Abstract
To investigate the functions of P-cadherin in vivo, we have mutated the gene encoding this cell adhesion receptor in mice. In contrast to E- and N-cadherin– deficient mice, mice homozygous for the P-cadherin mutation are viable. Although P-cadherin is expressed at high levels in the placenta, P-cadherin–null females are fertile. P-cadherin expression is localized to the myoepithelial cells surrounding the lumenal epithelial cells of the mammary gland. The role of the myoepithelium as a contractile tissue necessary for milk secretion is clear, but its function in the nonpregnant animal is unknown. The ability of the P-cadherin mutant female to nurse and maintain her litter indicates that the contractile function of the myoepithelium is not dependent on the cell adhesion molecule P-cadherin. The virgin P-cadherin–null females display precocious differentiation of the mammary gland. The alveolar-like buds in virgins resemble the glands of an early pregnant animal morphologically and biochemically (i.e., milk protein synthesis). The P-cadherin mutant mice develop hyperplasia and dysplasia of the mammary epithelium with age. In addition, abnormal lymphocyte infiltration was observed in the mammary glands of the mutant animals. These results indicate that P-cadherin–mediated adhesion and/or signals derived from cell–cell interactions are important determinants in negative growth control in the mammary gland. Furthermore, the loss of P-cadherin from the myoepithelium has uncovered a novel function for this tissue in maintaining the undifferentiated state of the underlying secretory epithelium.
Classical cadherins, such as E-, N-, and P-cadherin, play critical roles in tissue morphogenesis as evidenced by studies in Xenopus and mice (Kintner, 1992; Hermiston and Gordon, 1995). Cadherins are a family of glycoproteins involved in Ca++-dependent, homotypic cell–cell adhesion (Takeichi, 1995; Gumbiner, 1996). Classical cadherins have five extracellular domains, one transmembrane domain, and a highly conserved cytoplasmic domain. Two subclasses of cadherins, E- and P-cadherin, are detected in various epithelial tissues of mouse embryos (Nose and Takeichi, 1986). Antibody perturbation experiments have shown that E- and P-cadherin function cooperatively in the histogenesis of embryonic lung and lip skin in organ explant cultures (Hirai et al., 1989a , b ). In the case of the lung primordia, addition of anti-E-cadherin antibodies resulted in collapsed lobules with little luminal space, while anti–P-cadherin antibodies had a less dramatic effect. However, a mixture of both antibodies had a synergistic effect resulting in a severely distorted epithelium that could not undergo the normal branching process. In similar experiments performed on embryonic skin, P-cadherin appears to be more important compared with E-cadherin, but again disruption of both E- and P-cadherin produced the more dramatic effect on skin morphogenesis. Taken together, these studies suggest that both E- and P-cadherin play important roles in maintaining the structural integrity of epithelial tissues.
The cadherin cytoplasmic domain interacts with a group of proteins termed catenins, which link the cadherin to the actin cytoskeleton. Interaction with both the catenins and the actin cytoskeleton is necessary for full cadherin adhesive activity (Kemler, 1993). Either β-catenin or plakoglobin, which are members of the Armadillo family of proteins (Peifer, 1995), binds directly to the cadherin. In addition to playing structural roles in cell–cell adhesion, these two catenins, along with Armadillo, appear to have signaling roles, although the exact mechanism is not fully understood. Armadillo is the product of a Drosophila segment polarity gene and is part of the wingless signaling pathway, downstream of Zeste-White 3 kinase (Peifer et al., 1994). β-catenin and plakoglobin have been implicated in formation of mesoderm and the anterior-posterior axis in the Xenopus embryo (Heasman et al., 1994; Funayama et al., 1995). Recently, β-catenin was shown to interact with the transcription factor, LEF-1, providing evidence that β-catenin can regulate gene expression (Behrens et al., 1996). α-catenin, which shares homology with the cytoskeleton-associated protein vinculin, binds the cadherin indirectly through β-catenin or plakoglobin. Like vinculin, α-catenin binds to both α-actinin and actin (Knudsen et al., 1995; Rimm et al., 1995). Thus, α-catenin serves to link the cadherin/catenin complex to the actin cytoskeleton.
Cell adhesion molecules, including the cadherins, are known to play important roles in mammary gland morphogenesis. The mammary gland develops postnatally under the proper hormonal stimuli during puberty and adolescence. The morphogenesis of the mammary ductal tree occurs when the end buds invade the surrounding fatty stroma until they reach the edge of the fat pad. The end buds of the mammary ducts represent the growth points for ductal morphogenesis. The end buds consist of basally located cap cells and lumenal epithelial cells (Williams and Daniel, 1983). The cap cells are loosely adhering epithelial cells that lack cytoplasmic polarity and a well organized cytoskeleton. During early pregnancy lateral buds differentiate from the ducts and during the second half of pregnancy these alveoli develop into fully differentiated secretory lobules. These morphogenetic events are accompanied by cellular differentiation leading to development of secretory epithelial cells which are capable of synthesizing and secreting milk proteins.
The mammary duct consists of two main cell types, the lumenal epithelial cells and a surrounding monolayer of myoepithelial cells with a closely apposed basement membrane. The myoepithelial cells are thought to differentiate from the cap cells extending their cell processes laterally along the duct. In the pregnant animal, the myoepithelium is present all along the duct and in the alveoli, where myoepithelial cells are basket shaped resulting in space between the cells allowing direct contact between the alveolar epithelial cells and the basal lamina. In contrast with other tissues, the expression pattern of E- and P-cadherin in the mammary gland is very distinct. In the mouse, cap cells and myoepithelial cells express P-cadherin while the lumenal epithelial cells express E-cadherin (Daniel et al., 1995). Function-blocking antibodies were used in situ to examine the role of E- and P-cadherin in maintaining the tissue integrity of the end bud (Daniel et al., 1995). Antibody to E-cadherin induced disruption of the epithelium resulting in freely floating epithelial cells in the lumen. In contrast, antibody to P-cadherin had no effect on the lumenal layer but partially disrupted the basally located cap cell layer. These data show that E- and P-cadherin are important for maintaining the integrity of the different cell layers of the mammary duct.
The cell–cell and cell–matrix interactions of myoepithelial cells may play an important role in maintaining the structural integrity of the mammary duct. Myoepithelial cells are specialized contractile cells, whose ultrastructure is reminiscent of smooth muscle cells (Deugnier et al., 1995). They express smooth muscle contractile and cytoskeletal proteins such as α-smooth muscle actin (Radnor, 1972). However, they are true epithelial cells since cytokeratin is the major component of the intermediate filament system, they form desmosomes, hemidesmosomes, and adherens junctions, and are permanently separated from the connective tissue by the underlying basement membrane (Franke et al., 1980; Sonnenberg et al., 1986; Rasbridge et al., 1993).
Recent experiments suggest that myoepithelial cells may have an important role in branching morphogenesis in the mammary gland (Niranjan et al., 1995). Hepatocyte growth factor/scatter factor (HGF/SF)1 is produced by the fibroblasts in the breast. The lumenal epithelium and myoepithelium respond differently to HGF/SF; which acts as a mitogen for the lumenal cells while it behaves like a morphogenic factor for the myoepithelial cells. Human myoepithelial cells exposed to HGF/SF form extended branching tubules while lumenal epithelial cells did not show any morphological changes (Niranjan et al., 1995). Furthermore, overexpression of HGF/SF in the mammary gland of transgenic mice leads to precocious alveolar differentiation (Takayama et al., 1997). These data suggest that myoepithelial cells may play an important role in branching morphogenesis in the mammary gland.
To understand the role of the P-cadherin adhesion receptor in mouse development, we mutated the P-cadherin gene in embryonic stem (ES) cells and introduced the mutation into the mouse germ line (Capecchi, 1989). In contrast to E- and N-cadherin knockout mice, the P-cadherin–deficient mice are viable and fertile. However, the P-cadherin–null females exhibit precocious differentiation of the mammary gland and display mammary hyperplasia later in life. The P-cadherin–deficient mice provide us with a valuable model to determine the role of P-cadherin in cell proliferation and differentiation in the mammary gland.
Materials and Methods
Derivation of Mutant Mice
The genomic structure of the mouse P-cadherin gene was reported previously (Hatta et al., 1991). An EcoRI-EcoRI DNA fragment (8.5 kb) was isolated from lambda clone, PG21, and subcloned into Bluescript KS (Stratagene, La Jolla, CA). The neo expression cassette, pMC1neo (Thomas and Capecchi, 1987), was digested with XhoI-SalI, and subcloned into a unique XhoI site in exon 11. The BamHI-BamHI DNA fragment was subcloned into a plasmid containing the pMC1-HSVTK cassette (Mansour et al., 1988). The construct was linearized with NotI and electroporated into D3 ES cells (Doetschman et al., 1985), and colonies were selected with G418 and gancyclovir (a gift from Syntex Corp.) as described (George et al., 1993). Homologous recombination events were screened by Southern blot analysis (Sambrook et al., 1989). An EcoRI-BamHI genomic DNA fragment (0.7 kb) located immediately 5′ to the short arm of the targeting vector was used as a probe. The targeted ES clones were injected into blastocysts from C57Bl/6J mice (Bradley, 1987). The mice were genotyped by Southern blot analysis or PCR. One pair of primers corresponding to the intron/exon borders of exon 11 were used in the PCR reactions to amplify the wild-type and mutant alleles: P11F, 5′-TCCTTTCCAGCTACACCAT-3′ and P11R, 5′-AAGCTCTCACCACTGTCTGTG-3′. Unpurified tail DNA was used for the PCR reactions (Hanley and Merlie, 1991). Temperature cycling conditions for the Perkin Elmer 480 PCR machine were 94°C for 1 min; 65°C for 2 min; and 72°C for 3 min for 40 cycles. PCR products were resolved on a 1.6% agarose gel.
Western Immunoblotting
Decidual tissue from wild-type, heterozygous, and homozygous conceptuses was isolated on day 8 of gestation. The protein lysates were subjected to SDS-PAGE (Laemmli, 1970), and the resolved proteins were transferred electrophoretically to nitrocellulose. The blot was reacted with antibodies to P-cadherin (PCD-1; Nose and Takeichi, 1986) and protein bands were visualized using the ECL (Amersham Corp., Arlington Heights, IL) detection system.
Morphological and Histological Analysis
The thoracic no. 3 and/or inguinal no. 4 mammary glands were examined. Whole mount staining of the glands was performed as previously described (Williams and Daniel, 1983). The mammary glands were fixed overnight in Tellyesniczky's fixative (5% formalin, 5% acetic acid, 70% EtOH). The fixed glands were defatted in acetone, stained with: hematoxylin (0.65g FeCl3, 67.5 ml H2O, 8.7 ml stock hematoxylin [10% in 95% ethanol], and 1,000 ml 95% ethanol, adjust pH 1.25 with concentrated HCl), and then rinsed in tap water, dehydrated in increasing concentrations of ethanol to xylene, and photographed with a dissecting microscope.
For histology, mammary glands were fixed in 10% formalin, processed for paraffin sectioning, and stained with hematoxylin and eosin.
Immunohistochemistry
Immunostaining for P- and E-cadherin, α-smooth muscle actin, and caseins was performed on frozen sections of mammary tissue as described previously (Daniel et al., 1995). Tissue was embedded in Tissue-Tek OCT compound (Miles Diagnostic Division, Elkhart, IN) and frozen in an isopentane/dry ice bath. 8-μm sections were cut with a Zeiss cryotome, placed on Superfrost Plus slides (Fisher Scientific, Pittsburgh, PA), postfixed in 1:1 acetone/methanol at −20°C for 10 min, air dried, and stored at −20°C. Antibodies were diluted in 5% skim milk/PBS except for casein antibody (1% goat serum/PBS) as follows: rat monoclonal PCD-1 (1:200), rat monoclonal ECCD-2 (1:500; Shirayoshi et al., 1986), mouse monoclonal α-smooth muscle actin (clone 1A4, 1:400; Sigma Chemical Co., St. Louis, MO), and rabbit polyclonal casein (1:1,000; a gift from Charles Daniel, University of California, Santa Cruz). The samples were incubated overnight with primary antibodies except casein antibody (1 h), washed with PBS, incubated with species specific biotinylated secondary antibodies (Amersham), washed with PBS, and processed with Vectastain ABC reagents (Vector Labs, Burlingame, CA), regular or elite, and washed with PBS. The casein antibody washes contained 1% goat serum. The peroxidase substrate was applied, sections were lightly counterstained with hematoxylin, dehydrated, and coverslipped. The samples were photographed with a Nikon Optiphot microscope.
Results
Generation of P-Cadherin–deficient Mice
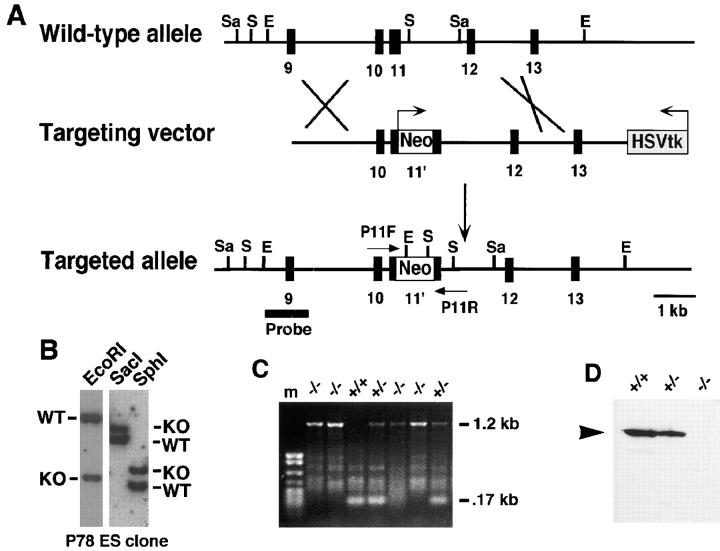
To construct a targeting vector for the P-cadherin gene (Fig. 1 A), a neomycin phosphotransferase (neo) expression cassette (pMC1neo; Thomas and Capecchi, 1987) was inserted into a unique XhoI site in exon 11 (Nose et al., 1987; Hatta et al., 1991). This disrupts the open reading frame of the P-cadherin gene in the extracellular domain of the protein. The neo cassette, containing a polyadenylation signal, was placed in the same transcriptional orientation as the P-cadherin gene thus serving to truncate any P-cadherin/neo fusion transcript. The vector includes 2.5 kb of genomic sequences 5′ of the neo gene, 5 kb on the 3′ side, and a flanking Herpes simplex virus (HSV) thymidine kinase expression cassette (pMC1-tk; Mansour et al., 1988). A similar construct was used successfully to inactivate the N-cadherin gene in mice (Radice et al., 1997).
Figure 1.
Targeted disruption of the P-cadherin gene. (A) Schematic representation of the expected gene replacement at the P-cadherin locus. Exons are represented as closed boxes. The MC1-neomycin resistance cassette and MC1-thymidine kinase cassette are designated Neo and HSVtk, respectively; arrows indicate the orientation of the genes. The flanking probe used for screening ES cell clones and genotyping mice is shown (Probe). Restriction endonuclease sites are abbreviated as follows: E, EcoRI; S, SphI; Sa, SacI. The PCR primers, P11F and P11R, used for genotyping are shown. (B) Southern blot analysis of a targeted ES cell clone. The wild-type (WT) and knockout (KO) genomic fragments are indicated. (C) PCR blot analysis of P-cadherin intercross progeny indicating the genotypes. The wild-type, 0.17 kb, and mutant, 1.2 kb, PCR products are shown. (D) Western analysis of placental lysates from P-cadherin intercross progeny. The arrowhead indicates the 118-kD P-cadherin protein recognized by the mAb PCD-1. No P-cadherin protein was detected in the homozygous mice. kb, kilobases; m, markers.
The linearized targeting vector was electroporated into D3 ES cells (Doetschman et al., 1985), and the electroporated cells were subjected to positive/negative selection (Mansour et al., 1988) using G418 and gancyclovir. One of 86 double-resistant ES cell clones (P78) underwent homologous recombination at the P-cadherin locus as determined by Southern blot analysis (Fig. 1 B). Hybridization with a neo probe failed to detect any additional sites of integration (data not shown). The targeted clone was injected into host C57BL/6 blastocysts, and the blastocysts were transferred to the uteri of pseudopregnant females. Germline transmission of the mutant allele was achieved with the targeted ES clone. The heterozygous mice did not display any obvious abnormalities in comparison with their wild-type littermates.
P-Cadherin Mutant Mice Are Viable and Fertile
To examine whether animals homozygous for the P-cadherin mutation were viable, heterozygous animals were intercrossed and genotypes of the progeny were determined by Southern blot or by PCR analysis. Mice homozygous for the P-cadherin mutation were detected among the intercross progeny (Fig. 1 C). The genotypes of the progeny showed a good fit to Mendelian distribution (107 +/+: 211 −/+:114 −/−). Homozygous P-cadherin–deficient mice did not show any overt developmental abnormalities and were indistinguishable from their heterozygous or wild-type littermates on the basis of size, activity, or fertility. To determine whether wild-type P-cadherin protein was present in homozygous mice, we examined the uterine decidua of pregnant mice since P-cadherin protein is very abundant in this maternal tissue. The decidual tissue without the embryo and extraembryonic membranes was isolated from day 8 conceptuses of wild-type, heterozygous, and homozygous mice. Western blot analysis of decidual lysates was performed using monoclonal antibody, PCD-1, which recognizes the amino terminus of the protein (Nose et al., 1990). No full-length or truncated P-cadherin protein was detected in the mutant, while a reduced amount of P-cadherin protein was present in the heterozygote (Fig. 1 D). In addition, a pan-cadherin polyclonal antibody (Takeichi et al., 1990) which recognizes the conserved cytoplasmic domain of classical cadherins did not detect P-cadherin in the mutant decidual tissue (data not shown).
To determine if loss of P-cadherin affected litter size, hybrid 129Sv/C57BL mutant males and females were mated. The P-cadherin–null females had litter sizes (average 8.8, 114 pups/13 litters) comparable to their wild-type littermates. The ability of the P-cadherin mutant females to nurse and maintain a normal size litter indicates that the contractile function of the myoepithelium is not dependent on the cell adhesion molecule P-cadherin.
Loss of P-Cadherin from Myoepithelial Cells Leads to Alveolar Differentiation in Virgin Females
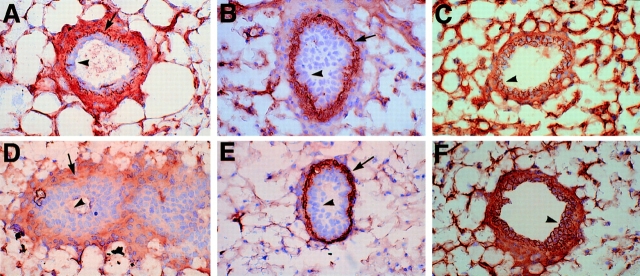
To examine cadherin expression in the mutant mammary duct, immunohistochemistry was performed on sections of mammary tissue. P-cadherin was localized to the myoepithelial cells surrounding the lumenal epithelial cells in the wild-type, but absent from the mutant duct (Fig. 2, A and D). The presence of myoepithelium in the mutant duct was confirmed by smooth muscle actin staining (Fig. 2, B and E), a marker for myoepithelial cells (Radnor, 1972). E-cadherin expression was similar in wild-type and mutant ducts (Fig. 2, C and F), thus demonstrating that E-cadherin expression was not affected by mutating the closely linked P-cadherin gene (Hatta et al., 1991).
Figure 2.
Cadherin expression in wild-type and mutant mammary ducts. Mammary gland sections of wild-type (A–C) and mutant (D– F) animals were immunostained for P-cadherin (A and D), smooth muscle actin (B and E), and E-cadherin (C and F). P-cadherin is present in the myoepithelial cells surrounding the lumenal epithelial cells in the wild-type duct (A) and absent in the mutant duct (D). A diffuse nonspecific background staining was observed in the mutant (D), but the characteristic dark linear staining of the myoepithelial cells (A) was absent in the mutant. Myoepithelial cells are present in both the wild-type (B) and mutant (E) as shown by the smooth muscle actin staining. E-cadherin expression appears similar in wild-type (C) and mutant (F) ducts. The arrowheads indicate lumenal epithelial cells and arrows indicate myoepithelial cells. Original magnification 125×.
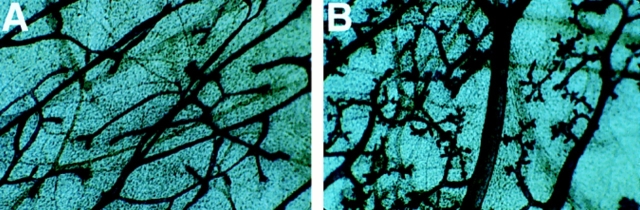
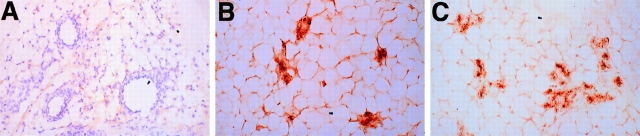
During puberty the end buds of the mammary ductal tree actively penetrate the surrounding adipose stroma, P-cadherin has been shown to be expressed in the cap cells of the terminal end bud (Daniel et al., 1995). No difference in the extent of branching morphogenesis was observed in the mammary glands of mutant mice compared with their wild-type littermates at 5 wk of age, before puberty is complete (data not shown). However, we observed an unexpected phenotype when mammary glands from postpubescent virgin females (10 wk old) were examined by whole-mount staining. Wild-type animals had normal ductal morphogenesis, while the mutant animals displayed precocious alveolar differentiation resembling an early pregnant gland (Fig. 3, A and B). The mammary glands from mutant male mice appeared normal; no alveolar differentiation was observed (data not shown). To determine whether the mutant mammary glands exhibited biochemical changes associated with differentiation, milk protein production was examined using a polyclonal antibody against caseins. The wild-type virgin animal did not express casein(s) while the mutant expressed casein(s) at levels similar to a pregnant animal (Fig. 4, A–C). These data indicate that loss of P-cadherin leads to precocious alveolar differentiation which is morphologically and biochemically similar to an early pregnant wild-type gland.
Figure 3.
Structure of normal and mutant mammary glands in virgin female mice. Whole-gland stain of (A) wild-type and (B) mutant mammary gland from 10-wk-old virgin animals. P-cadherin–deficient virgin females display precocious alveolar differentiation. Original magnification 10×.
Figure 4.
Expression of milk protein in P-cadherin–null virgin mice. Mammary gland sections of wild-type virgin (A) , mutant virgin (B), and wild-type pregnant day 14 (C) animals were immunostained with a polyclonal antibody against caseins. The P-cadherin–deficient virgin mammary gland express casein(s) similarly to a pregnant animal. The wild-type virgin gland was counterstained to distinguish the ducts. Original magnification 62×.
A Mutation in the P-Cadherin Gene Predisposes Mice to Focal Mammary Hyperplasia and Dysplasia
As demonstrated in various animal models of mammary tumorigenesis, precocious alveolar differentiation is often associated with neoplastic lesions, an initial step in tumor development (Cardiff and Muller, 1993). Therefore, the P-cadherin mutant mice and their heterozygous and wild-type littermates were maintained until 2 yr of age. No palpable tumors were observed in the aged P-cadherin mutant animals. However, upon histological examination of the mammary glands, focal alveolar hyperplasia and ductal dysplasia were observed in the mutants. Comparison of a 20-mo-old wild-type and mutant virgin female gland reveals the extent of alveolar hyperplasia observed in the mutants (Fig. 5, A and B). The clusters of alveoli are shown at higher magnification (Fig. 5 C). A 24-mo-old virgin mutant animal displays ductal dysplasia and stromal/fibroblastic hyperplasia (Fig. 5 D). Secretory vacuoles were observed in the alveoli of a 20-mo-old virgin animal consistent with a differentiated hyperplastic phenotype (Fig. 5 E). In addition, extensive periductal lymphocyte infiltration was observed in the interstitial space of the mutant glands (Fig. 5 F). Furthermore, several multiparous mutant females (15 mo old) exhibited extensive mammary hyperplasia and dysplasia, however, palpable mammary tumors were not observed (data not shown). It should be noted that P-cadherin is not expressed in the epithelium which becomes hyperplastic, but confined to the surrounding myoepithelium which appears normal in the mutant animals. In conclusion, mutation of the P-cadherin gene predisposes animals to focal hyperplasia and dysplasia as well as leading to an increase in lymphoid cells in the mammary gland.
Figure 5.
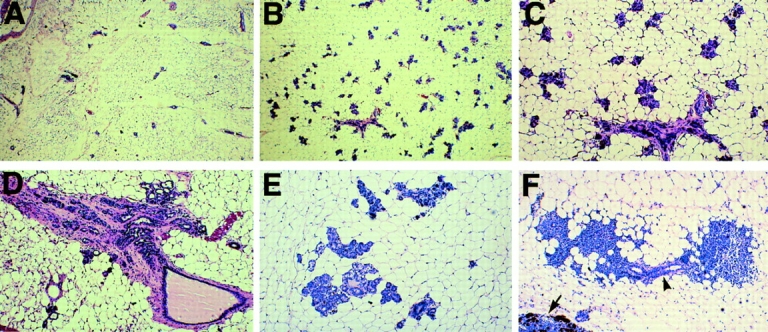
P-cadherin mutant mice develop focal hyperplasia and dysplasia with age. Histology of virgin mammary tissue from wild-type (A) and mutant (B–F) animals, 19–24 mo of age. The mutant mice (B) exhibit extensive alveolar differentiation compared with their control littermates (A). Higher magnification of the alveoli (C). P-cadherin mutant animals develop fibroblastic (D) and secretory hyperplasia (E) and exhibit extensive lymphocyte infiltration (F). The lymphocytes are concentrated around the ducts (arrowhead). A portion of the lymph node is shown (arrow). Original magnifications: (A and B) 12.5×; (C–F) 31×.
Discussion
Gene inactivation of the classical cadherins, E- and N-cadherin, results in embryonic lethality at the pre- and postimplantation stage, respectively (Larue et al., 1994; Riethmacher et al., 1995; Radice et al., 1997). Here we report the mutation of a third classical cadherin, P-cadherin, that is compatible with normal embryonic development. Although P-cadherin is expressed at high levels in placenta and testis, P-cadherin–null females and males are both fertile. E- and P-cadherin are often coexpressed in embryonic and adult tissues, for example, both cadherins are present in the notochord and basal layer of the epidermis. In these tissues, E-cadherin may functionally substitute for P-cadherin in the mutant animals thus explaining their viability. However, recently, a distinct pattern of expression of E- and P-cadherin was reported for the mouse mammary gland (Daniel et al., 1995). The mammary duct consists of two main cell types, the lumenal epithelial cells and an outer monolayer of myoepithelial cells with a closely apposed basement membrane. E-cadherin expression is restricted to the lumenal epithelial cells, while P-cadherin is expressed in the surrounding myoepithelial cells. We observed an unexpected phenotype in the mammary glands of the mutant animals. Loss of P-cadherin resulted in cell proliferation and differentiation of the mammary epithelium in virgin female animals.
Ductal morphogenesis in the mammary gland occurs during puberty when the end buds of the ducts invade the surrounding fatty stroma until they reach the edge of the fat pad. The cells surrounding the end buds, called cap cells, are thought to play an important role in ductal growth and branching morphogenesis. P-cadherin is expressed by the cap cells and function-blocking antibodies can disrupt the cellular integrity of the end bud (Daniel et al., 1995). However, ductal morphogenesis appears normal in the mutant mice, indicating that P-cadherin is not required by the cap cells for invasion of the surrounding fat pad. There are presumably other cell adhesion molecules expressed by the cap cells which can functionally substitute for P-cadherin in the mutant mammary glands. Normally, the mammary gland does not differentiate fully until the onset of pregnancy when lateral buds develop as side branches of the mammary tree. The alveoli develop further during the second half of pregnancy into fully differentiated secretory lobules capable of synthesizing and secreting milk proteins. The P-cadherin–deficient females display precocious differentiation of the mammary epithelium similar to an early pregnant animal. Furthermore, the mutant virgin glands synthesize milk protein (i.e., casein) indicative of a differentiated mammary epithelium.
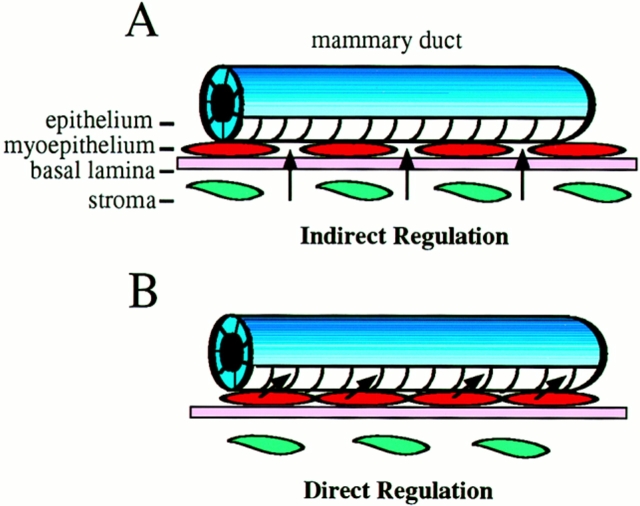
How might loss of P-cadherin cause the mammary gland phenotype? The possibility that P-cadherin is normally downregulated during early pregnancy leading to alveolar differentiation is intriguing. However, Northern blot analysis did not detect any change in P-cadherin expression between virgin and early, mid, or late pregnant mammary glands (D'Cruz, C.M., G.L. Radice, and L.A. Chodosh, unpublished data). The fact that the P-cadherin–null females lactate and nurse their pups implies that the myoepithelium can still perform its function as a contractile tissue, however, subtle defects in cell adhesion may be possible. While there is considerable evidence for inductive signals from the stroma and extracellular matrix (ECM) affecting the differentiation of the lumenal epithelium (Roskelley et al., 1995), there is very little information about the role of the myoepithelium in this process, even though it separates the stroma and basal lamina from the lumenal epithelial cells. The myoepithelium is situated in a unique position to regulate signals from the surrounding ECM and stroma to the underlying epithelium, the indirect regulation model (Fig. 6 A). The myoepithelium consists of a monolayer of cells that probably does not represent a physical barrier per se, but it may act as a filter to transmit specific signals to the underlying epithelium. P-cadherin–mediated adhesion may be important for the assembly of other junctional complexes. A cell adhesion defect may perturb intercellular communication (i.e., gap junction assembly) in the myoepithelium leading to altered growth signals. The loss of P-cadherin may affect the function of other cell adhesion molecules, for example, integrin- mediated ECM interactions may be compromised leading to changes in cell morphology of the myoepithelial cells.
Figure 6.
Possible models for regulation of mammary gland differentiation by P-cadherin. (A) The indirect regulation model is based on a cell adhesion defect. For example, inductive signals from the surrounding ECM (basal lamina) and/or stroma may more easily permeate a less cohesive myoepithelium leading to differentiation of the epithelium. (B) The direct regulation model is based on a P-cadherin–mediated cell signaling defect. For example, loss of P-cadherin may cause the myoepithelium to relay a positive signal(s), or alternatively, it may relieve an inhibitory signal(s) resulting in differentiation of the lumenal epithelium. The cell adhesion and cell signaling models are not mutually exclusive.
In Drosophila, armadillo, the β-catenin homologue, is a segment polarity gene acting in the wingless signaling pathway. As in vertebrates, it also interacts with cadherins and is localized to adherens junctions. In Drosophila, the functions of armadillo in adherens junctions and wingless signaling are competitive. The segment polarity defect of a weak armadillo allele can be partially rescued by making the flies heterozygous for DE-cadherin (Cox et al., 1996). The suppression of the armadillo mutation is thought to occur because there is a reduction in the amount of DE-cadherin thus freeing up some of the wild-type maternal store of armadillo protein, allowing it to function in the wingless pathway. Similar competitive interactions have been seen in Xenopus, where increases in cadherin levels can block action of β-catenin in Wnt signaling as monitored by inhibition of axis duplication (Fagotto et al., 1996). In both these cases, there appears to be a competition between the β-catenin bound to the cytoplasmic tail of cadherin and the free cytoplasmic β-catenin involved in the wingless/Wnt signaling pathway.
The cadherin/catenin adhesion complex and the Wnt signaling pathway share a common component, β-catenin. Data from transgenic and mutant mice suggest that the P-cadherin–null phenotype may be due to activation of the Wnt signaling pathway. Transgenic mice that overexpress Wnt-1 in the mammary gland develop extensive alveolar hyperplasia and adenocarcinomas (Tsukamoto et al., 1988). In addition, mutation of another member of the Wnt signaling pathway, Adenomatous polyposis coli (APC), predisposes mice to focal alveolar hyperplasia and carcinomas (Moser et al., 1993). APC is a negative regulator of the intracellular β-catenin pool, hence when APC is active the cytoplasmic pool of β-catenin is very low. When the Wnt pathway is activated, APC is turned off, and β-catenin accumulates in the cytoplasm. β-catenin can then interact with the DNA binding proteins of the T cell factor-lymphoid enhancer factor (Tcf-Lef) family to activate transcription of target genes (Behrens et al., 1996). The loss of P-cadherin may increase the available cytoplasmic β-catenin thus activating the Wnt signaling pathway resulting in transcription of growth factor genes whose gene products induce differentiation of the neighboring epithelial cells, the direct regulation model (Fig. 6 B). This scenario assumes that APC cannot efficiently inactivate the excess β-catenin present in the P-cadherin–deficient mammary gland.
Ectopic or overexpression of several proteins including Wnt1 (Tsukamoto et al., 1988), growth hormone (Bchini et al., 1991), HGF/SF (Takayama et al., 1997), and stromelysin1 (Sympson et al., 1994) lead to extensive alveolar hyperplasia and mammary tumors in transgenic mice. The extent of precocious alveolar differentiation observed in the P-cadherin–deficient mice appears less dramatic in comparison to these other transgenic strains. No palpable tumors were observed in the mutant animals, although, focal hyperplastic and dysplastic lesions were observed in the mutant mammary glands upon necropsy. Hyperplasia was also observed in the salivary gland where P-cadherin is normally expressed in the myoepithelial cells (Ferreira-Cornwell, M.C. and G.L. Radice, unpublished data). The finding that disruption of P-cadherin function alone is not sufficient to induce mammary tumors, suggests that additional genetic lesion(s) are necessary to progress beyond the hyperplastic phenotype.
P-cadherin is also expressed in myoepithelial cells of the human mammary gland suggesting that loss of function of this cell adhesion molecule may promote cell growth and differentiation in the human breast. While most attention has focused on the role of E-cadherin in breast cancer, our data suggest that P-cadherin may also be involved. Loss of heterozygosity (LOH) of 16q22.1 has been implicated in many types of cancer including breast (Birchmeier and Behrens, 1994). E- and P-cadherin are tandemly arranged at this locus separated by only 32 kb of genomic DNA (Bussemakers et al., 1994). While cadherin expression has been examined extensively in the tumor tissue itself, perturbation of cadherin function in the surrounding myoepithelium has not been addressed. Germline mutations in the human P-cadherin gene may predispose women to breast cancer since hyperplastic growth is an early step in tumor development.
Recently, a novel cadherin, H-cadherin, was found to be expressed in human mammary epithelial cells (Lee, 1996). H-cadherin lacks a cytoplasmic domain and is most similar to T-cadherin (Ranscht and Dours-Zimmermann, 1991). Its expression was found to be significantly reduced in human breast carcinoma cell lines and primary breast tumors (Lee, 1996). Furthermore, transfection of H-cadherin into the breast cancer cell lines led to a decreased cell growth rate and loss of anchorage-independent growth in soft agar. These data suggest that two distinct cadherins, H- and P-cadherin, are involved in negative growth control in the mammary gland.
Several loss of function mutations in the mouse result in disruption of mammary gland development and function. Mutations in cyclin D1 (Sicinski et al., 1995), activin/inhibin βB (Vassalli et al., 1994), progesterone (Lydon et al., 1995), and prolactin receptors (Ormandy et al., 1997), and Stat5a (Liu et al., 1997) all result in inhibition of lobuloalveolar outgrowth in the mammary gland. All these gene products are positive effectors in a signaling pathway(s) leading to alveolar cell proliferation and differentiation. In contrast, P-cadherin appears to act as a negative regulator of cell growth and differentiation in the mammary gland. Its absence leads to precocious alveolar differentiation in virgin animals.
In conclusion, gene targeting of the cell adhesion receptor, P-cadherin, has uncovered a novel function for this molecule in negative growth control of the mammary gland. The mechanism by which P-cadherin acts to control cell growth remains to be determined. No obvious cell adhesion defect has been observed in the myoepithelium of the mutant animals so far, suggesting the mammary gland phenotype may result from perturbation of cellular signals involved in negative growth control.
Acknowledgments
This work was supported in part by a Japan Society for Promotion of Science Fellowship (G.L. Radice), University of Pennsylvania's Cancer Center Pilot Project Program, IRG-135P from the American Cancer Society, National Institutes of Health (1R21CA66179); the Charles E. Culpeper Foundation (L.A. Chodosh); and a Program of Excellence grant (HL41484) from the National Heart Lung and Blood Institute and by the Howard Hughes Medical Institute. L.A. Chodosh is a Charles E. Culpeper Medical Scholar. R.O. Hynes is an Investigator of the Howard Hughes Medical Institute.
Footnotes
1. Abbreviations used in this paper: APC, adenomatous polyposis coli; ECM, extracellular matrix; ES, embryonic stem; HGF/SF, hepatocyte growth factor/scatter factor; HSV, Herpes simplex virus; PCD-1, P-cadherin antibody.
Special thanks to members of the Takeichi and Hynes labs for making this project possible. We thank Dr. Charles Daniel for the polyclonal casein antibody. We thank Nohelia Canales, Jose Carlos de Lima and Keisha Jones for technical assistance.
References
- Bchini O, Andres AC, Schubaur B, Mehtali M, LeMeur M, Lathe R, Gerlinger P. Precocious mammary gland development and milk protein synthesis in transgenic mice ubiquitously expressing human growth hormone. Endocrinology. 1991;128:539–546. doi: 10.1210/endo-128-1-539. [DOI] [PubMed] [Google Scholar]
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of β-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- Birchmeier W, Behrens J. Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta. 1994;1198:11–26. doi: 10.1016/0304-419x(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Bradley, A. 1987. Production and analysis of chimeric mice. In Teratocarcinomas and Embryonic Stem Cells: A Practical Approach. E.J. Robertson, editor. IRL Press, Oxford. 113–151.
- Bussemakers MJG, Bokhoven AV, Voller M, Smit FP, Schalken JA. The genes for the calcium-dependent cell adhesion molecules P- and E-cadherin are tandemly arranged in the human genome. Biochem Biophys Res Com. 1994;203:1291–1294. doi: 10.1006/bbrc.1994.2322. [DOI] [PubMed] [Google Scholar]
- Capecchi MR. Altering the genome by homologous recombination. Science. 1989;244:1288–1292. doi: 10.1126/science.2660260. [DOI] [PubMed] [Google Scholar]
- Cardiff RD, Muller WJ. Transgenic mouse models of mammary tumorigenesis. Cancer Surveys. 1993;16:97–137. [PubMed] [Google Scholar]
- Cox RT, Kirkpatrick C, Peifer M. Armadillo is required for adherens junction assembly, cell polarity, and morphogenesis during Drosophilaembryogenesis. J Cell Biol. 1996;134:133–148. doi: 10.1083/jcb.134.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel C, Strickland P, Friedmann Y. Expression and functional role of E- and P-cadherins in mouse mammary ductal morphogenesis and growth. Dev Biol. 1995;169:511–519. doi: 10.1006/dbio.1995.1165. [DOI] [PubMed] [Google Scholar]
- Deugnier M-A, Moiseyeva EP, Thiery JP, Glukhova M. Myoepithelial cell differentiation in the developing mammary gland: progressive acquisition of smooth muscle phenotype. Dev Dyn. 1995;204:107–117. doi: 10.1002/aja.1002040202. [DOI] [PubMed] [Google Scholar]
- Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exper Morph. 1985;87:27–45. [PubMed] [Google Scholar]
- Fagotto F, Funayama N, Gluck U, Gumbiner BM. Binding of cadherins antagonizes the signaling activity of β-catenin during axis formation in Xenopus. . J Cell Biol. 1996;132:1105–1114. doi: 10.1083/jcb.132.6.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke WW, Schmid E, Freudenstein C, Appelhans B, Osborn M, Weber K, Keenan TW. Intermediate-sized filaments of the prekaratin type in myoepithelial cells. J Cell Biol. 1980;84:633–654. doi: 10.1083/jcb.84.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funayama N, Fagotto F, McCrea P, Gumbiner BM. Embryonic axis induction by the armadillo repeat domain of β-catenin: evidence for intracellular signaling. J Cell Biol. 1995;128:959–968. doi: 10.1083/jcb.128.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George EL, Georges EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesodermal migration and vascular development in fibronectin-deficient mice. Development. 1993;119:173–183. [Google Scholar]
- Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- Hanley T, Merlie JP. Transgene detection in unpurified mouse tail DNA by polymerase chain reaction. Biotechniques. 1991;10:56. [PubMed] [Google Scholar]
- Hatta M, Miyatani S, Copeland NG, Gilbert DJ, Jenkins NA, Takeichi M. Genomic organization and chromosomal mapping of the mouse P-cadherin gene. Nucleic Acids Res. 1991;19:4437–4441. doi: 10.1093/nar/19.16.4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heasman J, Crawford A, Goldstone K, Gamer-Hamrick P, Gumbiner B, McCrea P, Kintner C, Yoshida NC, Wylie C. Overexpression of cadherins and underexpression of β-catenin inhibit dorsal mesoderm induction in early Xenopusembryos. Cell. 1994;79:791–803. doi: 10.1016/0092-8674(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Hermiston ML, Gordon JI. In vivo analysis of cadherin function in the mouse intestinal epithelium: essential roles in adhesion, maintenance of differentiation, and regulation of programmed cell death. J Cell Biol. 1995;129:489–506. doi: 10.1083/jcb.129.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai Y, Nose A, Kobayashi S, Takeichi M. Expression and role of E- and P-cadherin adhesion molecules in embryonic histogeneis: I. lung epithelial morphogenesis. Development. 1989a;105:263–270. doi: 10.1242/dev.105.2.263. [DOI] [PubMed] [Google Scholar]
- Hirai Y, Nose A, Kobayashi S, Takeichi M. Expression and role of E- and P-cadherin adhesion molecules in embryonic histogeneis: II. skin morphogenesis. Development. 1989b;105:271–277. doi: 10.1242/dev.105.2.271. [DOI] [PubMed] [Google Scholar]
- Kemler R. From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet. 1993;9:317–321. doi: 10.1016/0168-9525(93)90250-l. [DOI] [PubMed] [Google Scholar]
- Kintner C. Regulation of embryonic cell adhesion by the cadherin cytoplasmic domain. Cell. 1992;69:225–236. doi: 10.1016/0092-8674(92)90404-z. [DOI] [PubMed] [Google Scholar]
- Knudsen KA, Peralta A, Soler, Johnson KR, Wheelock MJ. Interaction of α-actinin with the cadherin/catenin cell–cell adhesion complex via α-catenin. J Cell Biol. 1995;130:67–77. doi: 10.1083/jcb.130.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (Lond) 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larue L, Ohsugi M, Hirchenhain J, Kemler R. E-cadherin null mutant embryos fail to form a trophectoderm epithelium. Proc Natl Acad Sci USA. 1994;91:8263–8267. doi: 10.1073/pnas.91.17.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SW. H-cadherin, a novel cadherin with growth inhibitory functions and diminished expression in human breast cancer. Nature Med. 1996;2:776–782. doi: 10.1038/nm0796-776. [DOI] [PubMed] [Google Scholar]
- Liu X, Robinson GW, Wagner K-U, Garrett L, Wynshaw-Boris A, Hennighausen L. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes & Dev. 1997;11:179–186. doi: 10.1101/gad.11.2.179. [DOI] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Shyamala G, Conneely OM, O'Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes & Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- Mansour SL, Thomas KR, Capecchi MR. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to nonselectable genes. Nature. 1988;336:348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- Moser RA, Mattes EM, Dove WF, Lindstrom MJ, Haag JD, Gould MN. APC/Min, a mutation in the murine Apc gene, predisposes to mammary carcinomas and focal alveolar hyperplasias. Proc Natl Acad Sci USA. 1993;90:8977–8981. doi: 10.1073/pnas.90.19.8977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niranjan B, Buluwela L, Yant J, Perusinghe N, Atherton A, Phippard D, Dale T, Gusterson B, Kamalati T. HGF/SF: a potent cytokine for mammary growth, morphogenesis and development. Development. 1995;121:2897–2908. doi: 10.1242/dev.121.9.2897. [DOI] [PubMed] [Google Scholar]
- Nose A, Takeichi M. A novel cadherin cell adhesion molecule: its expression patterns associated with implantation and organogenesis of mouse embryos. J Cell Biol. 1986;103:2649–2658. doi: 10.1083/jcb.103.6.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose A, Nagafuchi A, Takeichi M. Isolation of placental cadherin cDNA: identification of a novel gene family of cell-cell adhesion molecules. EMBO (Eur Mol Biol Organ) J. 1987;6:3655–3661. doi: 10.1002/j.1460-2075.1987.tb02698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose A, Tsuji K, Takeichi M. Localization of specificity determining sites in cadherin cell adhesion molecules. Cell. 1990;61:147–155. doi: 10.1016/0092-8674(90)90222-z. [DOI] [PubMed] [Google Scholar]
- Ormandy CJ, Camus A, Barra J, Damotte D, Lucas B, Buteau H, Edery M, Brousse N, Babinet C, Binart N, Kelly PA. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes & Dev. 1997;11:167–178. doi: 10.1101/gad.11.2.167. [DOI] [PubMed] [Google Scholar]
- Peifer M. Cell adhesion and signal transduction: the armadillo connection. Trends Cell Biol. 1995;5:224–229. doi: 10.1016/s0962-8924(00)89015-7. [DOI] [PubMed] [Google Scholar]
- Peifer M, Pai L-M, Casey M. Phosphorylation of the Drosophilaadherens junction protein Armadillo: roles for wingless signal and Zeste-White 3 kinase. Dev Biol. 1994;166:543–556. doi: 10.1006/dbio.1994.1336. [DOI] [PubMed] [Google Scholar]
- Radice GL, Rayburn H, Matsunami H, Knudsen KA, Takeichi M, Hynes RO. Developmental defects in mouse embryos lacking N-cadherin. Dev Biol. 1997;181:64–78. doi: 10.1006/dbio.1996.8443. [DOI] [PubMed] [Google Scholar]
- Radnor CJ. Myoepithelial cell differentiation in rat mammary glands. J Anat. 1972;111:381–398. [PMC free article] [PubMed] [Google Scholar]
- Ranscht B, Dours-Zimmermann MT. T-cadherin, a novel cadherin cell adhesion molecule in the nervous system lacks the conserved cytoplasmic region. Neuron. 1991;7:391–402. doi: 10.1016/0896-6273(91)90291-7. [DOI] [PubMed] [Google Scholar]
- Rasbridge SA, Gillet CE, Sampson SA, Walsh FS, Millis RR. Epithelial (E-) and placental (P-) cadherin cell adhesion molecule expression in breast carcinoma. J Pathol. 1993;169:245–250. doi: 10.1002/path.1711690211. [DOI] [PubMed] [Google Scholar]
- Riethmacher D, Brinkmann V, Birchmeier C. A targeted mutation in the mouse E-cadherin gene results in defective preimplantation development. Proc Natl Acad Sci USA. 1995;92:855–859. doi: 10.1073/pnas.92.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimm DL, Koslov ER, Kebriaei P, Cianci CD, Morrow JS. α(E)-catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc Natl Acad Sci USA. 1995;92:8813–8817. doi: 10.1073/pnas.92.19.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskelley CD, Srebrow A, Bissell MJ. A hierarchy of ECM-mediated signalling regulates tissue-specific gene expression. Curr Opin Cell Biol. 1995;7:736–747. doi: 10.1016/0955-0674(95)80117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J., E.F. Fritsch, and T. Maniatis. 1989. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York. p. 931.
- Shirayoshi Y, Nose A, Iwasaki K, Takeichi M. N-linked oligosaccharides are not involved in the function of a cell-cell binding glycoprotein E-cadherin. Cell Struct Funct. 1986;11:245–252. doi: 10.1247/csf.11.245. [DOI] [PubMed] [Google Scholar]
- Sicinski P, Donaher JL, Parker SB, Li T, Fazeli A, Gardner H, Haslam SZ, Bronson RT, Elledge SJ, Weinberg RA. CyclinD1 provides a link between development and oncogenesis in the retina and breast. Cell. 1995;82:621–630. doi: 10.1016/0092-8674(95)90034-9. [DOI] [PubMed] [Google Scholar]
- Sonnenberg A, Daamq H, Van der Valk MA, Hilkens J, Hilgers J. Development of mouse mammary gland: identification of stages of differentiation of luminal and myoepithelial cells using monoclonal antibodies and polyvalent antiserum against keratin. J Histochem Cytochem. 1986;34:1037–1046. doi: 10.1177/34.8.2426332. [DOI] [PubMed] [Google Scholar]
- Sympson CJ, Talhouk RS, Alexander CM, Chin JR, Clift SM, Bissell MJ, Werb Z. Targeted expression of stromelysin-1 in mammary gland provides evidence for a role of proteinases in branching morphogenesis and the requirement for an intact basement membrane for tissue-specific gene expression. J Cell Biol. 1994;125:681–693. doi: 10.1083/jcb.125.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama H, LaRochelle WJ, Sharp R, Otsuka T, Kriebel P, Anver M, Aaronson SA, Merlino G. Diverse tumorigenesis associated with aberrant development in mice overexpressing hepatocyte growth factor/scatter factor. Proc Natl Acad Sci USA. 1997;94:701–706. doi: 10.1073/pnas.94.2.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. Morphogenetic roles of classic cadherins. Curr Opin Cell Biol. 1995;7:619–627. doi: 10.1016/0955-0674(95)80102-2. [DOI] [PubMed] [Google Scholar]
- Takeichi, M., H. Inuzuka, K. Shimamura, T. Fujimori, and A. Nagafuchi. 1990. Cadherin subclasses: differential expression and their roles in neural morphogenesis. Cold Spring Harbor Symp. Quant. Biol. LV:319–325. [DOI] [PubMed]
- Thomas KR, Capecchi MR. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987;51:503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Tsukamoto AS, Grosschedl R, Guzman RC, Parslow T, Varmus HE. Expression of the int-1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice. Cell. 1988;55:619–625. doi: 10.1016/0092-8674(88)90220-6. [DOI] [PubMed] [Google Scholar]
- Vassalli A, Matzuk MM, Gardner HAR, Lee K-F, Jaenisch R. Activin/inhibin βB subunit gene disruption leads to defects in eyelid development and female reproduction. Genes & Dev. 1994;8:414–427. doi: 10.1101/gad.8.4.414. [DOI] [PubMed] [Google Scholar]
- Williams JM, Daniel CW. Mammary ductal elongation: differentiation of myoepithelium and basal lamina during branching morphogenesis. Dev Biol. 1983;97:274–290. doi: 10.1016/0012-1606(83)90086-6. [DOI] [PubMed] [Google Scholar]