Abstract
Introduction
Although previous studies report an association between erectile dysfunction (ED) and smoking, few have examined the impact of passive smoke exposure on ED. This analysis examines the association of active and passive smoking and ED and investigates a dose-response effect of smoking.
Methods
The Boston Area Community Heath (BACH) survey is a study of urologic symptoms in a racially and ethnically diverse population. BACH used a multistage stratified random sample to recruit 2301 men, aged 30–79 yr, from the city of Boston. ED was assessed using the five-item International Index of Erectile Function. Smoking and passive smoking were assessed by self-report. Analyses adjusted for sociodemographic and lifestyle factors and important chronic illnesses.
Results
An association between smoking and ED was observed with a significant trend in increased risk of ED with cumulative pack-years of smoking (adjusted odds ratio [OR] = 1.68; 95% confidence interval [CI], 1.03, 2.30 for ≥ 20 pack-years). Compared to never smokers not exposed to passive smoking, men who never smoked but were exposed to passive smoking had a moderate, statistically nonsignificant, increase in risk of ED (adjusted OR = 1.33; 95%CI: 0.69, 2.55) comparable to the OR observed for a cumulative exposure of 10–19 pack-years of active smoking (adjusted OR = 1.25; 95%CI, 0.68, 2.30).
Conclusions
Results indicate a dose-response association between smoking and ED with a statistically significant effect observed with ≥ 20 pack-years of exposure. Passive smoking is associated with a small, statistically nonsignificant increase in risk of ED comparable to approximately 10–19 pack-years of active smoking.
Keywords: Epidemiology, Erectile dysfunction, Passive smoking, Smoking
1. Introduction
Erectile dysfunction (ED) is a common problem among aging men [1–4]. A number of modifiable risk factors are associated with ED, including obesity, hypertension, unfavorable lipid levels, alcohol abuse, physical activity, and cigarette smoking [5–8]. The association between ED and current smoking has been reported in previous cross-sectional studies [9–14]. Additionally, longitudinal data from the Massachusetts Male Aging Study (MMAS) and the Health Professionals Followup Study (HPFS) have reported increased incidence of ED among smokers [7,15]. Experimental studies in animal models have linked exposure to passive smoking to endothelial function in animal models [16], and passive smoking was associated with coronary artery disease among individuals who had never smoked but lived with a former or current smoker [17]. Longitudinal data from the MMAS have shown a higher incidence of ED among both active smokers and those exposed to passive smoking; however, a dose-response relationship of active smoking and ED was not investigated [7].
The objectives of this analysis were to: (1) to confirm the association between smoking and ED assessed using the 5-item International Index of Erectile Dysfunction (IIEF-5) scale, an internationally validated self-administered questionnaire; (2) to determine whether a dose-response effect was present between smoking and ED by quantifying smoking using pack-years of smoking exposure; and (3) to investigate the association between passive smoking and ED.
2. Methods
The Boston Area Community Health (BACH) survey is a community-based epidemiologic survey of a broad range of urologic symptoms and risk factors conducted from 2002 to 2005. Detailed methods are given in a paper in this issue [18]. In brief, BACH used a multistage stratified random sample to recruit 5506 adults aged 30–79 yr in three racial/ethnic groups from the city of Boston (2301 men, 3205 women, 1770 black [African American], 1877 Hispanic, 1859 white respondents). Information about urologic symptoms, comorbidities, lifestyle, anthropometrics, and psychosocial attributes were collected via an interviewer-administered questionnaire; respondents used a self-administered questionnaire to answer questions about sexual function. All protocols and informed consent procedures were approved by New England Research Institute’s Institutional Review Board.
2.1. ED
ED was defined using the IIEF-5, a self-administered and validated instrument [19]. The five items assess erection confidence, erection firmness, maintenance ability, maintenance frequency, and satisfaction. Each item is scored on a 5-point ordinal scale where lower values represent poorer sexual function. The IIEF-5 score ranges between 5 and 25 with lower scores indicating increased severity of ED. Severity of ED is classified into five categories as severe (IIEF score 5–7), moderate (8–11), mild to moderate (12–16), mild (17–21), and no ED (22–25). Additionally, ED was defined as a dichotomous variable using a cut-off point of IIEF-5 < 17 (mild to moderate, moderate, and severe). This approach is similar to the definition of ED used in the MMAS study where ED was assessed using a single question and presence of ED was defined as moderate or severe ED [2].
2.2 Smoking
Smoking and passive smoking were assessed by self-report. The baseline questionnaire assessed whether men had smoked at least 100 cigarettes in their lifetime and if they were currently smoking. Smoking status was defined as never smokers (smoked < 100 cigarettes lifetime and not currently smoking), former smokers (smoked > 100 cigarettes lifetime and currently nonsmoker), and current smoker (smoked > 100 cigarettes and currently a smoker). Retrospective self-report of smoking behavior has been shown to be reliable with prevalence rates derived from retrospective data matching closely those from contemporaneous rates [20]. Both former and current smokers were asked to report the usual number of cigarettes smoked per day and for how many years they had smoked that amount. Pack-years of smoking were calculated by multiplying the number of packs (20 cigarettes in one pack) smoked per day by the number of years smoked. Pack-years were categorized as < 10, 10–19, and ≥ 20 pack-years. Categorizing retrospectively calculated pack-years has been shown to reduce misclassification bias [21]. Exposure to passive smoking either at home or at work was assessed by self-report. Passive smoking was defined as living with someone who smokes at home regularly or working or spending time on a daily basis with people who are smoking. To separate the effect of active smoking and passive smoking and assess a dose-response pattern, the smoking status, pack-years of smoking, and exposure to passive smoking variable were combined into a five-category variable defined as: never smokers not exposed to passive smoking; never smokers exposed to passive smoking; former or current smokers with < 10, 10–19, and ≥ 20 or more pack-years.
2.3. Covariates
The following covariates were considered as categorical variables: age, 30–39, 40–49, 50–59, 60–69, and 70–79 yr; racial/ethnic group (black [African American], Hispanic, white); body mass index (BMI): < 25, 25–30, ≥ 30+ kg/m2; physical activity as measured by the Physical Activity Scale for the Elderly (PASE) [22]: < 100,100–249, 250+ with increasing values indicating increasing activity; alcohol use: alcoholic drinks including beer, wine, and hard liquor consumed per day: 0, < 1, 1–3, > 3 drinks/d; self-reports of major comorbidities including cardiovascular disease (CVD; coronary artery bypass or angioplasty, myocardial infarction, angina pectoris, congestive heart failure, carotid artery surgery, intermittent claudication, surgery, or angioplasty for arterial disease of the leg, aortic aneurysm, Raynaud’s disease, peripheral vascular disease, stroke, or transient ischemic attack), hypertension, and diabetes. Depressive symptoms were assessed using the Center for Epidemiological Survey Depression Scale (CES-D; at least 5 of 8 symptoms on the abbreviated CES-D scale) [23].
2.4. Statistical analyses
Descriptive statistics, proportions for categorical variables, and means and standard errors (SEs) for continuous variables were used to describe the analysis sample. Using the IIEF-5 score as a continuous variable, linear regression was used to assess the association between ED and both smoking and exposure to passive smoking. Multiple linear regression models were used to adjust for potential confounding effect of covariates. Adjusted mean IIEF-5 scores and regression coefficients are reported. Analyses were repeated with ED defined as a dichotomous variable using a cut-off point of IIEF-5 < 17 (mild to moderate, moderate, and severe). Association of dichotomous ED and smoking was assessed using the odds ratio (OR) and 95% confidence interval (95%CI) estimated using logistic regression. Multiple logistic regression models were used to adjust for potential confounders. Because the association of smoking and ED was similar by race/ethnicity, results are presented for the whole sample and not stratified by race/ethnicity.
Multiple imputation was used to impute plausible values for missing data [24]. To be representative of the city of Boston, observations were weighted inversely proportional to their probability of selection [25]. Weights were post-stratified to the Boston population according to the 2000 census. Analyses were conducted in version 9.1 of SAS (SAS Institute, Cary, NC, USA) and version 9.0.1 of SUDAAN (Research Triangle Institute, Research Triangle Park, NC, USA).
3. Results
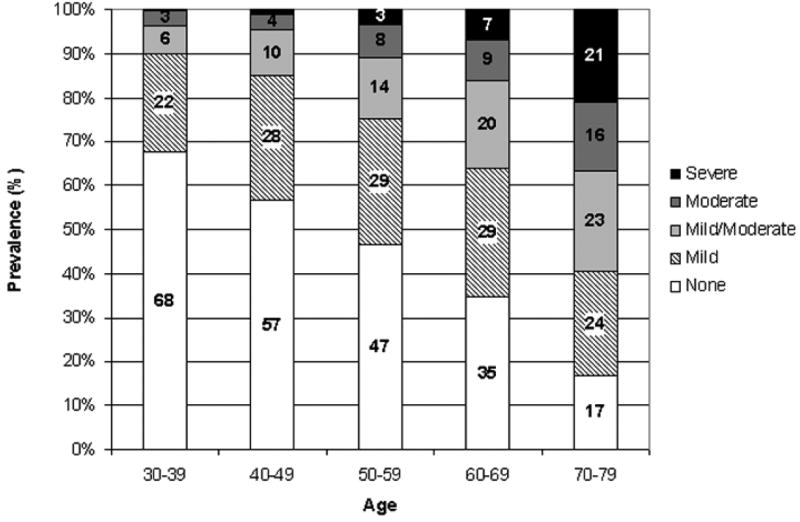
Characteristics of the 2301 men (835 white, 700 black [African American], and 766 Hispanic) in the BACH sample are presented in Table 1. The age range was 30–79 yr, with a mean of 47.6 yr. About 41% of men were overweight (BMI 25–30) and 33% were obese (BMI ≥ 30). CVD and hypertension were reported by 21% and 26%, respectively, with over half of the men who reported CVD also reporting hypertension. Prevalence of diabetes was 9.3%; depressive symptoms were reported by 14%. Prevalence of ED increased with age (Fig. 1). About 45% of participants were categorized as never smokers, 28.7% were former smokers, and 26.2% were current smokers. A total of 832 participants (33%) reported exposure to passive smoking, including 44% who were current smokers and 23.6% who were former smokers. Of the 832 men who reported exposure to passive smoking, only 205 (26.8%) men had no history of either current or past active and, therefore, could be classified as exposed to passive smoking alone.
Table 1.
Characteristics of the analysis sample
| Unweighted
|
Weighted | |||
|---|---|---|---|---|
| n | % | % | ||
| ED (IIEF-5) | None | 991 | 43.1 | 53.5 |
| Mild | 698 | 30.3 | 25.8 | |
| Mild/moderate | 351 | 15.3 | 11.5 | |
| Moderate | 164 | 7.1 | 5.9 | |
| Severe | 98 | 4.3 | 3.3 | |
| Smoking | Never | 963 | 41.9 | 45.1 |
| Former | 662 | 28.8 | 28.7 | |
| Current | 675 | 29.3 | 26.2 | |
| Pack-years | None | 963 | 41.9 | 45.1 |
| < 10 | 626 | 27.2 | 26.4 | |
| 10–19 | 261 | 11.3 | 11.9 | |
| 20+ | 451 | 19.6 | 16.6 | |
| Passive smoking | No | 1469 | 63.8 | 66.6 |
| Yes | 832 | 36.2 | 33.4 | |
| Age, yr | 30–39 | 615 | 26.7 | 37.2 |
| 40–49 | 659 | 28.6 | 25.8 | |
| 50–59 | 510 | 22.2 | 17.8 | |
| 60–79 | 517 | 14.3 | 11.3 | |
| 70–79 | 329 | 8.2 | 7.8 | |
| Race/ethnicity | White | 835 | 36.3 | 61.9 |
| Black | 700 | 30.4 | 25.1 | |
| Hispanic | 766 | 33.3 | 13.0 | |
| BMI, kg/m2 | < 25 | 596 | 25.9 | 26.6 |
| 25–29 | 904 | 39.3 | 40.7 | |
| 30+ | 801 | 34.8 | 32.7 | |
| Physical activity, PASE | < 100 | 695 | 30.2 | 26.8 |
| 100–250 | 1069 | 46.5 | 47.4 | |
| > 250 | 538 | 23.4 | 25.8 | |
| Alcohol use, drinks/d | None | 800 | 34.8 | 27.5 |
| < 1/d | 815 | 35.4 | 38.9 | |
| 1–3/d | 434 | 18.9 | 24.0 | |
| 4+/d | 252 | 11.0 | 9.6 | |
| CVD | 469 | 20.4 | 20.6 | |
| Hypertension | 735 | 31.9 | 26.2 | |
| Diabetes | 297 | 12.9 | 9.3 | |
| Depression | 391 | 17.0 | 14.0 | |
BMI = body mass index; PASE = Physical Activity Scale for the Elderly; CVD = cardiovascular disease.
Fig. 1. Prevalence of erectile dysfunction by age.
Weighted percentages.
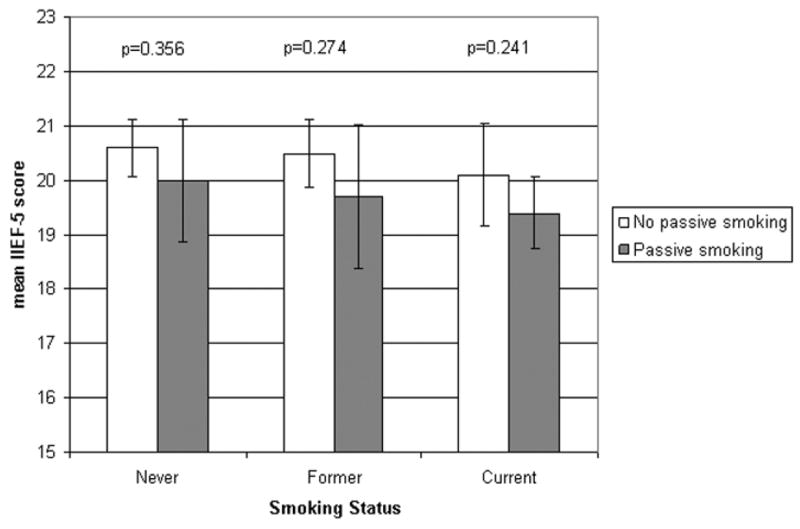
Figure 2 presents the age-adjusted mean score among never, former, and current smokers by passive smoking. Although age-adjusted mean IIEF-5 scores are consistently lower among those exposed to passive smoking, the differences are small and statistically nonsignificant. Similarly, the effect of passive smoking is not significant within pack-year categories (data not shown). Because information on time since smoking cessation was not collected, the effect of quitting smoking could not be directly assessed. Although mean IIEF-5 scores decrease with increasing pack-years (Table 2), only small nonsignificant differences are observed between former and current smokers with similar cumulative pack-years exposure to smoking (data not shown).
Fig. 2. Minimal impact of passive smoking on erectile function by active smoking status.
Age-adjusted mean five-item International Index of Erectile Function score and 95% confidence intervals.
Table 2.
Association between smoking and ED shows a dose-response pattern with increased pack-years
| IIEF-5 continuous
|
||||||
|---|---|---|---|---|---|---|
| Unadjusted | Age-adjusted | Multivariate adjusted* | ||||
| β† | 95%CI | β† | 95%CI | β† | 95%CI | |
| Constant | 21.02 | 22.63 | 21.51 | |||
| Never smoker/no passive | Reference | Reference | Reference | |||
| Never smoker/passive | −0.66 | −2.15, 0.83 | −0.55 | −1.74, 0.63 | −0.29 | −1.53, 0.95 |
| Pack-years < 10 | −0.28 | −1.07, 0.50 | −0.12 | −0.81, 0.58 | 0.03 | −0.68, 0.74 |
| Pack-years 10–19 | −0.99 | −2.08, 0.11 | −0.44 | −1.60, 0.71 | −0.42 | −1.54, 0.59 |
| −4.31, | −2.63, | −2.23, | ||||
| Pack-years ≥ 20 | −3.35 | −2.40 | −1.71 | −0.79 | −1.27 | −0.30 |
| Overall F-test p value | < 0.001 | 0.005 | 0.067 | |||
| Trend test p value‡ | < 0.001 | 0.001 | 0.013 | |||
|
| ||||||
|
ED defined as IIEF-5 <17
|
||||||
| Unadjusted | Age-adjusted | Multivariate adjusted* | ||||
| OR | 95%CI | OR | 95%CI | OR | 95%CI | |
|
| ||||||
| Never smoker/no passive | 1.00 | 1.00 | 1.00 | |||
| Never smoker/passive | 1.46 | 0.75, 2.85 | 1.43 | 0.77, 2.63 | 1.33 | 0.69, 2.55 |
| Pack-years < 10 | 1.23 | 0.81, 0.87 | 1.15 | 0.75, 1.77 | 1.10 | 0.68, 1.78 |
| Pack-years 10–19 | 1.46 | 0.87, 2.43 | 1.18 | 0.66, 2.11 | 1.25 | 0.68, 2.30 |
| Pack-years ≥ 20 | 3.15 | 2.04, 4.87 | 1.83 | 1.18, 2.83 | 1.68 | 1.03, 2.76 |
| Overall F-test p value | < 0.001 | 0.094 | 0.285 | |||
| Trend test p value‡ | < 0.001 | 0.009 | 0.032 | |||
Regression coefficient (β) or odd ratio (OR) and 95% confidence intervals (95%CI) shown.
ED = erectile dysfunction; IIEF-5 = 5-item International Index of Erectile Function;
Adjusted for age, race/ethnicity, physical activity, alcohol use, CVD, diabetes, depression.
Linear regression coefficient
The p value for linear trend test across pack-year categories with the never smokers/no passive group as the reference category.
Table 2 presents the unadjusted, age-adjusted, and multivariate adjusted association between ED (assessed using the continuous IIEF-5 score) and smoking (using the five-category variable combining smoking status, pack-years, and passive smoking information). Results show a dose-response effect of increased severity of ED with increased pack-years smoking, with the strongest effect seen among those with ≥ 20 pack-years of exposure to smoking. This effect remained significant (95%CI for regression coefficient excludes 0) after adjusting for age, lifestyle factors, and comorbidities. A test for trend for decreasing IIEF-5 scores across pack-years of smoking remained significant after adjusting for potential confounders (p value for trend = 0.013). A small, nonsignificant, effect of passive smoking was observed among never smokers. Although statistically nonsignificant, the magnitude of the effect of passive smoking (multivariate adjusted regression coefficient = −0.29; 95%CI, −1.53, 0.95) was comparable to that observed for 10–19 pack-years of smoking (multivariate adjusted regression coefficient = −0.42, 95%CI, −1.54, 0.59). Similar results were observed when analyses were repeated and ED was defined as a dichotomous variable using IIEF-5 < 17 as a cutoff. A dose-response effect was observed with increasing ORs with increasing pack-years (p value for trend = 0.032) and an OR of 1.68 (95%CI, 1.03, 2.76) for men with ≥ 20 pack-years of smoking exposure. The magnitude of the association for passive smoking among never smokers (OR = 1.33, 95%CI, 0.69, 2.55) was comparable to that of 10–19 pack-years (OR = 1.28, 95%CI, 0.71-2.32).
4. Discussion
Results from the BACH survey, a population-based random sample of 2301 men, reveal an association between smoking and ED. A dose-response effect was observed with increasing pack-years of smoking, with exposure to ≥ 20 pack-years being associated with ED after adjusting for major ED risk factors such as older age, CVD, and diabetes. The association between passive smoking and ED is not statistically significant; however, the magnitude of the effect of passive smoking is comparable to 10–19 pack-years of smoking exposure.
The observed association between smoking and ED and dose-response with increased cumulative exposure is consistent with reports from previous studies across different countries. Increased risk of ED among current smokers who smoke ≥ 20 cigarettes per day compared to never smokers was reported by studies conducted in Australia [11] (adjusted OR = 1.39; 95%CI, 1.05, 183), China [12] (adjusted OR = 1.47; 95%CI, 1.00, 2.16), Canada [13] (adjusted OR for 25–50 pack-years was 2.11, 95%CI, 1.02, 4.36), Italy (adjusted OR for 10–20 yr of smoking was 1.6, 95%CI, 1.2, 2.0, with a similar OR for ≥ 20 years of smoking), [14] and a cross-national survey of Brazil, Italy, Japan, and Malaysia [10] (adjusted OR for ≥ 30 cigarettes/d was 2.3, 95%CI, 1.19, 4.49). Findings from the BACH study show a similar effect of cumulative pack-years among both former and current smokers with an adjusted OR of 1.70 (95%CI, 1.04, 2.77) for ≥ 20 pack-years of smoking. Compared to never smokers, a significant trend of increasing ED risk was observed across pack-years of smoking (Table 2). Similar finding were reported by a study using data from the Olmsted County Study (OCS), with a statistically significant multivariate-adjusted OR of 1.60 (95%CI, 1.04, 2.46) for men with ≥ 29 pack-years of smoking, and a dose-response pattern with increasing pack-years [9]. Longitudinal data from the MMAS show that the incidence of ED is twice as high among smokers compared to nonsmokers over a 9-yr period [7]. Similarly, data from the HPFS show an increased risk of ED, over a follow-up period of 14 yr, for both former smokers (relative risk = 1.2; 95%CI, 1.1, 1.3) and current smokers (relative risk = 1.5; 95%CI, 1.3-1.7).
Few studies have reported on the effect of passive smoking on ED. Results from the present study indicate a moderate, statistically nonsignificant, increase in risk of ED with exposure to passive smoking. This effect was comparable to a cumulative exposure of 10–19 pack-years (Table 2). Longitudinal results from the MMAS study show that men exposed to passive smoking were at twice the risk of developing ED over a 9-yr follow-up period [7]. Although the effect of current smoking was adjusted for in the analysis of MMAS data, former smokers were not excluded from the passive smoking group, thus potentially overestimating the effect of passive smoking. These results suggest that although the increased risk in ED with passive smoking is small, long-term chronic exposure to passive smoking may have adverse effects on erectile function.
Although the association between smoking, ED, and CVD has been previously documented [7,26,27], indicating vascular damage as the likely pathway between smoking and ED, the role of passive smoking in the etiology of ED is not known. Animal models suggest that long-term exposure to passive smoke leads to impaired endothelial function, possibly due to reduced penile oxide synthase activity [16,28]. However, small studies of short-term exposure to passive smoke among healthy nonsmokers have not confirmed a direct effect of passive smoking on endothelial function [29,30], suggesting that assessment of chronic exposure to passive smoke is warranted.
This study has many strengths; however, there are some important limitations. The BACH survey is a community-based random sample across a broad age range (30–79 yr) and includes large numbers of minority participants representative of both the black (African American) and Hispanic populations. ED is assessed using the IIEF-5, a validated instrument for ED assessment widely used in both clinical and epidemiologic studies. A limitation of the use of the IIEF-5 instrument is recall bias among men who had not engaged in sexual activities in the 4 wk preceding completion of the questionnaire (about 40% of men), potentially resulting in greater misclassification in this group. Results of analyses restricted to sexually active men showed a similar pattern but were statistically nonsignificant, largely due to the decreased sample size. The BACH study is presently a cross-sectional design; thus, incidence of ED related to smoking could not be assessed. Additionally, because data on duration since smoking cessation were not collected, the impact of quitting cigarette smoking on erectile function could not be assessed. Full information on smoking cessation will be collected in BACH follow-up studies. Self-reported smoking status and retrospective pack-year calculation may also result in reporting and misclassification bias. Previous studies of the validity of self-reported smoking have shown that prevalence rates of self-reported smoking match well with those of from contemporaneous smoking behavior [20]. Although retrospective pack-year calculation may result in misclassification, categorizing pack-years into smoking groups reduces this bias [21]. The BACH study was limited geographically to the Boston area. However, comparison of sociodemographic and health-related variables from the BACH survey with other large regional (Boston BRFSS) and national (National Health and Nutrition Examination Survey, National Health Interview Survey, national BRFSS) studies have shown that BACH estimates are comparable on health-related variables.
5. Conclusion
In summary, data from the BACH study show an association between smoking and ED and supports a dose-response relationship between cumulative pack-years of smoking and risk of ED. These results confirm previous reports from the literature supporting the association between smoking and ED. The observed dose-response pattern between duration and intensity of smoking and increased risk of ED in conjunction with previous published research indicating beneficial effects of smoking cessation,[13,31] especially at a younger age [32], highlights the importance of abstaining from or quitting smoking. Finally, a moderate, statistically nonsignificant effect of passive smoking was also observed suggesting possible effects of long-term chronic exposure on erectile function.
Acknowledgments
This work was supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; National Institutes of Health DK 56842).
Funding sources: This work was supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (DK56842)
Footnotes
Take-home message: Results confirm an association of smoking and erectile dysfunction and demonstrate a dose-response effect with duration and intensity of smoking. The effect of passive smoking is comparable to approximately 10–19 pack-years of active smoking.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Saigal CS, Wessells H, Pace J, Schonlau M, Wilt TJ. Predictors and prevalence of erectile dysfunction in a racially diverse population. Arch Intern Med. 2006;166:207–12. doi: 10.1001/archinte.166.2.207. [DOI] [PubMed] [Google Scholar]
- 2.Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. 1994;151:54–61. doi: 10.1016/s0022-5347(17)34871-1. [DOI] [PubMed] [Google Scholar]
- 3.Ponholzer A, Temml C, Mock K, Marszalek M, Obermayr R, Madersbacher S. Prevalence and risk factors for erectile dysfunction in 2869 men using a validated questionnaire. Eur Urol. 2005;47:80–5. doi: 10.1016/j.eururo.2004.08.017. discussion 85–6. [DOI] [PubMed] [Google Scholar]
- 4.Papaharitou S, Athanasiadis L, Nakopoulou E, et al. Erectile dysfunction and premature ejaculation are the most frequently self-reported sexual concerns: profiles of 9,536 men calling a helpline. Eur Urol. 2006;49:557–63. doi: 10.1016/j.eururo.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 5.Wei M, Macera CA, Davis DR, Hornung CA, Nankin HR, Blair SN. Total cholesterol and high density lipoprotein cholesterol as important predictors of erectile dysfunction. Am J Epidemiol. 1994;140:930–7. doi: 10.1093/oxfordjournals.aje.a117181. [DOI] [PubMed] [Google Scholar]
- 6.Muller SC, el-Damanhoury H, Ruth J, Lue TF. Hypertension and impotence. Eur Urol. 1991;19:29–34. doi: 10.1159/000473574. [DOI] [PubMed] [Google Scholar]
- 7.Feldman HA, Johannes CB, Derby CA, et al. Erectile dysfunction and coronary risk factors: prospective results from the Massachusetts Male Aging study. Prev Med. 2000;30:328–38. doi: 10.1006/pmed.2000.0643. [DOI] [PubMed] [Google Scholar]
- 8.Barrett-Connor E. Heart disease risk factors predict erectile dysfunction 25 years later (the Rancho Bernardo Study) Am J Cardiol. 2005;96:3M–7M. doi: 10.1016/j.amjcard.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 9.Gades NM, Nehra A, Jacobson DJ, et al. Association between smoking and erectile dysfunction: a population-based study. Am J Epidemiol. 2005;161:346–51. doi: 10.1093/aje/kwi052. [DOI] [PubMed] [Google Scholar]
- 10.Nicolosi A, Glasser DB, Moreira ED, Villa M. Prevalence of erectile dysfunction and associated factors among men without concomitant diseases: a population study. Int J Impot Res. 2003;15:253–7. doi: 10.1038/sj.ijir.3901010. [DOI] [PubMed] [Google Scholar]
- 11.Millett C, Wen LM, Rissel C, et al. Smoking and erectile dysfunction: findings from a representative sample of Australian men. Tob Control. 2006;15:136–9. doi: 10.1136/tc.2005.015545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lam TH, Abdullah AS, Ho LM, Yip AW, Fan S. Smoking and sexual dysfunction in Chinese males: findings from men’s health survey. Int J Impot Res. doi: 10.1038/sj.ijir.3901436. In press. [DOI] [PubMed] [Google Scholar]
- 13.Polsky JY, Aronson KJ, Heaton JP, Adams MA. Smoking and other lifestyle factors in relation to erectile dysfunction. BJU Int. 2005;96:1355–9. doi: 10.1111/j.1464-410X.2005.05820.x. [DOI] [PubMed] [Google Scholar]
- 14.Austoni E, Mirone V, Parazzini F, et al. Smoking as a risk factor for erectile dysfunction: data from the Andrology Prevention Weeks 2001-2002 a study of the Italian Society of Andrology (s.I.a.) Eur Urol. 2005;48:810–7. doi: 10.1016/j.eururo.2005.03.005. discussion 817–8. [DOI] [PubMed] [Google Scholar]
- 15.Bacon CG, Mittleman MA, Kawachi I, Giovannucci E, Glasser DB, Rimm EB. A prospective study of risk factors for erectile dysfunction. J Urol. 2006;176:217–21. doi: 10.1016/S0022-5347(06)00589-1. [DOI] [PubMed] [Google Scholar]
- 16.Xie Y, Garban H, Ng C, Rajfer J, Gonzalez-Cadavid NF. Effect of long-term passive smoking on erectile function and penile nitric oxide synthase in the rat. J Urol. 1997;157:1121–6. [PubMed] [Google Scholar]
- 17.Steenland K. Passive smoking and the risk of heart disease. JAMA. 1992;267:94–9. [PubMed] [Google Scholar]
- 18.McKinlay JB, Link CL. Measuring the urologic iceberg: design and implementation of the Boston Area Community Health (BACH) survey. Eur Urol. doi: 10.1016/j.eururo.2007.03.013. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Pena BM. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11:319–26. doi: 10.1038/sj.ijir.3900472. [DOI] [PubMed] [Google Scholar]
- 20.Kenkel D, Lillard DR, Mathios A. Smoke or fog? The usefulness of retrospectively reported information about smoking. Addiction. 2003;98:130713. doi: 10.1046/j.1360-0443.2003.00445.x. [DOI] [PubMed] [Google Scholar]
- 21.Bernaards CM, Twisk JW, Snel J, Van Mechelen W, Kemper HC. Is calculating pack-years retrospectively a valid method to estimate life-time tobacco smoking? A comparison between prospectively calculated pack-years and retrospectively calculated pack-years. Addiction. 2001;96:1653–61. doi: 10.1046/j.1360-0443.2001.9611165311.x. [DOI] [PubMed] [Google Scholar]
- 22.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–62. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 23.Turvey CL, Wallace RB, Herzog R. A revised CES-D measure of depressive symptoms and a DSM-based measure of major depressive episodes in the elderly. Int Psychogeriatr. 1999;11:139–48. doi: 10.1017/s1041610299005694. [DOI] [PubMed] [Google Scholar]
- 24.Schafer J. Analysis of incomplete multivariate data. London, United Kingdom: Chapman and Hall; 1997. [Google Scholar]
- 25.Cochran W. Sampling techniques. 3. New York: John Wiley and Sons; 1977. [Google Scholar]
- 26.McVary KT, Carrier S, Wessells H. Smoking and erectile dysfunction: evidence based analysis. J Urol. 2001;166:1624–32. [PubMed] [Google Scholar]
- 27.Shiri R, Hakkinen J, Koskimaki J, Tammela TL, Auvinen A, Hakama M. Smoking causes erectile dysfunction through vascular disease. Urology. 2006;68:1318–22. doi: 10.1016/j.urology.2006.08.1088. [DOI] [PubMed] [Google Scholar]
- 28.Torok J, Gvozdjakova A, Kucharska J, et al. Passive smoking impairs endothelium-dependent relaxation of isolated rabbit arteries. Physiol Res. 2000;49:135–41. [PubMed] [Google Scholar]
- 29.Hausberg M, Mark AL, Winniford MD, Brown RE, Somers VK. Sympathetic and vascular effects of short-term passive smoke exposure in healthy nonsmokers. Circulation. 1997;96:282–7. [PubMed] [Google Scholar]
- 30.Kato M, Roberts-Thomson P, Phillips BG, et al. The effects of short-term passive smoke exposure on endothelium-dependent and independent vasodilation. J Hypertens. 1999;17:1395–401. doi: 10.1097/00004872-199917100-00006. [DOI] [PubMed] [Google Scholar]
- 31.Pourmand G, Alidaee MR, Rasuli S, Maleki A, Mehrsai A. Do cigarette smokers with erectile dysfunction benefit from stopping? A prospective study. BJU Int. 2004;94:1310–3. doi: 10.1111/j.1464-410X.2004.05162.x. [DOI] [PubMed] [Google Scholar]
- 32.Guay AT, Perez JB, Heatley GJ. Cessation of smoking rapidly decreases erectile dysfunction. Endocr Pract. 1998;4:23–6. doi: 10.4158/EP.4.1.23. [DOI] [PubMed] [Google Scholar]