Abstract
The heregulin receptor tyrosine kinase ErbB-4 is constitutively cleaved, in the presence or absence of ligand, by an exofacial proteolytic activity producing a membrane-anchored cytoplasmic domain fragment of 80 kD. Based on selective sensitivity to inhibitors, the proteolytic activity is identified as that of a metalloprotease. The 80-kD product is tyrosine phosphorylated and retains tyrosine kinase activity. Importantly, the levels of this fragment are controlled by proteasome function. When proteasome activity is inhibited for 6 h, the kinase-active 80-kD ErbB-4 fragment accumulates to a level equivalent to 60% of the initial amount of native ErbB-4 (∼106 receptors per cell). Hence, proteasome activity is essential to prevent the accumulation of a significant level of ligand-independent, active ErbB-4 tyrosine kinase generated by metalloprotease activity. Proteasome activity, however, does not act on the native ErbB-4 receptor before the metalloprotease-mediated cleavage, as no ErbB-4 fragments accumulate when metalloprotease activity is blocked. Although no ubiquitination of the native ErbB-4 is detected, the 80-kD fragment is polyubiquitinated. The data, therefore, describe a unique pathway for the processing of growth factor receptors, which involves the sequential function of an exofacial metalloprotease and the cytoplasmic proteasome.
When growth factor ligands bind to their cognate receptors, tyrosine kinase activity is activated, and results in the initiation of multiple signal transduction pathways. Coincidentally, activated ligand– receptor complexes are subject to less defined processes that alter their activity and cell surface distribution, and/or number. Most all ligand-occupied growth factor receptor tyrosine kinases are rapidly internalized by receptor-mediated endocytosis through clathrin-coated pits (Sorkin and Waters, 1993). Tyrosine-kinase activity, as well as internalization sequences in the receptor carboxyl terminus, are essential for this step in receptor trafficking. Internalized ligand–receptor complexes subsequently are sorted to lysosomes where both receptor and ligand are degraded. This process is thought to represent an attenuation mechanism necessary for the proper biological response, as it produces a dramatic decrease or downregulation in the number of surface receptors. It has been reported that growth factor binding to internalization-defective receptors leads to increased transforming potential, presumably due to persistent signaling at the cell surface (Wells et al., 1990; Masui et al., 1991).
Within the ErbB family of receptor tyrosine kinases (Earp et al., 1995), the activated EGF receptor or ErbB-1 is rapidly and extensively downregulated by this pathway (Carpenter and Cohen, 1976). However, other members of this family, which bind heregulin, are not subject to rapid internalization and downregulation (Baulida et al., 1996; Pinkas-Kramarski et al., 1996). This includes the two receptors, ErbB-3 and ErbB-4, which bind heregulin directly (Plowman et al., 1993; Carraway et al., 1994; Tzahar et al., 1994), as well as the ErbB-2/ErbB-3 heterodimer, which also constitutes a high affinity heregulin receptor (Sliwkowski et al., 1994). As heregulin is not trafficked to the internalization pathway by receptor-mediated endocytosis, it seems likely that other mechanisms of receptor regulation at the cell surface may control the function of these receptors. A recent study found that protein kinase C activation brings about the rapid and extensive proteolytic cleavage of ErbB-4, producing a soluble fragment (120 kD) representing the extracellular ligand-binding domain and a membrane-anchored fragment (80 kD) composed of the entire cytoplasmic and transmembrane domain (Vecchi et al., 1996).
This article focuses on a protein kinase C–independent basal or constitutive mechanism that generates a similar hydrolysis of ErbB-4. This hydrolysis is due to a metalloprotease and produces an active tyrosine kinase, whose levels are, in turn, controlled by proteasome activity.
Materials and Methods
Materials
EGF was prepared from mouse submaxillary glands as previously described (Savage and Cohen, 1972). Heregulin β1 was a generous gift of M. Sliwkowski (Genentech Inc., San Francisco, CA). Betacellulin, heparin-binding EGF, and heregulin α were obtained from R & D Systems Inc. (Minneapolis, MN). Neuregulin-2 was a gift from K. Carraway, III (Harvard Medical School, Cambridge, MA). Polyclonal IgG to the carboxyl terminus (residues 1291–1308) of ErbB-4 were purchased from Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Serum raised against the carboxyl-terminal sequence 1108–1264 of ErbB-4 was supplied by M. Kraus (Instituto Europeo di Oncologia, Milan, Italy) (Vecchi et al., 1996). Antisera to PLC-γ1 was described previously (Arteaga et al., 1991). Anti-phosphotyrosine purified IgG and HRP-conjugated protein A were purchased from Zymed Labs, Inc., South San Franscisco, CA). Polyclonal antibodies to Shc proteins were products of Transduction Laboratories (Lexington, KY). Rabbit anti–ubiquitin serum, protein A–Sepharose, and enhanced chemiluminescence (ECL)1 reagents were obtained from Sigma Chemical Co. (St. Louis, MO). 125I-protein A was a product of ICN Biomedicals, Inc. (Irvine, CA) and Immunobilon-P membranes were from MCI. PMA and wheat germ (WG) agarose were from Sigma Chemical Co., bisindolylmaleimide (GF109203X) and N-acetyl-l-leucinyl-l-leucinyl-l-norleucinal (ALLN) were from Calbiochem-Novabiochem Corp. (La Jolla, CA). The metalloprotease inhibitor BB-3103 was provided by A. Drummond (British Biotech Pharmaceuticals Limited, Oxford, England). The metalloprotease inhibitors N-(dl-[2-9{hydroxy-aminocarbonyl}methyl]-4-methypentanoyl)-l-3-terbutyl-l-alanine, 2-aminoethylamide (TAPI-2), and Batimastat (BB-94) were gifts of L. Matrisian and P. Dempsey (Vanderbilt University, Nashville, TN). The proteasome inhibitor lactacystin was purchased from J.E. Corey (Harvard University, Cambridge, MA), and N-carboxybenzyl-leucyl-leucyl-leucine (MG-132) and carboxybenzyl-leucyl-leucyl-leucine vinyl sulfone (Z-Leu3-VS) were gifts of S. Cohen (Vanderbilt University, Nashville, TN; through H. Ploegh, Harvard University). PMA, metalloprotease inhibitors, and proteasome inhibitors ALLN, Z-leu3-VS, and MG-132 were dissolved in DMSO.
Cell Culture
T47-14 cells, transfected NIH 3T3 cells that overexpress human ErbB-4 (∼106 receptors per cell), have been described elsewhere (Baulida et al., 1996; Vecchi et al., 1996). These cells were routinely grown in 5% CO2 at 37°C in DME containing 20 mM Hepes, pH 7.4, 50 μM Gentamycin (GIBCO BRL, Grand Island, NY), and 10% calf serum. Atrial tumor myocytes, AT-1 cells, derived from T antigen transgenic mice (Steinhelper et al., 1990; Delcarpio et al., 1991), were provided by D.M. Roden (Vanderbilt University). These cells, maintained as transplanted tumors, were prepared and grown in culture as previously described (Yang et al., 1994). Under these conditions, the cells maintain the phenotype properties of cardiac myocytes (Yang and Roden, 1996; Lanson et al., 1992). Experimental cultures were generally grown in 60- or 100-mm-diam culture dishes.
Immunoprecipitation and Immunoblotting
Cell lysates were obtained as previously described (Vecchi et al., 1996). Briefly, after overnight starvation in DME and 0.5% serum, monolayers were incubated for the indicated times at 37°C in basal medium (DME, 0.1% BSA, and 20 mM Hepes, pH 7.2) with indicated additions, i.e., inhibitors, growth factors. The cells were next washed with calcium and magnesium-free PBS and then solubilized for 20 min at 4°C in TGH buffer (1% Triton X-100, 10% glycerol, 20 mM Hepes, pH 7.2, 100 mM NaCl, 1 mM phenylmethylsulphonylfluoride, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 1 mM Na3VO4). Lysates were clarified by centrifugation (14,000 g, 10 min) at 4°C and protein concentration was determined by the modified method of Bradford (Bio-Rad Laboratories, Hercules, CA). The ErbB-4 protein was immunoprecipitated by adding ∼1 μg of ErbB-4 antibody per 200 μg of cell lysate for 2 h at 4°C and then incubating (1 h, 4°C) with protein A–Sepharose CL-4B. Subsequently, the immunocomplexes were extensively washed with TGH buffer and resuspended in 1× Laemmli buffer. After boiling, proteins in the samples were electrophoretically separated on 7.5% SDS-PAGE gels and transferred to nitrocellulose membranes for Western blotting. Membranes were blocked with 5% milk in PBS containing 0.05% Tween for 1 h before blotting with antibodies to anti–ErbB-4, –Shc, –PLC-γ1, and –ubiquitin. Before anti-phosphotyrosine blotting, membranes were blocked by incubating for 1 h with 3% BSA in TBST buffer (0.05% Tween, 150 mM NaCl, 50 mM Tris, pH 7.4). Membranes were then incubated with the appropriate antibody for 2 h at room temperature and washed with PBS or TBST buffer, incubated with 125I-protein A for 1 h at room temperature, and after five washes with PBS or TBST buffer, visualized by autoradiography (X-Omat AR film; Eastman Kodak, Rochester, NY). Where indicated, bound antibody was detected with HRP-protein A and ECL.
In Vitro Kinase Assay
T47-14 cells overexpressing ErbB-4 were washed and the cell monolayers solubilized at 4°C in TGH buffer without Na3VO4. Equal aliquots of cell lysates (100 μg protein) were immunoprecipitated by adding 0.5 μg of antibody to ErbB-4. After a 2-h incubation at 4°C, protein A–Sepharose was added for 1 h. The immunocomplexes were then washed twice with TGH buffer without Na3VO4 and twice with kinase buffer (20 mM Hepes, pH 7.4, 3 mM MnCl2, 20 mM MgCl2, 50 mM NaCl, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 100 μM Na3VO4). The immunocomplexes were resuspended in 50 μl of kinase buffer containing 20 μM cold ATP, and 4 μg of recombinant PLC-γ1 (Horstman et al., 1995) was added. The reaction mixtures were then incubated at room temperature for the indicated times before stopping the reaction by adding 50 μl of 2× Laemmli buffer and boiling for 5 min. Proteins were subsequently separated on a 7.5% SDS-PAGE gel and analyzed for phosphotyrosine content by Western blotting with antibody to phosphotyrosine. The amount of tyrosine phosphorylated PLC-γ1 was quantitated by densitometric scanning. For each phosphorylation sample, a parallel aliquot of lysate was analyzed for ErbB-4 protein by immunoprecipitation and Western blot, as described above.
Lectin Fractionation
WG agarose was used to selectively adsorb the highly glycosylated native ErbB-4 receptor, but did not react with the 80-kD ErbB-4 fragment expected to contain little, if any, carbohydrate. Lysates (100 μg) in TGH buffer were incubated for 4 h at 4°C with 50 μl WG agarose (cross-linked 4% beads) that had been prewashed in TGH buffer. After incubation, the mixture was centrifuged and the supernatant recovered. Anti–ErbB-4 was added to the supernatant for analysis of kinase activity and ErbB-4 protein as described above. The WG agarose beads were also analyzed directly for adsorbed kinase activity and ErbB-4 protein.
Results
Metalloprotease Cleavage of ErbB-4
Previous data demonstrated a PMA-stimulated proteolytic cleavage of ErbB-4, which produces a soluble extracellular domain and a membrane-anchored fragment that includes the tyrosine kinase cytoplasmic domain (Vecchi et al., 1996). To identify the type of protease involved in this cleavage, cells expressing ErbB-4 were preincubated with various protease inhibitors before the addition of PMA. The cleavage of ErbB-4 was then detected by assaying the 80-kD cytoplasmic domain with anti–ErbB-4. The results, shown in Table I, revealed that three metalloprotease inhibitors reduced the PMA-stimulated cleavage by 90%. Inhibitors of other types of proteases were relatively ineffective in this assay. Hence, these data implicate a metalloprotease as the likely enzyme that modulates ErbB-4 structure in response to PMA. Previously, a low level of a similarly sized cytoplasmic domain fragment was detected in the absence of PMA (Vecchi et al., 1996). As the amount of this 80-kD fragment constitutively released from ErbB-4 is small (∼4%) relative to native ErbB-4 and PMA-stimulated fragment levels (Table I), one cannot accurately judge inhibitor effects on the constitutive hydrolysis under these conditions.
Table I.
Influence of Protease Inhibitors on ErbB-4 Proteolysis
| Protease inhibitor | Primary specificity | 80-kD fragment (relative percent) | ||
|---|---|---|---|---|
| Control | — | 4 | ||
| PMA (100 ng/ml) | — | 100 | ||
| Phenylmethylsulfonylfluoride (2 mM) | Serine proteases | 66 | ||
| Aprotinin (1.5 μM) | Serine proteases | 87 | ||
| 3,4-dichloroisocoumarin (2 mM) | Serine proteases | 77 | ||
| E-64 (500 μM) | Cysteine proteases | 56 | ||
| Leupeptin (105 μM) | Trypsin, some cysteine proteases | 81 | ||
| Pepstatin (1 μM) | Some aspartic proteases | 92 | ||
| N-tosyl-l-phenylalanine chloromethyl ketone (100 μM) | Chymotrypsin | 131 | ||
| N-tosyl-l-lysyl chloromethyl ketone (100 μM) | Trypsin | 97 | ||
| Elastatinal (100 μM) | Elastase | 102 | ||
| EDTA (5 mM) | Metalloproteases | 104 | ||
| EGTA (5 mM) | Metalloproteases | 135 | ||
| BB-94 (1 μM) | Metalloproteases | 13 | ||
| BB-94 (5 μM) | Metalloproteases | 5 | ||
| BB-3103 (5 μM) | Metalloproteases | 24 | ||
| BB-3103 (10 μM) | Metalloproteases | 10 | ||
| TAPI-2 (10 μM) | Metalloproteases | 11 | ||
| TAPI-2 (40 μM) | Metalloproteases | 4 |
Cells were preincubated with the indicated concentration of inhibitor for 20 min before the addition of PMA (100 ng/ml). After a 30-min incubation with PMA, the cells were lysed and the amount of 80-kD ErbB-4 fragment quantitated as described in Materials and Methods. The amount of 80-kD fragment is expressed relative to what accumulated in cells similarly treated with PMA only.
Proteasome Function in ErbB-4 Cleavage
Previous studies (Vecchi et al., 1996) showed that the PMA-stimulated cleavage of ErbB-4 was independent of heregulin binding. Various ErbB-4 ligands (including heregulin-β and -α isoforms, betacellulin, heparin-binding EGF, and neuregulin-2), were assayed for their capacity to alter the basal level of receptor cleavage. However, none had a significant influence (data not shown).
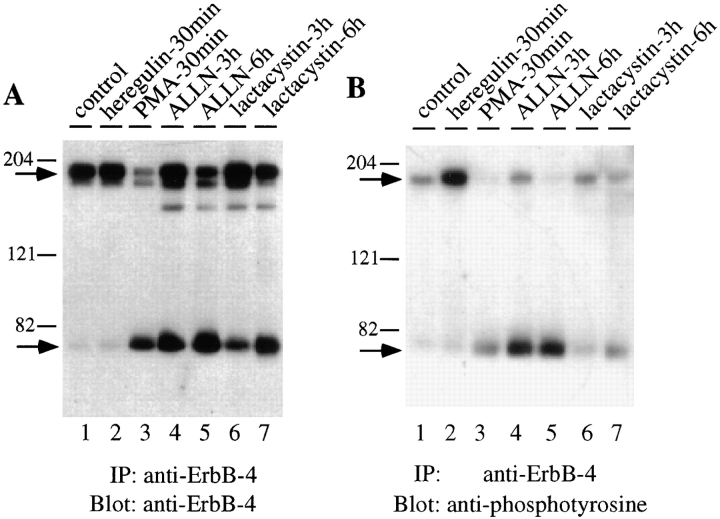
However, when ErbB-4–expressing cells are exposed to the proteasome inhibitors ALLN or lactacystin, the intracellular level of the 80-kD ErbB-4 fragment is slowly but dramatically increased (Fig. 1 A, lanes 4–7). The level of accumulation of 80-kD fragment under these conditions equals that previously reported (Vecchi et al., 1996) after PMA treatment (Fig. 1, lane 2). Lactacystin is considered the most specific inhibitor of proteasome activity (Fenteany et al., 1995). In addition, two peptide aldehyde proteasome inhibitors, MG-132/Z-Leu 3-H and Z-Leu 3-Vs (Bogyo et al., 1997) also enhanced accumulation of the 80-kD fragment (data not shown). Hence, proteasome activity normally limits the level of ErbB-4 80-kD fragment accumulation. Additional experiments have shown that, similar to the 80-kD fragment generated in the presence of PMA (Vecchi et al., 1996), the 80-kD fragment that accumulates in the absence of proteasome function is tyrosine phosphorylated (Fig. 1 B, lanes 3–7) and is membrane- localized, as determined by cell fractionation (data not shown).
Figure 1.
Detection of ErbB-4 fragment in the presence of proteasome inhibitors. Cells were treated with PMA (100 ng/ml), heregulin (100 ng/ml), ALLN (250 μM), or lactacystin (10 μM) for the indicated periods of time. After detergent lysis, duplicate aliquots (500 μg) of each lysate were immunoprecipitated with anti– ErbB-4. After SDS-PAGE and transfer to nitrocellulose, the samples were Western blotted with anti–ErbB-4 (A) or anti-phosphotyrosine (B). Bound antibody was detected with 125I-protein A and visualized by autoradiography, as described in Materials and Methods. Arrows indicate positions of the 180-kD native ErbB-4 and 80-kD ErbB-4 fragment.
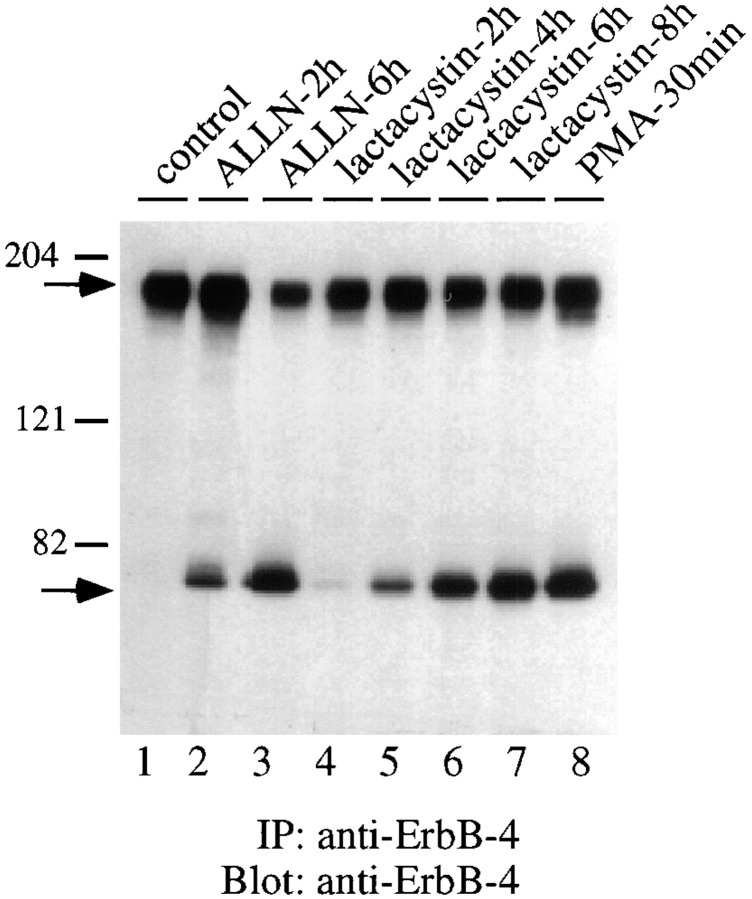
To determine the rate and extent of this basal level of ErbB-4 hydrolysis, the proteasome inhibitor ALLN was added to stabilize the 80-kD fragment and accumulation of the fragment was measured at various times thereafter. As shown in Fig. 2 A, accumulation occurred slowly for several hours, until 4–6 h when the amount of 80-kD fragment was readily detectable. At this time, the cellular pool of 80-kD ErbB-4 fragment was equal to ∼60% of the initial level of native ErbB-4 receptor. Since the cells used in this experiment overexpress ErbB-4 (∼106 receptors per cell), it is estimated that the basal rate of ErbB-4 cleavage is ∼105 receptors per hour. In a separate experiment, cells were incubated with ALLN for 6 h to accumulate an 80-kD ErbB-4 fragment. The ALLN was then removed by washing, which restores proteasome function within 60 min (Rock et al., 1994), and the half-life of the 80-kD fragment was determined (Fig. 2 B). Under these conditions, the fragment was degraded with a half-life of ∼4 h.
Figure 2.
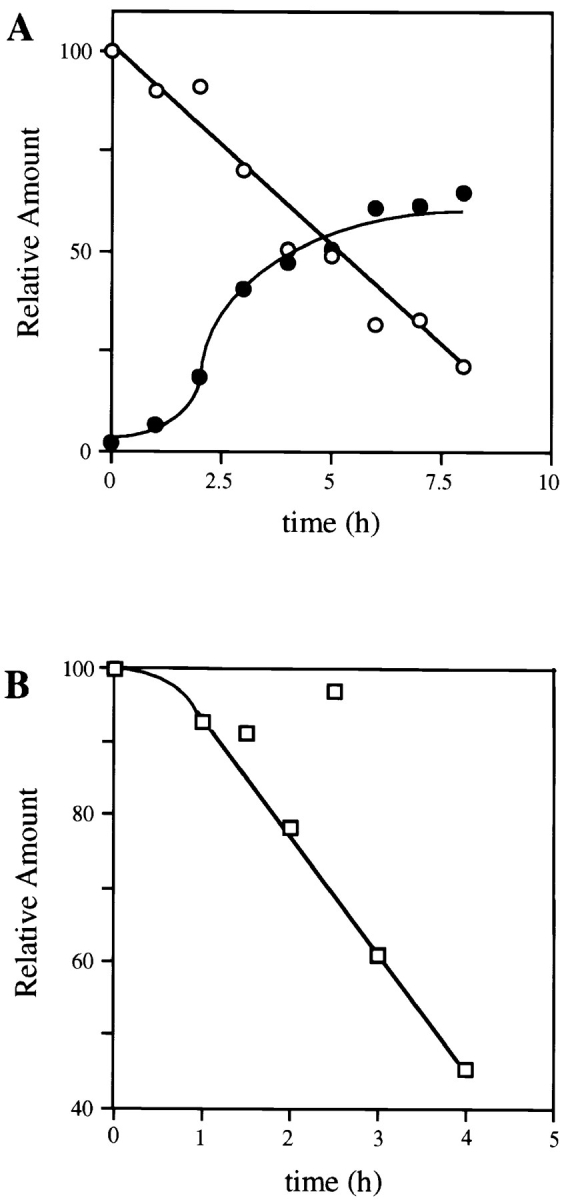
Time course of the accumulation and metabolic degradation of the 80-kD ErbB-4 fragment. (A) Cells were incubated at 37°C in the absence or presence of ALLN (250 μM) for the indicated periods of time. After detergent solubilization, cell lysates (100 μg) were analyzed by SDS-PAGE and Western blotting with anti–ErbB-4. Immunoreactive native ErbB-4 (open circles) and the 80-kD fragment (closed circles) were visualized with 125I-protein A autoradiography and quantitated by PhosphorImager. The amount of each molecule is expressed relative to the initial level of native ErbB-4 receptor in control cells at time zero, which is set at 100%. (B) Cells were incubated in the absence or presence of ALLN (250 μM) for 6 h at 37°C to maximize the intracellular pool of 80-kD ErbB-4 fragment. The cells were then washed once to remove ALLN and incubated at 37°C in media not containing the proteasome inhibitor. At the indicated times, cells were analyzed, as described above, for the amount of 80-kD ErbB-4 fragment remaining. The amount of fragment at each time point is expressed relative to the amount present after the 6-h ALLN incubation (100%).
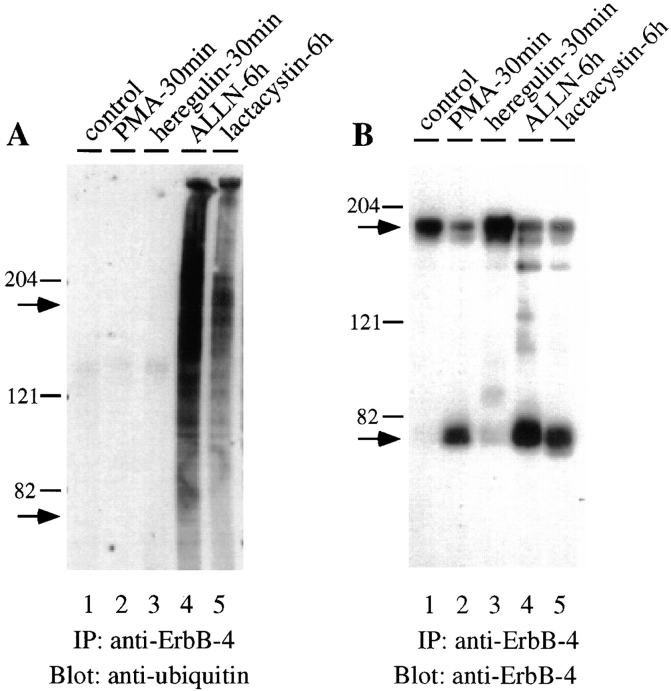
Ubiquitination is a frequent posttranslational marker for proteasome substrates. Therefore, we used antibody to ubiquitin to determine whether the 80-kD molecule is modified in this manner. Cells were treated with PMA, heregulin, ALLN, or lactacystin for the indicated times and then lysates were precipitated with antibody to the ErbB-4. Subsequent Western blotting demonstrates the specific polyubiquitination of the ErbB-4 fragment in cells treated with ALLN or lactacystin (Fig. 3 A, lanes 4 and 5). Heterogeneous ubiquitinated products were detected from 80 kD to the top of the gel. Using normal rabbit IgG, control precipitations of lysates from cells treated with proteosome inhibitors showed that the detection of ubiquitinated proteins is specific for anti–ErbB-4. Interestingly, no ubiquitination of the 80-kD ErbB-4 fragment generated by PMA treatment was detected (Fig. 3 A, lane 2) even though equivalent levels of 80-kD fragments were produced by PMA, ALLN, and lactacystin (Fig. 3 B, lanes 2, 4, and 5). Also, no ubiquitination of the native ErbB-4 receptor could be detected in the absence or presence of heregulin (Fig. 3 A, lanes 1 and 3). Addition of ALLN for 1 h also failed to reveal the presence of ubiquitinated native receptor (data not shown).
Figure 3.
Ubiquitination of ErbB-4 Fragment. (A) Cells were incubated for the indicated times with PMA (100 ng/ml), heregulin (100 ng/ml), ALLN (250 μM), or lactacystin (50 μM). The cells were then solubilized and lysates (1 μg each) were immunoprecipitated with anti-ErbB-4. After SDS-PAGE and transfer to nitrocellulose, each sample was blotted with anti-ubiquitin. Before blotting, the nitrocellulose membranes were autoclaved in water for 20 min to completely denature proteins (Lee et al., 1996; Mimnaugh et al., 1996). Bound anti-ubiquitin was detected by ECL. (B) Anti–ErbB-4 immunoprecipitated samples were stripped to remove anti-ubiquitin and then reprobed with anti–ErbB-4. Bound antibody was visualized with 125I-protein A. Arrows indicate positions of the native ErbB-4 and 80-kD ErbB-4 fragment.
The results described above were obtained with NIH 3T3 cells that overexpress the transfected ErbB-4 receptor. To ascertain that the proteolytic activities toward ErbB-4 are not a consequence of the exogenous expression of this receptor, AT-1 cardiac myocytes, which express endogenous ErbB-4, were assayed. As shown in Fig. 4, addition of PMA (lane 8), or the addition of proteasome inhibitors (lanes 2–7) produced accumulation of an 80-kD ErbB-4 fragment in these cells. It is probable that ErbB-4 is physiologically important in these cells, as targeted disruption of the ErbB-4 gene in mice produces, in null homozygotes, embryonic lethality due to abnormal heart development (Gassmann et al., 1995).
Figure 4.
ErbB-4 degradation in cells endogenously expressing ErbB-4. Cardiac myocytes (AT-1 cells) were treated with vehicle alone (control), ALLN (250 μM), or lactacystin (10 μM) for the indicated periods of time at 37°C. Cell lysates (500 μg) were then immunoprecipitated with anti–ErbB-4 and Western blotted with the same antibody. Bound antibody was detected by ECL. Arrows indicate positions of native ErbB-4 and the 80-kD ErbB-4 fragment.
Interrelationship of Metalloprotease and Proteasome Functions
The results cited above suggest both metalloprotease and proteasome activities are involved in a constitutive pathway of ErbB-4 cleavage. However, these data only suggest that the 80-kD fragment detected in the presence of proteasome inhibitors represents accumulation of the metalloprotease cleavage product of ErbB-4. It is plausible that the constitutive level of 80-kD ErbB-4 fragment is derived from cleavage of native ErbB-4 by another protease or splicing of ErbB-4 mRNA so as to produce an amino-terminal truncation of the native protein. Therefore, we tested whether the accumulation of 80-kD fragment in the presence of a proteasome inhibitor depends on metalloprotease activity.
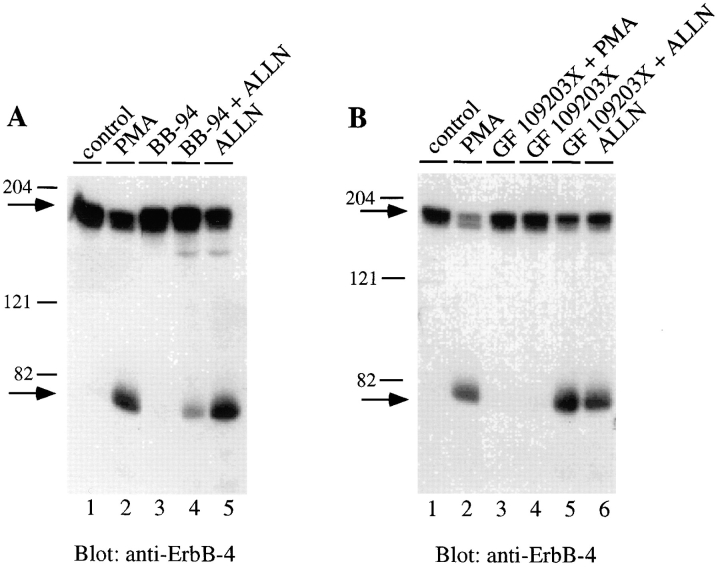
As shown in Fig. 5 A, cells were pretreated with the metalloprotease inhibitor BB-94 for 30 min before the addition of the proteasome inhibitor ALLN for an additional 4 h. The amount of 80-kD fragment was then compared to that detected in cells treated for the same time with ALLN only. The results show that inhibition of metalloprotease activity reduces by ∼80% the ALLN-dependent accumulation of the 80-kD ErbB-4 fragment (Fig. 5, compare lanes 4 and 5). This result indicates that the metalloprotease-generated 80-kD fragment is the substrate for proteasome activity.
Figure 5.
Influence of metalloprotease or protein kinase C inhibition on the accumulation of 80-kD ErbB-4 fragment in the absence of proteasome activity. (A) Cells were incubated at 37°C with vehicle alone (control), PMA (100 ng/ml) for 30 min, the metalloprotease inhibitor BB-94 (1 μM) for 4.5 h, ALLN (250 μM) for 4 h, or BB-94 and ALLN for 4.5 h. After cell solubilization, aliquots of cell lysate (100 μg) were separated by SDS-PAGE and Western blotted with anti–ErbB-4. Bound antibody was visualized by 125I-protein A and autoradiography. (B) Cells were incubated with vehicle alone, PMA (100 ng/ml) for 30 min, ALLN (250 μM) for 4 h, the protein kinase C inhibitor GF109203X (5 μM) for 4.5 h, or GF109203X and ALLN for 4.5 h. Native ErbB-4 and the 80-kD ErbB-4 fragment were detected as described in A. Arrows indicate positions of the native ErbB-4 and 80-kD ErbB-4 fragment.
Although the PMA-stimulated accumulation of the 80-kD ErbB-4 fragment is dependent on protein kinase C activity (Vecchi et al., 1996), it is unclear whether the constitutive hydrolysis may also depend on endogenous protein kinase C activity. This has been tested by adding the specific protein kinase C inhibitor GF109203X to cells and then measuring the accumulation of the fragment after the addition of either PMA or ALLN. As shown in Fig. 5 B, the protein kinase C inhibitor completely blocked the hydrolysis stimulated by PMA (Fig. 5, lane 3) but had no influence on accumulation of the 80-kD fragment in the presence of ALLN (Fig. 5, lane 5). This result indicates that the basal level of ErbB-4 proteolysis is not dependent on protein kinase C activity.
Tyrosine Kinase Activity of the 80-kD Fragment
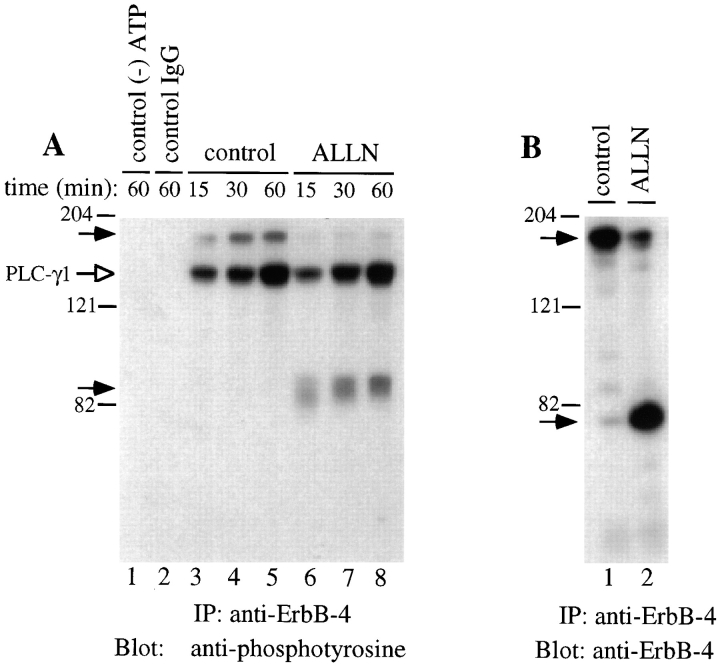
The 80-kD ErbB-4 fragment that accumulates in the presence of proteasome inhibitors represents the entire cytoplasmic domain of ErbB-4 and may, therefore, retain tyrosine kinase activity. This possibility has been tested by using in vitro kinase assays. After cell incubation in the absence or presence of ALLN, cell lysates were immunoprecipitated by antibody to the ErbB-4 carboxyl terminus. The immunoprecipitates were then incubated with kinase reaction components, including unlabeled ATP and recombinant tyrosine kinase substrate PLC-γ1 (Horstman et al., 1995). Phosphotyrosine blotting was used to detect phosphorylated PLC-γ1 and ErbB-4 blotting was used to assess the levels of native ErbB-4 and the 80-kD ErbB-4 fragment. Control experiments showed that the ErbB-4 receptor is equally active in vitro regardless of whether the cells are treated with or without heregulin (data not shown). The results, shown in Fig. 6 A, indicate that the native ErbB-4 (Fig. 6, lanes 3–5) and the ALLN-dependent 80-kD ErbB-4 fragment (Fig. 6, lanes 6–8) phosphorylate PLC-γ1 to an equivalent extent and at approximately the same rate under these conditions. In this experiment, ErbB-4 blots (Fig. 6 B) show comparable levels of native ErbB-4 from control cells and 80-kD fragment from ALLN-treated cells. Control assays showed that no kinase activity was detected in the absence of ATP or when an unrelated IgG was used for immunoprecipitation (Fig. 6, lanes 6–8). After incubation in the presence of ATP, the 80-kD fragment migrates at a slightly higher molecular mass indicative of autophosphorylation (Fig. 6, lanes 6–8).
Figure 6.
Tyrosine phosphorylation activity in vitro of the native ErbB-4 and 80-kD ErbB-4 fragment. Cells were incubated for 6 h with or without ALLN (250 μM). After solubilization, replicate aliquots (100 μg) of each lysate were precipitated with anti– ErbB-4 and assayed for tyrosine kinase activity toward PLC-γ1 by blotting with anti-phosphotyrosine (A), or the level of ErbB-4 protein by blotting with anti–ErbB-4 (B). Kinase assays were performed as described in Materials and Methods with the incubation times in vitro as indicated. Control assays were performed with extract from untreated cells and included an immunoprecipitation with an unrelated antibody (control IgG) or with ATP not included in the kinase reaction (−ATP). Closed arrows indicate positions of native ErbB-4 and the 80-kD ErbB-4 fragment, whereas the open arrow indicates the position of tyrosine phosphorylated PLC-γ1.
When lysates from ALLN-treated cells are immunoprecipitated with anti ErbB-4, a small amount of native ErbB-4 is detected together with the 80-kD fragment (Fig. 6 B). Although the amount of native ErbB-4 in the ALLN samples is low, ∼20% of that in untreated cells, it is plausible that it might significantly contribute to the observed phosphorylation activity in vitro. Two approaches have been used to ascertain the possible contribution of native ErbB-4 to the kinase activity measured in immunoprecipitates from ALLN-treated cells. First, to approximate the low level of native ErbB-4 in ALLN lysates, control lysates were diluted (1:3) to determine if that reduced level of receptor is sufficient to phosphorylate PLC-γ1 under these conditions. As shown in Fig. 7, dilution did reduce by ∼75% both the amount of native ErbB-4 (Fig. 7, lane 8) and the kinase activity toward PLC-γ1 (Fig. 7, lane 1) and does approximate the level of native ErbB-4 protein present in immunoprecipitates of ALLN lysates (Fig. 7, compare lanes 8 and 12). Nevertheless, this low level of native ErbB-4 does contribute to the kinase activity measured in ALLN lysates.
Figure 7.
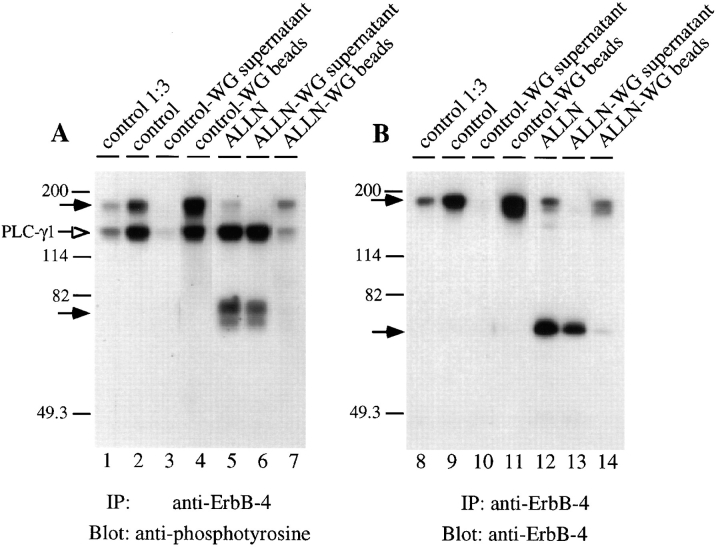
Separation of the native ErbB-4 and 80-kD ErbB-4 fragment by WG fractionation. Cells were left untreated (control) or incubated with ALLN (250 μM) for 6 h and solubilized as described previously. After treatments of the lysates as indicated below, samples were assayed for kinase activity toward PLC-γ1 (A), or the amount of ErbB-4 protein (B). Lanes 2 and 5 indicate the kinase activity recovered in ErbB-4 immunoprecipitates from aliquots of control and ALLN lysates (100 μg), whereas lanes 9 and 12 demonstrate the amount of ErbB-4 protein (native or 80-kD) present in immunoprecipitates from equivalent lysates (100 μg). Lanes 1 and 8 show the amount of kinase activity and ErbB-4 protein detected when control lysates were diluted 1:3 before precipitation with anti–ErbB-4. To test the capacity of WG agarose to separate native ErbB-4 from the 80-kD ErbB-4 fragment, aliquots (100 μg) of control and ALLN lysates were precleared with WG agarose, as described in Materials and Methods. The nonadsorbed supernatants were then precipitated with anti– ErbB-4 before kinase assays (lanes 3 and 6), or detection of ErbB-4 protein (lanes 10 and 13). Also, the amount of kinase activity (lanes 4 and 7), and ErbB-4 protein (lanes 11 and 14) adsorbed onto the WG agarose was measured. Solid arrows indicate positions of native ErbB-4 and 80-kD ErbB-4 fragment. The open arrow indicates tyrosine phosphorylated PLC-γ1.
To measure the kinase activity of the 80-kD fragment only, lysates from ALLN-treated cells were precleared with WG lectin to adsorb the highly glycosylated native ErbB-4. The 80-kD fragment is expected to contain little, if any, carbohydrate. These WG supernatants were then immunoprecipitated with anti ErbB-4 and assayed for kinase activity (Fig. 7 A) and ErbB-4 reactive protein (Fig. 7 B). The results show that the 80-kD fragment retains phosphorylation activity in the absence of detectable native ErbB-4 protein. WG agarose effectively removed, respectively, the native ErbB-4 protein (Fig. 7, lane 10) and its in vitro kinase activity (Fig. 7, lane 2) from control lysates. The kinase activity (Fig. 7, lane 4) and protein (Fig. 7, lane 11) of the native ErbB-4 from the control lysates were recovered on the WG beads. Hence, WG fractionation effectively removes detectable levels of native ErbB-4 protein and kinase activity before immunoprecipitation.
When ALLN samples were precleared with WG agarose, neither the amount of kinase activity (Fig. 7, lane 6) nor 80-kD protein (Fig. 7, lane 13) was significantly decreased. In the WG agarose supernatant from the ALLN lysate, the presence of even a small amount of native ErbB-4 was no longer detectable (Fig. 7, compare lanes 12 and 13). The low level of native ErbB-4 kinase activity and protein originally present in ALLN lysates was retained on the WG agarose beads (Fig. 7, lanes 7 and 14). These results demonstrate that the ErbB-4 80-kD fragment is, in fact, an active tyrosine kinase. We have used densitometric scanning of the antiphosphotyrosine signal, representing PLC-γ1 phosphorylation, and the anti–ErbB-4 signal, indicating the level of ErbB-4 native or 80-kD fragment, to approximate a kinase-specific activity. This analysis included shorter exposures of the data shown in Figs. 6 and 7. In all samples, the specific phosphorylation activity of the 80-kD fragment was equal to that of the native ErbB-4 receptor (data not shown).
Discussion
Numerous studies have demonstrated the ligand-dependent degradation of growth factor receptors through a mechanism involving internalization of coated pits and endosome sorting to the lysosome (Sorkin and Waters, 1993). In certain instances, recycling of growth factor receptors and ligands from endosomes to the cell surface has been noted. The cell surface ligand-dependent trafficking of EGF receptor-like ErbB receptors is novel in that the endocytic pathway is not used to rapidly internalize occupied receptors after ligand binding (Baulida et al., 1996; Pinkas-Kramarski et al., 1996). This may suggest alternate mechanisms exist to desensitize these surface ligand–receptor complexes as the mitogenic potency of EGF and heregulin are similar (Baulida et al., 1996).
A previous study has demonstrated that protein kinase C activation rapidly produces a cleavage of the ErbB-4 ectodomain (Vecchi et al., 1996). The ectodomain cleavage of several growth factor receptor tyrosine kinases has been observed in cells treated with PMA (Downing et al., 1989; Yee et al., 1993; Brizzi et al., 1994; Yee et al., 1994; O'Bryan et al., 1995; Cabrera et al., 1996; Vecchi et al., 1996; Jeffers et al., 1997); however, the PMA-sensitive protease(s) has not been identified. Based on sensitivity to several metalloprotease inhibitors, we conclude that protein kinase C activation directly or indirectly enhances the activity of a member of this protease family toward the ErbB-4 extracellular domain. Several reports indicate that PMA increases expression of metalloprotease genes (Birkedal-Hansen et al., 1993), but protein synthesis is not required for the PMA-induced cleavage of ErbB-4 (Vecchi et al., 1996). Hence, the action of protein kinase C in this system is most likely at the secretion and/or activation step of the metalloprotease latent precursor, known sites of regulation (Coussens and Werb, 1996). However, the latter seems unlikely given the intracellular localization of protein kinase C and the extracellular location of metalloproteases, which function mainly in the degradation of extracellular matrix components. A transmembrane disintegrin molecule could provide a means of communication, as recently reported with the cleavage of tumor necrosis factor-α precursor (Black et al., 1997; Moss et al., 1997).
The data in this report show that a constitutive cleavage of ErbB-4 also occurs in the absence of exogenous protein kinase C activation and produces an 80-kD transmembrane and cytoplasmic domain fragment of ErbB-4 that is very similar to the ErbB-4 fragment observed after PMA addition to cells (Vecchi et al., 1996). Most likely this basal receptor degradation represents a low level of metalloprotease activity in the extracellular environment. Although this ErbB-4 fragment is generally found at low levels in the cell, proteasome activity is crucial to prevent its accumulation. Blocking proteasome activity with specific proteasome inhibitors results in accumulation of this receptor fragment to significant levels, approaching the cellular levels of native ErbB-4. Since no ErbB-4 80-kD fragment accumulates in cells treated with both proteasome and metalloprotease inhibitors, the proteasome seems to degrade the 80-kD fragment but not the intact ErbB-4 receptor. This is reinforced by the finding that the 80-kD fragment is ubiquitinated, but no ubiquitin is detectable on the intact ErbB-4 molecule under several conditions including those comparable to the detection of ubiquitin on the PDGF (Mori et al., 1992; Yarden et al., 1986), EGF (Galcheva-Gargova et al., 1995; Mori et al., 1995), stem cell factor/c-kit (Miyazawa et al., 1994; Yee et al., 1994), and colony stimulating factor-1/c-fms (Mori et al., 1995) receptor tyrosine kinases. In each of these instances, however, ubiquitination occurred subsequent to growth factor binding. ErbB-4 receptor metabolism, therefore, represents a novel coupling of two protease activities acting in series to allow ligand-independent degradation of ErbB-4 and to prevent accumulation of cytoplasmic domain receptor fragment. If, in fact, the proteasome recognizes the 80-kD fragment but not the intact ErbB-4 receptor, this implies that lack of an extracellular domain in some way, perhaps involving topological distribution, leads to recognition of the ErbB-4 cytoplasmic domain by the ubiquitination system.
Recently the hepatocyte growth factor receptor, Met, has been shown to be ubiquitinated and subject to proteosome-mediated degradation in the absence or presence of its ligand (Jeffers et al., 1997). In this system, prior cleavage of the intact receptor was not reported to be required for ubiquitination. After proteasome inhibition a 50-kD fragment of Met was detected that represented the cytoplasmic tyrosine kinase domain. Interestingly, PMA also stimulated the accumulation of a similarly sized Met fragment. In this system, however, the 50-kD fragment accumulated to only a low level relative to the level of intact Met. Phosphotyrosine was detectable on the 50-kD fragment though kinase activity of the fragment was not assayed given the low amount of fragment produced. Analogously, PMA induces ectodomain cleavage of the NGF receptor TrkA resulting in a tyrosine phosphorylated cytoplasmic domain fragment with unreported kinase activity (Cabrera et al., 1996).
There are several reported instances where removal, by proteolysis or deletion mutagenesis, of the ectodomain of receptor tyrosine kinases has produced an active or activated kinase domain. This includes the insulin receptor (Ellis et al., 1987; Goren et al., 1987; Wang et al., 1987; Shoelson et al., 1988; Hsuan et al., 1989; Lebwohl et al., 1991), insulin-like growth factor I receptor (Liu et al., 1992, 1993), and the Drosophila Sevenless receptor (Basler et al., 1991). Oncogenic forms of the avian EGF receptor involve deletion of the extracellular domain as well as other more subtle changes, all of which contribute to its transforming potential (Carter et al., 1994).
Using an in vitro assay with recombinant SH2 domain-containing protein (PLC-γ1) as a substrate, we have compared the kinase activity of the 80-kD ErbB-4 fragment accumulated in proteasome-inhibited cells to that of the intact receptor recovered from control cells. The data show that both molecules tyrosine phosphorylate PLC-γ1 at comparable efficiencies. These data indicate that this ErbB-4 fragment has intrinsic tyrosine kinase activity. Therefore, it is possible that the fragment has ligand-independent biological activity, increasing the importance of proteasome degradation of the fragment. Unfortunately, it is not possible to test the kinase activity of the 80-kD fragment in intact cells due to its proteasome sensitivity and the toxicity of proteosome inhibitors. Regardless of kinase activity, the tyrosine phosphorylated fragment is constitutively generated in ErbB-4-expressing cells and may act not only as a kinase, but as a membrane-localized docking molecule for signaling molecules with SH2 domains. Coprecipitation data have shown the association of PLC-γ1 and Shc with the 80-kD fragment produced in PMA-treated cells (Vecchi et al., 1996). In some instances, docking at the cytoplasmic face of the plasma membrane may be more critical than tyrosine phosphorylation for the function of certain signaling molecules that associate with receptor autophosphorylation sites, for example PI-3 kinase and Grb-2 (Pawson and Schlessinger, 1993).
There are now examples of endogenous ectodomain cleavage of several growth factor receptor tyrosine kinases. If these results with ErbB-4 are generally applicable, proteasome function may also limit the accumulation of active tyrosine kinase fragments in other receptor systems.
Acknowledgments
The authors thank S. Carpenter for the manuscript preparation, L. Rudolph-Owen for reading the manuscript, S. Ermini for technical assistance, D. Horstman for the recombinant PLC-γ1, and H. Waldrop, and D. Roden for AT-1 cells (all from Vanderbilt University).
Abbreviations used in this paper
- ECL
enhanced chemiluminescence
- PLC-γ1
phospholipase c-γ1
- WG
wheat germ
Footnotes
This research was supported by a National Cancer Institute grant (CA24071).
Manuela Vecchi's current address is European Institute of Oncology, Via Ripamonte 435, 20141 Milan, Italy.
Address all correspondence to Graham Carpenter, Department of Biochemistry, 647 Light Hall, Vanderbilt University School of Medicine, Nashville, TN 37232-0146. Tel.: (615) 322-6678. Fax: (615) 322-2931.
References
- Arteaga CL, Johnson MD, Todderud G, Coffey RJ, Carpenter G, Page DL. Elevated content of the tyrosine kinase substrate phospholipase C-γ1 in primary human breast carcinomas. Proc Natl Acad Sci USA. 1991;88:10435–10439. doi: 10.1073/pnas.88.23.10435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basler K, Christen B, Hafen E. Ligand-independent activation of the sevenless receptor tyrosine kinase changes the fate of cells in the developing drosophila eye. Cell. 1991;64:1069–1081. doi: 10.1016/0092-8674(91)90262-w. [DOI] [PubMed] [Google Scholar]
- Baulida J, Kraus MH, Alimandi M, Di Fiore PP, Carpenter G. All ErbB receptors other than the epidermal growth factor receptor are endocytosis impaired. J Biol Chem. 1996;271:5251–5257. doi: 10.1074/jbc.271.9.5251. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H, Moore WGI, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MR, Castner BJ, Stocking KL, Reddy P, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-α from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- Bogyo M, McMaster JS, Gaczynska M, Tortorella D, Goldberg AL, Ploegh H. Covalent modification of the active site threonine of proteasomal β subunits and the Escherichia colihomologue HsIV by a new class of inhibitors. Proc Natl Acad Sci USA. 1997;94:6629–6634. doi: 10.1073/pnas.94.13.6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brizzi MF, Blechman JM, Cavalloni G, Givol D, Yarden Y, Pegoraro L. Protein kinase C-dependent release of a functional whole extracellular domain of the mast cell growth factor (MGF) receptor by MGF- dependent human myeloid cells. Oncogene. 1994;9:1583–1589. [PubMed] [Google Scholar]
- Cabrera N, Díaz-Rodríguez E, Becker E, Martín-Zanca D, Pandiella A. TrkA receptor ectodomain cleavage generates a tyrosine-phosphorylated cell-associated fragment. J Cell Biol. 1996;132:427–436. doi: 10.1083/jcb.132.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G, Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol. 1976;71:159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraway KL, III, Sliwkowski MX, Akita R, Platko JV, Guy PM, Nuijens A, Diamonti AJ, Vandlen RL, Cantley LC, Cerione RA. The erbB3gene product is a receptor for heregulin. J Biol Chem. 1994;269:14303–14306. [PubMed] [Google Scholar]
- Carter TH, Kung HJ. Tissue-specific transformation by oncogenic mutants of epidermal growth factor receptor. Crit Rev Oncog. 1994;5:389–428. doi: 10.1615/critrevoncog.v5.i4.40. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Matrix metalloproteinases and the development of cancer. Chem Biol (Lond) 1996;3:895–904. doi: 10.1016/s1074-5521(96)90178-7. [DOI] [PubMed] [Google Scholar]
- Delcarpio JB, Lanson NA, Jr, Filed LJ, Claycomb WC. Morphological characterization of cardiomyocytes isolated from a transplantable cardiac tumor derived from transgenic mouse atria (AT-1 Cells) Circ Res. 1991;69:1591–1600. doi: 10.1161/01.res.69.6.1591. [DOI] [PubMed] [Google Scholar]
- Downing JR, Roussel MF, Sherr CJ. Ligand and protein kinase C downmodulate the colony-stimulating factor 1 receptor by independent mechanisms. Mol Cell Biol. 1989;9:2890–2896. doi: 10.1128/mcb.9.7.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earp HS, Dawson TL, Li X, Yu H. Heterodimerization and functional interaction between EGF receptor family members: a new signaling paradigm with implications for breast cancer research. Breast Cancer Res Treat. 1995;35:115–132. doi: 10.1007/BF00694752. [DOI] [PubMed] [Google Scholar]
- Ellis L, Morgan DO, Clauser E, Roth RA, Rutter WJ. A membrane-anchored cytoplasmic domain of the human insulin receptor mediates a constitutively elevated insulin-independent uptake of 2-deoxyglycose. Mol Endocrinol. 1987;1:15–24. doi: 10.1210/mend-1-1-15. [DOI] [PubMed] [Google Scholar]
- Fenteany G, Standaert RF, Lane WS, Choi S, Corey EJ, Schreiber SL. Inhibition of proteasome activities and subunit-specific amino-terminal threonine modification by lactacystin. Science. 1995;268:726–731. doi: 10.1126/science.7732382. [DOI] [PubMed] [Google Scholar]
- Galcheva-Gargova Z, Therous SJ, Davis RJ. The epidermal growth factor receptor is covalently linked to ubiquitin. Oncogene. 1995;11:2649–2655. [PubMed] [Google Scholar]
- Gassmann M, Casagranda F, Orioli D, Simon H, Lai C, Klein R, Lemke G. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature (Lond) 1995;378:390–394. doi: 10.1038/378390a0. [DOI] [PubMed] [Google Scholar]
- Goren HJ, White MF, Kahn CR. Separate domains of the insulin receptor contain sites of autophosphorylation and tyrosine kinase activity. Biochemistry. 1987;26:2374–2382. doi: 10.1021/bi00382a044. [DOI] [PubMed] [Google Scholar]
- Horstman DA, Ball R, Carpenter G. Baculovirus expression and purification of the second messenger enzyme phospholipase C-γ1, a tyrosine kinase substrate. Protein Expr Purif. 1995;6:278–283. doi: 10.1006/prep.1995.1036. [DOI] [PubMed] [Google Scholar]
- Hsuan JJ, Downward J, Clark S, Waterfield MD. Proteolytic generation of constitutive tyrosine kinase activity of the human insulin receptor. Biochem J. 1989;259:519–527. doi: 10.1042/bj2590519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffers M, Taylor GA, Weidner KM, Omura S, Vande GF, Woude Degradation of the Met tyrosine kinase receptor by the ubiquitin-proteasome pathway. Mol Cell Biol. 1997;17:799–808. doi: 10.1128/mcb.17.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanson NA, Jr, Glembotski CC, Steinhelper ME, Field LJ, Claycomb WC. Gene expression and atrial natriuretic factor processing and secretion in cultured AT-1 cardiac myocytes. Circulation. 1992;85:1835–1841. doi: 10.1161/01.cir.85.5.1835. [DOI] [PubMed] [Google Scholar]
- Lebwohl DE, Nunez I, Chan M, Rosen OM. Expression of inducible membrane-anchored insulin receptor kinase enhances deoxyglucose uptake. J Biol Chem. 1991;266:386–390. [PubMed] [Google Scholar]
- Lee H-W, Smith L, Pettit GR, Vinitsky A, Smith JB. Ubiquitination of protein kinase C-α and degradation by the proteasome. J Biol Chem. 1996;271:20973–20976. [PubMed] [Google Scholar]
- Liu D, Rutter WJ, Wang L-H. Enhancement of transforming potential of human insulinlike growth factor 1 receptor by NH2-terminal truncation and fusion to avian sarcoma virus UR2 gagsequence. J Virol. 1992;66:374–385. doi: 10.1128/jvi.66.1.374-385.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Rutter WJ, Wang L-H. Modulating effects of the extracellular sequence of the human insulinlike growth factor I receptor on its transforming and tumorigenic potential. J Virol. 1993;67:9–18. doi: 10.1128/jvi.67.1.9-18.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui H, Wells A, Lazar CS, Rosenfeld MG, Gill G. Enhanced tumorigenesis of NR6 cells which express nondownregulating epidermal growth factor receptors. Cancer Res. 1991;51:6170–6175. [PubMed] [Google Scholar]
- Mimnaugh EG, Chavany C, Neckers L. Polyubiquitination and proteasomal degradation of the p185c-erbB-2receptor protein-tyrosine kinase induced by geldanamycin. J Biol Chem. 1996;271:22796–22801. doi: 10.1074/jbc.271.37.22796. [DOI] [PubMed] [Google Scholar]
- Miyazawa K, Toyama K, Gotoh A, Hendrie PC, Mantel C, Broxmeyer HE. Ligand-dependent polyubiquitination of c-kitgene product:a possible mechanism of receptor down modulation in M07e cells. Blood. 1994;83:137–145. [PubMed] [Google Scholar]
- Moss ML, Jin S-LC, Milla ME, Burkhart W, Carter HL, Chen W-J, Clay WC, Didsbury JR, Hassler D, Hoffman CR, et al. Cloning of a disintegrin metalloproteinase that processes precursor tumor-necrosis factor-α. Nature. 1997;385:733–736. doi: 10.1038/385733a0. [DOI] [PubMed] [Google Scholar]
- Mori S, Heldin C-H, Claesson-Welsh L. Ligand-induced polyubiquitination of the platelet-derived growth factor β-receptor. J Biol Chem. 1992;267:6429–6434. [PubMed] [Google Scholar]
- Mori S, Claesson-Welsh L, Okuyama Y, Saito Y. Ligand-induced polyubiquitination of receptor tyrosine kinases. Biochem Biophys Res Comm. 1995;213:32–39. doi: 10.1006/bbrc.1995.2094. [DOI] [PubMed] [Google Scholar]
- O'Bryan JP, Fridell Y-W, Koski R, Varnum B, Liu ET. The transforming receptor tyrosine kinase, Axl, is posttranslationally regulated by proteolytic cleavage. J Biol Chem. 1995;270:551–557. doi: 10.1074/jbc.270.2.551. [DOI] [PubMed] [Google Scholar]
- Pawson T, Schlessinger J. SH2 and SH3 domains. Curr Biol. 1993;3:434–442. doi: 10.1016/0960-9822(93)90350-w. [DOI] [PubMed] [Google Scholar]
- Pinkas-Kramarski R, Soussan L, Waterman H, Levkowitz G, Alroy I, Klapper L, Lavi S, Seger R, Ratzkin BJ, Sela M, Yarden Y. Diversification of Neu differentiation factor and epidermal growth factor signaling by combinatorial receptor interactions. EMBO (Eur Mol Biol Organ) J. 1996;15:2452–2467. [PMC free article] [PubMed] [Google Scholar]
- Plowman GD, Green JM, Culouscou J-M, Carlton GW, Rothwell VM, Buckley S. Heregulin induces tyrosine phosphorylation of HER4/ p180erbB4 . Nature. 1993;366:473–475. doi: 10.1038/366473a0. [DOI] [PubMed] [Google Scholar]
- Rock KL, Gramm C, Rothstein L, Clark K, Stein R, Dick L, Hwang D, Goldberg AL. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78:761–771. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- Savage CR, Cohen S. Epidermal growth factor and a new derivative. Rapid isolation procedures and biological and chemical characterization. J Biol Chem. 1972;247:7609–7611. [PubMed] [Google Scholar]
- Shoelson SE, White MF, Kahn CR. Tryptic activation of the insulin receptor. Proteolytic truncation of the α-subunit releases the β-subunit from inhibitory control. J Biol Chem. 1988;263:4852–4860. [PubMed] [Google Scholar]
- Sliwkowski MX, Schaefer G, Akita RW, Lofgren JA, Fitzpatrick VD, Nuijens A, Fendly BM, Cerione RA, Vandlen RL, Carraway KL., III Coexpression of erbB2 and erbB3 proteins reconstitutes a high affinity receptor for heregulin. J Biol Chem. 1994;269:14661–14665. [PubMed] [Google Scholar]
- Sorkin A, Waters CM. Endocytosis of growth factor receptors. BioEssays. 1993;6:375–382. doi: 10.1002/bies.950150603. [DOI] [PubMed] [Google Scholar]
- Steinhelper ME, Lanson NA, Jr, Dresdner KP, Delcarpio JB, Wit AL, Claycomb WC, Field LJ. Proliferation in vivo and in culture of differentiated adult atrial cardiomyocytes from transgenic mice. Am J Physiol. 1990;259:H1826–H1834. doi: 10.1152/ajpheart.1990.259.6.H1826. [DOI] [PubMed] [Google Scholar]
- Tzahar E, Levkowitz G, Karunagaran D, Yi L, Peles E, Lavi S, Chang D, Liu N, Yayon A, Wen D, Yarden Y. ErbB-3 and ErbB-4 function as the respective low and high affinity receptors of all Neu differentiation factor/heregulin isoforms. J Biol Chem. 1994;269:25226–25233. [PubMed] [Google Scholar]
- Vecchi M, Baulida J, Carpenter G. Selective cleavage of the heregulin receptor ErbB-4 by protein kinase C activation. J Biol Chem. 1996;271:18989–18995. doi: 10.1074/jbc.271.31.18989. [DOI] [PubMed] [Google Scholar]
- Wang L-H, Lin B, Jong S-MJ, Dixon D, Ellis L, Roth RA, Rutter WJ. Activation of transforming potential of the human insulin receptor gene. Proc Natl Acad Sci USA. 1987;84:5725–5729. doi: 10.1073/pnas.84.16.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells A, Welsh JB, Lazar CS, Wiley HS, Gill GN, Rosenfeld MG. Ligand-induced transformation by noninternalizing epidermal growth factor receptor. Science. 1990;247:962–964. doi: 10.1126/science.2305263. [DOI] [PubMed] [Google Scholar]
- Yang T, Roden DM. Regulation of sodium current development in cultured atrial tumor myocytes (AT-1 cells) Am J Physiol. 1996;271:H541–H547. doi: 10.1152/ajpheart.1996.271.2.H541. [DOI] [PubMed] [Google Scholar]
- Yang T, Wathen MS, Felipe A, Tamkun MM, Snyders DJ, Roden DM. K+ currents and K+channel mRNA in cultured atrial cardiac myocytes (AT-1 cells) Circ Res. 1994;75:870–878. doi: 10.1161/01.res.75.5.870. [DOI] [PubMed] [Google Scholar]
- Yarden Y, Escobedo JA, Kuang W-J, Yang-Feng TL, Daniel TO, Tremble PM, Chen EY, Ando ME, Harkins RN, Francke U, et al. Structure of the receptor for platelet-derived growth factor helps define a family of closely related growth factor receptors. Nature. 1986;323:226–232. doi: 10.1038/323226a0. [DOI] [PubMed] [Google Scholar]
- Yee NS, Langen H, Besmer P. Mechanism of kit ligand, phorbol ester, and calcium-induced down regulation of c-kitreceptors in mast cells. J Biol Chem. 1993;268:14189–14201. [PubMed] [Google Scholar]
- Yee NS, Hsiau C-WM, Serve H, Vosseller K, Besmer P. Mechanism of down-regulation of c-kitreceptor. J Biol Chem. 1994;269:31991–31998. [PubMed] [Google Scholar]