Abstract
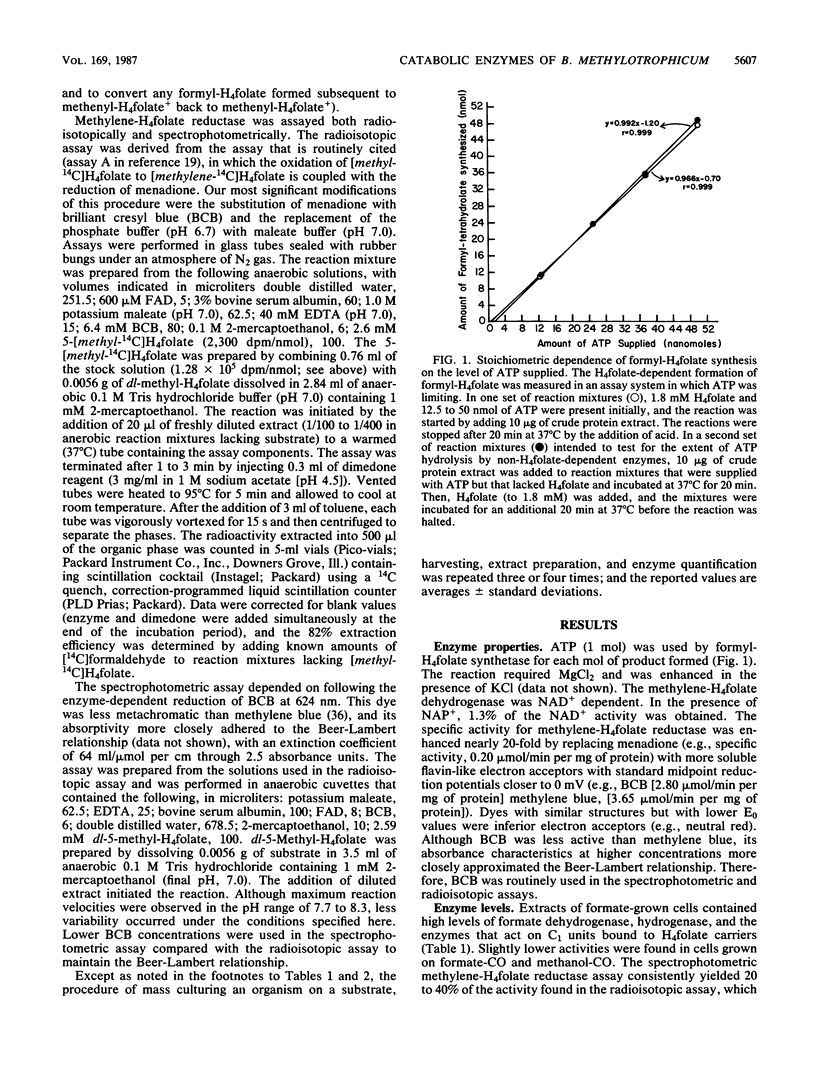
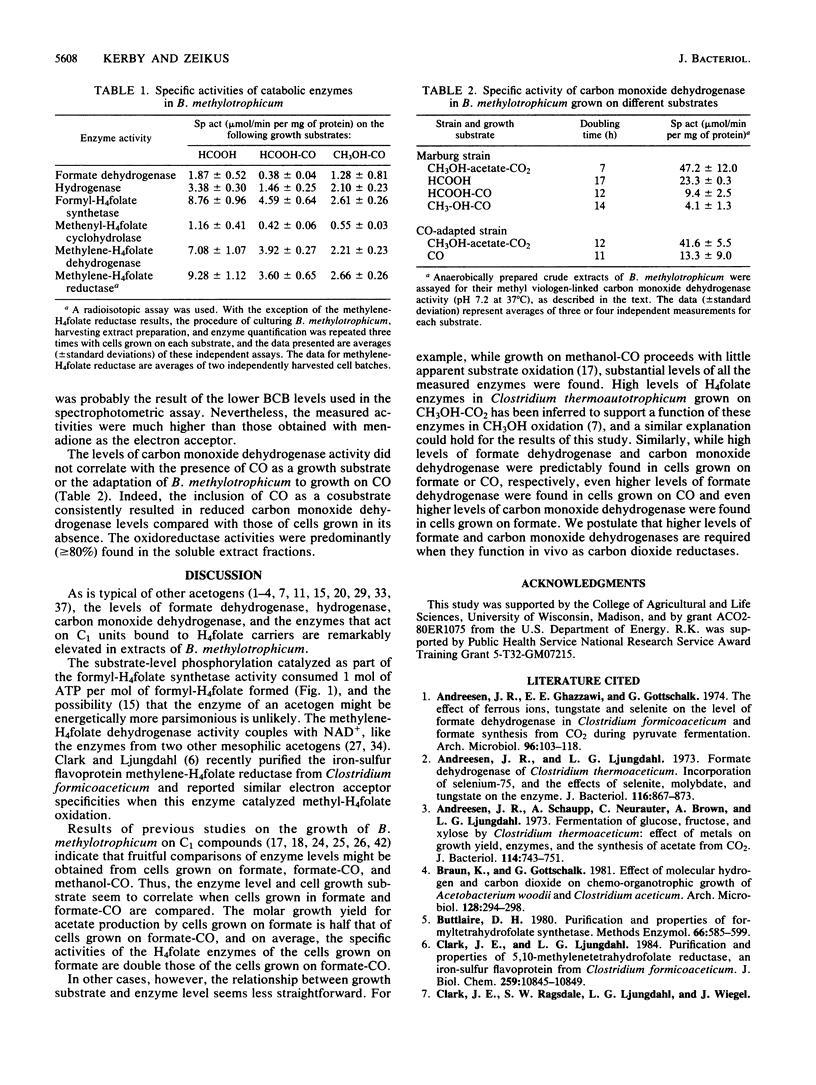
When grown on formate, formate-CO, and methanol-CO, Butyribacterium methylotrophicum contained high levels of tetrahydrofolate (H4folate) and required enzymes, carbon monoxide dehydrogenase, formate dehydrogenase, and hydrogenase. The activities of methylene-H4folate reductase were comparable to other H4 folate activities (which ranged from 0.55 to 9.28 mumol/min per mg of protein) when measured by an improved procedure. The H4folate activities in formate-grown cells were twice those found in formate-CO-grown cells. This result correlated with a growth yield on formate that was one-half that on formate-CO. The stoichiometry of the formyl-H4folate synthetase reaction was 1 mol of ATP per 1 mol of formate. The methylene-H4folate dehydrogenase was NAD+ dependent. We conclude that B. methylotrophicum utilizes these enzymes in homoacetogenic catabolism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreesen J. R., El Ghazzawi E., Gottschalk G. The effect of ferrous ions, tungstate and selenite on the level of formate dehydrogenase in Clostridium formicoaceticum and formate synthesis from CO2 during pyruvate fermentation. Arch Mikrobiol. 1974 Mar 4;96(2):103–118. doi: 10.1007/BF00590167. [DOI] [PubMed] [Google Scholar]
- Andreesen J. R., Ljungdahl L. G. Formate dehydrogenase of Clostridium thermoaceticum: incorporation of selenium-75, and the effects of selenite, molybdate, and tungstate on the enzyme. J Bacteriol. 1973 Nov;116(2):867–873. doi: 10.1128/jb.116.2.867-873.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreesen J. R., Schaupp A., Neurauter C., Brown A., Ljungdahl L. G. Fermentation of glucose, fructose, and xylose by Clostridium thermoaceticum: effect of metals on growth yield, enzymes, and the synthesis of acetate from CO 2 . J Bacteriol. 1973 May;114(2):743–751. doi: 10.1128/jb.114.2.743-751.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun K., Gottschalk G. Effect of molecular hydrogen and carbon dioxide on chemo-organotrophic growth of Acetobacterium woodii and Clostridium aceticum. Arch Microbiol. 1981 Jan;128(3):294–298. doi: 10.1007/BF00422533. [DOI] [PubMed] [Google Scholar]
- Buttlaire D. H. Purification and properties of formyltetrahydrofolate synthetase. Methods Enzymol. 1980;66:585–599. doi: 10.1016/0076-6879(80)66512-4. [DOI] [PubMed] [Google Scholar]
- Clark J. E., Ljungdahl L. G. Purification and properties of 5,10-methylenetetrahydrofolate reductase, an iron-sulfur flavoprotein from Clostridium formicoaceticum. J Biol Chem. 1984 Sep 10;259(17):10845–10849. [PubMed] [Google Scholar]
- Drake H. L. Demonstration of hydrogenase in extracts of the homoacetate-fermenting bacterium Clostridium thermoaceticum. J Bacteriol. 1982 May;150(2):702–709. doi: 10.1128/jb.150.2.702-709.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S. I., Drake H. L., Wood H. G. Synthesis of acetyl coenzyme A from carbon monoxide, methyltetrahydrofolate, and coenzyme A by enzymes from Clostridium thermoaceticum. J Bacteriol. 1982 Feb;149(2):440–448. doi: 10.1128/jb.149.2.440-448.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S. I., Pezacka E., Wood H. G. Acetate synthesis from carbon monoxide by Clostridium thermoaceticum. Purification of the corrinoid protein. J Biol Chem. 1984 Jul 25;259(14):8892–8897. [PubMed] [Google Scholar]
- Kellum R., Drake H. L. Effects of cultivation gas phase on hydrogenase of the acetogen Clostridium thermoaceticum. J Bacteriol. 1984 Oct;160(1):466–469. doi: 10.1128/jb.160.1.466-469.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenealy W., Zeikus J. G. Influence of corrinoid antagonists on methanogen metabolism. J Bacteriol. 1981 Apr;146(1):133–140. doi: 10.1128/jb.146.1.133-140.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerby R., Niemczura W., Zeikus J. G. Single-carbon catabolism in acetogens: analysis of carbon flow in Acetobacterium woodii and Butyribacterium methylotrophicum by fermentation and 13C nuclear magnetic resonance measurement. J Bacteriol. 1983 Sep;155(3):1208–1218. doi: 10.1128/jb.155.3.1208-1218.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerby R., Zeikus J. G. Anaerobic catabolism of formate to acetate and CO2 by Butyribacterium methylotrophicum. J Bacteriol. 1987 May;169(5):2063–2068. doi: 10.1128/jb.169.5.2063-2068.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutzbach C., Stokstad E. L. Mammalian methylenetetrahydrofolate reductase. Partial purification, properties, and inhibition by S-adenosylmethionine. Biochim Biophys Acta. 1971 Dec 15;250(3):459–477. doi: 10.1016/0005-2744(71)90247-6. [DOI] [PubMed] [Google Scholar]
- Leonhardt U., Andreesen J. R. Some properties of formate dehydrogenase, accumulation and incorporation of 185W-tungsten into proteins of Clostridium formicoaceticum. Arch Microbiol. 1977 Dec 15;115(3):277–284. doi: 10.1007/BF00446453. [DOI] [PubMed] [Google Scholar]
- Ljungdahl L. G., O'Brien W. E., Moore M. R., Liu M. T. Methylenetetrahydrofolate dehydrogenase from Clostridium formicoaceticum and methylenetetrahydrofolate dehydrogenase, methenyltetrahydrofolate cyclohydrolase (combined) from Clostridium thermoaceticum. Methods Enzymol. 1980;66:599–609. doi: 10.1016/0076-6879(80)66513-6. [DOI] [PubMed] [Google Scholar]
- Lynd L. H., Zeikus J. G. Metabolism of H2-CO2, methanol, and glucose by Butyribacterium methylotrophicum. J Bacteriol. 1983 Mar;153(3):1415–1423. doi: 10.1128/jb.153.3.1415-1423.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynd L., Kerby R., Zeikus J. G. Carbon monoxide metabolism of the methylotrophic acidogen Butyribacterium methylotrophicum. J Bacteriol. 1982 Jan;149(1):255–263. doi: 10.1128/jb.149.1.255-263.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M. R., O'Brien W. E., Ljungdahl L. G. Purification and characterization of nicotinamide adenine dinucleotide-dependent methylenetetrahydrofolate dehydrogenase from Clostridium formicoaceticum. J Biol Chem. 1974 Aug 25;249(16):5250–5253. [PubMed] [Google Scholar]
- Nixon P. F., Bertino J. R. Enzymic preparations of radiolabeled +-L-5-methyltetrahydrofolate and +-L-formyltetrahydrofolate. Methods Enzymol. 1980;66:547–553. doi: 10.1016/0076-6879(80)66504-5. [DOI] [PubMed] [Google Scholar]
- O'Brien W. E., Ljungdahl L. G. Fermentation of fructose and synthesis of acetate from carbon dioxide by Clostridium formicoaceticum. J Bacteriol. 1972 Feb;109(2):626–632. doi: 10.1128/jb.109.2.626-632.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezacka E., Wood H. G. Role of carbon monoxide dehydrogenase in the autotrophic pathway used by acetogenic bacteria. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6261–6265. doi: 10.1073/pnas.81.20.6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezacka E., Wood H. G. The synthesis of acetyl-CoA by Clostridium thermoaceticum from carbon dioxide, hydrogen, coenzyme A and methyltetrahydrofolate. Arch Microbiol. 1984 Jan;137(1):63–69. doi: 10.1007/BF00425809. [DOI] [PubMed] [Google Scholar]
- Ragsdale S. W., Ljungdahl L. G. Hydrogenase from Acetobacterium woodii. Arch Microbiol. 1984 Nov;139(4):361–365. doi: 10.1007/BF00408380. [DOI] [PubMed] [Google Scholar]
- Ragsdale S. W., Ljungdahl L. G. Purification and properties of NAD-dependent 5,10-methylenetetrahydrofolate dehydrogenase from Acetobacterium woodii. J Biol Chem. 1984 Mar 25;259(6):3499–3503. [PubMed] [Google Scholar]
- Ragsdale S. W., Wood H. G. Acetate biosynthesis by acetogenic bacteria. Evidence that carbon monoxide dehydrogenase is the condensing enzyme that catalyzes the final steps of the synthesis. J Biol Chem. 1985 Apr 10;260(7):3970–3977. [PubMed] [Google Scholar]
- Tanner R. S., Wolfe R. S., Ljungdahl L. G. Tetrahydrofolate enzyme levels in Acetobacterium woodii and their implication in the synthesis of acetate from CO2. J Bacteriol. 1978 May;134(2):668–670. doi: 10.1128/jb.134.2.668-670.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanoni M. A., Ballou D. P., Matthews R. G. Methylenetetrahydrofolate reductase. Steady state and rapid reaction studies on the NADPH-methylenetetrahydrofolate, NADPH-menadione, and methyltetrahydrofolate-menadione oxidoreductase activities of the enzyme. J Biol Chem. 1983 Oct 10;258(19):11510–11514. [PubMed] [Google Scholar]
- Zeikus J. G., Kerby R., Krzycki J. A. Single-carbon chemistry of acetogenic and methanogenic bacteria. Science. 1985 Mar 8;227(4691):1167–1173. doi: 10.1126/science.3919443. [DOI] [PubMed] [Google Scholar]


