Abstract
The conjunctive presence of mechanical stress and active transforming growth factor β1 (TGF-β1) is essential to convert fibroblasts into contractile myofibroblasts, which cause tissue contractures in fibrotic diseases. Using cultured myofibroblasts and conditions that permit tension modulation on the extracellular matrix (ECM), we establish that myofibroblast contraction functions as a mechanism to directly activate TGF-β1 from self-generated stores in the ECM. Contraction of myofibroblasts and myofibroblast cytoskeletons prepared with Triton X-100 releases active TGF-β1 from the ECM. This process is inhibited either by antagonizing integrins or reducing ECM compliance and is independent from protease activity. Stretching myofibroblast-derived ECM in the presence of mechanically apposing stress fibers immediately activates latent TGF-β1. In myofibroblast-populated wounds, activation of the downstream targets of TGF-β1 signaling Smad2/3 is higher in stressed compared to relaxed tissues despite similar levels of total TGF-β1 and its receptor. We propose activation of TGF-β1 via integrin-mediated myofibroblast contraction as a potential checkpoint in the progression of fibrosis, restricting autocrine generation of myofibroblasts to a stiffened ECM.
Introduction
The development of tension by myofibroblasts (Mfs) promotes physiological tissue repair for which the contracting granulation tissue of healing wounds is a paradigm (Hinz, 2007). However, the excessive ECM-secreting and contractile activities of Mfs contribute to progressive fibrosis in many organs, such as the heart, lung, liver, kidney, and skin (Gabbiani, 2003; Hinz et al., 2007). The high contractile activity of Mfs is generated by α smooth muscle actin (α-SMA) in stress fibers, which are hallmarks of a differentiated Mf (Tomasek et al., 2002). Interfering with α-SMA action by adding a membrane-penetrating fusion protein that contains the α-SMA–specific N-terminal sequence AcEEED (SMA-FP) significantly reduces tension generation by Mfs (Hinz et al., 2002).
Two factors, TGF-β1 and mechanical tension, are pivotal in promoting Mf differentiation from a variety of progenitors (Hinz et al., 2007). TGF-β1 induces Mf differentiation on two-dimensional culture substrates with a stiffness that corresponds to that of contracting fibrotic and granulation tissue but not on substrates exhibiting the compliance of normal connective tissue such as dermis (Goffin et al., 2006). TGF-β1 also induces Mf differentiation in three-dimensional collagen when gels are mechanically restrained (Arora et al., 1999) but not when the gels are free-floating and relaxed (Tomasek et al., 2002). Conversely, mechanical stress alone fails to induce Mf differentiation in the absence of active TGF-β1, as demonstrated when TGF-β1 antagonists are either applied to cells cultured on rigid substrates (Arora et al., 1999; Hinz et al., 2001a) or injected into stressed granulation tissues (Hinz et al., 2001b). Although it is unclear whether and how mechanical stress and TGF-β1 signaling converge to promote increased α-SMA expression and Mf differentiation, it is possible that intracellular and extracellular tension directly regulate TGF-β1 activation. Thus, the release of TGF-β1 from its latent complex by tension would produce a signaling molecule that induces Mf differentiation and α-SMA expression in a feed-forward manner.
In fibroblasts and Mfs, TGF-β1 is secreted as part of the large latent complex (LLC), which, in addition to TGF-β1, consists of latency associated protein (LAP) and latent TGF-β binding protein 1 (LTBP-1). LAP and TGF-β1 form the small latent complex (SLC; Miyazono et al., 1991; Annes et al., 2003). The LLC provides a reservoir of latent TGF-β1 in the ECM by binding to other ECM components like fibrillin-1 and fibronectin (FN; Unsold et al., 2001; Annes et al., 2003; Hyytiainen et al., 2004; Koli et al., 2005), of which ED-A FN is the major splice variant expressed by Mfs (Serini et al., 1998). Several cellular mechanisms have been described that activate latent TGF-β1 by promoting its dissociation from LAP. These activation processes include cleavage of LLC by proteases (Mu et al., 2002; Ge and Greenspan, 2006) such as plasmin as well as interaction of LAP with thrombospondin (for review see Annes et al., 2003). Binding of active TGF-β1 to TGF-β receptor type II (TGF-β RII) leads to the phosphorylation and recruitment of TGF-β RI. This heteromeric receptor complex phosphorylates Smad2 and 3, which bind to Smad4 and translocate into the nucleus to enhance transcription of Mf-specific genes such as α-SMA by cooperating with DNA transcription factors (for review see Hinz, 2007).
Recently, the epithelial integrin αvβ6 was demonstrated to activate latent TGF-β1 in vivo during development of lung fibrosis (Munger et al., 1998; Jenkins et al., 2006) and in vitro (Annes et al., 2004). Because activation by αvβ6 depends on incorporation of the TGF-β1 LLC into the ECM via binding of the LTBP-1 hinge domain, cell traction mediated by αvβ6 integrin has been proposed as part of the mechanism of latent TGF-β1 activation (Annes et al., 2004; Keski-Oja et al., 2004). However, no direct evidence has been provided that mechanical stress liberates TGF-β1 from the ECM-bound LLC. In addition, although latent TGF-β1 activation by αvβ6 integrin–mediated traction can be of physiological significance during initiation of lung and kidney fibrosis where epithelium is prominent (Jenkins et al., 2006; Kim et al., 2006), this is unlikely to occur during progressive fibrosis of organs with less abundant epithelium. In such conditions, αvβ6 integrin–negative Mfs are the major producers and consumers of TGF-β1.
We show here that both external stretching of Mf cultures and increasing Mf intracellular tension directly activate latent TGF-β1 from the ECM. This process requires α-SMA–positive stress fibers and integrin binding to LAP in the LLC. Latent TGF-β1 stress activation is limited to culture substrates with stiffness similar to that of fibroblast-populated early wound granulation tissue but does not occur on more compliant substrates. We propose that ECM stiffness modulated as a function of cell remodeling activity controls the level of TGF-β1 release by contraction and thus restricts autocrine maintenance of the Mf phenotype to the appropriate mechanical microenvironment.
Results
Mfs activate latent TGF-β1 from self-generated stores in the ECM
Autocrine TGF-β1 formation has been suggested as the stimulus for constitutive α-SMA expression in cultured rat lung Mfs (Hinz et al., 2001a). To determine whether a portion of this TGF-β1 is stored in the ECM as the LLC, we first assessed ECM incorporation of the latent TGF-β1 scaffolding protein LTBP-1. Increasing levels of LTBP-1 were incorporated in the ECM of lung Mfs over a 7 d period as visualized by immunofluorescence (Fig. 1, A–D, green) and confirmed by Western blotting of plasmin-digested lung Mf ECM (Fig. 1 E). Proteolytic processing was required to liberate LTBP-1 presumably because of covalent binding of LTBP-1 to other ECM proteins (Nunes et al., 1997). In Mf cultures, LTBP-1 colocalized with the Mf-characteristic FN splice variant ED-A FN (Fig. 1, A–D, red), that was secreted ∼1 d earlier as appreciated from the red to yellow/green color shift in confocal immunofluorescence over culture time (Fig. 1, A–D) and from Western blots (Fig. 1 E).
Figure 1.
Mfs activate latent TGF-β1 from self-generated ECM depots. (A–D and G) Lung Mfs were stained for LTBP-1 (green), ED-A FN (red), α-SMA (blue), and nuclei (insets) after a 2- (A), 3- (B), or 7-d (C) culture, and after extracting all cellular components with DOC at day 7 (D). (E) Expression of LTBP-1, ED-A FN, and α-SMA was further evaluated together with TGF-β RII and LAP by Western blotting; vimentin served as loading control. Whole culture extracts were compared with samples taken after both TX-100 (TX; preserves the cytoskeleton and ECM) and DOC (preserves ECM) extraction. LTBP-1 was blotted in nonreducing conditions after digesting DOC-insoluble ECM overnight with plasmin. (F) Lung Mf (2–7 d) and subcutaneous fibroblasts (7 d) were examined for total TGF-β1. Total TGF-β1 was measured by incubating TMLC with supernatants from 80°C heat-activated trypsinized cells (cellular TGF-β1), as well as from heat-activated, DOC-insoluble ECM (ECM-associated total TGF-β1). Function-blocking TGF-β1 antibody was added to test TGF-β isoform specificity (dashed lines). TGF-β1 concentration was calculated from a standard. (G) Cultures of subcutaneous fibroblasts were stained after 7 d as in A–D. (H, left) Total culture extracts were produced from lung Mfs, lung fibroblasts (lung F), subcutaneous fibroblasts (sub F), and subcutaneous Mfs (sub Mf) and blotted as in E. Gray lines indicate that intervening lanes have been spliced out. (H, right) The DOC-insoluble fraction of 7-d Mf was used as latent TGF-β1–containing ECM for co-culture of fibroblasts or Mfs with TMLC. TGF-β1 activated by cells during 24 h was measured as luminescence compared with 24-h control cultures on plastic culture dishes. Error bars represent the SD of the mean. Bars: (A–D and G) 20 μm; (insets) 200 μm.
To assess whether the detected LTBP-1 is associated with latent TGF-β, we quantified total TGF-β production after activating all latent TGF-β by heating 2–7-d-old Mf cultures to 80°C. The heat-released TGF-β was then measured using transformed mink lung reporter cells (TMLC), which produce luciferase as a function of active TGF-β (Abe et al., 1994). To distinguish between cellular and ECM-derived TGF-β, i.e., LLC, we first measured TGF-β in heated trypsinized cells, which represent the nonsecreted pool of TGF-β. This fraction of total TGF-β was increased as early as 3 d of culture (Fig. 1 F). Next, to evaluate only the amount of total TGF-β1 stored in the ECM, we removed the cells from cultures with deoxycholate (DOC); the effectiveness of extraction was confirmed by the loss of α-SMA and nuclei in immunofluorescence (Fig. 1 D) and the loss of vimentin in Western blots (Fig. 1 E). DOC treatment preserved the ECM proteins LTBP-1 and ED-A FN as well as LAP (Fig.1 E, DOC). When plasmin-digested ECM extracts were blotted under nonreducing conditions, the anti–LTBP-1 antibody stained two bands (Fig. 1 E). The ∼240-kD band corresponds to LTBP-1 bound to LAP–TGF-β1 and the ∼140-kD band represents unbound LTBP-1 (Koli et al., 2005). Consistently, the anti-LAP antibody under nonreducing conditions revealed one band at ∼240 kD (unpublished data), confirming the presence of the LLC in the ECM. Significant levels of TGF-β were liberated by heating Mf-derived ECM to 80°C (Fig. 1 F). The response of TMLC to this ECM-derived TGF-β was reduced by ∼90% using function-blocking antibodies against TGF-β1, which demonstrates that TGF-β1 is the predominant isoform stored in the ECM of Mfs as part of the LLC (Fig. 1 F). We will hereafter refer to DOC-extracted, 7-d-old ECM as Mf-derived ECM, which will be used as a latent TGF-β1–conditioned culture substrate.
We next wanted to compare latent TGF-β1 production and ECM storage between lung Mfs and rat subcutaneous fibroblasts, which under identical culture conditions express low levels of α-SMA (Fig. 1, G and H, left). Fibroblasts exhibited significantly lower amounts of total TGF-β1 in the cytosol (Fig. 1 F, trypsinized cells) and cell-derived ECM (Fig. 1 F) compared with Mfs. The ECM from these fibroblasts also contained lower amounts of LTBP-1 and ED-A FN compared with Mfs (Fig. 1, G and H, left). Because fibroblasts express considerable levels of TGF-β RII (Fig. 1 H, left), it appears that the low autocrine production and possibly lower activation of latent TGF-β1 account for low α-SMA expression in these cells. Indeed, fibroblasts expressed α-SMA when treated for 5 d with exogenous active TGF-β1 (Fig. 1 H, left). Inversely, lung Mfs dedifferentiated into fibroblasts within 5 d by blocking TGF-β1 with soluble TGF-β1 RII (Fig. 1 H, left). By these means, we produced fibroblasts and/or Mfs from different precursors.
We next evaluated the capacity of contractile Mfs to activate latent TGF-β1 from the same Mf-derived ECM compared with low contractile fibroblasts. Active TGF-β1 produced by the cells was quantified by directly co-culturing TMLC for 1 d on Mf-derived ECM. Interestingly, Mfs activated more latent TGF-β1 than did fibroblasts (1.5–2-fold) regardless of their origin (Fig. 1 H, right). TMLC alone did not activate TGF-β1 from Mf-derived ECM (unpublished data). The direct contact between the experimental and reporter cells was mandatory to detect cell-activated TGF-β1, confirming previous studies demonstrating that nonproteolytically activated TGF-β1 was poorly soluble (Munger et al., 1999). To elucidate how much of the active TGF-β1 had been liberated from the ECM as opposed to secreted, we compared latent TGF-β1 activation from Mf-derived ECM to that from cells cultured for the same time on tissue culture dishes. On culture dishes, Mfs produced approximately two- to threefold lower amounts of active TGF-β1 (Fig. 1 H, right). Collectively, these results demonstrate that Mfs not only deposit more latent TGF-β1 in the ECM than do fibroblasts but that Mfs are also more efficient in activating these stores.
Latent TGF-β1 activation from the ECM is promoted by Mf contraction
The capacity of fibroblastic cells to activate latent TGF-β1 from the ECM (Fig. 1 H, right) correlated with their contractile activity (Hinz et al., 2001a). To study further whether contraction can promote release of TGF-β1 from the ECM, we assessed latent TGF-β1 activation after treating Mfs with drugs that modulate cell tension development (Fig. S1 and Video 1, available at http://www.jcb.org/cgi/content/full/jcb.200704042/DC1). First, however, we monitored whether these drugs were compatible with the TMLC reporter system. When added to TMLC alone, Mf contraction agonists thrombin, angiotensin-II (AT-II), and endothelin-1 (ET-1), as well as contraction inhibitors cytochalasin D, ML-7, blebbistatin, and SMA-FP did not alter TMLC reporter activity in the presence or absence of TGF-β1 (Fig. 2 A). In contrast, nocodazole and staurosporin significantly interfered with TMLC luciferase production and were not further considered (Fig. 2 A). We excluded the possibility that the basal luminescence signal of TMLC was caused by TGF-β1 production by the reporter cells themselves because addition of a TGF-β1 function-blocking antibody did not alter basal TMLC reporter activity (Fig. 2 A, anti–TGF-β1).
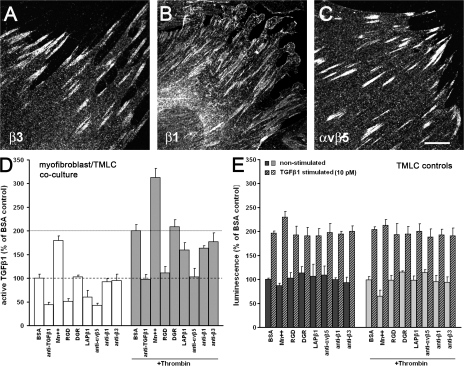
Figure 2.
Mf contraction activates latent TGF-β1. (A) In control experiments, TMLC were incubated with various drugs for 1 h in the presence (dashed bars) or absence (solid bars) of TGF-β1. The TGF-β1 function-blocking antibody was used to assess the contribution of this isoform to the baseline luciferase level in TMLC cultures. (B) The role of Mf contraction in activating latent TGF-β1 was tested by seeding TMLC directly on 7-d-old Mf cultures 4 h before the addition of contraction-promoting drugs. An anti–TGF-β1 antibody was used to test TGF-β1 contribution in the luciferase response in Mf/TMLC co-cultures. Cell contraction inhibitors were added to contraction-stimulated and control cultures. The dotted line indicates the level of latent TGF-β1 activation by the most potent contraction agonist thrombin; the broken line shows baseline latent TGF-β activation by Mfs. Results are expressed as the percentage of nonstimulated control (BSA) corrected for TMLC baseline reporter activity. (C) To control whether contraction agonists alone activate latent TGF-β1 from the ECM, lung Mf-derived ECM was treated for 1 h with thrombin and active TGF-β1 release into the supernatant was assessed with TMLC; ECM digested with plasmin for 1 h was used as a positive control. (D) TMLC were incubated with protease inhibitors together with contractile drugs for 1 h in the presence or absence of TGF-β1 to control interference with reporter activity. (E) Protease inhibitor cocktail was added simultaneously with contraction-promoting drugs to test whether proteases are involved in latent TGF-β1 activation by Mf contraction. Error bars represent the SD of the mean.
We next tested the effect of these drugs on Mfs. Lung Mfs in the presence of thrombin, AT-II, and ET-1 generated 1.6–2-fold higher levels of active TGF-β1 (Fig. 2 B, dotted line) when compared with the BSA control (broken line). Addition of a TGF-β1 function-blocking antibody to nonstimulated Mf/TMLC co-cultures reduced luciferase production to ∼40% of control (Fig. 2 B, BSA + anti–TGF-β1). When added to Mf in the presence of contraction agonist thrombin, AT-II, and ET-1, the TGF-β1 function-blocking antibody largely reduced TMLC reporter activity (Fig. 2 B), which confirms that TGF-β1 is the major isoform released upon Mf contraction. It is possible that residual luciferase production of TMLC/Mf co-cultured in the presence of TGF-β1 antibody is either caused by incomplete blocking by the antibody or other cytokines that activate the PAI-1 reporter in the TMLC. Consistent with the concept of stress-mediated latent TGF-β1 activation, the contraction inhibitors cytochalasin D, ML-7, blebbistatin, and SMA-FP decreased both thrombin-induced latent TGF-β1 activation (Fig. 2 B, + thrombin) and less strongly basal levels of TGF-β1 activation (Fig. 2 B). Collectively, these results show that the contractile activity of Mfs correlates with their capacity to activate latent TGF-β1.
Thrombin, the most potent latent TGF-β1–activating agonist in our experiments is also known to liberate active TGF-β1 from the LLC by proteolytic cleavage at 10-fold higher concentrations than used here (0.5 U/ml; Taipale et al., 1992). At 0.5 U/ml, thrombin did not release active TGF-β1 from cell-free, Mf-derived ECM (Fig. 2 C), excluding the contribution of proteolysis in contraction-mediated TGF-β1 activation. It was only at 10–25-fold higher concentrations (5–25 U/ml) that thrombin enzymatically activated latent TGF-β1; plasmin served as a positive control (Fig. 2 C; Taipale et al., 1992). To further eliminate the role of proteolysis in our system, we stimulated Mf contraction in the presence of a protease inhibitor cocktail. Stimulating Mf contraction in the presence of protease inhibitors did not interfere with TMLC reporter activity (Fig. 2 D). As expected, protease inhibition abolished thrombin-induced latent TGF-β1 activation by Mfs (Fig. 2 E) because thrombin induces Mf contraction through proteolytic cleavage of the protease-activated receptor 1 (Bogatkevich et al., 2003). In contrast, when added to Mf/TMLC co-cultures, protease-independent contraction of Mfs by ET-1 and AT-II elevated the amounts of active TGF- β1 by 1.8–2.5-fold compared with the BSA control in the presence of protease inhibitors (Fig. 2 E, dotted line). These results demonstrate that Mf contraction directly activates latent TGF-β1 in a protease-independent mechanism.
Contraction activation of latent TGF-β1 from the ECM is integrin mediated
The fibroblast integrins that bind to the LLC and are potentially involved in protease-independent latent TGF-β1 activation are αvβ3, α8β1, and αvβ5 (Levine et al., 2000; Lu et al., 2002; Mu et al., 2002; Ludbrook et al., 2003; Asano et al., 2005; Sheppard, 2005; Araya et al., 2006). As visualized by immunofluorescence, cultured lung Mfs do express β3 (Fig. 3 A) and β1 (Fig. 3 B) integrin subunits and αvβ5 integrin (Fig. 3 C). To investigate whether activation of latent TGF-β1 by Mf contraction was mediated by integrin binding to the LLC, we added integrin effectors to nonstimulated and thrombin-contraction–stimulated Mf/TMLC co-cultures (Fig. 3 D). Basal (broken line) and contraction-induced activation of latent TGF-β1 (dotted line) were strongly increased by the integrin activator Mn2+ (Fig. 3 D). In contrast, general integrin blocking with RGD peptides and specific inhibition of integrin binding to LAP in the LLC with a competitive LAP–TGF-β1 (LAPβ1) decapeptide inhibited basal and thrombin-induced activation of latent TGF-β1 (Fig. 3 D). Comparable results were obtained with exogenously added soluble SCL that competes with LTBP-bound SLC (unpublished data). Soluble SLC, however, severely increases the reporter activity of TMLC alone and was excluded here. At the concentration used, the RGD-containing peptides did not alter cell spreading or stress fiber formation (Fig. S2, available at http://www.jcb.org/cgi/content/full/jcb.200704042/DC1). Control DGR peptides had no effect (Fig. 3 E).
Figure 3.
Activation of latent TGF-β1 by Mf contraction is integrin mediated. Cultured rat lung Mfs express β3 (A), β1 (B), and αvβ5 integrin (C), as shown by confocal micrographs produced from immunostaining. (D) TMLC were seeded directly on 7-d-old Mf cultures 2 h before adding modulators of integrin binding for an additional 2 h; this was followed by another 1-h incubation in the absence or presence of 0.5 U/ml thrombin. Integrin binding was stimulated with Mn2+ and inhibited using RGD peptides, LAP–TGF-β1 (LAPβ1) decapeptide, and integrin function-blocking antibodies; scrambled DGR peptide was used as control. The broken line shows basal latent TGF-β1 activation by Mfs; the dotted line demonstrates active TGF-β1 levels after inducing contraction with thrombin only. Results are expressed as a percentage of nonstimulated control (BSA) corrected for TMLC baseline reporter activity. (E) Controls were performed to test the effect of integrin modulators on TMLC reporter activity with and without stimulation by TGF-β1 and 0.5 U/ml thrombin. Error bars represent the SD of the mean. Bar, 10 μm.
To identify the integrins involved in latent TGF-β1 activation by Mf contraction, we applied specific function-blocking antibodies. Addition of anti-αvβ5 strongly reduced both basal and contraction-induced latent TGF-β1 activation, whereas anti–β1 and –β3 integrin function-blocking antibodies only mildly inhibited latent TGF-β1 activation in both conditions (Fig. 3 D). All effectors were also tested on TLMC reporter activity in the absence of Mfs (Fig. 3 E). Together, these results demonstrate that integrin binding to LAP is required to activate latent TGF-β1 from the LLC and αvβ5 integrin is predominantly involved in latent TGF-β1 activation by Mf contraction.
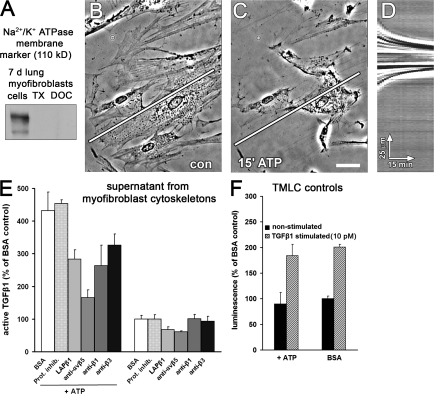
Stress activation of latent TGF-β1 requires the Mf cytoskeleton
Protease inhibition reduced activation of latent TGF-β1 in Mf cultures by ∼40%, which suggests that proteolysis is part of basal latent TGF-β1 activation (Fig. 2 G). To exclude a role for proteases in integrin-mediated latent TGF-β1 activation by Mf contraction, we extracted 7-d cultures of Mfs with Triton X-100 (TX-100). This treatment preserved the ECM, the α-SMA and vimentin cytoskeleton (Fig. 1 E), and focal adhesion integrity (Hinz et al., 2003). Cytosol and membranes were completely removed as shown by the loss of the membrane marker Na+/K+-ATPase (Fig. 4 A). Addition of ATP to such TX-100 cytoskeletons induced dramatic contraction of stress fibers (Fig. 4, B and C; and Video 2, available at http://www.jcb.org/cgi/content/full/jcb.200704042/DC1) clearly visualized by kymographic analysis over a line positioned along the extracted cell's axis and the substrate (Fig. 4 D). ATP-induced contraction increased active TGF-β1 by 4.5-fold compared with nonstimulated cytoskeletons in the presence and absence of protease inhibitors (Fig. 4 E). Latent TGF-β1 activation by ATP-induced contraction was reduced to approximately threefold upon incubation with either the LAP–TGF-β1 decapeptide or the anti-β1 or -β3 integrin antibodies and reduced to ∼1.5-fold upon incubation with the anti-αvβ5 integrin antibody; these inhibitors had little effect on basal TGF-β1 activity in the absence of ATP (Fig. 4 E). ATP did not activate latent TGF-β1 from Mf-derived ECM (unpublished data) and did not interfere with TMLC reporter activity (Fig. 4 F). Together, these data show that Mf contraction, mediated by β1, β3, and most strongly by αvβ5 integrin binding to LAP, directly activates latent TGF-β1 from the LLC by a protease-independent mechanism.
Figure 4.
Integrin-transmitted contraction of TX-100 cytoskeletons activates latent TGF-β1. Cytoskeletons from 7-d-old Mf cultures were produced by cell extraction with TX-100. (A) Extraction efficiency was monitored by Western blotting for the plasma membrane–associated Na+/K+-ATPase. Stress fibers remaining after TX-100 extraction (B) were contracted by adding ATP for 30 min (C). Kymograph lines, 150 μm. Bar, 25 μm. (D) Isolated stress fiber contraction was visualized by a kymograph produced along the overlaid white lines in A and B. (E) TX-100 cytoskeletons were incubated for 2 h with protease inhibitors and integrin antagonists and subsequently contracted with ATP for 1 h; supernatants were analyzed for their content in active TGF-β1. Results are expressed as the percentage of nonstimulated control (BSA) corrected for TMLC baseline reporter activity. (F) To control for the influence of ATP on TMLC reporter activity, TMLC were incubated with ATP for 1 h in the presence (dashed bars) or absence (solid bars) of TGF-β1. Error bars represent the SD of the mean.
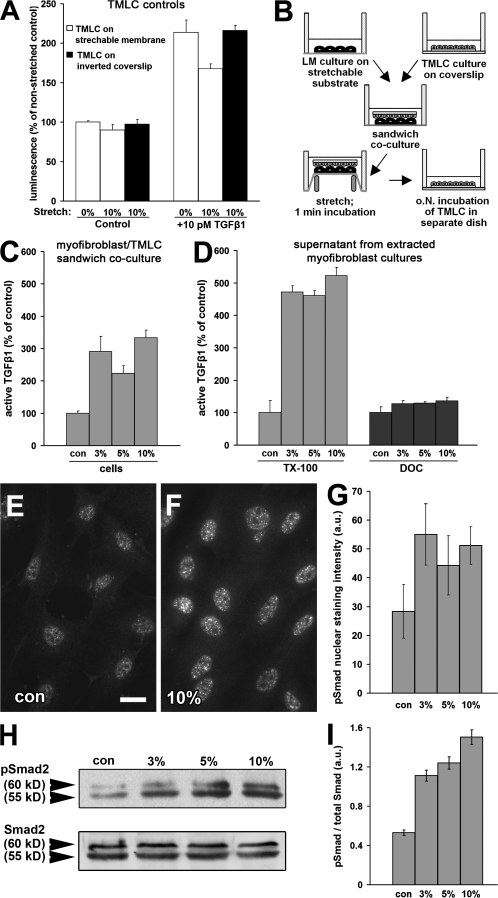
To directly test activation of latent TGF-β1 by mechanical forces, we subjected Mf cultures on stretchable silicone membranes to unique stretches of up to 10%. We first controlled whether stretching interfered with TMLC reporter activity (Fig. 5 A). When TMLC were grown directly on the silicone membrane, 10% stretching did not affect baseline reporter activity but attenuated the response to TGF-β1 (Fig. 5 A). To accomplish the necessary close contact with lung Mfs and simultaneously protect the TMLC from stretching, we developed the “TMLC sandwich” assay (Fig. 5 B). In this assay, TMLC grown on glass coverslips were placed upside-down on top of Mfs during stretching and removed shortly after (1 min) for separate analysis of luciferase production (Fig. 5 B). Control experiments without Mfs demonstrated no interference of the TMLC response to TGF-β1 in this configuration (Fig. 5 A).
Figure 5.
Latent TGF-β1 activation by mechanical stress requires a functional cytoskeleton. (A) To control whether stretching interferes with TGF-β1 reporting activity, TMLC were directly grown on a silicon membrane and stretched by 10% in the presence and absence of 10 pM TGF-β1. Reporter activity was compared with TMLC grown on coverslips to protect from stretching and placed upside-down on the membrane during 10% stretching. (B) This configuration was repeated in the sandwich assay, in which lung Mfs were cultured for 7 d on silicone membranes that were stretched 3–10%. After stretching, TMLC were cultured separately for further analysis. Active TGF-β1 was measured as a function of stretch applied to TMLC/Mf sandwich cultures (C), TX-100 cytoskeletons, and Mf-derived ECM (DOC; D). Active TGF-β1 is expressed as the percentage of nonstretched control (con) corrected for TMLC baseline reporter activity. Phosphorylation of Smad2 (pSmad2) was assessed by immunofluorescence detecting pSmad2 in nonstretched (E) and 10% stretched (F) conditions and by Western blotting of Mf culture extracts equilibrated for total Smad2 (H). Intensity of pSmad2 labeling in the nuclei (G) and of pSmad2 bands on the Western blot (I) was quantified by densitometric analysis. Error bars represent SD of the mean. Bar, 10 μm.
Stretching Mfs by 3% increased active TGF-β1 by threefold compared with controls (Fig. 5 C). No significant further augmentation of active TGF-β1 levels was observed at higher stretches (Fig. 5 C). Stretch-activated TGF-β1 exerted a stimulatory response on Mf differentiation as demonstrated by enrichment of phosphorylated Smad2 in the nuclei of Mfs within 1 h after stretching (Fig. 5, E–G), which indicates that TGF-β1 binds to its receptor (Heldin et al., 1997). This was confirmed by Western blotting, demonstrating increased Smad2/3 phosphorylation at unchanged levels of total Smad2/3 (Fig. 5, H and I).
To investigate whether the Mf cytoskeleton is required for activation of latent TGF-β1 by stretching, we preextracted Mfs with TX-100 or DOC (Fig. 5 D). In Mf cytoskeletons, 3% stretching increased active TGF-β1 in the supernatant by fivefold compared with nonstretched controls (Fig. 5 D, TX-100). In contrast, stretch failed to activate latent TGF-β1 from Mf-derived ECM after treatment with DOC (Fig. 5 D, DOC). Subsequent heat activation of stretched Mf-derived ECM to 80°C always confirmed the presence of ECM-bound latent TGF-β1 after DOC extraction (unpublished data). Hence, mechanical activation of TGF-β1 from the ECM requires the resistance of functional stress fibers.
Activation of latent TGF-β1 by Mf contraction requires mechano-resistant ECM
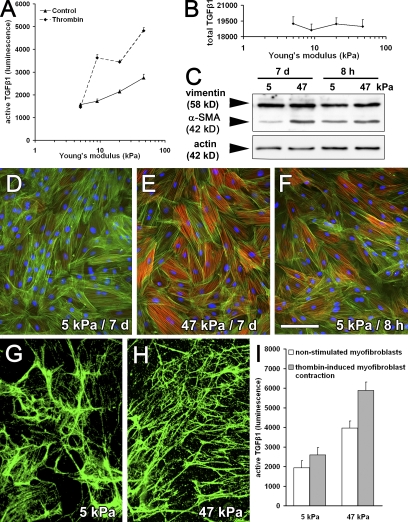
Mf differentiation and function depend on a threshold of ECM stiffness (Arora et al., 1999; Goffin et al., 2006). To investigate whether the mechanical properties of the ECM modulate the level of contraction-activated latent TGF-β1, we assessed latent TGF-β1 activation by Mfs grown on silicone culture substrates with tunable Young's modulus (5–47 kPa) in direct co-culture with TMLC (Fig. 6 A). Basal TGF-β1 activation increased by ∼1.5-fold between 5-kPa soft substrates that mimic the compliance of a normal dermis and substrates exhibiting a stiffness corresponding to that of fibrotic and late wound granulation tissue (47 kPa; Goffin et al., 2006). Induction of Mf contraction with thrombin enhanced latent TGF-β1 activation on 9–47-kPa substrates by approximately twofold but had no effect on 5-kPa soft substrates (Fig. 6 A). Substrate compliance only mildly reduced total TGF-β1 production as assessed by heating Mf-derived ECM to 80°C (Fig. 6 B). Cell numbers were comparable in all conditions and TMLC controls produced similar levels of luciferase on all substrates (unpublished data).
Figure 6.
Activation of latent TGF-β1 by Mf contraction increases with increasing ECM stiffness. (A) Lung Mfs were cultured for 7 d on silicone substrates with increasing stiffness (Young's modulus). TMLC reporter cells were added for a 4-h direct co-culture to assess active TGF-β1 with and without promoting Mf contraction using 0.5 U/ml thrombin. (B) Total TGF-β1 content was measured in the supernatant of heat-activated DOC-insoluble ECM produced from each substrate condition; values are expressed as luminescence in the TMLC reporter system. (C) Protein expression was evaluated by Western blotting normalized for vimentin expression and by immunostaining (D–F) for α-SMA (red), F-actin (green, phalloidin), and nuclei (blue) after 7 (D and E) and 8 h (F) of culture on 5-kPa compliant (D and F) and 47-kPa stiff (E) substrates. ECM was extracted with DOC from 7-d Mf cultures on compliant (G) and stiff (H) substrates and immunostained for LTBP-1. (I) Mfs were freshly seeded onto Mf-derived, DOC-insoluble ECM and cell-activated TGF-β1 was evaluated after 8 h of direct co-culture with TMLC as luminescence. Error bars represent the SD of the mean. Bar, 100 μm.
These results suggest that activation of latent TGF-β1 by Mf contraction requires a threshold ECM resistance. However, we have previously shown that incorporation of α-SMA into contractile stress fibers of rat embryonic fibroblasts is impaired by prolonged growth on soft substrates (Goffin et al., 2006), which is also observed for lung Mfs (Fig. 6, C–E). In contrast, after 8 h in culture, α-SMA expression and incorporation into lung Mf stress fibers was comparable between 5-kPa soft (Fig. 6, C and F) and 47-kPa stiff substrates (Fig. 6 C, incorporation similar to E). To obtain both sufficient amounts of ECM-stored TGF-β1 and cells incorporating α-SMA into stress fibers, Mfs were first precultured for 7 d on silicone substrates and then extracted with DOC. Next, fresh Mfs were seeded onto the Mf-derived ECM for 8 h and latent TGF-β1 activation was assessed. Substrate compliance did not alter LTBP-1 expression, as shown by confocal immunofluorescence (Fig. 6, G and H), and did not change total TGF-β1 in the ECM (Fig. 6 B). Importantly, even under these conditions that preserved α-SMA–positive stress fibers, activation of latent TGF-β1 by thrombin-induced Mf contraction was reduced on soft versus stiffer substrates (Fig. 6 I). Thus, to promote activation of latent TGF-β1 by Mf contraction, the LLC needs to be part of an ECM that resists the cell-generated mechanical stress.
Stretching of LTBP-1 fibers by Mf contraction is reduced on compliant ECM
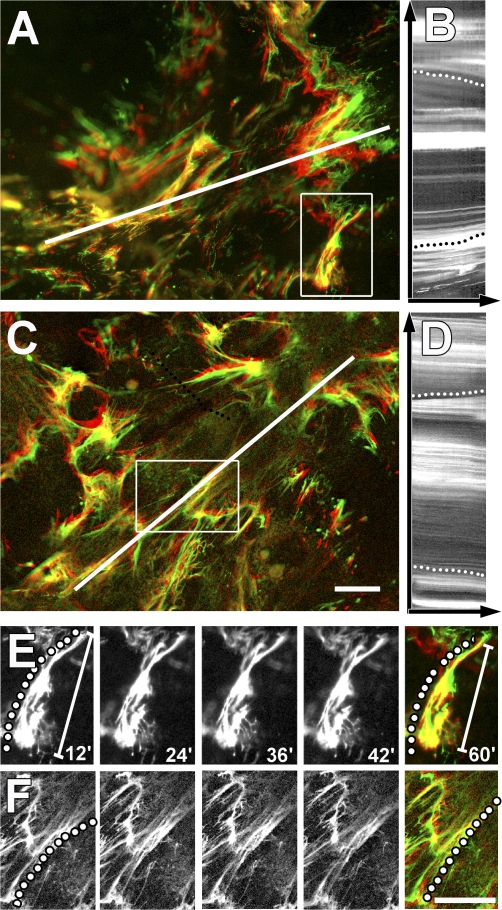
To analyze how substrate compliance affects LLC fiber organization during Mf contraction, we compared the dynamics of fluorescently labeled LTBP-1 between 5-kPa soft (Fig. 7, A, B, and E) and 47-kPa stiff (Fig. 7, C, D, and F) substrates. For this experiment, Mfs were cultured for 8 h on Mf-derived ECM. On soft ECM that provided little resistance against cell contraction, we noted significant displacement of LTBP-1 toward the center of Mfs upon contraction with thrombin (Fig. 7, A and B; and Video 3, available at http://www.jcb.org/cgi/content/full/jcb.200704042/DC1). Displacement is visualized by overlaying LTBP-1 position before (Fig. 7, green) and 1 h after stimulation of Mf contraction (red). Individual fiber aggregates were shortened by compression and bending (Fig. 7 E and Video 4). In contrast, on mechanically resisting ECM, little displacement was observed for the bulk ECM (Fig. 7, C and D; and Video 5). Here, LTBP-1 localization before and after cell contraction exhibited high overlap (Fig. 7 A, yellow). However, locally, individual fibers underwent significant straightening (Fig. 7 F), sometimes even leading to fiber rupture (Video 6). We concluded that contraction-mediated stretching of individual fibers against a mechano-resistant ECM anchor liberates TGF-β1.
Figure 7.
Stretching of LTBP-1 fibers by Mf contraction is reduced on compliant ECM. Mfs were grown for 8 h on 5-kPa soft (A, B, and E) and 47-kPa stiff (C, D, and F) silicone substrates provided with 7-d Mf-derived ECM. Living Mfs were incubated for 2 h with fluorescently labeled LTBP-1 antibody to follow LTBP-1 displacement by videomicroscopy. The image field includes the ECM associated with one cell. Displacement is visualized by overlaying initial LTBP-1 position (green) with LTBP-1 images taken 1 h after stimulating Mf contraction (red) with 0.5 U/ml thrombin. Dynamic deformation over 1 h was analyzed from Videos 3 and 5 (available at http://www.jcb.org/cgi/content/full/jcb.200704042/DC1) using kymographs (B and D) extracted along the white lines that span the long axis of one cell. Kymograph lines, 200 μm. Dotted lines on kymographs indicate fiber displacement toward the cell center on soft and rather static overall behavior on stiff substrates. Close-up frames taken every 12 min from selected fibers demonstrate fiber compression on soft (E, diagonal scale bars indicate the shortening of the fiber; Video 4) and stretching on stiff substrates (F, dotted line; Video 6). Bars, 25 μm.
Releasing rat wounds from mechanical tension decreases phosphorylation of Smad2/3
Finally, we evaluated the expression of Mf markers and components of the TGF-β1 signaling cascade in rat wound granulation tissue subjected to different levels of stress. In normally healing control wounds, α-SMA was neoexpressed by 9 d after wounding (Fig. S3 A, available at http://www.jcb.org/cgi/content/full/jcb.200704042/DC1). Expression of α-SMA was induced earlier and stronger by 6 d and enhanced after 9 d after wounding, when wounds were subjected to mechanical stress by splinting the edges with a plastic frame as described previously (Hinz et al., 2001b). Relaxation of prestressed wounds by removing the splint after 8 d for 2 d significantly reduced α-SMA expression levels compared with 10-d stressed wounds (Fig. S3 A). Expression of α-SMA correlated with the activation state of Smad2 and 3 in granulation tissue; phosphorylation of Smad3 and more significantly Smad2 was increased by stress and decreased by wound relaxation. However, expression of TGF-β1 RII, total Smad2, Smad3, and LAP was not altered by stress (Fig. S3 A). Similarly, total levels of TGF-β1 in the granulation tissue were similar between control (Fig. S3, A and B) and stressed wounds after 6 (Fig. S3, A and compare B to C) and 9 d (Fig. S3 A) and between 10-d stress-relaxed (Fig. S3A, D) and 10-d stressed wounds (Fig. S3, A and compare D to E). In all conditions, reepithelialization was negligible (Fig. S3, B–E). This result indicates that stress induces higher activity of TGF-β1 in the granulation tissue in the absence of a prominent epithelium (i.e., αvβ6-expressing cells) without significantly altering latent TGF-β1 expression levels.
Discussion
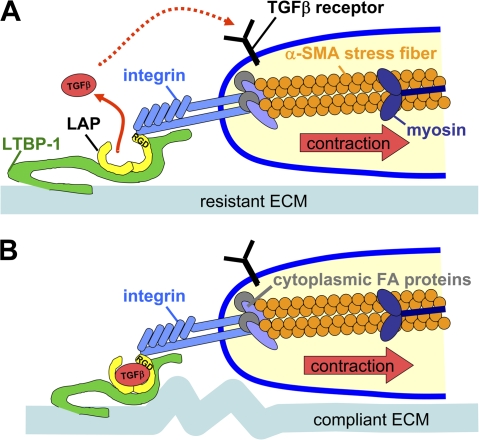
Mechanical tension in conjunction with the presence of active TGF-β1 is a prerequisite for the development of α-SMA–positive fibrogenic Mfs (Arora et al., 1999; Hinz et al., 2001b). However, a direct link between both factors has not been established. Here, we provide evidence that Mfs activate TGF-β1 from self-generated and predeposited ECM stores by a contraction-mediated mechanism. Three elements are mandatory to liberate TGF-β1 from this ECM: (1) high contractile activity generated by α-SMA–positive stress fibers; (2) stress transmission to LAP–TGF-β1 via integrins; and (3) integration of the LLC into a mechano-resistant ECM (Fig. 8). We propose that tension-mediated activation of TGF-β1 plays an important role in maintaining Mf differentiation in mechanically challenged environments, e.g., in the stiff ECM of fibrotic lesions (Hinz, 2007), during the contraction phase of wound healing (Goffin et al., 2006) and in the stiff stroma that surrounds epithelial tumors (Paszek and Weaver, 2004; Amatangelo et al., 2005).
Figure 8.
Model of Mf contraction-mediated TGF-β1 activation. The high contractile activity generated by α-SMA in stress fibers is transmitted at sites of integrins binding to RGD sites in the LAP protein as part of the LLC, which also includes TGF-β1 and LTBP-1. (A) When the LLC is anchored in a comparably stiff ECM, cell-mediated stress can induce allosteric changes in LTBP-1 and/or LAP conformation, leading to liberation of TGF-β1; such activated TGF-β1 possibly feeds back by binding to its receptor, which is located close by in the activating cell. (B) In the context of compliant ECM, the LLC is dragged toward the pulling cell but because of the lack of mechanical resistance, no conformation change occurs and TGF-β1 remains latent. Likewise, inhibition of high cell contraction and interaction of integrins with the LLC block mechanical activation of latent TGF-β1.
Several new findings establish Mf contraction as a novel mechanism to directly activate latent TGF-β1 from the ECM. Stimulation of Mf contraction with AT-II, ET-1, and thrombin increases the level of active TGF-β1 independently from protease activity. Thrombin was recently shown to enhance TGF-β1 activation by epithelial cells in an αvβ6 integrin–dependent manner, presumably via the receptor protease-activated receptor 1 and downstream activation of RhoA ( Jenkins et al., 2006). Moreover, adenoviral infection with a constitutively active form of RhoA, which is known to enhance actin reorganization, leads to increased TGF-β activation in β6 integrin–transfected fibroblasts (Jenkins et al., 2006). It has been suggested that subsequent cytoskeletal changes enhance cell traction without further evaluating this point (Jenkins et al., 2006). We reduced the activation of latent TGF-β1 by inhibiting Mf contractile activity generated in α-SMA–positive stress fibers. Low contractile fibroblasts were consistently less efficient in liberating active TGF-β1 from Mf-derived ECM compared with their α-SMA–positive counterparts. ATP-induced contraction of TX-100 cytoskeletons alone is sufficient to release active TGF-β1 from Mf-derived ECM independently from membrane-located secreted factors or proteases. Extracellular stress applied to Mfs and TX-100 cytoskeletons almost instantly liberates active TGF-β1 from the ECM, which indicates that neither synthesis nor secretion of TGF-β1 are involved.
One of our major findings is that the efficiency of latent TGF-β1 activation by Mf contraction increases with increasing ECM stiffness. We suggest that the transmission of Mf force to the LLC induces a conformational change in LAP that liberates active TGF-β1 (Fig. 8), which is similar to a recent model proposed for epithelial cells (Annes et al., 2003). Whereas compliant substrates appear to absorb the large deformations generated by Mf contraction and thus protect the LLC against conformational changes (Fig. 7 A), individual fibers become stretched on stiff substrates. In our experiments, stretching the ECM by 3–10% liberates active TGF-β1 from the LLC in the presence of the mechanically resistant Mf cytoskeleton; this stretch is in the same range as what has been described to reveal cryptic self-assembly sites in FN (Zhong et al., 1998) and to mediate mechanotransduction by various extracellular and cytoplasmic proteins (Giannone and Sheetz, 2006; Vogel, 2006). To allow deformation by cell traction, the LLC must be connected to an element that provides resistance to mechanical strain (Annes et al., 2004; Keski-Oja et al., 2004). Consistent with this idea, transformed fibroblasts overexpressing SLC (LAP–TGF-β1) do not induce Mf formation when implanted into mouse dermis (Campaner et al., 2006), presumably because of the lack of ECM fixation in the absence of LTBP-1.
The necessity for anchoring LTBP-1 with a resistant ECM offers the intriguing possibility that the level of ECM remodeling controls the availability of active TGF-β, e.g., during the course of wound healing. Normal dermis contains significant levels of LTBP-1 that potentially acts as a repository for the TGF-β1 LLC (Karonen et al., 1997; Raghunath et al., 1998), and the levels of total TGF-β1 are elevated during all stages of wound healing (Brunner and Blakytny, 2004). In contrast, active TGF-β1 is generally low and peaks during early inflammation as well as during the phase of wound contraction by Mfs (Yang et al., 1999). The question arises why α-SMA–negative fibroblasts, which are abundant during early wound remodeling, are not capable of activating existing latent TGF-β1 deposits from the ECM. Our in vivo data suggest that ECM stress makes the difference; wound granulation tissue between 6 and 10 d after wounding exhibits significantly higher activation of the TGF-β1 downstream targets Smad2/3 and higher expression of α-SMA in stressed compared with relaxed wounds, which indicates enhanced TGF-β1 signaling. This is achieved with similar levels of total TGF-β1. It remains to be investigated whether our concept of high Mf contraction and high ECM tension is applicable to αvβ6-mediated latent TGF-β1 activation that occurs with the low traction force generated by cultured epithelial cells. The fact that αvβ6-mediated latent TGF-β1 activation in an animal model of early lung fibrosis is augmented by high-tidal-volume ventilation, i.e., stress (Jenkins et al., 2006), seems to support the requirement for a mechanically loaded ECM in this system.
Stretching Mf-derived stiff ECM only liberated active TGF-β1 in the presence of the Mf cytoskeleton in our experiments, and depolymerization of the actin cytoskeleton completely blocked activation of latent TGF-β1 by epithelial cells (Munger et al., 1999). These results indicate that the proposed LLC conformation change requires a second, cellular anchor point. We show that integrin binding to LAP in the LLC is essential for the contraction-mediated activation process because RGD peptide competitors and the LAP–TGF-β1 decapeptide decrease contraction-mediated activation of TGF-β1. We can exclude integrin binding to other RGD sequences in the LTBP-1 because these epitopes are not present in the rat LTBP-1. Integrins αvβ5, β3, β1, and β8 are expressed in fibroblastic cells and bind to RGD in LAP (Levine et al., 2000; Lu et al., 2002; Mu et al., 2002; Ludbrook et al., 2003; Asano et al., 2005; Sheppard, 2005; Araya et al., 2006). Activation of latent TGF-β1 by binding of αvβ8 integrin to SLC involves proteolytic action of MT1–matrix metalloproteinase and does not require incorporation of LLC into the ECM (Mu et al., 2002). The generally low levels of active TGF-β1 in the culture supernatant and the activation of latent TGF-β1 by purified cytoskeletons all rule out αvβ8 integrin–assisted proteolytic release in our conditions.
In contrast to αvβ8, integrins αvβ5 and β3 are interesting candidates for latent TGF-β1 stress activation because they are involved in TGF-β1 activation by systemic sclerosis fibroblasts and their inhibition reverses the myofibroblastic phenotype by a yet undefined mechanism (Scaffidi et al., 2004; Asano et al., 2005, 2006). Function blocking of the β3 integrin in our hands moderately reduces latent TGF-β1 activation by Mf contraction. On the contrary, inhibition of αvβ5 integrin significantly reduces contraction-mediated latent TGF-β1 activation by Mfs and TX-100 cytoskeletons, which suggests that αvβ5 integrin predominantly mediates stress activation of latent TGF-β1 in Mfs.
Notably, by blocking β1 integrin in Mfs and TX-100 cytoskeletons, we also inhibit latent TGF-β1 activation in the presence of contraction agonists. Integrin α8β1 was previously shown to strongly interact with LAP–TGF-β1 (Lu et al., 2002) and is up-regulated in conjunction with Mf differentiation during heart (Bouzeghrane et al., 2004), pulmonary, and hepatic fibrosis (Levine et al., 2000). However, overexpression of α8β1 integrin in nonfibroblastic cells does not enhance levels of active TGF-β1 (Lu et al., 2002). This apparent discrepancy with our results can be explained by the low contractile potential of the transfected cells or by the involvement of another β1 integrin heterodimer that has not yet been shown to bind to LAP. Further studies along this line shall identify this candidate for contraction-mediated latent TGF-β1 activation and elucidate its respective contribution compared with αvβ5 integrin.
Until now, activation of latent TGF-β1 by cell traction has been suggested exclusively for the epithelial integrin αvβ6 (Annes et al., 2004; Yang et al., 2007), which is involved in the initiation of lung fibrosis (Jenkins et al., 2006; Munger et al., 1999). During fibrosis of epithelialized tissues, such as kidney and lung, Mfs are partly recruited through mesenchymal transition of epithelial cells (Hinz et al., 2007) involving αvβ6 integrin–mediated latent TGF-β1 activation (Kim et al., 2006). However, the evolution of pulmonary fibrosis toward end-stage fibrotic lung disease depends on the persistence of αvβ6-negative Mfs to express and activate latent TGF-β1 (Phan, 2002; Thannickal et al., 2004). Furthermore, Mfs drive fibrosis in a variety of other organs that neither exhibit a pronounced epithelium nor posses cells expressing αvβ6 integrin (Hinz et al., 2007). Our data strongly suggest that in these conditions, Mfs liberate and activate TGF-β1 from preexisting and self-generated deposits in the ECM by transmitting their high contractile force to the LLC through αvβ5 integrin and yet unidentified β1 and 3 integrins.
Materials and methods
Cell culture and reagents
Primary rat lung Mfs expressing constitutively high levels of α-SMA and low α-SMA–expressing subcutaneous fibroblasts were isolated and cultured as described previously (Hinz et al., 2001a). Subcutaneous Mf differentiation was induced with 5 ng/ml TGF-β1 (R&D Systems) for 5 d; to dedifferentiate lung Mfs, we applied 250 ng/ml of recombinant soluble TGF-β RII (provided by V. Koteliansky, Alnylam Pharmaceuticals, Cambridge, MA) for 5 d. Anti–TGF-β1 antibody (chicken, AF-101-NA; R&D Systems) was used to neutralize TGF-β1 activity (10 μg/ml). Contraction was induced with 0.5 U/ml thrombin, 100 μM ET-1, 5 μg/ml nocodazole, 10 μM AT-II, and 100 mM ATP (all obtained from Sigma-Aldrich). To inhibit contraction, we used 3 μg/ml of the Mf-specific SMA-FP (provided by C. Chaponnier, University of Geneva, Geneva, Switzerland), 100 μM blebbistatin (EMD), 10 μM cytochalasin D, and 50 nM staurosporin (both obtained from Sigma-Aldrich). Integrin binding was stimulated with 500 μM Mn2+ and antagonized using 100–500 mM RGD peptides (RGDS and control SDGR; Sigma-Aldrich). Function-blocking antibodies against β1 integrin (rabbit, AB1937; Millipore), β3 integrin (mouse clone F11; BD Biosciences), and αvβ5 integrin (mouse clone P1F6; Abcam) were added at 10 μg/ml for 3 h. Integrin binding to LAP was inhibited with 100 μg/ml of a custom-synthesized decapeptide containing the RGD sequence and flanking amino acids of rat LAP (KRRGDLSTIH). Protease activity was blocked with an inhibitor cocktail (Roche) plus 1 mM PMSF and 1 mM TAME.
Antibodies and microscopy
For immunofluorescence and Western blotting we used primary antibodies against α-SMA (mouse IgG2a and α-SM-1; provided by G. Gabbiani, University of Geneva; Skalli et al., 1986), LAP (goat, AF-246-NA; R&D Systems), LTBP-1 (rabbit, Ab39; provided by C.-H. Heldin, University of Uppsala, Uppsala, Sweden; Kanzaki et al., 1990), ED-A FN (mouse IgG1, IST-9; provided by L. Zardi, National Institute for Cancer Research, Genoa, Italy; Serini et al., 1998), TGF-β RII (rabbit, L-21; Santa Cruz Biotechnology, Inc.), vimentin (mouse IgG1, V9; Dako), vinculin (mouse IgG1, hVin-1; Sigma-Aldrich), phosphorylated and total Smad2 and 3 (Cell Signaling Technology), anti–Na+/K+-ATPase (mouse clone α6F), and anti-integrin antibodies (see Cell culture and reagents). For immunofluorescence, antibodies against integrins and LTBP-1 were added for 1 h to living cells before fixation; all other primary antibodies were added after cell fixation and permeabilization and probed with TRITC- and FITC-conjugated goat anti–mouse IgG2a (SouthernBiotech) and Alexa Fluor 488–conjugated goat anti–rabbit secondary antibodies (Invitrogen). DNA was detected with DAPI (Sigma-Aldrich) and F-actin was detected with phalloidin–Alexa 488 (Invitrogen). For Western blotting, we used HRP-conjugated secondary antibodies goat anti–mouse and goat anti–rabbit (Jackson ImmunoResearch Laboratories). LTBP-1 was digested from DOC-extracted and scraped ECM using 0.05 U/ml of human plasma plasmin (Sigma-Aldrich) overnight at 37°C and resuspended in nonreducing sample buffer.
Live microscopy was performed in F12/10% FCS with an oil immersion objective (Plan-Neofluar 63× 1.4 Ph3; Carl Zeiss, Inc.) mounted on an inverted microscope (Axiovert 135; Carl Zeiss, Inc.) with a 37°C heated CO2 incubator (Carl Zeiss, Inc.), EGFP filter set (Omega Optical) and digital charge-coupled device camera (C4742-95-12ERG; Hamamatsu). Image sequences were acquired with Openlab 3.1.2 software (PerkinElmer). Confocal images were acquired with a scanning confocal microscope (DM RXA2 with TCS SP2 AOBS head; Leica) using a 40× NA 1.25 objective (Leica). Fluorescence quantification of phospho-Smad staining in nuclei was automated with a self-developed routine in MetaMorph (Visitron Systems). In brief, nuclei were outlined by regions of interest by image thresholding and fluorescence was calculated per unit area, corrected for image background fluorescence. Mean values ± SD were calculated from 10 images per condition performed in triplicate (∼1,000 nuclei per condition). Kymographs were produced from image sequences as described previously (Hinz et al., 1999) using MetaMorph and figures were assembled with Photoshop (Adobe).
Stretchable culture membranes and tunable compliant and wrinkling culture substrates
Cells were stretched isoaxially by 3–10% in home-made culture devices with silicone membranes at the bottom (Goffin et al., 2006). Compliant culture substrates were generated and calibrated as described previously (Goffin et al., 2006) with a Young's modulus of ∼47 and 5 kPa, respectively, as determined by atomic force microscopy (Nanowizard II; JPK Instruments; Goffin et al., 2006). Wrinkling silicone substrates to visualize cell contraction were prepared as described previously (Hinz et al., 2001a). All surfaces were activated for cell attachment by covalent linking of 10 μg/ml of collagen type I (Sigma-Aldrich; Goffin et al., 2006).
Generation of Mf-derived ECM and TX-100–extracted cytoskeletons
To prepare ECM, cultures were extracted two times for 5 min with 0.5% DOC in radio immunoprecipitation assay buffer (50 mM Tris, 150 mM NaCl, and 1% Nonidet P-40) under gentle shaking (Unsold et al., 2001). Membrane- and cytosol-free cytoskeletons were produced by extracting cells two times for 15 min with TX-100–containing buffer (60 mM Pipes, 25 mM Hepes, 2 mM MgCl2, 1 mM sodium orthovanadate, 1 mM PMSF, and 0.5% TX-100, pH 6.9) under gentle shaking (Dugina et al., 2001); all steps were performed with ice-cold solutions at 4°C.
TGF-β bioassay
To quantify active TGF-β, we used TMLC to produce luciferase under control of the PAI-1 promoter in response to TGF-β (Abe et al., 1994). TMLC (50,000 cells/cm2) were grown for 4 h before being exposed to TGF-β1 in 0.1% BSA in serum-free medium. TMLC were immersed with culture-derived medium, directly co-cultured with experimental cells, or grown in a sandwich assay (Fig. 5 A). In selected experiments, the level of total TGF-β was assessed by heat-activating samples for 10 min at 80°C (Abe et al., 1994). After exposure to TGF-β1, TMLC were incubated for further 14 h and lysed, and luciferase activity was assessed by light production from a luciferin substrate (Promega) using a luminometer (Centro LB; Berthold Technologies). If not stated otherwise, all results were corrected for TMLC luciferase production in the absence of TGF-β1 (approximately five times lower than that produced by nonstimulated lung Mfs) and are presented as mean ± SD of one representative experiment performed in triplicate. Experiments were performed a minimum of five times.
Online supplemental material
Fig. S1 illustrates the evolution of tension generated by Mfs grown on silicone wrinkling substrates upon treatment with thrombin and SMA-FP. Video 1 shows the corresponding cell movements. Fig. S2 shows that 100 mM of DGR or RGD peptides preserves the integrity of cytoskeleton and focal adhesion organization. Video 2 demonstrates the contraction of TX-100–purified, Mf-derived cytoskeleton upon addition of ATP. Video 3 presents the deformation of LTBP-1 fibers in thrombin-stimulated Mf cultures grown on 5-kPa soft silicone substrate; a close-up of a single fiber is available in Video 5. The deformation of LTBP-1 fibers in thrombin-stimulated Mf cultures grown on 47-kPa hard silicone substrate is shown in Video 4; a close-up of a single fiber is available in Video 6. Fig. S3 shows that components of the TGF-β1 signaling cascade are differentially phosphorylated in rat wound granulation tissue subjected to different levels of stress. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200704042/DC1.
Supplementary Material
Acknowledgments
We acknowledge J. Smith-Clerc and D. Truan for technical assistance. We acknowledge Dr. G. Gabbiani, Dr. C. Chaponnier, C. Heldin, L. Zardi, and V. Koteliansky for providing antibodies and reagents. We acknowledge Drs. J. Hubbell and M. Mochizuki for synthesis and purification of LAP decapeptide. We are grateful to Dr. G. Gabbiani for carefully reading the manuscript.
This paper was supported by grants from the Swiss National Science Foundation (3100A0-102150/1 and 3100A0-113733/1), the Service Académique, Ecole Polytechnique Fédérale de Lausanne, and the Competence Centre for Materials Science and Technology of the Eidgenössische Technische Hochschule Board of Switzerland to B. Hinz; and by grants from the National Institutes of Health (AR49698 and CA34282) to D.B. Rifkin.
Abbreviations used in this paper: α-SMA, α smooth muscle actin; AT-II, angiotensin-II; DOC, deoxycholate; ET-1, endothelin-1; FN, fibronectin; LAP, latency associated protein; LLC, large latent complex; LTBP-1, latent TGF-β binding protein 1; Mf, myofibroblast; SLC, small latent complex; TGF-β RII, TGF-β receptor type II; TMLC, transformed mink lung reporter cells; TX-100, Triton X-100.
References
- Abe, M., J.G. Harpel, C.N. Metz, I. Nunes, D.J. Loskutoff, and D.B. Rifkin. 1994. An assay for transforming growth factor-beta using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal. Biochem. 216:276–284. [DOI] [PubMed] [Google Scholar]
- Amatangelo, M.D., D.E. Bassi, A.J. Klein-Szanto, and E. Cukierman. 2005. Stroma-derived three-dimensional matrices are necessary and sufficient to promote desmoplastic differentiation of normal fibroblasts. Am. J. Pathol. 167:475–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annes, J.P., J.S. Munger, and D.B. Rifkin. 2003. Making sense of latent TGFbeta activation. J. Cell Sci. 116:217–224. [DOI] [PubMed] [Google Scholar]
- Annes, J.P., Y. Chen, J.S. Munger, and D.B. Rifkin. 2004. Integrin αvβ6– mediated activation of latent TGF-β requires the latent TGF-β binding protein-1. J. Cell Biol. 165:723–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araya, J., S. Cambier, A. Morris, W. Finkbeiner, and S.L. Nishimura. 2006. Integrin-mediated transforming growth factor-beta activation regulates homeostasis of the pulmonary epithelial-mesenchymal trophic unit. Am. J. Pathol. 169:405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora, P.D., N. Narani, and C.A. McCulloch. 1999. The compliance of collagen gels regulates transforming growth factor-beta induction of alpha-smooth muscle actin in fibroblasts. Am. J. Pathol. 154:871–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano, Y., H. Ihn, K. Yamane, M. Jinnin, Y. Mimura, and K. Tamaki. 2005. Increased expression of integrin alpha(v)beta3 contributes to the establishment of autocrine TGF-beta signaling in scleroderma fibroblasts. J. Immunol. 175:7708–7718. [DOI] [PubMed] [Google Scholar]
- Asano, Y., H. Ihn, K. Yamane, M. Jinnin, and K. Tamaki. 2006. Increased expression of integrin alphavbeta5 induces the myofibroblastic differentiation of dermal fibroblasts. Am. J. Pathol. 168:499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogatkevich, G.S., E. Tourkina, C.S. Abrams, R.A. Harley, R.M. Silver, and A. Ludwicka-Bradley. 2003. Contractile activity and smooth muscle alpha- actin organization in thrombin-induced human lung myofibroblasts. Am. J. Physiol. Lung Cell. Mol. Physiol. 285:L334–L343. [DOI] [PubMed] [Google Scholar]
- Bouzeghrane, F., C. Mercure, T.L. Reudelhuber, and G. Thibault. 2004. Alpha8beta1 integrin is upregulated in myofibroblasts of fibrotic and scarring myocardium. J. Mol. Cell. Cardiol. 36:343–353. [DOI] [PubMed] [Google Scholar]
- Brunner, G., and R. Blakytny. 2004. Extracellular regulation of TGF-beta activity in wound repair: growth factor latency as a sensor mechanism for injury. Thromb. Haemost. 92:253–261. [DOI] [PubMed] [Google Scholar]
- Campaner, A.B., L.M. Ferreira, A. Gragnani, J.M. Bruder, J.L. Cusick, and J.R. Morgan. 2006. Upregulation of TGF-beta1 expression may be necessary but is not sufficient for excessive scarring. J. Invest. Dermatol. 126:1168–1176. [DOI] [PubMed] [Google Scholar]
- Dugina, V., L. Fontao, C. Chaponnier, J. Vasiliev, and G. Gabbiani. 2001. Focal adhesion features during myofibroblastic differentiation are controlled by intracellular and extracellular factors. J. Cell Sci. 114:3285–3296. [DOI] [PubMed] [Google Scholar]
- Gabbiani, G. 2003. The myofibroblast in wound healing and fibrocontractive diseases. J. Pathol. 200:500–503. [DOI] [PubMed] [Google Scholar]
- Ge, G., and D.S. Greenspan. 2006. BMP1 controls TGFβ1 activation via cleavage of latent TGFβ-binding protein. J. Cell Biol. 175:111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannone, G., and M.P. Sheetz. 2006. Substrate rigidity and force define form through tyrosine phosphatase and kinase pathways. Trends Cell Biol. 16:213–223. [DOI] [PubMed] [Google Scholar]
- Goffin, J.M., P. Pittet, G. Csucs, J.W. Lussi, J.J. Meister, and B. Hinz. 2006. Focal adhesion size controls tension-dependent recruitment of α-smooth muscle actin to stress fibers. J. Cell Biol. 172:259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin, C.H., K. Miyazono, and P. ten Dijke. 1997. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 390:465–471. [DOI] [PubMed] [Google Scholar]
- Hinz, B. 2007. Formation and function of the myofibroblast during tissue repair. J. Invest. Dermatol. 127:526–537. [DOI] [PubMed] [Google Scholar]
- Hinz, B., W. Alt, C. Johnen, V. Herzog, and H.W. Kaiser. 1999. Quantifying lamella dynamics of cultured cells by SACED, a new computer-assisted motion analysis. Exp. Cell Res. 251:234–243. [DOI] [PubMed] [Google Scholar]
- Hinz, B., G. Celetta, J.J. Tomasek, G. Gabbiani, and C. Chaponnier. 2001. a. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol. Biol. Cell. 12:2730–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz, B., D. Mastrangelo, C.E. Iselin, C. Chaponnier, and G. Gabbiani. 2001. b. Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am. J. Pathol. 159:1009–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz, B., G. Gabbiani, and C. Chaponnier. 2002. The NH2-terminal peptide of α–smooth muscle actin inhibits force generation by the myofibroblast in vitro and in vivo. J. Cell Biol. 157:657–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz, B., V. Dugina, C. Ballestrem, B. Wehrle-Haller, and C. Chaponnier. 2003. Alpha-smooth muscle actin is crucial for focal adhesion maturation in myofibroblasts. Mol. Biol. Cell. 14:2508–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz, B., S.H. Phan, V.J. Thannickal, A. Galli, M.L. Bochaton-Piallat, and G. Gabbiani. 2007. The myofibroblast: one function, multiple origins. Am. J. Pathol. 170:1807–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyytiainen, M., C. Penttinen, and J. Keski-Oja. 2004. Latent TGF-beta binding proteins: extracellular matrix association and roles in TGF-beta activation. Crit. Rev. Clin. Lab. Sci. 41:233–264. [DOI] [PubMed] [Google Scholar]
- Jenkins, R.G., X. Su, G. Su, C.J. Scotton, E. Camerer, G.J. Laurent, G.E. Davis, R.C. Chambers, M.A. Matthay, and D. Sheppard. 2006. Ligation of protease-activated receptor 1 enhances alpha(v)beta6 integrin-dependent TGF-beta activation and promotes acute lung injury. J. Clin. Invest. 116:1606–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzaki, T., A. Olofsson, A. Moren, C. Wernstedt, U. Hellman, K. Miyazono, L. Claesson-Welsh, and C.H. Heldin. 1990. TGF-beta 1 binding protein: a component of the large latent complex of TGF-beta 1 with multiple repeat sequences. Cell. 61:1051–1061. [DOI] [PubMed] [Google Scholar]
- Karonen, T., L. Jeskanen, and J. Keski-Oja. 1997. Transforming growth factor beta 1 and its latent form binding protein-1 associate with elastic fibres in human dermis: accumulation in actinic damage and absence in anetoderma. Br. J. Dermatol. 137:51–58. [PubMed] [Google Scholar]
- Keski-Oja, J., K. Koli, and H. von Melchner. 2004. TGF-beta activation by traction? Trends Cell Biol. 14:657–659. [DOI] [PubMed] [Google Scholar]
- Kim, K.K., M.C. Kugler, P.J. Wolters, L. Robillard, M.G. Galvez, A.N. Brumwell, D. Sheppard, and H.A. Chapman. 2006. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc. Natl. Acad. Sci. USA. 103:13180–13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koli, K., M. Hyytiainen, M.J. Ryynanen, and J. Keski-Oja. 2005. Sequential deposition of latent TGF-beta binding proteins (LTBPs) during formation of the extracellular matrix in human lung fibroblasts. Exp. Cell Res. 310:370–382. [DOI] [PubMed] [Google Scholar]
- Levine, D., D.C. Rockey, T.A. Milner, J.M. Breuss, J.T. Fallon, and L.M. Schnapp. 2000. Expression of the integrin alpha8beta1 during pulmonary and hepatic fibrosis. Am. J. Pathol. 156:1927–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, M., J.S. Munger, M. Steadele, C. Busald, M. Tellier, and L.M. Schnapp. 2002. Integrin alpha8beta1 mediates adhesion to LAP-TGFbeta1. J. Cell Sci. 115:4641–4648. [DOI] [PubMed] [Google Scholar]
- Ludbrook, S.B., S.T. Barry, C.J. Delves, and C.M. Horgan. 2003. The integrin alphavbeta3 is a receptor for the latency-associated peptides of transforming growth factors beta1 and beta3. Biochem. J. 369:311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazono, K., A. Olofsson, P. Colosetti, and C.H. Heldin. 1991. A role of the latent TGF-beta 1-binding protein in the assembly and secretion of TGF-beta 1. EMBO J. 10:1091–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu, D., S. Cambier, L. Fjellbirkeland, J.L. Baron, J.S. Munger, H. Kawakatsu, D. Sheppard, V.C. Broaddus, and S.L. Nishimura. 2002. The integrin αvβ8 mediates epithelial homeostasis through MT1-MMP–dependent activation of TGF-β1. J. Cell Biol. 157:493–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger, J.S., J.G. Harpel, F.G. Giancotti, and D.B. Rifkin. 1998. Interactions between growth factors and integrins: latent forms of transforming growth factor-beta are ligands for the integrin alphavbeta1. Mol. Biol. Cell. 9:2627–2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger, J.S., X. Huang, H. Kawakatsu, M.J. Griffiths, S.L. Dalton, J. Wu, J.F. Pittet, N. Kaminski, C. Garat, M.A. Matthay, et al. 1999. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 96:319–328. [DOI] [PubMed] [Google Scholar]
- Nunes, I., P.E. Gleizes, C.N. Metz, and D.B. Rifkin. 1997. Latent transforming growth factor-β binding protein domains involved in activation and transglutaminase-dependent cross-linking of latent transforming growth factor-β. J. Cell Biol. 136:1151–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszek, M.J., and V.M. Weaver. 2004. The tension mounts: mechanics meets morphogenesis and malignancy. J. Mammary Gland Biol. Neoplasia. 9:325–342. [DOI] [PubMed] [Google Scholar]
- Phan, S.H. 2002. The myofibroblast in pulmonary fibrosis. Chest. 122:286S–289S. [DOI] [PubMed] [Google Scholar]
- Raghunath, M., C. Unsold, U. Kubitscheck, L. Bruckner-Tuderman, R. Peters, and M. Meuli. 1998. The cutaneous microfibrillar apparatus contains latent transforming growth factor-beta binding protein-1 (LTBP-1) and is a repository for latent TGF-beta1. J. Invest. Dermatol. 111:559–564. [DOI] [PubMed] [Google Scholar]
- Scaffidi, A.K., N. Petrovic, Y.P. Moodley, M. Fogel-Petrovic, K.M. Kroeger, R.M. Seeber, K.A. Eidne, P.J. Thompson, and D.A. Knight. 2004. alpha(v)beta(3) integrin interacts with the transforming growth factor beta (TGFbeta) type II receptor to potentiate the proliferative effects of TGFbeta1 in living human lung fibroblasts. J. Biol. Chem. 279:37726–37733. [DOI] [PubMed] [Google Scholar]
- Serini, G., M.L. Bochaton-Piallat, P. Ropraz, A. Geinoz, L. Borsi, L. Zardi, and G. Gabbiani. 1998. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-β1. J. Cell Biol. 142:873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard, D. 2005. Integrin-mediated activation of latent transforming growth factor beta. Cancer Metastasis Rev. 24:395–402. [DOI] [PubMed] [Google Scholar]
- Skalli, O., P. Ropraz, A. Trzeciak, G. Benzonana, D. Gillessen, and G. Gabbiani. 1986. A monoclonal antibody against α-smooth muscle actin: a new probe for smooth muscle differentiation. J. Cell Biol. 103:2787–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipale, J., K. Koli, and J. Keski-Oja. 1992. Release of transforming growth factor-beta 1 from the pericellular matrix of cultured fibroblasts and fibrosarcoma cells by plasmin and thrombin. J. Biol. Chem. 267:25378–25384. [PubMed] [Google Scholar]
- Thannickal, V.J., G.B. Toews, E.S. White, J.P. Lynch III, and F.J. Martinez. 2004. Mechanisms of pulmonary fibrosis. Annu. Rev. Med. 55:395–417. [DOI] [PubMed] [Google Scholar]
- Tomasek, J.J., G. Gabbiani, B. Hinz, C. Chaponnier, and R.A. Brown. 2002. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 3:349–363. [DOI] [PubMed] [Google Scholar]
- Unsold, C., M. Hyytiainen, L. Bruckner-Tuderman, and J. Keski-Oja. 2001. Latent TGF-beta binding protein LTBP-1 contains three potential extracellular matrix interacting domains. J. Cell Sci. 114:187–197. [DOI] [PubMed] [Google Scholar]
- Vogel, V. 2006. Mechanotransduction involving multimodular proteins: converting force into biochemical signals. Annu. Rev. Biophys. Biomol. Struct. 35:459–488. [DOI] [PubMed] [Google Scholar]
- Yang, L., C.X. Qiu, A. Ludlow, M.W. Ferguson, and G. Brunner. 1999. Active transforming growth factor-beta in wound repair: determination using a new assay. Am. J. Pathol. 154:105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Z., Z. Mu, B. Dabovic, V. Jurukovski, D. Yu, J. Sung, X. Xiong, and J.S. Munger. 2007. Absence of integrin-mediated TGFβ1 activation in vivo recapitulates the phenotype of TGFβ1-null mice. J. Cell Biol. 176:787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, C., M. Chrzanowska-Wodnicka, J. Brown, A. Shaub, A.M. Belkin, and K. Burridge. 1998. Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J. Cell Biol. 141:539–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.