Abstract
Synaptic vesicles are concentrated in the distal axon, far from the site of protein synthesis. Integral membrane proteins destined for this organelle must therefore make complex targeting decisions. Short amino acid sequences have been shown to act as targeting signals directing proteins to a variety of intracellular locations. To identify synaptic vesicle targeting sequences and to follow the path that proteins travel en route to the synaptic vesicle, we have used a defective herpes virus amplicon expression system to study the targeting of a synaptobrevin-transferrin receptor (SB-TfR) chimera in cultured hippocampal neurons. Addition of the cytoplasmic domain of synaptobrevin onto human transferrin receptor was sufficient to retarget the transferrin receptor from the dendrites to presynaptic sites in the axon. At the synapse, the SB-TfR chimera did not localize to synaptic vesicles, but was instead found in an organelle with biochemical and functional characteristics of an endosome. The chimera recycled in parallel with synaptic vesicle proteins demonstrating that the nerve terminal efficiently sorts transmembrane proteins into different pathways. The synaptobrevin sequence that controls targeting to the presynaptic endosome was not localized to a single, 10– amino acid region of the molecule, indicating that this targeting signal may be encoded by a more distributed structural conformation. However, the chimera could be shifted to synaptic vesicles by deletion of amino acids 61–70 in synaptobrevin, suggesting that separate signals encode the localization of synaptobrevin to the synapse and to the synaptic vesicle.
Since synaptic vesicles are found at presynaptic specializations in the distal axon, far from the site of protein synthesis in the cell body, neurons must have high fidelity mechanisms that regulate the trafficking of proteins to this organelle during synaptic vesicle biogenesis. Short amino acid sequences in the cytoplasmic domains of transmembrane proteins have been found to encode targeting signals to organelles in many other cell types (Trowbridge et al., 1993; Hunziker and Geuze, 1996). Synaptic vesicle proteins might be expected to make multiple sorting decisions as they travel from the TGN to the nerve terminal; however, the signals that direct these transport steps and the organelle pathways leading to the synaptic vesicle in neurons remain ill defined.
Studies on the biogenesis of synapticlike microvesicles (SLMVs)1 in the neuroendocrine cell line PC12 suggest that synaptic vesicles are derived by endocytosis (Kelly et al., 1993; Régnier-Vigouroux and Huttner, 1993). In these cells, the synaptic vesicle protein synaptophysin leaves the TGN in constitutive secretory vesicles that fuse with the plasma membrane; upon internalization, synaptophysin colocalizes with fluid phase endocytic tracers before being sorted away to SLMVs (Régnier-Vigouroux et al., 1991). The immediate donor compartment for synaptic vesicles has been proposed to be either transferrin receptor–containing early endosomes (Johnston et al., 1989; Clift-O'Grady et al., 1990; Cameron et al., 1991; Linstedt and Kelly, 1991) or a specialized invagination of the plasma membrane that lacks the transferrin receptor (Schmidt et al., 1997). Cytoplasmic sequences were identified in the synaptic vesicle protein synaptobrevin that mediate two steps in this sorting pathway (endocytosis and synaptic vesicle targeting) in PC12 cells (Grote et al., 1995; Grote and Kelly, 1996).
As polarized cells, neurons have a more complex endosomal system than PC12 cells; they have heterogeneous subcompartments distributed throughout the axon, dendrites, and cell body (Parton et al., 1992; Augenbraun et al., 1993; Overly and Hollenbeck, 1996). The transferrin receptor is found only in endosomes of the dendrites and cell body (Cameron et al., 1991; Parton et al., 1992). The individual synaptic vesicle proteins are thought to travel independently from the TGN to the synapse through several precursor compartments, with final synaptic vesicle assembly occurring only at the nerve terminal (Mundigl et al., 1993; Mundigl and De Camilli, 1994; Okada et al., 1995). Synaptic vesicle assembly has been best studied through the vesicle recycling process that occurs after every round of release (Holtzman et al., 1971). Smooth tubular–vesicular, endosomal-like membranes containing small amounts of synaptic vesicle proteins are found in the nerve terminal in close proximity to synaptic vesicles (Kadota et al., 1994); however, the role of an endosomal intermediate in the recycling process is debated (Heuser and Reese, 1973; De Camilli and Takei, 1996; Koenig and Ikeda, 1996).
In this study we examined the mechanisms and pathways of synaptic vesicle biogenesis in cultured hippocampal neurons by following the sorting of a chimera of two proteins with distinct intracellular localizations. We have identified two distinct signal-dependent sorting steps that direct the synaptic vesicle protein synaptobrevin to its final destination. When added to the NH2-terminus of the human transferrin receptor (hTfR), the cytoplasmic domain of synaptobrevin is sufficient to target the synaptobrevin-transferrin receptor (SB-TfR) chimera to the synapse. This synapse-targeting signal is not confined to a single, 10– amino acid region of synaptobrevin, indicating that it depends on either a distributed signal or the overall conformation of the molecule. At the synapse, the chimera is sorted to a presynaptic endosome, through which it recycles in parallel with synaptic vesicle proteins, revealing that the nerve terminal is capable of sorting and maintaining separate populations of recycling membrane proteins. A distinct signal is required for sorting into synaptic vesicles, as the chimera can be targeted to this organelle only by the deletion of amino acids 61–70 in synaptobrevin; deletion of these amino acids also enhances targeting of synaptobrevin to SLMVs in PC12 cells (Grote et al., 1995). Together these data support the idea that synaptic vesicle proteins make a number of sorting decisions to reach the synaptic vesicle in neurons, and reinforce the importance of local events at the nerve terminal for synaptic vesicle biogenesis.
Materials and Methods
Antibodies
The primary antibodies used in this study were as follows: mAb against human transferrin receptor (H68.4) was provided by Dr. I. Trowbridge (Salk Institute, La Jolla, CA) or was purchased from Zymed Labs Inc. (South San Francisco, CA); cell line for the mAb against human transferrin receptor (OKT9) was purchased from American Type Culture Collection (ATCC) (Rockville, MD); polyclonal antibody against MAP2 was provided by Dr. R. Vallee (Worchester Foundation for Experimental Biology, Shrewsbury, MA); mAb against rat synaptobrevin II (C1 69.1) was provided by Dr. R. Jahn (Yale University, New Haven, CT); rabbit polyclonal antibody against the hemagglutinin (HA) epitope tag was purchased from MBL International (Watertown, MA); mAb against MAP2 (AP20) was purchased from Boehringer-Mannheim Corp. (Indianapolis, IN); rabbit polyclonal antibody against synaptophysin was provided by Dr. D. Cutler (Medical Research Council, London, England); mAb against synaptophysin (SY38) was purchased from Boehringer Mannheim Corp. (Indianapolis, IN); mAb against SV2 was as described (Buckley and Kelly, 1985); a cell line producing a mAb against the myc epitope (9E10) was purchased from ATCC; rabbit polyclonal antibody against SCAMP (SG7C12) was provided by Dr. D. Castle (University of Virginia, Charlottesville, VA); mAb against the a subunit of rat kidney Na/K-ATPase (Mck1) was provided by Dr. K. Sweadner (Massachusetts General Hospital, Boston, MA); rabbit polyclonal antibody against translocon-associated protein & subunit (TRAPα) was provided by Dr. T. Rapoport (Harvard Medical School, Boston, MA); mouse IgG1k (MOPC21) was purchased from Sigma Chemical Co. (St. Louis, MO).
Hippocampal Cell Culture
Primary cultures of rat hippocampal neurons were prepared from E18 rats (Sprague-Dawley; Taconic, Germantown, NY) as described (Banker and Cowan, 1977; Goslin and Banker, 1991). Neurons were plated onto coverslips at densities of 2,600 cells/cm2 for immunofluorescence. For gradients, the neurons were plated at 53,000 cells/cm2 onto poly-l-lysine–coated 60-mm tissue culture dishes. Coverslips were cocultured over glia, and dense cultures for gradients were fed with medium that had been conditioned over glia for 24 h. Neurons were grown for 5–9 d (stage 4–5 cells [Dotti et al., 1988]) before infection, fixation, or fractionation.
DNA Constructs
A human transferrin receptor cDNA (McClelland et al., 1984) was kindly provided by Dr. M. Birnbaum (University of Pennsylvania, Philadelphia, PA). A rat synaptobrevin II cDNA (Elferink et al., 1989) was isolated by oligonucleotide hybridization from a rat brain cDNA library in λgt11 (a gift of Dr. R. Joho, University of Texas, Southwestern Medical School, Dallas, TX). All PCR was performed with the Pfu enzyme (Stratagene, La Jolla, CA). A BglII restriction site was engineered by PCR immediately upstream of the start site of the transferrin receptor cDNA. The cDNA was cloned into Bluescript SK− (Stratagene) at the BamHI and XbaI sites, using the XbaI site that lies 45 bases past the stop codon. The synaptobrevin-transferrin receptor chimera was created by the technique of extension overlap PCR (Horton et al., 1993). Amino acids 1–93 of synaptobrevin were amplified in a PCR reaction that added a BglII site to the 5′ end of synaptobrevin and a region of overlap with transferrin receptor to the 3′ region. Amino acids 1–761 of transferrin receptor were amplified adding a region of overlap with amino acids 87–93 of synaptobrevin to the NH2-terminal end, and including the XbaI cloning site on the 3′ end. These PCR products were annealed, and a second amplification was performed with only the outside primers. A region of the transferrin receptor from NdeI (at 451 bases past the transferrin receptor translation start site) to XbaI (at the 3′ end) was replaced with an insert from a transferrin receptor cDNA that had not been amplified to eliminate possible mutations from this region. All constructs were fully sequenced to ensure that no unwanted mutations had been introduced during PCR. Deletions were generated in the chimera by the technique of PCR-ligation-PCR (Ali and Steinkasserer, 1995), in which blunt ended products are generated in the first reaction flanking the region to be deleted. The products are ligated and a second PCR reaction using the outside primers creates a single product across the ligation. For HA-SB, the HA epitope (MYPYDVPDYA) was synthesized as an oligonucleotide (ATGTACCCATACGATGTTCCGGATTACGCT) with EcoRI and BamHI ends, cloned into Bluescript SK− and attached in frame at the NH2-terminus of synaptobrevin to a BamHI site engineered immediately upstream of the synaptobrevin start site. For SV2-myc, the myc epitope (EQKLISEEDL) was attached in frame to the COOH-terminus of SV2 by PCR with an oligonucleotide (GAGCAGAAGCTCATCTCAGAAGAAGACCTC). All constructs were cloned into the defective herpes vector pHSVPrPUC (Geller et al., 1993) using the SalI and XbaI sites.
Herpes Virus Amplicon Packaging
Engineered constructs were packaged as defective herpes simplex virus–1 (HSV-1) particles using an amplicon-based vector as described (Geller and Breakefield, 1988; Lim et al., 1996). Briefly, cDNAs in the vector pHSVPrPUC were transfected into 2-2 cells (Smith et al., 1992) with lipofectamine (Life Technologies Inc., Gaithersburg, MD) and superinfected 1 d later with the helper virus strain 5dl 1.2 (McCarthy et al., 1989). Virus was harvested and passaged on fresh 2-2 cells three additional times to amplify the yield and to increase the ratio of vector to helper virus. Stocks were stored in small aliquots at −70°C and thawed a maximum of three times. Helper virus was titered in a plaque assay on 2-2 cells, and the vector-containing particles were titered by expression in PC12 cells. The titers for each stock of virus used in this study are listed here in units of infectious particles × 106/ml as vector (v), helper (h), and vector to helper ratio (v:h): hTfR, 56(v), 520(h), 0.1(v:h); HA-SB, 167(v), 92(h), 1.8(v:h); SB-TfR, 130(v), 180(h), 0.7(v:h); d3-18/TfR, 27(v), 100(h), 0.3(v:h); d11-20/SB-TfR, 36(v), 68(h), 0.5(v:h); d31-40/SB-TfR, 38(v), 85(h), 0.4(v:h); d41-50/ SB-TfR, 120(v), 370(h), 0.3(v:h); d61-70/SB-TfR, 158(v), 490(h), 0.3(v:h); SV2myc, 20(v), 138(h), 0.1(v:h).
Infection of Neurons
Coverslips were removed from the glial cocultures and placed cell side up in 1 ml of N2 medium (Goslin and Banker, 1991). Virus was added to the medium at a multiplicity of infection (based on the vector titer) of 0.1–1.0. Cells were incubated for 16–24 h before fixing and staining for protein expression. Dense cultures for gradients were infected by adding 3 ml fresh N2 medium plus virus at an multiplicity of infection of 0.1–0.3 to the culture. The cells were left for 24 h before homogenization.
Immunofluorescence
Cells were fixed in 4% paraformaldehyde, 0.05% glutaraldehyde in PBS for 10 min at 37°C. The cells were then blocked and permeabilized in a solution of 16% goat serum and 0.1% Triton X-100 in PBS, pH 7.4, for 1 h at room temperature. Primary antibodies were applied in the block/permeabilization solution at 4°C overnight. After washing twice with PBS containing 0.05% Triton X-100 (to minimize shear force on the neurons), goat anti–mouse IgG conjugated to fluorescein (Pierce Chemical Co., Rockford, IL) and goat anti–rabbit IgG conjugated to Texas red (Vector Labs, Inc., Burlingame, CA), secondary antibodies in the block/permeabilization solution were bound for 60 min at room temperature. Cells were mounted in Vectashield mounting medium (Vector Labs, Inc., Burlingame, CA) to resist bleaching. Neurons were viewed at ×40 (dry) or ×63 (oil) on a Zeiss axioskop (Carl Zeiss Inc., Thornwood, NY) equipped with epifluorescence and photographed on Kodak (Rochester, NY) asa 400 black and white print film or color slide film. All photographs were taken with 30-s exposures and treated equivalently during developing. Slides or negatives were scanned into Photoshop (Adobe Systems Corp., San Jose, CA) for display. All backgrounds were adjusted equally to ensure that the images of different constructs can be legitimately compared.
Fractionation of Intracellular Organelles in Glycerol Velocity Gradients
Neurons were washed with 3 ml buffer A (150 mM NaCl, 10 mM Hepes, pH 7.4, 1 mM EGTA, 1 mM MgCl2), scraped into 1 ml of buffer A, and spun down 5 min at 5,500 g. Pellets from four 60-mm dishes (about six million cells) were combined for one gradient. Cells were resuspended in 0.45 ml ice-cold ddH2O and homogenized in a 1 ml Teflon–glass tissue homogenizer for 10 strokes at 500 rpm. After homogenization the osmolarity of the solution was adjusted with 50 μl of 10× buffer A. Nuclei and unbroken cells were spun out at 1,000 g for 5 min, and a protease inhibitor cocktail was added to the supernatant (1 μg/ml pepstatin, 1 μg/ml aprotinin, 1 mM PMSF, 1 μg/ml leupeptin). Velocity gradients were run essentially as described (Clift-O'Grady et al., 1990). The low speed supernatant was loaded on top of a 4.5 ml continuous 5–25% (in buffer A) glycerol gradient with a 0.4 ml 50% sucrose pad. The gradient was spun for 66 min at 4°C at 48,000 rpm in an SW50.1 rotor (Beckman Instruments, Fullerton, CA) in a Sorvall ultracentrifuge. 16 0.3-ml fractions were taken from the top of the gradient, and every two fractions were combined to yield a total of eight fractions. To pellet membranes from the fractions, each 0.6 ml was mixed with 2.5 ml buffer A and placed in 3 ml polycarbonate ultracentrifuge tubes (Beckman Instruments). Membranes were pelleted at 150,000 g for 2 h at 4°C in a tabletop TLA100.4 rotor. Membrane pellets were resuspended directly into SDS sample buffer and loaded onto SDS-PAGE gels.
Western Blots
Proteins were separated on 10% SDS-PAGE minigels, and then transferred overnight at 50 V to nitrocellulose in transfer buffer (20 mM Tris, 150 mM glycine, 20% methanol). Proteins were visualized with Ponceau S to determine the fidelity of transfer. Blots were blocked for 1–8 h at room temperature in a solution of 5% milk, 5% goat serum in TBST (50 mM Tris, 150 mM NaCl, 0.05% Tween 20). Primary antibodies were applied overnight at 4°C in the block solution. Goat anti–mouse IgG conjugated to HRP (Pierce Chemical Co.) was applied at a dilution of 1:5,000 in the block solution for 60 min at room temperature. Blots were reacted for 5 min with “SuperSignal” ECL reagents (Pierce Chemical Co.) diluted 1:5 in ddH2O, and exposed to Kodak X-AR film. Bands were quantitated on a densitometer (LKB-Wallac, Gaithersburg, MD) with the linear range of the film determined by comparison to synaptosomal standards.
Transferrin Uptake
Coverslips were placed face up in 12-well dishes on a 37°C slide warmer. Cells were washed twice with Hepes-buffered solution (119 mM NaCl, 2.5 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 25 mM Hepes, pH 7.4, 30 mM glucose, 0.1% albumin). To visualize recycling, Cy3-transferrin (Cy3-Tf; provided by Dr. P. Leopold, Cornell University Medical School, New York) was added to 0.4 ml of the Hepes solution at 10 μg/ml and incubated for 20 min at 37°C. Cells were washed twice in HBSS (GIBCO BRL, Gaithersburg, MD) before fixing as above. Receptor-mediated uptake was assessed by blocking binding of the labeled Tf with an excess of unlabeled Tf at 1 μg/ml.
Results
Herpes Infection Does Not Alter Polarized Targeting of Synaptobrevin or the Transferrin Receptor
To uncover sequences acting as targeting signals in synaptic vesicle proteins, we have used a defective HSV-1 expression system to express mutations and chimeras of two proteins known to have a polarized distribution in neurons. The transferrin receptor, a marker of early endosomes, is restricted to the dendrites and strictly excluded from the axon (Cameron et al., 1991; Fig. 1 a). The synaptic vesicle protein synaptobrevin is concentrated in the distal axon (Fig. 1 c), although some immunoreactivity is present in the dendrites, especially in less mature neurons before synaptogenesis (Mundigl et al., 1993). We studied targeting of these proteins in cultured embryonic rat hippocampal neurons, which undergo a characteristic development of polarity in vitro (Dotti et al., 1988). We infected neurons after 5–7 d in vitro (DIV), which corresponds to stages 4–5 of development, when the axons and dendrites of these cells show clear evidence of morphological and molecular polarity. Each neuron at this stage has elaborated several short, tapered dendrites that are characterized by their expression of the microtubule-associated protein, MAP2 (Caceres et al., 1984), and a single, long, thin axon that can be identified by the absence of MAP2 staining and the accumulation of synaptic vesicle proteins (Fletcher et al., 1991).
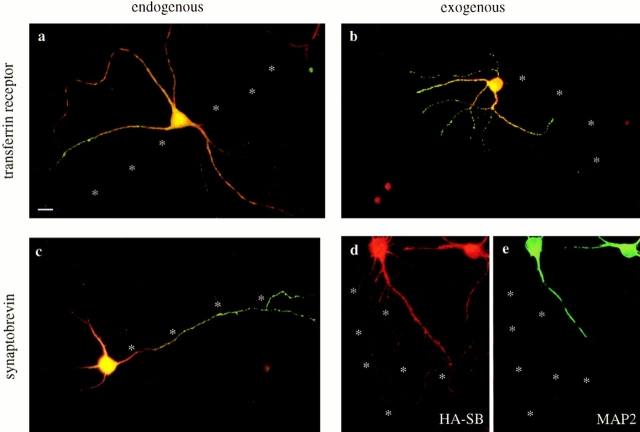
Figure 1.
The distribution of the transferrin receptor and synaptobrevin expressed from defective HSV-1 vectors matches the localization of the endogenous proteins in cultured hippocampal neurons. Neurons cultured for 5–7 d were either fixed immediately, or infected, and then fixed 20 h later. Asterisks indicate the axon. (a) The endogenous transferrin receptor (green) was colocalized with the dendritic marker MAP2 (red) and was strictly excluded from the axon. (b) Human transferrin receptor (green) expressed from a defective HSV-1 vector was also restricted to the dendrites (stained for MAP2 in red). (c) Endogenous synaptobrevin (green) in a control cell infected with the empty HSV-1 vector was concentrated in the distal axon although a small amount of protein colocalized with MAP2 (red) in the dendrites. (d and e) Synaptobrevin with an NH2-terminal HA epitope tag (red) expressed from a defective HSV-1 vector was transported well out into the distal axon (dendrites in e were stained for MAP2 in green). Bar, 20 μm.
To show that overexpression does not affect protein targeting, we compared the immunofluorescence patterns of these endogenous proteins to a recombinant human transferrin receptor and an HA epitope–tagged synaptobrevin expressed from viral vectors. As shown in Fig. 1 b, in cells infected with a vector encoding human transferrin receptor and stained 20 h later, the immunoreactivity for the expressed transferrin receptor matched exactly the distribution of the endogenous protein: both were found only in MAP2-positive dendrites and cell bodies, demonstrating that overexpression of this protein did not cause entry into the axon. The human transferrin receptor was detected with the OKT9 mAb, which binds the extracellular domain (Sutherland et al., 1981) and shows no cross-reactivity with rat transferrin receptor (data not shown). Epitope-tagged synaptobrevin, which was detected with a rabbit polyclonal anti-HA antiserum, was present in long, thin MAP2-negative axons (Fig. 1, d and e), where the endogenous synaptobrevin is known to be concentrated (Fig. 1 c). The localization of HA-tagged synaptobrevin in the distal axon indicated that a 20-h incubation was sufficient to target newly synthesized proteins to distant synaptic regions.
We saw little toxicity from the herpes virus within the time frame studied (up to 24 h after infection). To demonstrate that virus infection alone did not influence protein targeting or neuronal morphology, we infected cells with virus particles containing only the empty cloning vector and stained for markers of polarity. As shown in Fig. 1 c, in a cell infected with the empty vector, MAP2 was found in several short, tapered dendrites, and synaptobrevin was concentrated in a long, thin axon, indicating that herpes virus infection alone does not alter either the morphological or molecular polarity of these neurons.
The Cytoplasmic Domain of Synaptobrevin Is Sufficient to Target the Transferrin Receptor to Presynaptic Sites
To identify potential targeting sequences in synaptobrevin, we constructed a chimera consisting of the cytoplasmic domain of synaptobrevin (amino acids 1–93) added onto the NH2-terminus of the complete human transferrin receptor (Fig. 2 a, SB-TfR). Both molecules are topologically similar, with short (61 or 93 amino acids) cytoplasmic NH2-terminal domains and a single transmembrane domain (McClelland et al., 1984; Elferink et al., 1989). However, rat synaptobrevin has only two amino acids predicted to be on the lumenal side of the membrane, whereas the transferrin receptor has an extracellular domain of several hundred amino acids. Deletion of this extracellular domain did not affect the localization of the transferrin receptor, nor did addition of the transferrin receptor extracellular domain to synaptobrevin influence targeting (data not shown); thus relevant sorting signals must be found in either the transmembrane or cytoplasmic domains of these proteins.
Figure 2.
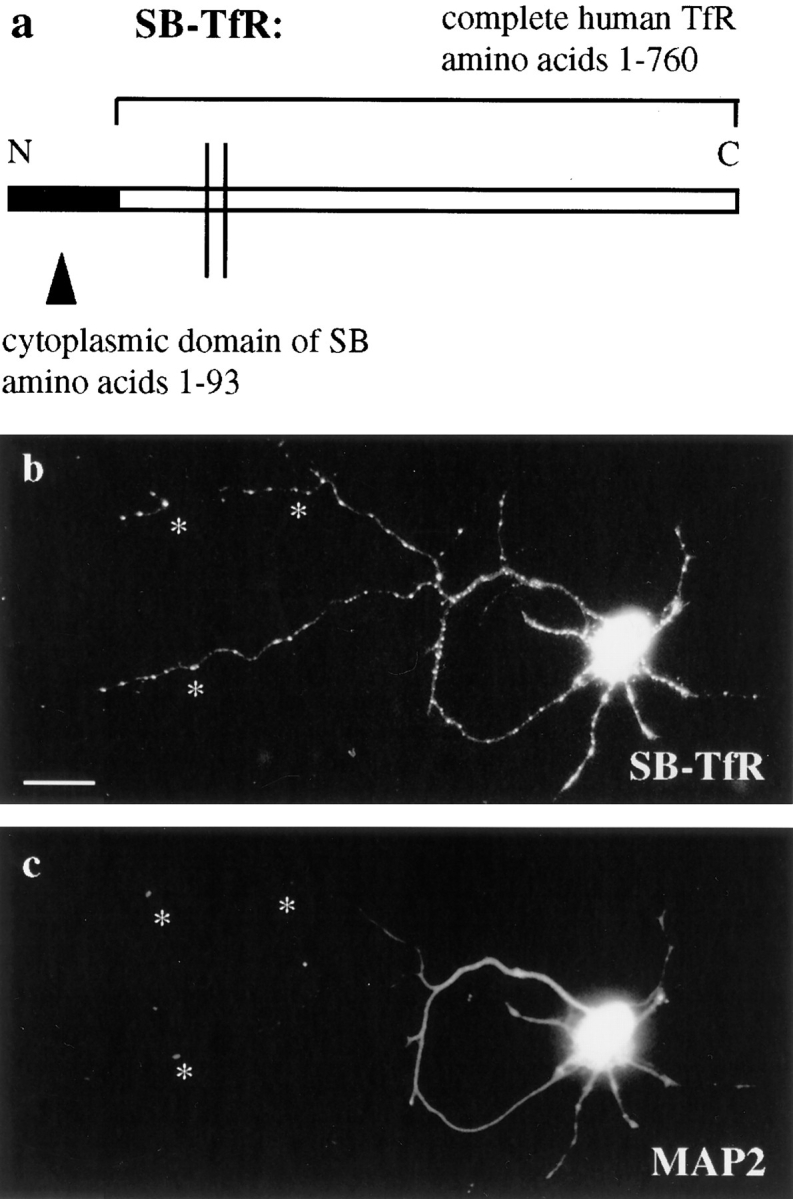
Expression of a SB-TfR chimera in the axon. A cDNA was constructed by PCR in which the cytoplasmic domain of rat synaptobrevin II (amino acids 1–93) was added onto the NH2-terminus of the complete human transferrin receptor (amino acids 1–760). Chimeric cDNAs were cloned into a defective HSV-1 vector, packaged, and then infected into neurons on day 5 in vitro. The cells were fixed 20 h later and the distribution of the chimera was compared to the dendritic marker MAP2. (a) Diagram of the SB-TfR chimera. (b) SB-TfR was evident as bright puncta well into the axon of the infected neuron (asterisks) as well as in the dendrites. (c) MAP2. Bar, 20 μm.
In striking contrast to the axonal exclusion of the transferrin receptor, the SB-TfR chimera expressed in 5 DIV neurons was found well out into the MAP2-negative axon (Fig. 2 b). Staining was concentrated in bright puncta, resembling the staining pattern of synaptic vesicle proteins (compare, for example, to the axonal staining for synaptobrevin in Fig. 1 c). More dendritic staining was seen for the chimera than for synaptic vesicle proteins at this stage of development (again compare to Fig. 1 c). The presence of the chimera in dendrites was not due to targeting sequences contributed by the transferrin receptor in the chimera, since SV2 (a synaptic vesicle protein) expressed from an HSV-1 vector also showed significant expression in the dendrites (data not shown). The elevated levels of dendritic staining may indicate that newly synthesized synaptic vesicle proteins follow the bulk flow of membrane throughout the cell before recognition of synapse and synaptic vesicle targeting signals locally at the nerve terminal (see Discussion).
The bright axonal puncta of SB-TfR were found at sites of synaptic vesicle clusters, as indicated by the colocalization of SB-TfR with the synaptic vesicle marker synaptophysin. Clusters of synaptic vesicles (as defined by their electron microscopic morphology, the presence of synaptic vesicle proteins, and calcium-dependent exocytosis) can be found in the axons of isolated neurons as early as 3 DIV, although these organelle clusters remain mobile in the axon until the time of synapse formation, which begins to occur just after 3 DIV in neurons that make intercellular contacts (Fletcher et al., 1994; Kraszewski et al., 1995). In cultures expressing the SB-TfR chimera, numerous bright spots of reactivity for synaptophysin were seen along the axon (Fig. 3 a, asterisks), indicating the location of synaptic vesicle clusters. SB-TfR reactivity colocalized precisely with many of these puncta (Fig. 3 b, asterisks correspond to those in Fig. 3 a), indicating that some significant fraction of the chimera was targeted to axonal regions where synaptic vesicles accumulate. Note that only a subset of the neurons in this field were infected with the chimera as shown by synaptophysin-positive, SB-TfR–negative processes.
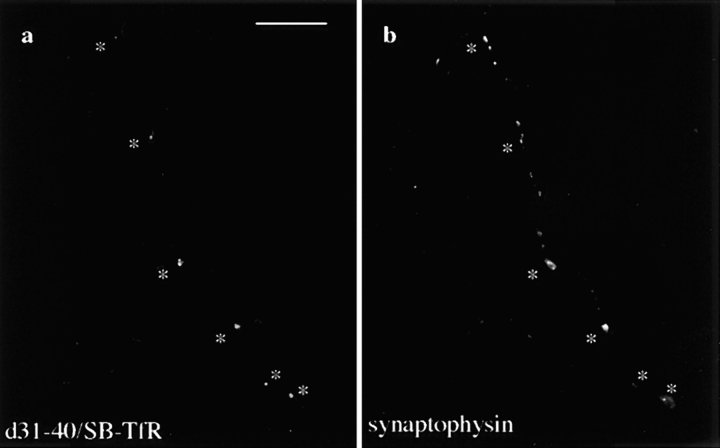
Figure 3.
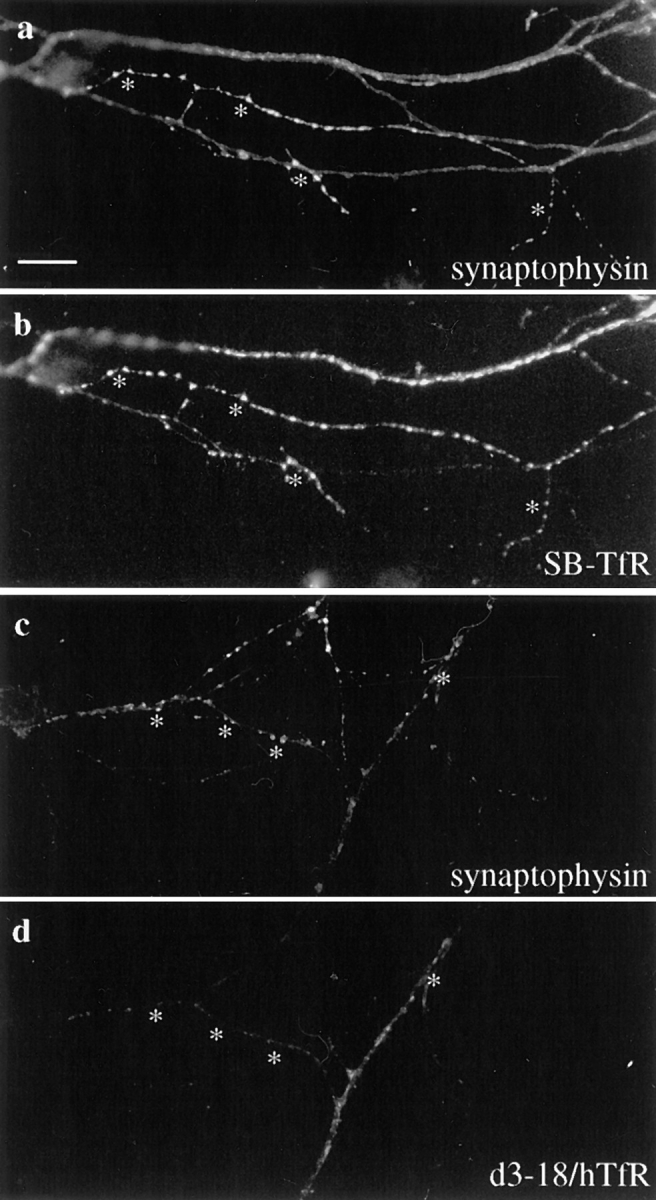
SB-TfR protein in the axon colocalized with the synaptic vesicle marker synaptophysin at synaptic vesicle clusters. Neurons infected with SB-TfR or d3–18/hTfR-defective HSV-1 vectors on day 5–7 were incubated for 20 h and then double-stained with antibodies against hTfR and synaptophysin. (a) Bright spots of synaptophysin staining indicate the location of synaptic vesicle clusters (asterisks). (b) The SB-TfR chimera was colocalized precisely with synaptophysin at many of the puncta. (c) Synaptophysin marks synaptic vesicle clusters in a cell infected with the d3–18/hTfR vector. (d) A mutant transferrin receptor (d3–18/ hTfR) that is not restricted to dendrites was not colocalized precisely with synaptophysin. Bar, 10 μm.
The cytoplasmic domain of the transferrin receptor contains a dendritic targeting signal; deletion of amino acids 3–18 (d3–18/hTfR) results in the appearance of the human transferrin receptor in the axon of rat hippocampal neurons (West et al., 1997). To demonstrate that the localization of the SB-TfR chimera to presynaptic sites in the axon was due to synaptobrevin sequences rather than inactivation of the dendritic targeting signal, we compared the immunofluorescence patterns of d3–18/hTfR and the SB-TfR chimera in the axon (Fig. 3, c and d). In contrast to the punctate, intracellular staining extending to the distal axon seen for SB-TfR, the staining for d3–18/hTfR diminished with distance from the cell body, labeled the plasma membrane, and was relatively uniform rather than brightly punctate (West et al., 1997). At high power, the d3–18/hTfR staining overlapped with, but did not appear similar to synaptophysin (Fig. 3 c). Thus although deletion of a dendritic targeting signal permitted axonal entry, sequences from the cytoplasmic domain of synaptobrevin were necessary for precise colocalization of the transferrin receptor with synaptic vesicle proteins. Synaptobrevin sequences therefore mediated a targeting step within the axon that localized the SB-TfR chimera to a specific axonal domain.
We were unable to disrupt presynaptic localization of the SB-TfR chimera by making consecutive deletions of 10 amino acids each through synaptobrevin, suggesting that the synapse-targeting signal is not a short linear sequence, but is instead encoded in a distributed signal or dependent on the global structure of the molecule. Proteins expressed from a series of nine SB-TfR constructs, each deleted for 10 amino acids in synaptobrevin (d2–10, d11–20, d31–40, etc. to d81–90) had nearly indistinguishable immunofluorescence patterns. Just like the full-length chimera, each deletion mutant was expressed as bright puncta well out into the distal axon in a pattern reminiscent of synaptic vesicle protein staining (Fig. 4 shows three of the constructs). Fig. 4 a shows a particularly striking example in which the d11–20/SB-TfR puncta delineate the axon of the infected cell as it climbs along the dendrites and cell body of a neighboring uninfected cell. Interestingly, deletion of amino acids 31–40 in synaptobrevin, which decreases targeting to SLMVs in PC12 cells without reducing endocytosis (Grote et al., 1995; Grote and Kelly, 1996), did not affect the presynaptic localization of the SB-TfR chimera. Fig. 5 shows that d31–40/SB-TfR colocalized precisely with synaptophysin at sites of synaptic vesicle clusters. These data indicate that the signal mediating targeting to the synapse in these neurons is distinct from the SLMV targeting signal in synaptobrevin identified in PC12 cells (Grote et al., 1995).
Figure 4.

Expression patterns for three deletion mutants of the SB-TfR chimera in the axons of infected cells. A series of nine SB-TfR constructs were built in the HSV-1 vector by PCR, each with a single, 10–amino acid deletion in synaptobrevin (d2–10, d11–20, etc. to d81–90). 5–7 DIV neurons were infected and stained 20 h later for the mutant chimeras. The nine constructs showed nearly indistinguishable distributions of bright puncta extending to the distal axon, and three examples are shown here. Asterisks mark the axon. (a) d11–20/SB-TfR. A particularly synaptic-looking pattern is seen where the axon of the infected cell wraps around the cell body of an adjacent uninfected cell (asterisks). (b) d31–40/SB-TfR. (c) d61–70/SB-TfR. Bar, 20 μm.
Figure 5.
Colocalization of d31–40/SB-TfR with synaptophysin. Neurons were infected with the d31–40/SB-TfR vector, fixed 20 h later, and double stained for the chimera and synaptophysin. (a) d31–40/SB-TfR staining in bright intracellular puncta (asterisks). (b) Bright puncta of synaptophysin mark synaptic vesicle clusters. Asterisks indicate the puncta that colocalize with d31–40/SB-TfR. Bar, 10 μm.
The SB-TfR Chimera Is Sorted to a Presynaptic Endosome
Although the SB-TfR chimera was concentrated at synapses, upon glycerol gradient fractionation of intracellular organelles we discovered that the chimera was not sorted into synaptic vesicles. Fig. 6 shows the results of glycerol gradient fractionation of hippocampal cultures. To promote the development of synapses and increase the yield of synaptic vesicles, neurons were plated at high density (53,000 cell/cm2) and cultured for 7 d before infection. 24 h after infection, the cells were homogenized and organelles were separated in a continuous 5–25% glycerol velocity gradient, a fractionation technique that has been used to isolate synaptic vesicles from brain and SLMVs from endocrine cells (Clift-O'Grady et al., 1990). 6 × 106 neurons were used per gradient (the yield of hippocampal neurons from about one full litter of rats).
Figure 6.
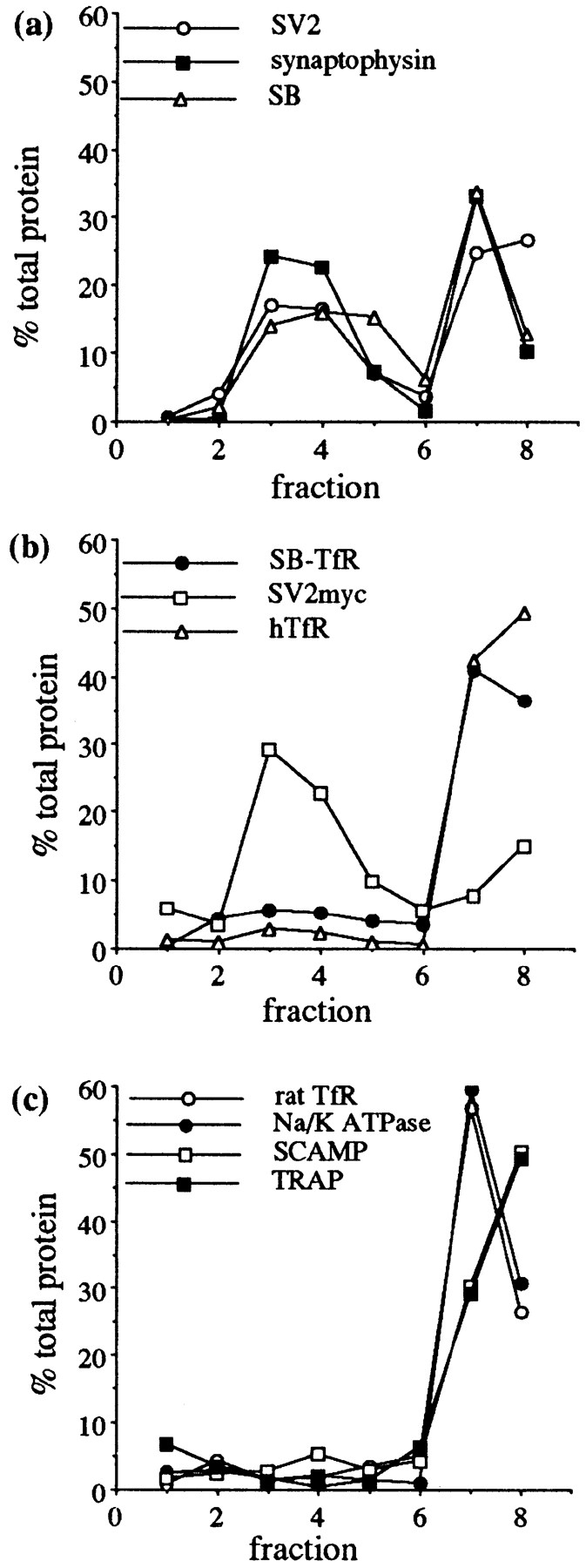

Distribution of synaptic vesicle proteins, virally expressed constructs, and markers of intracellular compartments from homogenized, cultured hippocampal neurons fractionated on a glycerol velocity gradient. Neurons were grown at high density (53,000/ cm2) for 7 d and then some were infected with virus at a multiplicity of infection of 0.35. 24 h later cells were collected, osmotically lysed, homogenized, and the organelles separated by velocity centrifugation in a continuous 5–25% glycerol gradient. Control cells were homogenized after 8 DIV. (a) Endogenous synaptic vesicle proteins showed a bimodal distribution, with a synaptic vesicle peak in lanes 3–5 and a second peak coincident with larger organelles in the bottom fractions. SV2 (○), synaptophysin (▪), and synaptobrevin (▵). (b) Virally expressed constructs. The SB-TfR chimera was found mainly in the bottom fractions (•). A myc-tagged SV2 construct matched the synaptic vesicle protein pattern (□). hTfR was present primarily in the bottom fractions (▵). (c) Markers of other intracellular compartments peaked in the bottom fractions of the gradient: the endogenous rat TfR (endosomes; ○), the Na/ K-ATPase (plasma membrane; •), SCAMPs (the recycling system; □), TRAPα (rough ER; ▪). (d) The distribution of the d61–70/SB-TfR chimera was identical to that of synaptic vesicle proteins. The results of two separate experiments are shown (d61–70/SB-TfR; ○ and d61–70/SB-TfR #2; ▵) along with synaptic vesicle protein distributions from the same gradients (synaptophysin; • and synaptobrevin; ▪). (e) Two other deletion mutants of the SB-TfR chimera were not targeted to synaptic vesicles: d11–20/SB-TfR (○) and d31–40/SB-TfR (▪).
Fig. 6 a shows the distribution of synaptic vesicle proteins in this gradient. For each of three integral membrane proteins of synaptic vesicles (SV2, synaptophysin, and synaptobrevin), a peak of immunoreactivity was present in the middle of the gradient (Fig. 6 a, lanes 3–5), corresponding to the small, light, homogeneous synaptic vesicles, which travel only a short distance into the gradient during centrifugation; a second large peak of immunoreactivity was present at the bottom of the gradient, corresponding to protein in endosomes, plasma membrane, or other large heterogeneous membranes such as Golgi or ER (Clift-O'Grady et al., 1990; Schmidt et al., 1997). Fig. 6 c demonstrates the distribution of a number of markers of these larger compartments on the gradients. The endogenous transferrin receptor, a marker of early endosomes (Hopkins and Trowbridge, 1983), the Na/K-ATPase, a plasma membrane protein (Hammerton et al., 1991), SCAMP (a general marker of the recycling system) (Brand et al., 1991; Brand and Castle, 1993), and TRAPα, a rough ER protein (Prehn et al., 1990), were all concentrated in the bottom fractions of the gradient. Fig. 6 b shows the distribution of three constructs expressed from defective HSV-1 vectors. The pattern of fractionation of the SB-TfR chimera matched that of proteins sorted to larger organelles rather than that of synaptic vesicle proteins (Fig. 6 b, •). The major peak of reactivity for SB-TfR was in the bottom two fractions, and although some protein was detectable in the lighter fractions, there was no peak of signal intensity in Fig. 6 b, lanes 3–5 as would be expected for synaptic vesicle proteins, which are greatly enriched in the middle fractions. Expression of proteins by viral infection did not alter protein targeting. A myc epitope–tagged SV2 protein expressed from a viral vector showed a peak of reactivity in fractions 3–5, indicating that it was targeted to synaptic vesicles, similar to its endogenous counterpart (Fig. 6 b, □), and the distribution of the expressed human transferrin receptor also matched that of the endogenous protein, concentrated in the bottom two fractions (Fig. 6 b, ▵).
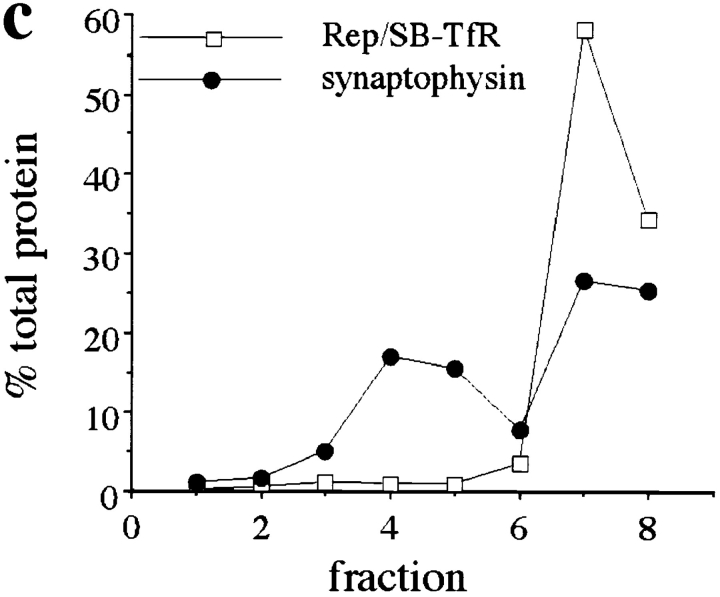
To ensure that the SB-TfR chimera was not excluded from synaptic vesicles because of competing targeting information in the cytoplasmic domain of TfR, we studied the sorting of another SB-TfR chimera in which the cytoplasmic domain of synaptobrevin was substituted for the cytoplasmic domain of the human transferrin receptor (Fig. 7 a). The targeting of the cytoplasmic replacement chimera was indistinguishable from that of SB-TfR both by immunofluorescence and by gradient fractionation (Fig. 7, b and c). Thus the cytoplasmic signals that localize SB-TfR in the axon must be contained within the synaptobrevin sequence.
Figure 7.
Like SB-TfR, a second chimera in which the cytoplasmic domain of the transferrin receptor was replaced with the cytoplasmic domain of synaptobrevin was concentrated in axonal puncta but did not reach synaptic vesicles. (a) Diagram of the Rep/SB-TfR chimera. A cDNA was constructed by PCR in which the cytoplasmic domain of synaptobrevin (amino acids 1–93) was attached to the transmembrane and extracellular domains of hTfR (amino acids 61–760). (b) Immunofluorescence of Rep/SB-TfR expressed from a defective herpes virus vector. Rep/ SB-TfR reactivity was found in bright puncta well out into the distal axon (asterisks). (c) Distribution of Rep/SB-TfR after fractionation of homogenates of infected neurons in glycerol gradients. Rep/SB-TfR reactivity (□) was concentrated only in the bottom two fractions of the gradient and did not colocalize with the peak of synaptophysin representing synaptic vesicles (•) in fractions 3–5. Bar, 20 μm.
By immunofluorescence the SB-TfR chimera was targeted to presynaptic sites, but by biochemical criteria the chimera was present in an organelle larger than synaptic vesicles. Since the SB-TfR chimera was sorted to a compartment that cofractionated with endosomes in the glycerol gradient, we asked if this organelle had other properties of a putative presynaptic sorting/recycling endosome. The axonal organelles containing the SB-TfR chimera were labeled by constitutive endocytotic uptake of Cy3-labeled human transferrin (Cy3-hTf) from the medium surrounding the cells, indicating that the organelle is derived by endocytosis, fulfilling one functional criterion of an endosome. Cy3-hTf was added for 20 min to the medium surrounding low density neurons expressing the SB-TfR chimera. Labeling in cells expressing the chimera was present well out into long, thin axonal processes, in puncta indistinguishable from those labeled by immunofluorescence for the chimera (Fig. 8 a, compare to Fig. 2 b). Under these conditions, uptake of the Cy3-hTf by uninfected cells was minimal, and labeling of axons of uninfected cells was undetectable (Fig. 8 b). Uptake of Cy3-hTf into both infected and uninfected cells was blocked by addition of a 100-fold excess of unlabeled hTf, indicating that cell labeling occurred by receptor-mediated endocytosis (data not shown). These data indicate that the SB-TfR chimera was present in an axonal organelle derived by endocytosis. Since the chimera was actively recycling in the axon of unstimulated neurons, these data are consistent with evidence from the glycerol gradients that the chimera was in an axonal organelle other than synaptic vesicles; depolarization is required to stimulate the exo-endocytic recycling of synaptic vesicles and to facilitate the labeling of synaptic vesicle proteins by extracellular markers (Kraszewski et al., 1995).
Figure 8.
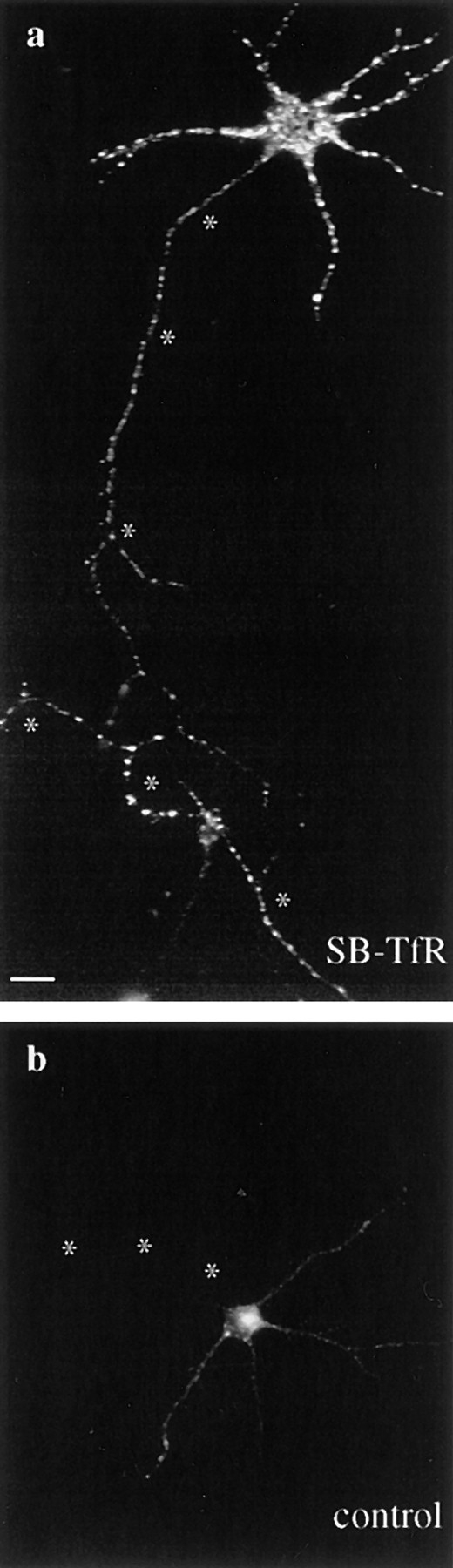
Endocytosis of Cy3-hTf by SB-TfR in axons. Cy3-hTf was added at 37°C to low density cultures of neurons that either had been infected with the SB-TfR vector or were uninfected. After 20 min of labeling, the cells were washed twice, fixed, and then mounted in glycerol for fluorescence microscopy. Asterisks mark the axons. (a) Endocytosed Cy3-hTf labeling of an SB-TfR– infected neuron. Bright puncta were present well out into the long, thin axon. (b) An uninfected neuron showed much less total uptake with no labeling of the axon. Bar, 20 μm.
Deletion of Amino Acids 61–70 in Synaptobrevin Enhances Synaptic Vesicle Targeting of the SB-TfR Chimera
Previous work in PC12 cells has shown that deletion of amino acids 61–70 in synaptobrevin increases the amount of protein targeted into SLMVs from the plasma membrane 50-fold over wild type (Grote et al., 1995). This sequence has been proposed to inhibit the interaction of synaptobrevin with a synaptic vesicle sorting protein (Grote et al., 1995). To determine if the same sequence regulated sorting of the SB-TfR chimera to synaptic vesicles in hippocampal neurons, we expressed the d61–70/SB-TfR chimera in dense cultures of neurons, and fractionated intracellular organelles on a glycerol gradient. In contrast to the SB-TfR chimera, d61–70/SB-TfR did show a peak of immunoreactivity in Fig. 6 d, lanes 3–5, cofractionating with the synaptic vesicle markers synaptophysin and synaptobrevin. Quantitation of the bands by densitometry demonstrated that d61–70/SB-TfR was targeted to the synaptic vesicle fractions with similar efficiency to synaptophysin and synaptobrevin. 35.3 and 16% of total d61–70/ SB-TfR was recovered in fractions 3–5 in two separate trials as compared to 30% of total synaptophysin and 35.1% of total synaptobrevin. This distribution differed from that of the SB-TfR chimera (Fig. 6 b, •) and other deletion chimeras, for example d11–20/SB-TfR and d31–40/SB-TfR (Fig. 6 e). Although immunoreactivity for these proteins was found in some of the upper and middle fractions of the gradient, they did not peak in Fig. 6 e, lanes 3–5 with the synaptic vesicle proteins, indicating that d11–20/SB-TfR and d31–40/SB-TfR were not targeted to synaptic vesicles.
Discussion
We have shown that multiple sorting steps are required to target proteins to the synaptic vesicle, and we have found evidence for cytoplasmic sequences in synaptobrevin that mediate at least two of these steps: to the synapse and to the synaptic vesicle. Targeting to the synapse is a distinct step from entry into the axon. At the synapse we have labeled a presynaptic endosomal compartment through which proteins recycle in parallel with synaptic vesicle proteins. Finally, we have presented data supporting the hypothesis that the synaptic vesicle targeting signal acts at the synapse rather than earlier in the pathway, pinpointing the nerve terminal as the most likely site to find the proteins that assemble synaptic vesicles.
Targeting to the Synapse
The cytoplasmic domain of synaptobrevin contains a signal for localization to the synapse. Addition of synaptobrevin amino acids 1–93 onto the NH2-terminus of the transferrin receptor, which is normally excluded from the axon, was sufficient to direct this chimera to presynaptic sites where it colocalized with the synaptic vesicle protein synaptophysin. Localization of the chimera to the synapse was not due to inactivation of the dendritic targeting signal in the transferrin receptor, because a mutated transferrin receptor lacking the dendritic targeting motif but without added synaptobrevin sequences entered the axon but did not accumulate at synapses (West et al., 1997). This observation suggests that synapse targeting is a separate event from axonal entry. The synapse-targeting signal was not restricted to a single, 10–amino acid region of synaptobrevin, which indicates that the signal is either (a) redundant, (b) not colinear, or (c) dependent on the global conformation of the molecule. Regardless of which interpretation is correct, it is clear that targeting to the synapse is distinct from sorting to the synaptic vesicle since amino acids 61–70 in synaptobrevin influence protein targeting to synaptic vesicles without affecting synaptic localization. Thus synaptobrevin makes at least three sorting decisions upon exit from the TGN: axonal entry, synapse localization, and synaptic vesicle targeting.
Protein targeting in neurons is frequently compared to that of another polarized cell type, the MDCK epithelial cell line. It has been proposed that the apical/axonal and basolateral/dendritic plasma membranes are similar and may share sorting mechanisms (Dotti and Simons, 1990). Apical targeting motifs have been identified in the extracellular or transmembrane domains of proteins (Simons and Wandinger-Ness, 1990; Fiedler and Simons, 1995); lipid and carbohydrate modifications of proteins also act as apical targeting signals (Lisanti and Rodriguez-Boulan, 1990; Fiedler and Simons, 1995). These sequences are proposed to act by sorting these proteins to distinct lipid domains in the TGN and plasma membrane (Simons and Wandinger-Ness, 1990). In cultured neurons, the polyimmunoglobulin receptor and the β-amyloid precursor protein are targeted to the axon by similar transmembrane, extracellular, and carbohydrate signals (de Hoop et al., 1995; Tienari et al., 1996).
In contrast to this model, our results show that the targeting sequences that guide the localization of synaptobrevin within the axon are found in the cytoplasmic domain of the molecule. Short cytoplasmic sequences have been identified as targeting signals for transmembrane proteins trafficking to a variety of intracellular destinations (Trowbridge et al., 1993), and are thought in many cases to function by interacting with coated vesicle-associated proteins (Heilker et al., 1996; Marks et al., 1997). Consistent with the idea that synapse-targeting signals differ from the apical/ axonal signals above, the synaptic vesicle protein synaptophysin expressed in MDCK cells recycles equally from both polar surfaces, indicating that it does not contain a recognizable apical targeting signal despite the fact that it is efficiently sorted to synapses in the axon of neurons (Cameron et al., 1993).
That different types of signals are used to sort axonal proteins suggests that the mechanism used to generate a restricted distribution of any particular transmembrane protein may depend on the nature of the compartment to which that protein is targeted at steady state. The endomembrane system is a dynamic network of intracellular membranes that flow throughout the cell (Hopkins et al., 1990). Transmembrane proteins internalized into endosomes will follow the path of bulk membrane flow through the cell unless recognition of a sorting signal causes them to be retained in a particular branch of the membrane network (Mayor et al., 1993; French and Lauffenburger, 1996). Steady-state maintenance of proper protein sorting from the endosomal system therefore requires the repeated recognition of sorting signals and retrieval to the proper destination upon each round of recycling. At the nerve terminal, the bulk of internalized membrane flows retrogradely toward the cell body (Gonatas et al., 1977). The synaptobrevin synapse-targeting signal may therefore act locally at the nerve terminal to actively encode axonal and synaptic enrichment of the chimera by repeatedly retrieving the protein to either the presynaptic endosome or the synaptic plasma membrane, trapping it in a local recycling circuit.
Presynaptic Endosomes
At the nerve terminal, the SB-TfR chimera was sorted into an organelle with biochemical and functional characteristics of an endosome. The chimera cycled constitutively on and off the plasma membrane at the synapse, but it was not detectable in synaptic vesicles, demonstrating that the nerve terminal can sort recycling membrane proteins in the endocytic pathway to different intracellular destinations. Protein sorting is one of the primary functions of endosomes; the majority of the membrane and the membrane proteins endocytosed are not degraded, but either recycle back to the plasma membrane or are targeted to other intracellular membranes from sorting/recycling endosomes, which shunt recycling membrane proteins away from those bound for the lysosomal pathway (Trowbridge et al., 1993; Gruenberg and Maxfield, 1995; Sofer et al., 1996). The nerve terminal is the site of the majority of endocytosis in axons of mature neurons (Parton et al., 1992), and proteins internalized here are eventually targeted to a wide variety of intracellular locations. For example, the β-amyloid precursor protein and tetanus toxin both follow transcytotic pathways from the axon to the dendrites (Schwab et al., 1979; Yamazaki et al., 1995), whereas wheat germ agglutinin taken up in the axon accumulates in a Golgi or para-Golgi compartment in the cell body (Gonatas et al., 1977; Matteoli et al., 1992). Amyloid precursor protein colocalizes with synaptic vesicle proteins in clathrin-coated vesicles purified from rat brain synaptosomes, but it is not present in purified synaptic vesicles (Ikin et al., 1996; Marquez-Sterling et al., 1997). Further characterization of presynaptic endosomes like the one labeled by SB-TfR may help to reveal the molecular components responsible for these regional sorting events.
Synaptic Vesicle Assembly at the Nerve Terminal
Although the SB-TfR chimera was present at synapses, it was not targeted to synaptic vesicles. The deletion of amino acids 61–70 in synaptobrevin allowed the chimera to enter synaptic vesicles with an efficiency similar to that of endogenous synaptophysin and synaptobrevin, indicating that this sequence negatively regulates entry to synaptic vesicles. In PC12 cells, this mutation enhances targeting of synaptobrevin to SLMVs from the plasma membrane 50-fold (Grote et al., 1995). Synaptobrevin is phosphorylated at serine 61 by calcium/calmodulin protein kinase II, which could provide a mechanism to physiologically modulate this regulatory signal and influence targeting (Hirling and Scheller, 1996).
In PC12 cells, amino acids 31–38 and 41–50 in synaptobrevin are proposed to contain SLMV targeting signals (Grote et al., 1995); however, in neurons, the SB-TfR chimera did not reach synaptic vesicles at detectable levels despite the presence of these sequences. This result may reflect saturation of the synaptic vesicle protein–targeting machinery by endogenous synaptobrevin. Alternatively, competing signals in the transferrin receptor domain could restrict entry of the chimera into synaptic vesicles unless the synaptic vesicle targeting signal was strengthened by the deletion of the negative regulatory domain from amino acids 61–70.
If the synapse-targeting signal functions to trap synaptobrevin in a local recycling circuit as described above, our data suggest that the synaptic vesicle targeting signal acts locally to remove synaptobrevin from this circuit into newly assembled synaptic vesicles. This two–step system would enable the presynaptic endosomal compartment to serve as a reservoir for different synaptic vesicle proteins arriving independently at the nerve terminal until they were present in the correct ratios for synaptic vesicle assembly to proceed. We observed that the immunofluorescence patterns for all of the deletion chimeras of SB-TfR were indistinguishable, regardless of whether the proteins reached synaptic vesicles. Notably, amino acids 31–38 are necessary for SLMV targeting in PC12 cells (Grote et al., 1995), but their deletion did not affect synapse targeting in neurons as indicated by the precise colocalization of d31– 40/SB-TfR with synaptophysin in the axon. This result supports the hypothesis that targeting to the synapse and to the synaptic vesicle are separate events mediated by distinct signals in synaptobrevin. In addition, it pinpoints the synapse as the site where the machinery that drives synaptic vesicle assembly is likely be found. Identification of proteins that interact with the synaptic vesicle targeting signals in synaptobrevin may help to resolve the debate over exactly where and how at the nerve terminal synaptic vesicle proteins are assembled into functional synaptic vesicles.
Acknowledgments
We thank C. Sadow and J. Cheung for technical assistance. We also thank R. Kelly and E. Grote for sharing their data on synaptobrevin in PC12 cells, M. Birnbaum for the initial idea, P. Leopold, P. Hollenbeck, and K. Overly for invaluable advice, and J.B. Miller, C. Provoda, and P. Purcell for comments on the manuscript.
This work was supported by National Institutes of Health Grant NS27536 (to K.M. Buckley), the Stuart H. and Victoria Quan Fellowship in Neurobiology and National Research Scientist Award 2 T32 NS07009-21 (to A.E. West).
Abbreviations used in this paper
- Cy3-hTf
Cy3-labeled human transferrin
- DIV
days in vitro
- HA
hemagglutinin
- HSV-1
herpes simplex virus–1
- hTfR
human transferrin receptor
- SB
synaptobrevin
- SB-TfR
synaptobrevin–transferrin receptor
- SLMV
synapticlike microvesicles
- TRAPα
translocon-associated protein subunit–α
Footnotes
Address all correspondence to K.M. Buckley, Department of Neurobiology, Harvard Medical School, 220 Longwood Avenue, Boston, MA 02115. Tel.: (617) 432-2288. Fax: (617) 734-7557. E-mail: kbuckley@warren.med.harvard.edu
References
- Ali SA, Steinkasserer A. PCR-Ligation-PCR mutagenesis: a protocol for creating gene fusions and mutations. Biotechniques. 1995;18:746–749. [PubMed] [Google Scholar]
- Augenbraun E, Maxfield FR, St. Jules R, Setlik W, Holtzman E. Properties of acidified compartments in hippocampal neurons. Eur J Cell Biol. 1993;61:34–43. [PubMed] [Google Scholar]
- Banker GA, Cowan WM. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977;126:397–425. doi: 10.1016/0006-8993(77)90594-7. [DOI] [PubMed] [Google Scholar]
- Brand SH, Castle JD. SCAMP 37, a new marker within the general cell surface recycling system. EMBO (Eur Mol Biol Organ) J. 1993;12:3753–3761. doi: 10.1002/j.1460-2075.1993.tb06053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand SH, Laurie SM, Mixon MB, Castle JD. Secretory carrier membrane proteins 31–35 define a common protein composition among secretory carrier membranes. J Biol Chem. 1991;266:18949–18957. [PubMed] [Google Scholar]
- Buckley KM, Kelly RB. Identification of a transmembrane glycoprotein specific for secretory vesicles of neural and endocrine cells. J Cell Biol. 1985;100:1284–1294. doi: 10.1083/jcb.100.4.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceres A, Banker G, Steward O, Binder L, Payne M. MAP2 is localized to the dendrites of hippocampal neurons which develop in culture. Dev Brain Res. 1984;13:314–318. doi: 10.1016/0165-3806(84)90167-6. [DOI] [PubMed] [Google Scholar]
- Cameron PL, Südhof TC, Jahn R, De Camilli P. Colocalization of synaptophysin with transferrin receptors: implications for synaptic vesicle biogenesis. J Cell Biol. 1991;115:151–164. doi: 10.1083/jcb.115.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron, P., O. Mundigl, and P. De Camilli. 1993. Traffic of synaptic vesicle proteins in polarized and nonpolarized cells. J. Cell Sci. 17(Suppl.):93–100. [DOI] [PubMed]
- Clift-O'Grady L, Linstedt AD, Lowe AW, Grote E, Kelly RB. Biogenesis of synaptic vesicle-like structures in a pheochromocytoma cell line PC12. J Cell Biol. 1990;110:1693–1703. doi: 10.1083/jcb.110.5.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Camilli P, Takei K. Molecular mechanisms in synaptic vesicle endocytosis and recycling. Neuron. 1996;16:481–486. doi: 10.1016/s0896-6273(00)80068-9. [DOI] [PubMed] [Google Scholar]
- de Hoop M, von Poser C, Lange C, Ikonen E, Hunziker W, Dotti CG. Intracellular routing of wild-type and mutated polymeric immunoglobulin receptor in hippocampal neurons in culture. J Cell Biol. 1995;130:1447–1459. doi: 10.1083/jcb.130.6.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotti CG, Simons K. Polarized sorting of viral glycoproteins to the axon and dendrites of hippocampal neurons in culture. Cell. 1990;62:63–72. doi: 10.1016/0092-8674(90)90240-f. [DOI] [PubMed] [Google Scholar]
- Dotti CG, Sullivan CA, Banker GA. The establishment of polarity by hippocampal neurons in culture. J Neurosci. 1988;8:1454–1468. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elferink LA, Trimble WS, Scheller RH. Two vesicle-associated membrane protein genes are differentially expressed in the rat central nervous system. J Biol Chem. 1989;264:11061–11064. [PubMed] [Google Scholar]
- Fiedler K, Simons K. The role of N-glycans in the secretory pathway. Cell. 1995;81:309–311. doi: 10.1016/0092-8674(95)90380-1. [DOI] [PubMed] [Google Scholar]
- Fletcher TL, Cameron P, De Camilli P, Banker G. The distribution of synapsin I and synaptophysin in hippocampal neurons developing in culture. J Neurosci. 1991;11:1617–1626. doi: 10.1523/JNEUROSCI.11-06-01617.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher TL, De Camilli P, Banker G. Synaptogenesis in hippocampal cultures: evidence indicating that axons and dendrites become competent to form synapses at different stages of neuronal development. J Neurosci. 1994;14:6695–6706. doi: 10.1523/JNEUROSCI.14-11-06695.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French AR, Lauffenburger DA. Intracellular receptor/ligand sorting based on endosomal retention components. Biotechnol Bioeng. 1996;51:281–297. doi: 10.1002/(SICI)1097-0290(19960805)51:3<281::AID-BIT4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Geller AI, Breakefield XO. A defective HSV-1 vector expresses Escherichia coli β-galactosidase in cultured peripheral neurons. Science. 1988;241:1667–1669. doi: 10.1126/science.241.4873.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller AI, During MJ, Haycock JW, Freese A, Neve R. Long-term increases in neurotransmitter release from neuronal cells expressing a constitutively active adenylate cyclase from a herpes simplex virus type 1 vector. Proc Natl Acad Sci USA. 1993;90:7603–7607. doi: 10.1073/pnas.90.16.7603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonatas NK, Kim SU, Stieber A, Avramens S. Internalization of lectins in neuronal GERL. J Cell Biol. 1977;73:1–13. doi: 10.1083/jcb.73.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goslin, K., and G. Banker. 1991. Rat hippocampal neurons in low density culture. In Culturing Nerve Cells. G. Banker, and K. Goslin, editors. MIT Press, Cambridge, MA. 251–282.
- Grote E, Kelly RB. Endocytosis of VAMP is facilitated by a synaptic vesicle targeting signal. J Cell Biol. 1996;132:537–547. doi: 10.1083/jcb.132.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote E, Hao JC, Bennett MK, Kelly RB. A targeting signal in VAMP regulating transport to synaptic vesicles. Cell. 1995;81:581–589. doi: 10.1016/0092-8674(95)90079-9. [DOI] [PubMed] [Google Scholar]
- Gruenberg J, Maxfield FR. Membrane transport in the endocytic pathway. Curr Opin Cell Biol. 1995;7:552–563. doi: 10.1016/0955-0674(95)80013-1. [DOI] [PubMed] [Google Scholar]
- Hammerton RW, Krzeminski KA, Mays RW, Ryan TA, Wollner DA, Nelson WJ. Mechanism for regulating cell surface distribution of Na+, K+-ATPase in polarized epithelial cells. Science. 1991;254:847–850. doi: 10.1126/science.1658934. [DOI] [PubMed] [Google Scholar]
- Heilker R, Manning-Kreig U, Zuber J-F, Spiess M. In vitro binding of clathrin adaptors to sorting signals correlates with endocytosis and basolateral sorting. EMBO (Eur Mol Biol Organ) J. 1996;15:2893–2899. [PMC free article] [PubMed] [Google Scholar]
- Heuser JE, Reese TS. Evidence for recycling of synaptic vesicle membrane during neurotransmitter release at the frog neuromuscular junction. J Cell Biol. 1973;57:315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirling H, Scheller RH. Phosphorylation of synaptic vesicle proteins—modulation of the α-SNAP interaction with the core complex. Proc Natl Acad Sci USA. 1996;93:11945–11949. doi: 10.1073/pnas.93.21.11945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman E, Freeman AR, Kashner LA. Stimulation-dependent alterations in peroxidase uptake at lobster neuromuscular junctions. Science. 1971;173:733–736. doi: 10.1126/science.173.3998.733. [DOI] [PubMed] [Google Scholar]
- Hopkins CR, Trowbridge IS. Internalization and processing of transferrin and the transferrin receptor in human carcinoma A431 cells. J Cell Biol. 1983;97:508–521. doi: 10.1083/jcb.97.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins CR, Gibson A, Shipman M, Miller K. Movement of internalized ligand-receptor complexes along a continuous endosomal reticulum. Nature. 1990;346:335–339. doi: 10.1038/346335a0. [DOI] [PubMed] [Google Scholar]
- Horton RM, Ho SN, Pullen JK, Hunt HD, Cai Z, Pease LR. Gene splicing by overlap extension. Methods Enzymol. 1993;217:270–279. doi: 10.1016/0076-6879(93)17067-f. [DOI] [PubMed] [Google Scholar]
- Hunziker W, Geuze HJ. Intracellular trafficking of lysosomal membrane proteins. Bioessays. 1996;18:379–389. doi: 10.1002/bies.950180508. [DOI] [PubMed] [Google Scholar]
- Ikin AF, Annaert WG, Takei K, De Camilli P, Jahn R, Greengard P, Buxbaum JD. Alzheimer amyloid protein precursor is localized in nerve terminal preparations to rab5-containing vesicular organelles distinct from those implicated in the synaptic vesicle pathway. J Biol Chem. 1996;271:31783–31786. doi: 10.1074/jbc.271.50.31783. [DOI] [PubMed] [Google Scholar]
- Johnston PA, Cameron PL, Stukenbrok H, Jahn R, De Camilli P, Südhof TC. Synaptophysin is targeted to similar microvesicles in CHO and PC12 cells. EMBO (Eur Mol Biol Organ) J. 1989;8:2863–2872. doi: 10.1002/j.1460-2075.1989.tb08434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadota T, Mizote M, Kadota K. Dynamics of presynaptic endosomes produced during transmitter release. J Electron Microsc. 1994;43:62–71. [PubMed] [Google Scholar]
- Kelly, R.B., F. Bonzelius, A. Cleves, L. Clift-O'Grady, E. Grote, and G. Herman. 1993. Biogenesis of synaptic vesicles. J. Cell Sci. 17(Suppl.):81–83. [DOI] [PubMed]
- Koenig J H, Ikeda K. Synaptic vesicles have two distinct recycling pathways. J Cell Biol. 1996;135:797–808. doi: 10.1083/jcb.135.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraszewski K, Mundigl O, Daniell L, Verderio C, Matteoli M, De Camilli P. Synaptic vesicle dynamics in living cultured hippocampal neurons visualized with CY3-conjugated antibodies directed against the lumenal domain of synaptotagmin. J Neurosci. 1995;15:4328–4342. doi: 10.1523/JNEUROSCI.15-06-04328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim F, Hartley D, Starr P, Lang P, Song S, Yu L, Wang Y, Geller AI. Generation of high-titer defective HSV-1 vectors using an IE 2 deletion mutant and quantitative study of expression in cultured cortical cells. Biotechniques. 1996;20:460–469. doi: 10.2144/19962003460. [DOI] [PubMed] [Google Scholar]
- Linstedt AD, Kelly RB. Synaptophysin is sorted from endocytic markers in neuroendocrine PC12 cells but not in transfected fibroblasts. Neuron. 1991;7:309–317. doi: 10.1016/0896-6273(91)90269-6. [DOI] [PubMed] [Google Scholar]
- Lisanti MP, Rodriguez-Boulan E. Glycophospholipid membrane anchoring provides clues to the mechanism of protein sorting in polarized epithelial cells. Trends Biochem Sci. 1990;15:113–118. doi: 10.1016/0968-0004(90)90195-h. [DOI] [PubMed] [Google Scholar]
- Marks MS, Ohno H, Kirchhausen T, Bonifacino JS. Protein sorting by tyrosine-based signals: adapting to the Ys and wherefores. Trends Cell Biol. 1997;7:124–128. doi: 10.1016/S0962-8924(96)10057-X. [DOI] [PubMed] [Google Scholar]
- Marquez-Sterling NR, Lo ACY, Sisodia SS, Koo EH. Trafficking of cell-surface β amyloid precursor protein: evidence that a sorting intermediate participates in synaptic vesicle recycling. J Neurosci. 1997;17:140–151. doi: 10.1523/JNEUROSCI.17-01-00140.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteoli M, Takei K, Perin MS, Südhof TC, De Camilli P. Exo-endocytotic recycling of synaptic vesicles in developing processes of cultured hippocampal neurons. J Cell Biol. 1992;117:849–861. doi: 10.1083/jcb.117.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor S, Presley JF, Maxfield FR. Sorting of membrane components from endosomes and subsequent recycling to the cell surface occurs by a bulk flow process. J Cell Biol. 1993;121:1257–1269. doi: 10.1083/jcb.121.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy AM, McMahan L, Schaffer P A. Herpes simplex virus type I ICP27 deletion mutants exhibit altered patterns of transcription and are DNA deficient. J Virol. 1989;63:18–27. doi: 10.1128/jvi.63.1.18-27.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland A, Kühn LC, Ruddle FH. The human transferrin receptor gene: genomic organization, and the complete primary structure of the receptor deduced from a cDNA sequence. Cell. 1984;39:267–274. doi: 10.1016/0092-8674(84)90004-7. [DOI] [PubMed] [Google Scholar]
- Mundigl O, De Camilli P. Formation of synaptic vesicles. Curr Opin Cell Biol. 1994;6:561–567. doi: 10.1016/0955-0674(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Mundigl O, Matteoli M, Daniell L, Thomas-Reetz A, Metcalf A, Jahn R, De Camilli P. Synaptic vesicle proteins and early endosomes in cultured hippocampal neurons: differential effects of Brefeldin A in axon and dendrites. J Cell Biol. 1993;122:1207–1221. doi: 10.1083/jcb.122.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Yamazaki H, Sekine-Aizawa Y, Hirokawa N. The neuron-specific kinesin superfamily protein KIF1A is a unique monomeric motor for anterograde axonal transport of synaptic vesicle precursors. Cell. 1995;81:769–780. doi: 10.1016/0092-8674(95)90538-3. [DOI] [PubMed] [Google Scholar]
- Overly CC, Hollenbeck PJ. Dynamic organization of endocytic pathways in axons of cultured sympathetic neurons. J Neurosci. 1996;16:6056–6064. doi: 10.1523/JNEUROSCI.16-19-06056.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton RG, Simons K, Dotti CG. Axonal and dendritic endocytic pathways in cultured neurons. J Cell Biol. 1992;119:123–137. doi: 10.1083/jcb.119.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehn S, Henz J, Hartmann E, Kurzchalia TV, Frank R, Remisch K, Dobberstein B, Rapoport TA. Structure and biosynthesis of the signal-sequence receptor. Eur J Biochem. 1990;188:439–445. doi: 10.1111/j.1432-1033.1990.tb15421.x. [DOI] [PubMed] [Google Scholar]
- Régnier-Vigouroux A, Huttner WB. Biogenesis of small synaptic vesicles and synaptic-like microvesicles. Neurochem Res. 1993;18:59–64. doi: 10.1007/BF00966923. [DOI] [PubMed] [Google Scholar]
- Régnier-Vigouroux A, Tooze SA, Huttner WB. Newly synthesized synaptophysin is transported to synaptic-like microvesicles via constitutive secretory vesicles and the plasma membrane. EMBO (Eur Mol Biol Organ) J. 1991;10:3589–3601. doi: 10.1002/j.1460-2075.1991.tb04925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Hannah MJ, Huttner WB. Synaptic-like microvesicles of neuroendocrine cells originate from a novel compartment that is continuous with the plasma membrane and devoid of transferrin receptor. J Cell Biol. 1997;137:445–458. doi: 10.1083/jcb.137.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab ME, Suda K, Thoenen H. Selective retrograde transsynaptic transfer of a protein, tetanus toxin, subsequent to its retrograde axonal transport. J Cell Biol. 1979;82:798–810. doi: 10.1083/jcb.82.3.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Wandinger-Ness A. Polarized sorting in epithelia. Cell. 1990;62:207–210. doi: 10.1016/0092-8674(90)90357-k. [DOI] [PubMed] [Google Scholar]
- Smith IL, Hardwicke MA, Sandri-Goldin RM. Evidence that the herpes simplex virus immediate early protein ICP27 acts post-transcriptionally during infection to regulate gene expression. Virology. 1992;186:74–86. doi: 10.1016/0042-6822(92)90062-t. [DOI] [PubMed] [Google Scholar]
- Sofer A, Schwarzmann G, Futerman AH. The internalization of a short acyl chain analogue of ganglioside GM1 in polarized neurons. J Cell Sci. 1996;109:2111–2119. doi: 10.1242/jcs.109.8.2111. [DOI] [PubMed] [Google Scholar]
- Sutherland DR, Delia D, Schneider C, Newman RA, Kemshead J, Greaves MF. Ubiquitous cell-surface glycoprotein on tumor cells is a proliferation associated receptor for transferrin. Proc Natl Acad Sci USA. 1981;78:4515–4519. doi: 10.1073/pnas.78.7.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tienari PJ, Destrooper B, Ikonen E, Simons M, Wiedemann A, Czech C, Hartman T, Ida N, Multhaup G, Masters CL, et al. The β-amyloid domain is essential for axonal sorting of amyloid precursor protein. EMBO (Eur Mol Biol Organ) J. 1996;15:5218–5229. [PMC free article] [PubMed] [Google Scholar]
- Trowbridge IS, Collawn JF, Hopkins CR. Signal-dependent membrane protein trafficking in the endocytic pathway. Annu Rev Cell Biol. 1993;9:129–161. doi: 10.1146/annurev.cb.09.110193.001021. [DOI] [PubMed] [Google Scholar]
- West AE, Neve RL, Buckley KM. Identification of a somatodendritic targeting signal in the cytoplasmic domain of the transferrin receptor. J Neurosci. 1997;17:6038–6047. doi: 10.1523/JNEUROSCI.17-16-06038.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T, Selkoe DJ, Koo EH. Trafficking of cell surface β amyloid precursor protein: retrograde and transcytotic transport in cultured neurons. J Cell Biol. 1995;129:431–442. doi: 10.1083/jcb.129.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]