Abstract
IL-1β converting enzyme (ICE) family cysteine proteases are subdivided into three groups; ICE-, CPP32-, and Ich-1–like proteases. In Fas-induced apoptosis, activation of ICE-like proteases is followed by activation of CPP32-like proteases which is thought to be essential for execution of the cell death. It was recently reported that two subfamily members of the mitogen-activated protein kinase superfamily, JNK/SAPK and p38, are activated during Fas-induced apoptosis. Here, we have shown that MKK7, but not SEK1/ MKK4, is activated by Fas as an activator for JNK/ SAPK and that MKK6 is a major activator for p38 in Fas signaling. Then, to dissect various cellular responses induced by Fas, we used several peptide inhibitors for ICE family proteases in Fas-treated Jurkat cells and KB cells. While Z-VAD-FK which inhibited almost all the Fas-induced cellular responses blocked the activation of JNK/SAPK and p38, Ac-DEVD-CHO and Z-DEVD-FK, specific inhibitors for CPP32-like proteases, which inhibited the Fas-induced chromatin condensation and DNA fragmentation did not block the activation of JNK/SAPK and p38. Interestingly, these DEVD-type inhibitors did not block the Fas-induced morphological changes (cell shrinkage and surface blebbing), induction of Apo2.7 antigen, or the cell death (as assessed by the dye exclusion ability). These results suggest that the Fas-induced activation of the JNK/SAPK and p38 signaling pathways does not require CPP32-like proteases and that CPP32-like proteases, although essential for apoptotic nuclear events (such as chromatin condensation and DNA fragmentation), are not required for other apoptotic events in the cytoplasm or the cell death itself. Thus, the Fas signaling pathway diverges into multiple, separate processes, each of which may be responsible for part of the apoptotic cellular responses.
Apoptosis, a mechanism of cell suicide, is an intrinsic biological event that plays an essential role in various developmental stages and also in immune systems. Apoptosis is characterized by dramatic morphological changes of the cell, including membrane blebbing, cell shrinkage, chromatin condensation, DNA cleavage, and fragmentation of the cell into apoptotic bodies. The apoptotic pathway could be divided into four steps, decision to die, execution of death, engulfment of apoptotic cells by macrophages, and degradation of apoptotic cells. An insight into the execution machinery of apoptosis has come from genetical studies in the nematode Caenorhabditis elegans. Three kinds of apoptosis-related genes have been cloned, ced-3, ced-4, and ced-9. The former two genes are required for execution of death (Ellis et al., 1991; Yuan and Horvitz, 1992), whereas ced-9 prevents cell death (Hengartner et al., 1992). Mammalian homologues of ced-3 and ced-9 have been identified as IL-1β converting enzyme (ICE)1 cysteine protease (Yuan et al., 1993) and oncogene bcl-2 (Hengartner and Horvitz, 1994), respectively. A number of ICE-related proteases have been cloned including ICE (caspase-1), ICH-1/Nedd-2 (caspase-2) (Kumar et al., 1994; Wang et al., 1995), CPP32/Yama/apopain (caspase-3) (Fernandes-Alnemri et al., 1994; Nicholson et al., 1995; Tewari, 1995), TX/ICErel-II/ICH-2 (caspase-4) (Faucheu et al., 1995; Kamens et al., 1995; Munday et al., 1995), ICErel-III/TY (caspase-5) (Faucheu et al., 1995; Munday et al., 1995), Mch2 (caspase-6) (Fernandes- Alnemri et al. 1995a), Mch3/ICE-LAP3/CMH-1 (caspase-7) (Fernandes-Alnemri et al., 1995b ; Duan et al., 1996a ; Lippke et al., 1996), FLICE/MACH/Mch5 (caspase-8) (Boldin et al., 1996; Fernandes-Alnemri et al., 1996; Muzio et al., 1996), ICE-LAP6/Mch6 (caspase-9) (Duan et al., 1996b ; Srinivasula et al., 1996), and Mch4 (caspase-10) (Fernandes- Alnemri et al., 1996). These ICE-related proteases are classified into three groups; ICE-, CPP32-, and ICH-1–like proteases.
Fas (Yonehara et al., 1989) is a type-I membrane protein and belongs to the TNF/NGF receptor superfamily (Itoh et al., 1991). The death domain localized in the cytoplasmic region of Fas is essential for transducing apoptotic signals to cytoplasm (Itoh and Nagata, 1993). Upon crosslinking with its ligand or anti-Fas antibody, Fas trimerizes and binds to FADD/MORT1 (Boldin et al., 1995; Chinnaiyan et al., 1995, 1996) through its death domain. FADD/MORT1 binds to the NH2-terminal region of FLICE/MACH/Mch5 (Boldin et al., 1996; Muzio et al., 1996). Then FLICE/MACH/Mch5 may be activated, and other members of ICE-related proteases might be sequentially activated. It has been shown that activation of CPP32-like proteases follows the activation of ICE-like proteases (Enari et al., 1995, 1996; Los et al., 1995) and inhibition of CPP32-like proteases prevents Fas-induced apoptosis (Schlegel et al., 1996). Therefore, CPP32-like proteases are thought to be essential for Fas-induced apoptosis. It remains, however, unclear how these ICE-related proteases are involved in each of the apoptotic cellular responses.
Two subfamily members of the MAP kinase superfamily, JNK/SAPK (Derijard et al., 1994; Kyriakis et al., 1994) and p38 (Han et al., 1994; Lee et al., 1994; Rouse et al., 1994), have recently been implicated in several types of apoptosis, such as apoptosis of differentiated PC12 cells induced by NGF-deprivation (Xia et al., 1995) and apoptosis of different kinds of cells induced by ceramide (Verheij et al., 1996), UV and γ irradiation (Chen et al., 1996). In the Fas-induced signaling pathway also, activation of JNK/SAPK and p38 has been observed (Latinis et al., 1996; Wilson et al., 1996; Juo et al., 1997; Lenczowski et al., 1997). However, little is known about upstream activators for these kinases in this pathway, and the relationship between these kinases and the ICE-related proteases remains unclear. Here we have shown first that MKK7 (= a newly identified JNK/SAPK activator; Moriguchi et al., 1997), but not SEK1/MKK4 (Sanchez et al., 1994; Derijard et al., 1995), is activated by Fas as an activator for JNK/ SAPK and that MKK6 (Cuenda et al., 1996; Han et al., 1996; Moriguchi et al., 1996a ; Raingeaud et al., 1996; Stein et al., 1996) acts as a major activator for p38 in Fas signaling. Then, to dissect various cellular responses induced by Fas, we used several kinds of inhibitors for ICE-related proteases with different specificities; Z-VAD-FK as an inhibitor for a broad spectrum of ICE-related proteases, Ac-DEVD-CHO and Z-DEVD-FK as specific inhibitors for CPP32-like proteases, and Ac-YVAD-CHO as a specific inhibitor for ICE-like proteases. Z-VAD-FK blocked both the cell death and the activation of JNK/SAPK and p38, but Ac-YVAD-CHO did not block either response. Importantly, Ac-DEVD-CHO and Z-DEVD-FK did block the chromatin condensation and DNA fragmentation almost completely, but did not block the activation of JNK/ SAPK and p38. Moreover, these inhibitors did not block the Fas-induced membrane blebbing, cell shrinkage or other apoptotic events of the cytoplasm. Thus, in the presence of the inhibitors for CPP32-like proteases, Fas causes the cell to die without any chromatin condensation. These results suggest that Fas can induce at least two separate signaling pathways; one is CPP32-like proteases-dependent and the other is independent and that the former pathway is essential for some of the apoptotic nuclear events such as the drastic changes in chromatin and DNA, but is dispensable for the apoptotic cytoplasmic events, the activation of JNK/SAPK and p38, or the cell death.
Materials and Methods
Cell Cultures
Jurkat cells were cultured in RPMI 1640 containing 10% fetal calf serum. KB cells were cultured in Dulbecco's modified Eagle's medium containing 10% fetal calf serum. These cells were maintained in 5% CO2 at 37°C. KB cells were transiently transfected using LipofectAMINE (GIBCO BRL, Gaithersburg, MD) as described previously (Moriguchi et al., 1996a ).
Reagents, Antibodies, and Recombinant Proteins
Ac-YVAD-CHO and Ac-DEVD-CHO were purchased from Peptide Institute, Inc. (Osaka, Japan) and Z-VAD-FK and Z-DEVD-FK from Kamiya Biomedical Co. (Tukwila, WA). Anti-Fas mAb (clone CH-11), Apo2.7 mAb, anti-actin mAb (A-4700), and anti-JNK1 and anti-p38 polyclonal antibodies were purchased from MBL (Nagoya, Japan), Immunotech (Luminy, France), Sigma Chem. Co. (St. Louis, MO), and Santa Cruz Biotechnology Inc., respectively. His-tagged wild-type SAPKα, kinase negative MPK2, c-Jun, and ATF-2 were expressed in Escherichia coli and purified using a Ni+ affinity column (Moriguchi et al., 1996b ). GST-kinase negative SAPKα, GST wild-type SAPKα, and GST-p38 were purified by affinity chromatography on glutathione–Sepharose 4B (Pharmacia LKB Biotech Inc., Piscataway, NJ) (Moriguchi et al., 1996b ).
Immunecomplex Kinase Assays
The cell lysate was prepared as described (Moriguchi et al., 1996b ). In brief, Jurkat cells were lysed in lysis buffer (20 mM Hepes, pH 7.35, 12.5 mM 2-glycerophosphate, 150 mM NaCl, 1.5 mM MgCl2, 2 mM EGTA, 10 mM NaF, 0.5% Triton X-100, 2 mM DTT, 1 mM sodium vanadate, 1 mM PMSF, and 20 μg/ml aprotinin) and then centrifuged at 15,000 rpm for 15 min. JNK/SAPK, p38, SEK1/MKK4/XMEK2, MKK3, MKK6, and MKK7 were precipitated from the cell lysate by incubation with specific antibodies and protein A–Sepharose beads (Pharmacia Biotech Inc.) as described previously (Moriguchi et al., 1996a ,b; Moriguchi et al., 1997). Each precipitate was washed three times with TBS-T (20 mM Tris, pH 7.5, 0.15 M NaCl, 0.2% Tween-20), and once with extraction buffer (20 mM Tris, pH 7.2, 25 mM 2-glycerophosphate, 2 mM EGTA, 2 mM DTT, 1 mM sodiume vanadate) and then mixed with 3 μg of each substrate (His-tagged c-Jun for JNK/SAPK, GST-kinase negative SAPK for SEK1/MKK4/XMEK2 and MKK7, His-tagged ATF2 for p38, and His-tagged kinase negative MPK2 for MKK3 and MKK6), 50 μM ATP, 15 mM MgCl2, and 2 μCi of [γ-32P]ATP and incubated for 20 min at 30°C in a final volume of 15 μl. The reaction was stopped by addition of Laemmli's sample buffer and boiling. The reaction mixture was resolved by SDS-PAGE analysis.
Column Chromatography
The cell lysate, obtained from Jurkat cells (200 ml culture) treated with anti-Fas mAb (CH-11, 150 ng/ml) for 5 h, were loaded onto a HiTrap Q (1 ml; Pharmacia Biotech Inc.) equilibrated with extraction buffer containing 0.01% Brij-35 (buffer A). The column was washed with 3 ml of buffer A and developed with a 18 ml NaCl gradient (0–0.3 M NaCl). To measure the activity to activate SAPKα, each fraction (7 μl) was first incubated for 30 min at 30°C with or without 1 μg His-tagged SAPKα, 100 μM ATP, and 20 mM MgCl2 and subsequently incubated for 20 min at 25°C with 3 μg of His-tagged c-Jun and 1 μCi [γ-32P]ATP (final volume, 15 μl). The reaction was stopped by addition of Laemmli's sample buffer and boiling.
Immunodepletion
Jurkat cells were treated with anti-Fas mAb (CH-11, 150 ng/ml) for 5 h and the cell lysate was prepared as described above. The lysate was incubated for 1 h at 4°C with increasing amounts of anti-MKK6 polyclonal antibody (Moriguchi et al., 1996b ) and protein A–Sepharose beads, and then centrifuged. To measure the activity of the supernatant to activate p38, the samples were first incubated for 30 min at 30°C with 1 μg GST-p38, 50 μM ATP, and 15 mM MgCl2 followed by incubation for 1 h at 4°C with glutathione–Sepharose 4B. The precipitated GST-p38 was washed three times with TBS-T and once with extraction buffer, and then incubated for 20 min at 20°C with 3 μg His-tagged ATF2, 50 μM ATP, and 15 mM MgCl2, and 2.5 μCi [γ-32P]ATP. The reaction was stopped by addition of Laemmli's sample buffer and boiling. Each of the supernatants was analyzed by immunoblotting with anti-MKK6 mAb (Moriguchi et al., 1996b ) and anti-MKK3 polyclonal antibody (C-19; Santa Cruz Biotechnology Inc.).
Jurkat cells were treated with anti-Fas mAb (CH-11, 200 ng/ml) for 5 h and the cell lysate was loaded onto a Hitrap Q column as described above. The flow-through fraction was incubated for 2 h at 4°C with anti-MKK7 polyclonal antibody (Moriguchi et al., manuscript in preparation) and protein A–Sepharose beads, and then centrifuged. The activity to activate JNK/SAPK, remaining in the supernatant, was measured by using 1 μg GST-SAPK and 3 μg His-tagged c-Jun.
Cell Staining
Jurkat cells were treated with anti-Fas mAb (CH-11, 150 ng/ml) for 20 h. Cells were fixed and stained with Apo2.7 mAb (Zhang et al., 1996) by the slightly modified method. In brief, cells were fixed in 1% paraformaldehyde solution in PBS for 2 min on ice and washed twice with PBS followed by incubation for 15 h on ice with Phycoerythrin-labeled Apo2.7 mAb (Immunotech), 40 μg/ml digitonin, and 0.1 mg/ml DAPI in blocking buffer (1 mg/ml goat IgG, 10 mg/ml BSA in PBS). KB cells were treated with anti-Fas mAb (CH-11, 150 ng/ml) for 8 h and fixed in 3.7% formalin solution in PBS for 10 min at 37°C and permeabilized by incubation in 0.5% Triton X-100 in PBS for 10 min. Cells were stained with 0.1 mg/ml DAPI and FITC-labeled phalloidin. Cells were observed under fluorescent microscope (Axiophot).
DNA Fragmentation Assay
Jurkat cells (106 cells) were treated with anti-Fas mAb (CH-11, 150 ng/ml) for 20 h. Cells were collected, washed once in PBS (20 mM NaH2PO4, 150 mM NaCl, pH 7.4), lysed in 100 μl TE-T buffer (10 mM Tris, 10 mM EDTA, 0.5% Triton X-100) and then centrifuged at 15,000 rpm for 20 min. The supernatant was subjected to digestion with ribonuclease A (0.4 mg/ml) for 1 h at 37°C followed by incubation with proteinase K (0.4 mg/ ml) for 1 h at 37°C. The sample was then extracted with isopropanol for overnight at −20°C. DNA was precipitated and resuspended in 20 μl of TE buffer and analyzed by electrophoresis on a 0.7% agarose gel in the presence of 0.5 μg/ml ethidium bromide.
Cell Death Assay
Cell death was measured by two assays, trypan blue staining and MTT (3- (4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. MTT assay was done as previously described (Mosmann, 1983). In brief, Jurkat cells (5 × 103 cells) were incubated overnight in 96-well plate and treated with anti-Fas mAb (CH-11, 150 ng/ml) for 20 h in the presence or absence of various inhibitors. 10 μl of a 5 mg/ml MTT (Sigma) stock solution was added per well and incubated for 4 h at 37°C. Cells were lysed and the difference between the absorbance at 562 and 650 nm of each sample was measured. The value of the sample without anti-Fas mAb was regarded as 100% survival. The percentage of cell death was expressed as: 100 − (% survival).
Results
Fas Induces Activation of the MKK7-JNK/SAPK and MKK6-p38 Protein Kinase Cascades
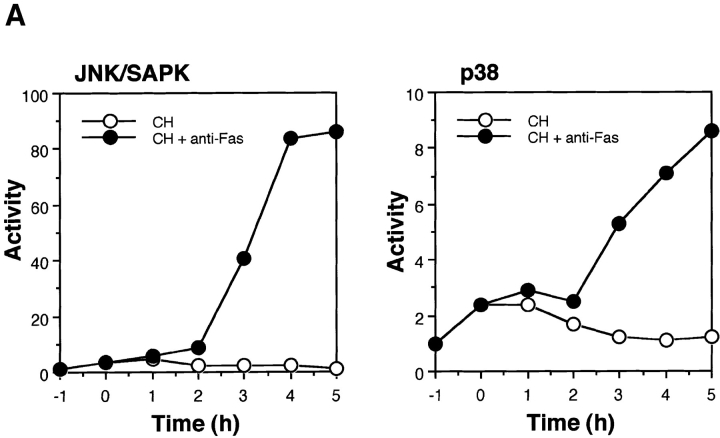
It was recently reported that two subfamily members of the MAP kinase superfamily, JNK/SAPK and p38, are activated in Fas-induced apoptosis (Latinis et al., 1996; Wilson et al., 1996; Juo et al., 1997; Lenczowski et al., 1997). We confirmed the activation of both JNK/SAPK and p38 in Fas-induced signaling by measuring the activities of endogenous JNK/SAPK and endogenous p38 in Jurkat T cells at various time points after treatment with anti-Fas mAb (CH-11). The activation was relatively slow but sustained; the increase in the kinase activities was detected 2 h after Fas stimulation and the maximal activation was attained at about 6 h (Fig. 1 A). In this series of experiments the apoptotic bodies began to appear under microscope 4 h after Fas stimulation.
Figure 1.
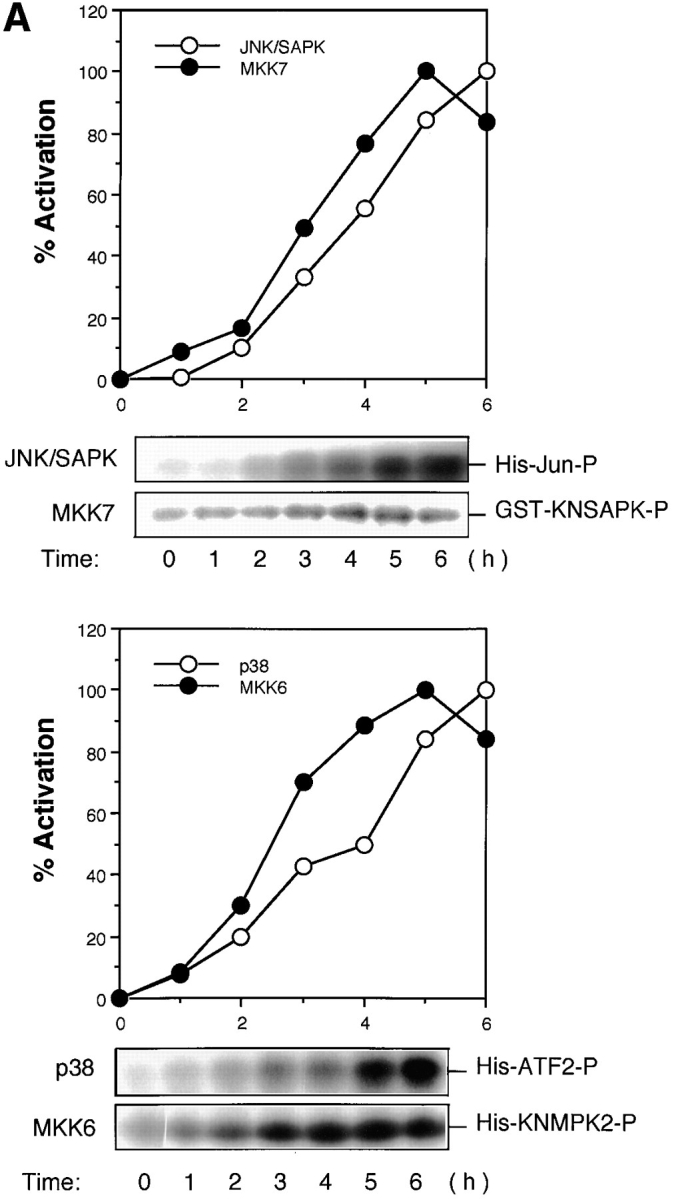
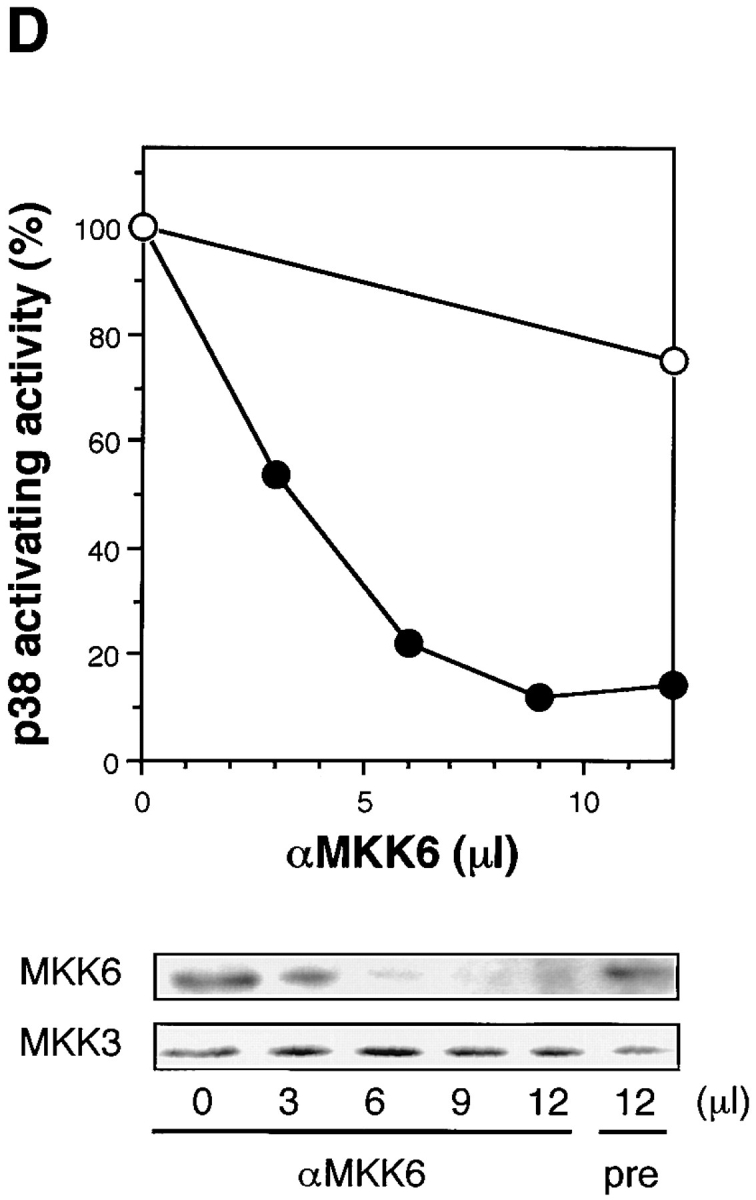
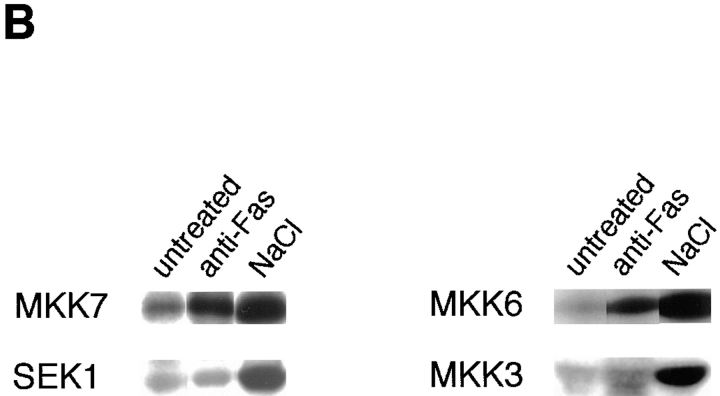
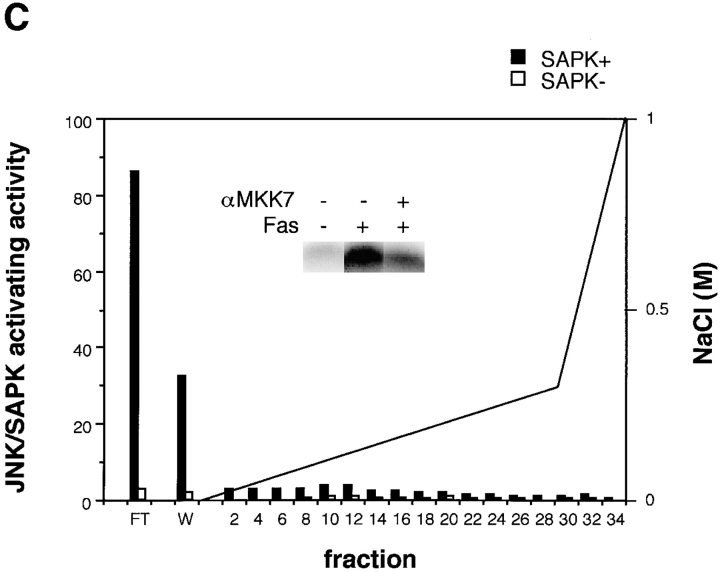
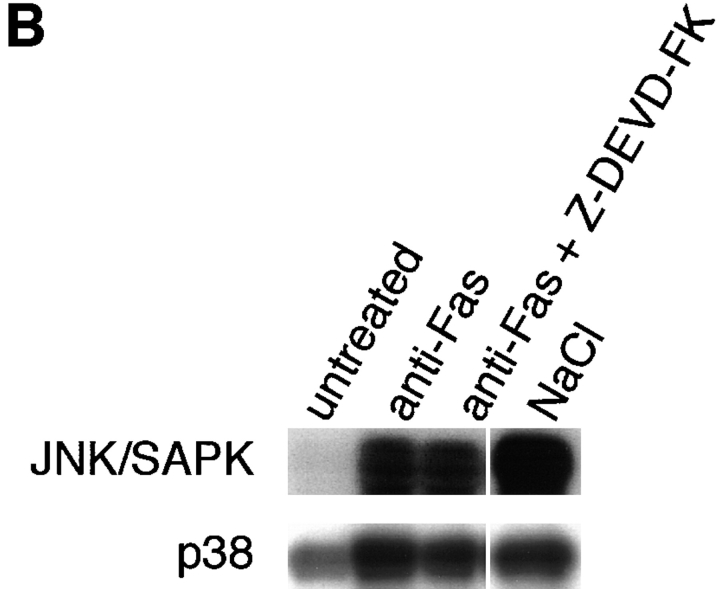
(A) Activation of JNK/SAPK and MKK7 (top) and p38 and MKK6 (bottom) during Fas-induced apoptosis. Jurkat cells were incubated with anti-Fas mAb (CH-11, 250 ng/ml) for various periods, and were collected and lysed. The activities of JNK/SAPK (top), MKK7 (top), p38 (bottom), and MKK6 (bottom) in the cell lysate were determined by immunecomplex kinase assays. Each kinase activity was expressed as percent activation, relative to the maximal activation. The autoradiographed images are presented beneath the graph. (B) The activities of SEK1/MKK4 and MKK3 during Fas-induced apoptosis. Jurkat cells were left untreated (untreated), or were incubated with anti-Fas (CH-11, 250 ng/ml) for 5 h (anti-Fas), or exposed to 0.7 M NaCl for 30 min (NaCl), and were collected and lysed. The activities of MKK7 (left), SEK1/MKK4(left), MKK6 (right), and MKK3 (right) in the cell lysate were determined by immunecomplex kinase assays. (C) Fractionation of JNK/SAPK activating activity. Jurkat cells (200 ml culture) were incubated with anti-Fas mAb (CH-11, 150 ng/ml) for 5 h, and collected and lysed. The cell lysate was subjected to Hitrap-Q chromatography, and each fraction was assayed for JNK/SAPK activating activity by measuring c-Jun phosphorylating activity in the presence (SAPK+) or absence (SAPK−) of His-tagged SAPK. (Inset) A portion (300 μl) of the unadsorbed fraction (FT) was subjected to immunoprecipitation without (−) or with (+) anti-MKK7 antibody (10 μl). JNK/SAPK activating activity remaining in the supernatant was measured by c-Jun phosphorylating activity in the presence of GST-SAPK. (D) Immunodepletion of MKK6. Jurkat cells were incubated with anti-Fas mAb (CH-11, 250 ng/ ml) for 5 h, and collected and lysed. A portion of the cell lysate was subjected to immunoprecipitation with increasing amounts of anti-MKK6 pAb. p38 activating activity remaining in the supernatant was measured by ATF-2 phosphorylating activity in the presence of GST-p38 (top, closed circles). Preimmune serum was used for a control experiment (top, open circles). Each of the supernatants was analyzed by immunoblotting with anti-MKK6 and anti-MKK3 antibodies (bottom).
As for the MAP kinase kinase (MAPKK) superfamily, we could not detect significant activation of SEK1/MKK4, a well-known activator of JNK/SAPK, during Fas-induced signaling (Fig. 1 B, left). We have recently identified another activator for JNK/SAPK designated MKK7 (Moriguchi et al., manuscript in preparation). Then, we measured the activity of endogenous MKK7 by the immunecomplex kinase assay and found that MKK7 was activated by Fas before the activation of JNK/SAPK (Fig. 1, A and B). To examine the possibility that MKK7, but not SEK1/MKK4, is activated by Fas as a major activator for JNK/SAPK, we subjected the cell lysate obtained from the anti-Fas– treated Jurkat cells to chromatography on Hitrap-Q (Fig. 1 C). The JNK/SAPK activating activity was measured by increased phosphorylation of c-Jun in the presence of recombinant SAPK. Two peaks of JNK/SAPK activating activity were observed; one large peak in the flow-through (FT) and wash (W) fractions and the other, small peak eluting at about 0.1 M NaCl (Fig. 1 C). We have previously shown that MKK7 was eluted in the flow-through and wash fractions and SEK1/MKK4 in the 0.1 M NaCl fractions on Hitrap-Q (Moriguchi et al., 1995). We fractionated this large peak of the unadsorbed fractions of Hitrap-Q by heparin–Sepharose and found that elution of the JNK/ SAPK activating activity coincided with the elution of the MKK7 polypeptide detected by immunoblotting (data not shown). Furthermore, >50% of the JNK/SAPK activating activity in the unadsorbed fraction of the Hitrap-Q chromatography could be immunoprecipitated with anti-MKK7 antibody (Fig. 1 C, inset). Although we could not rule out the possibility that there is another activator for JNK/ SAPK in the unadsorbed fraction since some activity remained in the antibody-treated supernatant, this might result from the low titer of the antibody. Taken together, these results suggested that MKK7, but not SEK1/MKK4, acts as a major activator for JNK/SAPK in Fas-induced signaling.
We found also that MKK6, a specific activator of p38, was activated markedly by Fas before the activation of p38 (Fig. 1 A and B). In contrast to relatively strong activation of MKK6, another activator of p38, MKK3, was activated only weakly (Fig. 1 B, right). To examine whether MKK6 acts as the major activator of p38 in Fas-induced signaling, we immunodepleted endogenous MKK6 in the cell lysate obtained from Fas-treated Jurkat cells by using a specific polyclonal anti-MKK6 antibody (Fig. 1 D). The p38 activating activity remaining in the supernatant was decreased to less than 15% of the original level by increasing the amount of anti-MKK6 antibody (Fig. 1 D, upper). Immunoblotting of the supernatants with anti-MKK6 and anti-MKK3 antibodies showed that while the protein level of MKK6 was decreased in parallel with the decrease in the p38 activating activity, the protein level of MKK3 in the supernatant was not decreased at all (Fig. 1 D, lower). Therefore, it may be concluded that the major activator of p38 in Fas-induced signaling is MKK6, and MKK3 constitutes only part of the p38 activating activity.
Fas-induced Activation of JNK/SAPK and p38 Is Inhibited by Z-VAD-FK but Not by Ac-DEVD-CHO or Ac-YVAD-CHO
To analyze the relationship between the ICE family proteases and the JNK/SAPK and p38 kinase cascades, we used several kinds of peptide inhibitors for ICE family proteases with different specificities. Z-VAD-FK, an inhibitor for a broad spectrum of ICE family proteases, blocked the Fas-induced activation of JNK/SAPK and p38 almost completely (Fig. 2 B, left and right). In contrast, Ac-YVAD-CHO, a specific inhibitor for ICE-like proteases, did not block the activation of either kinase (Fig. 2 C). This is not surprising because, as described below, Ac-YVAD-CHO did not influence any cellular responses induced by Fas. Importantly, Ac-DEVD-CHO, a specific inhibitor for CPP32-like proteases, hardly blocked the activation of JNK/SAPK and p38 (Fig. 2 A). This is not due to insufficiency of the amount of this inhibitor because the same concentration of the inhibitor blocked the Fas-induced chromatin condensation and DNA fragmentation (see Figs. 3 and 4). In addition, essentially the same results were obtained by using Z-DEVD-FK, the other specific inhibitor for CPP32-like proteases, that is more permeable than Ac-DEVD-CHO (data not shown).
Figure 2.
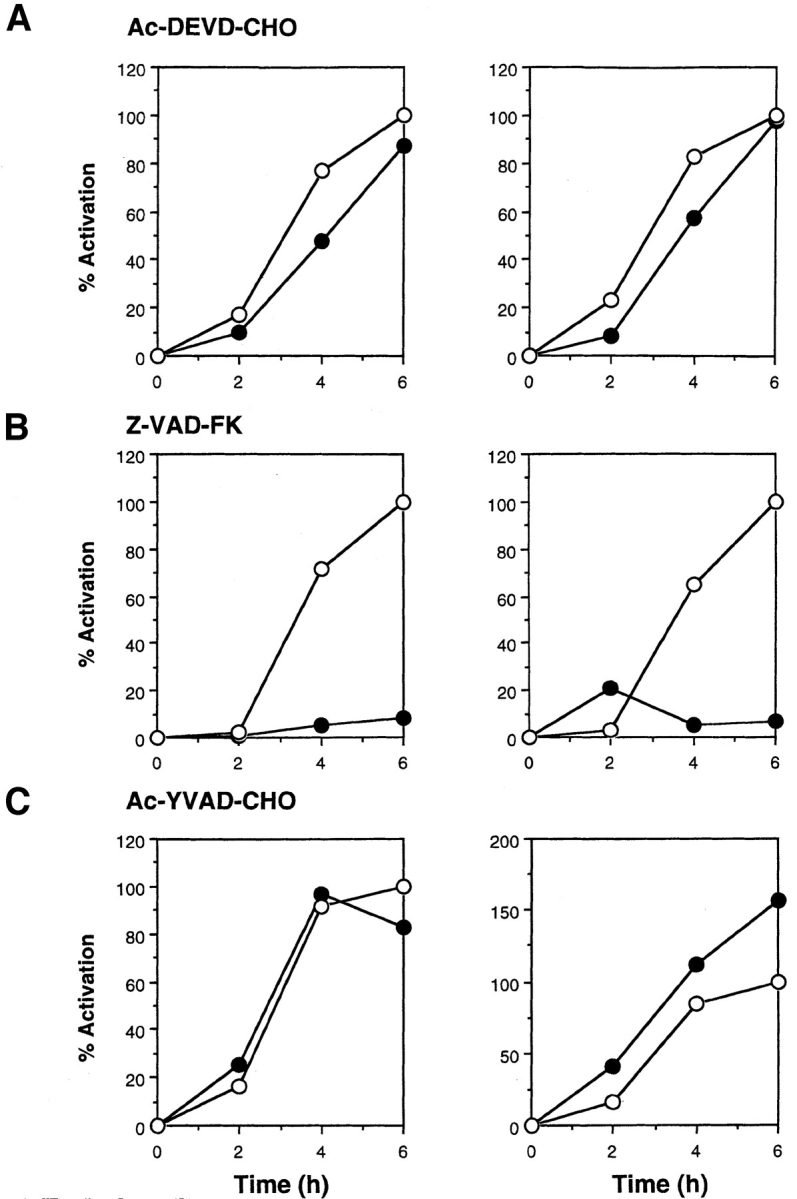
Effect of ICE-family protease inhibitors on Fas-induced activation of JNK/SAPK (left) and p38 (right). Jurkat cells were preincubated with ICE-family protease inhibitors (A, Ac-DEVD-CHO, 120 μM; B, Z-VAD-FK, 40 μM; C, Ac-YVAD-CHO, 120 μM) (closed circles) or with the solvent DMSO (open circles) as control for 1 h and then treated with anti-Fas mAb (CH-11, 150 ng/ml) for various periods. Cell lysates were prepared, and the activities of endogenous JNK/SAPK (left panels) and endogenous p38 (right panels) were determined by immunecomplex kinase assays. The kinase activity was expressed as percent activation, relative to the maximal activation in control cells at 6 h incubation as 100%.
Figure 3.
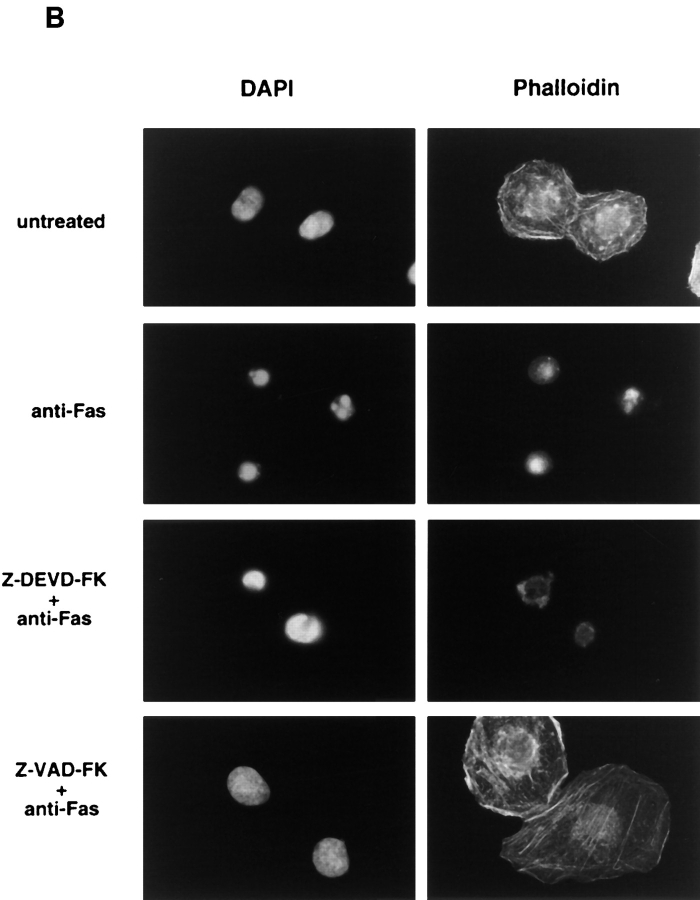
Effect of ICE-family protease inhibitors on Fas-induced cellular responses. Jurkat cells were treated with anti-Fas mAb (CH-11, 150 ng/ml) for 20 h after a 1-h preincubation without (d–f) or with ICE-family protease inhibitors; Ac-DEVD-CHO, 120 μM (g–i); Z-VAD-FK, 40 μM (j–l); Ac-YVAD-CHO, 120 μM (m–o). Cells were fixed and double stained with DAPI (middle panels) and Apo2.7 (right panels). Left panels show phase contrast images of cells before fixation.
Figure 4.
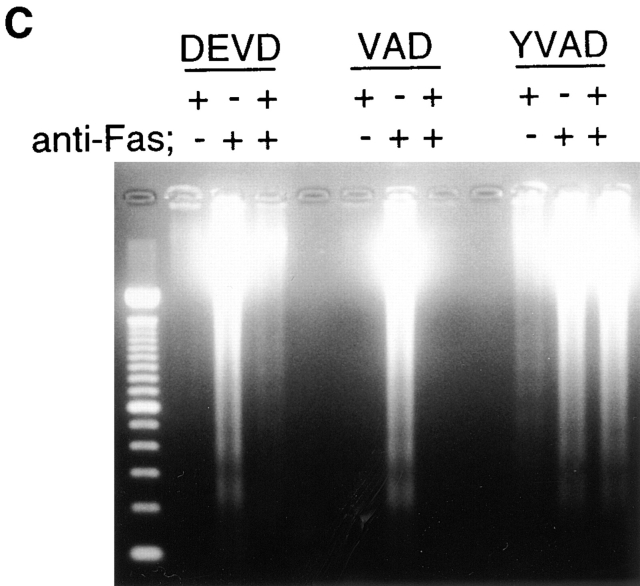
Inhibition of CPP32-like proteases did block Fas-induced chromatin condensation and DNA fragmentation but did not block induction of Apo2.7 staining. Jurkat cells were treated as in Fig. 3, and the cells that were stained with Apo2.7 (A) and the cells with apoptotic condensed nuclei (B) were counted. (C) Cellular DNA was extracted and analyzed by electrophoresis on a 1.7% agarose gel to detect DNA fragmentation.
Fas-induced Cellular Responses Are Totally Inhibited by Z-VAD-FK but Only Partly by Ac-DEVD-CHO
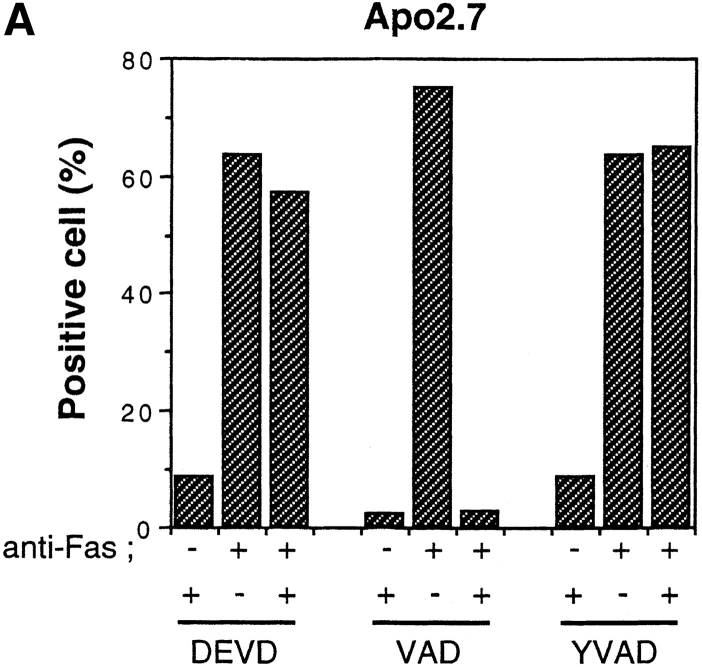
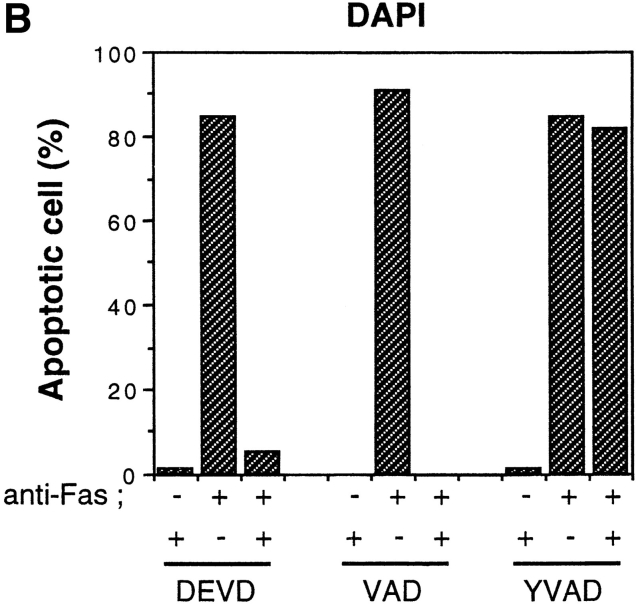
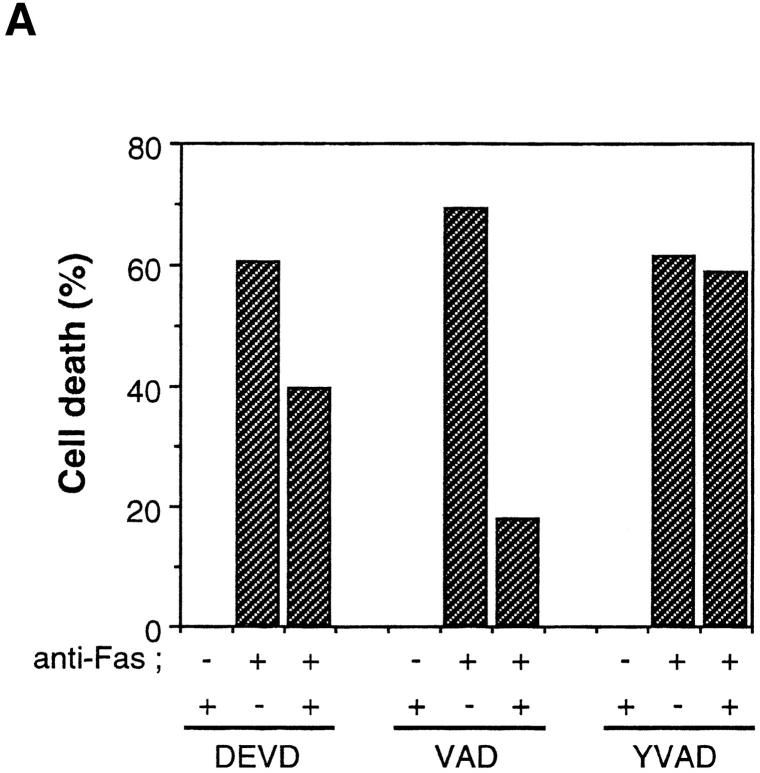
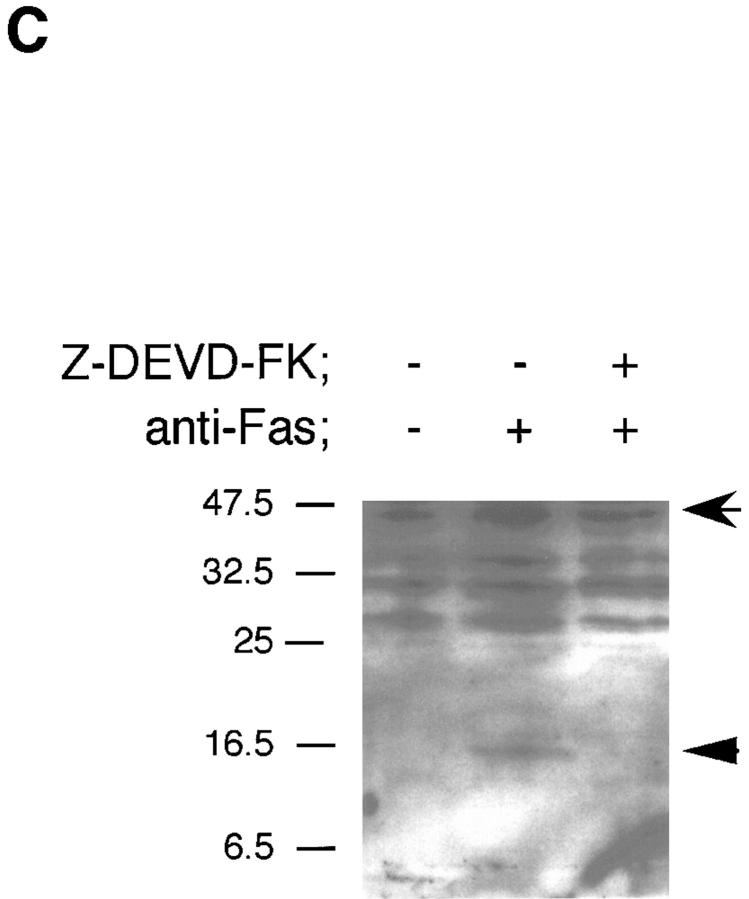
To dissect the Fas-induced apoptotic signaling pathways, we investigated the effects of several kinds of protease inhibitors on the Fas-induced cellular responses. We first examined chromatin condensaton and DNA fragmentation, typical nuclear phenomena in apoptosis. Jurkat cells were incubated with several kinds of inhibitors for ICE family for 1 h followed by treatment with anti-Fas mAb (150 ng/ ml) for 20 h. The cells were fixed and stained with DAPI (Fig. 3, b, e, h, k, and n). In the presence of Z-VAD-FK the Fas-induced chromatin condensation was completely inhibited (Fig. 3, k, and Fig. 4 B, VAD) and no change in cell morphology was induced by Fas (Fig. 3, a, d, and j). Cellular DNA was extracted and analyzed by electrophoresis to detect DNA fragmentation (Fig. 4 C). The Fas-induced DNA fragmentation was also inhibited by Z-VAD-FK (Fig. 4 C, VAD). In contrast, in the presence of Ac-YVAD-CHO, neither chromatin condensation nor DNA fragmentation was inhibited (Fig. 3, n, Fig. 4 B, YVAD, and Fig. 4 C, YVAD), and the cells were fragmented into apoptotic bodies by Fas (Fig. 3, m). Although Enari et al. (1995, 1996) and Los et al. (1995) showed previously that Fas-induced apoptosis in W4 cells or SKW 6.4 cells is blocked by YVAD-type inhibitors, we could not see any inhibitory effect of Ac-YVAD-CHO on the Fas-induced apoptosis in Jurkat cells even at the concentration of 200 μM. This discrepancy may be due to the difference of cell types used as several groups also demonstrated the lack of inhibitory effect of YVAD-type inhibitors on the Fas-induced apoptosis in Jurkat cells (Martin et al., 1996; Schlegel et al., 1996). The effect of Ac-DEVD-CHO was interesting. In the presence of Ac-DEVD-CHO, the Fas-induced chromatin condensation and DNA fragmentation were almost completely inhibited (Fig. 3, h, Fig. 4 B, DEVD, and Fig. 4 C, DEVD), but a marked change in the cell morphology, cell shrinkage, was induced by Fas (Fig. 3, g).
We next examined Apo2.7 staining. Apo2.7 is a monoclonal antibody obtained by immunizing mice with apoptotic Jurkat cells (Zhang et al., 1996). It has been shown that Apo2.7 recognizes a 38-kD protein which is expressed on mitochondrial membrane in apoptotic Jurkat cells and that Apo2.7 reacts only with apoptotic Jurkat cells but not with nonapoptotic cells (Zhang et al., 1996). In fact, Apo2.7 staining was detected in anti-Fas–treated Jurkat cells but not in untreated cells (Fig. 3, f and c, and Fig. 4 A). Z-VAD-FK inhibited the appearance of this Apo2.7 staining (Fig. 3, l and Fig. 4 A, VAD). In contrast, Ac-YVAD-CHO did not inhibit the induction of the Apo2.7 staining (Fig. 3, o and Fig. 4 A, YVAD) nor apoptotic morphological changes (Fig. 3, m and n), confirming that Ac-YVAD-CHO cannot inhibit Fas-induced apoptosis. Interestingly, Ac-DEVD-CHO-treated cells which, after Fas stimulation, exhibited normal nuclear morphology were strongly positive with Apo2.7 (Fig. 3, i and Fig. 4 A, DEVD). The same result was obtained by using Z-DEVD-FK, another inhibitor for CPP32-like proteases (data not shown). These results were rather surprising because a CPP32-like protease(s) was thought to be essential for Fas-induced apoptotic signaling. So we next examined whether these cells were dead or not.
Fas-induced Cell Death Is Not Inhibited by Ac-DEVD-CHO
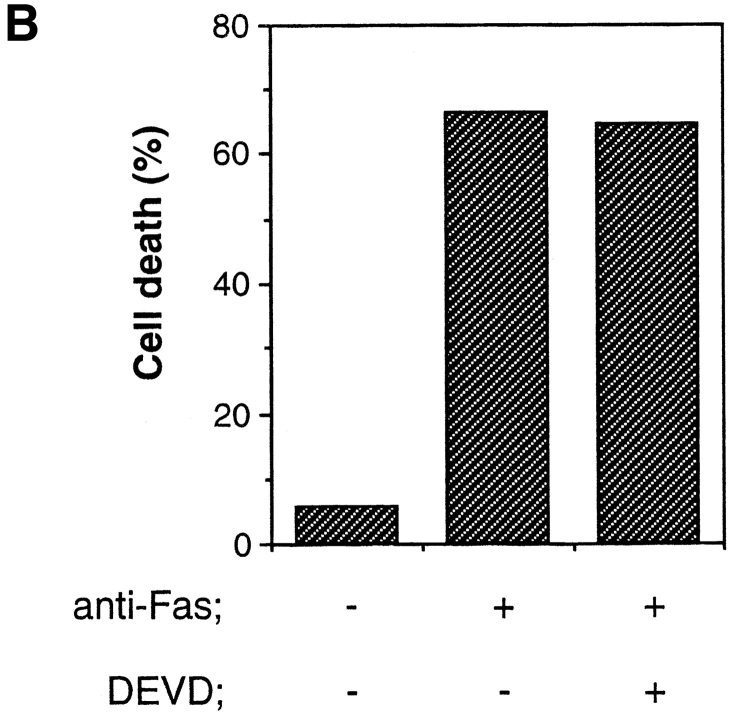
To examine the viability of the anti-Fas–treated cells, we first used the MTT assay which measures the activity of the mitochondrial enzyme. As shown in Fig. 5 A, the Fas-induced loss of mitochondrial activity was strongly inhibited by Z-VAD-FK but only weakly inhibited by Ac-DEVD-CHO. We next assayed membrane permeability of Ac-DEVD-CHO–treated cells by trypan blue dye exclusion. Even in the presence of Ac-DEVD-CHO, which inhibited the Fas-induced chromatin condensation and DNA fragmentation, anti-Fas–treated cells lost thier ability of dye exclusion (Fig. 5 B), suggesting that the Fas-induced cell death is not inhibited by Ac-DEVD-CHO.
Figure 5.
Inhibition of CPP32-like proteases did not block Fas-induced cell death. (A) Jurkat cells were treated as in Fig. 3, and the percentage of cell death was determined by MTT assay. (B) Jurkat cells were preincubated with or without Ac-DEVD-CHO (120 μM) and then treated with anti-Fas mAb (CH-11, 150 ng/ ml) for 20 h. Cell death was measured by using trypan blue dye exclusion, and is expressed as a percentage of cell death.
The Activation of JNK/SAPK and p38, the Changes in Cell Morphology, and the Cell Death Induced by Fas in KB Cells Are Not Blocked by Inhibition of CPP32-like Proteases
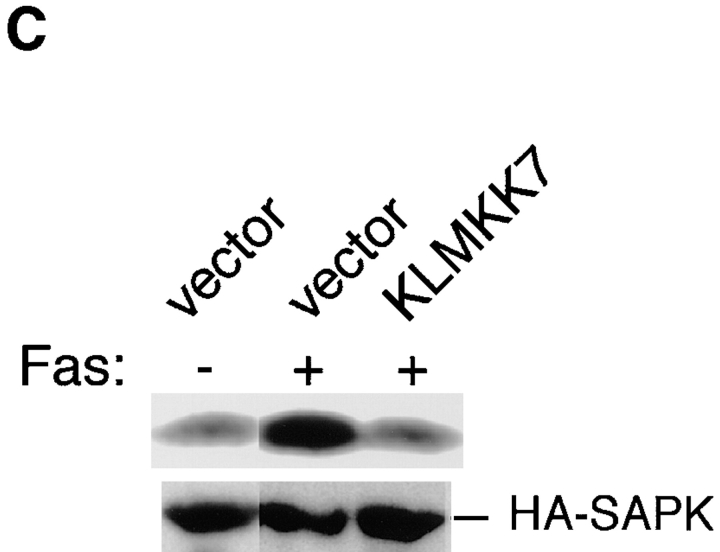
To generalize the above observation that a CPP32-like protease(s) may be specifically involved in the Fas-induced chromatin condensation and DNA fragmentation, but not in other apoptotic responses, we then used KB cells. It is known that the apoptosis of KB cells can be induced by Fas in the presence of cycloheximide. Cycloheximide treatment alone did not induce any apoptotic responses in KB cells (data not shown). We then examined the activities of JNK/SAPK and p38 during Fas stimulation in the presence of cycloheximide (Fig. 6). KB cells were preincubated with 50 μg/ml cycloheximide for 1 h, and then treated with anti-Fas mAb (CH-11, 200 ng/ml, at time 0 in Fig. 6 A). Cycloheximide is one of known activators of JNK/SAPK (Kyriakis et al., 1994) and p38 (Moriguchi et al., 1996b ), and here we detected slight activation of JNK/SAPK and p38, peaking at ∼1 or 2 h after the addition of cycloheximide in the absence of anti-Fas (Fig. 6 A, open circles). Then, strong activation of JNK/SAPK and p38 occurred 3 to 5 h after the treatment with anti-Fas (Fig. 6 A, closed circles), when the cycloheximide-induced activation of JNK/SAPK and p38 was decreased to the basal level (Fig. 6 A, open circles). Preincubation with Z-DEVD-FK, an inhibitor of CPP32-like proteases, did not block significantly the Fas-induced activation of JNK/SAPK and p38 in the cycloheximide-treated KB cells (Fig. 6 B). We then examined the effect of expression of dominant negative MKK7 (KL-MKK7) on the Fas-induced activation of JNK/SAPK (Fig. 6 C). KB cells were transiently cotransfected with HA-tagged SAPK/JNK and KL-MKK7 (or a vacant plasmid) and then treated with anti-Fas mAb (CH-11, 250 ng/ml). After 2 h, HA-tagged SAPK/JNK was precipitated with anti-HA antibody and assayed for the kinase activity. The Fas-induced activation of SAPK/JNK was almost completely inhibited by KL-MKK7 (Fig. 6 C). The result is consistent with our conclusion that the major activator of JNK/SAPK during Fas signaling is MKK7.
Figure 6.
(A) Fas induced the activation of JNK/SAPK and p38 in KB cells. KB cells were preincubated with 50 μg/ml cycloheximide for 1 h (−1 to 0 h) and then treated with (closed circles) or without (open circles) anti-Fas mAb (CH-11, 200 ng/ml) for various periods. Cell lysates were prepared, and the activities of endogenous JNK/SAPK (left panel) and endogenous p38 (right panel) were determined by immunecomplex kinase assays. The activity was expressed as fold-increase relative to that in time −1 h. (B) Fas-induced activation of JNK/SAPK and p38 is not dependent on CPP32-like proteases in KB cells. KB cells were preincubated with 50 μM Z-DEVD-FK for 1 h and then treated with anti-Fas mAb (CH-11, 200 ng/ml) for 4 h. As a control, cells were exposed to 0.7 M NaCl for 30 min (NaCl). The activities of endogenous JNK/SAPK and endogenous p38 were determined by immunecomplex kinase assays. Cycloheximide (50 μg/ml) was added in all samples. (C) Dominant negative effect of KL-MKK7 on the Fas-induced activation of JNK/SAPK in KB cells. KB cells were transiently cotransfected with HA-tagged SAPK/JNK and KL-MKK7 (a kinase-negative mutant of MKK7) (KLMKK7) or a vacant plasmid (vector), and then treated with anti-Fas mAb (CH-11, 250 ng/ml). After 2 h, the HA-tagged SAPK/JNK was precipitated by anti-HA antibody and assayed for the kinase activity (top). The immunoprecipitated HA-SAPK/JNK was analyzed by immunoblotting with anti-SAPK antibody (bottom). Cycloheximide (50 μg/ml) was added in all samples.
We next examined other apoptotic responses in KB cells. KB cells were preincubated with or without each of peptide inhibitors, Z-VAD-FK or Z-DEVD-FK, for 1 h in the presence of 50 μg/ml cycloheximide. The cells were then treated with anti-Fas mAb (CH-11, 150 ng/ml). In the absence of peptide inhibitors, dramatic morphological changes, rounding and shrinkage of the cell and membrane blebbing, were induced 3–4 h after Fas stimulation, and almost all the cells became detached from the dish and fragmented into apoptotic bodies within 8 h (Fig. 7 A, anti-Fas). In the presence of Z-VAD-FK, all of the Fas-induced cellular responses were completely inhibited (Fig. 7, A and B, Z-VAD-FK + anti-Fas). In the presence of Z-DEVD-FK, cellular shrinkage and membrane blebbing were induced by Fas stimulation, like in the absence of the inhibitor, but the cells were not detached from the dish and eventually died without any fragmentation of the cell (Fig. 7 A, Z-DEVD-FK + anti-Fas). When KB cells were fixed 8 h after Fas stimulation, and stained with DAPI and phalloidin, the Fas-induced chromatin condensation and disruption of actin network were clearly observed (Fig. 7 B, anti-Fas). Z-VAD-FK completely blocked either response; the cells exhibited normal nuclear morphology and normal actin network (Fig. 7 B, Z-VAD-FK + anti-Fas). Importantly, Z-DEVD-FK inhibited strongly the Fas-induced chromatin condensation but did not block the disruption of actin network; the cells thus showed only partial chromatin condensation and no actin network (Fig. 7 B, Z-DEVD-FK + anti-Fas). Essentially the same results were obtained with Ac-DEVD-CHO (data not shown).
Figure 7.
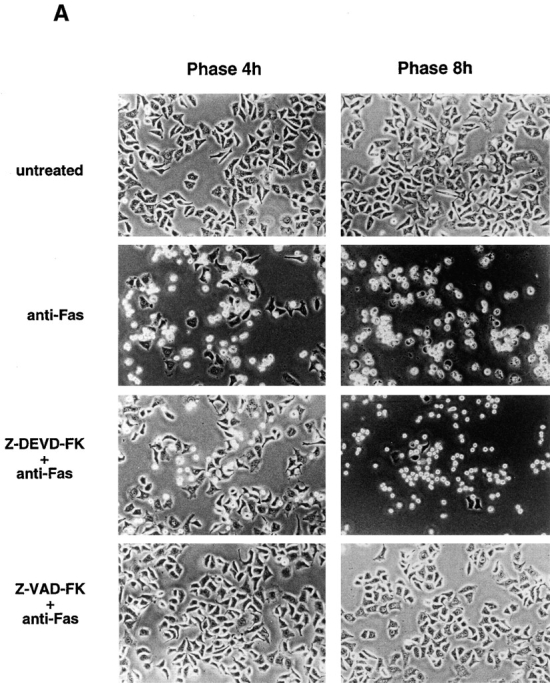
Inhibition of CPP32-like proteases did not block Fas-induced morphological changes in KB cells. (A) KB cells were preincubated for 1 h with or without ICE-family protease inhibitors; Z-DEVD-FK (50 μM) or Z-VAD-FK (50 μM) in the presence of 50 μg/ml cycloheximide. The cells were then treated with anti-Fas mAb (CH-11, 150 ng/ml) for 4 and 8 h. Phase contrast images are shown. (B) KB cells were treated as in A. After incubation for 8 h, the cells were fixed and stained with DAPI and phalloidin. (C) KB cells were preincubated for 1 h with or without Z-DEVD-FK (50 μM) in the presence of 50 μg/ml cycloheximide. The cells were then treated with anti-Fas mAb (CH-11, 150 ng/ml) for 8 h, and collected and lysed. A portion of the cell lysate was analyzed by immunoblotting with anti-actin mAb (A-4700; Sigma) that recognizes the COOH-terminal region of actin. An arrow and an arrowhead indicate an intact actin molecule and a 15-kD fragment of actin, respectively.
It was previously reported that actin (45 kD) was proteolytically cleaved by ICE family proteases into 15- and 30-kD fragments in several types of apoptosis (Mashima et al., 1995; McCarthy et al., 1997). To examine whether the Fas-induced disruption of actin network is associated with the cleavage of actin molecule, the cell lysate obtained from the anti-Fas–treated KB cells in the presence or absence of Z-DEVD-FK was subjected to immunoblotting with anti-actin mAb (A-4700) that recognizes the COOH-terminal region of actin. In the absence of Z-DEVD-FK, 15-kD COOH-terminal fragment of actin appeared after Fas stimulation (Fig. 7 C, middle lane), but in the presence of Z-DEVD-FK, this cleavage was not seen (Fig. 7 C, right lane). As the disruption of actin network occurs even in the presence of Z-DEVD-FK, the Fas-induced morphological changes may be independent of the cleavage of actin molecule.
Discussion
Apoptosis or a programmed cell death is a mechanism of cell suicide that is conserved in many kinds of species. It has been shown that ICE and/or ICE-related proteases play a central role in execution of cell death in many types of apoptosis. The ICE-related proteases, now designated caspase-1 to -10, are classified into three groups; ICE-, CPP32-, and Ich-1–like proteases. In Fas-induced apoptosis, it has been suggested that the activation of ICE-like proteases is followed by the activation of CPP32-like proteases and that CPP32-like proteases are essential for Fas-induced apoptotic responses. In addition, a number of molecules other than proteases have been suggested to be involved in Fas-induced apoptotic signaling, including ceramide, reactive oxygen species, protein kinases, protein phosphatases, and cytochrome c. But, the relationship between the ICE-related proteases and the other molecules has not been fully understood. Further, how these molecules are related to apoptotic cellular responses has not been analyzed in detail. To address these questions, we first focused on the MAP kinase superfamily molecules, and found that slow and sustained activation of both the MKK7-JNK/SAPK cascade and the MKK6-p38 cascade occurs during Fas-induced apoptosis in an ICE family protease(s)-dependent manner. This extends previous observations showing activation of JNK/SAPK (Kevin et al., 1996; Wilson et al., 1996; Juo et al., 1997; Lenczowski et al., 1997) and p38 (Juo et al., 1997). Rather surprisingly, the activation of both kinase cascades was not suppressed by specific inhibitors for CPP32-like proteases which were able to block almost completely the Fas-induced chromatin condensation and DNA fragmentation, indicating that the signaling pathway of the protein kinase cascades is independent, or lying upstream, of the activation of CPP32-like proteases. Since expression of a dominant negative form of JNK/SAPK activator that blocked the Fas-induced activation of JNK/SAPK had no effect on Fas-mediated DNA fragmentation and chromatin condensation (Lenczowski et al., 1997 and our unpublished data), the JNK/ SAPK activation pathway may not lie upstream of the activation of CPP32-like proteases that is essential for DNA fragmentation. Therefore, in Fas signaling, the activation of ICE-like proteases is followed by at least two separate pathways; one is the activation of CPP32-like proteases followed by CPP32-like proteases-dependent processes, and the other is independent of CPP32-like proteases, such as the activation of the MAP kinase superfamily molecules. Moreover, the above data clearly indicated that the MAP kinase superfamily molecules are not involved in the Fas-mediated chromatin condensation and DNA fragmentation.
The close examination of the anti-Fas–treated Jurkat cells in the presence of the inhibitor for CPP32-like proteases revealed that a number of apoptotic responses in cells occur independent of CPP32-like proteases, including cell shrinkage and induction of Apo2.7 staining in mitochondria. More importantly, even the cell death itself, as assessed by the dye exclusion ability, can be induced by Fas stimulation in the presence of the inhibitor for CPP32-like proteases. In this case, the cells die without chromatin condensation and DNA fragmentation.
In KB cells that are cultured as mono-layer on a dish, Fas stimulation induces apoptosis in the presence of cycloheximide. The Fas-induced morphological changes are quite conspicuous in KB cells and thus are easily detected. Inhibition of CPP32-like proteases did not block these dramatic morphological changes of KB cells such as rounding and shrinkage and membrane blebbing, but did inhibit chromatin condensation. These anti-Fas–treated KB cells with the inhibitor for CPP32-like proteases eventually died and became detached from the dish, although the detachment was markedly delayed as compared with the cells that were treated with anti-Fas in the absence of the inhibitor. Therefore, Fas stimulation is able to induce the death of KB cells without chromatin condensation when CPP32-like proteases are inhibited. Thus, in both Jurkat cells and KB cells there are CPP32-like proteases-dependent and -independent pathways; the former leads to chromatin condensation and DNA fragmentation and the latter may lead to other apoptotic responses such as morphological changes. A hypothetical model of our proposed signaling pathways in Fas-induced apoptosis is shown in Fig. 8. Differential involvement of different proteases in different apoptotic responses has also been suggested recently (Cryns et al., 1996). Moreover, a recent report of McCarthy et al. (1997) has shown that in the apoptosis induced by c-myc, etoposide, and Bak, the DNA fragmentation but not the cell shrinkage and membrane blebbing is blocked by the ICE-family protease inhibitor. It is interesting to speculate that in the apoptotic signaling the chromatin condensation and DNA fragmentation, one of the most characteristic events in the apoptotic nuclei, and the morphological change, a phenomenon outside the nucleus, are separately directed by different pathways. Most recently, Rudel and Bokoch (1997) reported that PAK2, a member of the p21 activated protein kinase family, is cleaved to be activated by Fas in a CPP32-like protease(s)-dependent manner. They have also shown that expression of dominant-negative PAK inhibits formation of apoptotic bodies during Fas-induced apoptosis but has no effect on Fas-induced DNA fragmentation. Our observations here showed that in the presence of the inhibitor for CPP32-like proteases, neither DNA fragmentation nor formation of apoptotic bodies occurred in Fas-treated Jurkat or KB cells (Fig. 3 g, and data not shown). So, not only DNA fragmentation but also formation of apoptotic bodies may lie downstream of a CPP32-like protease(s), which is not involved in Fas-induced membrane blebbing and cell shrinkage. It is interesting to examine whether Fas-induced cell shrinkage and membrane blebbing can occur in those cells expressing dominant-negative PAK. As some members of PAK family have been shown to activate JNK/SAPK signaling pathways, Fas-induced activation of PAK2 might lead to activation of JNK/SAPK. However, our observation clearly indicates that the activation of JNK/SAPK by Fas is independent of CPP32-like proteases. Additional experiments are necessary to elucidate the Fas-induced signaling pathway that leads to activation of JNK/SAPK and p38.
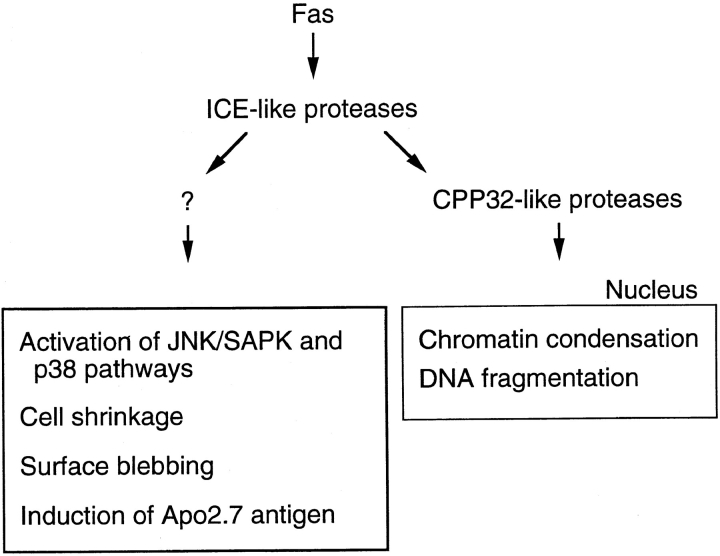
Figure 8.
A proposed model for Fas-induced signaling pathways.
The present finding that both the Fas-induced morphological changes (cell shrinkage and membrane blebbing) and the Fas-induced activation of the MAPK superfamily molecules (JNK/SAPK and p38) are independent of CPP32-like proteases might suggest the possibility of involvement of the MAPK superfamily in the morphological changes. In our preliminary experiments, however, expression of a dominant negative form of MKK7 or a MAPK phosphatase-1 (MKP-1 or CL100) did not affect the Fas-induced cell shrinkage and membrane blebbing in the presence of Z-DEVD-FK, the inhibitor for CPP32-like proteases. Thus, the MAPK superfamily molecules may not be directly responsible for the Fas-induced morphological responses. We may hypothesize that the Fas signaling pathway diverges into multiple, separate biochemical and cellular processes, each of which may be responsible for each part of the apoptotic cellular responses. The molecular mechanisms for the Fas-induced morphological changes as well as the cellular events elicited by the Fas-induced activation of the MAPK superfamily molecules are to be elucidated in future studies.
Acknowledgments
This work was supported by Grants-in-Aid from the Ministry of Education, Science and Culture of Japan to E. Nishida.
Footnotes
1. Abbreviation used in this paper: ICE, IL-1β converting enzyme.
References
- Boldin MP, Varfolomeev EE, Pancer Z, Mett IL, Camonis JH, Wallach D. A novel protein that interacts with the death domain of Fas/ APO1 containing a sequence motif related to the death domain. J Biol Chem. 1995;270:7795–7798. doi: 10.1074/jbc.270.14.7795. [DOI] [PubMed] [Google Scholar]
- Boldin M, Goncharov T, Goltsev Y, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- Chen Y-R, Wang X, Templeton D, Davis RJ, Tan T-H. The role of c-Jun N-terminal kinase (JNK) in apoptosis induced by ultraviolet C and γ radiation. J Biol Chem. 1996;271:31929–31936. doi: 10.1074/jbc.271.50.31929. [DOI] [PubMed] [Google Scholar]
- Chinnaiyan AM, O'Rourke K, Tewari M, Dixit VM. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- Chinnaiyan AM, Tepper CG, Seldin MF, O'Rourke K, Kischkel FC, Hellbardt S, Krammer PH, Peter ME, Dixit VM. FADD/ MORT1 is a common mediator of CD95 (Fas/APO-1) and tumor necrosis factor receptor-induced apoptosis. J Biol Chem. 1996;271:4961–4965. doi: 10.1074/jbc.271.9.4961. [DOI] [PubMed] [Google Scholar]
- Cryns VL, Bergeron L, Zhu H, Li H, Yuan J. Specific cleavage of α-fodrin during Fas- and tumor necrosis factor-induced apoptosis is mediated by an interleukin-1β-converting enzyme/Ced-3 protease distinct from the poly (ADP-ribose) polymerase protease. J Biol Chem. 1996;271:31277–31282. doi: 10.1074/jbc.271.49.31277. [DOI] [PubMed] [Google Scholar]
- Cuenda A, Alonso G, Morrice N, Jones M, Meier R, Cohen P, Nebreda AR. Purification and cDNA cloning of SAPKK3, the major activator of RK/p38 in stress- and cytokine- stimulated monocytes and epithelial cells. EMBO (Eur Mol Biol Organ) J. 1996;15:4156–4164. [PMC free article] [PubMed] [Google Scholar]
- Derijard B, Hibi M, Wu I-H, Barrett T, Su B, Deng T, Karin M, Davis RJ. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphotylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- Derijard B, Raingeaud J, Barret T, Wu I-H, Han J, Ulevitch RJ, Davis RJ. Independent human MAP kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267:682–685. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- Duan H, Chinnaiyan AM, Hudson PL, Wing JP, He WW, Dixit VM. ICE-LAP3, a novel mammalian homologue of the Caenorhabditis eleganscell death protein Ced-3 is activated during Fas- and tumor necrosis factor-induced apoptosis. J Biol Chem. 1996a;271:1621–1625. doi: 10.1074/jbc.271.3.1621. [DOI] [PubMed] [Google Scholar]
- Duan H, Orth K, Chinnaiyan AM, Poirier GG, Froelich CJ, He WW, Dixit VM. ICE-LAP6, a novel member of the ICE/Ced-3 gene family, is activated by the cytotoxic T cell protease granzyme B. J Biol Chem. 1996b;271:16720–16724. doi: 10.1074/jbc.271.28.16720. [DOI] [PubMed] [Google Scholar]
- Ellis RE, Yuan J, Horvitz HR. Mechanisms and functions of cell death. Annu Rev Cell Biol. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- Enari M, Hug H, Nagata S. Involvement of an ICE-like protease in Fas-mediated apoptosis. Nature. 1995;375:78–81. doi: 10.1038/375078a0. [DOI] [PubMed] [Google Scholar]
- Enari M, Talanian RV, Wong WW, Nagata S. Sequential activation of ICE-like and CPP32-like proteases during Fas-mediated apoptosis. Nature. 1996;380:723–726. doi: 10.1038/380723a0. [DOI] [PubMed] [Google Scholar]
- Faucheu C, Diu A, Chan AWE, Blanchet A-M, Miossec C, Herve F, Colard-Dutilleu V, Gu Y, Aldape RA, Lippke JA, et al. A novel human protease similar to the interleukin-1β converting enzyme induces apoptosis in transfected cells. EMBO (Eur Mol Biol Organ) J. 1995;14:1914–1922. doi: 10.1002/j.1460-2075.1995.tb07183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes-Alnemri T, Litwack G, Alnemri ES. CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegancecell death protein Ced-3 and mammalian interleukin-1β-converting enzyme. J Biol Chem. 1994;269:30761–30764. [PubMed] [Google Scholar]
- Fernandes-Alnemri T, Litwack G, Alnemri E. Mch2, a new member of the apoptotic ced-3/ICE cystein protease gene family. Cancer Res. 1995a;55:2737–2742. [PubMed] [Google Scholar]
- Fernandes-Alnemri T, Takahashi A, Armstrong R, Krebs J, Fritz L, Tomaselli K, Wang L, Yu Z, Croce C, Salveson G, et al. Mch3, a novel human cysteine protease highly related to CPP32. Cancer Res. 1995b;55:6045–6052. [PubMed] [Google Scholar]
- Fernandes-Alnemri T, Armstrong RC, Krebs J, Srinivasula SM, Wang L, Bullrich F, Fritz LC, Trapani JA, Tomaselli KJ, Litwack G, Alnemri ES. In vitro activation of CPP32 and Mch3 by Mch4, a novel human apoptotic cysteine protease containing two FADD-like domains. Proc Natl Acad Sci USA. 1996;93:7464–7469. doi: 10.1073/pnas.93.15.7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- Han J, Lee J-D, Jiang Y, Li Z, Feng L, Ulevitch RJ. Characterization of the structure and function of a novel MAP kinase kinase (MKK6) J Biol Chem. 1996;271:2886–2891. doi: 10.1074/jbc.271.6.2886. [DOI] [PubMed] [Google Scholar]
- Hengartner MO, Horvitz HR. C.elegans cell survival gene ced-9 encodes a functional homologue of the mammalian proto-oncogene bcl-2. . Cell. 1994;76:665–676. doi: 10.1016/0092-8674(94)90506-1. [DOI] [PubMed] [Google Scholar]
- Hengartner MO, Ellis RE, Horvitz HR. Caenorhabditis elegans gene ced-9protects cells from programmed cell death. Nature. 1992;356:494–499. doi: 10.1038/356494a0. [DOI] [PubMed] [Google Scholar]
- Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima S, Sameshima M, Hase A, Seto Y, Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediated apoptosis. Cell. 1991;66:233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- Itoh N, Nagata S. A novel protein domain required for apoptosis. J Biol Chem. 1993;268:10932–10937. [PubMed] [Google Scholar]
- Juo P, Kuo CJ, Reynolds SE, Konz RF, Raingeaud J, Davis RJ, Biemann H-P, Blenis J. Fas activation of the p38 mitogen-activated protein kinase signaling pathway requires ICE/CED-3 family proteases. Mol Cell Biol. 1997;17:24–35. doi: 10.1128/mcb.17.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamens J, Paskind M, Hugunin M, Talanian RV, Allen H, Banach D, Bump N, Hackett M, Johnston CG, Li P, et al. Identification and characterization of ICH-2, a novel member of the interleukin-1β-converting enzyme family of cysteine proteases. J Biol Chem. 1995;270:15250–15256. doi: 10.1074/jbc.270.25.15250. [DOI] [PubMed] [Google Scholar]
- Kumar S, Kinoshita M, Noda M, Copeland N, Jenkins N. Induction of apoptosis by the Nedd2 gene, which encodes a protein similar to the Caenorhabditis elegance cell death gene ced-3and the mammalian IL-1β-converting enzyme. Genes Dev. 1994;8:1613–1626. doi: 10.1101/gad.8.14.1613. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Banerjee P, Nikolakaki E, Dai T, Rubie EA, Ahmad MF, Avruch J, Woodgett JR. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- Latinis KM, Koretzky GA. Fas ligation induced apoptosis and Jun kinase activation independently of CD45 and Lck in human T cells. Blood. 1996;87:871–875. [PubMed] [Google Scholar]
- Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, McNulty D, Blumenthal MJ, Heys JR, Landvatter SW, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- Lenczowski JM, Dominguez L, Eder AM, King LB, Zacharchuk CM, Ashwell JD. Lack of a role for Jun kinase and AP-1 in Fas-induced apoptosis. Mol Cell Biol. 1997;17:170–181. doi: 10.1128/mcb.17.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippke JA, Gu Y, Samecki C, Caron PR, Su MS-S. Identification and characterization of CPP32/Mch-2 homolog 1, a novel cysteine protease similar to CPP32. J Biol Chem. 1996;271:1825–1828. doi: 10.1074/jbc.271.4.1825. [DOI] [PubMed] [Google Scholar]
- Los M, Craen MV, Penning LC, Schenk H, Weatendorp M, Baeuerle PA, Droge W, Krammer PH, Fiers W, Schulze-Osthoff K. Requirement of an ICE/CED-3 protease for Fas/APO-1-mediated apoptosis. Nature. 1995;375:81–83. doi: 10.1038/375081a0. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Finucane DM, Amarante-Mendes GP, O'Brien GA, Green DR. Phosphatidylserine externalization during CD95-induced apoptosis of cells and cytoplasts requires ICE/CED-3 protease activity. J Biol Chem. 1996;271:28753–28756. doi: 10.1074/jbc.271.46.28753. [DOI] [PubMed] [Google Scholar]
- Mashima T, Naito M, Fujita N, Noguchi K, Tsuruo T. Identification of actin as a substrate of ICE and an ICE-like protease and involvement of an ICE-like protease but not ICE in VP-16-induced U937 apoptosis. Biochem Biophys Res Commun. 1995;271:1185–1192. doi: 10.1006/bbrc.1995.2894. [DOI] [PubMed] [Google Scholar]
- McCarthy NJ, Whyte MKB, Gilbert CS, Evan GI. Inhibition of Ced-3/ICE-related protease does not prevent cell death induced by oncogenes, DNA damage, or the Bcl-2 homologue Bak. J Cell Biol. 1997;136:215–227. doi: 10.1083/jcb.136.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T, Kawasaki H, Matsuda S, Gotoh Y, Nishida E. Evidence for multiple activators for stress-activated protein kinases/c-Jun amino-terminal kinases. J Biol Chem. 1995;270:12969–12972. doi: 10.1074/jbc.270.22.12969. [DOI] [PubMed] [Google Scholar]
- Moriguchi T, Kuroyanagi N, Yamaguchi K, Gotoh Y, Irie K, Kano T, Shirakabe K, Muro Y, Shibuya H, Matsumoto K, et al. A novel kinase cascade mediated by mitogen-activated protein kinase kinase 6 and MKK3. J Biol Chem. 1996a;271:13675–13679. doi: 10.1074/jbc.271.23.13675. [DOI] [PubMed] [Google Scholar]
- Moriguchi T, Toyoshima F, Gotoh Y, Iwamatsu A, Irie K, Mori E, Kuroyanagi N, Hagiwara M, Matsumoto K, Nishida E. Purification and identification of a major activator for p38 from osmotically shocked cells. Activation of mitogen-activated protein kinase kinase 6 by osmotic shock, tumor necrosis factor-α, and H2O2 . J Biol Chem. 1996b;271:26981–26988. doi: 10.1074/jbc.271.43.26981. [DOI] [PubMed] [Google Scholar]
- Moriguchi, T., F. Toyoshima, N. Masuyama, H. Hanafusa, Y. Gotoh, and E. Nishida. 1997. A novel SAPK/JNK kinase, MKK7, stimulated by TNFα and cellular stresses. EMBO J. In press. [DOI] [PMC free article] [PubMed]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Munday NA, Vaillancourt JP, Ali A, Casano FJ, Miller DK, Molineaux SM, Yamin TT, Yu VL, Nicholson DW. Molecular cloning and pro-apoptotic activity of ICErel-II and ICErel-III, members of the ICE/ CED-3 family of cysteine proteases. J Biol Chem. 1995;270:15870–15876. doi: 10.1074/jbc.270.26.15870. [DOI] [PubMed] [Google Scholar]
- Muzio M, Chinnaiyan A, Kischkel F, O'Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz J, Zhang M, Gentz R, et al. FLICE, a novel FADD homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/Apo-1) death-inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- Nicholson D, Ali A, Thornberry N, Vaillancourt J, Ding C, Gallant M, Gareau Y, Griffin P, Labele M, Lazebnik Y, et al. Identification and inhibition of the ICE/CED-3-like proteases necessary for mammalian apoptosis. Nature. 1995;375:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- Raingeaud J, Whitmarsh AJ, Barrett T, Derijard B, Davis RJ. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol. 1996;16:1247–1255. doi: 10.1128/mcb.16.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, Hunt T, Nebreda AR. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78:1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- Rudel T, Bokoch GM. Membrane and morphological changes in apoptotic cells regulated by caspase-mediated activation of PAK2. Science. 1997;276:1571–1574. doi: 10.1126/science.276.5318.1571. [DOI] [PubMed] [Google Scholar]
- Sanchez I, Hughes RT, Mayer BJ, Yee K, Woodgett JR, Avruch J, Kyriakis JM, Zon LI. Role of SAPK/ERK kinase-1 in the stress-activated pathway regulating transcription factor c-Jun. Nature. 1994;372:794–798. doi: 10.1038/372794a0. [DOI] [PubMed] [Google Scholar]
- Schlegel J, Peters I, Orrenius S, Miller DK, Thornberry NC, Yamin TT, Nicholson DW. CPP32/Apopain is a key interleukin-1β converting enzyme-like protease involved in Fas-mediated apoptosis. J Biol Chem. 1996;271:1841–1844. doi: 10.1074/jbc.271.4.1841. [DOI] [PubMed] [Google Scholar]
- Srinivasula SM, Fernandes-Alnemri T, Zangrilli J, Robertson N, Armstrong RC, Wang L, Trapani JA, Tomaselli KJ, Litwack G, Alnemri ES. The Ced-3/interleukin-1β converting enzyme-like homolog Mch6 and the lamin-cleaving enzyme Mch2α are substrates for the apoptotic mediator CPP32. J Biol Chem. 1996;271:27099–27106. doi: 10.1074/jbc.271.43.27099. [DOI] [PubMed] [Google Scholar]
- Stein B, Brady H, Yang MX, Young DB, Barbosa MS. Cloning and characterization of MEK6, a novel member of the mitogen-activated protein kinase kinase cascade. J Biol Chem. 1996;271:11427–11433. doi: 10.1074/jbc.271.19.11427. [DOI] [PubMed] [Google Scholar]
- Tewari M, Quan L, O'Rourke K, Desnoyers S, Zeng Z, Beidler D, Poirer G, Salvesen G, Dixit V. Yama/CPP32β, a mammalian homolog of CED-3 is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-Ribose)polymerase. Cell. 1995;81:801–809. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- Verheij M, Bose R, Lin XH, Yao B, Jarvis WD, Grant S, Birrer MJ, Szabo E, Zon LI, Kyriakis JM, et al. Requirement for ceramide-initiated SAPK/JNK signaling in stress-induced apoptosis. Nature. 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- Wang X, Pai JT, Wiedenfeld EA, Medina JC, Slaughter CA, Goldstein JL, Brown MS. Purification of an interleukin-1β converting enzyme-related cystein protease that cleaves sterole regulatory element-binding proteins between the leucine zipper and transmembrane domains. J Biol Chem. 1995;270:18044–18050. doi: 10.1074/jbc.270.30.18044. [DOI] [PubMed] [Google Scholar]
- Wilson DJ, Fortner KA, Lynch DH, Mattingly RR, Macara IG, Posada JA, Budd RC. JNK, but not MAPK, activation is associated with Fas-mediated apoptosis in human T cells. Eur J Immunol. 1996;26:989–994. doi: 10.1002/eji.1830260505. [DOI] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effect of ERK and JNK-p38 MAP kinase on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Yonehara S, Ishii A, Yonehara M. A cell-killing monoclonal antibody (anti-Fas) to a cell surface antigen co-downregulated with the receptor of tumor necrosis factor. J Exp Med. 1989;169:1747–1756. doi: 10.1084/jem.169.5.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Horvitz HR. Caenorhabditis elegans cell death gene ced-4encodes a novel protein and is expressed during the periods of extensive programmed cell death. Development. 1992;116:309–320. doi: 10.1242/dev.116.2.309. [DOI] [PubMed] [Google Scholar]
- Yuan J, Shaham S, Ledouw S, Ellis HM, Horvitz HR. The C. elegance cell death gene ced-3encodes a protein similar to mammalian interleukin-1β-converting enzyme. Cell. 1993;75:641–652. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]
- Zhang C, Ao Z, Seth A, Schlossman SF. A mitochondrial membrane protein defined by a novel monoclonal antibody is preferentially detected in apoptotic cells. J Immunol. 1996;157:3980–3987. [PubMed] [Google Scholar]