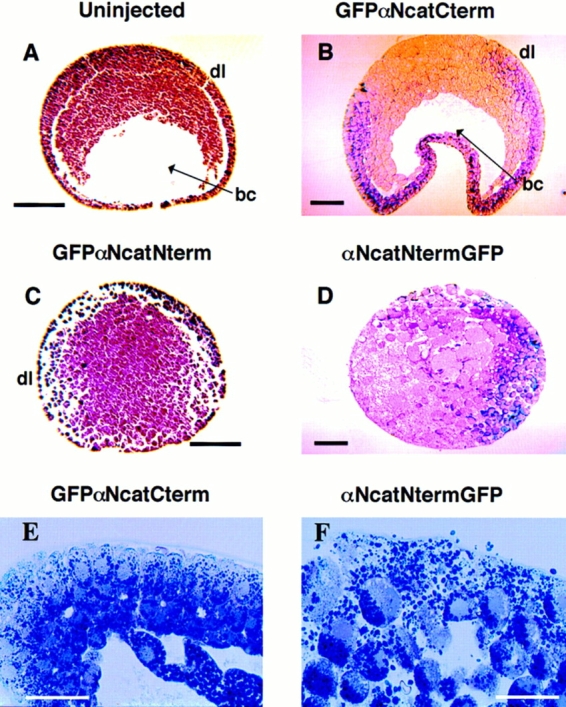
Histology of gastrulating embryos expressing α-catenin– GFP fusion proteins. Stage 10–11 embryos were fixed in MEMFA and imbedded in paraplast (A and C) or plastic (B, D, E, and F) for sectioning. Arrowheads point to the blastocoel (bc). The abbreviation dl denotes the presumptive dorsal side of the embryos. (A) An uninjected normal embryo. (B) An embryo injected with GFPαNcatCterm mRNA, (C) αNcatNtermGFP, and (D) GFPαNcatNterm. Embryos expressing αNcatNtermGFP completely lack the blastocoel and are disorganized compared to normal embryos. Also noticeable is the lack of integrity of the ectodermal layers in the two mutants. Higher magnification emphasizes the disorganization of cells in an embryo expressing αNcatNtermGFP compared to a normal embryo expressing GFPαNcatCterm. mRNA was injected into the animal pole of both cells of two cell embryos. Bars: (A–D) 200 μm; (E and F) 100 μm.
ΔArm Displaces αNcatNtermGFP and GFPαNcatNterm from Their Association with Cadherins to a Soluble Fraction
To provide evidence addressing the mechanism by which the β-catenin construct ΔArm, which lacks a cadherin binding site, rescued the defects caused by the expression of the COOH-terminal deletion αN-catenin constructs, cell fractionation methods were used. Following a protocol described by Fagotto et al. (1996), embryos were homogenized in a detergent-free buffer and fractionated by centrifugation. Most of α-catenin remains in solution after this fractionation (Hinck et al., 1994). The pellet obtained after high-speed centrifugation was extracted with a nonionic detergent (1% NP-40) and incubated with concanavalin A beads to obtain a glycoprotein-enriched fraction, where cadherins, and their associated proteins are found. After this procedure, a high majority of each of the α-catenin constructs was found in the soluble pool, but a significant portion was also found in the pellet, associated with cadherins. The distributions of these αN-catenin–GFP chimeras was visualized using anti-GFP, and results are presented in Fig. 7, A (supernatant) and B (pellet). From this experiment, it is again clear that GFPαNcatCterm, although highly expressed in the soluble fraction, is not associated with cadherins or other glycoproteins and therefore was not found in the glycoprotein-enriched pellet (Fig. 7, A and B, lane 3). GFPαNcat, the functional full-length αN-catenin construct, appeared in both fractions (Fig. 7, A and B, lane 8). When 0.3 ng of αNcatNtermGFP or GFPαNcatNterm was coinjected with 1.2 ng of GFP mRNA, a large amount of each αN-catenin–GFP chimera was found in the glycoprotein-enriched fraction of the precipitate (Fig. 7 B, lanes 4 and 6). However, when the same amount (1.2 ng) of ΔArm was coinjected, the levels in the pellet fraction of the αN-catenin–GFP chimeras decreased significantly (Fig. 7 B, lanes 5 and 7). In the case of αNcatNtermGFP, coinjection of ΔArm resulted in its displacement to the soluble fraction (compare Fig. 7, lanes 4 and 5 between A and B). Another type of experiment revealed similar results: embryos extracted in NP-40 buffer were subjected to immunoprecipitation with an antibody raised to C-cadherin and subsequently immunoblotted with anti-GFP. Again, when ΔArm was coinjected with αNcatNtermGFP, the binding of αNcatNtermGFP to C-cadherin was diminished (data not shown). Both of these experiments were performed several times and yielded consistent data. These results suggest a likely mechanism for how ΔArm rescued the dominant-negative effects of the COOH-terminal–deleted αN-catenin constructs. ΔArm is likely to bind avidly to the αN-catenin mutants and sequester them, thereby diminishing their binding to functional β-catenin in cadherin complexes.
Figure 7.
Redistribution of GFPαNcatNterm and αNcatNtermGFP from the glycoprotein fraction to the soluble fraction by ΔArm. (A) Soluble fractions immunoblotted with the anti-GFP polyclonal antibody. (B) Glycoprotein fractions immunoblotted with the anti-GFP polyclonal antibody. Lanes in both cases are 1, uninjected; 2, GFP injected; 3, GFPαNcatCterm injected; 4, αNcatNtermGFP + GFP; 5, αNcatNtermGFP + ΔArm; 6, GFPαNcatNterm + GFP; 7, GFPαNcatNterm + ΔArm; 8, GFPαNcat. Note the decrease in B between lanes 4 and 5 and lanes 6 and 7, implying that ΔArm acts by binding to the α-catenin mutants, keeping them from binding to the cadherin complex. When coinjections were done, 0.3 ng of the first mRNA was coninjected with 1.2 ng of the second mRNA.
Binding of Endogenous Xenopus α-Catenin to Cadherins Diminishes in Embryos Injected with Dominant-negative α-Catenin Constructs
To substantiate the model that the dominant-negative α-catenin mutants function by displacing endogenous α-catenin from binding to cadherin complexes, immunoprecipitations were performed with a monoclonal antibody to C-cadherin. The immune complexes were subsequently immunoblotted with an antibody raised against a peptide corresponding to the COOH-terminal 21 amino acids of chick αN-catenin. This antibody (CME) does not recognize αNcatNtermGFP or GFPαNcatNterm, both of which lack the COOH-terminal domain of αN-catenin. Results in Fig. 8 illustrate that the levels of endogenous α-catenin decreased significantly in embryos expressing αNcatNtermGFP or GFPαNcatNterm (lanes 3 and 4), as compared to embryos expressing GFP or GFPαNcatCterm (lanes 1 and 2). α-catenin was not detected in uninjected embryos subjected to immunoprecipitations with an anti-myc antibody (9E10) (Fig. 8, lane 5). The decrease of endogenous α-catenin bound to a cadherin- enriched fraction was also observed when embryo lysates were precipitated with conconavalin A (data not shown).
Figure 8.
Levels of endogenous Xenopus α-catenin bound to cadherins decrease in embryos expressing the dominant-negative mutants αNcatNtermGFP or GFPαNcatNterm. Embryos were lysed in NP-40 buffer and immunoprecipitated using the monoclonal antibody to C-cadherin 6B6. The immunoprecipitates were immunoblotted with the polyclonal anti–αN-catenin antibody (CME). The lanes correspond to embryos injected with mRNA for 1, uninjected; 2, GFP; 3, αNcatNtermGFP; 4, GFPαNcatNterm; 5, uninjected. Lane 5 represents uninjected embryos subjected to immunoprecipitation with an anti-myc epitope antibody (9E10) as a negative control. Embryos were injected into both cells at the two-cell stage to obtain protein expression in the highest possible number of cells. Notice that the levels of endogenous α-catenin decrease significantly in lanes 3 and 4 compared to lanes 1 and 2.
Animal Cap Assays Reveal that Embryos Injected with Dominant-negative α-Catenin Constructs Lack Calcium-dependent Cell Adhesion
To extend the results revealing disorganization of sectioned embryos (Fig. 6), we performed animal cap assays using embryos expressing the dominant-negative α-catenin COOH-terminal deletion constructs to reveal the degree of dissociation of blastomeres. Animal caps explanted from embryos expressing GFPαNcat, which developed normally, rounded up and healed in 1× MMR after an hour at room temperature (Fig. 9 A). However animal caps taken from embryos expressing either COOH-terminal truncation of αN-catenin did not undergo this process. Instead, blastomeres dissociated and were shed from the explants (Fig. 9 B).
Figure 9.
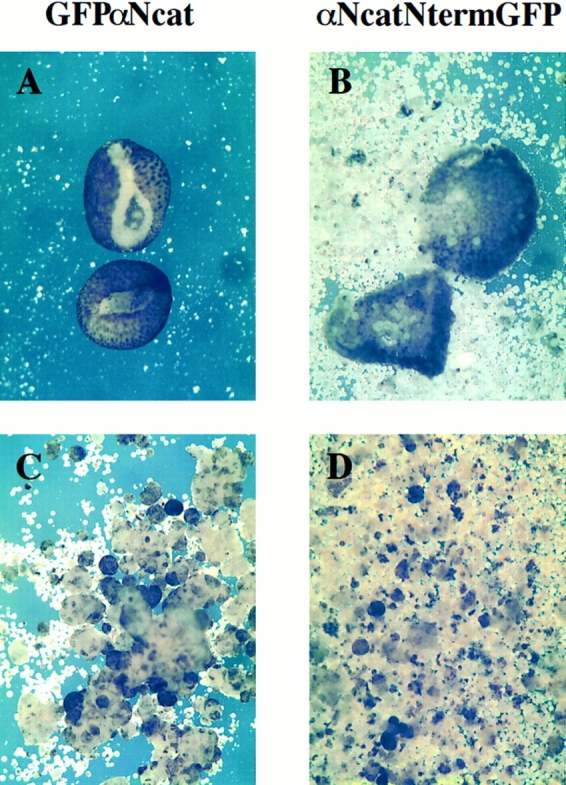
Animal cap assays reveal the dissociated nature of blastomeres expressing dominant-negative α-catenin. (A) Animal caps in 1× MMR normally heal and involute after 1 h as shown in GFPαNcat-injected embryos. (B) αNcatNtermGFP-expressing animal caps fail to involute, and dissociated blastomeres disperse after 1 h. (C) Dissociated GFP-expressing animal caps reaggregate in 2 mM Ca2+, whereas in D animal caps expressing αNcatNtermGFP do not. mRNA was injected into the animal pole of both cells of two-cell embryos.
Further experiments were performed to determine whether this disaggregation is based upon a calcium-dependent, and thus presumably cadherin-based, adhesion system. Both blastomeres at the two-cell stage were injected with control mRNA, or mRNAs encoding the dominant-negative α-catenin mutants. Animal caps were explanted from six stage 9 embryos and immediately dissociated by pipetting in calcium-free CMF medium. Calcium was added back to 2 mM, and the blastomeres were allowed to reaggregate for 1 h on a rotating table. Blastomeres expressing the COOH-terminal deletion constructs did not reaggregate, or they formed very small aggregates (Fig. 9 D), whereas blastomeres expressing GFP, GFPαNcatCterm, or GFPαNcat formed large cohesive aggregates (Fig. 9 C, not all data shown). The presence of GFP-derived fluorescence revealed that most of the blastomeres expressed αN-catenin–GFP chimeric proteins (data not shown).
αN-Catenin Antagonizes the Dorsalizing Effects of Xwnt-8 and β-Catenin in Ventral Blastomeres
Both Xwnt-8 and β-catenin have been established as potent dorsalizing factors that cause axis duplication after mRNA encoding these proteins is injected into ventral blastomeres of four-cell Xenopus embryos (Sokol et al., 1991; Funayama et al., 1995). Overexpression of C-cadherin in dorsal blastomeres results in ventralized embryos, and coinjection of C-cadherin with β-catenin in ventral blastomeres antagonizes the dorsalizing effects of β-catenin (Fagotto et al., 1996). To determine if overexpression of α-catenin, like C-cadherin, can affect the Wnt-signaling pathway, we first determined whether coinjection of the COOH-terminal αN-catenin deletion constructs could affect signaling induced by β-catenin injection into ventral blastomeres. In these experiments, at levels of expression where β-catenin normally caused axis duplication, αNcatNtermGFP, when coinjected with β-catenin, led to gastrulation defects indistinguishable from the phenotype when the former was injected alone. These experiments emphasize that it is difficult to observe formation of double axes if embryos die at gastrulation.
We next examined effects of coinjection of the full-length αN-catenin chimera (GFPαNcat) on Xwnt-8–activated signaling. 0.125 ng of Xwnt-8 mRNA was coinjected with increasing amounts of either GFP or GFPαNcat mRNA into a single ventral blastomere of four-cell embryos. When increasing amounts of GFP mRNA were coinjected, no inhibition of Xwnt-8–induced dorsalization was observed. At these concentrations, the embryos were severely dorsalized, radially symmetric with enlarged cement glands, and completely lacking in all trunk features (Fig. 10 C). In contrast, coinjection of increasing amounts of GFPαNcat mRNA significantly decreased the degree of Xwnt-8–induced dorsalization. Embryos developed normal head features, and in most cases simply had shortened, bent axes and reduced trunks (Fig. 10 D). Ventral blastomeres coinjected with 0.125 ng of GFP mRNA and high levels of GFPαNcat mRNA developed normally (Fig. 10 B). These data are summarized in Table III, using the Dorsoanterior index (DAI) developed by Kao and Elinson (1988). GFP mRNA did not reduce the dorsalizing effects of Xwnt-8, and DAI scores remained high, with all embryos more dorsalized than a score of 8. In contrast, increasing amounts of GFPαNcat resulted in less dorsalized embryos, and when 3 ng of GFPαNcat were coinjected with 0.125 ng Xwnt-8, the average DAI was reduced to 6.3.
Figure 10.
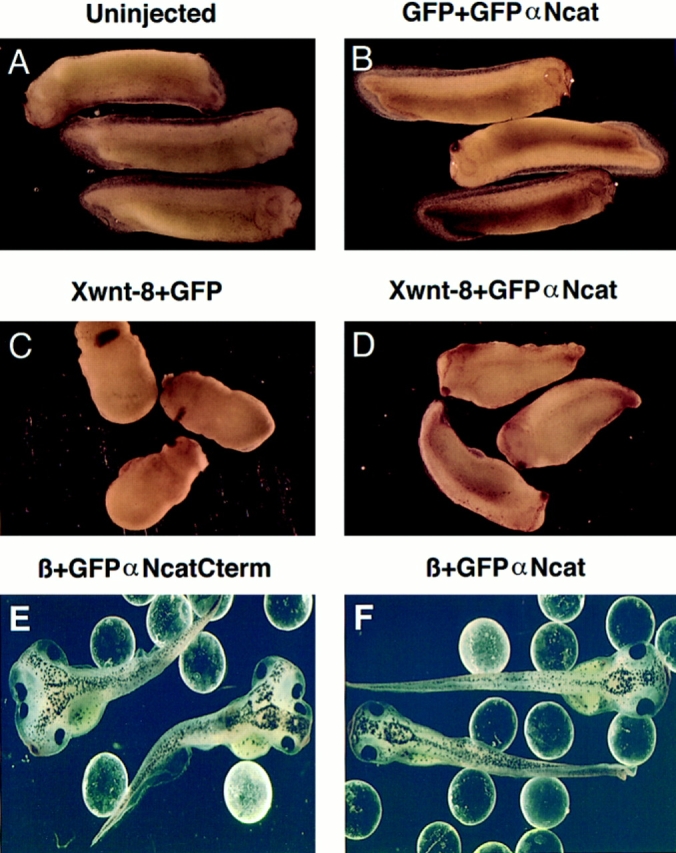
Coexpression of α-catenin diminishes the dorsalizing effects of Xwnt-8 and β-catenin. (A) Noninjected normal tadpoles. (B) Embryos expressing GFP + GFPαNcat. (C) Embryos expressing Xwnt-8 + GFP. (D) Xwnt-8 + GFPαNcat. (E) β-catenin + GFPαNcatCterm. (F) β-catenin + GFPαNcat. Embryos coexpressing α-catenin with Xwnt-8 develop longer trunks and are less dorsalized than embryos coexpressing GFP. Embryos ventrally expressing GFPαNcat + GFP develop normally (B). GFPαNcat antagonizes the induction of a secondary dorsoanterior axis by β-catenin (F). In B–D, embryos were coinjected with 0.125 ng of the first mRNA and 3 ng of the second. In E and F, 0.2 ng of β-catenin mRNA was coinjected with 3.0 ng of the αN-catenin constructs. One ventral blastomere was injected in each four-cell embryo. 1-mm glass beads hold the embryos upright.
Table III.
Full-Length α-Catenin Diminishes the Dorsalization caused by Xwnt-8 and the Axis Duplication Caused by β-Catenin
| DAI | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mRNA Injected | # of Embryos | >8 | 7 | 6 | 5 | Average DAI | ||||||
| 0.125 ng Xwnt8 + 0.85 ng GFP | 89 | 89 | 0 | 0 | 0 | >8 | ||||||
| 0.125 ng Xwnt8 + 3 ng GFP | 74 | 74 | 0 | 0 | 0 | >8 | ||||||
| 0.125 ng Xwnt8 + 0.85 GFPαNcat | 86 | 3 | 67 | 13 | 3 | 6.8 | ||||||
| 0.125 ng Xwnt8 + 3 ng GFPαNcat | 98 | 5 | 36 | 55 | 0 | 6.3 | ||||||
| 0.125 ng GFP + 3 ng GFPαNcat | 77 | 0 | 0 | 0 | 77 | 5 | ||||||
| No. with duplicated axes | Percent duplicated axes | |||||||||||
| 0.02 ng β-catenin + 3 ng GFPαNcatCterm | 142 | 67 | 47 | |||||||||
| 0.02 ng β-catenin + 3 ng GFPαNcat | 115 | 13 | 11 | |||||||||
Numbers shown are sums taken from four separate experiments. Embryos were injected in one ventral blastomere at the four-cell stage. The degree of dorsalization (dorsoanterior index [DAI]) and axis duplication were scored at early tadpole stages. A score of 5 is given to a normal embryo; 10 means the embryo is completely dorsalized (as described by Kao and Elinson, 1988). In addition, when duplicate axes were seen in embryos injected with β-catenin + GFPαNcat, the axes were very much reduced, with no complete head structures when compared to the axes duplications seen in embryos injected with β + GFPαNcatCterm.
Similar effects of GFPαNcat were observed on β-catenin–induced axis duplication. When 0.02 ng of β-catenin mRNA was coinjected with 3 ng of GFP mRNA, the duplicated axes and two heads are clearly visible (Fig. 10 E). Coninjecting 3 ng of GFPαNcat mRNA fully rescued these embryos, and they were indistinguishable from normal uninjected embryos (Fig. 10 F). Data in Table III illustrate that this effect was reproducible and that GFPαNcat consistently rescued the axis-duplicating effects of β-catenin. Thus, these data show that α-catenin can influence the β-catenin–stimulated Wnt-signaling pathway, presumably by binding and witholding free β-catenin from the signaling pool.
α-Catenin Expression in Dorsal Blastomeres Results in Ventralized Embryos
To test whether α-catenin can affect endogenous β-catenin signaling, GFPαNcat mRNA was injected into both dorsal blastomeres of four-cell embryos. Fig. 11 shows that embryos injected into dorsal blastomeres with mRNA encoding GFPαNcatCterm, which does not bind to β-catenin, developed normally. Embryos injected with the full-length αN-catenin construct often developed the ventral phenotype displayed here. Variability did occur, with some embryos more ventralized than others. But overall, the appearance of ventralized embryos suggests that α-catenin could play a regulatory role in endogenous β-catenin signaling, and in the establishment of a Nieuwkoop center.
Figure 11.
Full-length α-catenin can antagonize the endogenous induction of a dorsoanterior axis when expressed in dorsal blastomeres. 3.0 ng of either GFPαNcatCterm, which does not bind to β-catenin, or GFPαNcat, the full-length construct, were injected into both dorsal blastomeres of four-cell embryos. Embryos expressing GFPαNcat often develop the ventralized phenotypes illustrated here (B and D), with no anterior head structures present. Embryos expressing high levels of GFPαNcatCterm develop normally (A and C). Two stages are shown.
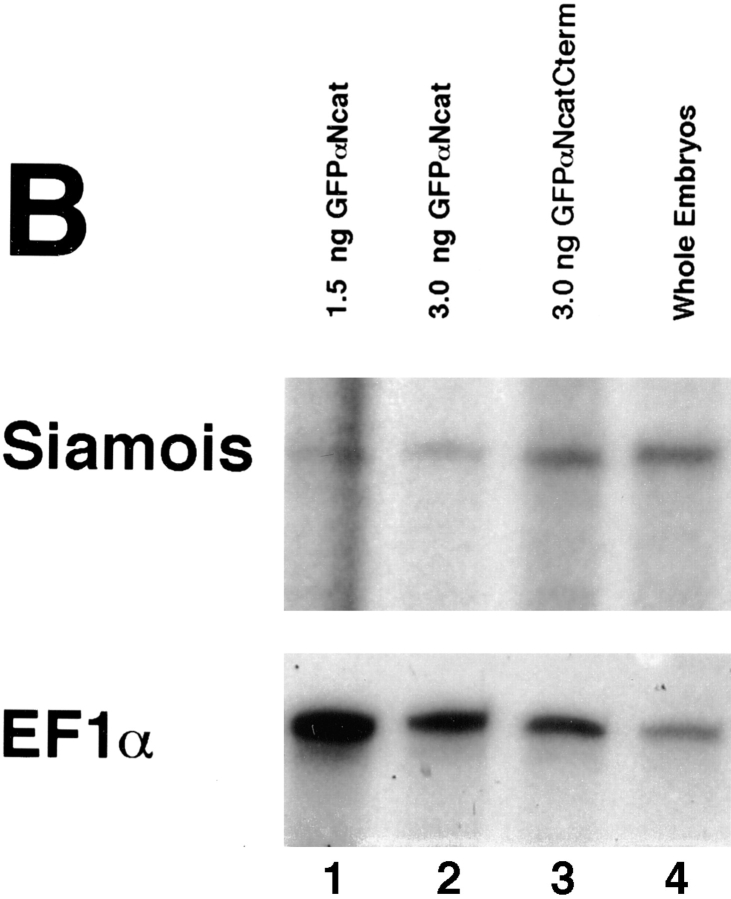
Siamois Expression Decreases with Increasing Levels of GFPαNcat in Xwnt-8–injected Animal Caps
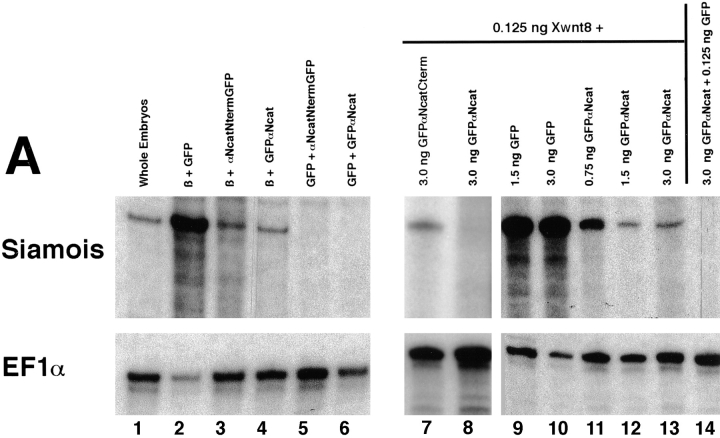
Expression of the homeobox gene Siamois in the Nieuwkoop center of early Xenopus embryos is dependent on β-catenin signaling (Fagotto et al., 1997). It is a target of the Wnt-signaling pathway, and its overexpression in ventral blastomeres also leads to dorsalized embryos with duplicated axes (Carnac et al., 1996; Lemaire et al., 1995; Brannon and Kimelman, 1996; Fagotto et al., 1997). We have used Siamois as a marker in RNAse protection assays to determine whether the phenotypes observed by coinjecting GFPαNcat mRNA with Xwnt-8 mRNA could be extended to the molecular level. When Xwnt-8 or β-catenin mRNAs were injected into the animal hemispheres of two cell embryos, Siamois was ectopically induced in animal caps of stage 9–10 embryos (Carnac et al., 1996; Fagotto et al., 1997). Results of RNAse protection assays presented in Fig. 12 show that the full-length αN-catenin construct, GFPαNcat, inhibited the ectopic induction of Siamois by β-catenin or Xwnt-8. mRNAs were injected into the animal hemisphere of one cell at the two-cell stage, and embryos were allowed to develop to stage 9, when animal cap explants were collected. Total RNA was made from the explants when control embryos reached stage 10.5. 10 μg of total RNA was protected with a probe for Siamois (pXSia BglII 350; Carnac et al., 1996) or the ubiquitously expressed elongation factor 1α (EF1α) (Cornell et al., 1995). Results revealed that β-catenin coinjected with GFP mRNA induced high levels of Siamois RNA, and coinjection of αNcatNtermGFP or GFPαNcat decreased these levels significantly (Fig. 12 A, lanes 2–4). GFPαNcat and αNcatNtermGFP did not induce Siamois on their own (Fig. 12 A, lanes 5–6). Increasing levels of GFP mRNA coinjected with Xwnt-8 did not affect Siamois expression, but increasing levels of GFPαNcat lowered levels of Siamois expression significantly (Fig. 12 A, lanes 9–13). Again, GFPαNcat did not induce Siamois by itself (Fig. 12 A, lane 14). In addition, coinjection of mRNA encoding GFPαNcat with Xwnt-8 reduces Siamois induction significantly compared to the coinjection of GFPαNcatCterm mRNA (which results in embryos that develop normally) (Fig. 12 A, lanes 7 and 8, taken from a separate experiment). Whole embryos (but not animal cap explants) of noninjected embryos expressed endogenous levels of Siamois (Fig. 12 A, lane 1). The levels of EF1α remained constant when compared to the corresponding decreases in Siamois expression.
Figure 12.
(A) α-Catenin inhibits the induction of the homeobox-containing gene, Siamois, by full-length β-catenin or Xwnt-8 in animal caps. One cell of two-cell embryos was injected in the animal hemisphere, and animal cap explants were taken at stage 9 and allowed to develop to stage 10.5, when total RNA was prepared. In lanes 2–6, coinjections were with 0.125 ng of the first mRNA and 3.0 ng of the second. Lane 1, uninjected whole embryos; lane 2, full-length β-catenin + GFP; lane 3, full-length β-catenin + αNcatNtermGFP; lane 4, full-length β-catenin + GFPαNcat; lane 5, GFP + αNcatNtermGFP; lane 6, GFP + GFPαNcat; lane 7, 0.125 ng Xwnt-8 + 3.0 ng GFPαNcatCterm; lane 8, 0.125 ng Xwnt-8 + 3.0 ng GFPαNcat; lane 9, 0.125 ng Xwnt-8 + 1.5 ng GFP; lane 10, 0.125 ng Xwnt-8 + 3 ng GFP; lane 11, 0.125 ng Xwnt-8 + 0.75 ng GFPαNcat; lane 12, 0.125 ng Xwnt-8 + 1.5 ng GFPαNcat; lane 13, 0.125 ng Xwnt-8 + 3 ng GFPαNcat; 14, 0.125 ng GFP + 3 ng GFPαNcat. The ubiquitously expressed elongation factor 1α (EF1α) was included as a loading control. RNAse protection assays show that either full-length αN-catenin (GFPαNcat) or the dominant-negative αN-catenin (αNcatNtermGFP) decrease the levels of Siamois mRNA induced in animal caps by β-catenin or Xwnt-8. Moreoever, increasing concentrations of GFPαNcat result in proportionate decreases in Siamois induction by Xwnt-8. (B) α-Catenin inhibits the endogenous expression of Siamois when injected into dorsal blastomeres at the four-cell stage. Both dorsal blastomeres were injected with mRNA, and embryos were extracted for total RNA at stage 10.5. Lane 1, 1.5 ng GFPαNcat; lane 2, 3.0 ng GFPαNcat; lane 3, 3.0 ng GFPαNcatCterm; lane 4, uninjected embryos. Embryos injected with GFPαNcat mRNA (which develop into ventralized embryos) express lower levels of Siamois than normal embryos or embryos injected with GFPαNcatCterm (which develop normally).
When high levels of GFPαNcat mRNA (3.0 ng) were injected into the two dorsal blastomeres of four-cell embryos (which causes the ventralized phenotype shown in Fig. 11), endogenous levels of Siamois RNA taken from whole embryos decreased when compared to noninjected embryos or embryos injected with GFPαNcatCterm (Fig. 12 B). Together, these results confirm that α-catenin can inhibit the Wnt-signaling pathway and provide evidence that regulation of α-catenin levels in the developing embryo may be crucial for normal morphogenesis.
Discussion
The cadherin cell adhesion system has been widely studied in the early development of Xenopus, but the role in this process of α-catenin, a protein that forms a link between cadherins and the cytoskeleton, has only recently begun to be examined. In the present study, we dissect the regions of α-catenin that are necessary for its proper function and find that certain molecular mutants can cause severe developmental defects. We also find that α-catenin can influence a signaling pathway in which there was no previous evidence for its participation. Our data point to a model where α-catenin is primarily used as a linker protein between the actin-based cytoskeleton and the cadherin-based intercellular adhesion system. The NH2 terminus of α-catenin is required for binding to β-catenin, and the COOH terminus is most likely essential for binding to cytoskeletal components. Since β-catenin is also known to be a linker protein in the same adhesion system but also has an important function in the Wnt-signaling pathway, our results further suggest that sequestration of β-catenin by α-catenin removes β-catenin from the Wnt-accessible signaling pool. By this means, α-catenin could modulate dorsoanterior axis induction.
We have found, using deletion constructs, that the NH2-terminal 210 amino acid residues of αN-catenin are essential for the binding and sequestration of β-catenin. This information extends previous data analyses assayed in a yeast two-hybrid system that illustrated that the NH2-terminal 606 amino acids are essential for this interaction (Jou et al., 1995).
Less is known regarding the functional interactions of the COOH-terminal domain of α-catenin, except that it is necessary for the proper function of the protein. Cosedimentation assays have indicated that the COOH-terminal 447 amino acids of α-catenin interact with F-actin (Rimm et al., 1995). α-catenin also appears to interact with α-actinin, although the precise domain needed for this interaction has not been determined (Knudsen et al., 1995). Consistent with its postulated function as a mediator of linkage to the cytoskeleton, a chimera of the E-cadherin extracellular and transmembrane domains fused to the α-catenin COOH-terminal domain has been shown to promote efficient cadherin-dependent cell aggregation, bypassing the normal requirements for the cytoplasmic domain of the cadherin and β-catenin (Nagafuchi et al., 1994). Moreover, embryos homozygous for a gene-trap mutation, where the LacZ reporter gene replaced the COOH-terminal third of α-catenin, have been shown to be deficient in the formation of the trophoblast epithelium that did not develop past the blastocyst stage (Torres et al., 1997). The COOH-terminal deletion in αN-catenin used in the present paper begins at amino acid residue 675, which is close to the truncation at position 632 of the gene trap mutant (Torres et al., 1997). The murine embryos homozygous for the α-catenin-βgal fusion protein did not develop a blastocoelic cavity; cultured blastocysts were shown to have altered cell adhesion, but heterozygous animals developed normally (Torres et al., 1997). Our data show that overexpressing the two α-catenin mutants lacking the COOH terminus disrupts function in a similar fashion, severely reducing cell adhesion with no blastocoel forming in embryos. In addition, our results illustrate that this type of mutation, when overexpressed, can disrupt endogenous α-catenin function. The data of Torres et al. (1997) suggest that at a 1:1 ratio, as would be found in animals heterozygous for the gene-trap fusion protein, the mutant protein is not at a high enough concentration to disrupt endogenous α-catenin. We have not been able to calculate the levels of mutant dominant-negative αN-catenin relative to endogenous Xenopus embryonic α-catenin, but it is probable that the levels of protein translated from synthetic mRNA injected into the embryos exceed endogenous levels. The fact that these defects can be rescued by full-length αN-catenin (GFPαNcat) indicates the relative amounts of normal and mutant proteins determine whether there is a phenotype.
In accordance with our model, GFPαNcatCterm, since it does not bind β-catenin (or presumably plakoglobin), does not cause a visible phenotype. Nor does the full-length αN-catenin construct (GFPαNcat) induce an adhesion phenotype since it, according to the model, can substitute and fully function for endogenous α-catenin because of its retention of both functional β-catenin and cytoskeletal binding domains. The fact that the expression of GFPαNcatNterm results in an identical phenotype to that induced by αNcatNtermGFP demonstrates that it is the deletion of the COOH-terminal domain, and not the addition of GFP, that causes these defects. These results suggest that associations with β-catenin (and plakoglobin) are limiting in linking cadherins to the cytoskeleton. The failure of overexpression of GFPαNcatCterm to cause a detectable phenotype suggests that sites for cytoskeletal association are not limiting.
The mechanism by which the β-catenin mutant ΔArm rescues the phenotype caused by the dominant-negative α-catenin constructs is not entirely clear. It has previously been shown that this construct, which lacks internal armadillo repeats, does not bind cadherins or APC (Funayama et al., 1995; Kypta et al., 1996). Previous results of others indicate that it almost certainly does not bind to HMG transcription factor family members or to the cytoskeletal protein fascin (Behrens et al., 1996; Tao et al., 1996). Expression of this protein in early Xenopus embryos caused no detectable phenotype, and this protein was found in the soluble pool, not associated with membrane-bound glycoproteins (Funayama et al., 1995). ΔArm has previously been shown to bind to α-catenin and to a LAR-family phosphatase (Kypta et al., 1996). In our experiments, ΔArm was shown to bind to the COOH-terminal deletion mutants GFPαNcatNterm and αNcatNtermGFP and reduce their associations through endogenous β-catenin with the membrane-bound cadherin complex (Fig. 7). Questions arise, however, about why ΔArm does not inhibit the association between endogenous α-catenin and the cadherin complex at the membrane and cause an adhesion phenotype on its own. One possible explanation is that endogenous β-catenin levels are limiting in establishing cadherin-based adhesion, but α-catenin is present in excess. As a result, overexpression of a dominant-negative mutant of α-catenin has stronger effects on endogenous β-catenin functions than the overexpression of the β-catenin mutant ΔArm on α-catenin functions.
It is not surprising that α-catenin dominant-negative mutants would cause such a striking phenotype in the early Xenopus embryo. Cadherin-based adhesion is known to be the primary adhesion system in early Xenopus, and several different cadherins are expressed (Huber et al., 1996a ). Since α-catenin appears to be a common linker between each of the different cadherins and the cytoskeleton, it is not surprising that disruptions in α-catenin have more severe phenotypes than disruptions in individual cadherins. The phenotype caused by overexpression of the cytoplasmic domain of N-cadherin (Kintner, 1992) exhibited many similarities to the phenotypes resulting from overexpression of the αN-catenin constructs in the present paper. In each instance, the primary defects occurred in gastrulation and resulted in lesions in the ectoderm layer caused by apparent deficiencies in cell adhesion. The embryos expressing the dominant-negative α-catenin constructs, however, seem to be more severely affected and have never developed past stage 11. In contrast, with low concentrations of dominant-negative cadherin mRNA, embryos had a weaker phenotype with the onset of visible defects delayed and integrity of the ectoderm maintained (Kintner, 1992). Since both the cadherin and α-catenin dominant-negative proteins are thought to function by titrating β-catenin (and plakoglobin) from functional complexes, it is understandable that the embryos have similar phenotypes. The higher degree of dissociation caused by the α-catenin dominant-negative mutants could reflect higher efficiency of synthetic mRNA translation, differences in protein stability, or both. On the other hand, Dufour et al. (1994) have presented evidence that the cytoplasmic domain of N-cadherin has properties differing from that of XB-cadherin. XB-cadherin binds β-catenin more efficiently than N-cadherin (Dufour et al., 1994). Moreover, it was found that the depletion of β-catenin by antisense oligonucleotides did not result in an adhesion phenotype but rather a phenotype more consistent with a disruption in signaling (Heasman et al., 1994). These results implicate plakoglobin, or another yet unknown linker protein, as a crucial component in the structural adhesion complex. Since α-catenin binds both plakoglobin and β-catenin (Hinck et al., 1994a ; Hülsken et al., 1994), the dominant-negative αN-catenin mutants could thus impart a more deleterious phenotype than the cytoplasmic domain of N-cadherin, which may not bind efficiently to β-catenin.
Very recently, a study by Kofron et al. (1997) showed that the injection of antisense oligonucleotides complementary to α-catenin mRNA specifically depleted maternal α-catenin mRNAs in Xenopus embryos. This reduction of mRNA resulted in less α-catenin protein translation, and the phenotype observed was a disruption of cell adhesion in blastula stage embryos. It was noticed that gastrulation was delayed, but did proceed normally, and embryos eventually developed through the neurula stages and formed normal axial structures. The results presented by Kofron et al. (1997) show that α-catenin is necessary for cell adhesion in early embryos, and this data is entirely consistent with the data presented here. However, the effects of the dominant-negative αN-catenin mutants were much more disruptive of gastrulation than the antisense experiments described by Kofron et al. (1997). The dominant-negative αN-catenin mutants are expressed highly and are present throughout early embryogenesis, as observed by the green fluorescent protein tag. They are perhaps more disruptive than the antisense oligonucleotides since antisense oligonucleotides are degraded within embryos and therefore can only reliably inhibit function until the midblastula transition at stage 8 when zygotic transcription begins. The results of the present study agree with the finding that cell adhesion is not entirely necessary for the survival of blastula stage embryos, but the data in this work show that cell–cell adhesion is in fact very necessary for the survival of Xenopus embryos. Our work also demonstrates that α-catenin plays an important role in maintaining the integrity of the cadherin-based cell adhesion system that is essential for gastrulation and the subsequent development of the embryo.
That α-catenin is important in maintaining cell adhesion in Xenopus embryos is consistent with previous observations in cell lines and recent data in mouse embryos (Hirano et al., 1992; Torres et al., 1997). However the effects of α-catenin on the Wnt-signaling pathway were unexpected. We have shown that α-catenin, when overexpressed in conjunction with Xwnt-8 or β-catenin, diminishes the degree of dorsalization induced by these signaling molecules. This was seen both phenotypically in embryos and at the molecular level in assays of mRNA encoding the homeobox-containing gene Siamois. In previous studies, overexpression of C-cadherin was observed to inhibit β-catenin– mediated signaling and was proposed to act by sequestering it to the cadherin complex at the plasma membrane (Fagotto et al., 1996, 1997). Since α-catenin inhibits β-catenin signaling, β-catenin, when bound to α-catenin, appears to be locked into a nonsignaling complex. This complex probably does not travel to the nucleus, and a mechanism for the release of β-catenin from the complex must be crucial for proper signaling. It is known that α-catenin forms soluble cytosolic complexes with β-catenin and APC (Hinck et al., 1994a ; Hülsken et al., 1994; Rubinfeld et al., 1993; Su et al., 1993). APC itself can induce an ectopic dorsoanterior axis in Xenopus, and C-cadherin inhibits this effect, implying that sequestration of β-catenin to the cadherin complex removes it from its association with cytosolic APC (Vleminckx et al., 1997). It would be interesting to determine whether α-catenin has the same effect as C-cadherin on APC signaling. Wnt-1 signaling stabilizes free β-catenin–APC cytosolic complexes (Papkoff et al., 1996) and does not affect levels of α-catenin in cell lines (Hinck et al., 1994b ), but the exact role of α-catenin in the Wnt-signaling pathway remains unclear. Since APC and the HMG transcription factor Lef-1 both bind to the armadillo repeats of β-catenin (Hülsken et al., 1994; Behrens et al., 1996), β-catenin must dissociate from APC and subsequently bind to Lef-1 to allow translocation to the nucleus and transcriptional activation. It is possible that α-catenin could abrogate monomeric β-catenin's association with Lef-1 or alternatively stabilize β-catenin's association in the APC complex. Adding another dimension is the speculation that v-src–mediated tyrosine phosphorylation of either β-catenin or an unknown cytoskeletal protein may regulate the interactions between α-catenin and β-catenin and thus cell adhesion (Papkoff, 1997). Whether the modulation of Wnt signaling by α-catenin is biologically relevant remains to be answered. It has been shown that an excess of free α-catenin is present in the early Xenopus embryo (Schneider et al., 1993), and this may play a role in regulating β-catenin levels. The conditions of dorsalization and its regulation that we created in the present study are artificial, but it seems possible that fluctuating levels or posttranslational modifications of α-catenin play a regulatory role in β-catenin–mediated signaling.
Recent results have suggested that β-catenin is itself an oncoprotein that can lead to the formation of colon cancers and melanomas (Korinek et al., 1997; Morin et al., 1997; Rubinfeld et al., 1997). The overexpression of full-length α-catenin seems to have no consequence on morphogenesis, but it can influence β-catenin signaling. It is thus possible that α-catenin could modulate β-catenin's oncogenic properties, and this may have important future therapeutic implications. Further research will determine how α-catenin removes β-catenin from the signaling pool and will identify the regulatory mechanisms mediating detachment of β-catenin from the cadherin adhesion complex.
Acknowledgments
This work has been supported by the Howard Hughes Medical Institute. L.F. Reichardt is an investigator of the Howard Hughes Medical Institute. R.N.M. Sehgal has been supported by a U.S. Public Health Service Training Grant No. T32 GM 08120.
Abbreviations used in this paper
- APC
adenomatous polyposis coli
- DAI
dorsalizing index
- GFP
green fluorescent protein
Footnotes
The authors are deeply grateful to Dr. R. Kypta and Dr. F. Fagotto for helpful comments on the manuscript; Dr. Tabitha Doniach for help in analyzing Xenopus development; Dr. Isabel Fariñas for instruction in histology; Dr. Cindy Sholes, Dr. Uli Müller, and Mr. Kuanhong Wang for the development of important antibodies; and members of the Gumbiner laboratory for support.
Address all correspondence to Louis Reichardt, HHMI, Room U-426, UCSF, Box 0724, San Francisco, CA 94143-0724. Tel.: (415) 476-3976. Fax: (415) 476-9914. E-mail: lfr@cgl.ucsf.edu
References
- Aberle H, Schwartz H, Hoschuetzky H, Kemler R. Single amino acid substitutions in proteins of the armadillo gene family abolish their binding to α-catenin. J Biol Chem. 1996;271:1520–1526. doi: 10.1074/jbc.271.3.1520. [DOI] [PubMed] [Google Scholar]
- Behrens J, von Kries JP, Kühl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of β-catenin with the transcription factor Lef-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- Bradley RS, Cowin P, Brown AMC. Expression of Wnt-1 in PC12 cells results in modulation of plakoglobin and E-cadherin and increased cellular adhesion. J Cell Biol. 1993;123:1857–1865. doi: 10.1083/jcb.123.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannon M, Kimelman D. Activation of Siamoisby the Wnt pathway. Dev Biol. 1996;180:344–347. doi: 10.1006/dbio.1996.0306. [DOI] [PubMed] [Google Scholar]
- Brieher WM, Gumbiner BM. Regulation of C-cadherin function during activin induced morphogenesis of Xenopusanimal caps. J Cell Biol. 1994;126:519–527. doi: 10.1083/jcb.126.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broders F, Thiery JP. Contribution of cadherins to directional cell migration and histogenesis in Xenopus embryos. Cell Adhes Commun. 1995;3:419–440. doi: 10.3109/15419069509081296. [DOI] [PubMed] [Google Scholar]
- Brunner E, Peter O, Schweizer L, Basler K. Pangolinencodes a Lef-1 homologue that acts downstream of armadillo to transduce the wingless signal in Drosophila. Nature. 1997;385:829–833. doi: 10.1038/385829a0. [DOI] [PubMed] [Google Scholar]
- Carnac G, Kodjabachian L, Gurdon JB, Lemaire P. The homeobox gene Siamoisis a target of the Wnt dorsalisation pathway and triggers organizer activity in the absence of mesoderm. Development (Camb) 1996;122:3055–3065. doi: 10.1242/dev.122.10.3055. [DOI] [PubMed] [Google Scholar]
- Cornell RA, Musci TJ, Kimelman D. FGF is a prospective competence factor for early activin-type signals in Xenopus mesoderm induction. Development (Camb) 1995;121:2429–2437. doi: 10.1242/dev.121.8.2429. [DOI] [PubMed] [Google Scholar]
- DeMarais AA, Moon R. The armadillo homologs β-catenin and plakoglobin are differently expressed during early development of Xenopus laevis. . Dev Biol. 1992;153:337–346. doi: 10.1016/0012-1606(92)90118-z. [DOI] [PubMed] [Google Scholar]
- Dominguez I, Itoh K, Sokol S. Role of glycogen synthase kinase 3β as a negative regulator of dorsoventral axis formation in Xenopus embryos. Proc Natl Acad Sci USA. 1995;92:8498–8502. doi: 10.1073/pnas.92.18.8498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour S, Saint-Jeannet JP, Broders F, Wedlich D, Thiery JP. Differential perturbations in the morphogenesis of anterior structures induced by overexpression of truncated XB- and N-cadherins in Xenopusembryos. J Cell Biol. 1994;127:521–535. doi: 10.1083/jcb.127.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagotto F, Funayama N, Glück U, Gumbiner BM. Binding to cadherins antagonizes the signaling activity of β-catenin during axis formation in Xenopus. . J Cell Biol. 1996;132:1105–1114. doi: 10.1083/jcb.132.6.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagotto F, Guger K, Gumbiner BM. Induction of the primary dorsalizing center in Xenopus by the Wnt/GSK/β-catenin signaling pathway, but not by Vg1, Activin or Noggin. Development (Camb) 1997;124:453–60. doi: 10.1242/dev.124.2.453. [DOI] [PubMed] [Google Scholar]
- Funayama N, Fagotto F, McCrea P, Gumbiner BM. Embryonic axis induction by the armadillo repeat domain of β-catenin: evidence for intracellular signaling. J Cell Biol. 1995;128:959–968. doi: 10.1083/jcb.128.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner BM. Signal transduction of β-catenin. Curr Opin Cell Biol. 1995;7:634–640. doi: 10.1016/0955-0674(95)80104-9. [DOI] [PubMed] [Google Scholar]
- Heasman J, Crawford A, Goldstone K, Garner-Hamrick P, Gumbiner B, McCrea P, Kintner C, Noro CY, Wylie C. Overexpression of cadherins and underexpression of β-catenin inhibit dorsal mesoderm induction in early Xenopus embryos. Cell. 1994;79:791–803. doi: 10.1016/0092-8674(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Herrenknecht K, Ozawa M, Eckerskorn C, Lottspeich F, Lenter M, Kemler R. The uvomorulin-anchorage protein α-catenin is a vinculin homologue. Proc Natl Acad Sci USA. 1991;88:9156–9160. doi: 10.1073/pnas.88.20.9156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinck L, Näthke IS, Papkoff J, Nelson W J. Dynamics of cadherin/catenin complex formation: novel protein interactions and pathways of complex assembly. J Cell Biol. 1994a;125:1327–1340. doi: 10.1083/jcb.125.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinck L, Nelson WJ, Papkoff J. Wnt-1 modulates cell–cell adhesion in mammalian cells by stabilizing β-catenin binding to the cell adhesion protein cadherin. J Cell Biol. 1994b;124:729–741. doi: 10.1083/jcb.124.5.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano S, Kimoto N, Shimoyama Y, Hirohashi S, Takeichi M. Identification of a neural α-catenin as a key regulator of cadherin function and multicellular organization. Cell. 1992;70:293–301. doi: 10.1016/0092-8674(92)90103-j. [DOI] [PubMed] [Google Scholar]
- Huber O, Bierkamp C, Kemler R. Cadherins and catenins in development. Curr Opin Cell Biol. 1996a;8:685–691. doi: 10.1016/s0955-0674(96)80110-4. [DOI] [PubMed] [Google Scholar]
- Huber O, Korn R, McLaughlin J, Ohsugi M, Herrmann BG, Kemler R. Nuclear localization of β-catenin by interaction with transcription factor LEF-1. Mech Dev. 1996b;59:3–10. doi: 10.1016/0925-4773(96)00597-7. [DOI] [PubMed] [Google Scholar]
- Hülsken J, Birchmeier W, Behrens J. E-Cadherin and APC compete for the interaction with β-catenin and the cytoskeleton. J Cell Biol. 1994;127:2061–2069. doi: 10.1083/jcb.127.6.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou TS, Stewart DB, Stappert J, Nelson WJ, Marrs JA. Genetic and biochemical dissection of protein linkages in the cadherin-catenin complex. Proc Natl Acad Sci USA. 1995;92:5067–5071. doi: 10.1073/pnas.92.11.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao KR, Elinson RP. The entire mesodermal mantle behaves as Spemann's organizer in dorsoanterior enhanced Xenopus laevisembryos. Dev Biol. 1988;127:64–77. doi: 10.1016/0012-1606(88)90189-3. [DOI] [PubMed] [Google Scholar]
- Karnovsky A, Klymkowsky M. Anterior axis duplication in Xenopus induced by the overexpression of the cadherin-binding protein plakoglobin. Proc Natl Acad Sci USA. 1995;92:4522–4526. doi: 10.1073/pnas.92.10.4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemler R. From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet. 1993;9:317–321. doi: 10.1016/0168-9525(93)90250-l. [DOI] [PubMed] [Google Scholar]
- Kintner C. Regulation of embryonic cell adhesion by the cadherin cytoplasmic domain. Cell. 1992;69:225–236. doi: 10.1016/0092-8674(92)90404-z. [DOI] [PubMed] [Google Scholar]
- Knudsen KA, Soler AP, Johnson KR, Wheelock MJ. Interaction of α-actinin with the cadherin/catenin cell–cell adhesion complex via α-catenin. J Cell Biol. 1995;130:67–77. doi: 10.1083/jcb.130.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofron M, Spagnuolo A, Klymkowsky M, Wylie C, Heasman J. The roles of maternal α-catenin and plakoglobin in the early Xenopus embryo. Development (Camb) 1997;124:1553–1560. doi: 10.1242/dev.124.8.1553. [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a β-catenin-Tcf complex in APC−/− colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- Kypta RM, Su H, Reichardt LF. Association between a transmembrane protein tyrosine phosphatase and the cadherin–catenin complex. J Cell Biol. 1996;134:1519–1529. doi: 10.1083/jcb.134.6.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire P, Garrett N, Gurdon JB. Expression cloning of Siamois, a Xenopus homeobox gene expressed in dorsal-vegetal cells of blastulae and able to induce a complete secondary axis. Cell. 1995;8:85–94. doi: 10.1016/0092-8674(95)90373-9. [DOI] [PubMed] [Google Scholar]
- Levine E, Lee CH, Kintner C, Gumbiner BM. Selective disruption of E-cadherin function in early Xenopus embryos by a dominant negative mutant. Development (Camb) 1994;120:901–909. doi: 10.1242/dev.120.4.901. [DOI] [PubMed] [Google Scholar]
- Miller JR, Moon R. Signal transduction through β-catenin and specification of cell fate during embryogenesis. Genes Dev. 1996;10:2527–2539. doi: 10.1101/gad.10.20.2527. [DOI] [PubMed] [Google Scholar]
- Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destree O, Clevers H. XTcf-3 transcription factor mediates β-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- Munemitsu S, Albert I, Souza B, Rubinfeld B, Polakis P. Regulation of intracellular β-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc Natl Acad Sci USA. 1995;92:3046–3050. doi: 10.1073/pnas.92.7.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi A, Takeichi M, Tsukita S. The 102 kd cadherin-associated protein: similarity to vinculin and posttranscriptional regulation of expression. Cell. 1991;65:849–857. doi: 10.1016/0092-8674(91)90392-c. [DOI] [PubMed] [Google Scholar]
- Nagafuchi A, Ishihara S, Tsukita S. The roles of catenins in the cadherin-mediated cell adhesion: functional analysis of E-cadherin–α catenin fusion molecules. J Cell Biol. 1994;127:235–245. doi: 10.1083/jcb.127.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop, P.D., and J. Faber. 1967. Normal Table of Xenopus laevis (Daudin). Elsevier North-Holland Biomedical Press, Amsterdam. 242 pp.
- Papkoff J. Regulation of complexed and free catenin pools by distinct mechanisms. J Biol Chem. 1997;272:4536–4543. [PubMed] [Google Scholar]
- Papkoff J, Rubinfeld B, Schryver B, Polakis P. Wnt-1 regulates free pools of catenins and stabilizes APC-catenin complexes. Mol Cell Biol. 1996;16:2128–2134. doi: 10.1128/mcb.16.5.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peifer M. β-catenin as oncogene: the smoking gun. Science. 1997;275:1752–1753. doi: 10.1126/science.275.5307.1752. [DOI] [PubMed] [Google Scholar]
- Riese J, Yu X, Munnerlyn A, Eresh S, Hsu S-C, Grosschedl R, Bienz M. Lef-1, a nuclear factor coordinating signaling inputs from wingless and decapentaplegic. Cell. 1997;88:777–787. doi: 10.1016/s0092-8674(00)81924-8. [DOI] [PubMed] [Google Scholar]
- Rimm DL, Koslov ER, Kebriaei P, Cianci CD, Morrow JS. α1(E)-catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc Natl Acad Sci USA. 1995;92:8813–8817. doi: 10.1073/pnas.92.19.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinfeld B, Souza B, Albert I, Müller O, Chamberlain SH, Masiarz FR, Munemitsu S, Polakis P. Association of the APC gene product with β-catenin. Science. 1993;262:1731–1734. doi: 10.1126/science.8259518. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Binding of GSK3β to the APC–β-catenin complex and regulation of complex assembly. Science. 1996;272:1023–1026. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P. Stabilization of β-catenin by genetic defects in melanoma cell lines. Science. 1997;275:1790–1792. doi: 10.1126/science.275.5307.1790. [DOI] [PubMed] [Google Scholar]
- Rupp RAW, Snider L, Weintraub H. Xenopusembryos regulate the nuclear localization of XmyoD. Genes Dev. 1994;8:1311–1323. doi: 10.1101/gad.8.11.1311. [DOI] [PubMed] [Google Scholar]
- Schneider S, Herrenknecht K, Butz S, Kemler R, Hausen P. Catenins in Xenopus embryogenesis and their relation to the cadherin-mediated cell–cell adhesion system. Development (Camb) 1993;118:629–640. doi: 10.1242/dev.118.2.629. [DOI] [PubMed] [Google Scholar]
- Smith WC, Harland RM. Injected Xwnt-8 RNA acts early in Xenopus embryos to promote formation of a vegetal dorsalizing center. Cell. 1991;67:753–765. doi: 10.1016/0092-8674(91)90070-f. [DOI] [PubMed] [Google Scholar]
- Sokol S, Christian JL, Moon RT, Melton DA. Injected Wnt RNA induces a complete body axis in Xenopus embryos. Cell. 1991;67:741–752. doi: 10.1016/0092-8674(91)90069-b. [DOI] [PubMed] [Google Scholar]
- Su L-K, Vogelstein B, Kinzler K. Association of APC tumor suppressor protein with catenins. Science. 1993;262:1734–1737. doi: 10.1126/science.8259519. [DOI] [PubMed] [Google Scholar]
- Tao YS, Edwards RA, Tubb B, Wang S, Bryan J, McCrea PD. β-Catenin associates with the actin-bundling protein fascin in a noncadherin complex. J Cell Biol. 1996;134:1271–1281. doi: 10.1083/jcb.134.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres M, Stoykova A, Huber O, Chowdhury K, Bonaldo P, Mansouri A, Butz S, Kemler R, Gruss P. An α-E-catenin gene trap mutation defines its function in preimplantation development. Proc Natl Acad Sci USA. 1997;94:901–906. doi: 10.1073/pnas.94.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Yang-Snyder JA, Purcell SM, DeMarais AA, McGrew LL, Moon RT. Activities of the Wnt-1 class of secreted signaling factors are antagonized by the Wnt-5A class and by a dominant negative cadherin in early Xenopusdevelopment. J Cell Biol. 1996;133:1123–1137. doi: 10.1083/jcb.133.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vleminckx K, Wong E, Guger K, Rubinfeld B, Polakis P, Gumbiner B. Adenomatous polyposis coli tumor suppressor protein has signaling activity in Xenopus laevisembryos resulting in the induction of an ectopic dorsoanterior axis. J Cell Biol. 1997;136:411–420. doi: 10.1083/jcb.136.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe M, Nagafuchi A, Tsukita S, Takeichi M. Induction of polarized cell–cell association and retardation of growth by activation of the E-cadherin–catenin adhesion system in a dispersed carcinoma line. J Cell Biol. 1994;127:247–256. doi: 10.1083/jcb.127.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wetering M, Cavallo R, Dooijes D, van Beest M, van Es J, Loureiro J, Ypma A, Hursh D, Jones T, Bejsovec A, et al. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell. 1997;88:789–799. doi: 10.1016/s0092-8674(00)81925-x. [DOI] [PubMed] [Google Scholar]
- Yost C, Torres M, Miller JR, Huang E, Kimmelman D, Moon RT. The axis-inducing activity, stability, and subcellular distribution of β-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–1454. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]