Abstract
We studied endocytosis in chromaffin cells with both perforated patch and whole cell configurations of the patch clamp technique using cell capacitance measurements in combination with amperometric catecholamine detection. We found that chromaffin cells exhibit two relatively rapid, kinetically distinct forms of stimulus-coupled endocytosis. A more prevalent “compensatory” retrieval occurs reproducibly after stimulation, recovering an approximately equivalent amount of membrane as added through the immediately preceding exocytosis. Membrane is retrieved through compensatory endocytosis at an initial rate of ∼6 fF/s. Compensatory endocytotic activity vanishes within a few minutes in the whole cell configuration. A second form of triggered membrane retrieval, termed “excess” retrieval, occurs only above a certain stimulus threshold and proceeds at a faster initial rate of ∼248 fF/s. It typically undershoots the capacitance value preceding the stimulus, and its magnitude has no clear relationship to the amount of membrane added through the immediately preceding exocytotic event. Excess endocytotic activity persists in the whole cell configuration. Thus, two kinetically distinct forms of endocytosis coexist in intact cells during perforated patch recording. Both are fast enough to retrieve membrane after exocytosis within a few seconds. We argue that the slower one, termed compensatory endocytosis, exhibits properties that make it the most likely mechanism for membrane recycling during normal secretory activity.
In the neuroendocrine chromaffin cells of the adrenal medulla, exocytosis of catecholamines and peptides through the fusion of large dense core vesicles is followed by specific endocytosis of vesicle membrane components (Kobayashi et al., 1978; Benchimol and Cantin, 1982; Lingg et al., 1983; Phillips et al., 1983; Patzak and Winkler, 1986). The process by which exocytosis in neuroendocrine cells takes place and its kinetic description have received much attention and study in the past, but just recently has the process by which the secretory granule membrane components become recycled been kinetically described on the subsecond time scale (Neher and Zucker, 1993; Heinemann et al., 1994; Thomas et al., 1994; Artalejo et al., 1995; Burgoyne, 1995; Smith and Betz, 1996; for review see Henkel and Almers, 1996). The process of evoked endocytosis has been difficult to quantitatively measure in chromaffin cells because of an apparent rapid loss of activity in the whole cell patch clamp configuration, here referred to as “wash-out” (Burgoyne, 1995; Fig. 1 of this study). The wash-out may be due to either the dialysis of molecules into or out of the patch pipette or a change in the physiological state of the cell due to the patch configuration. Such whole cell wash-out of endocytotic activity has also been reported in pancreatic β-cells (Ämmälä et al., 1993; Eliasson et al., 1996) and saccular hair cells (Parsons et al., 1994) and can be recognized in rat pituitary melanotrophs (Thomas et al., 1990; Figs. 1 and 4–6, but see also Fig. 7 for apparent exception) as well as cyclically secreting rat gonadotropes (Tse et al., 1993).
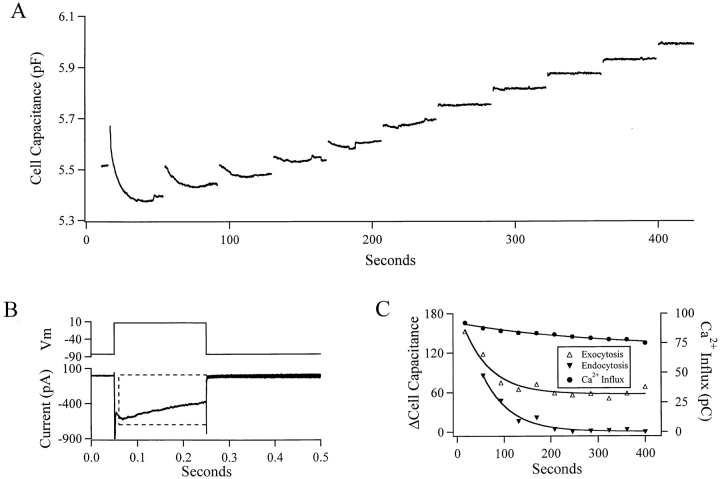
Figure 1.
Endocytotic activity decays in the whole cell patch clamp configuration. (A) Cell capacitance from a chromaffin cell held under the whole cell patch clamp configuration is plotted against time. The cell membrane was voltage clamped at −83 mV and depolarized to +7 mV for 200 ms every 39 s. The record began immediately after establishing the whole cell configuration. The first pulse resulted in a capacitance increase of 152.9 fF but was followed by a 295.3-fF capacitance decrease; the current trace measured during the first depolarization is plotted in B. The upper portion of the panel depicts the membrane voltage protocol and is in millivolts. The lower trace in the graph shows the membrane current. Immediately upon depolarization, a large initial inward current was measured, which presumably represents Na+ influx. After the decay of the Na+ current, the remainder of the current is assumed to be carried by Ca2+. The lines through the data in C represent the best fit exponential decays. The decay time constant for both exocytosis and endocytosis is ∼60 seconds. The first endocytotic response from the cell is not included in C for reasons that will be developed further.
Interestingly, in some studies a rapid form of excess membrane retrieval was seen in whole cell recordings after intense exocytosis, triggered by high levels of intracellular Ca2+. Excess endocytosis seems to be resistant to wash-out in chromaffin cells (Neher and Zucker, 1993; Heinemann et al., 1994; Artalejo et al., 1995), pituitary melanotrophs (Thomas et al., 1994), and pancreatic β-cells (Eliasson et al., 1996). A slightly slower form of excess endocytosis is even seen after large increases in cellular Ca2+ in nonexcitable cultured Chinese hamster ovary cells (Coorssen et al., 1996; Ninomiya et al., 1996). Excess endocytosis in neuroendocrine cells can be very fast, with time constants ranging between 300 ms and 3 s (Thomas et al., 1994; Artalejo et al., 1995). It was therefore suggested that it is responsible for rapid recycling of membrane during electrical activity in neuroendocrine cells. We were puzzled, however, by the fact that in whole cell recordings, using flash photolysis of caged-Ca2+, Ca2+ concentrations higher than 50 μM are required to elicit excess retrieval (Neher and Zucker, 1993; Heinemann et al., 1994; Thomas et al., 1994). We wondered whether such conditions are reached in physiological situations. Also, excess endocytosis often displays all-or-nothing behavior. For instance, in the work of Artalejo and collaborators (1995, 1996), many examples can be found where several identical, strong stimuli do not evoke membrane retrieval. Then, a single subsequent stimulus elicits endocytosis, which recovers membrane in excess to that accumulated during the preceding stimuli. We could not find any correlate for such all-or-nothing behavior in morphological or fluorometric studies performed under physiological recording conditions, and we wondered whether the high Ca2+ requirement and the “excess retrieval” property might be an artifact of whole cell recording. Particularly, we wanted to inquire whether the relatively rapid graded-endocytosis described in several preparations in the perforated patch configuration was actually modified in its properties in the whole cell configuration, thus appearing to wash-out by acquiring the high threshold and all-or-nothing features that are characteristic for the excess retrieval. For this reason, we characterized endocytosis in the perforated patch configuration. We found that two distinct forms of endocytosis coexist in this configuration, one of them displaying all the features of excess retrieval and the other one, termed “compensatory retrieval” (Engisch and Nowycky, 1998), occurring consistently only in perforated patch configuration and rapidly disappearing in whole cell configuration. Thus, the hypothesis of one form changing in characteristic into the other is unlikely to hold. We provide arguments why compensatory retrieval should be considered the mechanism responsible for membrane recycling during physiological secretory activity.
Materials and Methods
Chromaffin cells of the bovine adrenal medulla were used in this study. The cells were isolated from dissociated adrenal glands of adult cattle and kept in primary culture for 1–4 d. Cells were voltage clamped in either the whole cell (Hamill et al., 1981) or the perforated patch (Horn and Marty, 1988; Gillis et al., 1991; Rae et al., 1991) configuration of the patch clamp technique. Cells were stimulated by electrical depolarization, causing Ca2+ influx, fusion of secretory granules, and subsequent catecholamine exocytosis. Exocytosis was measured by monitoring changes in cell capacitance (Neher and Marty, 1982), a measure of granule–plasma membrane fusion, or by the direct electrochemical detection of secreted catecholamine (Wightmann et al., 1991; Chow et al., 1992). Endocytosis was measured as a postdepolarization decrease in cell capacitance. Such decreases act as an estimation of membrane retrieval since continued exocytosis is not expected to significantly influence the capacitance record after depolarization on the time scale relevant to this study (Chow et al., 1992; Smith and Betz, 1996).
Chromaffin Cell Culture
Adult bovine chromaffin cells were prepared by digestion in collagenase type I (0.5 mg/ml; Worthington Biochemical Corp., Freehold, NJ) and cultured for 1–4 d. Further details are described in Zhou and Neher (1993). After enrichment on a Percoll gradient, cells were plated in DMEM, 1× GMS-X (GIBCO BRL, Gaithersburg, MD), penicillin, and streptomycin at a density of approximately 4.4 × 103 mm−2. Cells were incubated at 37°C and 10% CO2.
Solutions
During recording, cells were constantly perfused at a rate of approximately 1 ml/min in a Ringer solution of the following composition (mM): 150 NaCl, 10 Hepes-H, 10 glucose, 10 CaCl2, 2.8 KCl, and 2 MgCl2. The osmolarity was adjusted to 310 mOsm with mannitol, and pH was adjusted to 7.2 with NaOH. The standard perforated patch solution was of the following composition (mM): 135 CsGlutamate, 10 Hepes-H, 9.5 NaCl, 0.5 TEA-Cl, and also included 0.5 mg/ml amphotericin B. The pH of the perforated patch solution was adjusted to 7.2 with CsOH; osmolarity was typically 300 mOsm. All chemicals were obtained from Sigma Chemical Co. (St. Louis, MO) with the exceptions of CsOH (Aldrich Chemical Co., Milwaukee, WI) and amphotericin B (Calbiochem-Novabiochem, La Jolla, CA), or as otherwise noted. An amphotericin B stock was prepared every week at 50 mg/ml in DMSO and stored at 4°C, protected from light. Fresh perforated patch pipette solution was prepared every day by addition of 10 μl stock amphotericin B to 1 ml CsGlutamate internal solution. The combined solution was sonicated thoroughly, protected from light and kept on ice. Pipettes were tip-dipped in amphotericin-free solution for 2–10 s and back-filled with freshly mixed amphotericin-containing solution. The liquid junction potential between the extracellular Ringer and the perforated patch solution was measured to be 13.7 mV, and all potentials are adjusted accordingly.
The intracellular solution for whole cell configuration was of the following composition (mM); 145 CsGlutamate, 10 Hepes-H, 8 NaCl, 2 MgATP, 1 MgCl2, 0.35 Na2GTP, 0.3 EGTA-H. The pH of the intracellular solution was adjusted to 7.2 with CsOH; osmolarity was typically 300 mOsm. Na2GTP was obtained from Boehringer Mannheim (Mannheim, Germany).
Electrophysiological Measurements
Carbon fiber electrodes for amperometric catecholamine detection were prepared as described by Schulte and Chow (1996). Catecholamine detection was performed as described by Chow and colleagues (1992). Briefly, an 800-mV potential was applied to the carbon fiber tip, and the carbon fiber was placed as close as possible to the cell, without exerting any mechanical force on the cell. The 800-mV potential on the fiber tip falls with a Debye constant of ∼0.9 nm and therefore does not exert any significant electrical influence on the cell (see Chow and von Rüden, 1995). As catecholamines are released from the secretory granule, they diffuse to the charged carbon fiber. The potential encountered causes oxidation of the catecholamine and the release of two electrons, which are recorded as a current through the carbon fiber. An EPC-7 patch clamp amplifier (HEKA Elektronik, Lambrecht, Germany) was used for the current detection. The signal was filtered at 1,000 Hz and stored on a digital tape recorder. The signal was later resampled onto an Apple Macintosh Quadra 800 computer (Cupertino, CA) using the “continuous” acquisition mode of the PULSE program (HEKA Elektronik).
Further electrophysiological measurements were carried out using an EPC-9 amplifier and PULSE software running on an Apple Macintosh. Pipettes of ∼2-MΩ resistance were pulled from borosilicate glass capillary tube, partially coated with a silicone compound (G.E. Silicones, Bergen Op Zoom, The Netherlands) and lightly fire polished. After seal formation and perforation, the cell capacitance was estimated by the “C-slow” compensation algorithm of the EPC-9 amplifier. Cell membrane capacitance (C m) changes were estimated by the Lindau-Neher technique (for review see Gillis, 1995) implemented through the “Sine + DC” feature of the Pulse lock-in module. A 700-Hz, 30-mV peak amplitude sine wave was applied to a holding potential of −83 mV, and the reversal potential of the lock-in module was set to 0 mV. The Sine + DC technique uses the DC current value in addition to the real and imaginary admittance to calculate the three elements of the cell's equivalent circuit (pipette series resistance, cell membrane resistance, and cell membrane capacitance). See Gillis (1995) for a discussion of the Lindau Neher technique for the calculation of C m in perforated patch configuration.
The capacitance data presented in all figures were recorded using the Xchart plug-in module of the pulse software. The Xchart module sampled all experimental parameters at ∼18 Hz. Cell capacitance, pipette series conductance and membrane conductance were measured at higher time resolution (700 Hz) immediately before and immediately after cell depolarization. DC current and amperometric current were sampled at 8.6 kHz immediately before, during, and after depolarizations. Capacitance increases due to depolarizations were determined from the high time resolution PULSE data as the difference between average cell capacitance measured in a 50-ms window 50 ms after the depolarization minus prestimulus call capacitance, also measured as a mean over a 50-ms window. The first 50 ms of the postdepolarization capacitance was neglected to avoid nonsecretory capacitance transients that have been observed in chromaffin cells because of rapid changes in membrane potential (Horrigan and Bookman 1994; Chow et al., 1996). The magnitude of the depolarization-induced capacitance increase is sometimes obscured by the rapid excess retrieval in the lower time resolution data presented in the figures. Decreases in C m, presumably caused by endocytosis, were calculated from the high time resolution capacitance as the difference between the postdepolarization cell capacitance minus the minimum capacitance within the time window until the subsequent stimulus.
Evoked Ca2+ currents were measured under conditions in which the majority of the potassium currents were blocked by intracellular Cs+. Tetrodotoxin was not used to block sodium conductances in these experiments because it has been shown to prolong nonsecretory capacitance transients in rat chromaffin cells (Horrigan and Bookman, 1994). Instead, the first 10 ms of evoked inward current was neglected in estimations of Ca2+ influx. Membrane currents were baseline subtracted and integrated to estimate the Ca2+ influx in terms of pC (see Figs. 1 and 2, the region within the dotted box in B). Experiments were carried out at 20–25°C.
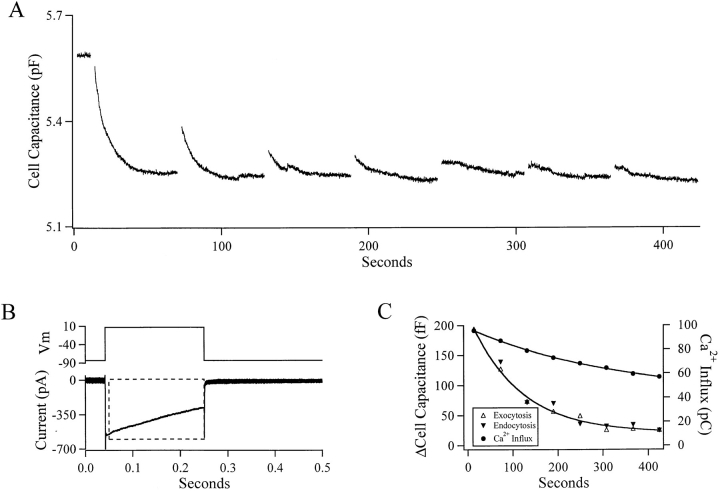
Figure 2.
Endocytotic activity is preserved in the perforated patch clamp configuration. A protocol like that applied to the cell in Fig. 1 was applied to a cell under the perforated patch configuration. The cell was voltage clamped at −83 mV and depolarized to +7 mV every 60 s. Cell capacitance was measured and is plotted against time in A. The evoked Ca2+ current from the first depolarization is presented in B and is qualitatively equivalent to that in Fig. 1 B. The endocytotic activity persisted much longer than in the cell in Fig. 1. Also, the decay of exocytosis and endocytosis proceed in an identical manner in both rate and magnitude (C). The first endocytotic response was not included in C for reasons that will be developed further.
Results
Diminished Endocytotic Activity in the Whole Cell Configuration
When a cell was stimulated repeatedly by 200-ms depolarizing pulses in the whole cell configuration, rapid endocytosis completely disappeared or washed out from the cell within 3 or 4 min (Fig. 1). Exocytotic activity also decreased during this period but stabilized at some intermediate level. Exocytotic activity decayed faster than Ca2+ influx. Ca2+ influx was quantified as the integrated membrane current elicited during a depolarization, with the first 10 ms of the evoked current blocked out in order not to contaminate the integral by the voltage-gated Na+ currents. The membrane current trace elicited from the first depolarization in Fig. 1 A is shown in B, with the dashed line outlining the window of current integration. 92 pC of Ca2+ entered during the first depolarization. Endocytotic activity was lost from the cell, totally disappearing by the fourth minute of recording, while exocytotic activity persisted, albeit in a depleted capacity. The first endocytosis event was the only one that retrieved more membrane than was added during the immediately preceding exocytotic episode. The capacitance changes from the same cell were measured and plotted (Fig. 1 C). Exocytosis would have to depend on Ca2+ influx raised to a power of 7.8 for the decay in Ca2+ influx to totally explain the decay in exocytotic activity. The actual power relationship of exocytosis to Ca2+ influx has been measured in chromaffin cells anywhere between 1.8 (Engisch and Nowycky, 1996) and 4 (Heinemann et al., 1994), falling as the submembrane Ca2+ increases in concentration (Chow et al., 1994). The very high Ca2+ power relationship predicted in Fig. 1 C indicates that some other factors in addition to Ca2+ may cause the decay. Possibilities include the loss of a cytoplasmic molecule or incomplete refilling of the releasable vesicle pool (complete refilling of releasable pool requires ∼60 s at normal cytosolic Ca2+ levels; Heinemann et al., 1993; von Rüden and Neher, 1993). Endocytotic activity disappeared totally in this experiment, indicating that it did not decrease as a sole consequence of the decay in exocytotic activity (Fig. 1 C) but that there are additional factors involved.
Compensatory Endocytosis in Perforated Patch Recordings
A pulse protocol similar to that represented in Fig. 1 A was applied to cells under voltage clamp in the perforated patch configuration. Measured cell capacitance and Ca2+ influx from one such cell are represented in Fig. 2. In the perforated patch configuration, endocytotic activity was seen to persist throughout the recordings. The absolute exocytotic and endocytotic activity in Fig. 2 A decreased at an identical rate (Fig. 2 C), while in other cells (see Fig. 3), both exo- and endocytosis persisted almost unattenuated during the recording. The differences in endocytotic behavior between whole cell and perforated patch recordings were not due to a change in the evoked Ca2+ current. The current elicited during the first depolarization of this cell is shown in Fig. 2 B and is qualitatively the same as the elicited current shown in Fig. 1 B. The Ca2+-exocytosis power relationship required to fit the decay in exocytotic activity to the decay in Ca2+ influx in Fig. 2 C is 3.1, a reasonable number. The value of the fitted Ca2+ power relationship indicates that the exocytotic activity probably decayed at least partly, and perhaps totally, because of a decrease in Ca2+ influx. An additional cause for the decay in exocytosis activity may have been an incomplete refilling of the releasable vesicle pool. Endocytotic activity probably decreased in a manner dependent on diminished exocytotic activity.
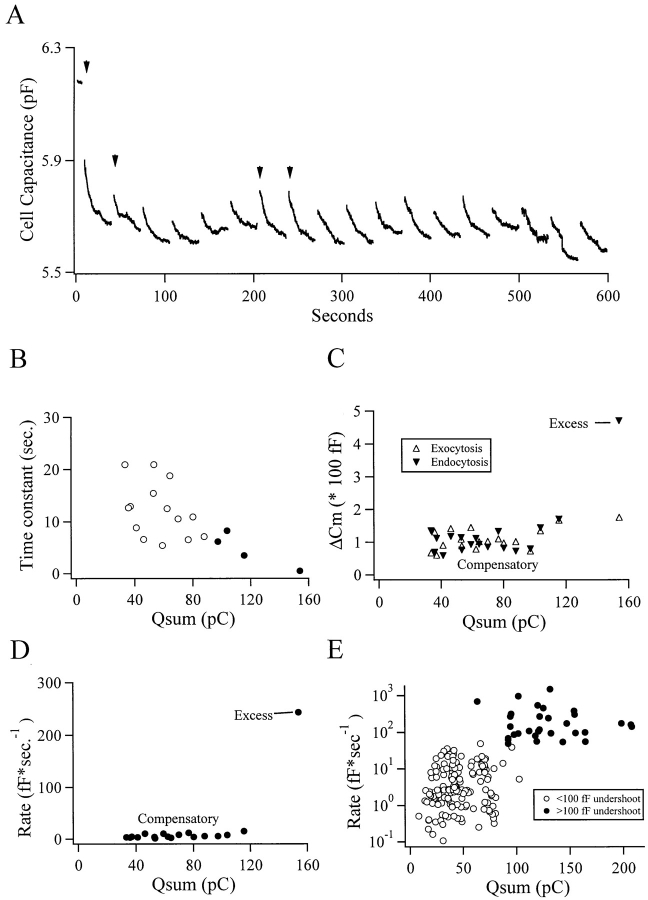
Figure 3.
Two kinetic forms of endocytosis are present in chromaffin cells. A chromaffin cell voltage clamped at −83 mV in the perforated patch configuration was depolarized to +7 mV every 34 s for durations of 50, 100, and 500 ms (arrows indicate 500-ms stimuli). The cell capacitance is plotted against time in A. The approximate time constants of capacitance decay were estimated by fitting the interpulse periods to a single exponential. The decay time constants are plotted against the integrated Ca2+ influx (Q sum, a quantification of stimulus strength) in B. Filled symbols correspond to the 500-ms depolarizations in A. The amount of membrane added through exocytosis and then retrieved through endocytosis for each pulse is plotted against Ca2+ influx in C. The initial rate of membrane retrieval after each depolarization was estimated and plotted against Ca2+ influx (D). Data like those in D were pooled for 40 cells and are plotted in E. The symbol type was determined by whether the cells exhibited excess endocytosis with a limiting value of 100 fF.
Sometimes a small, slowly decaying inward current (<10 pA, decay time constant of 1–2 s; see also Engisch and Nowycky, 1996) was observed after the depolarization. This current was determined not to have affected the accuracy of the capacitance calculations for two reasons. First, it was too small to have any substantial effect on the cell capacitance; by converting the conductance to the same units as the capacitance (G m/[2πf sine], where f sine is the frequency of the sine wave voltage command and G m is the membrane conductance), the conductance deflection was negligible with respect to the depolarization-evoked change in cell capacitance (data not shown). Second, when cells were stimulated heavily in rapid succession, leading to depletion of the secretory response, the slowly decaying conductance could be observed with no corresponding change in capacitance (data not shown).
Occasionally, step-wise decreases in cell capacitance were observed (such as following the second to last stimulus in Fig. 3 A). These steps may indicate the fission of larger endosomal bodies. Such step-like decreases in cell capacitance after exocytosis were originally reported in chromaffin cells (Neher and Marty, 1982), in pancreatic β-cells (Ämmälä et al., 1993), as well as in pituitary melanotrophs (Thomas et al., 1994). The capacitance steps may represent the equivalent of the cavicapture endocytotic vacuoles observed by Baker and Knight (1981) in chromaffin cells. 24 such steps were observed in the course of this study, occurring at an average 481 s after the beginning of an experiment, or on average after the 15th stimulus. The average size of the steps was 33 fF, corresponding to a single sphere ∼1 μm in diameter or else to the mass endocytosis of a larger number of small vesicles.
Intense Stimulation Triggers Excess Endocytosis
To study the modulatory effects of stimulation intensity on the rate and magnitude of endocytosis, we varied the depolarization duration in individual cells. Cell capacitance from such an experiment is shown in Fig. 3 A. Fig. 3 B, which represents data from the same cell as A, shows the relationship of the approximate time constant of cell capacitance decay after depolarizations as a function of stimulus strength. Stimulus strength was defined by the integrated Ca2+ influx during depolarization. While variable at any given stimulation intensity, increased stimulation strength tended to decrease the approximate decay time constant of the cell capacitance. Stimulus-evoked endocytotic responses from the same cell as in Fig. 3 B were further quantified. Fig. 3 C relates the magnitude of evoked exo- and endocytosis plotted against stimulus intensity for the same single cell. For all but one event, a roughly equivalent amount of membrane to that added through exocytosis was retrieved through endocytosis; these are the compensatory endocytotic events. In the exception, after the first depolarization in Fig. 3 A, causing the largest Ca2+ influx (154 pC), there was far more membrane endocytosed than had been added through exocytosis. The capacitance undershoot represents “excess” endocytosis.
To assign a velocity to the endocytotic events, the initial rates for all the endocytotic events were roughly approximated as the magnitude of retrieved membrane divided by the decay time constant of the monoexponential fits. These initial rates are plotted against the corresponding stimulus intensity in Fig. 3 D. Again, for the compensatory endocytotic events, the initial rate of membrane retrieval was similar (mean = 6.9 fF/s), although it became faster with increasing stimulus intensity. For one endocytotic event, corresponding to the excess endocytosis identified in Fig. 3 C, the initial rate of endocytosis was far greater (242 fF/s). Data from 40 cells were pooled, and the initial rates of endocytosis were plotted against the stimulus intensity (Fig. 3 E). Two kinetic forms of endocytosis emerged based on the initial rate of membrane retrieval: the majority of events retrieved membrane at an average of 6.41 ± 0.65 fF/s (mean ± SEM), while a few events retrieved membrane at a far more rapid rate of 248.91 ± 55.71 fF/s. In Fig. 3 E, the endocytotic events were assigned symbols according to whether or not they retrieved membrane in excess of at least 100 fF to the amount added through the immediately preceding exocytosis. The endocytosis events with slower initial rates correlated to the compensatory events, while the faster endocytosis correlated to the excess endocytotic events. Excess retrieval events were triggered only by stimuli that led to large Ca2+ influxes (132.6 ± 42.4 pC, mean ± SD). Therefore, excess endocytosis occurred in response to at least 90 pC Ca2+ entry and proceeded at a rate of at least 50 fF/s. For comparison, the Ca2+ influx due to a single action potential is ∼1 pC.
The intense stimulation required to trigger excess retrieval led us to wonder what approximate cytosolic Ca2+ concentration would result from 90 pC Ca2+ influx. The following equation represents free average internal Ca2+ shortly after a stimulus as a function of resting Ca2+, influx of free Ca2+, and subsequent buffering by the endogenous Ca2+ buffer:
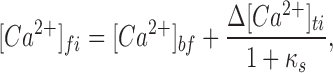 |
1 |
where [Ca2+]fi is the final free internal Ca2+ concentration and [Ca2+]bf is the internal basal total free Ca2+ concentration, here assumed to be 150 nM. Δ[Ca2+]ti represents the concentration change of bound and free intracellular Ca2+ due to evoked Ca2+ influx, and κs represents the binding capacity of the endogenous Ca2+ buffer. κs is further defined:
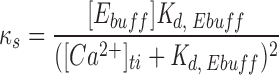 |
2 |
Here, [Ebuff] is the concentration of the endogenous Ca2+ buffer, and K d,Ebuff is the dissociation constant of the endogenous buffer for Ca2+. Since K d,Ebuff has been measured to be ∼100 μM (Xu et al., 1997), and since we expect [Ca2+]fi << K d,Ebuff (see Chow et al., 1994 for an approximation of [Ca2+] at the release site), the process represented by Eq. 2 becomes linear. Zhou and Neher (1993) measured κs to be ∼50 in chromaffin cells in the perforated patch configuration under conditions similar to those in this study. We can estimate the value Δ[Ca2+]ti simply from the integrated Ca2+ influx and resting cell capacitance, assuming that ∼70% of the cell volume is accessible as cytoplasm (Zhou and Neher, 1993). With these assumptions, Eq. 1 takes the reduced form
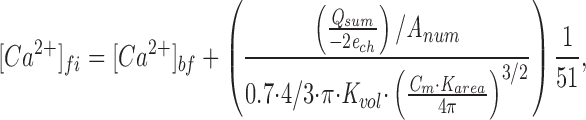 |
3 |
where e ch is the charge of an electron, C m is the resting capacitance of the cell (in Farads, present to correct for differences in individual cell size), and A num is Avagadro's number. Q sum is the integrated Ca2+ influx in Coulombs. The constant K area has the value 100 and converts cell capacitance (Farads) into surface area (square meters). Another constant (K vol = 1,000) converts cell volumes from cubic meters to liters. From Eq. 3, the average Ca2+ influx required to trigger excess retrieval predicts a lower cytoplasmic free [Ca2+] limit of ∼10 μM, a concentration ∼10 times higher than the threshold concentration for exocytosis. It should be pointed out that Eq. 3 gives [Ca2+]fi after diffusional equilibration throughout the cytoplasm. Local [Ca2+] at the membrane may be much higher for periods of several hundreds of milliseconds after depolarization.
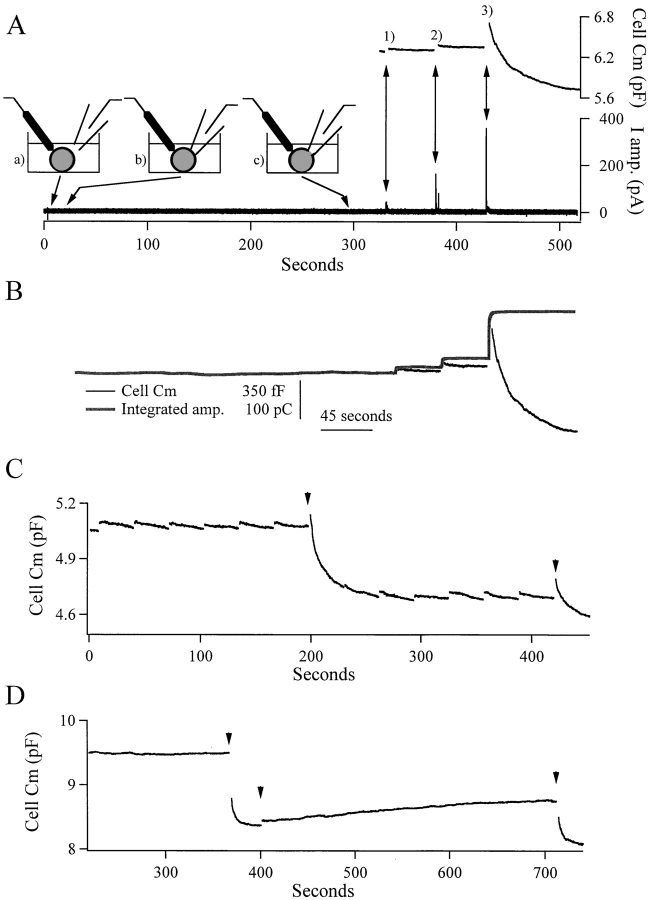
Thomas and colleagues (1994) hypothesized that the excess retrieval events measured in pituitary melanotrophs were caused by the retrieval of excess membrane stranded in the plasma membrane from exocytotic activity before the given stimulus. One possible source for this membrane would be the result of what effectively could be considered a patch clamp artifact, that is exocytosis caused by Cs+ leaking from the pipette tip during approach to the cell, thereby depolarizing the cell. Another possible patch artifact that could lead to accumulation of excess membrane would be through exocytosis triggered by mechanical perturbation and slight Ca2+ influx during seal formation. The excess membrane would reside in the plasma membrane, undetected, detained at some late step in the endocytotic process awaiting the proper Ca2+ signal. We used the amperometric catecholamine detection technique to monitor cell secretory activity during pipette approach, seal formation, patch perforation, and depolarization. One such recording is shown in Fig. 4 A; the result was reproduced in 11 cells. No catecholamine release was detected during seal formation or perforation. Catecholamine secretion from this cell and amperometric sensitivity were later confirmed through a series of three depolarizations, during which secretion was measured as catecholamine oxidation on the surface of a charged carbon fiber, giving rise to current spikes. In this cell, excess retrieval was delayed until the third depolarization, corresponding to the largest Ca2+ influx elicited (125 pC). The lack of current spikes in the amperometric record before the first depolarization was confirmed by base-line subtraction of the amperometric current trace and integration. The integrated trace was scaled to the capacitance trace at the first depolarization-evoked response. Cell capacitance and integrated amperometric current both are shown in Fig. 4 B. We conclude that the excess retrieval of membrane is not due to undetected exocytosis during the process of patching. Nevertheless, it may represent membrane constitutively “stranded” through spontaneous activity over a longer period of time.
Figure 4.
Excess endocytosis does not represent the retrieval of vesicle membrane added during patching of the cell. (A) Continuous amperometric current recordings from a cell during patch pipette approach (a), seal formation (b), and completed perforation (c), and subsequent cell stimulation (double arrows) shows that no catecholamine was released until the first depolarization. The cell was stimulated by depolarization from −83 to +7 mV for different durations (1, 50 ms; 2, 100 ms; 3, 700 ms). Additionally, cell capacitance recordings show that the same cell was capable of exhibiting excess endocytosis after the third depolarization, which led to a large Ca2+ influx (125 pC). The amperometric data from A were base-line subtracted and integrated. The first step increase in the integrated amperometric record was then scaled to the first capacitance step for presentation. (C) A cell held in the perforated patch clamp configuration was depolarized so that the threshold Ca2+ concentration was reached only rarely. It showed multiple excess retrieval events. All the depolarizations were 100 ms except those marked with an arrow, which were 500 ms. In D, a chromaffin cell was held in the whole cell configuration for 6 min and then depolarized for 650 ms (first arrow). This depolarization resulted in the influx of 194 pC of Ca2+ and was followed by excess retrieval. The cell was then depolarized for 100 ms 30 s later (second arrow), resulting in 75 pC Ca2+ influx, and showed no endocytotic response. After a further 360 s, the cell was depolarized for 650 ms, resulting in the influx of 169 pC of Ca2+, which again resulted in excess endocytotic retrieval.
The excess retrieval events could be delayed well into the course of an experiment, sometimes beyond the eighth depolarization (or presumably more), if the preceding stimuli were subthreshold (Fig. 4 C). The excess retrieval of membrane could be evoked on multiple occasions in single cells if sufficient time was allowed between superthreshold stimuli (Fig. 4 C). If two superthreshold stimuli were applied to a cell in succession, only the first would result in excess endocytosis (see Fig. 3 for an example). We observed that the likelihood of the second superthreshold stimulus resulting in excess endocytosis grew with time, although the second excess retrieval event was always smaller than the first.
Several studies have shown a form of excess endocytosis that is resistant to wash-out in chromaffin cells (Neher and Zucker, 1993; Heinemann et al., 1994; Artalejo et al., 1995), in pituitary melanotrophs (Thomas et al., 1994), in pancreatic β-cells (Eliasson et al., 1996), and in nonexcitable fibroblasts (Coorssen et al., 1996; Ninomiya et al., 1996). We examined excess retrieval as described here for its resistance to wash-out and found it too persisted in the whole cell configuration at times when compensatory endocytosis had washed-out (Fig. 4 D).
Differences between Excess and Compensatory Retrieval
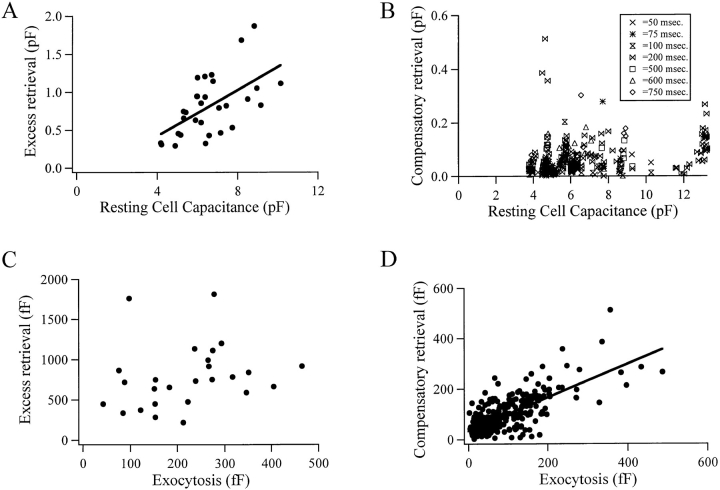
To better understand the differences and similarities between excess and compensatory endocytotic membrane retrieval, we studied the relationship between the two different forms of endocytosis and other characteristics, such as cell size and dependency on stimulus intensity. We found that excess retrieval and compensatory retrieval of membrane differ in their relationship to several parameters. The magnitude of membrane retrieved through excess retrieval events was analyzed for its relationship to the resting cell capacitance. Surprisingly, a dependency was found. The capacitance of membrane endocytosed through excess retrieval was approximately equal to 17% of the cell surface area (Fig. 5 A; only the first excess retrieval event from a given cell was analyzed to avoid underestimation due to smaller subsequent events). The same analysis applied to compensatory endocytotic events did not show a clear relationship to resting cell capacitance (Fig. 5 B).
Figure 5.
Excess and compensatory endocytosis can be further separated. (A) The magnitude of membrane recovered through excess endocytosis shows a linear relationship to the resting capacitance of the cell (slope = 0.17). (B) The same analysis as that represented in A, except performed for compensatory endocytosis. The membrane capacitance retrieved through compensatory endocytosis does not show the proportionality to the resting cell capacitance seen for excess retrieval events. The events in B were collected in response to a range of depolarization durations. The duration of each event is represented by the symbol. (C) The amount of membrane retrieved through excess retrieval is not related to the amount of membrane added through the immediately preceding exocytosis, while there is a relationship between the amount of membrane recovered through compensatory endocytosis and the amount of membrane added through the immediately preceding exocytosis (D, slope = 0.66).
The amount of membrane internalized through excess endocytosis was independent of the amount of membrane added immediately before (Fig. 5 C). When the same relationship was examined for compensatory endocytotic events, the capacitance of the endocytotic membrane was correlated to the capacitance added through the immediately preceding exocytotic event (Fig. 5 D), although on average the amount of membrane endocytosed was slightly less than that exocytosed. The slight under-retrieval of membrane described in Fig. 5 D may be due to restimulation before completion of the compensatory endocytosis in some cells.
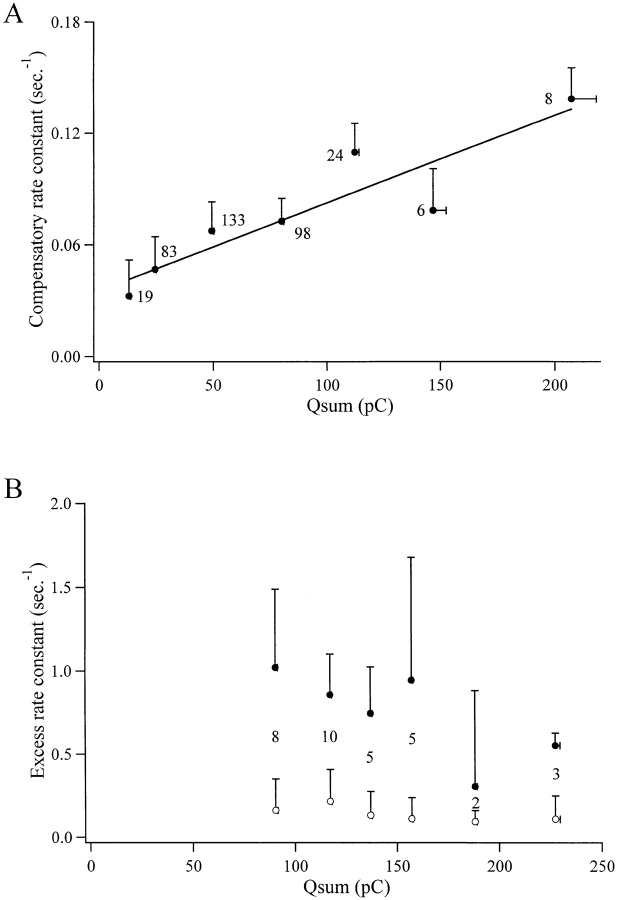
Data from single cells indicated that the rate constant of membrane retrieval may depend on stimulus intensity (see Fig. 3 B), becoming faster with more intense stimulation. To determine if this was a general phenomenon, 371 compensatory endocytotic events from 42 different cells and 33 excess endocytotic events from separate cells were pooled and analyzed. Under closer examination it was found that, like in the pituitary melanotrophs (Thomas et al., 1994) and calf chromaffin cells (Artalejo, 1995), the excess retrieval described here consisted of two kinetic components. The data were binned according to stimulus intensity, here represented by integrated Ca2+ influx, and the rate constants of endocytosis were plotted as a function of integrated Ca2+ influx (Fig. 6, A and B). The rate constant of membrane retrieval through compensatory endocytosis showed a linear relationship to the magnitude of total Ca2+ influx, with endocytosis becoming faster at more intense stimulus levels. Although exhibiting variable rates, neither the fast nor the slow components of excess endocytosis were related to the stimulus intensity. It should be pointed out that at Ca2+ concentrations in the range 70 μM to 1 mM, the time constant of excess endocytosis decreased (Heinemann et al., 1994); such high Ca2+ concentrations were probably not reached in our experiments. Excess retrieval followed an all-or-nothing dependency on Ca2+; that is, once the excess retrieval was triggered through a large Ca2+ influx, the rate constant of the retrieval was independent of Ca2+ concentration.
Figure 6.
The rate constant of excess retrieval and compensatory retrieval show different dependencies on stimulus intensity. The pooled data from 371 compensatory endocytotic events were binned according to Ca2+ influx. These data are plotted in A. Numbers next to the data points represent the number of responses averaged for the point. The rate constant for capacitance decay follows as linear dependency (solid line, ordinate intercept = 0.03, slope = 5.9 × 10−4) on stimulus intensity, becoming larger with higher Ca2+ influx. Error bars represent the standard error of the mean. The same analysis was performed on 33 excess retrieval events for comparison and is plotted in B. Filled symbols represent the fast component, and empty symbols represent the slow component of the excess retrieval. The numbers between the symbol pairs (filled and empty) represent the number of events averaged for each point. Neither the fast nor the slow components of excess retrieval showed a significant dependency on stimulus strength. Only responses up to and including the first excess retrieval event from a cell were analyzed to avoid underestimation resulting from exhaustion of the excess endocytosis mechanism in later, smaller events.
Discussion
Evidence for several exocytosis-coupled forms of endocytosis, from divergent cell types, exists. In cultured hippocampal neurons (Ryan et al., 1996), stimulus intensity was found to have little or no effect on the rate of endocytosis. In other cell types, such as the giant terminals of the goldfish retinal bipolar cell (von Gersdorff and Matthews, 1994) and terminals of the rat posterior pituitary (Hsu and Jackson, 1996), increased stimulus intensity seemed to slow exocytosis-coupled membrane retrieval. At the neuromuscular junction of the frog and the fly it has been suggested that endocytosis on the average slows, and possibly changes entirely in mechanism, in response to higher levels of nerve stimulation (Heuser and Reese, 1973; Ceccarelli and Hurlbut, 1980; Koenig and Ikeda, 1996; Wu and Betz, 1996), indicating parallel endocytotic pathways in the same cell.
We show here that two relatively rapid forms of endocytosis coexist in intact chromaffin cells studied with the perforated patch clamp technique. One form, termed compensatory retrieval, sequesters within several seconds after a stimulus an amount of membrane roughly equivalent to that added during the prior stimulus. Its time constant is in the range of 20 s for very weak stimuli and decreases to about 5 s for strong stimuli. This process is also observed in whole cell recordings in the very early phase of an experiment, but rapidly disappears. To the contrary, the other, faster form of endocytosis, termed excess retrieval, persists in prolonged whole cell recordings. It requires very strong stimuli and can be elicited under quite similar circumstances in both perforated patch and whole cell recordings. This form has been studied in detail by Artalejo and coworkers (1995, 1996) and has been shown not to be clathrin mediated and to depend on Ca2+ and calmodulin. It has been suggested (Artalejo et al., 1995; Henkel and Almers, 1996) that this form of endocytosis is responsible for membrane recycling during electrical activity of neuroendocrine cells because it is the fastest process of endocytosis known to occur after secretion.
We originally asked whether compensatory endocytosis changed in character during whole cell recordings to acquire the properties of excess retrieval. Two observations speak against this proposal. First, compensatory retrieval and excess retrieval coexist in perforated patch. Secondly, excess retrieval commonly and most prominently is observed in whole cell recordings during the first stimulus (if it is strong enough) before compensatory retrieval washes out (see Fig. 1 for an example).
However, separation of the two forms of endocytosis in perforated patch experiments is not trivial. One might argue that the kinetic distinction is not so clear, because the rate constant of compensatory retrieval increases with stimulus strength, such that at the high stimulus strengths required for what we call excess retrieval, the process may be fast enough to qualify for such. In fact, the plot of rate versus stimulation strength of Fig. 3 E gives no compelling distinction when rate constant is considered as the only criterion. However, if the data points are subdivided into two groups, one with >100 fF undershoot in amplitude and the other with 0–100 fF, it is clear that the former all have rates larger than 50 fF/s and require stimuli >90 pC. In contrast, the latter have rates <50 fF/s and extend down to very small stimuli. Furthermore, we identified four criteria in which the two forms (as defined by the undershoot criteria) are distinct. Only excess retrieval, not compensatory retrieval, shows a clear dependency of its amplitude on cell size. Only compensatory retrieval, not excess retrieval, shows a clear dependency of its amplitude on the amplitude of the immediately preceding exocytosis and only compensatory retrieval shows a dependence of its rate constant on stimulus strength within the accessible range. Furthermore, the question has to be asked why compensatory retrieval, but not excess retrieval should wash-out, if the distinction were artificial and the two phenomena represented the same mechanism under different stimulus strengths.
Thus, we conclude that the two mechanisms are distinct and that in addition to the well-known and well-characterized process of excess retrieval, there is a robust, relatively rapid mechanism of endocytosis in intact cells that is capable of retrieving the majority of membrane within seconds, no matter whether the stimulus is large or as small as a single action potential. According to our analysis, this mechanism recycles at least 60% of exocytosed membrane (possibly more, since our data in Fig. 5 D includes experiments where compensatory endocytosis has not come to an end). It may well be that a small fraction of exocytosed membrane (and maybe more so in whole cell recordings) gets stranded and accumulates, as suggested by Thomas and colleagues (1994). Once a sufficient amount has accumulated, a strong stimulus may trigger an avalanche-like event, which retrieves stranded membrane. Further experiments will have to clarify how often or how prominently this occurs during physiological activity of the cell. From our present data, however, it can be concluded that this should be a relatively rare event if stimuli >90 pC (corresponding to ∼100 action potentials) are avoided. Compensatory retrieval seems to be responsible for most of membrane recycling, but in contrast to excess retrieval, it has not been characterized in molecular terms yet.
Acknowledgments
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (SFB 406).
Footnotes
We would like to gratefully thank Dr. T. Xu for help in the Ca2+ estimation protocol. We would also like to thank Drs. H. von Gersdorff and C. Mathes for helpful discussions of this manuscript. F. Friedlein and M. Pilot furnished invaluable technical assistance. (All acknowledged are affiliated with the Max-Planck Institute for Biophysical Chemistry, Göttingen, Germany.)
Address all correspondence to Corey Smith, Department of Membrane Biophysics, Max-Planck-Institute for Biophysical Chemistry, Am Fassberg 11, D-37077 Göttingen, Germany. Tel.: +49-551-201-1629. Fax: +49-551-201-1688.
References
- Ämmälä C, Eliasson L, Bokvist K, Larsson O, Ashcroft F M, Rorsman P. Exocytosis elicited by action potentials and voltage-clamp calcium currents in individual mouse pancreatic B-cells. J Physiol. 1993;472:665–688. doi: 10.1113/jphysiol.1993.sp019966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artalejo CR, Elhamdani A, Palfrey HC. Calmodulin is the divalent cation receptor for rapid endocytosis, but not exocytosis, in adrenal chromaffin cells. Neuron. 1996;16:195–205. doi: 10.1016/s0896-6273(00)80036-7. [DOI] [PubMed] [Google Scholar]
- Artalejo CR, Henley JR, McNiven M A, Palfrey HC. Rapid endocytosis coupled to exocytosis in adrenal chromaffin cells involves Ca2+, GTP, and dynamin but not clathrin. Proc Natl Acad Sci USA. 1995;92:8328–8332. doi: 10.1073/pnas.92.18.8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker PF, Knight DE. Calcium control of exocytosis and endocytosis in bovine adrenal medullary cells. Philos Trans R Soc Lond Ser B Biol Sci. 1981;296:83–103. doi: 10.1098/rstb.1981.0174. [DOI] [PubMed] [Google Scholar]
- Benchimol S, Cantin M. Ultrastructural radioautography of synthesis and migration of proteins and catecholamines in the rat adrenal medulla. Cell Tissue Res. 1982;225:293–314. doi: 10.1007/BF00214683. [DOI] [PubMed] [Google Scholar]
- Burgoyne RD. Fast exocytosis and endocytosis triggered by depolarization in single adrenal chromaffin cells before rapid Ca2+current run-down. Pflüg Arch. 1995;430:213–219. doi: 10.1007/BF00374652. [DOI] [PubMed] [Google Scholar]
- Ceccarelli B, Hurlbut WP. Ca2+-dependent recycling of synaptic vesicles at the frog neuromuscular junction. J Cell Biol. 1980;87:297–303. doi: 10.1083/jcb.87.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow R, Klingauf J, Neher E. Time course of Ca2+concentration triggering exocytosis in neuroendocrine cells. Proc Natl Acad Sci USA. 1994;91:12765–12769. doi: 10.1073/pnas.91.26.12765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow, R.H., and L. von Rüden. 1995. Chapter 11. Electrochemical detection of secretion from single cells. In Single-Channel Recording, Second Edition. B. Sakmann and E. Neher, editors. Plenum Press, New York. 245–275.
- Chow RH, Klingauf J, Heinemann C, Zucker RS, Neher E. Mechanisms determining the time course of secretion in neuroendocrine cells. Neuron. 1996;16:369–736. doi: 10.1016/s0896-6273(00)80054-9. [DOI] [PubMed] [Google Scholar]
- Chow RH, von Rüden L, Neher E. Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature. 1992;356:60–63. doi: 10.1038/356060a0. [DOI] [PubMed] [Google Scholar]
- Coorssen JR, Schmitt H, Almers W. Ca2+-triggers massive exocytosis in Chinese hamster ovary cells. EMBO (Eur Mol Biol Organ) J. 1996;15:3787–3791. [PMC free article] [PubMed] [Google Scholar]
- Eliasson L, Proks P, Ämmälä C, Ashcroft FM, Bokvist K, Renström E, Rorsman P, Smith PA. Endocytosis of secretory granules in mouse pancreatic β-cells evoked by transient elevation of cytosolic calcium. J Physiol. 1996;493:755–767. doi: 10.1113/jphysiol.1996.sp021420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engisch KL, Nowycky MC. Calcium dependence of large dense-cored vesicle exocytosis evoked by calcium influx in bovine adrenal chromaffin cells. J Neurosci. 1996;16:1359–1369. doi: 10.1523/JNEUROSCI.16-04-01359.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engisch, K., and M. Nowycky. 1998. Compensatory and excess retrieval: two types of endocytosis following single step depolarizations in bovine adrenal chromaffin cells. J. Physiol. In press. [DOI] [PMC free article] [PubMed]
- Gillis K, Pun R, Misler S. Single cell assay of exocytosis from adrenal chromaffin cells using “perforated patch recording.” . Pflüg Arch. 1991;418:611–613. doi: 10.1007/BF00370579. [DOI] [PubMed] [Google Scholar]
- Gillis, K.D. 1995. Chapter 7. Techniques for membrane capacitance measurements. In Single-Channel Recording, Second Edition. B. Sakmann and E. Neher, editors. Plenum Press, New York. 155–198.
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflüg Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Heinemann C, von Rüden L, Chow RH, Neher E. A two-step model of secretion control in neuroendocrine cells. Pflüg Arch. 1993;424:105–112. doi: 10.1007/BF00374600. [DOI] [PubMed] [Google Scholar]
- Heinemann C, Chow R, Neher E, Zucker R. Kinetics of the secretory response in bovine chromaffin cells following flash photolysis of caged Ca++ . Biophys J. 1994;67:2546–2557. doi: 10.1016/S0006-3495(94)80744-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkel AW, Almers W. Fast steps in exocytosis and endocytosis studied by capacitance measurements in endocrine cells. Curr Opin Neurobiol. 1996;6:350–357. doi: 10.1016/s0959-4388(96)80119-x. [DOI] [PubMed] [Google Scholar]
- Heuser JE, Reese TS. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol. 1973;57:315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R, Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988;92:145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrigan FT, Bookman RJ. Releasable pools and the kinetics of exocytosis in adrenal chromaffin cells. Neuron. 1994;13:1119–1129. doi: 10.1016/0896-6273(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Kent C, Coupland R. Observations on the localization of labeled amino acid in mouse adrenal chromaffin cells after the injection of L-[4,5-3H] leucine. J Endocrinol. 1978;78:21–29. doi: 10.1677/joe.0.0780021. [DOI] [PubMed] [Google Scholar]
- Koenig JH, Ikeda K. Synaptic vesicles have two distinct recycling pathways. J Cell Biol. 1996;135:797–808. doi: 10.1083/jcb.135.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingg G, Fischer C R, Schmidt W, Winkler H. Exposure of an antigen of chromaffin granules on cell surface during exocytosis. Nature. 1983;301:610–611. doi: 10.1038/301610a0. [DOI] [PubMed] [Google Scholar]
- Neher E, Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci USA. 1982;79:6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E, Zucker R. Multiple calcium-dependent processes related to secretion in bovine chromaffin in cells. Neuron. 1993;10:21–30. doi: 10.1016/0896-6273(93)90238-m. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Kishimoto T, Miyashita Y, Kasai H. Ca2+-dependent exocytotic pathways in Chinese hamster ovary fibroblasts revealed by a caged-Ca2+compound. J Biol Chem. 1996;271:17751–17754. doi: 10.1074/jbc.271.30.17751. [DOI] [PubMed] [Google Scholar]
- Parsons TD, Lenzi D, Almers W, Roberts WM. Calcium-triggered exocytosis and endocytosis in an isolated presynaptic cell: capacitance measurements in saccular hair cells. Neuron. 1994;13:875–883. doi: 10.1016/0896-6273(94)90253-4. [DOI] [PubMed] [Google Scholar]
- Patzak A, Winkler H. Exocytotic exposure and recycling of membrane antigens of chromaffin granules: ultrastructural evaluation after. J Cell Biol. 1986;102:510–515. doi: 10.1083/jcb.102.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JH, Burridge K, Wilson SP, Kirshner N. Visualization of the exocytosis/endocytosis secretory cycle in culture adrenal chromaffin cells. J Cell Biol. 1983;97:1906–1917. doi: 10.1083/jcb.97.6.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae J, Cooper K, Gates P, Watsky M. Low access resistance perforated patch recordings using amphotericin C. J Neurosci Methods. 1991;37:15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- Ryan TA, Smith SJ, Reuter H. The timing of synaptic vesicle endocytosis. Proc Natl Acad Sci USA. 1996;93:5567–5571. doi: 10.1073/pnas.93.11.5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte A, Chow R. A simple method for insulating carbon-fiber microelectrodes using anodic electrophoretic deposition paint. Anal Chem. 1996;68:3054–3058. doi: 10.1021/ac960210n. [DOI] [PubMed] [Google Scholar]
- Seward EP, Nowycky MC. Kinetics of stimulus-coupled secretion in dialyzed bovine chromaffin cells in response to trains of depolarizing pulses. J Neurosci. 1996;16:553–562. doi: 10.1523/JNEUROSCI.16-02-00553.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CB, Betz WJ. Simultaneous independent measurement of endocytosis and exocytosis. Nature. 1996;380:531–534. doi: 10.1038/380531a0. [DOI] [PubMed] [Google Scholar]
- Thomas P, Surprenant A, Almers W. Cytosolic Ca2+, exocytosis, and endocytosis in single melanotrophs of the rat pituitary. Neuron. 1990;5:723–733. doi: 10.1016/0896-6273(90)90226-6. [DOI] [PubMed] [Google Scholar]
- Thomas P, Lee AK, Wong JG, Almers W. A triggered mechanism retrieves membrane in seconds after Ca2+-stimulated exocytosis in single pituitary cells. J Cell Biol. 1994;124:667–675. doi: 10.1083/jcb.124.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse A, Tse FW, Almers W, Hille B. Rhythmic exocytosis stimulated by GnRH-induced calcium oscillations in rat gonadotropes. Science. 1993;260:82–84. doi: 10.1126/science.8385366. [DOI] [PubMed] [Google Scholar]
- von Gersdorff H, Matthews G. Inhibition of endocytosis by elevated internal calcium in a synaptic terminal. Nature (Lond) 1994;370:652–655. doi: 10.1038/370652a0. [DOI] [PubMed] [Google Scholar]
- von Rüden L, Neher E. A Ca-dependent early step in the release of catecholamines from adrenal chromaffin cells. Science. 1993;262:1061–1065. doi: 10.1126/science.8235626. [DOI] [PubMed] [Google Scholar]
- Wightmann RM, Jankowski JA, Kennedy RT, Kawagoe KT, Schroeder TJ, Leszczyszyn DJ, Near JA, Dilberto E JJ, Viveros OH. Temporally resolved catecholamine spikes correspond to single vesicle release from individual chromaffin cells. Proc Natl Acad Sci USA. 1991;88:10754–10758. doi: 10.1073/pnas.88.23.10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L-G, Betz WJ. Nerve activity but not intracellular calcium determines the time course of endocytosis at the frog neuromuscular junction. Neuron. 1996;17:769–779. doi: 10.1016/s0896-6273(00)80208-1. [DOI] [PubMed] [Google Scholar]
- Xu T, Naraghi M, Kang H, Neher E. Kinetic studies of Ca2+ binding and Ca2+clearance in the cytosol of adrenal chromaffin cells. Biophys J. 1997;73:532–545. doi: 10.1016/S0006-3495(97)78091-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Neher E. Mobile and immobile calcium buffers in bovine adrenal chromaffin cells. J Physiol. 1993;469:245–273. doi: 10.1113/jphysiol.1993.sp019813. [DOI] [PMC free article] [PubMed] [Google Scholar]