Abstract
First-generation adenovirus can be engineered with powerful promoters to drive expression of therapeutic transgenes. Numerous clinical trials for glioblastoma multiforme using first generation adenoviral vectors have either been performed or are ongoing, including an ongoing, Phase III, multicenter trial in Europe and Israel (Ark Therapeutics, Inc.). Although in the absence of anti-adenovirus immune responses expression in the brain lasts 6–18 months, systemic infection with adenovirus induces immune responses that inhibit dramatically therapeutic transgene expression from first generation adenoviral vectors, thus, potentially compromising therapeutic efficacy. Here, we show evidence of an immunization threshold for the dose that generates an immune response strong enough to eliminate transgene expression from the CNS. For the systemic immunization to eliminate transgene expression from the brain, ≥1 × 107 infectious units (iu) of adenovirus need to be used as immunogen. Furthermore, this immune response eliminates >90% of transgene expression from 1 × 107–1 × 10³ iu of vector injected into the striatum 60 days earlier. Importantly, elimination of transgene expression is independent of the nature of the promoter that drives transgene expression and is accompanied by brain infiltration of CD8+ T cells and macrophages. In conclusion, once the threshold for systemic immunization (i.e. 1 × 107 iu) is crossed, the immune response eliminates transgene expression by >90% even from brains that receive as little as 1000 iu of adenoviral vectors, independently of the type of promoter that drives expression.
Keywords: Gene therapy, neuroimmunology, adenovirus, promoter, threshold
INTRODUCTION
First-generation adenovirus are powerful vectors for gene transfer into the brain in vitro and in vivo, and are employed currently in clinical trials for gene therapy. Experimentally, they are successful in gene therapy models of many brain diseases, including Parkinson’s disease (Bilang-Bleuel et al., 1997; Bjorklund et al., 2000; Do Thi et al., 2004; Hurtado-Lorenzo et al., 2004), Huntington’s disease (Bemelmans et al., 1999; Mittoux et al., 2000; Mittoux et al., 2002), brain tumors (Dewey et al., 1999; Sandmair et al., 2000; Maleniak et al., 2001; Biglari et al., 2004; Chiocca et al., 2004; Gondi et al., 2004; Immonen et al., 2004) and various pain syndromes (Cope et al., 2006). In comparative clinical trials of gene therapy for malignant brain tumors, first-generation adenoviral vectors are significantly more effective than retroviral vectors, utilizing the conditional cytotoxic gene HSV1-TK and ganciclovir (Immonen et al., 2004). Numerous clinical trails for glioblastoma multiforme using first generation adenoviral vectors have been performed or are ongoing (Sandmair et al., 2000; Trask et al., 2000; Eck et al., 2001; Ehtesham et al., 2004; Immonen et al., 2004), including an ongoing Phase III multicenter trial in Europe and Israel (see http://www.virtualtrials.com/news3.cfm?item = 3785) by Ark Therapeutics, Inc.
However, there is controversy on the extent of adenoviral-mediated transgene expression in the brain. Short-term expression (e.g. <1 month) results from the use of very high titers that are cytotoxic because they stimulate a strong innate immune response (Thomas et al., 2001a), the injection of vectors into the brain ventricles, which primes the systemic immune system (Lowenstein, 2002; Tada et al., 2005), or contaminated vector preparations. However, if injectionskeep the amount of injected vectors to ~107 iu, are delivered carefully into the brain parenchyma, and vector preparations are devoid of lipopolysaccharide and replication competent adenovirus, expression lasts between 6–18 months (Byrnes et al., 1995; Geddes et al., 1997; Kajiwara et al., 1997; Ideguchi et al., 2000; Thomas et al., 2000b; Zou et al., 2000; Amalfitano et al., 2002; Glover et al., 2003).
However, if animals become immunized against adenovirus, either before or following vector administration, stimulation of the adaptive immune response either silences or significantly reduces transgene expression (Byrnes et al., 1995; Byrnes et al., 1996a; Byrnes et al., 1996b; Thomas et al., 2000b; Thomas et al., 2001a; Thomas et al., 2001c). Loss of transgene expression is accompanied by infiltration of CD8+- and CD4+-T cells, NK cells and macrophages, and activation of local astrocytes and microglia. The role of these cells in eliminating transgene expression, and their molecular mechanisms of action are unknown.
Many different types of viruses infect the brain (Griffin, 2003). The pathological, functional and long-term consequences depend on the type of virus, route of infection, and the capacity of the immune system to clear the virus from the brain. In those cases in which virus is effectively cleared from the brain by the immune system, for example LCMV (Rall et al., 1995; Kagi et al., 1996) and Sindbis virus (Binder et al., 2003; Griffin, 2003), most authors postulate that viral clearing from the brain in vivo is achieved by non-cytolytic mechanisms, even if T cells are able to kill cells in vitro. The molecular details of such non-cytolytic mechanisms have not been elucidated. However, even the most accomplished researchers in the field, have not yet demonstrated that in vivo, CTLs effectively kill target brain cells. The inability to detect cell death of brain cells by CTLs in vivo despite their capacity to do so in vitro (Rall et al., 1995) remains unexplained. Further, the quantitative aspects of immunemediated elimination of transgene expression from the brain have never been studied. We believe these studies will aid development of a model to examine neuroimmune interactions at the single cell level in vivo.
OBJECTIVES
Herein, we address the quantitative analysis of systemic immune-mediated elimination of adenoviral transgene expression from the brain. Furthermore, we also evaluate the formation of immunological synapses, the microanatomical structure that mediates intercellular communication between T cells and transduced astrocytes. The quantitative aspects of loss of transgene expression from the brain have not been addressed before (Thomas et al., 2000b; Thomas et al., 2001c).
METHODS
Adenoviral vectors
All adenoviruses used in this study are first-generation E1a-deleted recombinant adenovirus vectors based on adenovirus type 5. The construction of Ad-hCMV-βgal and Ad-hCMV-HPRT have been described elsewhere (Akrigg et al., 1985; Wilkinson et al., 1992; Dewey et al., 1999; Cowsill et al., 2000; Southgate et al., 2000). Briefly, expression cassettes containing the gene for HSV1 thymidine kinase (RAdTK), β-galactosidase (Ad-hCMV-βgal) or hypoxanthine-guanine phosphoribosyltransferase (Ad-hCMV-HPRT) were inserted into the adenoviral genome in place of the E1 region. RAdTK, Ad-hCMV-βgal and Ad-hCMV-HPRT all expressed their transgenes from the human cytomegalovirus intermediate-early promoter. The adenovirus vector RAdRSVlacZ was constructed similarly to all other vectors in this study, and it expressed β-galactosidase from the Rous Sarcoma Virus (RSV) promoter. The adenovirus vector Ad-synapsin-lacZ (obtained from Dr. James Uney, University of Bristol, UK) expressed β-galactosidase from the synapsin promoter (Ralph et al., 2000). The vector Ad-βActsp1-lacZ was obtained from Dr. Frank Graham, McMaster University, Canada. Construction of this vector is described in Bett (1995). Ad-βActsp-1lacZ expressed lacZ from the human β-actin promoter.
All adenovirus vectors were propagated on 293 cells, purified by double caesium chloride gradient centrifugation followed by dialysis and finally titered for infectious units by end point dilution of 293 cells (Southgate et al., 2000b). All vector preparations were screened for replication-competent adenovirus contamination by serial amplification on HeLa cells as described (Dion et al., 1996). Replication-competent adenovirus contamination was below 1:1 × 109 iu for all virus preparations. Adenoviruses were diluted in sterile saline solution before injection.
Animals and surgical procedure
Adult, male, Sprague-Dawley rats of 250 g body weight (Charles River Breeding Laboratories) were used in all the experiments described bellow. All experiments were conducted according to the Declaration of Helsinki. All animal experiments were performed at either the University of Manchester, UK, under the authority of The Home Office, or following approval by the IACUC at Cedars-Sinai Medical Center.
To determine the threshold immunization dose necessary to silence transgene expression, three animals per experimental group were anaesthetized with halothane and were placed in a stereotactic apparatus that was modified for use with inhalational anaesthesia. Animals were injected unilaterally in the left striatum (0.6 mm forward and 3.4 mm lateral from bregma, and 5.0 mm vertical from the dura) with 1 × 107 iu of Ad-mCMV-βgal in a volume of 3 µl. Each injection was performed over 10 min. One month after the intrastriatal injections, rats were anaesthetized with halothane and injected intradermally in the back with increasing doses of Ad-hCMV-HPRT (10¹–108 iu of Ad-hCMV-HPRT). Sixty days after the immunization animals were injected intraperitoneally with pentobarbitone and transcardially perfused-fixed with heparinized and oxygenated tyrode solution followed by 4% paraformaldehyde. Brains were removed, post-fixed in 4% paraformaldehyde for 12 hours and stored in PBS, before sectioning and immunohistochemical analysis.
To determine the minimal dose of adenoviral vector injected in the brain that avoids immune-mediated elimination three animals per experimental group were anaesthetized with halothane and injected unilaterally in the left striatum, using the conditions described above, with increasing doses of Ad-mCMV-βgal (10¹–107 iu) in a volume of 3 µl. One month after the intrastriatal injections, rats were anaesthetized with halothane and injected intradermally in the back with 5 × 108 iu of Ad-hCMV-HPRT). Sixty days after the intradermal injection, animals were perfused-fixed and their brains removed and post-fixed as above before sectioning and immunohistochemical analysis.
To determine the stability of adenovirus-mediated β-galactosidase expression driven by different promoters vis-à-vis the immune system, rats were injected unilaterally in the left striatum with Ad-hCMV-βgal (n = 10), Ad-mCMV-βgal (n = 6), RAdRSVlacZ (n = 10), RAdsynapsinlacZ (n = 10) or AdβActsp1lacZ (n = 10). Six weeks after the intrastriatal injection, half the animals in each group were injected intradermally with 5 × 108 iu of Ad-hCMV-HPRT. Sixty days after the intradermal injection, animals were perfused-fixed and their brains removed and post-fixed as above before sectioning and immunohistochemical analysis.
To illustrate the formation of immunological synapses between virally infected astrocytes and T cells that infiltrate the brain, animals were injected unilaterally in the left striatum as described above with 1 × 107 iu of Ad-hCMV-TK. Four weeks after the intrastriatal injection animals were immunized intradermally with 5 × 108 iu of Ad-hCMV-HPRT. Fourteen days after the intradermal injection, animals were perfused-fixed and their brains removed and post-fixed as above before sectioning and immunohistochemical and confocal analysis.
Immunohistochemistry
Coronal brain sections (50 µm thick) were cut through the striatum using a vibratome. Free-floating immunohistochemistry was performed on serial brain sections as previously described (Thomas et al., 2000a), using the following primary antibodies: anti-β-galactosidase (1:1000, Promega); ED1 (1:1000, Serotec); anti-CD8 (1:500, Serotec); anti phosphorylated ZAP-70 (1:100, Cell Signaling); anti phosphorylated Lck (1:50, Cell Signaling); anti LAT (1:100, Cell Signaling); and anti-GFAP (1:500, Advanced Immunochemical), as described earlier (Barcia et al., 2006). The antibodies against TK were generated against peptides derived from HSV1-TK in chicken (raised by Aves Labs) and used at 1:100.
Immunostaining
Serial sections were used to detect transgene expression using either immunofluorescence or DAB. Immunofluorescence was carried out as described in detail previously (Barcia et al., 2006). For DAB staining, endogenous peroxidase activity was quenched with 0.3% H2O2 in PBS, non-specific Fc-binding sites were blocked with 10% horse serum and sections incubated 48 hours at room temperature with primary antibody diluted in PBS containing 1% hose serum and 0.5% Triton-X-100 (antibody solution). Sections were then incubated for 4 hours with appropriate biotin-conjugated secondary antibodies (DAKO, Cambridge, UK). Antibody binding was detected using the avidin-biotin peroxidase with diaminobenzidine as chromogen. These sections were mounted on gelatinized glass slides and dehydrated before coverslipping.
Quantification of immunohistochemical staining
Quantitative image analysis of (1) the area occupied by cells immunohistochemically stained with anti β-galactosidase, or ED1 antibodies and (2) the estimation of the number of β-gal and CD8 cells were performed with stereology software (StereoInvestigator®) using a computer-assisted image analysis system with a Zeiss microscope connected to a digital camera through a Zeiss zoom set, as described by us previously (Suwelack et al., 2004). (1) The area of transgene immmunoreactivity (β-galactosidase) was quantified in five 50 µm sections spaced at regular 250-µm intervals through the injected striatum as described (Thomas et al., 2000a; Thomas et al., 2001b; Thomas et al., 2001c). The area of transgene immunoreactivity in each of the five sections was summed for each brain. This sampling method covered >90% of the transduced area from each brain. Single brain sections containing the needle track (and, thus, displaying the highest levels of immunoreactivity) were used to quantify the area of ED1 immunoreactivity. (2) The stereological estimation of the number of CD8-immunoreactive cells was performed in the striatum and external capsule overlying the striatum, and anatomical locations were identified according to the atlas of the rat brain (Paxinos, 1986) in 50 µm brain sections spaced at regular, 250-µm intervals in immunized animals versus non-immunized animals. The estimation of the number of cells was performed using the principle of the optical fractionator (Sterio, 1984). The number of cells was measured in 200 µm-sided dissectors covering the whole surface area of the analysed regions (50 counting frames in each section). Positive cells were counted only when they cut the superior and left limit of the square. Results were expressed as an absolute number of positive cells in the anatomical regions analyzed. Data are expressed as the mean ± SEM.
Neutralizing antibody assay
Neutralizing antibody titers were measured in serum samples taken from animals that were killed at specific time-points following intraperitoneal injection of Ad-hCMV-HPRT. Serum was heat inactivated at 56°C for 30 min and diluted from 1:2-1:4096 in MEM containing 2% FBS. Serum dilutions were incubated with 3 × 105 iu of Ad-mCMV-βgal in a volume of 10 ml for 90 min at 37°C. 50 µl of each serum dilution was then added to wells of a 96-well plate in duplicate for each animal. An additional 50 µl of 10% FBS was added to each well and the cells incubated overnight (18 hours) at 37°C. Cells were fixed with 4% paraformaldehyde in PBS and stained with 5-bromo-4-chloro-indolyl-β-D-galactosidase (X-gal). Anti-adenoviral neutralizing antibody titer for each animal is given by the reciprocal of the highest dilution of serum at which 50% of Ad-hCMV-βgal-mediated staining was inhibited.
Statistical analysis
Viability data were expressed as mean ± SEM and evaluated by either two- or one-way ANOVA (followed by either Dunnet or Tukey multiple comparisons tests). Differenceswere considered significant if P<0.05. When the ANOVA test was not possible Kruskal-Wallis non-parametric test was used.
Confocal analysis
Brain sections were examined using a Leica DMIRE2 confocal microscope as described previously (Barcia et al., 2006). A series range for each section was determined by setting an upper and lower threshold using the Z/Y Position for Spatial Image Series setting. Confocal microscope settings have been established and maintained by Leica and local technicians for optimal resolution.
RESULTS
What is the threshold dose of RAd necessary to induce a systemic immunization that is sufficient to eliminate transgene expression from the CNS?
1 × 107 iu of Ad-mCMV-βgal (previously referred to as RAd36), were injected into adult striata of Sprague-Dawley rats. Double immunofluorescence staining demonstrated that β-galactosidase expressing cells were mostly astrocytes (Fig. 1A). Thirty days later, animals were immunized intradermally through a single injection of Ad-hCMV-HPRT (previously referred to as RAdHPRT), in doses of 10¹ to 107 iu. Ad-hCMV-HPRT was used as the immunogen so that the immune response would be generated against the viral vector, not the transgene. This paradigm has been described and published in detail previously (Thomas et al., 2000b). One month after immunization brains were processed to detect β-galactosidase expression by immunohistochemistry. Animals immunized intradermally with doses of ≤105 Ad-hCMV-HPRT did not eliminate expression of β-gal from the striatum (Fig. 1B, a,b). Quantification of the number of β-gal expressing cells showed no reduction in the level of striatal β-gal immunoreactivity when animals were immunized with doses of Ad-hCMV-HPRT of up to 105 (Fig. 1B, i); these doses did not increase serum neutralizing antibody titers (Fig. 1B, j). Further, animals injected intradermally with 107 iu of Ad-hCMV-HPRT lost all β-gal expression in the striatum (Fig. 1B, g,h), and these animals displayed high titers of neutralizing antibodies (Fig. 1B, j). Animals immunized intradermally with 1 × 106 iu of Ad-hCMV-HPRT showed variable expression of β-gal in the striatum (Fig. 1B, c–f), and variable levels of neutralizing antibodies (Fig. 1B, j). This demonstrates that the peripheral threshold of RAd needed to induce a systemic immune response that is strong enough to eliminate RAd-mediated expression from the CNS in 100% of animals is ≥1 × 107 iu.
Fig. 1. (A) RAd36 induces expression of β-galactosidase in astrocytes.

Rat brain sections injected with RAd36 were stained for β-galactosidase (green) and GFAP, a marker of astrocytes (magenta) and analyzed with confocal microscope. Confocal analysis demonstrated that β-galactosidase is expressed in astrocytes. Note the colocalization of β-galactosidase and GFAP in the merged image. Scale bar, 30 µm. (B) Determination of the dose of systemic immunization needed to silence transgene expression in the CNS. Panels a–h show β-gal immunohistochemistry in rats injected with 107 iu Ad-mCMV-βgal in the striatum and immunized 30 days later with 10¹–108 iu Ad-hCMV-HPRT. Only doses 105 to 107 iu are illustrated. Peripheral immunization with ≤105 iu did not reduce brain transgene expression (a,b,i) or increase serum neutralizing antibodies (j). Immunization with 106 iu induced loss of transgene expression in some animals and one animal from each group is illustrated (c–f,i,j). Following peripheral immunization of ≥107 iu transgene expression was lost in all animals (g,h,i), with all animals showing significant titers of neutralizing antibodies (j). Each dot in i and j represents one animal.
Is there a minimum dose of RAd injected into the brain that escapes elimination by the systemic immune response?
Animals injected in the brain with increasing doses of Ad-mCMV-βgal (i.e. 1 × 10¹–1 × 107 iu) have a proportional, dose-related increase in expression of β-galactosidase in the striatum of naïve animals (injected systemically with saline) (Fig 2 and Fig 3). However animals immunized systemically with 5 × 108 iu of Ad-mCMV-HPRT showed a reduction of β-galactosidase expressing cells even when very low amounts of vector (i.e. 10³ iu) were injected into the brain (Fig 2 and Fig 3). No statistically significant reductions were detected at either 10¹ iu or 10² iu doses (Fig 2 and Fig 3).
Fig. 2. β-gal immunohistochemistry of rat brain injected with increasing doses of Ad-mCMV-βgal in the striatum.

Animals were injected with 10¹–107 iu Ad-mCMV-βgal in the striatum and 30 days later systemically with either saline (left) or 108 iu Ad-hCMV-HPRT (right). Peripheral immunization with 108 iu Ad-hCMV-HPRT reduced brain transgene expression independently of the dose of adenovirus injected into the brain.
Fig. 3. Quantification of β-gal-positive cells after injection with Ad-mCMV-βgal in the striatum and Ad-hCMV-HPRT systemically.
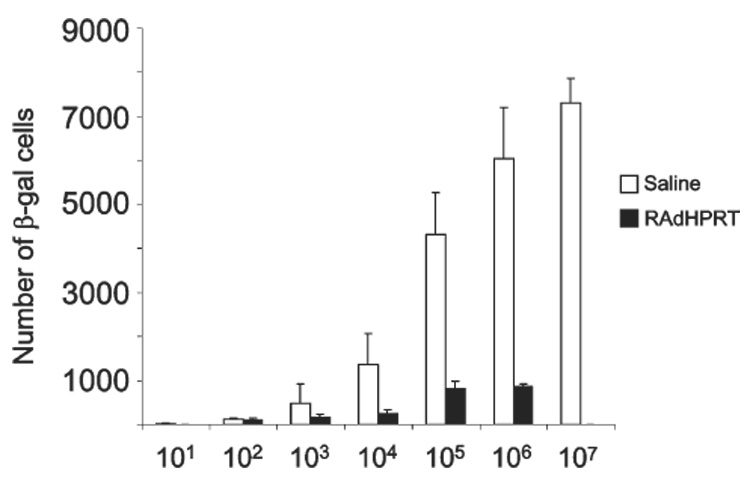
Rats were injected with increasing doses of Ad-mCMV-βgal in the striatum (10¹–107 iu) and injected systemically 30 days later with either saline or 108 iu Ad-hCMV-HPRT. Expression of β-gal was always reduced following immunization with 108 iu Ad-hCMV-HPRT despite the different doses injected intracranially.
Systemic anti-RAd immunization silences adenoviral β-gal expression from RAd driven by neuronal, housekeeping, and/or viral promoters
Experiments illustrated in Fig 1–Fig 3 demonstrated decreased expression of transgene expression from Ad-mCMV-βgal following systemic immunization. Therefore, we examined whether the immune response to adenovirus would selectively inhibit expression from the mCMV promoter, a viral promoter, or whether other viral, housekeeping and/or cellular promoters would not be affected by the systemic immune response. To this end, we injected into the striatum RAds encoding β-gal under the control of the hCMV promoter (Ad-hCMV-βgal; previously named RAd35), mCMV (Ad-mCMV-βgal), the RSV promoter (RAd122), the β-actin promoter (RAd-βactin), and a recombinant adenovirus expressing β-gal under the control of the neuronal-specific synapsin promoter (RAdsyn).
Expression of β-gal was observed in the striatum of all control animals (sham immunized with saline). Maximum expression, as expected, depends on individual promoters (Fig. 4a–c). However, all animals immunized systemically with Ad-hCMV-HPRT showed a dramatic decrease in expression (Fig. 4a–c). The decrease in transgene expression was independent of the promoter utilized to drive transgene expression. ED1 (lysosomal membrane antigen specific for brain macrophages and microglial cells) and CD8 immunostaining of serial adjacent sections revealed an increase of both cell types surrounding the injection site in all immunized animals. Quantification of the striatal area occupied by ED1 immunoreactivity was increased significantlyin animals immunized systemically with Ad-hCMV-HPRT compared with non-immunized rats (Fig. 4c3). Stereological quantification of the number of CD8+ T cells infiltrating the striatum in immunized animals also revealed a significant increase compared with controls (Fig. 4c2). Although animals were injected with the same amount of recombinant adenovirus, the level of expression detected depended on the individual promoter activity. Nevertheless, the inflammatory and immune response was identical in all animals because it is directed against viral-vector proteins rather then the amount of transgene expressed.
Fig. 4. Immunohistochemical detection of β-gal, CD8 and ED1.
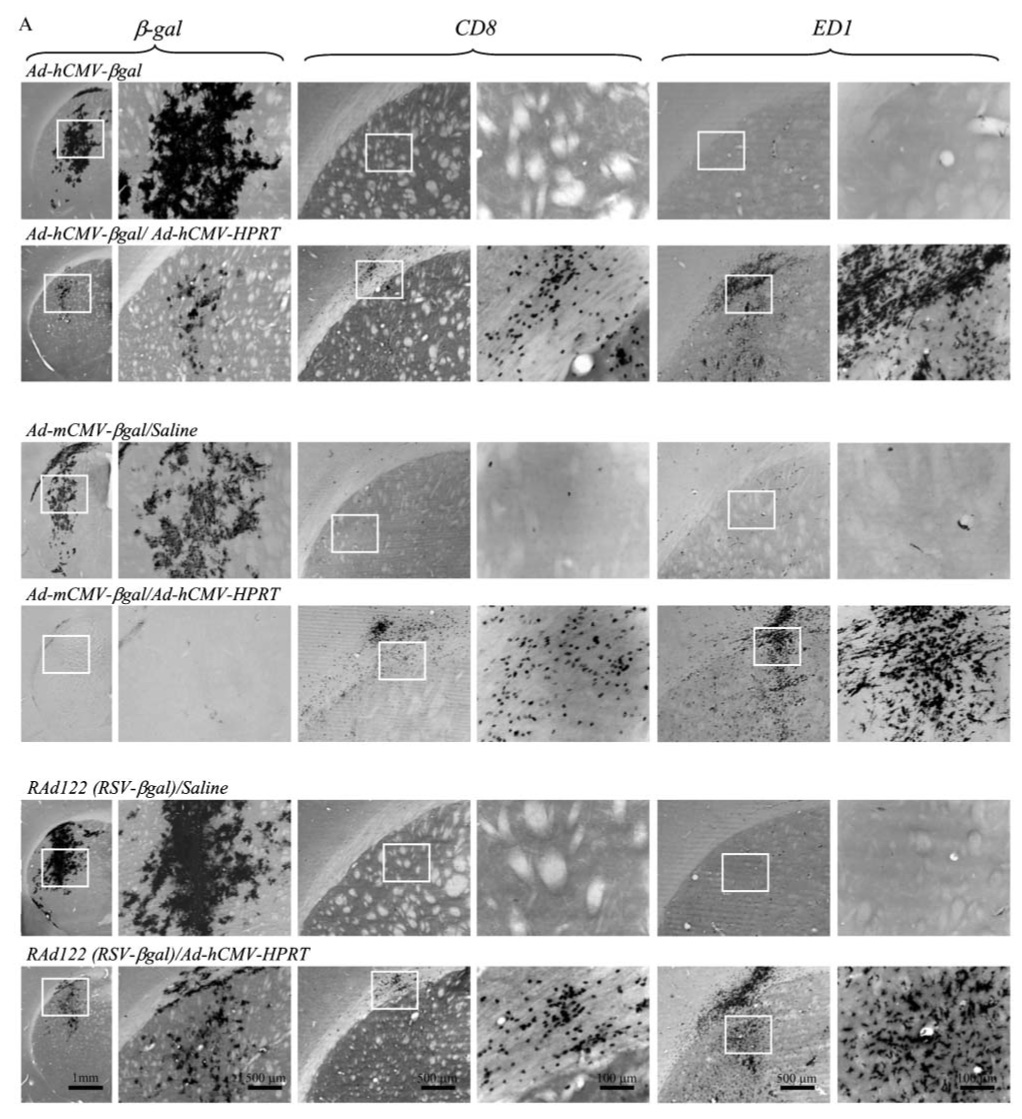

β-gal, CD8 and ED1 in brain sections from rats injected in the striatum with different virus (Ad-hCMV-βgal, Ad-mCMV-βgal, RAd122, RAdsyn and RAdβact) containing different promoters (hCMV, mCMV, RSV, syn and βact, respectively) to drive expression of β-gal. Thirty days after injection animals were injected intradermally with either Ad-hCMV-HPRT (immunized animals) or with saline (control group). Sixty days after intradermal injection, animals were sacrificed and perfused. The expression of β-gal, CD8 and ED1 is shown in immunized (Ad-hCMV-HPRT) and in control animals (saline). (A,B) 1st, 3rd and 5th columns show low magnification of β-gal, CD8 and ED1, respectively, and the boxed areas are shown at higher magnification (right columns). (C) The area occupied by β-gal is reduced significantly in immunized animals (Ad-hCMV-HPRT) compared to controls (C1). Stereological estimation of the number of CD8 cells increased significantly in animals immunized with Ad-hCMV-HPRT compared to controls (C2). The area occupied by ED1 also increased significantly in immunized animals compared to controls (C3). *P<0.05, **P<0.01 Student’s t-test.
Activated T cells expressing tyrosine kinases form immunological synapses with virally infected cells
Recently, we have demonstrated that activated antiviral T cells form specific immunological synapses with virally infected astrocytes. Immunological synapses are thought to mediate intercellular communication in the immune system and, thus, might mediate antiviral immune responses in the brain, as described above. The anatomical substrate of these interactions is illustrated in Fig. 5; these confocal images demonstrate the formation of immunological synapses between virally infected astrocytes and CD8+ T cells. This figure demonstrates, for the first time, that phosphorylated ZAP 70 (Fig. 5a), and phosphorylated LCK (Fig. 5b) are indeed polarized in T cells establishing immunological synapses with infected astrocytes (they are shown to express TK), thus, demonstrating that T cell receptor (TCR) signaling has been stimulated in response to antigen encounter. LAT (Fig. 5c), an adaptor protein that is involved in TCR signaling, is also localized within the contact area between T cells and infected astrocytes.
Fig. 5. Activated T cells that express tyrosine kinases form immunological synapses with virally infected cells.
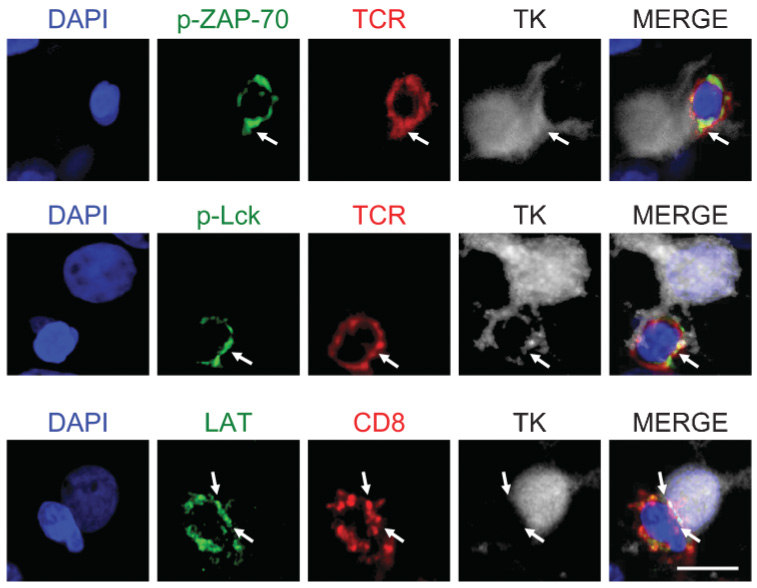
Confocal images show three immunological synapses formed between T cells and virally infected astrocytes in the striatum of rats injected with Ad-hCMV-TK and immunized systemically 30 days later with Ad-hCMV-HPRT. Activated T cells show expression, phosphorylation and polarization of tyrosine kinases when in close apposition with virally infected astrocytes, indicating the activation and signaling of TCR in response to antigen encounter. Top row: a T cell (red, stained for TCR) that expresses phosphorylated ZAP-70 (green) forms an immunological synapse with a virally infected cell (white, stained for TK). Middle row: a T cell (red, stained for TCR) expressing phosphorylated Lck (green) polarized to the area of contact with the target cell (white, stained for TK). Bottom row: a CD8+ T cell that expresses LAT (green) forms an immunological synapse with a virally infected cell (white, stained for TK). White arrows indicate the areas of close apposition between T cells and infected brain cells. Scale bar, 20 µm.
CONCLUSIONS
The minimum dose of replication-defective adenovirus type 5 required to induce a systemic immune response that can eliminate transgene expression from the brain in 100% of Sprague-Dawley rats is 1 × 107 infectious units.
The maximum dose of a replication-defective adenovirus type 5 injected into the striatum of Sprague-Dawley rats that avoids immune-mediated elimination is 100 infectious units. Transgene expression in the brain from all vector doses above 10² infectious units is eliminated.
The primed/activated adaptive immune response abolishes transgene expression in the brain driven by viral, housekeeping, or cell-type specific promoters.
Specific TCR cell signaling at the immunological synapse between CTLs and infected adenovirally infected brain cells induces the targeting of phosphorylated tyrosine kinases and LAT to the T cell:astrocyte interface.
DISCUSSION
First-generation adenoviral vectors are powerful vectors for gene transfer and gene therapy in the CNS (Bilang-Bleuel et al., 1997; Bemelmans et al., 1999; Dewey et al., 1999; Bjorklund et al., 2000; Mittoux et al., 2000; Sandmair et al., 2000; Maleniak et al., 2001; Mittoux et al., 2002; Biglari et al., 2004; Chiocca et al., 2004; Do Thi et al., 2004; Gondi et al., 2004; Hurtado-Lorenzo et al., 2004; Immonen et al., 2004). As a result, recent trials have shown adenoviral vectors encoding HSV1-TK to be effective in randomized clinical trials for brain glioblastoma multiforme, compared to patients treated with standard treatment. These encouraging clinical data have led to a large, European, Phase III, multicenter, clinical trial of adenovirus-TK (Shand et al., 1999).
In naïve rats not exposed previously to adenovirus, transgene expression from adenoviral vectors is stable for up to 13 months (Byrnes et al., 1995; Geddes et al., 1997; Kajiwara et al., 1997; Ideguchi et al., 2000; Thomas et al., 2000b; Zou et al., 2000; Amalfitano et al., 2002; Glover et al., 2003). However, systemic immunization of animals injected previously with adenovirus into the CNS with first-generation adenoviral vectors leads to immune responses in the CNS that substantially reduce and, in some cases, eliminate transgene expression from the brain (Byrnes et al., 1995; Byrnes et al., 1996a; Byrnes et al., 1996b; Wood et al., 1996; Kajiwara et al., 1997; Thomas et al., 2000; Zermansky et al., 2001). However, transgene expression from high-capacity adenoviral vectors remains stable; further, three days after intracranial injection of vector in animals previously immunized with a peripheral injection of adenoviral vector was identical to non-immunized animals. This indicates that neutralizing antibodies are ineffective at blocking adenoviral infection of brain tissue (Thomas et al., 2001c).
In these studies we determined the dose of adenovirus needed for systemic immunization to eliminate transgene expression from the brain. Systemic injections of increasing amounts of viral vectors demonstrated that doses of >1 × 106 iu eliminate transgene expression from the brains of all animals. In some cases, loss of transgene expression has been attributed to the shut-off of viral promoters. To ascertain whether expression from a viral promoter in our vectors was inhibited non-specifically, we injected into the brain vectors that express the transgene under various viral, housekeeping and neuronal promoters. In all cases, immunization reduced transgene expression, demonstrating that this phenomenon is not a consequence of promoter shut-off, but represents a general mechanism that abrogates expression from any promoter within the viral vector.
Previous experiments describing the shut-off of transgene expression utilized recombinant adenovirus vectors encoding the hCMV promoter. This promoter is influenced by several regulatory mechanisms, including pro-inflammatory cytokines such as IFN-γ (Shering et al., 1997). Our experiments indicate that the immune system downregulates expression form several promoters, including viral, housekeeping and cell type-specific promoters. However, the alternative hypothesis, that limited cytotoxicity occurs throughout the CNS, needs to be considered equally at this stage.
Specific downregulation of transcription from viral promoters might operate through inhibition of viral genome-specific mechanisms. These might, for example, involve reorganization of ND-10 domains (viral DNA docking sites) and sequestration of transcription factors in response to IFNγ (Griffin, 2003; Khanna et al., 2004), phosphorylation of the translation regulator ieFα, and activation of PKR-related multi-viral mechanisms (Scheuner et al., 2003; Baltzis et al., 2004; Bonizzi et al., 2004; Hsu et al., 2004; Isler et al., 2005). However, it remains to be seen whether more specifically gene expression is affected in transduced cells. It is interesting to note that regulation of transgene expression is mediated mostly bycellular elements of the adaptive immune response because the early innate inflammatory immune responses do not affect gene expression (Thomas et al., 2000b; Thomas et al., 2001b).
Furthermore, other mechanisms have been described by which the adaptive immune response might silence gene expression. In the CNS, antibodies and IFN-γ mediated mechanisms have been postulated to clear virus infection from neurons through non-cytolytic pathways (Patterson et al., 2002; Griffin, 2003). Non-cytolytic mechanisms might be more frequent in clearing virus from infected neurons, whereas cytotoxic mechanisms might be more common in clearing virus from infected astrocytes and oligodendrocytes (Griffin, 2003). Using quantitative PCR, we have previously demonstrated a reduction in vector genome copy numbers in animals immunized against adenovirus (Barcia et al., 2006). Although the copy numbers of the vector genome were reduced, our data did not rule out promoter shut-off completely. We believe that most elimination of transgene expression could be attributed to cytotoxic effects and that transcriptional down-regulation, if it occurs, is likely to make a minor contribution to the loss of transgene expression.
To date, however, cytotoxicity has only been demonstrated conclusively in in vitro models; in vivo direct killing and elimination of brain cells by lymphocytes is difficult to demonstrate (Rall et al., 1995). Immunological synapses are the anatomical substrate that underlies intercellular communication in the immune system, and recently we have demonstrated their existence in vivo (Barcia et al., 2006). Here, we provide further evidence that anti-viral CD8+ cytotoxic T cells that express phosphorylated tyrosine kinases form immunological synapses with virally infected brain cells preceding the elimination of transgene expression from virally infected astrocytes. We also demonstrate that LAT, the crucial linker between TCR engagement and T-cell activation also accumulates at the immunological synapses. Currently, we are evaluating a novel system to explore the consequences to virally-infected astrocytes of the formation of immunological synapses with anti-viral CD8+ cytotoxic T cells.
In summary, we have demonstrated that a primed adaptive immune response specifically inhibits transgene expression in the CNS independently of the promoter that drives transgene expression. Cellular elements of the systemically activated immune response infiltrate the CNS and cause the shut-off of expression from viral, housekeeping and cell-type specific promoters encoded with adenoviral vectors.
Understanding the mechanisms of action by which the immune system regulates transgene expression in the brain is crucial for safe, effective clinical trials. This is especially important if patients have been exposed previously to viruses from which the vectors used in gene therapy are derived (e.g. adenovirus, AAV and HSV-1). If the immune system regulates gene expression in the CNS mainly through noncytotoxic mechanisms, any downregulation of gene expression will block the efficiency of clinical trials that involve gene therapy. However, if the immune response physically eliminates transduced cells, it might make conditions in which the therapy aims to protect neurons from ongoing degeneration, such as Parkinson’s disease, worse. An optimal animal model of the immunological challenges associated with human clinical trials for gene therapy would involve immunization with a wild-type, replicating, human adenovirus. Unfortunately human adenovirus does not replicate in rodents and so we use a replication-deficient viral vector as the immunization agent.
These studies also establish the threshold input of non-replicating adenovirus vectors needed to prime a systemic immune response, and demonstrate that a threshold must be crossed before a systemic, anti-adenoviral immune response is unleashed. Furthermore, we demonstrate that once a systemic immune response has been primed it is highly efficient in recognizing and eliminating even very small numbers of transduced cells. We believe that our data demonstrate the need to establish vector dose thresholds for all viral-derived vector platforms that are used in gene therapy for neurological diseases.
Of specific interest to this special issue, pain is a potential target for gene therapy strategies, especially by modifying the function of either dorsal root ganglion neurons or neural pathways along the pain circuits. Preclinical studies of chronic pain have used several gene therapy vectors (Cope et al., 2006). Adenoviral, AAV, and HSV vectors have been used to deliver therapeutic genes including β-endorphin (Finegold et al., 1999), proopiomelanocortin (Lu et al., 2002), interleukin 2 (IL-2) (Yao et al., 2003), proenkephalin-A (Braz et al., 2001), IL-10 (Milligan et al., 2005a; Milligan et al., 2005b), glial-derived growth factor (Hao et al., 2003) and neurotrophin-3 (Pradat et al., 2001).
Currently gene therapy approaches for chronic pain are yet to be utilized in a human clinical trial but several research groups are in the early stages of implementation. It is important to consider the immunological consequences of delivering viral vectors systemically. Immune responsesmight, in some cases, be deleterious to either the transduced cells or the long-term expression of the therapeutic gene, an effect that is mediated through the establishment of immunological synapses (Barcia et al., 2006).
Our data indicate that the primed systemic adaptive immune response inhibits expression from any type of promoter, and efficiently suppresses expression from as few as 1000 transduced brain cells. The mechanisms by which the immune system regulates gene expression in the CNS needs to be explored and developed further to make clinical trials in neurological gene therapy safer and more effective. In addition, steps must be taken to avoid priming the systemic adaptive immune response.
ACKNOWLEDGEMENTS
Gene therapy projects for neurological diseases are funded by grants from the National Institutes of Health/National Institute of Neurological Disorders & Stroke Grant 1R01 NS44556.01, National Institute of Diabetes and Digestive and Kidney Diseases 1 RO3 TW006273-01 to M.G.C., National Institutes of Health/National Institute of Neurological Disorders & Stroke Grant 1 RO1 NS 42893.01, U54 NS045309-01, and 1R21 NS047298-01 to PRL, and The Bram and Elaine Goldsmith Chair In Gene Therapeutics (P.R.L.), as well as The Linda Tallen & David Paul Kane Annual Fellowship to M.G.C. and P.R.L. We appreciate the funding our Institute receives from the Board of Governors at Cedars Sinai Medical Center, and thank S. Melmed, R. Katzman and D. Meyer for their superb administrative and organizational support.
References
- Akrigg A, Wilkinson GW, Oram JD. The structure of the major immediate early gene of human cytomegalovirus strain AD169. Virus Research. 1985;2:107–121. doi: 10.1016/0168-1702(85)90242-4. [DOI] [PubMed] [Google Scholar]
- Amalfitano A, Parks RJ. Separating fact from fiction: assessing the potential of modified adenovirus vectors for use in human gene therapy. Current Gene Therapy. 2002;2:111–133. doi: 10.2174/1566523024605618. [DOI] [PubMed] [Google Scholar]
- Baltzis D, Qu LK, Papadopoulou S, Blais JD, Bell JC, Sonenberg N, et al. Resistance to vesicular stomatitis virus infection requires a functional cross talk between the eukaryotic translation initiation factor 2alpha kinases PERK and PKR. Journal of Virology. 2004;78:12747–12761. doi: 10.1128/JVI.78.23.12747-12761.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcia C, Thomas CE, Curtin JF, King GD, Wawrowsky K, Candolfi M, et al. In vivo mature immunological synapses forming SMACs mediate clearance of virally infected astrocytes from the brain. Journal of Experimental Medicine. 2006;203:2095–2107. doi: 10.1084/jem.20060420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemelmans AP, Horellou P, Pradier L, Brunet I, Colin P, Mallet J. Brain-derived neurotrophic factor-mediated protection of striatal neurons in an excitotoxic rat model of Huntington’s disease, as demonstrated by adenoviral gene transfer. Human Gene Therapy. 1999;10:2987–2997. doi: 10.1089/10430349950016393. [DOI] [PubMed] [Google Scholar]
- Bett AJ. PhD Thesis. Ontario, Canada: McMaster University, Hamilton; 1995. Construction and characterization of recombinant human adenovirus type 5 vectors. [Google Scholar]
- Biglari A, Bataille D, Naumann U, Weller M, Zirger J, Castro MG, et al. Effects of ectopic decorin in modulating intracranial glioma progression in vivo, in a rat syngeneic model. Cancer Gene Therapy. 2004;11:721–732. doi: 10.1038/sj.cgt.7700783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilang-Bleuel A, Revah F, Colin P, Locquet I, Robert JJ, Mallet J, et al. Intrastriatal injection of an adenoviral vector expressing glial-cell-line-derived neurotrophic factor prevents dopaminergic neuron degeneration and behavioral impairment in a rat model of Parkinson disease. Proceedings of the National Academy of Sciences of the U.S.A. 1997;94:8818–8823. doi: 10.1073/pnas.94.16.8818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder GK, Griffin DE. Immune-mediated clearance of virus from the central nervous system. Microbes and Infection. 2003;5:439–448. doi: 10.1016/s1286-4579(03)00047-9. [DOI] [PubMed] [Google Scholar]
- Bjorklund A, Kirik D, Rosenblad C, Georgievska B, Lundberg C, Mandel RJ. Towards a neuroprotective gene therapy for Parkinson’s disease: use of adenovirus, AAV and lentivirus vectors for gene transfer of GDNF to the nigrostriatal system in the rat Parkinson model. Brain Research. 2000;886:82–98. doi: 10.1016/s0006-8993(00)02915-2. [DOI] [PubMed] [Google Scholar]
- Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends in Immunology. 2004;25:280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Braz J, Beaufour C, Coutaux A, Epstein AL, Cesselin F, Hamon M, et al. Therapeutic efficacy in experimental polyarthritis of viral-driven enkephalin overproduction in sensory neurons. Journal of Neuroscience. 2001;21:7881–7888. doi: 10.1523/JNEUROSCI.21-20-07881.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes AP, MacLaren RE, Charlton HM. Immunological instability of persistent adenovirus vectors in the brain: peripheral exposure to vector leads to renewed inflammation, reduced gene expression, and demyelination. Journal of Neuroscience. 1966a;16:3045–3055. doi: 10.1523/JNEUROSCI.16-09-03045.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes AP, Rusby JE, Wood MJ, Charlton HM. Adenovirus gene transfer causes inflammation in the brain. Neuroscience. 1995;66:1015–1024. doi: 10.1016/0306-4522(95)00068-t. [DOI] [PubMed] [Google Scholar]
- Byrnes AP, Wood MJ, Charlton HM. Role of T cells in inflammation caused by adenovirus vectors in the brain. Gene Therapy. 1996b;3:644–651. [PubMed] [Google Scholar]
- Chiocca EA, Abbed KM, Tatter S, Louis DN, Hochberg FH, Barker F, et al. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-Attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Molecular Therapeutics. 2004;10:958–966. doi: 10.1016/j.ymthe.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Cope DK, Lariviere WR. Gene therapy and chronic pain. ScientificWorldJournal. 2006;6:1066–1074. doi: 10.1100/tsw.2006.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowsill C, Southgate TD, Morrissey G, Dewey RA, Morelli AE, Maleniak TC, et al. Central nervous system toxicity of two adenoviral vectors encoding variants of the herpes simplex virus type 1 thymidine kinase: reduced cytotoxicity of a truncated HSV1-TK. Gene Therapy. 2000;7:679–685. doi: 10.1038/sj.gt.3301147. [DOI] [PubMed] [Google Scholar]
- Dewey RA, Morrissey G, Cowsill CM, Stone D, Bolognani F, Dodd NJ, et al. Chronic brain inflammation and persistent herpes simplex virus 1 thymidine kinase expression in survivors of syngeneic glioma treated by adenovirus-mediated gene therapy: implications for clinical trials. Nature Medicine. 1999;5:1256–1263. doi: 10.1038/15207. [DOI] [PubMed] [Google Scholar]
- Dion LD, Fang J, Garver RI., Jr Supernatant rescue assay vs. polymerase chain reaction for detection of wild type adenovirus-contaminating recombinant adenovirus stocks. Journal of Virology Methods. 1996;56:99–107. doi: 10.1016/0166-0934(95)01973-1. [DOI] [PubMed] [Google Scholar]
- Do Thi NA, Saillour P, Ferrero L, Dedieu JF, Mallet J, Paunio T. Delivery of GDNF by an E1,E3/E4 deleted adenoviral vector and driven by a GFAP promoter prevents dopaminergic neuron degeneration in a rat model of Parkinson’s disease. Gene Therapy. 2004;11:746–756. doi: 10.1038/sj.gt.3302222. [DOI] [PubMed] [Google Scholar]
- Eck SL, Alavi JB, Judy K, Phillips P, Alavi A, Hackney D, et al. Treatment of recurrent or progressive malignant glioma with a recombinant adenovirus expressing human interferon-beta (H5.010CMVhIFN-beta): a phase I trial. Human Gene Therapy. 2001;12:97–113. doi: 10.1089/104303401451013. [DOI] [PubMed] [Google Scholar]
- Ehtesham M, Black KL, Yu JS. Recent progress in immunotherapy for malignant glioma: treatment strategies and results from clinical trials. Cancer Control. 2004;11:192–207. doi: 10.1177/107327480401100307. [DOI] [PubMed] [Google Scholar]
- Finegold AA, Mannes AJ, Iadarola MJ. A paracrine paradigm for in vivo gene therapy in the central nervous system: treatment of chronic pain. Human Gene Therapy. 1999;10:1251–1257. doi: 10.1089/10430349950018238. [DOI] [PubMed] [Google Scholar]
- Geddes BJ, Harding TC, Lightman SL, Uney JB. Long-term gene therapy in the CNS: reversal of hypothalamic diabetes insipidus in the Brattleboro rat by using an adenovirus expressing arginine vasopressin. Nature Medicine. 1997;3:1402–1404. doi: 10.1038/nm1297-1402. [DOI] [PubMed] [Google Scholar]
- Gerdes CA, Castro MG, Lowenstein PR. Strong promoters are the key to highly efficient, noninflammatory and noncytotoxic adenoviral-mediated transgene delivery into the brain in vivo. Molecular Therapeutics. 2000;2:330–338. doi: 10.1006/mthe.2000.0140. [DOI] [PubMed] [Google Scholar]
- Glover CP, Bienemann AS, Hopton M, Harding TC, Kew JN, Uney JB. Long-term transgene expression can be mediated in the brain by adenoviral vectors when powerful neuron-specific promoters are used. Journal of Gene Medicine. 2003;5:554–559. doi: 10.1002/jgm.381. [DOI] [PubMed] [Google Scholar]
- Gondi CS, Lakka SS, Yanamandra N, Olivero WC, Dinh DH, Gujrati M, et al. Adenovirus-mediated expression of antisense urokinase plasminogen activator receptor and antisense cathepsin B inhibits tumor growth, invasion, and angiogenesis in gliomas. Cancer Research. 2004;64:4069–4077. doi: 10.1158/0008-5472.CAN-04-1243. [DOI] [PubMed] [Google Scholar]
- Griffin DE. Immune responses to RNA-virus infections of the CNS. Nature Reviews Immunology. 2003;3:493–502. doi: 10.1038/nri1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao S, Mata M, Wolfe D, Huang S, Glorioso JC, Fink DJ. HSV-mediated gene transfer of the glial cell-derived neurotrophic factor provides an antiallodynic effect on neuropathic pain. Molecular Therapeutics. 2003;8:367–375. doi: 10.1016/s1525-0016(03)00185-0. [DOI] [PubMed] [Google Scholar]
- Hsu LC, Park JM, Zhang K, Luo JL, Maeda S, Kaufman RJ, et al. The protein kinase PKR is required for macrophage apoptosis after activation of Toll-like receptor 4. Nature. 2004;428:341–345. doi: 10.1038/nature02405. [DOI] [PubMed] [Google Scholar]
- Hurtado-Lorenzo A, Millan E, Gonzalez-Nicolini V, Suwelack D, Castro MG, Lowenstein PR. Differentiation and transcription factor gene therapy in experimental parkinson’s disease: sonic hedgehog and Gli-1, but not Nurr-1, protect nigrostriatal cell bodies from 6-OHDA-induced neurodegeneration. Molecular Therapeutics. 2004;10:507–524. doi: 10.1016/j.ymthe.2004.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ideguchi M, Kajiwara K, Yoshikawa K, Uchida T, Ito H. Local adenovirus-mediated CTLA4-immunoglobulin expression suppresses the immune responses to adenovirus vectors in the brain. Neuroscience. 2000;95:217–226. doi: 10.1016/s0306-4522(99)00402-9. [DOI] [PubMed] [Google Scholar]
- Immonen A, Vapalahti M, Tyynela K, Hurskainen H, Sandmair A, Vanninen R, et al. AdvHSV-tk gene therapy with intravenous ganciclovir improves survival in human malignant glioma: a randomised, controlled study. Molecular Therapeutics. 2004;10:967–972. doi: 10.1016/j.ymthe.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Isler JA, Skalet AH, Alwine JC. Human cytomegalovirus infection activates and regulates the unfolded protein response. Journal of Virology. 2005;79:6890–6899. doi: 10.1128/JVI.79.11.6890-6899.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagi D, Ledermann B, Burki K, Zinkernagel RM, Hengartner H. Molecular mechanisms of lymphocyte-mediated cytotoxicity and their role in immunological protection and pathogenesis in vivo. Annual Review of Immunology. 1996;14:207–232. doi: 10.1146/annurev.immunol.14.1.207. [DOI] [PubMed] [Google Scholar]
- Kajiwara K, Byrnes AP, Charlton HM, Wood MJ, Wood KJ. Immune responses to adenoviral vectors during gene transfer in the brain. Human Gene Therapy. 1997;8:253–265. doi: 10.1089/hum.1997.8.3-253. [DOI] [PubMed] [Google Scholar]
- Khanna KM, Lepisto AJ, Decman V, Hendricks RL. Immune control of herpes simplex virus during latency. Current Opinion in Immunology. 2004;16:463–469. doi: 10.1016/j.coi.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Lowenstein PR. Immunology of viral-vector-mediated gene transfer into the brain: an evolutionary and developmental perspective. Trends in Immunology. 2002;23:23–30. doi: 10.1016/s1471-4906(01)02063-4. [DOI] [PubMed] [Google Scholar]
- Lu CY, Chou AK, Wu CL, Yang CH, Chen JT, Wu PC, et al. Gene-gun particle with pro-opiomelanocortin cDNA produces analgesia against formalin-induced pain in rats. Gene Therapy. 2002;9:1008–1014. doi: 10.1038/sj.gt.3301774. [DOI] [PubMed] [Google Scholar]
- Maleniak TC, Darling JL, Lowenstein PR, Castro MG. Adenovirus-mediated expression of HSV1-TK or Fas ligand induces cell death in primary human glioma-derived cell cultures that are resistant to the chemotherapeutic agent CCNU. Cancer Gene Therapy. 2001;8:589–598. doi: 10.1038/sj.cgt.7700348. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Langer SJ, Sloane EM, He L, Wieseler-Frank J, O’Connor K, et al. Controlling pathological pain by adenovirally driven spinal production of the anti-inflammatory cytokine, interleukin-10. Eurropean Journal of Neuroscience. 2005a;21:2136–2148. doi: 10.1111/j.1460-9568.2005.04057.x. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Sloane EM, Langer SJ, Cruz PE, Chacur M, Spataro L, et al. Controlling neuropathic pain by adeno-associated virus driven production of the anti-inflammatory cytokine, interleukin-10. Molecular Pain. 2005b;1:9. doi: 10.1186/1744-8069-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittoux V, Joseph JM, Conde F, Palfi S, Dautry C, Poyot T, et al. Restoration of cognitive and motor functions by ciliary neurotrophic factor in a primate model of Huntington’s disease. Human Gene Therapy. 2000;11:1177–1187. doi: 10.1089/10430340050015220. [DOI] [PubMed] [Google Scholar]
- Mittoux V, Ouary S, Monville C, Lisovoski F, Poyot T, Conde F, et al. Corticostriatopallidal neuroprotection by adenovirus-mediated ciliary neurotrophic factor gene transfer in a rat model of progressive striatal degeneration. Journal of Neuroscience. 2002;22:4478–4486. doi: 10.1523/JNEUROSCI.22-11-04478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson CE, Lawrence DM, Echols LA, Rall GF. Immune-mediated protection from measles virus-induced central nervous system disease is noncytolytic and gamma interferon dependent. Journal of Virology. 2002;76:4497–4506. doi: 10.1128/JVI.76.9.4497-4506.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G. The Rat Brain in Stereotaxic Coordinates. Academic Press; 1986. [Google Scholar]
- Pradat PF, Kennel P, Naimi-Sadaoui S, Finiels F, Orsini C, Revah F, et al. Continuous delivery of neurotrophin 3 by gene therapy has a neuroprotective effect in experimental models of diabetic and acrylamide neuropathies. Human Gene Therapy. 2001;12:2237–2249. doi: 10.1089/10430340152710577. [DOI] [PubMed] [Google Scholar]
- Rall GF, Mucke L, Oldstone MB. Consequences of cytotoxic T lymphocyte interaction with major histocompatibility complex class I-expressing neurons in vivo. Journal of Experimental Medicine. 1995;182:1201–1212. doi: 10.1084/jem.182.5.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph GS, Bienemann A, Harding TC, Hopton M, Henley J, Uney JB. Targeting of tetracycline-regulatable transgene expression specifically to neuronal and glial cell populations using adenoviral vectors. Neuroreport. 2000;11:2051–2055. doi: 10.1097/00001756-200006260-00048. [DOI] [PubMed] [Google Scholar]
- Sandmair AM, Loimas S, Puranen P, Immonen A, Kossila M, Puranen M, et al. Thymidine kinase gene therapy for human malignant glioma, using replication-deficient retroviruses or adenoviruses. Human Gene Therapy. 2000;11:2197–2205. doi: 10.1089/104303400750035726. [DOI] [PubMed] [Google Scholar]
- Scheuner D, Gromeier M, Davies MV, Dorner AJ, Song B, Patel RV, et al. The double-stranded RNA-activated protein kinase mediates viral-induced encephalitis. Virology. 2003;317:263–274. doi: 10.1016/j.virol.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Shand N, Weber F, Mariani L, Bernstein M, Gianella-Borradori A, Long Z, et al. A phase 1-2 clinical trial of gene therapy for recurrent glioblastoma multiforme by tumor transduction with the herpes simplex thymidine kinase gene followed by ganciclovir. GLI328 European-Canadian Study Group. Human Gene Therapy. 1999;10:2325–2335. doi: 10.1089/10430349950016979. [DOI] [PubMed] [Google Scholar]
- Shering AF, Bain D, Stewart K, Epstein AL, Castro MG, Wilkinson GW, Lowenstein PR. Cell type-specific expression in brain cell cultures from a short human cytomegalovirus major immediate early promoter depends on whether it is inserted into herpesvirus or adenovirus vectors. Journal of General Virology. 1997;78:445–459. doi: 10.1099/0022-1317-78-2-445. [DOI] [PubMed] [Google Scholar]
- Smith-Arica JR, Morelli AE, Larregina AT, Smith J, Lowenstein PR, Castro MG. Cell-type-specific and regulatable transgenesis in the adult brain: adenovirus-encoded combined transcriptional targeting and inducible transgene expression. Molecular Therapeutics. 2000;2:579–587. doi: 10.1006/mthe.2000.0215. [DOI] [PubMed] [Google Scholar]
- Southgate T, Bain D, Fairbanks LD, Morelli A, Larregina A, Simmonds HA, et al. Adenoviruses encoding HPRT correct the biochemical abnormalities fully only in HPRT-deficient human cell lines: importance of species differences. Advances in Experimental and Medical Biology. 2000a;486:35–40. doi: 10.1007/0-306-46843-3_7. [DOI] [PubMed] [Google Scholar]
- Southgate T, Kingston P, Castro MG. Gene transer into neural cells in vivo using adenoviral vectors. In: Gerfen CR, McKay R, Rogawski MA, Sibley DR, Skolnick P, editors. Current Protocols in Neuroscience. John Wiley and Sons; 2000b. pp. 4.23.1–4.23.40.pp. 4.23.21–24.23.40. [Google Scholar]
- Sterio DC. The unbiased estimation of number and sizes of arbitrary particles using the disector. Journal of Microscopy. 1984;134:127–136. doi: 10.1111/j.1365-2818.1984.tb02501.x. [DOI] [PubMed] [Google Scholar]
- Suwelack D, Hurtado-Lorenzo A, Millan E, Gonzalez-Nicolini V, Wawrowsky K, Lowenstein PR, et al. Neuronal expression of the transcription factor Gli1 using the Talpha1 alpha-tubulin promoter is neuroprotective in an experimental model of Parkinson’s disease. Gene Therapy. 2004;11:1742–1752. doi: 10.1038/sj.gt.3302377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T, Nguyen JB, Hitoshi Y, Watson NP, Dunn JF, Ohara S, et al. Diffuse encephaloventriculitis and substantial leukoencephalopathy after intraventricular administration of recombinant adenovirus. Neurological Research. 2005;27:378–386. doi: 10.1179/016164105X22075. [DOI] [PubMed] [Google Scholar]
- Thomas CE, Abordo-Adesida E, Maleniak TC, Stone D, Gerdes G, Lowenstein PR. Gene transfer into rat brain using adenoviral vectors. In: Gerfen CR, McKay R, Rogawski MA, Sibley DR, Skolnick P, editors. Current Protocols in Neuroscience. John Wiley and Sons; 2000a. pp. 4.23.1–4.23.40.pp. 4.23.21–24.23.40. [DOI] [PubMed] [Google Scholar]
- Thomas CE, Birkett D, Anozie I, Castro MG, Lowenstein PR. Acute direct adenoviral vector cytotoxicity and chronic, but not acute, inflammatory responses correlate with decreased vector-mediated transgene expression in the brain. Molecular Therapeutics. 2001a;3:36–46. doi: 10.1006/mthe.2000.0224. [DOI] [PubMed] [Google Scholar]
- Thomas CE, Birkett D, Anozie I, Castro MG, Lowenstein PR. Acute direct adenoviral vector cytotoxicity and chronic, but not acute, inflammatory responses correlate with decreased vector-mediated transgene expression in the brain. Molecular Therapeutics. 2001b;3:36–46. doi: 10.1006/mthe.2000.0224. [DOI] [PubMed] [Google Scholar]
- Thomas CE, Schiedner G, Kochanek S, Castro MG, Lowenstein PR. Peripheral infection with adenovirus causes unexpected long-term brain inflammation in animals injected intracranially with first-generation, but not with high-capacity, adenovirus vectors: toward realistic long-term neurological gene therapy for chronic diseases. Proceedings of the National Academy of Sciences of the U.S.A. 2000b;97:7482–7487. doi: 10.1073/pnas.120474397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CE, Schiedner G, Kochanek S, Castro MG, Lowenstein PR. Preexisting antiadenoviral immunity is not a barrier to efficient and stable transduction of the brain, mediated by novel high-capacity adenovirus vectors. Human Gene Therapy. 2001c;12:839–846. doi: 10.1089/104303401750148829. [DOI] [PubMed] [Google Scholar]
- Trask TW, Trask RP, Aguilar-Cordova E, Shine HD, Wyde PR, Goodman JC, et al. Phase I study of adenoviral delivery of the HSV-tk gene and ganciclovir administration in patients with current malignant brain tumors. Molecular Therapeutics. 2000;1:195–203. doi: 10.1006/mthe.2000.0030. [DOI] [PubMed] [Google Scholar]
- Wilkinson GW, Akrigg A. Constitutive and enhanced expression from the CMV major IE promoter in a defective adenovirus vector. Nucleic Acids Research. 1992;20:2233–2239. doi: 10.1093/nar/20.9.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood MJ, Charlton HM, Wood KJ, Kajiwara K, Byrnes AP. Immune responses to adenovirus vectors in the nervous system. Trends in Neurosciences. 1996;19:497–501. doi: 10.1016/S0166-2236(96)10060-6. [DOI] [PubMed] [Google Scholar]
- Yao MZ, Gu JF, Wang JH, Sun LY, Liu H, Liu XY. Adenovirus-mediated interleukin-2 gene therapy of nociception. Gene Therapy. 2003;10:1392–1399. doi: 10.1038/sj.gt.3301992. [DOI] [PubMed] [Google Scholar]
- Zermansky AJ, Bolognani F, Stone D, Cowsill CM, Morrissey G, Castro MG, et al. Towards global and long-term neurological gene therapy: unexpected transgene dependent, high-level, and widespread distribution of HSV-1 thymidine kinase throughout the CNS. Molecular Therapeutics. 2001;4:490–498. doi: 10.1006/mthe.2001.0479. [DOI] [PubMed] [Google Scholar]
- Zou L, Zhou H, Pastore L, Yang K. Prolonged transgene expression mediated by a helper-dependent adenoviral vector (hdAd) in the central nervous system. Molecular Therapeutics. 2000;2:105–113. doi: 10.1006/mthe.2000.0104. [DOI] [PubMed] [Google Scholar]


